- 1Department of Reproductive Medicine Center, The Affiliated Guangdong Second Provincial General Hospital of Jinan University, Guangzhou, China
- 2Department of Gynecology, The Affiliated Guangdong Second Provincial General Hospital of Jinan University, Guangzhou, China
- 3The Second School of Clinical Medicine, Southern Medical University, Guangzhou, China
- 4Research Institute for Maternal and Child Health, The Affiliated Guangdong Second Provincial General Hospital of Jinan University, Guangzhou, China
Background: Obesity and metabolic syndrome are significant contributors to infertility in women and are closely associated with insulin resistance (IR). The metabolic score for insulin resistance (METS-IR) is a new, non-insulin-based fasting index used to measure IR. However, the potential of METS-IR as a predictive indicator of female infertility risk has not been established. This study aimed to explore the association between METS-IR and the risk of female infertility.
Methods: This cross-sectional study used data from the National Health and Nutrition Examination Survey (NHANES) from 2013 to 2018. We conducted multivariate logistic regression, restricted cubic spline (RCS), and threshold effect analyses to investigate the relationship between METS-IR and female infertility.
Results: According to the self-reported data, 188 (12.20%) participants were classified as infertile. A significantly higher proportion of participants with elevated METS-IR were found to have infertility. Multivariable logistic regression analysis revealed that METS-IR was significantly associated with increased risk of female infertility, irrespective of the independent variable analysis by continuous variables or tertiles in the fully adjusted model (Model 3, continuous variable: OR = 1.02, 95% confidence interval (CI):1.01–1.04, p = 0.005; tertile 3 vs. tertile 1: OR = 2.00, 95% CI = 1.21–3.28, p = 0.0128, p for trend =0.0126). RCS analysis indicated a linear correlation between METS-IR and the risk of infertility (p = 0.121), and threshold effect analysis further supported this linear association (p = 0.136). Moreover, above the inflection point of 32.94, the risk of infertility significantly increased with increasing METS-IR level (p < 0.0001).
Conclusion: Our results suggest that high levels of the METS-IR index are positively associated with infertility among reproductive-aged females in the United States.
Introduction
Infertility is defined as the inability of women to conceive after at least 1 year of regular unprotected sexual intercourse (1, 2). It represents a state of reduced fertility that affects both male and female reproductive health and has become a significant global public health issue (1). Epidemiological studies have shown that approximately 8–12% of couples of reproductive age face challenges in conceiving, with a significant proportion of cases attributed to female-specific factors, presenting major challenges to the field of reproductive health (3). Infertility has emerged as a critical issue that affects global human development. Hence, the Centers for Disease Control and Prevention (CDC) in the United States recommends prioritizing the diagnosis and treatment of infertility (4).
Epidemiological studies have suggested that infertility is a reproductive disorder caused by multiple factors, with advanced age being one of the most significant adverse factors affecting female fertility (1). Moreover, lifestyle factors, including diet, physical activity, psychological stress, smoking, alcohol consumption, and environmental exposures, such as radiation and chemical agents, are increasingly identified as significant contributors to infertility (5–8). Additionally, abnormalities in glucose levels and metabolic disorders, including obesity and metabolic syndrome, are frequently observed in infertile women (9–12). Insulin resistance (IR) reduces the sensitivity of muscle and fatty tissues to insulin and diminishes the sensitivity of the liver to suppress hepatic glucose production and output (13). IR can cause endocrine disturbances that affect follicular development, oocyte quality, and ovulatory patterns. These findings highlight the critical role of IR in infertility (14). IR is closely associated with polycystic ovary syndrome (PCOS), which is a common cause of infertility in women. Therefore, PCOS has garnered significant attention in the context of the association between IR and infertility. However, several studies have confirmed that IR is a distinct factor in infertile women and is potentially independent of PCOS (14–16).
The euglycemic-hyperinsulinemic clamp (EHC) is the gold standard for assessing peripheral tissue insulin sensitivity; however, its clinical application is limited owing to its complexity, invasiveness, and time requirements (17). Similarly, the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) and Quantitative Insulin Sensitivity Check Index (QUICKI) are commonly used in clinical settings. However, both methods depend on insulin measurements, making them less suitable for large-scale screening. Additionally, the techniques used for insulin assays can limit their accuracy and applicability, particularly in individuals with impaired β-cell function or those undergoing insulin therapy (18, 19). To overcome these issues, researchers have devised alternative fasting IR indices that do not depend on insulin measurements. These include the triglyceride-glucose index (TyG), triglyceride-glucose body mass index (TyG-BMI), and triglyceride to HDL-c ratio (TG/HDL-c ratio), which utilize fasting triglycerides, glucose, and lipoprotein measurements (13, 20, 21). Nevertheless, these indices, not insulin-based, have limitations in identifying IR associated with the pathophysiological aspects of metabolic syndrome (22). Metabolic syndrome is a cluster of pathological conditions characterized by IR, central obesity, high blood pressure, and elevated lipid levels. It significantly impairs female reproductive function, and metabolic disturbances can lead to abnormal regulatory mechanisms such as ovarian irregularities, hormone imbalances, and gonadal dysfunction in women, potentially increasing the likelihood of infertility (14, 23–25).
Recently, the metabolic score for insulin resistance (METS-IR) has emerged as a promising indirect method for detecting IR that correlates with the pathophysiological components of metabolic syndrome, garnering considerable interest among researchers and clinicians. METS-IR assesses IR using BMI, triglycerides, and fasting blood glucose instead of direct insulin measurements, making it ideal for large-scale screening and clinical practice (22). Numerous studies have demonstrated that METS-IR is an independent risk factor associated with several health issues, including type 2 diabetes mellitus (T2DM), kidney stones, frailty, psoriasis, and cardiovascular disease (26–30). Notably, Uysal et al. (31) identified a significant positive correlation between METS-IR and PCOS, suggesting its potential as a valuable index for comprehensive metabolic assessment of women with PCOS.
IR not only contributes to the development of various metabolic disorders but also affects female reproductive health and is significantly associated with infertility in women. To our knowledge, no studies have examined the potential association between METS-IR and female infertility. Therefore, in this cross-sectional study, we aimed to investigate the relationship between METS-IR and female infertility using a nationally representative sample of reproductive-aged women from the National Health and Nutrition Examination Survey (NHANES).
Methods
Data source
This study utilized data from NHANES, a comprehensive program that assesses the health and nutrition of the U.S. population, conducted by the NCHS. The survey implemented a sophisticated, multi-stage probability sampling strategy to ensure a representative sample of non-institutionalized individuals nationwide. Participants conducted household interviews to collect information on their health, socioeconomic conditions, and other pertinent factors. Physical examination and laboratory tests were conducted in mobile examination units.
The NCHS Ethics Review Committee conducted annual reviews and standardized the study protocols under Protocol #2011–17. All participants provided informed consent prior to data collection. Further information can be found at http://www.cdc.gov/nchs/nhanes/index.htm. Given that the NHANES data are publicly available, this study was exempt from the ethical approval and informed consent requirements. This cross-sectional study complied with the guidelines for improved reporting of epidemiological observational studies (32).
Study population
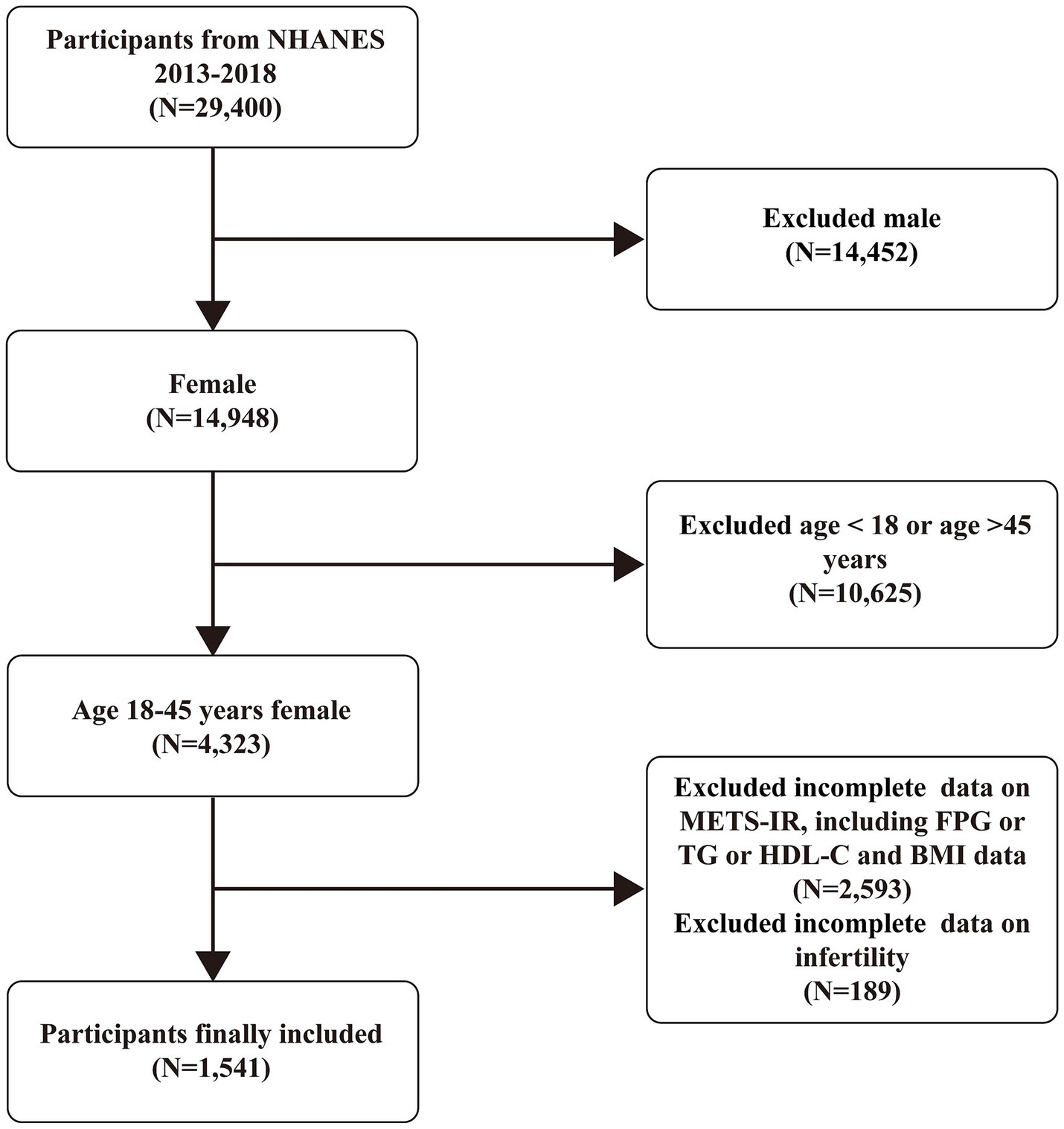
This study analyzed data on infertility-related health issues from NHANES cycles conducted between 2013 and 2018. The analysis included participants with complete information on infertility and the METS-IR scores. Initially, 29,400 participants were included in this study. However, we excluded male participants (14,452), those aged over 45 or under 18 years (10,625), and those with missing data on METS-IR (2,593) or infertility (189). The final analysis included 1,541 eligible participants (Figure 1).
Definitions of METS-IR and infertility
METS-IR was calculated as ln [(2 × fasting plasma glucose (FPG) (mg/dL) + fasting triglyceride (TG) (mg/dL)] × body mass index (BMI) (kg/m2))/ ln [high-density lipoprotein cholesterol (HDL-c) (mg/dL)] (22). All participants were categorized into three groups based on the tertiles of METS-IR: Tertile1 (19.73–32.78), Tertile2 (32.78–45.97), and Tertile3 (45.97–107.00). Quality control for laboratory tests is available at https://wwwn.cdc.gov/nchs/nhanes/Default.aspx. Infertility, the dependent variable, was assessed using questions from the Reproductive Health Questionnaire: “Have you ever attempted to become pregnant for at least 1 year without success?” (RHQ074) and “Have you ever consulted a doctor or other medical provider due to an inability to become pregnant?” (RHQ076). Participants who responded “yes” to either question were categorized as having infertility; those who responded “no” were categorized as not having infertility.
Covariates
The covariates included in the study were age, race, marital status, education level, poverty-income ratio (PIR), alcohol consumption status (yes/no), hypertension (yes/no), diabetes (yes/no), dyslipidemia (yes/no), age at menarche, history of pelvic infection or pelvic inflammatory disease (PID) (yes/no), contraceptive pill use (yes/no), female hormone use (yes/no), and smoking status (yes/no). For detailed information on the collection of these covariates, please refer to the official NHANES website.
Statistical analysis
All statistical analyses were conducted considering the complex, multistage clustered sampling design and appropriate NHANES sampling weights, according to the CDC guidelines. In the descriptive analysis, we compared the baseline characteristics of the participants based on their infertility status and METS-IR tertiles. Continuous variables are presented as means with 95% confidence intervals (CIs), while categorical variables are presented as percentages with 95% CIs. The baseline characteristics of the study population were assessed using weighted linear regression and chi-squared tests.
To handle missing data, continuous variables were imputed using either medians or means based on their distribution, and categorical variables were imputed using mode. We then applied weighted multivariate logistic regression models adjusted for known or potential confounders to explore the relationship between METS-IR and infertility risk. The potential nonlinear relationships between METS-IR and infertility were further evaluated using restricted cubic spline (RCS) curves based on multivariate logistic regression and threshold effect analysis. A recursive algorithm was employed to identify the inflection points, and a two-segment linear regression model was applied on either side of these inflection points. Additionally, subgroup analyses were conducted to examine the relationship between METS-IR and infertility according to age, BMI, educational level, smoking status, drinking status, hypertension, dyslipidemia, and diabetes. Statistical analyses were performed using R software, EmpowerStats, and Free Statistics. Statistical significance was set at p < 0.05.
Results
Baseline characteristics
In total, 29,400 participants were included in this study (Figure 1). Supplementary Table S1 compares the baseline characteristics of the infertile and non-infertile participants. Among them, 188 (12.20%) were categorized as infertile. Compared to non-infertile individuals, those in the infertile group were more likely to be older, consume alcohol, and have conditions such as hypertension, dyslipidemia, and diabetes. Additionally, there were differences in marital status, history of PID, use of contraceptive pills, and body mass index (BMI) between the two groups (all p < 0.05). Notably, the infertility group showed significantly higher METS-IR than the non-infertility group (46.87 vs. 41.22, p = 0.0005). This finding indicates that METS-IR may serve as a potential predictor of female infertility risk.
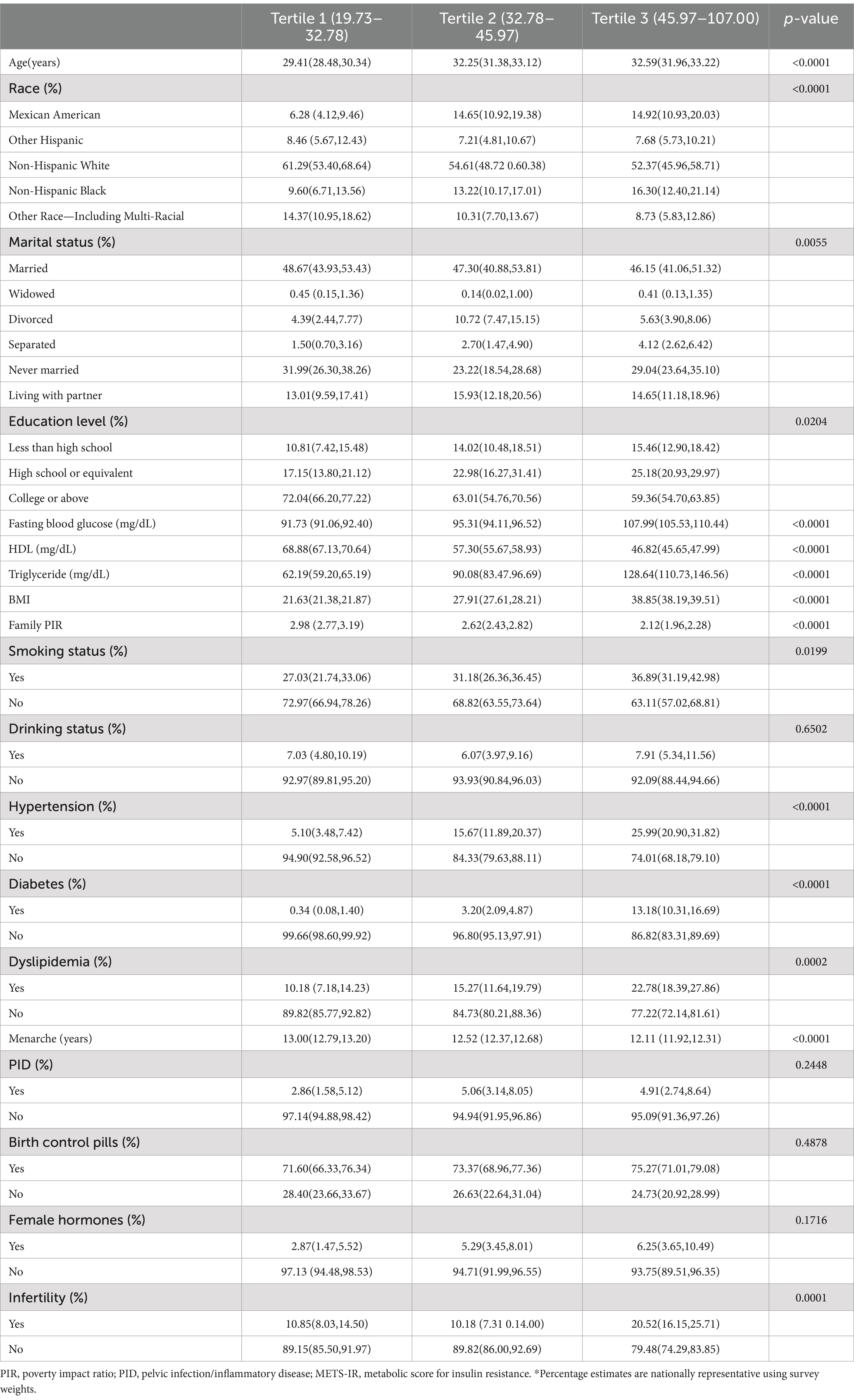
Subsequently, the study examined the clinical features of the participants by dividing them into tertiles based on their METS-IR scores. Compared with individuals in the lower METS-IR tertiles, those in the higher group were more likely to be older, of Mexican American descent, Non-Hispanic Black, and exhibit lower levels of education, HDL cholesterol, PIR, age at menarche, and higher levels of fasting blood glucose, triglycerides, BMI, smoking, hypertension, diabetes, and dyslipidemia (all p < 0.05, Table 1). Additionally, the percentage of participants experiencing infertility increased significantly from low to high (p = 0.0001; Table 1), with a notably higher rate in tertile 3 (20.52%) than in tertile1 and tertile 2 (10.85 and 10.18%, respectively). These differences indicate that the potential connection between METS-IR levels and infertility merits further investigation.
Association between METS-IR and infertility
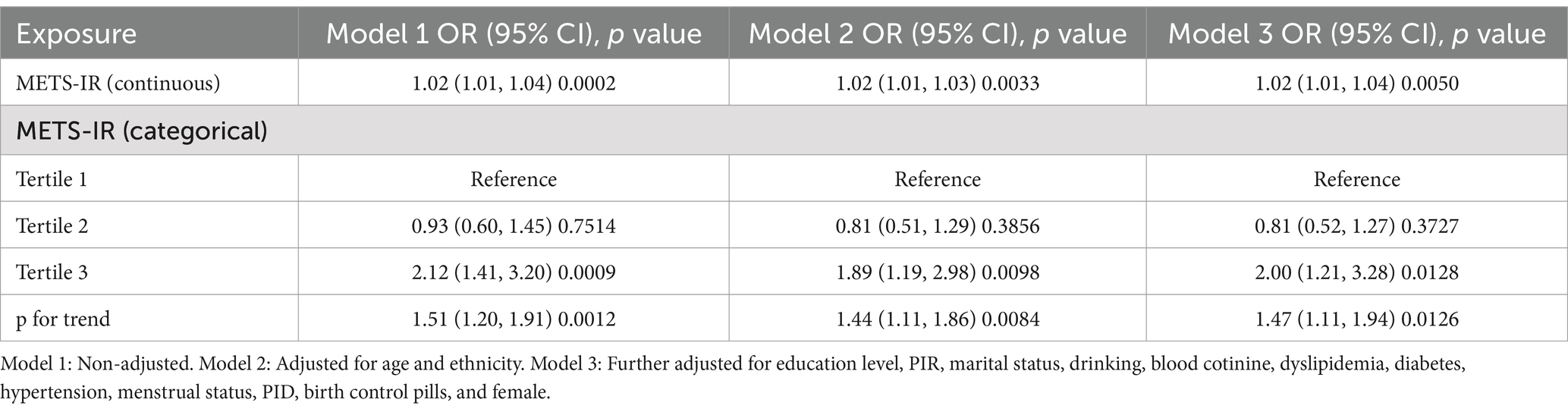
A weighted logistic regression analysis explored the relationship between METS-IR levels and female infertility. Across all three statistical models, the analysis consistently showed a positive association between METS-IR levels and infertility risk. In the fully adjusted model, each unit increase in METS-IR was associated with a 2% increase in the risk of infertility (Model 3: OR = 1.02, 95% CI: 1.01–1.04, p = 0.0050; Table 2). However, when METS-IR levels were categorized into tertiles, the risk of infertility in the highest tertile was significantly higher than that in the lowest tertile in Model 3, with a 100% increase in the risk of infertility (OR = 2.0, 95% CI: 1.21–3.28, p = 0.0128; Table 2). Additionally, trend tests across all models showed statistical significance, further supporting the significant association between higher METS-IR levels and an increased risk of infertility.
RCS and threshold analysis of the METS-IR and infertility correlation
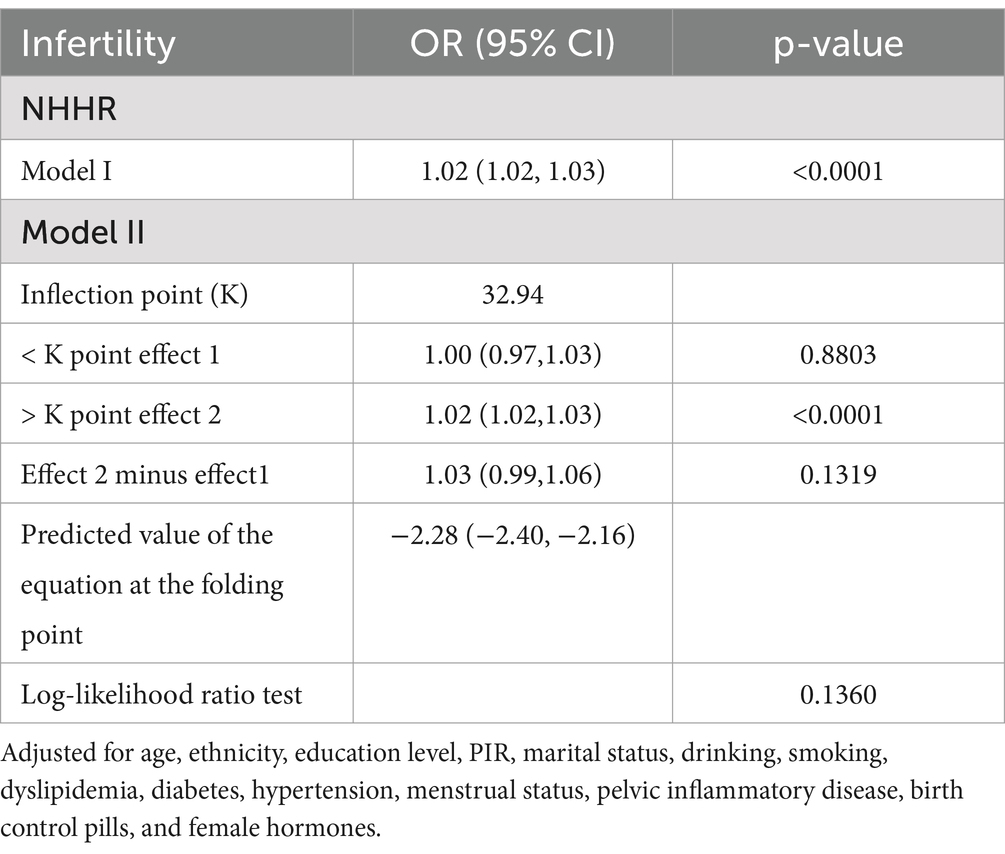
To better understand the relationship between METS-IR and infertility, we conducted RCS analysis using Model 3. The results indicated a positive linear relationship and dose–response effect between METS-IR and risk of infertility (p for nonlinearity = 0.121, Figure 2). In addition, we performed a threshold effect analysis using a weighted two-stage linear regression model and regression algorithm to analyze this relationship in more detail. The results showed that the calculated inflection point was 32.94, and the log-likelihood ratio test p-value was 0.136, indicating that there was indeed a linear relationship between METS-IR and infertility risk. Interestingly, the results also showed that above this threshold, the risk of infertility increased by 2% for every unit increase in METS-IR values (>32.94: OR = 1.02, 95% CI: 1.02–1.03, p < 0.0001; Table 3).
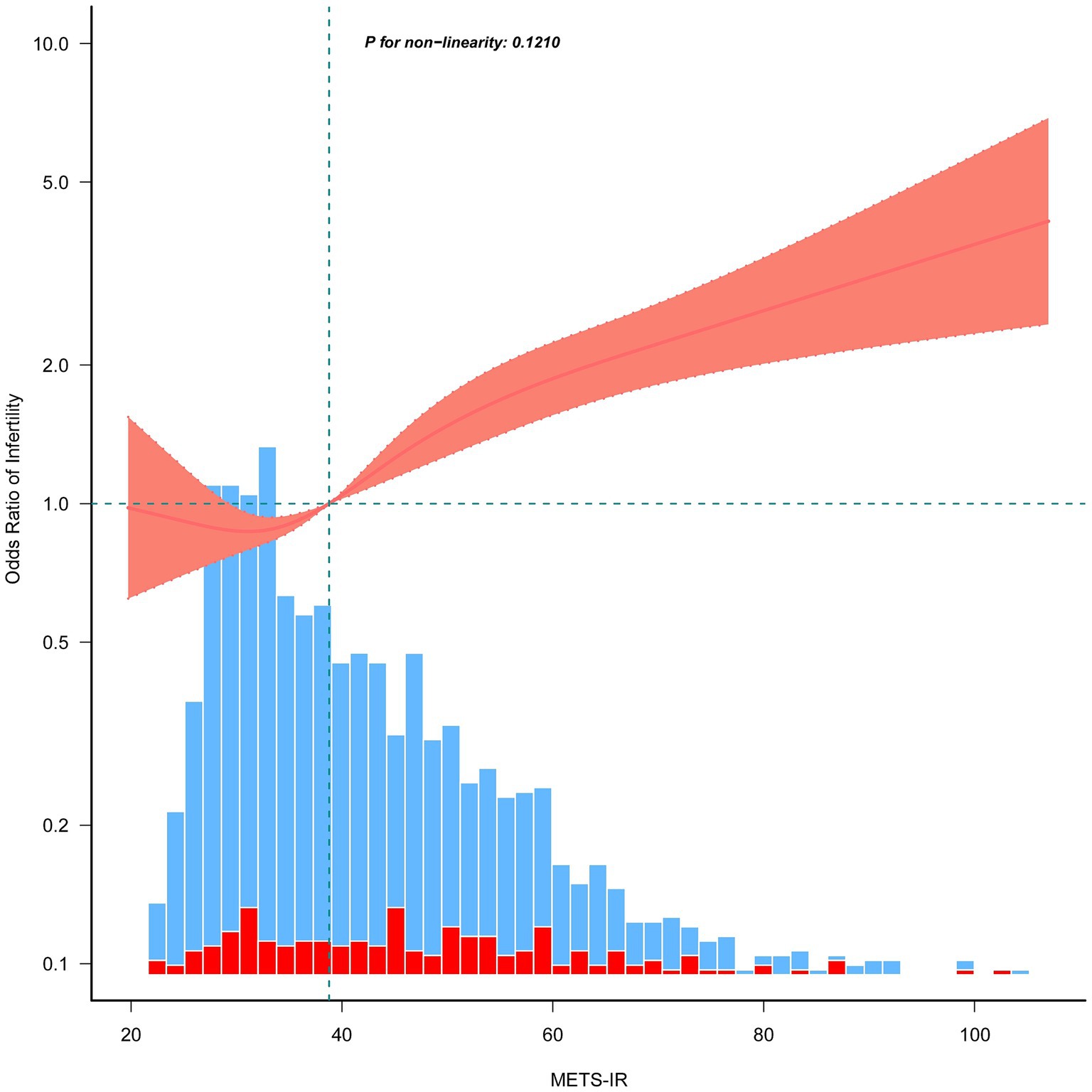
Figure 2. Restricted cubic spline plots for the association between METS-IR and female infertility after covariate adjustment. The red and blue histograms illustrate the percentage distributions of infertility and non-infertility cases within the study group at the corresponding METS-IR values. Thick lines in the center depict the calculated adjusted odds ratios, while the surrounding shaded areas indicate 95% confidence intervals. These calculations were adjusted in accordance with Model 3, as presented in Table 2.
Subgroup analysis
Subsequently, a subgroup analysis with interaction testing was conducted to assess whether the relationship between METS-IR and infertility risk remained consistent across the different demographic groups. In Model 3, all covariates were included in the analysis, except those used to define the subgroups. The results showed a significant interaction between METS-IR and age as well as dyslipidemia (p for interaction <0.05), indicating a potential modifying effect of these factors on the association. However, no significant interaction was found between METS-IR and BMI, education level, alcohol consumption, smoking status, hypertension, or diabetes (Figure 3).
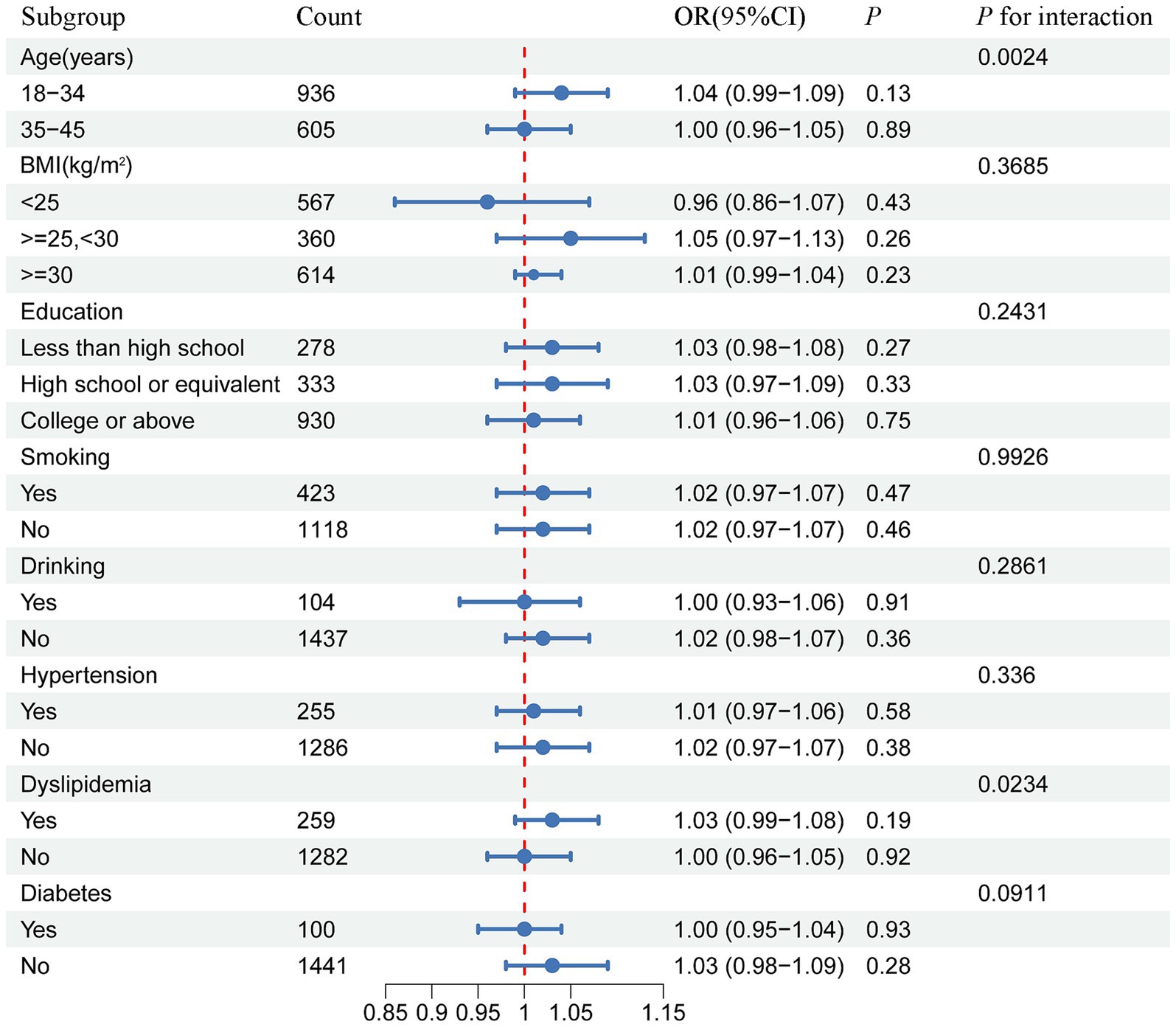
Figure 3. Forest plot of the stratified analysis and interaction effects on the association between METS-IR and female infertility.
Discussion
This large-scale cross-sectional study demonstrated that, after adjusting for potential confounding factors, METS-IR was positively associated with the risk of infertility. RCS and threshold effect analyses revealed a positive linear correlation between the METS-IR scores and infertility risk. Subgroup and interaction analyses showed that this association was influenced by age and dyslipidemia.
Obesity is closely linked to glucose intolerance and IR, both of which can negatively affect fertility and pregnancy outcomes (33). An increasing number of studies have indicated that IR and glucose intolerance are common underlying factors of obesity and PCOS. Additionally, PCOS exhibits several metabolic abnormalities that are commonly associated with obesity-related metabolic syndromes. Notably, IR is widely recognized as a key pathophysiological factor contributing to infertility in women with or without PCOS (33–35). A prospective cohort study conducted in China demonstrated that IR is associated with a reduced proportion of mature oocytes and lower embryo quality in lean infertile women without PCOS (16). Similarly, a retrospective analysis by Song et al., which involved 329 women undergoing in vitro fertilization, found that elevated Homeostasis Model Assessment of Insulin Resistance (HOMA-IR, an index of insulin resistance) and BMI significantly reduced clinical pregnancy rates, regardless of whether the women had PCOS (36).
Female reproductive function is negatively affected by IR through several pathways. One key mechanism involves the deterioration of oocyte quality due to IR disruption of the mitochondrial processes. Mitochondria play a crucial role as the main energy provider and a significant source of reactive oxygen species (ROS) in the oocyte cytoplasm, which is intimately connected to oocyte quality. OU et al. used a mouse model and revealed that maternal IR increased oxidative stress and disrupted mitochondrial function in mouse oocytes (37). Mitochondrial dysfunction leads to excessive ROS production, which triggers the release of inflammatory cytokines, such as TNF-α, interleukin-1β, and interleukin-6, further damaging pancreatic β-cell function and exacerbating IR. This creates a vicious cycle involving IR, mitochondrial damage, and inflammation (38). Moreover, IR disrupts energy metabolism in oocytes. Glucose transporter type 4 (GLUT4), which is essential for supplying energy to cells, shows reduced expression in patients with PCOS and IR. This reduction leads to decreased glucose uptake and utilization by granulosa cells, ultimately compromising the oocyte quality (39, 40). Interestingly, Wu et al. demonstrated that knockdown of insulin receptor (INSR) expression in ovarian membrane cells resulted in lower androgen levels and improved fertility in mice (41). Furthermore, IR negatively affects oocyte quality and reduces endometrial receptivity through several mechanisms. These mechanisms include disrupted energy metabolism, altered AMP-activated protein kinase (AMPK) signaling, impaired insulin receptor substrate (IRS)/PI3K/Akt pathways, and ongoing inflammation. Together, these factors contribute to a decline in female reproductive function (42–44).
The EHC is considered the gold standard for assessing metabolic IR in vivo. This method measures the impact of insulin on systemic glucose uptake by administering precise insulin infusions and adjusting the glucose infusion rates to maintain normal blood glucose levels. Frequent arterialized glucose measurements and feedback mechanisms are employed throughout this process (45, 46). Owing to the complexity and cost of EHC, there is an increasing need for easily accessible fasting glucose homeostasis parameters as an alternative method for diagnosing IR. Many studies have identified METS-IR as a reliable and practical biomarker for IR, making it a proposed tool for identifying individuals at high risk of T2DM and cardiovascular diseases (CVDs) at an early stage (22, 47). Recently, Xie et al. (15) studied the relationship between various IR surrogates (HOMA-IR, TyG, and TyG-BMI indices) and female infertility. They discovered that higher TyG-BMI levels were positively associated with infertility among reproductive-aged females in the U.S. However, the relationship between METS-IR and infertility remains unclear.
This study explored the relationship between METS-IR and female infertility using data from NHANES. Our findings indicate that elevated METS-IR levels are associated with an increased likelihood of infertility in women aged 18–45 years. Descriptive analysis showed that the mean METS-IR values were significantly higher in the infertility group than in the non-infertility group (Supplementary Table S1), suggesting that METS-IR could predict fertility status. When we categorized the participants into METS-IR tertiles, we observed a noticeable increase in the mean fasting blood glucose and infertility percentages from tertiles 1 to 3 (Table 1). Additionally, we found that the infertility percentage increased with increasing METS-IR among Mexican Americans and Non-Hispanic Black individuals (Table 1). Furthermore, we investigated the METS-IR and infertility relationships using weighted multivariate logistic regression models. As expected, viewing METS-IR as a categorical variable was a more informative independent risk factor for infertility than treating it as a continuous variable (Table 2). Subsequent RCS analysis and threshold effect analysis revealed a linear positive association between METS-IR and infertility (Figure 2 and Table 3). Significantly, the risk of infertility increased with higher METS-IR levels, notably when exceeding the threshold of 32.94 (p < 0.0001). These results suggest that METS-IR is particularly predictive of infertility risk in cases of insulin resistance and dyslipidemia. This finding is consistent with previous research showing that women with metabolic syndrome have a negative effect on fertility (48).
Female infertility is influenced by many factors, including age, lifestyle, metabolism, genetics, psychological and occupational stress, as well as experiences of familial abuse and socioeconomic status (49–52). This study examined whether METS-IR is a potentially effective indicator of the relationship between insulin resistance and infertility. Our findings highlight the importance of the METS-IR index as an effective tool for identifying women at an increased risk of infertility. However, this study has certain limitations. First, owing to the constraints of the NHANES database, the definition of female infertility as an outcome variable relied on self-reported data. Self-reports are a commonly used measurement method but may lack precision in specific contexts. For instance, the sample may have included women who had been trying to conceive for less than a year but had already sought medical assistance. Additionally, inadequate management of reproductive health can lead to ovulatory disorders, irregular menstrual cycles, or undiagnosed metabolic conditions, such as PCOS and insulin resistance, which can increase the risk of infertility. This study relied on self-reported data, which may not have fully reflected these issues. It is essential to acknowledge that the frequency of sexual intercourse contributes significantly to female infertility. Couples may face reduced chances of conception if they do not live together, have infrequent intercourse, or engage in intercourse only during nonovulatory periods. Furthermore, women with a family history of infertility may find it more challenging to conceive than those without such a background. Additionally, varying definitions of infertility, such as those based on medical records or time-to-conception derived from calendars, can influence the estimated prevalence (53, 54). Future studies should address the potential impacts of these definitions. It is important to note that this study relied on epidemiological survey data and did not include direct clinical validation, which may have impacted the accuracy and rigor of the findings. Second, as this was a cross-sectional study, we did not include a comparison group of age- and ethnicity-matched fertile women, which prevented us from establishing causal relationships. Finally, because this study focused exclusively on the U.S. population, it remains unclear whether our findings can be generalized to other countries or ethnicities, warranting further investigation.
Conclusion
In conclusion, this study indicated a positive linear relationship between METS-IR and the likelihood of infertility in females. Given the detrimental effect of IR on women’s fertility, METS-IR could serve as an effective tool for identifying high-risk individuals.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
Ethics statement
The studies involving humans were approved by the National Center for Health Statistics Ethics Review Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. HT: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. ZO: Data curation, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. XH: Data curation, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. YB: Data curation, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. TG: Conceptualization, Funding acquisition, Investigation, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. WH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was funded by the Science and Technology Projects of Guangzhou (2024A03J1059 and 2024A03J0990).
Acknowledgments
The authors thank all participants and researchers who contributed to data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1549525/full#supplementary-material
References
1. Carson, SA, and Kallen, AN. Diagnosis and Management of Infertility: a. Review. (2021) 326:65–76. doi: 10.1001/jama.2021.4788
2. Habbema, JD, Collins, J, Leridon, H, Evers, JL, Lunenfeld, B, and te Velde, ER. Towards less confusing terminology in reproductive medicine: a proposal. Hum Reprod. (2004) 19:1497–501. doi: 10.1093/humrep/deh303
3. Inhorn, MC, and Patrizio, P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. (2015) 21:411–26. doi: 10.1093/humupd/dmv016
4. Macaluso, M, Wright-Schnapp, TJ, Chandra, A, Johnson, R, Satterwhite, CL, Pulver, A, et al. A public health focus on infertility prevention, detection, and management. Fertil Steril. (2010) 93:16.e1–16.e10. doi: 10.1016/j.fertnstert.2008.09.046. PMID 18992879
5. Finelli, R, Mottola, F, and Agarwal, A. Impact of alcohol consumption on male fertility potential: a narrative review. Int J Environ Res Public Health. (2021) 19:328. doi: 10.3390/ijerph19010328. PMID 35010587
6. Wesselink, AK, Hatch, EE, Rothman, KJ, Mikkelsen, EM, Aschengrau, A, and Wise, LA. Prospective study of cigarette smoking and fecundability. Hum Reprod. (2019) 34:558–67. doi: 10.1093/humrep/dey372
7. Bala, R, Singh, V, Rajender, S, and Singh, K. Environment, lifestyle, and female infertility. Reprod Sci. (2021) 28:617–38. doi: 10.1007/s43032-020-00279-3
8. Zhu, L, Zhou, B, Zhu, X, Cheng, F, Pan, Y, Zhou, Y, et al. Association between body mass index and female infertility in the United States: Data from National Health and nutrition examination survey 2013-2018. Int J Gen Med. (2022) 15:1821–31. doi: 10.2147/IJGM.S349874
9. Vander Borght, M, and Wyns, C. Fertility and infertility: definition and epidemiology. Clin Biochem. (2018) 62:2–10. doi: 10.1016/j.clinbiochem.2018.03.012
10. Silvestris, E, de Pergola, G, Rosania, R, and Loverro, G. Obesity as disruptor of the female fertility. Reprod Biol Endocrinol. (2018) 16:22. doi: 10.1186/s12958-018-0336-z
11. Practice Committee of the American Society for Reproductive Medicine. Obesity and reproduction: a committee opinion. Fertil Steril. (2021) 116:1266–85. doi: 10.1016/j.fertnstert.2021.08.018. PMID 34583840
12. Zhuang, J, Wang, S, Wang, Y, Hu, R, and Wu, Y. Association between triglyceride glucose index and infertility in reproductive-aged women: a cross-sectional study. Int J Women's Health. (2024) 16:937–46. doi: 10.2147/IJWH.S461950
13. Er, LK, Wu, S, Chou, HH, Hsu, LA, Teng, MS, Sun, YC, et al. Triglyceride glucose-body mass index is a simple and clinically useful surrogate marker for Insulin resistance in nondiabetic individuals. PLoS One. (2016) 11:e0149731. doi: 10.1371/journal.pone.0149731
14. Lei, R, Chen, S, and Li, W. Advances in the study of the correlation between insulin resistance and infertility. Front Endocrinol. (2024) 15:1288326. doi: 10.3389/fendo.2024.1288326
15. Xia, W, Cai, Y, Zhang, S, and Wu, S. Association between different insulin resistance surrogates and infertility in reproductive-aged females. BMC Public Health. (2023) 23:1985. doi: 10.1186/s12889-023-16813-2
16. Wang, H, Zhang, Y, Fang, X, Kwak-Kim, J, and Wu, L. Insulin resistance adversely affect IVF outcomes in lean women without PCOS. Front Endocrinol. (2021) 12:734638. doi: 10.3389/fendo.2021.734638
17. Marcovina, S, Bowsher, RR, Miller, WG, Staten, M, Myers, G, Caudill, SP, et al. Standardization of insulin immunoassays: report of the American Diabetes Association workgroup. Clin Chem. (2007) 53:711–6. doi: 10.1373/clinchem.2006.082214
18. Porchia, LM, Ochoa-Precoma, R, Reyes-Albarracin, Y, Gonzalez-Mejia, ME, and Lopez-Bayghen, E. Age-related differences in fluctuations in insulin resistance evaluated with HOMA-IR and triglyceride and glucose-based indices during the menstrual cycle, as determined using the NHANES cross-sectional dataset. Maturitas. (2024) 188:108085. doi: 10.1016/j.maturitas.2024.108085
19. Katz, A, Nambi, SS, Mather, K, Baron, AD, Follmann, DA, Sullivan, G, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. (2000) 85:2402–10. doi: 10.1210/jcem.85.7.6661
20. Giannini, C, Santoro, N, Caprio, S, Kim, G, Lartaud, D, Shaw, M, et al. The triglyceride-to-HDL cholesterol ratio: association with insulin resistance in obese youths of different ethnic backgrounds. Diabetes Care. (2011) 34:1869–74. doi: 10.2337/dc10-2234
21. Guerrero-Romero, F, Simental-Mendia, LE, Gonzalez-Ortiz, M, Martinez-Abundis, E, Ramos-Zavala, MG, Hernandez-Gonzalez, SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. (2010) 95:3347–51. doi: 10.1210/jc.2010-0288. PMID 20484475
22. Bello-Chavolla, OY, Almeda-Valdes, P, Gomez-Velasco, D, Viveros-Ruiz, T, Cruz-Bautista, I, Romo-Romo, A, et al. METS-IR, a novel score to evaluate insulin sensitivity, is predictive of visceral adiposity and incident type 2 diabetes. Eur J Endocrinol. (2018) 178:533–44. doi: 10.1530/EJE-17-0883
23. Coviello, AD, Legro, RS, and Dunaif, A. Adolescent girls with polycystic ovary syndrome have an increased risk of the metabolic syndrome associated with increasing androgen levels independent of obesity and insulin resistance. J Clin Endocrinol Metab. (2006) 91:492–7. doi: 10.1210/jc.2005-1666
24. Ng, NYH, Jiang, G, Cheung, LP, Zhang, Y, Tam, CHT, Luk, AOY, et al. Progression of glucose intolerance and cardiometabolic risk factors over a decade in Chinese women with polycystic ovary syndrome: a case-control study. PLoS Med. (2019) 16:e1002953. doi: 10.1371/journal.pmed.1002953
25. Soares, EM, Azevedo, GD, Gadelha, RG, Lemos, TM, and Maranhao, TM. Prevalence of the metabolic syndrome and its components in Brazilian women with polycystic ovary syndrome. Fertil Steril. (2008) 89:649–55. doi: 10.1016/j.fertnstert.2007.03.081
26. Shen, Y, Zhu, Z, Bi, X, Shen, Y, Shen, A, Deng, B, et al. Association between insulin resistance indices and kidney stones: results from the 2015-2018 National Health and nutrition examination survey. Front Nutr. (2024) 11:1444049. doi: 10.3389/fnut.2024.1444049
27. Liu, H, Liu, C, Wang, T, and Fang, D. Association of METS-IR index with psoriasis in US adults: a cross-sectional study. Sci Rep. (2024) 14:26123. doi: 10.1038/s41598-024-77784-x
28. Hou, Y, Li, R, Xu, Z, Chen, W, Li, Z, Jiang, W, et al. Association of METS-IR index with type 2 diabetes: a cross-sectional analysis of national health and nutrition examination survey data from 2009 to 2018. PLoS One. (2024) 19:e0308597. doi: 10.1371/journal.pone.0308597
29. Li, Y, Li, H, Chen, X, and Liang, X. Association between various insulin resistance indices and cardiovascular disease in middle-aged and elderly individuals: evidence from two prospectives nationwide cohort surveys. Front Endocrinol. (2024) 15:1483468. doi: 10.3389/fendo.2024.1483468
30. Lai, T, Guan, F, Chen, Y, and Hu, K. Cross-sectional comparison of the association between three different insulin resistance surrogates and frailty: NHANES 1999-2018. Front Endocrinol. (2024) 15:1439326. doi: 10.3389/fendo.2024.1439326
31. Uysal, E, Tammo, O, Soylemez, E, Incebiyik, M, Filiz, D, and Alci, M. Significance of measuring anthropometric and atherogenic indices in patients with polycystic ovary syndrome. BMC Endocr Disord. (2024) 24:160. doi: 10.1186/s12902-024-01701-6
32. von Elm, E, Altman, DG, Egger, M, Pocock, SJ, Gotzsche, PC, Vandenbroucke, JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. (2007) 370:1453–7. doi: 10.1016/S0140-6736(07)61602-X
33. Cardozo, E, Pavone, ME, and Hirshfeld-Cytron, JE. Metabolic syndrome and oocyte quality. Trends Endocrinol Metab. (2011) 22:103–9. doi: 10.1016/j.tem.2010.12.002
34. Diamanti-Kandarakis, E, and Dunaif, A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. (2012) 33:981–1030. doi: 10.1210/er.2011-1034
35. Ding, H, Zhang, J, Zhang, F, Zhang, S, Chen, X, Liang, W, et al. Resistance to the Insulin and elevated level of androgen: a major cause of polycystic ovary syndrome. Front Endocrinol. (2021) 12:741764. doi: 10.3389/fendo.2021.741764
36. Song, H, Yu, Z, Li, P, Wang, Y, and Shi, Y. HOMA-IR for predicting clinical pregnancy rate during IVF. Gynecol Endocrinol. (2022) 38:33–8. doi: 10.1080/09513590.2021.1952976
37. Ou, XH, Li, S, Wang, ZB, Li, M, Quan, S, Xing, F, et al. Maternal insulin resistance causes oxidative stress and mitochondrial dysfunction in mouse oocytes. Hum Reprod. (2012) 27:2130–45. doi: 10.1093/humrep/des137
38. Yaribeygi, H, Farrokhi, FR, Butler, AE, and Sahebkar, A. Insulin resistance: review of the underlying molecular mechanisms. J Cell Physiol. (2019) 234:8152–61. doi: 10.1002/jcp.27603
39. Heber, MF, Ferreira, SR, Abruzzese, GA, Raices, T, Pignataro, OP, Vega, M, et al. Metformin improves ovarian insulin signaling alterations caused by fetal programming. J Endocrinol. (2019). doi: 10.1530/JOE-18-0520
40. Zhu, Q, Zuo, R, He, Y, Wang, Y, Chen, ZJ, Sun, Y, et al. Local regeneration of cortisol by 11beta-HSD1 contributes to Insulin resistance of the granulosa cells in PCOS. J Clin Endocrinol Metab. (2016) 101:2168–77. doi: 10.1210/jc.2015-3899
41. Wu, S, Divall, S, Nwaopara, A, Radovick, S, Wondisford, F, Ko, C, et al. Obesity-induced infertility and hyperandrogenism are corrected by deletion of the insulin receptor in the ovarian theca cell. Diabetes. (2014) 63:1270–82. doi: 10.2337/db13-1514
42. Hansda, SR, and Haldar, C. Uterine anomalies in cell proliferation, energy homeostasis and oxidative stress in PCOS hamsters, M. auratus: therapeutic potentials of melatonin. Life Sci. (2021) 281:119755. doi: 10.1016/j.lfs.2021.119755. PMID 34175318
43. Cabrera-Cruz, H, Orostica, L, Plaza-Parrochia, F, Torres-Pinto, I, Romero, C, and Vega, M. The insulin-sensitizing mechanism of myo-inositol is associated with AMPK activation and GLUT-4 expression in human endometrial cells exposed to a PCOS environment. Am J Physiol Endocrinol Metab. (2020) 318:E237–48. doi: 10.1152/ajpendo.00162.2019
44. Xiang, S, Xia, MF, Song, JY, Liu, DQ, and Lian, F. Effect of electro-acupuncture on expression of IRS-1/PI3K/GLUT4 pathway in ovarian granulosa cells of infertile patients with polycystic ovary syndrome-Insulin resistance of phlegm-dampness syndrome. Chin J Integr Med. (2021) 27:330–5. doi: 10.1007/s11655-020-3219-z
45. Bergman, RN, Finegood, DT, and Ader, M. Assessment of insulin sensitivity in vivo. Endocr Rev. (1985) 6:45–86. doi: 10.1210/edrv-6-1-45. PMID 3884329
46. DeFronzo, RA, Tobin, JD, and Andres, R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Phys. (1979) 237:E214–23. doi: 10.1152/ajpendo.1979.237.3.E214
47. Zhang, L, Yu, C, Wang, T, Zhou, W, Bao, H, and Cheng, X. Association of the metabolic score for insulin resistance with cardiovascular diseases, cardiovascular and all-cause mortality in Chinese hypertensive population. Front Endocrinol. (2023) 14:1326436. doi: 10.3389/fendo.2023.1326436
48. He, Y, Lu, Y, Zhu, Q, Wang, Y, Lindheim, SR, Qi, J, et al. Influence of metabolic syndrome on female fertility and in vitro fertilization outcomes in PCOS women. Am J Obstet Gynecol. (2019) 221:138.e1. doi: 10.1016/j.ajog.2019.03.011. PMID 30910544
49. Omidvar, S, Faramarzi, M, Hajian-Tilak, K, and Nasiri, AF. Associations of psychosocial factors with pregnancy healthy life styles. PLoS One. (2018) 13:e0191723. doi: 10.1371/journal.pone.0191723
50. Van Der Kelen, A, Okutman, O, Javey, E, Serdarogullari, M, Janssens, C, Ghosh, MS, et al. A systematic review and evidence assessment of monogenic gene-disease relationships in human female infertility and differences in sex development. Hum Reprod Update. (2023) 29:218–32. doi: 10.1093/humupd/dmac044
51. Domar, A, Gordon, K, Garcia-Velasco, J, La Marca, A, Barriere, P, and Beligotti, F. Understanding the perceptions of and emotional barriers to infertility treatment: a survey in four European countries. Hum Reprod. (2012) 27:1073–9. doi: 10.1093/humrep/des016
52. Malekpour, P, Hasanzadeh, R, Javedani Masroor, M, Chaman, R, and Motaghi, Z. Effectiveness of a mixed lifestyle program in couples undergoing assisted reproductive technology: a study protocol. Reprod Health. (2023) 20:112. doi: 10.1186/s12978-023-01652-6
53. Dick, ML, Bain, CJ, Purdie, DM, Siskind, V, Molloy, D, and Green, AC. Self-reported difficulty in conceiving as a measure of infertility. Hum Reprod. (2003) 18:2711–7. doi: 10.1093/humrep/deg504
Keywords: insulin resistance, metabolic syndrome, metabolic score for insulin resistance, female infertility, NHANES
Citation: Li H, Tan H, OuYang Z, Hu X, Bao Y, Gao T and Hua W (2025) Association between METS-IR and female infertility: a cross-sectional study of NHANES 2013–2018. Front. Nutr. 12:1549525. doi: 10.3389/fnut.2025.1549525
Edited by:
Macarena Lozano-Lorca, University of Granada, SpainReviewed by:
Zhong Xingming, Guangdong Provincial Family Planning Hospital, ChinaRobert A. Ngala, Kwame Nkrumah University of Science and Technology, Ghana
Copyright © 2025 Li, Tan, OuYang, Hu, Bao, Gao and Hua. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenfeng Hua, aHVhd2ZAZ2QyaC5vcmcuY24=; Tianyang Gao, Z2FvdGlhbnlhbmdzenl4QDE2My5jb20=
†These authors have contributed equally to this work
 Haiyan Li
Haiyan Li Hongxia Tan
Hongxia Tan Zhenbo OuYang
Zhenbo OuYang Xianyue Hu
Xianyue Hu Yanjing Bao
Yanjing Bao Tianyang Gao
Tianyang Gao Wenfeng Hua
Wenfeng Hua