- 1Department of Digestion, Zhejiang Hospital, Hangzhou, China
- 2Department of Nutrition, Zhejiang Hospital, Hangzhou, China
Background: Observational studies have assessed the association between total antioxidant capacity of the diet and risk of diabetes mellitus. However, results from these studies were not entirely consistent. In the current systematic review and dose-response meta-analysis, we aimed to determine the association between dietary total antioxidant capacity (TAC) and the risk of prediabetes and diabetes mellitus.
Methods: A systematic literature search of authentic electronic resources including PubMed/Medline, Embase, Scopus, ISI Web of Science and China National Knowledge Infrastructure (CNKI) was carried out to find the relevant articles published up to November 2024. Random-effects or fixed-effects models were used to aggregate the relative risks (RRs) and their 95% confidence intervals (CIs) where appropriate. Heterogeneity across the studies were determined using the Cochran’s Q test and I-square (I2) statistics.
Results: A total of 10 observational studies (five cohort, three case-control and two cross-sectional studies) were included in our meta-analysis. The pooled results indicated that higher dietary TAC was significantly associated with lower risk of prediabetes (RR = 0.58; 95% CI: 0.34–0.97; p = 0.039) and diabetes mellitus (RR = 0.71; 95% CI: 0.58–0.87, p = 0.001). In addition, dose-response analysis showed a linear trend association between dietary TAC and risk of diabetes mellitus (RR = 0.928; 95% CI: 0.842–1.023, pdose-response = 0.131, pnonlinearity = 0.078). Subgroup analyses showed the significant inverse association between dietary TAC and diabetes mellitus in mean age <50 and sample size <5,000 (RR = 0.26, 95% CI: 0.16–0.41, p < 0.001), and there was no evidence of heterogeneity (p = 0.939; I2 = 0.0%). Meanwhile, there was also an inverse association between dietary TAC and diabetes mellitus in Western countries (RR = 0.79; 95% CI: 0.68–0.92, p = 0.003), with less evidence of heterogeneity (p = 0.226; I2 = 36.7%).
Conclusion: Overall, higher dietary TAC was inversely associated with the risk of prediabetes and diabetes mellitus. Further well-designed prospective studies or randomized controlled trials are needed to validate the present findings.
Systematic Review Register: (PROSPERO), CRD42024611235.
Introduction
Globally, diabetes mellitus, as one of the most common and fastest growing chronic non-communicable diseases, has become a serious public health problem (1). According to the latest estimates from International Diabetes Federation (IDF) in 2021, the global prevalence of diabetes mellitus will reach to be 12.2%, projecting to affect 783.2 million adults (aged 20–79 years) by 2045 (2). Given the growing incidence and healthcare burden of diabetes mellitus, urgent public health preventive measures are of particular importance. As is known to all, diabetes mellitus is a chronic, multifactorial disorder that may be associated with various risk factors, including genetic predisposition and environmental factors, e.g., physical inactivity, cigarette smoking, and dietary factors (3, 4).
Over the past few decades, increasing evidence suggests that dietary factors, particularly those rich in antioxidants, may play a crucial role in the pathogenesis of diabetes mellitus (5). For example, a previous systematic review and meta-analysis revealed that greater intake of green leafy vegetables was associated with a 14% reduced risk of type 2 diabetes mellitus (6). The researchers speculated that the antioxidant vitamins in vegetables might play a key role in this protective effect (7). Antioxidants are known to inhibit the activity of free radicals and reduce oxidative stress (7), an important risk factor in the pathogenesis of diabetes mellitus (8). The majority of previous observational studies have commonly focused on the impact of individual antioxidants intake on diabetes mellitus risk (9–12). For example, Golmohamadi et al. (11), in a prospective cohort study, found that dietary vitamin E significantly decreased the risk of diabetes mellitus. However, research on individual antioxidants may not fully capture the cumulative or synergistic effects of various antioxidants in the overall diet (13). In view of this, dietary total antioxidant capacity (TAC) has been designed as a direct measurement tool for assessing the diet’s total antioxidant potential, considering the possible interactions between antioxidant nutrients in food (14).
Currently, dietary TAC is receiving extensive attention and interest in epidemiological research (15). Growing evidence shows that higher dietary TAC intake has been inversely related to various negative health outcomes, including cardiovascular diseases, pancreatic cancer and mortality (16–18). Notably, a more recent systematic review and meta-analysis shows that higher intake of dietary TAC is associated with a reduced risk of stroke (14). However, epidemiological studies on the correlation between dietary TAC and diabetes mellitus risk are quite limited. Up to now, only eight previous studies have reported the association between dietary TAC and risk of diabetes mellitus (7, 19–25), but their findings were not entirely consistent. Although several studies have shown the protective effect of dietary TAC against diabetes mellitus (7, 22), other studies found no significant association (19, 20, 23). Furthermore, to our knowledge, no previous systematic review and dose-response meta-analysis has been carried out to comprehensively evaluate the links between dietary TAC and prediabetes and diabetes mellitus risk. Therefore, to identify the exact associations between dietary TAC and prediabetes and diabetes mellitus risk, we performed this systematic review and dose-response meta-analysis to summarize the findings from observational studies published up to November 2024.
Methods
Protocol and registration
This study was performed in accordance with the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines (26), and the protocol has been registered on November 17, 2024 in the International Prospective Register of Systematic reviews (PROSPERO) database with registration number CRD42024611235.
Search strategy
A systematic search using PubMed/MEDLINE, ISI Web of Science, Embase, Scopus and CNKI databases was performed to find the studies published that have evaluated the associations between dietary TAC and prediabetes and diabetes mellitus up to November 2024. The search strategy included the predefined keywords: “dietary total antioxidant capacity,” “dietary TAC,” “dietary antioxidant capacity,” “non enzymatic antioxidant capacity,” “dietary antioxidant index,” “antioxidant capacity of diet” and “diabetes mellitus,” “diabetes,” “insulin resistance,” “hyperglycemia,” “prediabetes.” The detailed search strategy of each database is available in Supplementary Table 1.
Moreover, hand-searching from reference lists of all relevant articles, previous reviews and meta-analyses was performed to identify relevant studies. At the same time, unpublished studies or grey literature were not eligible in this meta-analysis.
Study selection
Two authors (RL and LS) independently examined and cross-checked the titles and abstracts of all published articles retrieved in the initial search, and removed duplicates and irrelevant articles. The full-text versions of these articles were then reviewed according to the inclusion and exclusion criteria for this study. In order to be included in our analyses, studies must meet all of the eligibility criteria: (1) observational studies, including cohort, case-control or cross-sectional studies; (2) those adult participants aged ≥18 years; (3) the main exposure of interest was dietary TAC; (4) the outcome of interest was prediabetes and/or diabetes mellitus; (5) provided the multivariable adjusted effect estimates in the form of HRs, RRs, or ORs with 95% CIs (or sufficient data to calculate them); (6) if the retrieved studies lacked sufficient detail, the corresponding author of eligible study were contacted by email. Additionally, exclusion criteria was as follows: (1) non-observational studies, such as reviews, case reports, letters and editorials; (2) did not provide the HRs, RRs or ORs with corresponding 95% CIs; (3): the exposure of interest was single antioxidant, such as vitamin C, vitamin E; (4) irrelevant articles. When the results of a study in men and women are reported separately, we treated each analysis as a separate study. Any discrepancy was resolved by discussion or in consultation with the corresponding author (DL). The study population, exposure, comparison, outcome, and study design (PECOS) information is illustrated in Table 1.
Data extraction
Two authors (RL and LS) performed data extraction independently, including first author’s last name, publication year, study design, study region, sample size, mean age/age range, follow-up time in cohort studies, methods for dietary assessment, numbers of participants and prediabetes/diabetes mellitus cases, confounding variables adjusted for the multivariate analyses, and reported risk estimates with their corresponding 95% CIs of prediabetes/diabetes mellitus across categories of dietary TAC.
Quality assessment
Two authors (QZ and LS) used the Newcastle–Ottawa Scale (NOS) to evaluate the quality of each selected article in this study. The NOS scale was designed for non-randomized studies in meta-analyses, composing of eight items in three domains: selection (4 points), comparability (2 points), and ascertaining of the outcome (3 points), with a maximum score of nine (27). Studies with NOS scores ≥7 points were recognized as high quality (28). Any discrepancies between two authors were resolved by the corresponding author to reach a consensus.
Definition of dietary TAC
Dietary TAC is designed as a direct measurement tool to evaluate total antioxidant potential of the whole diet using different chemical methods, such as the ferric reducing antioxidant potential (FRAP), the oxygen radical absorbance capacity (ORAC), the trolox equivalent antioxidant capacity (TEAC), the total radical-trapping antioxidant parameter (TRAP), and vitamin C equivalent antioxidant capacity (VCEAC) (14).
Data synthesis and statistical analyses
In main analyses, we used RR and 95% CI as the primary effect size. Additionally, we considered that HR was approximately equal to RR (29). OR was converted into RR using the following formula: RR = OR/[(1 − P0) + (P0 × OR)], where P0 shows the incidence of diabetes mellitus in the non-exposed group (30). We carried out a pairwise meta-analysis that pooled the RRs and 95% CIs of the highest and lowest categories of dietary TAC with prediabetes and diabetes mellitus risk. Between-study heterogeneity was assessed by Cochran’s Q test and quantified by the I2 statistics. A p-value of Q-test >0.10 or I2 < 50% showed an absence of heterogeneity among the included studies, and fixed-effects model was used to pool RRs. Due to expected heterogeneity between the included studies, RRs were calculated using the random-effects model (DerSimonian and Laird methods) (31). When results showed the significant heterogeneity, sensitivity and subgroup analyses were performed to identify the potential sources of heterogeneity. In our analyses, subgroup analyses were performed to explore potential effects attributable to variables such as study design (cohort or case-control/cross-sectional studies), sex (men or women), study region (Western or Asian countries), study quality (≥7 or <7), outcome (type 2 diabetes or gestational diabetes), mean age (≥50 years or <50 years), sample size (<5,000 or ≥5,000), and methods for dietary assessment (FFQ or others). A sensitivity analysis was performed to confirm whether the combined RRs had a robust or sensitive effect on a single study or a group of studies. Publication bias was assessed through visual inspection of the funnel plots and quantified by both Begg’s and Egger’s regression asymmetry tests (32). If there was publication bias, the trim and fill method was used to recalculate the combined RRs (33). At the same time, we performed a dose-response meta-analysis to estimate the trend from the correlated log RRs across the categories of dietary TAC scores. A two-stage GLST model based on generalized least squares was used to examine the linear or non-linear dose-response association between dietary TAC and diabetes mellitus risk (34). In this study, we used dietary TAC model and restricted cubic splines with three knots at fixed percentiles (10, 50, and 90%) distributions. Analyses were conducted using STATA/SE, version 14.0 (StataCorp, College Station, TX, United States). All statistical tests were two-sided, and p-values <0.05 were considered to be statistically significant unless otherwise specified.
Results
Search results
The flow chart of literature search process is shown in Figure 1. The systematic search and reference list screening yielded 7,851 articles. After omitting 2,793 duplicates, 5,058 articles were remained for further assessment. Subsequently, 5,033 articles were excluded based on the assessment of titles and abstracts of retrieved articles. Twenty-five full-text articles were assessed for eligibility. Of the remaining 25 articles, 15 were excluded because of the following reasons: outcomes of interest were not prediabetes or diabetes mellitus (n = 7), reported the same participants (n = 1), reported the association between dietary patterns and diabetes mellitus (n = 2), and reported the links between individual dietary antioxidants intake and diabetes mellitus (n = 5). Ultimately, 10 articles were included in the analyses (7, 19–25, 35, 36). The PECOS for the present meta-analysis is shown in Table 1.
Study characteristics
Characteristics of eligible studies were presented in Table 2. A total of 10 articles was included, with sample size of included studies ranging from 147 to 64,660. Of these included studies, five were cohort studies (7, 21–24), three were case-control studies (25, 35, 36) and two were cross-sectional studies (19, 20). With regards to the origin of the studies, four studies were carried out in Iran (24, 25, 36, 37), one in China (20), one in Korea (21), one in France (22), one in Japan (23), one in Netherlands (7), and one in Poland (19). All the included studies were published between 2018 and 2024. Seven studies included both men and women (7, 19–21, 23, 35, 36), and remaining three studies only included women (22, 24, 25). The follow-up duration for the cohort studies ranged from 1 to 15 years. The age of participants across studies ranged from ages 18 to above. Seven of included studies used FFQ to collect dietary data (7, 20, 21, 23, 24, 35, 36), two studies used 24-h dietary recall (19, 25), and one study used dietary questionnaire (22). To calculate the quantitative value of dietary TAC, six studies used FRAP assay (7, 19, 20, 22, 24, 35), one study used ORAC assay (36), one study used VCEAC (21) one study used FRAP, TRAP and TEAC assays (25), and one study used FRAP, TRAP and ORAC assays (23). Based on the NOS, from all included studies, nine studies were high quality (7, 19–24, 35, 36), and the remaining one study was medium quality (25). The quality assessment of included studies bases on NOS criteria is shown in Table 3.
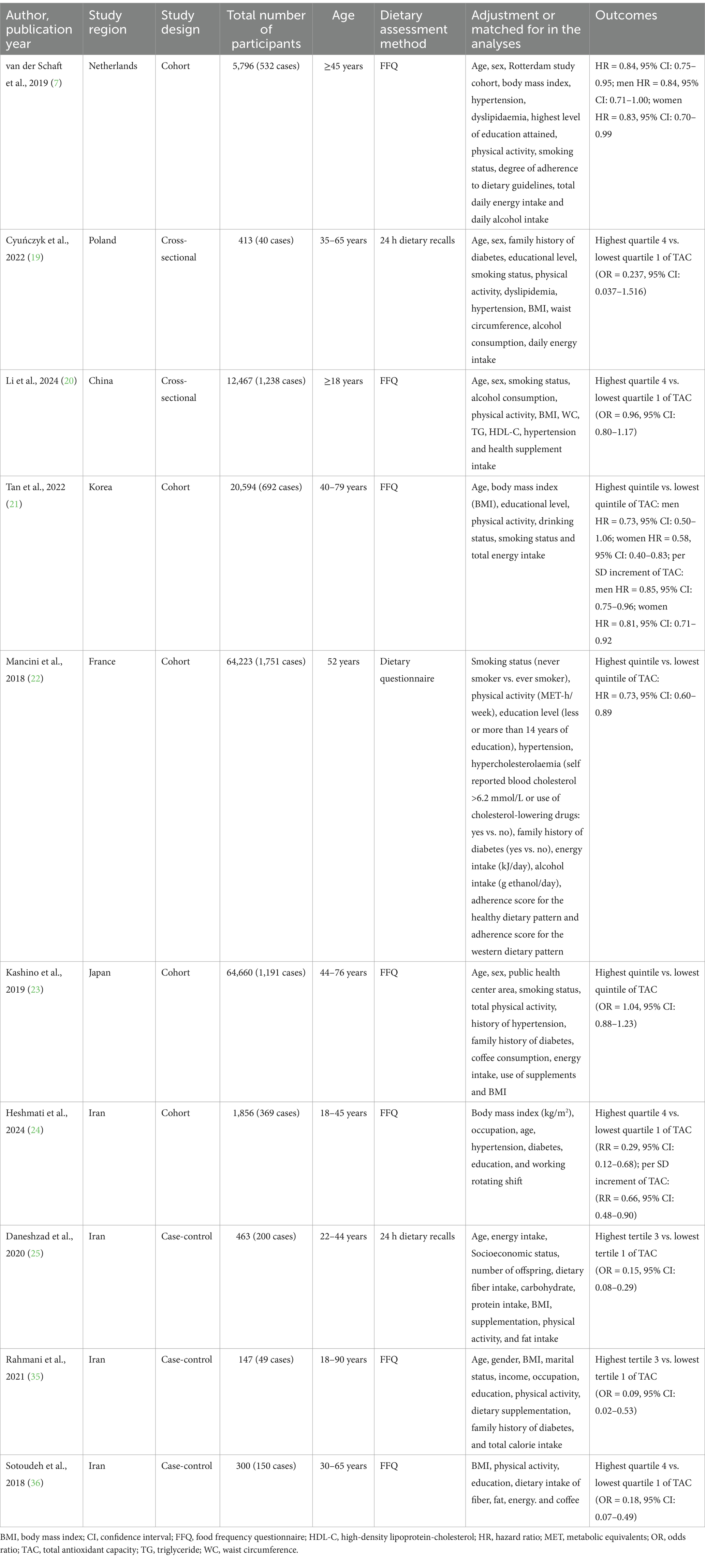
Table 2. Characteristics of the included studies on the association between dietary total antioxidant capacity and prediabetes and diabetes mellitus risk.
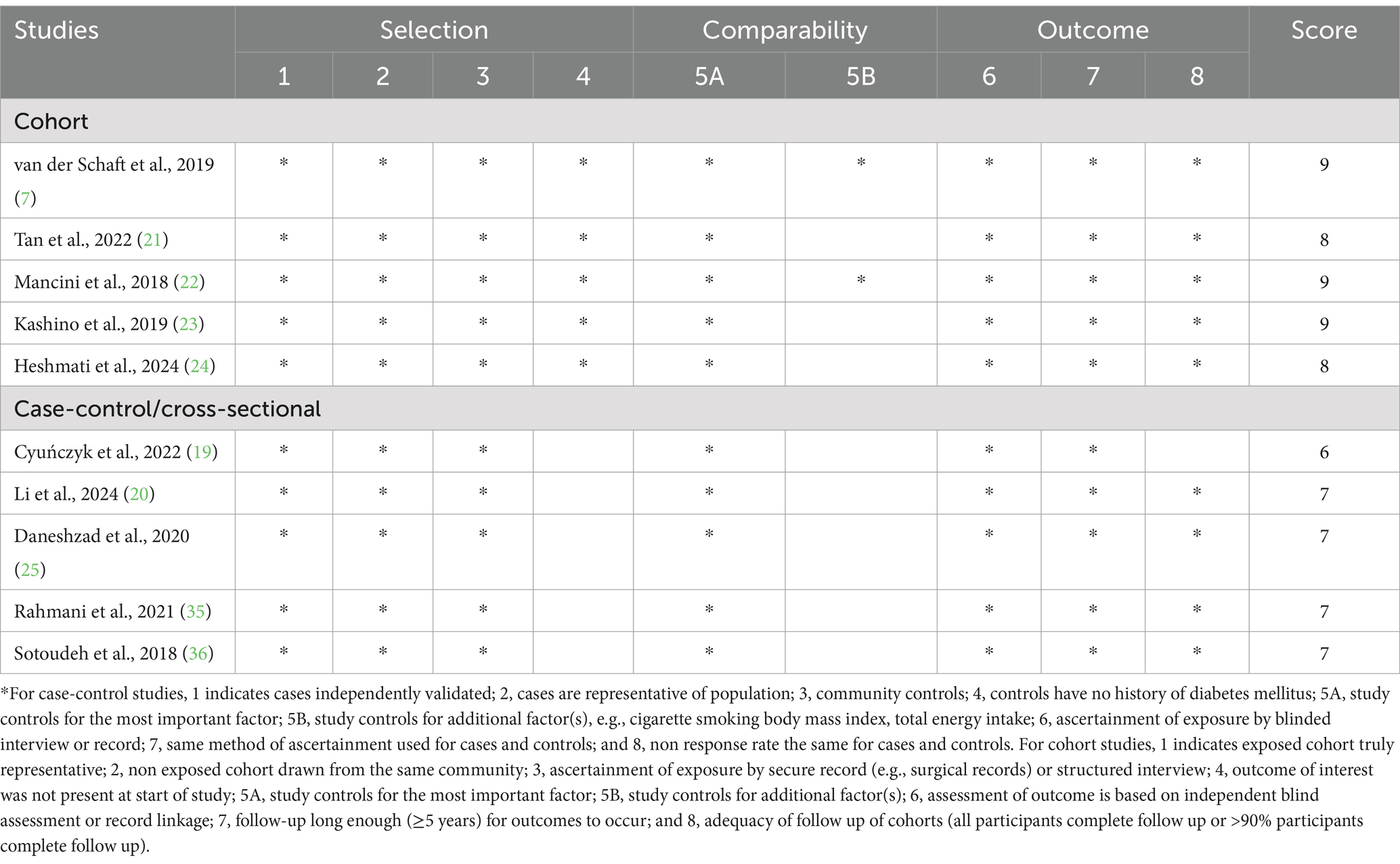
Table 3. Dietary total antioxidant capacity and risk of diabetes mellitus: assessment of study quality.
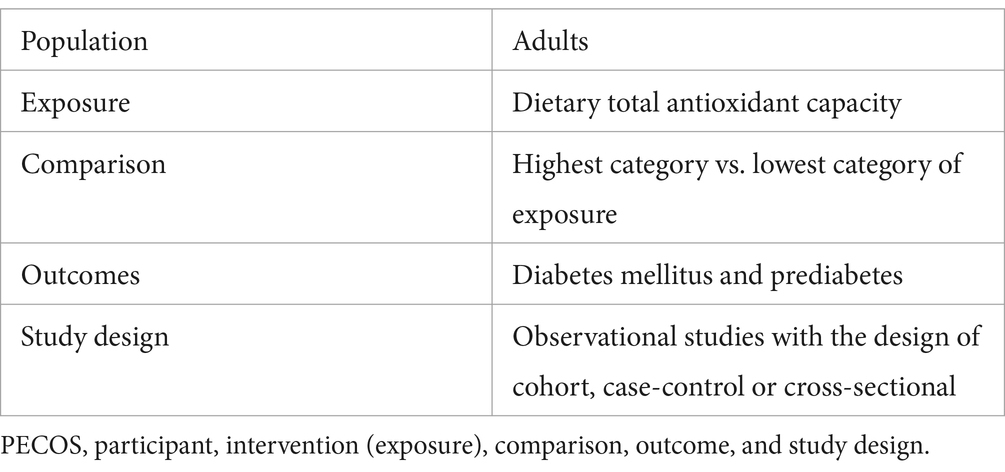
Dietary TAC and prediabetes risk
Four studies involving 5,817 participants and 1,195 cases, were included to evaluate the association between dietary TAC and prediabetes risk. Figure 2 showed that higher dietary TAC intake was associated with the reduced risk of prediabetes (RR = 0.58; 95% CI: 0.34–0.97; p = 0.039), with significant heterogeneity between included studies (I2 = 79.0%, p = 0.003). Thus, the effect size was evaluated using the random-effects models. Due to the limited data, we were unable to conduct subgroup analyses to explore potential sources of heterogeneity across studies.
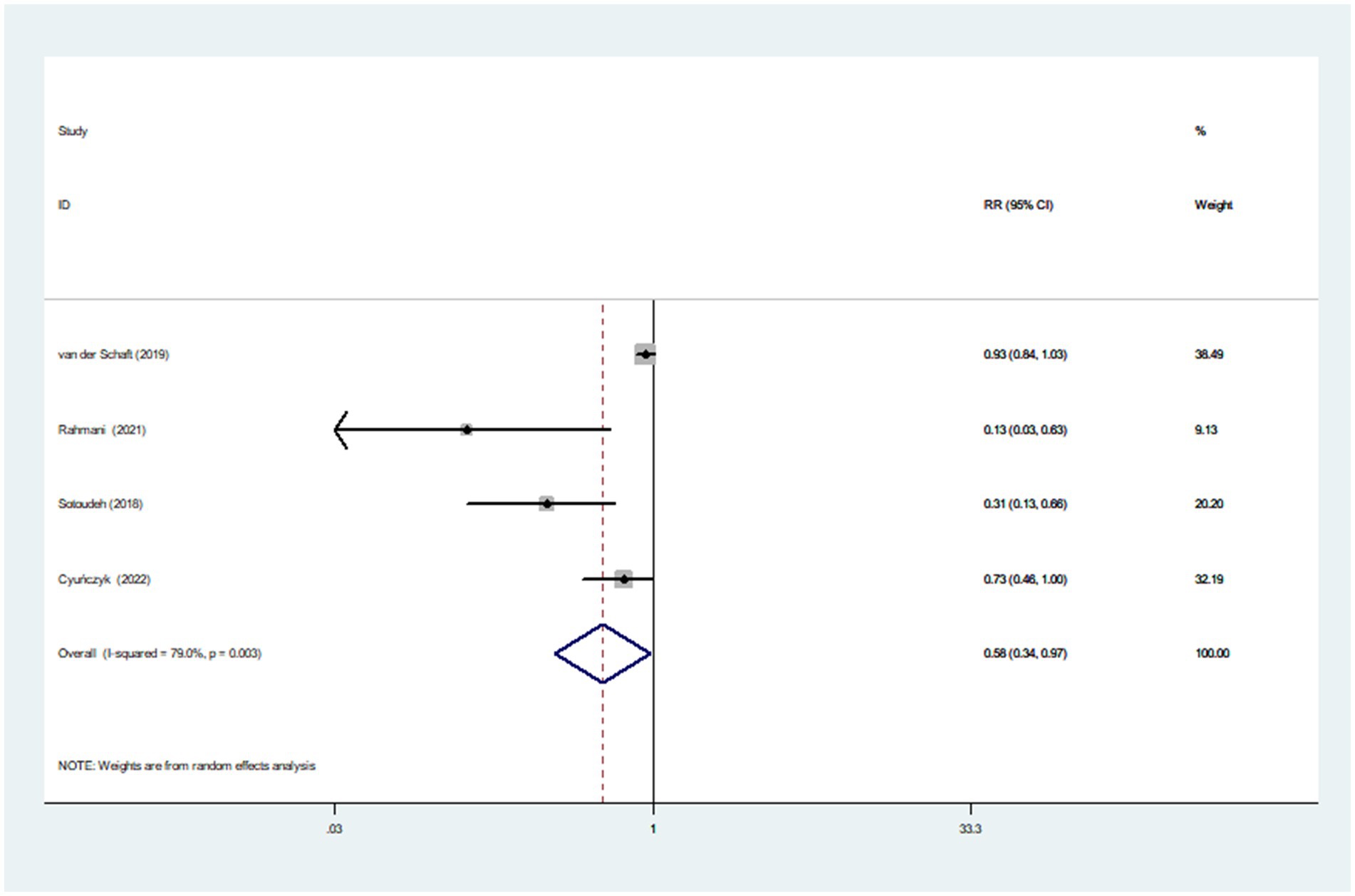
Dietary TAC and diabetes mellitus risk
Eight studies involving 170,472 participants and 6,013 cases, were included to evaluate the association between dietary TAC and diabetes mellitus risk. Combining nine effect sizes from eight studies, Figure 3 shows the evidence of a 29% lower risk of diabetes mellitus in the highest compared with the lowest categories of dietary TAC scores (RR = 0.71; 95% CI: 0.58–0.87, p = 0.001). The significant heterogeneity was found in the included studies (I2 = 80.2%; p < 0.001), thus a random-effects model was applied to pool RRs. In addition, Figure 4 showed that each SD increment in dietary TAC intake was associated with a 18% lower risk of diabetes mellitus (RR = 0.82; 95% CI: 0.77–0.89, p < 0.001; I2 = 0.0%; p = 0.687).
Figure 4. Forest plot of the association between each 1 SD increment in dietary TAC intake and risk of diabetes mellitus.
Dose-response analysis
Seven studies (four cohort, two case-control, and one cross-sectional studies) were included in the dose-response analysis for the association between dietary TAC and risk of diabetes mellitus (Figure 5). The dose-response analysis indicated a linear trend association between dietary TAC and risk of diabetes mellitus (pnonlinearity = 0.078). However, this dose-response association was not statistically significant (RR = 0.928; 95% CI: 0.842–1.023, pdose-response = 0.131), possibly because the number of studies is small.
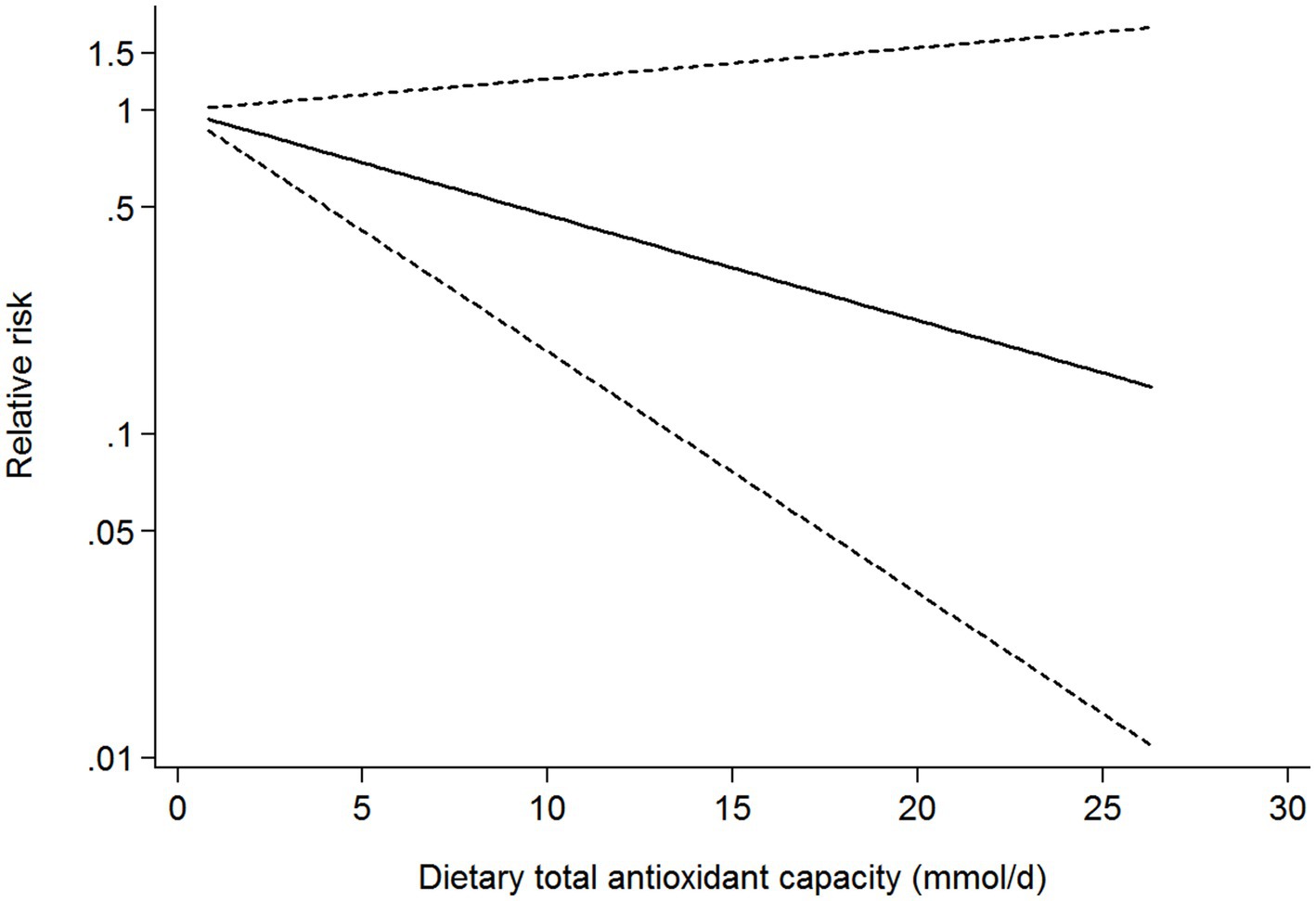
Figure 5. Dose-response analysis for the association between dietary TAC intake and risk of diabetes mellitus.
Subgroup analyses
To further identify the potential sources of heterogeneity across included studies, we conducted subgroup analyses basing on study design, sex, study region, study quality, outcome, mean age, sample size, and methods for dietary assessment (Table 4). The results showed a significant inverse association between dietary TAC and diabetes mellitus in studies with mean age <50 and sample size <5,000 (RR = 0.26, 95% CI: 0.16–0.41, p < 0.001), and there was no evidence of heterogeneity (p = 0.939; I2 = 0.0%). In addition, there was also an inverse association between dietary TAC intake and diabetes mellitus in Western countries (RR = 0.79; 95% CI: 0.68–0.92, p = 0.003), with less evidence of heterogeneity (p = 0.226; I2 = 36.7%).
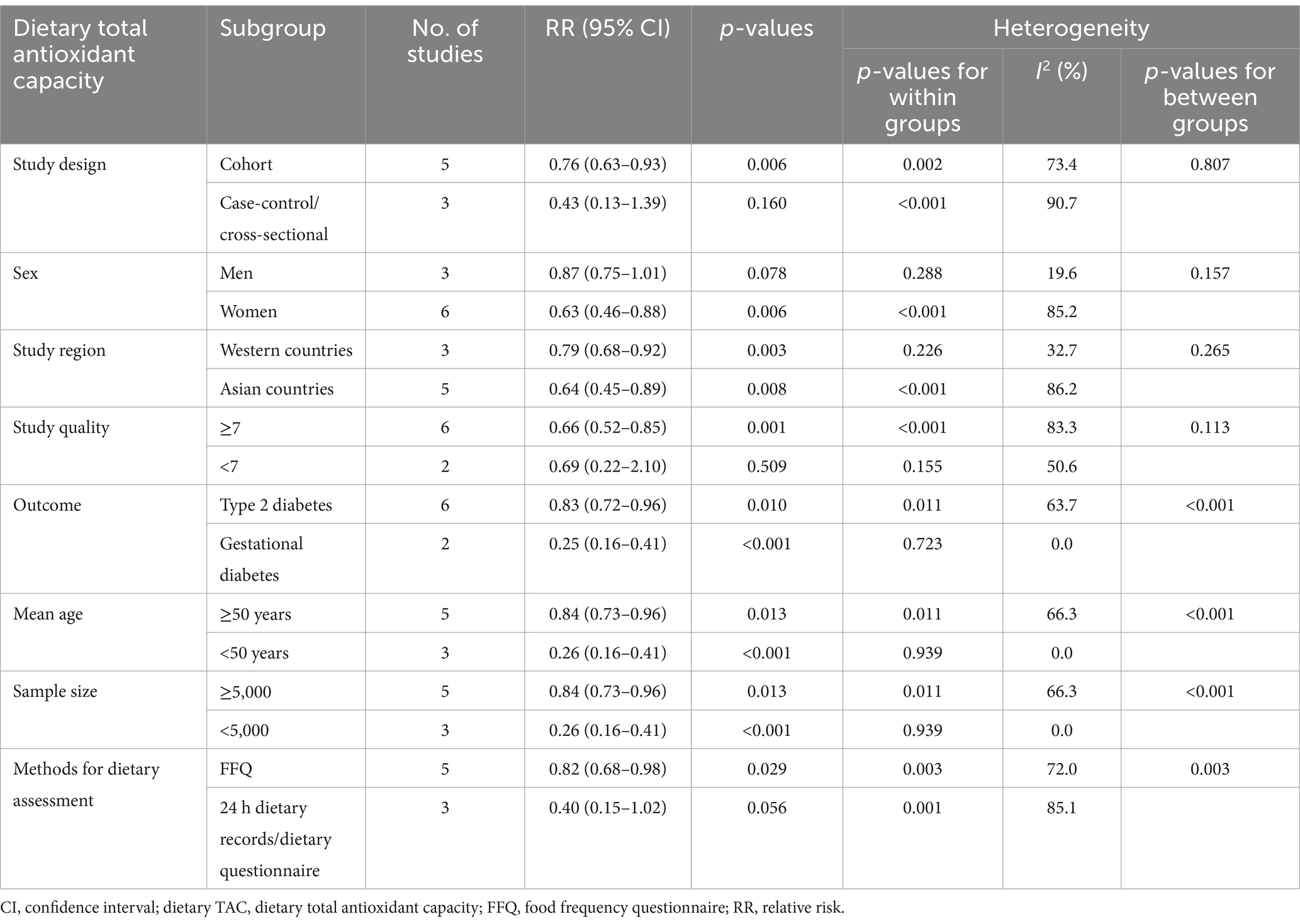
Table 4. Subgroup analyses of diabetes mellitus for the highest versus lowest categories of dietary TAC intake.
Publication bias
As shown in Supplementary Figure 1, inspection of funnel plots revealed little evidence of asymmetry. Begg’s test showed no evidence of publication bias had no statistical significance (highest compared with lowest categories of dietary TAC: p = 0.076). However, Egger’s test for publication bias had statistical significance (p = 0.021). Thus, we applied the trim and fill analysis to re-estimate the pooled RRs (Supplementary Figure 2). After performing trim and fill analysis, the results showed that no study was added to the funnel plot and no change in the overall RRs was found (RR = 0.71; 95% CI: 0.58–0.87, p < 0.01).
Sensitivity analysis
In sensitivity analysis (Supplementary Figure 3), the results showed that the association between dietary TAC and diabetes mellitus risk was robust and not affected by any single study or a couple of studies.
Discussion
To the best of our knowledge, this is the first systematic review and dose-response meta-analysis to comprehensively evaluate the association between dietary TAC and risk of prediabetes and diabetes mellitus. In this study, our results indicated that higher dietary TAC intake was significantly associated with lower risk of prediabetes and diabetes mellitus. Moreover, the dose-response analysis also indicated a linear trend association between dietary TAC and risk of diabetes mellitus. Similarly, sensitivity analysis did not show the significant impact of any single study on the pooled results. Our findings validate the results of previous observational studies and underscore the clinical importance of higher dietary TAC intake in the prevention of prediabetes and diabetes mellitus.
In the past few decades, the global epidemic of diabetes mellitus has continued to increase (37). According to the latest estimates from IDF in 2021, the global prevalence of diabetes mellitus will reach to be 12.2%, projecting to affect 783.2 million adults by 2045 (2). Considering the enormous burden to public healthcare systems, identifying risk factors and implementing prevention are the main strategies for controlling diabetes mellitus. It is well established that among modifiable factors for diabetes mellitus, dietary factors have attracted more attention. However, many observational studies have highlighted the important role of single dietary antioxidants (e.g., polyphenols, vitamin C and minerals) in the prevention of diabetes mellitus (9–11, 38, 39), and examining the antioxidant capacity of the overall diet remains limited. To date, few epidemiological studies have investigated dietary TAC in relation to prediabetes and diabetes mellitus (7, 19–25), but the results are inconsistent. For example, in a French E3N-European Prospective Investigation into Cancer and Nutrition (EPIC) cohort, the total antioxidant capacity of the diet was inversely associated with type 2 diabetes in middle-aged women (22). Similarly, van der Schaft et al. (7) also observed that higher total dietary antioxidant capacity was associated with a reduced risk of type 2 diabetes among the total population. In contrast, Kashino et al. (23), in the Japan Public Health Center-based Prospective Study, failed to observe any significant association between dietary NEAC and type 2 diabetes. It is worth noting that in this meta-analysis, we found that higher dietary TAC intake was inversely associated with the risk of prediabetes and diabetes mellitus. The variations in these published studies may be ascribed to the following several reasons. First, the difference in dietary habits may contribute the inconsistent results. In all included studies, four studies were carried out in Iran, where dietary habits are different to the Western countries, such as France. Second, methods for measuring the dietary TAC are also different. For example, six of included studies used FRAP assay to measure the dietary TAC (7, 19, 20, 22, 24, 35), one used ORAC assay (36), and one used VCEAC (21). Thus, different methods might affect the true antioxidant capacity of overall diet. Third, the major contributors to dietary TAC were inconsistent across countries and regions. For instance, in Bialystok Polish Longitudinal University Study (PLUS) population, Cyuńczyk et al. (19), reported that the principal food sources of dietary TAC were coffee infusion, fruits and juices, tea infusion, nuts and seeds and vegetables without potatoes. However, in middle-aged French women, Mancini et al. (22) reported that the main contributors to dietary TAC were fruit, vegetables, alcoholic beverages and hot beverages without coffee. Notably, coffee has been reported to be the main contributor of dietary TAC in many countries (7, 22). Fourth, discrepancies in dietary assessment methods may contribute to the differing results. Daneshzad et al. (25) and Cyuńczyk et al. (19), used 24-h dietary recalls to obtain dietary intake information, Mancini et al. (22), used dietary questionnaire, and the remaining seven studies used FFQs (7, 20, 21, 23, 24, 35, 36). Taken together, variabilities in the dietary habits across countries, methods for measuring dietary TAC, dietary assessment methods, and types of foods may contribute to the different results.
Although evidence on the correlation between dietary TAC and risk of diabetes mellitus remains inconsistent, several potential mechanisms could be put forward to explain the protective effect. Firstly, previous studies have documented that dietary antioxidants can prevent oxidative stress (40), an important contributing factor in the pathogenesis of prediabetes and type 2 diabetes mellitus (41). Secondly, available evidence indicates that antioxidants, e.g., vitamin C, E and carotenoids, rich in fruits and vegetables are associated with a decreased risk of hypertension, which is implicated in the development of type 2 diabetes (42). Simultaneously, a recent updated systematic review and meta-analysis also showed that higher intake of dietary TAC was associated with reduced fasting blood sugar (43). Thirdly, higher dietary TAC intake has been reported to be related to the lower plasma concentration of high-sensitivity C-reactive protein (44). The evidence shows that increased inflammation can impair the secretion of insulin, leading to insulin resistance (45), a well-established risk factor for type 2 diabetes mellitus (46). Fourthly, as mentioned above, coffee is a major contributor of dietary TAC. Prior studies have confirmed the beneficial effect of coffee intake on the prevention of type 2 diabetes (47). Taken together, the above mechanisms may explain the beneficial effect of high dietary TAC intake against diabetes mellitus.
Our meta-analysis showed the protective associations between high dietary TAC intake and the risk of prediabetes and diabetes mellitus, but significant heterogeneity was also observed for prediabetes (I2 = 79.0%; p = 0.003) and diabetes mellitus (I2 = 80.2%; p < 0.001), respectively. For this reason, we carried out subgroup analyses to explore the possible sources of heterogeneity. In our analyses, subgroup analyses were performed based on study design, sex, study region, study quality, outcome, mean age, sample size, and methods for dietary assessment. The results showed that significant heterogeneity could be explained by the differences in study region, sample size, mean age, and outcome. When subgroup analyses were performed in a subgroup of mean age <50 and sample size <5,000, heterogeneity of this study decreased from 80.2 to 0.0%. Indeed, several plausible explanations have been put forward. First, RRs, HRs or ORs were from highest category vs. lowest category. However, different studies measuring the dietary TAC used different methods, such as FRAP assay or ORAC assay, and divided dietary TAC range into different intervals. These might result in significant heterogeneity. Second, all included studies were performed in Iran (24, 25, 36, 37), China (20), Korea (21), France (22), Japan (23), Netherlands (7), and Poland (19), respectively. These countries often have their own unique eating habits, which may lead to significant heterogeneity. Third, different confounding variables adjusted in the eligible studies may contribute to significant heterogeneity. Fourth, five of included studies were case-control and cross-sectional studies, which were more prone to recall and selection biases. We therefore could not determine the causality of observed relationship. Thus, the significant inverse associations observed in subgroups with small sample sizes (<5,000) should be taken with caution. Additionally, information on dietary intake were collected through FFQs and 24 h dietary recall, which might also lead to recall and selection biases. Finally, significant heterogeneity remained in the other subgroup analyses, indicating that there may be other unknown confounding factors.
Strengths and limitations
This meta-analysis has several strengths and limitations. First, this is the first systematic review and dose-response meta-analysis that has assessed the association between dietary TAC and risk of prediabetes and diabetes mellitus. Our findings add the available evidence for the favorable effect of higher dietary TAC intake on diabetes mellitus, and underscore the importance of higher dietary TAC intake in the prevention of diabetes mellitus. Second, all eligible articles have been strictly screened basing on our inclusion and exclusion criteria. Third, incident cases of prediabetes and diabetes mellitus were ascertained through medical records review, reducing possibility of misdiagnosis. Fourth, subgroup and sensitivity analyses further improved the accuracy of our findings. Finally, we also conducted a dose-response analysis to strengthen the relationship between dietary TAC and risk of diabetes mellitus. Nonetheless, several possible limitations should be acknowledged when interpreting the findings. First, due to the observational nature of included studies, no causal relationship can be drawn from the results. Meanwhile, half of included studies were case-control and cross-sectional studies. Thus, we could not fully rule out recall and selection biases. Further prospective cohort studies or randomized controlled trials are necessary to validate the results of this study. Second, in this study, dietary data was collected by using the self-completed FFQ or 24 h dietary records, which might lead to the under-or over-estimations of dietary TAC. As such, measurement errors were inevitable. Third, even though adjusting for some established potential confounders, residual confounding from undetected or unknown factors could not be excluded. Moreover, differences in adjustment for potential confounders (e.g., dietary patterns, physical activity, or socioeconomic status) across studies might affect the reliability of our findings. Fourth, significant heterogeneity was observed in our analyses. Although subgroup and sensitivity analyses were carried out to find the possible sources of heterogeneity, we were unable to fully explain the sources of inter-study heterogeneity. Finally, more than half of included studies were performed in Asian and Middle Eastern countries, limiting the generalizability of our findings to the broader population of Western countries.
Conclusion
In conclusion, this study showed that higher dietary TAC intake was inversely associated with the risk of prediabetes and diabetes mellitus. Our findings confirmed the existing evidence on the beneficial effects of dietary TAC on prediabetes and diabetes mellitus, and underscored the importance of higher dietary TAC intake in the prevention of diabetes mellitus. Moreover, our findings also support public health recommendations to encourage the consumption of dietary TAC. Nevertheless, further well-designed prospective studies or randomized controlled trials are needed to confirm these findings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
RL: Conceptualization, Methodology, Validation, Writing – original draft. LS: Formal analysis, Investigation, Methodology, Writing – original draft. QZ: Funding acquisition, Investigation, Methodology, Validation, Writing – original draft. DL: Conceptualization, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was funded by the National Natural Science Foundation of China (Grant No. 82004040).
Acknowledgments
The authors are grateful to Dr. Shu for his important contribution to data extraction and analysis in this study. In addition, the authors are also grateful to Dr. Zhu for providing additional data for this meta-analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1541734/full#supplementary-material
References
1. Cole, JB, and Florez, JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol. (2020) 16:377–90. doi: 10.1038/s41581-020-0278-5
2. Sun, H, Saeedi, P, Karuranga, S, Pinkepank, M, Ogurtsova, K, Duncan, BB, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. (2022) 183:109119. doi: 10.1016/j.diabres.2021.109119
3. Hemmingsen, B, Gimenez-Perez, G, Mauricio, D, Figuls, RI, Metzendorf, MI, and Richter, B. Diet, physical activity or both for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database Syst Rev. (2017) 2017:CD003054. doi: 10.1002/14651858.CD003054.pub4
4. Sullivan, VK, Kim, H, Caulfield, LE, Steffen, LM, Selvin, E, and Rebholz, CM. Plant-based dietary patterns and incident diabetes in the atherosclerosis risk in communities (ARIC) study. Diabetes Care. (2024) 47:803–9. doi: 10.2337/dc23-2013
5. Montonen, J, Knekt, P, Järvinen, R, and Reunanen, A. Dietary antioxidant intake and risk of type 2 diabetes. Diabetes Care. (2004) 27:362–6. doi: 10.2337/diacare.27.2.362
6. Carter, P, Gray, LJ, Troughton, J, Khunti, K, and Davies, MJ. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: systematic review and meta-analysis. BMJ. (2010) 341:c4229. doi: 10.1136/bmj.c4229
7. van der Schaft, N, Schoufour, JD, Nano, J, Kiefte-de Jong, JC, Muka, T, Sijbrands, EJG, et al. Dietary antioxidant capacity and risk of type 2 diabetes mellitus, prediabetes and insulin resistance: the Rotterdam study. Eur J Epidemiol. (2019) 34:853–61. doi: 10.1007/s10654-019-00548-9
8. Yaribeygi, H, Sathyapalan, T, Atkin, SL, and Sahebkar, A. Molecular mechanisms linking oxidative stress and diabetes mellitus. Oxid Med Cell Longev. (2020) 2020:8609213–3. doi: 10.1155/2020/8609213
9. Liu, C, Zhong, C, Chen, R, Zhou, X, Wu, J, Han, J, et al. Higher dietary vitamin C intake is associated with a lower risk of gestational diabetes mellitus: a longitudinal cohort study. Clin Nutr. (2020) 39:198–203. doi: 10.1016/j.clnu.2019.01.015
10. Bjørklund, G, Dadar, M, Pivina, L, Doşa, MD, Semenova, Y, and Aaseth, J. The role of zinc and copper in insulin resistance and diabetes mellitus. Curr Med Chem. (2020) 27:6643–57. doi: 10.2174/0929867326666190902122155
11. Golmohamadi, M, Hosseinpour-Niazi, S, Hadaegh, P, Mirmiran, P, Azizi, F, and Hadaegh, F. Association between dietary antioxidants intake and the risk of type 2 diabetes mellitus in a prospective cohort study: Tehran lipid and glucose study. Br J Nutr. (2024) 131:1452–60. doi: 10.1017/S0007114523002854
12. Das, UN. Vitamin C for type 2 diabetes mellitus and hypertension. Arch Med Res. (2019) 50:11–4. doi: 10.1016/j.arcmed.2019.05.004
13. Wang, S, and Zhu, F. Dietary antioxidant synergy in chemical and biological systems. Crit Rev Food Sci Nutr. (2017) 57:2343–57. doi: 10.1080/10408398.2015.1046546
14. Huang, Y, Ni, Y, Yu, L, Shu, L, Zhu, Q, and He, X. Dietary total antioxidant capacity and risk of stroke: a systematic review and dose-response meta-analysis of observational studies. Front Nutr. (2024) 11:1451386. doi: 10.3389/fnut.2024.1451386
15. Nascimento-Souza, MA, Paiva, PG, Martino, HSD, and Ribeiro, AQ. Dietary total antioxidant capacity as a tool in health outcomes in middle-aged and older adults: A systematic review. Crit Rev Food Sci Nutr. (2018) 58:905–12. doi: 10.1080/10408398.2016.1230089
16. Wang, P, Jiang, X, Tan, Q, Du, S, and Shi, D. Meal timing of dietary total antioxidant capacity and its association with all-cause, CVD and cancer mortality: the US national health and nutrition examination survey, 1999–2018. Int J Behav Nutr Phys Act. (2023) 20:83. doi: 10.1186/s12966-023-01487-1
17. Lucas, AL, Bosetti, C, Boffetta, P, Negri, E, Tavani, A, Serafini, M, et al. Dietary total antioxidant capacity and pancreatic cancer risk: an Italian case-control study. Br J Cancer. (2016) 115:102–7. doi: 10.1038/bjc.2016.114
18. Henríquez-Sánchez, P, Sánchez-Villegas, A, Ruano-Rodríguez, C, Gea, A, Lamuela-Raventós, RM, Estruch, R, et al. Dietary total antioxidant capacity and mortality in the PREDIMED study. Eur J Nutr. (2016) 55:227–36. doi: 10.1007/s00394-015-0840-2
19. Cyuńczyk, M, Zujko, ME, Jamiołkowski, J, Zujko, K, Łapińska, M, Zalewska, M, et al. Dietary total antioxidant capacity is inversely associated with prediabetes and insulin resistance in Bialystok PLUS population. Antioxidants. (2022) 11:283. doi: 10.3390/antiox11020283
20. Li, X, Xue, Y, Zhang, Y, Wang, Q, Qiu, J, Zhang, J, et al. Association between dietary antioxidant capacity and type 2 diabetes mellitus in Chinese adults: a population-based cross-sectional study. Nutr Metab. (2024) 21:16. doi: 10.1186/s12986-024-00786-z
21. Tan, LJ, Hwang, SB, Jun, S, Joung, H, and Shin, S. Dietary antioxidant consumption and the risk of type 2 diabetes in South Korean adults: a prospective cohort study based on the health examinees study. BMJ Open. (2022) 12:e065073. doi: 10.1136/bmjopen-2022-065073
22. Mancini, FR, Affret, A, Dow, C, Balkau, B, Bonnet, F, Boutron-Ruault, MC, et al. Dietary antioxidant capacity and risk of type 2 diabetes in the large prospective E3N-EPIC cohort. Diabetologia. (2018) 61:308–16. doi: 10.1007/s00125-017-4489-7
23. Kashino, I, Serafini, M, Kurotani, K, Akter, S, Mizoue, T, Ishihara, J, et al. Relationship between dietary non-enzymatic antioxidant capacity and type 2 diabetes risk in the Japan Public Health Center-based Prospective Study. Nutrition. (2019) 66:62–9. doi: 10.1016/j.nut.2019.03.011
24. Heshmati, S, Moludi, J, Nachvak, SM, Pirjani, R, Heshmati, J, and Sepidarkish, M. The association of dietary total antioxidant capacity and gestational diabetes: a prospective cohort study from the mothers and their children’s health (MATCH). Nutr Diabetes. (2024) 14:78. doi: 10.1038/s41387-024-00333-y
25. Daneshzad, E, Tehrani, H, Bellissimo, N, and Azadbakht, L. Dietary total antioxidant capacity and gestational diabetes mellitus: a case-control study. Oxid Med Cell Longev. (2020) 2020:5471316. doi: 10.1155/2020/5471316
26. Stroup, DF, Berlin, JA, Morton, SC, Olkin, I, Williamson, GD, Rennie, D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
27. Stang, A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
28. Zhu, Q, Shu, L, Zhou, F, Chen, LP, and Feng, YL. Adherence to the Mediterranean diet and risk of gastric cancer: a systematic review and dose-response meta-analysis. Front Nutr. (2023) 10:1259453. doi: 10.3389/fnut.2023.1259453
29. Symons, MJ, and Moore, DT. Hazard rate ratio and prospective epidemiological studies. J Clin Epidemiol. (2002) 55:893–9. doi: 10.1016/s0895-4356(02)00443-2
30. Grant, RL. Converting an odds ratio to a range of plausible relative risks for better communication of research findings. BMJ. (2014) 348:f7450. doi: 10.1136/bmj.f7450
31. Higgins, JP, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
32. Begg, CB, and Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
33. Duval, S, and Tweedie, R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x
34. Greenland, S, and Longnecker, MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. (1992) 135:1301–9. doi: 10.1093/oxfordjournals.aje.a116237
35. Rahmani, J, Parastouei, K, Taghdir, M, Santos, HO, Hosseini Balam, F, and Saberi, IM. Healthy Eating Index-2015 and dietary total antioxidant capacity as predictors of prediabetes: a case-control study. Int J Endocrinol. (2021) 2021:2742103–7. doi: 10.1155/2021/2742103
36. Sotoudeh, G, Abshirini, M, Bagheri, F, Siassi, F, Koohdani, F, and Aslany, Z. Higher dietary total antioxidant capacity is inversely related to prediabetes: a case-control study. Nutrition. (2018) 46:20–5. doi: 10.1016/j.nut.2017.08.005
37. Pearson-Stuttard, J, Cheng, YJ, Bennett, J, Vamos, EP, Zhou, B, Valabhji, J, et al. Trends in leading causes of hospitalisation of adults with diabetes in England from 2003 to 2018: an epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol. (2022) 10:46–57. doi: 10.1016/S2213-8587(21)00288-6
38. Grosso, G, Stepaniak, U, Micek, A, Kozela, M, Stefler, D, Bobak, M, et al. Dietary polyphenol intake and risk of type 2 diabetes in the Polish arm of the Health, Alcohol and Psychosocial factors in Eastern Europe (HAPIEE) study. Br J Nutr. (2017) 118:60–8. doi: 10.1017/S0007114517001805
39. Zamora-Ros, R, Forouhi, NG, Sharp, SJ, González, CA, Buijsse, B, Guevara, M, et al. Dietary intakes of individual flavanols and flavonols are inversely associated with incident type 2 diabetes in European populations. J Nutr. (2014) 144:335–43. doi: 10.3945/jn.113.184945
40. Basu, A, Alman, AC, and Snell-Bergeon, JK. Associations of dietary antioxidants with glycated hemoglobin and insulin sensitivity in adults with and without type 1 diabetes. J Diabetes Res. (2022) 2022:4747573–8. doi: 10.1155/2022/4747573
41. Lee, SH, Park, SY, and Choi, CS. Insulin resistance: from mechanisms to therapeutic strategies. Diabetes Metab J. (2022) 46:15–37. doi: 10.4093/dmj.2021.0280
42. Livesey, G, Taylor, R, Livesey, HF, Buyken, AE, Jenkins, DJA, Augustin, LSA, et al. Dietary glycemic index and load and the risk of type 2 diabetes: a systematic review and updated meta-analyses of prospective cohort studies. Nutrients. (2019) 11:1280. doi: 10.3390/nu11061280
43. Farhangi, MA, and Mohammad-Rezaei, A. Higher dietary total antioxidant capacity (TAC) reduces the risk of cardio-metabolic risk factors among adults: an updated systematic review and meta-analysis. Int J Vitam Nutr Res. (2023) 93:178–92. doi: 10.1024/0300-9831/a000708
44. Brighenti, F, Valtueña, S, Pellegrini, N, Ardigò, D, del, D, Salvatore, S, et al. Total antioxidant capacity of the diet is inversely and independently related to plasma concentration of high-sensitivity C-reactive protein in adult Italian subjects. Br J Nutr. (2005) 93:619–25. doi: 10.1079/bjn20051400
45. Luc, K, Schramm-Luc, A, Guzik, TJ, and Mikolajczyk, TP. Oxidative stress and inflammatory markers in prediabetes and diabetes. J Physiol Pharmacol. (2019) 70:809–824. doi: 10.26402/jpp.2019.6.01
46. Cruz, NG, Sousa, LP, Sousa, MO, Pietrani, NT, Fernandes, AP, and Gomes, KB. The linkage between inflammation and type 2 diabetes mellitus. Diabetes Res Clin Pract. (2013) 99:85–92. doi: 10.1016/j.diabres.2012.09.003
47. Grosso, G, Godos, J, Galvano, F, and Giovannucci, EL. Coffee, caffeine, and health outcomes: an umbrella review. Annu Rev Nutr. (2017) 37:131–56. doi: 10.1146/annurev-nutr-071816-064941
Glossary
ABTS – 2,2′-azino-bis (3-ethylbenzthiazoline) 6-sulfonic acid
CI – Confidence interval
CNKI – China National Knowledge Infrastructure
CVD – Cardiovascular disease
EPIC – European Prospective Investigation into Cancer and Nutrition
FFQ – Food frequency questionnaire
FRAP – Ferric reducing antioxidant potential
GBD – Global Burden of Disease Study
HR – Hazard ratio
IDF – International Diabetes Federation
NEAC – Non-enzymatic antioxidant capacity
NOS – Newcastle–Ottawa Quality Scale
OR – Odds ratio
ORAC – Oxygen radical absorbance capacity
PRISMA – Preferred Reporting Items for Systematic Reviews and Meta-Analyses
RR – Relative risk
TAC – Total antioxidant capacity
TEAC – Trolox equivalent antioxidant capacity
TRAP – Total radical-trapping antioxidant parameter
VCEAC – Vitamin C equivalent antioxidant capacity
Keywords: dietary total antioxidant capacity, prediabetes, diabetes mellitus, systematic review, dose-response meta-analysis
Citation: Li R, Shu L, Zhu Q and Lu D (2025) Dietary total antioxidant capacity and risk of prediabetes and diabetes mellitus: a systematic review and dose-response meta-analysis of 170,919 participants. Front. Nutr. 12:1541734. doi: 10.3389/fnut.2025.1541734
Edited by:
Neha Garg, Banaras Hindu University, IndiaReviewed by:
Antonella Al Refaie, University of Siena, ItalyGulzar Ahmed Rather, Sathyabama Institute of Science and Technology, India
Copyright © 2025 Li, Shu, Zhu and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dan Lu, MTUzODIzODEwODZAMTYzLmNvbQ==
 Ruomeng Li1
Ruomeng Li1 Long Shu
Long Shu Qin Zhu
Qin Zhu Dan Lu
Dan Lu