
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Nutr., 12 March 2025
Sec. Nutritional Epidemiology
Volume 12 - 2025 | https://doi.org/10.3389/fnut.2025.1539665
This article is part of the Research TopicNutrition, Inflammation and Oxidative Stress in Obstetrics and GynecologyView all 12 articles
Endometriosis is an estrogen-dependent chronic inflammatory disease which causes dysmenorrhea, chronic pelvic pain, and infertility in women of childbearing age, significantly impacting their quality of life and physical and mental health. The etiology of endometriosis remains unclear, with oxidative stress and inflammation currently thought to play pivotal roles in its pathophysiology. Epidemiological studies and clinical trials indicate that varying dietary patterns and specific nutrient supplementation can influence oxidative stress markers and levels of inflammatory factors and related pathways, potentially impacting the progression of endometriosis. In this review, we summarize the roles of oxidative stress and inflammation in endometriosis and thoroughly examine the current understanding of the effect of dietary patterns and nutrient supplementation in treating endometriosis. This study suggests that nutrients may prevent the occurrence of endometriosis by modulating levels of inflammatory factors, regulating angiogenesis, and influencing the metabolism of estrogen pathways. The findings might provide new insights into the treatment of endometriosis patients and the potential benefits of dietary patterns and nutrient supplementation in patients with endometriosis.
Endometriosis is a common gynecological disorder characterized by the presence of endometrial tissue outside the uterus (1), typically presenting with severe pelvic pain and infertility, which affects 5–15% of reproductive-age women and as much as 3–5% of postmenopausal women (2). Endometriosis is a multifaceted, heterogeneous disease with no clear pathogenesis yet identified. Current research suggests that oxidative stress and inflammation play important roles in endometriosis. In the peritoneal fluid of patients with endometriosis, the concentration of numerous inflammatory factors, angiogenesis-related factors, and adhesion factors is elevated (3), and the activation of inflammation accelerates the progression of endometriosis (4). Endometriosis lesions stimulate the production of inflammatory factors and growth factors in the abdominal cavity. The persistent inflammatory response interacts with the central nervous system, resulting in chronic pain (5). Chronic pelvic pain significantly affects endometriosis patients’ quality of life and social functioning (6).
Dietary intervention and specific dietary patterns can influence the onset and outcomes of inflammation-related diseases, including cardiovascular disease, cancer, diabetes, and obesity (7). Different dietary patterns alter the levels of inflammatory factors and inflammation pathways in the body, affecting the occurrence of endometriosis. Ingestion of specific foods affects the levels of inflammatory factors, regulates angiogenesis, modulates the estrogen metabolism pathway in the body (8), and promotes or inhibits the development of endometriosis (9). The risk of endometriosis can be reduced by adjusting dietary intake, such as consuming nutrients with anti-inflammatory and antioxidant properties. Current research generally suggests that a high intake of green vegetables and fresh fruits (10), dairy products (11), legumes (12), polyphenols, and fish oil (13) significantly reduces the risk of endometriosis. Those with a high intake of red meat (14) and trans-fatty acids (15) have an increased risk of endometriosis. Women with endometriosis resort to non-medical methods to manage symptoms and enhance daily living (16). Self-care activities, complementary therapies, and positive doctor-patient relationships are critical components of endometriosis self-management.
However, a few studies have yielded inconsistent results, and there is still a lack of large-scale, high-quality studies to verify the relationship among dietary patterns, nutrients, and endometriosis. The role of different dietary patterns and nutrients in endometriosis is still ambiguous. This review aims to provide an overview of oxidative stress and inflammation in endometriosis, and comprehensively summarize the effects and potential mechanisms of various dietary patterns and nutrients in endometriosis (Figure 1).
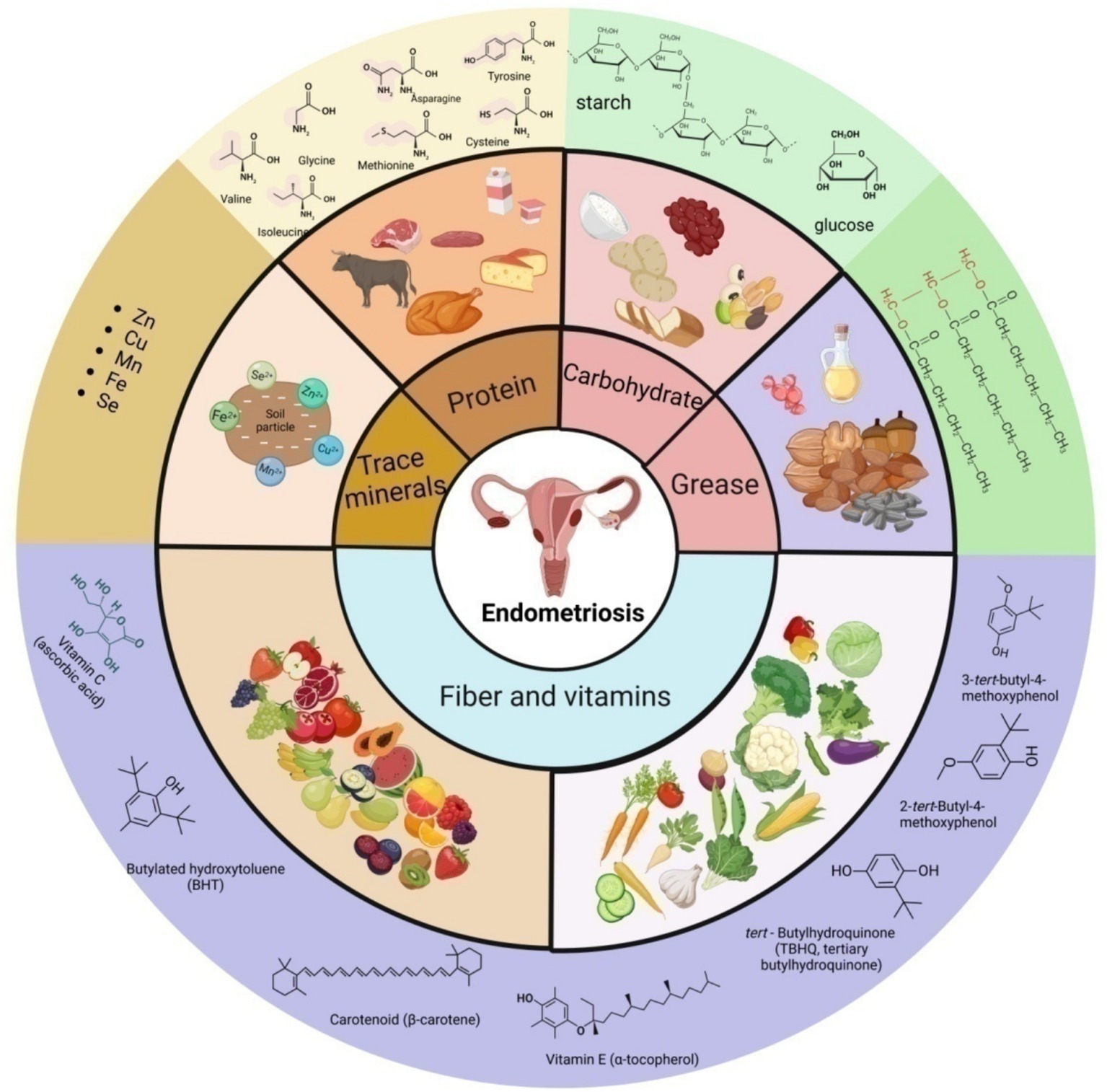
Figure 1. Schematic presentation of the main source, classification and some representative components of nutrition in endometriosis.
There is evidence that endometriosis can be caused by retrograde menstruation (17), endometrial implantation, epithelial transformation (18), hormonal effects (19), and immune system dysfunction (20). Moreover, peritoneal fluid from people with endometriosis has unusual amounts of angiogenic and adhesion factors, as well as numerous inflammatory markers. This suggests that long-term inflammation plays a part in how endometriosis starts and gets worse (3). Endometriosis lesions stimulate the production of inflammatory cytokines and growth factors within the abdominal cavity, where the activation of inflammation further advances the progression of endometriosis (4).
Currently, it is believed that dietary ingredients with anti-proliferative, anti-inflammatory, antioxidant, analgesic, and estrogen-reducing properties can reduce the incidence of endometriosis. Nevertheless, the exact modus operandi remains obscure. Consequently, we have meticulously updated and comprehensively rearticulated these two mechanisms, with a particular focus on innovative and all-encompassing methodologies (Figure 2).
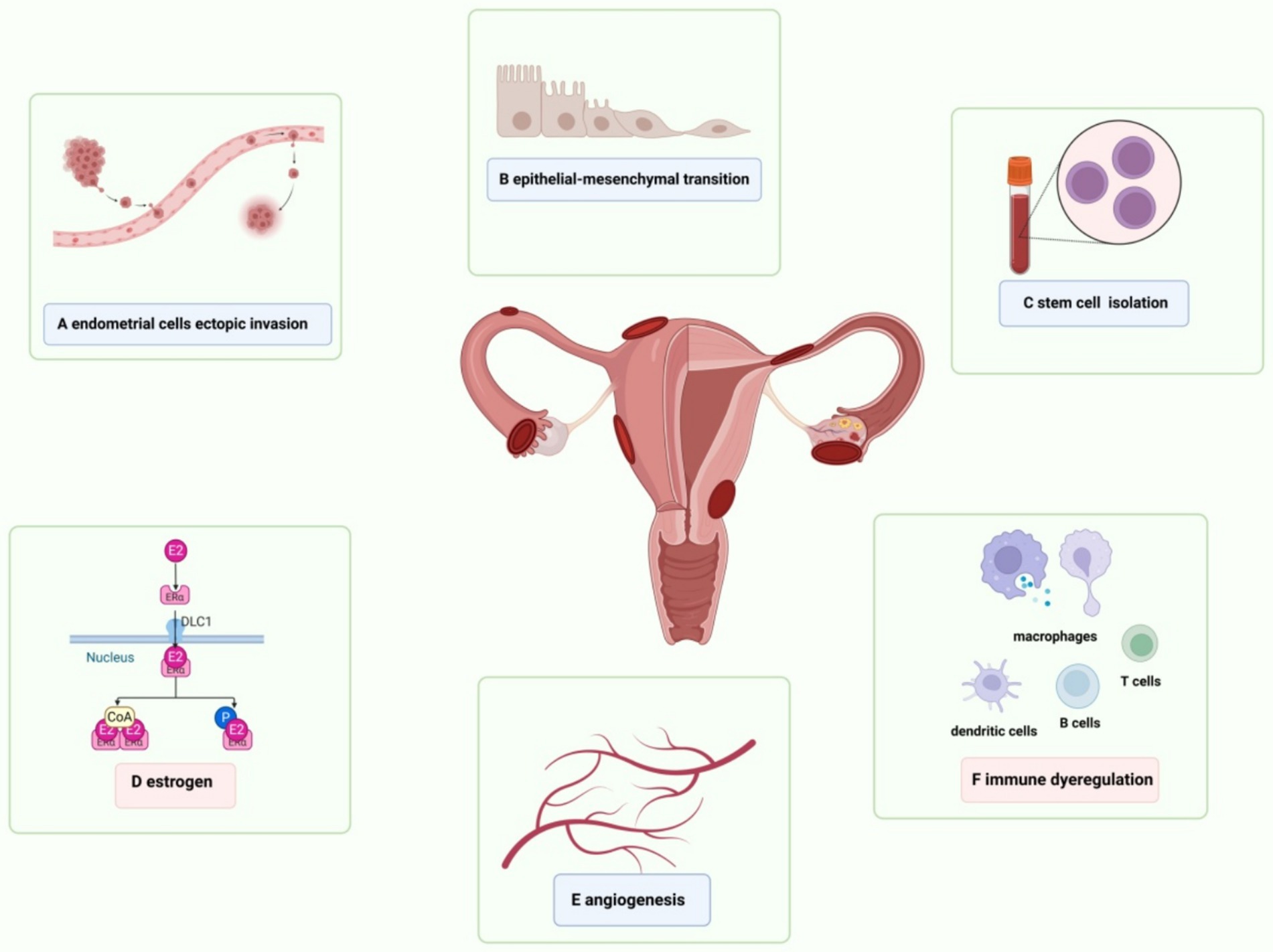
Figure 2. Schematic presentation of risk factors for endometriosis. (A) The exfoliated endometrial cells retrograde into the pelvic cavity and are implanted into the peritoneum and abdominal organs. (B) Epithelial-mesenchymal transformation is involved in the process of local inflammatory damage, repair, fibrosis, cell invasion and metastasis in endometriosis, as well as the formation of local lesions. (C) Adult stem cells are detected in endometrial suspension, which drive physiological endometrial regeneration and participate in the pathogenesis of endometriosis. (D) VEGF induces endothelial cell proliferation, migration and angiogenesis in endometriosis. (E) Elevated estrogen levels and the increased activity of estrogen receptors regulate immunity, inflammation and angiogenesis, and promote the continuous progression of endometrial lesions. (F) Abnormal activation of immune response and changes in peritoneal immune microenvironment are the basis of endometriosis.
Macrophages, red blood cells, and apoptotic endometrial tissue transplanted into the peritoneal cavity via retrograde menstruation serve as inducers of oxidative stress (21). The release of hemoglobin and iron from red blood cells activates macrophages, leading to the production of inflammatory factors including interleukin-1, interleukin-6, and tumor necrosis factor-alpha (22) and fostering the formation of damaging reactive oxygen species (23). The iron storage levels (ferritin load) in the peritoneal macrophages of patients with endometriosis are significantly elevated compared to the control group (24). The continuous increase in iron content triggers oxidative stress, resulting in local damage to the peritoneal mesothelium and creating adhesion sites for ectopic endometrial cells (25). Iron overload further exacerbates the condition by stimulating cell proliferation, thereby advancing endometriosis (26, 27).
ROS are intermediates generated during normal oxygen metabolism in the human body, with a delicate balance maintained between pro-oxidants and antioxidants in healthy individuals. Mitochondria generate ROS via the electron transport chain, a process termed oxidative phosphorylation (OXPHOS) (28). An excess of oxidants triggers oxidative stress (29), leading to a significant elevation in mitochondrial superoxide levels within ectopic endometrial tissue (30), which alters mitochondrial structure, particularly by expanding the surface area of the cristae. This influences OXPHOS function and leads to increased ROS production (31). Excess ROS regulates cell proliferation (32) and leads to the overactivation of nuclear factor kappa-B (NF-κB) by IL-1 and TNF-α. NF-κB enhances the invasive and adhesive capacities of endometriotic cells to the peritoneal surface by regulating the expression of matrix metalloproteinases, thereby stimulating angiogenesis and inflammation (33). Although mitochondria are the primary ROS producers, they are vulnerable to ROS attacks (30).
The coordinated action of macrophages, endometrial cells, endothelial cells, and activated lymphocytes, along with cytokines and chemokines, alters the abdominal immune microenvironment, facilitating pathological processes including cell proliferation, angiogenesis, lesion adhesion, growth, and invasion (34). Macrophage numbers significantly rise in peritoneal fluid and ectopic endometrium (35), and co-culturing macrophages with endometrial stromal cells (ESCs) enhances the proliferative and invasive capacities of ESCs (36). Co-culturing ESCs with monocyte-derived macrophages and NK cells from endometriosis patients showed that the interaction between ESCs and macrophages downregulates NK cell cytotoxicity by enhancing the release of IL-10 and TNF-β (37), potentially allowing endometrial fragments to evade immune clearance (38). Studies have reported that patients with endometriosis have an increased presence or activation of B lymphocytes (39). Anti-endometrial antibodies have been detected in the serum and peritoneal fluid of patients with endometriosis (40). These autoantibodies can stimulate the immune system, causing persistent inflammation, advancing endometriosis, and contributing to the formation of the inflammatory microenvironment.
The endometriosis microenvironment may enhance ERK activity in endometriotic cells. TNFα and IL-1β are capable of activating ERK and inducing the expression of IL-8 and IL-6 (41). The chemokine MCP1 also greatly increases the production of PGE2 (42), VEGF, IL-8, and MCP-1 in human endometriotic cells through a pathway specific to ERK (43).
The PI3K/AKT/mTOR pathway regulates cell growth, proliferation, differentiation, and apoptosis (44). Membrane-bound phosphoinositide 3-kinase (PI3K) is the most common mediator of mTOR activation, forming the core of the PI3K/AKT/mTOR pathway along with AKT (45). When PI3K is turned on, it causes PDK1 to be phosphorylated by AKT. This then turns on downstream mTOR receptors by interacting with TSC2 (46). The TSC complex, consisting of TSC1 and TSC2, interacts with GTPase-Ras, acting as a negative regulator of mTOR activity (47). When TSC2 is phosphorylated by kinases such as AKT, it dissociates from the complex, leading to the inactivation of the complex and the subsequent activation of mTORC1 by Rheb, which is bound to GTP (48). When activated, mTORC1 activates numerous downstream proteins, promoting protein synthesis and cell growth. Additionally, mTORC1 activates HIF-alpha through VEGF, serving as the primary angiogenic switch (49), thereby inducing new angiogenesis. LncRNA IGF2-AS facilitates the progression of endometriosis by targeting the miR-370-3p/IGF2 axis and activating the PI3K/AKT/mTOR signaling pathway (50). Interventions targeting HIF-1α and mTOR pathways may emerge as potential therapeutic targets for endometriosis (51).
Additionally, E2 regulates protein and DNA synthesis in uterine epithelial cells via the PKC/ERK/mTOR pathway, ultimately controlling cell proliferation (52). In endometrial stromal cells (ESC), TNFα-induced activation of the estrogen receptor ER leads to heightened ERK activation (53). Lipoprotein A4 suppresses inflammation and enhances autophagy via the AhR/mTOR/AKT pathway, thereby inhibiting endometriosis (54).
IL-37b, a unique member of the IL-1 family, may inhibit lesion growth by regulating proliferation, invasion, angiogenesis, and inflammation through the AKT and ERK1/2 signaling pathways (55). Recombinant human IL-37 enhances the Th1/Th2 ratio by inducing dendritic cell maturation, thus curbing the progression of endometriosis in mouse models (56). Exogenous IL-1β increases the production of nerve growth factor in primary endometrial stromal cells in endometriosis. This is associated with the onset of endometriosis and the concomitant pain (57). Interleukin-33 is a member of the IL-1β family. In mouse models that did not have IL-33, the size of endometriotic lesions was greatly reduced (58). When injected into a monkey model of endometriosis with its long-acting recovery antibody (AMY109), IL-8, a potent chemoattractant for angiogenic factors and immune cells, can inhibit neutrophil recruitment to endometrial lesions and suppress their production of monocyte chemotactic protein-1, thereby ameliorating endometriosis inflammation and fibrosis (59). IL-17 is upregulated in the serum, peritoneal fluid (PF), and endometrial lesions of patients with endometriosis (60), regulating the recruitment and M2 polarization of peritoneal macrophages in endometriosis (61).
HSP-70, a chaperone protein within the heat shock protein family, is produced by macrophages, smooth muscle cells, endometrial cells, dendritic cells, and vascular endothelial cells. It significantly stimulates the production of vascular endothelial growth factor, interleukin-6, and TNF-α in women with endometriosis. Furthermore, HSP-70 facilitates Toll-like receptor 4 (TLR4)-mediated growth of endometrial cells (62). The decrease in HSP-70 may facilitate unrestricted nuclear translocation of NF-κB, resulting in targeted transcription (Figure 3).
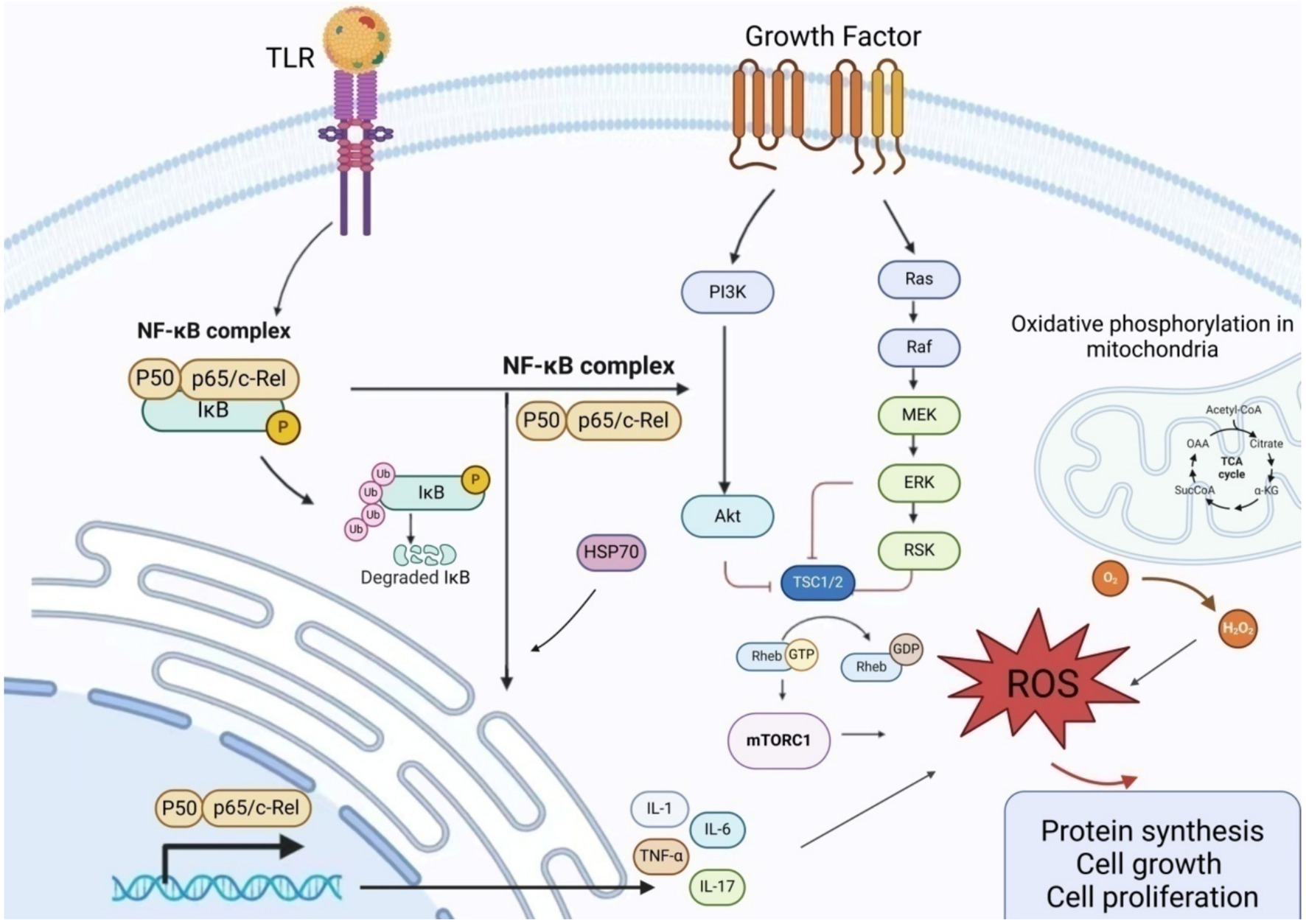
Figure 3. Schematic presentation of the mechanism of action of inflammatory factors in endometriosis NF-κB promotes the production and release of inflammatory factors by regulating the expression of matrix metalloproteinases. Excessive ROS regulates cell proliferation and induces excessive activation of NF-κB by IL-1 and TNF-α, thereby attacking mitochondria. The PI3K/AKT/mTOR pathway regulates cell growth, proliferation, differentiation, and apoptosis. Upon PI3K activation, AKT phosphorylation of PDK1 is stimulated, and the interaction between AKT and TSC2 activates downstream mTOR receptors. The TSC complex, consisting of TSC1 and TSC2, interacts with GTPase-Ras to serve as a negative regulator of mTOR activity. When TSC2 is phosphorylated by kinases (such as AKT), it dissociates from the complex, rendering it inactive. This leads to Rheb binding to GTP, activating mTORC1, and triggering an oxidative stress response. AKT, Protein Kinase B; ERK, Extracellular Signal-Regulated Kinase; GDP, Guanosine DiPhosphate; GTP, Guanosine TriPhosphate; MEK, Mitogen-Activated Protein Kinase; mTORC1, Mechanistic Target of Rapamycin Complex 1; NF-κB, Nuclear factor kappa-B; PI3K, Phosphatidylinositol 3-Kinase; RAF, RAF kinase; RAS, Rat Sarcoma Virus Oncogene; ROS, Reactive oxygen species; TRL, Toll-like receptor; TSC, Tuberous Sclerosis Complex.
Endometriosis is characterized by hormone dependency and a high recurrence rate, necessitating lifelong management. Dietary intervention is currently one of the main methods for self-management for endometriosis patients, with numerous studies indicating that a higher intake of vitamin-rich foods reduces the risk of endometriosis (63–65). Excessive intake of red meat is associated with increased levels of estrogen sulfate, leading to higher levels of steroids and inflammatory factors that influence the progression of endometriosis (66, 67). Consuming dairy products, fruits, legumes, red meat, and potatoes is significantly associated with a reduced risk of endometriosis. Consuming fried potatoes, on the other hand, has a positive correlation with the risk of endometriosis. Intake of animal proteins, EPA, MUFA, and oleic acid is associated with a reduced risk of endometriosis (68). Those in the top fifth with the highest intake of trans-unsaturated fats have a 48% higher likelihood of being diagnosed with endometriosis (15). In the absence of ovarian dysfunction and insulin resistance in mice, high dietary fat intake increases the risk of endometriosis (69).
Most of the aforementioned studies focus on the association between specific foods and endometriosis, failing to establish a link between the participants’ overall dietary nutrient intake and the condition. When other factors are taken into account, the findings may be contradictory. Schwarz (70) found that the beneficial effects associated with fruit fiber vanished after adjusting for the healthy diet index. Furthermore, an elevated risk of endometriosis was noted when combining the total intake of vegetables and cruciferous fiber. Currently, there are no specific dietary recommendations for endometriosis patients. We have outlined the key features of prevalent dietary patterns and explored the connections between various dietary patterns and endometriosis.
Mediterranean diet (MedDiet) includes eating a lot of fresh, seasonal, minimally processed plant-based foods, like vegetables, fruits, legumes, potatoes, bread, nuts, seeds, and other grains; a lot of olive oil (OO), especially virgin olive oil (VOO) and extra-virgin olive oil (EVOO), which is the main source of fat; some dairy products, like cheese and yogurt; and some poultry and fish, which are main sources of long-chain polyunsaturated fatty acids (PUFAs) (71), especially omega-3 fatty acids. Typically, the caloric intake from fat should not exceed 30%, with less than 8–10% from saturated fats.
The various nutrients in MedDiet, such as vitamins, minerals, polyphenols, fibers, nitrates, PUFAs, and monounsaturated fatty acids (MUFAs), are biologically active compounds that are beneficial to health (72, 73). They have synergistic and interactive effects in reducing inflammation (73) and affect different inflammatory markers in the body, such as IL-6 or TNF-α (74, 75). Flavonoids in grains, vegetables, fruits, and olive oil have potentially beneficial effects, including free radical scavenging, anti-inflammatory effects, and anti-Aβ neurotoxicity. Dietary polyphenols can also effectively combat ROS, reduce oxidative damage to genetic material, and enhance the antioxidant capacity of endothelial cells (76). The polyphenols in olive oil regulate the inflammation by inhibiting NF-κB (77). Resveratrol can improve the antioxidant status of Parkinson’s disease rats and reduce dopamine loss (78). When several polyphenols are used in combination, they exhibit stronger antioxidant capacity in terms of their activity (79). Hydroxytyrosol, which is found in extra virgin olive oil, can lower inflammation by stopping cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase from working (80). HT can also reduce superoxide ions and inhibit the excessive secretion of the important inflammatory mediator prostaglandin E2 in the human body (81).
Olive oil that is high in MUFA is crucial for keeping the lipid profile of mitochondrial membranes in check so that they can fight oxidative damage and age-related problems (82). People with a higher intake of olive oil have more MUFA in their mitochondrial membranes, while those who mainly consume sunflower oil have more omega-6 PUFA. These changes are consistent with the level of oxidative damage; namely, compared to those who consume sunflower oil, those who consume OO have fewer hydroperoxides in their body tissues. Therefore, a diet rich in olive oil produces membranes with less PUFA, which weakens the increase in lipid peroxidation (83). HT and oleuroside can reduce oxidative stress and optimize mitochondrial function (84). Fish oil is a beneficial source of omega-3 PUFAs. Giving older mice fish oil for 21 days increased the amount of omega-3 PUFA derivatives in their brains, which led to better mitochondrial function and ATP production (85). Fish oil omega-3 supplementation may reduce endometrial implant growth and inflammatory factor production, particularly in patients with stage III or IV endometriosis (13). Supplementing omega-3 polyunsaturated fatty acids alleviates pain symptoms related to endometriosis (86).
It is now believed that the long-term Mediterranean diet has a potential protective effect on cardiovascular diseases, stroke, obesity, diabetes, hypertension, malignant tumors, allergic diseases, as well as Alzheimer’s disease and Parkinson’s disease (87). Patients with endometriosis who follow the Mediterranean diet can reduce inflammatory factors in their bodies and alleviate pain symptoms (88).
The characteristic of a low-carbon ketogenic diet is the consumption of extremely high-fat and low-carbohydrate diets, with carbohydrate intake ≤10% of energy consumption (89). This dietary pattern forces the system to shift from glucose metabolism to fatty acid metabolism, thereby producing ketones. A ketogenic diet increases low-density lipoprotein cholesterol in healthy, young, normal-weight women (90). Low carbohydrate intake causes physiological ketosis in patients with polycystic ovary syndrome, which reduces circulating insulin levels, lowers IGF-1 levels, and inhibits stimulation of androgen production, including in the ovaries and adrenal glands. Reducing circulating lipids, low-grade inflammation, and oxidative stress also helps prevent cardiovascular complications (91, 92). Low-fat and low-carbohydrate diets significantly reduce the concentrations of several serum inflammatory markers, such as TNF-α, IL-6, IL-8, MCP-1, etc. (93). After 21 days on a carefully formulated ketogenic diet (WFKD), women show improvements in body composition, blood pressure, and blood sugar; increased ketone bodies; and improvements in some, but not all, cholesterol markers (94). WFKD can improve fasting blood glucose, insulin resistance, weight, and body composition in women with stage IV metastatic breast cancer. WFKD serves as an adjunctive therapy, playing a positive role (95).
A low-carbohydrate ketogenic diet can alleviate chronic musculoskeletal pain, improve blood biomarkers, and enhance the quality of life of patients (96). The ketogenic diet regulates gut microbiota and short-chain fatty acids, which are associated with Alzheimer’s disease markers in subjects with mild cognitive impairment (97).
FODMAP stands for fermentable oligosaccharides, disaccharides, monosaccharides, and polyols. These osmotic carbohydrates are non-absorbable and undergo bacterial fermentation in the small intestine, leading to functional gastrointestinal symptoms such as cramps, bloating, and diarrhea (98). A short-term moderate-low-FODMAP diet can significantly alleviate gastrointestinal symptoms, with significant reductions in pain, bloating, diarrhea, and fullness scores (99).
The symptoms of endometriosis are closely related to other chronic diseases such as irritable bowel syndrome (IBS) (100), inflammatory bowel disease (IBD), and celiac disease (CD) (101). A low-FODMAP diet reduces irritable bowel symptoms in patients with endometriosis (102, 103). After 6 months on a low-FODMAP diet, women with endometriosis reported reduced pain and improved quality of life (104). After 3 months on a low-FODMAP diet, significant improvements were observed in all chronic pelvic pain, dysmenorrhea, and both intestinal and extraintestinal symptoms (105).
GFD involves avoiding foods containing gluten (such as wheat, barley, spelled kham, rye, and triticale). The diet typically includes carbohydrates without gluten (GF) sources (such as rice, quinoa, corn, buckwheat, and legumes) and/or industrial GF products. Gluten-free products are typically high in saturated fats, sugars, and salts (106) and low in protein, fiber, and vitamins (107). Pain symptoms of endometriosis were alleviated after 12 months on a gluten-free diet (108). Celiac disease (CD) is an autoimmune disorder affecting 1% of the population, causing reversible inflammation of the small intestine mucosa and accompanied by acute symptoms such as diarrhea, constipation, bloating, nausea, and vomiting. A lifelong gluten-free diet (GFD) is a treatment for Crohn’s disease (109). However, some studies suggest that the increased gut side effects of a gluten-free diet may lead to adverse health outcomes (Table 1) (101).
The pathogenesis of endometriosis remains under investigation, prompting many affected women to adopt dietary interventions to manage symptoms and enhance daily living (16). A cross-sectional study revealed a dietary shift among endometriosis patients, with increased consumption of vegetables, fruits, grains, legumes, and fish, coupled with a decrease in dairy products, soy-based foods, and high saturated fat intake (110). An Italian study reported that 76% of women with endometriosis employed self-management strategies, with 44% opting for dietary changes, achieving a high score in dietary management effectiveness (111).
Various dietary patterns influence the onset of endometriosis by modifying the levels of inflammatory factors and the inflammation pathways within the body. Consuming specific foods influences the levels of inflammatory factors, regulates angiogenesis, affects the estrogen metabolism pathway (8), and can promote or inhibit the progression of endometriosis. Supplementation with antioxidant vitamins can effectively reduce dysmenorrhea severity, ameliorate chronic pelvic pain, and enhance the quality of life for these patients (112). The risk of endometriosis can be mitigated by dietary adjustments, particularly the consumption of nutrients with anti-inflammatory and antioxidant properties (113). We summarized the antioxidant capacity of various nutrients and their roles in animal models of endometriosis and endometriosis patients.
VC can inhibit ROS and AKT/mTOR signaling, thereby preventing mesenchymal stem cell aging (114). Additionally, VC inhibits HIF1a transcription and increases HIF1 alpha-hydroxylase activity, leading to mitochondrial activation (115) and antioxidant effects. In mouse models, VC contributes to anti-ovarian aging by influencing collagen synthesis, angiogenesis, aging, cell proliferation, and differentiation (116). Intravenous vitamin C treatment prevents the induction of endometrial implants while inhibiting the regression of existing ones in mouse models (117). VE significantly lowers serum levels of CRP and IL-6 (118). Vitamin E enhances antioxidant status by increasing TAC levels in postmenopausal women, offering greater anxiety relief compared to the placebo group (119). Vitamin C and E supplementation alters the expression and production of vascular endothelial growth factor genes in patients with endometriosis (120), effectively reducing the severity and improving pelvic pain (121).
VD can inhibit the activation of nuclear factor kappa B, reduce the expression of IL-1β, TNF-α, and IL-8, and decrease prostaglandin activity (122). In a mouse model, supplementation with VD induces an anti-inflammatory phenotype in macrophages and exhibits anti-proliferative properties (123). Serum levels of 25-hydroxyvitamin D3 negatively correlate with endometriosis (124). People with endometriosis who take vitamin D have much less pelvic pain and lower levels of hs-CRP, TAC, and total cholesterol/high-density lipoprotein cholesterol ratio (125). Supplementation with VD reduces pelvic pain in young patients with endometriosis (126) and ameliorates immune-inflammatory biomarkers in young postmenopausal women (127).
The antioxidant and anti-inflammatory properties of tea polyphenols may have potential therapeutic effects on endometriosis (128). The amount of mRNA for nuclear factor kappa B and mitogen-activated protein kinase 1 goes up when epigallocatechin-3-gallate (EGCG) is present (129). Furthermore, in mice, EGCG selectively inhibits angiogenesis and blood perfusion in endometriotic lesions, leading to their regression (130). Pro-EGCG greatly slows down the development, growth, and formation of new blood vessels in experimental endometriosis, showing that it can both protect cells from damage and stop the growth of new blood vessels (131). Some genes, like vascular endothelial growth factor C (VEGFC) and the tyrosine kinase receptor VEGF receptor 2 (VEGFR2), are turned down by EGCG (132). EGCG downregulates VEGFC/VEGFR2 signaling through pathways involving c-JUN, interferon-γ, matrix metalloproteinase-9, and chemokine ligand 3, thereby inhibiting endothelial proliferation, inflammation, and cell migration. EGCG prevents the activation of MAPK and Smad signaling pathways stimulated by TGF-β1 in both endometrial and endometriotic stromal cells by a large amount. Animal experiments indicate that EGCG can prevent the progression of fibrosis in endometriosis (133).
In endometriosis, there is an increased expression of melatonin receptors (134). Melatonin mitigates oxidative stress, inflammation processes, and cell apoptosis by upregulating the Nrf2 signaling pathway and downregulating COX-2 protein levels (135), as well as SOD, GPx, CAT, and Bcl-2 activities (136). Melatonin inhibits the development of endometriosis by disrupting mitochondrial function and regulating siRNA expression in mouse models (137). Melatonin treatment reduces MMP-3 activity in mouse endometriosis models and facilitates endometriosis regression through caspase-3-mediated pathways, enhancing endometriotic cell apoptosis (138). Melatonin lowers the activity and expression of proMMP-9, which has anti-inflammatory effects and lessens the damage caused by peritoneal endometriosis in mice (139). A clinical study suggests that oral administration of 10 mg of melatonin before bedtime during menstruation provides better analgesic relief for dysmenorrheal (140).
Quercetin can reduce the expression of IL-6, IL-1β, and TNFα (141). Quercetin induces p21 CDK inhibitors that decrease pRb phosphorylation by capturing E2F1, thus inhibiting G1/S cell cycle progression. Low doses of quercetin induce mild DNA damage and activate Chk2, a key regulator of quercetin-induced p21 expression. Quercetin also lowers the levels of cyclin B1 and CDK1, which prevent transcription. This suggests that quercetin can stop the cell cycle from moving forward in physiologically relevant doses (142). Quercetin treatment effectively suppresses the growth of endometrial lesions in mice with endometriosis (143).
Quercetin induces the downregulation of ERK1/2, P38 MAPK, and AKT signaling molecules, leading to DNA fragmentation, mitochondrial membrane potential loss, and ROS production, thereby inducing cell apoptosis (144). Following intraperitoneal quercetin injection in mice, Ccnd1 mRNA expression was significantly reduced compared to the control group, leading to G0/G1 cell cycle arrest and reduced cell proliferation, accompanied by increased apoptosis of VK2/E6E7 and End1/E6E7 cells. In mouse models, the activity of oxidative stress markers is reduced in vivo through the Nrf2 signaling pathway (145). For example, it can prevent LPS from causing oxidative stress in the jejunum by activating the MAPK/Nrf2 signaling pathway. This can fix the damage that LPS does to the mitochondria in the jejunum and increase the expression of mitochondrial DNA copy number-related genes like COX1, ATP6, and ND1 (146).
Resveratrol (RSV), a polyphenol found in red wine, regulates NF-κB activity (147). It inhibits Th17 cells, decreasing the production of the inflammatory factor IL-17 (148). When resveratrol is added to the stromal cells of women with endometriosis, the levels of IGF-1 and HGF go down (149).
Resveratrol decreases the concentrations of MCP1, VEGF (150), IL-6, IL-8, and TNF α in peritoneal fluid in vitro and in animal models (151), inhibiting angiogenesis and inflammation to regress endometriotic lesions (152). It reduces the invasiveness of endometrial stromal cells and curbs the progression of endometriosis in nude mouse models (153). Metastasis-associated protein 1 (MTA1) increases epithelial-mesenchymal transition (EMT) through its connection with ZEB2. Resveratrol suppresses ectopic lesions’ growth and MTA1 and ZEB2 expression. MTA1 may be a target for resveratrol (154). Taking 400 mg of resveratrol every day for 12–14 weeks lowers the amounts of matrix metalloproteinases MMP-2 and MMP-9 in endometriosis patients’ serum and peritoneal fluid, which lowers inflammation (155).
E2 levels in endometriosis lesions are significantly elevated; supplementing with curcumin can notably decrease estradiol levels, thereby inhibiting the growth rate of endometriosis (156). Curcumin can additionally suppress endometrial cells in endometriosis by downregulating the vascular endothelial growth factor (157). Curcumin contributes to the treatment of mouse models for endometriosis by modulating the HIF signaling pathway, ameliorating local hypoxia, and diminishing inflammation (158). Curcumin therapy reduces endometriosis in mice by preventing NFκB translocation and suppressing MMP-3 expression. It primarily enhances cell apoptosis in endometriosis via the cytochrome c-mediated mitochondrial pathway (159). Curcumin slows the progression of endometriosis in mice by inhibiting MMP-2 activity (160). Curcumin blocks endometriosis by reducing matrix metalloproteinase-9 activity (161). Curcumin supplementation can ameliorate oxidative stress levels (MDA and TAC) in postmenopausal women and lower inflammatory biomarkers (hs-CRP) (Table 2) (119).
Oxidative stress and inflammatory factors play crucial roles in endometriosis. This article summarizes the roles of oxidative stress and inflammatory factors in endometriosis and reviews the risk factors of endometriosis. Additionally, we have summarized the current mainstream dietary patterns and the impact of common nutrients on endometriosis. Supplementing nutrients can inhibit the progression of endometriosis in various ways, such as reducing levels of inflammatory factors, decreasing oxidative stress responses, inhibiting cell proliferation, and reducing angiogenesis. The Mediterranean dietary paradigm, renowned as a quintessential anti-inflammatory dietary regimen, is capable of curtailing the levels of inflammatory mediators within the body and mitigating the symptoms experienced by patients afflicted with endometriosis. Nutrients endowed with anti-inflammatory properties, including vitamins (such as vitamin C and vitamin E), quercetin, resveratrol, curcumin, and epigallocatechin-3-gallate (EGCG), hold the potential to diminish the lesions associated with endometriosis and thus exhibit latent therapeutic implications. Analyzing endometriosis patients’ dietary traits and formulating new preventive dietary strategies (162) promise to mitigate disease incidence and lessen the patients’ burden. The anti-inflammatory and antioxidant properties of nutrients may be key areas of focus. Currently, there are no dietary guidelines for endometriosis patients, and previous studies were limited in sample size and primarily descriptive. Existing research on dietary patterns and the role of nutrients in endometriosis is insufficiently comprehensive. There is a pressing need to delve deeper into the pathogenesis of endometriosis and the impact of varied dietary patterns, aiming to identify optimal dietary regimes for these patients. This approach holds significant promise for alleviating symptoms and halting disease progression.
LZ: Data curation, Writing – original draft, Writing – review & editing. BL: Investigation, Writing – original draft. XJ: Formal analysis, Methodology, Writing – original draft. LJ: Conceptualization, Visualization, Writing – review & editing. KL: Project administration, Resources, Visualization, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. Scientific research funding project of Liaoning Provincial Department of Science and Technology (No. 2020JH2/10300050).
The authors thank the support of the funding “Scientific research funding project of Liaoning Provincial Department of Science and Technology (No. 2020JH2/10300050)” to this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Upson, K. Environmental risk factors for endometriosis: a critical evaluation of studies and recommendations from the epidemiologic perspective. Curr Epidemiol Rep. (2020) 7:149–70. doi: 10.1007/s40471-020-00236-3
2. Vigano, P, Parazzini, F, Somigliana, E, and Vercellini, P. Endometriosis: epidemiology and aetiological factors. Best Pract Res Clin Obstet Gynaecol. (2004) 18:177–200. doi: 10.1016/j.bpobgyn.2004.01.007
3. Agarwal, A, Aponte-Mellado, A, Premkumar, BJ, Shaman, A, and Gupta, S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. (2012) 10:49. doi: 10.1186/1477-7827-10-49
4. Capobianco, A, Monno, A, Cottone, L, Venneri, MA, Biziato, D, di Puppo, F, et al. Proangiogenic Tie2(+) macrophages infiltrate human and murine endometriotic lesions and dictate their growth in a mouse model of the disease. Am J Pathol. (2011) 179:2651–9. doi: 10.1016/j.ajpath.2011.07.029
5. McKinnon, BD, Bertschi, D, Bersinger, NA, and Mueller, MD. Inflammation and nerve fiber interaction in endometriotic pain. Trends Endocrinol Metab. (2015) 26:1–10. doi: 10.1016/j.tem.2014.10.003
6. Kalfas, M, Chisari, C, and Windgassen, S. Psychosocial factors associated with pain and health-related quality of life in endometriosis: a systematic review. Eur J Pain. (2022) 26:1827–48. doi: 10.1002/ejp.2006
7. Koene, RJ, Prizment, AE, Blaes, A, and Konety, SH. Shared risk factors in cardiovascular disease and cancer. Circulation. (2016) 133:1104–14. doi: 10.1161/CIRCULATIONAHA.115.020406
8. Vennberg Karlsson, J, Patel, H, and Premberg, A. Experiences of health after dietary changes in endometriosis: a qualitative interview study. BMJ Open. (2020) 10:e032321. doi: 10.1136/bmjopen-2019-032321
9. Nirgianakis, K, Egger, K, Kalaitzopoulos, DR, Lanz, S, Bally, L, and Mueller, MD. Effectiveness of dietary interventions in the treatment of endometriosis: a systematic review. Reprod Sci. (2022) 29:26–42. doi: 10.1007/s43032-020-00418-w
10. Parazzini, F, Chiaffarino, F, Surace, M, Chatenoud, L, Cipriani, S, Chiantera, V, et al. Selected food intake and risk of endometriosis. Hum Reprod. (2004) 19:1755–9. doi: 10.1093/humrep/deh395
11. Qi, X, Zhang, W, Ge, M, Sun, Q, Peng, L, Cheng, W, et al. Relationship between dairy products intake and risk of endometriosis: a systematic review and dose-response meta-analysis. Front Nutr. (2021) 8:701860. doi: 10.3389/fnut.2021.701860
12. Samaneh, Y, ShahidehJahanian, S, Azadeh, M, and Anoshirvan, K. The association of food consumption and nutrient intake with endometriosis risk in Iranian women: a case-control study. Int J Reprod Biomed. (2019) 17:661–70. doi: 10.18502/ijrm.v17i9.5102
13. Khanaki, K, Nouri, M, Ardekani, AM, Ghassemzadeh, A, Shahnazi, V, Sadeghi, MR, et al. Evaluation of the relationship between endometriosis and omega-3 and omega-6 polyunsaturated fatty acids. Iran Biomed J. (2012) 16:38–43. doi: 10.6091/ibj.1025.2012
14. Yamamoto, A, Harris, HR, Vitonis, AF, Chavarro, JE, and Missmer, SA. A prospective cohort study of meat and fish consumption and endometriosis risk. Am J Obstet Gynecol. (2018) 219:178.e1–178.e10. doi: 10.1016/j.ajog.2018.05.034
15. Missmer, SA, Chavarro, JE, Malspeis, S, Bertone-Johnson, ER, Hornstein, MD, Spiegelman, D, et al. A prospective study of dietary fat consumption and endometriosis risk. Hum Reprod. (2010) 25:1528–35. doi: 10.1093/humrep/deq044
16. O'Hara, R, Rowe, H, and Fisher, J. Self-management in condition-specific health: a systematic review of the evidence among women diagnosed with endometriosis. BMC Womens Health. (2019) 19:80. doi: 10.1186/s12905-019-0774-6
17. Sampson, JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol. (1927) 3:93–110.43.
18. Ferguson, BR, Bennington, JL, and Haber, SL. Histochemistry of mucosubstances and histology of mixed mullerian pelvic lymph node glandular inclusions. Evidence for histogenesis by mullerian metaplasia of coelomic epithelium. Obstet Gynecol. (1969) 33:617–25.
19. Parente Barbosa, C, Bentes de Souza, AM, Bianco, B, and Christofolini, DM. The effect of hormones on endometriosis development. Minerva Ginecol. (2011) 63:375–86.
21. Donnez, J, Binda, MM, Donnez, O, and Dolmans, MM. Oxidative stress in the pelvic cavity and its role in the pathogenesis of endometriosis. Fertil Steril. (2016) 106:1011–7. doi: 10.1016/j.fertnstert.2016.07.1075
22. Simoni, J, Simoni, O, Lox, CD, McGunegle, DE, and Feola, M. Cytokines and PAF release from human monocytes and macrophages: effect of hemoglobin and contaminants. Artif Cells Blood Substit Immobil Biotechnol. (1994) 22:525–34. doi: 10.3109/10731199409117880
23. Van Langendonckt, A, Casanas-Roux, F, and Donnez, J. Oxidative stress and peritoneal endometriosis. Fertil Steril. (2002) 77:861–70. doi: 10.1016/S0015-0282(02)02959-X
24. Lousse, JC, Defrère, S, van Langendonckt, A, Gras, J, González-Ramos, R, Colette, S, et al. Iron storage is significantly increased in peritoneal macrophages of endometriosis patients and correlates with iron overload in peritoneal fluid. Fertil Steril. (2009) 91:1668–75. doi: 10.1016/j.fertnstert.2008.02.103
25. Arumugam, K, and Yip, YC. De novo formation of adhesions in endometriosis: the role of iron and free radical reactions. Fertil Steril. (1995) 64:62–4. doi: 10.1016/S0015-0282(16)57655-9
26. Defrère, S, Langendonckt, AV, Vaesen, S, Jouret, M, González, RR, et al. Iron overload enhances epithelial cell proliferation in endometriotic lesions induced in a murine model. Hum Reprod. (2006) 21:2810–6. doi: 10.1093/humrep/del261
27. Wyatt, J, Fernando, SM, Powell, SG, Hill, CJ, Arshad, I, Probert, C, et al. The role of iron in the pathogenesis of endometriosis: a systematic review. Hum Reprod Open. (2023) 2023:hoad033. doi: 10.1093/hropen/hoad033
28. Staniek, K, Gille, L, Kozlov, AV, and Nohl, H. Mitochondrial superoxide radical formation is controlled by electron bifurcation to the high and low potential pathways. Free Radic Res. (2002) 36:381–7. doi: 10.1080/10715760290021225
29. Lu, J, Wang, Z, Cao, J, Chen, Y, and Dong, Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod Biol Endocrinol. (2018) 16:80. doi: 10.1186/s12958-018-0391-5
30. Ngô, C, Chéreau, C, Nicco, C, Weill, B, Chapron, C, Batteux, F, et al. Reactive oxygen species controls endometriosis progression. Am J Pathol. (2009) 175:225–34. doi: 10.2353/ajpath.2009.080804
31. Assaf, L, Eid, AA, and Nassif, J. Role of AMPK/mTOR, mitochondria, and ROS in the pathogenesis of endometriosis. Life Sci. (2022) 306:120805. doi: 10.1016/j.lfs.2022.120805
32. Scutiero, G, Iannone, P, Bernardi, G, Bonaccorsi, G, Spadaro, S, Volta, CA, et al. Oxidative stress and endometriosis: a systematic review of the literature. Oxidative Med Cell Longev. (2017) 2017:7265238. doi: 10.1155/2017/7265238
33. Kaponis, A, Iwabe, T, Taniguchi, F, Ito, M, Deura, I, Decavalas, G, et al. The role of NF-kappaB in endometriosis. Front Biosci (Schol Ed). (2012) 4:1213–34. doi: 10.2741/s327
34. Symons, LK, Miller, JE, Kay, VR, Marks, RM, Liblik, K, Koti, M, et al. The immunopathophysiology of endometriosis. Trends Mol Med. (2018) 24:748–62. doi: 10.1016/j.molmed.2018.07.004
35. Berbic, M, Schulke, L, Markham, R, Tokushige, N, Russell, P, and Fraser, IS. Macrophage expression in endometrium of women with and without endometriosis. Hum Reprod. (2009) 24:325–32. doi: 10.1093/humrep/den393
36. Chan, RWS, Lee, CL, Ng, EHY, and Yeung, WSB. Co-culture with macrophages enhances the clonogenic and invasion activity of endometriotic stromal cells. Cell Prolif. (2017) 50:e12330. doi: 10.1111/cpr.12330
37. Yang, HL, Zhou, WJ, Chang, KK, Mei, J, Huang, LQ, Wang, MY, et al. The crosstalk between endometrial stromal cells and macrophages impairs cytotoxicity of NK cells in endometriosis by secreting IL-10 and TGF-beta. Reproduction. (2017) 154:815–25. doi: 10.1530/REP-17-0342
38. Macer, ML, and Taylor, HS. Endometriosis and infertility: a review of the pathogenesis and treatment of endometriosis-associated infertility. Obstet Gynecol Clin N Am. (2012) 39:535–49. doi: 10.1016/j.ogc.2012.10.002
39. Riccio, LGC, Baracat, EC, Chapron, C, Batteux, F, and Abrão, MS. The role of the B lymphocytes in endometriosis: a systematic review. J Reprod Immunol. (2017) 123:29–34. doi: 10.1016/j.jri.2017.09.001
40. Randall, GW, Gantt, PA, Poe-Zeigler, RL, Bergmann, CA, Noel, ME, Strawbridge, WR, et al. Serum antiendometrial antibodies and diagnosis of endometriosis. Am J Reprod Immunol. (2007) 58:374–82. doi: 10.1111/j.1600-0897.2007.00523.x
41. Yoshino, O, Osuga, Y, Hirota, Y, Koga, K, Hirata, T, Harada, M, et al. Possible pathophysiological roles of mitogen-activated protein kinases (MAPKs) in endometriosis. Am J Reprod Immunol. (2004) 52:306–11. doi: 10.1111/j.1600-0897.2004.00231.x
42. Carli, C, Metz, CN, Al-Abed, Y, Naccache, PH, and Akoum, A. Up-regulation of cyclooxygenase-2 expression and prostaglandin E2 production in human endometriotic cells by macrophage migration inhibitory factor: involvement of novel kinase signaling pathways. Endocrinology. (2009) 150:3128–37. doi: 10.1210/en.2008-1088
43. Veillat, V, Carli, C, Metz, CN, Al-Abed, Y, Naccache, PH, Akoum, A, et al. Macrophage migration inhibitory factor elicits an angiogenic phenotype in human ectopic endometrial cells and triggers the production of major angiogenic factors via CD44, CD74, and MAPK signaling pathways. J Clin Endocrinol Metab. (2010) 95:E403–12. doi: 10.1210/jc.2010-0417
44. Hennessy, BT, Smith, DL, Ram, PT, Lu, Y, and Mills, GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. (2005) 4:988–1004. doi: 10.1038/nrd1902
45. McKinnon, BD, Kocbek, V, Nirgianakis, K, Bersinger, NA, and Mueller, MD. Kinase signalling pathways in endometriosis: potential targets for non-hormonal therapeutics. Hum Reprod Update. (2016) 22:382–403. doi: 10.1093/humupd/dmv060
46. Manning, BD. Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. J Cell Biol. (2004) 167:399–403. doi: 10.1083/jcb.200408161
47. Li, Y, Corradetti, MN, Inoki, K, and Guan, KL. TSC2: filling the GAP in the mTOR signaling pathway. Trends Biochem Sci. (2004) 29:32–8. doi: 10.1016/j.tibs.2003.11.007
48. Laplante, M, and Sabatini, DM. mTOR signaling at a glance. J Cell Sci. (2009) 122:3589–94. doi: 10.1242/jcs.051011
49. Karar, J, and Maity, A. PI3K/AKT/mTOR pathway in angiogenesis. Front Mol Neurosci. (2011) 4:51. doi: 10.3389/fnmol.2011.00051
50. Jin, X, Feng, J, and Cheng, X. LncRNA IGF2-AS promotes endometriosis progression through targeting miR-370-3p/IGF2 axis and activating PI3K/AKT/mTOR signaling pathway. J Assist Reprod Genet. (2022) 39:2699–710. doi: 10.1007/s10815-022-02638-2
51. Badary, DM, Abou-Taleb, HA, and Ibrahim, M. Hypoxia-inducible factor-1alpha and mTOR as a potential therapeutic target in endometriosis: an immunohistochemical study. Appl Immunohistochem Mol Morphol. (2023) 31:629–34. doi: 10.1097/PAI.0000000000001148
52. Wang, Y, Zhu, L, Kuokkanen, S, and Pollard, JW. Activation of protein synthesis in mouse uterine epithelial cells by estradiol-17beta is mediated by a PKC-ERK1/2-mTOR signaling pathway. Proc Natl Acad Sci USA. (2015) 112:E1382–91. doi: 10.1073/pnas.1418973112
53. Gori, I, Pellegrini, C, Staedler, D, Russell, R, Jan, C, and Canny, GO. Tumor necrosis factor-alpha activates estrogen signaling pathways in endometrial epithelial cells via estrogen receptor alpha. Mol Cell Endocrinol. (2011) 345:27–37. doi: 10.1016/j.mce.2011.06.043
54. Huang, ZX, He, XR, Ding, XY, Chen, JH, Lei, YH, Bai, JB, et al. Lipoxin A4 depresses inflammation and promotes autophagy via AhR/mTOR/AKT pathway to suppress endometriosis. Am J Reprod Immunol. (2023) 89:e13659. doi: 10.1111/aji.13659
55. He, Y, Xiong, T, Guo, F, du, Z, Fan, Y, Sun, H, et al. Interleukin-37b inhibits the growth of murine endometriosis-like lesions by regulating proliferation, invasion, angiogenesis and inflammation. Mol Hum Reprod. (2020) 26:240–55. doi: 10.1093/molehr/gaaa014
56. Li, L, Liao, Z, Ye, M, and Jiang, J. Recombinant human IL-37 inhibited endometriosis development in a mouse model through increasing Th1/Th2 ratio by inducing the maturation of dendritic cells. Reprod Biol Endocrinol. (2021) 19:128. doi: 10.1186/s12958-021-00811-3
57. Peng, B, Alotaibi, FT, Sediqi, S, Bedaiwy, MA, and Yong, PJ. Role of interleukin-1beta in nerve growth factor expression, neurogenesis and deep dyspareunia in endometriosis. Hum Reprod. (2020) 35:901–12. doi: 10.1093/humrep/deaa017
58. Kato, T, Yasuda, K, Matsushita, K, Ishii, KJ, Hirota, S, Yoshimoto, T, et al. Interleukin-1/−33 signaling pathways as therapeutic targets for endometriosis. Front Immunol. (2019) 10:2021. doi: 10.3389/fimmu.2019.02021
59. Nishimoto-Kakiuchi, A, Sato, I, Nakano, K, Ohmori, H, Kayukawa, Y, Tanimura, H, et al. A long-acting anti-IL-8 antibody improves inflammation and fibrosis in endometriosis. Sci Transl Med. (2023) 15:eabq5858. doi: 10.1126/scitranslmed.abq5858
60. Shi, JL, Zheng, ZM, Chen, M, Shen, HH, Li, MQ, and Shao, J. IL-17: an important pathogenic factor in endometriosis. Int J Med Sci. (2022) 19:769–78. doi: 10.7150/ijms.71972
61. Miller, JE, Ahn, SH, Marks, RM, Monsanto, SP, Fazleabas, AT, Koti, M, et al. IL-17A modulates peritoneal macrophage recruitment and M2 polarization in endometriosis. Front Immunol. (2020) 11:108. doi: 10.3389/fimmu.2020.00108
62. Khan, KN, Kitajima, M, Imamura, T, Hiraki, K, Fujishita, A, Sekine, I, et al. Toll-like receptor 4-mediated growth of endometriosis by human heat-shock protein 70. Hum Reprod. (2008) 23:2210–9. doi: 10.1093/humrep/den195
63. Harris, HR, Eke, AC, Chavarro, JE, and Missmer, SA. Fruit and vegetable consumption and risk of endometriosis. Hum Reprod. (2018) 33:715–27. doi: 10.1093/humrep/dey014
64. Darling, AM, Chavarro, JE, Malspeis, S, and Harris, HR. A prospective cohort study of vitamins B, C, E, and multivitamin intake and endometriosis. J Endometr. (2013) 5:17–26. doi: 10.5301/je.5000151
65. Mier-Cabrera, J, Aburto-Soto, T, Burrola-Méndez, S, Jiménez-Zamudio, L, Tolentino, MC, Casanueva, E, et al. Women with endometriosis improved their peripheral antioxidant markers after the application of a high antioxidant diet. Reprod Biol Endocrinol. (2009) 7:54. doi: 10.1186/1477-7827-7-54
66. Aris, A, and Paris, K. Hypothetical link between endometriosis and xenobiotics-associated genetically modified food. Gynecol Obstet Fertil. (2010) 38:747–53. doi: 10.1016/j.gyobfe.2010.08.030
67. Afrin, S, AlAshqar, A, el Sabeh, M, Miyashita-Ishiwata, M, Reschke, L, Brennan, JT, et al. Diet and nutrition in gynecological disorders: a focus on clinical studies. Nutrients. (2021) 13:1747. doi: 10.3390/nu13061747
68. Arab, A, Karimi, E, Vingrys, K, Kelishadi, MR, Mehrabani, S, and Askari, G. Food groups and nutrients consumption and risk of endometriosis: a systematic review and meta-analysis of observational studies. Nutr J. (2022) 21:58. doi: 10.1186/s12937-022-00812-x
69. Heard, ME, Melnyk, SB, Simmen, FA, Yang, Y, Pabona, JMP, and Simmen, RCM. High-fat diet promotion of endometriosis in an immunocompetent mouse model is associated with altered peripheral and ectopic lesion redox and inflammatory status. Endocrinology. (2016) 157:2870–82. doi: 10.1210/en.2016-1092
70. Schwartz, NRM, Afeiche, MC, Terry, KL, Farland, LV, Chavarro, JE, Missmer, SA, et al. Glycemic index, glycemic load, fiber, and gluten intake and risk of laparoscopically confirmed endometriosis in premenopausal women. J Nutr. (2022) 152:2088–96. doi: 10.1093/jn/nxac107
71. Román, GC, Jackson, RE, Gadhia, R, Román, AN, and Reis, J. Mediterranean diet: the role of long-chain omega-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev Neurol (Paris). (2019) 175:724–41. doi: 10.1016/j.neurol.2019.08.005
72. Shannon, OM, Stephan, BCM, Minihane, AM, Mathers, JC, and Siervo, M. Nitric oxide boosting effects of the Mediterranean diet: a potential mechanism of action. J Gerontol A Biol Sci Med Sci. (2018) 73:902–4. doi: 10.1093/gerona/gly087
73. Tosti, V, Bertozzi, B, and Fontana, L. Health benefits of the Mediterranean diet: metabolic and molecular mechanisms. J Gerontol A Biol Sci Med Sci. (2018) 73:318–26. doi: 10.1093/gerona/glx227
74. Estruch, R, Martínez-González, MÁ, Corella, D, Salas-Salvadó, J, Ruiz-Gutiérrez, V, Covas, MI, et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med. (2006) 145:1–11. doi: 10.7326/0003-4819-145-1-200607040-00004
75. Salas-Salvadó, J, Garcia-Arellano, A, Estruch, R, Marquez-Sandoval, F, Corella, D, Fiol, M, et al. Components of the Mediterranean-type food pattern and serum inflammatory markers among patients at high risk for cardiovascular disease. Eur J Clin Nutr. (2008) 62:651–9. doi: 10.1038/sj.ejcn.1602762
76. Zambonin, L, Caliceti, C, Vieceli Dalla Sega, F, Fiorentini, D, Hrelia, L, Landi, S, et al. Dietary phenolic acids act as effective antioxidants in membrane models and in cultured cells, exhibiting proapoptotic effects in leukaemia cells. Oxidative Med Cell Longev. (2012) 2012:839298. doi: 10.1155/2012/839298
77. Andreo-López, MC, Contreras-Bolívar, V, Muñoz-Torres, M, and García-Fontana, B. Influence of the Mediterranean diet on healthy aging. Int J Mol Sci. (2023) 24:4491. doi: 10.3390/ijms24054491
78. Khan, MM, Ahmad, A, Ishrat, T, Khan, MB, Hoda, MN, Khuwaja, G, et al. Resveratrol attenuates 6-hydroxydopamine-induced oxidative damage and dopamine depletion in rat model of Parkinson's disease. Brain Res. (2010) 1328:139–51. doi: 10.1016/j.brainres.2010.02.031
79. Kurin, E, Mucaji, P, and Nagy, M. In vitro antioxidant activities of three red wine polyphenols and their mixtures: an interaction study. Molecules. (2012) 17:14336–48. doi: 10.3390/molecules171214336
80. Zhang, X, Cao, J, and Zhong, L. Hydroxytyrosol inhibits pro-inflammatory cytokines, iNOS, and COX-2 expression in human monocytic cells. Naunyn Schmiedeberg's Arch Pharmacol. (2009) 379:581–6. doi: 10.1007/s00210-009-0399-7
81. Rosignoli, P, Fuccelli, R, Fabiani, R, Servili, M, and Morozzi, G. Effect of olive oil phenols on the production of inflammatory mediators in freshly isolated human monocytes. J Nutr Biochem. (2013) 24:1513–9. doi: 10.1016/j.jnutbio.2012.12.011
82. Fernández del Río, L, Gutiérrez-Casado, E, Varela-López, A, and Villalba, JM. Olive oil and the hallmarks of aging. Molecules. (2016) 21:163. doi: 10.3390/molecules21020163
83. Jilge, B, and Meyer, H. Coprophagy-dependant changes of the anaerobic bacterial flora in stomach and small intestine of the rabbit. Z Versuchstierkd. (1975) 17:308–14.
84. Schaffer, S, Podstawa, M, Visioli, F, Bogani, P, Müller, WE, and Eckert, GP. Hydroxytyrosol-rich olive mill wastewater extract protects brain cells in vitro and ex vivo. J Agric Food Chem. (2007) 55:5043–9. doi: 10.1021/jf0703710
85. Afshordel, S, Hagl, S, Werner, D, Röhner, N, Kögel, D, Bazan, NG, et al. Omega-3 polyunsaturated fatty acids improve mitochondrial dysfunction in brain aging – impact of Bcl-2 and NPD-1 like metabolites. Prostaglandins Leukot Essent Fatty Acids. (2015) 92:23–31. doi: 10.1016/j.plefa.2014.05.008
86. Abokhrais, IM, Denison, FC, Whitaker, LHR, Saunders, PTK, Doust, A, Williams, LJ, et al. A two-arm parallel double-blind randomised controlled pilot trial of the efficacy of Omega-3 polyunsaturated fatty acids for the treatment of women with endometriosis-associated pain (PurFECT1). PLoS One. (2020) 15:e0227695. doi: 10.1371/journal.pone.0227695
87. Ciebiera, M, Esfandyari, S, Siblini, H, Prince, L, Elkafas, H, Wojtyła, C, et al. Nutrition in gynecological diseases: current perspectives. Nutrients. (2021) 13:1178. doi: 10.3390/nu13041178
88. Cirillo, M, Argento, FR, Becatti, M, Fiorillo, C, Coccia, ME, and Fatini, C. Mediterranean diet and oxidative stress: a relationship with pain perception in endometriosis. Int J Mol Sci. (2023) 24:14601. doi: 10.3390/ijms241914601
89. Vinciguerra, F, Graziano, M, Hagnäs, M, Frittitta, L, and Tumminia, A. Influence of the Mediterranean and ketogenic diets on cognitive status and decline: a narrative review. Nutrients. (2020) 12:1019. doi: 10.3390/nu12041019
90. Burén, J, Ericsson, M, Damasceno, NRT, and Sjödin, A. A ketogenic low-carbohydrate high-fat diet increases LDL cholesterol in healthy, young, Normal-weight women: a randomized controlled feeding trial. Nutrients. (2021) 13:814. doi: 10.3390/nu13030814
91. Paoli, A, Mancin, L, Giacona, MC, Bianco, A, and Caprio, M. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J Transl Med. (2020) 18:104. doi: 10.1186/s12967-020-02277-0
92. Bueno, NB, de Melo, ISV, de Oliveira, SL, and da Rocha Ataide, T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br J Nutr. (2013) 110:1178–87. doi: 10.1017/S0007114513000548
93. Forsythe, CE, Phinney, SD, Fernandez, ML, Quann, EE, Wood, RJ, Bibus, DM, et al. Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids. (2008) 43:65–77. doi: 10.1007/s11745-007-3132-7
94. Saenz, C, Hooper, S, Orange, T, Knight, A, Barragan, M, Lynch, T, et al. Effect of a free-living ketogenic diet on feasibility, satiety, body composition, and metabolic health in women: the grading level of optimal carbohydrate for women (GLOW) study. J Am Coll Nutr. (2021) 40:295–306. doi: 10.1080/07315724.2021.1875338
95. Buga, A, Harper, DG, Sapper, TN, Hyde, PN, Fell, B, Dickerson, R, et al. Feasibility and metabolic outcomes of a well-formulated ketogenic diet as an adjuvant therapeutic intervention for women with stage IV metastatic breast cancer: the keto-CARE trial. PLoS One. (2024) 19:e0296523. doi: 10.1371/journal.pone.0296523
96. Field, R, Pourkazemi, F, and Rooney, K. Effects of a low-carbohydrate ketogenic diet on reported pain, blood biomarkers and quality of life in patients with chronic pain: a pilot randomized clinical trial. Pain Med. (2022) 23:326–38. doi: 10.1093/pm/pnab278
97. Nagpal, R, Neth, BJ, Wang, S, Craft, S, and Yadav, H. Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer's disease markers in subjects with mild cognitive impairment. EBioMedicine. (2019) 47:529–42. doi: 10.1016/j.ebiom.2019.08.032
98. Syed, K, and Iswara, K. Low-FODMAP diet In: StatPearls. Treasure Island (FL): StatPearls Publishing (2024)
99. van Megen, F, Skodje, GI, Lergenmuller, S, Zühlke, S, Aabakken, L, Veierød, MB, et al. A low FODMAP diet reduces symptoms in treated celiac patients with ongoing symptoms-a randomized controlled trial. Clin Gastroenterol Hepatol. (2022) 20:2258–2266.e3. doi: 10.1016/j.cgh.2022.01.011
100. Algera, JP, Demir, D, Törnblom, H, Nybacka, S, Simrén, M, and Störsrud, S. Low FODMAP diet reduces gastrointestinal symptoms in irritable bowel syndrome and clinical response could be predicted by symptom severity: a randomized crossover trial. Clin Nutr. (2022) 41:2792–800. doi: 10.1016/j.clnu.2022.11.001
101. Brouns, F, van Haaps, A, Keszthelyi, D, Venema, K, Bongers, M, Maas, J, et al. Diet associations in endometriosis: a critical narrative assessment with special reference to gluten. Front Nutr. (2023) 10:1166929. doi: 10.3389/fnut.2023.1166929
102. Varjú, P, Farkas, N, Hegyi, P, Garami, A, Szabó, I, Illés, A, et al. Low fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) diet improves symptoms in adults suffering from irritable bowel syndrome (IBS) compared to standard IBS diet: a meta-analysis of clinical studies. PLoS One. (2017) 12:e0182942. doi: 10.1371/journal.pone.0182942
103. Moore, JS, Gibson, PR, Perry, RE, and Burgell, RE. Endometriosis in patients with irritable bowel syndrome: specific symptomatic and demographic profile, and response to the low FODMAP diet. Aust N Z J Obstet Gynaecol. (2017) 57:201–5. doi: 10.1111/ajo.12594
104. van Haaps, AP, Wijbers, JV, Schreurs, AMF, Vlek, S, Tuynman, J, de Bie, B, et al. The effect of dietary interventions on pain and quality of life in women diagnosed with endometriosis: a prospective study with control group. Hum Reprod. (2023) 38:2433–46. doi: 10.1093/humrep/dead214
105. Borghini, R, Porpora, MG, Casale, R, Marino, M, Palmieri, E, Greco, N, et al. Irritable bowel syndrome-like disorders in endometriosis: prevalence of nickel sensitivity and effects of a low-nickel diet. An open-label pilot study. Nutrients. (2020) 12:341. doi: 10.3390/nu12020341
106. Pellegrini, N, and Agostoni, C. Nutritional aspects of gluten-free products. J Sci Food Agric. (2015) 95:2380–5. doi: 10.1002/jsfa.7101
107. Vici, G, Belli, L, Biondi, M, and Polzonetti, V. Gluten free diet and nutrient deficiencies: a review. Clin Nutr. (2016) 35:1236–41. doi: 10.1016/j.clnu.2016.05.002
108. Marziali, M, Venza, M, Lazzaro, S, Lazzaro, A, Micossi, C, and Stolfi, VM. Gluten-free diet: a new strategy for management of painful endometriosis related symptoms? Minerva Chir. (2012) 67:499–504.
109. Itzlinger, A, Branchi, F, Elli, L, and Schumann, M. Gluten-free diet in celiac disease-forever and for all? Nutrients. (2018) 10:1796. doi: 10.3390/nu10111796
110. Mazza, E, Troiano, E, Mazza, S, Ferro, Y, Abbinante, A, Agneta, MT, et al. The impact of endometriosis on dietary choices and activities of everyday life: a cross-sectional study. Front Nutr. (2023) 10:1273976. doi: 10.3389/fnut.2023.1273976
111. Armour, M, Sinclair, J, Chalmers, KJ, and Smith, CA. Self-management strategies amongst Australian women with endometriosis: a national online survey. BMC Complement Altern Med. (2019) 19:17. doi: 10.1186/s12906-019-2431-x
112. Zheng, SH, Chen, XX, Chen, Y, Wu, ZC, Chen, XQ, and Li, XL. Antioxidant vitamins supplementation reduce endometriosis related pelvic pain in humans: a systematic review and meta-analysis. Reprod Biol Endocrinol. (2023) 21:79. doi: 10.1186/s12958-023-01126-1
113. Abramiuk, M, Mertowska, P, Frankowska, K, Świechowska-Starek, P, Satora, M, Polak, G, et al. How can selected dietary ingredients influence the development and progression of endometriosis? Nutrients. (2024) 16:154. doi: 10.3390/nu16010154
114. Yang, M, Teng, S, Ma, C, Yu, Y, Wang, P, and Yi, C. Ascorbic acid inhibits senescence in mesenchymal stem cells through ROS and AKT/mTOR signaling. Cytotechnology. (2018) 70:1301–13. doi: 10.1007/s10616-018-0220-x
115. Fujisawa, K, Hara, K, Takami, T, Okada, S, Matsumoto, T, Yamamoto, N, et al. Evaluation of the effects of ascorbic acid on metabolism of human mesenchymal stem cells. Stem Cell Res Ther. (2018) 9:93. doi: 10.1186/s13287-018-0825-1
116. Abdollahifar, MA, Azad, N, Sajadi, E, Shams Mofarahe, Z, Zare, F, Moradi, A, et al. Vitamin C restores ovarian follicular reservation in a mouse model of aging. Anat Cell Biol. (2019) 52:196–203. doi: 10.5115/acb.2019.52.2.196
117. Erten, OU, Ensari, TA, Dilbaz, B, Cakiroglu, H, Altinbas, SK, Çaydere, M, et al. Vitamin C is effective for the prevention and regression of endometriotic implants in an experimentally induced rat model of endometriosis. Taiwan J Obstet Gynecol. (2016) 55:251–7. doi: 10.1016/j.tjog.2015.07.004
118. Asbaghi, O, Sadeghian, M, Nazarian, B, Sarreshtedari, M, Mozaffari-Khosravi, H, Maleki, V, et al. The effect of vitamin E supplementation on selected inflammatory biomarkers in adults: a systematic review and meta-analysis of randomized clinical trials. Sci Rep. (2020) 10:17234. doi: 10.1038/s41598-020-73741-6
119. Farshbaf-Khalili, A, Ostadrahimi, A, Mirghafourvand, M, Ataei-Almanghadim, K, Dousti, S, Iranshahi, AM, et al. Clinical efficacy of curcumin and vitamin E on inflammatory-oxidative stress biomarkers and primary symptoms of menopause in healthy postmenopausal women: a triple-blind randomized controlled trial. J Nutr Metab. (2022) 2022:6339715. doi: 10.1155/2022/6339715
120. Ansariniya, H, Hadinedoushan, H, Javaheri, A, and Zare, F. Vitamin C and E supplementation effects on secretory and molecular aspects of vascular endothelial growth factor derived from peritoneal fluids of patients with endometriosis. J Obstet Gynaecol. (2019) 39:1137–42. doi: 10.1080/01443615.2019.1601167
121. Amini, L, Chekini, R, Nateghi, MR, Haghani, H, Jamialahmadi, T, Sathyapalan, T, et al. The effect of combined vitamin C and vitamin E supplementation on oxidative stress markers in women with endometriosis: a randomized, triple-blind placebo-controlled clinical trial. Pain Res Manag. (2021) 2021:5529741. doi: 10.1155/2021/5529741
122. Miyashita, M, Koga, K, Izumi, G, Sue, F, Makabe, T, Taguchi, A, et al. Effects of 1,25-dihydroxy vitamin D3 on endometriosis. J Clin Endocrinol Metab. (2016) 101:2371–9. doi: 10.1210/jc.2016-1515
123. Lopez, A, Cruz, ML, Chompre, G, Hernández, S, Isidro, RA, Flores, I, et al. Influence of stress on the vitamin D-vitamin D receptor system, macrophages, and the local inflammatory milieu in endometriosis. Reprod Sci. (2020) 27:2175–86. doi: 10.1007/s43032-020-00235-1
124. Xie, B, Liao, M, Huang, Y, Hang, F, Ma, N, Hu, Q, et al. Association between vitamin D and endometriosis among American women: national health and nutrition examination survey. PLoS One. (2024) 19:e0296190. doi: 10.1371/journal.pone.0296190
125. Mehdizadehkashi, A, Rokhgireh, S, Tahermanesh, K, Eslahi, N, Minaeian, S, and Samimi, M. The effect of vitamin D supplementation on clinical symptoms and metabolic profiles in patients with endometriosis. Gynecol Endocrinol. (2021) 37:640–5. doi: 10.1080/09513590.2021.1878138
126. Nodler, JL, DiVasta, AD, Vitonis, AF, Karevicius, S, Malsch, M, Sarda, V, et al. Supplementation with vitamin D or omega-3 fatty acids in adolescent girls and young women with endometriosis (SAGE): a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. (2020) 112:229–36. doi: 10.1093/ajcn/nqaa096
127. Bueloni-Dias, FN, Orsatti, CL, Cangussu, LM, Poloni, PF, Spadoto-Dias, D, Nahas-Neto, J, et al. Isolated vitamin D supplementation improves the immune-inflammatory biomarkers in younger postmenopausal women: a randomized, double-blind, placebo-controlled trial. Menopause. (2018) 25:897–903. doi: 10.1097/GME.0000000000001106
128. Chen, X, Man, GCW, Hung, SW, Zhang, T, Fung, LWY, Cheung, CW, et al. Therapeutic effects of green tea on endometriosis. Crit Rev Food Sci Nutr. (2023) 63:3222–35. doi: 10.1080/10408398.2021.1986465
129. Xu, H, Lui, WT, Chu, CY, Ng, PS, Wang, CC, and Rogers, MS. Anti-angiogenic effects of green tea catechin on an experimental endometriosis mouse model. Hum Reprod. (2009) 24:608–18. doi: 10.1093/humrep/den417
130. Laschke, MW, Schwender, C, Scheuer, C, Vollmar, B, and Menger, MD. Epigallocatechin-3-gallate inhibits estrogen-induced activation of endometrial cells in vitro and causes regression of endometriotic lesions in vivo. Hum Reprod. (2008) 23:2308–18. doi: 10.1093/humrep/den245
131. Wang, CC, Xu, H, Man, GCW, Zhang, T, Chu, KO, Chu, CY, et al. Prodrug of green tea epigallocatechin-3-gallate (pro-EGCG) as a potent anti-angiogenesis agent for endometriosis in mice. Angiogenesis. (2013) 16:59–69. doi: 10.1007/s10456-012-9299-4
132. Xu, H, Becker, CM, Lui, WT, Chu, CY, Davis, TN, Kung, AL, et al. Green tea epigallocatechin-3-gallate inhibits angiogenesis and suppresses vascular endothelial growth factor C/vascular endothelial growth factor receptor 2 expression and signaling in experimental endometriosis in vivo. Fertil Steril. (2011) 96:1021–1028.e1. doi: 10.1016/j.fertnstert.2011.07.008
133. Matsuzaki, S, and Darcha, C. Antifibrotic properties of epigallocatechin-3-gallate in endometriosis. Hum Reprod. (2014) 29:1677–87. doi: 10.1093/humrep/deu123
134. Mosher, AA, Tsoulis, MW, Lim, J, Tan, C, Agarwal, SK, Leyland, NA, et al. Melatonin activity and receptor expression in endometrial tissue and endometriosis. Hum Reprod. (2019) 34:1215–24. doi: 10.1093/humrep/dez082
135. Güney, M, Oral, B, and Karahan, N. Regression of endometrial explants in a rat model of endometriosis treated with melatonin. Fertil Steril. (2008) 89:934–42. doi: 10.1016/j.fertnstert.2007.04.023
136. Koc, O, Gunduz, B, Topcuoglu, A, Bugdayci, G, Yilmaz, F, and Duran, B. Effects of pinealectomy and melatonin supplementation on endometrial explants in a rat model. Eur J Obstet Gynecol Reprod Biol. (2010) 153:72–6. doi: 10.1016/j.ejogrb.2010.06.012
137. Park, S, Ham, J, Yang, C, Park, W, Park, H, An, G, et al. Melatonin inhibits endometriosis development by disrupting mitochondrial function and regulating tiRNAs. J Pineal Res. (2023) 74:e12842. doi: 10.1111/jpi.12842
138. Paul, S, Bhattacharya, P, das Mahapatra, P, and Swarnakar, S. Melatonin protects against endometriosis via regulation of matrix metalloproteinase-3 and an apoptotic pathway. J Pineal Res. (2010) 49:156–68. doi: 10.1111/j.1600-079X.2010.00780.x
139. Paul, S, Sharma, AV, Mahapatra, PD, Bhattacharya, P, Reiter, RJ, and Swarnakar, S. Role of melatonin in regulating matrix metalloproteinase-9 via tissue inhibitors of metalloproteinase-1 during protection against endometriosis. J Pineal Res. (2008) 44:439–49. doi: 10.1111/j.1600-079X.2007.00547.x
140. Söderman, L, Edlund, M, and Böttiger, Y. Adjuvant use of melatonin for pain management in dysmenorrhea - a randomized double-blinded, placebo-controlled trial. Eur J Clin Pharmacol. (2022) 78:191–6. doi: 10.1007/s00228-021-03234-6
141. Bournival, J, Plouffe, M, Renaud, J, and Provencher, C. Quercetin and sesamin protect dopaminergic cells from MPP+-induced neuroinflammation in a microglial (N9)-neuronal (PC12) coculture system. Oxidative Med Cell Longev. (2012) 2012:921941. doi: 10.1155/2012/921941
142. Jeong, JH, An, JY, Kwon, YT, Rhee, JG, and Lee, YJ. Effects of low dose quercetin: cancer cell-specific inhibition of cell cycle progression. J Cell Biochem. (2009) 106:73–82. doi: 10.1002/jcb.21977
143. Zhang, L, Mohankumar, K, Martin, G, Mariyam, F, Park, Y, Han, SJ, et al. Flavonoids quercetin and Kaempferol are NR4A1 antagonists and suppress endometriosis in female mice. Endocrinology. (2023) 164:bqad133. doi: 10.1210/endocr/bqad133
144. Park, S, Lim, W, Bazer, FW, Whang, KY, and Song, G. Quercetin inhibits proliferation of endometriosis regulating cyclin D1 and its target microRNAs in vitro and in vivo. J Nutr Biochem. (2019) 63:87–100. doi: 10.1016/j.jnutbio.2018.09.024
145. Zhao, P, Hu, Z, Ma, W, Zang, L, Tian, Z, and Hou, Q. Quercetin alleviates hyperthyroidism-induced liver damage via Nrf2 signaling pathway. Biofactors. (2020) 46:608–19. doi: 10.1002/biof.1626
146. Sun, L, Xu, G, Dong, Y, Li, M, Yang, L, and Lu, W. Quercetin protects against lipopolysaccharide-induced intestinal oxidative stress in broiler chickens through activation of Nrf2 pathway. Molecules. (2020) 25:1053. doi: 10.3390/molecules25051053
147. Leiro, J, Arranz, JA, Fraiz, N, Sanmartín, ML, Quezada, E, and Orallo, F. Effect of cis-resveratrol on genes involved in nuclear factor kappa B signaling. Int Immunopharmacol. (2005) 5:393–406. doi: 10.1016/j.intimp.2004.10.006
148. Lanzilli, G, Cottarelli, A, Nicotera, G, Guida, S, Ravagnan, G, and Fuggetta, MP. Anti-inflammatory effect of resveratrol and polydatin by in vitro IL-17 modulation. Inflammation. (2012) 35:240–8. doi: 10.1007/s10753-011-9310-z
149. Arablou, T, Delbandi, AA, Khodaverdi, S, Arefi, S, Kolahdouz-Mohammadi, R, Heidari, S, et al. Resveratrol reduces the expression of insulin-like growth factor-1 and hepatocyte growth factor in stromal cells of women with endometriosis compared with nonendometriotic women. Phytother Res. (2019) 33:1044–54. doi: 10.1002/ptr.6298
150. Ergenoğlu, AM, Yeniel, AÖ, Erbaş, O, Aktuğ, H, Yildirim, N, Ulukuş, M, et al. Regression of endometrial implants by resveratrol in an experimentally induced endometriosis model in rats. Reprod Sci. (2013) 20:1230–6. doi: 10.1177/1933719113483014
151. Bayoglu Tekin, Y, Guven, S, Kirbas, A, Kalkan, Y, Tumkaya, L, and Guvendag Guven, ES. Is resveratrol a potential substitute for leuprolide acetate in experimental endometriosis? Eur J Obstet Gynecol Reprod Biol. (2015) 184:1–6. doi: 10.1016/j.ejogrb.2014.10.041
152. Ozcan Cenksoy, P, Oktem, M, Erdem, O, Karakaya, C, Cenksoy, C, Erdem, A, et al. A potential novel treatment strategy: inhibition of angiogenesis and inflammation by resveratrol for regression of endometriosis in an experimental rat model. Gynecol Endocrinol. (2015) 31:219–24. doi: 10.3109/09513590.2014.976197
153. Bruner-Tran, KL, Osteen, KG, Taylor, HS, Sokalska, A, Haines, K, and Duleba, AJ. Resveratrol inhibits development of experimental endometriosis in vivo and reduces endometrial stromal cell invasiveness in vitro. Biol Reprod. (2011) 84:106–12. doi: 10.1095/biolreprod.110.086744
154. Kong, X, Xu, X, Zhou, L, Zhu, M, Yao, S, Ding, Y, et al. MTA1, a target of resveratrol, promotes epithelial-mesenchymal transition of endometriosis via ZEB2. Mol Ther Methods Clin Dev. (2020) 19:295–306. doi: 10.1016/j.omtm.2020.09.013
155. Kodarahmian, M, Amidi, F, Moini, A, Kashani, L, Shabani Nashtaei, M, Pazhohan, A, et al. The modulating effects of resveratrol on the expression of MMP-2 and MMP-9 in endometriosis women: a randomized exploratory trial. Gynecol Endocrinol. (2019) 35:719–26. doi: 10.1080/09513590.2019.1576612
156. Zhang, Y, Cao, H, Yu, Z, Peng, HY, and Zhang, CJ. Curcumin inhibits endometriosis endometrial cells by reducing estradiol production. Iran J Reprod Med. (2013) 11:415–22.
157. Cao, H, Wei, YX, Zhou, Q, Zhang, Y, Guo, XP, and Zhang, J. Inhibitory effect of curcumin in human endometriosis endometrial cells via downregulation of vascular endothelial growth factor. Mol Med Rep. (2017) 16:5611–7. doi: 10.3892/mmr.2017.7250
158. Ding, J, Mei, S, Cheng, W, Ni, Z, and Yu, C. Curcumin treats endometriosis in mice by the HIF signaling pathway. Am J Transl Res. (2022) 14:2184–98.
159. Jana, S, Paul, S, and Swarnakar, S. Curcumin as anti-endometriotic agent: implication of MMP-3 and intrinsic apoptotic pathway. Biochem Pharmacol. (2012) 83:797–804. doi: 10.1016/j.bcp.2011.12.030
160. Jana, S, Rudra, DS, Paul, S, and Snehasikta, S. Curcumin delays endometriosis development by inhibiting MMP-2 activity. Indian J Biochem Biophys. (2012) 49:342–8.
161. Swarnakar, S, and Paul, S. Curcumin arrests endometriosis by downregulation of matrix metalloproteinase-9 activity. Indian J Biochem Biophys. (2009) 46:59–65.
162. Piecuch, M, Garbicz, J, Waliczek, M, Malinowska-Borowska, J, and Rozentryt, P. I am the 1 in 10-what should I eat? A research review of nutrition in endometriosis. Nutrients. (2022) 14:5283. doi: 10.3390/nu14245283
163. Black, CJ, Staudacher, HM, and Ford, AC. Efficacy of a low FODMAP diet in irritable bowel syndrome: systematic review and network meta-analysis. Gut. (2022) 71:1117–26. doi: 10.1136/gutjnl-2021-325214
164. Melini, V, and Melini, F. Gluten-free diet: gaps and needs for a healthier diet. Nutrients. (2019) 11:170. doi: 10.3390/nu11010170
165. Nonn, L, Peng, L, Feldman, D, and Peehl, DM. Inhibition of p38 by vitamin D reduces interleukin-6 production in normal prostate cells via mitogen-activated protein kinase phosphatase 5: implications for prostate cancer prevention by vitamin D. Cancer Res. (2006) 66:4516–24. doi: 10.1158/0008-5472.CAN-05-3796
166. Amzajerdi, A, Keshavarz, M, Ghorbali, E, Pezaro, S, and Sarvi, F. The effect of vitamin D on the severity of dysmenorrhea and menstrual blood loss: a randomized clinical trial. BMC Womens Health. (2023) 23:138. doi: 10.1186/s12905-023-02284-5
167. Kraujalytė, V, Venskutonis, PR, Pukalskas, A, Česonienė, L, and Daubaras, R. Antioxidant properties and polyphenolic compositions of fruits from different European cranberrybush (Viburnum opulus L.) genotypes. Food Chem. (2013) 141:3695–702. doi: 10.1016/j.foodchem.2013.06.054
168. Saltan, G, Süntar, I, Ozbilgin, S, Ilhan, M, Demirel, MA, Oz, BE, et al. Viburnum opulus L.: a remedy for the treatment of endometriosis demonstrated by rat model of surgically-induced endometriosis. J Ethnopharmacol. (2016) 193:450–5. doi: 10.1016/j.jep.2016.09.029
169. Mozaffarian, D. Trans fatty acids - effects on systemic inflammation and endothelial function. Atheroscler Suppl. (2006) 7:29–32. doi: 10.1016/j.atherosclerosissup.2006.04.007
170. Zemel, MB, and Sun, X. Dietary calcium and dairy products modulate oxidative and inflammatory stress in mice and humans. J Nutr. (2008) 138:1047–52. doi: 10.1093/jn/138.6.1047
171. Trabert, B, Peters, U, de Roos, AJ, Scholes, D, and Holt, VL. Diet and risk of endometriosis in a population-based case-control study. Br J Nutr. (2011) 105:459–67. doi: 10.1017/S0007114510003661
COX2 - Cyclo-oxygenase2
ESCs - Endometrial stromal cells
ERK - Extracellular regulated protein kinases
HIF-α - Hypoxia inducible factor-1 α
HT - Hydroxytyrosol
MAPK - Mitogen-activated protein kinases
MCP-1 - Monocyte chemotactic protein-1
MUFA - Monounsaturated fatty acids
mtDNA - Mitochondria DNA
NGF - Nerve growth factor
NF-κB - Nuclear factor kappa-B
OO - Olive oil
OS - Oxidative stress
OXPHOS - Oxidative Phosphorylation
PKC - Phosphokinase C
PI3K - Phosphate inositol 3 kinase
PUFA - Polyunsaturated fatty acids
RSV - Resveratrol
ROS - Reactive oxygen species
TSC - Tuberculous protein sclerosis complex
TLR4 - Toll like receptor 4
VEGF - Vascular endothelial growth factor
WFKD - Well-formulated ketogenic diet
Keywords: endometriosis, inflammation, oxidative stress, dietary patterns, nutrition
Citation: Zhou L, Liu B, Jian X, Jiang L and Liu K (2025) Effect of dietary patterns and nutritional supplementation in the management of endometriosis: a review. Front. Nutr. 12:1539665. doi: 10.3389/fnut.2025.1539665
Received: 04 December 2024; Accepted: 21 February 2025;
Published: 12 March 2025.
Edited by:
Maria Luisa Mizgier, University of the Andes, ChileReviewed by:
Małgorzata Mizgier, Poznan University of Physical Education, PolandCopyright © 2025 Zhou, Liu, Jian, Jiang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lili Jiang, amlhbmdsbDMxMUAxNjMuY29t; Kuiran Liu, bGl1a3IwNDEyQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.