
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 25 March 2025
Sec. Nutritional Epidemiology
Volume 12 - 2025 | https://doi.org/10.3389/fnut.2025.1538289
This article is part of the Research TopicNutritional Epidemiology: Advances in the Analysis of Healthy and Sustainable Dietary PatternsView all 5 articles
 Chengcheng Wei1†
Chengcheng Wei1† Qian Yang2,3†
Qian Yang2,3† Jingke He1†
Jingke He1† Yu Luo1
Yu Luo1 Kun Han1
Kun Han1 Junkun Li1
Junkun Li1 Shuai Su1,4*
Shuai Su1,4* Jindong Zhang1*
Jindong Zhang1* Hongxing Wang1,5*
Hongxing Wang1,5* Delin Wang1*
Delin Wang1*Background: This study aims to investigate the comprehensive association between healthy dietary patterns, biological aging, and kidney stones in a large population-based cohort.
Methods: We analyzed data from 6 cycles of NHANES surveys from 2007 to 2018 and included a total of 26,755 participants. The association was examined using logistic regression, restricted cubic splines, and a mediation model. Machine learning with Shapley Additive Explanations (SHAP) was used to determine the relative importance of dietary factors. Sensitivity analysis was conducted to validate the stability of the results.
Results: A higher healthy dietary score was significantly associated with a reduced risk of kidney stone prevalence, as indicated by the Alternative Healthy Eating Index (AHEI) (OR = 0.76, 95% CI 0.69–0.84), the Dietary Approaches to Stop Hypertension Index (DASHI) (OR = 0.67, 95% CI 0.58–0.77), the Healthy Eating Index 2020 (HEI-2020) (OR = 0.80, 95% CI 0.72–0.89), and the Mediterranean Diet Index (MEDI) (OR = 0.81, 95% CI 0.73–0.89). Conversely, higher aging indicators were associated with an increased risk of kidney stones, including the following: Klemera-Doubal Method Age (KDMAge) (OR = 2.40, 95% CI 1.70–3.37) and Phenotypic Age (PhenoAge) (OR = 2.36, 95% CI 1.75–3.19). Mediation analyses suggested that aging indicators significantly mediated the relationship between healthy dietary patterns and kidney stones. Machine learning with SHAP revealed the relative importance of dietary patterns and specific dietary components in this association. The sensitivity analysis was largely consistent with the primary analyses.
Conclusion: These findings provide valuable insights into the complex interplay between dietary patterns, biological aging, and the risk of kidney stone. Promoting healthy dietary patterns may be an effective strategy for kidney stone prevention, potentially through the modulation of biological aging processes.
Kidney stones are common urological disorders with a rising global incidence, particularly in developed countries (1–3). It is estimated that approximately 1 in 10 individuals will experience this ailment during their lifetime (4, 5). Beyond causing severe pain, kidney stones impose a substantial financial burden on healthcare systems worldwide, accounting for billions of dollars in annual medical costs and lost productivity (6, 7).
The pathogenesis of kidney stones is driven by a complex interplay of environmental and genetic factors, with dietary components playing a pivotal role (8). High sodium and protein diets, combined with inadequate water intake, are recognized as significant contributors to kidney stone formation (9–11). Consequently, dietary modifications, such as increased hydration and adjusted intakes of calcium and oxalate, have been recognized as fundamental strategies for reducing recurrence rates (12–14). Moreover, nutritional research favors dietary scores over discrete dietary components, as they provide a comprehensive understanding of dietary patterns and their health impacts (15). Globally, various dietary scores—including the Alternative Healthy Eating Index (AHEI), the DASH Index in serving sizes from the DASH trial (DASHI), the Healthy Eating Index 2020 (HEI-2020), and the and MED Index in serving sizes from the PREDIMED trial (MEDI)—are used to assess the complex interactions between dietary nutrition, disease risks, and metabolic disorders, with an emphasis on dietary recommendations (16). These findings highlight the necessity of rigorous research into the diet–kidney stone relationships to develop robust preventive and therapeutic interventions.
Moreover, the pathogenesis of kidney stones is closely related to chronic conditions such as obesity (17), diabetes (18), and cardiovascular disease (19), necessitating multifaceted health interventions for prevention and management. Age notably influences the progression of these conditions, as declining metabolic rates with advancing age change the body’s ability to process dietary minerals, potentially increasing the risk of kidney stone. Research confirms that biological age provides a more accurate representation of an individual’s physiological state than chronological age. Algorithms such as KDMAge and PhenoAge, which assess biological age based on clinical markers, have proven highly accurate in predicting morbidity and mortality rates (20–25). KDMAge and PhenoAge were chosen for their comprehensive assessment of biological aging and predictive power for chronic diseases. Their associated biomarkers, such as creatinine and albumin, are linked to kidney function and metabolic disturbances, suggesting potential relationships with kidney stone formation through mechanisms such as inflammation and oxidative stress. Despite data indicating a higher occurrence of kidney stones among individuals aged 20–40 years (26), large-scale studies examining the influence of biological age on kidney stones remain limited. Therefore, exploring the specific roles of dietary patterns and biological age in the risk of kidney stone is important for enhancing preventive and therapeutic approaches.
In this context, our study used the US National Health and Nutrition Examination Survey (NHANES) database to systematically investigate the association between healthy dietary patterns, biological aging, and the prevalence of kidney stones. The development of kidney stones is influenced by a variety of environmental factors, with dietary components playing a crucial role. However, the association between dietary factors and kidney stones remains unclear. This study aims to investigate the relationship between healthy dietary patterns, biological aging, and the prevalence of kidney stones in a large population-based cohort.
The National Center for Health Statistics (NCHS) conducts the NHANES research project, a nationwide study assessing the health and nutritional status of both adults and children in the United States. The study is overseen by the Centers for Disease Control and Prevention (CDC). The NHANES survey data are publicly accessible on the official webpage: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm. Our analysis includes cross-sectional data gathered from six NHANES study cycles (2007–2018), which initially comprised 58,842 participants. A total of 24,163 individuals were excluded due to incomplete kidney stone questionnaire data. Subsequently, we excluded 7,924 individuals with dietary survey data. After these exclusions, a final sample of 26,755 individuals remained for additional research (Figure 1). Finally, a subsample was analyzed based on indicator data that were available again, comprising 16,143 individuals for KDMAge and homeostatic dysregulation and 16,563 individuals for PhenoAge. Since NHANES data were used for our investigation, and the NHANES procedures were previously approved by the NCHS Research Ethics Review Board (ERB) and the NHANES Institutional Review Board (IRB), no further approvals were required. The study adhered to the ethical principles outlined in the Declaration of Helsinki and was reported in accordance with the Strengthening The Reporting Of Cohort Studies in Surgery (STROCSS) criteria.
We measured biological aging utilizing the “BioAge” package with information from NHANES (27). This package computes three biological aging metrics: Klemera-Doubal Method (KDM) biological age (KDMAge), phenotypic age (PhenoAge), and homeostatic dysregulation, based on established biomarker algorithms. The KDM biological age forecast for an individual indicates the age at which their physiology is expected to align with typical norms. The KDM was assessed using the “Levine Original” KDM algorithm, which relies on chronological age and 10 biomarkers, namely total cholesterol, systolic blood pressure, albumin, alkaline phosphatase, blood urea nitrogen, creatinine, C-reactive protein, cytomegalovirus optical density, hemoglobin A1C (HbA1c), and forced expiratory volume in 1 s (FEV1). Nine biomarkers comprise the “Levine Original” PhenoAge, alongside chronological age: albumin, alkaline phosphatase, creatinine, C-reactive protein, fasting glucose, white blood cell count, lymphocyte percentage, mean cell volume, and red cell distribution width (28). The homeostatic dysregulation value for an individual indicates the extent to which their physiology deviates from that of a healthy reference. Higher levels of homeostatic dysregulation suggest an accelerated stage of biological aging and an increased risk of illness, disability, and mortality. Conversely, lower levels of homeostatic dysregulation signify a delayed biological aging process and a reduced likelihood of illness, incapacity, and death. Homeostatic dysregulation is calculated using the Mahalanobis distance, which evaluates the deviation of a set of biomarkers from a reference sample. The Mahalanobis distance formula is structured as follows (29):
This is equivalent to normalizing each biomarker by its variance and then summing the squared deviance for each observation, assuming that all variables are uncorrelated:
Kidney stone status was extracted from the “Kidney Conditions—Urology” section of the NHANES Questionnaire Data. Trained interviewers used the Computer-Assisted Personal Interview (CAPI) system to ask interview subjects at home whether they had ever experienced kidney stones (KIQ026). Individuals who responded “yes” were classified as having a history of kidney stones.
A validated semiquantitative food frequency questionnaire (FFQ) consisting of over 130 questions was used to collect dietary data, distributed every 2–4 years. Detailed descriptions of the FFQs’ validity and repeatability are available, demonstrating a strong correlation between the nutrients measured by the FFQs and multiple weeks of food records or dietary biomarkers. Using the food and nutrient components, we calculated the dietary scores for the AEHI, DASHI, HEI-2020, and MEDI to reflect adherence to healthy eating. For these four scores, a better diet is indicated by a higher score, and a less healthy diet is indicated by a lower score. In 2002, the Alternate Healthy Eating Index (AHEI) was introduced as an alternative to the HEI, which was based on dietary components and nutrients that were indicative of the risk of chronic diseases (30). Emphasizing increased consumption of fruits, vegetables, and low-fat dairy products while reducing total and saturated fats, the DASH diet significantly lowers blood pressure and is recommended for the prevention of cardiovascular diseases (31). The HEI-2020 is a revised and updated version of the HEI based on the 2020–2025 Dietary Guidelines for Americans (DGA), which includes 13 components aiming to maintain a healthy body weight and minimize the risk of chronic diseases (32). The Mediterranean diet is recognized for its ability to identify overall dietary patterns rather than just single foods or nutrients, which is considered effective in providing a considerable defense against mortality, the development of heart diseases, and major chronic degenerative diseases (33).
Data from the laboratory, questionnaires, and demographics were used to collect covariates. Using a standardized questionnaire, we included variables such as age, sex, race, marital status, education level, poverty-income ratio, obesity, smoking, alcohol use, diabetes, high blood pressure, food insecurity, water consumption, serum calcium, and sitting time. Age, sex, and obesity were identified as adjustment factors due to their roles as confounders in the relationship between dietary habits and kidney stone formation. Prior studies and literature reviews confirm the significance of these variables in influencing the risk of kidney stone. A body mass index of 30 kg/m2 was considered obesity. Participants were defined as smokers if they had smoked at least 100 cigarettes in their lifetime. Participants were defined as alcohol users if they had consumed at least 12 alcoholic drinks in 1 year. In evaluating food security, we utilized data from the “Food Security” questionnaire. The assessment was conducted using the 10-item U.S. Food Security Survey Module available at https://aspe.hhs.gov/topics/poverty-economic-mobility/poverty-guidelines/prior-hhs-poverty-guidelines-federal-register-references. An acknowledgment of six or more affirmative responses signifies significant food insecurity, whereas fewer than six responses indicate marginal food security or food security. Diagnosed diabetes was defined as a fasting glucose level of 7 mmol/L (126 mg/dL), an HbA1c level of 6.5%, or a diabetes diagnosis. A systolic blood pressure of 130 mm Hg, a diastolic blood pressure of 80 mm Hg, or the use of antihypertensive drugs was considered high blood pressure. Water consumption data were obtained from the dietary questionnaire. Serum calcium was obtained from the laboratory data of the standard biochemistry profile. Sitting time was derived from the questionnaire data on physical activity. Each of these variables is described on the NHANES website.
Sample weights, clustering, and stratification were all considered while evaluating the data due to the complex sampling method of the NHANES. We evaluated the relationships between kidney stones, aging indicators, and dietary scores using multivariable logistic regression models. Non-linear relationships were analyzed with restricted cubic splines. The relationship between healthy dietary scores and aging indicators was investigated using multiple linear regression models. We utilized the R package “mediation” to assess the direct effect (DE), indirect effect (IE), and total effect (TE) for causal mediation research (34). The “shapviz” package was used to build the machine learning model to predict the relative importance of dietary patterns and components. R software (version 4.0.1) was used for all statistical analyses, with a significance threshold set at p < 0.05 (two-sided).
Table 1 provides an overview of the baseline characteristics of participants classified by kidney stone status, while Supplementary Table S1 presents the survey-weighted descriptive statistics for these participants. We included 26,755 participants from the NHANES survey conducted between 2007 and 2018, all of whom met the research criteria for our study. The median age of participants was 50.14 ± 17.61 years. Among these participants, 2,577 were kidney stone patients, while 24,178 were participants without kidney stones. We observed varying dietary scores, including the Alternative Healthy Eating Index (AHEI), the DASH Index from the DASH trial (DASHI), the HEI-2020, and the MED Index from the PREDIMED trial (MEDI), all of which displayed different distribution patterns between kidney stone and non-kidney stone participants. Significantly lower dietary scores were noted among kidney stone participants. Moreover, kidney stone participants exhibited higher levels of aging indicators, including KDMAge, PhenoAge, KDMAge accelerated aging, PhenoAge accelerated aging, and indicators of homeostatic dysregulation. We also found notable differences across several variables between kidney stone participants and non-participants, including age, sex, race, marital status, obesity, smoking, alcohol use, diabetes, high blood pressure, food insecurity, and water consumption (p < 0.05).
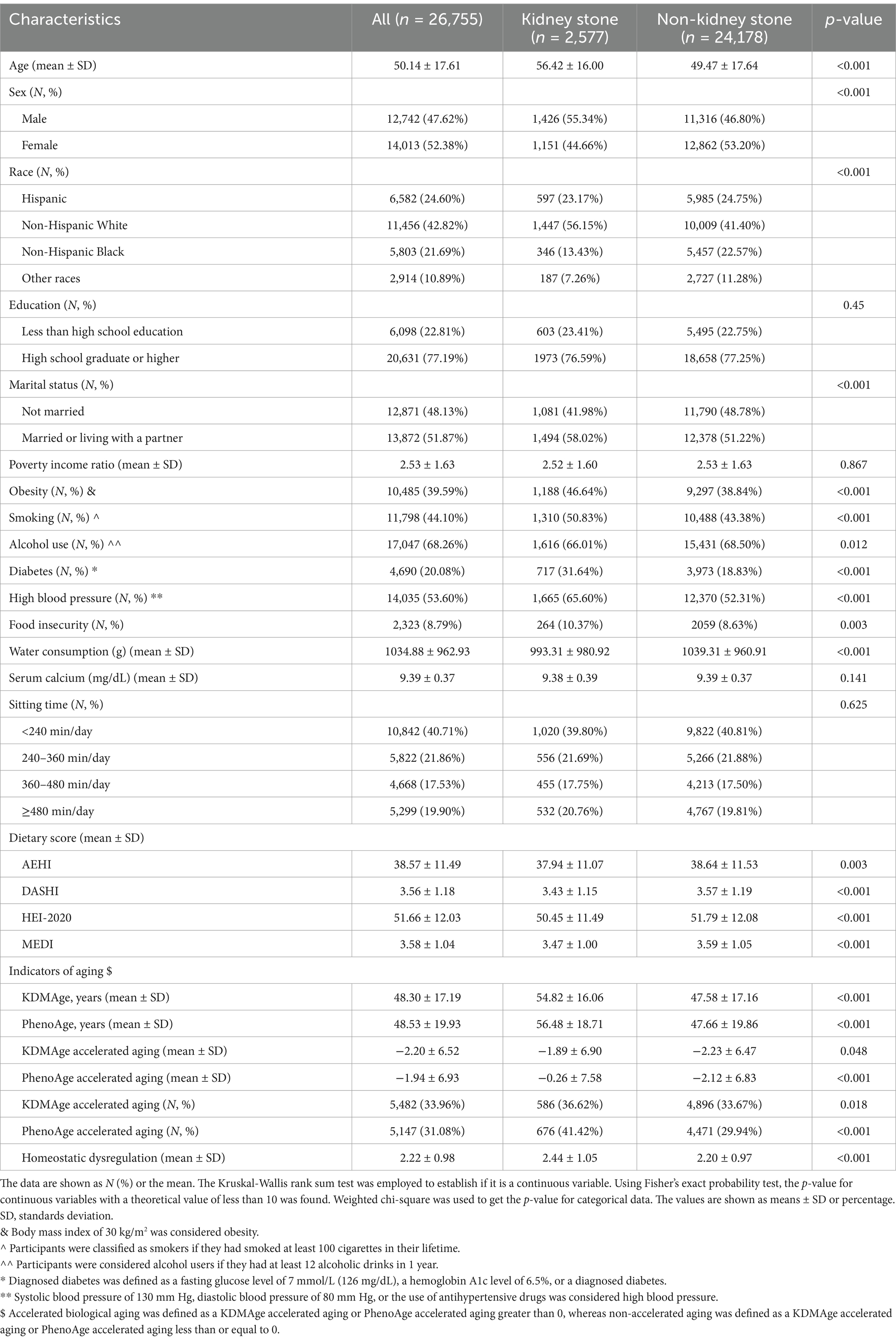
Table 1. Basic characteristics of participants by kidney stone among U.S. adults in NHANES 2007–2018.
We used the multiple logistic regression analysis to explore the potential relationship between healthy dietary scores and kidney stones (Figure 2A). The log2-transformed exposures, including the AHEI (OR = 0.76, 95% CI 0.69–0.84, p < 0.001), DASHI (OR = 0.67, 95% CI 0.58–0.77, p < 0.001), HEI-2020 (OR = 0.90, 95% CI 0.72–0.89, p < 0.001), and MEDI (OR = 0.81, 95% CI 0.73–0.89, p < 0.001), showed a statistically significant negative correlation with kidney stones in the fully adjusted models. The fourth quartile of dietary scores indicated significantly lower odds of kidney stones compared to the first quartile. In all models, a monotonically increasing trend in kidney stone prevalence risk was observed as the quartiles of healthy dietary scores increased (all p for trend <0.01) (Figure 2A). Additionally, to explore the potential non-linear association between healthy dietary scores and the occurrence of kidney stones, we conducted a restricted cubic spline model. The results indicate linear relationships between healthy dietary scores and kidney stones (P-overall <0.05 and p for non-linearity >0.05) (Figures 2B–E). We also analyzed the characteristics of specific dietary components (Supplementary Table S9) and the association of these components with kidney stones (Supplementary Table S10).
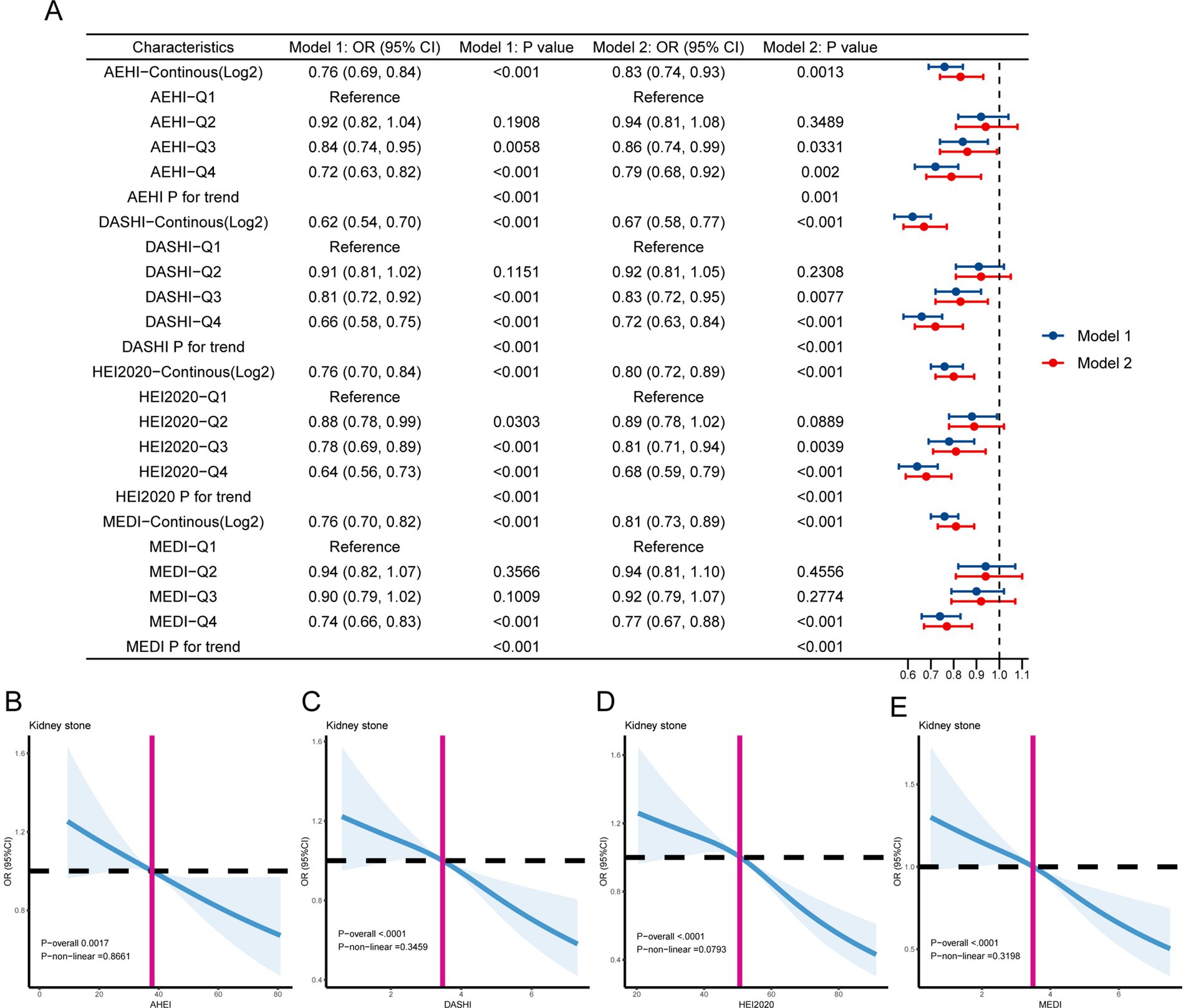
Figure 2. (A) The association between healthy dietary scores and kidney stones is depicted using forest plots. Model 1 was adjusted for age, sex, race, marital status, education level, and poverty income ratio. Model 2 was adjusted for age, sex, race, marital status, education level, poverty income ratio, obesity, smoking, alcohol use, diabetes, high blood pressure, food insecurity, water consumption, serum calcium, and sitting time. (B–E) The linear associations between kidney stones and healthy dietary scores are depicted using restricted cubic splines. The solid lines represent the odds ratios (ORs) based on the restricted cubic splines of healthy dietary scores. The top and lower 95% confidence interval (CI) boundaries are shaded. The adjustment factors are the same as those used in the expanded model. The model is adjusted for age, sex, race, marital status, educational level, poverty income ratio, obesity, smoking, alcohol use, diabetes, high blood pressure, food insecurity, water consumption, serum calcium, and sitting time.
In Table 2, a multiple logistic regression analysis was used to demonstrate the associations between biological aging and kidney stone incidence. We found significant positive associations between kidney stones and aging indicators, including KDMAge (OR = 2.40, 95% CI 1.70–3.37, p < 0.0001) and PhenoAge (OR = 2.36, 95% CI 1.75–3.19, p < 0.0001) in Model 2. However, no statistical difference was detected regarding homeostatic dysregulation in the fully adjusted model (OR = 1.11, 95% CI 0.97–1.27, p = 0.1421). The risk of kidney stones increased monotonically with higher quartiles of KDMAge and PhenoAge. The results indicated that accelerated aging was associated with a greater risk of kidney stones: KDMAge (OR = 1.20, 95% CI 1.04–1.38, p = 0.0104) and PhenoAge (OR = 1.89, 95% CI 1.16–3.06, p = 0.0104). Additionally, the linear association between kidney stones and aging markers was examined using cubic spline curves (Supplementary Figure S1), which demonstrated non-linear correlations. However, no association was found between kidney stones and homeostatic dysregulation (p overall >0.05).
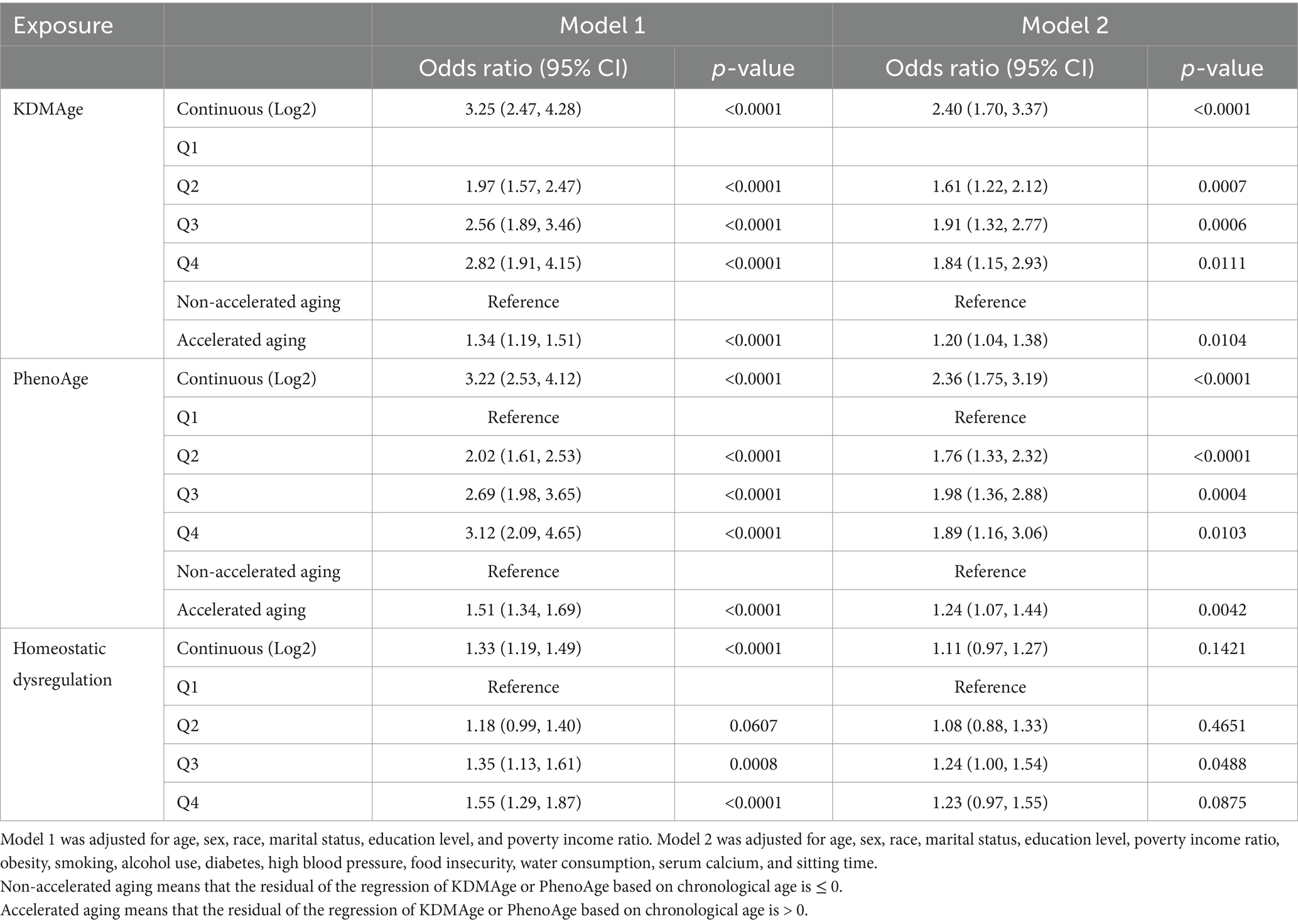
Table 2. Multiple logistic regression analysis revealed the associations of aging indicators with kidney stones in adults.
We conducted an analysis to examine the association between healthy dietary scores and aging indicators listed in Supplementary Table S2. After adjusting for all covariates, we observed significant negative associations between healthy dietary scores (the AHEI, DASHI, HEI-2020, and MEDI) and aging indicators (KDMAge, PhenoAge, homeostatic dysregulation, KDMAge accelerated aging, and PhenoAge accelerated aging). Additionally, the results of the multiple logistic regression analysis indicated that higher healthy dietary scores were associated with a decreased risk of accelerated aging, as shown by KDMAge and PhenoAge. Restricted cubic splines in Figure 3 visualize both linear and non-linear relationships between healthy dietary scores and aging indicators. KDMAge, PhenoAge, KDMAge accelerated aging, and PhenoAge accelerated aging all exhibited linear associations with the four dietary scores.
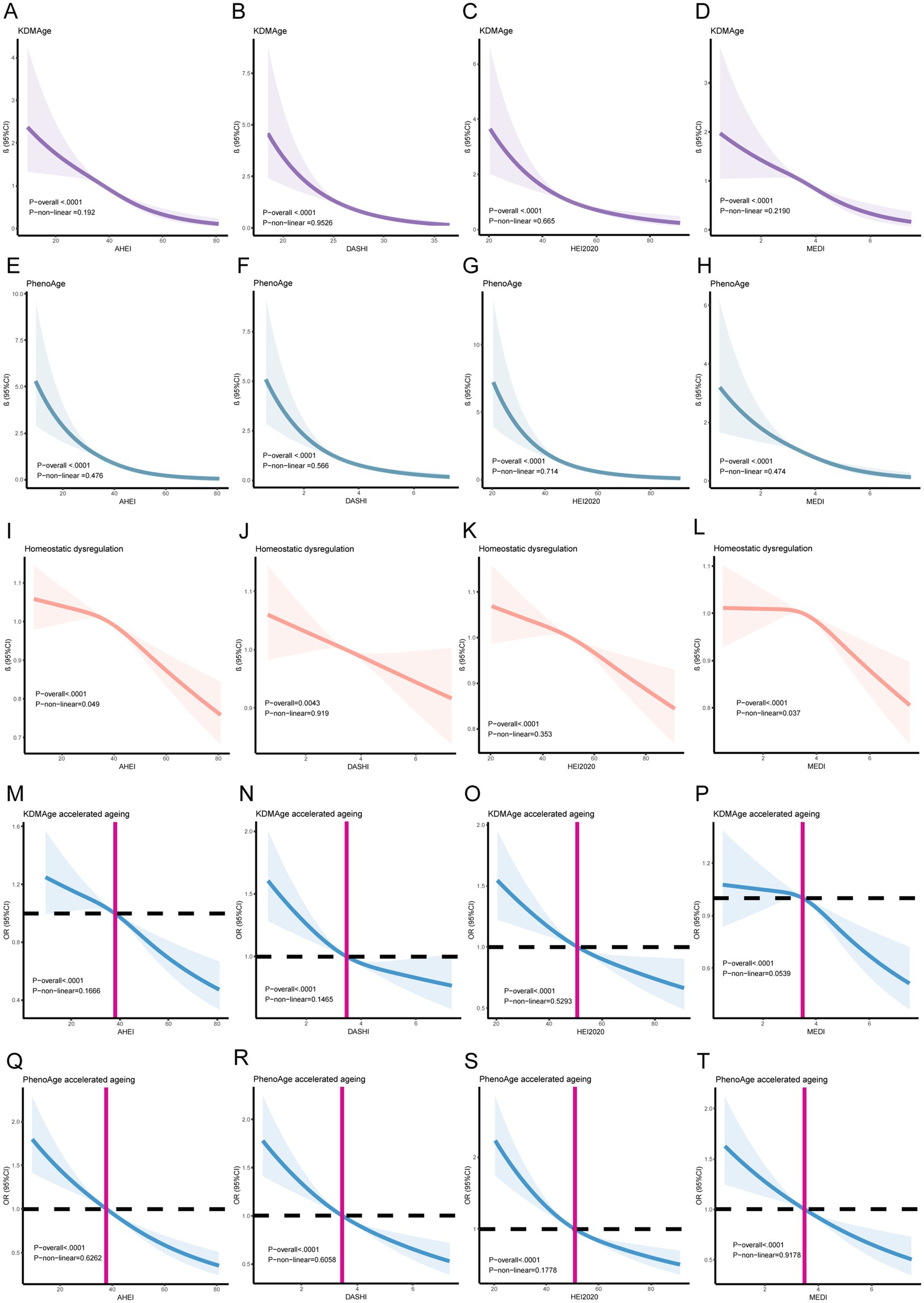
Figure 3. Linear and Non-linear relationships between healthy dietary scores and aging indicators/accelerating aging using restricted cubic splines. (A) The AHEI and KDMAge; (B) DASHI and KDMAge; (C) the HEI-2020 and KDMAge; (D) the MEDI and KDMAge; (E) the AHEI and PhenoAge; (F) DASHI and PhenoAge; (G) the HEI-2020 and PhenoAge; (H) the MEDI and PhenoAge; (I) the AHEI and Homeostatic dysregulation; (J) the DASHI and Homeostatic dysregulation; (K) the HEI-2020 and Homeostatic dysregulation; (L) the MEDI and Homeostatic dysregulation; (M) the HEI-2020 and KDMAge accelerated aging; (N) the DASHI and KDMAge accelerated aging; (O) the HEI-2020 and KDMAge accelerated aging; (P) the MEDI and KDMAge accelerated aging; (Q) the AHEI and PhenoAge accelerated aging; (R) DASHI and PhenoAge accelerated aging; (S) the HEI-2020 and PhenoAge accelerated aging; (T) the MEDI and PhenoAge accelerated aging. Generalized-linear models and logistic regression models for age, sex, race, marital status, education level, poverty income ratio, obesity, smoking, alcohol use, diabetes, high blood pressure, food insecurity, water consumption, serum calcium, and sitting time.
We assessed the mediating effect in the association between kidney stones and a healthy diet using mediation analysis, with aging indicators as mediators (Supplementary Table S3). Our results suggest that the aging indicators KDMAge, PhenoAge, KDMAge accelerated aging, and PhenoAge accelerated aging significantly mediated the associations between healthy dietary patterns (the AHEI, DASHI, HEI-2020, and MEDI) and the risk of kidney stones (Figure 4). For KDMAge and healthy dietary patterns (the AHEI, DASHI, HEI-2020, and MEDI), the mediation proportions were 7.00, 3.21, 2.98, and 3.02%, respectively. For PhenoAge and healthy dietary patterns (the AHEI, DASHI, HEI-2020, and MEDI), the mediation proportions were 9.63, 4.60, 4.52, and 3.87%, respectively. For PhenoAge accelerated aging and healthy dietary patterns (the AHEI, DASHI, HEI-2020, and MEDI), the mediation proportions were 10.42, 4.74, 4.78, and 3.45%, respectively. Nevertheless, no significant mediation effects were observed in the associations between healthy dietary scores and kidney stones mediated by homeostatic dysregulation (all p > 0.05).
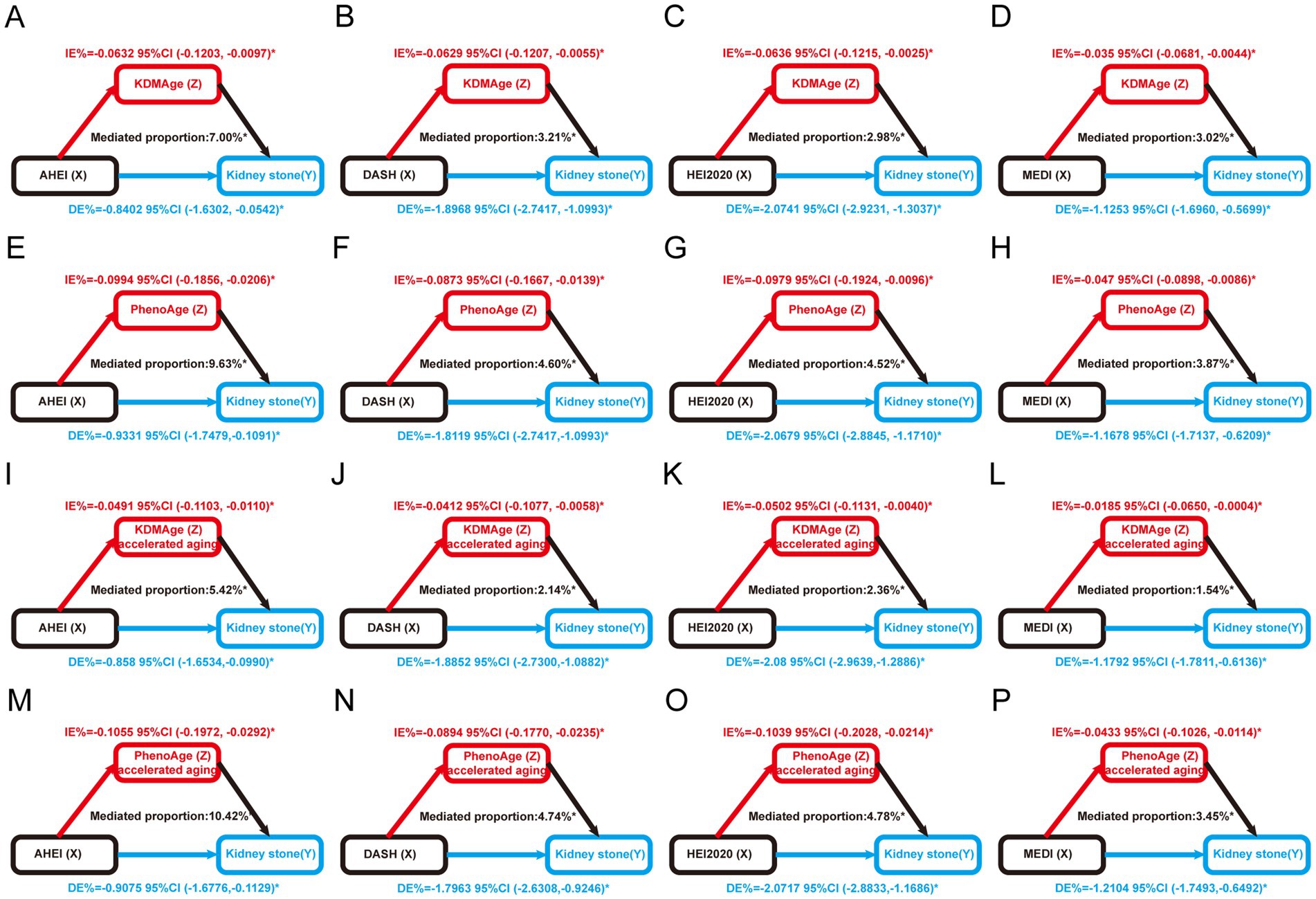
Figure 4. Estimated proportion of the association between healthy dietary scores and kidney stones mediated by aging markers. (A) The AHEI mediated by KDMAge; (B) the DASHI mediated by KDMAge; (C) the HEI-2020 mediated by KDMAge; (D) the MEDI mediated by KDMAge; (E) the AHEI mediated by PhenoAge; (F) the DASHI mediated by PhenoAge; (G) the HEI-2020 mediated by PhenoAge; (H) the MEDI mediated by PhenoAge; (I) the AHEI mediated by KDMAge accelerated aging; (J) the DASHI mediated by KDMAge accelerated aging; (K) the HEI-2020 mediated by KDMAge accelerated aging; (L) the MEDI mediated by KDMAge accelerated aging; (M) the AHEI mediated by PhenoAge accelerated aging; (N) the DASHI mediated by PhenoAge accelerated aging; (O) the HEI-2020 mediated by PhenoAge accelerated aging; (P) the MEDI mediated by PhenoAge accelerated aging. Age, sex, race, marital status, education level, poverty income ratio, obesity, smoking, alcohol use, diabetes, high blood pressure, food insecurity, water consumption, serum calcium, and sitting time were all considered while adjusting the models. IE, indirect effect; DE, direct effect; Proportion of mediation = IE / (DE + IE); *p < 0.05.
Furthermore, we constructed the SHAP model for machine learning to identify the relative importance of dietary patterns and specific dietary components (HEI-2020). We found that HEI-2020 was the most important dietary score for kidney stones compared to other dietary scores. Regarding specific dietary components, saturated fatty acids were the most important component for kidney stones (Figure 5).
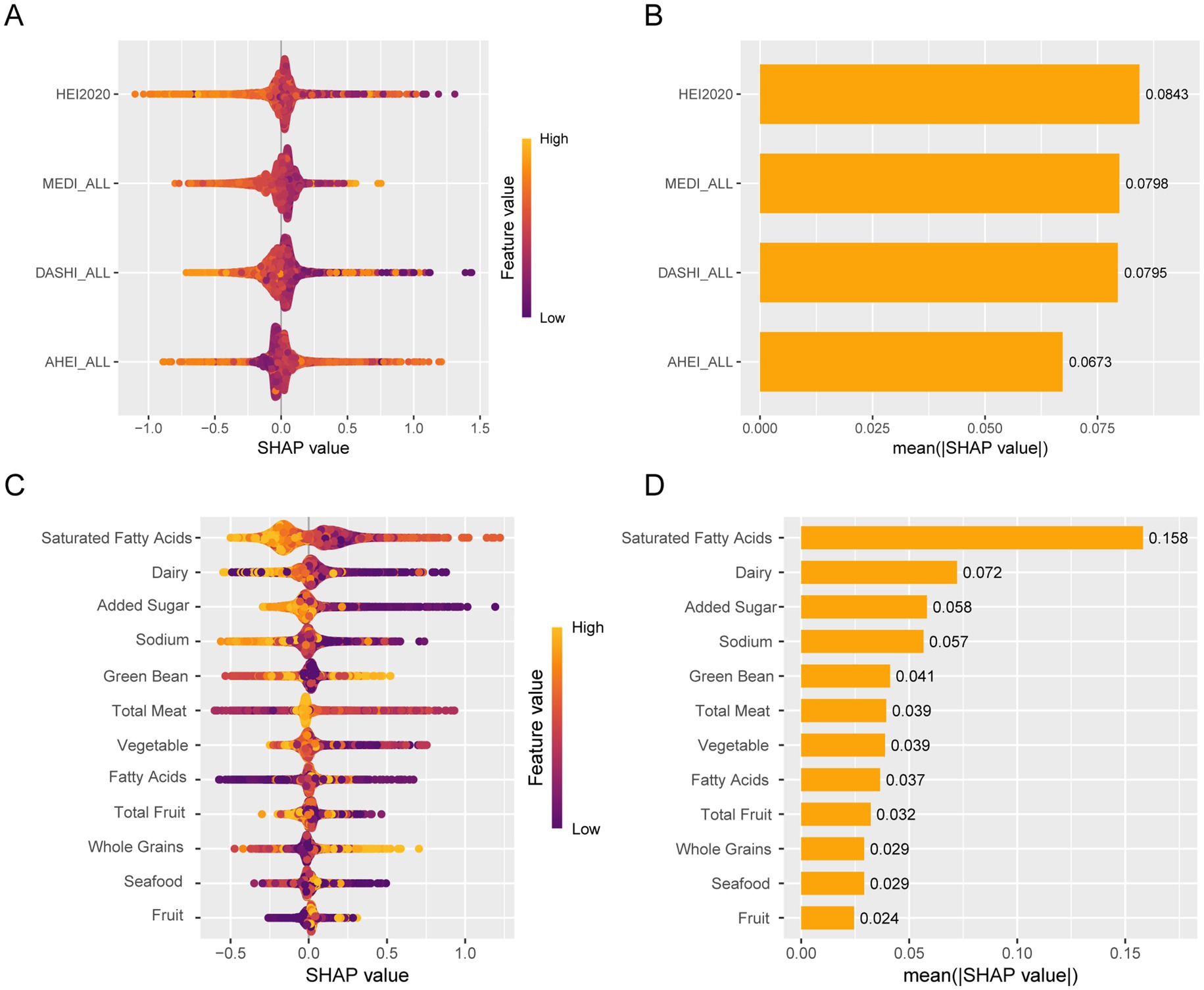
Figure 5. Machine learning using SHAP identifies relative importance. (A) Bee warm SHAP analysis of dietary patterns. (B) Importance of the SHAP representation of dietary patterns. (C) Bee warm SHAP analysis of specific dietary components. (D) Bee warm SHAP analysis of specific dietary components.
Initially, we conducted multiple imputations to complete the cases. We analyzed the association between healthy dietary scores and the risk of prevalent kidney stones using complete cases (Supplementary Table S4). Additionally, we carried out multiple logistic regression to identify the associations between indicators of aging and kidney stones in adults (Supplementary Table S5) and further examined the associations between indicators of aging and kidney stones within the complete cases (Supplementary Table S6). The relationships observed in the complete case analysis were largely consistent with those from the primary analyses. Subsequently, we conducted a sensitivity analysis stratified by two 6-year survey periods (2007–2012 and 2013–2018) to evaluate the consistency of associations between healthy dietary scores, indicators of aging, and kidney stones (Supplementary Tables S7, S8).
In this study, we used the NHANES database to elucidate the relationship between healthy dietary practices and the prevalence of kidney stones. Our analysis revealed a robust linear negative association between healthy diet scores and the risk of kidney stone prevalence. Conversely, biological aging and accelerated aging showed a significant association with a higher prevalence of kidney stones, exhibiting linear correlations. Notably, the aging indicators KDMAge and PhenoAge showed significant statistical differences when mediated by the four dietary scores (the AHEI, HEI-2020, MEDI, and DASHI), highlighting the mediating role of KDMAge and PhenoAge in the relationship between healthy diets and kidney stones. A similar association was also observed in cases of accelerated aging. This finding highlights the potential for a healthy diet to reduce the risk of kidney stone and slow disease progression by decelerating biological aging. Machine learning SHAP identified saturated fatty acids as the most significant component associated with kidney stones.
Our findings align with prior research, which consistently demonstrates that healthier dietary patterns, as represented by dietary indices such as the AEHI, DASHI, HEI-2020, and MEDI, are associated with a reduced risk of kidney stones, emphasizing the critical role of dietary habits in prevention and management (35, 36). These dietary indices prioritize a balanced intake of vegetables, nuts, whole grains, and low-fat dairy products, which are crucial for preventing both chronic metabolic disorders and kidney stone formation (30, 31, 32, 33). Diets rich in fruits and vegetables provide key nutrients such as citrate, potassium, and magnesium, which lower urinary calcium levels and inhibit the formation of oxalate crystals, thereby reducing the risk of kidney stone (37, 38, 39, 40). In contrast, high-protein diets, particularly those with excessive red meat and seafood, elevate the urinary levels of calcium, uric acid, and oxalate, increasing the risk of kidney stone due to their high purine content, which promotes uric acid production during metabolism (41, 42, 43).
Additionally, healthy dietary patterns, assessed by the HEI-2020, show a significant negative correlation with biological aging indicators such as KDMAge and PhenoAge, suggesting that healthier diets may reduce the risk of kidney stones by slowing biological aging processes. This effect is achieved through mechanisms such as reduced oxidative stress and inflammation, improved metabolic pathways, and better management of metabolic syndrome-related parameters such as BMI, insulin sensitivity, and serum uric acid levels (35, 36). Healthy dietary patterns also regulate the excretion of stone-forming components in urine. For instance, moderate calcium intake binds dietary oxalate in the gut, reducing its urinary excretion, while alkaline diets decrease the risk of uric acid stone formation (10, 44). Furthermore, dietary choices, such as reducing sugar-sweetened beverages and increasing fiber-rich foods, improve gut microbiota composition, thereby promoting the growth of probiotics and reducing oxalate absorption and excretion (37, 38). Collectively, these mechanisms highlight the importance of dietary habits in kidney stone prevention, highlighting the interaction of multiple pathways, including the regulation of metabolic processes, inflammation, oxidative stress, and gut microbiota, in reducing the risk of kidney stone.
The direct association between aging and kidney stone formation is less explored; however, existing literature suggests that various physiological and lifestyle changes associated with aging may significantly contribute to stone formation (45, 46, 47). As metabolic rates decline with age, not only is energy expenditure affected, but the excretion processes of metabolites such as calcium, oxalate, and uric acid are also altered. Thus, these changes may promote stone formation, particularly among the elderly (48, 49). Our comprehensive assessment of whole-body aging using various markers consistently indicates a positive correlation between biological aging and the risk of kidney stone. Specifically, the biological age markers KDMAge and PhenoAge show promise in identifying individuals with accelerated biological aging, which could aid in preventing kidney stone development.
The mediation analysis further substantiated the mediating role of biological age in the relationship between a healthy diet and the risk of kidney stone. Our findings indicate that the aging indicators KDMAge, PhenoAge, KDMAge accelerated aging, and PhenoAge accelerated aging significantly mediated the associations between a healthy dietary pattern (the AHEI, DASHI, HEI-2020, and MEDI) and the risk of kidney stones. This pivotal finding indicates that healthy dietary patterns can reduce the risk of kidney stones and their progression by modulating biological age, underscoring the significance of biological age as a physiological marker in assessing the risk of kidney stone. Previous studies have shown that dietary patterns rich in vegetables and fruits, such as the HEI-2020, may slow the aging process due to their high levels of antioxidant and anti-inflammatory agents found in these foods (50). This finding not only supports the hypothesis that dietary modifications can slow the biological aging process but also provides a scientific foundation for dietary guidelines in clinical settings. Such dietary patterns could indirectly reduce the risk of kidney stone by ameliorating metabolic disorders and optimizing mineral metabolism, encouraging further research into how biological age influences kidney stone formation and progression and facilitating the development of personalized and targeted preventive strategies.
This study leveraged the extensive dataset provided by the NHANES to thoroughly examine the interplay between healthy dietary patterns, biological age, and kidney stone incidence. However, several limitations warrant mention. First, the use of self-reported data for kidney stones may introduce potential biases, such as memory inaccuracies, with no medical records available for validation, which could compromise the reliability of the results. Second, while efforts were made to adjust for known confounders, residual and unmeasured confounding factors might still have influenced the findings. Additionally, measurement errors, particularly in responses to the food frequency questionnaire, could have impacted study outcomes. Data on biological aging indicators were only collected at baseline, limiting our ability to analyze their temporal dynamics and effects on the risk of kidney stone, thus constraining our understanding of the influence of fluctuations in biological age. Finally, the cross-sectional nature of this study restricts our capacity to establish causality, as it captures data at a single point rather than over time, necessitating further validation through prospective or interventional studies.
Our analysis revealed a strong negative association between healthy dietary scores and kidney stone prevalence, while biological aging was significantly associated with an increased risk of kidney stones. Notably, the biological aging markers KDMAge, PhenoAge, KDMAge accelerated aging, and PhenoAge accelerated aging served as significant mediators in this association. These findings suggest that maintaining healthy dietary patterns may help reduce the prevalence of kidney stones and may delay their onset by decelerating biological aging. General dietary suggestions include increased fluid intake, appropriate calcium consumption, reduced high-oxalate foods, controlled animal protein intake, increased dietary fiber, and limited salt and sugar. For elderly individuals, dietary adjustments should be made gradually, with a focus on maintaining hydration and consuming easily digestible foods. High-risk groups require personalized dietary plans, strict limitations on high-oxalate foods, weight management, and regular monitoring of urine and blood tests. This study highlights the importance of dietary management in controlling biological age, thereby providing a strategic approach to the prevention and management of kidney stones.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
CW: Conceptualization, Data curation, Formal analysis, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. QY: Conceptualization, Data curation, Formal analysis, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. JH: Conceptualization, Methodology, Writing – review & editing. YL: Conceptualization, Methodology, Writing – review & editing. KH: Conceptualization, Methodology, Writing – review & editing. JL: Writing – review & editing. SS: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing. JZ: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing. HW: Project administration, Writing – review & editing DW: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
We would like to thank Chengcheng Wei and Qi Xiong for all their support and help.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1538289/full#supplementary-material
1. Singh, P, Harris, PC, Sas, DJ, and Lieske, JC. The genetics of kidney stone disease and nephrocalcinosis. Nat Rev Nephrol. (2022) 18:224–40. doi: 10.1038/s41581-021-00513-4
2. Geraghty, RM, Cook, P, Walker, V, and Somani, BK. Evaluation of the economic burden of kidney stone disease in the UK: a retrospective cohort study with a mean follow-up of 19 years. BJU Int. (2020) 125:586–94. doi: 10.1111/bju.14991
3. Crivelli, JJ, Maalouf, NM, Paiste, HJ, Wood, KD, Hughes, AE, Oates, GR, et al. Disparities in kidney stone disease: a scoping review. J Urol. (2021) 206:517–25. doi: 10.1097/JU.0000000000001846
4. Scales, CD Jr, Smith, AC, Hanley, JM, and Saigal, CS. Prevalence of kidney stones in the United States. Eur Urol. (2012) 62:160–5. doi: 10.1016/j.eururo.2012.03.052
5. Khan, SR, Pearle, MS, Robertson, WG, Gambaro, G, Canales, BK, Doizi, S, et al. Kidney stones. Nat Rev Dis Primers. (2016) 2:16008. doi: 10.1038/nrdp.2016.8
6. Thongprayoon, C, Krambeck, AE, and Rule, AD. Determining the true burden of kidney stone disease. Nat Rev Nephrol. (2020) 16:736–46. doi: 10.1038/s41581-020-0320-7
7. Watson, G, Payne, SR, Kunitsky, K, Natchagande, G, Mabedi, C, and Scotland, KB. Stone disease in low- and middle-income countries: could augmented reality have a role in its management? BJU Int. (2022) 130:400–7. doi: 10.1111/bju.15877
8. Siener, R. Nutrition and kidney stone disease. Nutrients. (2021) 13:1917. doi: 10.3390/nu13061917
9. Coe, FL, Worcester, EM, and Evan, AP. Idiopathic hypercalciuria and formation of calcium renal stones. Nat Rev Nephrol. (2016) 12:519–33. doi: 10.1038/nrneph.2016.101
10. Ferraro, PM, Bargagli, M, Trinchieri, A, and Gambaro, G. Risk of kidney stones: influence of dietary factors, dietary patterns, and vegetarian-vegan diets. Nutrients. (2020) 12:779. doi: 10.3390/nu12030779
11. Ticinesi, A, Nouvenne, A, Borghi, L, and Meschi, T. Water and other fluids in nephrolithiasis: state of the art and future challenges. Crit Rev Food Sci Nutr. (2017) 57:963–74. doi: 10.1080/10408398.2014.964355
12. Peerapen, P, and Thongboonkerd, V. Kidney stone prevention. Adv Nutr. (2023) 14:555–69. doi: 10.1016/j.advnut.2023.03.002
13. Moe, OW. Kidney stones: pathophysiology and medical management. Lancet. (2006) 367:333–44. doi: 10.1016/S0140-6736(06)68071-9
14. Alexander, RT, Fuster, DG, and Dimke, H. Mechanisms underlying calcium nephrolithiasis. Annu Rev Physiol. (2022) 84:559–83. doi: 10.1146/annurev-physiol-052521-121822
15. Wang, J, Chen, S, Zhao, J, Liang, J, Gao, X, Gao, Q, et al. Association between nutrient patterns and hyperuricemia: mediation analysis involving obesity indicators in the NHANES. BMC Public Health. (2022) 22:1981. doi: 10.1186/s12889-022-14357-5
16. Tian, T, Zhang, J, Xie, W, Ni, Y, Fang, X, Liu, M, et al. Dietary quality and relationships with metabolic dysfunction-associated fatty liver disease (MAFLD) among United States adults, results from NHANES 2017-2018. Nutrients. (2022) 14:4505. doi: 10.3390/nu14214505
17. Wollin, DA, Skolarikos, A, and Preminger, GM. Obesity and metabolic stone disease. Curr Opin Urol. (2017) 27:422–7. doi: 10.1097/MOU.0000000000000427
18. Aune, D, Mahamat-Saleh, Y, Norat, T, and Riboli, E. Body fatness, diabetes, physical activity and risk of kidney stones: a systematic review and meta-analysis of cohort studies. Eur J Epidemiol. (2018) 33:1033–47. doi: 10.1007/s10654-018-0426-4
19. Zisman, AL, Evan, AP, Coe, FL, and Worcester, EM. Do kidney stone formers have a kidney disease? Kidney Int. (2015) 88:1240–9. doi: 10.1038/ki.2015.254
20. Gao, X, Geng, T, Jiang, M, Huang, N, Zheng, Y, Belsky, DW, et al. Accelerated biological aging and risk of depression and anxiety: evidence from 424,299 UK biobank participants. Nat Commun. (2023) 14:2277. doi: 10.1038/s41467-023-38013-7
21. Chen, L, Wu, B, Mo, L, Chen, H, Zhao, Y, Tan, T, et al. Associations between biological ageing and the risk of, genetic susceptibility to, and life expectancy associated with rheumatoid arthritis: a secondary analysis of two observational studies. Lancet Healthy Longev. (2024) 5:e45–55. doi: 10.1016/S2666-7568(23)00220-9
22. Luo, S, Wong, ICK, Chui, CSL, Zheng, J, Huang, Y, Schooling, CM, et al. Effects of putative metformin targets on phenotypic age and leukocyte telomere length: a mendelian randomisation study using data from the UK biobank. Lancet Healthy Longev. (2023) 4:e337–44. doi: 10.1016/S2666-7568(23)00085-5
23. Gao, X, Huang, N, Guo, X, and Huang, T. Role of sleep quality in the acceleration of biological aging and its potential for preventive interaction on air pollution insults: findings from the UK biobank cohort. Aging Cell. (2022) 21:e13610. doi: 10.1111/acel.13610
24. Belsky, DW, Moffitt, TE, Cohen, AA, Corcoran, DL, Levine, ME, Prinz, JA, et al. Eleven telomere, epigenetic clock, and biomarker-composite quantifications of biological aging: do they measure the same thing? Am J Epidemiol. (2018) 187:1220–30. doi: 10.1093/aje/kwx346
25. Li, X, Ploner, A, Wang, Y, Magnusson, PK, Reynolds, C, Finkel, D, et al. Longitudinal trajectories, correlations and mortality associations of nine biological ages across 20-years follow-up. eLife. (2020) 9:1507. doi: 10.7554/eLife.51507
27. Kwon, D, and Belsky, DW. A toolkit for quantification of biological age from blood chemistry and organ function test data: BioAge. Geroscience. (2021) 43:2795–808. doi: 10.1007/s11357-021-00480-5
28. Levine, ME, Lu, AT, Quach, A, Chen, BH, Assimes, TL, Bandinelli, S, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging. (2018) 10:573–91. doi: 10.18632/aging.101414
29. Cohen, AA. Complex systems dynamics in aging: new evidence, continuing questions. Biogerontology. (2016) 17:205–20. doi: 10.1007/s10522-015-9584-x
30. Chiuve, SE, Fung, TT, Rimm, EB, Hu, FB, McCullough, ML, Wang, M, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. (2012) 142:1009–18. doi: 10.3945/jn.111.157222
31. Rai, SK, Fung, TT, Lu, N, Keller, SF, Curhan, GC, and Choi, HK. The dietary approaches to stop hypertension (DASH) diet, Western diet, and risk of gout in men: prospective cohort study. BMJ. (2017) 357:j1794. doi: 10.1136/bmj.j1794
32. Shams-White, MM, Pannucci, TE, Lerman, JL, Herrick, KA, Zimmer, M, Meyers Mathieu, K, et al. Healthy Eating Index-2020: Review and Update Process to Reflect the Dietary Guidelines for Americans,2020-2025. J Acad Nutr Diet. (2023) 123:1280–8. doi: 10.1016/j.jand.2023.05.015
33. Sofi, F, Abbate, R, Gensini, GF, and Casini, A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr. (2010) 92:1189–96. doi: 10.3945/ajcn.2010.29673
34. VanderWeele, TJ. Explanation in causal inference: developments in mediation and interaction. Int J Epidemiol. (2016) 45:1904–8. doi: 10.1093/ije/dyw277
35. Goldfarb, DS. Empiric therapy for kidney stones. Urolithiasis. (2019) 47:107–13. doi: 10.1007/s00240-018-1090-6
36. Jian, Z, Wang, M, Jin, X, Li, H, and Wang, K. Diet-derived antioxidants and risk of kidney stone disease: results from the NHANES 2007-2018 and Mendelian randomization study. Front Nutr. (2021) 8:738302. doi: 10.3389/fnut.2021.738302
37. Pearle, MS, Goldfarb, DS, Assimos, DG, Curhan, G, Denu-Ciocca, CJ, Matlaga, BR, et al. Medical management of kidney stones: AUA guideline. J Urol. (2014) 192:316–24. doi: 10.1016/j.juro.2014.05.006
38. Cheng, JW, Wagner, H, Asplin, JR, Hodgkin, G, Schlaifer, A, Fargusson, M, et al. The effect of lemonade and diet lemonade upon urinary parameters affecting calcium urinary stone formation. J Endourol. (2019) 33:160–6. doi: 10.1089/end.2018.0623
39. Haleblian, GE, Leitao, VA, Pierre, SA, Robinson, MR, Albala, DM, Ribeiro, AA, et al. Assessment of citrate concentrations in citrus fruit-based juices and beverages: implications for management of hypocitraturic nephrolithiasis. J Endourol. (2008) 22:1359–66. doi: 10.1089/end.2008.0069
40. Trinchieri, A, Lizzano, R, Marchesotti, F, and Zanetti, G. Effect of potential renal acid load of foods on urinary citrate excretion in calcium renal stone formers. Urol Res. (2006) 34:1–7. doi: 10.1007/s00240-005-0001-9
41. Perinpam, M, Ware, EB, Smith, JA, Turner, ST, Kardia, SL, and Lieske, JC. Effect of demographics on excretion of key urinary factors related to kidney stone risk. Urology. (2015) 86:690–6. doi: 10.1016/j.urology.2015.07.012
42. Dongre, AR, Rajalakshmi, M, Deshmukh, PR, Thirunavukarasu, MR, and Kumar, R. Risk factors for kidney stones in rural Puducherry: case-control study. J Clin Diagn Res. (2017) 11:Lc01-lc05. doi: 10.7860/JCDR/2017/29465.10561
43. Asoudeh, F, Talebi, S, Jayedi, A, Marx, W, Najafi, MT, and Mohammadi, H. Associations of Total protein or animal protein intake and animal protein sources with risk of kidney stones: a systematic review and dose-response Meta-analysis. Adv Nutr. (2022) 13:821–32. doi: 10.1093/advances/nmac013
44. Ferraro, PM, Taylor, EN, Gambaro, G, and Curhan, GC. Soda and other beverages and the risk of kidney stones. Clin J Am Soc Nephrol. (2013) 8:1389–95. doi: 10.2215/CJN.11661112
45. Lee, YC, Huang, SP, Juan, YS, Huang, TY, and Liu, CC. Impact of metabolic syndrome and its components on kidney stone in aging Taiwanese males. Aging Male. (2016) 19:197–201. doi: 10.1080/13685538.2016.1174987
46. Ye, Z, Wu, C, Xiong, Y, Zhang, F, Luo, J, Xu, L, et al. Obesity, metabolic dysfunction, and risk of kidney stone disease: a national cross-sectional study. Aging Male. (2023) 26:2195932. doi: 10.1080/13685538.2023.2195932
47. Khan, J, and Shaw, S. Risk of multiple lower and upper urinary tract problems among male older adults with type-2 diabetes: a population-based study. Aging Male. (2023) 26:2208658. doi: 10.1080/13685538.2023.2208658
48. Kang, HW, Seo, SP, Kwon, WA, Woo, SH, Kim, WT, Kim, YJ, et al. Distinct metabolic characteristics and risk of stone recurrence in patients with multiple stones at the first-time presentation. Urology. (2014) 84:274–8. doi: 10.1016/j.urology.2014.02.029
49. Wang, K, Ge, J, Han, W, Wang, D, Zhao, Y, Shen, Y, et al. Risk factors for kidney stone disease recurrence: a comprehensive meta-analysis. BMC Urol. (2022) 22:62. doi: 10.1186/s12894-022-01017-4
Keywords: healthy dietary pattern, biological aging, kidney stone, epidemiology, prospective cohort study
Citation: Wei C, Yang Q, He J, Luo Y, Han K, Li J, Su S, Zhang J, Wang H and Wang D (2025) Healthy dietary patterns, biological aging, and kidney stones: evidence from NHANES 2007–2018. Front. Nutr. 12:1538289. doi: 10.3389/fnut.2025.1538289
Received: 02 December 2024; Accepted: 06 March 2025;
Published: 25 March 2025.
Edited by:
Solange Parra-Soto, Universidad del Bío-Bío, ChileReviewed by:
Ziye Huang, The Second Affiliated Hospital of Kunming Medical University, ChinaCopyright © 2025 Wei, Yang, He, Luo, Han, Li, Su, Zhang, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Delin Wang, ZGVsaW53YW5nX2NxbXVAMTYzLmNvbQ==; Hongxing Wang, aG9uZ3hpbmd3cHJvQDE2My5jb20=; Jindong Zhang, emhhbmdqaW5kb25nQGhvc3BpdGFsLmNxbXUuZWR1LmNu; Shuai Su, c3VzaHVhaTkzMDgwOUAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.