
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Nutr. , 19 February 2025
Sec. Nutrition and Microbes
Volume 12 - 2025 | https://doi.org/10.3389/fnut.2025.1537610
 Pin Li1
Pin Li1 Uma Mageswary2
Uma Mageswary2 Adli Ali3
Adli Ali3 Fahisham Taib4
Fahisham Taib4 Thai Hau Koo4
Thai Hau Koo4 Azianey Yusof5
Azianey Yusof5 Hua Jiang6
Hua Jiang6 Hanglian Lan7
Hanglian Lan7 Weilian Hung8
Weilian Hung8 Min-Tze Liong2
Min-Tze Liong2 Yumei Zhang1*
Yumei Zhang1*Objectives: This study aimed to examine the effects of Bifidobacterium longum subsp. infantis YLGB-1496 (B. infantis YLGB-1496) on the frequency of respiratory illness symptoms and immunity profiles among toddlers.
Methods: In this double-blind, randomized, placebo-controlled, 12-week intervention study, toddlers with at least 2 respiratory illness symptoms were randomly assigned into the probiotic (YLGB-1496) or placebo group at a 1:1 ratio. Follow-up examinations were conducted at baseline (week 0) and at weeks 6 and 12 of the intervention. The frequency of respiratory illness symptoms was assessed at these time points using validated questionnaires. Oral swabs and fecal samples were collected from participants at weeks 0, 6, and 12 to examine inflammatory cytokines.
Results: Among the 120 toddlers initially included in the study, 115 completed the 12-week intervention (58 in the YLGB-1496 group and 57 in the placebo group). The risk of antibiotic use or clinical visits was significantly lower in the YLGB-1496 group than in the placebo group (antibiotic use odds ratio (OR) = 0.37 [0.369, 0.372]; clinical visit, OR = 0.743 [0.741, 0.744]), but these differences were nonsignificant after adjusting for other potential confounders (p > 0.05). The YLGB-1496 group presented a lower incidence of several respiratory symptoms than the placebo group, including fever (p < 0.001), cough (p < 0.001), sneezing (p = 0.012), nose block (p = 0.001), and runny nose (p < 0.001). The results also revealed that the salivary cortisol concentration was significantly lower in the YLGB-1496 group than in the placebo group (p = 0.026), but no effects on INF-γ, IL-1β, IL-13, IL-4, or IL-10 were detected.
Conclusion: Bifidobacterium infantis YLGB-1496 may serve as a potential natural, nonpharmacological strategy for the safe management of respiratory tract issues in toddlers.
Clinical trial registration: The trial was registered at ClinicalTrials.gov (identifier number NCT05794815).
Probiotics are active microorganisms that colonize the human body and confer various benefits to the host. This concept was first proposed by Stillwell R. H. and Lilly D. M. in 1965 (1). The Food and Agriculture Organization of the United Nations, in conjunction with the World Health Organization, defines probiotics as living microorganisms that provide health benefits to their host when they are administered in adequate quantities (2). The most widely recognized probiotics include various strains of Lactobacillus and Bifidobacterium. Notably, Bifidobacterium longum subsp. infantis (B. infantis) may play a crucial role during early life stages. Previous studies have demonstrated that this specific subspecies enhances immune function in infants and toddlers (3–5).
Respiratory diseases represent a significant global health issue, especially among infants and toddlers (6). Common respiratory symptoms in these age groups include fever, chills, sore throat, headache, cough, and runny nose. Children who experience respiratory tract infections usually have immunity responses such as high levels of changes in cytokines signal inflammation and intestinal barrier disruption due to invasions of pathogens. The use of antibiotics has been proven to be effective in treating respiratory infections, but their overuse is a widespread concern among affected individuals (7). Previous studies have shown that supplementing Bifidobacterium probiotics early in life can effectively reduce the frequency of respiratory symptoms (8, 9).
The gut serves as the largest immune organ within the body. In the gut, stimulation from food, environmental antigens, and the intestinal flora postnatally is essential for developing an effective intestinal immune system (10). The surface components of B. infantis, such as S-protein, lipoteichoic acid (LTA), and exopolysaccharides (EPS), exhibit stimulatory effects similar to those induced by pathogenic microorganisms (11). In addition to contributing to biological barriers, these components influence immune barriers (intestinal-associated lymphoid tissue and secretory antibodies), thereby modulating intestinal immunity.
Bifidobacterium longum subsp. infantis YLGB-1496 (B. infantis YLGB-1496) is isolated from human breast milk. It is able to maintain a high survival rate in acidic or bile salts mimics artificial gastrointestinal environments, and it strongly adheres to Caco-2 cells derived from the human intestinal epithelium. Furthermore, this probiotic has shown significant immunoregulatory capabilities (12).
However, most relevant studies were cell-based or animal studies, and there is a paucity of data regarding human subjects among toddlers. Consequently, this study aimed primarily to assess differences in the frequency of respiratory illness symptoms between toddlers receiving B. infantis YLGB-1496 and those receiving placebo as well as to evaluate variations in immunity profiles between these two groups.
Subjects were recruited from the Universiti Kebangsaan Malaysia (UKM) campus as well as from the community. The inclusion criteria were as follows: (a) aged 1–3 years, and (b) exhibiting at least two of the following signs and symptoms: fever, nasal obstruction, chills, sore throat, headache, cough, runny nose, olfactory disturbances, or taste disturbances. The exclusion criteria were as follows: (a) taking long-term medication (>6 months) for any disease; (b) any deformities or metabolic/chronic diseases; (c) conditions or interventions that may interfere with the study(judged by professional pediatricians); (d) consumption of nutritional supplements containing probiotics within 2 weeks before the intervention; (e) intake of foods for special medical purposes or nonstandard formula powders due to lactose intolerance and galactosemia; and (f) participation in other clinical studies within 4 weeks before the intervention. Informed consent was obtained from the parents or legal guardians of the children before the commencement of the study.
Both probiotic and placebo products (2 g/bag) were manufactured under HACCP conditions and were stored at a temperature below 4°C to ensure that the probiotics remained active as much as possible. According to previous researches, a high dose of B. infantis (1.8–2.8*1010 CFU/day) was safe and well-tolerated in healthy breastfed and/or formula-fed infants (13, 14). The probiotic product contains about 1.5*1010 colony-forming units (CFU)/g viable B. infantis YLGB-1496 during production. After bagging, transportation, and storage, three batches of placebo products are randomly selected for viable bacteria counting. After storage for 3–6 months, each probiotic product contains about 1*1010CFU B. infantis YLGB-1496.
In this double-blind, randomized, placebo-controlled, 12-week intervention study, qualified subjects were randomly assigned into the probiotic (YLGB-1496) or placebo group at a 1:1 ratio. Randomization was performed by the study statistician, who had no contact with the participants, using a computer-generated process in Microsoft Excel (version 2016) to ensure unbiased allocation of participants to experimental groups. The allocation sequence was not revealed to any research team member until the study’s completion. This study was conducted in accordance with the Declaration of Helsinki, where all procedures involving human subjects were approved by the UKM Research Ethics Committee (No. UKM/PPI/111/8/JEP-2023-074) and the Medical Ethics Research Board of Peking University (No. IRB00001052-22166). The trial was registered at ClinicalTrials.gov (identifier number NCT05794815).
The probiotic group received one sachet(2 g/bag) of the probiotic product only contains B. infantis YLGB-1496 and maltodextrin per day in plain water or milk at a temperature not exceeding 40°C. The placebo group received one sachet of placebo (maltodextrin, 2 g/bag) per day in the same package. The intervention probiotic powder and placebo had the same outer packaging and were only distinguished by different production lot numbers. The grouping codes were revealed once the statistical analysis was completed. Follow-up surveys were conducted at baseline (week 0) and at weeks 6 and 12 of the intervention.
The sample size was calculated for a parallel group study design based on power design analysis. Previous studies have shown that for an intervention in which probiotics are used to reduce clinical visits in children for respiratory diseases, a standard deviation of 0.46 times within the group was observed, accompanied by a 0.32-fold reduction between the treatment and placebo groups (15). This calculation was based on the need for a continuous response variable from independent control and experimental subjects, with a ratio of power to subject fixed at 1:1, a probability of 0.95, and a type-I error probability associated with this test of the null hypothesis of 0.05. Considering a potential dropout rate of 10%, each group required a sample size of 60, and a total of 120 subjects was needed for this study.
Eligible subjects who met all the inclusion and exclusion criteria were asked to complete two types of questionnaires: (a) a demographic questionnaire (collected at baseline week 0) and (b) a respiratory health questionnaire (collected at weeks 0, 6, and 12). All the questionnaires were validated and translated into the Malay language (16). The short Food Frequency Questionnaire was used to investigate the children’s dietary status during the intervention, which mainly assessed the consumption rates of various foods and nutritional supplements. Adverse events (AEs) and serious adverse events (sAEs) that occurred during the trial were recorded, and their correlation with the tested products was evaluated by doctors.
At weeks 0, 6, and 12, oral swab samples were collected from each toddler from the inside of both the left and right cheeks, one immediately after the other. At the same time points, fecal samples were also collected in fecal collection tubes and glass beads by the subjects. All the tubes were capped tightly and stored at −80°C until further analyses. These samples were collected by our medical team.
The fecal samples were analyzed for the concentrations of cytokines, including inter-feron-γ (IFN-γ), interleukin-1β (IL-1β), IL-4, IL-10, and IL-13. The oral swab samples were collected for the analysis of cortisol. All the biomarkers were tested via commercial enzyme-linked immunosorbent assay (ELISA) kits in accordance with the manufacturer’s instructions (Sunlong Biotech, Hangzhou, China).
Intention-to-treat analysis was performed via R software version 4.2.3. For basic information, the data are presented as the mean value ± standard error unless otherwise stated. Differences in continuous variables between groups were compared via Student’s t-test, whereas categorical variables were compared via the chi-square test. Poisson regression was used to analyze the effect of the intervention on the incidence of respiratory symptoms. A linear or generalized linear mixed model was constructed to analyze the impact of the intervention on the outcomes. All tests were two-sided, with p < 0.05 considered to indicate statistical significance.
A total of 130 volunteers were recruited for the study, and 120 met the inclusion and exclusion criteria and signed the informed consent form after the initial screening. A total of 115 people ultimately completed the study (58 in the experimental group and 57 in the placebo group; Figure 1). All 115 subjects provided oral samples and answers to the questionnaires. No adverse effects were reported throughout the study, and no subjects dropped out due to any complications. The sociodemographic characteristics of the participants in the different groups are summarized in Table 1. The proportion of children who were delivered by cesarean section and who were mainly cared for by family was greater in the placebo group than in the YLGB-1496 group. There was no significant difference in other sociological demographic factors between the two groups.
The consumption rates of various food and nutritional supplements during the intervention are shown in Supplementary Table S1. At baseline, the use of breast milk was significantly greater in the YLGB-1496 group than in the placebo group, and the consumption rate of vegetables in the YLGB-1496 group was lower at baseline and 12 weeks. The consumption rates of other food types and nutrient supplements were not significantly different between the two groups and did not change over time during the intervention period (p > 0.05).
A generalized linear mixed model was used to analyze the risk of antibiotic use and clinical visits due to respiratory illnesses during the intervention between groups. In Model 1, which was adjusted for only baseline values, time, group, and interaction terms com-prising time and group, the risk of antibiotic use or clinical visit in the YLGB-1496 group was significantly lower than that in the placebo group (antibiotic use odds ratio, OR = 0.37 [0.369, 0.372]; clinical visit odds ratio, OR = 0.743 [0.741, 0.744]), but there was no statistically significant difference between the two groups after further adjustment for other possible confounders (Table 2).
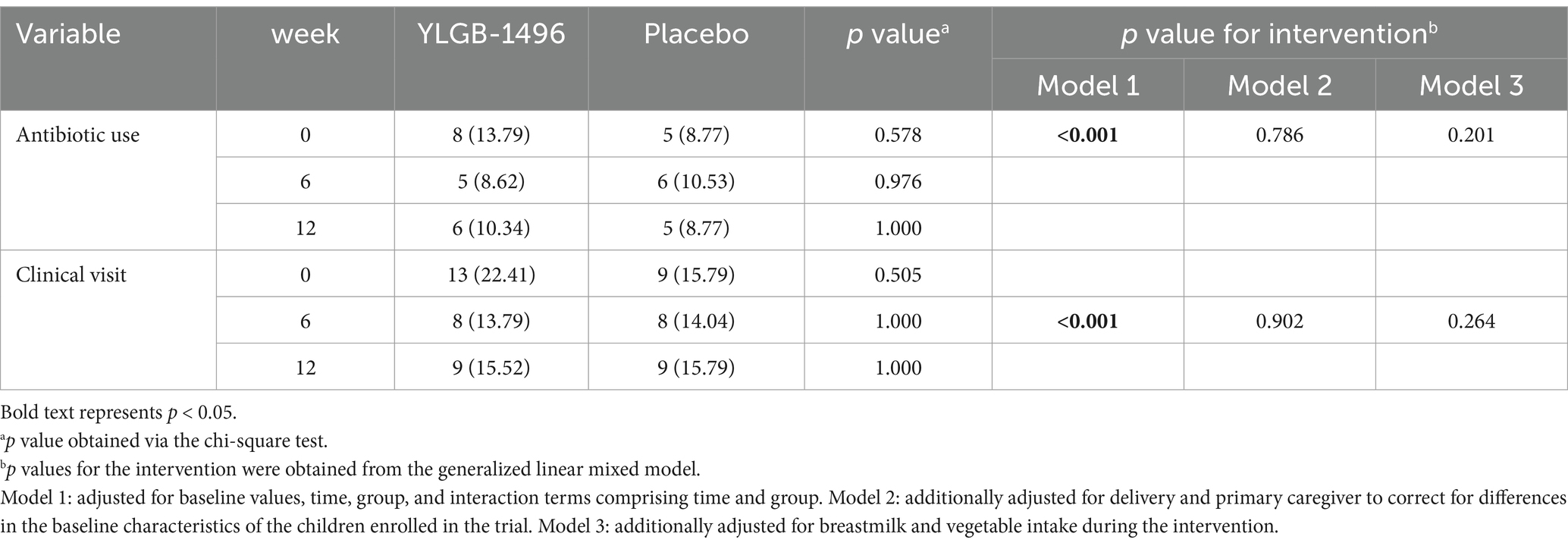
Table 2. Antibiotic use and clinical visits due to respiratory illnesses during the intervention (n/%).
After further adjustment for possible confounders, the YLGB-1496 group presented a lower incidence of several respiratory symptoms than the placebo group (Table 3), including fever, cough, sneezing, nose block, and runny nose. Although other symptoms were not significantly different between the two groups according to the generalized linear mixed model, the incidence rate ratio (IRR) also showed a decreasing trend during the intervention. Despite having a greater incidence of symptoms of wheezing, poor appetite, and vomiting at baseline (week 0) than the placebo group did, the YLGB-1496 group eventually had a significantly lower incidence rate than the placebo group did at week 6 or week 12.
Tests of cortisol in saliva samples revealed that the YLGB-1496 group had higher levels of cortisol at baseline than the placebo group. However, the cortisol concentration in the YLGB-1496 group significantly decreased at week 6 and week 12 (p < 0.05). After adjusting for possible confounders through the linear mixed-effects model, the salivary cortisol concentration in the YLGB-1496 group was significantly lower than that in the placebo group during the intervention period (Table 4).
The use of probiotics to treat or prevent respiratory diseases in children is common, and probiotics are often considered a safe nonpharmaceutical tool to enhance children’s immunity (17, 18). Probiotics may influence respiratory health in children through the gut-lung axis, with the theoretical basis rooted in their ability to modulate the gut microbiota while exerting antagonistic effects against intestinal pathogens (19). This process occurs through various mechanisms, including competition for nutrients and binding sites, acidification of the intestinal milieu, synthesis of bioactive compounds, and enhancement of both specific and non-specific immune responses (20). Probiotics have demonstrated efficacy in promoting pulmonary health across diverse age groups and environments. However, the effects of different strains on health vary, and the combination, content, supplementation method, and intake time of probiotics may also affect their health effects. Various meta-analyses have shown conflicting results, with some studies showing no significant beneficial effects on children’s respiratory diseases (21).
The various cytokines induced by probiotics are closely related to inflammatory responses. When probiotics are recognized by nonspecific immune cells, they can promote cytokine secretion or directly act on specific immune cells, further affecting cellular immunity and humoral immunity. This study analyzed the ability of B. infantis YLGB-1496 to relieve respiratory symptoms in children and explored its impact on salivary cortisol and fecal immunochemical compounds. The results revealed that the incidence of various respiratory symptoms (fever, cough, sneezing, nasal congestion, runny nose) in children who consumed B. infantis YLGB-1496 was significantly lower than that in the placebo group, and the salivary cortisol concentration was significantly lower; however, no effects on INF-γ, IL-1β, IL-13, IL-4, or IL-10 were detected.
The results of a study conducted by Cazzola et al. (22) in Spain revealed that the addition of Bifidobacterium longum. Subsp. infantis R0033 (B. infantis R0033) to a synbiotic preparation significantly reduced the risk of common infectious diseases. Sanctuary et al. (23) reported that after a 12-week intervention with Bifidobacterium longum. Subsp. infantis UCD272 (B. infantis UCD272), some subjects presented lower levels of the proinflammatory cytokines IL-5 and IL-13. A trial using Bifidobacterium longum. Subsp. infantis EVC001 (B. infantis EVC001) also confirmed that a 60-day intervention could significantly reduce the levels of multiple inflammatory cytokines in infant feces (24).
Type B Streptococcus infection can cause severe disease and even death in newborns. Chinese scholars compared the inhibitory effects of 42 strains of probiotics, including B. infantis YLGB-1496, on type B Streptococcus under heat-inactivated death conditions. The experimental results demonstrated that among the 42 heat-inactivated probiotic strains, B. infantis YLGB-1496 exhibited the strongest antibacterial effect (25). B. infantis can promote the proliferation and differentiation of T cells, weaken the response of Th2-type cells, and regulate the balance between Th1 and Th2 cells (26, 27). Some scholars compared the immunomodulatory functions of 18 strains of bifidobacteria derived from human milk and reported that B. infantis YLGB-1496 exhibited strong immunomodulatory capabilities, manifested as increased induction of the Th1-related cytokines IFN-γ and TGF-β and decreased production of Th2-related cytokines. This effect aids in the shift of the immune response from Th2 to Th1 polarization (12). Previous studies have confirmed that a shift toward Th2 polarization in the Th1/Th2 balance promotes the secretion of IL-5 and IL-13 (26, 28). Therefore, it is speculated that B. infantis YLGB-1496 has immunomodulatory effects in humans, protecting the host from inflammatory diseases.
No statistically significant differences in the risks of clinical visits and antibiotic use were detected between the two groups after adjusting for some possible confounding factors; however, the clinical visit and antibiotic use rates in the YLGB-1496 group generally showed a downward trend, whereas there was no decrease in the placebo group. Furthermore, our study considered the use of antibiotic treatment as an outcome, without further investigating the frequency or dosage of antibiotic administration. Follow-up studies with larger sample sizes and mor detailed information are still needed in the future.
The main advantage of this study is that it is the first to evaluate the effects of B. infantis YLGB-1496 application in children with respiratory diseases through a randomized controlled trial. This study measured changes in salivary cortisol concentrations and various anti-inflammatory and proinflammatory cytokines in feces while also considering the influence of dietary factors during data analysis. However, this study has notable limitations. First, owing to ethical considerations, no blood tests were conducted, as the participants were young. The concentration of cytokines in feces is influenced by numerous factors, which may not accurately reflect the body’s inflammatory state. Additionally, the incidence of respiratory symptoms was primarily self-reported by parents, which may have introduced reporting or recall bias into the statistical results. Finally, this study focused solely on the alleviation of adverse respiratory symptoms. Since a variety of diseases can produce similar symptoms, the findings of this study have limited applicability in clinical practice. To determine the effect and value of B. infantis YLGB-1496 in clinical practice, more research with larger sample sizes, different probiotic doses, and detection of blood biomarkers is still needed.
In conclusion, the administration of probiotics has beneficial effects on respiratory symptoms and anxiety in toddlers. Thus, the use of B. infantis YLGB-1496 may serve as a potential natural, nonpharmacological strategy for the safe management of respiratory tract issues in this age group. Further studies are required to explore the rational clinical utilization of B. infantis YLGB-1496, including the incorporation of serum-related indicators and the experimentation with varying doses of probiotics.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
The studies involving humans were approved by the UKM Research Ethics Committee (No. UKM/PPI/111/8/JEP-2023-074) and the Medical Ethics Research Board of Peking University (No. IRB00001052-22166). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
PL: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing – original draft. UM: Data curation, Investigation, Project administration, Writing – review & editing. AA: Investigation, Project administration, Writing – review & editing. FT: Investigation, Project administration, Writing – review & editing. TK: Investigation, Project administration, Writing – review & editing. AY: Investigation, Project administration, Writing – review & editing. HJ: Writing – review & editing. HL: Writing – review & editing. WH: Writing – review & editing. M-TL: Conceptualization, Investigation, Project administration, Writing – review & editing. YZ: Conceptualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
HL is an employee of the National Center of Technology Innovation for Dairy. WH is an employee of the Inner Mongolia Dairy Technology Research Institute Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1537610/full#supplementary-material
1. Lilly, DM, and Stillwell, RH. Probiotics: growth-promoting factors produced by microorganisms. Science (New York, NY). (1965) 147:747–8. doi: 10.1126/science.147.3659.747
2. FAO/WHO (2001). “Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria,” Report of a joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria, córdoba, 1-4.Available at: http://www.who.int/foodsafety/publications/fs_management/en/probiotics.pdf.
3. Chichlowski, M, Shah, N, Wampler, JL, Wu, SS, and Vanderhoof, JA. Bifidobacterium longum subspecies infantis (B. infantis) in pediatric nutrition: current state of knowledge. Nutrients. (2020) 12:1581. doi: 10.3390/nu12061581
4. Moreno-Muñoz, JA, Martín-Palomas, M, and Jiménez, LJ. Bifidobacterium longum subsp. infantis CECT 7210 (B. infantis IM-1®) shows activity against intestinal pathogens. Nutr Hosp. (2022) 39:65–8. doi: 10.20960/nh.04315
5. Barratt, MJ, Nuzhat, S, Ahsan, K, Frese, SA, Arzamasov, AA, Sarker, SA, et al. Bifidobacterium infantis treatment promotes weight gain in Bangladeshi infants with severe acute malnutrition. Sci Transl Med. (2022) 14:eabk1107. doi: 10.1126/scitranslmed.abk1107
6. Unger, SA, and Bogaert, D. The respiratory microbiome and respiratory infections. J Inf Secur. (2017) 74:S84–8. doi: 10.1016/S0163-4453(17)30196-2
7. van, C, Cohen, A, Engelhard, D, Hays, JP, Karlsson, R, Moore, E, et al. Antibiotic misuse in respiratory tract infections in children and adults-a prospective, multicentre study (TAILORED treatment). Eur J Clin Microbiol Infect Dis. (2019) 38:505–14. doi: 10.1007/s10096-018-03454-2
8. Li, KL, Wang, BZ, Li, ZP, Li, YL, and Liang, JJ. Alterations of intestinal flora and the effects of probiotics in children with recurrent respiratory tract infection. World J Pediatr. (2019) 15:255–61. doi: 10.1007/s12519-019-00248-0
9. Ozen, M, Kocabas Sandal, G, and Dinleyici, EC. Probiotics for the prevention of pediatric upper respiratory tract infections: a systematic review. Expert Opin Biol Ther. (2015) 15:9–20. doi: 10.1517/14712598.2015.980233
10. Kusumo, PD, Bela, B, Wibowo, H, Munasir, Z, and Surono, IS. Lactobacillus plantarum IS-10506 supplementation increases faecal sIgA and immune response in children younger than two years. Benefic Microbes. (2019) 10:245–52. doi: 10.3920/BM2017.0178
11. Dasgupta, S, Erturk-Hasdemir, D, Ochoa-Reparaz, J, Reinecker, HC, and Kasper, DL. Plasmacytoid dendritic cells mediate anti-inflammatory responses to a gut commensal molecule via both innate and adaptive mechanisms. Cell Host Microbe. (2014) 15:413–23. doi: 10.1016/j.chom.2014.03.006
12. Chiu, YH, Tsai, JJ, Lin, SL, Chotirosvakin, C, and Lin, MY. Characterisation of bifidobacteria with immunomodulatory properties isolated from human breast milk. J Funct Foods. (2014) 7:700–8. doi: 10.1016/j.jff.2013.12.015
13. Capeding, MRZ, Phee, LCM, Ming, C, Noti, M, Vidal, K, le, G, et al. Safety, efficacy, and impact on gut microbial ecology of a Bifidobacterium longum subspecies infantis LMG11588 supplementation in healthy term infants: a randomized, double-blind, controlled trial in the Philippines. Front Nutr. (2023) 10:10. doi: 10.3389/fnut.2023.1319873
14. Smilowitz, JT, Moya, J, Breck, MA, Cook, C, Fineberg, A, Angkustsiri, K, et al. Safety and tolerability of Bifidobacterium longum subspecies infantis EVC001 supplementation in healthy term breastfed infants: a phase I clinical trial. BMC Pediatr. (2017) 17:133. doi: 10.1186/s12887-017-0886-9
15. Weizman, Z, Asli, G, and Alsheikh, A. Effect of a probiotic infant formula on infections in child care centers: comparison of two probiotic agents. Pediatrics. (2005) 115:5–9. doi: 10.1542/peds.2004-1815
16. Lau, ASY, Yusoff, MSB, Lee, YY, Choi, SB, Rashid, F, Wahid, N, et al. Development, translation and validation of questionnaires for diarrhea and respiratory-related illnesses during probiotic administration in children. Educ Med J. (2017) 9:19–30. doi: 10.21315/eimj2017.9.4.3
17. Zhao, Y, Dong, BR, and Hao, Q. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev. (2022) 8:Cd006895. doi: 10.1002/14651858.CD006895.pub4
18. Wang, Y, Li, X, Ge, T, Xiao, Y, Liao, Y, Cui, Y, et al. Probiotics for prevention and treatment of respiratory tract infections in children: a systematic review and meta-analysis of randomized controlled trials. Medicine. (2016) 95:e4509. doi: 10.1097/MD.0000000000004509
19. Zhu, W, Wu, Y, Liu, H, Jiang, C, and Huo, L. Gut–lung Axis: microbial crosstalk in pediatric respiratory tract infections. Front Immunol. (2021) 12:12. doi: 10.3389/fimmu.2021.741233
20. Budden, KF, Gellatly, SL, Wood, DLA, Cooper, MA, Morrison, M, Hugenholtz, P, et al. Emerging pathogenic links between microbiota and the gut–lung axis. Nat Rev Microbiol. (2016) 15:55–63. doi: 10.1038/nrmicro.2016.142
21. Esposito, S, Rigante, D, and Principi, N. Do children's upper respiratory tract infections benefit from probiotics? BMC Infect Dis. (2014) 14:194. doi: 10.1186/1471-2334-14-194
22. Cazzola, M, Pham-Thi, N, Kerihuel, JC, Durand, H, and Bohbot, S. Efficacy of a synbiotic supplementation in the prevention of common winter diseases in children: a randomized, double-blind, placebo-controlled pilot study. Ther Adv Respir Dis. (2010) 4:271–8. doi: 10.1177/1753465810379010
23. Sanctuary, MR, Kain, JN, Chen, SY, Kalanetra, K, Lemay, DG, Rose, DR, et al. Pilot study of probiotic/colostrum supplementation on gut function in children with autism and gastrointestinal symptoms. PLoS One. (2019) 14:e0210064. doi: 10.1371/journal.pone.0210064
24. Henrick, BM, Chew, S, Casaburi, G, Brown, HK, Frese, SA, Zhou, Y, et al. Colonization by B. infantis EVC001 modulates enteric inflammation in exclusively breastfed infants. Pediatr Res. (2019) 86:749–57. doi: 10.1038/s41390-019-0533-2
25. Shan, LY, Shan, HP, Ho, HH, Wu, JF, and Huang, CC. Bacteriostatic abilities of viable and heat-killed lactic acid bacteria against group B streptococcus. Basic & Clinical Pharmacology & Toxicology (2011).
26. Wang, W, Luo, X, Zhang, Q, He, X, Zhang, Z, and Wang, X. Bifidobacterium infantis relieves allergic asthma in mice by regulating Th1/Th2. Med Sci Monit. (2020) 26:e920583. doi: 10.12659/MSM.920583
27. Henrick, BM, Rodriguez, L, Lakshmikanth, T, Pou, C, Henckel, E, Arzoomand, A, et al. Bifidobacteria-mediated immune system imprinting early in life. Cell. (2021) 184:3884–98.e11. doi: 10.1016/j.cell.2021.05.030
Keywords: Bifidobacterium longum subsp. infantis YLGB-1496, respiratory symptoms, immunological effects, toddlers, randomized controlled trial
Citation: Li P, Mageswary U, Ali A, Taib F, Koo TH, Yusof A, Jiang H, Lan H, Hung W, Liong M-T and Zhang Y (2025) Clinical effects of Bifidobacterium Longum Subsp. Infantis YLGB-1496 on children with respiratory symptoms. Front. Nutr. 12:1537610. doi: 10.3389/fnut.2025.1537610
Received: 01 December 2024; Accepted: 04 February 2025;
Published: 19 February 2025.
Edited by:
Zhengze Li, North Dakota State University, United StatesReviewed by:
Mongkol Thirabunyanon, Maejo University, ThailandCopyright © 2025 Li, Mageswary, Ali, Taib, Koo, Yusof, Jiang, Lan, Hung, Liong and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yumei Zhang, emhhbmd5dW1laUBiam11LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.