
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Nutr., 19 February 2025
Sec. Sport and Exercise Nutrition
Volume 12 - 2025 | https://doi.org/10.3389/fnut.2025.1537291
Introduction: Sarcopenic obesity (SO) is a condition characterized by the coexistence of age-related obesity and sarcopenia. This systematic review and network meta-analysis (NMA) aimed to compare the effects of different training modalities, such as aerobic training (AT), resistance training (RT), combined resistance with AT (CT), and multicomponent training (MCT) on body composition, muscle strength, and physical performance in elderly patients with SO.
Methods: We electronically searched randomized controlled trials, published from inception to March 2024 in PubMed, Embase, Cochrane Library, Web of Science and Scopus. Effect estimates were presented as mean differences (MD) or Standard Mean Difference (SMD) with 95% confidence interval (CI). The comprehensive effects of all treatments were ranked by the surface under the cumulative ranking (SUCRA) probabilities.
Results: 14 trials enrolling 955 participants were included. The body fat percentage (BFP) in MCT (MD= −6.37, 95% CI: −8.67, −4.07), CT (MD = −2.08, 95% CI: −4.00, −0.16), and RT (MD = −1.85, 95% CI: −3.25, −0.44) was significantly lower than in the normal control group, with MCT showing superior effects compared to CT and RT. Furthermore, only MCT significantly improved fat-free mass (FFM; MD = 5.21, 95% CI:1.51, 8.91), as well as in body mass index (BMI; MD = 0.74, 95% CI:0.08, 1.40). In addition, handgrip strength (HGS) significantly improved under both MCT (SMD = 0.87, 95% CI: 0.19, 1.5) and RT(SMD = 0.84, 95% CI: 0.43, 1.25). The performance on the 30s chair stand test also yielded better outcomes for MCT (MD = 3.10, 95% CI: 1.33, 4 0.86), CT(MD = 2 0.50, 95% CI: 0.18, 5.18), and RT(MD = 3.91, 95% CI: 2.30, 5.52) when compared to the control group. Lastly, gait speed was enhanced by both MCT (MD = 0.35, 95% CI: 0.30, 0.41) and CT(MD = 0.14, 95% CI: 0.06, 0.21). The ranking results indicated that MCT was superior to other training modalities in enhancing body composition and gait speed. In contrast, RT appears to be more advantageous in the 30-second chair standing test and in improving HGS.
Conclusion: MCT outperformed other training modalities in improving body composition and gait speed. RT was more beneficial for the 30-second chair standing test and enhancing HGS. These findings provide valuable insights for clinicians and researchers to customize exercise prescriptions for older patients with SO.
Systematic review registration: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42024544962.
Sarcopenic obesity (SO) is a complex clinical and functional condition characterized by the concurrent presence of obesity, marked by excessive fat mass (FM), and sarcopenia (1, 2). Sarcopenia, defined by diminished skeletal muscle mass and impaired physical function, is acknowledged as a geriatric syndrome with its incidence signifcantly increasing as individuals age (3, 4). Given the global trend toward population aging (5), the persistent prevalence of obesity (6), and the increasingly refined diagnostic criteria for sarcopenia, the incidence of sarcopenic obesity is rising at an alarming rate (7–9). SO is associated with numerous adverse health outcomes, including frailty, fractures, cardiovascular diseases, cancer, and increased hospitalization rates and mortality, thereby imposing a substantial social and economic burden (10, 11). Consequently, the prevention and treatment of SO in elderly individuals have become critical priorities in both research and clinical practice.
Although multiple pathogenesis mechanisms are involved, physical inactivity is a common and critical pathophysiological factor contributing to both sarcopenia and obesity (12, 13). Therefore, exercise training (ET) is widely acknowledged as one of the most cost-effective strategies for the prevention and management of SO (14–16). ET encompasses a variety of modalities, including resistance training (RT) (17), aerobic training (AT) (18, 19), flexibility (20, 21), and balance/gait training (22). Different exercise regimens induce distinct physiological adaptations. Specifically, AT is generally considered the most effective mode for enhancing cardiorespiratory fitness and reducing fat mass (19, 21, 23), while RT significantly improves muscle mass and strength (24, 25). Balance exercises contribute to improved stabilit, thereby reducing the risk of falls and enhancing daily functional performance (26). These improvements collectively enhance overall health and patient outcomes. However, not all exercise modalities uniformly enhance every aspect of muscle strength and physical performance in older adults with SO (27). Elderly patients with SO frequently experience multiple concurrent impairments, such as reduced muscle strength, diminished cardiorespiratory fitness, and impaired balance (28, 29). Consequently, exercise strategies that can address all these aspects within a single session may be more efficient for potential exercisers (30–32). This has resulted in the development of multicomponent training (MCT), which integrates at least three types of training, such as strength, aerobic, balance, and/or flexibility training (33–36). Research has demonstrated that MCT positively influences muscle mass, strength, gait, balance, and cardiorespiratory fitness in elderly individuals (33, 35, 37). Various position statements and consensus guidelines for physical activity in healthy older adults advocate for a multimodal exercise prescription (38, 39). Several recent clinical studies have highlighted the benefits of the MCT program on physical fitness in older adults with SO (40–44). However, it remains unclear whether MCT is more effective than single exercise protocols in treating SO.
Several systematic reviews have investigated the effects of exercise interventions on improving body fat, muscle mass, muscle strength, and physical performance in individuals with SO (27, 45–52). However, these traditional meta-analyses predominantly focus on comparing data from specific training modes against non-exercise control groups. While they can validate the efficacy of particular interventions, they lack a comprehensive analysis and comparison of various exercise modalities, thus failing to provide a clear understanding of the relative merits of different approaches. To date, no study has systematically compared these intervention types or comprehensively ranked various exercise interventions based on their efficacy in preventing and treating SO. Consequently, the optimal exercise type prescription for preventing or treating SO remains uncertain.
This study aimed to conduct a network meta-analysis (NMA) to comprehensively compare and evaluate the relative efficacy of different exercise modalities, such as AT, RT, combined training (CT, combined RT with AT), and MCT, on body composition and physical performance in elderly individuals with SO. This analysis will also provide evidence-based research support for formulating exercise prescriptions and bridge the gap between research and clinical implementation in exercise prescriptions for SO.
This NMA was performed following the PRISMA 2020 principles (53) and registered in PROSPERO (CRD42024544962).
We electronically searched the studies published from inception to March 2024 in the following electronic databases: PubMed, Embase, Cochrane Library, Web of Science, and Scopus. We conducted the search on Boolean logic using the following terms: (“sarcopenia” OR “sarcopenic”) AND (“obese” OR “obesity” OR “overweight”) AND (“exercise” OR “training” OR “physical activity”). All retrieved references were exported to the EndNote (version X9) software to exclude duplicates as well as facilitate the initial screening and article selection as per the criteria. The detailed search strategy for each database is mentioned in Supplementary Appendix 1. Additionally, we also scanned the references of the included articles to segregate those who met the inclusion criteria. Two independent reviewers (WX and QR) screened the articles’ titles and abstracts to exclude ineligible articles. Subsequently, the chosen full-text articles were further screened to ensure that they met the inclusion criteria. Any disagreements between the reviewers were resolved either by discussion or the input of a third assessor (XH).
The inclusion criteria were as follows: randomized and/or controlled clinical trials; participants ≥60 years with SO; and intervention: any ET mode alone without incorporating other treatments was one of the interven-tion arms. ET mode included AT, RT, RT, CT, and MCT. The control group included either educational or psychological intervention or no intervention. Outcome measurements encompassed at least one aspect of body composition [e.g., body fat percentage [BFP], body mass index [BMI], or fat-free mass (53)], muscle strength (assessed in upper or lower extremities), or physical performance (measured by gait speed and the 30-s chair stand test).
The exclusion criteria were as follows: animal studies, case studies, cross-sectional or retrospective studies, and review articles; sarcopenia or obesity alone as well as osteosarcopenic obesity; other medical complications, such as cancer, liver cirrhosis, or renal failure; absence of a standard control group; exercise intervention with other supportive treatments (e.g., nutritional supplements, medication, or calorie-restricted diet); and outcome measurements without any one of the body composition aspects, muscle function, or physical performance.
Two reviewers (WX and X) independently extracted data using predefined data forms based on the Cochrane Handbook. Additionally, each study provided the following data: author details, publication year, criteria for diagnosis of SO, sample sizes, the exercise intervention details, the body composition [bio-impedance analysis (BIA) or dual-energy X-ray absorptiometry (DXA)] measurement techniques, and participants’ baseline characteristics in each arm. For outcome measures, the number of patients, mean values, and standard deviations were extracted. When raw data was missing, we contacted the authors by email to request the data. Disagreements were resolved by discussion between the two reviewers and the trial information review.
The Cochrane Risk of Bias Tool RoB 2.0 was employed to assess the quality (54). The risk of bias in the studies was assessed by two independent authors (H and QR) as per the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (55). Moreover, disagreements were resolved by a third reviewer (XH). Review Manager (RevMan) version 5.4.1 software was used to summarize the results of the bias risk assessment. Potential publication bias was investigated through visual inspection of funnel plots using the criterion of symmetry (56) and was assessed by Egger’s regression asymmetry test.
All analyses were performed using Stata version 15.1 (Stata Corp LP, College Station, TX, United States), and p values <0.05 indicated statistical significance. Mean difference (MD) was used to estimate the changes in baseline and post-intervention outcome measurements. In the case of variable study units, we calculated the standard mean difference (SMD) to determine the effect size. The MD or SMD was calculated with a 95% confidence interval (CI) for determining efficacy outcomes. The heterogeneity across trial results was tested with Cochran’s Q test and I2 statistic (57). If the Q test p > 0.1 and I2 50%, indicating low inconsistency between the results of individual trials, the pooled effect was calculated using a fixed-effects model; If I2 > 50% or p < 0.1, considered as high heterogeneity between studies and a random-effects model was used. Then subgroup analysis on intervention modalities and intervention duration were conducted in outcomes that had a sufficient number of studies and the results were subsequently presented in forest plots.
NMAs were conducted using the Stata 15.1 “mvmeta” and “network” packages based on a frequentist analysis framework for all outcome measures. Initially stored in a long format (one record per treatment per study), the raw data were imported into an augmented format. Firstly, a network diagram with nodes and lines was constructed to summarize the comparative relationships among exercise interventions and controls. If a closed loop connecting different interventions existed, we used an inconsistency model and the node-splitting method for global and local inconsistencies, respectively. The results were subsequently presented in forest plots and league tables. Once the comparative effectiveness of the treatments had been evaluated, the treatments were ranked to identify their superiority. The interventions’ relative ranking was estimated based on the surface under the cumulative ranking curve (SUCRA), ranging from 0 to 100%. A higher SUCRA value denoted that the therapy was in the top rank (56, 58).
The initial electronic search identified 1979 studies. After duplicate removal, 1,366 records were included for title and abstract screening. After the exclusion of irrelevant titles and abstracts, the full texts of 23 studies were further screened. Consequently, 12 eligible RCTs were included in our systematic review. Following an updated search conducted via Google Scholar and references in August 2024, two additional eligible papers were identified and included. Thus, 14 studies (40–42, 44, 59–68) were included in the final analysis. Detailed information is provided in the PRISMA flow diagram (Figure 1).
A total of 955 participants with SO and involvement in the exercise program were found in 14 studies published between 2016 and 2023. The characteristics of the included studies are displayed in Table 1.
As seen in Figure 2, the risk of bias was high, unclear, and low in 2, 10, and 2 studies, respectively. Since one trial adopted the nonrandomized health interventions’ evaluations, it was judged as a high risk of bias for selection and performance. Moreover, one trial was assessed as high risk of bias due to its single-blind program. Additionally, 10 trials were assessed as having unclear risk of bias owing to insufficient data on the random sequence generation, allocation concealment, or the outcome measurement. The detail annotation for clarity is presented in Supplemental Appendix 2.
Overall, we did not find any publication bias across the included studies. All outcome measurements’ funnel plots were visually symmetrically distributed around the mean estimated treatment effect. The p-values for Egger’s test were: 0.076 for BFP, 0.275 for BMI, 1.31 for FFM, 0.977 for handgrip strength (HGS), 0.321 for 30-s chair stand test, and 0.99 for gait speed (Supplementary Appendix 3).
A total of four different interventions and control arms were included in our NMA. The primary outcomes’ network geometry is shown in Figure 3. Among the 14 eligible studies, most studies reported at least two indicators each: 12 studies focused on BFP, 8 assessed BMI, 7 examined FFM, 9 evaluated HGS, 5 investigated the 30-s chair stand test,and 8 examined gait speed. The characteristics of the intervention methods for each outcomes are displayed in Supplementary Appendix 4.
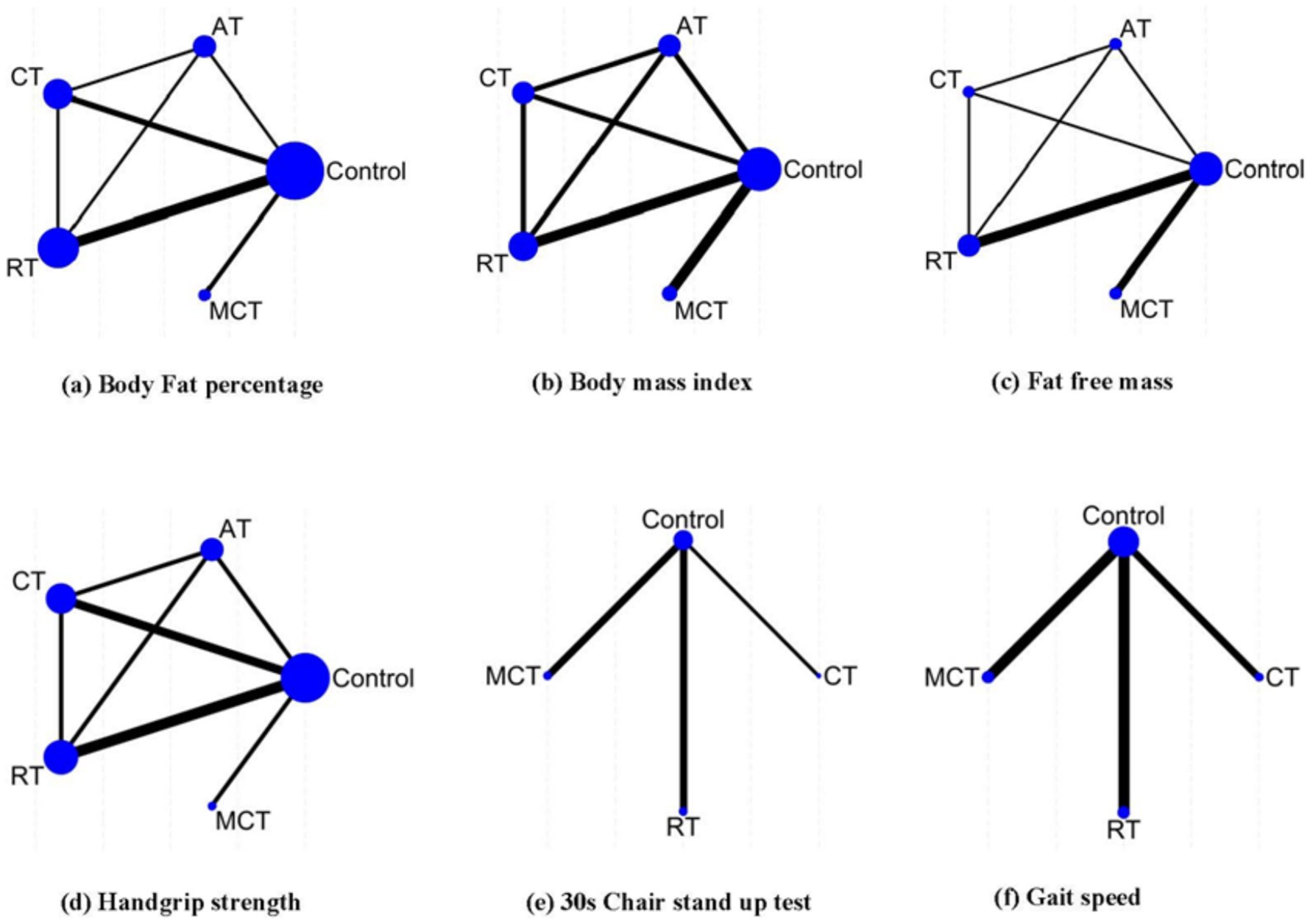
Figure 3. Network geometry summary. The size of the edges is proportional to the number of studies, and the size of the nodes is proportional to the number of each intervention. AT, aerobic training; RT, resistance training; CT, combined resistance with aerobic training; MCT, multiple component training.
The NMA-based inconsistency test showed no statistically significant differences in global inconsistency (BFP: p = 0.7268; BMI: p = 0.6657; FFM: p = 0.9597, and HGS: p = 0.3634). The closed-loop network evaluation revealed no statistically significant differences in inconsistency between direct and indirect outcomes (Supplementary Appendix 5).
We included 12 studies and 711 subjects. The results showed that MCT [MD = −6.37, 95% CI-8.67, −4.07)], CT [(MD = −2.08, 95% CI (−4.00, −0.16)], and RT [MD = −1.85, 95% CI (−3.25, −0.44)] were superior to the control group. Additionally, MCT was significantly better than RT [MD = −4.52, 95% CI (−7.17, −1.88)] and CT [MD = −4.29, 95% CI (−7.25, −1.33)] groups, respectively. However, no significant difference was observed between the AT and the control group or between other exercise interventions (Table 2). The ranked results showed that MCT was superior to CT and RT; MCT was the most effective technique in reducing BFP in older adults with SO (Figure 4; Table 3).
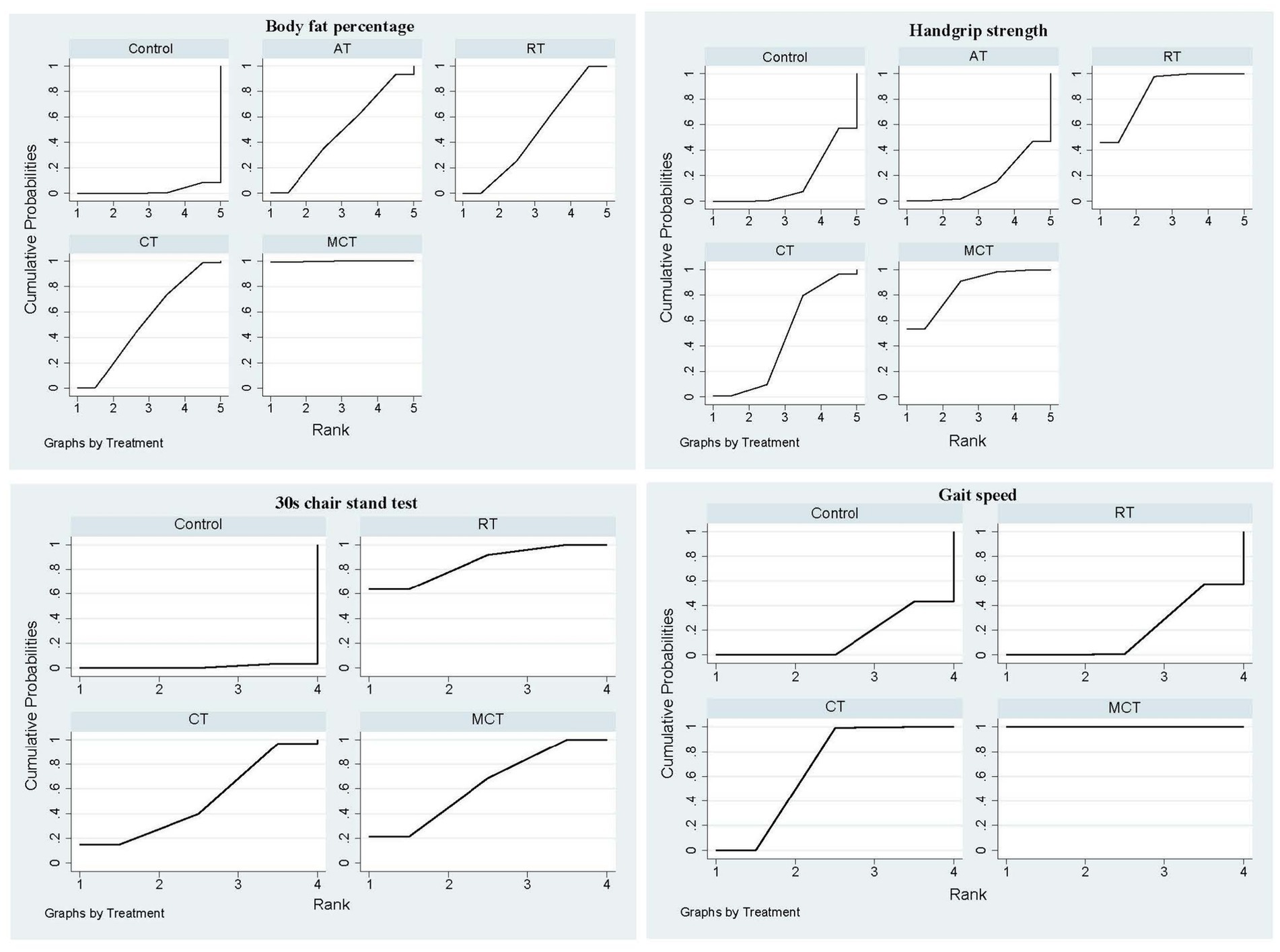
Figure 4. The primary outcome’s cumulative ranking probability plot. AT, Aerobic training; CT, Combined resistance with aerobic training; MCT, Multicomponent training; RT, Resistance training.
The ranked results demonstrated that MCT outperformed CT and RT, establishing MCT as the most effective technique for reducing BFP in older adults with SO (Figure 4; Table 3). Furthermore, subgroup analysis based on intervention duration revealed that MCT consistently ranked as the optimal treatment for BFP across both short-term (≤12 weeks) and long-term (>12 weeks) intervention periods (Supplementary Appendix 6).
We included eight studies and 642 subjects. The NMA results showed that only MCT intervention was superior to the control group [MD = 0.74, 95% CI (0.08, 1.40)]. Nonetheless, no significant difference was observed between the other interventions and the control group or between interventions (p > 0.05; Table 2).
A total of seven studies and 315 subjects were utilized. Our results showed that only MCT intervention was better than the control group [MD = 5.21, 95% CI (1.51, 8.91)]. However, there was no significant difference between the other interventions and the control group or between interventions (Table 2).
We used nine studies and 515 subjects. HGS with RT [SMD = 0.84, 95% CI (0.43, 1.25)] and MCT 0.87 (0.19, 1.55) [MD = 0.87, 95% CI (0.19, 1.55)] were superior to the control group. No significant differences were observed between the other interventions and the control group or between interventions (p > 0.05; Table 2). The ranked results showed that RT was the most effective intervention in improving HGS in older adults with SO (Figure 4; Table 3).
A total of five studies and 408 participants were utilized (Table 1). Although 30s chair stand repetitions improved in MCT [MD = 3.10, 95% CI (1.33, 4.86)] and RT [MD = 3.91, 95% CI (2.30, 5.52)] when compared with control groups; however, the MCT and RT values were similar [MD = −0.82, 95% CI (−3.26, 1.63)]. There was no significant difference between the other interventions and the control group or between interventions (Table 2). The ranked results showed that while RT was superior to MCT, it was the most effective modality in improving 30s chair stand repetitions in older adults with SO (Figure 4; Table 3).
A total of eight studies and 555 subjects were involved. Our results showed that MCT [MD = 0.35, 95% CI (0.30, 0.41)] and CT [MD = 0.14, 95%CI (0.06, 0.21)] exerted better effects than the control group; however, MCT was superior to CT [MD = 0.22, 95% CI (0.12, 0.31)]. No significant differences were observed between the RT and the control group [MD = 0.01, 95% CI (−0.0.07, 0.08), Table 2]. While MCT was superior to CT, MCT was the most effective technique in improving gait speed in older adults with SO (Figure 4; Table 3).
Furthermore, subgroup analysis based on intervention duration revealed that MCT was the sole modality that significantly enhanced speed in the short-term intervention group. However, in the long-term intervention group, MCT demonstrated a superior effect compared to CT (Supplementary Appendix 6).
This systematic review and NMA on exercise interventions for elders adults with SO included data from 14 clinical trials involving 955 participants. To our knowledge, this is the first NMA to explore the relative efficacy of different exercise modes on body composition and physical performance in older adults with SO. Our results confirm the beneficial effects of exercise interventions on body composition and physical performance, and highlighting MCT may as the most promising exercice strategy for addressing SO.
The body composition of SO patients is characterized by increased adipose tissue and decreased muscle mass. Optimal results require simultaneously increasing skeletal muscle mass and reducing body fat (69). The latest study from Liu et al. indicated that a 12-week CT intervention improved muscle strength and cardiopulmonary fitness in older adults with sarcopenia, while body composition remained unchanged (70). Our results showed that both RT and CT significantly reduced BFP, but neither of them decreased BMI nor improved FFM. RT is considered the most effective intervention for improving muscle mass; however, its efficacy is compromised in individuals with obesity (71). These findings align with the previous meta-analyses (27, 47, 49). Notably, MCT not only significantly reduced BFP more effectively than RT and CT but also increased FFM simultaneously. This is consistent with prior studies showing that MCT improves almost all body composition parameters in middle-aged and older women, particularly in overweight participants (72, 73). Therefore, MCT may be the optimal exercise strategy for improving body composition in older individuals with SO. A considerable body of literature has established that low-grade inflammation is a key factor in the progressive loss of muscle mass and increased fat accumulation (74–76). Extensive research has demonstrated that physical exercise exhibits anti-inflammatory properties (77), with different exercise modalities exerting varying effects on inflammatory biomarkers (74). Jung et al. reported that after 12 weeks of MCT, elevated high-sensitivity CRP (hs-CRP) and IL-6 levels in SO patients were significantly reduced (44). Gargallo et al. found that MCT decreased inflammatory status by downregulating CRP in obese subjects, whereas RT did not have this effect (78). Chen et al. also identified MCT as the most effective exercise modality for ameliorating IL-6, TNF-α, and IL-10, while RT had the least effect compared to other exercise types (74). It is widely accepted that significant positive correlations were observed between reductions in body fat and the effect sizes of hs-CRP, TNF-α, and IL-10 (73–78). These findings suggest that the reduction in body fat and enhancement of FFM following MCT may be attributed to improvements in inflammatory markers (44).
HGS serves as a primary indicator of upper limb strength, and diminished HGS is a robust and independent predictor of sarcopenia (3, 4, 79). In line with previous studies (78, 80, 81), our findings demonstrate that RT significantly enhances HGS. Moreover, our NMA revealed that MCT has a comparable positive effect on HGS to that of RT, which aligns with the findings of Labott et al. (82). Several mechanisms are likely responsible for the substantial improvement in HGS following RT. These include alterations in muscle fiber type composition (83, 84), activation and proliferation of satellite cells (85), increased rates of mitochondrial protein synthesis (86), and enhanced motor unit recruitment (87, 88).
Chair stand tests are widely acknowledged as a reliable indicator of lower limb strength (89) and are frequently utilized in the diagnosis of sarcopenia (90). Consistent with the HGS findings, the 30-s chair stand test also exhibited significant improvements after both RT and MCT interventions, thereby validating the efficacy of these methods in enhancing lower extremity strength. Consequently, our results align with the studies by Poli et al. (91, 92) and the systematic review by Labata-Lezaun et al. (34), which collectively demonstrated that MCT significantly increases strength in both upper and lower extremities. In this study, the intervention duration for the RT group in literature related to HGS and the 30-s chair stand test ranged from 8 to 12 weeks, whereas that for the MCT group ranged from 12 to 24 weeks. However, the ranked results indicated that RT performed better than MCT in both measures. This finding is consistent with prior research that has demonstrated the efficacy of RT in enhancing muscle strength, even over relatively brief periods (93).
Gait speed is the most widely utilized assessment tool for evaluating physical performance in individuals with sarcopenia (3, 4, 79). Consistent with the meta-analyses of Hsu et al. (49) and Zhuang et al. (45), our study demonstrated that CT significantly improved gait speed, whereas RT did not yield significant improvements. Furthermore, we found that multicomponent training (MCT) also significantly enhanced gait speed in sarcopenic older (SO) patients, with MCT showing superior efficacy compared to CT. Cadore et al. conducted a systematic review of exercise interventions for gait ability in frail elderly individuals and concluded that MCT can significantly improve gait performance, while RT alone has limited efficacy (94). The study by Wang et al. demonstrated that a two-week MCT intervention significantly improved gait speed in very old inpatients with sarcopenia (95). Collectively, these studies highlight the advantages of MCT in improving gait speed, which aligns with our findings. Reduced gait speed has been associated with age-related declines in lower extremity muscle strength, endurance, balance, motor control, and cognition (96–99). Additionally, walking speed has been shown to have an inverse relationship with the proportion of adipose tissue in the quadriceps muscle (80) and a positive correlation with with FFM (97). Previous research has shown that MCT is highly beneficial for reducing fat infiltration and enhancing muscle strength, endurance, and balance in older adults (100, 101), particularly in improving cognitive function (102). Our results further confirm that MCT is the only type of exercise that improves both muscle composition and function. Therefore, MCT demonstrates a significant advantage in enhancing gait speed compared to other forms of exercise, and we recommend MCT as the primary intervention for treating SO, particularly in individuals with pronounced weakness and physical performance impairments.
This study exhibits several significant strengths. Firstly, to the best of our knowledge, this is the first NMA that systematically compared and quantitatively summarized the efficacy of various exercise modalities on SO. Secondly, we adhered strictly to a rigorous inclusion criterion by exclusively incorporating randomized controlled trials (RCTs), which are regarded as the gold standard in clinical research.
Our NMA had several limitations that merit attention. Firstly, the number of studies included was limited to 14. In particular, with only two RCTs involving 35 participant focusing on AT. This relatively small sample size restricts the generalizability of the findings to broader populations of older adults with SO. Given that 80.75% of the study participants were female, the applicability of the treatment across all genders requires further investigation. Therefore, future research should prioritize conducting more RCTs with larger and more diverse sample sizes, including a broader representation of genders. Secondly, among the included studies, only two utilized multi-arm designs, neither of which involved MCT. Consequently, many effect size estimates relied heavily on indirect comparisons. Additionally, this NMA focused exclusively on the effects of different exercise modalities and did not include dose–response analysis.
Publication bias and small sample size bias were assessed using Egger’s test and visually examined via a funnel plot of effect size (ES) relative to standard error. No evidence of publication bias was detected across all outcomes in the included studies, indicating a minimal likelihood of publication bias or small sample effects. Heterogeneity among studies was assessed using the Q-test and the I2 statistic. The results indicated substantial heterogeneity (I2>75% and p < 0.05) across all outcomes. Subgroup analyses based on intervention modalities revealed that, apart from the MCT group, there was no significant heterogeneity within the other subgroups (Supplementary Appendix 7).This suggests that the primary source of statistical heterogeneity may be attributed to variations in exercise modality. Given that MCT incorporates a diverse array of components, this inherently leads to variability in the combinations and sequences of training programs (31, 103–105). Any inconsistencies in the integration strategies for these modalities, the sequencing of its components, or the duration of each phase, can significantly impact the overall effectiveness of MCT. Therefore, the heterogeneity observed in the MCT group may stem from variations in practical implementation. Consequently, further research is imperative to identify the specific exercise parameters—including components, sequences, intensity, duration, and frequency—that optimize the benefits of MCT for patients with SO.
The current NMA demonstrated that MCT outperformed other exercise intervention models in enhancing body composition and gait speed. Moreover, RT showed a significant advantage in enhancing muscle strength, while MCT’s efficacy in strength improvement was comparable to that of RT. Given that MCT has been shown to significantly enhance both morphology and function in patients with SO, it appears to be the most optimal and efficacious exercise strategy for addressing this condition. However, due to the limited number of studies in this field, future research should prioritize conducting more high-quality RCTs to validate the positive effects of MCT on individuals with SO. Additionally, future investigations should aim to determine the optimal combination of exercise types and dosages for MCT programs to maximize their beneficial impacts on individuals with SO.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
HQ: Writing – original draft, Conceptualization. WZ: Writing – original draft, Investigation, Methodology. XiZ: Writing – original draft, Investigation, Methodology. QL: Writing – original draft, Data curation, Formal analysis. XuZ: Conceptualization, Data curation, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1537291/full#supplementary-material
1. Scott, D, Blyth, F, Naganathan, V, le Couteur, DG, Handelsman, DJ, Waite, LM, et al. Sarcopenia prevalence and functional outcomes in older men with obesity: comparing the use of the EWGSOP2 sarcopenia versus ESPEN-EASO sarcopenic obesity consensus definitions. Clin Nutr. (2023) 42:1610–8. doi: 10.1016/j.clnu.2023.07.014
2. Donini, LM, Busetto, L, Bischoff, SC, Cederholm, T, Ballesteros-Pomar, MD, Batsis, JA, et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Clin Nutr. (2022) 41:990–1000. doi: 10.1016/j.clnu.2021.11.014
3. Cruz-Jentoft, AJ, Bahat, G, Bauer, J, Boirie, Y, Bruyère, O, Cederholm, T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:601. doi: 10.1093/ageing/afz046
4. Cruz-Jentoft, AJ, Baeyens, JP, Bauer, JM, Boirie, Y, Cederholm, T, Landi, F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. (2010) 39:412–23. doi: 10.1093/ageing/afq034
5. López-Otín, C, Blasco, MA, Partridge, L, Serrano, M, and Kroemer, G. Hallmarks of aging: an expanding universe. Cell. (2023) 186:243–78. doi: 10.1016/j.cell.2022.11.001
6. Tian, X, and Wang, H. Projecting National-Level Prevalence of general obesity and abdominal obesity among Chinese adults with aging effects. Front Endocrinol (Lausanne). (2022) 13:849392. doi: 10.3389/fendo.2022.849392
7. Donini, LM, Busetto, L, Bischoff, SC, Cederholm, T, Ballesteros-Pomar, MD, Batsis, JA, et al. Definition and diagnostic criteria for Sarcopenic obesity: ESPEN and EASO consensus statement. Obes Facts. (2022) 15:321–35. doi: 10.1159/000521241
8. Ji, T, Li, Y, and Ma, L. Sarcopenic obesity: an emerging public health problem. Aging Dis. (2022) 13:379–88. doi: 10.14336/AD.2021.1006
9. Gao, Q, Mei, F, Shang, Y, Hu, K, Chen, F, Zhao, L, et al. Global prevalence of sarcopenic obesity in older adults: a systematic review and meta-analysis. Clin Nutr. (2021) 40:4633–41. doi: 10.1016/j.clnu.2021.06.009
10. Benz, E, Pinel, A, Guillet, C, Capel, F, Pereira, B, de Antonio, M, et al. Sarcopenia and Sarcopenic obesity and mortality among older people. JAMA Netw Open. (2024) 7:e243604. doi: 10.1001/jamanetworkopen.2024.3604
11. Park, MJ, and Choi, KM. Interplay of skeletal muscle and adipose tissue: sarcopenic obesity. Metabolism. (2023) 144:155577. doi: 10.1016/j.metabol.2023.155577
12. Choi, S, Chon, J, Yoo, MC, Shim, GY, Kim, M, Kim, M, et al. The impact of the physical activity level on Sarcopenic obesity in community-dwelling older adults. Healthcare (Basel). (2024) 12:349. doi: 10.3390/healthcare12030349
13. Prado, CM, Batsis, JA, Donini, LM, Gonzalez, MC, and Siervo, M. Sarcopenic obesity in older adults: a clinical overview. Nat Rev Endocrinol. (2024) 20:261–77. doi: 10.1038/s41574-023-00943-z
14. Reiter, L, Bauer, S, Traxler, M, Schoufour, JD, Weijs, PJM, Cruz-Jentoft, A, et al. Effects of nutrition and exercise interventions on persons with Sarcopenic obesity: an umbrella review of Meta-analyses of randomised controlled trials. Curr Obes Rep. (2023) 12:250–63. doi: 10.1007/s13679-023-00509-0
15. Mirzai, S, Carbone, S, Batsis, JA, Kritchevsky, SB, Kitzman, DW, and Shapiro, MD. Sarcopenic obesity and cardiovascular disease: an overlooked but high-risk syndrome. Curr Obes Rep. (2024) 13:532–44. doi: 10.1007/s13679-024-00571-2
16. Tian, H, Li, H, Zhang, X, Liu, H, Huang, L, Yu, H, et al. Non-pharmacological treatment strategies for anthropometric, physical capacity and physiological indicators among sarcopenic obesity patients: a systematic review of rigorous randomized controlled trials. Age Ageing. (2024) 53:afae 278. doi: 10.1093/ageing/afae278
17. Zhao, H, Cheng, R, Song, G, Teng, J, Shen, S, Fu, X, et al. The effect of resistance training on the rehabilitation of elderly patients with sarcopenia: a Meta-analysis. Int J Environ Res Public Health. (2022) 19:15491. doi: 10.3390/ijerph192315491
18. Brellenthin, AG, Bennie, JA, and Lee, DC. Aerobic or muscle-strengthening physical activity: which is better for health? Curr Sports Med Rep. (2022) 21:272–9. doi: 10.1249/JSR.0000000000000981
19. Franklin, BA. Physical activity, cardiorespiratory fitness, and cardiovascular health: a clinical practice statement of the American Society for Preventive Cardiology Part II: physical activity, cardiorespiratory fitness, minimum and goal intensities for exercise training, prescriptive methods, and special patient populations. Am J Prev Cardiol. (2022) 12:100425. doi: 10.1016/j.ajpc.2022.100425
20. American College of Sports Medicine Position Stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. (1998) 30:992–1008. doi: 10.1249/00005768-199806000-00033
21. Donnelly, JE, Blair, SN, Jakicic, JM, Manore, MM, Rankin, JW, Smith, BK, et al. American College of Sports Medicine position stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. (2009) 41:459–71. doi: 10.1249/MSS.0b013e3181949333
22. Abou, L, Fliflet, A, Zhao, L, du, Y, and Rice, L. The effectiveness of exercise interventions to improve gait and balance in individuals with lower limb amputations: a systematic review and Meta-analysis. Clin Rehabil. (2022) 36:857–72. doi: 10.1177/02692155221086204
23. Oppert, JM, Bellicha, A, van Baak, MA, Battista, F, Beaulieu, K, Blundell, JE, et al. Exercise training in the management of overweight and obesity in adults: synthesis of the evidence and recommendations from the European Association for the Study of obesity physical activity working group. Obes Rev. (2021) 22:e13273. doi: 10.1111/obr.13273
24. Hurst, C, Robinson, SM, Witham, MD, Dodds, RM, Granic, A, Buckland, C, et al. Resistance exercise as a treatment for sarcopenia: prescription and delivery. Age Ageing. (2022) 51:afac003. doi: 10.1093/ageing/afac003
25. Hurst, C, and Sayer, AA. Improving muscle strength and physical function in older people living with sarcopenia and physical frailty: not all exercise is created equal. J R Coll Physicians Edinb. (2022) 52:166–71. doi: 10.1177/14782715221104859
26. Dunsky, A. The effect of balance and coordination exercises on quality of life in older adults: a Mini-review. Front Aging Neurosci. (2019) 11:318. doi: 10.3389/fnagi.2019.00318
27. da Silva Gonçalves, L, Santos Lopes da Silva, L, Rodrigues Benjamim, CJ, Tasinafo, MF Jr, Bohn, L, Ferreira Abud, G, et al. The effects of different exercise training types on body composition and physical performance in older adults with Sarcopenic obesity: a systematic review and Meta-analysis. J Nutr Health Aging. (2023) 27:1076–90. doi: 10.1007/s12603-023-2018-6
28. Nilsson, MI, Xhuti, D, de Maat, NM, Hettinga, BP, and Tarnopolsky, MA. Obesity and metabolic disease impair the anabolic response to protein supplementation and resistance exercise: a retrospective analysis of a randomized clinical trial with implications for aging, Sarcopenic obesity, and weight management. Nutrients. (2024) 16:4407. doi: 10.3390/nu16244407
29. Virgolici, B, Dobre, MZ, and Stefan, DCA. Bridging the gap: supplements strategies from experimental research to clinical applications in Sarcopenic obesity. Curr Issues Mol Biol. (2024) 46:13418–30. doi: 10.3390/cimb46120800
30. Hurst, C, Weston, KL, McLaren, SJ, and Weston, M. The effects of same-session combined exercise training on cardiorespiratory and functional fitness in older adults: a systematic review and meta-analysis. Aging Clin Exp Res. (2019) 31:1701–17. doi: 10.1007/s40520-019-01124-7
31. Cadore, EL, and Izquierdo, M. How to simultaneously optimize muscle strength, power, functional capacity, and cardiovascular gains in the elderly: an update. Age (Dordr). (2013) 35:2329–44. doi: 10.1007/s11357-012-9503-x
32. Azizan, A, Justine, M, and Kuan, CS. Effects of a behavioral program on exercise adherence and exercise self-efficacy in community-dwelling older persons. Curr Gerontol Geriatr Res. (2013) 2013:282315. doi: 10.1155/2013/282315
33. Bouaziz, W, Lang, PO, Schmitt, E, Kaltenbach, G, Geny, B, and Vogel, T. Health benefits of multicomponent training programmes in seniors: a systematic review. Int J Clin Pract. (2016) 70:520–36. doi: 10.1111/ijcp.12822
34. Labata-Lezaun, N, González-Rueda, V, Llurda-Almuzara, L, López-de-Celis, C, Rodríguez-Sanz, J, Bosch, J, et al. Effectiveness of multicomponent training on physical performance in older adults: a systematic review and meta-analysis. Arch Gerontol Geriatr. (2023) 104:104838. doi: 10.1016/j.archger.2022.104838
35. Labata-Lezaun, N, Canet-Vintró, M, López-de-Celis, C, Rodríguez-Sanz, J, Aiguadé, R, Cuadra-Llopart, L, et al. Effectiveness of a multicomponent training program on physical performance and muscle quality in older adults: a quasi-experimental study. Int J Environ Res Public Health. (2022) 20:222. doi: 10.3390/ijerph20010222
36. Izquierdo, M, Merchant, RA, Morley, JE, Anker, SD, Aprahamian, I, Arai, H, et al. International exercise recommendations in older adults (ICFSR): expert consensus guidelines. J Nutr Health Aging. (2021) 25:824–53. doi: 10.1007/s12603-021-1665-8
37. Lin, TY, Chen, JJJ, Lin, LL, Ou Yang, WT, Chen, MY, and Tsai, YC. Effects of multicomponent exercise training on muscle oxygenation in young and older adults. J Exerc Sci Fit. (2023) 21:138–46. doi: 10.1016/j.jesf.2022.12.002
38. Izquierdo, M, and Cadore, EL. Multicomponent exercise with power training: a vital intervention for frail older adults. J Nutr Health Aging. (2024) 28:100008. doi: 10.1016/j.jnha.2023.100008
39. Bauman, ACommentary on Izquierdo. Where next for exercise recommendations for healthy longevity in older adults? J Nutr Health Aging. (2024) 2024:100421. doi: 10.1016/j.jnha.2024.100421
40. Ferhi, H, Gaied Chortane, S, Durand, S, Beaune, B, Boyas, S, and Maktouf, W. Effects of physical activity program on body composition, physical performance, and neuromuscular strategies during walking in older adults with Sarcopenic Obesity: Randomized Controlled Trial. Healthcare (Basel). (2023) 11:2294. doi: 10.3390/healthcare11162294
41. Magtouf, E, Chortane, SG, Chortane, OG, Boyas, S, Beaune, B, Durand, S, et al. Influence of concurrent exercise training on ankle muscle activation during static and proactive postural control on older adults with Sarcopenic obesity: a multicenter, randomized, and controlled trial. Eur J Investig Health Psychol Educ. (2023) 13:2779–94. doi: 10.3390/ejihpe13120192
42. Marcos-Pardo, PJ, González-Gálvez, N, Gea-García, GM, López-Vivancos, A, Espeso-García, A, and Gomes de Souza Vale, R. Sarcopenia as a mediator of the effect of a Gerontogymnastics program on cardiorespiratory fitness of overweight and obese older women: a randomized controlled trial. Int J Environ Res Public Health. (2020) 17:7064. doi: 10.3390/ijerph17197064
43. Lee, HB, and Lee, HK. The effects of circuit training on the indexes of sarcopenia and the risk factors of metabolic syndrome in aged obese women. J Exerc Rehabil. (2018) 14:666–70. doi: 10.12965/jer.1836232.116
44. Jung, W-S, Kim, YY, Kim, JW, and Park, HY. Effects of circuit training program on cardiovascular risk factors, vascular inflammatory markers, and insulin-like growth Factor-1 in elderly obese women with sarcopenia. RCM. (2022) 13:917525. doi: 10.31083/j.rcm2304134
45. Zhuang, M, Jin, M, Lu, T, Lu, L, Ainsworth, BE, Liu, Y, et al. Effects of three modes of physical activity on physical fitness and hematological parameters in older people with sarcopenic obesity: a systematic review and meta-analysis. Front Physiol. (2022) 13:917525. doi: 10.3389/fphys.2022.917525
46. Xu, J, Hu, Q, Li, J, Zhou, Y, and Chu, T. Effects of non-pharmacological interventions on patients with sarcopenic obesity: a meta-analysis. PLoS One. (2023) 18:e0290085. doi: 10.1371/journal.pone.0290085
47. Eglseer, D, Traxler, M, Schoufour, JD, Weijs, PJM, Voortman, T, Boirie, Y, et al. Nutritional and exercise interventions in individuals with sarcopenic obesity around retirement age: a systematic review and meta-analysis. Nutr Rev. (2023) 81:1077–90. doi: 10.1093/nutrit/nuad007
48. Hita-Contreras, F, Bueno-Notivol, J, Martínez-Amat, A, Cruz-Díaz, D, Hernandez, AV, and Pérez-López, FR. Effect of exercise alone or combined with dietary supplements on anthropometric and physical performance measures in community-dwelling elderly people with sarcopenic obesity: a meta-analysis of randomized controlled trials. Maturitas. (2018) 116:24–35. doi: 10.1016/j.maturitas.2018.07.007
49. Hsu, KJ, Liao, CD, Tsai, MW, and Chen, CN. Effects of exercise and nutritional intervention on body composition, metabolic health, and physical performance in adults with Sarcopenic obesity: a Meta-analysis. Nutrients. (2019) 11:2163. doi: 10.3390/nu11092163
50. Poggiogalle, E, Parrinello, E, Barazzoni, R, Busetto, L, and Donini, LM. Therapeutic strategies for sarcopenic obesity: a systematic review. Curr Opin Clin Nutr Metab Care. (2021) 24:33–41. doi: 10.1097/MCO.0000000000000714
51. Martínez-Amat, A, Aibar-Almazán, A, Fábrega-Cuadros, R, Cruz-Díaz, D, Jiménez-García, JD, Pérez-López, FR, et al. Exercise alone or combined with dietary supplements for sarcopenic obesity in community-dwelling older people: a systematic review of randomized controlled trials. Maturitas. (2018) 110:92–103. doi: 10.1016/j.maturitas.2018.02.005
52. Ghiotto, L, Muollo, V, Tatangelo, T, Schena, F, and Rossi, AP. Exercise and physical performance in older adults with sarcopenic obesity: a systematic review. Front Endocrinol (Lausanne). (2022) 13:913953. doi: 10.3389/fendo.2022.913953
53. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
54. Sterne, JAC, Savović, J, Page, MJ, Elbers, RG, Blencowe, NS, Boutron, I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
55. Higgins, JP. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
56. Shim, S, Yoon, BH, Shin, IS, and Bae, JM. Network meta-analysis: application and practice using Stata. Epidemiol Health. (2017) 39:e2017047. doi: 10.4178/epih.e2017047
57. Huedo-Medina, TB, Sánchez-Meca, J, Marín-Martínez, F, and Botella, J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. (2006) 11:193–206. doi: 10.1037/1082-989X.11.2.193
58. Mbuagbaw, L, Rochwerg, B, Jaeschke, R, Heels-Andsell, D, Alhazzani, W, Thabane, L, et al. Approaches to interpreting and choosing the best treatments in network meta-analyses. Syst Rev. (2017) 6:79. doi: 10.1186/s13643-017-0473-z
59. Chen, HT, Chung, YC, Chen, YJ, Ho, SY, and Wu, HJ. Effects of different types of exercise on body composition, muscle strength, and IGF-1 in the elderly with Sarcopenic obesity. J Am Geriatr Soc. (2017) 65:827–32. doi: 10.1111/jgs.14722
60. Chiu, SC, Yang, RS, Yang, RJ, and Chang, SF. Effects of resistance training on body composition and functional capacity among sarcopenic obese residents in long-term care facilities: a preliminary study. BMC Geriatr. (2018) 18:21. doi: 10.1186/s12877-018-0714-6
61. Gadelha, AB, Paiva, FML, Gauche, R, de Oliveira, RJ, and Lima, RM. Effects of resistance training on sarcopenic obesity index in older women: a randomized controlled trial. Arch Gerontol Geriatr. (2016) 65:168–73. doi: 10.1016/j.archger.2016.03.017
62. Huang, SW, Ku, JW, Lin, LF, Liao, CD, Chou, LC, and Liou, TH. Body composition influenced by progressive elastic band resistance exercise of sarcopenic obesity elderly women: a pilot randomized controlled trial. Eur J Phys Rehabil Med. (2017) 53:556–63. doi: 10.23736/S1973-9087.17.04443-4
63. Kim, H, Kim, M, Kojima, N, Fujino, K, Hosoi, E, Kobayashi, H, et al. Exercise and nutritional supplementation on community-dwelling elderly Japanese women with Sarcopenic obesity: a randomized controlled trial. J Am Med Dir Assoc. (2016) 17:1011–9. doi: 10.1016/j.jamda.2016.06.016
64. Liao, CD, Tsauo, JY, Huang, SW, Ku, JW, Hsiao, DJ, and Liou, TH. Effects of elastic band exercise on lean mass and physical capacity in older women with sarcopenic obesity: a randomized controlled trial. Sci Rep. (2018) 8:2317. doi: 10.1038/s41598-018-20677-7
65. Liao, CD, Tsauo, JY, Lin, LF, Huang, SW, Ku, JW, Chou, LC, et al. Effects of elastic resistance exercise on body composition and physical capacity in older women with sarcopenic obesity: a CONSORT-compliant prospective randomized controlled trial. Medicine (Baltimore). (2017) 96:e7115. doi: 10.1097/MD.0000000000007115
66. Park, J, Kwon, Y, and Park, H. Effects of 24-week aerobic and resistance training on carotid artery intima-media thickness and flow velocity in elderly women with Sarcopenic obesity. J Atheroscler Thromb. (2017) 24:1117–24. doi: 10.5551/jat.39065
67. Vasconcelos, KS. Effects of a progressive resistance exercise program with high-speed component on the physical function of older women with sarcopenic obesity: a randomized controlled trial. Braz J Phys Ther. (2016) 20:432–40. doi: 10.1590/bjpt-rbf.2014.0174
68. Wang, LZ, Guo, YB, and Lou, JH. Effects of home exercise on sarcopenia obesity for aging people. Chin J Rehabil Theory Pract. (2019) 25:90–6. doi: 10.3969/j.issn.1006-9771.2019.01.012
69. Koliaki, C, Liatis, S, Dalamaga, M, and Kokkinos, A. Sarcopenic obesity: epidemiologic evidence, pathophysiology, and therapeutic perspectives. Curr Obes Rep. (2019) 8:458–71. doi: 10.1007/s13679-019-00359-9
70. Liu, M, Li, J, Xu, J, Chen, Y, Chien, C, Zhang, H, et al. Graded progressive home-based resistance combined with aerobic exercise in community-dwelling older adults with sarcopenia: a randomized controlled trial. Clin Interv Aging. (2024) 19:1581–95. doi: 10.2147/CIA.S473081
71. Al-Horani, RA, Alsays, KM, and Abo Alrob, O. Obesity blunts insulin sensitivity improvements and attenuates strength gains following resistance training in nondiabetic men. Eur J Appl Physiol. (2024) 124:1425–37. doi: 10.1007/s00421-023-05370-6
72. Hsu, WH, Hsu, RWW, Lin, ZR, and Fan, CH. Effects of circuit exercise and tai chi on body composition in middle-aged and older women. Geriatr Gerontol Int. (2015) 15:282–8. doi: 10.1111/ggi.12270
73. Villareal, DT, Smith, GI, Sinacore, DR, Shah, K, and Mittendorfer, B. Regular multicomponent exercise increases physical fitness and muscle protein anabolism in frail, obese, older adults. Obesity (Silver Spring). (2011) 19:312–8. doi: 10.1038/oby.2010.110
74. Chen, C, Zhang, D, Ye, M, You, Y, Song, Y, and Chen, X. Effects of various exercise types on inflammatory response in individuals with overweight and obesity: a systematic review and network meta-analysis of randomized controlled trials. Int J Obes. (2024) 25:e13666. doi: 10.1038/s41366-024-01649-6
75. Jimenez-Gutierrez, GE, Martínez-Gómez, LE, Martínez-Armenta, C, Pineda, C, Martínez-Nava, GA, and Lopez-Reyes, A. Molecular mechanisms of inflammation in sarcopenia: diagnosis and therapeutic update. Cells. (2022) 11:2359. doi: 10.3390/cells11152359
76. Pan, L, Xie, W, Fu, X, Lu, W, Jin, H, Lai, J, et al. Inflammation and sarcopenia: a focus on circulating inflammatory cytokines. Exp Gerontol. (2021) 154:111544. doi: 10.1016/j.exger.2021.111544
77. Gleeson, M, Bishop, NC, Stensel, DJ, Lindley, MR, Mastana, SS, and Nimmo, MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. (2011) 11:607–15. doi: 10.1038/nri3041
78. Gargallo, P, Tamayo, E, Jiménez-Martínez, P, Juesas, A, Casaña, J, Benitez-Martinez, JC, et al. Multicomponent and power training with elastic bands improve metabolic and inflammatory parameters, body composition and anthropometry, and physical function in older women with metabolic syndrome: a 20-week randomized, controlled trial. Exp Gerontol. (2024) 185:112340. doi: 10.1016/j.exger.2023.112340
79. Jung, HJ, Lee, YM, Kim, M, Uhm, KE, and Lee, J. Suggested assessments for sarcopenia in patients with stroke who can walk independently. Ann Rehabil Med. (2020) 44:20–37. doi: 10.5535/arm.2020.44.1.20
80. Visser, M, Kritchevsky, SB, Goodpaster, BH, Newman, AB, Nevitt, M, Stamm, E, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. (2002) 50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x
81. Zeng, D, Ling, XY, Fang, ZL, and Lu, YF. Optimal exercise to improve physical ability and performance in older adults with sarcopenia: a systematic review and network meta-analysis. Geriatr Nurs. (2023) 52:199–207. doi: 10.1016/j.gerinurse.2023.06.005
82. Labott, BK, Bucht, H, Morat, M, Morat, T, and Donath, L. Effects of exercise training on handgrip strength in older adults: a Meta-analytical review. Gerontology. (2019) 65:686–98. doi: 10.1159/000501203
83. Jacob, CDS, Barbosa, GK, Rodrigues, MP, Pimentel Neto, J, Rocha, LC, and Ciena, AP. Stretching prior to resistance training promotes adaptations on the postsynaptic region in different myofiber types. Eur J Histochem. (2022) 66:3356. doi: 10.4081/ejh.2022.3356
84. Verdijk, LB, Gleeson, BG, Jonkers, RA, Meijer, K, Savelberg, HH, Dendale, P, et al. Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. J Gerontol A Biol Sci Med Sci. (2009) 64:332–9. doi: 10.1093/gerona/gln050
85. Erskine, RM, Fletcher, G, and Folland, JP. The contribution of muscle hypertrophy to strength changes following resistance training. Eur J Appl Physiol. (2014) 114:1239–49. doi: 10.1007/s00421-014-2855-4
86. Perry, CGR, and Hawley, JA. Molecular basis of exercise-induced skeletal muscle mitochondrial biogenesis: Historical advances, current knowledge, and future challenges. Cold Spring Harb Perspect Med. (2018) 8:a029686. doi: 10.1101/cshperspect.a029686
87. Balshaw, TG, Massey, GJ, Maden-Wilkinson, TM, Morales-Artacho, AJ, McKeown, A, Appleby, CL, et al. Changes in agonist neural drive, hypertrophy and pre-training strength all contribute to the individual strength gains after resistance training. Eur J Appl Physiol. (2017) 117:631–40. doi: 10.1007/s00421-017-3560-x
88. Škarabot, J, Balshaw, TG, Maeo, S, Massey, GJ, Lanza, MB, Maden-Wilkinson, TM, et al. Neural adaptations to long-term resistance training: evidence for the confounding effect of muscle size on the interpretation of surface electromyography. J Appl Physiol (1985). (2021) 131:702–15. doi: 10.1152/japplphysiol.00094.2021
89. Lord, SR, Murray, SM, Chapman, K, Munro, B, and Tiedemann, A. Sit-to-stand performance depends on sensation, speed, balance, and psychological status in addition to strength in older people. J Gerontol A Biol Sci Med Sci. (2002) 57:M539–43. doi: 10.1093/gerona/57.8.M539
90. Cruz-Jentoft, AJ, Bahat, G, Bauer, J, Boirie, Y, Bruyère, O, Cederholm, T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
91. Poli, L, Greco, G, Cataldi, S, Ciccone, MM, de Giosa, A, and Fischetti, F. Multicomponent versus aerobic exercise intervention: effects on hemodynamic, physical fitness and quality of life in adult and elderly cardiovascular disease patients: a randomized controlled study. Heliyon. (2024) 10:e36200. doi: 10.1016/j.heliyon.2024.e36200
92. Manh Nguyen, H. The effects of long-term yoga program on blood pressure and physical fitness of older adults with stage 1 hypertension. Annals of App Sport Sci. (2024) 12. doi: 10.61186/aassjournal.1414
93. Borde, R, Hortobágyi, T, and Granacher, U. Dose-response relationships of resistance training in healthy old adults: a systematic review and Meta-analysis. Sports Med. (2015) 45:1693–720. doi: 10.1007/s40279-015-0385-9
94. Cadore, EL, Sáez de Asteasu, ML, and Izquierdo, M. Multicomponent exercise and the hallmarks of frailty: considerations on cognitive impairment and acute hospitalization. Exp Gerontol. (2019) 122:10–4. doi: 10.1016/j.exger.2019.04.007
95. Wang, R, Liang, Y, Jiang, J, Chen, M, Li, L, Yang, H, et al. Effectiveness of a short-term mixed exercise program for treating sarcopenia in hospitalized patients aged 80 years and older: a prospective clinical trial. J Nutr Health Aging. (2020) 24:1087–93. doi: 10.1007/s12603-020-1429-x
96. Hicks, GE, Shardell, M, Alley, DE, Miller, RR, Bandinelli, S, Guralnik, J, et al. Absolute strength and loss of strength as predictors of mobility decline in older adults: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. (2012) 67:66–73. doi: 10.1093/gerona/glr055
97. Osawa, Y, Chiles Shaffer, N, Shardell, MD, Studenski, SA, and Ferrucci, L. Changes in knee extension peak torque and body composition and their relationship with change in gait speed. J Cachexia Sarcopenia Muscle. (2019) 10:1000–8. doi: 10.1002/jcsm.12458
98. Osawa, Y, Studenski, SA, and Ferrucci, L. Knee extension rate of velocity development affects walking performance differently in men and women. Exp Gerontol. (2018) 112:63–7. doi: 10.1016/j.exger.2018.09.005
99. Bohannon, RW. Comfortable and maximum walking speed of adults aged 20-79 years: reference values and determinants. Age Ageing. (1997) 26:15–9. doi: 10.1093/ageing/26.1.15
100. Li, Y, Gao, Y, Hu, S, Chen, H, Zhang, M, Yang, Y, et al. Effects of multicomponent exercise on the muscle strength, muscle endurance and balance of frail older adults: a meta-analysis of randomised controlled trials. J Clin Nurs. (2023) 32:1795–805. doi: 10.1111/jocn.16196
101. Zhang, Y, Zhang, Y, du, S, Wang, Q, Xia, H, and Sun, R. Exercise interventions for improving physical function, daily living activities and quality of life in community-dwelling frail older adults: a systematic review and meta-analysis of randomized controlled trials. Geriatr Nurs. (2020) 41:261–73. doi: 10.1016/j.gerinurse.2019.10.006
102. Yan, J, Li, X, Guo, X, Lin, Y, Wang, S, Cao, Y, et al. Effect of multicomponent exercise on cognition, physical function and activities of daily life in older adults with dementia or mild cognitive impairment: a systematic review and Meta-analysis. Arch Phys Med Rehabil. (2023) 104:2092–108. doi: 10.1016/j.apmr.2023.04.011
103. Wilson, JM, Marin, PJ, Rhea, MR, Wilson, SMC, Loenneke, JP, and Anderson, JC. Concurrent training: a meta-analysis examining interference of aerobic and resistance exercises. J Strength Cond Res. (2012) 26:2293–307. doi: 10.1519/JSC.0b013e31823a3e2d
104. Lundberg, TR, Feuerbacher, JF, Sünkeler, M, and Schumann, M. The effects of concurrent aerobic and strength training on muscle Fiber hypertrophy: a systematic review and Meta-analysis. Sports Med. (2022) 52:2391–403. doi: 10.1007/s40279-022-01688-x
105. Eddens, L, van Someren, K, and Howatson, G. The role of intra-session exercise sequence in the interference effect: a systematic review with Meta-analysis. Sports Med. (2018) 48:177–88. doi: 10.1007/s40279-017-0784-1
Keywords: aging, exercise training, multicomponent training, obesity, sarcopenia, sarcopenic obesity
Citation: Qiu H, Zheng W, Zhou X, Liu Q and Zhao X (2025) Training modalities for elder sarcopenic obesity: a systematic review and network meta-analysis. Front. Nutr. 12:1537291. doi: 10.3389/fnut.2025.1537291
Received: 30 November 2024; Accepted: 29 January 2025;
Published: 19 February 2025.
Edited by:
Donny Michael Camera, Swinburne University of Technology, AustraliaReviewed by:
Seyed Morteza Tayebi, Allameh Tabataba’i University, IranCopyright © 2025 Qiu, Zheng, Zhou, Liu and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuehong Zhao, eHVlaG9uZ196aGFvQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.