- 1Department of Community Medicine, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
- 2Health Collaborative Center (HCC), Jakarta, Indonesia
- 3Danone Specialized Nutrition, Jakarta, Indonesia
- 4Department of Epidemiology, Faculty of Public Health, Universitas Indonesia, Jakarta, Indonesia
- 5Medical Study Program, Faculty of Medicine, Brawijaya University, Malang, Indonesia
- 6Master Program of Biomedical Science, Faculty of Medicine, Brawijaya University, Malang, Indonesia
- 7Medical Research Center of Indonesia, Surabaya, Indonesia
- 8Bachelor of Medicine, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia
- 9Medical Study Program, Faculty of Medicine, Airlangga University, Surabaya, Indonesia
Iron Deficiency Anemia (IDA) remains a pervasive global health challenge, disproportionately affecting vulnerable populations such as women and children. This review explores the cutting-edge interplay between microRNAs (miRNAs) and proteins in erythropoiesis, highlighting novel therapeutic strategies for IDA. Emerging evidence underscores the pivotal role of miRNAs—such as miR-15a, miR-24, miR-150, and miR-223—in regulating erythropoiesis, with dysregulation linked to hematologic and systemic diseases. Proteins, acting as modulators of miRNA activity, present innovative pathways for intervention by influencing erythropoiesis at multiple stages, from stem cell proliferation to red blood cell maturation. Our synthesis highlights key molecular mechanisms: miR-15a suppresses erythropoiesis by inhibiting c-Myb, miR-24 impairs heme biosynthesis through ALK4 regulation, while miR-150 and miR-223 modulate critical hematopoietic pathways affecting cell differentiation and apoptosis. These miRNA-protein interactions suggest targeted therapies such as protein-based miRNA modulators could optimize erythropoiesis, advancing IDA management. Additionally, the review emphasizes the potential of leveraging protein-miRNA interactions for precision medicine, especially in resource-limited settings where anemia’s burden is profound. By bridging current knowledge gaps, our proposed strategies offer personalized and scalable therapeutic solutions. This comprehensive perspective lays the groundwork for future interventions addressing one of the world’s most widespread public health crises.
1 Introduction
Anemia is characterized by hemoglobin levels falling below the threshold required to support the physiological demand for oxygen transport by circulating red blood cells. It remains a significant global health challenge. In 2021, the global prevalence of anemia across all age groups was 24.3%, equating to approximately 1.92 billion people worldwide (1). This prevalence varies across countries, with the highest rates in South Asia, Western Sub-Saharan Africa, and Central Sub-Saharan Africa (2). Anemia affects approximately one-third of the global population and is linked to increased morbidity and mortality, particularly among women and children. It contributes to adverse birth outcomes, reduced work productivity in adults, and impaired cognitive and behavioral development in children. Preschool children (PSC) and women of reproductive age (WRA) are especially vulnerable to the effects of anemia (3). Despite extensive public health efforts, the global burden of anemia has persisted, prompting a deeper exploration of its underlying biological mechanisms. While iron deficiency remains the most recognized cause, emerging research points to complex molecular processes driving impaired red blood cell production. Recent scientific advances have identified the regulatory role of microRNAs (miRNAs) in erythropoiesis, highlighting them as key molecular players in anemia pathogenesis.
Managing anemia continues to be a considerable challenge, particularly in Indonesia. Despite various interventions, such as iron and folic acid supplementation, the prevalence of anemia has not significantly declined (4). One of the key factors contributing to anemia is nutritional deficiency, particularly protein. During protein malnutrition, structural and cellular changes in the hematopoietic microenvironment contribute to bone marrow atrophy and impair hematopoietic stem cell formation, disrupting hematopoietic homeostasis and leading to anemia (5). Furthermore, animal studies have demonstrated that protein deficiency can induce anemia by inhibiting effective erythropoiesis through reduced protein synthesis in erythroid cells and decreased erythropoietin production, even when iron stores are sufficient (6).
Proteins, serving as modulators or co-factors of miRNA activity, further shape this regulatory network. Understanding these intricate miRNA-protein interactions opens new avenues for therapeutic interventions aimed at correcting dysregulated erythropoiesis. At the biomolecular level, the red blood cell formation process, or erythropoiesis, is regulated by various molecular mechanisms, including microRNA (miRNA) modulation. Notably, miRNAs such as miRNA-15a, miRNA-24, miRNA-150, and miRNA-223 play critical roles in this process. Dysregulation of these miRNAs has been linked to diseases like thalassemia, leukemia, sickle cell disease, and cancer. Moreover, miRNAs are crucial in erythropoiesis as they regulate gene expression in erythroid progenitor cells’ proliferation, differentiation, and apoptosis. For example, miRNA-15a suppresses erythropoiesis by inhibiting the expression of the MYB protein, which is essential for the kinetics of mature erythroid cells, while miRNA-24 impedes terminal differentiation by regulating the ALK4 gene (7). By shifting the focus from traditional iron supplementation to biomolecular targets such as miRNAs and their associated protein regulators, innovative strategies can be developed to combat anemia more effectively. This molecular perspective not only enhances the understanding of anemia’s root causes but also paves the way for precision medicine applications tailored to individual patient profiles.
In recent years, scientific research has increasingly focused on biomolecular aspects to understand the fundamental mechanisms behind red blood cell formation and anemia. A key area of interest is the role of proteins in this process, as proteins play a pivotal role in regulating various biochemical pathways involved in red blood cell production and maintenance. For instance, proteins such as erythropoietin promote cell survival and drive terminal erythroid maturation (8), while transcription factors like GATA1 regulate all aspects of erythroid maturation at the transcriptional and functional levels (9). However, comprehensive studies exploring the relationship between proteins and miRNAs in anemia are still lacking despite the potential of this area in developing anemia management strategies. This review aims to bridge this gap by examining the modulation of miRNAs by proteins and exploring how these interactions could offer novel therapeutic strategies to combat anemia, particularly in populations most affected by this condition.
This opinion paper uniquely explores the interplay between proteins and miRNAs in erythropoiesis, offering a novel biomolecular perspective on combating iron deficiency anemia. By integrating recent evidence and highlighting therapeutic potentials, this discussion not only advances the scientific understanding of anemia management but also paves the way for globally scalable interventions aimed at mitigating one of the most prevalent and impactful health challenges worldwide.
2 Search strategy and study selection criteria
To identify relevant studies, a comprehensive literature search was conducted using major scientific databases including PubMed, Scopus, Google Scholar and Web of Science. The search strategy employed combinations of key terms such as “microRNA,” “miRNA modulation,” “iron deficiency anemia,” “erythropoiesis,” and “protein regulation.” Only peer-reviewed articles published in English were included to ensure up-to-date insights.
The inclusion criteria were: (1) original research or systematic reviews focused on miRNA and protein interactions in erythropoiesis; (2) studies highlighting therapeutic applications for IDA; and (3) articles reporting molecular mechanisms with experimental or clinical evidence. Exclusion criteria included non-English publications, studies lacking relevance to IDA, and conference abstracts or editorial pieces. Titles and abstracts were screened for eligibility, followed by full-text evaluations of selected articles. Data extraction focused on molecular pathways, key findings, and potential therapeutic implications. This systematic approach ensured a robust and focused analysis of the current landscape of miRNA and protein-based interventions in IDA management.
3 The health beneficial of protein
3.1 The fundamental role of protein in anemia
Proteins are biopolymers made up of amino acids. In the human body, they serve many crucial roles, such as providing the body’s building blocks, function as hormones, enzymes, precursors of several biologically relevant molecules, and initiators of cellular death (10). One of the key roles of protein is its involvement in the development of anemia. Hemoglobin (Hb), an important protein responsible for transporting oxygen to body tissues, serves as one of the markers for diagnosing anemia in humans (11). A reduction in hemoglobin levels below 13.5 g/dl in men and 12.0 g/dl in non-pregnant women, regardless of the underlying cause, is indicative of anemia. Structural abnormalities in hemoglobin, such as those seen in conditions like thalassemia and sickle cell disorders, can lead to anemia because of premature destruction of red blood cells (12).
Iron is an essential component of hemoglobin and its metabolism is regulated by various proteins, including ferritin (which stores iron), transferrin (which transports iron), and hepcidin (a negative regulator of iron absorption and recycling), along with other proteins that modulate iron metabolism at different physiological levels (13, 14). Disruptions in iron metabolism can result in iron deficiency anemia (IDA), the most common form of anemia worldwide (15). The involvement of proteins in anemia is particularly evident in anemia of inflammation (AI), also called anemia of chronic disease (ACD), the second most prevalent form of anemia globally (16). Inflammatory cytokines, key proteins in this process, contribute to a reduced lifespan of red blood cells, likely by activating macrophages, while also disrupting erythropoietin (EPO) production and function, and inhibiting the proliferation and differentiation of normal erythroid progenitor cells (3). Erythropoietin (EPO) is a glycoprotein hormone synthesized by the kidneys that is essential for stimulating red blood cell production in response to low partial pressure of oxygen (pO2), making it important in the context of anemia. A reduction in erythropoietin levels, commonly seen in individuals with chronic kidney disease, can also lead to the development of anemia (17). Figure 1 shows the involving of protein in anemia.
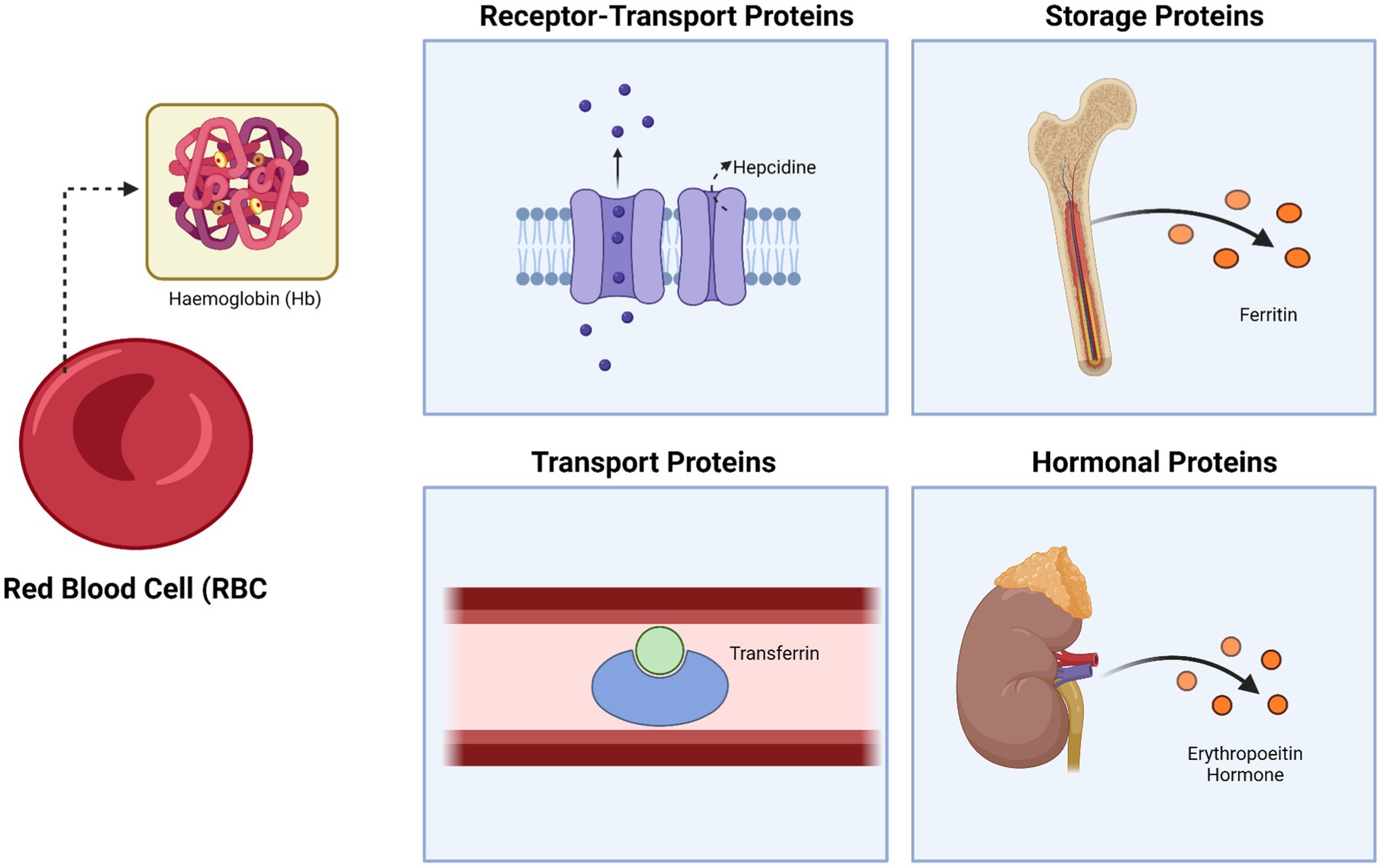
Figure 1. The fundamental role of proteins in anemia: protein as receptor-transport, storage, and hormonal proteins. Created with BioRender.com.
Protein deficiencies disrupt erythropoietic pathways by impairing key molecular processes essential for red blood cell formation (18). Specifically, inadequate protein intake compromises the synthesis of hemoglobin, the main oxygen-carrying protein in erythrocytes, and reduces the availability of erythropoietin, a hormone critical for stimulating erythroid progenitor proliferation (19). Furthermore, protein-related transcription factors like GATA1 are essential for the differentiation of erythroid cells, while transporter proteins such as transferrin and ferritin regulate iron homeostasis required for effective erythropoiesis (20). These disruptions collectively impair red blood cell production, leading to various anemia-related conditions.
3.2 Differences in protein types for Anemia management
Anemia has numerous etiologies which fundamentally revolve around two core issues: inadequate production of red blood cells or hemoglobin (supply) and excessive demand for them (either physiological or pathological). The management strategy for anemia focuses on addressing the specific etiology in each patient, with the goal of achieving a balance between the two (3). In cases of iron deficiency, the most common cause of anemia, therapy focuses on restoring normal iron levels in the body, through pharmacological iron supplementation (oral or IV, if necessary) (Table 1) (21). In more complex cases, such as anemia due to chronic diseases like cancer, management options are more varied, including vitamin B12, folic acid, erythropoiesis-stimulating agents, and even blood transfusions, taking into account the appropriateness of each option, as well as their respective risks and benefits. While it is important to manage anemia as an individual disease case, this disease can cause significant burden throughout the world. Thus, WHO has formulated key strategies for global anemia prevention and control. These strategies require collaboration from various sectors, including the nutrient and food sector which encompass the provision, utilization, and education about healthy and nutrient-rich foods. The goal is enhancing the intake of essential micronutrients, particularly iron, folate, vitamin B12, vitamin A, and riboflavin, along with other micronutrients, that can be achieved through dietary diversification, food fortification, and supplementation strategies (22).
The consumption of nutritious food should not only meet daily requirements but also provide high-quality nutrients (Table 1). One of the most important components is protein, particularly for obtaining various essential amino acids (23). There are numerous types of protein sources, generally classified as animal-based and plant-based proteins. Animal-based proteins tend to contain complete proteins and essential amino acids, which are well absorbed by the digestive system (24). High consumption of animal-based protein plays a role in anemia management due to its positive effects on iron status in the body, including blood hemoglobin levels. On the other hand, plant-based proteins typically lack complete essential amino acids in one type source and are less efficiently absorbed due to their fibrous structure, which is more difficult to digest. Therefore, individuals who follow a plant-based protein diet require a more diverse range of sources and larger quantities to meet their nutritional needs (25).
Several micronutrients required for normal erythropoiesis, such as iron, folic acid (Vitamin B9), and Vitamin B12, can be obtained from various protein sources, with their content differing across these sources. Animal-based protein sources, such as meat from four-legged animals and two-legged animals, contain good amounts of iron, particularly in the form of heme iron, which is readily absorbed by the body. Fish meat is also rich in iron, and a diet high in such sources can fully meet the daily dietary iron requirements. Plant-based protein sources (beans, nuts, peas, legumes, etc.) tend to contain non-heme iron, which has lower bioavailability. Moreover, these sources often contain iron absorption inhibitors, such as phytates (26). Folic acid, a vital micronutrient, is primarily found in animal-based protein sources like liver and kidney. In contrast, among plant-based foods, it is most abundant in leafy green vegetables, which are not typically recognized as significant sources of protein (27). Lastly, in the case of vitamin B12, there are no plant-based sources. This vitamin can be absorbed by the digestive system after being separated from the protein in meat. It is abundant in red meat (beef, lamb), all types of fish, and to a lesser extent in poultry products (28).
4 The role of miRNA in biomolecular process
The single-stranded structure of short nonprotein coding RNA or microRNA (miRNA), transcribed from DNA sequences, plays an essential role in gene expression. The miRNA interacts with messenger RNA (mRNA) to regulate transcription through the coding sequence as an independent gene promoter, producing a pattern that alters overall cellular processes. In this way, intracellular and extracellular miRNA act as messengers to cells, organs and systems that could alter the physiological and pathological homeostasis (29). A possible pathway to alter metabolism or paradoxically reprogramming the metabolism involves directly or indirectly diverse mechanisms, such as targeting key enzymes, transporters, transcription factors, and signalling pathways (30).
However, in order to interact with distant cells/organs, miRNA should remain stable and be able to interact and modulate transporters, enzymes and receptors. Exosomes or extracellular vesicles are mediators protecting miRNA stability and integrity. The miRNA exerts an influence on the modulation and inhibition of the inflammatory state, may result in alterations to physiological conditions in hematoimmunological processes. Furthermore the miRNA influences the modulation and inhibition of the inflammatory state (i.e., polarization of macrophages to two phenotypes M1 and M2), signalling pathway (NF-κB, TLR, STAT-3), and regulates the function of receptor cells (closely related to hypoxia and inflammation) (31). The hitchhiking of miRNA affects the motility of the transporter (vesicle), thereby enabling it to be exploited in an inactive form for the purpose of transporting from the post-synaptic (32).
MiRNA genes are transcribed by RNA polymerase II, producing a primary transcript known as pri-miRNA. These pri-miRNAs are long sequences that contain multiple miRNA segments, which fold into characteristic hairpin structures. Within the nucleus, the pri-miRNA is processed by the Drosha enzyme, in collaboration with the Dgcr8 protein, resulting in a double-stranded precursor miRNA (pre-miRNA) approximately 70 nucleotides in length. This pre-miRNA is then exported to the cytoplasm through the XPO5-GTP complex. Once in the cytoplasm, the enzyme Dicer, assisted by the cofactor TRBP, further processes the pre-miRNA into a miRNA duplex, which is about 21–24 nucleotides long. The miRNA duplex is subsequently incorporated into the RNA-induced silencing complex (RISC), where one strand is selected to become the mature miRNA, typically around 22 nucleotides in length. This mature miRNA contains a critical “seed region” of approximately 7 nucleotides at the 5′-end, essential for recognizing target mRNAs. The miRNA binds to partially complementary sites, usually located in the 3′ untranslated regions (3’UTRs) of target mRNAs, leading to either mRNA degradation or suppression of its translation. Figure 2 illustrates the miRNA processing pathway (33).
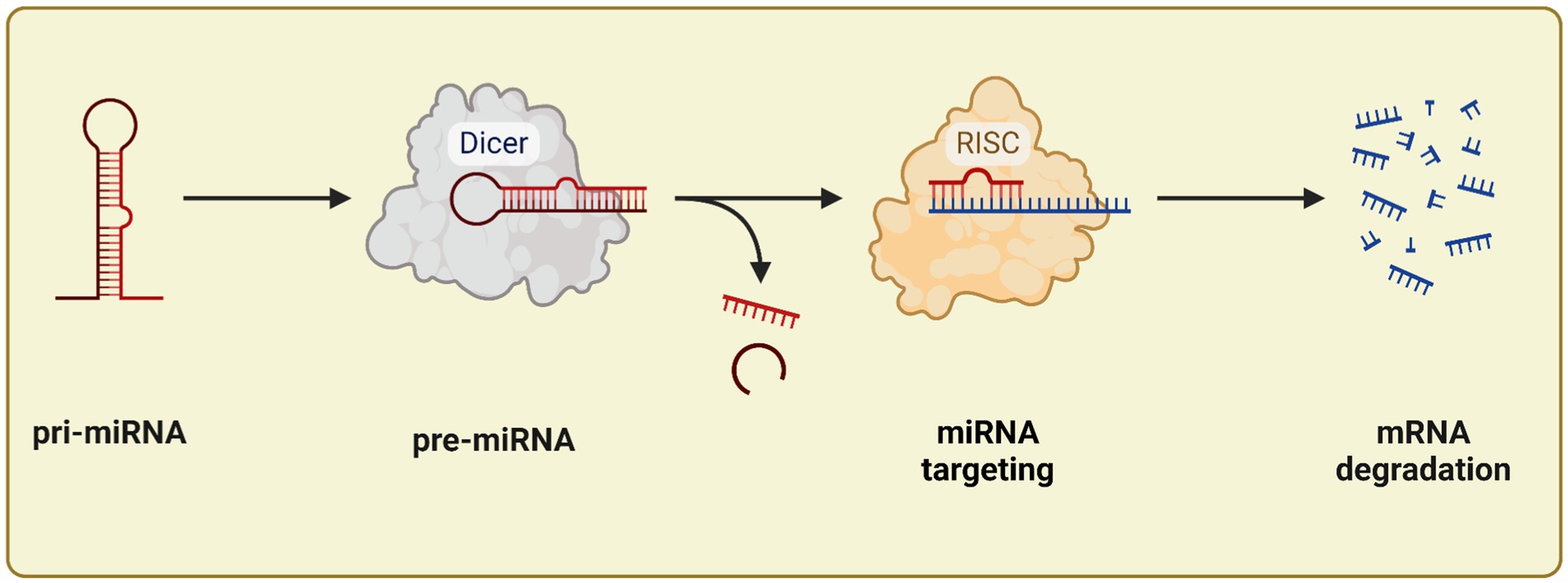
Figure 2. miRNA processing: start from pri-miRNA until mRNA degradation. Created with BioRender.com.
5 Proteins targeting miRNA modulation in red blood cell formation
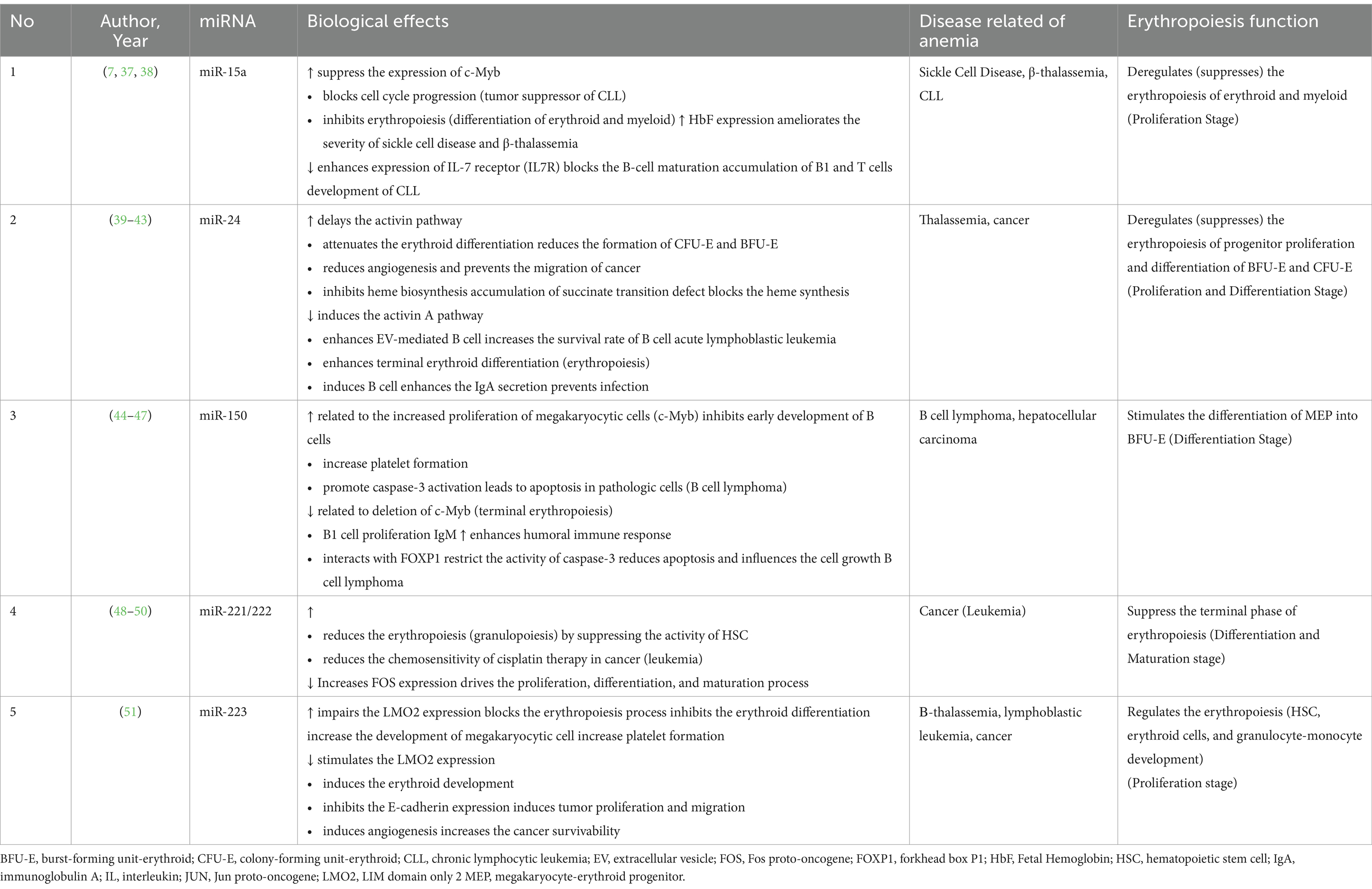
The clinical evidence of targeting miRNA in anemia for the past 10 years is summarized in Table 2. Proteins influence miRNA biogenesis, stability, and functionality through various mechanisms (34), including transcriptional regulation of miRNA-encoding genes, post-transcriptional processing by key enzymes such as Drosha and Dicer, modulation of miRNA maturation through co-factors, and interactions with RNA-binding proteins that can alter miRNA stability, localization, and target recognition (35, 36). Table 2 presents a summary of several studies conducted over the past 10 years on related to miRNA in erythropoiesis. The majority of several studies demonstrate that various types of miRNAs influence anemia through erythropoiesis. Erythropoiesis is part of hematopoiesis that plays a crucial part in erythrocyte equilibrium to maintain the physiological term. The origin of erythrocyte or red blood cell (RBC) results from the differentiation of hematopoietic stem cells (HSC) into multi-potent progenitor cells (MPP) and identified erythroid lineage called burst-forming unit erythroid (BFU-E). The BFU-E proliferates into several types of cells, such as proerythroblast. Below the regulation of erythropoietin hormone (Epo), the proerythroblast is amplified into a reticulocyte with erythrocyte as the final form of erythropoiesis. Several miRs contribute significantly to erythropoiesis, such as miR-15a, miR-24, miR-150, and miR-223. However, our tabulated studies also report several alteration targets, such as c-Myb, FOXP-1, and LMO2. Relative of several miRs (miR-15a, miR-24, miR-223) deregulated the erythropoiesis that can be found in several diseases such as thalassemia, leukemia, sickle cell disease, and cancer. The impaired erythropoiesis causes the common phenomenon of megakaryocytic cells that, in some cases, could alleviate the severity of the disease or be implicated with disease progressivity that leads to anemia condition.
6 Future directions of using protein in anemia
Previous research has identified the potential of miRNA targeting in the management of anemia, but the number of studies in this field remains limited. Moreover, variations in the markers used to assess reductions in disease incidence across studies complicate the ability to detect significant effects of miRNA specifically on anemia. Despite consistent findings over the past decade showing a strong correlation between miRNA and hematological processes such as erythropoiesis, the efficacy and efficiency of miRNA in modulating anemia-specific outcomes require further investigation.
This article underscores the need for additional clinical research to explore the connection between miRNA and anemia, particularly in identifying specific miRNAs involved in this condition. Although this paper provides indirect evidence of miRNA’s involvement in anemia through its regulation of erythropoiesis, future studies should aim to clarify its role in anemic patients, ultimately enhancing therapeutic efficacy.
6.1 Future directions
Advancing research into miRNA-protein interactions for IDA treatment necessitates a multidisciplinary approach involving molecular biology, bioinformatics, and clinical sciences. Proposed methodologies include:
1. Preclinical studies: conduct in vitro studies using erythroid progenitor cell lines to explore the effects of specific miRNA inhibitors or protein modulators. CRISPR-Cas9 technology could be employed to knock out target miRNAs, enabling precise mechanistic evaluations.
2. Animal models: utilize transgenic mouse models with altered expression of relevant miRNAs and proteins to assess phenotypic changes in erythropoiesis. Longitudinal studies could measure red blood cell counts, hemoglobin levels, and survival outcomes.
3. Omics integration: employ multi-omics approaches, including transcriptomics, proteomics, and metabolomics, to uncover new molecular targets and validate miRNA-protein interaction networks.
4. Clinical trial designs:
5. • Phase I/II trials: design early-phase clinical trials focusing on safety, dosage optimization, and proof-of-concept efficacy of miRNA-targeting therapeutics.
6. • Randomized controlled trials (RCTs): conduct RCTs comparing standard iron supplementation with novel protein-based miRNA modulators.
7. • Biomarker-driven studies: incorporate predictive biomarkers such as circulating miRNA signatures to stratify patient populations and personalize treatments.
8. Implementation science: evaluate the scalability and cost-effectiveness of miRNA-based therapies in low-resource settings. This could involve partnership models with global health organizations.
By integrating these strategies, future research can accelerate the development of transformative treatments for IDA, bridging the gap between molecular discoveries and clinical applications.
Author contributions
RW: Conceptualization, Funding acquisition, Investigation, Project administration, Supervision, Validation, Writing – original draft. TS: Funding acquisition, Supervision, Validation, Writing – review & editing. DP: Data curation, Funding acquisition, Resources, Writing – review & editing. NA: Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. YT: Data curation, Investigation, Software, Writing – original draft. MS'a: Formal analysis, Methodology, Software, Writing – original draft. GU: Data curation, Writing – review & editing. MA: Data curation, Formal analysis, Investigation, Writing – original draft. FN: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Refferences
1. Gardner, WM, Razo, C, McHugh, TA, Hagins, H, Vilchis-Tella, VM, Hennessy, C, et al. Prevalence, years lived with disability, and trends in anaemia burden by severity and cause, 1990–2021: findings from the global burden of disease study 2021. Lancet Haematol. (2023) 10:e713–34. doi: 10.1016/S2352-3026(23)00160-6
2. Safiri, S, Kolahi, AA, Noori, M, Nejadghaderi, SA, Karamzad, N, Bragazzi, NL, et al. Burden of anemia and its underlying causes in 204 countries and territories, 1990–2019: results from the global burden of disease study 2019. J Hematol Oncol. (2021) 14:185–6. doi: 10.1186/s13045-021-01202-2
3. Chaparro, CM, and Suchdev, PS. Anemia epidemiology, pathophysiology, and etiology in low-and middle-income countries. Ann N Y Acad Sci. (2019) 1450:15–31. doi: 10.1111/nyas.14092
4. Pibriyanti, K, Zahro, L, Ummah, SK, and Sari, FK. Macronutrient, nutritional status, and anemia incidence in adolescents at Islamic boarding school. Jurnal Gizi Klinik Indonesia. (2021) 18:97–103. doi: 10.22146/ijcn.63122
5. Utami, A, Margawati, A, Pramono, D, Julianti, HP, Adespin, DA, and Wulandari, DR. The effectiveness of iron-folic acid supplementation and education intervention to hemoglobin level, knowledge, and compliance among adolescent girls in Islamic boarding school. Open access Macedonian. J Med Sci. (2022) 10:1141–6. doi: 10.3889/oamjms.2022.9688
6. Bianchi, VE. Role of nutrition on anemia in elderly. Clin Nut ESPEN. (2016) 11:e1–e11. doi: 10.1016/j.clnesp.2015.09.003
7. Apple, CG, Miller, ES, Kannan, KB, Stortz, JA, Loftus, TJ, Lopez, MC, et al. The role of bone marrow microRNA (miR) in erythropoietic dysfunction after severe trauma. Surgery. (2021) 169:1206–12. doi: 10.1016/j.surg.2020.11.029
8. Tsiftsoglou, AS. Erythropoietin (EPO) as a key regulator of erythropoiesis, bone remodeling and endothelial transdifferentiation of multipotent mesenchymal stem cells (MSCs): implications in regenerative medicine. Cells. (2021) 10:2140. doi: 10.3390/cells10082140
9. Gutiérrez, L, Caballero, N, Fernández-Calleja, L, Karkoulia, E, and Strouboulis, J. Regulation of GATA1 levels in erythropoiesis. IUBMB Life. (2020) 72:89–105. doi: 10.1002/iub.2192
10. LaPelusa, A, and Kaushik, R. Physiology, proteins. Treasure Island (FL): StatPearls Publishing (2024).
11. Farid, Y, Bowman, NS, and Lecat, P. Biochemistry, hemoglobin synthesis. Treasure Island (FL): StatPearls Publishing (2024).
12. Turner, J, Parsi, M, and Badireddy, M. Anemia. Treasure Island (FL): StatPearls Publishing (2024).
13. Wallace, DF. The regulation of iron absorption and homeostasis. Clin Biochemist Rev. (2016) 37:51–62.
14. Waldvogel-Abramowski, S, Waeber, G, Gassner, C, Buser, A, Frey, BM, Favrat, B, et al. Physiology of iron metabolism. Transfus Med Hemother. (2014) 41:213–21. doi: 10.1159/000362888
15. Warner, MJ, and Kamran, MT. Iron deficiency anemia. Treasure Island (FL): StatPearls Publishing (2024).
16. Weiss, G, Ganz, T, and Goodnough, LT. Anemia of inflammation. Blood. (2019) 133:40–50. doi: 10.1182/blood-2018-06-856500
17. Schoener, B, and Borger, J. Erythropoietin stimulating agents. Treasure Island (FL): StatPearls Publishing (2024).
18. Kuhn, V, Lukas Diederich, TC, Stevenson Keller, IV, Kramer, CM, Lückstädt, W, Panknin, C, et al. Red blood cell function and dysfunction: redox regulation, nitric oxide metabolism, anemia. Antioxid Redox Signal. (2017) 26:718–42. doi: 10.1089/ars.2016.6954
19. Feret, W, Safranow, K, Ciechanowski, K, and Kwiatkowska, E. How is body composition and nutrition status associated with erythropoietin response in Hemodialyzed patients? A single-center prospective cohort study. J Clin Med. (2022) 11:2426. doi: 10.3390/jcm11092426
20. Tang, P, and Wang, H. Regulation of erythropoiesis: emerging concepts and therapeutic implications. Hematology. (2023) 28:2250645. doi: 10.1080/16078454.2023.2250645
21. Ning, S, and Zeller, MP. Management of iron deficiency. Hematology. (2019) 2019:315–22. doi: 10.1182/hematology.2019000034
22. Aapro, M, Beguin, Y, Bokemeyer, C, Dicato, M, Gascón, P, Glaspy, J, et al. Management of anaemia and iron deficiency in patients with cancer: ESMO clinical practice guidelines. Ann Oncol. (2018) 29:iv96–110. doi: 10.1093/annonc/mdx758
23. Chatterjee, S, Sarkar, A, and Boland, MJ. The world supply of food and the role of dairy protein. Milk Prot. (2014) 29:1–18. doi: 10.1016/B978-0-12-405171-3.00001-5
24. Ajomiwe, N, Boland, M, Phongthai, S, Bagiyal, M, Singh, J, and Kaur, L. Protein nutrition: understanding structure, digestibility, and bioavailability for optimal health. Food Secur. (2024) 13:1771. doi: 10.3390/foods13111771
25. Jackson, J, Williams, R, McEvoy, M, MacDonald-Wicks, L, and Patterson, A. Is higher consumption of animal flesh foods associated with better Iron status among adults in developed countries? A systematic review. Nutrients. (2016) 8:89. doi: 10.3390/nu8020089
26. Cohen, CT, and Powers, JM. Nutritional strategies for managing Iron deficiency in adolescents: approaches to a challenging but common problem. Adv Nutr. (2024) 15:100215. doi: 10.1016/j.advnut.2024.100215
27. Shulpekova, Y, Nechaev, V, Kardasheva, S, Sedova, A, Kurbatova, A, Bueverova, E, et al. The concept of folic acid in health and disease. Molecules. (2021) 26:3731. doi: 10.3390/molecules26123731
28. Herrmann, W. Vitamin B12 deficiency in vegetarians In: Vegetarian and plant-based diets in health and disease prevention : Academic Press (2017) 9:791–808.
29. O'Brien, J, Hayder, H, Zayed, Y, and Peng, C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol. (2018) 9:402. doi: 10.3389/fendo.2018.00402
30. Pedroza-Torres, A, Romero-Córdoba, SL, Justo-Garrido, M, Salido-Guadarrama, I, Rodríguez-Bautista, R, Montaño, S, et al. MicroRNAs in tumor cell metabolism: roles and therapeutic opportunities. Front Oncol. (2019) 9:1404. doi: 10.3389/fonc.2019.01404
31. Shapouri-Moghaddam, A, Mohammadian, S, Vazini, H, Taghadosi, M, Esmaeili, SA, Mardani, F, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. (2018) 233:6425–40. doi: 10.1002/jcp.26429
32. Corradi, E, Dalla Costa, I, Gavoci, A, Iyer, A, Roccuzzo, M, Otto, TA, et al. Axonal precursor mi RNA s hitchhike on endosomes and locally regulate the development of neural circuits. EMBO J. (2020) 39:e102513. doi: 10.15252/embj.2019102513
33. Cirillo, F, Lazzeroni, P, Catellani, C, Sartori, C, Amarri, S, and Street, ME. MicroRNAs link chronic inflammation in childhood to growth impairment and insulin-resistance. Cytokine Growth Factor Rev. (2018) 39:1–8. doi: 10.1016/j.cytogfr.2017.12.004
34. Shang, R, Lee, S, Senavirathne, G, and Lai, EC. MicroRNAs in action: biogenesis, function and regulation. Nat Rev Genet. (2023) 24:816–33. doi: 10.1038/s41576-023-00611-y
35. Mencia, R, Gonzalo, L, Tossolini, I, and Manavella, PA. Keeping up with the MiRNAs: current paradigms of the biogenesis pathway. J Exp Bot. (2023) 74:2213–27. doi: 10.1093/jxb/erac322
36. de Rooij, LA, Mastebroek, DJ, Ten Voorde, N, van der Wall, E, van Diest, PJ, and Moelans, CB. The MicroRNA lifecycle in health and cancer. Cancer. (2022) 14:5748. doi: 10.3390/cancers14235748
37. Underbayev, C, Kasar, S, Ruezinsky, W, Degheidy, H, Schneider, JS, Marti, G, et al. Role of mir-15a/16-1 in early B cell development in a mouse model of chronic lymphocytic leukemia. Oncotarget. (2016) 7:60986–99. doi: 10.18632/oncotarget.11290
38. Hutter, K, Rülicke, T, Szabo, TG, Andersen, L, Villunger, A, and Herzog, S. The miR-15a/16-1 and miR-15b/16-2 clusters regulate early B cell development by limiting IL-7 receptor expression. Front Immunol. (2022) 13:967914. doi: 10.3389/fimmu.2022.967914
39. Morianos, I, Papadopoulou, G, Semitekolou, M, and Xanthou, G. Activin-A in the regulation of immunity in health and disease. J Autoimmun. (2019) 104:102314. doi: 10.1016/j.jaut.2019.102314
40. Licari, E, Cricrì, G, Mauri, M, Raimondo, F, Dioni, L, Favero, C, et al. ActivinA modulates B-acute lymphoblastic leukaemia cell communication and survival by inducing extracellular vesicles production. Sci Rep. (2024) 14:16083. doi: 10.1038/s41598-024-66779-3
41. Portale, F, Beneforti, L, Fallati, A, Biondi, A, Palmi, C, Cazzaniga, G, et al. Activin a contributes to the definition of a pro-oncogenic bone marrow microenvironment in t (12, 21) preleukemia. Exp Hematol. (2019) 73:7–12.e4. doi: 10.1016/j.exphem.2019.02.006
42. Ries, A, Schelch, K, Falch, D, Pany, L, Hoda, MA, and Grusch, M. Activin a: an emerging target for improving cancer treatment? Expert Opin Ther Targets. (2020) 24:985–96. doi: 10.1080/14728222.2020.1799350
43. Detraux, D, Caruso, M, Feller, L, Fransolet, M, Meurant, S, Mathieu, J, et al. A critical role for heme synthesis and succinate in the regulation of pluripotent states transitions. eLife. (2023) 12:12. doi: 10.7554/eLife.78546
44. Hu, Y-Z, Li, Q, Wang, P-F, Li, X-P, and Hu, Z-L. Multiple functions and regulatory network of miR-150 in B lymphocyte-related diseases. Front Oncol. (2023) 13:1140813. doi: 10.3389/fonc.2023.1140813
45. Wang, X, Kan, Y, Chen, L, Ge, P, Ding, T, Zhai, Q, et al. miR-150 is a negative independent prognostic biomarker for primary gastrointestinal diffuse large B-cell lymphoma. Oncol Lett. (2020) 19:3487–94. doi: 10.3892/ol.2020.11452
46. van Keimpema, M, Grüneberg, LJ, Mokry, M, van Boxtel, R, Koster, J, Coffer, PJ, et al. FOXP1 directly represses transcription of proapoptotic genes and cooperates with NF-κB to promote survival of human B cells. Blood. (2014) 124:3431–40. doi: 10.1182/blood-2014-01-553412
47. Voskaridou, E, Ntanasis-Stathopoulos, I, Christoulas, D, Dimopoulou, M, Komninaka, V, Repa, K, et al. Activin-A is elevated in patients with thalassemia major and double heterozygous sickle cell/beta-thalassemia and correlates with markers of hemolysis and bone mineral density. Ann Hematol. (2019) 98:1583–92. doi: 10.1007/s00277-019-03695-x
48. Jani, PK, Petkau, G, Kawano, Y, Klemm, U, Guerra, GM, Heinz, GA, et al. MicroRNA-221/222-expression in HSC and MPP safeguards their quiescence and multipotency by downregulating stress-independent and dependent expression of IEG and of several myelo/granulopoiesis-enhancing target genes. bioRxiv. (2023):2023.01.30.526397. doi: 10.1101/2023.10.01.560397
49. Di Martino, MT, Arbitrio, M, Caracciolo, D, Cordua, A, Cuomo, O, Grillone, K, et al. miR-221/222 as biomarkers and targets for therapeutic intervention on cancer and other diseases: a systematic review. Mol Ther Nucleic Acids. (2022) 27:1191–224. doi: 10.1016/j.omtn.2022.02.005
50. Nassiri, SM, Ahmadi Afshar, N, and Almasi, P. Insight into microRNAs’ involvement in hematopoiesis: current standing point of findings. Stem Cell Res Ther. (2023) 14:282. doi: 10.1186/s13287-023-03504-3
Keywords: miRNA, microRNA, anemia, erythropoiesis, protein, iron deficiency anemia, erythrocyte
Citation: Wagiu Basrowi R, Sundjaya T, Pratiwi D, Amalia N, Tandi YYP, Syafa’atulloh MY, Utomo GNP, Albarok MAR and Nurkolis F (2025) Harnessing the power of proteins in modulation of miRNAs for targeting Iron deficiency Anemia: Opinion for future implications and strategies. Front. Nutr. 12:1535498. doi: 10.3389/fnut.2025.1535498
Edited by:
Gomathi Ramaswamy, All India Institute of Medical Sciences, Bibinagar, IndiaReviewed by:
Jose Manuel Miranda, University of Santiago de Compostela, SpainAparna Varma Bhongir, All India Institute of Medical Sciences, Bibinagar, India
Copyright © 2025 Wagiu Basrowi, Sundjaya, Pratiwi, Amalia, Tandi, Syafa’atulloh, Utomo, Albarok and Nurkolis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fahrul Nurkolis, ZmFocnVsLm51cmtvbGlzLm1haWxAZ21haWwuY29t; ZmFocnVsLm51cmtvbGlzLTIwMjRAZmsudW5haXIuYWMuaWQ=
 Ray Wagiu Basrowi
Ray Wagiu Basrowi Tonny Sundjaya3,4
Tonny Sundjaya3,4 Nurlinah Amalia
Nurlinah Amalia Yosi Yohanes Putra Tandi
Yosi Yohanes Putra Tandi Muhammad Abdir Rahman Albarok
Muhammad Abdir Rahman Albarok Fahrul Nurkolis
Fahrul Nurkolis