
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 13 February 2025
Sec. Nutritional Immunology
Volume 12 - 2025 | https://doi.org/10.3389/fnut.2025.1519140
This article is part of the Research TopicNew Progress of Nutritional Immunity on Aquatic Animals by Functional Feed Additives under the Condition of Low Fish MealView all 13 articles
 Wonsuk Choi1†‡
Wonsuk Choi1†‡ Mohammad Moniruzzaman2†§
Mohammad Moniruzzaman2†§ Seunghan Lee3§
Seunghan Lee3§ Jinho Bae4
Jinho Bae4 Sungchul C. Bai1,5§
Sungchul C. Bai1,5§ Taesun Min6*§
Taesun Min6*§ Seunghyung Lee1,7*
Seunghyung Lee1,7*A basal diet without feed additives was used as a control (CON) and three diets were formulated by supplementing with Bacillus subtilis WB60 at 1 × 108 CFU/g (Pro-A), B. subtilis SJ10 at 1 × 108 CFU/g (Pro-B), Enterococcus faecium SH30 at 1 × 107 CFU/g (Pro-C), and two other diets supplementing with antibiotics such as amoxicillin (AMO) at 4 g/kg and oxytetracycline (OTC) at 4 g/kg of the basal diet. A total of 450 fish averaging 12.1 ± 0.09 g (mean ± SD) were fed one of the six experimental diet groups in triplicates for 8 weeks. In disease resistance test, 45 fish from each group were intraperitoneally injected with the pathogenic bacteria, Edwardsiella tarda, and mortality was recorded for 15 days. At the end of 8-week feeding trial, weight gain, specific growth rate and feed efficiency of fish fed the Pro-A diet were significantly greater than those of fish fed the CON, OTC and AMO diets (p < 0.05). Furthermore, feeding efficiency and protein efficiency ratio of fish fed the Pro-A diet were significantly greater than those of fish fed the CON, OTC and AMO diets. Serum aspartate aminotransferase levels were significantly greater in fish fed the Pro-B diet than in those fed the Pro-A diet. The lysozyme activity of fish fed the Pro-A, Pro-B and Pro-C diets was significantly greater than that of the CON, OTC and AMO diets. The myeloperoxidase activity of fish fed the Pro-A diet was significantly greater than that of the fish fed the CON and AMO diets. The flounder growth hormone levels of fish fed the Pro-A, Pro-B, Pro-C and AMO diets were significantly greater than that of the fish fed the CON diet. The interleukin 1β gene expression levels in fish fed the Pro-B and Pro-C diets were significantly greater than those in fish fed the CON, OTC and AMO diets. The interleukin 10 gene expression levels in fish fed the Pro-A, Pro-B, Pro-C and OTC diets were significantly greater than those of fish fed the CON and AMO diets. Intestinal histology revealed that the average villi length of fish fed the Pro-A, Pro-B, and Pro-C diets were significantly greater than that of fish fed the CON, OTC and AMO diets. The cumulative survival rates of fish fed the Pro-A, Pro-B and Pro-C diets were significantly greater than those of fish fed the CON diet after the 15th day of the challenge test. Overall, the results demonstrated that the supplementation of fish-derived bacteria, B. subtilis (1 × 108 CFU/g diet) or E. faecium (1 × 107 CFU/g diet) in the diet could be the ideal probiotics to replace antibiotics in olive flounder fed FM reduced diet.
Olive flounder (Paralichthys olivaceus Temminck and Schlegel, 1846) is a flat fish native to the temperate coastal waters of East Asia that represents both an important capture as well as aquaculture industry. Olive flounder is most popular in Northeast Asian nations such as Korea, China, and Japan (1, 2). In South Korea, it is the most cultured species at more than 46,000 MT and accounts for more than 50% of the country’s overall production, which was 91,000 MT in 2022 (3). As olive flounder culture exceeds the capture fishery, there is an ever increasing pressure to reduce its reliance on fish meal (FM). FM accounts for up to 50% of the cost of olive flounder aqua feed. Although FM is an ideal source of essential amino acids (EAAs) and fatty acids (FAs), it comes at the expense of increased production for producers and great pressure on wild fisheries. Since the world’s production of FM is in decline and will unlikely increase in the future (4), the sustainability of the aquaculture industry is dependent on reducing reliance on this commodity moving forward. In olive flounder aquaculture, pathogenic bacteria Edwardsiellatarda infection (Edwardsiellosis) have become a major problem which cause severe economic losses. For this, farmers are mostly depend on the uses of antibiotics or vaccines for disease outbreak.
Fish meal replacement has been a major objective for aquaculture nutritionists for several decades. There are many candidates for FM replacement, such as meat and bone meal (5), poultry by-product meal (6), blood meal (7) and plant-based protein sources such as soybean meal (8). Proteins obtained from animal sources have great potential with some of the most popular sources being poultry byproduct meals, blood meals, and meat and bone meal (9). However, fish fed diets with low FM often suffer from issues related to growth, digestibility, palatability, and disease resistance. To overcome these problems, fish farmers have started to rely on various feed additives and antibiotics. The excessive application of the antibiotics is becoming a major health and environmental hazard since antibiotic-resistant strains are becoming increasingly prevalent (10, 11). Thus, the development of safe and sustainable alternatives to these pharmaceuticals has become an ever expanding field of research.
One of the most promising tools for reducing antibiotic reliance in the aquaculture industry is probiotics. Probiotics are microbes that help to correct imbalances in the microbiota of the intestines and confer benefits to the host organism’s health status when consumed in sufficient amounts (12). Some of the specific benefits of probiotics include their ability to retard and outcompete the growth of pathogens (13), aid in digestion by contributing to enzymatic activities (14), antiviral properties, enhancing immune responses (15, 16), reproductive performance (17), gut health and disease resistance (18) in fish. Recently, there have been tremendous interests in dietary intervention of host-associated or fish-derived probiotics research within the aquaculture industries (19–26). This kind of probiotic bacteria are generally isolated from fish intestine which would be more suitable in terms of enhanced growth and immune system in target animals than the so called probiotics using in aquaculture (13–18). In previous studies reported the positive single effect of the probiotics Bacillus subtilis WB60, Bacillus subtilis SJ10 and Enterococcus faecium in olive flounder. However, very few studies reported the efficacy of fish-derived multiple strains of probiotic bacteria in aquaculture. Therefore, in this study, we aimed to evaluate the comparison of dietary supplementation of the three fish-derivedprobiotics, Bacillus subtilis WB60, Bacillus subtilis SJ10 and Enterococcus faecium as well as two most widely used antibiotics in flounder aquaculture such as oxytetracycline and amoxicillin (27) based on the growth, immunity and disease resistance against E. tarda infection in juvenile olive flounder.
The candidate probiotics, B. subtilis WB60 was isolated from the intestines of healthy Japanese eel (Anguilla japonica) and was identified by cluster analysis via 16S rDNA sequencing. The B. subtilis WB60 was isolated according to Lee et al. (28) and incubated at 30°C for 72 h in Luria-Bertani broth (LB broth; Sigma-Aldrich, St. Louis, USA), after which the optical density (OD600) was measured at 600 nm using spectrophotometry. The B. subtilis SJ10 was isolated from jeotgal, a traditional Korean fermented dish made from salt-preserved seafood such as squid, pollock roe, and shrimp, according to Hasan et al. (29) and it was incubated from a single colony on lysogeny broth (LB, USB Corporation, USA) agar, and was subsequently cultured in 10 mL of LB broth for 16 h at 37°C in a shaking incubator. Furthermore, E. faecium SH30 was isolated from the intestine of healthy Nile tilapia (Oreochromis niloticus), and the bacteria were grown in MRS (deMan, Rogosa, and Sharpe) broth at 36°C for 48 h according to Wang et al. (30). All probiotics were washed in sterile saline and the concentration of the final suspension was calculated to be 1 × 108 CFU/g for WB60 and SJ10, and 1 × 107 CFU/g for SH30 in the diets (28–30).
Juvenile olive flounder were obtained from a private farm (JUNGANG Fisheries, Chungcheongnam-do, Taean-gun, Republic of Korea). Prior to the start of the feeding trial, the apparent health status of the fish was checked visually, and the fish were starved for 24 h. All the fish were then fed a commercial diet for two weeks prior to the start of the feeding trial to acclimatize to the laboratory conditions. On average, 12.1 ± 0.09 g (mean ± SD) of fish were weighed, divided into triplicate groups of 25 fish corresponding to the dietary treatment, and randomly distributed into 18 indoor fiberglass tanks (40 L each) receiving a constant flow (1.2 L/min) of filtered seawater. During the experiment, supplemental aeration was provided in each tank to maintain adequate dissolved oxygen. The temperature was maintained at 19.0 ± 1.0°C throughout the experiment by electric heaters in a concrete reservoir. Fish were fed twice a day (09:00 and 19:00) for 8 weeks at a rate of 2.5 ~ 5% body weight per day. Dead fish were immediately removed and weighed, after which the amount of feed provided to the remaining fish was adjusted. The uneaten feed was siphoned 1 h after feeding. The inside of the tanks was scrubbed once per week to minimize algal and fungal growth.
The basal or control diet (CON) formulation is shown in Table 1. Anchovy fish meal (68.75% CP, crude protein) and soybean meal (47.04% CP) were used as the main protein sources, while fish oil was used as the main lipid source. The feed additives (probiotics) used in this experiment were Pro-A: B. subtills WB60 (1 × 108 CFU/g) based on Lee et al. (28), Pro-B: B. subtills SJ10 (1 × 108 CFU/g) based on Hasan et al. (29), Pro-C: E. faecium SH30 (1 × 107 CFU/g) based on Wang et al. (30), as well as OTC: oxytetracycline, 4 g/kg diet and AMO: amoxicillin, 4 g/kg diet which were based on Won et al. (31). The procedures for feed manufacturing and preparation were performed as previously described by Lee et al. (28). According to the feed formulation table, all fine powdered ingredients were mixed thoroughly with an electric mixer (HYVM-1214, Hanyoung Food Machinery, Republic of Korea). Then, a stiff dough was formed by adding fish oil and the desired amount of water (~10%). The dough was passed through a pellet machine (SFD-GT, Shinsung, Republic of Korea) with a 0.2 cm die. The prepared diets were air-dried in a drying room for 48 h, broken into smaller pieces and stored at -20°C. According to the proximate composition analysis, shown in Table 2, all the diets were iso-nitrogenous and iso-lipidic.
At the end of the feeding trial, fish were starved for 24 h prior to sample and data collection. The fish were subsequently counted and weighed to calculate the final weight (FW), weight gain (WG), specific growth rate (SGR), feed efficiency (FE) protein efficiency ratio (PER) and survival (SR). Four fish from each tank were selected at random, weighed individually, and dissected to obtain liver and visceral metrics for calculation of hepatosomatic index (HSI) and visceral somatic index (VSI); thereafter, the same intestinal samples were used for histological observation and enzyme activity. Three additional fish per tank were captured at random and anesthetized with ethylene glycol phenyl ether (200 mg/L for 5–10 min). After this, blood was drawn from the caudal vein, which was subsequently centrifuged at 5000 × g for 10 min to obtain the serum. Serum samples were then stored at −70°C for the analysis of non-specific immune responses, such as lysozyme, and myeloperoxidase (MPO) activities, in addition to biochemical parameters, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), glucose and total protein (TP) levels. The serum levels of AST, ALT, glucose, and total protein were determined by a chemical analyzer (Fuji DRI-CHEM 3500i, Fuji Photo Film Ltd., Tokyo, Japan) following the manufacturer’s instructions.
Three additional fish from each tank were collected for whole-body proximate composition analysis. Proximate composition analyses of both whole fish and experimental diets were performed by the standard methods of AOAC (32). Whole fish and diet samples were dried at 105°C to a constant weight to determine their moisture content. The ash content was determined by incinerating the samples at 550°C. The protein concentration was determined by using the Kjeldahl method (N × 6.25) after acid digestion. Crude lipids were measured by Soxhlet extraction using Soxhlet system 1,046 (Tacator AB, Hoganas, Sweden) after the samples were freeze-dried for 20 h.
Olive flounder serum lysozyme activity was analyzed as follows: 0.1 mL of test serum was added to 2 mL of a suspension of Micrococcus lysodeikticus (0.2 mg/mL) in 0.05 M sodium phosphate buffer (pH 6.2). The reactions were carried out at 20°C, and the absorbance was measured at 530 nm. Measurements were taken between 0.5 min and 4.5 min on a spectrophotometer. One lysozyme activity unit was defined as the amount of enzyme that produced a decrease in absorbance corresponding to 0.001/min. Myeloperoxidase activity was measured according to the method described by Quade and Roth (33). Briefly, 20 μL of serum was diluted with Hank’s balanced salt solution (HBSS) without Ca2+ or Mg2+ (Sigma- Aldrich) in 96-well plates. Then, 35 μL of 3, 3′, 5, 5′ tetramethylbenzidine hydrochloride (TMB, 20 mM) (Sigma-Aldrich) and H2O2 (5 mM) were added. The color change reaction was stopped after 2 min by adding 35 μL of 4 M sulfuric acid. Finally, the optical density was read at 450 nm in a microplate reader.
Tissue fragments from intestine of fish were obtained and immediately stored at −80°C in TRIzol reagent (Thermo Fisher Scientific) for RNA extraction. Total RNA was extracted from 0.5 g of olive flounder tissue using TRIzol Reagent (Thermo Fisher Scientific, San Jose, CA, USA). Afterwards, the RNA was quantified and the purity was assessed spectrophotometrically. The RNA was then treated with DNase I (Cosmogenetech, Seoul, Republic of Korea) to remove genomic DNA contamination. Complementary DNA (cDNA) was synthesized using M-MuLV reverse transcriptase (Cosmogenetech). The expressions of three selected immune-related genes were analyzed by real-time quantitative polymerase chain reaction (RT-qPCR), which was performed with a Bio-Rad CFX96 (Bio-Rad, Hercules, CA, USA) using SYBR Green PCR Core Reagents (Cosmogenetech). The relative expression levels of the target gene transcripts such as flounder growth hormone (FGH), interleukin-1beta (IL-1β) and interleukin-10 (IL-10) were measured with β-actin as an internal control were using CFX Manager software version 2.0 (Bio-Rad) (Table 3). In all the cases, each PCR was performed with triplicate samples.
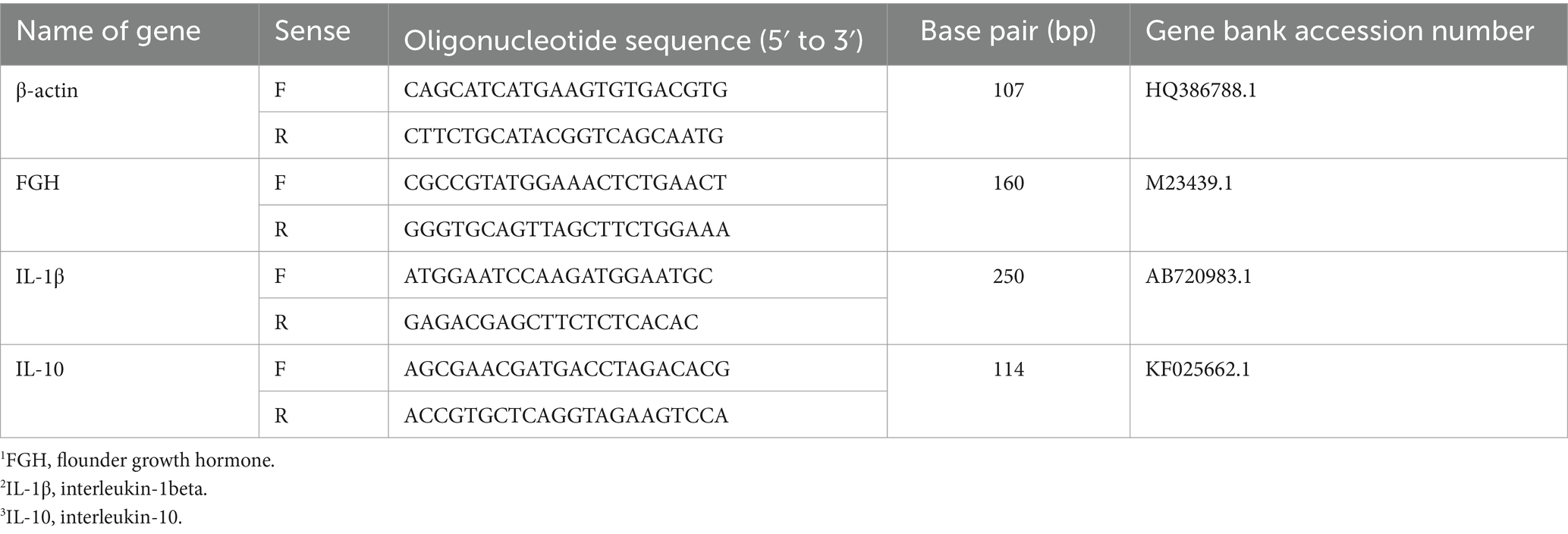
Table 3. Gene specific primers, amplicon lengths and gene bank accession numbers of immune and growth-related genes used in this study.
The anterior intestinal tissues from the fish (n = 3) were dissected and fixed in 10% neutral buffered formalin, dehydrated in a graded ethanol series and embedded in paraffin. The tissue blocks were sectioned by a microtome machine (HistoCore AUTOCUT, Leica Biosystems, Germany) each of 4 μm thick and stained with hematoxylin and eosin (H&E). At least 6 tissue sections from each sample were examined for intestinal villi length (small fingerlike projections protruded from the intestinal wall) under a light microscope (AX70 Olympus, Tokyo, Japan) equipped with a digital camera (DIXI Optics, Daejeon, Republic of Korea), and an image analysis software (Image J 1.32j, National Institute of Health, USA). Data are presented as means ± SE.
After sampling, seven fish from each tank were redistributed into 18 tanks in a non-recirculating system without water renewal to perform the 15 days of challenge test. The pathogenic bacterium, Edwardsiella tarda (E. tarda) FSW910410 was obtained from the Department of Biotechnology, Pukyong National University, Busan, Rep. Korea. The bacteria were originally sourced from diseased olive flounder and cultured on tryptic soy agar (TSA, Sigma) plates (24 h at 27°C). All the fish were subjected to intraperitoneal injection of 50 μL of active E. tarda (3 × 108 CFU/mL) solution. The water temperature was maintained at 19 ± 1.0°C (mean ± SD) during 15 days of challenge test and fish mortalities were recorded daily from each tank. Dead fish were necropsied and kidney samples were taken and streaked on Salmonella-Shigella agar (SS agar, Difco). The presence of black pigments confirmed E. tarda infection.
The results are presented as means ± SE (number of replicates as indicated). All the data were analyzed by one-way ANOVA (Statistix 3.1; Analytical Software, St. Paul, MN, USA) to test the effects of the dietary treatments. Prior to the one-way ANOVA, normality and homogeneity of variances were checked. When a significant treatment effect was observed, an LSD post hoc test was used to compare the means. Treatment effects were considered significant at the p < 0.05 level.
Table 4 shows the growth performance and feed utilization of juvenile olive flounder fed different experimental diets for 8 weeks. At the end of the feeding trial, WG and SGR of fish fed the Pro-A diet were significantly higher greater those of fish fed the CON diet (p > 0.05). The FE of fish fed the Pro-B diet was significantly greater than that of fish fed the CON, OTC, or AMO diets (p > 0.05). The PER of fish fed the Pro-A diet was significantly greater than that of fish fed the CON and AMO diets (p > 0.05). Moreover, probiotics supplemented diets were not significantly different from fish fed the Pro-A, Pro-B and Pro-C diets (p > 0.05) in terms of growth performance. Furthermore, there were no significant differences (p > 0.05) in terms of SR, CF, HSI or VSI among fish fed the experimental diets.
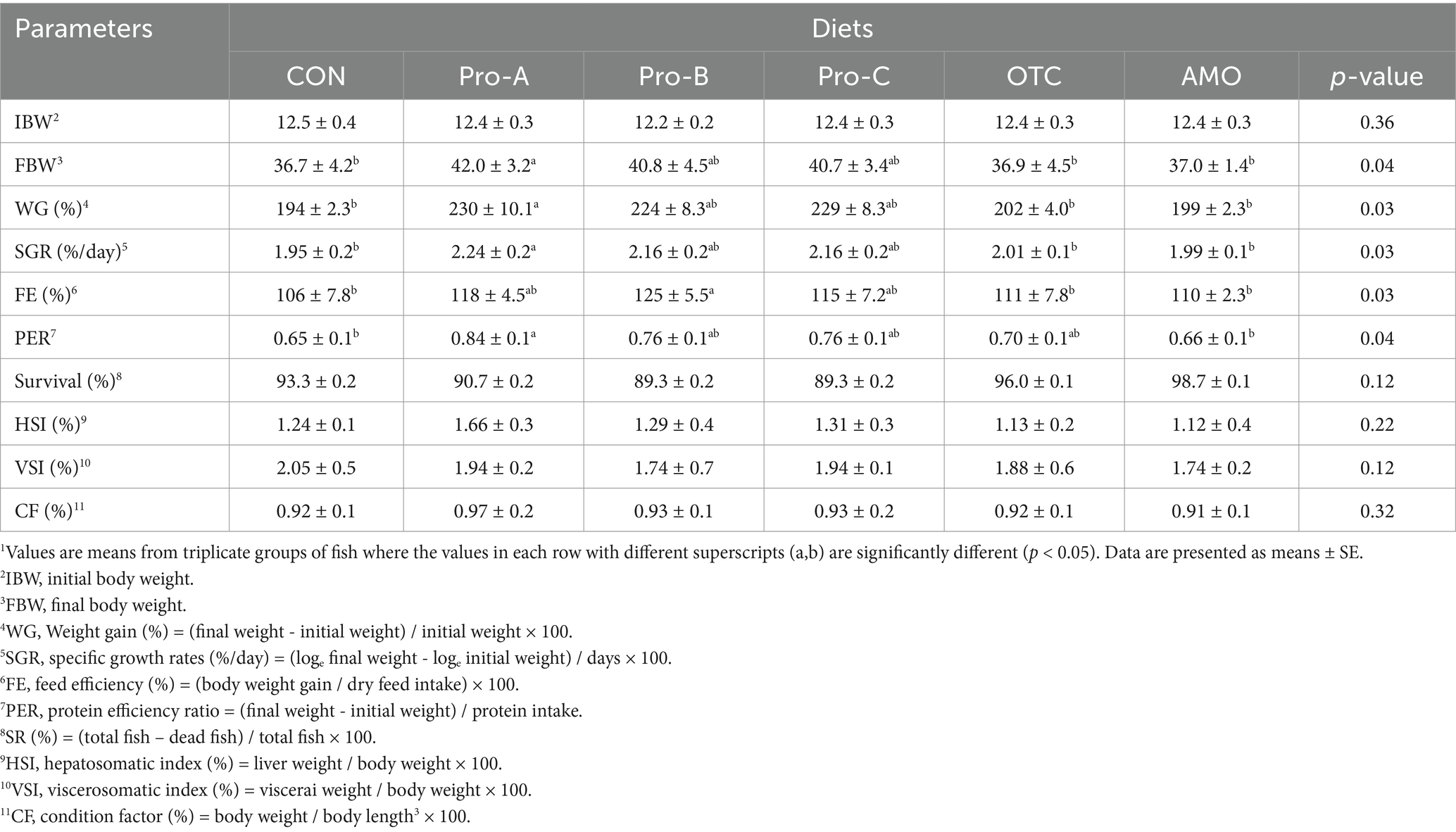
Table 4. Growth performance and feed utilization of olive flounder fed the six experimental diets for 8 weeks.1
There were no significant differences (p > 0.05) in terms of crude protein, lipid, moisture or ash content among any of the group of fish fed the experimental diets for 8 weeks (Table 5).
The blood parameters of the juvenile olive flounder fed the experimental diets are shown in Figure 1. The serum ALT level in the fish fed the Pro-A diet was significantly lower than those of fish fed the Pro-A, Pro-C and AMO diets (p < 0.05). There were no significant differences in AST, glucose, or total protein content among any of the group of fish fed the experimental diets for 8 weeks (p > 0.05).
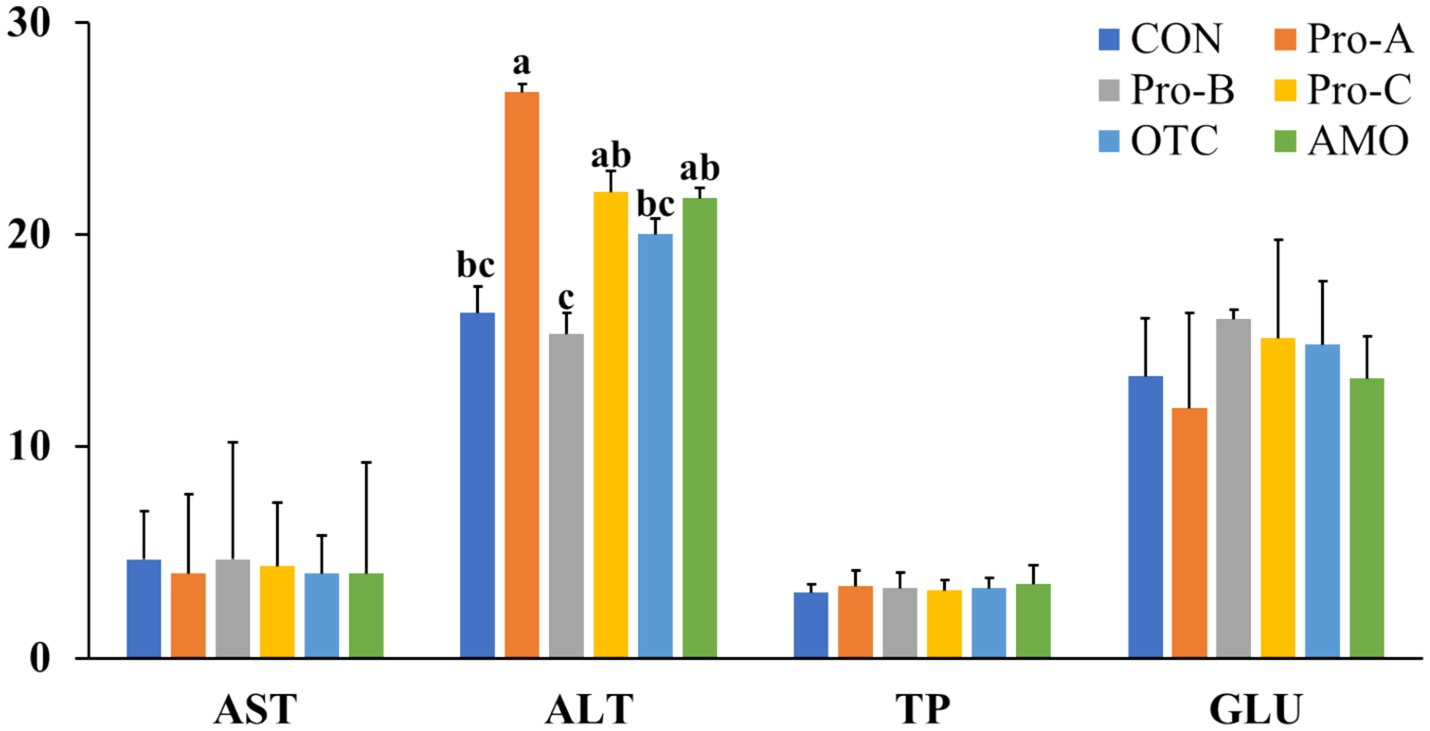
Figure 1. Hematological parameters of olive flounder fed the six experimental diets. Values are means from triplicate groups of fish (n = 3) where the values in each row with different superscripts (a,b,c) are significantly different (p < 0.05). AST: Aspartate aminotransferase (U/l), ALT, alanine aminotransferase (U/l), GLU, glucose (mg/dl), TP: Total protein (g/dl). Data are presented as means ± SE.
Figure 2 shows the non-specific immune responses of juvenile olive flounder fed different experimental diets for 8 weeks. The serum lysozyme activities of fish fed the Pro-A, Pro-B and Pro-C diets were significantly greater than that of fish fed CON,OTC and AMO diets (p > 0.05). Myeloperoxidase activity of fish fed Pro-A diet was significantly higher than those from the CON and AMO diets (p > 0.05). However, there were no significant differences in MPO activities among fish fed the CON, Pro-B, Pro-C, OTC and AMO diets.
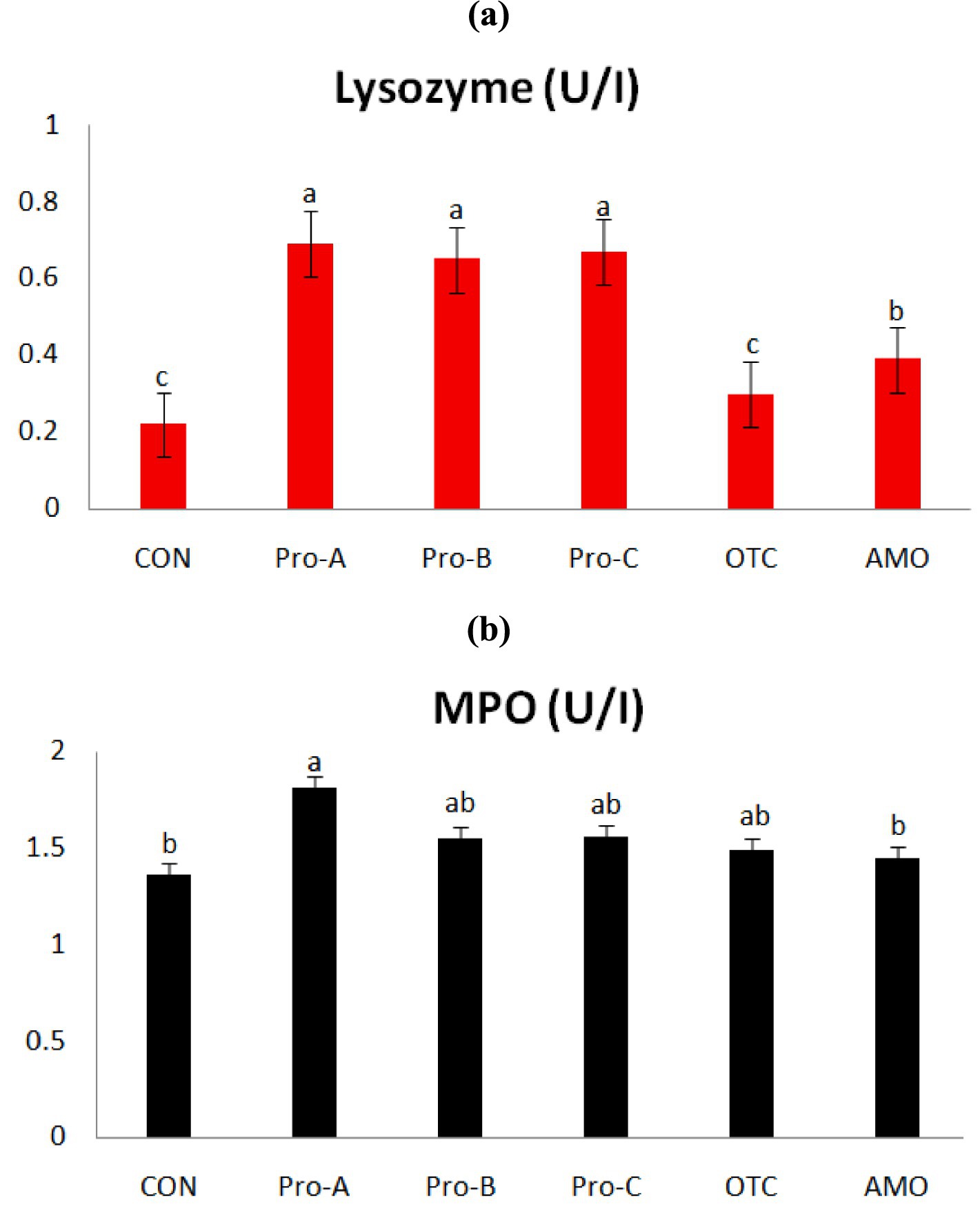
Figure 2. Non-specific immune responses of olive flounder fed the six experimental diets. Values are means from triplicate groups of fish (n = 3) where the values in each row with different superscripts (a,b,c) are significantly different (p < 0.05); (A) Lysozyme (U/ml): lysozyme activity and (B) MPO (absorbance): myeloperoxidase (OD at 450 nm). Data are presented as means ± SE.
The gene expression profiles of the immunological parameters in the intestine of olive flounder fed diets supplemented with probiotics are presented in Figure 3. The mRNA expression levels of the FGH in fish fed the Pro-A and Pro-B diets were significantly greater than those in fish fed the CON, Pro-C, OTC and AMO diets (P < 0.05). However, there were no significant differences in FGH concentrations in fish fed the CON and OTC diets (p > 0.05). The IL-1β expressions in fish fed the Pro-A, Pro-B and Pro-C diets were significantly greater than those in fish fed the CON and AMO diets (P < 0.05). The IL-10 expressions in fish fed the Pro-A and Pro-C diets were significantly greater than those in fish fed the CON, OTC and AMO diets (P < 0.05). However, there were no significant differences in IL-10 mRNA expressions in fish fed the CON and AMO diets (p > 0.05).

Figure 3. Relative mRNA gene expression levels of (A) flounder growth hormone (FGH), (B) interleukin-1 beta (IL-1β) and (C) interleukin-10 (IL-10) of intestine from olive flounder fed the experimental diets for 8 weeks. Data are presented as means ± SE.
Histological analysis of the anterior intestine of olive flounder fed different experimental diets for 8 weeks is shown in Figure 4. The intestinal villi length in fish fed the Pro-A, Pro-B and Pro-C diets had significantly greater villi lengths than the fish fed the CON, OTC and AMO diets (Figure 4A). The fish fed the Pro-A, Pro-B, Pro-C, OTC and AMO diets clearly exhibited better intestinal histomorphology with more massive villi in comparison to the CON diet (Figure 4B). In addition, the images which corresponds to the CON group, shows certain irregular shape and improper arrangement of villi in fish fed the CON, OTC and AMO diets compared to those of the Pro-A, Pro-B and Pro-C groups.
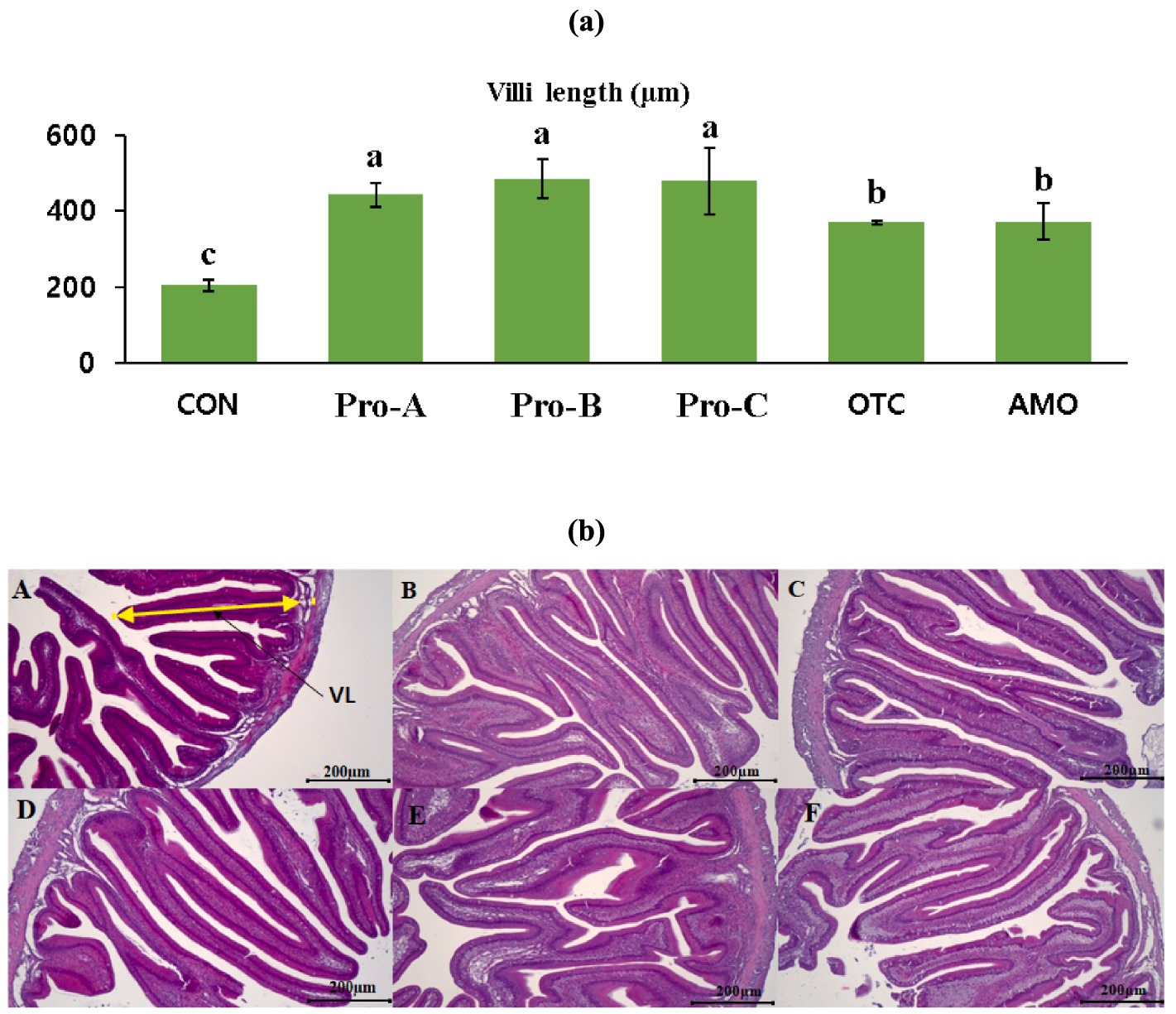
Figure 4. Intestinal histology of juvenile olive flounder fed the experimental diets for 8 weeks; (a) villi lengths in fish fed the different diets; (b) histological photomicrographs of (A) CON (B) Pro-A (C) Pro-B (D) Pro-C (E) OTC (F) AMO diet groups; (scale bar = 200 μm; original magnification×40).
The percent cumulative survival of juvenile olive flounder challenged with E. tarda for 15 days is shown in Figure 5. During the challenge test, the first mortalities occurred on the second day. At the end of the 15 day challenge, the percent cumulative survivals of fish fed the Pro-A, Pro-B, and Pro-C diets were significantly greater than that of fish fed the CON diet (p < 0.05). However, there were no significant differences in cumulative survival rates in fish fed the Pro-A, Pro-B, Pro-C, OTC, and AMO diets (p > 0.05).
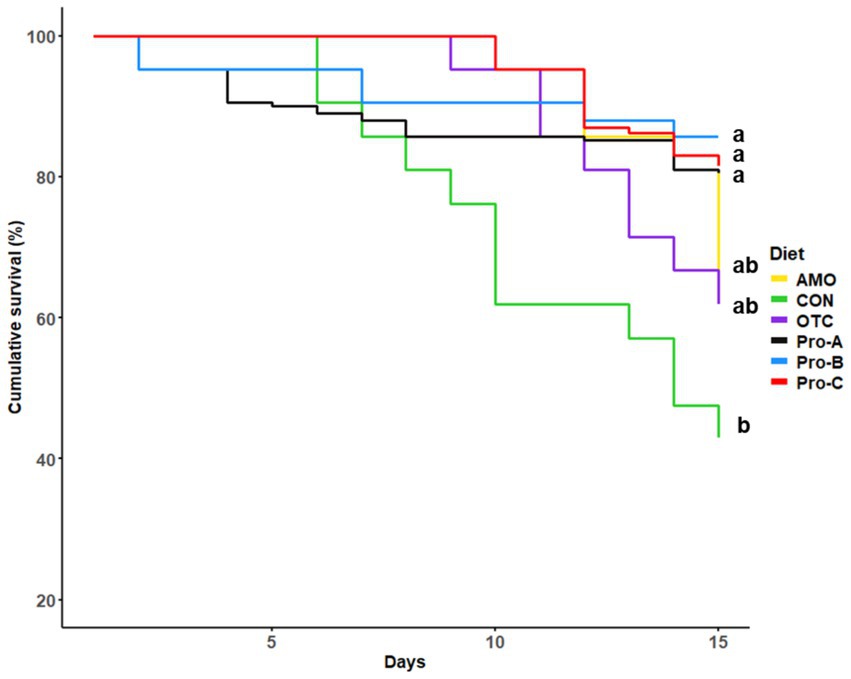
Figure 5. Cumulative survival (%) of olive flounder during 15 days challenge test. For the survival analysis, we used R 4.4.1 (R Development Core Team, 2023) with the survival and survminer packages to perform Kaplan–Meier survival analysis. This approach allowed us to estimate survival curves and compare survival rates among groups using the log-rank test. Specifically, the Kaplan–Meier method (via the survfit function) was employed to analyze survival data across different dietary treatments.
Research into the use of probiotics in aquaculture nutrition has attracted much interest due to their health benefits and because they are considered environmentally friendly (34). In recent years, probiotic effects have been studied in different fish species (35). Additionally, many trials have investigated the extraction of probiotic strains from the intestines of various fish, which are subsequently added to aquafeeds. This strategy of sourcing and using probiotics from these species has improved growth performance, feed efficiency and immune response (36, 37). Therefore, to build on this growing body of knowledge, the present study utilized three different probiotics according to the results of previous experiments.
The two probiotics used were Bacillus subtilis extracted from the intestines of Japanese eel and jeotgal (28, 29), because most other probiotic studies have focused on the use of Bacillus spp. (34–41). The other probiotics used was E. faecium isolated from the intestine of healthy Nile tilapia (18). In this study, we used dietary live or active probiotics during feeding of fish to ensure direct interaction with fish physiology in terms of gut microbiome and mucosal immunity. Moreover, a previous experiment in which Pirarucu, Arapaima gigas was fed diets containing live probiotics, E. faecium 1 × 108 CFU/g showed increased weight gain compared to that in the control treatment group (42). The results of the present study showed that all the experimental diets containing the probiotics were equally effective and resulted in increased weight gain and feed efficiency compared with the control diet. These results are likely due to the increased secretion of proteolytic enzymes, which increase feed efficiency, similar to the findings of probiotic experiments in olive flounder (35). Likewise, beneficial effects of probiotics have also been reported on growth performance in terms of improving weight gain, specific growth rate, and feed efficiency in tilapia (18, 30, 43, 44), whiteleg shrimp (31), Japanese eel (28, 36), starry flounder (39), red sea bream (26), rainbow trout (16, 37, 40), Pirarucu (42) and olive flounder (29, 35). In agreement of the present study, Won et al. (31, 41) reported that B. subtilis WB60 at 1 × 108 CFU/g can enhance growth and feed utilization in whiteleg shrimp and Nile tilapia. Furthermore, probiotic bacteria, E. faecium at 1 × 107 CFU/mL in water showed significantly better final weight and daily weight gain in tilapia which endorsed the results of the present study (30).
Modulation of the immune system is one of the most common benefits of probiotics (44). Lysozyme activity is frequently used as an indicator of non-specific immune functions and is the principle means of responding infections in fish (28). This enzyme not only has bacteriolytic activity against gram-positive and gram-negative bacteria (45), but also has anti-inflammatory and antiviral properties. The MPO is another important enzyme that utilizes oxidative radicals to produce hypochlorous acid, which kills pathogens (29). In the present study, the immune parameters, lysozyme including MPO activities were measured and the beneficial effects of both probiotic bacteria on nonspecific immune related enzyme responses, were clearly shown to be greatest for the olive flounder fed B. subtilis, at the 1 × 108 CFU/g (Pro-A and Pro-B) and E. faecium, at the 1 × 107 CFU/g (Pro-C). In agreement of the present study, previous research findings reported that dietary supplementation of BSWB60, BSSJ10 and EFSH30 in the diets can improve the lysozyme and MPO activities in olive flounder (28–30).
Growth hormone is a hormone that stimulates the secretion of IGF-1 in the liver, increases the concentration of glucose and vitreous acid (46), produces IGF-1 induced protein synthesis (47), and is reported to be an indicator of growth factors in fish such as promoting cell division (48). In the present study, olive flounder fed probiotics supplemented diets exhibited significantly greater FGH expression than did those fed the control diet (Figure 3A). Similarly, previous studies in which probiotics were added showed high FGH value (49, 50). These studies have reported that probiotic supplementation can affect growth hormone-related gene expression in fish (28, 29). In consistent of the present study, Jang et al. (26) found that dietary supplementation of Bacillus sp. as a host-associated probiotic in red sea bream can enhance the growth hormone in fish which is also reflected in the growth performance of fish.
The IL-1β is one of the earliest expressed pro-inflammatory cytokines and enables organisms to respond promptly to infection by inducing a cascade of reactions leading to inflammation (51). Many of the effector roles of IL-1β are mediated through the up- or down-regulation of the expressions of other cytokines and chemokines (52). The IL-1β was the first interleukin to be characterized in fish and has since been identified in a number of fish species, such as rainbow trout (53), carp (54), seabass (55), gilt head seabream (56), haddock (57), tilapia (58). The IL-10 on the other hand, is an anti-inflammatory cytokine that down-regulates the expression of pro-inflammatory cytokines (59). Additionally, IL-10 was initially discovered to be an inhibitory factor for the production of Th1 cytokines. Subsequently, pleiotropic inhibitory and stimulatory effects of IL-10 on various types of blood cells were described, including its role as a survival and differentiation factor for B cells. The IL-10, which is produced by activated monocytes, T cells and other cell types, such as keratinocytes, appears to be a crucial factor for at least some forms of peripheral tolerance and a major suppressor of the immune response and inflammation. The inhibitory function of IL-10 is mediated by the induction of regulatory T cells (60). In the present study, the activities of IL-1β and IL-10 in the intestine of the fish that were administered probiotics were significantly greater than those in the control with low FM diet. Therefore, dietary probiotics appears to increase the immune function of fish even though fish were offered with low FM diets. The results suggest that dietary supplementation of probiotics might be attenuated the inflammation in fish through up-regulating the cytokine gene (IL-1β and IL-10) expressions in fish. In agreement of the present study, Lara-Flores et al. (43) and Back et al. (50) reported that probiotics supplemented in low protein or low FM diets can enhance the immunity in tilapia and olive flounder, respectively without compromising the health status of fishes.
Intestinal morphological parameters (villus length and muscular layer thickness) are indicative of a healthy gut in fish. The intestine is very important for the digestion and absorption of nutrients. The length of the intestinal villi determines the absorption of nutrients in the GI tract (gastrointestinal tract) (61, 62). Thus, digestive function is associated with intestinal development (62). In this study, the beneficial effects of probiotics on intestinal morphology were clearly observed. The length of the villi increased in a dose dependent manner and villi length was significantly highest for the olive flounder fed the Pro-A, Pro-B, and Pro-C diets (Figure 4A). In the same manner, Lee et al. (28) reported that probiotics are capable of increasing the villus length in the proximal intestine of Japanese eel. Furthermore, Won et al. (31, 41) postulated that dietary Bacillus subtilis WB60 can increase the villus length and muscular thickness in the intestine of whiteleg shrimp and Nile tilapia which supported the results of the present study.
With regard to disease resistance, olive flounder fed with probiotics, Pro-A and Pro-B at 1 × 108 CFU/g as well as Pro-C at 1 × 107 CFU/g supplemented in the diet exhibited the highest disease resistance compared to the control group. However, there were no significant differences in cumulative survival rates in fish fed the Pro-A, Pro-B, Pro-C, OTC and AMO diets suggesting that the three fish-derived probiotics were equally effective to the antibiotics. In this study, the mode of probiotic action is attributed to follow the host-specific (63) and strain-specific (64) properties. The major factors that affecting the disease resistance in fish perhaps the origin, source, viability and dose of the probiotics and their duration of administration (65–68). Likewise, in recent studies, it is reported that dietary supplementation of host-associated probiotic bacteria B. subtilis and E. faecium could enhance the disease or stress resistance in Japanese eel, shrimp, hybrid yellow catfish, Chinese perch, hybrid grouper, olive flounder, rainbow trout, red sea bream and tilapia (20–31, 41). In consistent with the present study, it has been reported that the higher levels of lysozyme improved disease resistance in infected fish such as Atlantic salmon challenged with Aeromonas salmonicida (69) and sheatfish challenged with Edwardsiella tarda (70).
In conclusion, the present study revealed the potential benefits of supplementation with the bacteria species, B. subtilis and E. faecium as probiotics in the diet of olive flounder. Therefore, B. subtilis at 1 × 108 CFU/g and E. faecium at 1 × 107 CFU/g could be ideal probiotics for improving growth performance, immune responses, enzyme activity and disease resistance, while replacing the dietary supplementation of antibiotics in juvenile olive flounder fed a reduced FM diet. The utilization of these probiotics could help to further enhance the olive flounder production in the farm level and to replace indiscriminate use of antibiotics without compromising health status in fish as well as to reduce environmental pollution in terms of antimicrobial resistance with higher consumer acceptance. However, further research is warranted to evaluate the effects of the current probiotics on the diversity of intestinal microbiota and immunity on gut-brain axis in fish.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/genbank/, HQ386788.1, https://www.ncbi.nlm.nih.gov/genbank/, M23439.1, https://www.ncbi.nlm.nih.gov/genbank/, AB720983.1, https://www.ncbi.nlm.nih.gov/genbank/, KF025662.1.
The animal study was approved by the Institutional Animal Care and Use Committee, Pukyong National University, Busan, Republic of Korea. The study was conducted in accordance with the local legislation and institutional requirements.
WC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. MM: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. SAL: Formal Analysis, Software, Writing – review & editing. JB: Formal Analysis, Software, Writing – review & editing. SB: Conceptualization, Methodology, Project administration, Writing – review & editing. TM: Funding acquisition, Resources, Supervision, Validation, Visualization, Writing – review & editing. SYL: Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Pukyong National University Research Fund in 2021 (CD20210975). This work was also supported by the Basic Science Research Program (Grant No. 2019R1A6A1A11052070) funded by the Ministry of Education and the Basic Science Research Program (Grant No. 2022R1A2B5B02001711) funded by the Ministry of Science and ICT through the National Research Foundation of Korea (NRF) to Taesun Min. This work was supported by the Brain Pool Program (Grant No. RS-2024-00445420) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT to Mohammad Moniruzzaman.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Fuji, K, Kobayashi, K, Hasegawa, O, Coimbra, MRM, Sakamoto, T, and Okamoto, N. Identification of a single major genetic locus controlling the resistance to lymphocystis disease in Japanese flounder (Paralichthys olivaceus). Aquaculture. (2006) 254:203–10. doi: 10.1016/j.aquaculture.2005.11.024
2. Hamidoghli, A, Won, S, Lee, S, Lee, S, Farris, NW, and Bai, SC. Nutrition and feeding of olive flounder Paralichthys olivaceus: a review. Rev Fish Sci Aquac. (2020) 28:340–57. doi: 10.1080/23308249.2020.1740166
3. Statistics Korea. Results of the year 2022 fish farming trend survey. Text by Department of Agriculture and Fisheries Trends Department, Fish Farming Trend Survey. Daejeon. (2023). Available at: https://kostat.go.kr/board.es?mid=a10301080400&bid=225&act=view&list_no=424510 (Accessed February 20, 2024).
4. Boyd, CE. Overview of aquaculture feeds: Global impacts of ingredient use In: Feed and feeding practices in aquaculture : Elsevier (2015). 3–25. doi: 10.1016/B978-0-08-100506-4.00001-5
5. Bharadwaj, AS, Brignon, WR, Gould, NL, Brown, PB, and Wu, YV. Evaluation of meat and bone a.S. Meal in practical diets fed to juvenile hybrid striped bass Morone chrysops × M. saxatilis. J World Aquac Soc. (2002) 33:448–57. doi: 10.1111/j.1749-7345.2002.tb00024.x
6. Markey, JC, Amaya, EA, and Davis, DA. Replacement of poultry by-product meal in production diets for the Pacific white shrimp, Litopenaeus vannamei. J World Aquac Soc. (2010) 41:893–902. doi: 10.1111/j.1749-7345.2010.00432.x
7. Kang, YJ, Lee, SM, Yang, SG, and Bai, SC. Effects of meat meal, blood meal or soybean meal as a dietary protein source replacing fish meal in parrot fish, Oplegnathus fasciatus. J Aquacult. (1999) 12:205–12.
8. McGoogan, BB, and Gatlin, DM III. Effects of replacing fish meal with soybean meal in diets for red drum Sciaenops ocellatus and potential for palatability enhancement. J World Aquac Soc. (1997) 28:374–85. doi: 10.1111/j.1749-7345.1997.tb00284.x
9. Tacon, AGJ. Feed ingredients for warmwater fish: fish meal and other processed feedstuffs. Food and Agriculture Organization of the United Nations, 1993 FAO Fish. Circ (FAO). No. 856.
10. Cabello, FC, Godfrey, HP, Tomova, A, Ivanova, L, Dölz, H, Millanao, A, et al. Antimicrobial use in aquaculture re-examined: its relevance to antimicrobial resistance and to animal and human health. Environ Microbiol. (2013) 15:1917–42. doi: 10.1111/1462-2920.12134
11. Done, HY, Venkatesan, AK, and Halden, RU. Does the recent growth of aquaculture create antibiotic resistance threats different from those associated with land animal production in agriculture? AAPSJ. (2015) 17:513–24. doi: 10.1208/s12248-015-9722-z
12. Balcázar, JL, De Blas, I, Ruiz-Zarzuela, I, Cunningham, D, Vendrell, D, and Múzquiz, JL. The role of probiotics in aquaculture. Vet Microbiol. (2006) 114:173–86. doi: 10.1016/j.vetmic.2006.01.009
13. Balcázar, JL, Vendrell, D, De Blas, I, Ruiz-Zarzuela, I, and Muzquiz, JL. Probiotics: a tool for the future of fish and shellfish health management. J Aquac Trop. (2004) 19:239–42.
14. Garriques, D. An evaluation of the production and use of a live bacterial isolate to manipulate the microbial flora in the commercial production of Penaeus vennamei postlarvae in Ecuador., swim. Through Troubl Water Proc Spec Sess Shrimp Farming, Aquac World Aquac Soc (1995). pp. 53–59.
15. Direkbusarakom, S, Yoshimizu, M, Ezura, Y, Ruangpan, L, and Danayadol, Y. Vibrio spp., the dominant flora in shrimp hatchery against some fish pathogenic viruses. J Mar Biotechnol. (1998) 6:266–7.
16. Habibnia, M, Bahrekazemi, M, Bahram, S, Javadian, SR, Hedayatifard, M, and Abdel-Tawwab, M. Growth performance, hematological and immune parameters, and mRNA levels of cytokines and antioxidant-related genes in rainbow trout (Oncorhynchus mykiss) fed on Pediocuccus pentosaceus and/or ferulic acid. Anim Feed Sci Technol. (2024) 308:115872. doi: 10.1016/j.anifeedsci.2023.115872
17. Eissa, EH, El-Sayed, AFM, Hendam, BM, Ghanem, SF, Elnabi, HEA, El-Aziz, YMA, et al. The regulatory effects of water probiotic supplementation on the blood physiology, reproductive performance, and its related genes in red Tilapia (Oreochromis niloticus X O. mossambicus). BMC Vet Res. (2024) 20:351. doi: 10.1186/s12917-024-04190-w
18. Xia, Y, Wang, M, Gao, F, Lu, M, and Chen, G. Effects of dietary probiotic supplementation on the growth, gut health and disease resistance of juvenile Nile tilapia (Oreochromis niloticus). Anim Nutri. (2020) 6:69–79. doi: 10.1016/j.aninu.2019.07.002
19. Lazado, CC, Caipang, CMA, and Estante, EG. Prospects of host-associated microorganisms in fish and penaeids as probiotics with immunomodulatory functions. Fish Shell Immunol. (2015) 45:2–12. doi: 10.1016/j.fsi.2015.02.023
20. Ji, Z, Lu, X, Xue, M, Fan, Y, Tian, J, Dong, L, et al. The probiotic effects of host-associated Bacillus velezensis in diets for hybrid yellow catfish (Pelteobagrusfulvidraco × Pelteobagrusvachelli). Anim Nutri. (2023) 15:114–25. doi: 10.1016/j.aninu.2023.08.004
21. Liao, Z, Liu, Y, Wei, H, He, X, Wang, Z, Zhuang, Z, et al. Effects of dietary supplementation of Bacillus subtilis DSM 32315 on growth, immune response and acute ammonia stress tolerance of Nile tilapia (Oreochromis niloticus) fed with high or low protein diets. Anim Nutri. (2023) 15:375–85. doi: 10.1016/j.aninu.2023.05.016
22. Büyükdeveci, ME, Cengizler, I, Balcazar, JL, and Demirkale, I. Effects of two host-associated probiotics Bacillus mojavensis B191 and Bacillus subtilis MRS11 on growth performance, intestinal morphology, expression of immune-related genes and disease resistance of Nile tilapia (Oreochromis niloticus) against Streptococcus iniae. Dev Comp Immunol. (2023) 138:104553. doi: 10.1016/j.dci.2022.104553
23. Lee, SJ, Da-In Noh, DI, Lee, YS, Hasan, MT, Hur, SW, Lee, S, et al. Effects of host-associated low-temperature probiotics in olive founder (Paralichthysolivaceus) aquaculture. Sci Rep. (2024) 14:2134. doi: 10.1038/s41598-024-52491-9
24. Ji, Z, Zhu, C, Zhu, X, Ban, S, Yu, L, Tian, J, et al. Dietary host-associated Bacillus subtilis supplementation improves intestinal microbiota, health and disease resistance in Chinese perch (Siniperca chuatsi). Anim Nutri. (2023) 13:197–205. doi: 10.1016/j.aninu.2023.01.001
25. Amoah, K, Tan, B, Zhang, S, Chi, S, Yang, Q, Liu, H, et al. Host gut-derived Bacillus probiotics supplementation improves growth performance, serum and liver immunity, gut health, and resistive capacity against Vibrio harveyi infection in hybrid grouper (Epinephelus fuscoguttatus × Epinephelus lanceolatus). Anim Nutri. (2023) 14:163–84. doi: 10.1016/j.aninu.2023.05.005
26. Jang, WJ, Lee, KB, Jeon, MH, Lee, SJ, Hur, SW, Lee, S, et al. Characteristics and biological control functions of Bacillus sp. PM8313 as a host-associated probiotic in red sea bream (Pagrus major) aquaculture. Anim Nutri. (2023) 12:20–31. doi: 10.1016/j.aninu.2022.08.011
27. Choi, S, Sim, W, Jang, D, Yoon, Y, Ryu, J, Oh, J, et al. Antibiotics in coastal aquaculture waters: occurrence and elimination efficiency in oxidative water treatment processes. J Hazard Mat. (2020) 396:122585. doi: 10.1016/j.jhazmat.2020.122585
28. Lee, S, Katya, K, Hamidoghli, A, Hong, J, Kim, DJ, and Bai, SC. Synergistic effects of dietary supplementation of Bacillus subtilis WB60 and mannanoligosaccharide (MOS) on growth performance, immunity and disease resistance in Japanese EEL, Anguilla japonica. Fish Shell Immunol. (2018) 83:283–91. doi: 10.1016/j.fsi.2018.09.031
29. Hasan, MT, Jang, WJ, Kim, H, Lee, BJ, Kim, KW, Hur, SW, et al. Synergistic effects of dietary Bacillus sp. SJ-10 plus β-glucooligosaccharides as a synbiotic on growth performance, innate immunity and streptococcosis resistance in olive flounder (Paralichthys olivaceus). Fish Shell Immunol. (2018) 82:544–53. doi: 10.1016/j.fsi.2018.09.002
30. Wang, YB, Tian, ZQ, Yao, JT, and Li, WF. Effect of probiotics, Enteroccus faecium, on tilapia (Oreochromis niloticus) growth performance and immune response. Aquaculture. (2008) 277:203–7. doi: 10.1016/j.aquaculture.2008.03.007
31. Won, S, Hamidoghli, A, Choi, W, Park, Y, Jang, WJ, Kong, IS, et al. Effects of Bacillus subtilis WB60 and Lactococcus lactis on growth, immune responses, histology and gene expression in Nile Tilapia, Oreochromis niloticus. Microorganisms. (2020) 8:67. doi: 10.3390/microorganisms8010067
32. AOAC. Association of Official Analytical Chemists Official Methods of Analysis, Aquac. Res. Sixteenth (1995).
33. Quade, MJ, and Roth, JA. A rapid, direct assay to measure degranulation of bovine neutrophil primary granules. Vet Immunol Immunopathol. (1997) 58:239–48. doi: 10.1016/S0165-2427(97)00048-2
34. Kuebutornye, FKA, Abarike, ED, and Lu, Y. A review on the application of Bacillus as probiotics in aquaculture. Fish Shell Immunol. (2019) 87:820–8. doi: 10.1016/j.fsi.2019.02.010
35. Cha, JH, Yang, SY, Woo, SH, Song, JW, Oh, DH, and Lee, KJ. Effects of dietary supplementation with Bacillus sp. on growth performance, feed utilization, innate immunity and disease resistance against Streptococcus iniae in olive flounder Paralichthys olivaceus. Korean J Fish Aquat Sci. (2012) 45:35–42. doi: 10.5657/KFAS.2012.0035
36. Park, Y, Kim, H, Won, S, Hamidoghli, A, Hasan, MT, Kong, IS, et al. Effects of two dietary probiotics (Bacillus subtilis or licheniformis) with two prebiotics (mannan or fructo oligosaccharide) in Japanese EEL, Anguilla japonica. Aquac Nutr. (2020) 26:316–27. doi: 10.1111/anu.12993
37. Vazirzadeh, A, Roosta, H, Masoumi, H, Farhadi, A, and Jeffs, A. Long-term effects of three probiotics, singular or combined, on serum innate immune parameters and expressions of cytokine genes in rainbow trout during grow-out. Fish Shell Immunol. (2020) 98:748–57. doi: 10.1016/j.fsi.2019.11.023
38. Kim, YR, Kim, EY, Lee, JM, Kim, JK, and Kong, IS. Characterisation of a novel Bacillus sp. SJ-10 β-1,3–1,4-glucanase isolated from jeotgal, a traditional Korean fermented fish. Bioprocess Biosyst Eng. (2013) 36:721–7. doi: 10.1007/s00449-013-0896-4
39. Park, Y, Moniruzzaman, M, Lee, S, Hong, J, Won, S, Lee, JM, et al. Comparison of the effects of dietary single and multi-probiotics on growth, non-specific immune responses and disease resistance in starry flounder. Platichthysstellatus Fish Shell Immunol. (2016) 59:351–7. doi: 10.1016/j.fsi.2016.11.006
40. Park, Y, Lee, S, Hong, J, Kim, D, Moniruzzaman, M, and Bai, SC. Use of probiotics to enhance growth, stimulate immunity and confer disease resistance to Aeromonas salmonicida in rainbow trout (Oncorhynchus mykiss). Aquac Res. (2017) 48:2672–82. doi: 10.1111/are.13099
41. Won, S, Hamidoghli, A, Choi, W, Bae, J, Jang, WJ, Lee, S, et al. Evaluation of potential probiotics Bacillus subtilis WB60, Pediococcus pentosaceus, and Lactococcus lactis on growth performance, immune response, gut histology and immune-related genes in Whiteleg shrimp, Litopenaeus vannamei. Microorganisms. (2020) 8:281. doi: 10.3390/microorganisms8020281
42. da Costa, SN, do Couto, MVS, Abe, HA, Paixao, PEG, Cordeiro, CAM, Lopes, EM, et al. Effects of an Enterococcus faecium-based probiotic on growth performance and health of Pirarucu, Arapaima gigas. Aquac Res. (2019) 50:3720–8. doi: 10.1111/are.14332
43. Lara-Flores, MM, and Olvera-Novoa, A. The use of lactic acid bacteria isolated from intestinal tract of Nile tilapia (Oreochromis niloticus), as growth promoters in fish fed low protein diets. Lat Am J Aquat Res. (2013) 41:490–7. doi: 10.3856/vol41-issue3-fulltext-12
44. Aly, SM, Mohamed, MF, and John, G. Effect of probiotics on the survival, growth and challenge infection in Tilapia nilotica (Oreochromis niloticus). Aquac Res. (2008) 39:647–56. doi: 10.1111/j.1365-2109.2008.01932.x
45. Helal, R, and Melzig, MF. In vitro effects of selected saponins on the production and release of lysozyme activity of human monocytic and epithelial cell lines. Sci Pharm. (2011) 79:337–49. doi: 10.3797/scipharm.1012-15
46. Greenwood, FC, and Landon, J. Growth hormone secretion in response to stress in man. Nature. (1966) 210:540–1. doi: 10.1038/210540a0
47. Bornfeldt, KE, Arnqvist, HJ, Dahlkvist, HH, Skottner, A, and Wikberg, JES. Receptors for insulin-like growth factor-I in plasma membranes isolated from bovine mesenteric arteries. Eur J Endocrinol. (1988) 117:428–34. doi: 10.1530/acta.0.1170428
48. Clemmons, DR, Busby, W, Clarks, JB, Parker, A, Duan, C, and Nam, TJ. Modifications of insulin-like growth factor binding proteins and their role in controlling IGF actions. Endocr J. (1998) 45:S1–8. doi: 10.1507/endocrj.45.Suppl_S1
49. Kuebutornye, FKA, Tang, J, Cai, J, Yu, H, Wang, Z, Abarike, ED, et al. In vivo assessment of the probiotic potentials of three host-associated Bacillus species on growth performance, health status and disease resistance of Oreochromis niloticus against Streptococcus agalactiae. Aquaculture. (2020) 527:735440. doi: 10.1016/j.aquaculture.2020.735440
50. Back, SJ, Park, SJ, Moon, JS, Lee, SB, Jo, SJ, Nam, TJ, et al. The effects of dietary heat-killed probiotics bacteria additives in low-fishmeal feed on growth performance, immune responses, and intestinal morphology in juvenile olive flounder Paralichthys olivaceus. Aquac Rep. (2020) 18:100415. doi: 10.1016/j.aqrep.2020.100415
51. Reyes-Cerpa, S, Maisey, K, Reyes-López, F, Toro-Ascuy, D, Sandino, AM, and Imarai, M. Fish cytokines: current research and application. Fish Sci. (2021) 87:1–9. doi: 10.1007/s12562-020-01476-4
52. Dinarello, CA. Interleukin-1 and its biologically related cytokines. Adv Immunol. (1989) 44:153–205. doi: 10.1016/S0065-2776(08)60642-2
53. Pleguezuelos, O, Zou, J, Cunningham, C, and Secombes, CJ. Cloning, sequencing, and analysis of expression of a second IL-1 β gene in rainbow trout (Oncorhynchus mykiss). Immunogenetics. (2000) 51:1002–11. doi: 10.1007/s002510000240
54. Fujiki, K, Shin, DH, Nakao, M, and Yano, T. Molecular cloning and expression analysis of carp (Cyprinus carpio) interleukin-1β, high affinity immunoglobulin E fc receptor γ subunit and serum amyloid a. Fish Shell Immunol. (2000) 10:229–42. doi: 10.1006/fsim.1999.0253
55. Scapigliati, G, Buonocore, F, Bird, S, Zou, J, Pelegrin, P, Falasca, C, et al. Phylogeny of cytokines: molecular cloning and expression analysis of sea bass Dicentrarchus labrax interleukin-1β. Fish Shell Immunol. (2001) 11:711–26. doi: 10.1006/fsim.2001.0347
56. Pelegrín, P, García-Castillo, J, Mulero, V, and Meseguer, J. Interleukin-1β isolated from a marine fish reveals up-regulated expression in macrophages following activation with lipopolysaccharide and lymphokines. Cytokine. (2001) 16:67–72. doi: 10.1006/cyto.2001.0949
57. Corripio-Miyar, Y, Bird, S, Tsamopoulos, K, and Secombes, CJ. Cloning and expression analysis of two pro-inflammatory cytokines, IL-1β and IL-8, in haddock (Melanogrammus aeglefinus). Mol Immunol. (2007) 44:1361–73. doi: 10.1016/j.molimm.2006.05.010
58. Lee, DS, Hong, SH, Lee, HJ, Jun, LJ, Chung, JK, Kim, KH, et al. Molecular cDNA cloning and analysis of the organization and expression of the IL-1β gene in the Nile tilapia, Oreochromis niloticus. Comp Biochem Physiol Part A Mol Integr Physiol. (2006) 143:307–14. doi: 10.1016/j.cbpa.2005.12.014
59. Huo, HJ, Chen, SN, Li, L, and Nie, P. Functional characterization of IL-10 and its receptor subunits in a perciform fish, the mandarin fish, Siniperca chuatsi. Dev Comp Immunol. (2019) 97:64–75. doi: 10.1016/j.dci.2019.03.017
60. Moore, KW, de Waal, MR, Coffman, RL, and O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. (2001) 19:683–765. doi: 10.1146/annurev.immunol.19.1.683
61. Gao, YJ, Yang, HJ, Liu, Y, Chen, SJ, Guo, DQ, Yu, Y, et al. Effects of graded levels of threonine on growth performance, biochemical parameters and intestine morphology of juvenile grass carp Ctenopharyngodon idella. Aquaculture. (2014) 424-425:113–9. doi: 10.1016/j.aquaculture.2013.12.043
62. Klurfeld, DM. Nutritional regulation of gastrointestinal growth. Front Biosci. (1999) 4:D299–302. doi: 10.2741/klurfeld
63. Madsen, K. Probiotics and the immune response. J Clin Gastroenterol. (2006) 40:232–4. doi: 10.1097/00004836-200603000-00014
64. Ibnou-Zekri, N, Blum, S, Schiffrin, E, and von der Weid, T. Divergent patterns of colonization and immune response elicited from two intestinal lactobacillus strains that display similar properties in vitro. Infec Immun. (2003) 71:428–36. doi: 10.1128/IAI.71.1.428-436.2003
65. Donnet-Hughes, A, Rochat, F, Serrant, P, Aeschlimann, JM, and Schiffrin, EJ. Modulation of nonspecific mechanisms of defense by lactic acid bacteria: effective dose. J Dairy Sci. (1999) 82:863–9. doi: 10.3168/jds.S0022-0302(99)75304-X
66. Gill, HS, Rutherfurd, KJ, Cross, ML, and Gopal, PK. Enhancement of immunity in the elderly by dietary supplementation with the probiotic Bifidobacterium lactis HN019. Am J Clin Nutr. (2001) 74:833–9. doi: 10.1093/ajcn/74.6.833
67. Sharifuzzaman, SM, and Austin, B. Kocuria SM1 controls vibriosis in rainbow trout (Oncorhynchus mykiss, Walbaum). J Appl Microbiol. (2010) 108:2162–70. doi: 10.1111/j.1365-2672.2009.04618.x
68. Vollstad, D, Bogwald, J, Gaserod, O, and Dalmo, RA. Influence of high-M alginate on the growth and survival of Atlantic cod (Gadus morhua L.) and spotted wolf fish (Anarhichas minor Olafsen) fry. Fish Shell Immunol. (2006) 20:548–61. doi: 10.1016/j.fsi.2005.07.004
69. Moyner, K, Roed, KH, Sevatdal, S, and Heum, M. Changes in non-specific immune parameters in Atlantic salmon, Salmo salar (L.), induced by Aeromonas salmonicida infection. Fish Shell Immunol. (1993) 3:253–65. doi: 10.1006/fsim.1993.1025
Keywords: beneficial bacteria, growth performance, innate immunity, gastrointestinal tract, challenge test, olive flounder
Citation: Choi W, Moniruzzaman M, Lee S, Bae J, Bai SC, Min T and Lee S (2025) Evaluation of three fish-derived probiotic bacteria replacing antibiotics on growth, immunity, gut morphology and disease resistance in juvenile olive flounder Paralichthys olivaceus fed reduced fish meal diets. Front. Nutr. 12:1519140. doi: 10.3389/fnut.2025.1519140
Received: 29 October 2024; Accepted: 29 January 2025;
Published: 13 February 2025.
Edited by:
Samad Rahimnejad, University of Murcia, SpainReviewed by:
Ishtiyaq Ahmad, Sher-e-Kashmir University of Agricultural Sciences and Technology, IndiaCopyright © 2025 Choi, Moniruzzaman, Lee, Bae, Bai, Min and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Taesun Min, dHNtaW5AamVqdW51LmFjLmty; Seunghyung Lee, c2hsZWVAcGtudS5hYy5rcg==
‡Present address: Wonsuk Choi, CJ Feed and Care, AN R&D Center, Seoul, Republic of Korea
†These authors have contributed equally to this work
§ORCID: Mohammad Moniruzzaman, orcid.org/0000-0002-5266-2087
Seunghan Lee, orcid.org/0000-0002-7624-0954
Sungchul C. Bai, orcid.org/0000-0001-7984-2267
Taesun Min, orcid.org/0000-0002-3998-7493
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.