
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 20 March 2025
Sec. Clinical Nutrition
Volume 12 - 2025 | https://doi.org/10.3389/fnut.2025.1516003
Background: Many studies have used Body Mass Index (BMI) to define obesity and examine its potential link to adult asthma. However, BMI overlooks body fat distribution, which may significantly impact health. Unlike BMI, the Body Roundness Index (BRI) can more accurately reflect body fat distribution. Therefore, this study examined BRI’s relationship with asthma prevalence in U.S. adults.
Methods: This study was based on data from the National Health and Nutrition Examination Survey (NHANES) between 2001 and 2018 and covered 40,052 adult participants. Participants were categorized into four quartile groups based on their BRI levels: Quartile 1 (1.05, 3.80); Quartile 2 (3.80, 5.06); Quartile 3 (5.06, 6.61); Quartile 4 (6.61, 23.48). The association between BRI and asthma prevalence was assessed via weighted multivariate logistic regression, smoothed curve fitting, threshold effects, subgroup, and sensitivity analysis. BRI’s predictive power was compared to BMI and waist circumference using z-scores.
Results: Of the study population, 5,605 participants had asthma (13.99% prevalence). After adjusting for possible confounders, the results showed that higher BRI was linked to greater asthma prevalence (OR = 1.41, 95% CI:1.27, 1.56, p < 0.0001). A J-shaped relationship between BRI and asthma prevalence (p-nonlinearity = 0) was found, with asthma prevalence rising significantly when BRI surpassed 4.34. BRI outperformed BMI and waist circumference in predicting asthma (BRI: OR = 1.180; BMI: OR = 1.169; W.C.: OR = 1.166). Subgroup and sensitivity analyses confirmed our results’ robustness.
Conclusion: Adult asthma prevalence increases with increasing BRI levels, showing a J-shaped relationship. Keeping BRI under 4.34 is vital for lowering asthma prevalence, especially for overweight or obese individuals. In addition, BRI outperformed BMI and waist circumference in predicting asthma occurrence.
Asthma is a complex chronic respiratory disease whose primary symptoms include wheezing, coughing, and breathlessness. Nevertheless, the precise pathogenesis of asthma remains unclear (1, 2). According to the Global Initiative for Asthma (GINA) 2024 guidelines (3), approximately 300 million people worldwide are suffering from asthma, and it kills about 1,000 people every day. The World Health Organization (WHO) also predicts that the number of people with asthma could increase by 100 million by 2025 (4). Asthma not only adds to the global disease burden but also contributes to rising healthcare costs, loss of labor, and reduced ability to perform daily activities. Asthma affects adults in particular, potentially making chronic diseases like cardiovascular disease and diabetes more difficult to manage, and is especially common in older populations (5). While childhood asthma is usually associated with allergies (6), adult asthma can be caused by a variety of factors and often responds poorly to standard treatment regimens (7). Therefore, identifying the risk factors for adult asthma and taking preventive measures are crucial to improving patients’ quality of life and reducing healthcare expenditure.
The prevalence of obesity has increased rapidly in recent years. It is estimated that over one billion people around the world are affected by obesity (8). Body Mass Index (BMI) is commonly used to assess the degree of obesity in adults, with a BMI of 30 kg/m2 or more defined as obese. Numerous studies have confirmed that the risk of developing asthma increases with increasing obesity (9–12). BMI is a commonly used measure of obesity in studies exploring the relationship between obesity and asthma (13, 14). However, BMI does not reflect body fat distribution (15, 16). Therefore, more precise methods are needed to explore the relationship between obesity and asthma risk. Measures of body fat distribution have shown better predictive value than BMI in predicting diabetes and cardiovascular disease (17, 18). In order to reflect fat distribution more accurately, Thomas et al. (19) proposed a new anthropometric index, the Body Roundness Index (BRI). This index calculates body roundness based on an elliptical model of body shape. It uses eccentricity to estimate the percentage of visceral and total body fat. Unlike BMI, BRI considers waist circumference and provides a more complete picture of visceral fat distribution. Studies have shown that visceral fat has a greater effect on asthma development (20), so we hypothesized that BRI may be a better predictor of asthma incidence than BMI. BRI has a wide range of clinical applications. Previous studies have shown that the BRI has demonstrated superior predictive ability to traditional anthropometric methods in assessing the risk of diseases such as cardiometabolic diseases (21–23), kidney disease (24), and cancer (25), providing a more accurate tool for predicting and preventing obesity-related health problems in clinical practice. Until now, no studies have explicitly examined the association between the Body Roundness Index (BRI) and asthma.
To fill a knowledge gap about whether BRI is associated with prevalence in asthmatics, this study aims to analyze the data from the National Health and Nutrition Examination Survey (NHANES) in the United States from 2001 to 2018 and explore the relationship between BRI and asthma in U.S. adults, with the expectation of providing a new approach to the prevention and treatment of asthma.
The data used in this study originated from the 2001–2018 National Health and Nutrition Examination Survey (NHANES). This national survey assesses the nutritional and health status of adults and children in the U.S. The survey is unique in that it combines interviews and physical examinations and is published by the National Center for Health Statistics (NCHS). It uses a stratified multistage sampling design to produce a nationally representative sample of U.S. residents. The study was approved by the Research Ethics Review Board of the National Center for Health Statistics, and informed consent was obtained from all participants (26). For more information about the National Center for Health Statistics Institutional Review Board, please visit the website.1 Therefore, no other ethics committee approval was required for this study. The data from NHANES are publicly accessible.
Our analyses included participants with comprehensive information on asthma and the Body Roundness Index. Initially, a total of 91,351 participants were recruited. After excluding participants with no asthma diagnostic data (n = 4,033), lacking BRI data (n = 11,114), under 20 years of age (n = 31,255), and missing covariates (n = 4,897), our final analysis included 40,052 eligible participants, as shown in Figure 1.

Figure 1. Cohort flow diagram in NHANES 2001–2018. NHANES, National Health and Nutrition Examination Survey.
The Body Roundness Index (BRI) is calculated through the following formula: BRI = 364.2–365.5 × √ [1 – (W.C./(2π))2/ (0.5 × height)2] (19), where W.C. stands for the waist circumference. Both the waist circumference and the height are measured in meters. To guarantee data consistency and analysis accuracy, the height and waist circumference of the participants were measured at the mobile examination center using standardized procedures. Then, the BRI of each participant was calculated using these data. Based on the BRI values, the participants were divided into four quartile groups: Quartile 1 (1.05, 3.80); Quartile 2 (3.80, 5.06); Quartile 3 (5.06, 6.61); and Quartile 4 (6.61, 23.48). This classification method facilitates an in-depth study of the correlation between BRI levels and adult asthma prevalence in different populations.
This study relied on patient self-reported diagnostic information from the NHANES database. The diagnostic criteria for asthma are based on data from questionnaires in the NHANES data about two questions: (1) “Has a doctor or other health professional told you that you have asthma?”; (2) “Do you still have asthma?” Only respondents who answered “yes” to both questions were diagnosed with asthma. In contrast, respondents who answered “no” to either question were not included as asthmatics (27–29). According to several peer-reviewed studies (30–32), self-reporting to diagnose asthma is highly accurate.
To reduce the potential bias, we have referred to previous studies (29, 33–37) and incorporated covariates such as age (20–39; 40–59; 60–79; 80+), gender (Male; Female), race (White; Black; Mexican American; Others), marital status (Married/Living with partner; Widowed/divorced/separated; Never married) and education level (Less than high school; High school; More than high school). The poverty income ratio (PIR) was divided into Low (PIR <1.3), Middle (PIR 1.3–3.5), and High (PIR >3.5). The number of cigarettes smoked is used as a measure of an individual’s smoking status. Smoking status is classified into three categories: (1) never smokers, defined as individuals who have not smoked more than 100 cigarettes; (2) former smokers, comprising those who have smoked more than 100 cigarettes but have ceased smoking; and (3) current smokers, including those who have smoked up to 100 cigarettes and continue to smoke. The diagnosis of diabetes is based on the following criteria: whether the subject is using insulin, has doctor-diagnosed diabetes, or is taking medication for blood sugar control. A subject is recognized as having diabetes if they test positive for any of the above. The diagnosis of cardiovascular disease requires a positive diagnosis by a physician based on a history of myocardial infarction, angina pectoris, coronary heart disease, or stroke. Criteria for determining hypertension included that the patient had a documented diagnosis of hypertension at each of their multiple visits to the doctor, that the doctor had recommended that they take antihypertensive medication, or that the average of three consecutive blood pressure measurements was not less than 140 mm Hg for systolic blood pressure and 90 mm Hg for diastolic blood pressure.
To correct possible bias caused by oversampling, this study followed the NHANES guidelines and used a sample weighting method. Based on the NHANES guidelines for analysis, we divided the weights for each 2-year cycle by 9 to generate new sample weights for the combined survey cycle. In descriptive analyses, we reported means and standard deviations (S.D.) for baseline characteristics of continuous variables. In contrast, categorical variables were presented as proportions. Student’s t-test assessed differences in means of continuous variables. Differences in the proportions of categorical variables were rated using Pearson’s chi-square test. We used multivariate logistic regression models to analyze the relationship between different Body Roundness Index (BRI) levels and adult asthma prevalence. Model 1 was not adjusted for covariates. In Model 2, we adjusted for age, gender, race, marital status, education level, and PIR. In Model 3, we additionally adjusted for smoking status, diabetes, cardiovascular disease, and hypertension based on Model 2. To verify the stability of the findings, we classify the continuous variable BRI into categorical variables (quartiles). To deal with the possible nonlinear relationship between BRI and asthma risk, we used generalized additive modeling (GAM) and smoothed curve fitting techniques. In addition, we used segmented linear regression models, also known as two-stage linear regression models, to fit stages in the data and identify threshold effects in nonlinear relationships. When nonlinear associations were found, we identified threshold effects in each stage through the segmented linear regression model. We applied a two-step recursive approach to finding the optimal breakpoint K value, which was determined based on the model that best produced maximum likelihood. To facilitate the comparison of BRI with other obesity indicators in terms of the odds ratio, we standardized these indicators, calculated their z-scores, and included them in the linear model analysis. Finally, we conducted subgroup and sensitivity analyses to ensure the results’ reliability. All statistical analyses used Empower software2 (X&Y Solutions, Inc., Boston, MA) and R version 4.2.33 (R Foundation). In assessing the statistical significance of the results, we used the criterion of a p-value of less than 0.05.
Table 1 demonstrates the demographics and characteristics of the study participants. A total of 40,052 participants were included in this study, of which 5,605 (13.99%) had asthma. Participants ranged from 20 to 59 (78.43%) and women (58.55%). Of all participants, 71.26% were Non-Hispanic White. The asthma population tended to be younger, female, Non-Hispanic White, married or living with a partner, with a higher education level and higher income, and former or never smokers. In addition, asthmatics had relatively lower rates of diabetes, cardiovascular disease, and hypertension. Notably, the asthma group had significantly higher Body Roundness Index, Body Mass Index, waist circumference, and weight than the non-asthma group (p < 0.0001).
Table 2 demonstrates the correlation between BRI and asthma. Our data analysis revealed that an increase in BRI was associated with an increase in asthma risk. Models 1, 2, and 3 showed a positive association between BRI and asthma risk. After fully adjusting for covariates, we found that for every one-unit increase in BRI, participants were 7% more likely to develop asthma (Model 3: OR = 1.07, 95% CI: 1.06–1.09). This positive correlation remained statistically significant when the BRI was further analyzed by dividing it into quartiles. Participants with a BRI in the highest quartile had a 41% higher risk of developing asthma than those with a BRI in the lowest quartile (OR = 1.41, 95% CI: 1.27–1.56; p for trend <0.0001).
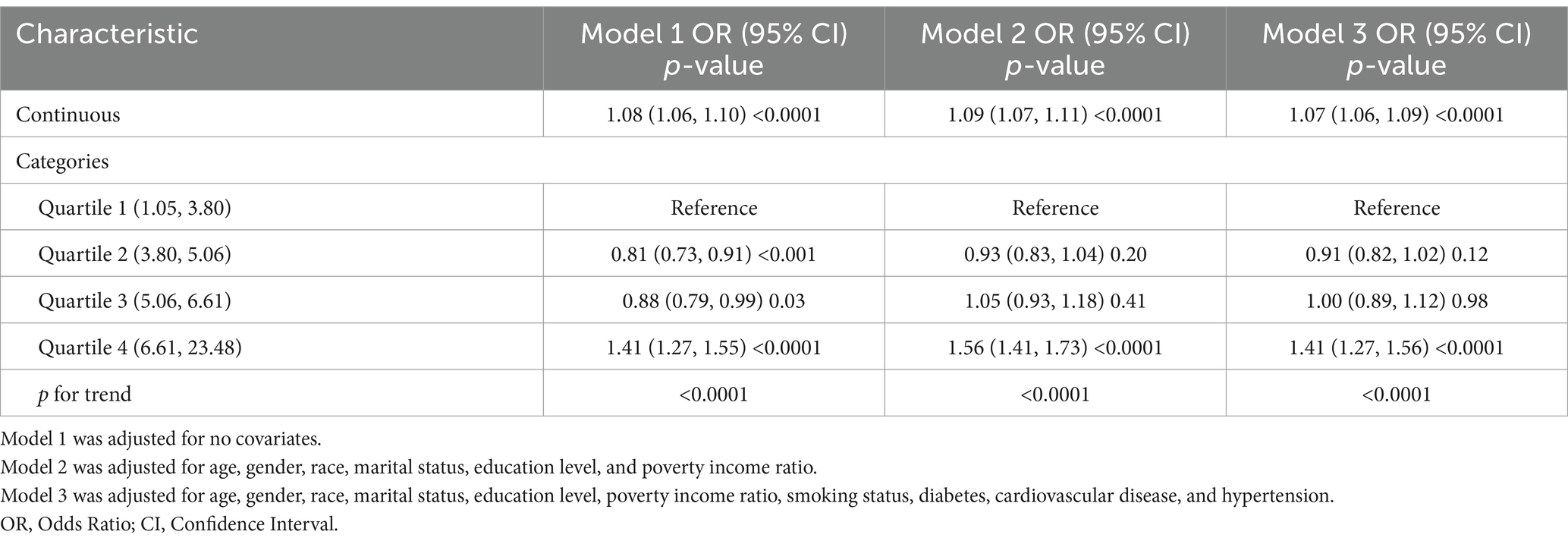
Table 2. The relationship between Body Roundness Index and Asthma prevalence among participants from the NHANES 2001–2018.
In addition, we used a smoothed curve-fitting technique to delve deeper into the association between BRI and asthma risk, and our analysis revealed a positive nonlinear association (Figure 2).
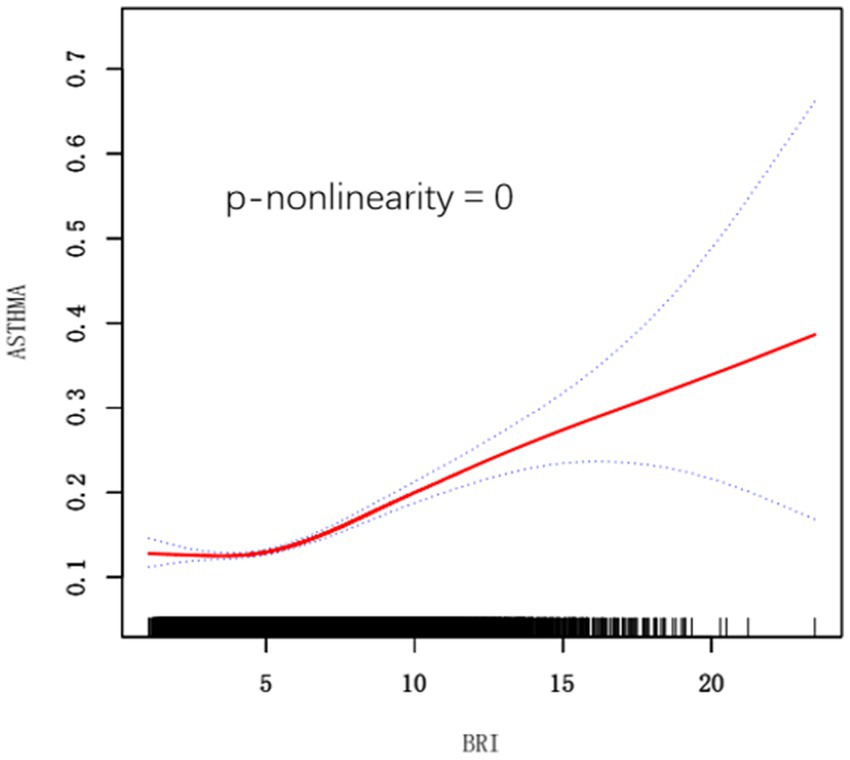
Figure 2. Smooth curve fitting for BRI and Asthma. BRI, Body Roundness Index. Adjusted for age, gender, race, marital status, education level, poverty income ratio, smoking status, diabetes, cardiovascular disease, and hypertension.
Smoothed curve analyses revealed nonlinear associations between obesity indicators other than BRI (e.g., BMI and waist circumference) and asthma risk (Figure 3). Following this, we applied a segmented regression model to fit the individual data intervals and assessed the threshold effect accordingly (Table 3).
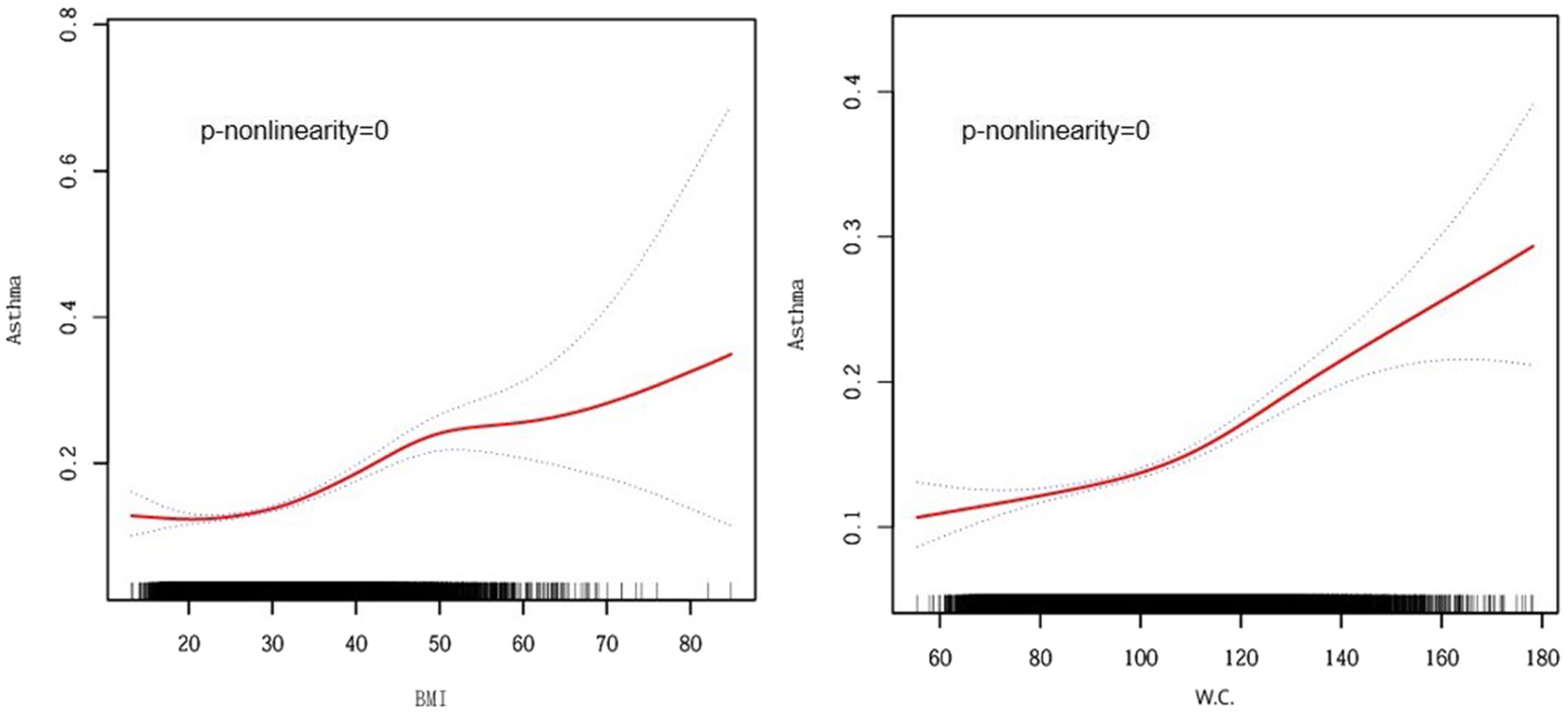
Figure 3. Smooth curve fitting for BMI, W.C., and Asthma. BMI, Body Mass Index, W.C., Waist Circumference. Adjusted for age, gender, race, marital status, education level, poverty income ratio, smoking status, diabetes, cardiovascular disease, and hypertension.
To compare the OR of BRI with other obesity indicators, we standardized these indicators, calculated their z-scores, and included them in a linear model analysis (Table 4). The results showed that BRI was more significantly associated with asthma risk than other obesity indicators such as Body Mass Index (BMI) and Waist Circumference (W.C.) (BRI: OR = 1.180; BMI: OR = 1.169; W.C.: OR = 1.166), which suggests that BRI may be a superior predictor of asthma risk.
Data from subgroup analyses revealed a consistent trend: the positive correlation between BRI and asthma risk remained stable in subjects with various characteristics. However, the more significant population characteristics were 60–79 years old, women, other races, widowed/divorced/separated, diabetes, cardiovascular disease, and hypertension (Figure 4). To avoid potential selection bias caused by the large number of missing values in the covariates of diabetes and the poverty income ratio, in the sensitivity analysis, we excluded these two variables from the analysis and re-included the study population. Subsequently, we again used the weighted multivariate logistic regression model to assess the association between BRI levels and the onset of asthma. The analysis results were consistent with the results shown in Table 5, verifying the stability of our research results.
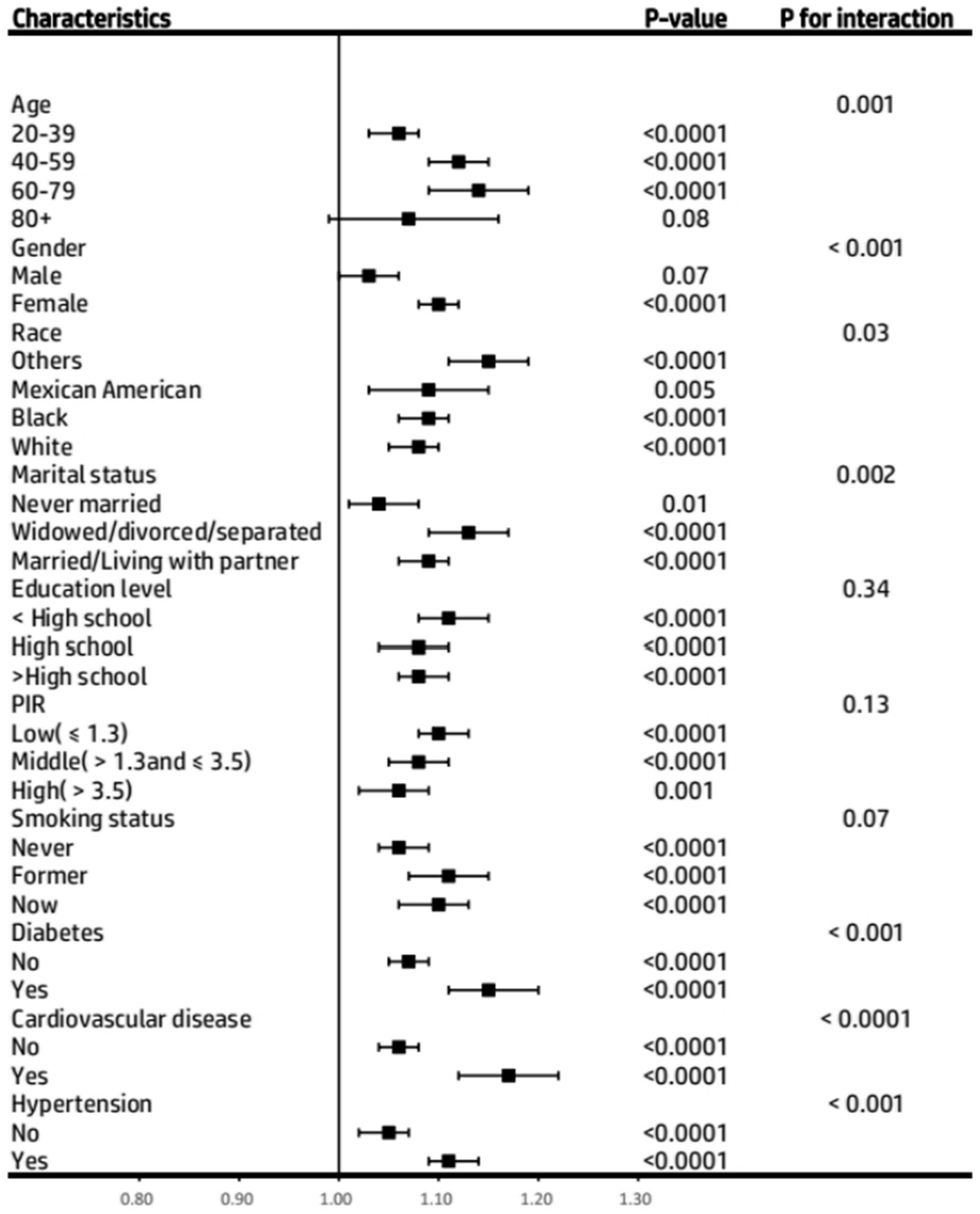
Figure 4. Subgroup analysis of the association between Body Roundness Index and Asthma prevalence among participants from the NHANES 2001–2018. PIR, Poverty Income Ratio.
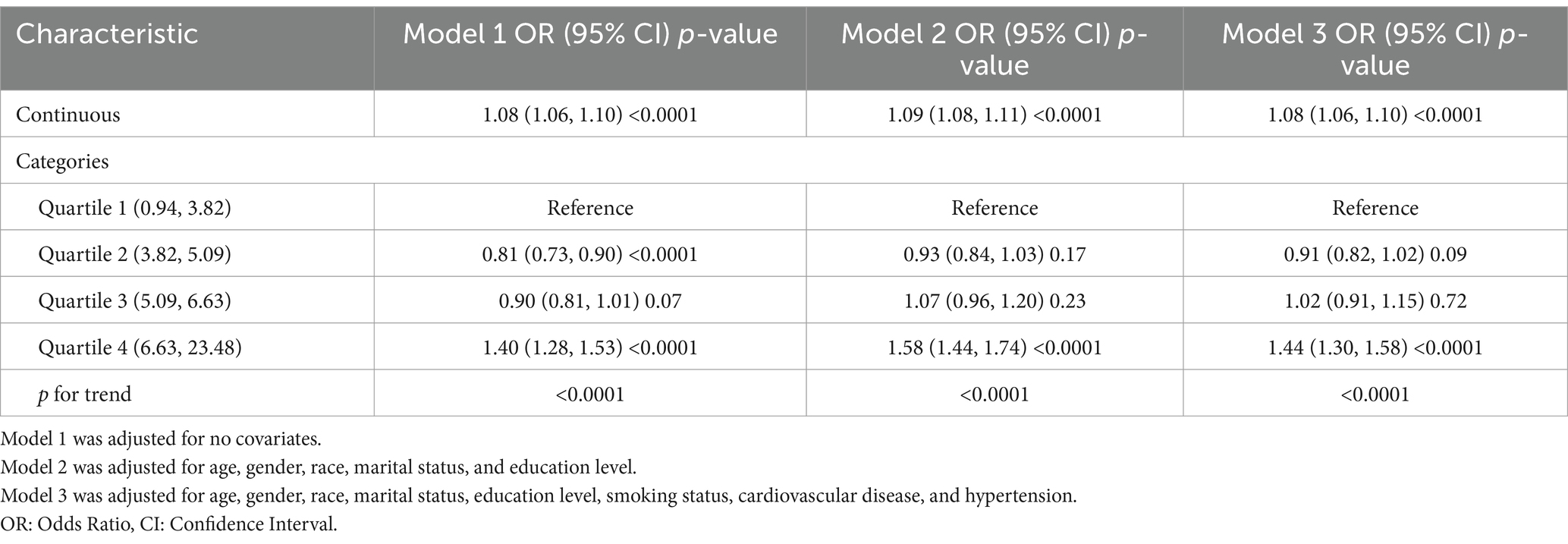
Table 5. The relationship between the Body Roundness Index and Asthma prevalence among participants from the NHANES 2001–2018 (Covariates did not include poverty income ratio and diabetes).
This study is the first to quantitatively analyze the relationship between Body Roundness Index (BRI) and asthma in U.S. adults. After adjusting for relevant covariates and based on cross-sectional data from the NHANES from 2001 to 2018, we used weighted logistic regression analysis to find that higher BRI was associated with an increased risk of asthma in adults. The findings suggest a J-shaped association between BRI and asthma risk, where changes in BRI have little effect on asthma risk below a certain threshold. However, once that threshold is exceeded, asthma risk rises as BRI increases. We found that the critical tipping point was 4.34. In addition, we found that BRI was more effective in predicting asthma risk than traditional obesity indicators such as BMI and waist circumference. Subgroup analyses suggest that management strategies for obese populations identified based on BRI should focus specifically on patients 60–79 years of age, women, nonwhite race, divorced/widowed/separated, diabetes, cardiovascular disease, and hypertension. These findings may lead to the development of new clinical guidelines.
Primary asthma prevention has been a challenge in research and clinical practice compared to timely and effective treatment options (38). The link between asthma and obesity is particularly complex, with obese asthmatics more likely to have persistent symptoms, increased absenteeism, increased medication use, and other related problems. Moreover, obese asthmatics are less likely to experience symptom relief and are more likely to develop severe persistent asthma (39). However, accurately assessing the actual extent to which obesity affects asthma is also a challenge. Although Body Mass Index (BMI) is a commonly used measure of obesity and is easy to measure, it has limitations. For example, a study of more than 40,000 U.S. adults by Tomiyama et al. found that half of the overweight population and nearly one-third of the obese population were metabolically healthy. In contrast, over 30% of the normal-weight population had metabolic abnormalities (40). The prevailing view is that the distribution of body fat and body composition cannot be described by BMI alone and that significant differences exist even among individuals with the same BMI (41). Prillaman suggests that the definition of obesity needs to be updated (42). He argues that BMI ignores essential factors such as visceral fat, which may significantly impact health. Therefore, in this study, we used the Body Roundness Index (BRI) to assess the association between obesity and asthma in adults more accurately.
Obesity can be categorized as visceral obesity, subcutaneous fat accumulation in the trunk and extremities, and fat ectopic in skeletal muscle (43). Visceral adipose tissue, in particular, being metabolically active, produces a variety of cytokines that are negatively correlated with one-second forceful expiratory volume (FEV1), FEV1% prediction, and forced lung capacity (FVC) (43, 44). Studies have noted an association between visceral adipose tissue (VAT) and the development of asthma and decreased lung function in adolescents and adults (20), whereas peripheral obesity has not been significantly associated with these health problems. A large-scale study conducted in Vienna found that a significantly higher proportion of asthmatics were obese compared to controls. There was a significant increase in visceral adipose tissue (VAT), especially in adult and elderly asthmatics (45). Indicators of abdominal obesity, such as waist circumference and waist-to-height ratio, are positively correlated with the prevalence of asthma. However, these indicators do not accurately reflect visceral adiposity (9, 46–49). Although magnetic resonance imaging (MRI) and abdominal computed tomography (C.T.) can assess visceral fat, both methods are costly and not easily accessible (44, 50). In contrast, the Body Roundness Index (BRI) is less costly and more accessible to patients.
The mechanisms linking asthma and visceral adipose tissue are not fully understood. Possible pathways of association include: (1) Adipose tissue may secrete adipokines such as leptin and adiponectin, as well as cytokines such as IL-6, IL-8, and TNFα, which triggers an inflammatory response, which in turn may lead to airway inflammation and asthma (51). (2) Airway hyperreactivity may be induced by the release of adipokines into serum during adipose tissue inflammation (52). (3) The link between obesity and asthma may also include chronic low-grade systemic inflammation, insulin resistance, and dyslipidemia (51). Studies have shown that visceral adipose tissue is more likely to be associated with adverse inflammation, blood glucose, and lipid levels than subcutaneous adipose tissue (53). Visceral fat, not subcutaneous fat, is associated with asthma (54). Further studies have also confirmed that abnormalities in visceral fat are associated with decreased lung function and increased inflammation in patients with obesity-related asthma (55). Increased visceral fat is associated with elevated levels of IL-6 (56, 57), which can disrupt the balance of fatty acid metabolism and trigger an active inflammatory state in the body (58, 59). Researchers have realized the link between IL-6 and the development of asthma (60). These findings support the general view that visceral fat is associated with a more active inflammatory state in the body. This view provides a plausible explanation for our findings: adults with a higher Body Roundness Index (BRI) are more likely to have more visceral fat, which triggers a robust inflammatory response that increases their risk of developing asthma.
This study has several strengths. First, the NHANES survey conducted between 2001 and 2018 used scientific sampling methods based on a representative sample of the U.S. general population and followed a rigorous study design with comprehensive quality control measures to ensure the reliability and scientific validity of the data. Second, the study meticulously adjusted for socioeconomic status, co-morbidities, and other possible confounders based on clinical knowledge and historical research, further ensuring the robustness of the findings. Finally, to compare the correlation between BRI and other obesity indicators, the corresponding Z-scores were calculated and included in the linear model analysis, thus ensuring the accuracy of the results. The study results showed that the correlation between BRI and asthma exceeded that of traditional obesity indicators such as waist circumference and BMI. Therefore, from a public health perspective, BRI, as a non-invasive and easy-to-administer screening tool, effectively assesses asthma risk in adults.
However, this study has its limitations. Firstly, given the study’s cross-sectional nature, we were unable to establish a causal association between BRI and asthma in adults, and future studies will need to validate this relationship with the help of cohort studies. Second, the study’s results may apply to specific countries and ethnic groups, and their generalizability has yet to be verified. Differences in fat distribution in different populations due to genetic and environmental factors may lead to differences in fat distribution, which in turn may affect the accuracy of BRI in predicting asthma prevalence. Therefore, future studies should include multinational and multiethnic samples to validate our findings further. Finally, the lack of detailed clinical variables in the database, such as medication history or specific type of asthma, is missing information that limits the depth of the study and needs to be supplemented by further research. Despite its limitations, this study demonstrated its value by revealing an association between BRI and asthma in adults. It was particularly innovative in exploring this relationship using National Health and Nutrition Examination Survey (NHANES) data.
In sum, our findings support an association between Body Roundness Index (BRI) and adult asthma risk, where higher BRI values may predict an increased risk of asthma in adults. This finding is essential for deepening our understanding of the relationship between obesity and asthma. It suggests that BRI has the potential to serve as an indicator of asthma prevention. Future studies need to further validate the efficacy of the BRI as a predictive tool for assessing adult asthma risk in different countries and ethnicities to expand its applicability to the population.
Publicly available datasets were analyzed in this study. This data can be found here: all data are freely available in NHANES. This data can be found at: https://www.cdc.gov/nchs/nhanes/index.htm.
The studies involving humans were approved by Research Ethics Review Board of the National Center for Health Statistics. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
KS: Conceptualization, Methodology, Software, Writing – original draft, Formal analysis, Investigation. YC: Investigation, Software, Visualization, Writing – review & editing, Methodology. JJ: Investigation, Validation, Writing – review & editing. CW: Funding acquisition, Writing – review & editing, Supervision. YG: Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Jilin Provincial Department of Science and Technology project (20220303001SF) and the Special Support for Health Talents in Jilin Province (JLSWSRCZX2021-031).
The authors would like to thank the First Hospital of Jilin University staff.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Wu, TD, Brigham, EP, and McCormack, MC. Asthma in the primary care setting. Med Clin North Am. (2019) 103:435–52. doi: 10.1016/j.mcna.2018.12.004
2. Huang, AA, and Huang, SY. Use of feature importance statistics to accurately predict asthma attacks using machine learning: a cross-sectional cohort study of the US population. PLoS One. (2023) 18:e0288903. doi: 10.1371/journal.pone.0288903
3. Global Initiative for Asthma. (2024). Global strategy for asthma management and prevention. Available at: http://www.ginasthma.org/ (Accessed at May 7, 2024).
4. Asher, MI, García-Marcos, L, Pearce, NE, and Strachan, DP. Trends in worldwide asthma prevalence. Eur Respir J. (2020) 56:2002094. doi: 10.1183/13993003.02094-2020
5. Lommatzsch, M, Buhl, R, and Korn, S. The treatment of mild and moderate asthma in adults. Dtsch Arztebl Int. (2020) 117:434–44. doi: 10.3238/arztebl.2020.0434
6. Zhou, Y, Li, L, Yu, Z, Gu, X, Pan, R, Li, Q, et al. Dermatophagoides pteronyssinus allergen Der p 22: cloning, expression, IgE-binding in asthmatic children, and immunogenicity. Pediatr Allergy Immunol. (2022) 33:e13835. doi: 10.1111/pai.13835
7. Blüher, M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. (2019) 15:288–98. doi: 10.1038/s41574-019-0176-8
8. Zhang, Y, Yang, J, Hou, W, and Arcan, C. Obesity trends and associations with types of physical activity and sedentary behavior in US adults: National Health and nutrition examination survey, 2007-2016. Obesity (Silver Spring). (2021) 29:240–50. doi: 10.1002/oby.23043
9. Brumpton, B, Langhammer, A, Romundstad, P, Chen, Y, and Mai, X-M. General and abdominal obesity and incident asthma in adults: the HUNT study. Eur Respir J. (2013) 41:323–9. doi: 10.1183/09031936.00012112
10. Ma, J, Xiao, L, and Knowles, SB. Obesity, insulin resistance and the prevalence of atopy and asthma in US adults. Allergy. (2010) 65:1455–63. doi: 10.1111/j.1398-9995.2010.02402.x
11. Beuther, DA, and Sutherland, ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. (2007) 175:661–6. doi: 10.1164/rccm.200611-1717OC
12. Swed, S, Sawaf, B, Al-Obeidat, F, Hafez, W, Rakab, A, Alibrahim, H, et al. Asthma prevalence among United States population insights from NHANES data analysis. Sci Rep. (2024) 14:8059. doi: 10.1038/s41598-024-58429-5
13. Akinbami, LJ, and Fryar, CD. Current asthma prevalence by weight status among adults: United States, 2001-2014. NCHS Data Brief. (2016) 239:1–8. Available at: https://pubmed.ncbi.nlm.nih.gov/27019018
14. Chen, Z, Salam, MT, Alderete, TL, Habre, R, Bastain, TM, Berhane, K, et al. Effects of childhood asthma on the development of obesity among school-aged children. Am J Respir Crit Care Med. (2017) 195:1181–8. doi: 10.1164/rccm.201608-1691OC
16. Alwadeai, KS, and Alhammad, SA. Asthma and its relationship with anthropometric markers among adults. PLoS One. (2023) 18:e0281923. doi: 10.1371/journal.pone.0281923
17. Kang, PS, and Neeland, IJ. Body fat distribution, diabetes mellitus, and cardiovascular disease: an update. Curr Cardiol Rep. (2023) 25:1555–64. doi: 10.1007/s11886-023-01969-5
18. Zhang, L, Xia, Z, Li, Z, Zhang, J, Wang, K, and Wang, W. Influence of body fat tissue on outcomes in patients undergoing hepatectomy or liver transplantation. Int J Surg. (2024) 111:1167–81. doi: 10.1097/JS9.0000000000001864
19. Thomas, DM, Bredlau, C, Bosy-Westphal, A, Mueller, M, Shen, W, Gallagher, D, et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity. (2013) 21:2264–71. doi: 10.1002/oby.20408
20. Kim, JY, Choi, J, Vella, CA, Criqui, MH, Allison, MA, and Kim, NH. Associations between weight-adjusted waist index and abdominal fat and muscle mass: multi-ethnic study of atherosclerosis. Diabetes Metab J. (2022) 46:747–55. doi: 10.4093/dmj.2021.0294
21. Cai, X, Song, S, Hu, J, Zhu, Q, Yang, W, Hong, J, et al. Body roundness index improves the predictive value of cardiovascular disease risk in hypertensive patients with obstructive sleep apnea: a cohort study. Clin Exp Hypertens. (2023) 45:2259132. doi: 10.1080/10641963.2023.2259132
22. Rico-Martín, S, Calderón-García, JF, Sánchez-Rey, P, Franco-Antonio, C, Martínez Alvarez, M, and Sánchez Muñoz-Torrero, JF. Effectiveness of body roundness index in predicting metabolic syndrome: a systematic review and meta-analysis. Obes Rev. (2020) 21:e13023. doi: 10.1111/obr.13023
23. Wu, L, Pu, H, Zhang, M, Hu, H, and Wan, Q. Non-linear relationship between the body roundness index and incident type 2 diabetes in Japan: a secondary retrospective analysis. J Transl Med. (2022) 20:110. doi: 10.1186/s12967-022-03321-x
24. Zhang, Y, Gao, W, Ren, R, Liu, Y, Li, B, Wang, A, et al. Body roundness index is related to the low estimated glomerular filtration rate in Chinese population: a cross-sectional study. Front Endocrinol. (2023) 14:1148662. doi: 10.3389/fendo.2023.1148662
25. Gao, W, Jin, L, Li, D, Zhang, Y, Zhao, W, Zhao, Y, et al. The association between the body roundness index and the risk of colorectal cancer: a cross-sectional study. Lipids Health Dis. (2023) 22:53. doi: 10.1186/s12944-023-01814-2
26. Zhang, X, Ma, N, Lin, Q, Chen, K, Zheng, F, Wu, J, et al. Body roundness index and all-cause mortality among US adults. JAMA Netw Open. (2024) 7:e2415051. doi: 10.1001/jamanetworkopen.2024.15051
27. Lin, Z, Huang, J, Xie, S, Zheng, Z, Tang, K, Li, S, et al. The association between insulin use and asthma: an epidemiological observational analysis and Mendelian randomization study. Lung. (2023) 201:189–99. doi: 10.1007/s00408-023-00611-z
28. Odebeatu, CC, Taylor, T, Fleming, LE, and Osborne, JN. Phthalates and asthma in children and adults: US NHANES 2007-2012. Environ Sci Pollut Res Int. (2019) 26:28256–69. doi: 10.1007/s11356-019-06003-2
29. Wang, K, Chen, Z, Wei, Z, He, L, and Gong, L. Association between body fat distribution and asthma in adults: results from the cross-sectional and bidirectional Mendelian randomization study. Front Nutr. (2024) 11:1432973. doi: 10.3389/fnut.2024.1432973
30. Torén, K, Brisman, J, and Järvholm, B. Asthma and asthma-like symptoms in adults assessed by questionnaires. A literature review. Chest. (1993) 104:600–8.
31. de Marco, R, Cerveri, I, Bugiani, M, Ferrari, M, and Verlato, G. An undetected burden of asthma in Italy: the relationship between clinical and epidemiological diagnosis of asthma. Eur Respir J. (1998) 11:599–605. doi: 10.1183/09031936.98.11030599
32. Kilpeläinen, M, Terho, EO, Helenius, H, and Koskenvuo, M. Validation of a new questionnaire on asthma, allergic rhinitis, and conjunctivitis in young adults. Allergy. (2001) 56:377–84. doi: 10.1034/j.1398-9995.2001.056005377.x
33. Wang, K, Zhao, L, Luo, H, Deng, C, Gong, L, and Chen, Z. Association of serum vitamin C levels with asthma in adults: results of NHANES 2003-2006 and mendelian randomization study. BMC Pulm Med. (2024) 24:4. doi: 10.1186/s12890-023-02821-w
34. Zhang, Q, Xiao, S, Jiao, X, and Shen, Y. The triglyceride-glucose index is a predictor for cardiovascular and all-cause mortality in CVD patients with diabetes or pre-diabetes: evidence from NHANES 2001-2018. Cardiovasc Diabetol. (2023) 22:279. doi: 10.1186/s12933-023-02030-z
35. Chin, HL, and Cheong, KK. Association between asthma and headache: findings from the NHANES 2001-2004. Clin Respir J. (2023) 17:799–804. doi: 10.1111/crj.13664
36. Ding, L, Zhang, H, Dai, C, Zhang, A, Yu, F, Mi, L, et al. The prognostic value of the stress hyperglycemia ratio for all-cause and cardiovascular mortality in patients with diabetes or prediabetes: insights from NHANES 2005-2018. Cardiovasc Diabetol. (2024) 23:84. doi: 10.1186/s12933-024-02172-8
37. Wang, X, Wen, J, Gu, S, Zhang, L, and Qi, X. Frailty in asthma-COPD overlap: a cross-sectional study of association and risk factors in the NHANES database. BMJ Open Respir Res. (2023) 10:e001713. doi: 10.1136/bmjresp-2023-001713
38. Castillo, JR, Peters, SP, and Busse, WW. Asthma exacerbations: pathogenesis, prevention, and treatment. J Allergy Clin Immunol Pract. (2017) 5:918–27. doi: 10.1016/j.jaip.2017.05.001
39. Taylor, B, Mannino, D, Brown, C, Crocker, D, Twum-Baah, N, and Holguin, F. Body mass index and asthma severity in the National Asthma Survey. Thorax. (2008) 63:14–20. doi: 10.1136/thx.2007.082784
40. Tomiyama, AJ, Hunger, JM, Nguyen-Cuu, J, and Wells, C. Misclassification of cardiometabolic health when using body mass index categories in NHANES 2005-2012. Int J Obes. (2016) 40:883–6. doi: 10.1038/ijo.2016.17
41. Khan, I, Chong, M, Le, A, Mohammadi-Shemirani, P, Morton, R, Brinza, C, et al. Surrogate adiposity markers and mortality. JAMA Netw Open. (2023) 6:e2334836. doi: 10.1001/jamanetworkopen.2023.34836
42. Prillaman, M. Why BMI is flawed - and how to redefine obesity. Nature. (2023) 622:232–3. doi: 10.1038/d41586-023-03143-x
43. Fenger, RV, Gonzalez-Quintela, A, Vidal, C, Gude, F, Husemoen, LL, Aadahl, M, et al. Exploring the obesity-asthma link: do all types of adiposity increase the risk of asthma? Clin Exp Allergy. (2012) 42:1237–45. doi: 10.1111/j.1365-2222.2012.03972.x
44. Goudarzi, H, Konno, S, Kimura, H, Makita, H, Matsumoto, M, Takei, N, et al. Impact of abdominal visceral adiposity on adult asthma symptoms. J Allergy Clin Immunol Pract. (2019) 7:1222–1229.e5. doi: 10.1016/j.jaip.2018.11.014
45. Schiffers, C, Wouters, EFM, Breyer-Kohansal, R, Buhl, R, Pohl, W, Irvin, CG, et al. Asthma prevalence and phenotyping in the general population: the LEAD (lung, hEart, sociAl, boDy) study. J Asthma Allergy. (2023) 16:367–82. doi: 10.2147/JAA.S402326
46. Miller, DR, Ingersoll, MA, Chou, Y-W, Kosmacek, EA, Oberley-Deegan, RE, and Lin, M-F. Dynamics of antioxidant heme oxygenase-1 and pro-oxidant p66Shc in promoting advanced prostate cancer progression. Free Radic Biol Med. (2022) 193:274–91. doi: 10.1016/j.freeradbiomed.2022.10.269
47. Kapoor, N, Lotfaliany, M, Sathish, T, Thankappan, KR, Thomas, N, Furler, J, et al. Obesity indicators that best predict type 2 diabetes in an Indian population: insights from the Kerala diabetes prevention program. J Nutr Sci. (2020) 9:e15. doi: 10.1017/jns.2020.8
48. Leone, N, Courbon, D, Thomas, F, Bean, K, Jégo, B, Leynaert, B, et al. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med. (2009) 179:509–16. doi: 10.1164/rccm.200807-1195OC
49. Lv, N, Xiao, L, Camargo, CA, Wilson, SR, Buist, AS, Strub, P, et al. Abdominal and general adiposity and level of asthma control in adults with uncontrolled asthma. Ann Am Thorac Soc. (2014) 11:1218–24. doi: 10.1513/AnnalsATS.201405-214OC
50. Kapoor, N, Jiwanmall, SA, Nandyal, MB, Kattula, D, Paravathareddy, S, Paul, TV, et al. Metabolic score for visceral fat (METS-VF) estimation - a novel cost-effective obesity Indicator for visceral adipose tissue estimation. Diabetes Metab Syndr Obes. (2020) 13:3261–7. doi: 10.2147/DMSO.S266277
51. Peters, U, Suratt, BT, Bates, JHT, and Dixon, AE. Beyond BMI: obesity and lung disease. Chest. (2018) 153:702–9. doi: 10.1016/j.chest.2017.07.010
52. Leone, N, Courbon, D, Berr, C, Barberger-Gateau, P, Tzourio, C, Alpérovitch, A, et al. Abdominal obesity and late-onset asthma: cross-sectional and longitudinal results: the 3C study. Obesity (Silver Spring). (2012) 20:628–35. doi: 10.1038/oby.2011.308
53. Fox, CS, Massaro, JM, Hoffmann, U, Pou, KM, Maurovich-Horvat, P, Liu, C-Y, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham heart study. Circulation. (2007) 116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355
54. Mensink-Bout, SM, Santos, S, van Meel, ER, Oei, EHG, de Jongste, JC, Jaddoe, VWV, et al. General and organ fat assessed by magnetic resonance imaging and respiratory outcomes in childhood. Am J Respir Crit Care Med. (2020) 201:348–55. doi: 10.1164/rccm.201905-0942OC
55. Collins, LC, Hoberty, PD, Walker, JF, Fletcher, EC, and Peiris, AN. The effect of body fat distribution on pulmonary function tests. Chest. (1995) 107:1298–302. doi: 10.1378/chest.107.5.1298
56. Hildebrand, S, Stümer, J, and Pfeifer, A. PVAT and its relation to Brown, beige, and white adipose tissue in development and function. Front Physiol. (2018) 9:70. doi: 10.3389/fphys.2018.00070
57. Kiefer, FW. The significance of beige and brown fat in humans. Endocr Connect. (2017) 6:R70–9. doi: 10.1530/EC-17-0037
58. Wolsk, E, Mygind, H, Grøndahl, TS, Pedersen, BK, and van Hall, G. IL-6 selectively stimulates fat metabolism in human skeletal muscle. Am J Physiol Endocrinol Metab. (2010) 299:E832–40. doi: 10.1152/ajpendo.00328.2010
59. Kwon, H, and Pessin, JE. Adipokines mediate inflammation and insulin resistance. Front Endocrinol. (2013) 4:71. doi: 10.3389/fendo.2013.00071
Keywords: asthma prevalence, Body Roundness Index, obesity, cross-sectional study, NHANES
Citation: Sun K, Chang Y, Jie J, Wang C and Gu Y (2025) The J-shaped relationship between body roundness index and adult asthma: insights from NHANES 2001–2018. Front. Nutr. 12:1516003. doi: 10.3389/fnut.2025.1516003
Received: 23 October 2024; Accepted: 10 March 2025;
Published: 20 March 2025.
Edited by:
Liang Gong, First Affiliated Hospital of Chongqing Medical University, ChinaReviewed by:
Samuel Huang, Virginia Commonwealth University, United StatesCopyright © 2025 Sun, Chang, Jie, Wang and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yue Gu, eWd1QGpsdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.