
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 31 March 2025
Sec. Clinical Nutrition
Volume 12 - 2025 | https://doi.org/10.3389/fnut.2025.1514209
Background: Given the global changes in environmental and dietary habits, understanding the potential impact of dietary factors and diet-related inflammation on skeletal muscle diseases, including sarcopenia, is crucial. Investigating these relationships can aid in the development of more effective prevention strategies. This study used the Dietary Index for Gut Microbiota (DI-GM) and the Dietary Inflammatory Index (DII) as diet-related variables. DI-GM is a scoring system used to assess the influence of diet on Gut Microbiota health. Additionally, DII quantifies the inflammatory potential of a diet. This study explores the association between DI-GM and sarcopenia and evaluates whether DII moderates this relationship.
Methods: This study conducted a cross-sectional analysis of 9,470 participants from the 2011–2018 NHANES database. Multivariable logistic regression, restricted cubic splines (RCS), and subgroup analysis were employed to examine the association between DI-GM and the prevalence of sarcopenia. Additionally, mediation analysis was performed to investigate the potential associations between DII, DI-GM, and sarcopenia.
Results: A total of 9,470 participants were included in this study, of whom 823 (7%) had sarcopenia. After adjusting for all variables using multivariable logistic regression, each one-unit increase in DI-GM was associated with a 15% decrease in sarcopenia prevalence (OR = 0.85, 95% CI: 0.77, 0.94), while each one-unit increase in DII was associated with a 28% increase in sarcopenia prevalence (OR = 1.28, 95% CI: 1.17, 1.41). Furthermore, the results remained robust when DI-GM and DII were divided into tertiles. RCS analysis revealed a significant linear relationship between DI-GM and sarcopenia. The results of the subgroup analysis also showed that the above relationships were robust. Mediation analysis showed that 55% of the association between DI-GM and sarcopenia was mediated by DII (P < 0.001).
Conclusion: Adhering to dietary recommendations based on DI-GM may reduce the prevalence of sarcopenia. Additionally, DII appears to mediate this relationship, suggesting that an anti-inflammatory diet could offer potential benefits.
Sarcopenia is defined as a progressive and systemic skeletal muscle disease characterized by the loss of muscle mass and function (1). Studies have shown that its prevalence reaches 5–13% among individuals aged 60–70 and rises to 11–50% in those aged 80 and older, posing a significant challenge to healthcare costs. In 2000, the direct medical costs associated with sarcopenia in the United States were estimated at $18.5 billion. A 10% reduction in sarcopenia prevalence could save the U.S. healthcare system $1.1 billion annually (2, 3). Beyond its association with increased mortality risk, sarcopenia has been identified as a critical prognostic indicator for survival and clinical complications in patients with cancer, kidney dysfunction, liver disease, and metabolic disorders (4). Preventing sarcopenia has been a key area of focus, with research identifying age, physical inactivity, metabolic imbalances, and neuromuscular dysfunction as major risk factors (1, 5). Regarding lifestyle factors, numerous studies have explored muscle outcomes related to dietary patterns, suggesting that physical activity and nutritional status, influenced by dietary intake or supplementation, are linked to sarcopenia risk (2, 4).
The composition of the gut microbiota is associated with various aspects of human health, with diet serving as a key determinant of the structure and function of gut microbial communities (6, 7). Research has indicated that dysbiosis can lead to significant alterations in skeletal muscle metabolism through the “gut-muscle axis” and the subsequent loss of microbiota-derived metabolites (8). An animal study suggested that probiotics may regulate age-related muscle damage, and other studies have highlighted that different gut microbial taxa may have varying effects on muscle (9, 10). Recently, a novel DI-GM was proposed (11). Unlike existing indices, DI-GM incorporates specific foods rather than food groups and includes fermented dairy products, which are a unique dietary component critical for gut microbiota health (11).
The Dietary Inflammatory Index (DII) was introduced in 2014 to quantify the effect of dietary components on six inflammatory biomarkers (IL-1β, IL-4, IL-6, IL-10, TNF-α, and C-reactive protein) (12). Chronic inflammation is linked to the development of several non-communicable chronic diseases, and diet may influence these diseases through mechanisms such as gut microbiota regulation (13). One study investigating the relationship between DII and musculoskeletal issues in adults found that pro-inflammatory diets may be associated with such problems (14). This could be due to elevated levels of serum inflammatory cytokines, which are linked to lower muscle mass and poorer muscle function (15). Inflammation may increase the risk of sarcopenia by activating the ubiquitin-proteasome system, enhancing oxidative stress and NF-κB activity, and impairing skeletal muscle protein synthesis (16–18). However, despite the growing research on DI-GM and DII, systematic studies exploring the association between DI-GM, DII, and sarcopenia remain scarce. Investigating these potential associations could not only offer new directions for sarcopenia prevention but also promote the adoption of healthier and more sustainable diets.
This study aims to investigate the association between DI-GM and sarcopenia, with a specific focus on the potential mediating role of DII in this relationship. Given the growing evidence suggesting that diet influences sarcopenia both directly and indirectly through its impact on gut microbiota and systemic inflammation, we hypothesize that DI-GM is inversely associated with sarcopenia and that DII partially mediates this association. To test these hypotheses, we utilized data from the 2005 to 2018 National Health and Nutrition Examination Survey (NHANES), a nationally representative database, to explore these relationships in a large sample.
This cross-sectional study utilized data from the National Health and Nutrition Examination Survey (NHANES) conducted by the National Center for Health Statistics (NCHS). The survey aims to collect demographic information regarding the health and nutritional intake of U.S. citizens. The NCHS Research Ethics Review Board approved all NHANES study protocols, and written informed consent was obtained from all participants. Our secondary analysis adhered to the STROBE guidelines for cross-sectional studies and did not require additional Institutional Review Board approval. For more details on NHANES methods and ethical considerations, please refer to the Centers for Disease Control and Prevention (CDC) and the National Health and Nutrition Examination Survey website: https://www.cdc.gov/nchs/nhanes/index.htm.
This cross-sectional study utilized nationally representative data from the National Health and Nutrition Examination Survey (NHANES). Among the 39,156 participants across four NHANES cycles from 2011 to 2018, 16,539 individuals aged 20 years and older and not pregnant were identified. Participants with incomplete data for the Dietary Gut Microbiota Index (DI-GM) and Dietary Inflammatory Index (DII) (n = 4,307) were excluded, as were those with incomplete data on sarcopenia (n = 8,840). Ultimately, 9,470 participants were included in the study (Supplementary Figure S1).
Total appendicular lean mass (ALM) was assessed using dual-energy X-ray absorptiometry (DEXA) in the NHANES program. Sarcopenia was diagnosed using the Sarcopenia Index, calculated by dividing the total mass of appendicular skeletal muscle (in kg/m2) by body mass index (BMI, in kg/m2) (19). According to the guidelines from the Foundation for the National Institutes of Health (FNIH), cut-off values for the Sarcopenia Index were set at < 0.789 for men and < 0.512 for women to diagnose sarcopenia (20).
The Dietary Inflammatory Index (DII) serves as a key metric for assessing the inflammatory potential of various food components, including vitamins and minerals. A DII score of ≥ 0 indicates a pro-inflammatory diet, while a score of < 0 reflects an anti-inflammatory diet (21). Furthermore, higher DII scores suggest harmful dietary habits, whereas lower DII scores are indicative of healthier dietary practices (22). Supplementary material include a detailed algorithm for calculating the DII score.
According to Kase et al. (11), the Dietary Index for Gut Microbiota (DI-GM) comprises 14 food items or nutrients. Beneficial components include fermented dairy products, chickpeas, soy, whole grains, fiber, cranberries, avocados, broccoli, coffee, and green tea, while adverse components consist of red meat, processed meats, refined grains, and diets high in fat (≥40% of energy from fat). Each component is scored as 0 or 1 based on gender-specific median intake levels, and the scores are summed to derive the overall DI-GM score. The specific calculation method for DI-GM is detailed in Supplementary Table S1.
The covariates for this study included age, sex, education level, marital status, poverty-income ratio (PIR), race, smoking, alcohol consumption, physical activity, hypertension, diabetes, and hyperlipidemia. Detailed information regarding these covariates can be found in Supplementary Table S2.
To ensure that the data represents the national population, all analyses employed sampling weights. The weight variable used in our study was the 2-day dietary sample weight (WTDR2D), and we calculated new weights for the 2011–2018 period as 1/4 × WTDR2D. Continuous variables are presented as means ± standard deviation (SD), while categorical variables are presented as frequencies (percentages). Weighted t-tests were used to compare continuous variables, and weighted chi-square tests were employed for categorical variables. The relationship between DI-GM and sarcopenia was examined using weighted logistic regression. Three logistic regression models were established: Model 1 was unadjusted for potential confounding factors; Model 2 adjusted for covariates including age, sex, education level, marital status, PIR, and race; and Model 3 further adjusted for smoking, alcohol consumption, physical activity, hypertension, diabetes, and hyperlipidemia based on Model 2. Additionally, in Model 3, DI-GM was treated as a continuous variable, and restricted cubic splines (RCS) were used to illustrate the linear or non-linear association between DI-GM and Sarcopenia. RCS is a flexible regression method that allows for modeling curved trends without assuming a strict linear relationship, providing a more precise representation of the association. Compared to traditional linear regression, RCS can detect threshold effects or dose-response relationships, making the results more biologically meaningful. Subsequently, a stratified subgroup analysis was conducted based on model 3 to examine covariates. Interaction analyses were then performed to explore potential variations in associations among subgroups.
Based on the premises that “DI-GM is statistically significantly associated with DII” and “DII is statistically significantly associated with sarcopenia,” a mediation analysis was conducted to investigate whether the effect of DI-GM on sarcopenia is mediated by DII. The mediation effect was calculated using the “mediation” package in R software (23). Data processing was performed using R statistical software (version 4.3.1). Two-sided p < 0.05 were considered statistically significant.
This study included 9,470 participants aged 20 and older, inferring approximately 82.93 million U.S. adults. The prevalence of sarcopenia was found to be 7%, corresponding to around 6.18 million individuals. The average score of DI-GM was 5.00 (SD:1.54). In addition, DI-GM scores were lower in patients with sarcopenia compared to those without sarcopenia (5.00 vs. 5.30). Statistically significant differences (all p < 0.05) were observed between sarcopenic patients and non-sarcopenic individuals concerning age, race, education level, PIR, alcohol consumption, physical activity, hypertension, blood glucose levels, and hyperlipidemia. Patients with sarcopenia present as older, male, white, married, highly educated, and hyperlipidemic. Additionally, DII was higher in sarcopenic patients compared to non-sarcopenic individuals, also showing a statistically significant difference (p < 0.05). For more detailed information, refer to Table 1.
As shown in Table 2, three different logistic regression models were employed to evaluate the association between DI-GM scores and the prevalence of sarcopenia, all indicating a negative correlation (p < 0.001). In Model 3, after adjusting for various covariates, a one standard deviation increase in the DI-GM score was associated with a 15% reduction in the prevalence of sarcopenia [odds ratio (OR): 0.85 (95% confidence interval: 0.77, 0.94)]. Furthermore, when DI-GM was categorized into tertiles, the group with the highest DI-GM scores (T3) showed a 51% lower prevalence of sarcopenia compared to the group with the lowest scores (T1) [OR: 0.49 (95% confidence interval: 0.31, 0.79)].
Additionally, the relationship between DII and sarcopenia was assessed, revealing a positive correlation across all three models (all p < 0.001). Higher DII scores were associated with an increased prevalence of sarcopenia, and the results were statistically significant (p < 0.05). Since dietary factors influence sarcopenia, we further included protein intake and total caloric intake in Model 4. The results remained robust. As shown in the RCS results (Figure 1), after adjusting for relevant variables, a significant negative correlation between DI-GM scores and the prevalence of sarcopenia was further confirmed (overall P < 0.001; non-linear P = 0.893).
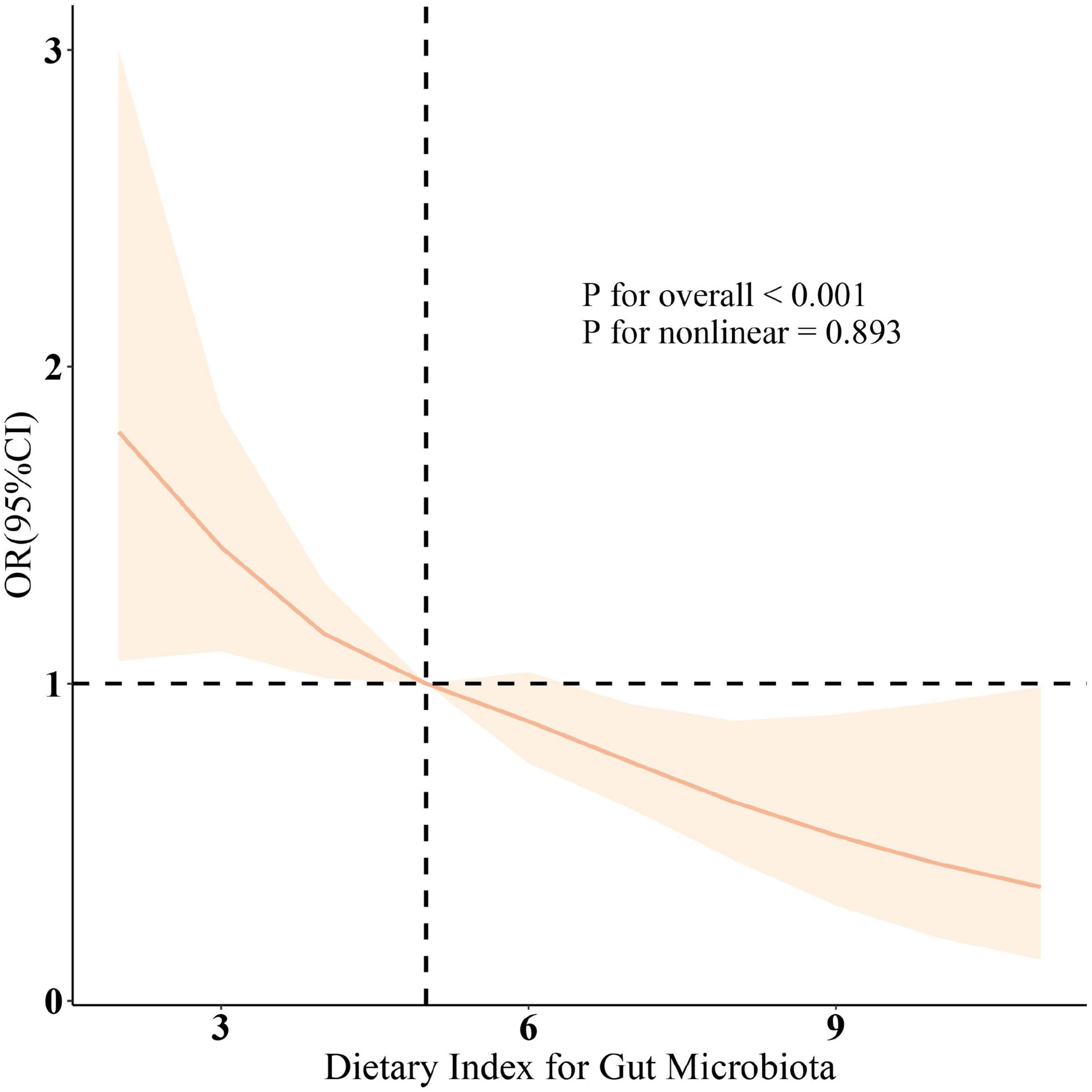
Figure 1. The restricted cubic spline (RCS) analysis between DI-GM and sarcopenia. OR (solid lines) and 95% confidence levels (shaded areas) were adjusted for age, gender, education level, marital, PIR, race, smoking, drinking, physical activity, hypertension, diabetes, and hyperlipidemia. RCS was used to illustrate the linear or nonlinear association between DI-GM and Sarcopenia. RCS is a flexible regression method that allows for modeling curved trends without assuming a strict linear relationship, providing a more precise representation of the association.
As shown in Figure 2, subgroup analysis was conducted based on age, sex, race, marital status, education level, PIR, smoking, alcohol consumption, physical activity, hypertension, diabetes, and hyperlipidemia. The results indicated a negative correlation between DI-GM scores and sarcopenia prevalence in most subgroups. After adjusting for various confounding factors, no significant interaction effects were observed, suggesting that the association between DI-GM and sarcopenia is stable across different demographic and health-related factors. Specifically, a higher DI-GM was associated with a lower risk of sarcopenia (OR = 0.85, 95% CI: 0.77–0.94). The protective effect was significant in individuals aged 20–30 years (OR = 0.83, 95% CI: 0.70–0.98) and > 40 years (OR = 0.85, 95% CI: 0.75–0.96). The association was stronger in females (OR = 0.81, 95% CI: 0.73–0.89) than in males. Low-income individuals (OR = 0.75, 95% CI: 0.65–0.85) benefited more than non-poor individuals. Never-smokers and former drinkers had a stronger protective effect. DI-GM remained significant in individuals with hypertension and hyperlipidemia but not in those with diabetes.
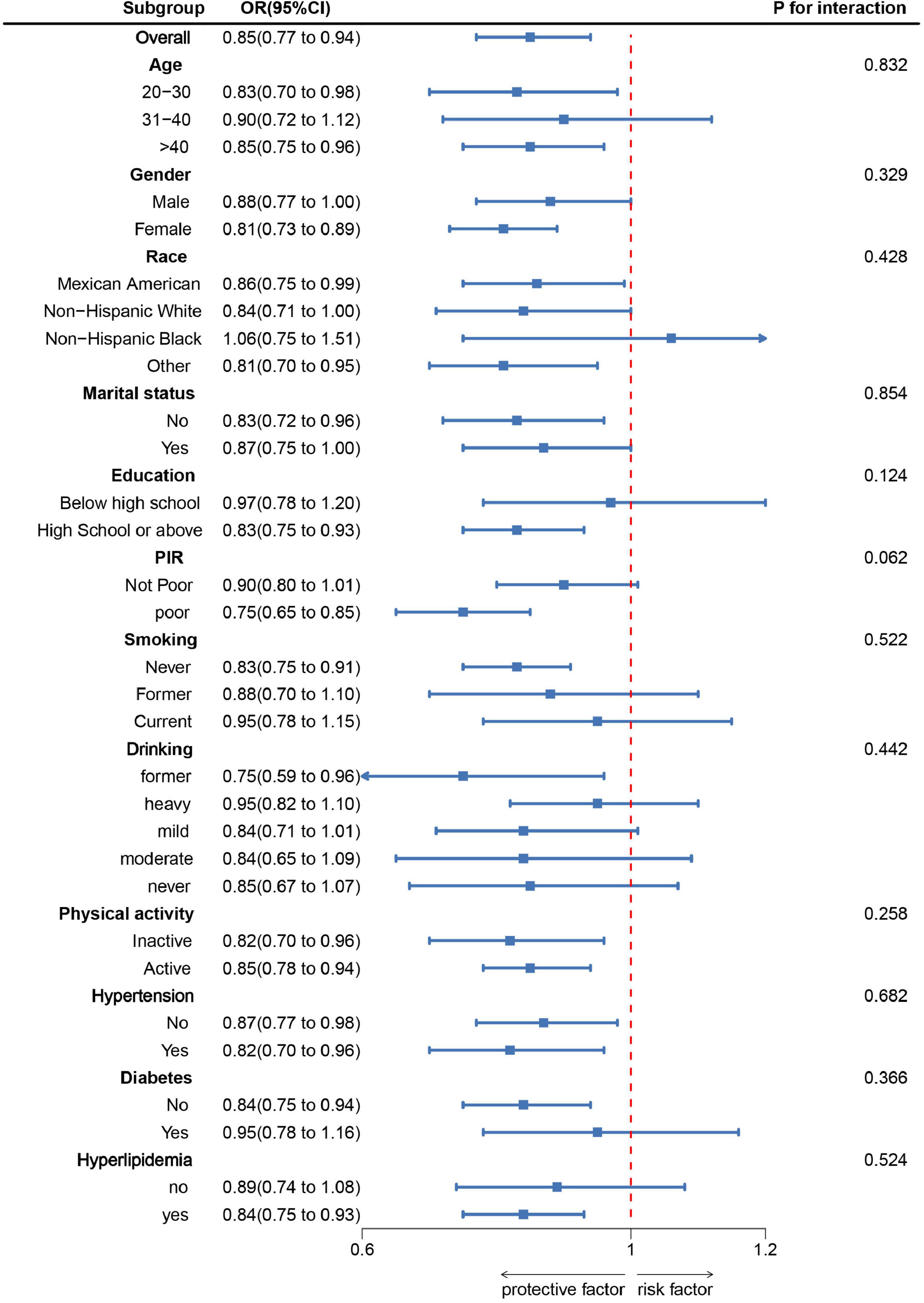
Figure 2. Subgroup analysis between DI-GM and Sarcopenia. ORs were calculated for each 1-unit increase in DI-GM. Analyses were adjusted for age, gender, education level, marital, PIR, race, smoking, drinking, physical activity, hypertension, diabetes, and hyperlipidemia.
The mediation model and pathways are illustrated in Figure 3, with DI-GM as the independent variable, sarcopenia as the dependent variable, and DII as the mediating variable. As shown in Table 3, a significant correlation was observed between DI-GM and DII after adjusting for other covariates (β = −0.46, 95% CI: −0.49, −0.43). After adjusting for all covariates, the mediating effect of DII was identified (Figure 3) (indirect effect = −0.011, P < 0.001; direct effect = −0.009, P = 0.014). Therefore, DII can be considered a mediating factor in the association between DI-GM and the likelihood of sarcopenia.
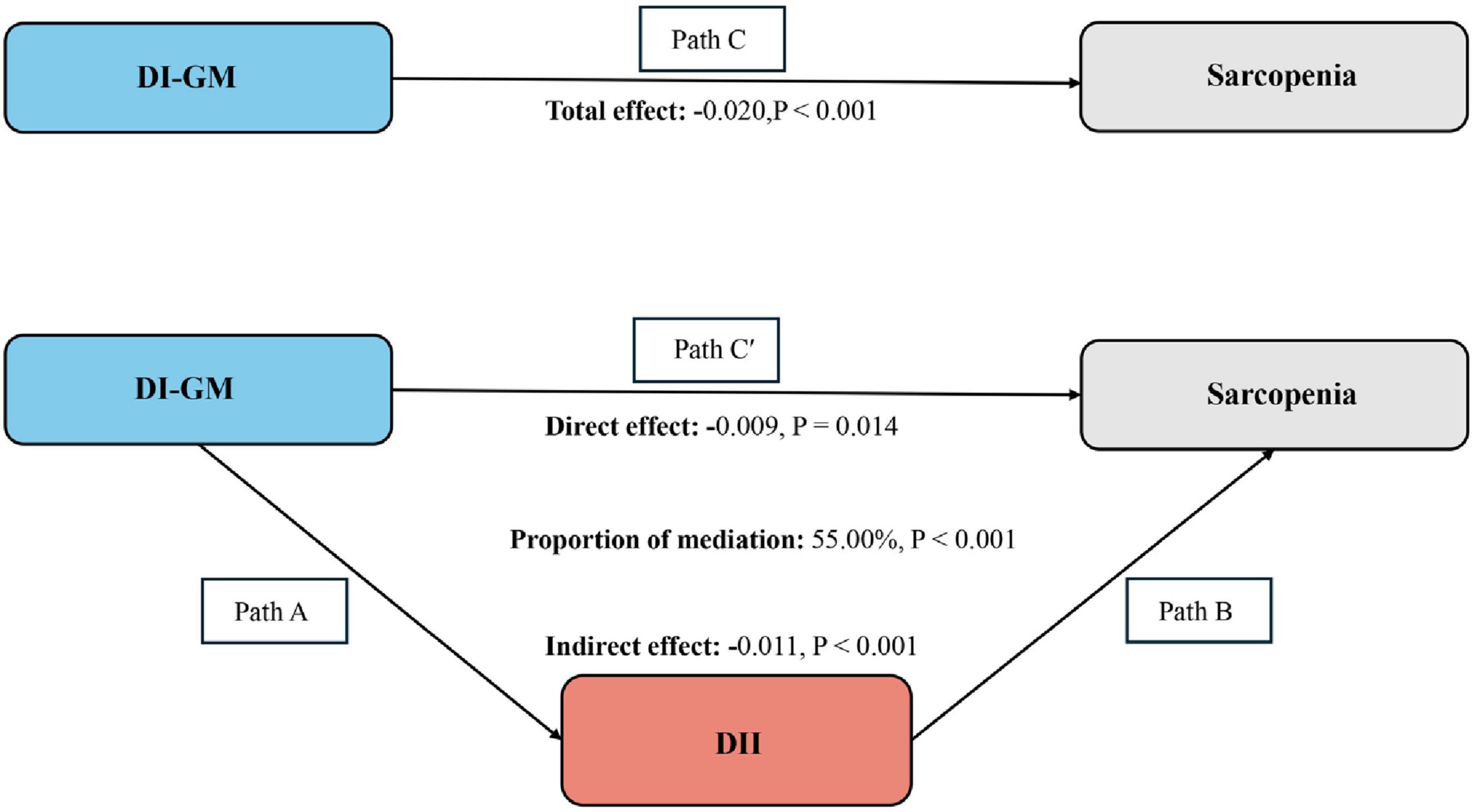
Figure 3. Schematic diagram of the mediation effect analysis. Path C indicates the total effect; path C’ indicates the direct effect. The indirect effect is estimated as the multiplication of paths A and B (path A*B). The mediated proportion is calculated as indirect effect/ (indirect effect + direct effect) × 100%. DI-GM, Dietary Index for Gut Microbiota; DII, Dietary Inflammatory Index. Analyses were adjusted for age, gender, education level, marital, PIR, race, smoking, drinking, physical activity, hypertension, diabetes, and hyperlipidemia. Mediation analysis was conducted to investigate whether the effect of DI-GM on sarcopenia is mediated by DII. The mediation effect was calculated using the “mediation” package in R software.
This cross-sectional analysis reveals a negative correlation between DI-GM and the prevalence of sarcopenia, while DII shows a positive correlation with sarcopenia prevalence. To our knowledge, this is the first cross-sectional study to explore the relationship between DI-GM and sarcopenia. Additionally, mediation analysis indicates that DII mediates the relationship between DI-GM and sarcopenia. As the understanding of the connections between diet and inflammation, as well as inflammation and health, advances, DII can be utilized to assess the impact of diet on health outcomes (24). Therefore, dietary management for sarcopenia holds significant implications for clinical practitioners. This study suggests that adherence to a DI-GM dietary pattern may influence the development of sarcopenia by reducing the inflammatory potential of the diet.
Changes in the composition of the gut microbiota may actually promote chronic inflammation and metabolic resistance, ultimately leading to reduced muscle size, impaired muscle function, and adverse clinical outcomes (25). Compared to existing dietary indices, the Dietary Gut Microbiota Index (DI-GM) can effectively identify dietary patterns that are beneficial or harmful to gut microbiota, potentially serving as a standardized tool for assessing a balanced diet for gut health (26). One unique aspect of DI-GM is the inclusion of fermented dairy products, which can modulate gut microbiota. The metabolites produced by the gut microbiota are believed to have potential effects on skeletal muscle, potentially through mechanisms such as the action of short-chain fatty acids (SCFAs). SCFAs, particularly butyrate, propionate, and acetate, exert anti-inflammatory effects by inhibiting histone deacetylases (HDACs) and activating GPR41/GPR43, reducing pro-inflammatory cytokines (27). Studies have shown that SCFAs enhance muscle mass and function by activating the mTOR signaling pathway in muscle-reducing mice to mitigate age-related muscle loss and dysfunction (28). SCFAs also inhibit the production of inflammatory markers via the NF-κB pathway (29), and chronic inflammation is a hallmark of skeletal muscle disorders (30). Furthermore, dysbiosis may reduce secondary bile acids, which are associated with a nuclear receptor (FXR). Animal studies have indicated that bile acids, FXR signaling, and the expression of FGF15/19 (fibroblast growth factors 15/19) may influence host skeletal muscle metabolism by promoting muscle growth and mitigating muscle atrophy (8, 31, 32). Additionally, one of the metabolites, branched-chain amino acids (BCAAs), enhances insulin-mediated glucose metabolism in liver and muscle cells, which may also be a potential mechanism by which gut microbiota influence skeletal muscle (33). A study suggested that the downregulation of certain key species in the gut of sarcopenia patients may affect muscle mass and physical function through the combined effects of various metabolites (34). Another cohort study indicated that reduced gut microbial diversity in men is associated with a low skeletal muscle mass index (SMI) (35), which aligns with our findings on the association between DI-GM and sarcopenia.
Chronic low-grade inflammation is known to be associated with the pathogenesis of several chronic non-communicable diseases (13). A low dietary quality score is often correlated with elevated levels of plasma IL-6, E-selectin, and soluble ICAM-1 (36). Furthermore, a randomized controlled trial demonstrated that the intake of fermented foods increased the diversity of the microbiome while reducing several pro-inflammatory cytokines and chemokines (37). Anti-inflammatory dietary patterns can decrease inflammation and thus prevent muscle loss (38). The Dietary Inflammatory Index (DII), which is based on six inflammatory biomarkers, effectively reflects the relationship between diet and inflammation (12). Several studies have indicated a correlation between DII and muscle mass (14, 15). The relationship between the inflammatory index and skeletal muscle mass may be attributed to inflammatory factors (including CRP, IL-6, and TNF-α) that inhibit the activity of the insulin-like growth factor 1 (IGF-1), activating the ubiquitin-proteasome system and leading to metabolic resistance and the loss of muscle homeostasis (39). Moreover, inflammation is associated with early malnutrition. In experimental models, the abundance of key taxa related to inflammation regulation (such as Escherichia coli) has been positively correlated with satiety and satiety hormone levels, suggesting that the microbiome may mediate muscle wasting by promoting malnutrition through inflammation (25, 40). On the other hand, a healthy gut microbiome regulates immune homeostasis, maintaining a balance between pro-inflammatory and anti-inflammatory responses while enhancing barrier function (32). Consequently, dysbiosis and changes in the gut barrier can lead to increased absorption of lipopolysaccharides (LPS), termed metabolic endotoxemia. LPS significantly increases NF-κB binding activity in skeletal muscle and JNK phosphorylation, collectively suppressing insulin signaling (41). Animal experiments have also shown that LPS injection induces various changes, including a reduced abundance of Bacteroidetes, increased serum concentrations of pro-inflammatory cytokines, and impaired mitochondrial morphology (41). The immune stress mediated by these changes can adversely affect muscle growth in weaned piglets (32, 42). Additionally, another study indicated that the administration of TAK-242, a Toll-like receptor 4 (TLR4) specific signaling inhibitor, reversed muscle atrophy induced by endotoxemia in mice (43). Understanding how gut microbiota interacts with host muscle provides a foundation for therapeutic microbial interventions aimed at combating or preventing disease.
This study provides further insights into the potential mechanisms by which dietary patterns influence the development of sarcopenia. Specifically, diets with high DII scores, such as those rich in red and processed meats, high-fat, and high-sugar foods, are closely associated with pro-inflammatory states (44, 45). In contrast, diets with low DII scores, including those rich in fruits, vegetables, and nuts, exhibit anti-inflammatory properties (44, 45). Additionally, diets with high DI-GM scores, characterized by foods rich in dietary fiber and beneficial components (e.g., whole grains, legumes, vegetables, and fermented dairy) (11), can modulate gut microbiota composition and function, indirectly influencing inflammation levels. Diets high in dietary fiber and beneficial components serve as substrates for gut microbiota fermentation, leading to the production of short-chain fatty acids (SCFAs), such as butyrate, acetate, and propionate (46). These SCFAs exert anti-inflammatory effects by modulating immune cell activity (47). These dietary patterns may play a protective role against sarcopenia by reducing inflammation and improving gut microbiota health. Based on these findings, we recommend adopting anti-inflammatory and prebiotic-rich dietary patterns, such as the Mediterranean diet (48) and the DASH diet (49), as potential strategies for sarcopenia prevention and treatment. Combining these dietary interventions with protein supplementation and resistance training (50, 51)may further enhance their effectiveness. Furthermore, this study provides new directions for future research. Prospective studies are needed to validate the causal relationships between DI-GM, DII, and sarcopenia. Animal experiments could further explore the gut microbiota-inflammation-muscle metabolism axis to provide direct evidence for the underlying mechanisms. Additionally, future research could investigate personalized dietary intervention strategies based on DI-GM and DII to evaluate their practical applications in sarcopenia prevention and treatment. These studies will help optimize dietary strategies for sarcopenia management and provide a scientific basis for public health policies. Therefore, our study indicates that there are associations among gut microbiota, diet, and sarcopenia. Given that multiple studies have linked gut microbiota to inflammatory bowel disease, epilepsy, and cancer (52–54), this research provides new insights into the prevention and management of sarcopenia through dietary management aimed at modulating gut microbiota. This study found that DI-GM was negatively associated with sarcopenia prevalence (OR = 0.85, 95% CI: 0.77, 0.94), while DII was positively associated with sarcopenia prevalence (OR = 1.28, 95% CI: 1.17, 1.41). These findings further support the aforementioned hypothesis.
Our study has several advantages. First, it is based on the NHANES database, which provides a large sample size and allows for the analysis of confounding factors, subgroup analysis, and mediation analysis, yielding stable results. This study demonstrates a negative correlation between DI-GM and the prevalence of sarcopenia, suggesting that DII may mediate this relationship. Second, as a newly proposed Dietary Index, DI-GM has the potential to serve as a standardized tool for assessing balanced diets that support gut microbiota. Its visual approach to evaluating the relationship between diet and sarcopenia offers a more convenient and implementable solution for clinical guidance. Additionally, we conducted a mediation analysis to explore the relationships among DII, DI-GM, and sarcopenia.
Despite the significant contributions of this study, several limitations should be noted: (1) As a cross-sectional study, it cannot establish a causal relationship between DI-GM and the prevalence of sarcopenia. Further prospective studies with larger and more diverse samples are needed to elucidate the causal relationship between DI-GM and sarcopenia. (2) The data for this study were sourced from the NHANES dataset covering 2011–2018, which means that the results apply only to U.S. adults and may have limited applicability to populations in other countries. (3) The dietary data in this study relied on questionnaires recorded by NHANES, and participants’ responses may be subject to recall bias. (4) Although this study has adjusted for many potential confounding factors, it is still unable to exclude the possibility of confounding effects from unmeasured or unidentified factors.
In conclusion, our study enhances the understanding of the association between DI-GM dietary patterns and sarcopenia. This knowledge is beneficial for future dietary recommendations aimed at preventing diseases related to skeletal muscle health. By tracking an individual’s dietary habits, the use of DI-GM scoring can help assess the health impacts of diet on the gut microbiota. This approach is particularly valuable for clinical nutritionists, public health practitioners, and researchers, providing important strategies for the clinical prevention and management of sarcopenia. Finally, the mediation analysis involving DII indicates that an anti-inflammatory diet can contribute to the reduction of sarcopenia prevalence through its effects on DI-GM.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://wwwn.cdc.gov/nchs/nhanes/.
HG: Conceptualization, Data curation, Investigation, Methodology, Software, Supervision, Writing – original draft, Writing – review & editing. SD: Conceptualization, Data curation, Formal Analysis, Investigation, Software, Writing – original draft, Writing – review & editing. XL: Conceptualization, Formal Analysis, Investigation, Project administration, Software, Supervision, Writing – original draft, Writing – review & editing. SH: Conceptualization, Formal Analysis, Funding acquisition, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
We sincerely appreciate the NHANES database for all the data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1514209/full#supplementary-material
DI-GM, Dietary Index for Gut Microbiota; DII, Dietary inflammatory Index; NHANES, National Health and Nutrition Examination Survey.
1. Cruz-Jentoft A, Sayer A. Sarcopenia. Lancet. (2019) 393:2636–46. doi: 10.1016/S0140-6736(19)31138-9
2. Dennison E, Sayer A, Cooper C. Epidemiology of sarcopenia and insight into possible therapeutic targets. Nat Rev Rheumatol. (2017) 13:340–7. doi: 10.1038/nrrheum.2017.60
3. Liu C, Cheung W, Li J, Chow S, Yu J, Wong S, et al. Understanding the gut microbiota and sarcopenia: A systematic review. J Cachexia Sarcopenia Muscle. (2021) 12:1393. doi: 10.1002/jcsm.12784
4. Yuan S, Larsson S. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism. (2023) 144:155533. doi: 10.1016/j.metabol.2023.155533
5. Huang Q, Wan J, Nan W, Li S, He B, Peng Z. Association between manganese exposure in heavy metals mixtures and the prevalence of sarcopenia in US adults from NHANES 2011-2018. J Hazard Mater. (2024) 464:133005. doi: 10.1016/j.jhazmat.2023.133005
6. Bowyer R, Jackson M, Pallister T, Skinner J, Spector T, Welch A, et al. Use of dietary indices to control for diet in human gut microbiota studies. Microbiome. (2018) 6:77. doi: 10.1186/s40168-018-0455-y
7. Zmora N, Suez J, Elinav E. You are what you eat: Diet, health and the gut microbiota. Nat Rev Gastroenterol Hepatol. (2019) 16:35–56. doi: 10.1038/s41575-018-0061-2
8. Mancin L, Wu G, Paoli A. Gut microbiota-bile acid-skeletal muscle axis. Trends Microbiol. (2023) 31:254–69. doi: 10.1016/j.tim.2022.10.003
9. Chen L, Chang S, Chang H, Wu C, Pan C, Chang C, et al. Probiotic supplementation attenuates age-related sarcopenia via the gut-muscle axis in SAMP8 mice. J Cachexia Sarcopenia Muscle. (2022) 13:515–31. doi: 10.1002/jcsm.12849
10. Zhao J, Liang R, Song Q, Song S, Yue J, Wu C. Investigating association between gut microbiota and sarcopenia-related traits: A Mendelian randomization study. Precis Clin Med. (2023) 6:bad010. doi: 10.1093/pcmedi/pbad010
11. Kase B, Liese A, Zhang J, Murphy E, Zhao L, Steck S. The development and evaluation of a literature-based dietary index for gut microbiota. Nutrients. (2024) 16:1045. doi: 10.3390/nu16071045
12. Shivappa N, Steck S, Hurley T, Hussey J, Hébert J. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. (2014) 17:1689–96. doi: 10.1017/S1368980013002115
13. Marx W, Veronese N, Kelly J, Smith L, Hockey M, Collins S, et al. The Dietary inflammatory index and human health: An umbrella review of meta-analyses of observational studies. Adv Nutr. (2021) 12:1681–90. doi: 10.1093/advances/nmab037
14. Khamoushi F, Soleimani D, Najafi F, Ahmadi N, Heidarzadeh-Esfahani N, Anvari B, et al. Association between dietary inflammatory index and musculoskeletal disorders in adults. Sci Rep. (2023) 13:20302. doi: 10.1038/s41598-023-46429-w
15. Su Y, Yeung S, Chen Y, Leung J, Kwok T. The associations of dietary inflammatory potential with musculoskeletal health in Chinese community-dwelling older people: The Mr/Ms. OS (Hong Kong) cohort study. J Bone Miner Res Off J Am Soc Bone Miner Res. (2022) 37:1179. doi: 10.1002/jbmr.4556
16. Nardone O, de Sire R, Petito V, Testa A, Villani G, Scaldaferri F, et al. Inflammatory bowel diseases and sarcopenia: The role of inflammation and gut microbiota in the development of muscle failure. Front Immunol. (2021) 12:694217. doi: 10.3389/fimmu.2021.694217
17. Liu D, Wang S, Liu S, Wang Q, Che X, Wu G. Frontiers in sarcopenia: Advancements in diagnostics, molecular mechanisms, and therapeutic strategies. Mol Aspects Med. (2024) 97:101270. doi: 10.1016/j.mam.2024.101270
18. Diao H, Yan F, He Q, Li M, Zheng Q, Zhu Q, et al. Association between dietary inflammatory index and sarcopenia: A meta-analysis. Nutrients. (2023) 15:219. doi: 10.3390/nu15010219
19. Zhou H, Su H, Gong Y, Chen L, Xu L, Chen G, et al. The association between weight-adjusted-waist index and sarcopenia in adults: A population-based study. Sci Rep. (2024) 14:10943. doi: 10.1038/s41598-024-61928-0
20. Xu W, Mu D, Wang Y, Wang Y, Wang C, Zhang X. Association between oxidative balance score and sarcopenia in US adults: NHANES 2011–2018. Front Nutr. (2024) 11:1342113. doi: 10.3389/fnut.2024.1342113
21. Zuercher M, Harvey D, Au L, Shadyab A, Santiago-Torres M, Liu S, et al. Energy-adjusted dietary inflammatory index and diabetes risk in postmenopausal hispanic women. J Acad Nutr Diet. (2024) 124:1431–9. doi: 10.1016/j.jand.2023.08.002
22. Qing L, Zhu Y, Yu C, Zhang Y, Ni J. Exploring the association between dietary inflammatory index and chronic pain in US adults using NHANES 1999-2004. Sci Rep. (2024) 14:8726. doi: 10.1038/s41598-024-58030-w
23. Huang S, He Q, Wang X, Choi S, Gong H. Associations of the planetary health diet index (PHDI) with asthma: The mediating role of body mass index. BMC Public Health. (2024) 24:2305. doi: 10.1186/s12889-024-19856-1
24. Hébert J, Shivappa N, Wirth M, Hussey J, Hurley T. Perspective: The dietary inflammatory index (DII)-lessons learned, improvements made, and future directions. Adv Nutr. (2019) 10:185–95. doi: 10.1093/advances/nmy071
25. Ticinesi A, Nouvenne A, Cerundolo N, Catania P, Prati B, Tana C, et al. Gut microbiota, muscle mass and function in aging: A focus on physical frailty and sarcopenia. Nutrients. (2019) 11:1633. doi: 10.3390/nu11071633
26. Zhang X, Yang Q, Huang J, Lin H, Luo N, Tang H. Association of the newly proposed dietary index for gut microbiota and depression: The mediation effect of phenotypic age and body mass index. Eur Arch Psychiatry Clin Neurosci. (2024): doi: 10.1007/s00406-024-01912-x Online ahead of print.
27. Vinolo M, Rodrigues H, Nachbar R, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. (2011) 3:858–76. doi: 10.3390/nu3100858
28. Liu C, Wong P, Wang Q, Wong H, Huang T, Cui C, et al. Short-chain fatty acids enhance muscle mass and function through the activation of mTOR signalling pathways in sarcopenic mice. J Cachexia Sarcopenia Muscle. (2024) 15:2387–401. doi: 10.1002/jcsm.13573
29. Boulangé C, Neves A, Chilloux J, Nicholson J, Dumas M. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. (2016) 8:42. doi: 10.1186/s13073-016-0303-2
30. Wu K, Shieh J, Qin L, Guo J. Mitochondrial mechanisms in the pathogenesis of chronic inflammatory musculoskeletal disorders. Cell Biosci. (2024) 14:76. doi: 10.1186/s13578-024-01259-9
31. Aslam H, Marx W, Rocks T, Loughman A, Chandrasekaran V, Ruusunen A, et al. The effects of dairy and dairy derivatives on the gut microbiota: A systematic literature review. Gut Microbes. (2020) 12:1799533. doi: 10.1080/19490976.2020.1799533
32. Li T, Yin D, Shi R. Gut-muscle axis mechanism of exercise prevention of sarcopenia. Front Nutr. (2024) 11:1418778. doi: 10.3389/fnut.2024.1418778
33. Daniel N, Nachbar R, Tran T, Ouellette A, Varin T, Cotillard A, et al. Gut microbiota and fermentation-derived branched chain hydroxy acids mediate health benefits of yogurt consumption in obese mice. Nat Commun. (2022) 13:1343. doi: 10.1038/s41467-022-29005-0
34. He Y, Cui W, Fang T, Zhang Z, Zeng M. Metabolites of the gut microbiota may serve as precise diagnostic markers for sarcopenia in the elderly. Front Microbiol. (2023) 14:1301805. doi: 10.3389/fmicb.2023.1301805
35. Park C, Lee E, Kim H, Lee Y, Yoon K, Kim H. Sex-specific associations between gut microbiota and skeletal muscle mass in a population-based study. J Cachexia Sarcopenia Muscle. (2022) 13:2908–19. doi: 10.1002/jcsm.13096
36. Gill P, Inniss S, Kumagai T, Rahman F, Smith A. The role of diet and gut microbiota in regulating gastrointestinal and inflammatory disease. Front Immunol. (2022) 13:866059. doi: 10.3389/fimmu.2022.866059
37. Armet A, Deehan E, O’Sullivan A, Mota J, Field C, Prado C, et al. Rethinking healthy eating in light of the gut microbiome. Cell Host Microbe. (2022) 30:764–85. doi: 10.1016/j.chom.2022.04.016
38. Ker A, Kao P. Methodological considerations on the association between dietary inflammatory potential and musculoskeletal health. J Bone Miner Res. (2022) 37:2678–9. doi: 10.1002/jbmr.4635
39. Xie H, Wang H, Wu Z, Li W, Liu Y, Wang N. The association of dietary inflammatory potential with skeletal muscle strength, mass, and sarcopenia: A meta-analysis. Front Nutr. (2023) 10:1100918. doi: 10.3389/fnut.2023.1100918
40. Wells J, Sawaya A, Wibaek R, Mwangome M, Poullas M, Yajnik C, et al. The double burden of malnutrition: Aetiological pathways and consequences for health. Lancet. (2020) 395:75–88. doi: 10.1016/S0140-6736(19)32472-9
41. Grosicki G, Fielding R, Lustgarten M. Gut microbiota contribute to age-related changes in skeletal muscle size, composition, and function: Biological basis for a gut-muscle axis. Calcif Tissue Int. (2018) 102:433–42. doi: 10.1007/s00223-017-0345-5
42. Yu J, Zheng C, Guo Q, Yin Y, Duan Y, Li F. LPS-related muscle loss is associated with the alteration of Bacteroidetes abundance, systemic inflammation, and mitochondrial morphology in a weaned piglet model. Sci China Life Sci. (2024) 67:1970–88. doi: 10.1007/s11427-023-2552-7
43. Ono Y, Maejima Y, Saito M, Sakamoto K, Horita S, Shimomura K, et al. TAK-242, a specific inhibitor of Toll-like receptor 4 signalling, prevents endotoxemia-induced skeletal muscle wasting in mice. Sci Rep. (2020) 10:694. doi: 10.1038/s41598-020-57714-3
44. Szypowska A, Regulska-Ilow B, Zatońska K, Szuba A. Comparison of intake of food groups based on dietary inflammatory index (DII) and cardiovascular risk factors in the middle-age population of lower Silesia: Results of the PURE poland study. Antioxidants. (2023) 12:285. doi: 10.3390/antiox12020285
45. Szypowska A, Zatońska K, Szuba A, Regulska-Ilow B. Dietary inflammatory index (DII)® and metabolic syndrome in the selected population of polish adults: Results of the PURE poland sub-study. Int J Environ Res Public Health. (2023) 20:1056. doi: 10.3390/ijerph20021056
46. Liu X, Shao J, Liao Y, Wang L, Jia Y, Dong P, et al. Regulation of short-chain fatty acids in the immune system. Front Immunol. (2023) 14:1186892. doi: 10.3389/fimmu.2023.1186892
47. Portincasa P, Bonfrate L, Vacca M, De Angelis M, Farella I, Lanza E, et al. Gut microbiota and short chain fatty acids: Implications in glucose homeostasis. Int J Mol Sci. (2022) 23:1105. doi: 10.3390/ijms23031105
48. Casas R, Sacanella E, Estruch R. The immune protective effect of the Mediterranean diet against chronic low-grade inflammatory diseases. Endocr Metab Immune Disord-Drug Targets. (2014) 14:245–54. doi: 10.2174/1871530314666140922153350
49. Soltani S, Chitsazi M, Salehi-Abargouei A. The effect of dietary approaches to stop hypertension (DASH) on serum inflammatory markers: A systematic review and meta-analysis of randomized trials. Clin Nutr. (2018) 37:542–50. doi: 10.1016/j.clnu.2017.02.018
50. Mende E, Moeinnia N, Schaller N, Weiß M, Haller B, Halle M, et al. Progressive machine-based resistance training for prevention and treatment of sarcopenia in the oldest old: A systematic review and meta-analysis. Exp Gerontol. (2022) 163:111767. doi: 10.1016/j.exger.2022.111767
51. Vikberg S, Sörlén N, Brandén L, Johansson J, Nordström A, Hult A, et al. Effects of resistance training on functional strength and muscle mass in 70-year-old individuals with pre-sarcopenia: A randomized controlled trial. J Am Med Dir Assoc. (2019) 20:28–34. doi: 10.1016/j.jamda.2018.09.011
52. Li W, Deng Y, Chu Q, Zhang P. Gut microbiome and cancer immunotherapy. Cancer Lett. (2019) 447:41–7. doi: 10.1016/j.canlet.2019.01.015
53. Dahlin M, Prast-Nielsen S. The gut microbiome and epilepsy. EBioMedicine. (2019) 44:741–6. doi: 10.1016/j.ebiom.2019.05.024
Keywords: Dietary Index for Gut Microbiota, Dietary Inflammatory Index, sarcopenia, NHANES, mediation analysis
Citation: Gong H, Duan S, Lin X and Huang S (2025) The association between Dietary Index for Gut Microbiota and sarcopenia: the mediating role of Dietary Inflammatory Index. Front. Nutr. 12:1514209. doi: 10.3389/fnut.2025.1514209
Received: 20 October 2024; Accepted: 17 March 2025;
Published: 31 March 2025.
Edited by:
Carlo Pedrolli, Azienda Provinciale per i Servizi Sanitari (APSS), ItalyReviewed by:
Jiatong Shan, National University of Singapore, SingaporeCopyright © 2025 Gong, Duan, Lin and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaoqun Huang, c3FodWFuZ0BmanRjbS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.