- 1Department of Obstetrics and Gynecology, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, China
- 2Department of Obstetrics and Gynecology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
Background: Few studies have explored the link between nutritional status and prognosis in patients with epithelial ovarian cancer (EOC), and existing findings are controversial. Thus, this study aimed to explore the effects of pre-treatment nutrition-related indicators on the prognosis of patients with newly diagnosed EOC.
Methods: In this ambispective cohort study, 1,020 patients with EOC diagnosed by pathology examination were enrolled and followed-up until December 31, 2023. Univariate and multivariable analyses were conducted on nutrition-related indicators, including body mass index (BMI), albumin (ALB), hemoglobin (Hb), diabetes mellitus (DM), and hyperlipidemia, along with clinicopathological characteristics that might affect patients’ first-line chemotherapy response, progression-free survival (PFS), and overall survival (OS). Survival curves were created using the Kaplan–Meier method. A Cox proportional hazards model was established to obtain hazard ratios (HRs) and 95% confidence intervals (CIs).
Results: The median follow-up duration was 48 months. Compared with patients having normal nutritional indicators, those with hypoalbuminemia had poorer first-line chemotherapy responses. The proportions of those with complete response (CR), partial response (PR), and stable disease or progressive disease (SD/PD) for the ≤30 g/L, 30 < ALB<35 g/L and normal ALB groups were 57.2, 20.6, and 22.2% vs. 62.0, 22.5, and 15.5% vs.79.5, 13.6, and 6.9%. Patients with hypoalbuminemia had shorter median PFS (mPFS): 15 vs. 19 vs. 57 months in the three groups, respectively; and shorter median OS (mOS): 36 vs. 51 vs. 124 months. Patients with hyperlipidemia also exhibited poorer first-line chemotherapy responses; CR, PR, and SD/PD rates for the hyperlipidemia and non-hyperlipidemia groups were 68.9, 19.5, and 11.6% vs. 76.4, 14.7, and 8.9%, respectively, and shorter mPFS (17 vs. 57 months) and mOS (40 vs. 119 months). Patients with anemia had poorer first-line chemotherapy responses; CR, PR, and SD/PD rates for the anemia and non-anemia groups were 68.4, 19.7, and 11.9% vs. 76.2, 14.9, and 8.9%, respectively. All differences were statistically significant (p < 0.05). Multivariable analysis identified hyperlipidemia as an independent risk factor for PFS (hazard ratio [HR] = 2.083; 95% CI:1.726–2.514; p < 0.001) and OS (HR = 2.158; 95% CI:1.746–2.666; p < 0.001), whereas hypoalbuminemia and anemia were not confirmed as independent prognostic factors. This study found no effect of BMI or DM on patient prognosis.
Conclusion: Pre-treatment hypoalbuminemia, hyperlipidemia, and anemia negatively affected the prognosis of patients with newly diagnosed EOC, with hyperlipidemia being an independent risk factor for shorter survival.
1 Introduction
Epidemiological data show that in 2022, the global incidence and mortality of ovarian cancer (OC) ranked eighth among female cancers; in China, both ranked ninth (1). The mortality rate of OC ranks first among all gynecological cancers. Epithelial ovarian cancer (EOC) accounts for approximately 90% of all cases of OC. The standard treatment is based on comprehensive staging surgery or cytoreductive surgery, followed by platinum-based chemotherapy. Approximately 70% of patients with EOC relapse within 3 years and often have a poor prognosis owing to platinum resistance or refractoriness (2). The Warburg effect (3), amino acid metabolism (4) and lipid metabolism (5) have been proved to be closely related to tumor growth, metastasis and the regulation of anti-tumor immunity. In patients with cancer, the balance of nutrient metabolism between tumor cells and immune cells in the tumor microenvironment affects the occurrence, development, treatment efficacy, and prognosis of tumors.
Malnutrition is associated with an increased risk of complications and reduced efficacy and tolerance to antitumor therapies (6). Overnutrition, such as a high-fat diet and obesity, can promote metastasis and weaken antitumor immunity in certain types of cancers (7). Many studies have shown that nutritional status is closely related to the prognosis of digestive system cancers (8), breast cancer (9), and lung cancer (10); however, studies related to EOC are few and remain controversial (11–15).
In this ambispective cohort study, we aimed to explore the clinical significance of nutritional status and identify independent nutrition-related risk factors for the prognosis of patients with newly diagnosed EOC to provide a theoretical basis for a better prognosis by improving the nutritional status.
2 Materials and methods
2.1 Study population and data sources
This study included 1,020 patients newly diagnosed with EOC at the Department of Obstetrics and Gynecology of the First Affiliated Hospital of Xi’an Jiaotong University between January 2010 and December 2021. The inclusion criteria were: pathologically confirmed primary EOC; received comprehensive staging surgery or cytoreductive surgery, followed by regular platinum-based chemotherapy; complete clinicopathological data, pre-treatment laboratory examination data and follow-up data; age ≥ 18 years; and life expectancy≥12 weeks at diagnosis. The exclusion criteria were the presence of other primary malignant tumors, pregnancy, liver and kidney diseases that seriously affect nutrition-related indicators, acute or chronic infectious diseases, hematological diseases, and major trauma. This study was approved by the Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University. Given that this study was observational in nature and the patients were enrolled retrospectively and anonymously, the requirement for obtaining consent was waived.
2.2 Follow-up and outcome assessment
In this ambispective cohort study, clinicopathological data and pre-treatment nutrition-related indicators were collected from the electronic medical record system, and patients were divided into groups according to each pre-treatment nutrition-related indicator. All patients were followed up from the date of surgery to December 31, 2023, using the outpatient system. The primary endpoints were overall survival (OS) and progression-free survival (PFS), and the secondary endpoint was first-line chemotherapy response. Figure 1 presents a flowchart of the study.
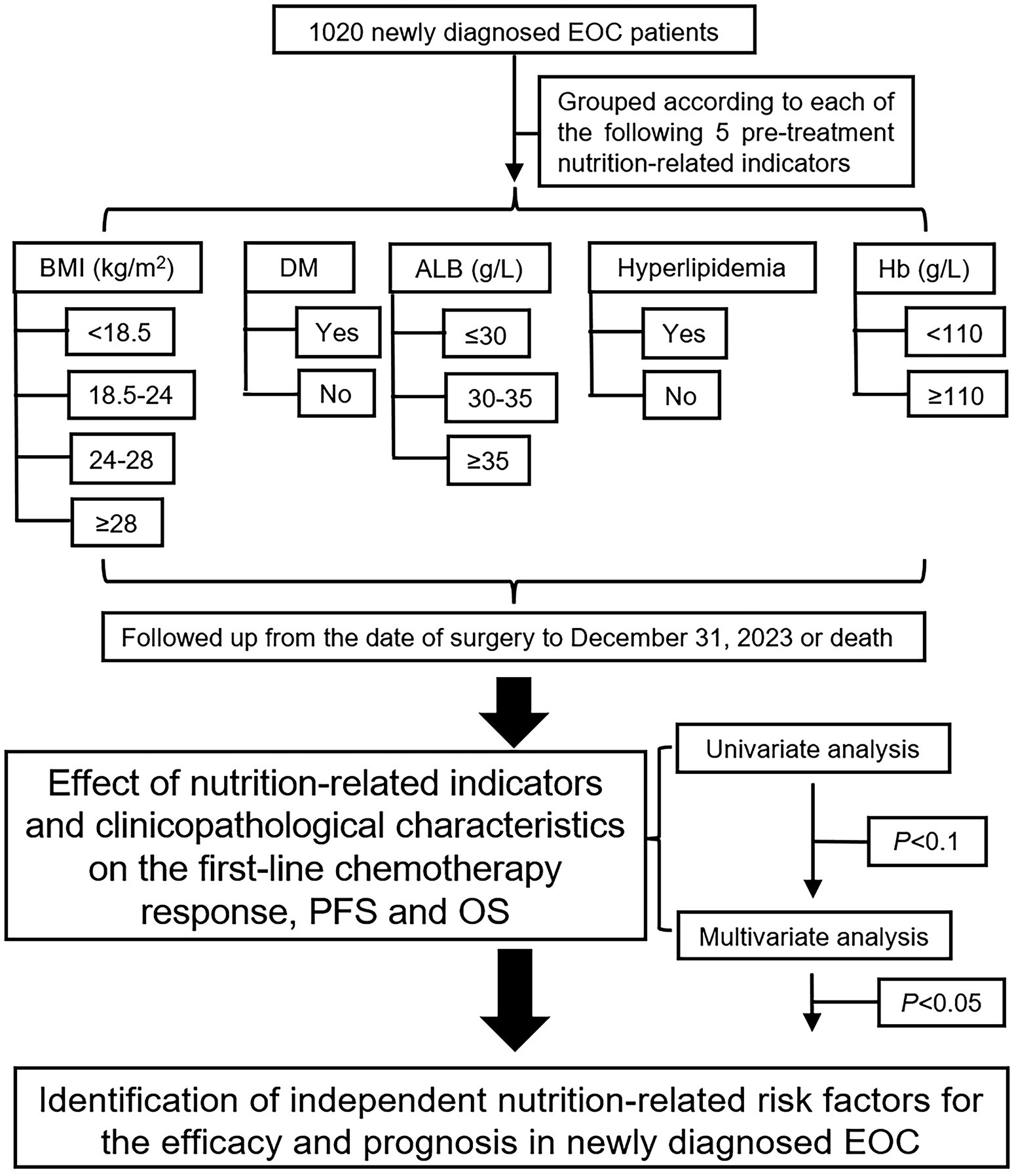
Figure 1. Research flowchart for five nutrition-related indicators to affect the EOC prognosis. EOC, epithelial ovarian cancer; BMI, body mass index; DM, diabetes mellitus; ALB, albumin; Hb, hemoglobin.
2.3 Definition of related indicators
Five pre-treatment nutrition-related indicators were included in this study. Body mass index (BMI) was calculated as weight/height2 (kg/m2) and, according to the Chinese BMI standard (16), patients were divided into four groups: BMI < 18.5 kg/m2 (underweight), 18.5 ≤ BMI < 24 kg/m2 (normal weight), 24 ≤ BMI < 28 kg/m2 (overweight), and BMI ≥ 28 kg/m2 (obese). Diabetes mellitus (DM) was diagnosed according to the 1999 World Health Organization DM diagnostic criteria (17): patients with typical DM symptoms and random blood glucose≥11.1 mmol/L, or fasting plasma glucose≥7.0 mmol/L, or blood glucose≥11.1 mmol/L at 2-h post glucose load in the oral glucose tolerance test, or glycosylated hemoglobin (HbA1c) ≥ 6.5%. The patients were divided into two groups according to the presence or absence of DM. Patients with EOC complicated by DM included those who had been diagnosed with DM before hospitalization (with a history of DM) and those newly diagnosed with DM after hospitalization (patients whose fasting blood glucose or random blood glucose levels were found to be elevated according to laboratory examinations and who were then diagnosed with DM after consultation with endocrinologists). The normal concentration of serum albumin (ALB) is 35–50 g/L and ALB<35 g/L is diagnosed as hypoalbuminemia; in clinical practice, the indication for ALB administration to patients with cancer is serum ALB≤30 g/L (18); thus, patients were divided into three groups; ALB≤30 g/L, 30 < ALB<35 g/L and ALB≥35 g/L. Patients were divided into anemia and normal groups according to their hemoglobin (Hb) level (19): <110 g/L or > 110 g/L. According to the criteria established by the Joint Committee on the Chinese Guidelines for Lipid Management (20), hyperlipidemia can be diagnosed by the presence of ≥1 of the following four criteria: total cholesterol (TC) > 6.20 mmol/L, triglyceride (TG) > 2.30 mmol/L, low-density lipoprotein (LDL) > 4.10 mmol/L, high-density lipoprotein (HDL) < 1.00 mmol/L. In this study, patients were divided into two groups according to whether they had hyperlipidemia before treatment.
According to the Response Evaluation Criteria in Solid Tumors 1.1 (21) for evaluating the response to first-line chemotherapy in patients with EOC, we divided the patients into three groups: complete response (CR), partial response (PR), and stable disease/progressive disease (SD/PD). OS is the time from the date of surgery to death from any cause, and PFS is the time from the date of surgery to the first time of disease progression or death from any cause.
2.4 Statistical analysis
Descriptive statistics of categorical and ordinal categorical variables were expressed as frequency (n) and percentage (%), and descriptive statistics of continuous variables were presented as medians. The Mann–Whitney U test was used for two-group comparisons for univariate analysis of ordinal categorical variables, the Kruskal–Wallis test was used for multiple-group comparisons, with two-by-two comparisons of groups using Bonferroni correction, univariate survival analysis was performed using the Kaplan–Meier method, and the log-rank test was used for comparisons between groups. Factors with p < 0.1 in univariate analysis were included in multivariable analysis to screen the independent factors affecting efficacy and prognosis. Ordered logistic regression analysis was used for multivariable analysis of ordinal categorical variables to calculate the odds ratio (OR), and the Cox proportional hazards model was used for multivariable survival analysis to calculate the hazard ratio (HR) and 95% confidence interval (95% CI). Statistical analyses were performed using IBM SPSS Statistics for Windows, version 27.0 (IBM Corp., Armonk, NY, United States). All p-values were two-sided, and p < 0.05 was considered statistically significant.
3 Results
3.1 Nutritional status and clinicopathological characteristics
Of the 1,020 patients, 59 (5.8%) were underweight, 569 (55.8%) were of normal weight, 319 (31.3%) were overweight, and 73 (7.1%) were obese. Seventy-five (7.4%) patients had DM. There were 263 (25.8%) patients with hypoalbuminemia, including 63 (6.2%) patients with ALB≤30 g/L and 200 (19.6%) patients with 30 < ALB<35 g/L. Hyperlipidemia was diagnosed in 225 (22.1%) patients. A total of 193 (18.9%) patients had anemia (Table 1).
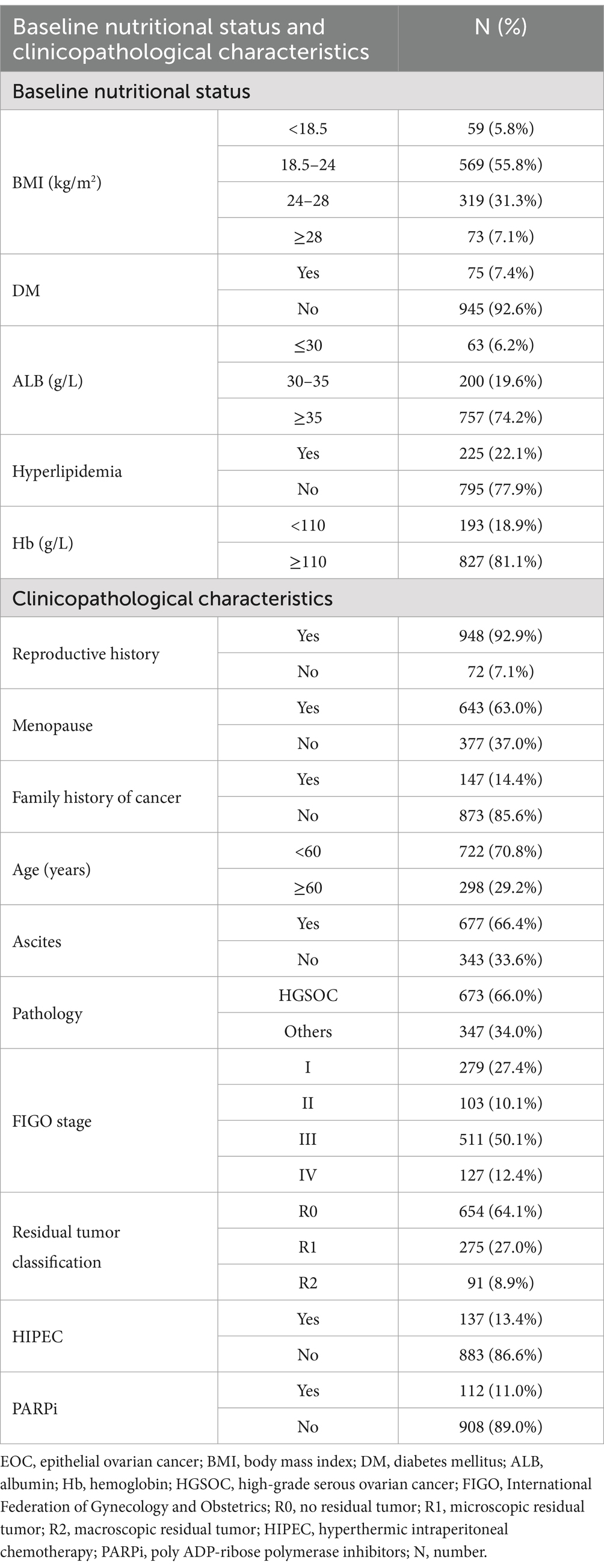
Table 1. Baseline nutritional status and clinicopathological characteristics of 1,020 newly diagnosed EOC patients.
Ascites was found in 677 (66.4%) patients and 673 (66.0%) patients were diagnosed with high-grade serous ovarian cancer (HGSOC). According to the International Federation of Gynecology and Obstetrics (FIGO) staging classification, 279 (27.4%) patients were stage I, 103 (10.1%) patients were stage II, 511 (50.1%) patients were stage III, and 127 (12.4%) patients were stage IV. Surgery achieved no residual tumor (R0) in 654 (64.1%) patients, microscopic residual tumor (R1) in 275 (27.0%), and macroscopic residual tumor (R2) in 91 (8.9%) patients. Among the 1,020 patients with EOC included in this study, 137 (13.4%) received hyperthermic intraperitoneal chemotherapy (HIPEC), and 112 (11.0%) received first-line poly ADP-ribose polymerase inhibitor (PARPi) maintenance therapy (Table 1).
3.2 First-line chemotherapy response
3.2.1 Patients with hypoalbuminemia, hyperlipidemia, or anemia demonstrated poorer first-line chemotherapy response compared with those having normal nutritional indicators
After receiving first-line chemotherapy, 762 patients (74.7%) achieved CR, 161 (15.8%) achieved PR, and 97 (9.5%) achieved SD/PD. Univariate analysis showed that patients with hypoalbuminemia had poorer first-line chemotherapy response than those with normal ALB (p < 0.001), but there was no significant difference in response between patients with ALB≤30 g/L and 30 < ALB<35 g/L (p > 0.05). The proportions of CR, PR, and SD/PD in the ALB≤30 g/L, 30 < ALB<35 g/L and normal ALB groups were 57.2% (n = 36), 20.6% (n = 13), and 22.2% (n = 14) vs. 62.0% (n = 124), 22.5% (n = 45), and 15.5% (n = 31) vs. 79.5% (n = 602), 13.6% (n = 103), and 6.9% (n = 52). Patients with hyperlipidemia had poorer responses than those without hyperlipidemia (p = 0.025). The proportions of patients in the two groups were 68.9% (n = 155), 19.5% (n = 44), and 11.6% (n = 26) vs. 76.4% (n = 607), 14.7% (n = 177), and 8.9% (n = 71), respectively. Patients with anemia had poorer responses than those with normal Hb levels (p = 0.026). The proportions of patients in the two groups were 68.4% (n = 132), 19.7% (n = 38), and 11.9% (n = 23), vs. 76.2% (n = 630), 14.9% (n = 123), and 8.9% (n = 74), respectively. The effects of BMI and DM on the first-line chemotherapy response in patients were not statistically significant (p > 0.05; Table 2).
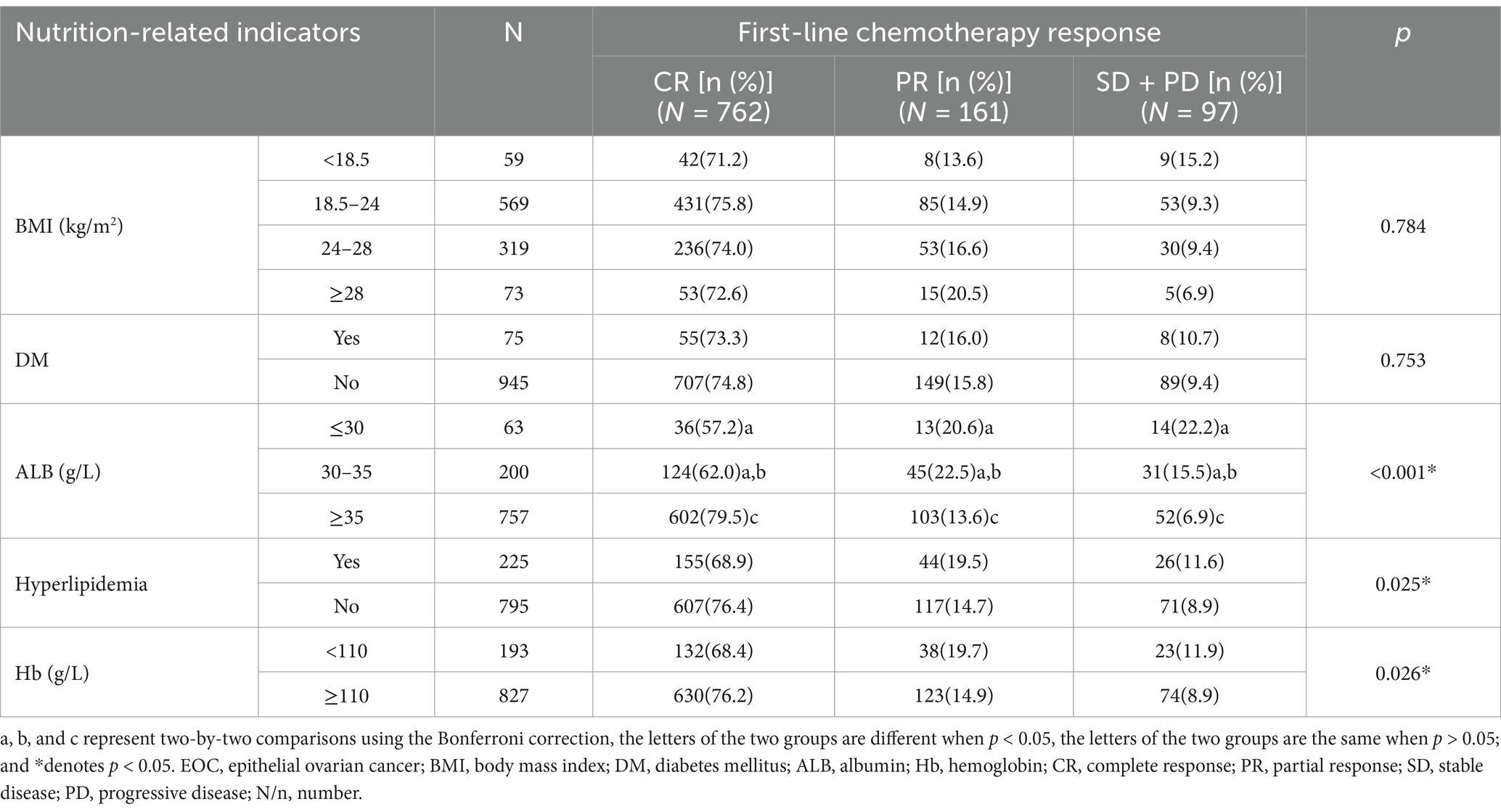
Table 2. Univariate analysis of pre-treatment nutrition-related indicators affecting the first-line chemotherapy response in newly diagnosed EOC patients (N = 1,020).
After undergoing standard first-line chemotherapy, the proportion of patients with FIGO stage I disease achieving CR, PR, and SD/PD were 97.8, 1.8, and 0.4%, respectively. These proportions were 85.4, 6.8, and 7.8%, respectively, in patients with stage II disease, 68.7, 19.6, and 11.7% in patients with stage III disease, and 39.4, 38.6, and 22.0% in patients with stage IV disease. Generally, the higher the stage, the poorer the response to first-line chemotherapy (p < 0.001), however, there was no statistically significant difference between stages I and II (p > 0.05; Supplementary Table 1).
3.2.2 Multivariable logistic regression analysis did not identify any independent risk factors for the first-line chemotherapy response
The factors with p < 0.1 in the univariate analysis and potentially affecting the first-line chemotherapy response in patients (Table 2; Supplementary Table 1), namely ALB, hyperlipidemia, Hb, reproductive history, menopausal status, age, ascites, pathology, FIGO stage, residual tumor classification, and HIPEC were included in the multivariable logistic regression analysis. Although univariate analysis revealed that pre-treatment hypoalbuminemia, hyperlipidemia, and anemia negatively affected the first-line chemotherapy response in patients with newly diagnosed EOC (p < 0.05), multivariable logistic regression analysis failed to confirm these as independent risk factors (p > 0.05; Figure 2).
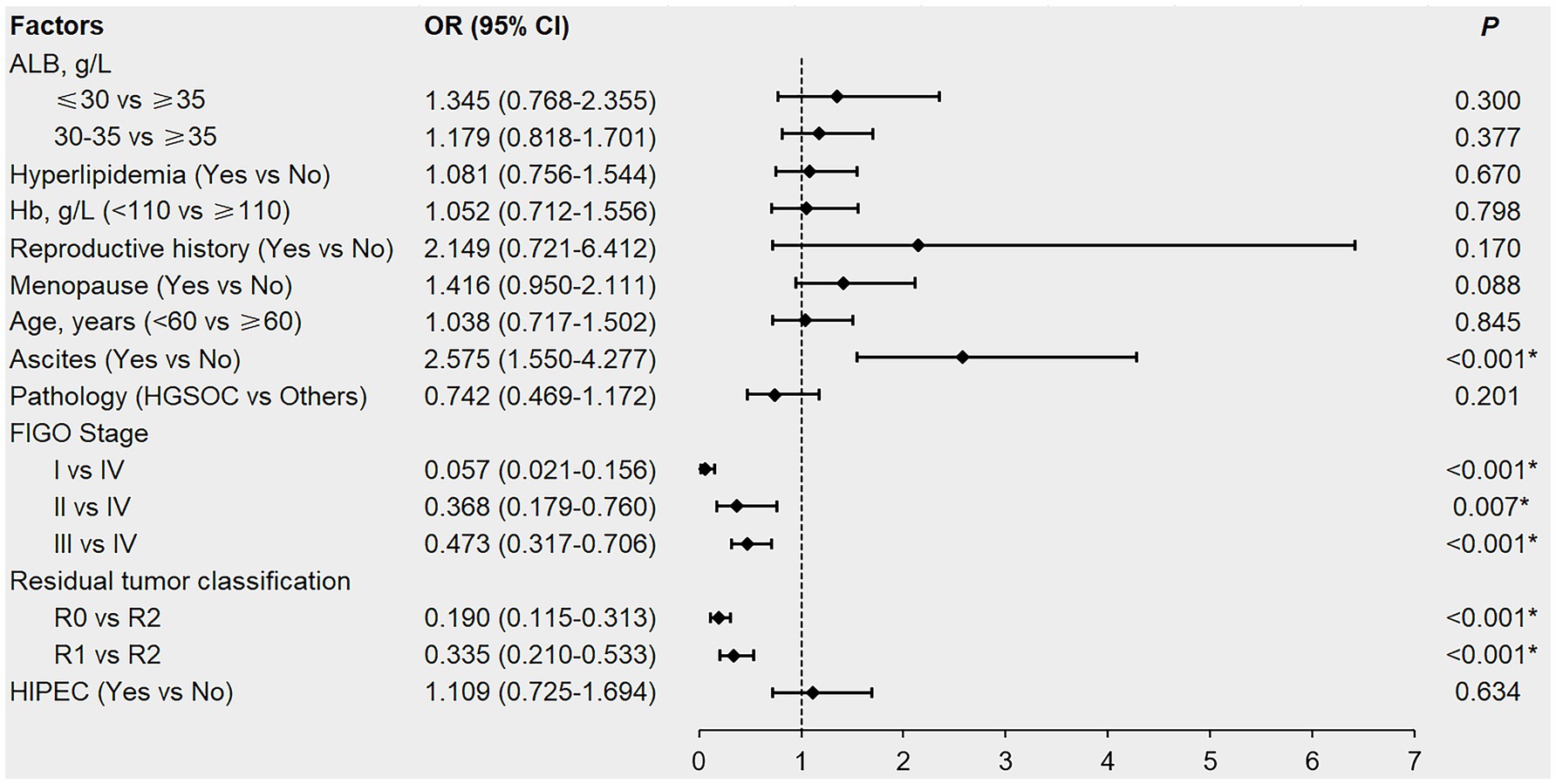
Figure 2. Forest plot of multivariable logistic regression analysis affecting the first-line chemotherapy response in newly diagnosed EOC patients. EOC, epithelial ovarian cancer; ALB, albumin; Hb, hemoglobin; HGSOC, high-grade serous ovarian cancer; FIGO, International Federation of Gynecology and Obstetrics; R0, no residual tumor; R1, microscopic residual tumor; R2, macroscopic residual tumor; HIPEC, hyperthermic intraperitoneal chemotherapy; OR, odds ratio; CI, confidence interval. *denotes p < 0.05.
3.3 Survival
3.3.1 Patients with hypoalbuminemia or hyperlipidemia had shorter PFS and OS compared with those having normal nutritional indicators
The median follow-up for the 1,020 patients was 48 months, during which 580 (56.9%) patients had disease progression and 442 (43.3%) died. Kaplan–Meier survival analysis found that patients with hypoalbuminemia had shorter PFS and OS than those with normal ALB (p < 0.001), and patients with ALB≤30 g/L had shorter OS than those with 30 < ALB<35 g/L (p = 0.037), but there was no significant difference in the PFS between patients with ALB≤30 g/L and 30 < ALB<35 g/L (p > 0.05). The mPFS and mOS of patients in ALB≤30 g/L, 30 < ALB<35 g/L and normal ALB groups were 15 vs. 19 vs. 57 months and 36 vs. 51 vs. 124 months, respectively (Table 3; Figures 3A,B). Patients with hyperlipidemia had shorter PFS and OS than those without hyperlipidemia (p < 0.001); the mPFS and mOS of the patients in the two groups were 17 vs. 57 months and 40 vs. 119 months, respectively (Table 3; Figures 3C,D). The effects of BMI, DM, and Hb on PFS and OS were not statistically significant (p > 0.05; Table 3).
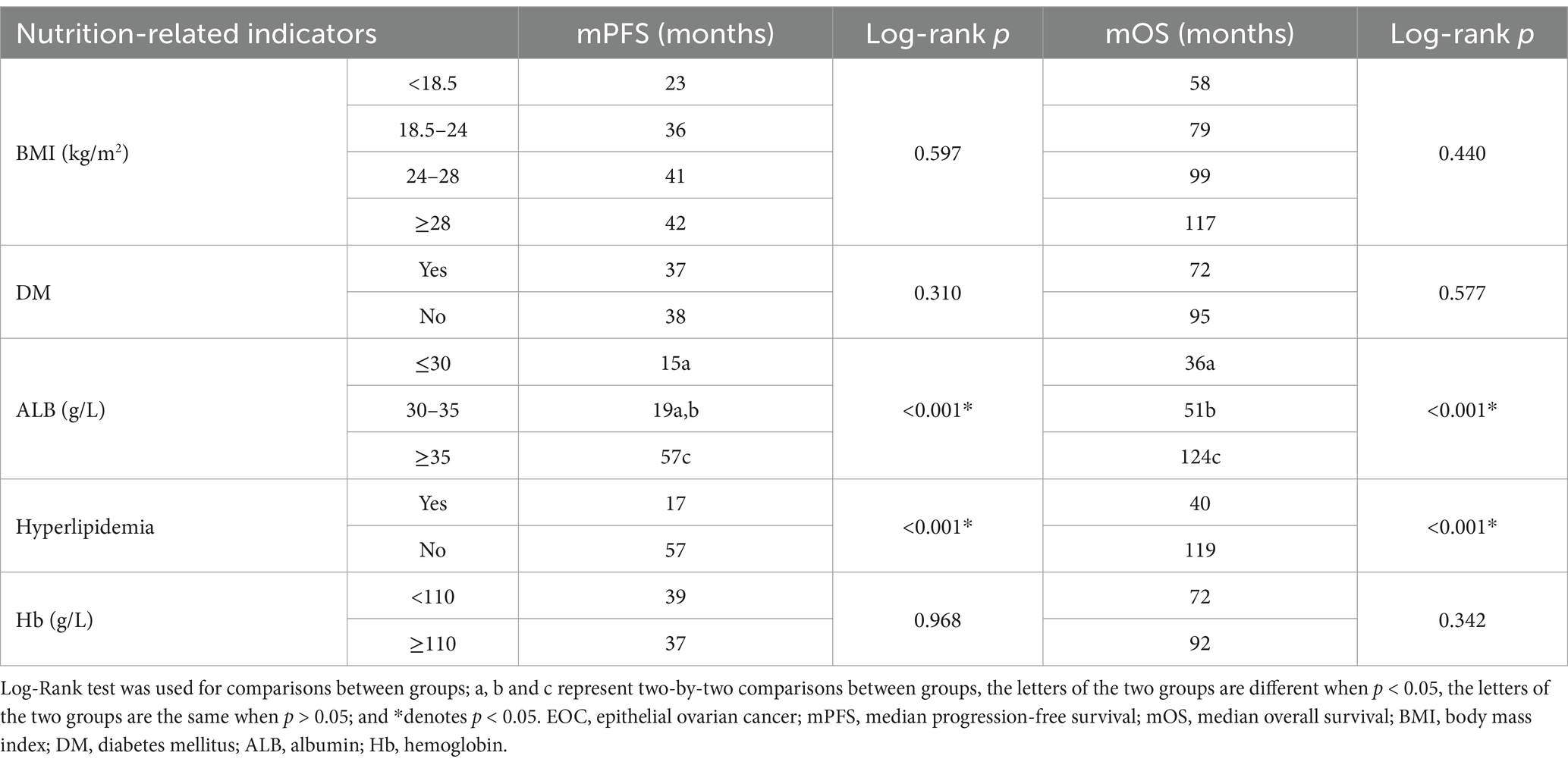
Table 3. Kaplan–Meier survival analysis of pre-treatment nutrition-related indicators affecting the PFS and OS in newly diagnosed EOC patients (N = 1,020).
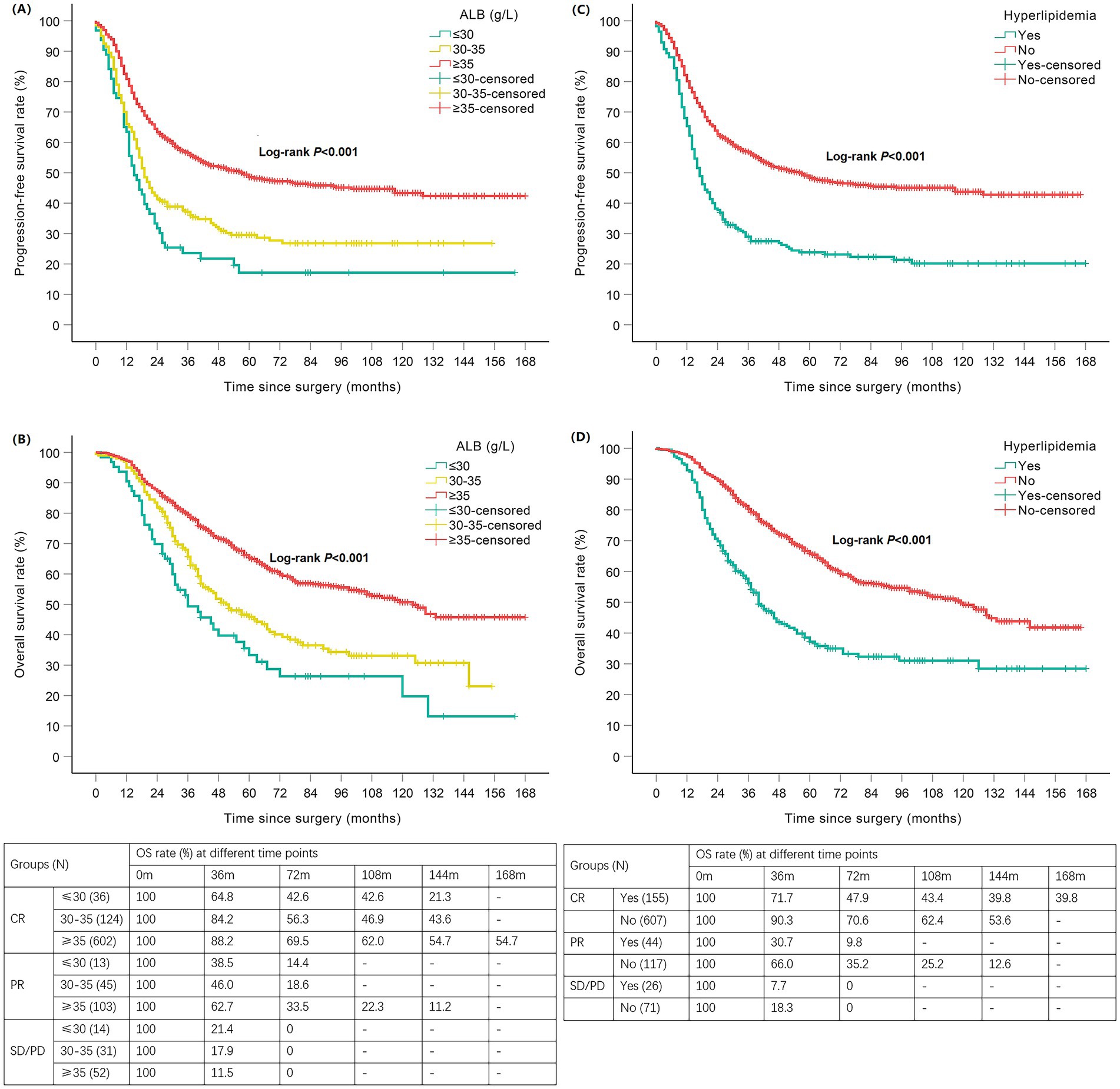
Figure 3. Kaplan–Meier curves of nutrition-related indicators with PFS/OS in newly diagnosed EOC patients. Hypoalbuminemia negatively affected PFS (A) and OS (B); Hyperlipidemia negatively affected PFS (C) and OS (D). EOC, epithelial ovarian cancer; ALB, albumin; PFS, progression-free survival; OS, overall survival; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; N, number; m, months.
The mPFS of patients with FIGO stages I, II, III, and IV were not reached (NR), NR, 19 months, and 13 months, respectively. Correspondingly, the mOS was 53 months, and 31 months in the NR group. In general, the later the stage, the shorter the PFS and OS (p < 0.001). However, there was no statistically significant difference in PFS or OS between patients with stage I and II disease (p > 0.05; Supplementary Table 2).
3.3.2 Multivariable cox regression analysis verified hyperlipidemia as an independent risk factor for shorter survival
The factors with p < 0.1 in the Kaplan–Meier survival analysis and potentially affecting the PFS/OS in patients (Table 3; Supplementary Table 2), namely ALB, hyperlipidemia, reproductive history, menopausal status, age, ascites, pathology, FIGO stage, residual tumor classification, cancer antigen 125 (CA125)-negative time, response to first-line chemotherapy and PARPi were included in the multivariable Cox regression analysis. In patients with newly diagnosed EOC, pre-treatment hyperlipidemia was an independent risk factor for shorter PFS (Figure 4A, HR = 2.083; 95% CI:1.726–2.514; p < 0.001) and OS (Figure 4B, HR = 2.158; 95% CI:1.746–2.666; p < 0.001). Although univariate analysis showed that pre-treatment hypoalbuminemia negatively affected patient survival (p < 0.05), multivariable Cox regression analysis failed to confirm it as an independent risk factor (p > 0.05; Figures 4A,B).
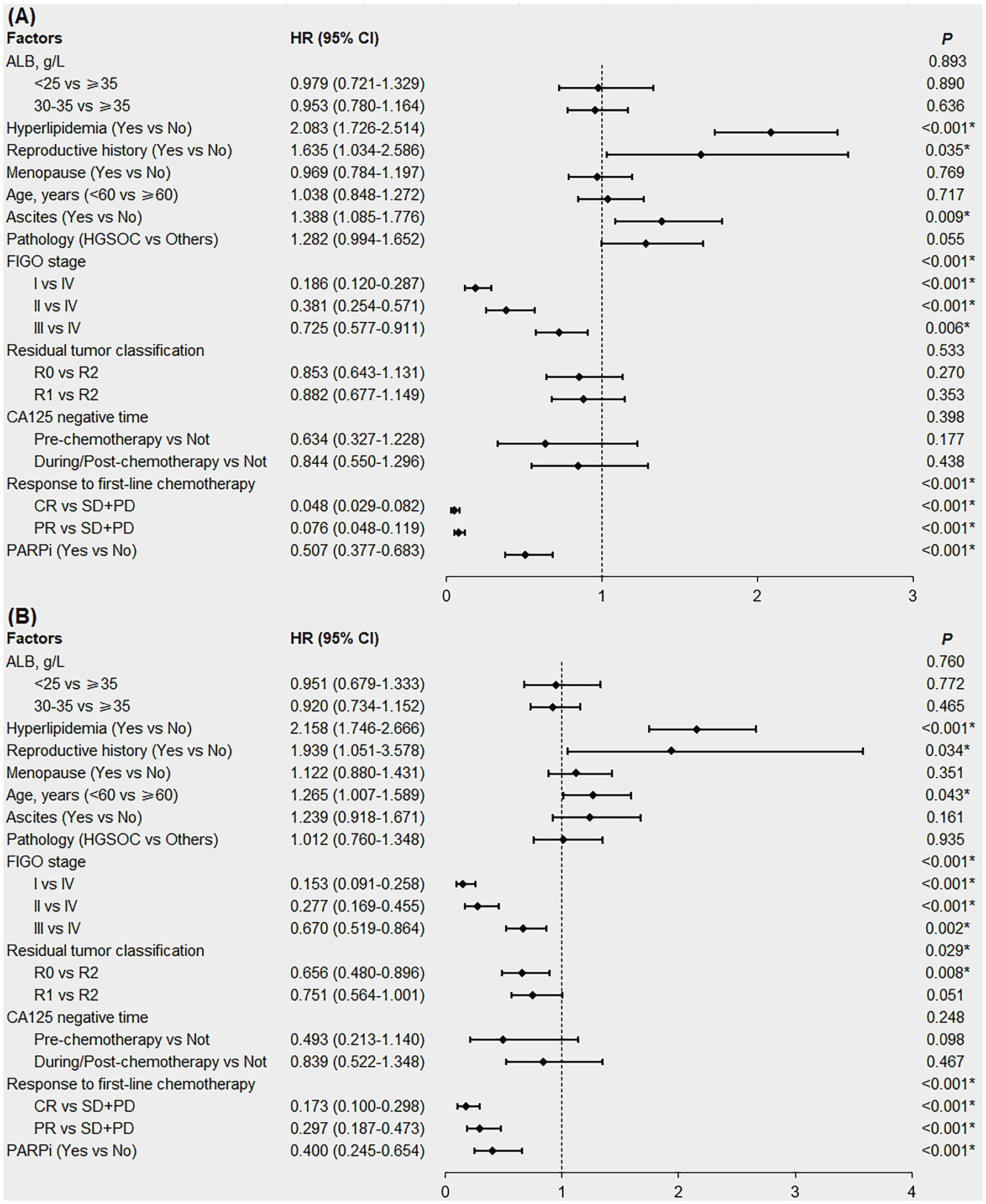
Figure 4. Forest plot of multivariable Cox regression analysis affecting the PFS (A) and OS (B) in newly diagnosed EOC patients. EOC, epithelial ovarian cancer; PFS, progression-free survival; OS, overall survival; ALB, albumin; HGSOC, high-grade serous ovarian cancer; FIGO, International Federation of Gynecology and Obstetrics; R0, no residual tumor; R1, microscopic residual tumor; R2, macroscopic residual tumor; CA125, cancer antigen 125; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; PARPi, poly ADP-ribose polymerase inhibitors; HR, hazard ratio; CI, confidence interval. *denotes p < 0.05.
We also conducted subgroup analyses according to FIGO stage. The results showed that in patients with early stage EOC (FIGO stages I and II), pre-treatment hyperlipidemia was an independent risk factor for shorter PFS (HR = 1.958; 95% CI:1.179–3.250; p = 0.009), but not for OS (p = 0.064). In patients with advanced EOC (FIGO stages III and IV), pre-treatment hyperlipidemia was an independent risk factor for shorter PFS (HR = 2.025; 95% CI:1.658–2.473; p < 0.001) and OS (HR = 2.118; 95% CI:1.700–2.639; p < 0.001; Supplementary Figures 1, 2).
4 Discussion
Cancer is a metabolism-related chronic wasting disease. There is growing evidence that the prognosis of patients with cancer is not only related to tumor factors, systemic inflammation, and immune status, but also to nutritional status (22). Malnutrition is found in 32% of patients with cancer, caused by tumor-related anorexia, inflammation, or metabolic changes (6). Several clinical studies have shown that both malnutrition and overnutrition in patients with cancer can affect disease progression and efficacy of anticancer therapy (7, 23). Therefore, evaluation and intervention of the nutritional status of patients with cancer may improve their efficacy and prognosis. There are few studies on the nutritional status and prognosis of patients with EOC; the factors studied are often single indicators or scores, and the results remain controversial. In this study, we used five common pre-treatment nutrition-related indicators, BMI, DM, hyperlipidemia, and ALB and Hb levels, to explore their effects on the prognosis of 1,020 patients with newly diagnosed EOC and identified hyperlipidemia as an independent nutrition-related risk factor for shorter survival.
Globally, there has been a gradual increase in the incidence of being overweight and obese. The Ovarian Cancer Association Consortium (OCAC) combined the data of 12,390 patients with OC from 21 studies and found that a higher BMI was associated with shorter OS and PFS (15). However, a Scottish study involving 1,067 patients with OC found no statistically significant correlation between BMI and survival (11); another study involving patients with EOC reached similar conclusions (24). One study reported that pre-diagnosis BMI ≥ 35 kg/m2 was associated with a lower survival rate in patients with stage I-II EOC, but in stage IV patients with BMI ≥ 35 kg/m2, the survival rate was higher; this correlation weakened when factors such as ascites and intestinal obstruction were adjusted for (25). However, the relationship between BMI and prognosis in patients with EOC remains controversial. In our study, 319 patients were overweight and 73 patients were obese; however, there was no statistically significant effect of BMI on the prognosis of patients with EOC, which is consistent with most existing studies (11, 24). Obesity is associated with a higher likelihood of comorbid DM, hypertension, and coronary heart disease, while underlying diseases increase the risk of perioperative and chemotherapy complications in patients with EOC, which is not conducive to a good prognosis (26). However, the fat metabolic reserve in obese patients with advanced cancer can help them resist the physiological challenges of surgery and chemotherapy, so those with a high BMI may even have a better prognosis, the “obesity paradox,” this has not been demonstrated in patients with EOC. In addition, although BMI is a commonly used indicator for evaluating the overall nutritional status, body weight is often affected by ascites and cachexia, making BMI unreliable as an appropriate indicator in some patients with EOC. The effect of BMI on the prognosis of patients with EOC, therefore, is uncertain, which may be due to differences in the time of weight measurement, definition of the BMI cutoff, body fat proportion, volume of ascites, and obesity-related underlying diseases. Prospective clinical studies related to overall nutritional status in the future should incorporate BMI, body fat proportion, abdominal circumference, and skinfold thickness simultaneously and choose fasting weight, excluding the effect of ascites, to reduce the error of evaluation and obtain more accurate correlation analysis data.
Glycolytic reprogramming of tumor cells not only provides a large amount of energy but also produces a large number of intermediate metabolites, such as lactic acid, which promote tumor proliferation, invasion, and metastasis (27–29). The Warburg effect also strengthens tumor immune escape and suppresses anti-tumor immunity through local hypoxia, inhibiting the monitoring and lethality of tumor-infiltrating lymphocytes. Inhibiting aerobic glycolysis-related pathways can eliminate tumor growth advantage and immune escape, thereby suppressing the occurrence and development of tumors and promoting anti-tumor immunity (30). A retrospective study has shown that DM is an independent risk factor for the prognosis of patients with EOC (30). However, data from 15 studies conducted by the OCAC showed no correlation between DM and PFS in patients with OC (31). Metformin reduces the production of insulin, insulin-like growth factor, inflammatory cytokines, and vascular endothelial growth factor and has antimitotic, anti-inflammatory, and anti-angiogenic effects (32). In a clinical study, metformin reduced the risk of EOC and prolonged the survival of patients with EOC and DM (33). These findings support the hypothesis that DM influences morbidity and survival in patients with EOC. Of the 1,020 patients included in this study, 75 had DM. No statistically significant correlation was found between DM and the prognosis of patients with EOC. Since metformin use is associated with prolonged survival in EOC patients with DM (33), glycemic control status may affect the prognosis. A history of DM, and levels of blood glucose, HbA1C, and glycated albumin are commonly used to evaluate the nutritional status in relation to carbohydrates. HbA1C reflects glycemic control over the preceding the 3 months, is more stable than other indicators, and is not affected by the external environment, emotion, or current diet; therefore, it is the best indicator for evaluating the status of recent glycemic control. HbA1C has been found to be associated with a risk of recurrence and death in early-stage breast (34) and colon cancer (8). Therefore, the results of this study suggest that, when HbA1C data cannot be used as a reference, the presence of DM does not affect the prognosis of patients with EOC.
In tumor cells, amino acids not only directly participate in the growth and proliferation of tumor cells as raw materials for the synthesis of various proteins or as intermediates for energy metabolism but are also necessary to activate the mammalian target of rapamycin (mTOR) pathway involved in tumor cell proliferation and migration. Additionally, amino acids are indirectly involved in antitumor immunity by regulating immune cell function (35). Amino acid metabolic reprogramming has been shown to be closely related to the development of various cancers (4), so it is particularly important to study the effect of protein nutritional status on patients with cancer. ALB has the highest concentration in human plasma, accounting for more than 60% of the total protein in healthy adults, and not only effectively reflects the nutritional status of patients with EOC but also plays an important role in regulating inflammation, oxidative stress, and innate immunity, and thus affecting the growth of tumor cells (36). Several studies have shown that ALB levels are related to the prognosis of patients with EOC (37–39), however, several questions remain unanswered. First, it is unclear whether hypoalbuminemia is an independent risk factor for the prognosis of patients with EOC. Anorexia in patients with advanced cancer can lead to inadequate amino acid intake and decreased ALB synthesis. High catabolism in patients with cancer leads to increased ALB consumption, and with the progression of the disease, increased capillary permeability leads to the infiltration of ALB into the interstitial space, resulting in the formation of exudates in the serosal cavities. All of the above can cause hypoalbuminemia in patients with cancer; therefore, pre-treatment ALB levels are usually associated with several clinical features. Our study found that compared to the normal ALB group, patients with hypoalbuminemia had a worse prognosis, but this was not an independent predictor of prognosis, which may be because ALB levels were also affected by ascites, body weight, and other nutrition-related indicators. In the Chinese clinical guidelines, the indication for human serum albumin supplementation in patients with cancer is ALB≤30 g/L, while hypoalbuminemia is defined as ALB≤35 g/L (18). Whether ALB in the range 30–35 g/L g/L affects the efficacy and prognosis of patients is clear. Our study revealed that patients with ALB≤30 g/L had shorter OS than those with ALB in the range 30–35 g/L, but the first-line chemotherapy response and PFS were not statistically different between the two groups, which demonstrated that hypoalbuminemia, especially when the ALB level dropped below 30 g/L, might severely affect the long-term survival of patients with EOC. Future clinical trials are needed to evaluate the effect of human serum ALB supplementation on the efficacy and prognosis of patients with EOC, especially those with ALB<30 g/L.
Lipids play a key role in the occurrence and development of tumors, with increased lipid levels supporting the high energy demand of growing tumor cells. Tumor cells reprogram lipid metabolism, mainly by affecting lipid uptake, synthesis, and catabolism. Studies have shown that lipid metabolism-related genes are overexpressed in a variety of cancers to adapt to the high energy demand of tumor cells, and their overexpression has been shown to be associated with poor cancer prognosis. In addition, excessive lipids can affect the function of various immune cells in the tumor microenvironment, thereby inhibiting antitumor immunity (7, 40). The accumulation of unsaturated fatty acids supports the growth and migration of OC cells, leading to worse prognosis. In addition, increased lipogenesis and lipid uptake promote chemotherapy resistance and suppress the immune response required to eliminate tumors (41). Blood lipid levels are commonly used as clinical indicators of lipid nutrition. Although there have been a few clinical studies on the correlation between hyperlipidemia and EOC prognosis, the use of statins after OC diagnosis prolongs survival (14, 42). Our study directly evaluated the effect of pre-treatment hyperlipidemia on the treatment efficacy and prognosis of patients with newly diagnosed EOC. We found that hyperlipidemia was an independent risk factor for poor prognosis, predicting shorter PFS and OS. Patients with hyperlipidemia also had poorer first-line chemotherapy responses than those without hyperlipidemia. However, in the subgroup analysis of patients with early stage EOC, hyperlipidemia was not an independent risk factor for OS. This suggests that pre-treatment hyperlipidemia only affects the short-term survival of patients with early stage EOC but does not affect their long-term survival. A study involving 249 patients with EOC found that the HDL-cholesterol (C)/TC ratio was significantly correlated with chemoresistance and that the HDL-C/LDL-C ratio was an independent protective factor for survival (43). The results regarding hyperlipidemia in our study are consistent with those of that study. Some patients were included in our study retrospectively. Specifically, for patients who had been diagnosed with hyperlipidemia before hospitalization, only their medical history regarding hyperlipidemia was collected during data gathering. Additionally, the lipid profiles of patients without hyperlipidemia were not available. In future prospective studies of patients with EOC, the associations between HDL-C/LDL-C or HDL-C/TC ratios and prognosis should be explored, especially for those with SD/PD.
Anemia is common in patients with cancer. Hb carries oxygen, and anemia is associated with many symptoms such as fatigue, depression, and dyspnea, which seriously affect the quality of life. Hypoxia promotes the metastatic potential and growth of tumor cells, decreases the cellular response to apoptotic signals, and generates therapeutic resistance (44). In addition, anemia itself may induce a feedback mechanism that promotes angiogenesis and leads to a higher proliferation rate of tumor cells (45). Existing studies on Hb levels and prognosis in patients with EOC have small sample sizes, and the results are inconsistent (46). Our study not only analyzed the effect of Hb levels on prognosis but also analyzed the response to first-line chemotherapy. Although the Hb level had no statistically significant effect on PFS or OS, patients with anemia had poorer first-line chemotherapy responses than those with normal Hb levels. Clinical practice should focus on the effects of increased Hb levels during treatment to improve the prognosis of patients with EOC.
The retrospective inclusion of patients, along with the changes in clinical guidelines and testing technologies, has led to certain selection biases and information biases, which in turn have contributed to the limitations of the results in this study. However, our study included patients with newly diagnosed EOC, with a large sample size of 1,020 cases and a median follow-up period of 48 months. Five nutrition-related indicators and three treatment-efficacy or prognostic indicators were analyzed, allowing the effect of nutritional status on patients to be comprehensively considered. Multivariable analysis eliminated the interactions between different types of nutrition-related indicators, as well as between indicators and clinicopathological characteristics, and thus more accurately identified the independent nutrition-related indicator risk factors affecting the treatment efficacy and prognosis of patients with EOC. Our research demonstrated that pre-treatment hypoalbuminemia, hyperlipidemia, and anemia negatively affected the response to first-line chemotherapy in patients with newly diagnosed EOC. Additionally, pre-treatment hypoalbuminemia and hyperlipidemia negatively affected survival, with hyperlipidemia being an independent risk factor for shorter survival. However, BMI and DM did not affect the prognosis of patients with EOC. These five nutritional indicators are easy to assess even in less-developed and resource-limited regions. In future clinical practice, nutritional status intervention during anti-tumor treatment of patients with EOC might improve treatment efficacy and prognosis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University (No. XJTU1AF2021LSL-024). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this study was an observational study and patients were included retrospectively and anonymously, the consent was waived.
Author contributions
SW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JiZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. NiW: Data curation, Methodology, Supervision, Writing – review & editing. NaW: Formal analysis, Supervision, Validation, Writing – review & editing. XD: Validation, Visualization, Writing – review & editing. MZ: Investigation, Project administration, Writing – review & editing. JuZ: Resources, Visualization, Writing – review & editing. DM: Conceptualization, Writing – review & editing. XY: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Natural Science Basic Research Program of Shaanxi (Number: 2023-JC-YB-683) and the Clinical Research Fund of the First Affiliated Hospital of Xi’an Jiaotong University (Number: XJTU1AF2021CRF-025).
Acknowledgments
We thank all the participants and thank the Department of Obstetrics and Gynecology, the First Affiliated Hospital of Xi’an Jiaotong University for clinical data related to this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1489934/full#supplementary-material
References
1. Bray, F, Laversanne, M, Sung, H, Ferlay, J, Siegel, RL, Soerjomataram, I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Lheureux, S, Gourley, C, Vergote, I, and Oza, AM. Epithelial ovarian cancer. Lancet. (2019) 393:1240–53. doi: 10.1016/s0140-6736(18)32552-2
3. Heiden, MGV, Cantley, LC, and Thompson, CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. (2009) 324:1029–33. doi: 10.1126/science.1160809
4. Wang, D, and Wan, X. Progress in research on the role of amino acid metabolic reprogramming in tumour therapy: a review. Biomed Pharmacother. (2022) 156:113923. doi: 10.1016/j.biopha.2022.113923
5. Broadfield, LA, Pane, AA, Talebi, A, Swinnen, JV, and Fendt, S-M. Lipid metabolism in cancer: new perspectives and emerging mechanisms. Dev Cell. (2021) 56:1363–93. doi: 10.1016/j.devcel.2021.04.013
6. Gangadharan, A, Choi, SE, Hassan, A, Ayoub, NM, Durante, G, Balwani, S, et al. Protein calorie malnutrition, nutritional intervention and personalized cancer care. Oncotarget. (2017) 8:24009–30. doi: 10.18632/oncotarget.15103
7. Martin-Perez, M, Urdiroz-Urricelqui, U, Bigas, C, and Benitah, SA. The role of lipids in cancer progression and metastasis. Cell Metab. (2022) 34:1675–99. doi: 10.1016/j.cmet.2022.09.023
8. Castro, M-J, Jimenez, J-M, Lopez, M, Cao, M-J, Santos-Torres, J, Lopez, A, et al. Impact of preoperative Total proteins and glycated hemoglobin on recurrences after early colorectal Cancer. Nutrients. (2021) 13:13. doi: 10.3390/nu13020711
9. Chan, DSM, Vieira, AR, Aune, D, Bandera, EV, Greenwood, DC, McTiernan, A, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. (2014) 25:1901–14. doi: 10.1093/annonc/mdu042
10. Kichenadasse, G, Miners, JO, Mangoni, AA, Rowland, A, Hopkins, AM, and Sorich, MJ. Association between body mass index and overall survival with immune checkpoint inhibitor therapy for advanced non-small cell lung Cancer. JAMA Oncol. (2020) 6:512–8. doi: 10.1001/jamaoncol.2019.5241
11. Barrett, SV, Paul, J, Hay, A, Vasey, PA, Kaye, SB, Glasspool, RM, et al. Does body mass index affect progression-free or overall survival in patients with ovarian cancer? Results from SCOTROC I trial. Ann Oncol. (2008) 19:898–902. doi: 10.1093/annonc/mdm606
12. Gadducci, A, Sartori, E, Landoni, F, Zola, P, Maggino, T, Colombo, N, et al. Pre-chemotherapy hemoglobin levels and survival in patients with advanced epithelial ovarian cancer who received a first-line taxane/platinum-based regimen: results of a multicenter retrospective Italian study. Gynecol Oncol. (2005) 98:118–23. doi: 10.1016/j.ygyno.2005.04.012
13. Lin, Q, Liu, W, Xu, S, and Sun, L. Associations of preoperative serum high-density lipoprotein cholesterol and low-density lipoprotein cholesterol levels with the prognosis of ovarian cancer. Arch Gynecol Obstet. (2022) 305:683–91. doi: 10.1007/s00404-021-06215-3
14. Majidi, A, Na, R, Jordan, SJ, De Fazio, A, Webb, PM, and Grp, OS. Statin use and survival following a diagnosis of ovarian cancer: a prospective observational study. Int J Cancer. (2021) 148:1608–15. doi: 10.1002/ijc.33333
15. Nagle, CM, Dixon, SC, Jensen, A, Kjaer, SK, Modugno, F, deFazio, A, et al. Obesity and survival among women with ovarian cancer: results from the ovarian Cancer association consortium. Br J Cancer. (2015) 113:817–26. doi: 10.1038/bjc.2015.245
16. Chen, C, and Lu, FC. Department of Disease Control Ministry of health, PR China. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci. (2004) 17:1–36.
17. Alberti, K, and Zimmet, PZ. Definition, diagnosis and classification of diabetes mellitus and its complications part 1: diagnosis and classification of diabetes mellitus - provisional report of a WHO consultation. Diabet Med. (1998) 15:539–53. doi: 10.1002/(sici)1096-9136(199807)15:7<539::Aid-dia668>3.0.Co;2-s
18. Hospital Pharmacy Professional Committee of Chinese Pharmaceutical Association, Compilation Group of Chinese Expert Consensus on Clinical Application Management of Human Albumin. Expert consensus on clinical application management of human albumin. Chin J Hosp Pharm. (2024) 44:739–51. doi: 10.13286/j.1001-5213.2024.07.01
19. Ge, J, and Xu, Y. Internal Medicine. 9th ed. Beijing: People's Medical Publishing House (2018). 536 p.
20. Joint committee on the Chinese guidelines for lipid management. Chinese guidelines for lipid management. Chinese Circulation J. (2023) 38:237–71. doi: 10.3969/j.issn.1000-3614.2023.03.001
21. Eisenhauer, EA, Therasse, P, Bogaerts, J, Schwartz, LH, Sargent, D, Ford, R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
22. Ogino, S, Galon, J, Fuchs, CS, and Dranoff, G. Cancer immunology-analysis of host and tumor factors for personalized medicine. Nat Rev Clin Oncol. (2011) 8:711–9. doi: 10.1038/nrclinonc.2011.122
23. Mayne, ST, Playdon, MC, and Rock, CL. Diet, nutrition, and cancer: past, present and future. Nat Rev Clin Oncol. (2016) 13:504–15. doi: 10.1038/nrclinonc.2016.24
24. Hew, KE, Bakhru, A, Harrison, E, Turan, MO, MacDonald, R, Im, DD, et al. The effect of obesity on the time to recurrence in ovarian Cancer: a retrospective study. Clinical Ovarian and Other Gynecologic Cancer. (2013) 6:31–5. doi: 10.1016/j.cogc.2014.02.002
25. Bandera, EV, Lee, VS, Qin, B, Rodriguez-Rodriguez, L, Powell, CB, and Kushi, LH. Impact of body mass index on ovarian cancer survival varies by stage. Br J Cancer. (2017) 117:282–9. doi: 10.1038/bjc.2017.162
26. Afshin, A, Forouzanfar, MH, Reitsma, MB, Sur, P, Estep, K, Lee, A, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. (2017) 377:13–27. doi: 10.1056/NEJMoa1614362
27. Abdel-Wahab, AF, Mahmoud, W, and Al-Harizy, RM. Targeting glucose metabolism to suppress cancer progression: prospective of anti-glycolytic cancer therapy. Pharmacol Res. (2019) 150:104511. doi: 10.1016/j.phrs.2019.104511
28. Faubert, B, Solmonson, A, and DeBerardinis, RJ. Metabolic reprogramming and cancer progression. Science. (2020) 368:152. doi: 10.1126/science.aaw5473
29. Shi, Q, Shen, Q, Liu, Y, Shi, Y, Huang, W, Wang, X, et al. Increased glucose metabolism in TAMs fuels O-GlcNAcylation of lysosomal Cathepsin B to promote cancer metastasis and chemoresistance. Cancer Cell. (2022) 40:1207–1222.e10. doi: 10.1016/j.ccell.2022.08.012
30. Akhavan, S, Ghahghaei-Nezamabadi, A, Modaresgilani, M, Mousavi, AS, Sepidarkish, M, Tehranian, A, et al. Impact of diabetes mellitus on epithelial ovarian cancer survival. BMC Cancer. (2018) 18:1246. doi: 10.1186/s12885-018-5162-3
31. Minlikeeva, AN, Freudenheim, JL, Cannioto, RA, Szender, JB, Eng, KH, Modugno, F, et al. History of hypertension, heart disease, and diabetes and ovarian cancer patient survival: evidence from the ovarian cancer association consortium. Cancer Causes Control. (2017) 28:469–86. doi: 10.1007/s10552-017-0867-1
32. Gadducci, A, Biglia, N, Tana, R, Cosio, S, and Gallo, M. Metformin use and gynecological cancers: a novel treatment option emerging from drug repositioning. Crit Rev Oncol Hematol. (2016) 105:73–83. doi: 10.1016/j.critrevonc.2016.06.006
33. Li, L, Qi, X, Xu, M, Ding, X, Zhou, X, Zhang, C, et al. The effects of metformin on ovarian cancer: an updated systematic review and meta-analysis. Int J Clin Exp Med. (2016) 9:17559–68.
34. Erickson, K, Patterson, RE, Flatt, SW, Natarajan, L, Parker, BA, Heath, DD, et al. Clinically defined type 2 diabetes mellitus and prognosis in early-stage breast Cancer. J Clin Oncol. (2011) 29:54–60. doi: 10.1200/jco.2010.29.3183
35. Yang, L, Chu, Z, Liu, M, Zou, Q, Li, J, Liu, Q, et al. Amino acid metabolism in immune cells: essential regulators of the effector functions, and promising opportunities to enhance cancer immunotherapy. J Hematol Oncol. (2023) 16:59. doi: 10.1186/s13045-023-01453-1
36. Belinskaia, DA, Voronina, PA, Shmurak, VI, Jenkins, RO, and Goncharov, NV. Serum albumin in health and disease: esterase, antioxidant, transporting and signaling properties. Int J Mol Sci. (2021) 22:22. doi: 10.3390/ijms221910318
37. Conrad, LB, Awdeh, H, Acosta-Torres, S, Conrad, SA, Bailey, AA, Miller, DS, et al. Pre-operative core muscle index in combination with hypoalbuminemia is associated with poor prognosis in advanced ovarian cancer. J Surg Oncol. (2018) 117:1020–8. doi: 10.1002/jso.24990
38. Ayhan, A, Gunakan, E, Alyazici, I, Haberal, N, Altundag, O, and Dursun, P. The preoperative albumin level is an independent prognostic factor for optimally debulked epithelial ovarian cancer. Arch Gynecol Obstet. (2017) 296:989–95. doi: 10.1007/s00404-017-4511-9
39. Asher, V, Lee, J, and Bali, A. Preoperative serum albumin is an independent prognostic predictor of survival in ovarian cancer. Med Oncol. (2012) 29:2005–9. doi: 10.1007/s12032-011-0019-5
40. Li, Z, and Kang, Y. Lipid metabolism fuels Cancer's spread. Cell Metab. (2017) 25:228–30. doi: 10.1016/j.cmet.2017.01.016
41. Chaudhry, S, Thomas, SN, and Simmons, GE Jr. Targeting lipid metabolism in the treatment of ovarian cancer. Oncotarget. (2022) 13:768–83. doi: 10.18632/oncotarget.28241
42. Feng, J-L, Dixon-Suen, SC, Jordan, SJ, and Webb, PM. Statin use and survival among women with ovarian cancer: an Australian national data-linkage study. Br J Cancer. (2021) 125:766–71. doi: 10.1038/s41416-021-01460-4
43. Li, Y, Shang, C, Liang, H, Zhang, K, Wu, Y, and Guo, H. Associations of novel serum lipid index with epithelial ovarian cancer chemoresistance and prognosis. Front. Oncologia. (2023) 13:13. doi: 10.3389/fonc.2023.1052760
44. Chen, Z, Han, F, Du, Y, Shi, H, and Zhou, W. Hypoxic microenvironment in cancer: molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther. (2023) 8:70–69. doi: 10.1038/s41392-023-01332-8
45. Van Belle, SJP, and Cocquyt, W. Impact of haemoglobin levels on the outcome of cancers treated with chemotherapy. Crit Rev Oncol Hematol. (2003) 47:1–11. doi: 10.1016/s1040-8428(03)00093-3
46. Pergialiotis, V, Daskalakis, G, Thomakos, N, Bellos, I, Haidopoulos, D, Loutradis, D, et al. Prechemotherapy hemoglobin levels as a predictive factor of ovarian Cancer survival a systematic review and Meta-analysis. Am J Clin Oncol-Cancer Clin Trials. (2019) 42:725–31. doi: 10.1097/coc.0000000000000570
Keywords: epithelial ovarian cancer, nutrition-related indicators, first-line chemotherapy response, progression-free survival, overall survival
Citation: Wang S, Zhu J, Wu N, Wang N, Dang X, Zhao M, Zhao J, Ma D and Yang X (2025) Pre-treatment nutrition-related indicators and the prognosis of patients with newly diagnosed epithelial ovarian cancer: an ambispective cohort study. Front. Nutr. 12:1489934. doi: 10.3389/fnut.2025.1489934
Edited by:
Lucilla Crudele, University of Bari Aldo Moro, ItalyCopyright © 2025 Wang, Zhu, Wu, Wang, Dang, Zhao, Zhao, Ma and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaofeng Yang, eXhmNzNAMTYzLmNvbQ==
 Shirui Wang
Shirui Wang Jingyu Zhu
Jingyu Zhu Ningjuan Wu
Ningjuan Wu Nannan Wang
Nannan Wang Xiaohe Dang1
Xiaohe Dang1 Xiaofeng Yang
Xiaofeng Yang