- 1Department of Neurology, Affiliated People's Hospital of Jiangsu University, Zhenjiang, China
- 2Department of Neurology, Affiliated Hospital of Jiangsu University, Zhenjiang, China
Background: Vitamins are essential micronutrients for the prevention and treatment of neurodegenerative diseases. The objectives of the present study were to evaluate the association between dietary vitamin intake and cognitive function in elderly adults and to explore the potential impact of serum neurofilament light chain (NfL) concentration.
Methods: Data from 468 elderly individuals, including information on the dietary consumption of 10 vitamins, were used. Cognitive performance was assessed according to a composite Z-score of the Animal Fluency Test (AFT), Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), and Digit Symbol Substitution Test (DSST). Serum NfL levels were measured using a highly sensitive immunoassay. Bayesian kernel machine regression (BKMR) models were used to estimate the combined effects of vitamin mixtures on cognitive function.
Results: In both single- and multiple-vitamin models, individuals with a higher intake of dietary vitamin K exhibited greater global cognitive function, compared to those with a lower vitamin intake. BKMR revealed positive associations between vitamin mixtures and global cognitive function, AFT Z-scores, and DSST Z-scores. Individuals in the third vitamin K intake tertile exhibited lower serum NfL levels than those in the first tertile (regression coefficient, β = −0.16 [95% confidence interval −0.29 to −0.02]; p = 0.023). Serum NfL levels mediated the association between higher vitamin K intake and global cognitive function (8.73%).
Conclusion: Vitamin mixtures were positively associated with global cognitive function in elderly participants. The association between vitamin K intake and cognitive function may be mediated by serum NfL concentration.
1 Introduction
Cognitive function is a crucial factor for maintaining quality of life and can be affected by aging and neurodegenerative disease(s). One of the most common diseases attributed to age-related decline in cognitive function is dementia (1). Dementia is a primary cause of death and disability among elderly adults worldwide (2). It has been estimated that the global number of cases of dementia was 57.4 million in 2019 and is projected to be 152.8 million in 2050 (3). Generally, dementia evolves from cognitive decline or impairment, and the transition to the disease is irreversible (4). Cognitive decline can be triggered by various pathological, behavioral, and environmental factors (3). As such, identifying modifiable risk factors and developing interventional strategies to prevent or, at least mitigate, cognitive decline in elderly individuals have significant public health implications.
Diet can act as a modifiable risk factor and nutrients may have an impact(s) on the decline of cognitive function (5). Vitamins are essential micronutrients for normal nervous system functioning and neurological wellbeing. By regulating DNA synthesis and methylation, and maintaining phospholipids, vitamins exert pleiotropic effects on neuronal structure, function, and neuroendocrine pathways (6). The neurofilament light chain (NfL), a component of the axonal cytoskeleton, has been identified as a marker of neuro-axonal damage in various neurological disorders, including Alzheimer’s disease (7, 8). The correlation between NfL in the cerebrospinal fluid and blood suggests that blood-based NfL can serve as a biomarker for monitoring neurological disorders (9).
Epidemiological studies have demonstrated that the antioxidant and neuroprotective properties of vitamins may contribute to neural integrity and cognitive preservation (10, 11). Several studies have generated consistent evidence supporting folate (vitamin B9) (12), and putative evidence for other vitamins associated with cognitive impairment in the general population (13, 14). However, the effects of vitamin combinations on cognitive function remain unclear. As such, we hypothesized that serum NfL levels may be involved in the association between individual vitamin intake and cognitive function. The aims of the present study were, therefore, to examine the associations between the intake of multiple vitamins and cognitive function among elderly participants, and to evaluate the potential impact of NfL on these associations.
2 Methods
2.1 Study design and population
The current analysis sourced publicly available data from the National Health and Nutrition Examination Survey (NHANES) database. Among the critical objectives of the NHANES was to evaluate the nutritional status and health of a large, representative sample of the United States population. Given the availability of data, the NHANES 2013–2014 cycle was used (15). Participants provided written informed consent before enrollment, and as such, additional consent requirements were waived. The study protocol (No. #2011-17) was reviewed and approved by the NCHS Research Ethics Review Board.
A total of 10,175 participants were enrolled in the NHANES 2013–2014 cycle, and data from elderly participants ≥60 years of age (n = 1,841) were extracted and included in the present study. Participants who lacked cognitive assessment data were excluded (n = 268), as were those without dietary information (n = 133), and/or serum NfL measurement data (n = 972) (Supplementary Figure S1).
2.2 Calculation of vitamin intake(s)
For NHANES participants, two 24-h dietary recall interviews were conducted (16): the first interview was in-person at the mobile examination center, and the second was via telephone after 3–10 days. The vitamin intake of each individual was calculated based on two 24-h dietary intakes and dietary supplements according to the United States Department of Agriculture Food and Nutrient Database (17). This database contains extensive information used for coding individual foods and beverages, as well as the portion sizes reported by participants. It also provides the nutrient values necessary for calculating nutrient intake(s). Dietary vitamin intake was assessed by averaging the data from two 24-h dietary and supplementation recalls. A panel of 10 vitamins was incorporated into the present analysis.
2.3 Serum NfL concentration
Serum samples were collected and frozen for further analysis. A high-sensitivity immunoassay was used to quantify serum NfL concentrations (15). Briefly, the samples were incubated with antibodies labeled with acridinium ester (AE), which specifically binds to the NfL antigen. Paramagnetic particles (PMPs) coated with a capture antibody were added to the sample, thus forming complexes formed by the antigen bound to both the AE-labeled antibodies and PMPs. Any unbound AE-labeled antibodies were separated and eliminated. Finally, acids and bases were added to generate chemiluminescence, enabling light emission measurements. As reported on the NHANES website, the analysis followed strict quality control and assurance procedures. The limit of quantification for NfL was 3.9 pg./mL.
2.4 Cognitive function
During the survey, the Consortium to Establish a Registry for Alzheimer’s Disease Word Learning test (CERAD W-L), the Animal Fluency test (AFT), and the Digit Symbol Substitution test (DSST) were administered to participants ≥60 years of age. As previously reported (18, 19), a combination of these tests has been widely used to assess individual cognitive performance, including verbal, memory, and executive abilities. The CERAD W-L, AFT, and DSST scores were standardized using the Z-score method (20), and a composite sum Z-score was used to assess individual global cognitive function (21, 22).
2.5 Covariates
Known or suspected influential factors of vitamin consumption and cognitive function were screened based on the previous literature (23, 24) and the directed acyclic graph method (Supplementary Figure S2). The final models included sex, age, ethnicity, education, family income-to-poverty ratio (PIR), body mass index (BMI), smoking status, alcohol consumption, physical activity levels, diabetes, hypertension, and energy intake. Smoking status was assessed using a cutoff of 100 cigarettes in life as never smoked, former smoker, or current smoker, while 12 alcoholic beverages/year were used as the cutoff to define alcohol consumption. Physical activity was measured according to moderate/vigorous recreational activity for ≥10 min continuously per week (25). Diabetes was evaluated by self-reported diabetes history and relevant medicine taken in combination with laboratory investigations, including a glycated hemoglobin (HbA1c) level > 6.5%, fasting blood glucose level ≥ 7.0 mmol/L, and a 2 h plasma glucose level ≥ 11.1 mmol/L (26). Similarly, hypertension was assessed according to measurements of systolic blood pressure (SBP) and diastolic blood pressure (DBP), self-reported history of hypertension, and the use of antihypertensive medication (27).
2.6 Statistical analysis
Participant characteristics are expressed as mean (standard deviation [SD]) and frequency (proportion). Vitamin intake was transformed into categorical variables (tertiles) due to non-linear dose–response relationships, as described previously (28). Serum NfL concentrations were natural logarithm (ln)-transformed due to skewed distribution. Multiple imputations with chained equations were applied to the small number of missing covariates.
Multivariable linear regression models were used to explore the associations between vitamin intake, sum Z-score, and serum NfL concentrations. The variance inflation factor (VIF) was used to assess collinearity in the linear regression models with no multicollinearity. Multivariable generalized additive models (GAMs) were used to estimate potential non-linear associations between vitamin consumption and cognitive function. The Wald test was used to test the significance of the models. Bayesian kernel machine regression (BKMR) models were used to assess the combined effects of the vitamin mixtures on cognitive function (29). BKMR is a novel approach for assessing dose–response relationships, joint effects of vitamin mixtures, and individual effects among vitamin mixtures (29). Mediation analysis was performed to assess the potential mediating role of serum NfL levels in the relationship between vitamin intake and cognitive function (22).
In the sensitivity analysis, several factors were considered to confirm the robustness of the results, including potential confounders such as beta-carotene, the relationship between serum NfL and global cognitive function, and single vitamin intake in the source population.
Statistical analyses were performed using R version 4.3.2 (R Foundation for Statistical Computing, Vienna, Austria). Differences with a p-value of < 0.05 were considered to be statistically significant.
3 Results
3.1 Participant characteristics
The general characteristics of the 468 elderly individuals (219 male [46.8%]) included in this study are summarized in Table 1. The mean (± SD) age and BMI were 66.3 ± 4.4 years and 29.6 ± 6.8 kg/m2, respectively. Among the participants, 228 (48.7%) were non-Hispanic white, and 249 (53.2%) had a college degree or above. The proportions of participants with diabetes and hypertension were 34.4 and 76.3%, respectively.
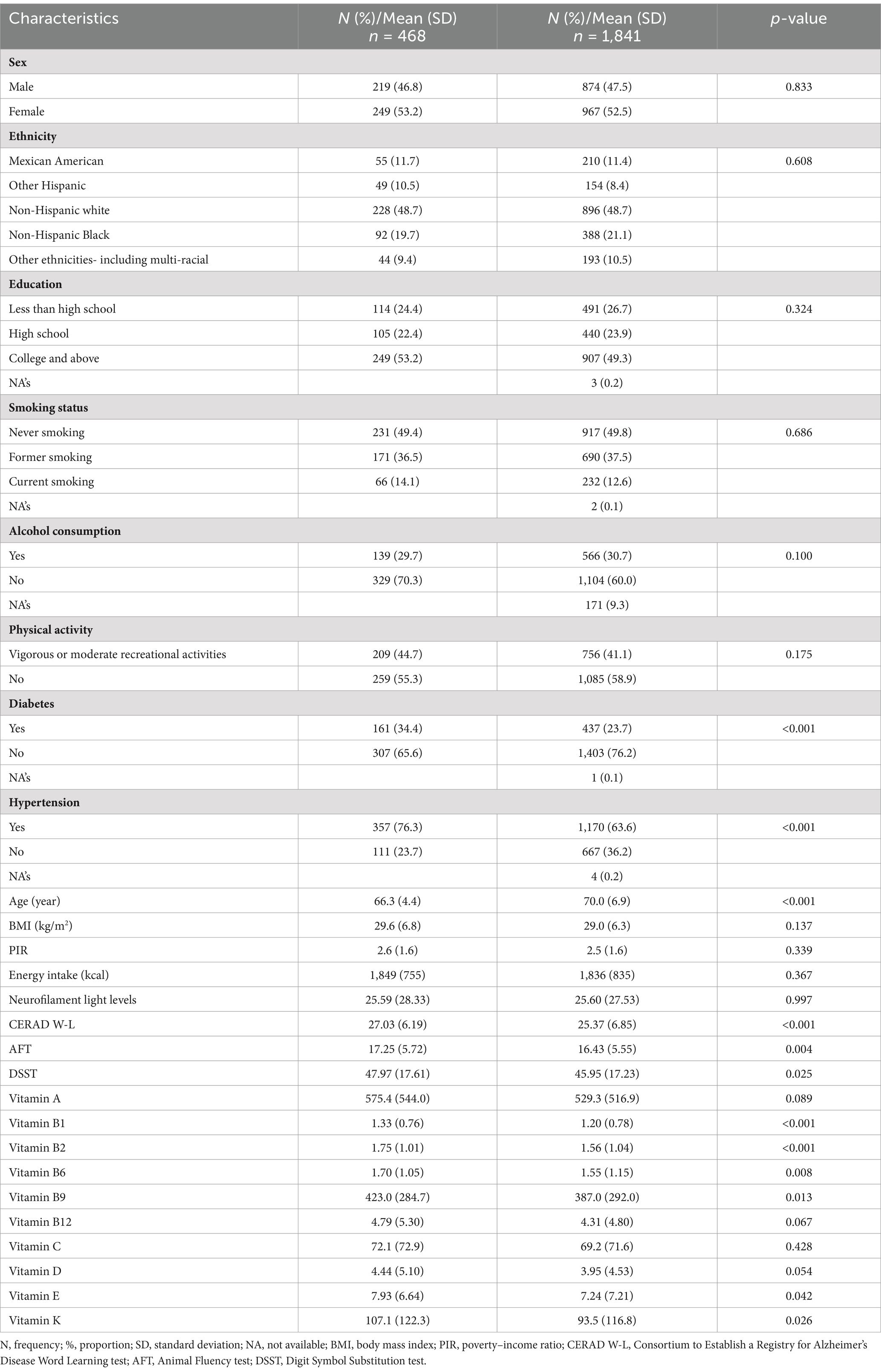
Table 1. Descriptive statistics of general characteristics of 468 participants from NHANES 2013–2014.
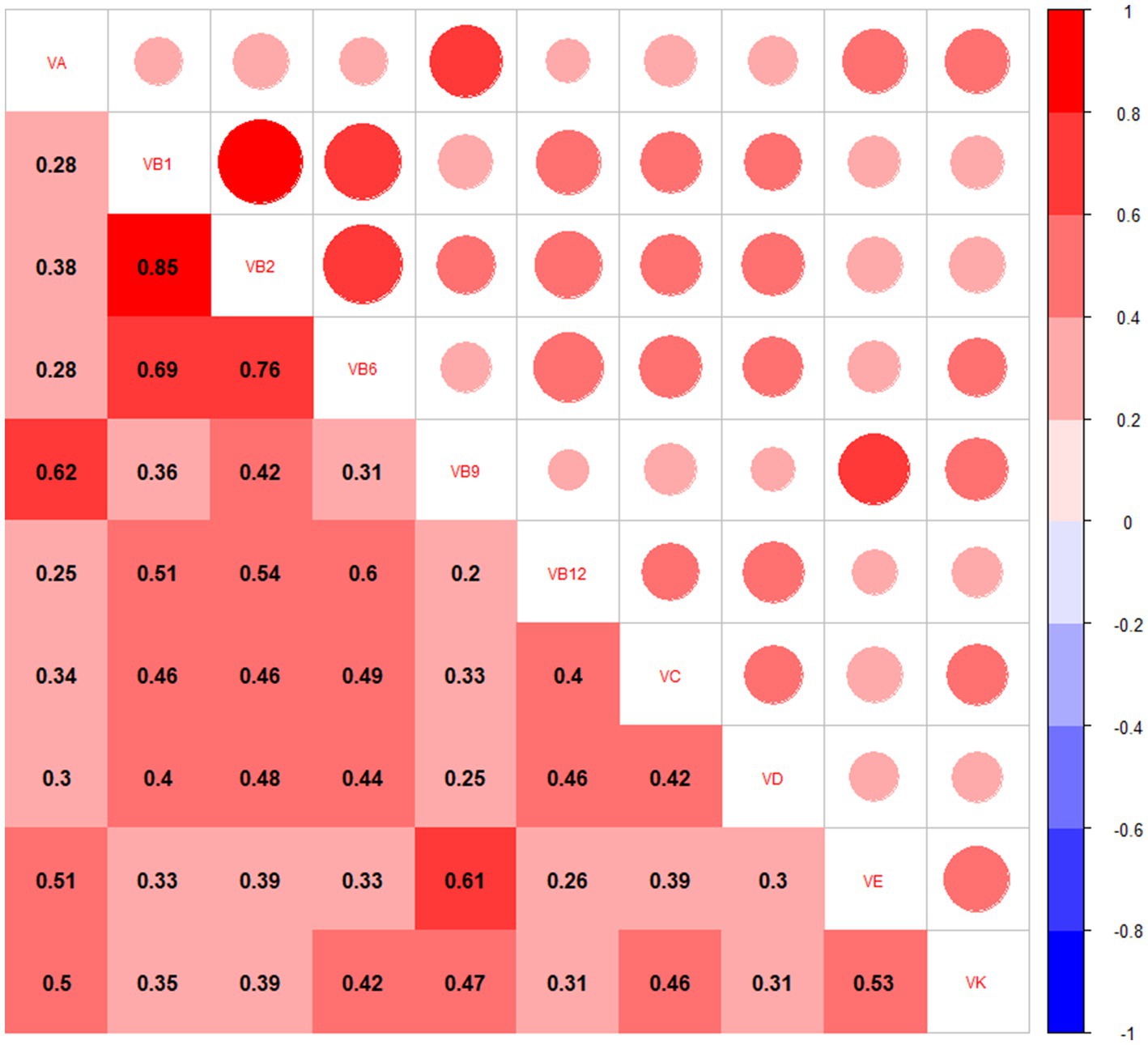
The mean CERAD, AFT, and DSST scores were 27.4 ± 6.0, 17.4 ± 5.7, and 48.7 ± 17.4, respectively. The correlations of vitamin intake ranged from low to high based on Pearson’s correlation analyses (Figure 1).
3.2 Single vitamin intake and cognitive function
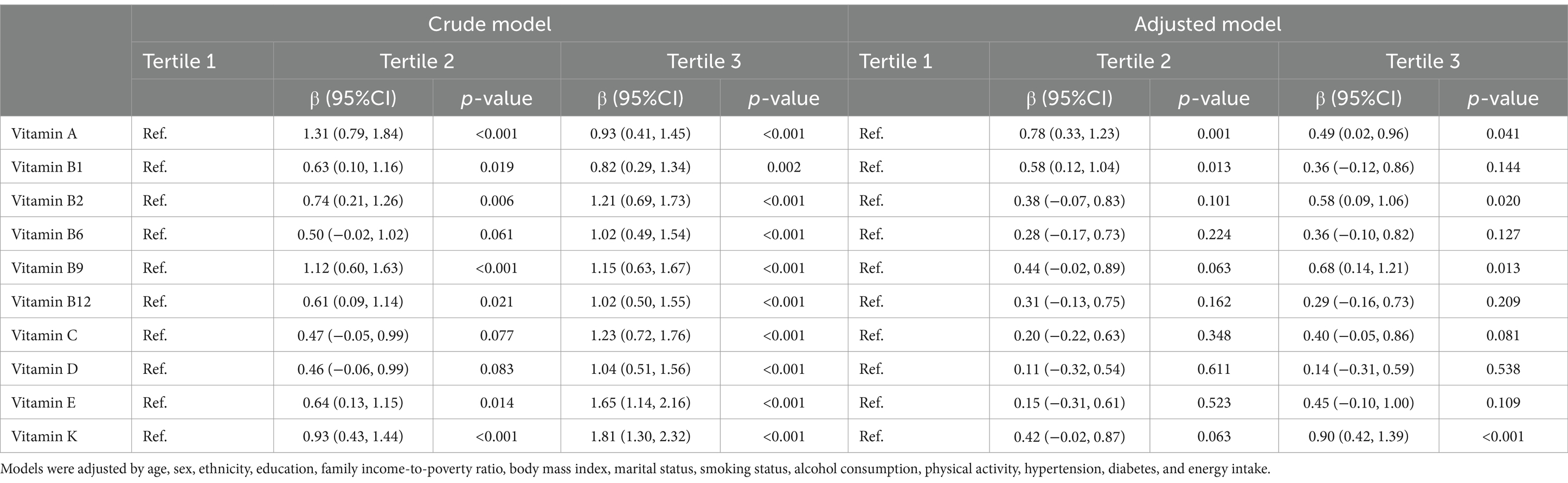
After adjusting for covariates (Table 2), global cognitive function in the third tertile (regression coefficient, β = 0.49 [95% confidence interval (CI) 0.02–0.96]; p = 0.041) and the second tertile (β = 0.78 [95% CI 0.33–1.23]; p = 0.001) of vitamin A intake were higher than in the first tertile. Global cognitive function in the second vitamin B1 intake tertile was significantly higher compared to that in the first tertile (β = 0.58 [95% CI 0.12–1.04]; p = 0.013). Higher vitamin B2 intake predicted increased global cognitive function (β = 0.58 [95% CI 0.09–1.06]; p = 0.020). Global cognitive function was higher in the third vitamin B9 intake tertile than that of the first tertile (β = 0.68 [95% CI 0.14–1.21]; p = 0.013). Compared to individuals in the first vitamin K intake tertile, those in the third tertile exhibited higher global cognitive function (β = 0.90 [95% CI 0.42–1.39]; p < 0.001).
Individuals with higher vitamin A and K intakes exhibited higher CERAD Z-scores (Supplementary Table S1). Higher vitamin A, B1, B2, B9, C, and K intake predicted higher AFT Z-scores. The DSST Z-score was higher in participants with a higher intake of vitamins A, B1, B2, B9, and K than in those with a lower intake.
3.3 Mixture of vitamin intake and impact on cognitive function
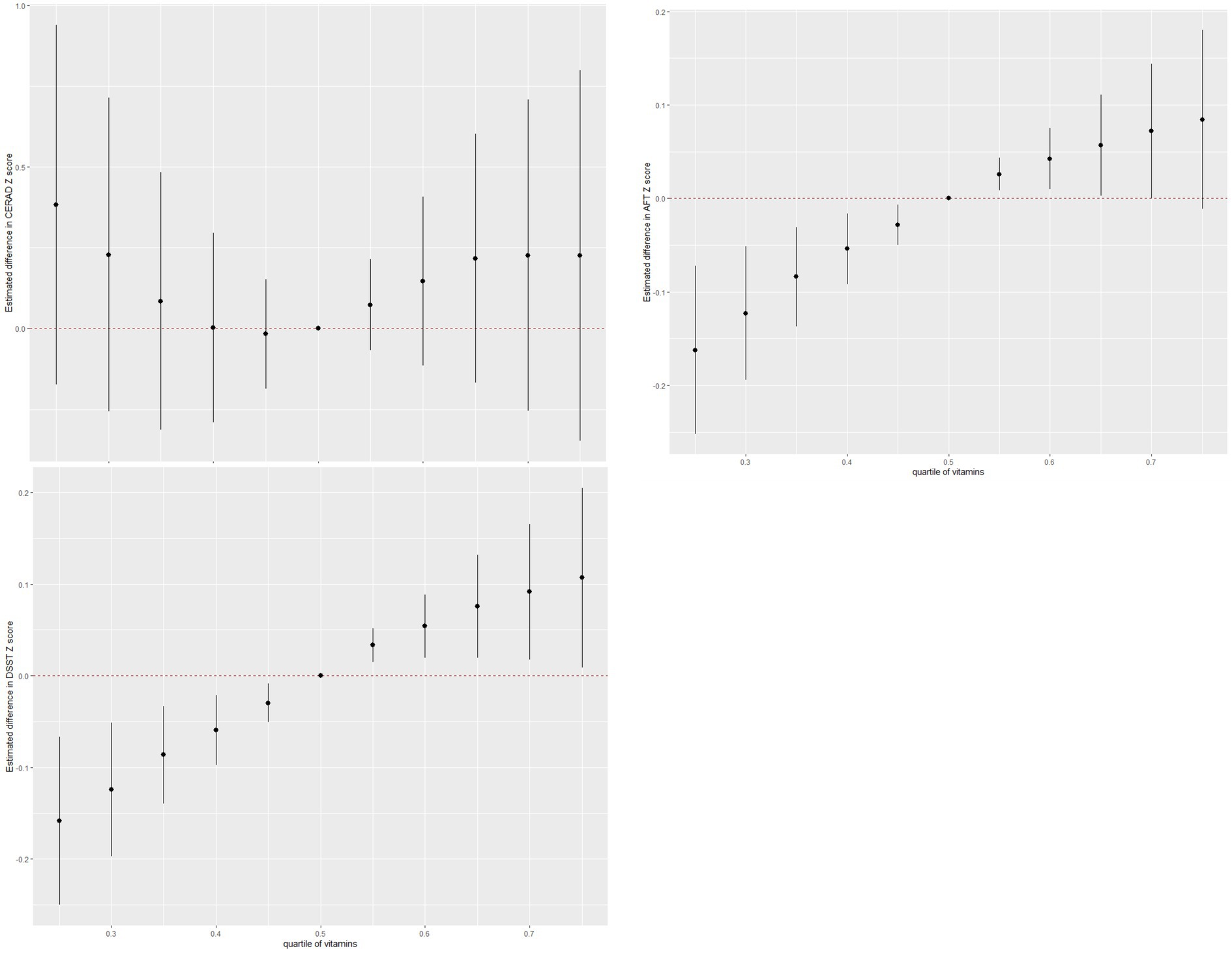
As shown in Figure 2, the associations of vitamins A, B1, B12, and K intake and global cognitive function appeared to be non-linear. These non-linear associations were further verified using GAMs (p < 0.05) (Supplementary Figure S3). Notably, vitamin intake was positively associated with global cognitive function in the BKMR model. When other vitamins were fixed at the 50th percentile, vitamins A and K levels were marginally and significantly associated with an increase in global cognitive function.
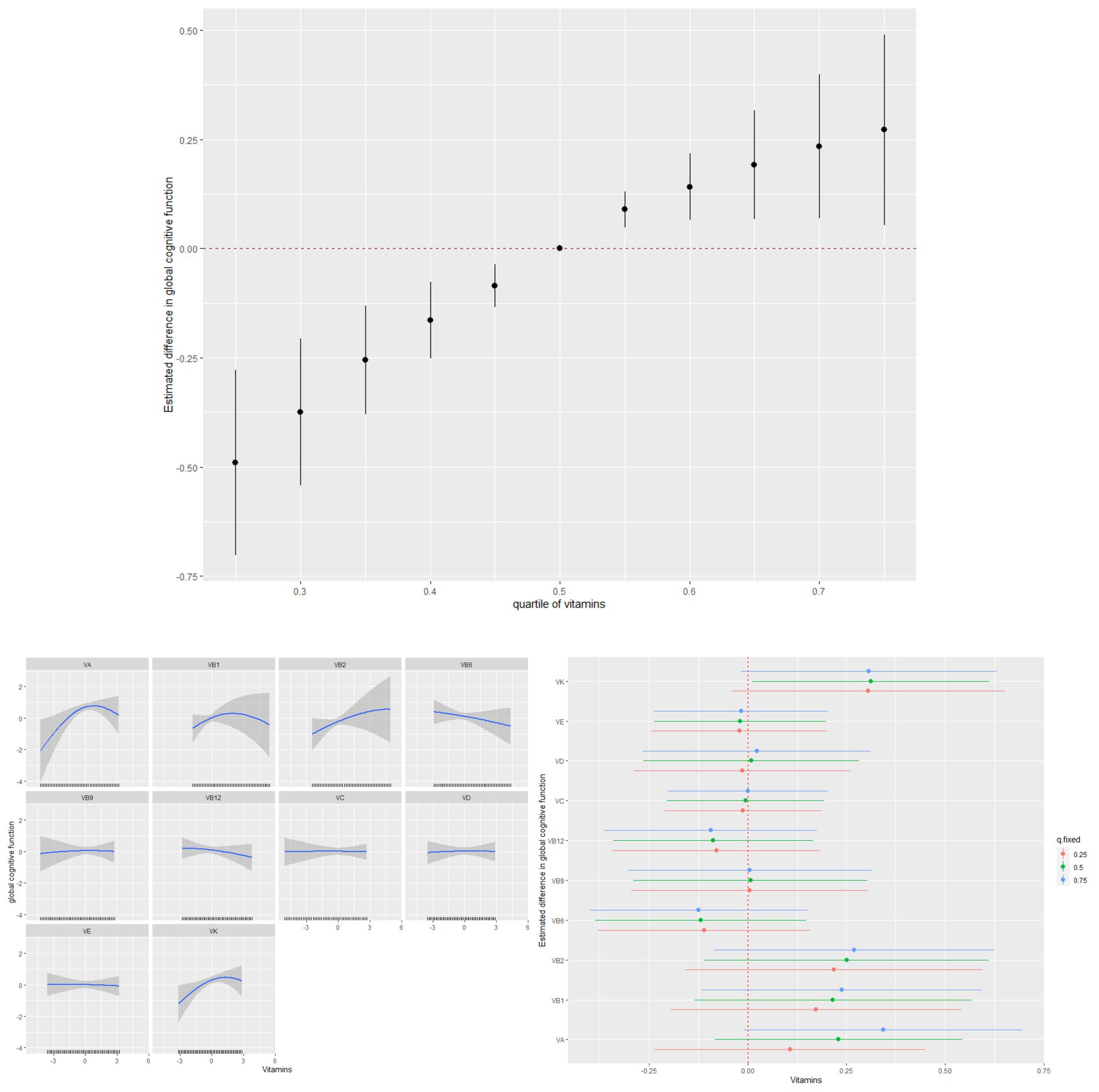
Figure 2. Individual and combined effects of vitamins on cognitive function and dose–response relationships in Bayesian kernel machine regression models.
A combination of vitamins was associated with an increase in AFT and DSST Z-scores but not with the CERAD Z-score (Figure 3).
3.4 Individual vitamin intake and serum NfL levels
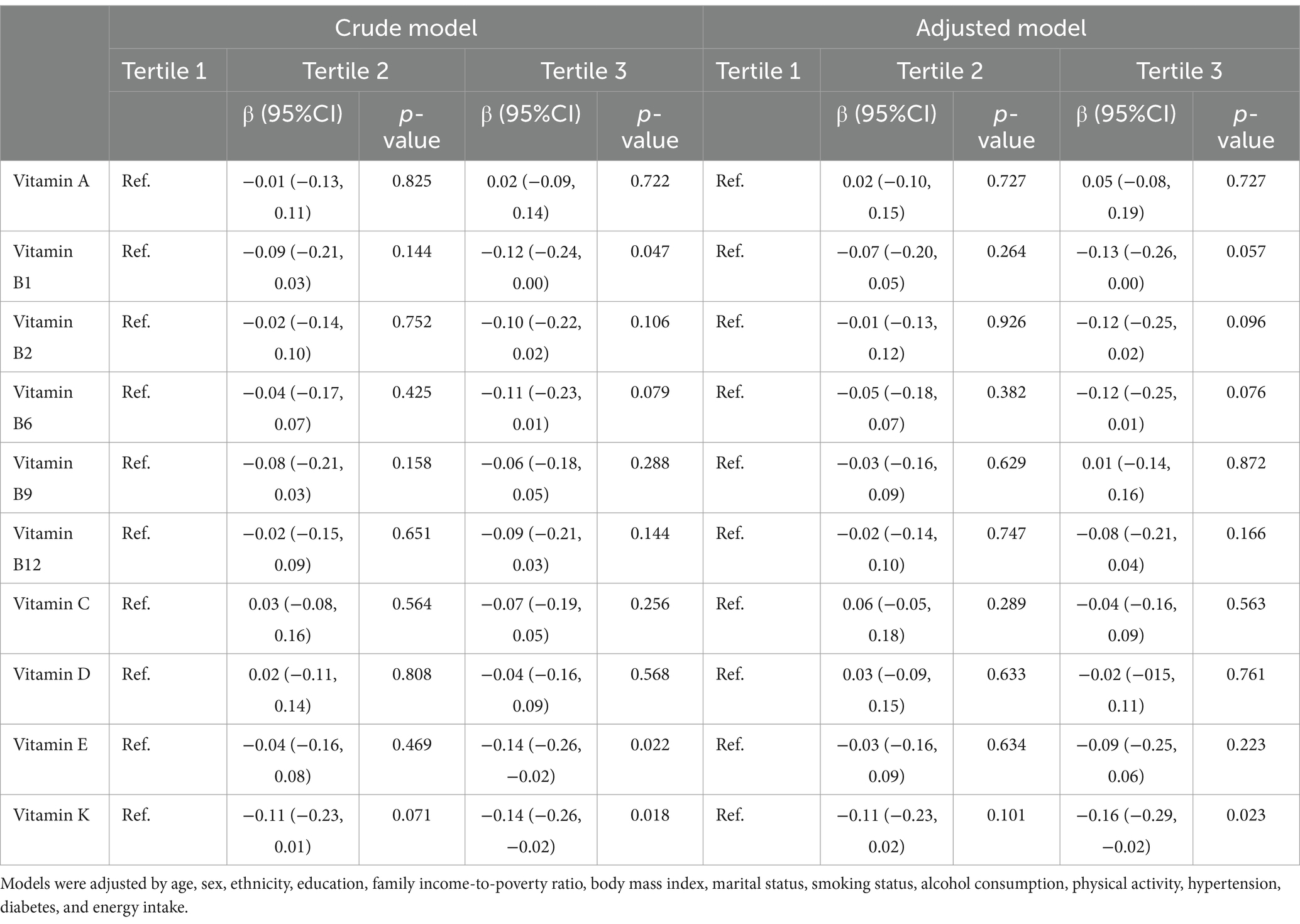
After adjusting for potential confounders (Table 3), individuals in the third vitamin K intake tertile exhibited lower serum concentrations of NfL than those in the first tertile (β = −0.16 [95% CI −0.29 to −0.02]; p = 0.023).
3.5 Mediation analysis
As shown in Figure 4, serum NfL concentration mediated the association between vitamin K intake (the third tertile compared to the first tertile) and global cognitive function, with a mediating effect of 8.73% (95% CI 1.30–19.2%; p = 0.002).
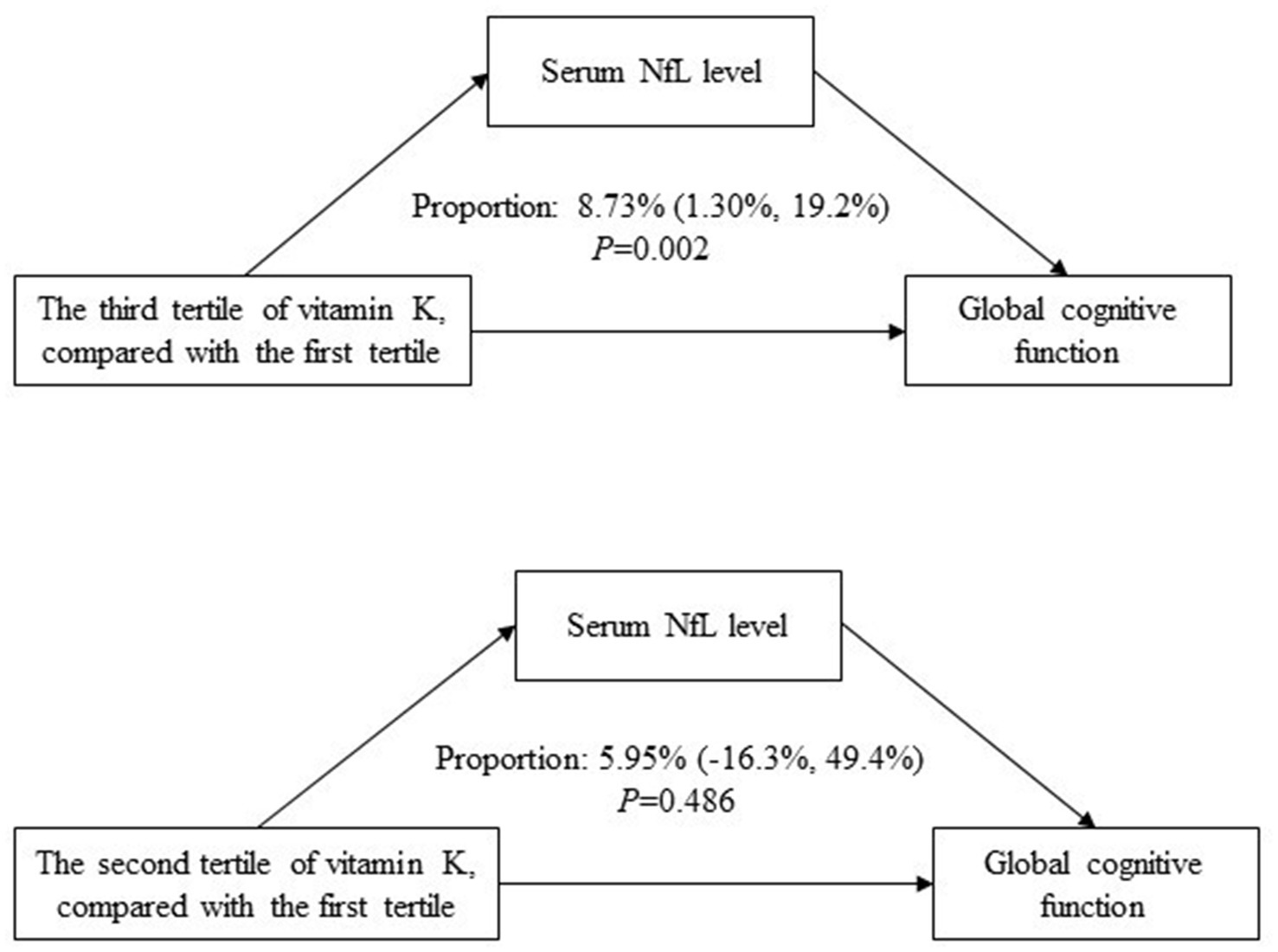
Figure 4. Mediating effects of serum NfL concentration on the association between vitamin K and global cognitive function.
3.6 Sensitivity analysis
After adjusting for beta-carotene, the main findings were, in large part, consistent with those obtained without adjustment (Supplementary Table S2). The associations between serum NfL levels or global cognitive function and single vitamin intake in the source population were similar to those in the analyzed population (Supplementary Table S3).
4 Discussion
In this cross-sectional analysis, we observed that in both single and mixed vitamin models, vitamin A and vitamin K intakes were associated with higher global cognitive function. The most remarkable finding of the current study was that the intake of the 10 vitamin mixtures was associated with an increase in global cognitive function. Higher vitamin K intake predicted a decrease in serum NfL concentration. Serum NfL concentration mediates the relationship between vitamin K intake and global cognitive function. To the best of our knowledge, this is the first study to explore the potential relationship between multiple vitamin intake and cognitive function among elderly individuals in the United States.
Vitamin B plays a crucial role in preventing cognitive decline during various neurological processes (10). Previous studies have demonstrated that the B vitamins can reduce the risk of dementia by regulating homocysteine (Hcy) levels or affecting amino acids linked to neurodegenerative diseases (12). Vitamins B1, B6, and B12 are involved in the synthesis of myelin and neurotransmitters that exert their neuroprotective effects (30). Regarding epidemiological evidence, Wang et al. (12) summarized 95 studies comprising 46,175 participants and showed that vitamin B supplementation may reduce the risk for cognitive decline and even incident dementia. Among these vitamins, the associations between vitamins B6, B9, and B12 and cognitive function have been extensively investigated (12, 24). However, the neurological effects of vitamins B1 and B2 are relatively limited. The latest studies using the NHANES database reported that dietary vitamin B1 and vitamin B2 intakes were associated with better cognitive performance among elders in the United States (23, 31). Consistent with previous findings, we observed that higher vitamin B1, B2, and B9 intake was associated with increased cognitive function in single vitamin models.
Antioxidant vitamins, such as vitamins A, C, and E, were included in the calculation of novel indices to assess the relationship between antioxidant indicators and human health. A cross-sectional analysis revealed that the composite dietary antioxidant index, which is calculated from three minerals and three vitamins (A, C, and E), was associated with an elevation in global cognitive function among elders in the United States (21). Similarly, another study found that dietary total antioxidant capacity—including vitamins A, C, and E—was associated with higher IRT, AFT, and DSST scores (32). The oxidative balance score, generated from 16 nutrients and 4 lifestyle components, was positively correlated with global cognitive function in elderly adults (33). Our findings suggest that higher vitamin A intake predicted better cognitive performance in both single- and multiple-vitamin models than in lower vitamin A intake.
Evidence supporting the association between vitamin K intake and cognitive function has recently increased. Sphingolipid metabolism is a crucial pathway through which vitamin K affects age-related brain function (34). Similar to other vitamins, vitamin K has antioxidant and anti-inflammatory effects related to the regulation of cognitive function. In a multicenter interventional study involving 5,533 older participants, higher dietary vitamin K intake was associated with better cognitive function (35). Menaquinone-4, a form of vitamin K in the brain, is associated with a lower risk for dementia and mild cognitive impairment, implying its involvement of vitamin K in the neuropathology of cognitive decline (36). Serum phylloquinone concentration, a marker of vitamin K, is associated with higher cognitive performance in older adults, especially memory (37). Similarly, our findings indicate that vitamin K is associated with higher global cognitive function in elderly individuals in the United States in single- and multiple-vitamin models. Although the exact mechanism by which vitamin K affects cognitive function is not yet clear, several potential mechanisms have been proposed to account for the protective effect of vitamin K on cognitive function, including participation in sphingolipid metabolism (38), anti-inflammation (39), anti-oxidative stress (40), and the activation of vitamin K-dependent proteins such as growth-arrest specific 6 (Gas6) and protein S (41).
Vitamin D can exert neurological effects on cognitive function through various biological mechanisms including oxidative stress, calcium homeostasis, and inflammatory pathways (42). Evidence from interventional and observational studies has shown inconsistent associations between vitamin D and cognitive function (13). In the Framingham Heart Study, no association between serum 25-hydroxyvitamin D concentration and Alzheimer’s disease was observed (43). Similarly, in the current study, we did not observe any association between vitamin D intake and cognitive function among elderly individuals.
To date, studies investigating the association between vitamin intake and serum NfL concentration have been limited. Similar to our findings, a previous study using data from the 2013–2014 cycle of the NHANES cycles also observed that higher vitamin K intake was associated with decreased serum NfL concentrations (44). Several clinical trials involving patients with multiple sclerosis reported a relationship between vitamin D supplementation and serum NfL concentration with conflicting findings (45). Insufficient intake of vitamin K may influence the activation of vitamin K-dependent on vitamin K (such as Gas 6 and protein S) and the synthesis of sphingolipids within the nervous system (46–48). Both Gas 6 and protein S exhibit diverse neuroprotective attributes, including the promotion of cell proliferation, prevention of apoptosis, and reduction of inflammation, which may mitigate neuronal damage and a subsequent decline in NfL levels. In the current study, we found that the serum NfL concentration acted as a mediator between vitamin K intake and global cognitive function. Our findings highlight that vitamin K intake may be involved in neurological protection, although the exact biological mechanisms require further investigation.
The primary strength of our study was the assessment of a panel of 10 vitamins, which may provide a basis for future studies investigating the impact of vitamin combinations in relation to cognitive function. Another advantage of the present study is that we investigated the potential involvement of serum NfL concentration in the association between vitamin intake and cognitive function. However, this study also had several limitations, the first of which was its cross-sectional design, which precluded us from drawing causal inferences. In addition, vitamin intake was obtained through dietary evaluation, which may have caused misclassification due to various starting values, especially for vitamin D. Meanwhile, we cannot exclude the possibility of residual confounding factors, such as the state of the intestine (49), creatine level (50), or air pollution (51), although a series of potential confounders were adjusted for in the statistical models. As such, the neurological effects of these combinations remain unclear. Finally, using a 24-h dietary recall interview to assess vitamin intake in exposure assessments may have been subject to recall bias.
5 Conclusion
A higher intake of vitamin K is associated with increased cognitive function among elderly individuals in the United States. A mixture of 10 vitamins was associated with an increase in global cognitive function. The association between vitamin K intake and cognitive function may be mediated by serum NfL concentration. These findings emphasize the importance of balanced vitamin intake in maintaining cognitive wellbeing in an aging population. These results highlight the need to develop interventional strategies to prevent cognitive decline in the geriatric population.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.cdc.gov/nchs/nhanes/index.htm.
Ethics statement
The studies involving humans were approved by NCHS Research Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ZZ: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. BF: Conceptualization, Methodology, Validation, Writing – original draft. QC: Formal analysis, Writing – original draft. XL: Supervision, Writing – review & editing. XK: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Social Development Plan of Zhenjiang (No. SH2021074).
Acknowledgments
We thanked the participants and research team of the NHANES 2013–2014 cycle.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1485648/full#supplementary-material
References
1. Sakaniwa, R, Shirai, K, Cador, D, Saito, T, Kondo, K, Kawachi, I, et al. Socioeconomic status transition throughout life and risk of dementia. JAMA Netw Open. (2024) 7:e2412303. doi: 10.1001/jamanetworkopen.2024.12303
2. Prince, M, Ali, G-C, Guerchet, M, Prina, AM, Albanese, E, and Wu, Y-T. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res Ther. (2016) 8:23. doi: 10.1186/s13195-016-0188-8
3. Collaborators, GBDDF. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the global burden of disease study 2019. Lancet. Public Health. (2022) 7:e105–25. doi: 10.1016/S2468-2667(21)00249-8
4. Zhang, XX, Tian, Y, Wang, ZT, Ma, YH, Tan, L, and Yu, JT. The epidemiology of Alzheimer’s disease modifiable risk factors and prevention. J Prev Alzheimers Dis. (2021) 8:313–21. doi: 10.14283/jpad.2021.15
5. TDL, K, Agron, E, and Chew, EYAREDS and AREDS2 Research Groups. Dietary nutrient intake and cognitive function in the age-related eye disease studies 1 and 2. Alzheimers Dement. (2023) 19:4311–24. doi: 10.1002/alz.13033
6. Suh, SW, Kim, HS, Han, JH, Bae, JB, Oh, DJ, Han, JW, et al. Efficacy of vitamins on cognitive function of non-demented people: a systematic review and meta-analysis. Nutrients. (2020) 12:1168. doi: 10.3390/nu12041168
7. Fan, Z, Liu, X, Liu, J, Chen, C, and Zhou, M. Neurofilament light chain as a potential biomarker in plasma for Alzheimer's disease and mild cognitive impairment: a systematic review and a Meta-analysis. J Integr Neurosci. (2023) 22:85. doi: 10.31083/j.jin2204085
8. Preische, O, Schultz, SA, Apel, A, Kuhle, J, Kaeser, SA, Barro, C, et al. Serum Neurofilament dynamics predicts neurodegeneration and clinical progression in Presymptomatic Alzheimer's disease. Nat Med. (2019) 25:277–83. doi: 10.1038/s41591-018-0304-3
9. Kakumoto, T, Matsukawa, T, Ishiura, H, Mori, H, Tsuji, S, and Toda, T. Neurofilament light chain levels in cerebrospinal fluid as a sensitive biomarker for cerebral Adrenoleukodystrophy. Ann Clin Transl Neurol. (2023) 10:1230–8. doi: 10.1002/acn3.51818
10. Fekete, M, Lehoczki, A, Tarantini, S, Fazekas-Pongor, V, Csipo, T, Csizmadia, Z, et al. Improving cognitive function with nutritional supplements in aging: a comprehensive narrative review of clinical studies investigating the effects of vitamins, minerals, antioxidants, and other dietary supplements. Nutrients. (2023) 15:5116. doi: 10.3390/nu15245116
11. Huang, SK, Lu, CW, Lin, TY, and Wang, SJ. Neuroprotective role of the B vitamins in the modulation of the central glutamatergic neurotransmission. CNS Neurol Disord Drug Targets. (2022) 21:292–301. doi: 10.2174/1871527320666210902165739
12. Wang, Z, Zhu, W, Xing, Y, Jia, J, and Tang, Y. B vitamins and prevention of cognitive decline and incident dementia: a systematic review and Meta-analysis. Nutr Rev. (2022) 80:931–49. doi: 10.1093/nutrit/nuab057
13. Gall, Z, and Szekely, O. Role of vitamin D in cognitive dysfunction: new molecular concepts and discrepancies between animal and human findings. Nutrients. (2021) 13:3672. doi: 10.3390/nu13113672
14. McCleery, J, Abraham, RP, Denton, DA, Rutjes, AW, Chong, LY, Al-Assaf, AS, et al. Vitamin and mineral supplementation for preventing dementia or delaying cognitive decline in people with mild cognitive impairment. Cochrane Database Syst Rev. (2018) 11:CD011905. doi: 10.1002/14651858.CD011905.pub2
15. CDC. National Health and Nutrition Examination Survey (NHANES). (2024). Available at: https://www.cdc.gov/nchs/nhanes/index.htm.
16. Va, P, Dodd, KW, Zhao, L, Thompson-Paul, AM, Mercado, CI, Terry, AL, et al. Evaluation of measurement error in 24-hour dietary recall for assessing sodium and potassium intake among us adults - National Health and nutrition examination survey (Nhanes), 2014. Am J Clin Nutr. (2019) 109:1672–82. doi: 10.1093/ajcn/nqz044
17. USDA. Food and nutrient database for dietary studies (2024). Available at: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/fndds/.
18. Fillenbaum, GG, van Belle, G, Morris, JC, Mohs, RC, Mirra, SS, Davis, PC, et al. Consortium to establish a registry for Alzheimer's disease (Cerad): the first twenty years. Alzheimers Dement. (2008) 4:96–109. doi: 10.1016/j.jalz.2007.08.005
19. Qin, B, Xun, P, Jacobs, DR Jr, Zhu, N, Daviglus, ML, Reis, JP, et al. Intake of niacin, folate, vitamin B-6, and vitamin B-12 through young adulthood and cognitive function in midlife: the coronary artery risk development in young adults (cardia) study. Am J Clin Nutr. (2017) 106:1032–40. doi: 10.3945/ajcn.117.157834
20. Smagula, SF, Zhang, G, Gujral, S, Covassin, N, Li, J, Taylor, WD, et al. Association of 24-hour activity pattern phenotypes with depression symptoms and cognitive performance in aging. JAMA Psychiatry. (2022) 79:1023–31.
21. Mao, J, Hu, H, Zhao, Y, Zhou, M, and Yang, X. Association between composite dietary antioxidant index and cognitive function among aging Americans from Nhanes 2011-2014. J Alzheimers Dis. (2024) (1875-8908 (Electronic))) 98:1377–89. doi: 10.3233/JAD-231189
22. Xu, Y, Chen, A, Chen, R, and Zheng, W. Association between depressive symptoms and cognitive function in the older population, and the mediating role of Neurofilament light chain: evidence from Nhanes 2013-2014. J Affect Disord. (2024) 360:221–8. doi: 10.1016/j.jad.2024.05.165
23. Jia, W, Wang, H, Li, C, Shi, J, Yong, F, and Jia, H. Association between dietary vitamin B1 intake and cognitive function among older adults: a cross-sectional study. J Transl Med. (2024) 22:165. doi: 10.1186/s12967-024-04969-3
24. Zhang, C, Luo, J, Yuan, C, and Ding, D. Vitamin B12, B6, or folate and cognitive function in community-dwelling older adults: a systematic review and Meta-analysis. J Alzheimers Dis. (2020) 77:781–94. doi: 10.3233/JAD-200534
25. Chen, H, Chen, L, and Hao, G. Exercise attenuates the association between household pesticide exposure and depressive symptoms: Evidence from Nhanes, 2005–2014. Environ Res. (2020) 188:109760. doi: 10.1016/j.envres.2020.109760
26. Xie, X, Lu, C, Wu, M, Liang, J, Ying, Y, Liu, K, et al. Association between Triclocarban and Triclosan exposures and the risks of type 2 diabetes mellitus and impaired glucose tolerance in the National Health and nutrition examination survey (Nhanes 2013-2014). Environ Int. (2020) 136:105445. doi: 10.1016/j.envint.2019.105445
27. Miao, H, Liu, Y, Tsai, TC, Schwartz, J, and Ji, JS. Association between blood Lead level and uncontrolled hypertension in the us population (Nhanes 1999-2016). J Am Heart Assoc. (2020) 9:e015533. doi: 10.1161/JAHA.119.015533
28. Ding, Z, Luo, L, Guo, S, Shen, Q, Zheng, Y, and Zhu, S. Non-linear association between folate/vitamin B12 status and cognitive function in older adults. Nutrients. (2022) 14:2443. doi: 10.3390/nu14122443
29. Bobb, JF, Valeri, L, Claus Henn, B, Christiani, DC, Wright, RO, Mazumdar, M, et al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics. (2015) 16:493–508. doi: 10.1093/biostatistics/kxu058
30. Calderon-Ospina, CA, and Nava-Mesa, MO. B vitamins in the nervous system: current knowledge of the biochemical modes of action and synergies of thiamine, pyridoxine, and cobalamin. CNS Neurosci Ther. (2020) 26:5–13. doi: 10.1111/cns.13207
31. Zhou, L. Association of vitamin B2 intake with cognitive performance in older adults: a cross-sectional study. J Transl Med. (2023) 21:870. doi: 10.1186/s12967-023-04749-5
32. Peng, M, Liu, Y, Jia, X, Wu, Y, Zou, X, Ke, M, et al. Dietary Total antioxidant capacity and cognitive function in older adults in the United States: the Nhanes 2011-2014. J Nutr Health Aging. (2023) 27:479–86. doi: 10.1007/s12603-023-1934-9
33. Song, L, Li, H, Fu, X, Cen, M, and Wu, J. Association of the Oxidative Balance Score and Cognitive Function and the mediating role of oxidative stress: evidence from the National Health and nutrition examination survey (Nhanes) 2011-2014. J Nutr. (2023) 153:1974–83. doi: 10.1016/j.tjnut.2023.05.014
34. Ferland, G. Vitamin K and brain function. Semin Thromb Hemost. (2013) 39:849–55. doi: 10.1055/s-0033-1357481
35. Camacho-Barcia, L, Garcia-Gavilan, J, Martinez-Gonzalez, MA, Fernandez-Aranda, F, Galie, S, Corella, D, et al. Vitamin K dietary intake is associated with cognitive function in an older adult Mediterranean population. Age Ageing. (2022) 51:afab246. doi: 10.1093/ageing/afab246
36. Booth, SL, Shea, MK, Barger, K, Leurgans, SE, James, BD, Holland, TM, et al. Association of Vitamin K with cognitive decline and neuropathology in community-dwelling older persons. Alzheimers Dement (NY). (2022) 8:e12255. doi: 10.1002/trc2.12255
37. Presse, N, Belleville, S, Gaudreau, P, Greenwood, CE, Kergoat, MJ, Morais, JA, et al. Vitamin K status and cognitive function in healthy older adults. Neurobiol Aging. (2013) 34:2777–83. doi: 10.1016/j.neurobiolaging.2013.05.031
38. Denisova, NA, and Booth, SL. Vitamin K and sphingolipid metabolism: evidence to date. Nutr Rev. (2005) 63:111–21. doi: 10.1111/j.1753-4887.2005.tb00129.x
39. Zheng, X, Hou, Y, He, H, Chen, Y, Zhou, R, Wang, X, et al. Synthetic vitamin K analogs inhibit inflammation by targeting the Nlrp3 Inflammasome. Cell Mol Immunol. (2021) 18:2422–30. doi: 10.1038/s41423-020-00545-z
40. Westhofen, P, Watzka, M, Marinova, M, Hass, M, Kirfel, G, Muller, J, et al. Human vitamin K 2,3-epoxide reductase complex subunit 1-like 1 (Vkorc1l1) mediates vitamin K-dependent intracellular antioxidant function. J Biol Chem. (2011) 286:15085–94. doi: 10.1074/jbc.M110.210971
41. Ferland, G. Vitamin K, an emerging nutrient in brain function. Biofactors. (2012) 38:151–7. doi: 10.1002/biof.1004
42. Bivona, G, Gambino, CM, Iacolino, G, and Ciaccio, M. Vitamin D and the nervous system. Neurol Res. (2019) 41:827–35. doi: 10.1080/01616412.2019.1622872
43. Karakis, I, Pase, MP, Beiser, A, Booth, SL, Jacques, PF, Rogers, G, et al. Association of Serum Vitamin D with the risk of incident dementia and subclinical indices of brain aging: the Framingham heart study. J Alzheimers Dis. (2016) 51:451–61. doi: 10.3233/JAD-150991
44. Luo, J, and Lin, S. Dietary vitamin K intake is associated with decreased Neurofilament light chain among middle-aged and older adults from the Nhanes. Front Nutr. (2024) 11:1396707. doi: 10.3389/fnut.2024.1396707
45. Holmoy, T, Rosjo, E, Zetterberg, H, Blennow, K, Lindstrom, JC, Steffensen, LH, et al. Vitamin D supplementation and Neurofilament light chain in multiple sclerosis. Acta Neurol Scand. (2019) 139:172–6. doi: 10.1111/ane.13037
46. Gruber, RC, Ray, AK, Johndrow, CT, Guzik, H, Burek, D, de Frutos, PG, et al. Targeted Gas6 delivery to the Cns protects axons from damage during experimental autoimmune encephalomyelitis. J Neurosci. (2014) 34:16320–35. doi: 10.1523/JNEUROSCI.2449-14.2014
47. Guo, H, Barrett, TM, Zhong, Z, Fernandez, JA, Griffin, JH, Freeman, RS, et al. Protein S blocks the extrinsic apoptotic Cascade in tissue plasminogen activator/N-methyl D-aspartate-treated neurons via Tyro3-Akt-Fkhrl1 signaling pathway. Mol Neurodegener. (2011) 6:13. doi: 10.1186/1750-1326-6-13
48. Yagami, T, Ueda, K, Asakura, K, Sakaeda, T, Nakazato, H, Kuroda, T, et al. Gas6 rescues cortical neurons from amyloid Beta protein-induced apoptosis. Neuropharmacology. (2002) 43:1289–96. doi: 10.1016/s0028-3908(02)00333-7
49. Said, HM, and Mohammed, ZM. Intestinal absorption of water-soluble vitamins: an update. Curr Opin Gastroenterol. (2006) 22:140–6. doi: 10.1097/01.mog.0000203870.22706.52
50. Xu, C, Bi, S, Zhang, W, and Luo, L. The effects of Creatine supplementation on cognitive function in adults: a systematic review and Meta-analysis. Front Nutr. (2024) 11:1424972. doi: 10.3389/fnut.2024.1424972
Keywords: cognitive function, Bayesian kernel machine regression, vitamins, vitamin K, neurofilament light chain
Citation: Zhou Z, Fan B, Chen Q, Li X and Ke X (2025) Individual and combined effects of dietary vitamin intake on cognitive function in elderly adults: the potential mediating role of serum neurofilament light chain levels. Front. Nutr. 12:1485648. doi: 10.3389/fnut.2025.1485648
Edited by:
Xiaoran Liu, Rush University Medical Center, United StatesReviewed by:
Roberto Cannataro, University of Calabria, ItalySong Lin, The Affiliated Huaian No.1 People’s Hospital of Nanjing Medical University, China
Copyright © 2025 Zhou, Fan, Chen, Li and Ke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianjin Ke, a3hqX2t4ajE3QG91dGxvb2suY29t
 Zhikui Zhou1
Zhikui Zhou1 Xianjin Ke
Xianjin Ke