
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 06 February 2025
Sec. Nutrition, Psychology and Brain Health
Volume 12 - 2025 | https://doi.org/10.3389/fnut.2025.1484344
Introduction: Depression is a major global mental health challenge. Previous research suggests a link between magnesium consumption and depression, but the dose–response relationship remains unclear. This study investigates the relationship between dietary magnesium intake and depression risk among American adults.
Methods: Data from the 2005–2020 National Health and Nutrition Examination Survey (NHANES) were examined. Depression was measured with the Patient Health Questionnaire-9 (PHQ-9), and dietary magnesium consumption was calculated from two 24-h meal recalls. We used restricted cubic spline models, logistic regression, and sensitivity analyses to assess the connection.
Results: Among 35,252 participants (mean age: 49.5 ± 17.6 years; 49.9% women), we observed a nonlinearity in the relationship between dietary magnesium intake and depression. Below the inflection point (366.7 mg/day), the odds ratio (OR) was 0.998 (95% CI: 0.997–0.999, p < 0.001). Above this point, the OR was 1.001 (95% CI: 1.000–1.002, p = 0.007). In participants aged ≥60 years, the association was inverse L-shaped, with magnesium intake ≥270.7 mg/day increasing depression incidence by 0.1% per 1 mg/d increase.
Conclusion: A nonlinear dose–response relationship exists between dietary magnesium intake and depression risk among US adults. Age significantly moderates this association, suggesting dietary recommendations should be tailored to different age groups.
Depression is the leading cause of mental health-related impairment worldwide (1), affecting almost 300 million people. Depression reduces human capital, prevents people from realizing their full potential, and is linked to early death from illnesses and suicide. According to a recent analysis, there is a connection between depression and certain nutrients (2),which may be linked to either a decreased incidence of depression (3) or a higher chance of getting depression (4–6). In order to help prevent or treat depression, it is imperative to investigate other dietary components that may be linked to this illness.
Magnesium is the fourth most abundant element on Earth and a necessary cofactor for over 600 enzymes involved in several vital catalytic events, which play an important role in biological processes (7). Magnesium is generally obtained via the consumption of green leafy vegetables, whole grains, nuts, and fish. It is absorbed in the gastrointestinal and renal systems and helps with calcium (Ca2) absorption. Both ions are regulated by parathyroid hormone; however, free ion concentrations do not always match total concentrations (8). Magnesium is required for proper neurotransmission and is involved in the synthesis of membrane phospholipids, which play an important role in brain function and emotional regulation (9). Recent research (10, 11)have shown that nutritional habits have a considerable impact on mental health, particularly in stressful or challenging circumstances, implying that dietary patterns can influence psychological well-being. Magnesium’s antidepressant effects may be mediated through a variety of methods. The most prominent appears to be the inhibition of glutamatergic N-methyl-D-aspartate (NMDA) receptors (12); some mediation also occurs through serotonin system control (13). Interestingly, research in rats have demonstrated that magnesium-deficient diets are related with abnormalities in the gut microbiota, which eventually leads to disruptions in the gut-brain axis and the development of depression-like behaviors (14). This rising body of research emphasizes magnesium’s multidimensional involvement in mental health, as well as its potential as a modifiable dietary element for depression reduction.
Despite overwhelming evidence of magnesium’s role in mental health, the majority of published research focuses on qualitative linkages, with minimal investigation into the dose–response relationship between dietary magnesium intake and depression risk. This study analyzes data from the National Health and Nutrition Examination Survey (NHANES) to assess the relationship between dietary magnesium consumption and depression in the whole US population, including dose–response dynamics. These studies aim to provide new insights into the role of dietary magnesium in depression risk across various population subgroups.
This cross-sectional analysis used data from the CDC’s 2005 ~ 2020 National Health and Nutrition Examination Survey (NHANES) (15). NHANES is a nationally representative survey that uses a stratified, multistage probability sampling design to assess the health and nutritional status of noninstitutionalized Americans (16). Data collection includes household interviews, physical examinations, and laboratory tests at mobile examination centers (MECs), ensuring comprehensive health assessments. The survey collects data on a wide range of variables, including demographic information, health conditions, lifestyle behaviors, and dietary intake, which are crucial for understanding health trends across the population. The ethical clearance for this study follows the protocols established by the NHANES program, which is conducted by the National Center for Health Statistics (NCHS). The NHANES procedures are reviewed and approved by the Ethics Review Board of the NCHS. All participants in NHANES provide written informed consent before participation, ensuring compliance with ethical guidelines. As our study is a secondary analysis of publicly available NHANES data, further Institutional Review Board (IRB) approval is not required (17). NHANES data is freely available on the NHANES website.1 Our study included interviewees aged 20 and over. We excluded pregnant women and individuals with missing data on the PHQ-9 questionnaire, dietary magnesium intake, and covariates.
Depression symptoms were assessed using the PHQ-9, a validated, dependable, and useful measure that combines the DSM-IV diagnostic criteria for depression and is appropriate for use in both clinical and research contexts (18). Participants rated each item based on their experiences in the 2 weeks preceding the questionnaire, with response categories scored as follows: 0 (not at all), 1 (a few days), 2 (more than half of the days), and 3 (almost every day). The total score varies between 0 and 27. In line with prior research, we classified the participants’ PHQ-9 scores as <10 (with no depression) or ≥10 (with depression) for this study (19).
Dietary data, including total energy, carbohydrate, and magnesium levels, were gathered during two 24-h dietary recalls. The first dietary recall interview was held at the Mobile Examination Center (MEC), and the second was completed over the phone after 3–10 days. Participants were asked to recall all foods and beverages ingested within the first 24 h before the interview (from midnight to midnight). Throughout both interviews, participants were given a set of measuring instructions and a food model booklet to help them report food quantities (20). The average values for total calories, carbohydrate, and dietary magnesium intake were computed using 24-h recall data. The 24-h dietary supplementation session included an interview on the use of nutritional supplements and over-the-counter antacids. The average daily consumption of dietary supplements was calculated by adding all supplemental nutrients and dividing by 30. Dietary magnesium intake was calculated using the average value, and total magnesium intake was defined as the sum of dietary magnesium intake and average daily supplement intake. Subjects were assigned to quintiles based on their dietary magnesium intake.
Based on the research (21–24), age, sex, marital status, race/ethnicity, education level, family income, smoking status, hypertension, diabetes, stroke, coronary heart disease, body mass index (BMI), caloric intake, and carbohydrate intake were all considered. Race and ethnicity were classified as White, Black, or Other. Marital status was defined as married, living with a partner, or living alone. There were two schooling levels: ≤12 years and >12 years. A US government research (25) categorized family income into three categories based on the poverty income ratio (PIR): low (PIR ≤ 1.3), intermediate (PIR > 1.3 to 3.5), and high (PIR > 3.5). According to prior research, the smoking status was classified as never smokers (fewer than 100 cigarettes smoked), current smokers, and former smokers (those who quit after smoking more than 100 cigarettes). Past ailments (hypertension, diabetes, stroke, and coronary heart disease) were determined using survey replies as to whether a doctor had ever told the participant about the condition. BMI was estimated using established methods based on weight and height.
The study was meticulously constructed in accordance with the STROBE standards (26). Categorical variables were presented as percentages (%), whereas continuous variables were presented as means (SD) or medians (IQR). To examine differences across groups, single-factor analysis of variance (for normally distributed data), Kruska-Wallis test (for skewed distributed data), and chi-square test (for categorical variables) were applied. Logistic regression models were used to calculate the odds ratios (OR) and 95% confidence intervals (95% CI) for the association between dietary magnesium consumption and depression. Model 1 accounts for sociodemographic factors such as age, gender, race/ethnicity, marital status, education level, and household income. Model 2 adjusted for covariates with p-values <0.05 in univariate analysis and sociodemographic parameters. Model 3 made a full adjustment for sociodemographic variables, smoking status, hypertension, diabetes, stroke, coronary heart disease, body mass index (BMI), calorie consumption, and carbohydrate intake.
Based on the variables adjusted in Model 3, restricted cubic spline (RCS) regression was performed with knots placed at the 5th, 35th, 65th, and 95th percentiles of dietary magnesium intake and divided into four segments to assess linearity and investigate the dose–response curve between dietary magnesium intake and depression.
We used a smooth two-piece logistic regression model to examine the threshold relationship between dietary magnesium consumption and depression after controlling for the factors in Model 3. The inflection point was determined using likelihood ratio tests and bootstrapping resampling.
We analyzed the relationship between dietary magnesium and depression according to gender, age (20–60 years vs. ≥60 years), education level (≤12 vs. >12 years), marital status (married or cohabiting vs. living alone), family income (low vs. medium or high), race, BMI (<25 vs. ≥25 kg/m2), smoking status, hypertension, diabetes, stroke, and coronary heart disease. Heterogeneity among categories was assessed using multivariable logistic regression, and the relationships between subgroups and dietary magnesium intake were investigated using likelihood ratio tests. We conducted sensitivity analyses by removing subjects with excessive energy consumption (<500 or >5,000 kcal/day) to evaluate the robustness of our findings.
Sample size was determined solely on the provided data; therefore, no a priori statistical power estimation was performed. All analyses were conducted using R version 4.2.22 and Free Statistics software version 1.9.2. Descriptive statistics were performed for all participants. A two-tailed p-value of <0.05 was declared significant.
The interviews were completed by 86,844 individuals, 38,453 of whom were under the age of 20. Individuals who met the following criteria were excluded: pregnant women (n = 1,076); missing PHQ-9 questionnaire data (n = 6,809); missing dietary magnesium intake data (n = 1,205); and missing covariate data (n = 4,049). This cross-sectional study included 35,252 NHANES participants from 2005 to 2020. Figure 1 illustrates the detailed inclusion and exclusion processes.
Table 1 presents the baseline characteristics of all participants by quartile of dietary magnesium consumption. A total of 2,964 people (8.4%) experienced depression. The average age of the participants was 49.5 (17.6) years, with 17,589 (49.9%) women and 17,663 (50.1%) men. Those who consumed more magnesium were more likely to be male, married or living with a partner, non-Hispanic White, never smokers, more educated; had a higher family income, a lower prevalence of hypertension, diabetes, stroke, and coronary heart disease and higher energy and carbohydrate consumption.
A univariate analysis revealed significant associations between depression and age, gender, education level, marital status, family income, BMI, smoking status, hypertension, diabetes, stroke, coronary heart disease, caloric intake, and carbohydrate intake (Table 2).
When dietary magnesium consumption was separated into quartiles and potential confounders were controlled for, there was a substantial negative correlation between magnesium intake and depression. In the unadjusted model, the ORs for depression in Q2 (192.5–252.0 mg/day), Q3 (252.1–314.4 mg/day), Q4 (314.5–406.9 mg/day), and Q5 (≥407.0 mg/day) were reduced by 31% (OR = 0.69 [95% CI 0.62, 0.77]), 39% (OR = 0.61 [95% CI 0.54, 0.68]), 54% (OR = 0.46 [95% CI 0.41, 0.52]), and 51% (OR = 0.49 [95% CI 0.43, 0.55]). After adjusting for the variables described in Table 1, the adjusted ORs were 0.83 (95% CI 0.74, 0.93), 0.82 (95% CI 0.72, 0.93), 0.70 (95% CI 0.61, 0.81), and 0.80 (95% CI 0.68, 0.94; p < 0.001; Table 3). Using Q4 as the reference group, in the unadjusted model, persons with low dietary magnesium intake had a 64% higher risk of depression (OR = 1.64 [95% CI 1.48, 1.83]), whereas those with high dietary magnesium intake had a 5% higher risk. After adjusting for the variables described in Table 1, the adjusted ORs were 1.23 (95% CI 1.09, 1.38) and 1.16 (95% CI 1.01, 1.34; Table 4).
After adjusting for various covariates, Figure 2 demonstrates a nonlinear relationship between dietary magnesium intake and the risk of depression. For magnesium intake levels below 366.7 mg/day, each 1 mg increase in magnesium intake is associated with a 0.2% decrease in the risk of depression (OR = 0.998, 95% CI: 0.997–0.999). In contrast, for magnesium intake levels ≥366.7 mg/day, the risk of depression increases by 0.1% for each 1 mg increase in daily magnesium consumption (OR = 1.001, 95% CI: 1.000–1.002; Table 5).
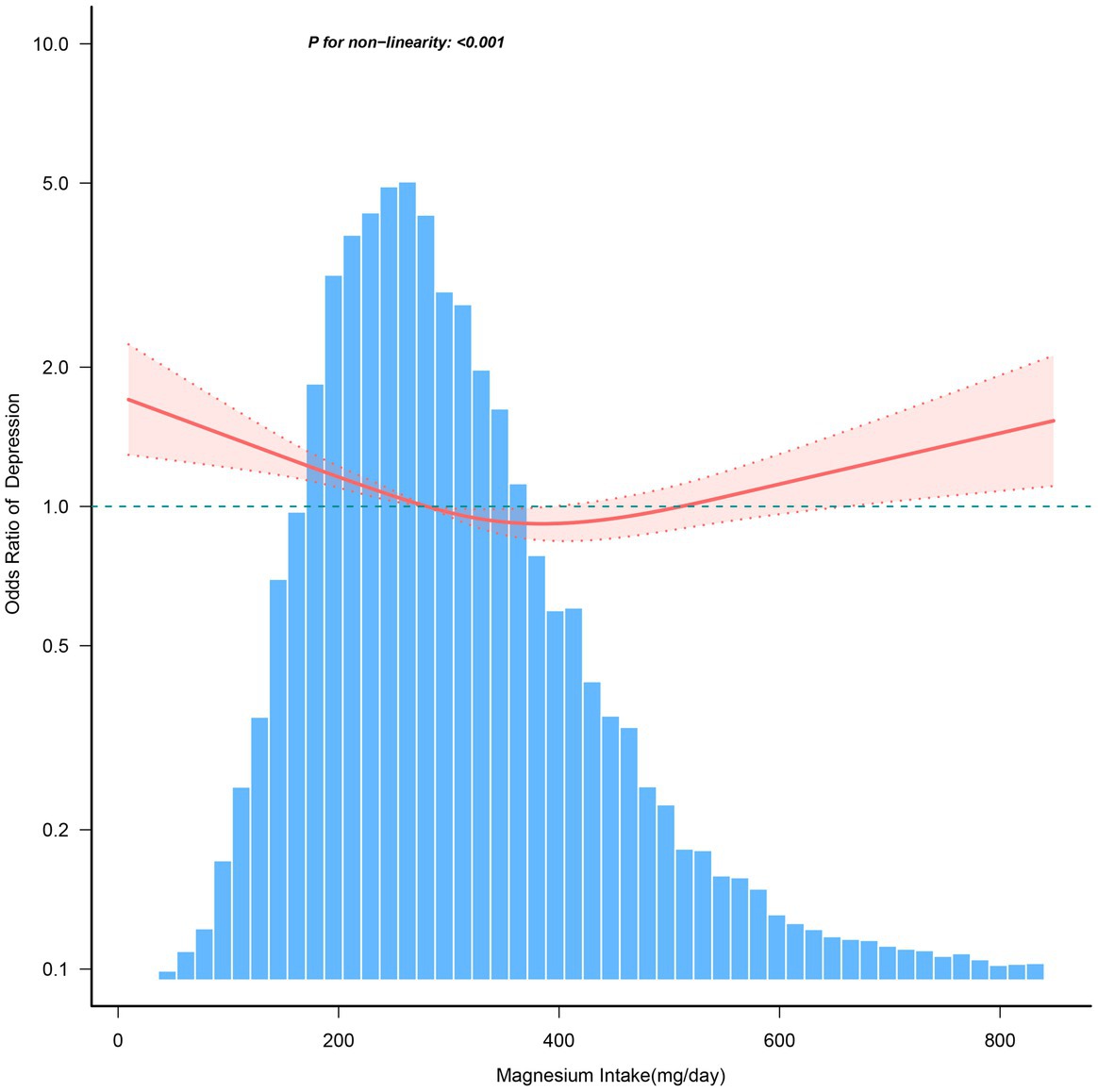
Figure 2. The dose–response relationship between dietary magnesium intake and the risk of depression. Solid and dashed lines represent the predicted value and 95% confidence intervals. They were adjusted for age, sex, race/ethnicity, education level, family income, marital status, smoking status, hypertension, diabetes, stroke, coronary heart disease, body mass index, calorie consumption, and carbohydrate consumption. Only 99% of the data is shown.
A stratified analysis was performed across multiple subgroups to assess the potential moderating effect of dietary magnesium on the association with depression. After stratifying by age, gender, race, education level, family income, marital status, smoking status, hypertension, diabetes, stroke, coronary heart disease, and body mass index, a significant interaction was observed between dietary magnesium intake and age (p-value for the likelihood ratio test for the interaction was p = 0.002; Figure 3).
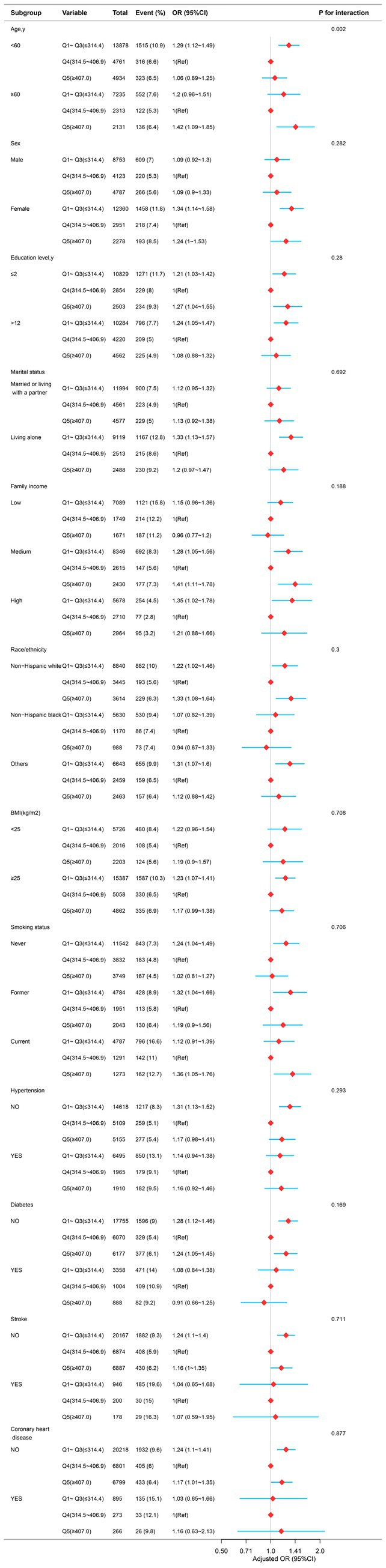
Figure 3. The relationship between dietary magnesium intake and depression in different subgroups. Except for the stratification component itself, each stratification factor was adjusted for all other variables (age, sex, race/ethnicity, education level, family income, marital status, smoking status, hypertension, diabetes, stroke, coronary heart disease, body mass index, calorie consumption, and carbohydrate consumption).
Figure 4 illustrates a nonlinear relationship between magnesium consumption and depression in individuals aged ≥60 years (p < 0.001). And the link is inverse L-shaped. For individuals with magnesium intake ≥270.7 mg/day, each 1 mg/day increase in magnesium consumption is associated with a 0.1% increase in the incidence of depression (Table 6).
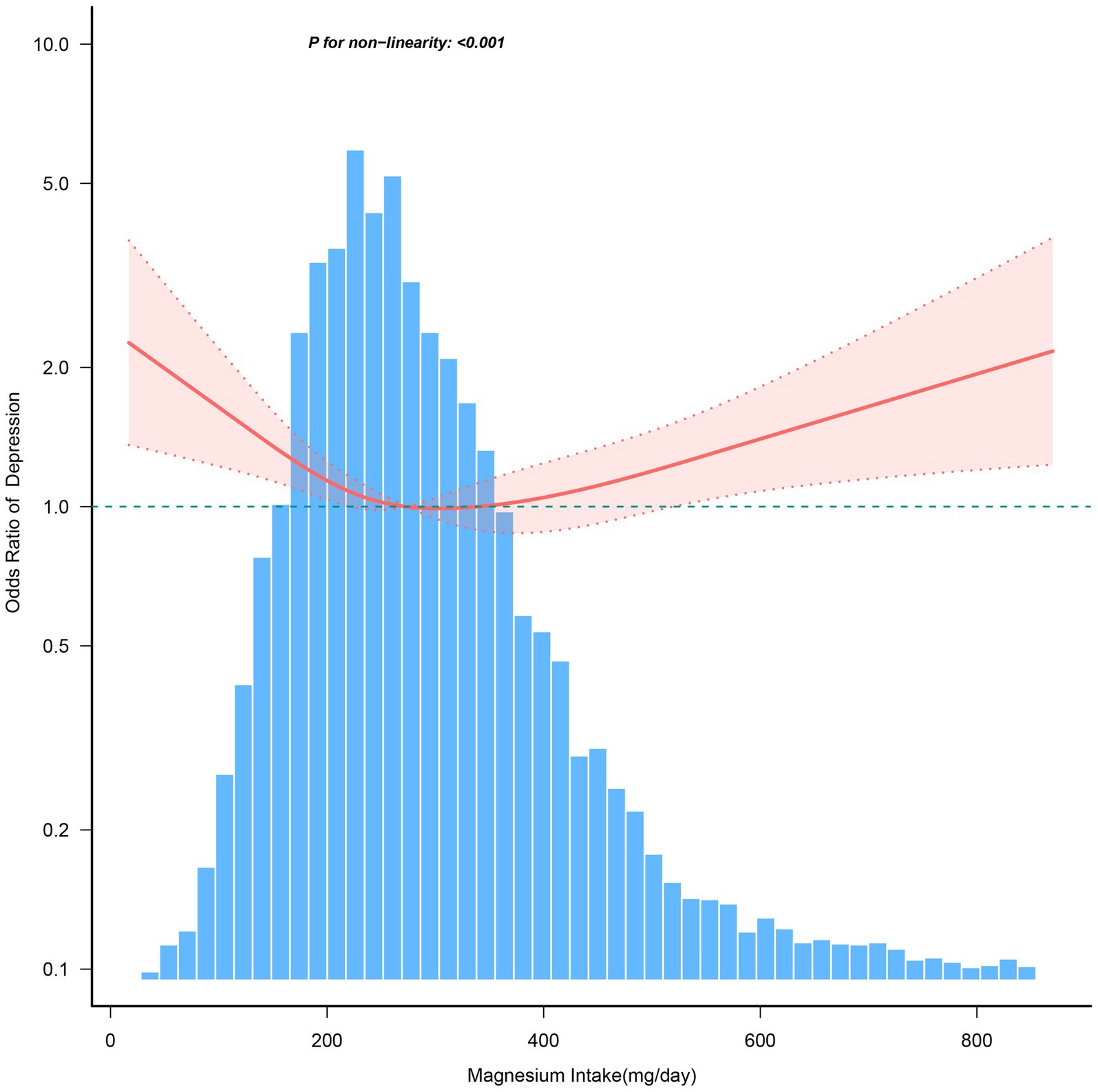
Figure 4. The dose–response relationship between dietary magnesium intake and the risk of depression among individuals over 60. Solid and dashed lines represent the predicted value and 95% confidence intervals. They were adjusted for age, sex, race/ethnicity, education level, family income, marital status, smoking status, hypertension, diabetes, stroke, coronary heart disease, body mass index, calorie consumption, and carbohydrate consumption. Only 99% of the data is shown.
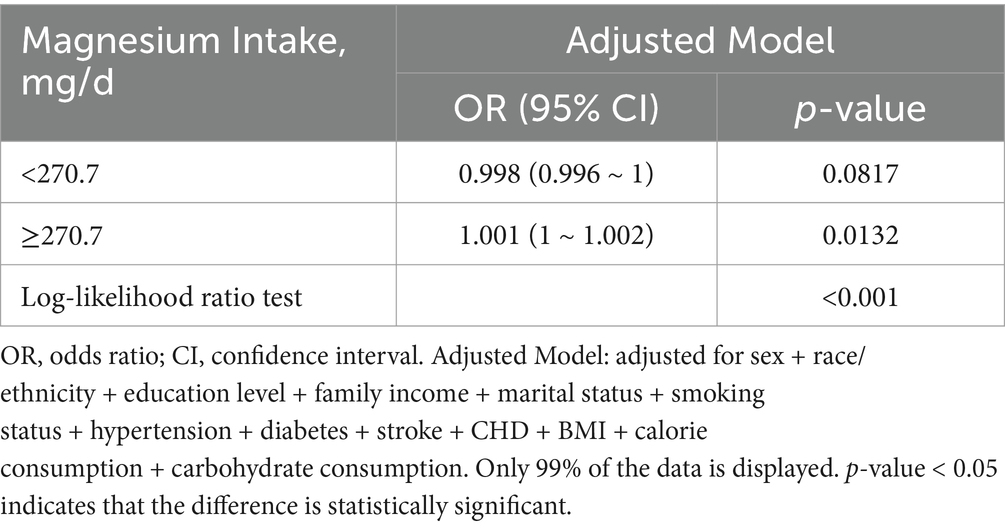
Table 6. Threshold effect analysis of the relationship of magnesium intake with depression among individuals over 60.
After excluding 412 individuals with excessive calorie intake, the relationship between dietary magnesium intake and depression remained unchanged. When compared to the Q4 group (314.5–406.9 mg/day), the adjusted odds ratios for depression were 1.23 (95% CI: 1.09–1.38, p = 0.001) for participants in Q1–Q3 (≤314.4 mg/day) and 1.16 (95% CI: 1.00–1.33, p = 0.049) for participants in Q5 (≥407.0 mg/day; Table 7).
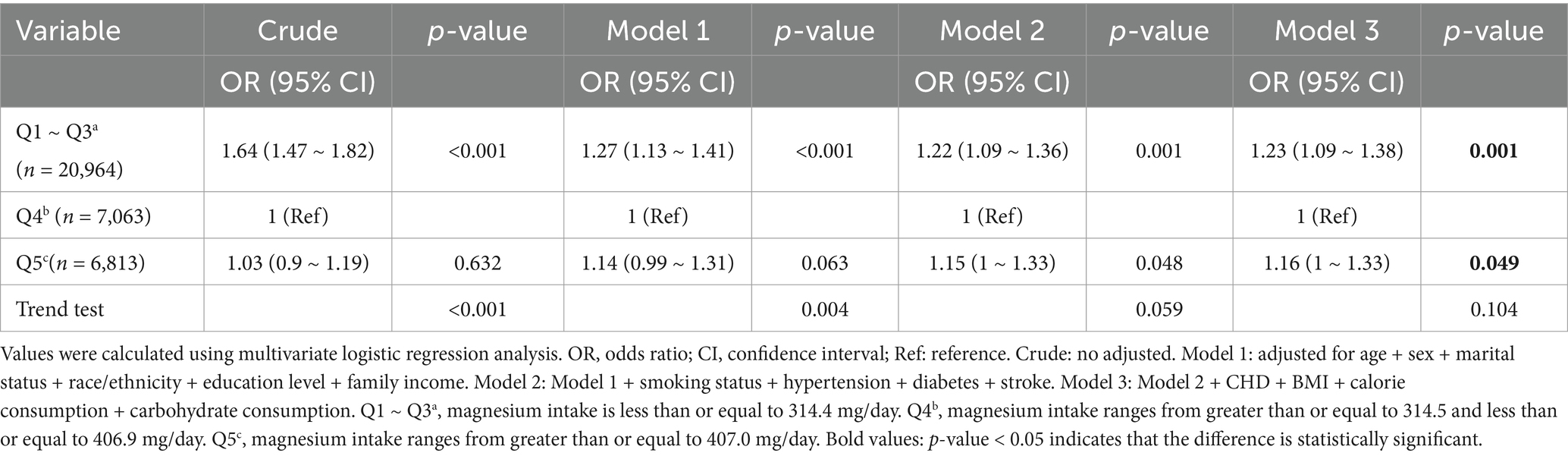
Table 7. Association between dietary magnesium intake and depression among individuals with a daily caloric intake between 500 and 5,000 kcal.
This large retrospective cross-sectional research study of US adults found a substantial, nonlinear relationship between magnesium consumption and depression risk, along with a nonlinear dose–response curve. Moderate magnesium intake was protective, whereas low and high intake levels increased the risk of depression. In addition, age emerged as a key moderator in this relationship. Higher magnesium consumption has been associated to an increased risk of depression in adults aged 60 and up. These findings highlight the complex function of magnesium in mental health, implying that focused dietary interventions may need to account for age-related physiological variations. This is especially important for East Asian communities, as magnesium intake is often lower than in Caucasian populations, potentially impacting depression prevention efforts in these countries.
Our findings align partially with prior studies suggesting the protective role of magnesium against depression but differ in detailing the nonlinear dose–response relationship. For instance, Chou et al. (24) reported no association between dietary magnesium and depressive symptoms in a Taiwanese cohort. Similarly, a prospective study among Spanish university graduates found no significant relationship (23). These discrepancies could stem from differences in study design, dietary assessment methods, or population characteristics. A review (27) found that magnesium shortage may increase vulnerability to stress. Some studies (28, 29) have showed that insufficient dietary magnesium is a primary cause of treatment-resistant depression (TRD), and magnesium supplementation may prevent depression and serve as an additional treatment. More research is needed to validate our findings, explore the detailed correlations, and identify potential processes.
The precise processes by which magnesium effects mental diseases remain unknown. However, numerous routes are thought to contribute to its effects on depression. Magnesium is essential for brain function, stress response control, and neurotransmission (30, 31). Magnesium ions play a crucial role in regulating glutamate transmission by affecting the activation of NMDA receptors (12). Magnesium shortage may result in overactivation of NMDA receptors, causing neurotoxicity and raising the risk of depression. Magnesium plays a crucial role in neurotransmitter control, affecting serotonin and dopamine synthesis and release, improving neuronal function, and preventing excitotoxicity, all of which impact mood and cognition (13). Magnesium offers anti-inflammatory properties. It reduces the secretion of pro-inflammatory cytokines such IL-6 and TNF-α (31), which can alleviate chronic inflammation. Chronic inflammation is regarded as a possible pathogenic cause for depression. Recent animal investigations suggest that magnesium deprivation can disrupt gut-brain axis communication and impact mood regulation (14).
Our study indicated that persons aged ≥60 years have a higher risk of depression with increased dietary magnesium intake. These data are congruent with those of Tarleton et al. (21) and it emphasizes the crucial moderating effect of age on the association between dietary magnesium consumption and depression risk. This interaction mechanism is believed to involve several components. To begin, as individuals age, their metabolic function and mineral absorption capabilities alter (32–34), potentially resulting in variable magnesium requirements and utilization efficiency in older adults compared to younger populations (35–38). Furthermore, older persons may have more chronic conditions and use more drugs (39–41), which may affect magnesium metabolism and its effect on the neurological system. Excess magnesium intake in older individuals may result in negative health effects, such as arrhythmias or gastrointestinal discomfort (42–44), indirectly influencing mental health. This finding is consistent with our findings from the nonlinear association study, which showed that moderate magnesium consumption protects against depression risk, whereas high magnesium intake may raise the risk. Therefore, nutritional recommendations should be adjusted to different age groups.
Our study also has limitations. First, while this study is cross-sectional, we cannot demonstrate a causal relationship between dietary magnesium intake and depression. Additional well-designed cohort studies are required to resolve this shortcoming. Second, self-reported dietary intake data may include recollection bias, reducing the precision of the findings. Finally, residual confounding factors, such as genetic predispositions or other lifestyle behaviors, may contribute to the risk of depression but were not adequately accounted for in this study.
This study demonstrated a complex relationship between dietary magnesium intake and the risk of depression, with age playing an important role. These findings provide significant scientific information for future nutritional therapies and depression prevention efforts. The results also provide possibilities for future research into the mechanisms of magnesium impact in various groups.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by National Center for Health Statistics Research Ethics Review Board (NCHS ERB). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
YH: Conceptualization, Methodology, Writing – original draft. SR: Data curation, Writing – original draft. YY: Formal analysis, Writing – original draft. HL: Writing – review & editing. SC: Data curation, Writing – review & editing. QC: Data curation, Writing – original draft.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors want to express their sincere gratitude to all participants and the schools for their participating in the study. In addition, we gratefully thank Jie Liu of Department of Vascular and Endovascular Surgery, Chinese PLA General Hospital for his contribution to the statistical support, study deign consultations and comments regarding the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Herrman, H, Kieling, C, McGorry, P, Horton, R, Sargent, J, and Patel, V. Reducing the global burden of depression: A lancet–world psychiatric association commission. Lancet. (2019) 393:e42–3. doi: 10.1016/S0140-6736(18)32408-5
2. Kris-Etherton, PM, Petersen, KS, Hibbeln, JR, Hurley, D, Kolick, V, Peoples, S, et al. Nutrition and behavioral health disorders: depression and anxiety. Nutr Rev. (2021) 79:247–60. doi: 10.1093/nutrit/nuaa025
3. Gangwisch, JE, Hale, L, Garcia, L, Malaspina, D, Opler, MG, Payne, ME, et al. High glycemic index diet as a risk factor for depression: analyses from the women’s health Initiative1. Am J Clin Nutr. (2015) 102:454–63. doi: 10.3945/ajcn.114.103846
4. Guo, X, Park, Y, Freedman, ND, Sinha, R, Hollenbeck, AR, Blair, A, et al. Sweetened beverages, Boffee, and tea and depression risk among older US adults. PLoS One. (2014) 9:e94715. doi: 10.1371/journal.pone.0094715
5. Knüppel, A, Shipley, MJ, Llewellyn, CH, and Brunner, EJ. Sugar intake from sweet food and beverages, common mental disorder and depression: prospective findings from the Whitehall II study. Sci Rep. (2017) 7:6287. doi: 10.1038/s41598-017-05649-7
6. Sanchez-Villegas, A, Zazpe, I, Santiago, S, Perez-Cornago, A, Martinez-Gonzalez, MA, and Lahortiga-Ramos, F. Added sugars and sugar-sweetened beverage consumption, dietary carbohydrate index and depression risk in the seguimiento universidad de Navarra (SUN) project. Br J Nutr. (2018) 119:211–21. doi: 10.1017/S0007114517003361
7. Adomako, EA, and Yu, ASL. Magnesium disorders: Core curriculum 2024. Am J Kidney Dis. (2024) 83:803–15. doi: 10.1053/j.ajkd.2023.10.017
8. Botturi, A, Ciappolino, V, Delvecchio, G, Boscutti, A, Viscardi, B, and Brambilla, P. The role and the effect of magnesium in mental disorders: A systematic review. Nutrients. (2020) 12:1661. doi: 10.3390/nu12061661
9. Veronese, N, Demurtas, J, Pesolillo, G, Celotto, S, Barnini, T, Calusi, G, et al. Magnesium and health outcomes: an umbrella review of systematic reviews and meta-analyses of observational and intervention studies. Eur J Nutr. (2020) 59:263–72. doi: 10.1007/s00394-019-01905-w
10. Taheri, M, Esmaeili, A, Irandoust, K, Mirmoezzi, M, Souissi, A, Laher, I, et al. Mental health, eating habits and physical activity levels of elite iranian athletes during the COVID-19 pandemic. Sci Sports. (2023) 38:527–33. doi: 10.1016/j.scispo.2023.01.002
11. Taheri, M, Saad, HB, Washif, JA, Reynoso-Sánchez, LF, Mirmoezzi, M, Youzbashi, L, et al. Comparative study of the long-term impact of the COVID-19 pandemic on mental health and nutritional practices among international elite and sub-elite athletes: A sample of 1420 participants from 14 countries. Sports Med. (2023) 9:104. doi: 10.1186/s40798-023-00653-w
12. Gao, J, Duan, B, Wang, D-G, Deng, X-H, Zhang, G-Y, Xu, L, et al. Coupling between NMDA receptor and acid-sensing ion channel contributes to ischemic neuronal death. Neuron. (2005) 48:635–46. doi: 10.1016/j.neuron.2005.10.011
13. Fatima, G, Dzupina, A, Alhmadi, H, Magomedova, A, Siddiqui, Z, Mehdi, A, et al. Magnesium matters: A comprehensive review of its vital role in health and diseases. Cureus. (2024) 16:e71392. doi: 10.7759/cureus.71392
14. Gudrun, W. Dietary magnesium deficiency alters gut microbiota and leads to depressive-like behaviour. Acta Neuropsychiatr. (2015) 27:168–76. doi: 10.1017/neu.2015.7
15. NHANES (n.d.). NHANES survey methods and analytic guidelines. Available at: https://wwwn.cdc.gov/nchs/nhanes/AnalyticGuidelines.aspx (Accessed June 11, 2024)
16. Zipf, G, Chiappa, M, Porter, KS, Ostchega, Y, Lewis, BG, and Dostal, J. National health and nutrition examination survey: plan and operations, 1999-2010. Vital Health Stat. (2013) 1:1–37.
17. US Department of Health & Human Services (n.d.). Office of Extramural Research. Available at: http://grants.nih.gov/grants/policy/hs/hs_policies.htm (Accessed June 11, 2024)
18. Spitzer RL, Kroenke K, Williams JBW, and the Patient Health Questionnaire Primary Care Study Group. Validation and utility of a self-report version of PRIME-MDThe PHQ primary care study. JAMA. (1999) 282:1737–44. doi: 10.1001/jama.282.18.1737
19. Levis, B, Sun, Y, He, C, Wu, Y, Krishnan, A, Bhandari, PM, et al. Accuracy of the PHQ-2 alone and in combination with the PHQ-9 for screening to detect major depression: systematic review and meta-analysis. JAMA. (2020) 323:2290–300. doi: 10.1001/jama.2020.6504
20. USDA (n.d.). Agriculture research service FSRG food and nutrient database for dietary Studies, 5.0. Available at: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/ (Accessed June 19, 2024).
21. Tarleton, EK, and Littenberg, B. Magnesium intake and depression in adults. J Am Board Fam Med. (2015) 28:249–56. doi: 10.3122/jabfm.2015.02.140176
22. Sun, C, Wang, R, Li, Z, and Zhang, D. Dietary magnesium intake and risk of depression. J Affect Disord. (2019) 246:627–32. doi: 10.1016/j.jad.2018.12.114
23. Derom, M-L, Martínez-González, MAÁ, Sayón-Orea, M, Bes-Rastrollo, M, Beunza, J, and Sánchez-Villegas, A. Magnesium intake is not related to depression risk in spanish university graduates. J Nutr. (2012) 142:1053–9. doi: 10.3945/jn.111.155572
24. Chou, M-H, Yang, YK, Wang, J-D, Lin, C-Y, and Lin, S-H. The association of serum and dietary magnesium with depressive symptoms. Nutrients. (2023) 15:774. doi: 10.3390/nu15030774
25. Agricultural ruralural Research Service; US Department of Agriculture (n.d.). What We Eat in America: Data Tables. Available at: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/wweia-data-tables/ (Accessed June 19, 2024).
26. von Elm, E, Altman, DG, Egger, M, Pocock, SJ, Gøtzsche, PC, Vandenbroucke, JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. (2007) 147:573–7. doi: 10.7326/0003-4819-147-8-200710160-00010
27. Pickering, G, Mazur, A, Trousselard, M, Bienkowski, P, Yaltsewa, N, Amessou, M, et al. Magnesium status and stress: the vicious circle concept revisited. Nutrients. (2020) 12:3672. doi: 10.3390/nu12123672
28. Derom, M-L, Sayón-Orea, C, Martínez-Ortega, JM, and Martínez-González, MA. Magnesium and depression: A systematic review. Nutr Neurosci. (2013) 16:191–206. doi: 10.1179/1476830512Y.0000000044
29. Eby, GA, and Eby, KL. Magnesium for treatment-resistant depression: A review and hypothesis. Med Hypotheses. (2010) 74:649–60. doi: 10.1016/j.mehy.2009.10.051
30. Xu, R, Wang, L, Sun, L, and Dong, J. Neuroprotective effect of magnesium supplementation on cerebral ischemic diseases. Life Sci. (2021) 272:119257. doi: 10.1016/j.lfs.2021.119257
31. Maier, JAM, Locatelli, L, Fedele, G, Cazzaniga, A, and Mazur, A. Magnesium and the brain: A focus on neuroinflammation and neurodegeneration. Int J Mol Sci. (2022) 24:223. doi: 10.3390/ijms24010223
32. Bitar, K, Greenwood-Van Meerveld, B, Saad, R, and Wiley, J. Aging and gastrointestinal neuromuscular function: insights from within and outside the gut. Neurogastroenterol Motil. (2011) 23:490–501. doi: 10.1111/j.1365-2982.2011.01678.x
33. Ross, AC, Taylor, CL, Yaktine, CL, and Del Valle, HB. Food and nutrition board In: Dietary reference intakes for calcium and vitamin D. Editors: A. Catharine Ross, Christine L. Taylor, Ann L. Yaktine, and Heather B. Del Valle. Washington, D.C: The National Academies Press (n.d.)
34. Soenen, S, Rayner, CK, Jones, KL, and Horowitz, M. The ageing gastrointestinal tract. Curr Opin Clin Nutr Metab Care. (2016) 19:12–8. doi: 10.1097/MCO.0000000000000238
35. Paolisso, G. Hypertension, diabetes mellitus, and insulin resistance the role of intracellular magnesium. Am J Hypertens. (1997) 10:346–55. doi: 10.1016/S0895-7061(96)00342-1
36. Barbagallo, M, and Dominguez, L. Magnesium and aging. CPD Dent. (2010) 16:832–9. doi: 10.2174/138161210790883679
37. Maguire, ME, and Cowan, JA. Magnesium chemistry and biochemistry. Biometals. (2002) 15:203–10. doi: 10.1023/A:1016058229972
38. Rude, RK, and Gruber, HE. Magnesium deficiency and osteoporosis: animal and human observations. J Nutr Biochem. (2004) 15:710–6. doi: 10.1016/j.jnutbio.2004.08.001
39. Marengoni, A, Angleman, S, Melis, R, Mangialasche, F, Karp, A, Garmen, A, et al. Aging with multimorbidity: A systematic review of the literature. Ageing Res Rev. (2011) 10:430–9. doi: 10.1016/j.arr.2011.03.003
40. Qato, DM, Wilder, J, Schumm, LP, Gillet, V, and Alexander, GC. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med. (2016) 176:473–82. doi: 10.1001/jamainternmed.2015.8581
41. Fried, TR, O’Leary, J, Towle, V, Goldstein, MK, Trentalange, M, and Martin, DK. Health outcomes associated with polypharmacy in community-dwelling older adults: A systematic review. J Am Geriatr Soc. (2014) 62:2261–72. doi: 10.1111/jgs.13153
42. Rosanoff, A, Weaver, CM, and Rude, RK. Suboptimal magnesium status in the United States: are the health consequences underestimated? Nutr Rev. (2012) 70:153–64. doi: 10.1111/j.1753-4887.2011.00465.x
43. Elin, RJ. Assessment of magnesium status for diagnosis and therapy. Magnes Res. (2010) 23:S194–8. doi: 10.1684/mrh.2010.0213
Keywords: depression, magnesium, mental health, cross-sectional study, nonlinear
Citation: Huang Y, Ruan S, Yang Y, Liang H, Chen S and Chang Q (2025) Impact of dietary magnesium intake on depression risk in American adults: a cross-sectional study of the National Health and Nutrition Examination Survey 2005–2020. Front. Nutr. 12:1484344. doi: 10.3389/fnut.2025.1484344
Received: 21 August 2024; Accepted: 20 January 2025;
Published: 06 February 2025.
Edited by:
Maria S. Garcia-Gutierrez, Miguel Hernández University of Elche, SpainReviewed by:
Morteza Taheri, University of Tehran, IranCopyright © 2025 Huang, Ruan, Yang, Liang, Chen and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanping Huang, ZG9jdG9yeWFucGluZ2h1YW5nQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.