
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr., 07 April 2025
Sec. Clinical Nutrition
Volume 12 - 2025 | https://doi.org/10.3389/fnut.2025.1454431
Aim: To investigate the relationship between unsaturated fatty acids (UFAs) intake and the risk of all-cause mortality in osteoarthritis (OA) patients.
Methods: This cohort study included the data of 3,271 participants with OA using data from the National Health and Nutrition Examination Survey (NHANES). Univariate and multivariable weighted Cox regression models were applied to analyze the relationship between UFAs intake and the risk of mortality in patients with OA. Subgroup analysis was used in age, gender, cardiovascular disease (CVD), hypertension, and diabetes. Hazard ratio (HR), and 95% confidence interval (CI) were calculated.
Results: The median follow-up time was 38.00 (69.00, 104.00) months, with 2,670 participants survived and 601 died. Monounsaturated fatty acids (MUFAs) ≥31.30 was associated with reduced risk of all-cause mortality in OA patients (HR = 0.48, 95% CI: 0.32–0.73). Lowered risk of all-cause mortality in OA patients was observed in patients with octadecenoic acid ≥29.14 (HR = 0.50, 95% CI: 0.34–0.72). Eicosenoic acid of 0.15–0.30 (HR = 0.70, 95% CI: 0.55–0.90) or eicosenoic acid ≥0.30 (HR = 0.62, 95% CI: 0.46–0.84) was related to decreased risk of all-cause mortality in OA patients. Polyunsaturated fatty acids (PUFAs) ≥20.33 was associated with reduced risk of all-cause mortality in OA patients (HR = 0.72, 95% CI: 0.54–0.96). Omega-3 fatty acid ≥1.98 was correlated with decreased risk of all-cause mortality in OA patients (HR = 0.60, 95% CI: 0.45–0.81). Decreased risk of all-cause mortality was found in people with alpha-linolenic acid (ALA) of 1.00–1.83 (HR = 0.75, 95% CI: 0.59–0.96) or ALA ≥1.83 (HR = 0.65, 95% CI: 0.46–0.92) in OA patients. Omega-6 fatty acid ≥18.04 (HR = 0.68, 95% CI: 0.51–0.92) or linoleic acid ≥17.89 (HR = 0.67, 95% CI: 0.50–0.90) were related to decreased risk of all-cause mortality in people with OA.
Conclusion: Total MUFAs and PUFAs, octadecenoic acid, eicosenoic acid, omega-3 fatty acid, ALA, omega-6 fatty acid and linoleic acid were correlated with decreased risk of all-cause mortality in OA patients, which might suggest the importance of specific UFAs supplement in OA patients.
Osteoarthritis (OA) is one of the most important chronic diseases that leads to the degeneration and loss of cartilage within joints, resulting in a huge burden to patients and society (1). A study based on Global Burden of Disease (GBD) data revealed that OA had an annual global increase of 0.32% in age standardized incidence rate or approximately 9% increase over the 28-year period from 1990 to 2017 (2). Compared with the general population, patients with OA have a significantly increased risk of all-cause mortality (3). A number of observational studies have reported that people with OA are at increased risk of premature mortality compared to the general population (4, 5). The primary management of OA may necessitate sequential treatment (6), thus, to identify more reliable biomarkers associated with the prognosis of OA patients is of great value.
Previous studies highlighted the significance of joint-specific inflammation and oxidative stress in the progression and prognosis of OA (7–9), indicating that incorporating nutrients with anti-inflammatory properties into nutritional interventions may confer benefits. Unsaturated fatty acids (UFAs) are essential fatty acids for human body (10). Polyunsaturated fatty acids (PUFAs), especially omega-3 fatty acids, have been found to help improve pain, cartilage loss and joint function in OA patients due to their antioxidant and anti-inflammatory properties (11). Omega-3 fatty acids have been proposed as potential therapeutic agents for individuals with OA owing to their capacity to attenuate the systemic inflammatory response and foster an environment that inhibits cartilage degradation (11). In addition, studies have found that monounsaturated fatty acids (MUFAs) and PUFA might alleviate the progression of knee OA (12). However, whether UFAs played roles on the prognosis of patients with OA was still unclear.
This study aimed to investigate the relationship between UFAs intake and the risk of all-cause mortality in OA patients based on the data from National Health and Nutrition Examination Survey (NHANES). Subgroup analysis was performed in terms of age, gender, complicated with cardiovascular disease (CVD), diabetes, or hypertension.
In this cohort study, the records of 3,642 participants with OA were extracted from NHANES between 2007–2018. NHANES is an ongoing survey of non-institutionalized civilians residing in the United States, utilizing a complex sampling design that oversamples specific minority groups, income brackets, and age ranges (13). Data from NHANES are publicly available from https://wwwn.cdc.gov/nchs/nhanes/Default.aspx. Participants included should meet the following criteria: (1) ≥18 years old; (2) OA based on self-reported personal interview data. Participants were asked if they had ever been told by their doctor or another health professional that they had OA. The exclusion criteria of participants were: (1) missing survival data; (2) information of unsaturated fatty acid intake. Finally, 3,271 participants were analyzed.
Main variables were MUFAs including total MUFAs, hexadecenoic acid, octadecenoic acid (g), eicosenoic acid (g), and docosenoic acid (g), and PUFAs including total PUFAs, total omega-3 [eicosapentaenoic acid (EPA) + docosahexaenoic acid (DHA) + docosapentaenoic acid (DPA) + alpha-linolenic acid (ALA) + stearidonic acid (SDA)], EPA + DHA, DPA, ALA, SDA, total omega-6 (linoleic acid +arachidonic acid), linoleic acid, and arachidonic acid.
The food and nutrient intake of each participant in the NHANES database was recorded through a 24-h dietary recall interview. The 24-h dietary recall interview is comprised of two parts, the 24-h recall and a short set of post-dietary recall questions. Information collected from the 24-h recall interview will be coded and linked to a database of nutrient composition of foods. Calculations of total daily intakes of energy and 51 dietary components, including 19 individual fatty acids will be derived from these data. Following the dietary recall, the second part of the dietary interview will consist of a short questionnaire. The Food and Nutrient Database for Dietary Studies provided by the United States Department of Agriculture was utilized to calculate the consumption of UFAs (14). The intake of dietary supplements during the previous month, including dosage, frequency, and duration of consumption, was determined based on the questionnaire interview (15). The daily total intake of UFAs was determined by calculating the average of the two 24-h dietary intake records and dietary supplements intake.
All-cause mortality was the outcome in our study. The median follow-up time was 38.00 (69.00, 104.00) months.
Age (years), gender (males or females), race (White, Black or other races), education status (high school and below, or above high school), poverty to income ratio (PIR) (≤1.3, 1.3–1.85, or >1.85) (16, 17), marital status (married, widowed, divorced, separated, never married, or living with partner), smoking status (yes or no), drinking status (yes or no), physical activity [<450 metabolic equivalent (MET) × min/week or ≥450 MET × min/week], duration of arthritis, osteoporosis (yes or no), fracture (yes or no), hypertension (yes or no), diabetes (yes or no), dyslipidemia (yes or no), cardiovascular disease (CVD) (yes or no), chronic kidney disease (CKD) (yes or no), cancer (yes or no), BMI (<25 kg/m2, 25–30 kg/m2, or ≥30 kg/m2), energy, white blood cell count, uric acid, and non-steroidal anti-inflammatory agents (yes or no) were variables analyzed.
All the data were analyzed in a weighted manner. The masked variance unit pseudo-stratum was sdmvstra, and the masked variance unit pseudo-primary sampling units was sdmvpsu. The confidence interval (CI) was applied for evaluating the reliability of an estimate. WTDRD1 was used as the dietary data weight. Continuous data were described as mean and standard error (S.E.), and the weighted t-test was used for comparison between groups. Enumeration data were described as the number and percentages of cases [n (%)], and χ2 test was used for comparison between groups. Univariate weighted Cox regression model was established to identify potential covariates associated the mortality of OA patients. Univariate and multivariable weighted Cox regression models were applied to analyze the relationship between UFAs intake and the risk of mortality in patients with OA. Missing values were manipulated via multiple imputation (Supplementary Table 1), and the results were compared between data with and without missing values imputation (Supplementary Table 2). Subgroup analysis was used in age, gender, CVD, hypertension, and diabetes. Hazard ratio (HR), and 95% confidence interval (CI) were calculated. Alpha was set as 0.05. Data analysis was generated using SAS 9.4.
In total, the data of 3,642 participants were extracted from NHANES. Among them, participants without survival information were excluded (n = 9). Also, those without data on UFAs were not analyzed (n = 362). Finally, 3,271 subjects were involved in our study. There were 2,670 participants survived and 601 died. The screen process of the participants was depicted in Figure 1.
The mean MUFAs levels (27.88 g vs. 23.64 g, p < 0.001), hexadecenoic acid level (1.04 g vs. 0.91 g, p < 0.001), eicosenoic acid level (0.30 g vs. 0.22 g, p < 0.001), docosenoic acid level (0.04 g vs. 0.02 g, p = 0.005) in the survival group were higher than the death group, the mean omega-3 fatty acid level in the survival group was higher than the death group (1.88 g vs. 1.53 g, p < 0.001). Higher omega-6 fatty acid levels were observed in the survival group compared to the death group (16.29 g vs. 13.35 g, p < 0.001) (Table 1).
As exhibited in Table 2, age, gender, education, PIR, marital status, smoking status, physical activity, duration of arthritis, osteoporosis, hypertension, diabetes, CVD, CKD, cancer, energy, WBC, uric acid, and non-steroidal anti-inflammatory agents were covariates associated with all-cause mortality in OA patients.
The results delineated that total MUFAs ≥31.30 (HR = 0.56, 95% CI: 0.41–0.76), hexadecenoic acid ≥1.14 (HR = 0.69, 95% CI: 0.52–0.93) might be associated with decreased risk of all-cause mortality in OA patients. Octadecenoic acid ≥29.14, eicosenoic acid of 0.15–0.30 (HR = 0.70, 95% CI: 0.55–0.89) or eicosenoic acid ≥0.30 might be correlated with reduced risk of all-cause mortality in OA patients. Docosenoic acid ≥0.013 (HR = 0.76, 95% CI: 0.58–0.99), PUFAs ≥20.33 (HR = 0.67, 95% CI: 0.53–0.84), omega-3 fatty acid ≥1.98 (HR = 0.61, 95% CI: 0.47–0.79), ALA ≥1.83 (HR = 0.66, 95% CI: 0.51–0.86), omega-6 fatty acid ≥18.04 (HR = 0.64, 95% CI: 0.50–0.81), or linoleic acid ≥17.89 (HR = 0.63, 95% CI: 0.49–0.80) might be related to lowered risk of all-cause mortality in OA patients (Table 3).
After adjusting for confounding factors, MUFAs ≥31.30 was associated with reduced risk of all-cause mortality in OA patients (HR = 0.48, 95% CI: 0.32–0.73). Lowered risk of all-cause mortality in OA patients was observed in patients with octadecenoic acid ≥29.14 (HR = 0.50, 95% CI: 0.34–0.72). Eicosenoic acid of 0.15–0.30 (HR = 0.70, 95% CI: 0.55–0.90) or eicosenoic acid ≥0.30 (HR = 0.62, 95% CI: 0.46–0.84) was related to decreased risk of all-cause mortality in OA patients. PUFAs ≥20.33 was associated with reduced risk of all-cause mortality in OA patients (HR = 0.72, 95% CI: 0.54–0.96). Omega-3 fatty acid ≥1.98 was correlated with decreased risk of all-cause mortality in OA patients (HR = 0.60, 95% CI: 0.45–0.81). Decreased risk of all-cause mortality was found in people with ALA of 1.00–1.83 (HR = 0.75, 95% CI: 0.59–0.96) or ALA ≥1.83 (HR = 0.65, 95% CI: 0.46–0.92) in OA patients. Omega-6 fatty acid ≥18.04 (HR = 0.68, 95% CI: 0.51–0.92) or linoleic acid ≥17.89 (HR = 0.67, 95% CI: 0.50–0.90) were related to decreased risk of all-cause mortality in people with OA (Table 3).
In people aged ≥65 years, MUFAs ≥31.30 (HR = 0.53, 95% CI: 0.33–0.86), hexadecenoic acid ≥1.14 (HR = 0.65, 95% CI: 0.47–0.91), octadecenoic acid ≥29.14 (HR = 0.56, 95% CI: 0.36 = 0.87), eicosenoic acid of 0.15–0.30 (HR = 0.75, 95% CI: 0.59–0.96) or eicosenoic acid ≥0.30 (HR = 0.58, 95% CI: 0.41–0.82) were associated with decreased risk of all-cause mortality in people with OA (Figures 2, 3).
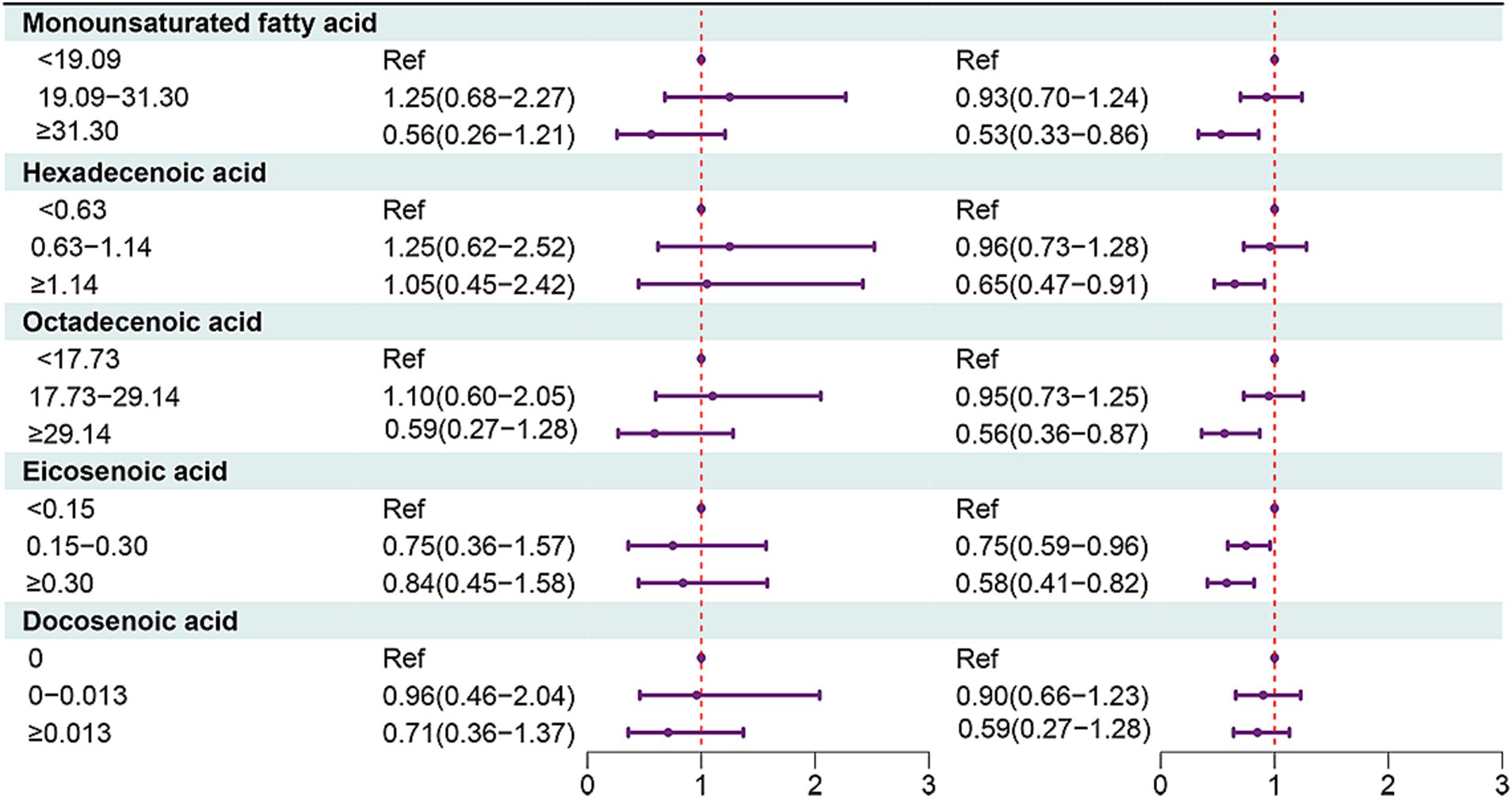
Figure 2. Forest plot showing the associations between MUFAs with all-cause mortality in OA patients in different age groups.
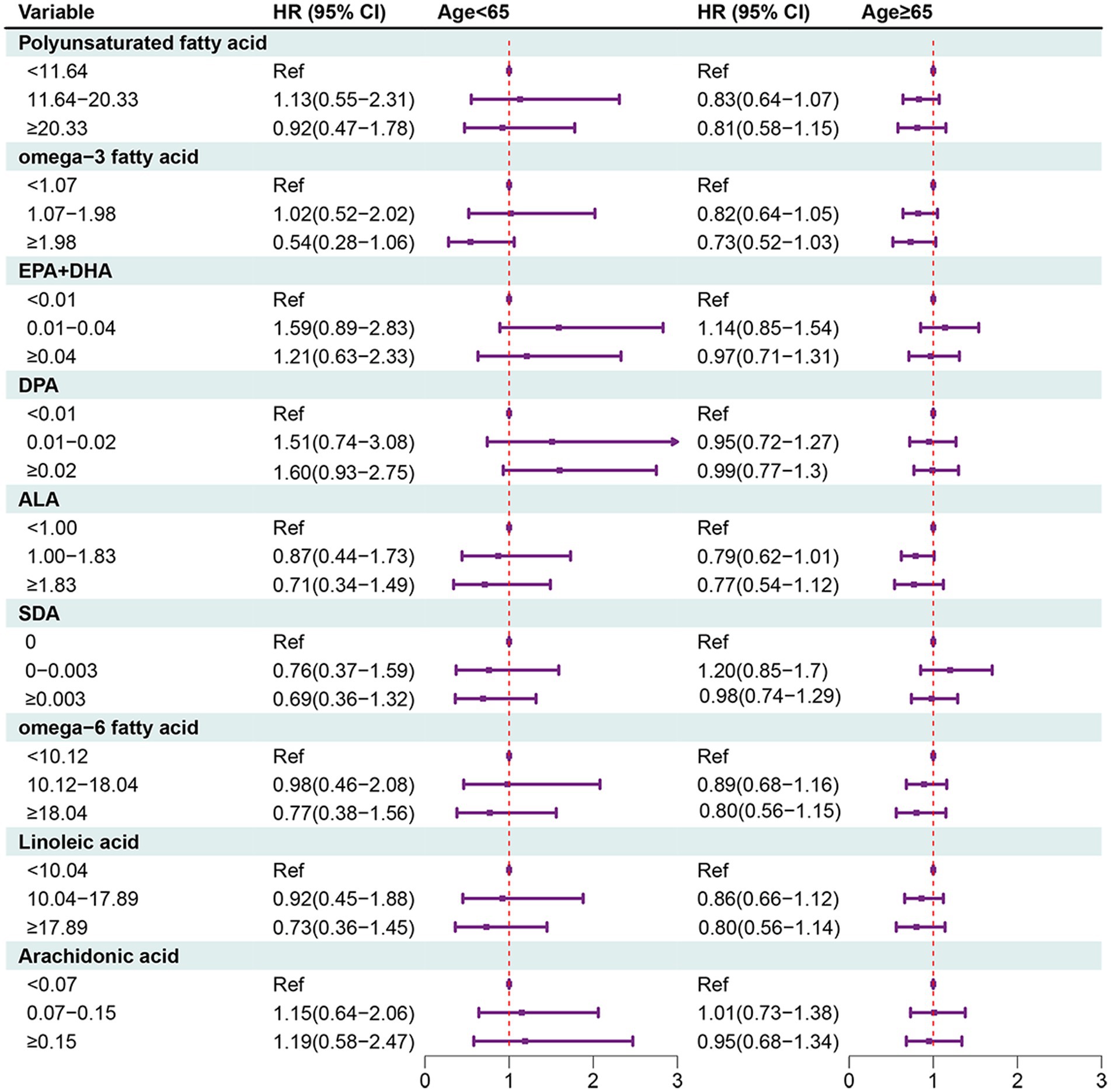
Figure 3. Forest plot showing the associations between PUFAs with all-cause mortality in OA patients in different age groups.
In females, MUFAs ≥31.30 (HR = 0.38, 95% CI: 0.23–0.64), octadecenoic acid ≥29.14 (HR = 0.39, 95% CI: 0.24–0.63), eicosenoic acid ≥0.30 (HR = 0.58, 95% CI: 0.40–0.84), docosenoic acid ≥0.013 (HR = 0.74, 95% CI: 0.56–0.99). PUFAs ≥20.33 (HR = 0.59, 95% CI: 0.39–0.90), omega-3 fatty acid ≥1.98 (HR = 0.50, 95% CI: 0.33–0.76), omega-6 fatty acid ≥18.04 (HR = 0.57, 95% CI: 0.37–0.87), or linoleic acid ≥17.89 (HR = 0.55, 95% CI: 0.36–0.87) (Figures 4, 5).
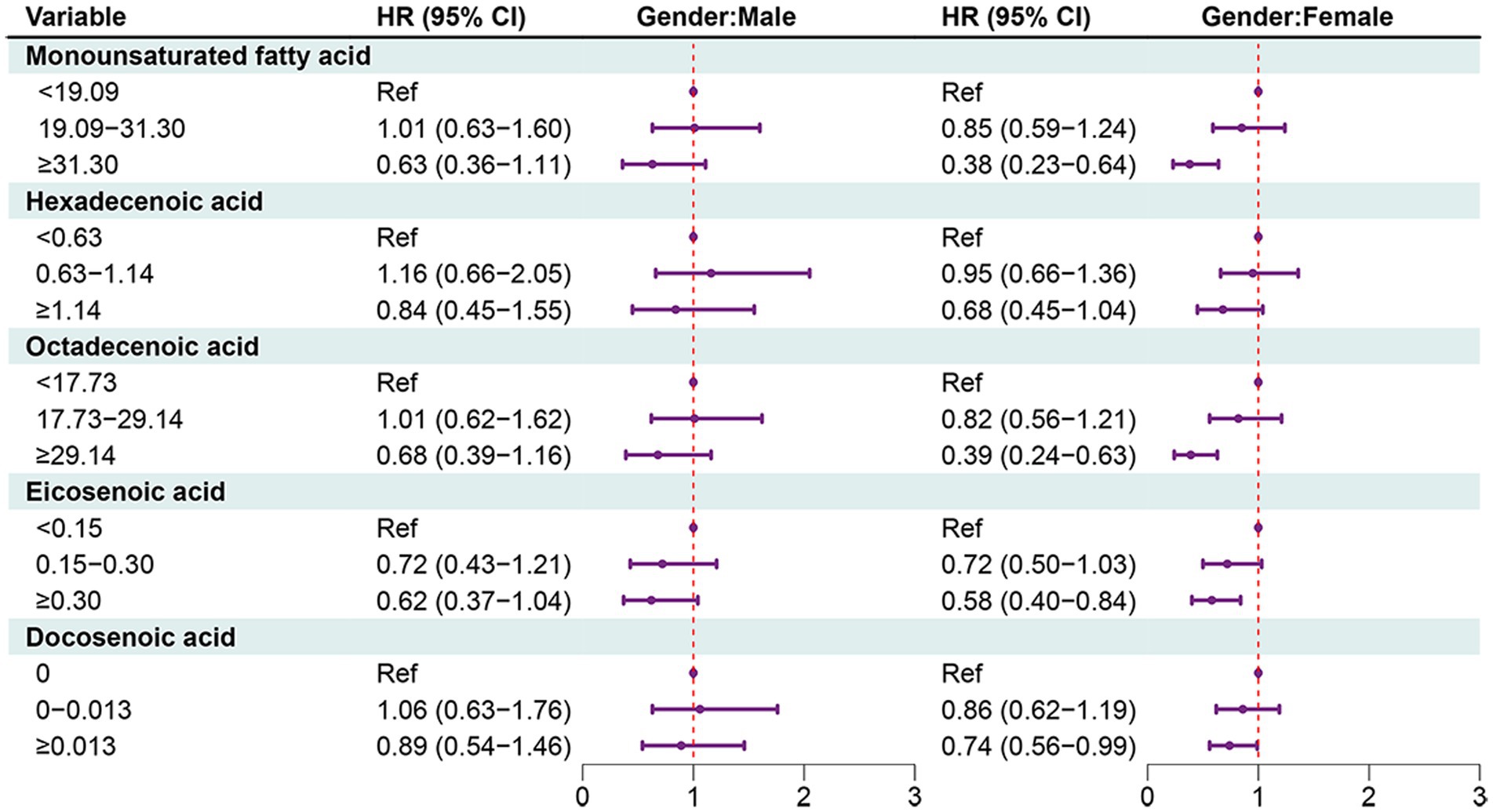
Figure 4. Forest plot showing the associations between MUFAs with all-cause mortality in OA patients in different gender groups.
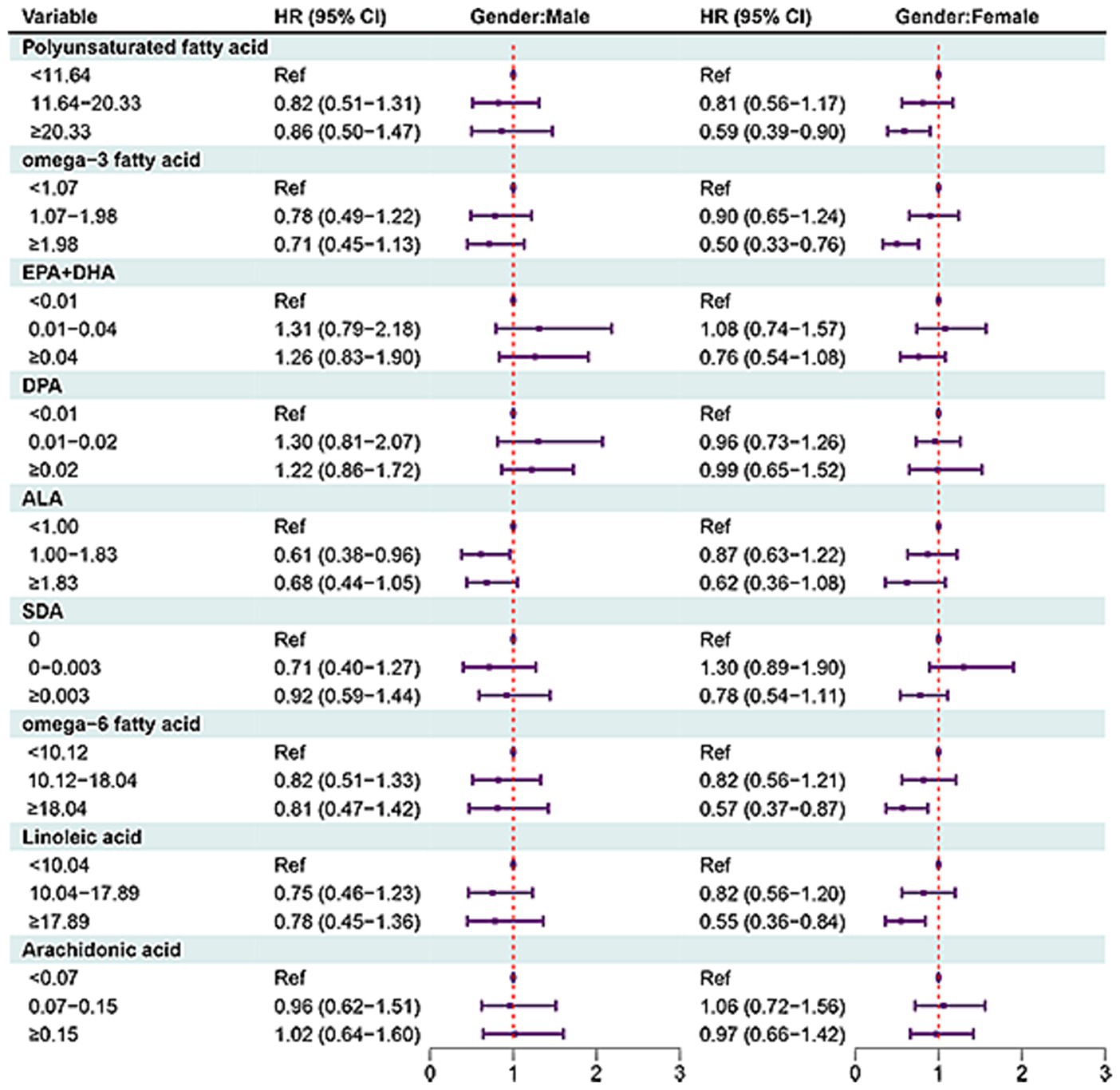
Figure 5. Forest plot showing the associations between PUFAs with all-cause mortality in OA patients in different gender groups.
The present study investigated the relationship between the levels of MUFAs and PUFAs intake and the risk of all-cause mortality in OA patients. The results depicted that total MUFAs ≥31.30, octadecenoic acid ≥29.14, and eicosenoic acid ≥0.15 as well as PUFAs ≥20.33, omega-3 fatty acid ≥1.98, ALA ≥1, omega-6 fatty acid ≥18.04 and linoleic acid ≥17.89 were related to decreased risk of all-cause mortality in OA patients. The finding showed directions for further studies to analyze the biologic and prognostic properties of MUFAs and PUFAs in OA patients.
In a previous study, a significant positive correlation of the consumption of saturated fatty acids, MUFAs, and PUFAs with bone mineral density was confirmed (18). MUFAs and PUFAs were reported to reduce radiographic progression of knee OA (12). Matsumoto et al. (19) revealed that MUFA, as a component of the Mediterranean diet score, was significantly lower in the rheumatoid arthritis, than in the control group and the ratio of consumed MUFA/SFA significantly differed within the rheumatoid arthritis group, which might suppress the disease activity in rheumatoid arthritis patients. Paunescu et al. (20) identified that MUFAs levels were positively associated with the stiffness index, and the status of MUFAs was associated with the strength of bone. Another cohort study identified that the HRs for total mortality when comparing the extreme quintiles of specific dietary fats were 0.81 (95% CI, 0.78–0.84) for PUFAs and 0.89 (95% CI, 0.84–0.94) for MUFAs. Replacing 5% of energy from saturated fats with an equivalent amount of energy from PUFAs and MUFAs was associated with estimated reductions in total mortality by 27% (HR, 0.73; 95% CI, 0.70–0.77) and 13% (HR, 0.87; 95% CI, 0.82–0.93), respectively (21). These findings might provide some support to the results in the current study. We found that total MUFAs ≥31.30, octadecenoic acid ≥29.14 and eicosenoic acid ≥0.15 were correlated with decreased risk of all-cause mortality of patients with OA, which suggested that that high MUFAs, octadecenoic acid, and and eicosenoic acid might help lower the mortality risk of OA patients.
Also, the relationship of PUFAs and bone health was illustrated in several studies. The potential therapeutic use of omega-3 fatty acids was postulated for individuals with OA, owing to their recognized anti-inflammatory properties, which could be beneficial in the context of OA by effectively moderating pro-inflammatory markers and mitigating cartilage loss (11). There was evidence indicated that the resolvin D1 (RvD1), a derivatives of omega-3 fatty acids, might participate in the pathogenesis of OA (22). The anti-inflammatory properties of omega-3 fatty acids are well recognized, which could be advantageous in the context of OA for modulating pro-inflammatory markers and mitigating cartilage loss (23, 24). A meta-analysis of randomized controlled trials revealed that the supplementation of omega-3 fatty acids has been proven effective in alleviating pain and enhancing joint function among patients with OA (25). The omega-3 fatty acids were reported to participate in certain mechanistic pathways involved in inflammation (26). The potential mechanisms underlying the beneficial effects of omega-3 fatty acids on OA are multifactorial. As previously reported, joint-specific inflammation plays a crucial role in the pathogenesis of OA (7), indicating that nutritional interventions incorporating anti-inflammatory nutrients may be beneficial. Omega-3 fatty acids are well-established for their potent anti-inflammatory properties (27). The alteration of cell membrane fatty acid composition, induced by the consumption of foods or supplements rich in omega-3 fatty acids, appears to play a crucial role in the anti-inflammatory effects attributed to omega-3 fatty acids (26). The omega-3 fatty acids were reported to participate in certain mechanistic pathways involved in inflammation (25). The alteration of cell membrane fatty acid composition, induced by the consumption of foods or supplements rich in omega-3 fatty acids, appears to play a crucial role in the anti-inflammatory effects attributed to omega-3 fatty acids (26). Omega-3 fatty acids exert anti-inflammatory effects by inhibiting inflammatory markers such as interleukin-1 beta (IL-1β) and inducible nitric oxide synthase (iNOS). Additionally, they suppress the expression of metalloproteinase 13, inhibit chondrocyte apoptosis, and restrain bone remodeling and angiogenesis within the osteochondral unit (28). The supplementation form of ALA significantly increased the content of bone-specific alkaline phosphatase, which was associated with bone health (29). A Mendelian randomization study indicated that there was causal relationship between PUFAs and OA susceptibility, and offered a novel insight that high omega-6 fatty acids might reduce the risk of knee OA and hip OA (30). These results were allied with the findings in our study, which delineated that PUFAs ≥20.33, omega-3 fatty acid ≥1.98, ALA ≥1, omega-6 fatty acid ≥18.04 and inoleic acid ≥17.89 were correlated with decreased risk of all-cause mortality in OA patients. High PUFAs including omega-3 fatty acid ALA omega-6 fatty acid and inoleic acid were potentially associated with the prognosis of OA patients. The causal associations still require validation in future studies.
Our study has a few notable advantages. This is the first study to examine the association between the intake of UFAs and the risk of all-cause mortality in patients with OA. The samples of this study was obtained from the NHANES database through multi-stage complex sampling, which represented the local population. The sample size was large and the follow-up time was long to ensure a sufficient number of outcome events. These results underscore the importance of maintaining the levels of UFAs in OA patients. Dietary recommendations advocating the role of UFAs and dietary guidelines should carefully consider the health effects of recommendations for UFAs in OA patients. The first limitation of this study was that this was a retrospective cohort study, and recall bias might exist. Second, due to the limitation of the NHANES database, to distinguish patients with OA at different sites was not possible. The association between UFAs intake and the risk of death in patients with OA at different sites needs to be further studied.
The associations of MUFAs and PUFAs intake with the risk of all-cause mortality in OA patients were investigated in the present study. The results depicted that total MUFAs and PUFAs, octadecenoic acid, eicosenoic acid, omega-3 fatty acid, ALA, omega-6 fatty acid and linoleic acid were correlated with decreased risk of all-cause mortality in OA patients. The finding might suggest the importance of specific UFAs supplement in OA patients.
Publicly available datasets were analyzed in this study. This data can be found here: NHANES database, https://wwwn.cdc.gov/nchs/nhanes/.
The requirement of ethical approval for the studies involving humans was waived by the Fourth Affiliated Hospital to Soochow University (Suzhou Dushu Lake Hospital). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
TL: Conceptualization, Project administration, Supervision, Writing – original draft, Writing – review & editing. ZT: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. SL: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. WJ: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. ML: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1454431/full#supplementary-material
1. Salman, LA, Ahmed, G, Dakin, SG, Kendrick, B, and Price, A. Osteoarthritis: a narrative review of molecular approaches to disease management. Arthritis Res Ther. (2023) 25:27. doi: 10.1186/s13075-023-03006-w
2. Jin, Z, Wang, D, Zhang, H, Liang, J, Feng, X, Zhao, J, et al. Incidence trend of five common musculoskeletal disorders from 1990 to 2017 at the global, regional and national level: results from the Global Burden of Disease Study 2017. Ann Rheum Dis. (2020) 79:1014–22. doi: 10.1136/annrheumdis-2020-217050
3. Constantino de Campos, G, Mundi, R, Whittington, C, Toutounji, MJ, Ngai, W, and Sheehan, B. Osteoarthritis, mobility-related comorbidities and mortality: an overview of meta-analyses. Ther Adv Musculoskelet Dis. (2020) 12:1759720x20981219. doi: 10.1177/1759720x20981219
4. Nüesch, E, Dieppe, P, Reichenbach, S, Williams, S, Iff, S, and Jüni, P. All cause and disease specific mortality in patients with knee or hip osteoarthritis: population based cohort study. BMJ. (2011) 342:d1165. doi: 10.1136/bmj.d1165
5. Liu, R, Kwok, WY, Vliet Vlieland, TP, Kroon, HM, Meulenbelt, I, Houwing-Duistermaat, JJ, et al. Mortality in osteoarthritis patients. Scand J Rheumatol. (2015) 44:70–3. doi: 10.3109/03009742.2014.922213
6. Healey, EL, Afolabi, EK, Lewis, M, Edwards, JJ, Jordan, KP, Finney, A, et al. Uptake of the NICE osteoarthritis guidelines in primary care: a survey of older adults with joint pain. BMC Musculoskelet Disord. (2018) 19:295. doi: 10.1186/s12891-018-2196-2
7. Stannus, O, Jones, G, Cicuttini, F, Parameswaran, V, Quinn, S, Burgess, J, et al. Circulating levels of IL-6 and TNF-α are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthr Cartil. (2010) 18:1441–7. doi: 10.1016/j.joca.2010.08.016
8. Marchev, AS, Dimitrova, PA, Burns, AJ, Kostov, RV, Dinkova-Kostova, AT, and Georgiev, MI. Oxidative stress and chronic inflammation in osteoarthritis: can Nrf2 counteract these partners in crime? Ann N Y Acad Sci. (2017) 1401:114–35. doi: 10.1111/nyas.13407
9. Knights, AJ, Redding, SJ, and Maerz, T. Inflammation in osteoarthritis: the latest progress and ongoing challenges. Curr Opin Rheumatol. (2023) 35:128–34. doi: 10.1097/bor.0000000000000923
10. Harwood, JL. Polyunsaturated fatty acids: conversion to lipid mediators, roles in inflammatory diseases and dietary sources. Int J Mol Sci. (2023) 24:8838. doi: 10.3390/ijms24108838
11. Cordingley, DM, and Cornish, SM. Omega-3 fatty acids for the management of osteoarthritis: a narrative review. Nutrients. (2022) 14:3362. doi: 10.3390/nu14163362
12. Lu, B, Driban, JB, Xu, C, Lapane, KL, McAlindon, TE, and Eaton, CB. Dietary fat intake and radiographic progression of knee osteoarthritis: data from the osteoarthritis initiative. Arthritis Care Res. (2017) 69:368–75. doi: 10.1002/acr.22952
13. Linakis, MW, Gustafson, P, Allen, BC, Bachand, AM, van Landingham, C, Keast, DR, et al. Is the cholesterol-perfluoroalkyl substance association confounded by dietary fiber intake?: a Bayesian analysis of NHANES data with adjustment for measurement error in fiber intake. Environ Health. (2022) 21:114. doi: 10.1186/s12940-022-00923-2
14. Ahuja, JK, Moshfegh, AJ, Holden, JM, and Harris, E. USDA food and nutrient databases provide the infrastructure for food and nutrition research, policy, and practice. J Nutr. (2013) 143:241S–9S. doi: 10.3945/jn.112.170043
15. Kantor, ED, Rehm, CD, Du, M, White, E, and Giovannucci, EL. Trends in dietary supplement use among us adults from 1999–2012. JAMA. (2016) 316:1464–74. doi: 10.1001/jama.2016.14403
16. Liu, T, Wang, Y, Meng, T, Ren, Q, Shi, H, and Lin, C. Association between cardiovascular health and all-cause mortality risk in patients with osteoarthritis. BMC Musculoskelet Disord. (2024) 25:641. doi: 10.1186/s12891-024-07729-y
17. Lin, Y, Zeng, G, and Sun, Y. The joint effect of vitamin-D status and tobacco exposure on overweight and obesity in children. Br J Nutr. (2024) 132:1386–93. doi: 10.1017/S0007114524002071
18. Fang, ZB, Wang, GX, Cai, GZ, Zhang, PX, Liu, DL, Chu, SF, et al. Association between fatty acids intake and bone mineral density in adults aged 20–59: NHANES 2011–2018. Front Nutr. (2023) 10:1033195. doi: 10.3389/fnut.2023.1033195
19. Matsumoto, Y, Sugioka, Y, Tada, M, Okano, T, Mamoto, K, Inui, K, et al. Monounsaturated fatty acids might be key factors in the Mediterranean diet that suppress rheumatoid arthritis disease activity: the tomorrow study. Clin Nutr. (2018) 37:675–80. doi: 10.1016/j.clnu.2017.02.011
20. Paunescu, AC, Ayotte, P, Dewailly, E, and Dodin, S. Saturated and monounsaturated fatty acid status is associated with bone strength estimated by calcaneal ultrasonography in Inuit women from Nunavik (Canada): a cross-sectional study. J Nutr Health Aging. (2014) 18:663–71. doi: 10.1007/s12603-014-0498-0
21. Wang, DD, Li, Y, Chiuve, SE, Stampfer, MJ, Manson, JE, Rimm, EB, et al. Association of specific dietary fats with total and cause-specific mortality. JAMA Intern Med. (2016) 176:1134–45. doi: 10.1001/jamainternmed.2016.2417
22. Benabdoune, H, Rondon, EP, Shi, Q, Fernandes, J, Ranger, P, Fahmi, H, et al. The role of resolvin D1 in the regulation of inflammatory and catabolic mediators in osteoarthritis. Inflamm Res. (2016) 65:635–45. doi: 10.1007/s00011-016-0946-x
23. Calder, PC. Mechanisms of action of (n-3) fatty acids. J Nutr. (2012) 142:592s–9s. doi: 10.3945/jn.111.155259
24. Calder, PC. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans. (2017) 45:1105–15. doi: 10.1042/bst20160474
25. Deng, W, Yi, Z, Yin, E, Lu, R, You, H, and Yuan, X. Effect of omega-3 polyunsaturated fatty acids supplementation for patients with osteoarthritis: a meta-analysis. J Orthop Surg Res. (2023) 18:381. doi: 10.1186/s13018-023-03855-w
26. Surette, ME. The science behind dietary omega-3 fatty acids. CMAJ. (2008) 178:177–80. doi: 10.1503/cmaj.071356
27. Richter, CK, Bowen, KJ, Mozaffarian, D, Kris-Etherton, PM, and Skulas-Ray, AC. Total long-chain n-3 fatty acid intake and food sources in the United States compared to recommended intakes: NHANES 2003–2008. Lipids. (2017) 52:917–27. doi: 10.1007/s11745-017-4297-3
28. Phitak, T, Boonmaleerat, K, Pothacharoen, P, Pruksakorn, D, and Kongtawelert, P. Leptin alone and in combination with interleukin-1-beta induced cartilage degradation potentially inhibited by EPA and DHA. Connect Tissue Res. (2018) 59:316–31. doi: 10.1080/03008207.2017.1385605
29. Dou, Y, Wang, Y, Chen, Z, Yu, X, and Ma, D. Effect of n-3 polyunsaturated fatty acid on bone health: a systematic review and meta-analysis of randomized controlled trials. Food Sci Nutr. (2022) 10:145–54. doi: 10.1002/fsn3.2655
Keywords: unsaturated fatty acid, death, osteoarthritis, NHANES, omega-6
Citation: Li T, Tang Z, Li S, Jiang W and Lu M (2025) Association of unsaturated fatty acid intake with risk of all-cause death in patients with osteoarthritis. Front. Nutr. 12:1454431. doi: 10.3389/fnut.2025.1454431
Received: 25 June 2024; Accepted: 24 March 2025;
Published: 07 April 2025.
Edited by:
Amir-Hossein Mousavian, Tehran University of Medical Sciences, IranReviewed by:
Lishi Zhang, Sichuan University, ChinaCopyright © 2025 Li, Tang, Li, Jiang and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Minhua Lu, bWluaHVhbGxsQG91dGxvb2suY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.