- 1Department of Pharmacy, Loudi Hospital of Traditional Chinese Medicine, Loudi, Hunan, China
- 2Department of Pharmacy, Changde Hospital, Xiangya School of Medicine, Central South University (The First People’s Hospital of Changde City), Changde, Hunan, China
- 3College of Traditional Chinese Medicine, Changsha Medical University, Changsha, Hunan, China
- 4Department of Hematology, Hunan Provincial People’s Hospital, The First Affiliated Hospital of Hunan Normal University, Changsha, Hunan, China
Background: We aimed to assess the global impact of chronic kidney disease (CKD) attributable to dietary risk factors.
Methods: The research utilized data from the Global Burden of Disease Study 2021 to evaluate age-standardized mortality rates (ASMR), disability-adjusted life years (DALYs), and estimated annual percentage changes (EAPCs) linked to CKD resulting from dietary risk factors.
Results: From 1990 to 2021, both the ASMR and age-standardized DALY rate (ASDR) for CKD attributable to dietary risk factors exhibited an overall increasing trend globally. The mortality EAPC was 0.65, while the EAPC for DALYs stood at 0.39. Among dietary risk factors examined, a diet high in sugar-sweetened beverages was associated with the most substantial increase in CKD burden. Notably, Central sub-Saharan Africa bore the highest burden of CKD due to dietary risk factors, with an ASMR of 10.24 and an ASDR of 229.23. The increases in ASMR and ASDR were more pronounced in high-income regions, particularly in Latin America and the Caribbean, where the EAPC values for ASMR were 1.45 and 1.05, respectively, and for ASDR were 1.08 and 0.96. Furthermore, the burden of CKD was notably higher among middle-aged and elderly individuals, especially men aged 65 and above.
Conclusion: The global disease burden attributed to dietary risk factors for CKD is increasing. A diet high in sugar-sweetened beverages exerted the most significant impact on CKD. There is a high incidence in Central sub-Saharan Africa, as well as in high-income regions and Latin America and the Caribbean.
1 Introduction
Chronic Kidney Disease (CKD) is an escalating global health issue. If left untreated, CKD can progressively worse, potentially leading to end-stage renal disease, necessitating dependence on dialysis or kidney transplantation (1). A 2023 study reports that the global prevalence of CKD stands at approximately 9.1%, with a rising trend, particularly in low- and middle-income countries where the issue is more pronounced (2). The two primary risk factors for CKD are hypertension and type 2 diabetes mellitus (3, 4). The incidence of both diabetes mellitus and hypertension is increasing exponentially and is anticipated to continue its upward trajectory in relation to CKD (5). The disability-adjusted life years of CKD are statistically forecasted to be the fifth most common cause of life expectancy loss by 2040 (6). However, the majority of cases are attributed to nutritional factors and are largely preventable, underscoring the critical importance of early prevention of CKD.
Dietary patterns represent a significant modifiable risk factor for CKD and are increasingly garnering attention (7). Research has demonstrated that adherence to healthy dietary habits is correlated with a reduced risk of CKD progression and overall mortality in patients with CKD (8). For instance, healthy dietary patterns such as the Dietary Approaches to Stop Hypertension (DASH) and Mediterranean diets (9), diets rich in whole grains (10), vegetables (11), and legumes (12), as well as lower consumption of red and processed meats (13), sodium (14), and sugar-sweetened beverages (15) have been linked to a decreased incidence of new-onset CKD and albuminuria. Furthermore, the selection of various nutrients and dietary patterns (16) can impact the progression of CKD in patients. Consequently, the investigation and promotion of healthy dietary patterns are crucial for the prevention and management of CKD.
Current comprehensive studies have examined the correlation between dietary patterns and the progression of CKD, focusing on disease progression deceleration and symptom alleviation through monitoring key nutrient intake, including protein, calcium, phosphorus, potassium, and sodium (17, 18). However, there is a paucity of research on the global burden of CKD attributable to dietary factors (19).
This study aims to provide an in-depth analysis of the specifics of CKD attributable to dietary risk factors and their global trends. It assesses data on the burden of CKD and the attributable dietary risk factors in 204 countries and territories, utilizing the GBD2021 database, a comprehensive and reliable source for assessing global health losses. The objective is to gain a profound understanding of the relationship between dietary risk factors and CKD, identify and quantify the contribution of these risk factors to the burden of CKD, and provide a scientific foundation for global dietary policy formulation and public health interventions.
2 Methods
2.1 Data sources
This study aims to conduct a comprehensive and systematic analysis of the global dynamics of disease burden attributable to dietary risk factors for CKD, based on the most recent research findings from the Global Burden of Disease 2021 (GBD 2021) database.1 The GBD project compiles and harmonizes data from a wide range of sources, including vital registration systems (such as death certificates), population-based surveys (e.g., household health surveys and nutrition surveys), disease registries, administrative data (hospital records, health insurance data), published literature (peer-reviewed articles, reports), and other relevant data repositories. By systematically analyzing and adjusting these data sources for biases and variations in data quality, the GBD provides comparable estimates of disease burden across different populations, time periods, and geographical regions. As a globally recognized health data platform, GBD 2021’s cause-of-death analysis encompasses 204 countries and regions, spanning the period from 1990 to 2021. It provides an in-depth analysis of mortality rates and years of life lost (YLLs) for 288 causes of death, disaggregated by age, sex, geographic location, and year. The dataset also includes information from 811 sub-administrative regions, ensuring a comprehensive analysis (20). In this study, annual statistics on CKD mortality, disability-adjusted life years (DALYs), and their corresponding age-standardized rates (ASRs) were extracted from the GBD 2021 dataset for the period 1990 to 2021. These statistics are accompanied by exhaustive 95% uncertainty intervals (UIs), which will be used to assess the role of dietary risk factors in this global health challenge.
The study identified cases of CKD attributable to dietary risk factors using the International Classification of Diseases, 10th Edition (ICD-10) and 9th Edition (ICD-9) codes. The ICD-10 codes utilized were N02-N02.9, N07-N07.9, Q60-Q63.2, Q63.8-Q63.9, and Q64.2-Q64.9, while the ICD-9 codes included 753.0–753.4 and 753.6–753.9. For the use of identified data in GBD study, a waiver of informed consent has been approved by the University of Washington Institutional Review Board. This study did not involve individual participants. The ethics approval can be found at https://www.healthdata.org/.
2.2 Estimates of the burden of CKD
In this research, we utilized DALY as the primary composite measure. DALYs are calculated as the sum of years of life lost (YLLs) due to premature mortality and years lived with disability (YLDs) (21). YLLs are derived by multiplying the number of deaths at each age by the remaining standard life expectancy at that age, while YLDs are calculated by multiplying the prevalence of a certain condition by a corresponding disability weight that reflects the severity of health loss. Furthermore, to accurately capture the influence of dietary risk factors on CKD’s disease burden, we employed the age-standardized mortality rate (ASMR) and age-standardized DALY rate (ASDR) as pivotal metrics. ASMR is computed by taking the total number of deaths from a given cause per 100,000 individuals in a population and adjusting for differences in the age structure using a standard population age distribution. Similarly, ASDR is calculated by taking the total DALYs due to a certain cause per 100,000 individuals, also standardized to a reference age structure. The age-standardization process ensures that observed differences in mortality or DALY rates between populations are not driven by differences in their age distributions, allowing for fair comparisons across countries, regions, and time periods.
2.3 Socio-demographic index
The Socio-demographic Index (SDI) is a composite measure that captures the overall socio-demographic development of a country or region. It is derived from a combination of key indicators, typically including average income per person (lag-distributed income), average educational attainment (mean years of schooling in the population aged 15 years and older), and total fertility rates under the age of 25. The SDI ranges from 0 to 1, with higher values reflecting greater socio-demographic development. This measure allows for analyzing how different socio-demographic conditions might influence disease burden and health outcomes globally.
2.4 Attributable dietary risk factors
Our research utilized DALYs related to CKD and corresponding data, employing dismod-mr 2.1 and spatio-temporal Gaussian process regression to quantify the influence of these risk factors on disease (22). This study encompassed seven dietary risk factors: diet low in fruits, diet low in vegetables, diet low in whole grains, diet high in red meat, diet high in processed meat, diet high in sugar-sweetened beverages, and diet high in sodium. All data on dietary risk factors were sourced directly from the GBD 2021 database, which integrates information from various national and international dietary surveys, food consumption databases, and nutritional studies. We adhered strictly to the GBD’s standardized definitions and thresholds for classifying diets as poor or excessive in specific food types, based on global dietary guidelines and expert consensus. Cultural differences and regional consumption patterns were inherently considered in the GBD’s data collection and modeling processes, ensuring that dietary risk factor classifications accurately reflect diverse dietary behaviors across different countries and regions.
2.5 Statistical analysis
In order to assess the disease burden stemming from dietary risk factors linked with CKD, we employed a directly standardized method to compute ASRs of mortality and DALY per 100,000 individuals, along with their respective 95% UIs. Furthermore, we utilized the estimated annual percentage change (EAPC) to quantify the temporal variation in age-standardized CKD mortality and DALY rates attributable to dietary risk factors over the period from 1990 to 2021. This was achieved by fitting a regression line to the natural logarithm of the rate (y = α + βx + ε), where y denotes the natural logarithm of the rate and x signifies the calendar year. The EAPC was derived by multiplying 100 by (exp[β]-1), and its 95% UI was obtained from a linear regression model (23). All statistical analyses conducted in this study were executed using R software (version 4.3.2), and p values below 0.05 were deemed statistically significant.
3 Results
3.1 Global trends in burden of CKD due to dietary risks factors
From 1990 to 2021, the age-standardized mortality rates for CKD attributable to dietary risk factors and the corresponding DALYs exhibited an increasing trend across 21 global regions. The EAPC in mortality rates was 0.65 (95% CI: 0.58 to 0.71), while the EAPC for DALYs stood at 0.39 (95% CI, 0.35 to 0.43). A detailed examination revealed that the ASMRs consistently rose from 3.23% in 1990 to 3.83% in 2021, marking a rise of 0.60 percentage points over three decades—a trend warranting attention. Concurrently, the age-standardized rate of DALYs demonstrated a varied upward trajectory, accumulating an increase of 9.4 percentage points since 1990, advancing from 84.12 to 93.52% (Table 1; Figure 1).
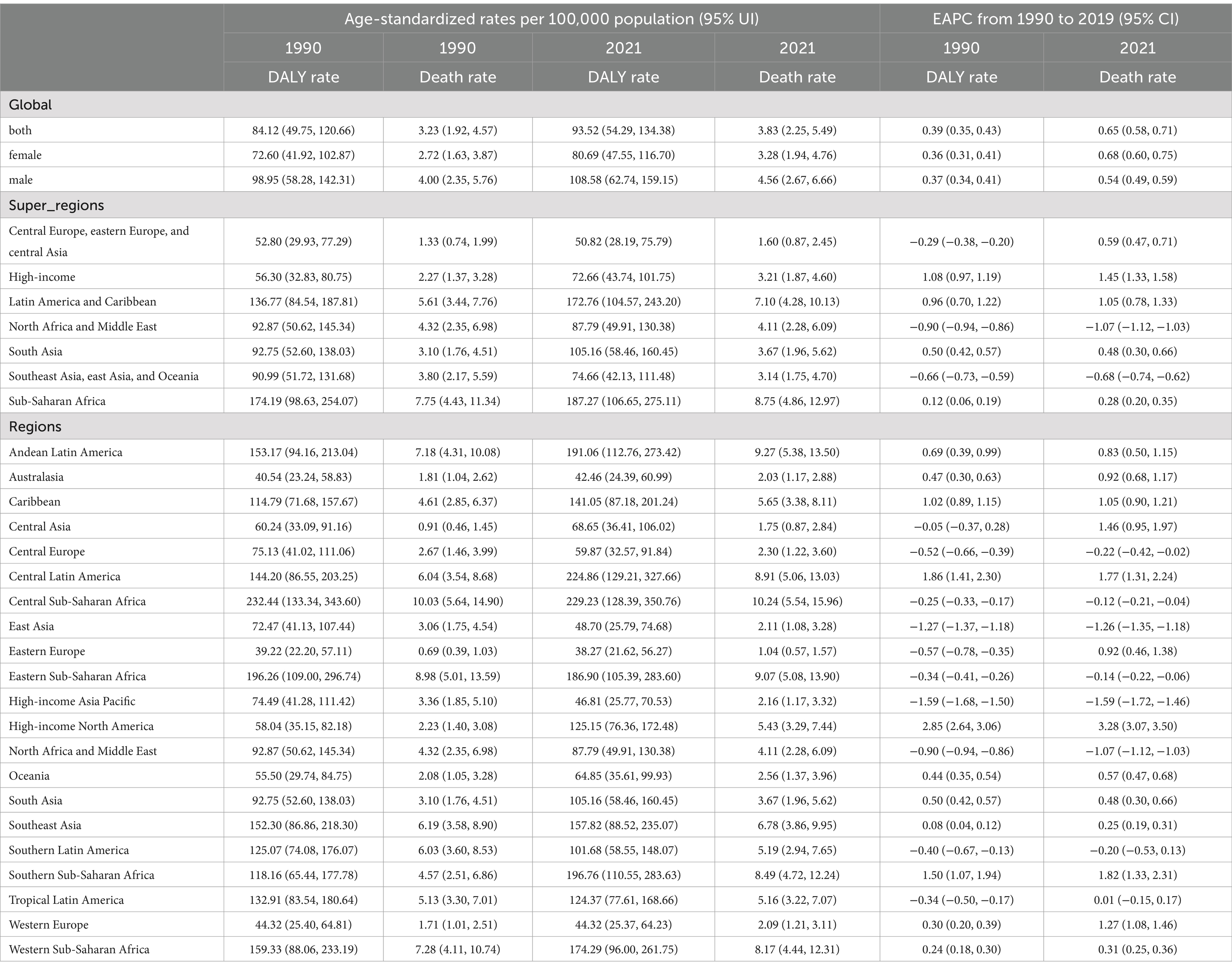
Table 1. Age-standardized mortality and DALY rates (ASMR and ASDR) and estimated annual percentage change (EAPC) for chronic kidney disease (CKD) attributable to dietary risk factors by super-region and region, 1990–2021.
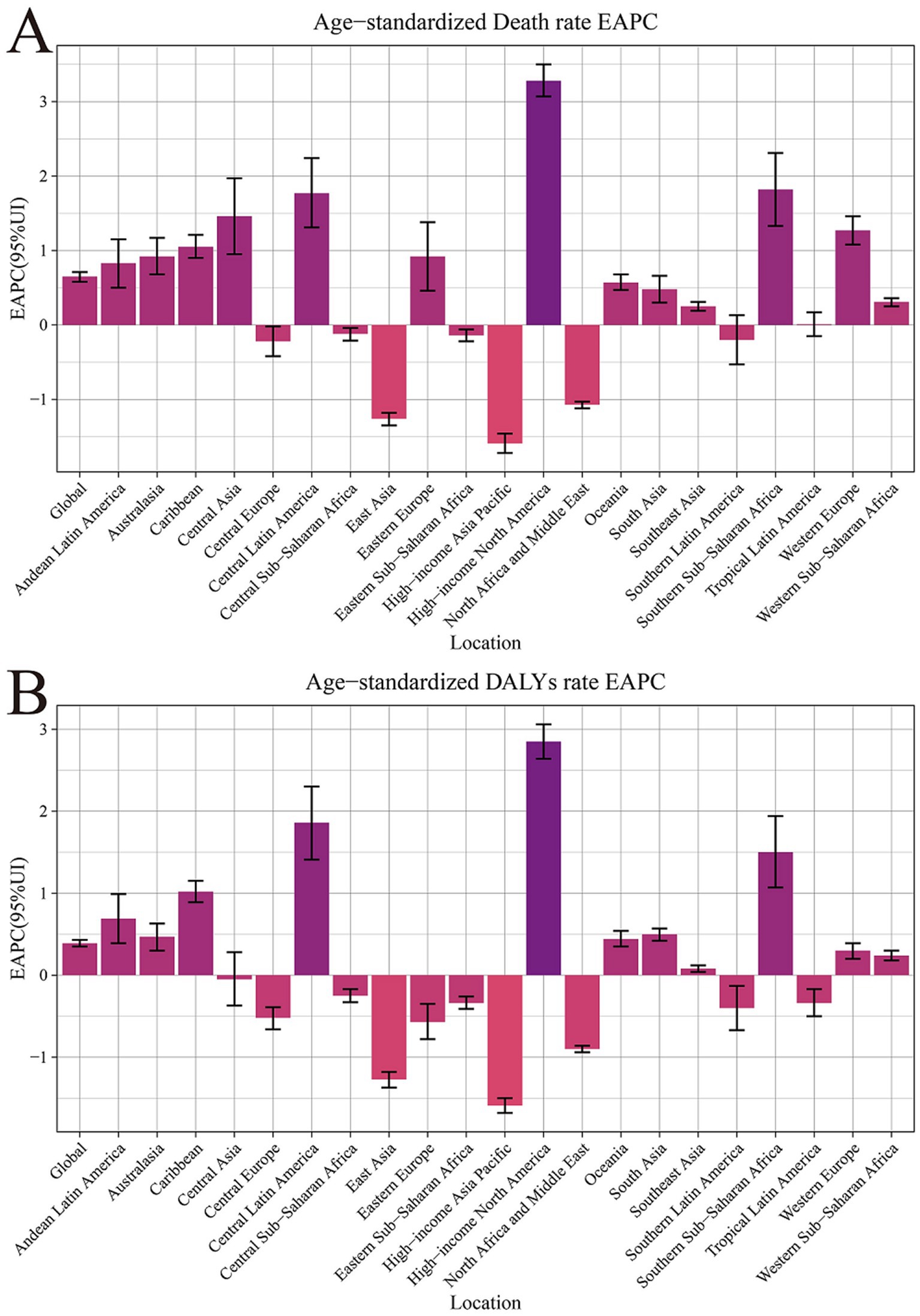
Figure 1. Age-standardized rates and estimated annual percentage change for chronic kidney disease due to dietary factors by 21 regions, from 1990 to 2021. (A) Age-standardized mortality rates (ASMR). (B) Age-standardized DALY rates (ASDR).
Among the various factors contributing to dietary risk, a diet high in sugar-sweetened beverages had a particularly significant impact on CKD, with a mortality EAPC of 2.17 (95% CI: 1.98 to 2.36). This was followed by an EAPC for DALYs of 2.14 (95% CI: 1.99 to 2.29), highlighting the severe threat that sugar-sweetened beverages pose to kidney health. Additionally, dietary habits such as high consumption of red meat and processed meat were also identified as high-risk factors. The former had EAPCs of 1.33 (95% CI: 1.20 to 1.46) and 1.07 (95% CI: 0.96 to 1.18) in mortality and DALYs respectively, while the latter had EAPCs of 1.23 (95% CI: 1.07 to 1.40) and 0.74 (95% CI: 0.60 to 0.88; Table 2).
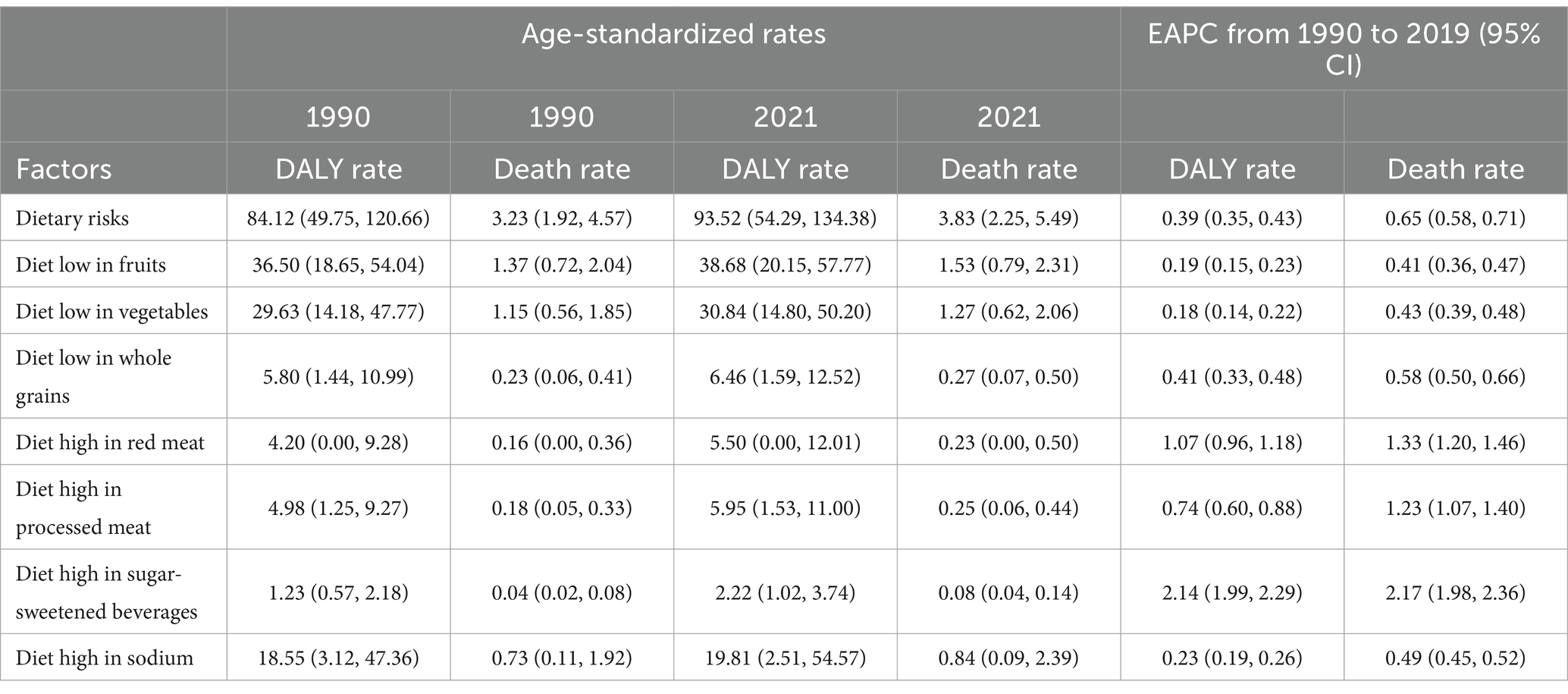
Table 2. Global age-standardized mortality and DALY rates (ASMR and ASDR) and rate changes attributable to dietary risk factors for chronic kidney disease (CKD), 1990 and 2021.
3.2 Burden of CKD in different countries and regions
Significant disparities exist in the ASMR and ASDR of CKD across various super regions and areas globally. The ASMR for five super regions, namely Central Europe, Eastern Europe, Central Asia, High Income, Latin America and the Caribbean, South Asia, and Sub-Saharan Africa, has demonstrated a consistent upward trend annually (EAPC>0). Notably, the High Income and Latin America and Caribbean regions exhibit particularly high EAPC values of 1.45 (95% CI: 1.33 to 1.58) and 1.05 (95% CI: 0.78 to 1.33) respectively, indicating a significant surge in CKD burden within these regions. Conversely, North Africa and the Middle East, Southeast Asia, East Asia, and Oceania have observed a downward trend in ASMR (EAPC<0). Southeast Asia, East Asia, and Oceania have shown the most substantial decline, with an EAPC value of −0.68 (95% CI: −0.74 to −0.62), suggesting that these regions have made considerable progress in CKD prevention and control(Table 1; Figures 1, 2).
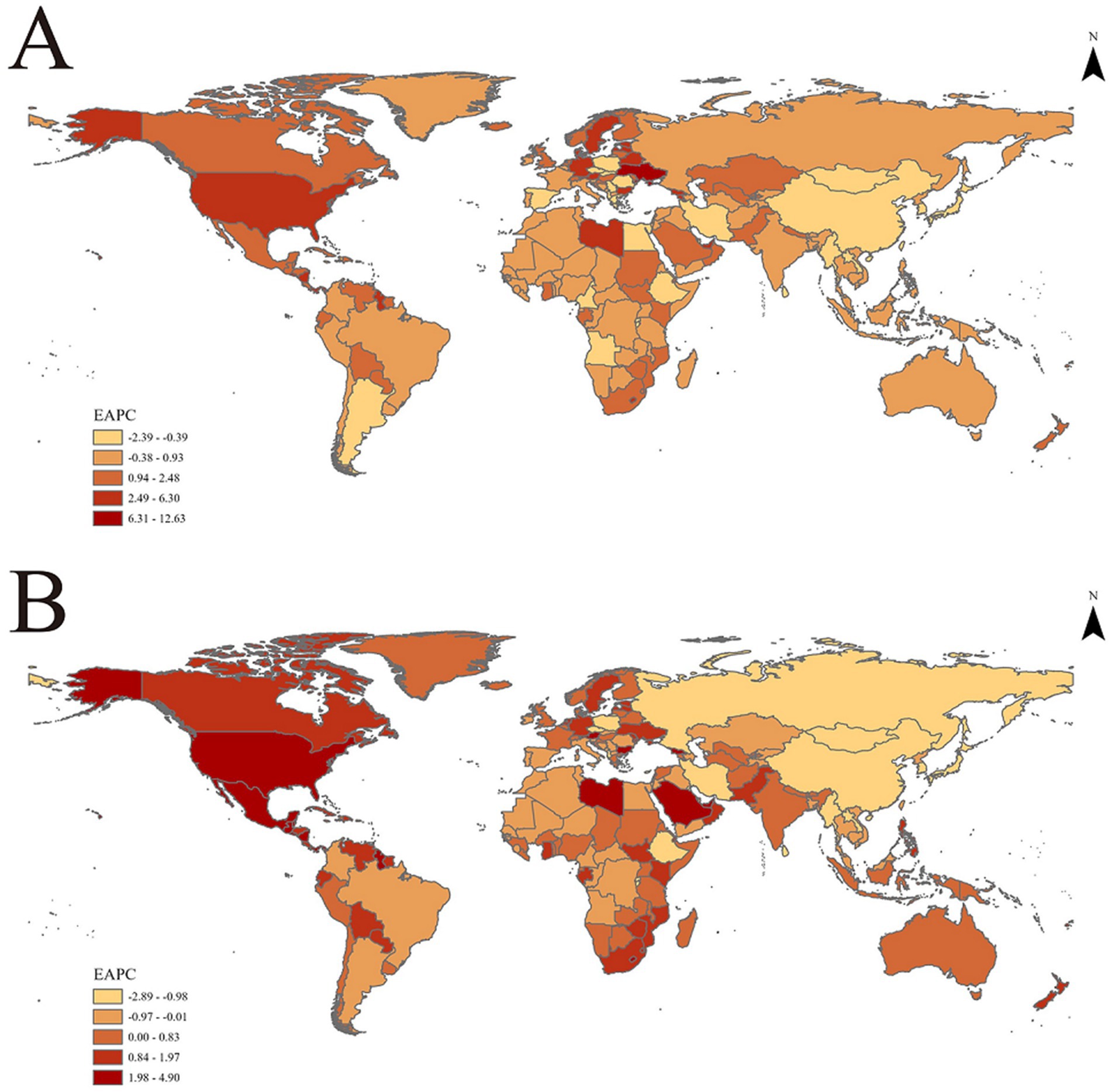
Figure 2. EAPC of age-standardized rates of chronic kidney disease due to dietary factors from 1990 to 2021, by locations. (A) Age-standardized mortality rates (ASMR). (B) Age-standardized DALY rates (ASDR).
In the trend of ASDR changes, regions such as High Income, Latin America and Caribbean, South Asia, and Sub-Saharan Africa demonstrated an upward trajectory. This was particularly noticeable in the High Income and Latin America and Caribbean regions, where the EAPC values for ASDR reached 1.08 (95% CI: 0.97 to 1.19) and 0.96 (95% CI: 0.70 to 1.22), respectively. Additionally, among the remaining 21 regions, 11 regions (including Andean Latin America, Australasia, Caribbean, etc.) exhibited an upward trend in both ASMR and ASDR. A particularly significant increase was observed in High Income North America, further underscoring the complexity and urgency of CKD prevention and control efforts worldwide (Table 1; Figures 1, 2).
Refined further at the national level, the United Arab Emirates is among the top countries in terms of increased ASMR and ASDR, with an EAPC value of 6.30 (95% Confidence Interval: 5.29 to 7.31) for ASMR and 4.90 (95% Confidence Interval: 4.13 to 5.67) for ASDR. This underscores the significant challenges the country faces in preventing and controlling CKD. Armenia and Georgia also rank highly in terms of rising ASMR, while Lesotho and American Samoa stand out in terms of increasing ASDR (Supplementary Table 1).
3.3 Burden and trends of CKD due to dietary risk factors in various countries and regions
From 1990 to 2021, the High income North America region exhibited the most pronounced increase in CKD mortality rates (EAPC = 3.28, 95% CI: 3.07 to 3.50) and DALYs (EAPC = 2.85, 95% CI: 2.64 to 3.06) attributed to various dietary risk factors globally. This suggests that high-income countries also grapple with addressing this public health challenge. Notably, the Central sub Saharan Africa region bears the greatest burden of CKD due to dietary risk factors. This is evident not only in its ASMR of 10.24 (95% UI: 5.54, 15.96), but also its leading position in ASDR with a rate of 229.23 (95% UI: 128.39, 350.76; Table 1; Figures 1, 2).
At the national level, Mauritius bears the most significant burden of CKD attributable to dietary risk factors, as evidenced by its high ASMR and ASDR. Conversely, Belarus exhibits the most favorable CKD ASMR, while Tajikistan demonstrates a comparatively lower ASDR. These findings offer potential benchmarks for the prevention and control of CKD in other nations (Supplementary Table 1).
Further examination of the influence of seven dietary risk factors on CKD mortality demonstrated that the Central sub-Saharan Africa region had a notably high CKD mortality rate, primarily due to diets low in fruits and vegetables. The ASMRs were 4.86 (95% UI: 2.44, 7.72) and 5.46 (95% UI: 2.58, 9.41), respectively. Conversely, the other five types of dietary risk factors - diets low in whole grains, high in red meat, high in processed meat, high in sugar-sweetened beverages, and high in sodium - resulted in higher CKD mortality rates in the Andean Latin America, Southern Latin America, High-income North America, Central Latin America, and Southeast Asia regions, respectively. In terms of ASDR, despite Central Latin America facing a high burden of CKD due to various dietary risk factors such as high sodium intake, high sugar-sweetened beverage consumption, and low vegetable intake, Central sub-Saharan Africa still holds a significant position in CKD high ASDR caused by factors such as low whole grain intake, high red meat consumption, and high processed meat consumption in Tropical Latin America. Additionally, the Southern Latin America region also faces a higher risk of CKD due to specific dietary risk factors (Supplementary Table 2).
3.4 Trends in burden of CKD due to dietary risk factors across genders and ages
The impact of dietary risk factors on the prevalence of CKD is particularly pronounced among the elderly, especially men aged 65 and above. This suggests that the health risks associated with CKD, attributable to dietary risk factors, escalate with age. In 2021, there was a marked increase in CKD deaths caused by dietary risk factors among women, starting from the 45–49 age group, which continued to rise until the 90–94 age group, followed by a decline in the oldest age group. Conversely, men experienced this threat earlier, with an increase in deaths from the 40–44 age group. However, it is important to note that after reaching the 75–79 age group, the death toll began to gradually decline. Further analysis of DALYs indicators reveals a sharp increase in DALYs among females within the 40–69 age range, followed by a gradual decrease, while among males, DALYs continued to accumulate within the 35–69 age range before showing a downward trend. Additionally, the global disease burden is lowest among individuals aged 25–29, and highest among those aged 65 and above (Figure 3).

Figure 3. The mortality and DALY rates and number of chronic kidney disease due to dietary factors in different age groups. (A) Mortality rate and number. (B) DALY rate and number.
3.5 The relationship between SDI and burden of CKD disease due to dietary risk factors
After a comprehensive analysis of the correlation between demographic indices and CKD ASR across 204 countries and regions, we observed that CKD ASMR attributable to dietary risk factors demonstrated significant regional disparities globally (Figure 4). Specifically, Central Asia, Eastern Europe, and High-income Asia Pacific regions were among the top three in ASMR rankings. Conversely, North Africa and the Middle East, Southeast Asia, and Western Europe showed lower levels of CKD ASMR. Further examination of ASDR rankings revealed that Western sub-Saharan Africa, Tropical Latin America, and Eastern sub-Saharan Africa were among the top three regions, underscoring the substantial burden of CKD-related health risks in these areas.
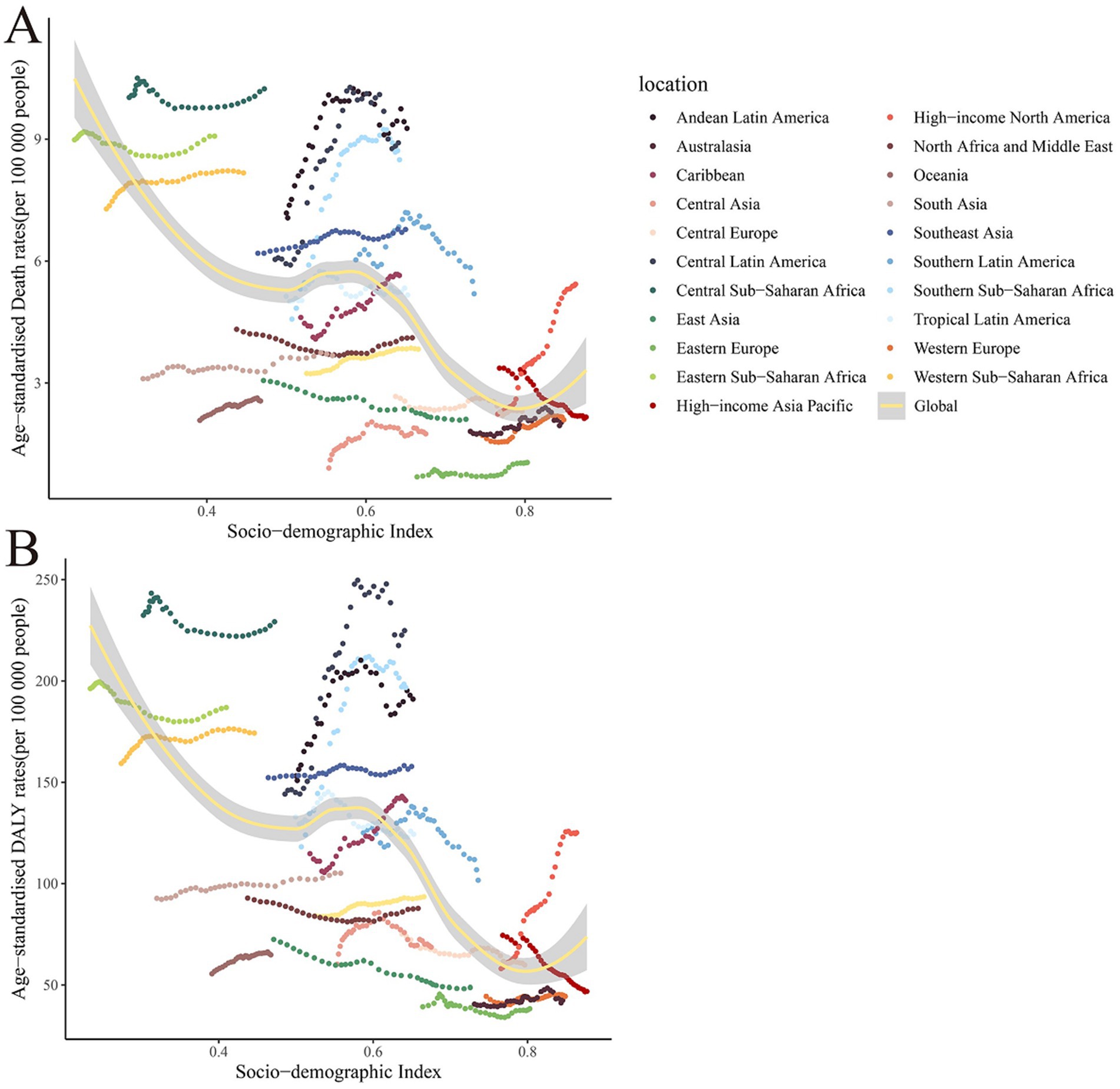
Figure 4. The relationship between age-standardized rates and SDI of global burden of chronic kidney disease due to dietary factors, by 21 regions. (A) Age-standardized mortality rates (ASMR). (B) Age-standardized DALY rates (ASDR).
4 Discussion
The global disease burden of CKD attributable to dietary risk factors has shown an upward trend, as indicated by increases in both ASMR and ASDR. This observed trend may be factors such as the prevalence of unhealthy dietary habits, an aging population, socio-economic development, lifestyle changes, and unequal access to healthcare resources (24, 25). Among the dietary risk factors examined, a diet high in sugar-sweetened beverages was associated with the most substantial increase in CKD burden. The burden of CKD varies significantly across countries and regions, with the highest burden attributable to dietary risk factors observed in Central sub-Saharan Africa and Mauritius. Additionally, the increase in ASMR and ASDR was more pronounced in high-income regions, as well as in Latin America, and the Caribbean. The burden of CKD was particularly higher among middle-aged and elderly individuals, especially men aged 65 years and older. These findings suggest the need for targeted public health policies and interventions to mitigate the rising burden of CKD associated with dietary risk factors.
Research has demonstrated a significant correlation between sugar-sweetened beverages (SASB) intake and CKD. Meta-analyses have confirmed this positive association, indicating that the risk of CKD in populations with high SBS consumption is 1.30 times greater than in low-consuming groups (26, 27). The potential for SASB to increase CKD risk may be attributed to several mechanisms, including body weight gain, altered blood glucose levels, elevated urate concentrations, and increased dietary phosphorus acid load (28). Furthermore, SASB consumption elevates the risk of diabetes, a primary risk factor for CKD. Persistently high blood glucose levels can overburden the kidney’s filtration system, leading to renal damage (29). Fructose metabolism in high-sugar beverages places stress on the liver and kidneys, and sustained high intake can result in insulin resistance, a precursor to diabetes (30). Concurrently, high-sugar beverages may cause elevated uric acid levels in the body, increasing the risk of gout and kidney damage (29). These beverages often contain artificial colors and additives, which may impair kidney function with prolonged intake (31). Therefore, reducing high-sugar beverage consumption is a crucial strategy for CKD prevention and management.
The prevalence of CKD attributable to dietary risk factors is notably high in Central sub-Saharan Africa and Mauritius. This can be attributed to a dietary and nutritional transition, propelled by rapid urbanization, economic development, and globalization. This transition has precipitated a dual burden of over-nutrition and under-nutrition, as well as unhealthy dietary habits such as insufficient intake of fruits, vegetables, grains, and an overconsumption of fatty foods high in red meat. These dietary habits escalate the risk of disability and mortality from CKD. Conversely, high fiber foods like grains, legumes, fruits, and vegetables mitigate renal load and inflammation, thereby decelerating the loss of renal function (32). In addition, malnutrition, driven by poverty, social injustice, and poor sanitation in these regions, may compromise immunity and increase susceptibility to various diseases, thereby impeding healthy physical development. In Central sub-Saharan Africa and Mauritius, the public healthcare system grapples with limited resources and extended waiting times, potentially resulting in diagnostic and treatment delays. Furthermore, Mauritius exhibits a high prevalence of non-communicable diseases (NCDs), particularly marked by elevated ASMR and DALY for CKD due to type 2 diabetes (33). The widespread occurrence of diabetes significantly contributes to the high burden of CKD. Therefore, targeted and comprehensive prevention and control strategies—including improved dietary habits, chronic disease management, enhanced accessibility to healthcare resources, and health education—are imperative to alleviate the burden of CKD.
Poor dietary habits in high-income and Latin America and Caribbean regions significantly contribute to the increased burden of CKD. The diets in these regions are characterized by a high intake of red meat, animal fats, sugar, and highly processed foods, while the consumption of vegetables and fruits is low. Such a Western-like dietary pattern has been linked to elevated levels of inflammation (34). In Latin America and the Caribbean, poor dietary habits primarily involve excessive consumption of high-salt foods and insufficient intake of vegetables and fruits, can elevate blood pressure. and chronic hypertension is a major risk factor for CKD (35), thereby increasing the burden on the kidneys. Furthermore, the consumption of high-sugar and highly processed foods is associated with an increased risk of obesity, diabetes, and CKD (36). The high sugar content and artificial additives in these foods pose a threat to kidney health. Red and processed meats contain high levels of sodium and potential carcinogens, and their excessive intake may lead to overnutrition or undernutrition, further increasing the risk of CKD (37). A significant portion of the population in Latin America and the Caribbean cannot afford a healthy diet due to economic constraints. Leading to hunger, childhood chronic malnutrition, and a high prevalence of non-communicable diseases, indirectly increase the CKD burden. Additional factors that exacerbate the risk of CKD include a lack of adequate physical activity, biased nutritional choices due to socioeconomic factors, and inadequate health awareness (32, 35). Therefore, improving dietary habits, reducing the intake of high-salt, sugar, and fat foods, and promoting healthy eating patterns are essential to reduce the burden of CKD.
The burden of CKD associated with dietary factors is notably pronounced in middle-aged and older adults, particularly men aged 65 and above. This increased susceptibility can be attributed to the natural decline in renal function as one ages (38). Furthermore, due to diminishing resistance, older adults frequently present with multiple comorbid chronic diseases such as diabetes and hypertension, which are significant risk factors for CKD. Concurrently, this demographic may encounter challenges in accessing healthcare resources and exhibit low awareness regarding CKD. Such barriers can hinder their ability to receive appropriate CKD treatment and might contribute to delays in early diagnosis and intervention. Biological and metabolic distinctions, coupled with a higher prevalence of chronic disease risk factors, may render men more susceptible to CKD (39). Specifically, diets high in protein and fat exacerbate this risk. Consequently, CKD prevention and management strategies must prioritize the middle-aged and older male population.
A correlation exists between the SDI and both the ASMR and ASDR of CKD attributable to dietary factors. As the SDI escalates, the trend in ASMR for CKD linked to dietary elements generally diminishes, with the lowest ASMR and ASDR observed in regions with a high SDI, and the highest in areas with a low SDI. This pattern may be attributed to superior medical resources and heightened health awareness in countries with a high SDI, facilitating earlier diagnosis and intervention for CKD, thereby reducing mortality rates and DALYs (2, 25). Conversely, both CKD mortality and DALYs are elevated in nations with a low SDI.
While our study highlights significant associations between dietary risk factors and the burden of CKD, it is important to acknowledge several limitations inherent in our analysis. Firstly, the observational nature of the study limits our ability to infer causality between dietary factors and CKD. The associations identified may be influenced by unmeasured confounding variables such as genetic predispositions, physical activity levels, socioeconomic status, and access to healthcare, which were not directly accounted for in our analysis. Secondly, the reliance on secondary data from the GBD 2021 database introduces potential biases related to data quality and availability, which can vary significantly across regions. Although the GBD employs rigorous statistical methods to adjust for these biases, some residual confounding and data inaccuracies may still affect the estimates. Additionally, accurately assessing dietary consumption patterns is inherently challenging due to variations in dietary assessment methods, reporting accuracy, and cultural differences in food consumption. The grouping of food types into broad categories may obscure specific interactions or effects of individual dietary components on CKD risk. Furthermore, certain regions may have limited data availability, leading to greater uncertainty in the estimates for those areas. Future research should aim to incorporate more granular dietary data, utilize longitudinal study designs, and account for a broader range of confounding factors to enhance the precision and validity of the associations between dietary risk factors and CKD.
In assessing the global burden of CKD disease attributable to dietary risk factors from 1990 to 2021, this study presents both strengths and limitations. Primarily, the study’s reliance on the GBD database means its accuracy is contingent upon the quality of the underlying data. This is particularly problematic in developing nations where data scarcity may introduce information bias. Additionally, CKD risk factors are multifaceted, encompassing metabolic, behavioral, and environmental dimensions. The intricate interplay of these factors might not be fully represented in current datasets, complicating the analytical process. Furthermore, while the study documents temporal trends in CKD disease burden, such trends can be shaped by a myriad of influences, including socioeconomic shifts, medical technological advancements, and lifestyle modifications, thereby complicating trend interpretation. Consequently, it is imperative to exercise caution when interpreting the findings, bearing in mind the inherent biases and uncertainties, to achieve a more nuanced understanding of CKD’s disease burden.
5 Conclusion
The global burden of CKD attributed to dietary risk factors is on the rise, with notable disparities observed across various countries and regions. The pronounced CKD burden in Central sub-Saharan Africa, coupled with the rising trend in high-income regions and Latin America and the Caribbean, underscores the need for tailored interventions. Moreover, the high-risk demographic of men aged 65 years further emphasizes the urgency for such preventive measures. Such a trend necessitates the implementation of targeted and comprehensive prevention and control strategies. These include rectifying poor dietary habits, advocating for healthy eating patterns to manage chronic diseases, and enhancing access to healthcare resources and health education.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: Global Health Data Exchange database (GHDx) (http://ghdx.healthdata.org/gbd-results-tool).
Ethics statement
The requirement of ethical approval was waived by University of Washington Institutional Review Board for the studies involving humans because this study did not involve individual participants. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board also waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because for the use of identified data in GBD study, a waiver of informed consent has been approved by the University of Washington Institutional Review Board.
Author contributions
LY: Conceptualization, Writing – original draft, Writing – review & editing. MK: Data curation, Writing – original draft, Writing – review & editing. ZL: Data curation, Formal analysis, Writing – review & editing. BZ: Formal analysis, Writing – review & editing. PW: Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to thank everyone who took part in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1522555/full#supplementary-material
Footnotes
References
1. Thomas, R, Kanso, A, and Sedor, JR. Chronic kidney disease and its complications. Prim Care. (2008) 35:329–44. doi: 10.1016/j.pop.2008.01.008
2. Stanifer, JW, Muiru, A, Jafar, TH, and Patel, UD. Chronic kidney disease in low- and middle-income countries. Nephrol Dial Transplant. (2016) 31:868–74. doi: 10.1093/ndt/gfv466
3. Tedla, FM, Brar, A, Browne, R, and Brown, C. Hypertension in chronic kidney disease: navigating the evidence. Int J Hypertens. (2011) 2011:132405:1–9. doi: 10.4061/2011/132405
4. Shen, Y, Cai, R, Sun, J, Dong, X, Huang, R, Tian, S, et al. Diabetes mellitus as a risk factor for incident chronic kidney disease and end-stage renal disease in women compared with men: a systematic review and meta-analysis. Endocrine. (2017) 55:66–76. doi: 10.1007/s12020-016-1014-6
5. Coresh, J, Selvin, E, Stevens, LA, Manzi, J, Kusek, JW, Eggers, P, et al. Prevalence of chronic kidney disease in the United States. JAMA. (2007) 298:2038–47. doi: 10.1001/jama.298.17.2038
6. Li, PKT, Garcia-Garcia, G, Lui, SF, Andreoli, S, Fung, WWS, Hradsky, A, et al. Kidney health for everyone everywhere-from prevention to detection and equitable access to care. Hong Kong Med J. (2020) 26:8–9. doi: 10.12809/hkmj198292
7. Jain, N, and Reilly, RF. Effects of dietary interventions on incidence and progression of CKD. Nat Rev Nephrol. (2014) 10:712–24. doi: 10.1038/nrneph.2014.192
8. Gutiérrez, OM, Muntner, P, Rizk, DV, McClellan, WM, Warnock, DG, Newby, PK, et al. Dietary patterns and risk of death and progression to ESRD in individuals with CKD: a cohort study. Am J Kidney Dis. (2014) 64:204–13. doi: 10.1053/j.ajkd.2014.02.013
9. Joshi, S, Kalantar-Zadeh, K, Chauveau, P, and Carrero, JJ. Risks and benefits of different dietary patterns in CKD. Am J Kidney Dis. (2023) 81:352–60. doi: 10.1053/j.ajkd.2022.08.013
10. Balk, EM, Lichtenstein, AH, Chung, M, Kupelnick, B, Chew, P, and Lau, J. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis. (2006) 189:19–30. doi: 10.1016/j.atherosclerosis.2006.02.012
11. Bahadoran, Z, Mirmiran, P, Momenan, AA, and Azizi, F. Allium vegetable intakes and the incidence of cardiovascular disease, hypertension, chronic kidney disease, and type 2 diabetes in adults: a longitudinal follow-up study. J Hypertens. (2017) 35:1909–16. doi: 10.1097/HJH.0000000000001356
12. Haring, B, Selvin, E, Liang, M, Coresh, J, Grams, ME, Petruski-Ivleva, N, et al. Dietary protein sources and risk for incident chronic kidney disease: results from the atherosclerosis risk in communities (ARIC) study. J Ren Nutr. (2017) 27:233–42. doi: 10.1053/j.jrn.2016.11.004
13. Barba, C, Benoit, B, Bres, E, Chanon, S, Vieille-Marchiset, A, Pinteur, C, et al. A low aromatic amino-acid diet improves renal function and prevent kidney fibrosis in mice with chronic kidney disease. Sci Rep. (2021) 11:19184. doi: 10.1038/s41598-021-98718-x
14. Garofalo, C, Borrelli, S, Provenzano, M, De Stefano, T, Vita, C, Chiodini, P, et al. Dietary salt restriction in chronic kidney disease: a Meta-analysis of randomized clinical trials. Nutrients. (2018) 10:10. doi: 10.3390/nu10060732
15. Kramer, H, and Shoham, D. The millennial physician and the obesity epidemic: a tale of sugar-sweetened beverages. Clin J Am Soc Nephrol. (2019) 14:4–6. doi: 10.2215/CJN.13851118
16. Naber, T, and Purohit, S. Chronic kidney disease: role of diet for a reduction in the severity of the disease. Nutrients. (2021) 13:3277. doi: 10.3390/nu13093277
17. van Westing, AC, Küpers, LK, and Geleijnse, JM. Diet and kidney function: a literature review. Curr Hypertens Rep. (2020) 22:14. doi: 10.1007/s11906-020-1020-1
18. Jhee, JH, Kee, YK, Park, JT, Chang, TI, Kang, EW, Yoo, TH, et al. A diet rich in vegetables and fruit and incident CKD: a community-based prospective cohort study. Am J Kidney Dis. (2019) 74:491–500. doi: 10.1053/j.ajkd.2019.02.023
19. Ke, C, Liang, J, Liu, M, Liu, S, and Wang, C. Burden of chronic kidney disease and its risk-attributable burden in 137 low-and middle-income countries, 1990-2019: results from the global burden of disease study 2019. BMC Nephrol. (2022) 23:17. doi: 10.1186/s12882-021-02597-3
20. Collaborators. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the global burden of disease study 2021. Lancet. (2024) 403:2100–32. doi: 10.1016/S0140-6736(24)00367-2
22. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
23. Zhao, G, Zhu, S, Zhang, F, Zhang, X, Zhang, X, Li, T, et al. Global burden of osteoarthritis associated with high body mass index in 204 countries and territories, 1990-2019: findings from the global burden of disease study 2019. Endocrine. (2023) 79:60–71. doi: 10.1007/s12020-022-03201-w
24. Ameh, OI, Ekrikpo, UE, and Kengne, AP. Preventing CKD in low- and middle-income countries: a call for urgent action. Kidney Int Rep. (2020) 5:255–62. doi: 10.1016/j.ekir.2019.12.013
25. Zhao, Y, Yan, H, Marshall, RJ, Dang, S, Yang, R, Li, Q, et al. Trends in population blood pressure and prevalence, awareness, treatment, and control of hypertension among middle-aged and older adults in a rural area of Northwest China from 1982 to 2010. PLoS One. (2013) 8:e61779. doi: 10.1371/journal.pone.0061779
26. Yuzbashian, E, Asghari, G, Mirmiran, P, Zadeh-Vakili, A, and Azizi, F. Sugar-sweetened beverage consumption and risk of incident chronic kidney disease: Tehran lipid and glucose study. Nephrology (Carlton). (2016) 21:608–16. doi: 10.1111/nep.12646
27. Lo, WC, Ou, SH, Chou, CL, Chen, JS, Wu, MY, and Wu, MS. Sugar- and artificially-sweetened beverages and the risks of chronic kidney disease: a systematic review and dose-response meta-analysis. J Nephrol. (2021) 34:1791–804. doi: 10.1007/s40620-020-00957-0
28. Gonzalez-Palacios, S, Navarrete-Muñoz, EM, García-de-la-Hera, M, Torres-Collado, L, Santa-Marina, L, Amiano, P, et al. Sugar-containing beverages consumption and obesity in children aged 4-5 years in Spain: the INMA study. Nutrients. (2019) 11:1772. doi: 10.3390/nu11081772
29. Nettleton, JA, Lutsey, PL, Wang, Y, Lima, JA, Michos, ED, and Jacobs, DR Jr. Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the multi-ethnic study of atherosclerosis (MESA). Diabetes Care. (2009) 32:688–94. doi: 10.2337/dc08-1799
30. Zheng, Z, Harman, JL, Coresh, J, Köttgen, A, McAdams-DeMarco, MA, Correa, A, et al. The dietary fructose:vitamin C intake ratio is associated with hyperuricemia in African-American adults. J Nutr. (2018) 148:419–26. doi: 10.1093/jn/nxx054
31. Banerjee, T, Crews, DC, Wesson, DE, Tilea, AM, Saran, R, Ríos-Burrows, N, et al. High dietary acid load predicts ESRD among adults with CKD. J Am Soc Nephrol. (2015) 26:1693–700. doi: 10.1681/ASN.2014040332
32. Goraya, N, Simoni, J, Jo, CH, and Wesson, DE. A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin J Am Soc Nephrol. (2013) 8:371–81. doi: 10.2215/CJN.02430312
33. Kang, S, Kang, M, and Lim, H. Global and regional patterns in noncommunicable diseases and dietary factors across National Income Levels. Nutrients. (2021) 13:3595. doi: 10.3390/nu13103595
34. Anand, S, Zheng, Y, Montez-Rath, ME, Wei, WJ, Perico, N, Carminati, S, et al. Do attributes of persons with chronic kidney disease differ in low-income and middle-income countries compared with high-income countries? Evidence from population-based data in six countries. BMJ Glob Health. (2017) 2:e000453. doi: 10.1136/bmjgh-2017-000453
35. Kramer, H. Diet and chronic kidney disease. Adv Nutr. (2019) 10:S367–79. doi: 10.1093/advances/nmz011
36. Banerjee, T, Carrero, JJ, McCulloch, C, Burrows, NR, Siegel, KR, Morgenstern, H, et al. Dietary factors and prevention: risk of end-stage kidney disease by fruit and vegetable consumption. Am J Nephrol. (2021) 52:356–67. doi: 10.1159/000514754
37. Odermatt, A. The Western-style diet: a major risk factor for impaired kidney function and chronic kidney disease. Am J Physiol Ren Physiol. (2011) 301:F919–31. doi: 10.1152/ajprenal.00068.2011
38. Yu, MK, Lyles, CR, Bent-Shaw, LA, and Young, BA. Risk factor, age and sex differences in chronic kidney disease prevalence in a diabetic cohort: the pathways study. Am J Nephrol. (2012) 36:245–51. doi: 10.1159/000342210
Keywords: chronic kidney disease, incidence rate, disability-adjusted life years, disease burden, adult
Citation: Yin L, Kuai M, Liu Z, Zou B and Wu P (2025) Global burden of chronic kidney disease due to dietary factors. Front. Nutr. 11:1522555. doi: 10.3389/fnut.2024.1522555
Edited by:
Elma Izze da Silva Magalhães, Federal University of Rio Grande do Sul, BrazilReviewed by:
Vânia Oliveira, Universidade Federal de Pelotas, BrazilLaura Moreira Goularte, Federal University of Pelotas, Brazil
Copyright © 2025 Yin, Kuai, Liu, Zou and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Wu, d3VwY2R5eTEyM0AxNjMuY29t
 Lingtao Yin1
Lingtao Yin1 Binbin Zou
Binbin Zou Ping Wu
Ping Wu