- Department of Neurology, The Fourth Affiliated Hospital of Guangxi Medical University, Liuzhou, China
Background: Global health issues related to obesity are growing. Visceral adipose tissue (VAT) significantly contributes to complications associated with obesity. Reducing adipose tissue accumulation can improves inflammation. However, it is still unknown how the systemic immune-inflammation index (SII) and VAT area are related.
Methods: With the help of multivariate linear regression and smooth curve fitting, the relationship between SII and VAT area was explored with data from the 2013 and 2014 National Health and Nutrition Examination Survey (NHANES). Analyzing subgroups and testing for interaction were used to investigate whether the relationship was accurate across demographics.
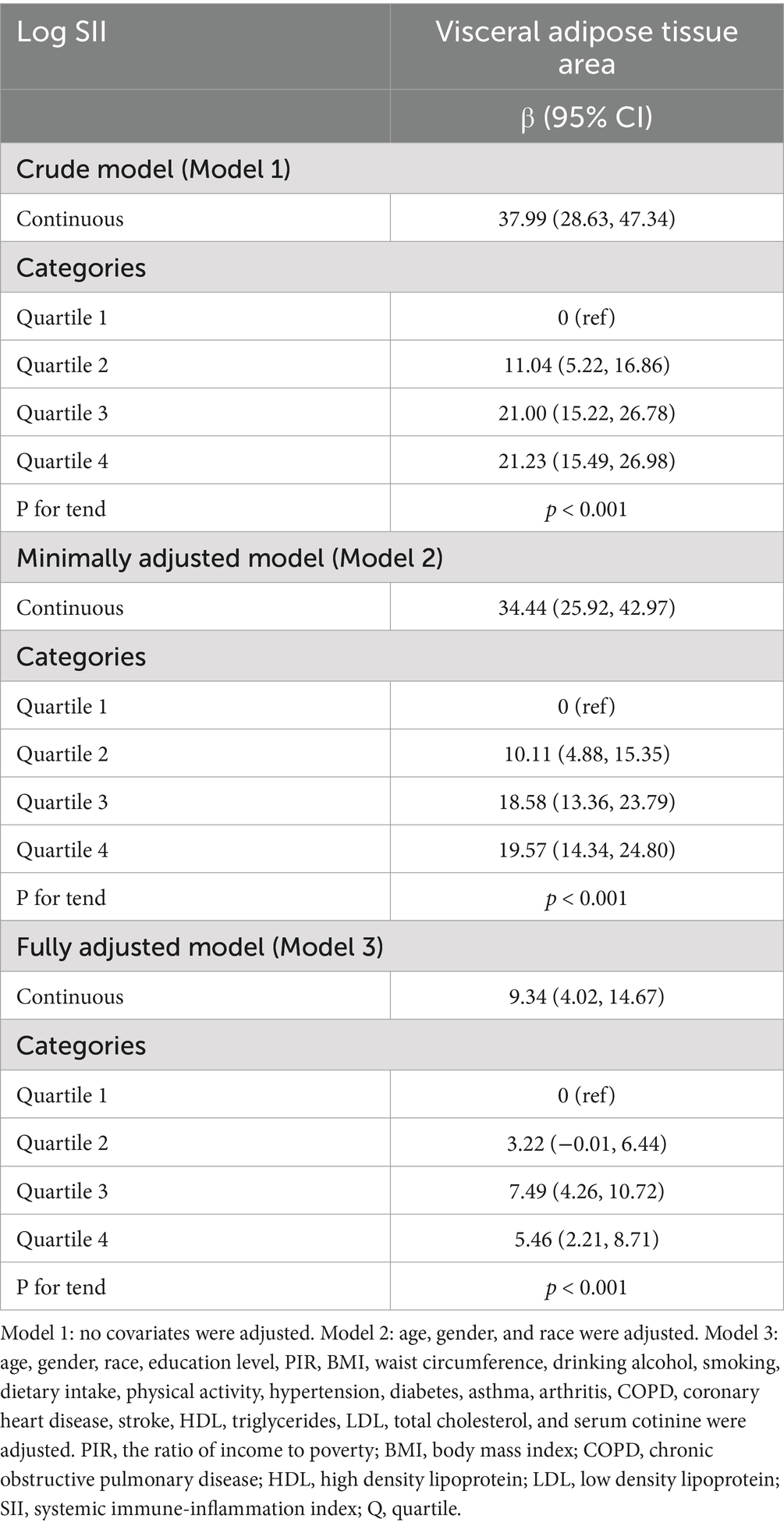
Results: From 20 to 59 years of age, 3,290 individuals were observed to have a positive correlation between SII and VAT area. In accordance with the fully adjusted model, the VAT area increased by 9.34 cm2 for every unit increase in log SII [β = 9.34, 95% CI (4.02, 14.67)]. In the highest quartile of SII, the VAT area was 5.46 cm2 [β = 5.46, 95% CI (2.21, 8.71)] higher than that in the lowest quartile. Additionally, the population that was overweight or obese showed a stronger positive correlation.
Conclusion: SII has a positive correlation with VAT area in US adults. SII may be valuable in clinical applications to evaluate the severity of VAT area.
1 Introduction
Obesity has emerged as a major global health concern that poses significant risks to human health overall, lowers life expectancy, and increases mortality rates (1). In the US, the prevalence of obesity is sharply rising. National Health and Nutrition Examination Survey data report that the age-adjusted obesity rate among adults increased from 30.5% in 1999–2000 to 42.4% in 2017–2018 (2). One important factor in the complications associated with obesity is visceral adipose tissue (3). An overabundance of visceral adipose tissue serves as a risk factor for numerous health issues, such as diabetes (4), cardiovascular disease (5), metabolic syndrome (6), non-alcoholic fatty liver (7), and various cancers (8). Additionally, a major cohort study has previously suggested a possible causal link between the buildup of excessive VAT and the onset of these diseases (9). Thus, both the prevention of excessive VAT accumulation and the management of disease progression are vital.
In contrast to the composite index, individual blood cell counts may be affected by factors such as changes in body fluids. Hu et al. introduced the systemic immune inflammation index (SII), a novel inflammatory biomarker and a powerful predictor of unfavorable outcomes for patients with hepatocellular carcinoma, which is based on the combination of platelets, neutrophils, and lymphocytes (10). SII is more responsive to the inflammatory state and thrombosis than traditional indicators such as PLR and NLR (11, 12). In addition, SII is a better predictor of coronary heart disease than PLR, NLR, and CRP (13). Both the systemic inflammation throughout the body and the local immune response are accurately represented by this index (14–16). Numerous earlier studies have illustrated that SII is utilized to assess and forecast tumor prognoses in various cancers, including gastric cancer (17), non-small cell lung cancer (18), colorectal cancer (19), esophageal cancer (20), and pancreatic cancer (21). Furthermore, a notable correlation exists between SII and various conditions such as cardiovascular disease (22), hepatic steatosis (23), rheumatoid arthritis (24), and kidney stones (25).
Overweight is associated with and exacerbates adipose tissue inflammation, particularly in visceral adipose tissue (VAT). There was a correlation found between VAT and levels of IL-6, INF-α, and C-reactive protein (26). However, the relationship between SII and VAT area is unclear. With data from the National Health and Nutrition Examination Survey (NHANES) conducted in 2013–2014, we conducted a cross-sectional study to look into the relationship between SII and VAT area.
2 Materials and methods
2.1 Study population
The Centers for Disease Control and Prevention conduct the NHANES survey, which is nationally representative (27). Research ethics review board approval was granted for the study procedures by the National Center for Health Statistics (NCHS). Written comments were provided by all participants at recruitment (28). The survey lasted for 2 years (2013–2014), and there was a total survey cycle. Participants missing visceral adipose tissue area (4748), incomplete or missing SII data (319 participants), and missing or outliers in the independent variables (1,818 participants) were excluded from the analysis. In total, 3,290 individuals were enrolled in the study (Figure 1).
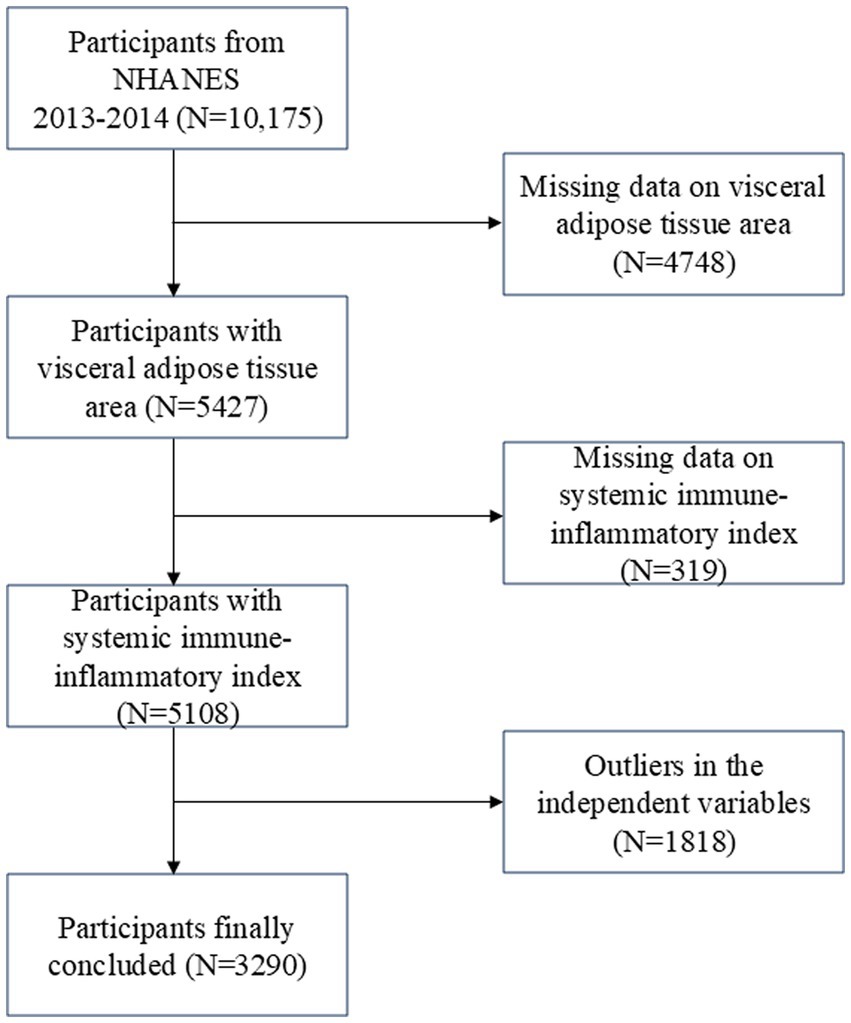
Figure 1. Flow chart of participants selection. NHANES, National Health and Nutrition Examination Survey.
2.2 Systemic immune-inflammation index
Standardized sampling protocols are followed when analyzing blood samples to ensure data validity and comparability. This is how SII was derived from the samples. Typically, survey vehicles or designated sampling sites are used to gather blood samples, which are then processed and tested in a laboratory. NHANES uses a precise formula to calculate SII, which is SII = platelet count × neutrophil count/lymphocyte count. The inflammatory status of the respondents is ascertained using this formula (29).
2.3 Visceral adipose tissue area
To calculate the visceral adipose tissue area, dual energy X-ray absorptiometry, or DXA, was utilized. In 2013 and 2014, the NHANES Mobile Examination Center conducted whole-body DXA scans. During scan analysis, VAT was accurately defined with the use of Hologic APEX software. In the abdominal cavity, the adipose tissue area was measured, especially in the vicinity of the L4 and L5 centrums. The DXA data collection and scan analysis procedure adhered to a stringent quality control framework, which included enforcing a strict schedule for phantom scanning, in order to maintain a high degree of accuracy and precision.
2.4 Covariates
Covariates were selected that might potentially alter the relationship between SII and VAT area. The following were typically included: age, gender, race, education level, income-to-poverty ratio (PIR), waist circumference, body mass index (BMI), smoking and drinking status, serum cotinine, energy intake, protein intake, total fat intake, physical activity, total cholesterol, low-density lipoprotein, high-density lipoprotein, triglyceride, hypertension, diabetes, stroke, coronary heart disease, asthma, arthritis, and chronic obstructive pulmonary disease (COPD). Individual self-report interviews provided the demographic data. There were four categories for race: non-Hispanic Black, non-Hispanic White, Mexican American, and Other. Three categories for education were established: less than high school, high school, and more than high school. The BMI is obtained by dividing the weight in kilograms by the square of the height in meters. According to the World Health Organization standards (30), BMI is divided into the following groups: underweight (< 18.5 kg/m2), normal weight (≥ 18.5 and < 25 kg/m2), overweight (≥ 25 and < 30 kg/m2), obesity class I (≥ 30 and < 35 kg/m2), obesity class II (≥ 35 and < 40 kg/m2), severe obesity (≥ 40 kg/m2). A lifetime of at least 100 cigarettes was considered smoking. Alcohol consumption was defined as an average of more than 1 drink during the previous 12 months on days in which alcoholic beverages were consumed. Physical activity means doing any moderate-intensity exercise, fitness, or recreational activity that causes a small increase in breathing or heart rate for at least 10 consecutive minutes in a typical week. If a respondent satisfied at least one of the following requirements, they were identified as having diabetes: (1) participants with a self-reported diagnosis of diabetes; (2) the glycated hemoglobin level was at least 6.5%; (3) the fasting plasma glucose level was at least 126 mg per deciliter; (4) use of insulin or glucose-lowering medications. If a respondent satisfied even one of the following requirements, they were identified as hypertensive: (1) participants with a self-reported diagnosis of hypertension; (2) the systolic blood pressure was at least 140 mmHg; (3) the diastolic blood pressure was at least 90 mmHg; (4) taking prescription drugs to treat high blood pressure.
2.5 Statistical analyses
Utilizing the chi-square test and t-test, the participant’s demographics were ascertained according to the SII quartile. To examine the linear relationship between SII and VAT area, multiple linear regression was employed. An analysis of the linear association trend between SII and VAT area was done using a trend test following the conversion of SII from a continuous variable to a categorical variable (quartile). The relationship between SII and VAT area in relation to various demographic variables, such as age, gender, BMI, diabetes, and hypertension, was examined using subgroup analysis. Subsequently, the consistency with which the associations held true across subgroups was assessed using interaction tests. To investigate the nonlinear relationship between SII and VAT area, smooth curve fitting was employed. R (version 4.2) or Empowerstats (version 5.0) were utilized for every analysis. The statistical significance threshold was set at a two-sided p-value of less than 0.05.
3 Results
3.1 Baseline characteristics
The study included 3,290 participants in total, ranging in age from 20 to 59. There were 49.73% males and 50.27% females among them, with a mean (SD) age of 39.66 (11.30) years. The mean (SD) VAT area of all participants was 104.61 (57.93) cm2. All participants had a mean (SD) SII of 502.96 (298.32), with the following interquartile range: quartile 1: < 318.6; quartile 2: ≥ 318.6 and < 443.7; quartile 3: ≥ 443.7 and ≤ 610.0; quartile 4: > 610.0. Comparing those with the lowest SII quartile to those with a higher SII, the former group was more likely to be female, non-Hispanic White, and to have a higher risk of developing asthma, arthritis, COPD, diabetes, and stroke (Table 1). Furthermore, as indicated by Table 1, individuals with higher SII frequently also had higher waist circumference, BMI, VAT area, and serum cotinine.
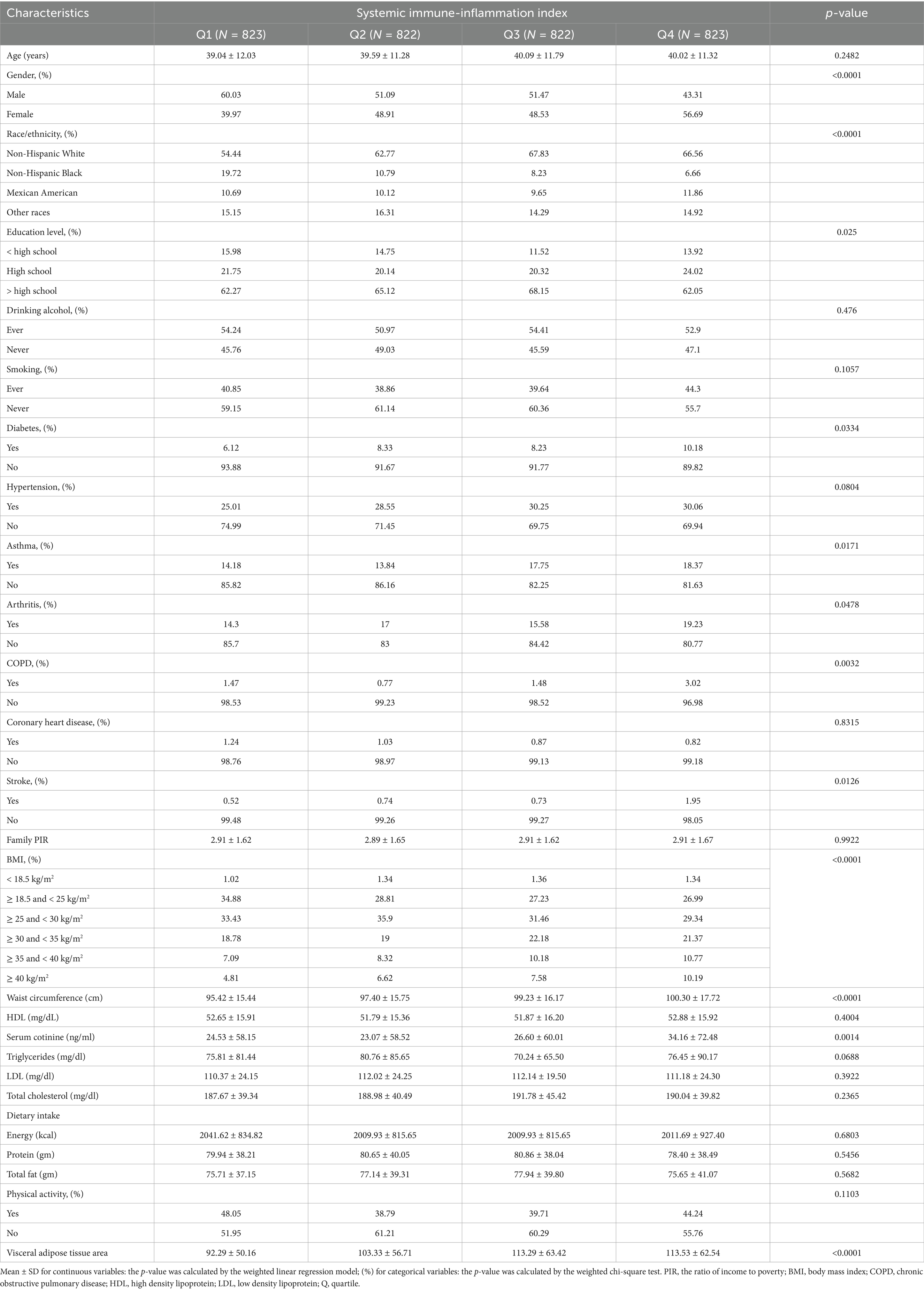
Table 1. Basic characteristics of participants by systemic immune-inflammation index among U.S. adults.
3.2 Association between SII and VAT area
Table 2 shows how SII and VAT area are correlated. Due to the extremely little influence per unit SII for the VAT area, we looked at the linear relationship between log SII and VAT. In all three models—the crude [β = 37.99, 95% CI (28.63, 47.34)], the partially adjusted [β = 34.44, 95% CI (25.92, 42.97)], and the fully adjusted [β = 9.34, 95% CI (4.02, 14.67)]—a significant positive association between SII and VAT area was established. This association was statistically significant (all P for trend <0.001) even after quartile-dividing SII. When comparing the VAT area in the highest quartile of SII to the lowest quartile, the difference was 5.46 cm2 [β = 5.46, 95% CI (2.21, 8.71)]. Furthermore, the nonlinear positive correlation between SII and VAT area was further confirmed by the smooth curve fitting results (Figure 2). Additional research revealed that the threshold effect has an inflection point of 2.74. Log SII and VAT area have a considerable positive connection before the inflection point as Table 3 demonstrates.
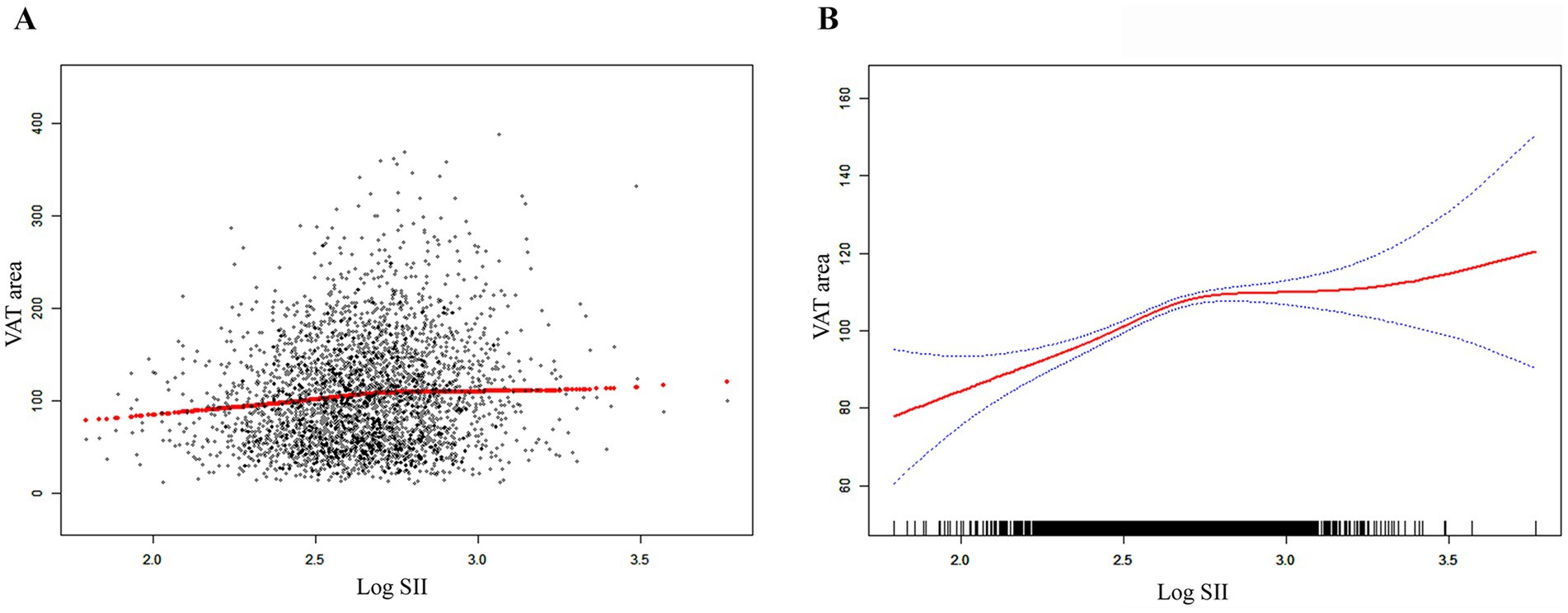
Figure 2. The association between SII and VAT area. (A) Each black point represents a sample. (B) The solid red line represents the smooth curve fit between variables. Blue bands represent the 95% of confidence interval from the fit. SII, systemic immune-inflammation index; VAT, visceral adipose tissue.
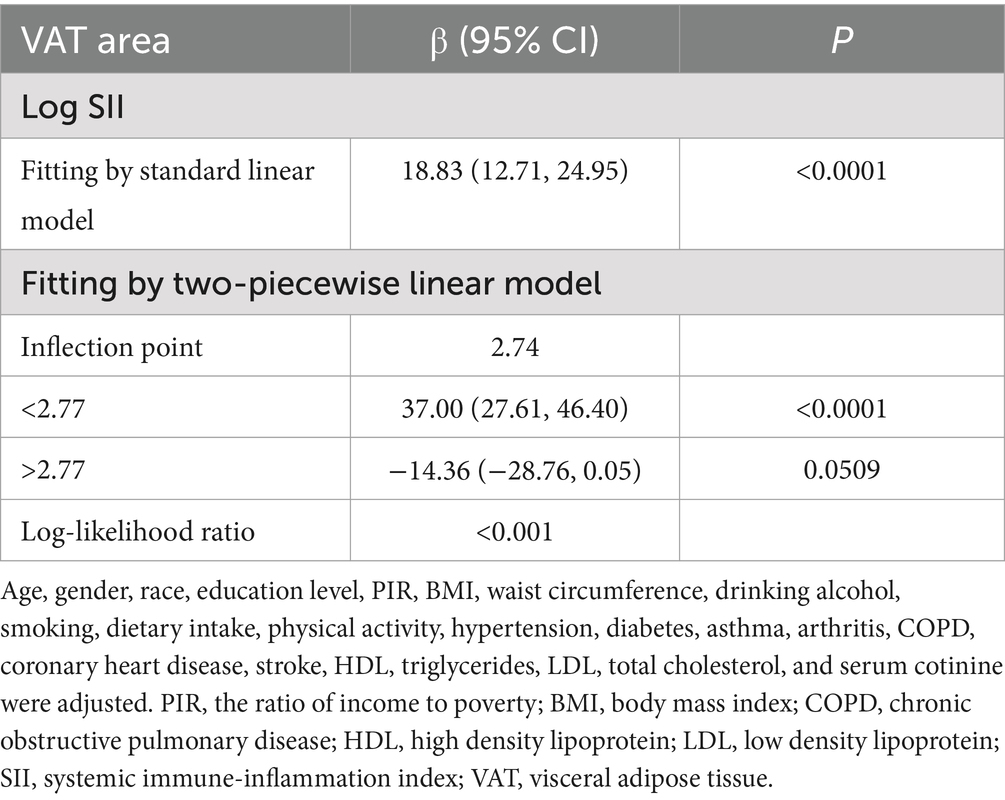
Table 3. Threshold effect analysis of SII on VAT area using a two piecewise linear regression model.
3.3 Subgroup analyses
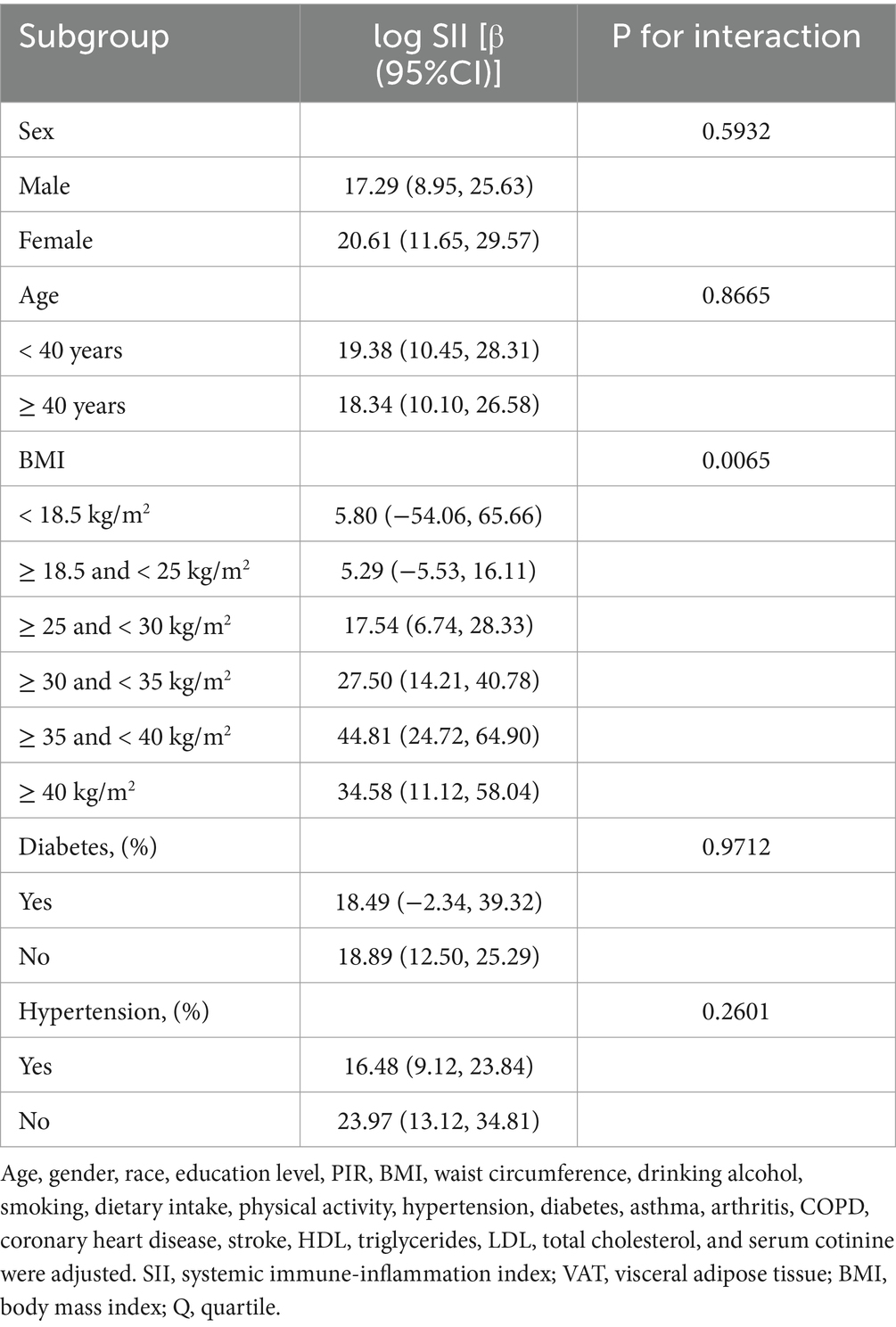
To ascertain whether the association between SII and VAT area was consistent in the general population and to identify any potentially different population settings, subgroup analyses and interaction tests were conducted, stratified by age, gender, BMI, diabetes, and hypertension (Table 4). Our results demonstrated that, in the overweight and obesity subgroup, there were significantly different associations between SII and VAT area. In overweight, obesity class I, obesity class II and severe obesity participants, each 1-unit increase in log SII was associated with an increase in VAT area of 17.54 cm2 [β = 17.54, 95% CI (6.74, 28.33)], 27.50 cm2 [β = 27.50, 95% CI (14.21, 40.78)], 44.81 cm2 [β = 44.81, 95% CI (24.72, 64.90)] and 34.58 cm2 [β = 34.58, 95% CI (11.12, 58.04)], respectively (Table 4). There was no significant correlation between SII and VAT area in normal weight, underweight participants.
4 Discussion
In a cross-sectional study with 3,290 representative participants, a significant positive correlation that was dependent on BMI was discovered between SII and VAT area. This suggests that systemic inflammation may be elevated with increased VAT area, especially in overweight and obese populations. When assessing the severity of the VAT area, SII may be clinically useful.
The association between SII and VAT area has not been examined in any other study, to our knowledge. Although immune, metabolic, and endocrine factors are involved in the effects of excess adiposity, persistent, low-grade inflammation is increasingly thought to be a major contributor to insulin resistance and obesity-related diseases (31, 32). According to recent studies, adipokines like interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) are overexpressed in the adipose tissue of obese patients, possibly as a result of macrophage infiltration (33, 34). In population-based studies of healthy adults, VAT is positively associated with hs-CRP, and VAT is the strongest indicator of elevated IL-6 and INF-α levels (26). Furthermore, a cohort study conducted in Korea with 150 patients with diabetes revealed a significant positive correlation between elevated serum hs-CRP and VAT (35). According to Boronat-Toscano et al., anti-tumor necrosis factor therapy suppressed immune cell infiltration in visceral adipose tissue in areas of inflamed gut and restored adipose tissue morphology in a cohort of 14 Crohn’s disease patients receiving anti-tumor necrosis factor biologic agents (36). Beyond blood markers, dietary intake also reflects the relationship between inflammation and VAT risk. According to research by Lozano et al. (37), there is a positive overall effect between the inflammatory index of an energy-adjusted diet (E-DII) and VAT. Consistent with earlier research, we observed a positive correlation in the current study between the VAT area and the composite inflammation index SII, indicating that individuals with elevated systemic inflammation also had higher VAT area.
Compared to blood cell counts alone, the composite index SII, which incorporates platelets, neutrophils, and lymphocytes, is more stable. SII, which is not affected by a number of variables like dehydration and fluid overload, is able to measure the inflammatory process and immune function of the body more thoroughly than a single inflammatory index (38, 39). Currently, SII is still not frequently utilized in clinical prognosis. Epidemiological research has connected elevated SII to hyperlipidemia, metabolic syndrome, diabetic nephropathy, and nonalcoholic fatty liver disease (40–43). In a retrospective study, Lee et al. discovered that visceral obesity and systemic inflammatory response were significant prognostic factors in patients receiving immune checkpoint inhibitor treatment for metastatic or unresectable melanoma, and that visceral fat’s impact on prognosis depended on the state of systemic inflammation (44). Additionally, in adult US citizens, SII is positively correlated with both high risk of obesity and abdominal obesity (45). SII and system inflammation response index (SIRI) may develop into a useful biomarker for the treatment of obesity, as Zhou et al. discovered a positive association between SII and obesity (46). In addition, according to the results of our subgroup analysis, SII had a more significant effect on the VAT region in overweight and obese participants. And this effect further increased with increasing obesity.
Visceral adipose tissue and inflammation show a positive correlation, although the underlying processes remain unclear. An increasing amount of research indicates that metabolic dysregulation associated with obesity plays a crucial role in the pathogenesis of chronic inflammatory diseases (47). Elevated blood levels of the proinflammatory marker CRP are linked to excess fat mass in obese individuals (48). Weight-loss interventions reduce levels of proinflammatory proteins, including CRP and IL-6 (49). The expansion of VAT is significantly accelerated by obesity, and the shape and inflammation of VAT are altered by alterations in adipocytes, their constitutive substrates, and immune cells such as neutrophil infiltration (50, 51). Adipocyte metabolic homeostasis disturbances lead to local inflammation and the subsequent recruitment of large numbers of macrophages into adipose tissue (33). Insulin resistance and systemic inflammation are associated with this process (52). Lipid overload results in increased NF-kB expression and the production of inflammatory signals like IL-6 and IL-8, which makes hypertrophic adipocytes incapable of preserving the metabolic balance between lipolysis and lipid storage (53, 54). Large amounts of proinflammatory cytokines, such as interferon-γ, are released by activated immune cells during obesity, enhancing the proinflammatory microenvironment of adipose tissue (55). In addition, local adipocyte enlargement causes hypoxia and induces mature adipocytes to secrete pro-inflammatory mediators including cytokines and chemokines such as CCL5, PAI-1, IL-6, and microRNAs (56–59).
Our study contains a number of limitations. We were unable to establish a causal association between SII and VAT area because of the nature of the cross-sectional investigation. Furthermore, we were unable to maintain a sufficiently large sample size because of database constraints in the United States that prevented us from including data on all covariates that affect inflammation levels and cardiovascular health. The correlation between SII and VAT area, however, is currently stable enough that it is unlikely to be greatly impacted by factors that are not included.
5 Conclusion
In conclusion, SII and VAT area were found to positively correlate, with a larger correlation being seen in overweight and obese individuals. SII may be useful in medical applications to evaluate the severity of VAT area.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the NCHS Ethics Review Committee approved the portion of this study that involved human subjects, human material, or human data. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YL: Methodology, Software, Writing – original draft. KZ: Investigation, Methodology, Writing – review & editing. BL: Investigation, Writing – review & editing. SD: Investigation, Writing – review & editing. BW: Investigation, Writing – review & editing. LP: Conceptualization, Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We appreciate everyone who participated in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Collaboration NCDRF. Worldwide trends in body-mass index, underweight, overweight, and obesity from. To 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. (1975) 390:2627–42. doi: 10.1016/S0140-6736(17)32129-3
2. Hales, CM, Carroll, MD, Fryar, CD, and Ogden, CL. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief. (2020) 360:1–8.
3. Zhang, X, Ha, S, Lau, HC, and Yu, J. Excess body weight: novel insights into its roles in obesity comorbidities. Semin Cancer Biol. (2023) 92:16–27. doi: 10.1016/j.semcancer.2023.03.008
4. Xu, F, Earp, JE, Riebe, D, Delmonico, MJ, Lofgren, IE, and Greene, GW. The relationship between fat distribution and diabetes in US adults by race/ethnicity. Front Public Health. (2024) 12:1373544. doi: 10.3389/fpubh.2024.1373544
5. West, HW, Siddique, M, Williams, MC, Volpe, L, Desai, R, Lyasheva, M, et al. Deep-learning for epicardial adipose tissue assessment with computed tomography: implications for cardiovascular risk prediction. JACC Cardiovasc Imaging. (2023) 16:800–16. doi: 10.1016/j.jcmg.2022.11.018
6. Kwon, H, Kim, D, and Kim, JS. Body fat distribution and the risk of incident metabolic syndrome: a longitudinal cohort study. Sci Rep. (2017) 7:10955. doi: 10.1038/s41598-017-09723-y
7. Tao, M, Zhou, G, Liu, J, He, M, Wang, C, Luo, X, et al. Visceral adipose tissue and risk of nonalcoholic fatty liver disease: a Mendelian randomization study. Clin Endocrinol. (2023) 99:370–7. doi: 10.1111/cen.14953
8. Lu, Y, Tang, H, Huang, P, Wang, J, Deng, P, Li, Y, et al. Assessment of causal effects of visceral adipose tissue on risk of cancers: a Mendelian randomization study. Int J Epidemiol. (2022) 51:1204–18. doi: 10.1093/ije/dyac025
9. Karlsson, T, Rask-Andersen, M, Pan, G, Höglund, J, Wadelius, C, Ek, WE, et al. Contribution of genetics to visceral adiposity and its relation to cardiovascular and metabolic disease. Nat Med. (2019) 25:1390–5. doi: 10.1038/s41591-019-0563-7
10. Hu, B, Yang, XR, Xu, Y, Sun, YF, Sun, C, Guo, W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. (2014) 20:6212–22. doi: 10.1158/1078-0432.CCR-14-0442
11. Zhou, YX, Li, WC, Xia, SH, Xiang, T, Tang, C, Luo, JL, et al. Predictive value of the systemic immune inflammation index for adverse outcomes in patients with acute ischemic stroke. Front Neurol. (2022) 13:836595. doi: 10.3389/fneur.2022.836595
12. Yang, YL, Wu, CH, Hsu, PF, Chen, SC, Huang, SS, Chan, WL, et al. Systemic immune-inflammation index (SII) predicted clinical outcome in patients with coronary artery disease. Eur J Clin Investig. (2020) 50:e13230. doi: 10.1111/eci.13230
13. Xu, M, Chen, R, Liu, L, Liu, X, Hou, J, Liao, J, et al. Systemic immune-inflammation index and incident cardiovascular diseases among middle-aged and elderly Chinese adults: the Dongfeng-Tongji cohort study. Atherosclerosis. (2021) 323:20–9. doi: 10.1016/j.atherosclerosis.2021.02.012
14. Ma, R, Cui, L, Cai, J, Yang, N, Wang, Y, Chen, Q, et al. Association between systemic immune inflammation index, systemic inflammation response index and adult psoriasis: evidence from NHANES. Front Immunol. (2024) 15:1323174. doi: 10.3389/fimmu.2024.1323174
15. Ye, C, Yuan, L, Wu, K, Shen, B, and Zhu, C. Association between systemic immune-inflammation index and chronic obstructive pulmonary disease: a population-based study. BMC Pulm Med. (2023) 23:295. doi: 10.1186/s12890-023-02583-5
16. Luo, S, Liu, Z, Jiao, R, Li, W, Sun, J, Ma, S, et al. The associations of two novel inflammation indexes, systemic immune-inflammation index (SII) and system inflammation response index (SIRI), with periodontitis: evidence from NHANES 2009-2014. Clin Oral Investig. (2024) 28:129. doi: 10.1007/s00784-024-05529-1
17. Yang, X, and Wu, C. Systemic immune inflammation index and gastric cancer prognosis: a systematic review and meta-analysis. Exp Ther Med. (2024) 27:122. doi: 10.3892/etm.2024.12410
18. Chen, D, Qin, H, Deng, G, Wang, Q, Wang, H, and Liu, X. Pre-radiotherapy systemic immune inflammation index associated with overall survival in patients with advanced EGFR mutant non-small cell lung cancer receiving thoracic radiotherapy. Clin Transl Oncol. (2023) 25:226–35. doi: 10.1007/s12094-022-02936-2
19. Menyhart, O, Fekete, JT, and Győrffy, B. Inflammation and colorectal cancer: a meta-analysis of the prognostic significance of the systemic immune-inflammation index (SII) and the systemic inflammation response index (SIRI). Int J Mol Sci. (2024) 25:25. doi: 10.3390/ijms25158441
20. Yang, J, Zheng, J, Qiu, J, Zhang, M, Liu, L, Wang, Z, et al. Systemic immune-inflammatory index, tumor-infiltrating lymphocytes, and clinical outcomes in esophageal squamous cell carcinoma receiving concurrent chemoradiotherapy. J Immunol Res. (2023) 2023:4275998–12. doi: 10.1155/2023/4275998
21. Li, M, Li, Z, Wang, Z, Yue, C, Hu, W, and Lu, H. Prognostic value of systemic immune-inflammation index in patients with pancreatic cancer: a meta-analysis. Clin Exp Med. (2022) 22:637–46. doi: 10.1007/s10238-021-00785-x
22. Xia, Y, Xia, C, Wu, L, Li, Z, Li, H, and Zhang, J. Systemic immune inflammation index (SII), system inflammation response index (SIRI) and risk of all-cause mortality and cardiovascular mortality: a 20-year follow-up cohort study of 42,875 US adults. J Clin Med. (2023) 12:12. doi: 10.3390/jcm12031128
23. Xie, R, Xiao, M, Li, L, Ma, N, Liu, M, Huang, X, et al. Association between SII and hepatic steatosis and liver fibrosis: a population-based study. Front Immunol. (2022) 13:925690. doi: 10.3389/fimmu.2022.925690
24. Liu, B, Wang, J, Li, YY, Li, KP, and Zhang, Q. The association between systemic immune-inflammation index and rheumatoid arthritis: evidence from NHANES 1999-2018. Arthritis Res Ther. (2023) 25:34. doi: 10.1186/s13075-023-03018-6
25. Di, X, Liu, S, Xiang, L, and Jin, X. Association between the systemic immune-inflammation index and kidney stone: a cross-sectional study of NHANES 2007-2018. Front Immunol. (2023) 14:1116224. doi: 10.3389/fimmu.2023.1116224
26. Schlecht, I, Fischer, B, Behrens, G, and Leitzmann, MF. Relations of visceral and abdominal subcutaneous adipose tissue, body mass index, and waist circumference to serum concentrations of parameters of chronic inflammation. Obes Facts. (2016) 9:144–57. doi: 10.1159/000443691
27. Zhang, Y, Xie, R, and Ou, J. A U-shaped association between serum albumin with total triiodothyronine in adults. J Clin Lab Anal. (2022) 36:e24473. doi: 10.1002/jcla.24473
28. Xie, R, Liu, Y, Wang, J, Zhang, C, Xiao, M, Liu, M, et al. Race and gender differences in the associations between cadmium exposure and bone mineral density in US adults. Biol Trace Elem Res. (2023) 201:4254–61. doi: 10.1007/s12011-022-03521-y
29. Tang, Y, Peng, B, Liu, J, Liu, Z, Xia, Y, and Geng, B. Systemic immune-inflammation index and bone mineral density in postmenopausal women: a cross-sectional study of the national health and nutrition examination survey (NHANES) 2007-2018. Front Immunol. (2022) 13:975400. doi: 10.3389/fimmu.2022.975400
30. Weir, CB, and Jan, A. BMI classification percentile and cut off points. Treasure Island, FL: StatPearls (2024).
31. Valcarcel-Ares, MN, Tucsek, Z, Kiss, T, Giles, CB, Tarantini, S, Yabluchanskiy, A, et al. Obesity in aging exacerbates neuroinflammation, dysregulating synaptic function-related genes and altering eicosanoid synthesis in the mouse Hippocampus: potential role in impaired synaptic plasticity and cognitive decline. J Gerontol A Biol Sci Med Sci. (2019) 74:290–8. doi: 10.1093/gerona/gly127
32. Cox, AJ, West, NP, and Cripps, AW. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. (2015) 3:207–15. doi: 10.1016/S2213-8587(14)70134-2
33. Weisberg, SP, McCann, D, Desai, M, Rosenbaum, M, Leibel, RL, and Ferrante, AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. (2003) 112:1796–808. doi: 10.1172/JCI19246
34. Mohamed-Ali, V, Pinkney, JH, and Coppack, SW. Adipose tissue as an endocrine and paracrine organ. Int J Obes Relat Metab Disord. (1998) 22:1145–58. doi: 10.1038/sj.ijo.0800770
35. Park, JS, Cho, MH, Nam, JS, Ahn, CW, Cha, BS, Lee, EJ, et al. Visceral adiposity and leptin are independently associated with C-reactive protein in Korean type 2 diabetic patients. Acta Diabetol. (2010) 47:113–8. doi: 10.1007/s00592-009-0125-4
36. Boronat-Toscano, A, Monfort-Ferré, D, Menacho, M, Caro, A, Bosch, R, Espina, B, et al. Anti-TNF therapies suppress adipose tissue inflammation in Crohn's disease. Int J Mol Sci. (2022) 23:23. doi: 10.3390/ijms231911170
37. Lozano, CP, Wilkens, LR, Shvetsov, YB, Maskarinec, G, Park, SY, Shepherd, JA, et al. Associations of the dietary inflammatory index with total adiposity and ectopic fat through the gut microbiota, LPS, and C-reactive protein in the multiethnic cohort-adiposity phenotype study. Am J Clin Nutr. (2022) 115:1344–56. doi: 10.1093/ajcn/nqab398
38. Tekesin, A, and Tunç, A. Inflammatory markers are beneficial in the early stages of cerebral venous thrombosis. Arq Neuropsiquiatr. (2019) 77:101–5. doi: 10.1590/0004-282X20190001
39. Azab, B, Zaher, M, Weiserbs, KF, Torbey, E, Lacossiere, K, Gaddam, S, et al. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am J Cardiol. (2010) 106:470–6. doi: 10.1016/j.amjcard.2010.03.062
40. Zhao, Y, Shao, W, Zhu, Q, Zhang, R, Sun, T, Wang, B, et al. Association between systemic immune-inflammation index and metabolic syndrome and its components: results from the National Health and nutrition examination survey 2011-2016. J Transl Med. (2023) 21:691. doi: 10.1186/s12967-023-04491-y
41. Zhao, E, Cheng, Y, Yu, C, Li, H, and Fan, X. The systemic immune-inflammation index was non-linear associated with all-cause mortality in individuals with nonalcoholic fatty liver disease. Ann Med. (2023) 55:2197652. doi: 10.1080/07853890.2023.2197652
42. Guo, W, Song, Y, Sun, Y, Du, H, Cai, Y, You, Q, et al. Systemic immune-inflammation index is associated with diabetic kidney disease in type 2 diabetes mellitus patients: evidence from NHANES 2011-2018. Front Endocrinol. (2022) 13:1071465. doi: 10.3389/fendo.2022.1071465
43. Mahemuti, N, Jing, X, Zhang, N, Liu, C, Li, C, Cui, Z, et al. Association between systemic immunity-inflammation index and hyperlipidemia: a population-based study from the NHANES (2015-2020). Nutrients. (2023) 15:1177. doi: 10.3390/nu15051177
44. Lee, JH, Hyung, S, Lee, J, and Choi, SH. Visceral adiposity and systemic inflammation in the obesity paradox in patients with unresectable or metastatic melanoma undergoing immune checkpoint inhibitor therapy: a retrospective cohort study. J Immunother Cancer. (2022) 10:e005226. doi: 10.1136/jitc-2022-005226
45. Qiu, L, Ren, Y, Li, J, Li, M, Li, W, Qin, L, et al. Association of systemic immune inflammatory index with obesity and abdominal obesity: a cross-sectional study from NHANES. Nutr Metab Cardiovasc Dis. (2024) 34:2409–19. doi: 10.1016/j.numecd.2024.06.003
46. Zhou, Y, Wang, Y, Wu, T, Zhang, A, and Li, Y. Association between obesity and systemic immune inflammation index, systemic inflammation response index among US adults: a population-based analysis. Lipids Health Dis. (2024) 23:245. doi: 10.1186/s12944-024-02240-8
47. Hotamisligil, GS. Inflammation and metabolic disorders. Nature. (2006) 444:860–7. doi: 10.1038/nature05485
48. Visser, M, Bouter, LM, McQuillan, GM, Wener, MH, and Harris, TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. (1999) 282:2131–5. doi: 10.1001/jama.282.22.2131
49. Esposito, K, Pontillo, A, Di Palo, C, Giugliano, G, Masella, M, Marfella, R, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. (2003) 289:1799–804. doi: 10.1001/jama.289.14.1799
50. Revelo, XS, Luck, H, Winer, S, and Winer, DA. Morphological and inflammatory changes in visceral adipose tissue during obesity. Endocr Pathol. (2014) 25:93–101. doi: 10.1007/s12022-013-9288-1
51. Uribe-Querol, E, and Rosales, C. Neutrophils actively contribute to obesity-associated inflammation and pathological complications. Cells. (2022) 11:11. doi: 10.3390/cells11121883
52. Xu, H, Barnes, GT, Yang, Q, Tan, G, Yang, D, Chou, CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. (2003) 112:1821–30. doi: 10.1172/JCI19451
53. Stenkula, KG, and Erlanson-Albertsson, C. Adipose cell size: importance in health and disease. Am J Physiol Regul Integr Comp Physiol. (2018) 315:R284–95. doi: 10.1152/ajpregu.00257.2017
54. Lemmer, IL, Willemsen, N, Hilal, N, and Bartelt, A. A guide to understanding endoplasmic reticulum stress in metabolic disorders. Mol Metab. (2021) 47:101169. doi: 10.1016/j.molmet.2021.101169
55. Winer, S, Chan, Y, Paltser, G, Truong, D, Tsui, H, Bahrami, J, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. (2009) 15:921–9. doi: 10.1038/nm.2001
56. Mori, MA, Ludwig, RG, Garcia-Martin, R, Brandão, BB, and Kahn, CR. Extracellular miRNAs: from biomarkers to mediators of physiology and disease. Cell Metab. (2019) 30:656–73. doi: 10.1016/j.cmet.2019.07.011
57. Skurk, T, Mack, I, Kempf, K, Kolb, H, Hauner, H, and Herder, C. Expression and secretion of RANTES (CCL5) in human adipocytes in response to immunological stimuli and hypoxia. Horm Metab Res. (2009) 41:183–9. doi: 10.1055/s-0028-1093345
58. Hosogai, N, Fukuhara, A, Oshima, K, Miyata, Y, Tanaka, S, Segawa, K, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. (2007) 56:901–11. doi: 10.2337/db06-0911
Keywords: systemic immune-inflammation index, visceral adipose tissue area, obesity, cross-sectional study, NHANES
Citation: Liao Y, Zhou K, Lin B, Deng S, Weng B and Pan L (2025) Associations between systemic immune-inflammatory index and visceral adipose tissue area: results of a national survey. Front. Nutr. 11:1517186. doi: 10.3389/fnut.2024.1517186
Edited by:
Juliana Lauar Gonçalves, Centro Universitário Una, BrazilReviewed by:
Jianping Wu, Southeast University, ChinaSolange S. Pereira, Universidade Federal de Viçosa, Brazil
Copyright © 2025 Liao, Zhou, Lin, Deng, Weng and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liya Pan, UGFubHlhQDEyNi5jb20=
†These authors have contributed equally to this work
 Yayun Liao†
Yayun Liao† Kejian Zhou
Kejian Zhou Baoquan Lin
Baoquan Lin Liya Pan
Liya Pan