- 1Medical College of Jinzhou Medical University, Jinzhou, Liaoning, China
- 2Medical College of Yangzhou University, Yangzhou, Jiangsu, China
- 3Medical College of Nantong University, Nantong, Jiangsu, China
- 4Medical College of Shaanxi University of Chinese Medicine, Xianyang, Shanxi, China
- 5Department of Urology, The First Affiliated Hospital of Jinzhou Medical University, Jinzhou, China
- 6Department of Urology, The Third Affiliated Hospital of Jinzhou Medical University, Jinzhou, Liaoning, China
Background: Urinary incontinence (UI), particularly urge urinary incontinence (UUI), is a prevalent condition that worsens with age and negatively affects quality of life. Antioxidants, measured by the composite dietary antioxidant index (CDAI), have been linked to inflammation and other diseases, but their relationship with UUI remains uncertain. The purpose of this study is to investigate the relationship between UUI prevalence and CDAI.
Materials and methods: Data for this cross-sectional study were obtained from the National Health and Nutrition Examination Survey’s four cycles (2011–2018). The odds ratio (OR) and 95% confidence interval (95% CI) of the relationship between CDAI and male UUI were ascertained by the use of weighted univariate analysis, multivariate logistic regression, restricted cubic spline regression, and subgroup analysis. PSM and sensitivity analyses were performed to assess the robustness of the findings.
Results: A total of 7,735 participants took part in this study. After adjusting for potential confounders, CDAI was found to be negatively associated with the prevalence of UUI in those with lower CDAI (about half overall). This relationship lost significance in populations with higher CDAI. The negative correlation between zinc and the prevalence of UUI was more significant in populations with low antioxidant diets. The results remained consistent, with subgroup analyses finding a significant interaction effect for race only after PSM (p = 0.043), with no significant interaction effect observed for the rest.
Conclusion: This study showed a negative correlation between CDAI and UUI incidence in the group of men with low CDAI levels (about half of the population). Thus, effective prevention or treatment of UUI requires dietary changes aimed at the male population with poor antioxidant diets.
1 Introduction
Urinary incontinence (UI) is a common disorder in the population, usually increasing in prevalence with age (1). The unintentional loss of pee during urine storage is classified as urinary incontinence (2). Senior age, High BMI, menopause, and childbearing have all been linked to an increased risk of UI, according to previous research (3–6). Stress UI (SUI), Urge UI (UUI), and mixed UI (MUI) are the three most prevalent forms of UI (7). The ageing of the world’s population is making UI a serious public health issue.
The sudden, overwhelming urge to urinate followed right away by involuntary incontinence is known as urge urinary incontinence (UUI) (8). According to estimates, UUI affects a significant percentage of people in the US population—2.6 to 20.9% of males and 9.3 to 30.8% of women—and its incidence rises sharply with age (9, 10). UUI can be a debilitating illness that causes significant deficits in self-confidence and psychological well-being in addition to a reduction in social contacts and interpersonal connections (11). These symptoms have a substantial negative influence on the quality of life and frequently call for behavioural, pharmaceutical, or surgical therapies.
Diet as a part of behavioural therapy is now being considered a promising treatment for many diseases (12–15). The composite dietary antioxidant index (CDAI) is based on a range of dietary vitamins and minerals with antioxidant properties, including carotenoids, zinc, selenium, and vitamins C, E, and A. This summary score is used to assess a person’s dietary total antioxidant capacity (TAC) (16, 17). Recent research indicates a link between CDAI and inflammatory biomarkers (18). It is widely recognized by scholars that considering a composite diet is more representative of actual daily nutrient intake in humans, rather than focusing on individual nutrients (19). Therefore, this study explores the relationship between the combination of the six trace nutrients mentioned above and UUI.
Numerous investigations have now demonstrated the connection between illnesses and CDAI (20–24). It’s still unclear, though, how CDAI and UUI are related. Previous studies have focused on the relationship between inflammation-related indices and female UUI, but there is relatively little research on inflammation and UUI in men. The National Health and Nutrition Examination Survey (NHANES) 2011–2018 data are used in this cross-sectional study to investigate any possible relationship between CDAI (and its components) and UUI prevalence in men. We also examined the potential pathways, and our findings should serve as a foundation for an exogenous antioxidant diet that prevents UUI.
2 Materials and methods
2.1 Information sources consulted for this study
One of the most important programs of the National Center for Health Statistics (NCHS) is the NHANES. Data that is indicative of the nation on the general health of the US population has been collected (25). With the use of a sophisticated multistage probability methodology, this program produces a nationally representative sample of non-institutionalized Americans (26). Since 1999, it has used a sophisticated, stratified, multi-stage probability sampling method to gather data from about five thousand people each year (27). IN addition, its survey cycle lasts 2 years. All procedures were approved by the NCHS Research Ethics Committee, and each participant gave their informed permission (28). Data from questionnaires, laboratory test indicators, physical examination characteristics, and sociodemographic status were also collected. There are extensive details about the program on the website.1
2.2 Study population in this investigation
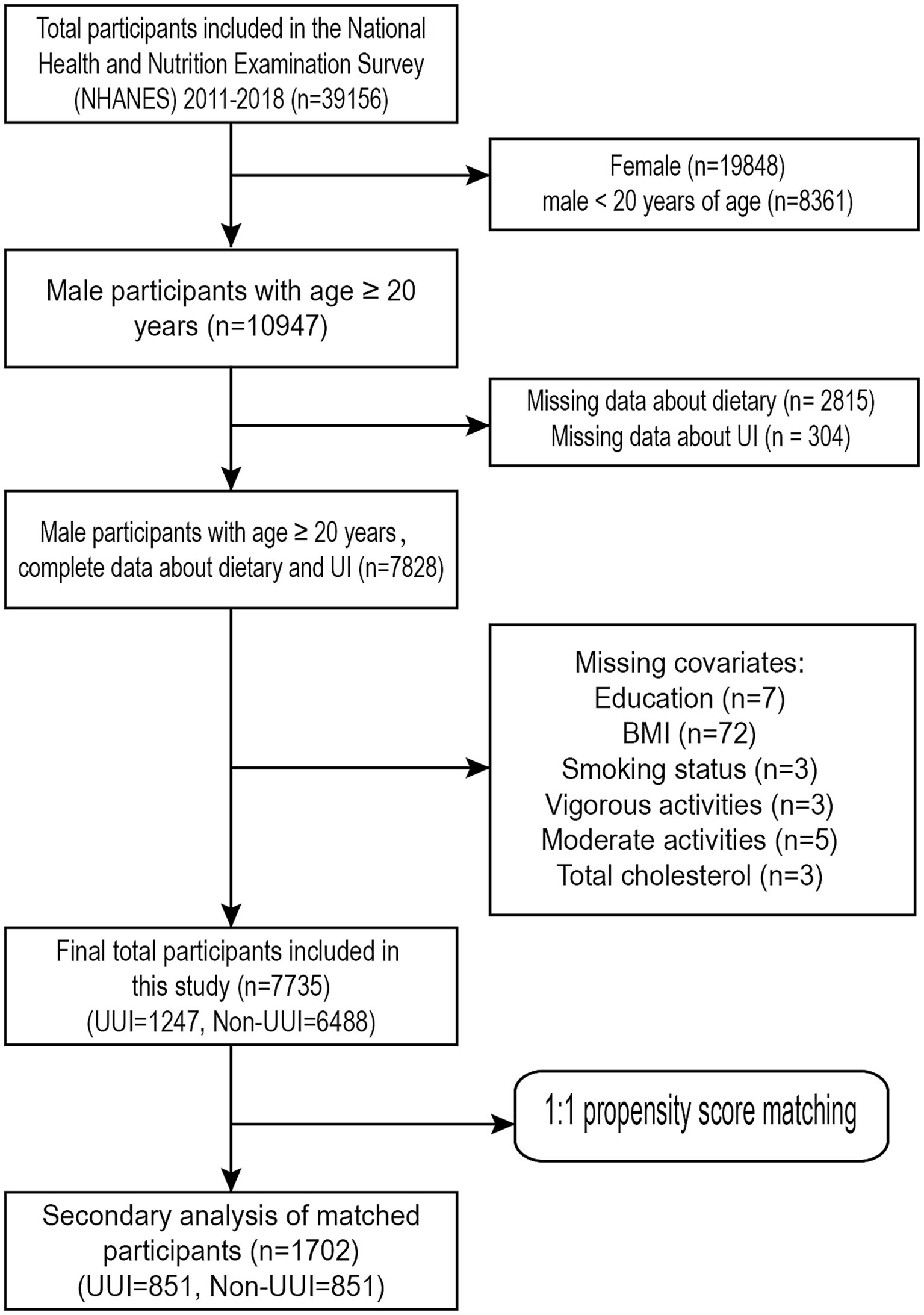
This study analysed data from four NHANES cycles, spanning from 2011 to 2018. The survey was completed by 39,156 people in total (Representing an actual population of 103,877,855). Firstly, we excluded females (n = 19,848) and males under 20 years of age (n = 8,361). The subsequent exclusion criteria were as follows: (1) information about dietary (n = 2,815) is unknown; (2) interviewees who had not finished the UI survey (n = 304); (3) data about education status (n = 7) is unknown; (4) body mass index (n = 72) is unknown; (5) data about smoking status (n = 3) is unknown; (6) information about vigorous activities (n = 3) is unknown; (7) information about moderate activities (n = 5) is unknown; and (8) Total cholesterol (n = 3) is unknown. Finally, the study comprised 7,735 individuals in total after screening procedures, including 1,247 men with UUI and the remaining without UUI. Figure 1 shows the screening procedures.
2.3 Measurement of CDAI
The 24 h dietary recall interview is the current section of the NHANES nutritional evaluation. Dietary interviewers with training who are fluent in both Spanish and English perform dietary recall interviews in person. Through discontinuous two-day, 24 h dietary recall interviews, the NHANES gathered data on participants’ food intake. A mobile examination center (MEC) served as the venue for the initial interview. Every mobile examination center had a nutritional interview room with a common set of measurement guidelines. The second dietary recall interview took place over the phone three to ten days later (29, 30).
We used the average of two measurements to minimize bias and increase the reliability of the results. A modified version developed by previous researchers was utilized to calculate each participant’s CDAI (31). Zinc, selenium, carotenoids, and vitamins A, C, and E are the six dietary antioxidants that make up CDAI. We standardized each micronutrient by subtracting the mean of the six dietary vitamins and minerals and dividing by the total standard deviation to calculate a Z-score. Their Z-scores were then summed to obtain the composite value of CDAI. The following is the calculating formula:
2.4 Evaluation of UI
In MECs (Mobile Examination Centres), only those who were ≥ 20 years old answered questions on urine incontinence. “During the past 12 months, have you leaked or lost control of even a small amount of urine with activity like coughing, lifting, or exercise?” asked participants, if they selected “yes.” grouped under “stress UI.” A positive response to the question, “During the past 12 months, have you leaked or lost control of even a small amount of urine with an urge or pressure to urinate and you could not get to the toilet fast enough?” formed the basis for the definition of “urgency UI.” If a participant answered “yes” to both the stress and urgency UI questions, they were categorized as mixed UI participants.
2.5 Evaluation of covariate
The following potential confounding variables were selected for this investigation based on prior research findings that may affect UI results. These variables included demographics, medical history, physical examination findings, and personal circumstances (6, 8, 32–35). Race, age, education, PIR, smoking status, alcohol use, diabetes, hypertension, total cholesterol levels, and exercise status were all considered categorical factors.
We then grouped these covariates. Two groups were formed from the participants according to their age, which was fifty. The age range of the first group was under 50, whereas the age range of the second group was over or equal to 50. Three categories—below high school diploma, high school diploma, and above high school diploma—were used to classify people’s educational positions. PIR < 2 was classified as low PIR, and PIR ≥ 2 as high PIR. We divided BMI into three groups: the first group was less than 25, the second group was greater than or equal to 25 and less than 30, and the third group was less than 30. When asked if they smoked, participants were divided as smokers if they replied “yes” (SMQ020). Answer the query “In the past 12 months, on those days that you drank alcoholic beverages, on average, how many drinks did you have?” to distinguish between drinkers and non-drinkers. Those who had less than 12 drinks were categorized as non-drinkers, while those who had more than or equal to 12 drinks were considered drinkers.
Those who responded “yes” to the inquiry “Have you been told you have hypertension?,” those who were taking antihypertensive medication, and those whose average of three measurements of systolic blood pressure (BP) was ≥140 mmHg and whose average of three measurements of diastolic blood pressure (BP) was ≥90 mmHg were all considered patients with hypertension. Participants were considered to have diabetes if they answered “yes” when asked if they had diabetes or if they used glucose-lowering drugs or insulin. Meanwhile, their fasting blood glucose (≥126 mg/dL) and glycosylated haemoglobin (≥6.5%) were used to diagnose diabetes. Total cholesterol <240 mg/dL was defined as a low cholesterol level and ≥ 240 mg/dL was defined as a high cholesterol level.
2.6 Statistical analysis
During the processing phase, NHANES sample weights were applied to guarantee the study population’s national representation. The baseline qualities were explained after the participants were classified according to the four CDAI categories. Categorical variables, expressed as weighted percentages (%), were compared using a chi-square test. Continuous variables were compared using weighted linear regression, and the results were shown as mean (± standard deviation).
To reduce confounding bias, we performed PSM processing based on UUI results. To ensure that the distribution of covariates was comparable between the UUI and non-UUI groups, we matched for the following factors: age, smoking, alcohol consumption, diabetes, hypertension, and total cholesterol (36–40). Propensity score matching (PSM) was carried out 1:1 using the R Software “MatchIt” program. The sample population was re-examined after PSM in order to validate the findings.
To determine whether there were any noteworthy trends or differences, a univariate analysis was performed. The study then developed three adjustment models in order to use multivariate regression analysis to examine the relationships between CDAI and UUI. Model 1 was the baseline model that did not alter any covariate variables. Race, age, education, and PIR were added to model 2. Model 3 included BMI, diabetes, hypertension, smoking, alcohol consumption, total cholesterol level, moderate activity, and vigorous activity on the basis of Model 2. A generalized additive model (GAM) was then developed to validate the dose–response relationship. A threshold effect analysis was first performed. Then, restricted cubic spline (RCS) and smooth curve fitting were used to describe the dose–response relationship between CDAI and UUI. The study used RCS to investigate whether there was a nonlinear association between CDAI and UUI, with the node set to 3. Smooth curve fitting was performed under the fully modified model. After obtaining the threshold value, the distribution of CDAI in the population was made into a violin plot for the next analysis.
We then categorized the population according to the thresholds and then regressed the CDAI and its components on the UUI separately to explore whether certain elements had a greater effect. Then, using subgroup analysis, the stratified association between CDAI and UUI was examined. Additionally, interaction tests were performed to assess the way in which relationships between subgroups interacted.
For all statistical studies, R (version 4.4.0) was utilized. When the two-sided p-value was less than 0.05, it was deemed statistically significant.
3 Results
3.1 Population characteristics
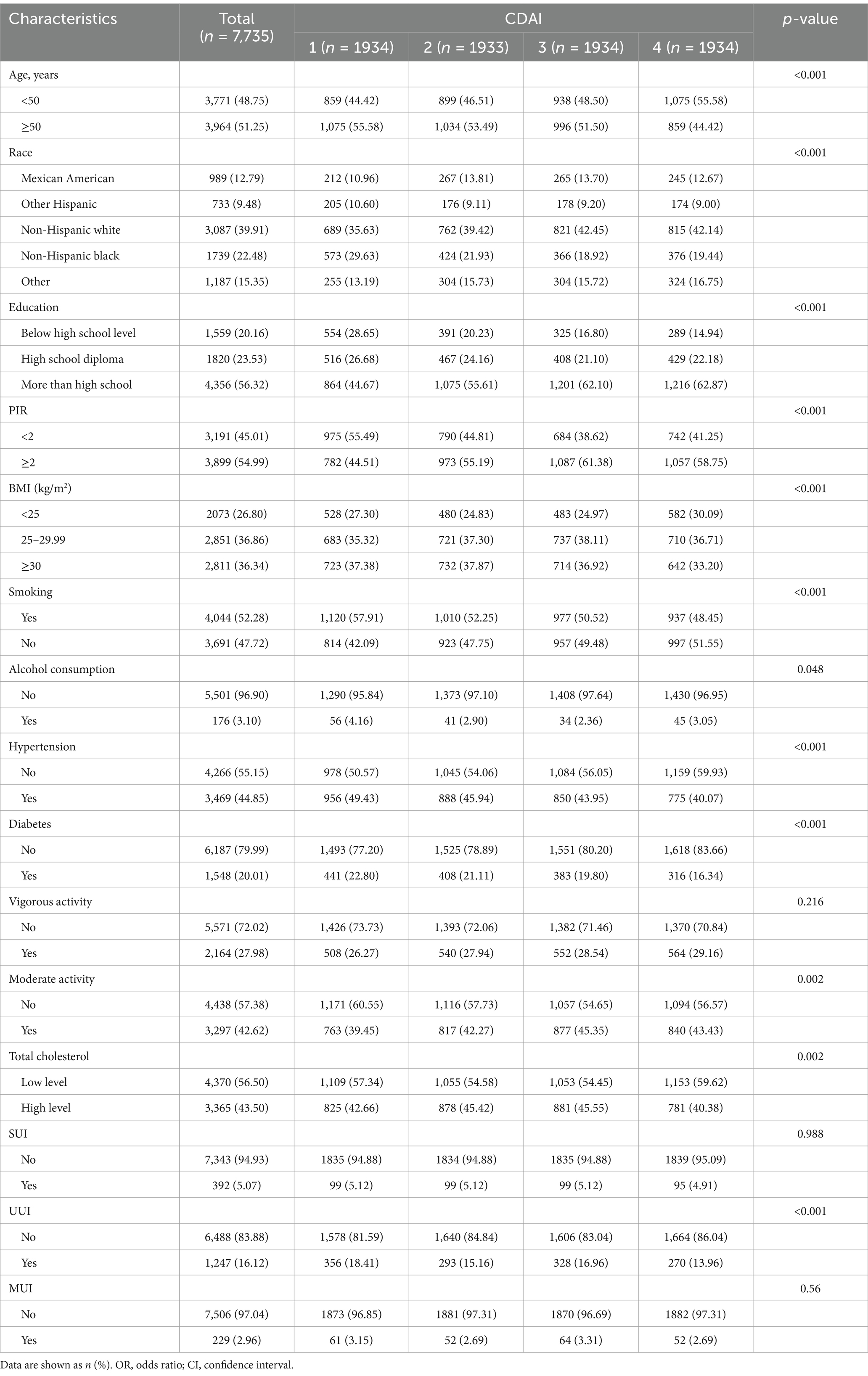
Based on the screening criteria, a total of 7,735 eligible participants from NHANES 2011–2018 were selected (Figure 1). Among them, 1,247 had UUI and 6,488 did not. The weighted estimates for the baseline characteristics of the study population are shown in Table 1. Study participants with a higher CDAI were found to be more heavily represented under the age of 50. In addition, those with higher levels of CDAI were more likely to be non-Hispanic white, more educated, have a higher PIR, lower smoking rates, lower alcohol consumption rates, lower body mass index, moderate exercise intensity, lower probability of having a history of diabetes and hypertension, and lower total cholesterol levels than those with lower levels of CDAI.
We found that differences in CDAI levels differed significantly in the presence or absence of UUI and that participants with UUI had lower CDAI levels. However, no significant CDAI level differences were observed in SUI and MUI.
Afterwards, the CDAI distribution was analysed and visualized as a violin plot (Figure 2). Many people have CDAI values clustered in the lower range, even less than 0 in a large proportion, while the number of people with higher antioxidant indices is smaller (median CDAI: 0.776).
We then balanced the effect of potential confounders associated with UUI through propensity score matching (PSM) analysis. In this investigation, a 1:1 PSM analysis was conducted. The standardized mean difference is visualized in Supplementary Figure S1, and the distributional balance plot of PSM is shown in Supplementary Figure S2. Following PSM, 1702 participants were enrolled in the study; 851 of them had UUI, while the remaining individuals did not (Figure 1). The weighted essential attributes of the research subjects were displayed in Supplementary Table S1 following PSM. Differences in variables between the two groups were managed to some degree. After PSM, age, race, education, PIR, hypertension, total cholesterol, vigorous activity, and UUI were found to be significantly associated with CDAI.
3.2 Univariate analysis of UUI
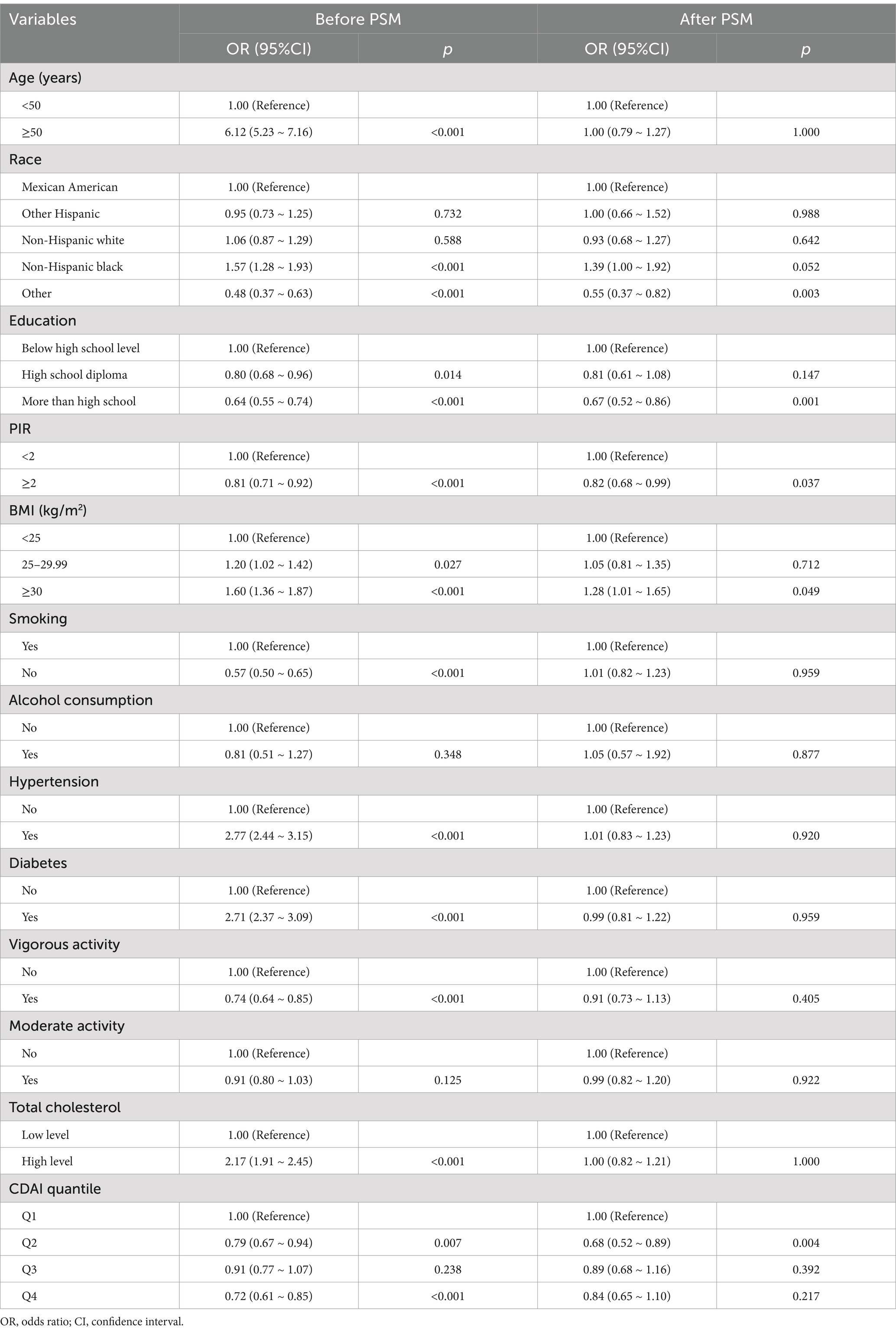
Preliminary exploration of pre- and post-PSM data with a univariate analysis of UUI. We found the following: prior to PSM, UUI was positively associated with age ≥ 50 years, body mass index ≥25, non-Hispanic black, hypertension, diabetes mellitus, and high cholesterol. In addition, UUI was negatively associated with other races, high school and higher education, high PIR, nonsmokers, vigorous activity, and Q2 and Q4 in the CDAI quartiles. After PSM, UUI was not associated with covariates such as age, high school education, 25 ≤ BMI < 30, smoking, hypertension, diabetes mellitus, level of exercise, and cholesterol level.
After PSM, only BMI ≥ 30 was positively associated with UUI. In contrast, other races, higher than high school education level, high PIR, and Q2 in CDAI were negatively associated with UUI (Table 2). We found that Q2 in CDAI was negatively correlated with UUI both before and after PSM (p = 0.007 and p = 0.004).
3.3 The relationships between CDAI and UUI
To further explore the relationship between CDAI and UUI, a weighted logistic regression analysis of the three models was conducted (Figure 3). The CDAI was converted (Q1-4; Q1 was used as a reference point) and examined by quaternity. In Model 1, no variables have been added. In Model 2, race, Age, education, and PIR adjustments were made. Model 3 was built using Model 2, modified to account for BMI, smoking, alcohol consumption, hypertensive disease, diabetes, total cholesterol, moderate activity, and vigorous activity.
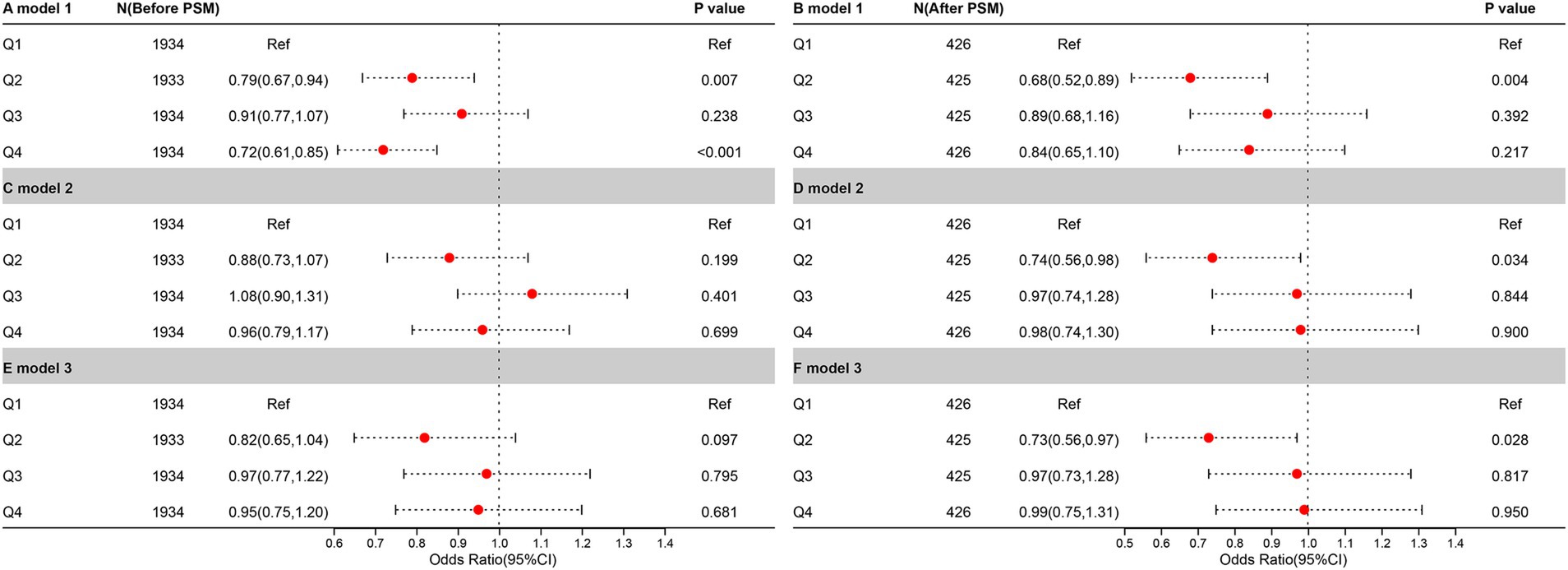
Figure 3. The relationship between UUI prevalence and CDAI before and after PSM. It is denoted by the letters “A,” “C,” and “E,” before PSM. Meanwhile, it is denoted by the letters “B,” “D,” and “F,” after PSM.
In the pre-PSM analysis, only Q2 (p = 0.007) and Q4 (p < 0.001) were negatively correlated with UUI in the crude model, and this relationship was lost in the other models. In the analysis following the PSM, a negative correlation between Q2 and UUI was found in all three models (p = 0.004 in the crude model, p = 0.034 in model 2, and p = 0.028 in model 3).
3.4 Connectivity between CDAI and UUI in terms of dose–response
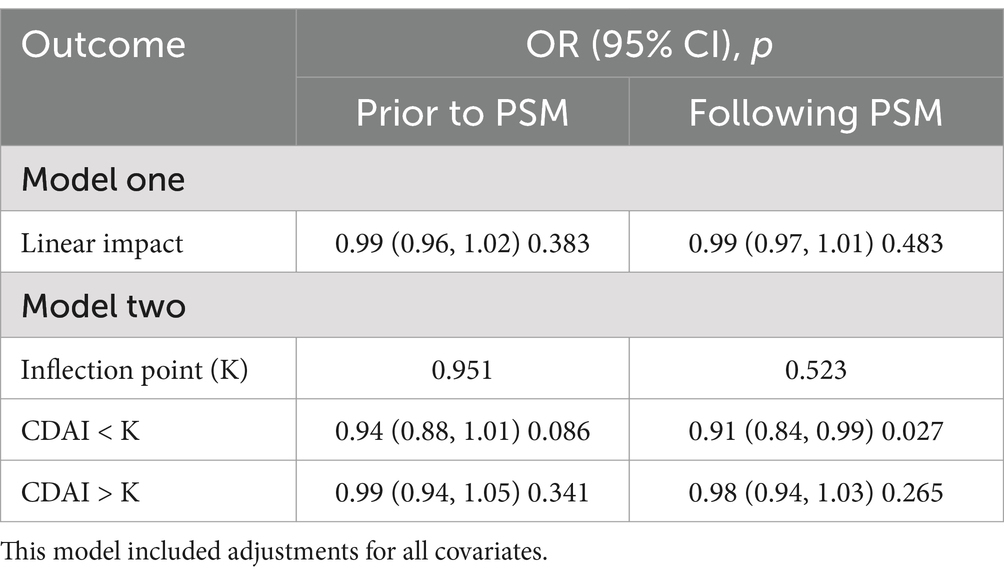
In the threshold effect analysis, we found no linear relationship between CDAI and UUI either before or after PSM (p = 0.383 and p = 0.483). Moreover, the negative correlation between CDAI greater than or less than the K value and UUI was not significant before PSM (p = 0.086 and p = 0.341). After PSM, CDAI was negatively correlated with UUI when CDAI was <K value (p = 0.027), and the relationship was not significant when it was > K value (Table 3).
Figure 4 displays the results of the dose–response relationship for the Restricted Cubic Spline (RCS) (node setting of 4 before and after PSM). Under the fully adjusted model, CDAI and UUI prevalence showed a nonlinear relationship before and after PSM (nonlinear p of 0.010 before PSM, nonlinear p of 0.007 after PSM). At OR = 1, the reference values for CDAI before and after PSM were 0.776 and 0.523, respectively. The overall relationship between CDAI and UUI was significant (p for overall = 0.024 before PSM and p for overall = 0.018 after PSM).
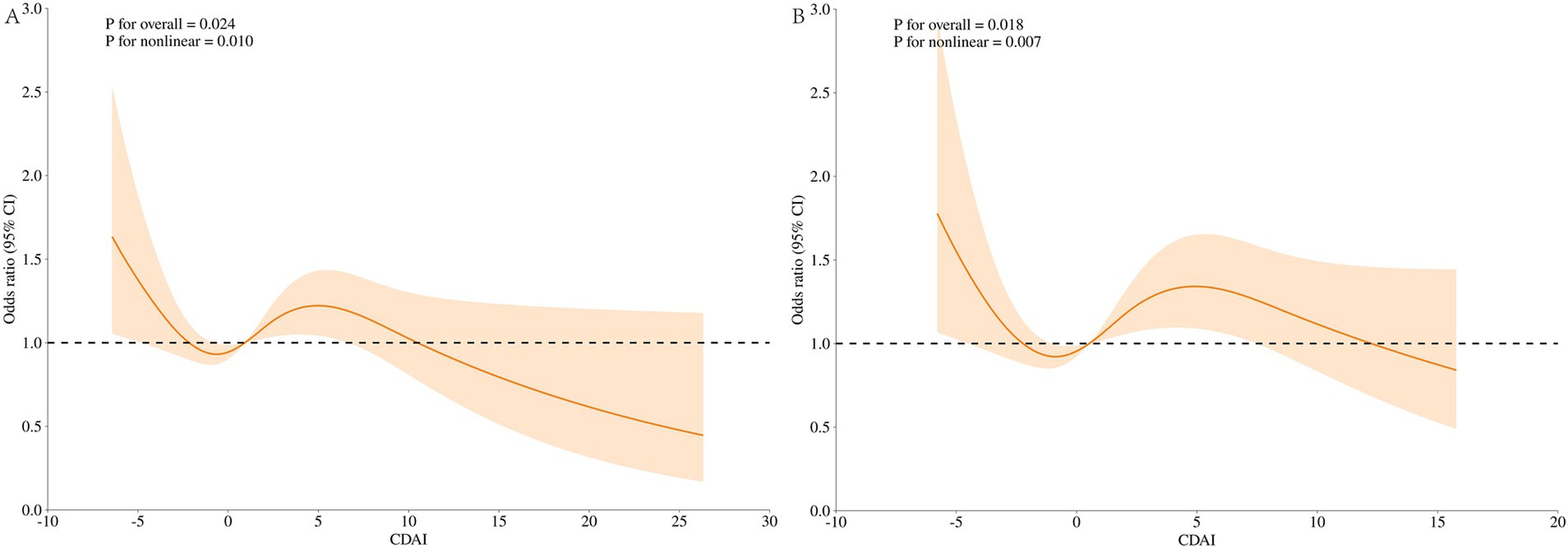
Figure 4. Dose–response analysis of UUI incidence (RCS). (A) Whole modified model, before PSM; (B) Whole modified model, after PSM. Terminologies: RCS, restricted cubic splines.
3.5 Segmented regression analysis and sensitivity analysis
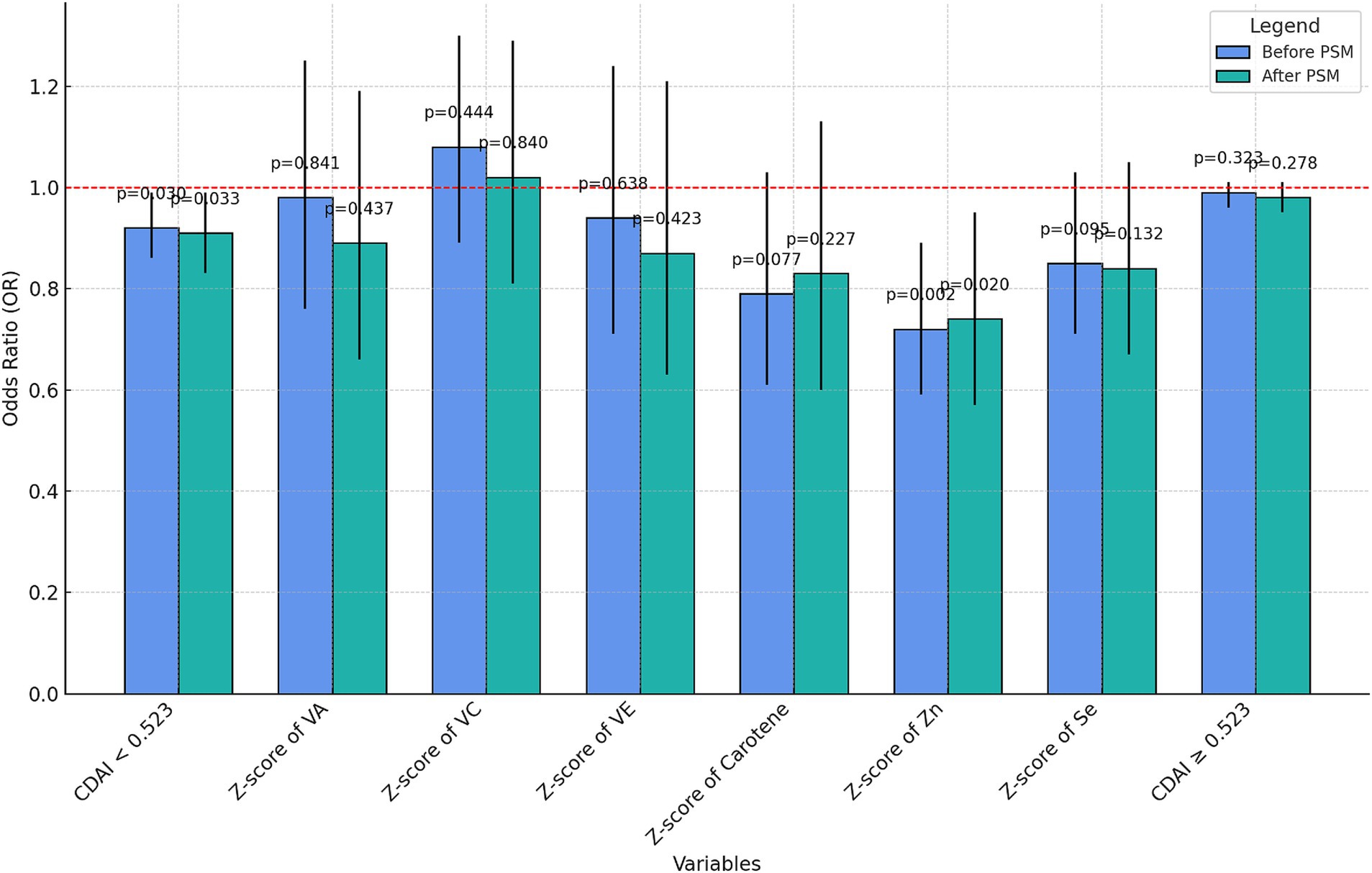
The K value after PSM (K = 0.523) was used to distinguish the two groups of people and the multiple regression analysis of CDAI and UUI was performed before and after PSM, respectively. The nutrient elements of each component in the CDAI<0.523 group were subjected to sensitivity analysis (Supplementary Table S2) and visualized (Figure 5).
The negative correlation with UUI was not significant for CDAI ≥0.523 either before or after PSM (p = 0.323 before PSM, p = 0.278 after PSM). However, at a CDAI less than 0.523, the CDAI was negatively correlated with UUI incidence both before and after PSM (OR = 0.92, p = 0.03 before PSM and OR = 0.91, p = 0.033 after PSM). Moreover, among the components, only the z-score of zinc showed a relatively significant negative correlation with UUI (OR = 0.72, p = 0.002 before PSM and OR = 0.74, p = 0.02 after PSM), and no significant correlation was found between the rest of the components and the incidence of UUI.
To further explore the relationship between CDAI and zinc with UUI, we performed RCS plots for CDAI and zinc, respectively, under the fully adjusted model with the node setting of 3 before PSM (Figure 6). Neither the CDAI nor the z score of Zinc showed a nonlinear relationship with UUI (CDAI, nonlinear p of 0.754, Zinc, nonlinear p of 0.262). At OR = 1, the reference values were − 1.672 and − 0.287, respectively. The overall p-value for the RCS plotted for the relationship between CDAI and UUI was 0.042.
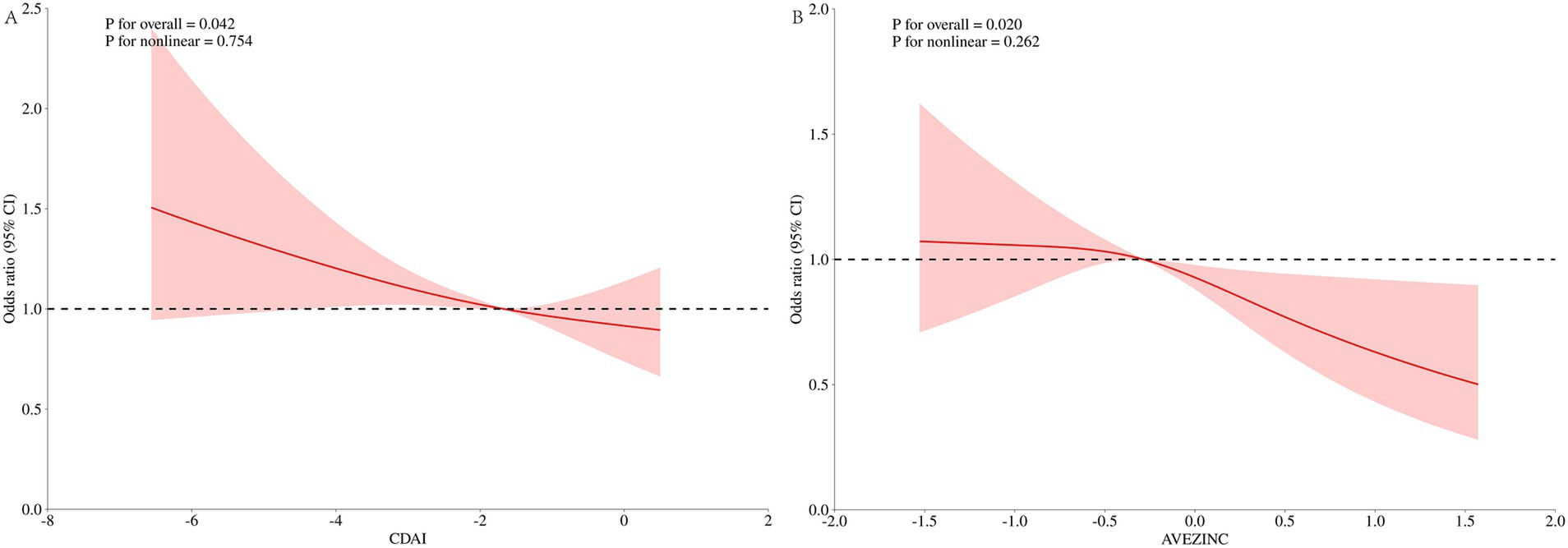
Figure 6. Dose–response analysis of UUI incidence (RCS). (A) Whole modified model, CDAI; (B) whole modified model, Zinc. Terminologies: RCS, restricted cubic splines.
3.6 Subgroup analysis between CDAI and incidence of UUI
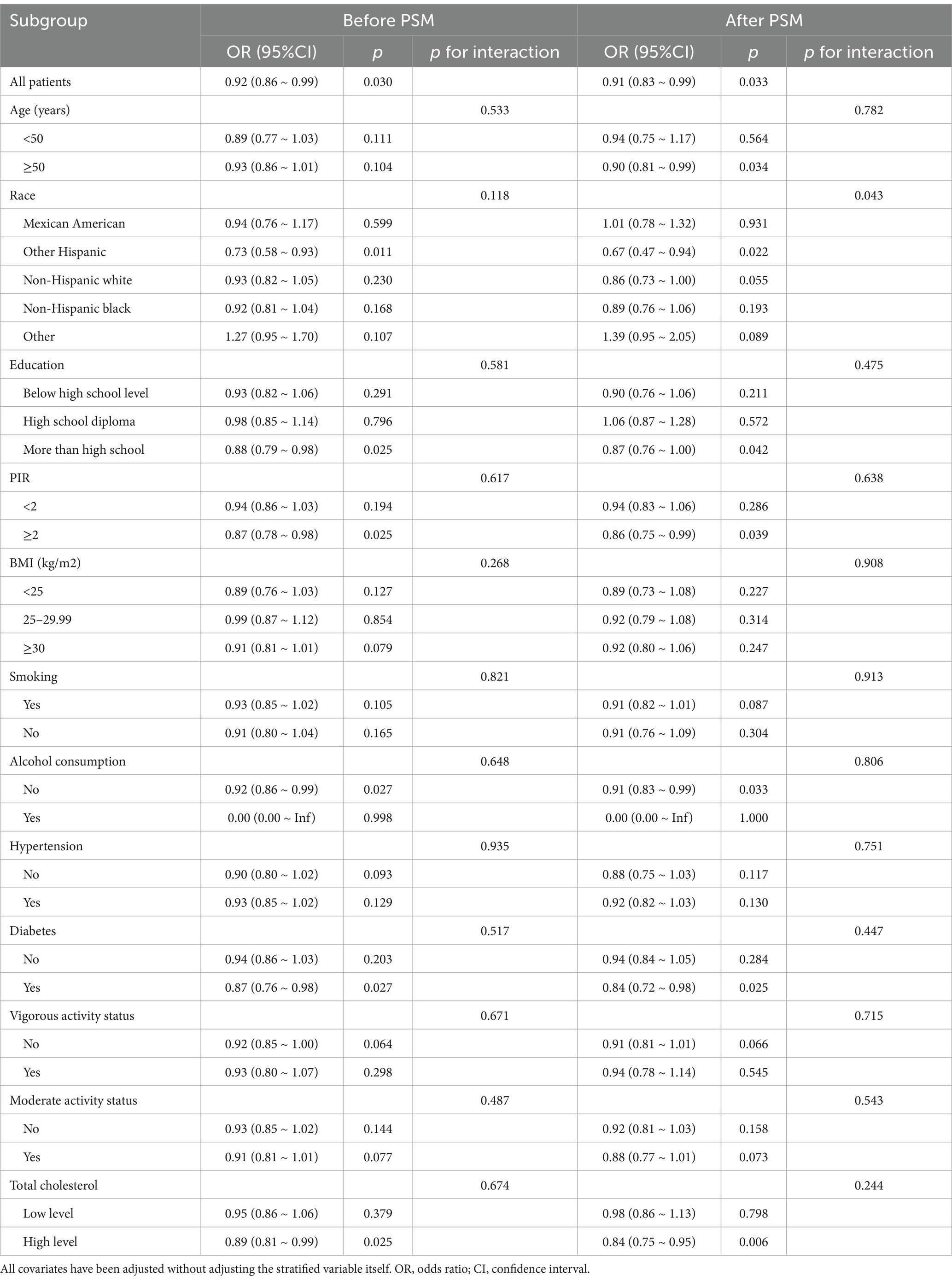
The study stratified the first subsection by race, age, education, PIR, body mass index, diabetes mellitus, hypertension, whether or not they smoked, whether or not they drank alcohol, total cholesterol, and moderate and vigorous activity (Table 4). Analyses were statistically significant regardless of pre- and post-PSM (p = 0.03 before PSM and p = 0.033 after PSM). Before and after PSM, CDAI was significantly negatively associated with UUI among those whose race was other Hispanic, higher than high school education, high PIR, non-drinkers, diabetics, and high cholesterol levels. In addition, after PSM, CDAI was negatively associated with UUI among those aged ≥50 years. Moreover, race played a significant moderating role in the effect after PSM, p for interaction = 0.043. It suggests a significant difference between racial subgroups. Apart from this, no significant interaction effects were found in other subgroups regardless of before or after PSM, suggesting that our results apply to almost all types of male populations.
3.7 Investigating possible mechanistic connections
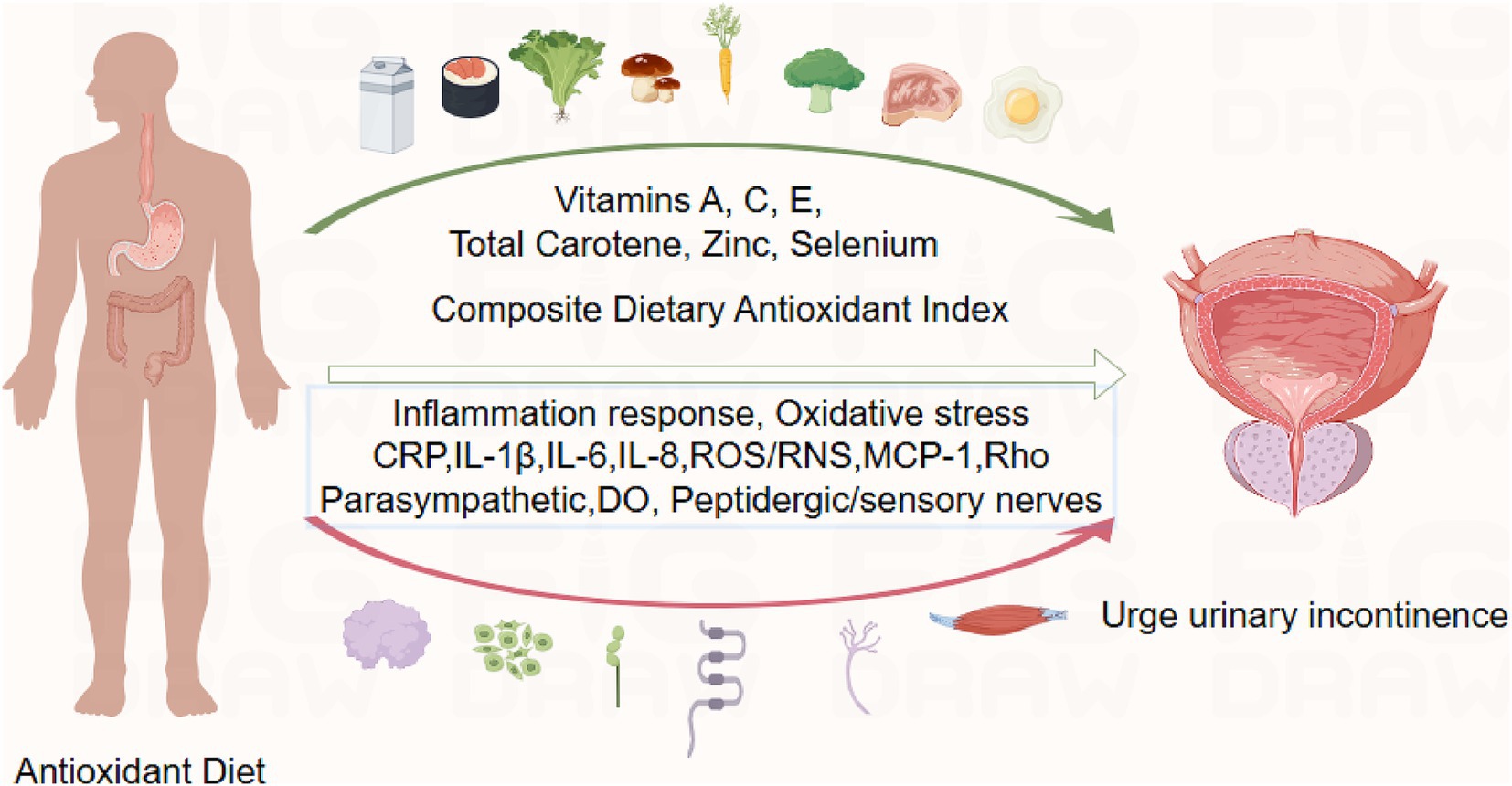
Based on Figdraw, an intuitive diagram is presented in Figure 7. We discuss the mechanisms by which CDAI affects the prevalence of UUI in Part 4. In the figure, the green arrows represent typical foods rich in the Composite Dietary Antioxidant Index (CDAI), which are beneficial for reducing the risk of UUI. The red arrows indicate biological mechanisms discussed in the article that contribute to the occurrence of UUI. These include markers of inflammation and oxidative stress: CRP (C-reactive protein), IL-1β, IL-6, IL-8 (cytokines), ROS/RNS (reactive oxygen and nitrogen species), and MCP-1 (monocyte chemoattractant protein-1). Other involved factors include Rho (Ras homologous), parasympathetic nerves, detrusor overactivity (DO), and peptidergic/sensory nerves (For more details, please refer to the discussion section).
4 Discussion
In this cross-sectional study of a nationally representative sample of U.S. men, greater consumption of antioxidant micronutrients was connected to a reduced likelihood of UUI in men with subthreshold CDAI intake (K = 0.523). However, the CDAI was not significantly associated with SUI or MUI.
Overactive bladder (OAB) syndrome is frequently accompanied by UUI. Prior research has indicated that 82.9% of patients with OAB have UUI (41). The connection between inflammation and overactive bladder (OAB) has been the subject of several investigations. Increased CRP levels were consistently linked to OAB in men, according to a study that investigated people in Boston between the ages of 30 and 79 (42). The idea that inflammatory infections may be an undervalued factor in the etiology of certain OAB patients is supported by a review article that discusses important findings from recent clinical and laboratory studies, including the relationship between bladder inflammation, urinary tract infections, and OAB pathogenesis (43).
Studies of the pathophysiology of urolithiasis have found that UUI involves molecular mechanisms: signalling and inflammation (44) (Figure 7). Increased serum C-reactive protein (CRP) levels in patients with UUI compared to non-patients have been demonstrated in several studies (45). Studies examining ischemia-modified albumin (IMA) have found that IMA levels are significantly elevated in patients with UUI compared to non-UUI patients (46). IMA levels are elevated in Systemic Inflammatory Response Syndrome (SIRS) and some inflammatory diseases. This may be related to the large number of free radicals released during the inflammatory process. Serum levels of interleukin-1β, −6 and − 8 have also been found to be elevated in UUI patients (47). IL-6 and IL-1β: elevated levels in a variety of chronic inflammatory diseases drive long-term inflammatory responses. IL-8 attracts primarily neutrophils to sites of inflammation and promotes infiltration of inflammatory cells. Urine levels of monocyte chemotactic protein 1 (MCP-1) in patients with UUI showed a trend toward higher levels compared to non-UUI patients (46). The pathophysiology of bladder dysfunction and the control of connexin expression are significantly influenced by inflammatory cytokines (48). Additionally, the relationship between local immune cells and overactive bladder parasympathetic and peptidergic/sensory innervation is mediated by inflammatory cytokines (49).
A “vicious cycle” can be created when oxidative stress triggers an inflammatory response by triggering inflammatory signalling pathways, which can further intensify oxidative stress. In an acute inflammatory response, neutrophils and macrophages kill pathogens by releasing large amounts of ROS through an oxidative burst (respiratory burst). The accumulation of reactive oxygen species (ROS) leads to the oxidation of DNA, proteins, carbohydrates lipids, and apoptosis (50). Diet controls the plasma redox state as an external factor and shields the body from reactive oxygen and reactive nitrogen species. Scavenging oxidants and antioxidants prevent oxidative damage by preserving a stable cellular redox state (51). Exogenous intake of antioxidants may prevent urinary incontinence and bladder ischemia (52, 53).
Our research revealed that CDAI was negatively and linearly correlated with UUI in men when CDAI was below 0.523 (median CDAI: 0.776). However, this relationship lost its significance when the CDAI value was greater than 0.523. This may be related to the saturating effect of dietary antioxidants. According to a study, dietary antioxidants and depression in persons who are overweight or obese are negatively correlated. However, in the group that was overweight, saturation effects were noted (54). In the study of dietary antioxidants and coronary heart disease, a threshold effect of complex dietary antioxidants was also found (55). A study examining dietary anti-inflammation and cognitive dysfunction in older adults also found a saturating effect of complex dietary antioxidants (56). In addition, excessive vitamin C intake is positively associated with the development of urinary incontinence (52). Because of the threshold effect, antioxidants have been shown to exhibit opposite effects under certain conditions (57).
More crucially, in the study of oxidative balance fractions and urinary incontinence, behavioural oxidative balance fractions were found to have a much greater effect on urinary incontinence than dietary oxidative balance fractions. And the behavioural oxidative balance score included physical activity, body mass index, alcohol consumption, and cotinine (58). All these four variables were included in our covariates, which had a significant impact on the prevalence of CDAI and UUI. As a result, at a CDAI greater than 0.523, the negative connection with UUI prevalence is no longer significant. In our stratified analyses, we found that the relationship between the prevalence of CDAI and UUI did not differ significantly in populations differing in BMI, smoking, and vigorous or moderate activity, regardless of before or after PSM. The relationship between the prevalence of UUI and CDAI was more significant only in those who did not drink alcohol.
We further stratified by each covariate and found no significant interaction effects before or after PSM except for the variable race after PSM, suggesting that our findings are applicable to almost all types of male populations. In subgroup analyses, there was greater sensitivity to the protective effects of CDAI among those whose race was other Hispanic, higher than high school education level, PIR ≥2, did not consume alcohol, had diabetes, and had high cholesterol levels. An article examining racial differences in overactive bladder found that urge incontinence has a high prevalence among Hispanics (59). Alcohol intake is positively associated with lower urinary tract disorders such as OAB (60). Several studies have confirmed that the prevalence of UI is increased in poor populations and exacerbated by complex social, cultural and psychological influences (61). It has been shown that the correlation between diabetes and OAB is more significant through systemic inflammation as a mediator (62). Research on the relationship between hypercholesterolemia and UUI is still lacking, but a study in rats found that hypercholesterolemia was positively associated with detrusor overactivity (DO) (63). In summary, we have identified specific beneficiary populations most likely to benefit from increased antioxidant dietary micronutrients to reduce the prevalence of UUI. In adult males with CDAI levels below 0.523, increasing dietary antioxidant intake is associated with a decrease in the incidence of UUI. This may provide some theoretical basis for the prevention or dietary treatment of UUI in clinical as well as public health settings.
In our compositional analysis, we found that the negative correlation between zinc and the incidence of UUI was more pronounced. The study found that zinc plays a significant role in antioxidant defense and inflammation regulation (64). Zinc acts as a cofactor for the antioxidant enzyme superoxide dismutase (SOD1), facilitating the conversion of superoxide radicals into less harmful molecules, thereby reducing oxidative stress and preventing cellular damage. In addition, zinc plays an important role in neural function by regulating neurotransmitter release, supporting synaptic plasticity, and protecting neurons from oxidative damage (65). Zinc regulates inflammation by modulating the production of pro-inflammatory cytokines (such as TNF-α and IL-6) and promoting an anti-inflammatory immune response, primarily through the inhibition of NF-κB activation (66). Therefore, zinc, as an important component of CDAI, plays a significant protective role in the risk of UUI. This study utilized a large, representative sample of adult males from NHANES and followed a carefully designed research protocol. To the best of our knowledge, this is the first study to explore the association between CDAI and UUI in men using a large sample size. We used univariate regression, multivariate regression, threshold effects analysis, sensitivity analysis, and subgroup analysis to enhance our understanding of their relationship. We investigated the relationship between composite dietary trace elements, which better reflect real-life scenarios, and the incidence of UUI in men. Additionally, we identified the threshold of CDAI that influences UUI in men. This threshold could be beneficial in clinical practice for preventing UUI and predicting the risk of UUI in adult men. Nevertheless, there are certain unavoidable limits to our study. First, since the study was cross-sectional, it was impossible for us to establish causality. Therefore, future longitudinal studies or randomized controlled trials are needed to better understand the causal relationship. Second, the potential bias easily introduced by dietary data from interviews leads to potentially biased results. Finally, we are unable to determine the residual confounding effects that may arise from unmeasured factors. Therefore, more research is needed in the future to address these limitations and provide further insights.
5 Conclusion
This study sought to increase understanding of the role antioxidant diets play in UUI prevalence among men. The results showed that when CDAI was below the threshold, the incidence of UUI was negatively correlated with CDAI. Therefore, CDAI can be used to predict the risk of UUI in men and guide the prevention of UUI in men. Men with lower dietary antioxidant micronutrient intake (approximately half of the adult male population in the United States) experience a reduced risk of UUI as their dietary antioxidant intake increases.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
NHANES was approved by National Center for Health Statistics Research Ethic Review Board. All subjects signed the informed consent during the recruitment period.
Author contributions
XJ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. WT: Supervision, Visualization, Writing – review & editing. LS: Data curation, Visualization, Writing – review & editing. SL: Supervision, Validation, Writing – review & editing. TX: Supervision, Validation, Writing – review & editing. PS: Supervision, Validation, Writing – review & editing. YL: Formal analysis, Funding acquisition, Supervision, Writing – review & editing. HL: Funding acquisition, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1514320/full#supplementary-material
Footnotes
References
1. Milsom, I, and Gyhagen, M. The prevalence of urinary incontinence. Climacteric. (2019) 22:217–22. doi: 10.1080/13697137.2018.1543263
2. Gacci, M, Sakalis, VI, Karavitakis, M, Cornu, JN, Gratzke, C, Herrmann, TRW, et al. European Association of Urology guidelines on male urinary incontinence. Eur Urol. (2022) 82:387–98. doi: 10.1016/j.eururo.2022.05.012
3. Mitteness, LS. Knowledge and beliefs about urinary incontinence in adulthood and old age. J Am Geriatr Soc. (1990) 38:374–8. doi: 10.1111/j.1532-5415.1990.tb03525.x
4. Khullar, V, Sexton, CC, Thompson, CL, Milsom, I, Bitoun, CE, and Coyne, KS. The relationship between BMI and urinary incontinence subgroups: results from EpiLUTS. Neurourol Urodyn. (2014) 33:392–9. doi: 10.1002/nau.22428
5. Russo, E, Caretto, M, Giannini, A, Bitzer, J, Cano, A, Ceausu, I, et al. Management of urinary incontinence in postmenopausal women: an EMAS clinical guide. Maturitas. (2021) 143:223–30. doi: 10.1016/j.maturitas.2020.09.005
6. Nazzal, Z, Khatib, B, Al-Quqa, B, Abu-Taha, L, and Jaradat, A. The prevalence and risk factors of urinary incontinence among women with type 2 diabetes in the North West Bank: a cross-sectional study. Lancet. (2021) 398:S42. doi: 10.1016/s0140-6736(21)01528-2
7. Zordani, A, Pisciotta, A, Bertoni, L, Bertani, G, Vallarola, A, Giuliani, D, et al. Regenerative potential of human dental pulp stem cells in the treatment of stress urinary incontinence: in vitro and in vivo study. Cell Prolif. (2019) 52:e12675. doi: 10.1111/cpr.12675
8. Lee, JA, Johns, TS, Melamed, ML, Tellechea, L, Laudano, M, Stern, JM, et al. Associations between socioeconomic status and urge urinary incontinence: an analysis of NHANES 2005 to 2016. J Urol. (2020) 203:379–84. doi: 10.1097/ju.0000000000000542
9. Bartoli, S, Aguzzi, G, and Tarricone, R. Impact on quality of life of urinary incontinence and overactive bladder: a systematic literature review. Urology. (2010) 75:491–500. doi: 10.1016/j.urology.2009.07.1325
10. Ko, Y, Lin, SJ, Salmon, JW, and Bron, MS. The impact of urinary incontinence on quality of life of the elderly. Am J Manag Care. (2005) 11:S103–11.
11. Lee, HY, Rhee, Y, and Choi, KS. Urinary incontinence and the association with depression, stress, and self-esteem in older Korean women. Sci Rep. (2021) 11:9054. doi: 10.1038/s41598-021-88740-4
12. Verrotti, A, Iapadre, G, Di Francesco, L, Zagaroli, L, and Farello, G. Diet in the treatment of epilepsy: what we know so far. Nutrients. (2020) 12:2645. doi: 10.3390/nu12092645
13. Jiang, Y, Jarr, K, Layton, C, Gardner, CD, Ashouri, JF, Abreu, MT, et al. Therapeutic implications of diet in inflammatory bowel disease and related immune-mediated inflammatory diseases. Nutrients. (2021) 13:890. doi: 10.3390/nu13030890
14. Xu, H, Li, X, Adams, H, Kubena, K, and Guo, S. Etiology of metabolic syndrome and dietary intervention. Int J Mol Sci. (2019) 20:128. doi: 10.3390/ijms20010128
15. Schwingshackl, L, Chaimani, A, Hoffmann, G, Schwedhelm, C, and Boeing, H. A network meta-analysis on the comparative efficacy of different dietary approaches on glycaemic control in patients with type 2 diabetes mellitus. Eur J Epidemiol. (2018) 33:157–70. doi: 10.1007/s10654-017-0352-x
16. Zheng, Y, Liu, W, Zhu, X, Xu, M, Lin, B, and Bai, Y. Associations of dietary inflammation index and composite dietary antioxidant index with preserved ratio impaired spirometry in US adults and the mediating roles of triglyceride-glucose index: NHANES 2007-2012. Redox Biol. (2024) 76:103334. doi: 10.1016/j.redox.2024.103334
17. van der Schaft, N, Trajanoska, K, Rivadeneira, F, Ikram, MA, Schoufour, JD, and Voortman, T. Total dietary antioxidant capacity and longitudinal trajectories of body composition. Antioxidants (Basel). (2020) 9:728. doi: 10.3390/antiox9080728
18. Luu, HN, Wen, W, Li, H, Dai, Q, Yang, G, Cai, Q, et al. Are dietary antioxidant intake indices correlated to oxidative stress and inflammatory marker levels? Antioxid Redox Signal. (2015) 22:951–9. doi: 10.1089/ars.2014.6212
19. Massey, PB. Dietary supplements. Med Clin North Am. (2002) 86:127–47. doi: 10.1016/s0025-7125(03)00076-2
20. Liu, C, Lai, W, Zhao, M, Zhang, Y, and Hu, Y. Association between the composite dietary antioxidant index and atherosclerotic cardiovascular disease in postmenopausal women: a cross-sectional study of NHANES data, 2013-2018. Antioxidants (Basel). (2023) 12:1740. doi: 10.3390/antiox12091740
21. Teng, TQ, Liu, J, Hu, FF, Li, QQ, Hu, ZZ, and Shi, Y. Association of composite dietary antioxidant index with prevalence of stroke: insights from NHANES 1999-2018. Front Immunol. (2024) 15:1306059. doi: 10.3389/fimmu.2024.1306059
22. Wang, M, Huang, ZH, Zhu, YH, He, P, and Fan, QL. Association between the composite dietary antioxidant index and chronic kidney disease: evidence from NHANES 2011-2018. Food Funct. (2023) 14:9279–86. doi: 10.1039/d3fo01157g
23. Zhao, L, Sun, Y, Cao, R, Wu, X, Huang, T, and Peng, W. Non-linear association between composite dietary antioxidant index and depression. Front Public Health. (2022) 10:988727. doi: 10.3389/fpubh.2022.988727
24. Xiong, B, Wang, J, He, R, and Qu, G. Composite dietary antioxidant index and sleep health: a new insight from cross-sectional study. BMC Public Health. (2024) 24:609. doi: 10.1186/s12889-024-18047-2
25. Paulose-Ram, R, Graber, JE, Woodwell, D, and Ahluwalia, N. The National Health and nutrition examination survey (NHANES), 2021-2022: adapting data collection in a COVID-19 environment. Am J Public Health. (2021) 111:2149–56. doi: 10.2105/ajph.2021.306517
26. Sun, H, Huang, J, Tang, H, and Wei, B. Association between weight-adjusted-waist index and urge urinary incontinence: a cross-sectional study from NHANES 2013 to 2018. Sci Rep. (2024) 14:478. doi: 10.1038/s41598-024-51216-2
27. Ahluwalia, N, Dwyer, J, Terry, A, Moshfegh, A, and Johnson, C. Update on NHANES dietary data: focus on collection, release, analytical considerations, and uses to inform public policy. Adv Nutr. (2016) 7:121–34. doi: 10.3945/an.115.009258
28. Cheng, TD, Ferderber, C, Kinder, B, and Wei, YJ. Trends in dietary vitamin a intake among US adults by race and ethnicity, 2003-2018. JAMA. (2023) 329:1026–9. doi: 10.1001/jama.2023.0636
29. Xu, Q, Qian, X, Sun, F, Liu, H, Dou, Z, and Zhang, J. Independent and joint associations of dietary antioxidant intake with risk of post-stroke depression and all-cause mortality. J Affect Disord. (2023) 322:84–90. doi: 10.1016/j.jad.2022.11.013
30. He, H, Chen, X, Ding, Y, Chen, X, and He, X. Composite dietary antioxidant index associated with delayed biological aging: a population-based study. Aging (Albany NY). (2024) 16:15–27. doi: 10.18632/aging.205232
31. Wright, ME, Mayne, ST, Stolzenberg-Solomon, RZ, Li, Z, Pietinen, P, Taylor, PR, et al. Development of a comprehensive dietary antioxidant index and application to lung cancer risk in a cohort of male smokers. Am J Epidemiol. (2004) 160:68–76. doi: 10.1093/aje/kwh173
32. Cao, S, Meng, L, Lin, L, Hu, X, and Li, X. The association between the metabolic score for insulin resistance (METS-IR) index and urinary incontinence in the United States: results from the National Health and nutrition examination survey (NHANES) 2001-2018. Diabetol Metab Syndr. (2023) 15:248. doi: 10.1186/s13098-023-01226-3
33. Li, J, Xie, R, Tian, H, Wang, D, Mo, M, Yang, J, et al. Association between triglyceride glucose body mass index and urinary incontinence: a cross-sectional study from the National Health and nutrition examination survey (NHANES) 2001 to 2018. Lipids Health Dis. (2024) 23:304. doi: 10.1186/s12944-024-02306-7
34. Di, X, Yuan, C, Xiang, L, Wang, G, and Liao, B. Association between sitting time and urinary incontinence in the US population: data from the National Health and nutrition examination survey (NHANES) 2007 to 2018. Heliyon. (2024) 10:e27764. doi: 10.1016/j.heliyon.2024.e27764
35. Liu, L, Xu, M, Zhou, H, Hao, X, Chen, X, and Liu, X. Association of serum 25-hydroxyvitamin D with urinary incontinence in elderly men: evidence based on NHANES 2007-2014. Front Endocrinol (Lausanne). (2023) 14:1215666. doi: 10.3389/fendo.2023.1215666
36. Searcy, JAR. Geriatric urinary incontinence. Nurs Clin North Am. (2017) 52:447–55. doi: 10.1016/j.cnur.2017.04.002
37. Bump, RC, and McClish, DK. Cigarette smoking and urinary incontinence in women. Am J Obstet Gynecol. (1992) 167:1213–8. doi: 10.1016/s0002-9378(11)91691-3
38. Lee, AH, and Hirayama, F. Alcohol consumption and female urinary incontinence: a community-based study in Japan. Int J Urol. (2012) 19:143–8. doi: 10.1111/j.1442-2042.2011.02889.x
39. Chai, TC, Asfaw, TS, Baker, JE, Clarkson, B, Coleman, P, Hoffstetter, S, et al. Future directions of research and Care for Urinary Incontinence: findings from the National Institute of Diabetes and Digestive and Kidney Diseases summit on urinary incontinence clinical research in women. J Urol. (2017) 198:22–9. doi: 10.1016/j.juro.2016.10.133
40. Batmani, S, Jalali, R, Mohammadi, M, and Bokaee, S. Prevalence and factors related to urinary incontinence in older adults women worldwide: a comprehensive systematic review and meta-analysis of observational studies. BMC Geriatr. (2021) 21:212. doi: 10.1186/s12877-021-02135-8
41. Cheung, WW, Khan, NH, Choi, KK, Bluth, MH, and Vincent, MT. Prevalence, evaluation and management of overactive bladder in primary care. BMC Fam Pract. (2009) 10:8. doi: 10.1186/1471-2296-10-8
42. Kupelian, V, Rosen, RC, Roehrborn, CG, Tyagi, P, Chancellor, MB, and McKinlay, JB. Association of overactive bladder and C-reactive protein levels. Results from the Boston area community health (BACH) survey. BJU Int. (2012) 110:401–7. doi: 10.1111/j.1464-410X.2011.10769.x
43. Mansfield, KJ, Chen, Z, Moore, KH, and Grundy, L. Urinary tract infection in overactive bladder: an update on pathophysiological mechanisms. Front Physiol. (2022) 13:886782. doi: 10.3389/fphys.2022.886782
44. Post, WM, Ruiz-Zapata, AM, Grens, H, de Vries, RBM, Poelmans, G, Coenen, MJH, et al. Genetic variants and expression changes in urgency urinary incontinence: a systematic review. Neurourol Urodyn. (2020) 39:2089–110. doi: 10.1002/nau.24512
45. Hsiao, SM, Lin, HH, and Kuo, HC. The role of serum C-reactive protein in women with lower urinary tract symptoms. Int Urogynecol J. (2012) 23:935–40. doi: 10.1007/s00192-012-1715-1
46. Farhan, B, Chang, H, Ahmed, A, Zaldivair, F, and Ghoniem, G. Characterisation of urinary monocyte chemoattractant protein 1: potential biomarker for patients with overactive bladder. Arab J Urol. (2019) 17:58–60. doi: 10.1080/2090598x.2019.1589932
47. Liu, HT, Jiang, YH, and Kuo, HC. Increased serum adipokines implicate chronic inflammation in the pathogenesis of overactive bladder syndrome refractory to antimuscarinic therapy. PLoS One. (2013) 8:e76706. doi: 10.1371/journal.pone.0076706
48. Wang, Z, Cheng, Z, Cristofaro, V, Li, J, Xiao, X, Gomez, P, et al. Inhibition of TNF-α improves the bladder dysfunction that is associated with type 2 diabetes. Diabetes. (2012) 61:2134–45. doi: 10.2337/db11-1763
49. Tyagi, P, Barclay, D, Zamora, R, Yoshimura, N, Peters, K, Vodovotz, Y, et al. Urine cytokines suggest an inflammatory response in the overactive bladder: a pilot study. Int Urol Nephrol. (2010) 42:629–35. doi: 10.1007/s11255-009-9647-5
50. Roumeliotis, S, Roumeliotis, A, Dounousi, E, Eleftheriadis, T, and Liakopoulos, V. Dietary antioxidant supplements and uric acid in chronic kidney disease: a review. Nutrients. (2019) 11:1911. doi: 10.3390/nu11081911
51. Demmig-Adams, B, and Adams, WW 3rd. Antioxidants in photosynthesis and human nutrition. Science. (2002) 298:2149–53. doi: 10.1126/science.1078002
52. Maserejian, NN, Giovannucci, EL, McVary, KT, and McKinlay, JB. Intakes of vitamins and minerals in relation to urinary incontinence, voiding, and storage symptoms in women: a cross-sectional analysis from the Boston area community health survey. Eur Urol. (2011) 59:1039–47. doi: 10.1016/j.eururo.2011.03.008
53. Wu, YH, Chueh, KS, Chuang, SM, Long, CY, Lu, JH, and Juan, YS. Bladder hyperactivity induced by oxidative stress and bladder ischemia: a review of treatment strategies with antioxidants. Int J Mol Sci. (2021) 22:6014. doi: 10.3390/ijms22116014
54. Zhao, L, Zhang, X, Guo, S, Han, K, Sun, Y, Li, X, et al. Relationship between composite dietary antioxidant index and depression among overweight and obese adults. J Affect Disord. (2023) 341:358–65. doi: 10.1016/j.jad.2023.08.140
55. Ma, R, Zhou, X, Zhang, G, Wu, H, Lu, Y, Liu, F, et al. Association between composite dietary antioxidant index and coronary heart disease among US adults: a cross-sectional analysis. BMC Public Health. (2023) 23:2426. doi: 10.1186/s12889-023-17373-1
56. Mao, J, Hu, H, Zhao, Y, Zhou, M, and Yang, X. Association between composite dietary antioxidant index and cognitive function among aging Americans from NHANES 2011-2014. J Alzheimers Dis. (2024) 98:1377–89. doi: 10.3233/jad-231189
57. Young, AJ, and Lowe, GM. Antioxidant and prooxidant properties of carotenoids. Arch Biochem Biophys. (2001) 385:20–7. doi: 10.1006/abbi.2000.2149
58. Yuan, Y, Tan, W, Huang, Y, Huang, H, Li, Y, Gou, Y, et al. Association between oxidative balance score and urinary incontinence in females: results from the national health and nutrition examination survey in 2005-2018. Int Urol Nephrol. (2023) 55:2145–54. doi: 10.1007/s11255-023-03665-3
59. Akbar, A, Liu, K, Michos, ED, Brubaker, L, Markossian, T, Bancks, MP, et al. Racial differences in urinary incontinence prevalence, overactive bladder and associated bother among men: the multi-ethnic study of atherosclerosis. J Urol. (2021) 205:524–31. doi: 10.1097/ju.0000000000001353
60. Robinson, D, Hanna-Mitchell, A, Rantell, A, Thiagamoorthy, G, and Cardozo, L. Are we justified in suggesting change to caffeine, alcohol, and carbonated drink intake in lower urinary tract disease? Report from the ICI-RS 2015. Neurourol Urodyn. (2017) 36:876–81. doi: 10.1002/nau.23149
61. Ansari, Z, and White, S. Managing incontinence in low-and middle income-countries: a qualitative case study from Pakistan. PLoS One. (2022) 17:e0271617. doi: 10.1371/journal.pone.0271617
62. He, Q, Wu, L, Deng, C, He, J, Wen, J, Wei, C, et al. Diabetes mellitus, systemic inflammation and overactive bladder. Front Endocrinol (Lausanne). (2024) 15:1386639. doi: 10.3389/fendo.2024.1386639
63. Son, H, Choi, WS, Paick, JS, and Park, WH. Detrusor Overactivity in Hyperchoelsterolemia rats. Low Urin Tract Symptoms. (2012) 4:16–20. doi: 10.1111/j.1757-5672.2011.00124.x
64. Skrajnowska, D, and Bobrowska-Korczak, B. Role of zinc in immune system and anti-Cancer defense mechanisms. Nutrients. (2019) 11:2273. doi: 10.3390/nu11102273
65. Prasad, AS. Zinc in human health: effect of zinc on immune cells. Mol Med. (2008) 14:353–7. doi: 10.2119/2008-00033.Prasad
Keywords: NHANES, UUI (urge urinary incontinence), composite dietary antioxidant index (CDAI), propensity score matching (PSM), exogenous antioxidants
Citation: Jin X, Tong W, Sun L, Lu S, Xu T, Sun P, Liu Y and Li H (2024) Composite dietary antioxidant index in relation to urge urinary incontinence in US men. Front. Nutr. 11:1514320. doi: 10.3389/fnut.2024.1514320
Edited by:
Esma Nur Okatan, University of Istinye, TürkiyeReviewed by:
Oyku Gonul Geyik, University of Istinye, TürkiyeZeynep Tokcaer Keskin, Acıbadem University, Türkiye
Copyright © 2024 Jin, Tong, Sun, Lu, Xu, Sun, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hangxu Li, aGFuZ3h1bGk5MTNAZm94bWFpbC5jb20=; Yan Liu, bGl1eWFuZm9yZXN0QDE2My5jb20=
 Xuefeng Jin
Xuefeng Jin Wenhui Tong2
Wenhui Tong2 Hangxu Li
Hangxu Li