- 1Yunnan Agricultural University, Kunming, China
- 2Yunnan Provincial High Court Characteristic Agricultural Industry Research Institute, Kunming, China
- 3College of Food Science and Technology, Yunnan Agricultural University, Kunming, China
The determination of allergenic proteins in Moringa oleifera leaves, which is the main components of immune activity, has enabled the development of a more effective method for evaluating the activity of extracted Moringa oleifera leaves protein. In this study, the extraction process of Moringa oleifera leaves protein was optimized based on a single factor experiment. The hemagglutination-related properties of Moringa oleifera leaves protein, such as (thermal, acid–base) stability, sugar binding specificity, ion binding characteristics, and hemolytic activity, were detected. The optimal combination of extraction process was: extraction time of 6 h, material-liquid ratio of 1:8, and ammonium sulfate saturation of 60%. The extraction rate of moringa leaf protein under this condition was 14.37 mg/g. The molecular weight of moringa leaf protein was analyzed by SDS-PAGE, and the molecular weight was mainly concentrated around 23 kDa~70 kDa, with the highest content of 35 kDa (major allergen). The study of the hemagglutination characteristics of Moringa oleifera leaves protein revealed that the protein exhibited high stability at temperatures below 60°C, with complete loss of activity occurring at temperatures above 110°C for 20 min. The effect of different pH conditions on the hemagglutination capacity of Moringa oleifera leaves protein was readily discernible. The hemagglutination activity of Moringa oleifera leaves protein was 104 in a pH value from 3.7 to 7.8, and the hemagglutination activity was completely lost at a pH value higher than 11.9. D(+) anhydrous glucose is the specific inhibitory sugar of Moringa oleifera leaves protein lectin. Moringa oleifera leaves protein exhibits hemolytic activity at a concentration of at least 20 mg/mL, and α-methyl-mannoside, galactoside, raffinose and Al3+ can inhibit the hemolysis of Moringa oleifera leaves protein. The present study identified the effects of different factors on the coagulation activity and hemolytic ability of Moringa oleifera leaves protein, thereby providing a theoretical basis for further purification and application of Moringa oleifera lectin. However, it should be noted that the results of the mixture have certain limitations, and further purification of lectin is needed to obtain more targeted research results.
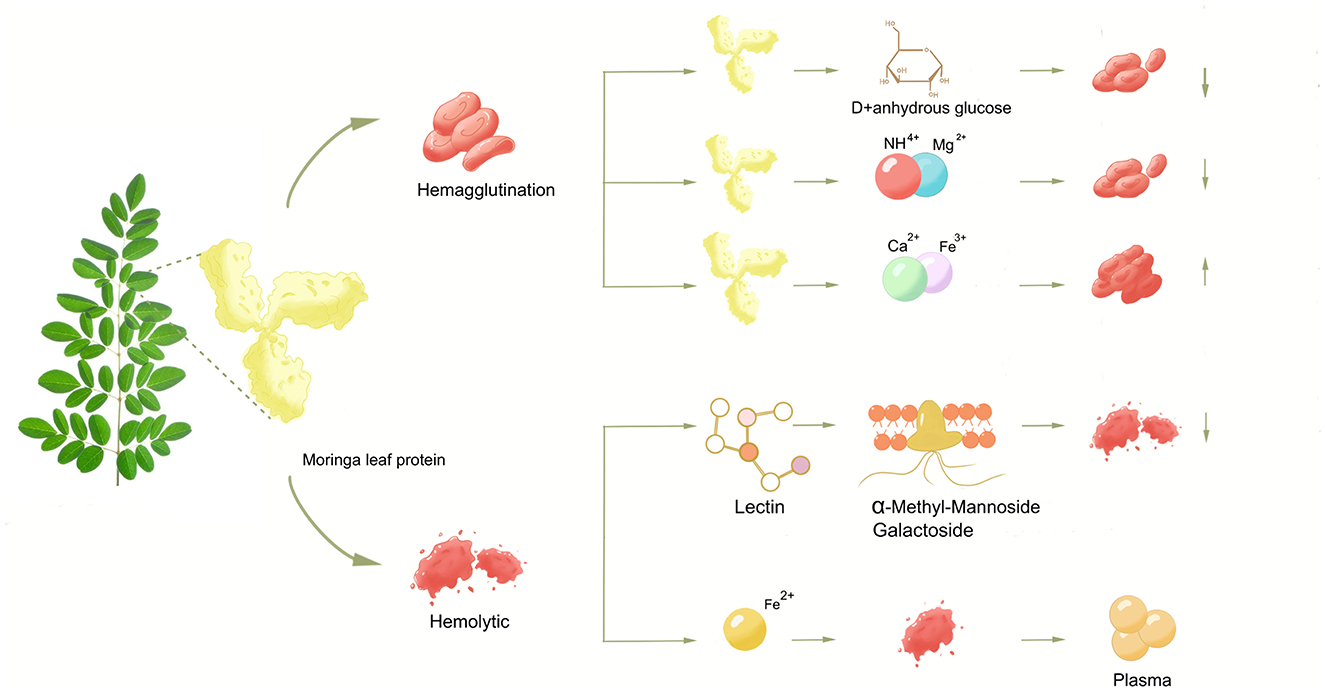
Graphical Abstract. The effects of different factors on the coagulation activity and hemolytic ability of Moringa oleifera leaves protein.
1 Introduction
Moringa oleifera (M. oleifera), is commonly known as drumstick tree. This crop grows rapidly, has strong adaptability and is widely planted in tropical and subtropical areas of Asia and Africa (1, 2). M. oleifera leaves were approved as a new resource food in 2012 by China National Food Industry Association due to their rich nutritional value (3) and have been widely consumed as a special food in many regions. M. oleifera is a versatile tree species. Its roots and bark can be used as a traditional medicinal materials, fresh leaves rich nutrition, can be used as edible vegetables, seeds are rich in fat, which can be used to press oil (4). M. oleifera is a rich source of vitamin A, vitamin B, vitamin C, vitamin E, as well as other nutrients, including potassium, calcium, iron, and various minerals (5). In addition, it is rich in essential amino acids and trace elements required by the human body, and its nutritional value is comparable to that of Spirulina, which enjoys the reputation of “the micro-treasure house of human nutrition” (5, 6). People in many countries and regions of the world have the habit of eating M. oleifera to promote health care. For example, in India, M. oleifera leaves are said to have the effect of lowering cholesterol and are used to treat heart disease and obesity (7, 8).
M. oleifera leaves are sources of high-quality plant protein. Satish et al. (9) also found the hydrolysis activity of casein in M. oleifera leaves. M. oleifera is an important source of protease inhibitors. Bijina et al. (10) extracted and purified a protease with a molecular weight of 51 kDa from M. oleifera leaves. The protease exhibited optimal activity at pH 8 and 37°C, with the highest casein hydrolysis activity observed under these conditions. The identified proteins are mainly involved in energy exchange and metabolism and in carbohydrate and protein metabolism. Among the identified proteins, proteases are mainly involved in the curd activity of milk. Pusztai et al. (11) used M. oleifera protein and alum for the treatment of turgor water and found that M. oleifera protein is better than alum in removing turgor water and E. coli.
M. oleifera leaves are rich in high-quality protein, but in the process of M. oleifera promotion, it is found that some people will have rash, diarrhea, abdominal pain, dizziness, nausea, vomiting and other symptoms after eating M. oleifera leaves, especially fresh leaves, which is particularly obvious (7). These adverse symptoms are thought to be caused by disorder of immune allergic reaction (12). Xi et al. (13) and Zhang et al. (14) haved successfully sensitized BALB/c mice to M. oleifera leaves protein through oral administration. D'Auria et al. identified potential M. oleifera leaves allergens, including morintides and nsLTP, through proteomic analysis (15). Iddagoda et al. determined the molecular weights of the M. oleifera leaves allergens to be 14, 23, 35, 43, and 48 kDa using Western blotting with serum from patients with M. oleifera leaves allergy (16). Most plant lectins in digestive system can enzyme degradation resistance, resistance to degradation of lectins in combination with surface receptor in the digestive tract, swallowing and lectin receptors was within the cell, through the epithelial cells into the circulation of the blood, produce anticoagulant set IgE (allergic reaction) and IgG antibody (9–12). In this study, we hypothesized that the major allergenic protein in M. oleifera leaves protein is M. oleifera lectin, and studying the basic properties of lectins in M. oleifera leaves protein was the first step to confirm this hypothesis, so its properties were investigated. Hev b 6 (Hevein, a lectin-like protein and a major allergen of latex) was predicted as the most similar allergen to the Morintides mO1 and mO2 proteins (15). That will support our hypothesis that “the major allergenic protein in M. oleifera leaves protein is Moringa oleifera lectin”. The existing methods for the detection and analysis of lectins are mainly carried out by using the properties of lectins, including cell agglutination, sugar binding properties and protein properties. Plant lectins have the ability to agglutinate red blood cells from rabbits and humans, and the hemagglutination activity (agglutination potency) is linearly related to the amount of lectins (9–11). Use lectins, therefore, all natural or enzyme treatment of people or animals to the characteristics of red blood cells or red blood cell agglutinate method (blood clot) (Hemagglutination) to test the lectin. Lectins can also be detected on the basis of their characteristic of binding specifically to sugars, glycoproteins, or sugar-binding proteins, namely the sugar-complex method (11, 12). Based on the above analysis, it can be seen that the hemagglutination method has the advantages of simple operation, rapid reaction, obvious effect, and less influence by conditions. In this experiment, M. oleifera leaf protein (lectin) was detected by hemagglutination method, and the hemagglutination activity of lectin was used to measure the activity of lectin. In this study, the thermal stability, acid-base stability, ion binding properties, sugar binding specificity and hemolytic activity of M. oleifera leaves protein or lectin were studied to provide theoretical basis for the development and utilization of M. oleifera protein and its agglutination active substances.
2 Materials and methods
2.1 Materials and chemicals
Fresh M. oleifera leaves were obtained from Tianyou Technology Co. of Dehong State. Phosphate-buffered saline was purchased from Beijing Solarbio Technology Co. Ammonium sulfate and methyl alcohol were purchased from Beijing Chemical Reagent Factory, and a BCA (Bicinchoninic Acid) protein quantification kit was purchased from Beyotime. Dialysis bag (3,000 Da) were purchased from Shanghai Yuanye Biotechnology Co., Ltd., and two-color prestained markers were obtained from Yase Biotechnology Co., Ltd. Defibrinated rabbit blood from Beijing Solaibao Technology Co.
2.2 Extraction of M. oleifera leaves protein
The protein of M. oleifera leaves was extracted according to the improved operation method described by Feng and Wang (8) and Satish et al. (9). Fresh M. oleifera leaves were used, and phosphate buffer solution was added at a material-to-liquid ratio of 1:6. M. oleifera leaves were broken and homogenized, placed in a refrigerator at 4°C for extraction for 4 h, and stirred every 1 h. The residue was then filtered through gauze, and the filtrate was centrifuged at 1,200g for 20 min using a centrifuge to collect the supernatant. Subsequently, 472 g of ammonium sulfate crystals was added to each 1 L of sample solution and allowed to precipitate overnight, and the ammonium sulfate concentration was 70% to completely dissolve the ammonium sulfate crystals (15, 16). The samples were centrifuged again at 1,200g for 20 min, and the supernatant was discarded, leaving the precipitate. Subsequently, PBS (phosphate buffer saline) was added to dissolve the precipitate, and the precipitate was placed into the dialysis bag for dialysis for 2–4 days until the dialysis water became clear. The dialyzed M. oleifera leaves protein was then placed into a freeze-drying dish for vacuum freeze-drying to obtain M. oleifera leaves protein.
2.3 Single-factor test
Using the M. oleifera leaves protein extraction rate as the index to be investigated, various types of extract (normal saline, PBS, ultrapure water, Tris-HCl), different ammonium sulfate saturation levels (60, 70, 80%), different solid–liquid ratios (1:6, 1:8, 1:10), and various extraction times (6, 8, 10 h) were studied. The effects of these four factors on the extraction yield of M. oleifera leaves protein were assessed to determine the level of each factor in the optimal process.
2.4 Orthogonal experimental design
Through single-factor tests, the single-factor extraction conditions that yielded the best M. oleifera leaves protein yield were obtained. To optimize the protein extraction content, an orthogonal experimental design was selected as shown in Table 1.
2.5 Determination of the M. oleifera leaves protein concentration
According to the method described by Dai et al. (27) and Huang et al. (28), the absorbance value was measured at a wavelength of 595 nm, and bovine serum protein was used as the standard protein to obtain the standard curve, where Y is the protein concentration and X is the absorbance value. The protein extraction rate in solution was used as the reference to determine the best conditions for each process.
2.6 SDS–PAGE analysis
The experiment was performed according to the method described by Zenteno et al. (12) with a few modifications. The loading volume was calculated according to the M. oleifera leaves protein concentration. M. oleifera leaves protein and 5 μL of sample loading solution were added into a 0.2-mL centrifuge tube, and the sample was boiled and denatured for 10 min at 95°C. A 10% separation gel and 4% concentration gel were used. After preparation of the gel, the prepared M. oleifera leaves protein sample was added to the gel for electrophoresis. After electrophoresis, the gel blocks were removed, immersed in fixative solution for 30 min and then stained with Coomassie brilliant blue staining solution in a shaker. After staining, the dye was decolorized until the gel background was clean. WD-9403 gel rapid imager (Beijing Liuyi Biotechnology Co., Ltd) was used for photo analysis.
2.7 Determination of agglutination activity of M. oleifera leaves protein
2.7.1 Determination of the hemagglutination titer
A 2% rabbit blood cell suspension was prepared as previously described (13). Using a total of 6 wells of type V blood clots, 25 μL of 0.9% saline was successively added to each well using a pipette gun. Twenty-five microliters of M. oleifera leaves protein was added to the first well of each row. Double dilution was performed (except in the last well of each row, which was used as a blank control), and 25 μL of 2% rabbit red blood cell suspension was then added to each well. The plate was shaken on a micro-oscillator for 1 min to ensure that the mixture was fully mixed. Hemagglutination was observed visually after 2 h at room temperature and denoted by 2n (where n is the number of holes).
2.7.2 Determination of the thermal stability of M. oleifera leaves protein
Lyophilized M. oleifera leaves protein was dissolved in PBS (pH 7.4, 0.01 mol/L) to obtain a solution with an initial concentration of 5 mg/mL. The solution was heated in a water bath, and the temperature was increased starting from 20°C and maintained constant for 5 min every 10°C. The M. oleifera leaves protein solution was removed, cooled at room temperature, and diluted in a 96-well “V” type hemagglutination plate. The hemagglutination activity was measured 2 h later.
2.7.3 Determination of the sugar-binding specificity of M. oleifera protein
The test sugar was prepared in 0.5 mol/L mother liquor, 50 μL was added to well No. 1 of a 96-well “V” type hemagglutinin plate, and 50 μL of PBS buffer (pH 7.4, 0.01 mol/L) was added to wells No. 2–9. To each well,50 μL of Moringa leaves protein solution was added, and after 30 min at room temperature, 50 μL of red blood cell suspension was appended. The plate was allowed to stand for 2 h before the hemagglutination activity was measured. The following saccharides were used: α-methyl-D-glucopyranoside, maltose-hydrate, D-mannose, D(+) anhydrous glucose, D-raffinose pentahydrate, methyl-α-D-glucopyranoside, lactose, and methyl-β-D-galactoside.
2.7.4 Determination of the ion-binding properties of M. oleifera leaves protein
Moringa oleifera leaves protein solution was prepared with 0.01 mol/L PBS buffer (pH 7.4) and set aside. Fifty microliters of 11ion solutions (0.1 mol/L) were added to each well of the 96-well “V” hemagglutination plate and then diluted with PBS buffer (pH 7.4, 0.01 mol/L). Subsequently, 50 μL of Moringa oleifera leaves protein solution was added to each well, and the hemagglutination activity was detected.
2.7.5 Determination of the acid–base stability of Moringa oleifera leaves protein
Moringa oleifera leaves protein solution with an initial concentration of 5 mg/mL was prepared in a series of buffer systems with pH values from 3.7 to 13.0. The solution was left at room temperature for 2 h, and the hemagglutination activity under different pH conditions was measured after multiple dilution. The following buffers were used: NaCl-HAc buffer (0.1 mol/L) at pH 3.7–5.8, 0.1 mol/L NaH2PO4-Na2HPO4 buffer at pH 6.2–7.8, 0.1 mol/L Tris-HCl buffer at pH 8.3–9.1, 0.1 mol/L Na2CO3-NaHCO3 buffer at pH 9.5–10.8, 0.1 mol/L Na2HPO4-NaOH buffer at pH 11.2–11.9, and 0.1 mol/L KCl-NaOH buffer at pH 12.2–13.0.
2.8 Determination of the hemolytic activity of M. oleifera leaves protein
2.8.1 Effect of M. oleifera leaves protein at different concentrations on hemolytic activity
The hemolytic activity was determined using the assay (19). Briefly, PBS-washed rabbit erythrocytes were prepared in 0.1 M PBS (pH 7.2, 1:9 v/v) containing 10 mM calcium chloride. Fifty microliters of various concentrations of M. oleifera leaves protein (5, 10, 20, and 40 mg/mL) was incubated with 950 μL of rabbit red blood cells (1:20 v/v) and centrifuged (2,000 rpm, 5 min). The absorbance of the supernatant (erythrocyte lysate) was read at 540 nm using a microplate reader. Erythrocytes incubated in PBS (pH 7.2) were used as a negative control, and 20% Triton X-100 was used as a positive control. The percentage of hemolysis was calculated using the following formula:
where a is the percentage of hemolysis, Z is the erythrocyte hemolysis rate, At is the absorbance of the test group, Anc is the absorbance of the negative control group, and Apc is the absorbance of the positive control group.
2.8.2 Effect of sugar on the hemolytic activity of M. oleifera leaves protein
M. oleifera leaves protein (20 mg/mL) was incubated with 0.2 M maltose-hydrate, α-methyl-D-glucopyranoside, D-mannose, D(+) anhydrous glucose, D-lactate pentahydrate, lactose and methyl-β-D-galactoside for 2 h at 25°C and then with 10% freshly prepared rabbit red blood cell suspension (200 μL) at 30-min intervals with gentle shaking for 4 h. The samples were centrifuged (2,000 rpm, 5 min), and the absorbance values of red cell lysates were analyzed at 540 nm. To calculate the percentage of hemolysis, erythrocytes incubated in PBS (pH 7.2) were used as a negative control, and 20% Triton X-100 was used as a positive control.
2.8.3 Effect of ions on the hemolytic activity of M. oleifera leaves protein
M. oleifera leaves protein (20 mg/mL) was incubated with MgCl2, KCl, FeCl3, CaCl2, NaCl, FeCl2, NH4Cl, BaCl2, and AlCl3 stock solutions for 2 h at 25°C and then with 10% freshly prepared rabbit red blood cell suspension (200 μL) with gentle shaking at 30-min intervals for 4 h. Samples were centrifuged (2,000 rpm, 5 min), and the absorbance values of red cell lysates were analyzed at 540 nm. To calculate the percentage of hemolysis, erythrocytes incubated with PBS (pH 7.2) were used as a negative control, and 20% Triton X-100 was used as a positive control.
2.9 Statistical analysis
All the data in this study are presented as the means from three or more independent experiments ± the standard deviations (SDs). The data were analyzed using GraphPad Prism software (7.0.0), and one-way analysis of variance (ANOVA) was used for comparisons among groups. *P < 0.05 indicates a statistically significant difference.
3 Results
3.1 Single-factor experiments
3.1.1 Effect of the extractant type on the protein extraction rate
With an extraction time of 6 h, a saturation level of ammonium sulfate equal to 60%, and a solid-to-liquid ratio of 1:8, the effect of the type of extract on the protein extraction rate of M. oleifera leaves is shown in Figure 1A. The protein extraction rates of the Tris-HCl and PBS groups were significantly higher than those of the other groups, and that of the PBS group was higher. This finding may be related to the strong buffering effect of PBS buffer, which can better maintain the structure and activity of the protein; this finding is consistent with the results reported (14). Therefore, PBS was selected as the optimal extract for M. oleifera leaves protein.
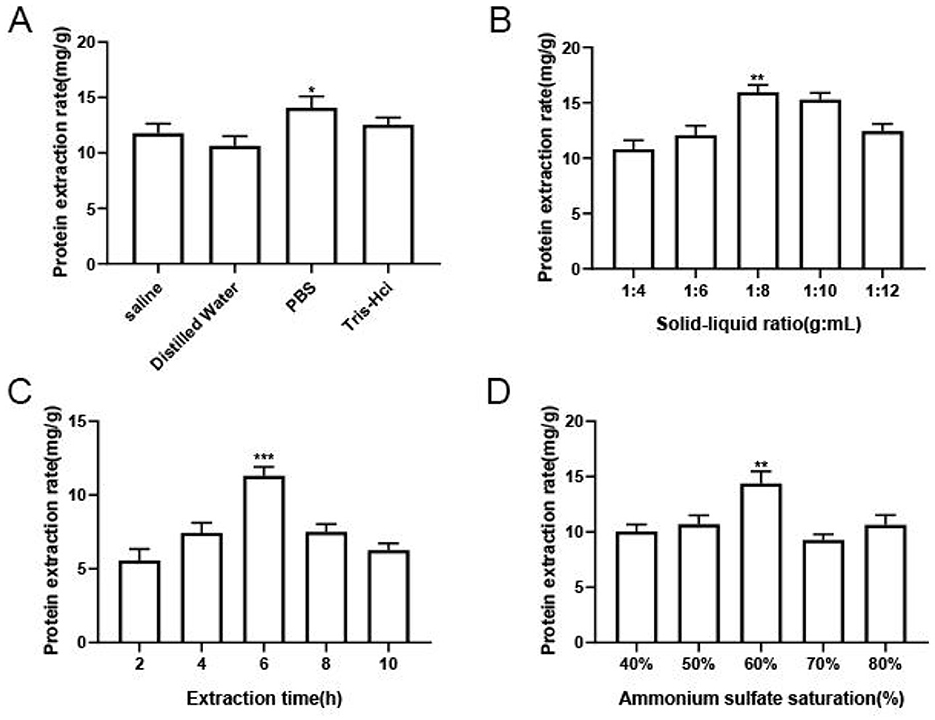
Figure 1. Results of the single-factor experiments. (A) Effect of the extractant type on the protein extraction rate. (B) Effect of the extraction time on the protein extraction rate. (C) Effect of the solid-to-liquid ratio on the extraction rate of M. oleifera leaves protein. (D) Effect of the ammonium sulfate saturation level on the extraction rate of M. oleifera leaves protein. The significance of the differences between groups is indicated as follows *P < 0.05, **P < 0.01, and ***P < 0.001.
3.1.2 Effect of the extraction time on the protein extraction rate
The effect of extraction time on the extraction rate of M. oleifera leaves protein was studied under the condition of 60% ammonium sulfate saturation and solid-to-liquid ratio of 1:8. As shown in the Figure 1B, an increase in the extraction time from 2 to 6 h increased the extraction rate of M. oleifera leaves protein from 5.57 to 11.3 mg/g. However, increasing the extraction time to 10 h decreased the protein extraction rate. This finding was probably observed because intracellular protein-degrading enzymes also are dissolved during the longer extraction time, and these protein-degrading enzymes degraded part of the dissolved proteins, thereby reducing the protein extraction rate (15). The optimal M. oleifera leaves protein extraction rate of 11.3 mg/g that was obtained with an extraction time of 6 h, which was 2.03 times that obtained with an extraction time of 2 h.
3.1.3 effect of the solid-to-liquid ratio on the extraction rate of M. oleifera leaves protein
Next, the effect of solid-liquid ratio on the protein extraction yield of M. oleifera leaves was explored at an extraction time of 6 h and ammonium sulfate saturation of 60%.leaves. As shown in the Figure 1C, an increase in the liquid-to-solid ratio from 1:4 to 1:8 increased the protein extraction rate of M. oleifera leaves from 10.89 to 15.96 mg/g. This rapid enhance in the protein extraction rate may be due to an appropriate growth in the solvent volume to improve the contact area for M. oleifera and augment its dispersion, which enhances the diffusion effect and thus advance the extraction rate (16). However, with a further increase in the solid-to-liquid ratio to 1:12, the protein in plant cells becomes completely dissolved, and the amount of solvent has no effect on the protein extraction rate (15). The results showed that once the soluble protein concentration reached its maximum value, an increase in the solid-to-liquid ratio decreased the protein concentration. Therefore, the optimal solid-to-liquid ratio for extracting M. oleifera leaves protein was determined to equal 1:8.
3.1.4 Effect of the ammonium sulfate saturation level on M. oleifera leaves protein extraction
The effect of ammonium sulfate saturation on the extraction rate of M. oleifera leaves at a solid-to-liquid ratio of 1:8 with an extraction time of 6 h is shown in Figure 1D. An increase in the ammonium sulfate saturation level from 40 to 60% raised the extraction yield of M. oleifera leaves protein from 10.03 to 14.37 mg/g, which indicated that M. oleifera leaves protein mainly consisted of salt-soluble proteins. However, further improve in the saturation level of ammonium sulfate increased to 80% decreased the extraction rate of M. oleifera leaves protein by 0.28 times compared with that obtained with an ammonium sulfate saturation level of 60%; this finding may be due to the salt-out of protein in a solution containing a high salt concentration, which would reduce the extraction rate of M. oleifera leaves protein (17). Therefore, the optimal ammonium sulfate saturation level for M. oleifera leaves protein extraction was determined to equal 60%.
3.2 Results from the orthogonal experiment of the M. oleifera leaves protein extraction process
Based on the results of the single-factor tests, the M. oleifera leaves protein extraction process was optimized, and a visual analysis of the orthogonal test (Table 2) was obtained. As shown in Table 2, the primary and secondary relationship of factors affecting the extraction rate of M. oleifera leaves protein identified in the orthogonal experimental design was the following: B > A > C, that is, solid–liquid ratio > extraction time > ammonium sulfate saturation. The optimal extraction process combination was thus as follows: an extraction time of 6 h, a solid-to-liquid ratio of 1:8, and an ammonium sulfate saturation level of 60%.
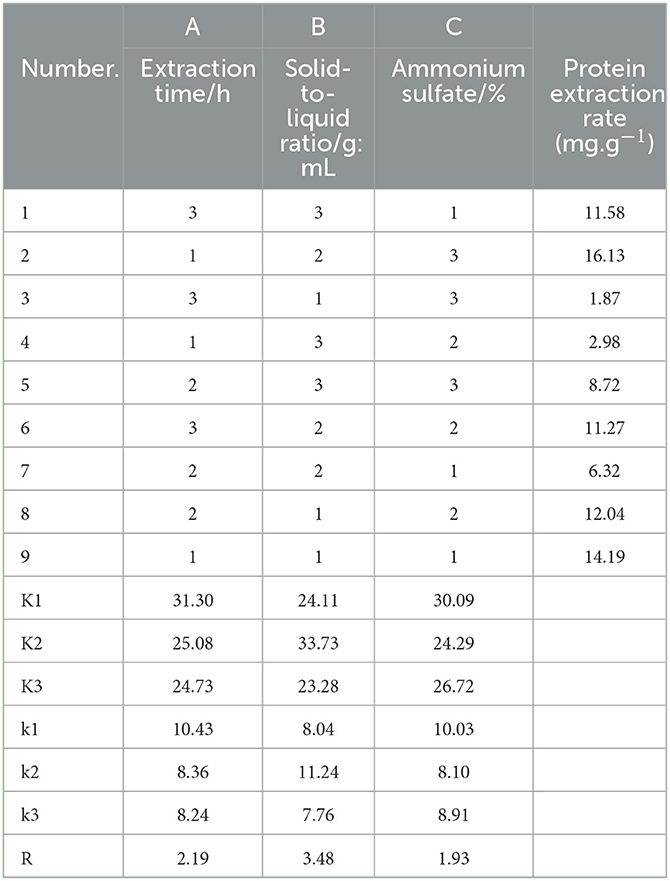
Table 2. Visual analysis of the orthogonal experiment for M. oleifera leaves protein extraction process optimization.
3.3 SDS–PAGE analysis of M. oleifera leaves protein
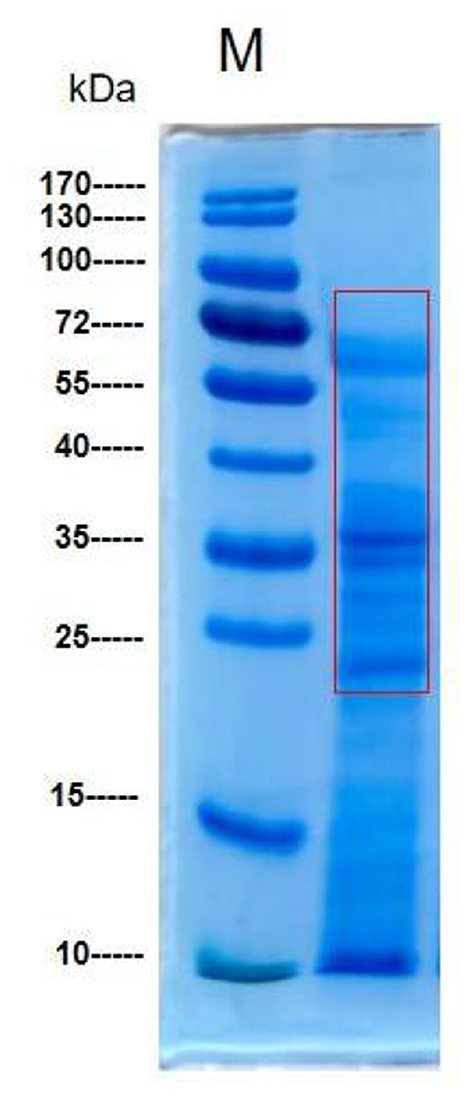
The protein bands of M. oleifera leaves obtained using the ammonium sulfate precipitation method were analyzed by SDS–PAGE (23). As shown in Figure 2, the protein bands were mainly concentrated at ~23 to 70 kDa, and the rest had a molecular weight of approximately 10 kDa; among all the observed bands, the highest abundance was observed for that at 35 kDa.
3.4 Hemagglutination titer of M. oleifera leaves protein
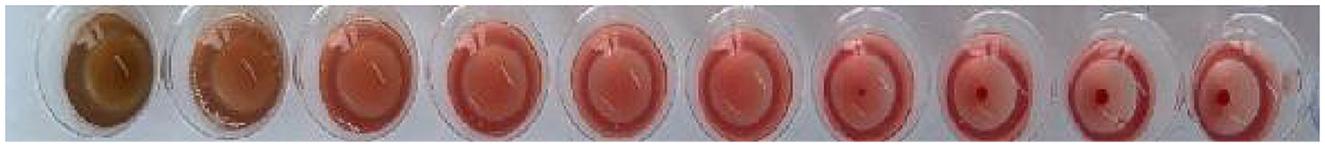
The comparison between the agglutination activity of M. oleifera leaves protein and the effect of rabbit erythrocyte agglutinin is shown in Figure 3 and Table 3. The results showed that crude M. oleifera leaves protein can agglutinate rabbit red blood cells, and the agglutination titer of M. oleifera leaves protein was 28.
3.4.1 Thermal stability of M. oleifera leaves protein
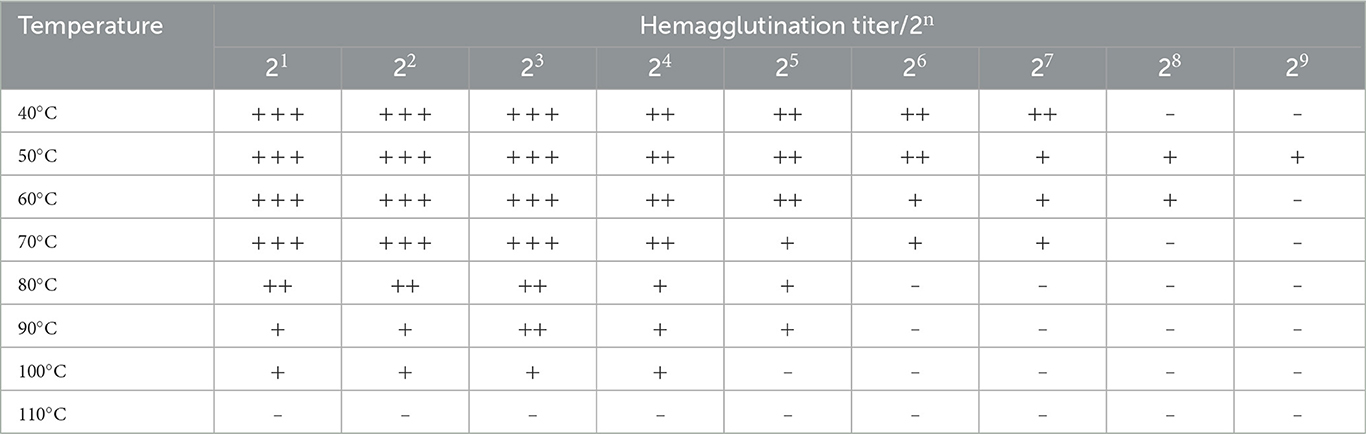
As shown in Table 4, when the temperature was below 60°C, the M. oleifera leaves protein was very stable, maintaining its full hemagglutination activity, and the hemagglutination titer was 28. Increasing the temperature gradually decreased the agglutination activity of M. oleifera leaves protein until a temperature of 110°C, which resulted in almost complete loss of hemagglutination activity. These findings show that temperature has a marked effect on M. oleifera leaves protein. The hemagglutination activity could be maintained under cooling conditions but might be reduced or even completely lost by increasing the temperature (28, 29).
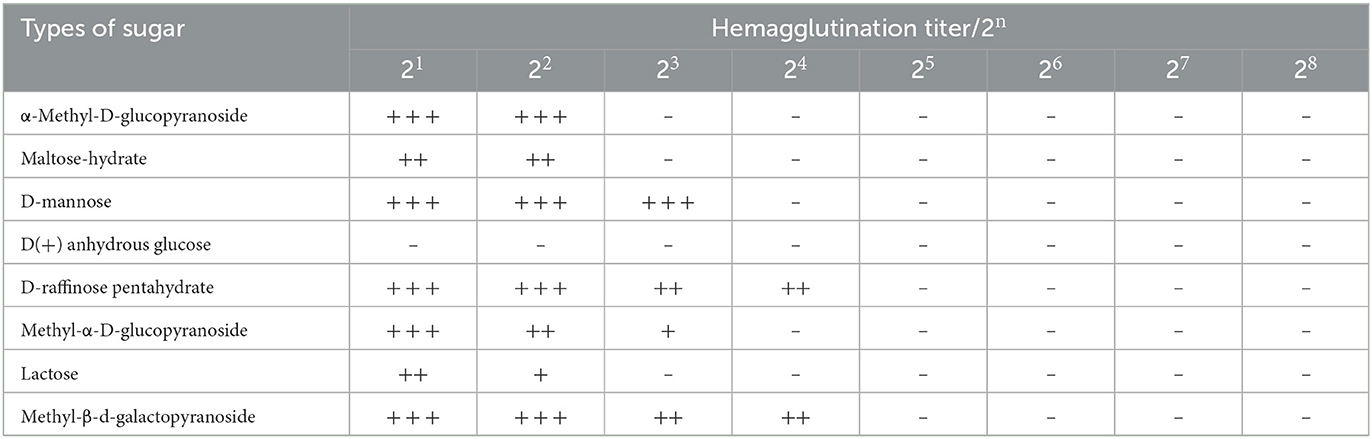
3.4.2 Sugar-inhibition/binding specificity of M. oleifera leaves protein
The inhibitory effects of eight sugars on M. oleifera leaves protein are shown in Table 5. The hemagglutination titer of α-methyl-D-glucopyranoside was 22, and those of maltose-hydrate, D-mannose, D(+) anhydrous glucose and were 22, 23, 20, and 24, respectively. In addition, the hemagglutination titer of methyl-α-D-glucopyranoside was 23, that of lactose was 22, and that of methyl-β-D-galactoside was 24. In the reactive state of hapten inhibition, the sugar with the best inhibitory effect is called the specific sugar of lectins. The results showed that D(+) anhydrous glucose was the specific inhibitory sugar of M. oleifera leaves lectin because it can better inhibit the agglutination reaction of rabbit red blood cells and M. oleifera leaves protein. α-Methyl-D-glucopyranoside, lactose and maltose-hydrate also have a partial inhibitory effect on the hemagglutination reaction (30–33).
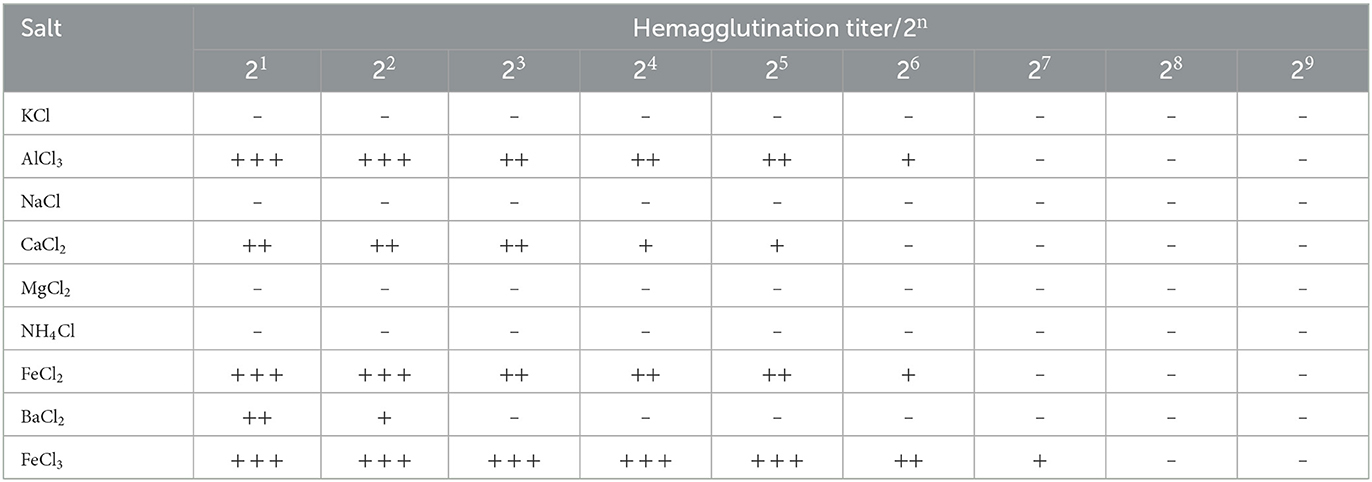
3.4.3 Ion-binding properties of M. oleifera leaves protein
Ions exert an important effect on protease activity and thermal stability (34, 35, 39). Most legume lectins are ions -binding proteins or glycoproteins. Ions stabilize the structure and increase the resistance of lectins to heat and thus inactivate proteolytic enzymes (40). The effect of ions on the hemagglutination activity of M. oleifera leaves protein was determined, and the results are shown in Table 6. As revealed by the results, M. oleifera lectin is aion-dependent lectin. K+, Na+, Mg2+, and NH4+ induced loss of the hemagglutination activity of M. oleifera, whereas M. oleifera leaves protein was found to be dependent on Ca2+, Fe3+, Fe2+, and Al3+. After Ca2+, Fe3+, Fe2+, and Al3+ treatment, the protein continued to exhibit high hemagglutination activity, and its hemagglutination titer was 25-27.
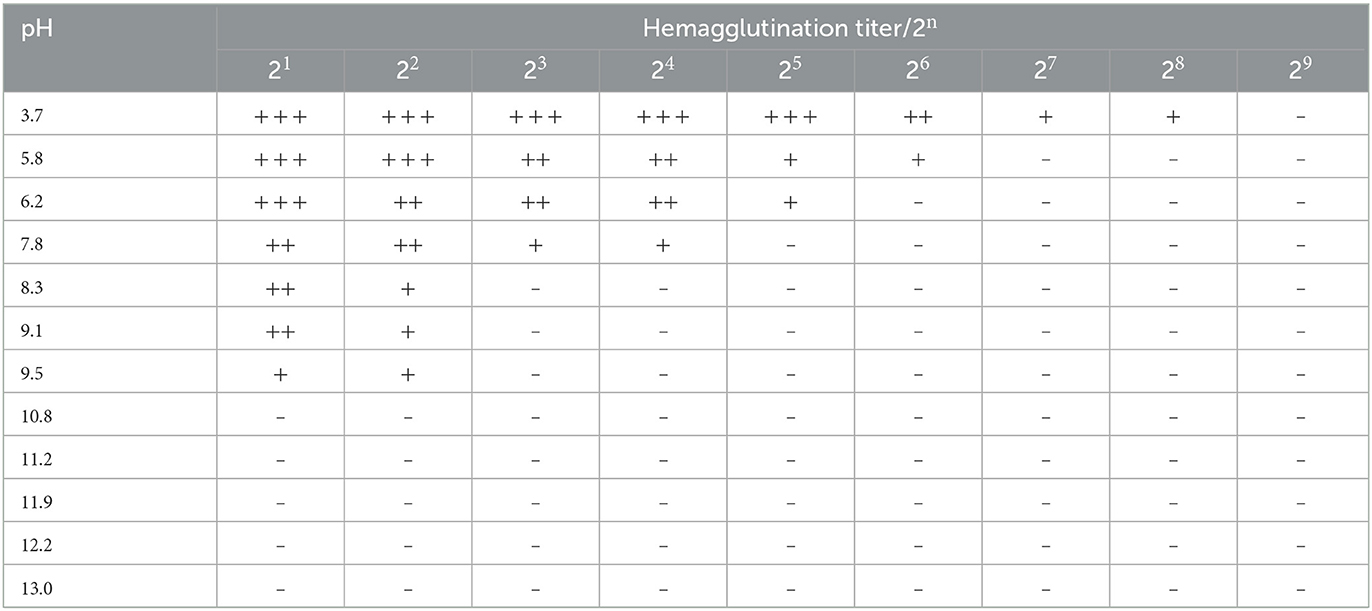
3.4.4 Acid/base stability of M. oleifera leaves protein
Lectins are generally more stable to acidic and basic conditions (41). Different pH values have obvious effects on the hemagglutination activity of M. oleifera leaves protein. The experimental results are shown in Table 7. The highest hemagglutination activity of M. oleifera leaves protein was observed at pH 3.7, and the hemagglutination activity of M. oleifera leaves protein was completely lost at a pH value of 11.9. The reasons for this finding are as follows: at near-neutral pH, the calm electric repulsion energy is smaller than the energy of other stable protein interactions, and the M. oleifera leaves protein is thus stable (42, 43). However, the strong molecular electrostatic repulsion caused by the high electrostatic charge at extreme pH leads to the swelling and expansion of M. oleifera leaves protein molecules and the ionization of carboxyl, phenolic hydroxyl and sulfhydryl groups partially buried in the protein molecules (43, 44). These ionic groups expose themselves to the water environment, resulting in the dispersion of polypeptide chains, which fundamentally changes the spatial structure of M. oleifera leaves protein and thus leads to irreversible denaturation and loss of vitality.
3.5 Determination of the hemolytic activity of M. oleifera leaves protein
3.5.1 Effect of M. oleifera leaves protein at different concentrations on hemolytic activity
The erythrocyte is considered a simple mimic of the endosome membrane and is thus a model for the study of protein-membrane interactions and for the in vitro assessment of the biocompatibility of important biotherapies (36). Intravenous therapeutic agents can trigger hemolysis, which is characterized by the release of hemoglobin into plasma as a result of the destruction, interference, or breakdown of red cells, which makes the search for specific proteins as carrier molecules imperative (37, 38). As shown in Figure 4, the cytocompatibility and endolytic activity of M. oleifera leaves proteins on rabbit red blood cells were studied using the hemolysis test, and the percentage of hemolysis was found to increase in a concentration-dependent manner. The hemolysis rate of M. oleifera protein at a concentration of 20 mg/mL was 5.68%, whereas that of the control group was 0.1% (P < 0.001).
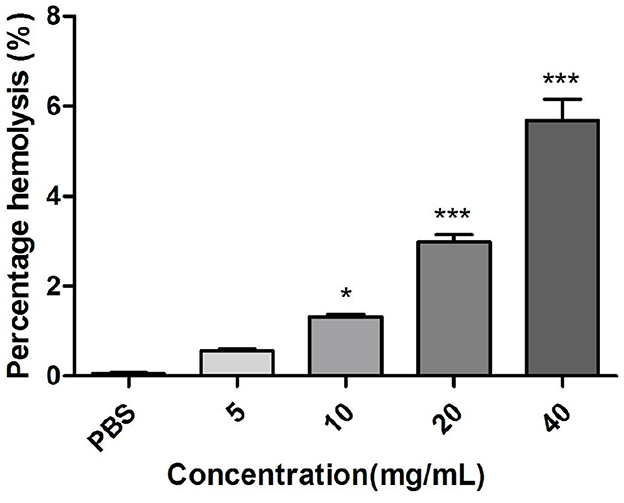
Figure 4. Effects of different concentrations of M. oleifera leaves protein on hemolytic activity. The data were compared with those of the blank control group (PBS), and the significance of the differences between groups is indicated as follows *P < 0.05 and ***P < 0.001.
3.5.2 Effect of sugars on the hemolytic activity of M. oleifera leaves protein
As shown in Figure 5, a significant difference in the hemolysis rate was found between M. leaves-treated erythrocytes and erythrocytes preincubated with α-methyl-mannoside, galactoside, and raffinose (P < 0.001). The strong inhibitory activity of lactose may indicate that the lectin possesses an extended binding site. Furthermore, the decreased hemolytic activity of M. oleifera leaves protein in the presence of α-methyl-mannoside, galactoside, and raffinose further confirmed the previously demonstrated fact that M. oleifera leaves lectins have an affinity for ketose sugars, which inhibits the hemolytic activity of M. oleifera leaves protein. To improve the efficiency and reduce the side effects of specific therapeutic agents, protein-related targeted drug delivery systems have become a favorable strategy (18, 19).
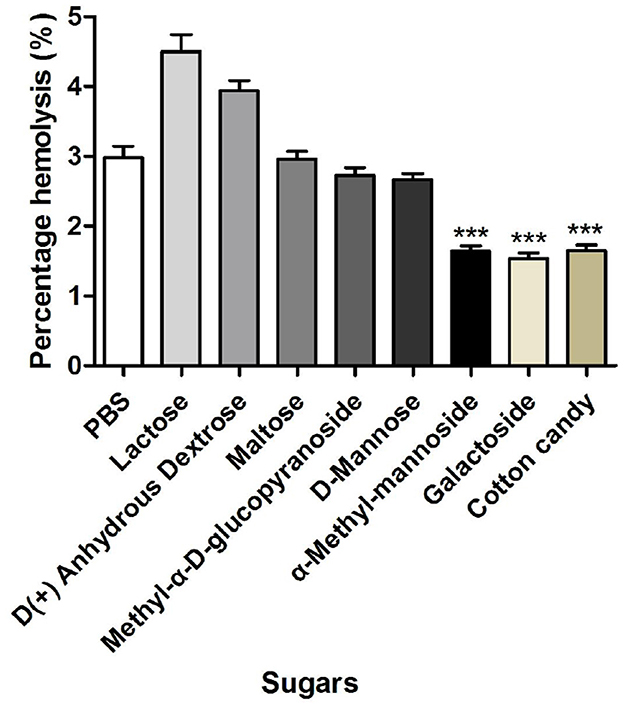
Figure 5. Effects of different sugars on the hemolytic activity of M. oleifera leaves protein. The data were compared with those of the blank control group (PBS), and the significance of the differences between groups is indicated as follows: ***P < 0.001.
3.5.3 Effect of ions on the hemolytic activity of M. oleifera leaves protein
As shown in Figure 6, among K+, Na+, Mg2+, NH4+, Fe2+, Ca2+, Fe3+, and Al3+ ions, the higher hemolysis rate was observed with erythrocytes treated with Fe2+ and M. oleifera leaves protein, indicating that Fe2+ caused erythrocytes to be destroyed, interfered or decomposed and released into plasma. However, Al3+ inhibited the hemolytic activity of M. oleifera protein, and similar results were reported for calcium-dependent hemolysin purified from Cucumis acanthopanthus (20) and Granella anadarensis (21).
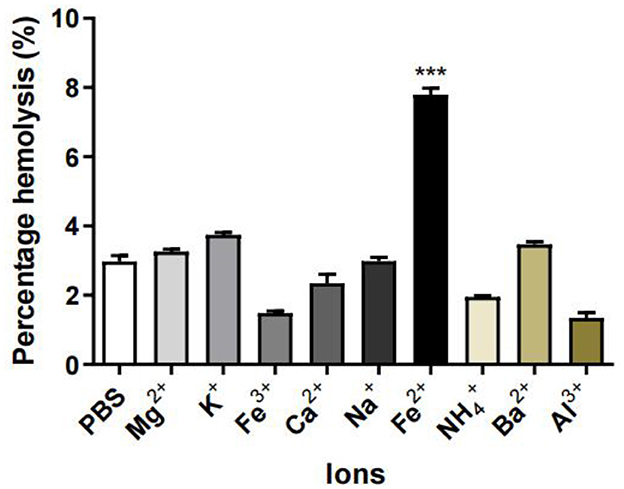
Figure 6. Effects of ions on the hemolytic activity of M. oleifera leaves protein. The data were compared with those of the blank control group (PBS), and the significance of the differences between groups is indicated as follows: ***P < 0.001.
4 Discussion and conclusion
The total amount of amino acids in M. oleifera leaves reaches 20.49%, and these leaves contain right types of essential amino acids that the human body cannot synthesize by itself or produces at a synthesis rate that cannot meet the body's needs (45–47). The most common methods for protein extraction include ultrafiltration, alkali-soluble acid precipitation, heating, and salting out, and the most traditional method is alkali-soluble acid precipitation (22, 48, 49). Based on the analysis of several extraction methods (50, 51), salting out method is the most suitable method for M. oleifera leaves protein extraction. The salting out method is used to separate and purify plant leaves proteins by using the characteristics of protein precipitation in a solution with a certain salt concentration (52). This method has the advantages of simple operation, low cost, and high leaves protein activity. Chen et al. (31) found that the salting out method was the most suitable method for extracting protein from leaves of Wedelia sinensis due to its high extraction efficiency, simple operation, good protein activity, and suitability for laboratory extraction and industrial production (53, 54). Therefore, in this study, fresh M. oleifera leaves were used as raw materials for the extraction of protein by the salting out method, and the effects of the extraction time, ammonium sulfate saturation and solid-to-liquid ratio on the protein extraction rate were studied to determine the best process conditions and thus lay a theoretical foundation for the deep processing of M. oleifera leaves protein products and promote the healthy development of the M. oleifera industry.
Most researchers believe that protein in food is the main cause of food allergy (24–26). Studies have found that plant-derived food allergens such as peanut, soybean, and wheat contain lectins, and these lectins may be inextricably related to allergy (55, 56). In this study, it was speculated that M. oleifera leaves lectin or leaves protein is the main protein responsible for the induction of allergy, and studying its basic characteristics is the first step for understanding the nature of the lectin. This study investigated various properties of M. oleifera lectin, particularly thermal stability, acid–base stability, ion-binding characteristics, sugar-binding specificity and hemolytic activity, to provide a theoretical basis for the development and utilization of M. oleifera lectin and agglutination active substances, which will pave the way for further understanding of protein anti-nutrients in M. oleifera lectins derived from different organisms or different types of lectins differ in their physical and chemical properties, and their biological characteristics are the basis for the wide application of lectins in different industries (57). Among them, sugar specificity is the most basic property of lectins. This property determines the hemocyte binding specificity, mitogenesis, cell recognition and agglutination properties of lectins. For legume lectins, the mutual recognition mechanism between rhizobia and host plants is also based on sugar specificity (58). Lectins are expected to become widely used in biology, biomedicine and gene engineering of disease resistance.
This study performed a preliminary exploration of the allergy-related activity of M. oleifera leaves protein. It was only demonstrated that the M. oleifera leaves protein has lectin related activity. We also identified the sequences of M. oleifera allergens. We took the three proteins and predicted that the sequences we identified are not homologous to the mO1 and mO2 proteins, but the 36 kd we identified is a homologous protein to Fructose 1,6 bisphosphate aldolase, FBA, and the substrate of FBA is a sugar, which has a sugar-binding sequence. According to the methodology of Nguyen et al. (59) and Maurer-Stroh et al. (60), we took the three previous sequences and used AllerCatpro to predict and study the allergenic potential of the proteins. Here we used AllerCatpro to predict that we can prove that the allergen is minimal, but our previous Western Blot experiment done on mouse serum and human serum bound to M. oleifera leaves protein, the bands are obvious, which is a strong evidence. But at the moment this relevant result is not published.
In the future, the purifi-cation of M. oleifera leaves protein allergens, the elimination of M. oleifera leaves protein allergens and the treatment of allergic diseases sensitized by M. oleifera leaves protein will be studied.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
JL: Conceptualization, Methodology, Writing – original draft. XL: Software, Validation, Writing – original draft, Writing – review & editing. WL: Formal analysis, Investigation, Resources, Writing – original draft. CX: Data curation, Writing – original draft. DF: Data curation, Writing – original draft. SS: Funding acquisition, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Natural Science Foundation of China, grant number 31960508 and Science and Technology Plan Project of Science and Technology Department of Yunnan Province, grant number 202401BBD070001-034.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gong D-y, Zuo T, Class X, Liu QG. Moringa oleifera cultivation and utilization. Guizhou Forest. Sci. Technol. (2006) 34:3.
2. Li S, Li Q. Application value and development prospect of Moringa oleifera in Guizhou. Agric Technol Serv. (2007) 24:1.
3. Changfen L, Liu C, Guo-Hua L, Li GH. Nutritional value of Moringa oleifera. Proc China Food Ind Assoc. (2011) 23:13.
5. Goldstein I, Hayes RC, Monsugny M, Osawa T, Sharon N. What should be called a lectin? Nature. (1980) 285:66. doi: 10.1038/285066b0
6. Wang Z, Li X, Guo S. Plant lectins and insect-resistant genetic engineering. Biotechnol Bull. (1998) 5–10.
7. Friedman M, Brandon DL. Nutritional and health benefits of soy proteins. J Agric Food Chem. (2001) 49:1069–86. doi: 10.1021/jf0009246
8. Feng D, Wang Z. Research progress on anti-trophic factors and their treatment. Progr Anim Nutr Res. (2001) 3:93–110.
9. Satish A, Sairam S, Ahmed F, Urooj A. Moringa oleifera Lam.: protease activity against blood coagulation cascade. Pharmacognosy Res. (2012) 4:44–9. doi: 10.4103/0974-8490.91034
10. Bijina B, Chellappan S, Krishna JG, Basheer SM, Elyas KK, Bahkali AH, et al. Protease inhibitor from Moringa oleifera Lam. with potential for use as therapeutic drug and as seafood preservative. Saudi J Biol Sci. (2011) 18:273–81. doi: 10.1016/j.sjbs.2011.04.002
11. Pusztai A, Grant G, Spencer RJ, Duguid TJ, Bardocz S. Kidney bean lectin-induced Escherichia coli overgrowth in the small intestine is blocked by GNA, a mannose-specific lectin. J Appl Microbiol. (2010) 75:360–8. doi: 10.1111/j.1365-2672.1993.tb02788.x
12. Zenteno R, Vázquez L, Martínez-Cairo S, Bouquelet S, Agundis C, Zenteno E. Identification of lectin isoforms in juvenile freshwater prawns Macrobrachium rosenbergii. Glycoconjugate, (2000) 17:339–47.
13. Xi C, Li W, Liu X, Xie J, Li S, Tian Y, et al. The potential role of Moringa oleifera Lam leaf proteins in moringa allergy by functionally activating murine bone marrow-derived dendritic cells and inducing their differentiation toward a Th2-polarizing phenotype Nutrients. (2023) 16:17. doi: 10.3390/nu16010007
14. Zhang J, Liu X, Wang Z, Zhang H, Gao J, Wu Y, et al. Potential allergenicity response to Moringa oleifera leaf proteins in BALB/c Mice. Nutrients. (2022) 14:4700. doi: 10.3390/nu14214700
15. D'Auria G, Nitride C, Nicolai MA, Mamone G, Montesano D, Mills SNC, et al. Identification of allergen encoding sequences in a novel food ingredient from Moringa oleifera leaves. Food Chem. (2023) 401:134185. doi: 10.1016/j.foodchem.2022.134185
16. Iddagoda J, Gunasekara P, Handunnetti S, et al. Identification of allergens in Artocarpus heterophyllus, Moringa oleifera, Trianthema portulacastrum and Syzygium samarangense. Clin Mol Allergy. (2023) 21:6. doi: 10.1186/s12948-023-00187-2
17. Nozdrachev AD, Akkuratov EG, Fateev MM. The distribution pattern of galactose-specific lectin receptors in sensory ganglia of mature white rats. Doklady Biol Sci. (2002) 386:445–7. doi: 10.1023/A:1020770402912
18. Edelman GM, Rutishauser U, Millette CF. Cell fractionation and arrangement on fibers, beads and surfaces. Proc Natl Acad Sci USA. (1971) 68:2153–7. doi: 10.1073/pnas.68.9.2153
19. Gabius HJ, Gabius S. Concepts of tumor lectinology. Cancer Invest. (1997) 15:454–64. doi: 10.3109/07357909709047585
21. Campos FV, Chanda B, Beirao PSL, Bezanilla F. α-scorpion toxin impairs a conformational change that leads to fast inactivation of muscle sodium channels. J Gen Physiol. (2008) 132:251. doi: 10.1085/jgp.200809995
22. Xu C, Peng K, Wang W-s, Yu M, Sun L, Yang B. Effect of mannose-binding lectin on barrier function of intestinal epithelial cells. J Third Milit Med Univ. (2015) 37:5.
23. Pan L, Qin G, Zhao Y, Wang J, Liu F, Che D. Effects of soybean agglutinin on mechanical barrier function and tight junction protein expression in intestinal epithelial cells from piglets international. J Mol Sci. (2013) 14:21689–704. doi: 10.3390/ijms141121689
24. Zhao Y, Qin G, Sun Z, Che D, Bao N, Zhang X. Effects of soybean agglutinin on intestinal barrier permeability and tight junction protein expression in weaned piglets. Int J Mol Sci. (2011) 12:8502–12. doi: 10.3390/ijms12128502
25. Zhao X, Fu Y, Zhao Q, Ning F, Xiong H. Preliminary study on isolation of selenium-rich soybean protein by ammonium sulfate precipitation. Sci Technol Food Ind. (2016) 37:5.
26. Roy UK, Lavignac N, Rahman AM, Nielsen BV. Purification of lectin and Kunitz trypsin inhibitor from soya seeds. J Chromatogr Sci. (2018) 12:3.
27. Dai J, Luo X, Shi C-y, Zhao C, Sheng J, Tian Y, et al. Optimization of water-soluble protein extraction from Moringa oleifera leaves by response surface method. J Yunnan Agri Univ. (2020) 35:9.
28. Huang Q, Peng X, Huang HF. Preliminary study on ultrasound-assisted salt extraction of Moringa oleifolia leaves protein. Agric Res Appl. (2016) 4.
29. Wen C-w, Zhao Y, Shi L, Ouyang Z. Regularity of polyethylene glycol precipitation of egg white protein and its application in ovalbumin separation. Food Sci. (2018) 39:7.
30. Jie LS, Zhou Z, Guoyong L, Jie Q, Yueling Z, Biology DO, et al. Investigation of agglutinative activity of human hemoglobin. J Shantou Univ. (2017) 21:1.
31. Chen X, Hu Y-p, Lin Y-k, Liu Z-d, Song L-y. Extraction and screening of protein from the leaves of Wedelia South America. Acta Agric Sinica. (2017) 48:1274–9.
32. Sun YY Li X, Shao N, Huang G, Su X, Zheng YM. Optimization of ultrasound-assisted extraction of polyphenols from Moringa oleifera leaves by Box-Behnken response surface method. Subtrop Plant Sci. (2018) 47:6.
33. Zhang L, Yu G, Qi W, Liu M, Cai X, Yue CH. Optimization of extracting protein from Auricularia auricularia by alkali dissolution and acid precipitation. Food Ind. (2015) 36:4.
34. Shao T, Liu X, Li C, Zhong J-f, Qin XL. Ultrasonic assisted efficient extraction of protein from Moringa oleifera seeds. Food Ferment Ind. (2019) 45:7.
35. Lohcharoenkal W, Wang L, Chen YC, Rojanasakul Y. Protein nanoparticles as drug delivery carriers for cancer therapy. Biomed Res Int. (2014) 2014:180549. doi: 10.1155/2014/180549
36. Xie J, Li Y, Cao Y, Xu C, Xia M, Qin M, et al. Photo synthesis of protein-based drug-delivery nanoparticles for active tumor targeting. Biomater Sci. (2013) 1:1216. doi: 10.1039/c3bm60174a
37. Nagao T, Masaki R, Unno H, Goda S, Hatakeyama T. Effects of amino acid mutations in the pore-forming domain of the hemolytic lectin CEL-III. Biosci Biotechnol Biochem. (2016) 1−4. doi: 10.1080/09168451.2016.1176520
38. Adhya M, Singha B. Gal/GalNAc specific multiple lectins in marine bivalve Anadara granosa. Fish Shellfish Immunol. (2016) 54:123. doi: 10.1016/j.fsi.2016.01.036
39. Pan LJ, Liu XJ. Research progress on cultivation, development and utilization of Moringa oleifera. Guangdong For Sci Technol. (2010) 26:71–7.
40. Sánchez-Machado DI, Núez-Gastélum JA, Reyes-Moreno C, Ramírez-Wong B, López-Cervantes J. Nutritional quality of edible parts of Moringa oleifera. Food Anal Methods. (2010) 3:175–80. doi: 10.1007/s12161-009-9106-z
41. Machida T, Novoa A, Gillon É, Zheng S, Claudinon J, Eierhoff T, et al. Dynamic cooperative glycan assembly blocks the binding of bacterial lectins to epithelial cells. Angewandte Chemie (2017) 129:6866–70. doi: 10.1002/ange.201700813
42. Gao Q, Jin X, Liu Z. Wang X-w, Zhang J, Xue YL. Advances in leaves protein research. Preserv Process. (2020) 23:98.
43. Chen X, Hu Y-p, Lin Y, Liu Z, Song LY. Extraction and screening of protein from the leaves of Wedelia sp. J South Agric. (2017) 48:6.
44. Sun C-z, Min T, Liu Y, Zhu J-h, Wu H. Comparison of functional properties of mulberry leaves proteins prepared by different methods. Mod Food Sci Technol. (2015) 8.
45. Pi X, Sun Y, Cheng J, Fu G, Guo M. A review on polyphenols and their potential application to reduce food allergenicity. Crit. Rev. Food Sci. (2022) 63:10014–31. doi: 10.1080/10408398.2022.2078273
46. Silva ACD, Moura S, Constant P. Sistema imunológico e principais alimentos envolvidos Food allergy: system immunologic and main food involved. Agric Res Appl. (2022).
47. Maciorkowska E, Panasiuk A, Kaczmarski M. Concentrations of gastric mucosal cytokines in children with food allergy and Helicobacter pylori infection. World J Gastroenterol. (2005) 11:6751. doi: 10.3748/wjg.v11.i43.6751
48. Umeoguaju FU, Ephraim-Emmanuel BC, Patrick-Iwuanyanwu KC, Zelikoff JT, Orisakwe OE. Plant-derived food grade substances (PDFGS) active against respiratory viruses: a systematic review of non-clinical studies. Front Nutr. (2021) 8:606782. doi: 10.3389/fnut.2021.606782
49. Skerritt JH, Hill AS, Sashidhar Rao RB, Beasley HL, Rani BEA, Udaya Kumari CG, et al. Sample matrix interference in immunoassays for organochlorine residues in plant-derived foods and some strategies for their removal. Food Agric Immunol. (2003) 15:17–34. doi: 10.1080/0954010031000138078
50. Astwood JD, Leach JN, Fuchs RL. Stability of food allergens to digestion in vitro. Nat Biotechnol. (1996) 14:1269–73. doi: 10.1038/nbt1096-1269
51. Jamil SA, Naseer SA, Noreen K. Comparison in effect of different ions, pH and reducing agent on the protease activity in human hyper mature and mature cataract. J Zhejiang Univ. (2007) 69–73. doi: 10.1631/jzus.2007.B0599
52. Rottensteiner H, Kaufmann S, Rathgeb A, Kink B, Plaimauer B, Matthiessen P, et al. Temperature-dependent irreversible conformational change of recombinant ADAMTS13 upon ion chelation. J Thromb Haemost. (2019) 17:995–1002. doi: 10.1111/jth.14440
53. Su A, Lin Y. A large umbrella lectin purification and properties of the. J Appl Environ Biol. (2004) 10:497–501.
54. Wong JH, Ng TB. Purification of a trypsin-stable lectin with antiproliferative and HIV-1 reverse transcriptase inhibitory activity. Biochem Biophys Res Commun. (2003) 301:545–50. doi: 10.1016/S0006-291X(02)03080-2
55. Wong JH, Ng TB. Isolation and characterization of a glucose/mannose/ rhamnose-specific lectin from the knife bean Canavalia gladiata. Arch Biochem Biophys. (2005) 439:91–8. doi: 10.1016/j.abb.2005.05.004
56. Zhu J, Wang H. Study on the INACTIVATION of Concanthus bean lectin. J Zhengzhou Inst Eng. (2002) 23:89–93.
57. Lavelle EC, Grant G, Pusztai A, Pfüller U, O'Hagan DT. Mucosal immunogenicity of plant lectins in mice. Immunology. (2000) 99:30–7. doi: 10.1046/j.1365-2567.2000.00932.x
58. Wood SD, Wright LM, Reynolds CD, et al. Structure of the native (unligated) mannose-specific bulb lectin from Scilla campanulata (bluebell) at 17 Resolution. Acta Cryst. (1999) 55:1264–72. doi: 10.1107/S0907444999005326
59. Nguyen MN, Krutz NL, Limviphuvadh V, Lopata AL, Gerberick GF, Maurer-Stroh S. AllerCatPro 2.0: a web server for predicting protein allergenicity potential. Nucl Acids Res. (2022) 50:W36–43. doi: 10.1093/nar/gkac446
Keywords: Moringa oleifera leaves protein, allergy, allergy-related activities, hemagglutination, hemolytic activity
Citation: Lu J, Liu X, Li W, Xi C, Feng D and Song S (2025) Analysis of the sensitization activity of Moringa oleifera leaves protein. Front. Nutr. 11:1509343. doi: 10.3389/fnut.2024.1509343
Received: 12 October 2024; Accepted: 18 December 2024;
Published: 22 January 2025.
Edited by:
Mostafa Gouda, National Research Centre, EgyptReviewed by:
Xiaolong Ji, Zhengzhou University of Light Industry, ChinaLee Sin Chang, USCI University, Malaysia
Minh Nguyen, Bioinformatics Institute (A*STAR), Singapore
Copyright © 2025 Lu, Liu, Li, Xi, Feng and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuang Song, c29uZ3NodWFuZ0B5bmF1LmVkdS5jbg==; Xiaoxue Liu, bGl1eGlhb3h1ZTA3MjZAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Juan Lu1,2†
Juan Lu1,2† Shuang Song
Shuang Song