- Department of Anesthesiology, Renmin Hospital, Hubei University of Medicine, Shiyan, Hubei, China
Background: Carotenoids are well-established for their potent antioxidant properties; however, their potential association with severe headaches or migraines remains largely unexamined. This study was conducted to explore the relationship between serum carotenoid levels and the prevalence of severe headaches or migraines within the US population.
Methods: We utilized data from the 2001–2004 National Health and Nutrition Examination Survey (NHANES), which comprised a total of 8,910 participants. Serum carotenoid levels—specifically α-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin, and lycopene—were quantified using high-performance liquid chromatography. Migraine status was determined based on a questionnaire. The research methodologies employed included multivariate logistic regression, subgroup analysis, and restricted cubic spline (RCS) models.
Results: The prevalence of migraines in the study population was 22.37%. Multivariate logistic regression analysis indicated that serum concentrations of α-carotene (OR = 0.91, 95% CI: 0.85–0.97), β-carotene (OR = 0.88, 95% CI: 0.81–0.94), β-cryptoxanthin (OR = 0.83, 95% CI: 0.76–0.90), lutein/zeaxanthin (OR = 0.75, 95% CI: 0.67–0.85), and total carotenoids (OR = 0.79, 95% CI: 0.70–0.90) were significantly inversely correlated with severe headaches or migraines; however, no significant association was found for lycopene levels. RCS analysis showed that β-cryptoxanthin had an L-shaped non-linear relationship with migraine prevalence at a threshold of approximately 9.392 μg/dL, while subgroup analyses confirmed the consistent inverse association between total serum carotenoid concentrations and migraine prevalence across various groups.
Conclusion: Serum concentrations of α-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin, and total serum carotenoids were inversely correlated with the incidence of severe headaches or migraines in US adults. This evidence indicates that carotenoids may provide a protective effect against migraines; however, further investigation is warranted to substantiate these associations and to elucidate the underlying mechanisms.
1 Background
Migraine is a highly prevalent and debilitating neurological disorder, affecting more than 1.5 billion people worldwide, with a higher prevalence in women (1–3). The most common symptom of a migraine is a throbbing headache that comes on repeatedly. Other symptoms include light or sound sensitivity, nausea, and vomiting (4, 5). These debilitating symptoms not only cause significant suffering for patients but also result in substantial economic burdens due to absenteeism and reduced productivity. In the U.S. alone, the direct and indirect costs associated with migraines are estimated to exceed $20 billion annually (6). While the precise pathophysiological mechanisms underlying migraines are still unknown, an increasing body of research indicates that oxidative stress is a major factor in the etiology of migraines (7, 8). Free radicals and the body’s antioxidant defenses are out of balance, which leads to oxidative stress, and can lead to cellular damage, inflammatory responses, and abnormal vasoconstriction and vasodilation, which are thought to trigger migraines (9, 10). Studies have shown that oxidative stress markers are elevated in individuals with migraines, while antioxidant levels tend to be lower (11). Therefore, nutrients with antioxidant properties, such as carotenoids, may potentially exert a protective effect by reducing the occurrence or severity of migraines.
Carotenoids are natural pigments found in plants that possess strong antioxidant and anti-inflammatory properties. Their physiological functions are primarily achieved by scavenging free radicals, reducing oxidative stress, and enhancing immune function (12, 13). Multiple research studies indicate that carotenoids have a negative correlation with the likelihood of developing chronic illnesses such as cardiovascular diseases, specific forms of cancer, and age-related macular degeneration (14–16). One example is the significant association between elevated serum levels of β-carotene and a decreased likelihood of developing cardiovascular disease (17). Additionally, Carotenoids like lutein and zeaxanthin have been shown to protect the eyes, particularly by defending against age-related ocular diseases (18). Although these health benefits are well-established, the specific relationship between carotenoid levels and neurological disorders, including severe headaches and migraines, remains insufficiently understood.
Our research aims to analyze the correlation between levels of serum carotenoids and the prevalence of severe headaches or migraines using data from the 2001–2004 NHANES, offering new insights into the potential role of nutritional interventions in migraine management.
2 Materials and methods
2.1 Data sources and study population
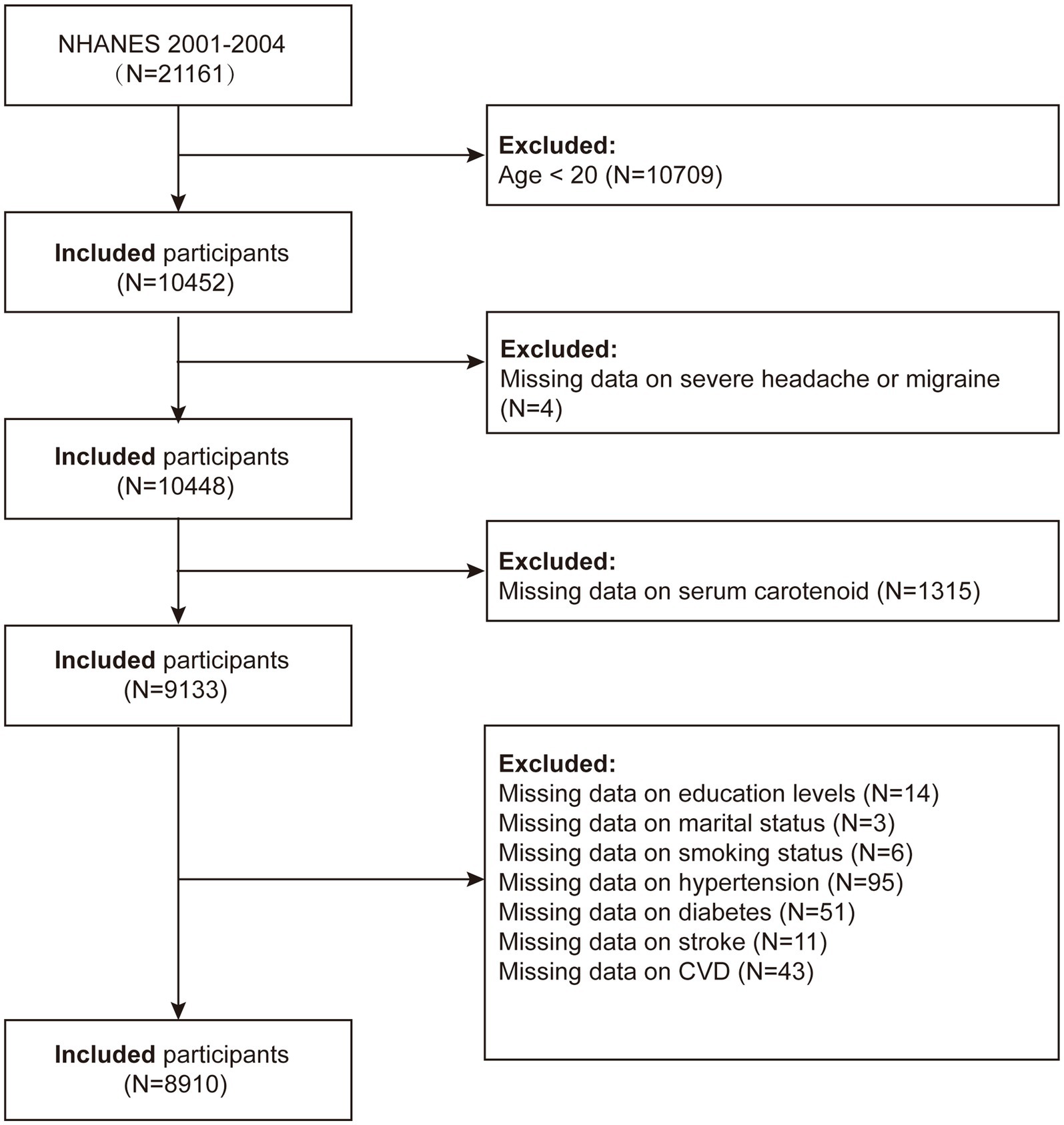
NHANES is a thorough survey conducted across the entire United States by the National Center for Health Statistics (NCHS) with the goal of precisely evaluating the health and nutritional status of the American population. We used data from NHANES 2001–2004, initially including 21,161 participants in the study. The criteria for exclusion were the following: (1) participants under 20 years of age (n = 10,709); (2) participants missing migraine data (n = 4); (3) participants missing carotenoid data (n = 1,315); (4) participants missing educational attainment data (n = 14); (5) participants with incomplete marital status information (n = 3); (6) participants with incomplete smoking status information (n = 6); (7) participants missing hypertension data (n = 95); (8) participants missing diabetes data (n = 51); (9) participants missing stroke data (n = 11); and (10) participants missing cardiovascular disease data (n = 43). After applying these criteria, the final analysis included a total of 8,910 individuals (Figure 1).
2.2 Measurement of serum carotenoids
Fasting blood samples were obtained using red-top or royal blue-top Vacutainer® tubes, frozen at −70°C, and transported with dry ice to the National Center for Environmental Health for analysis of serum carotenoid levels. High-performance liquid chromatography (HPLC) with photodiode array detection was utilized to measure serum carotenoids including α-carotene, β-carotene, β-cryptoxanthin, lycopene, and lutein/zeaxanthin. We then quantified and summed the five serum carotenoids to define total carotenoids and assess their overall effect (19, 20). National Health and Nutrition Examination Survey. Laboratory Procedure Manual: Fat Soluble Micronutrients, 2008. Available at: https://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/l45vit_c_met_vitae_carotenoids.pdf.
2.3 Assessment of severe headache or migraine
The criteria for including migraines were based on the pain section of the NHANES self-assessment questionnaire. In the pain questionnaire (MPQ-090), individuals who answered affirmatively to the question, “Have you experienced severe headaches or migraines in the past 3 months?” were classified as migraine patients. Previous studies have validated the robustness of these findings (21–23).
2.4 Covariates
In this research, the variables considered were gender (male/female), age, and ethnicity (Mexican American, other Hispanic, non-Hispanic white, non-Hispanic Black, and other races). Educational achievement was categorized as less than high school, high school graduate, and more than high school. Marital status was grouped as never married, married/living with a partner, or widowed/divorced/separated. The poverty income ratio (PIR) was divided into three categories: <1.3 (low income), 1.3–3.5 (middle income), and > 3.5 (high income). Body mass index (BMI) was classified into three groups: <25 kg/m2, 25–30 kg/m2, and ≥ 30 kg/m2. Smoking status was identified as current smokers (≥100 cigarettes smoked and currently smoking) and never smokers (≤100 cigarettes smoked or never having smoked). A drinker was defined as an individual who had consumed at least 12 alcoholic beverages in any given year of their life. Diabetes was defined as either a doctor-diagnosed condition or a fasting blood glucose level ≥ 126 mg/dL. Hypertension was characterized by a history of diagnosis, current use of antihypertensive medication, or an average of three blood pressure readings ≥140/90 mmHg. Stroke status was recorded as “yes” or “no.” CVD encompassed a history of congestive heart failure coronary artery disease angina pectoris or myocardial infarction.
2.5 Statistical analyses
Statistical analyses were performed following the data analysis guidelines provided by NHANES, utilizing appropriate NHANES complex multistage sampling weights. Survey-weighted means (95% CI) were reported for continuous variables, and survey-weighted percentages (95% CI) were reported for categorical variables. Weighted linear regression or weighted chi-square tests were utilized to compare the migraine and non-migraine groups. Skewed serum carotenoid levels were transformed using a natural logarithm (ln) to approximate a normal distribution.
This study employed three weighted logistic regression models to investigate the association between serum carotenoids and severe headaches or migraines. Model 1 was unadjusted. Model 2 was adjusted for age, sex, and race/ethnicity. Model 3 was further adjusted, in addition to the covariates in Model 2, for education level, marital status, BMI, PIR, smoking, alcohol consumption, diabetes, hypertension, cardiovascular disease, and stroke. After adjusting for these covariates, the study employed restricted cubic splines (RCS) to examine the nonlinear association between serum carotenoids and migraines. Subgroup analyses and interaction tests were conducted to assess the potential confounding factors listed in the baseline table, exploring variations in the associations across subgroups. Statistical analyses were performed using R (version 4.3.3), with significance set at p < 0.05.
3 Results
3.1 Baseline characteristics of the study population
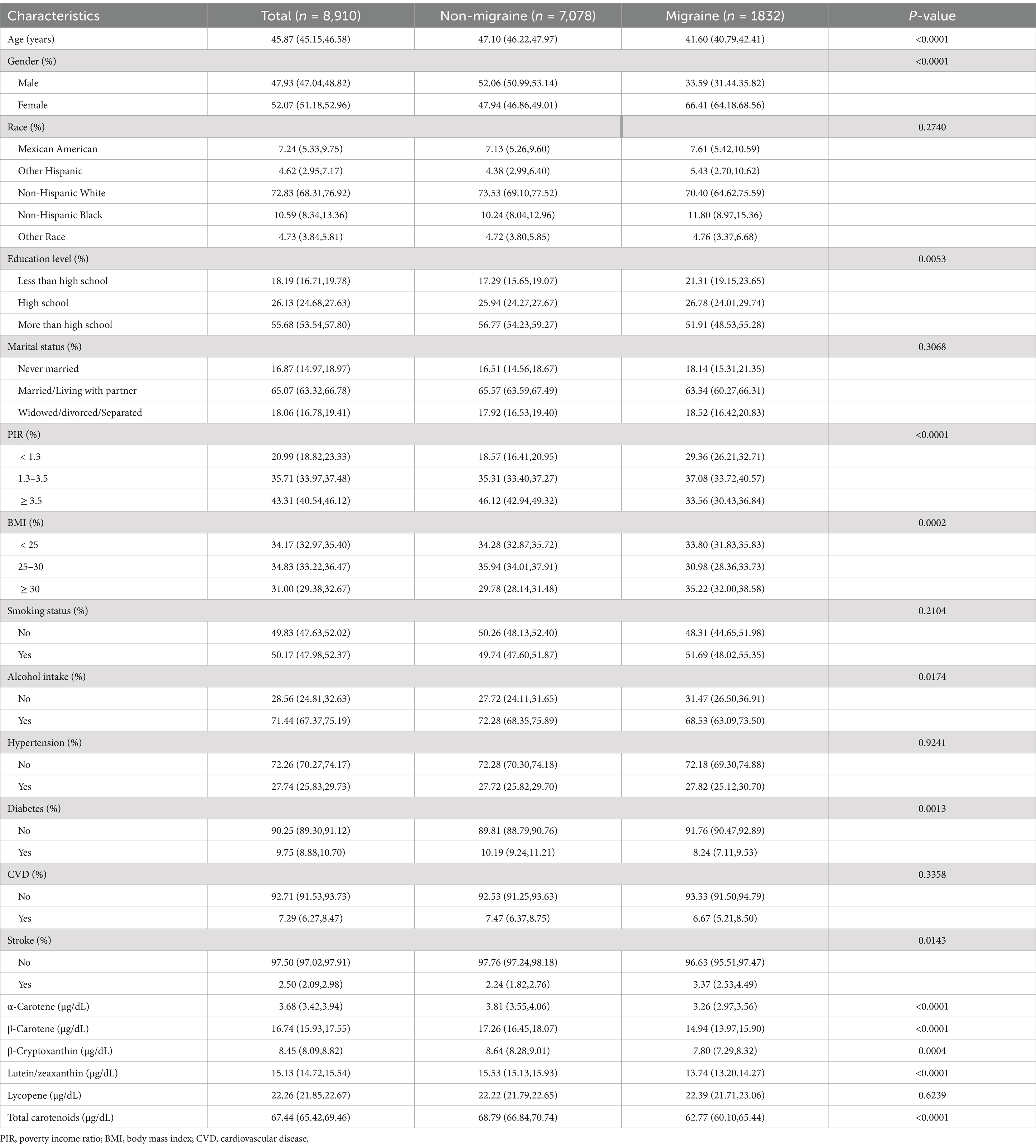
A study was conducted with a total of 8,910 individuals participating, with a weighted prevalence of migraine at 22.37% (20.84, 23.97%). Compared with non-migraine patients, migraine patients had significantly lower levels of α-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin, and total carotenoids, with respective levels of 3.26 (2.97, 3.56) μg/dL, 14.94 (13.97, 15.90) μg/dL, 7.80 (7.29, 8.32) μg/dL, 13.74 (13.20, 14.27) μg/dL, and 62.77 (60.10, 65.44) μg/dL, all showing significant differences between groups (p < 0.01). Additionally, the migraine group had a higher proportion of male patients, individuals with primary or high school education, those who were widowed/divorced/separated, PIR <3.5, BMI ≥30, smokers, and those with hypertension, with all showing significant differences between groups (p < 0.01) (Table 1).
3.2 Relationship between serum carotenoids and migraine
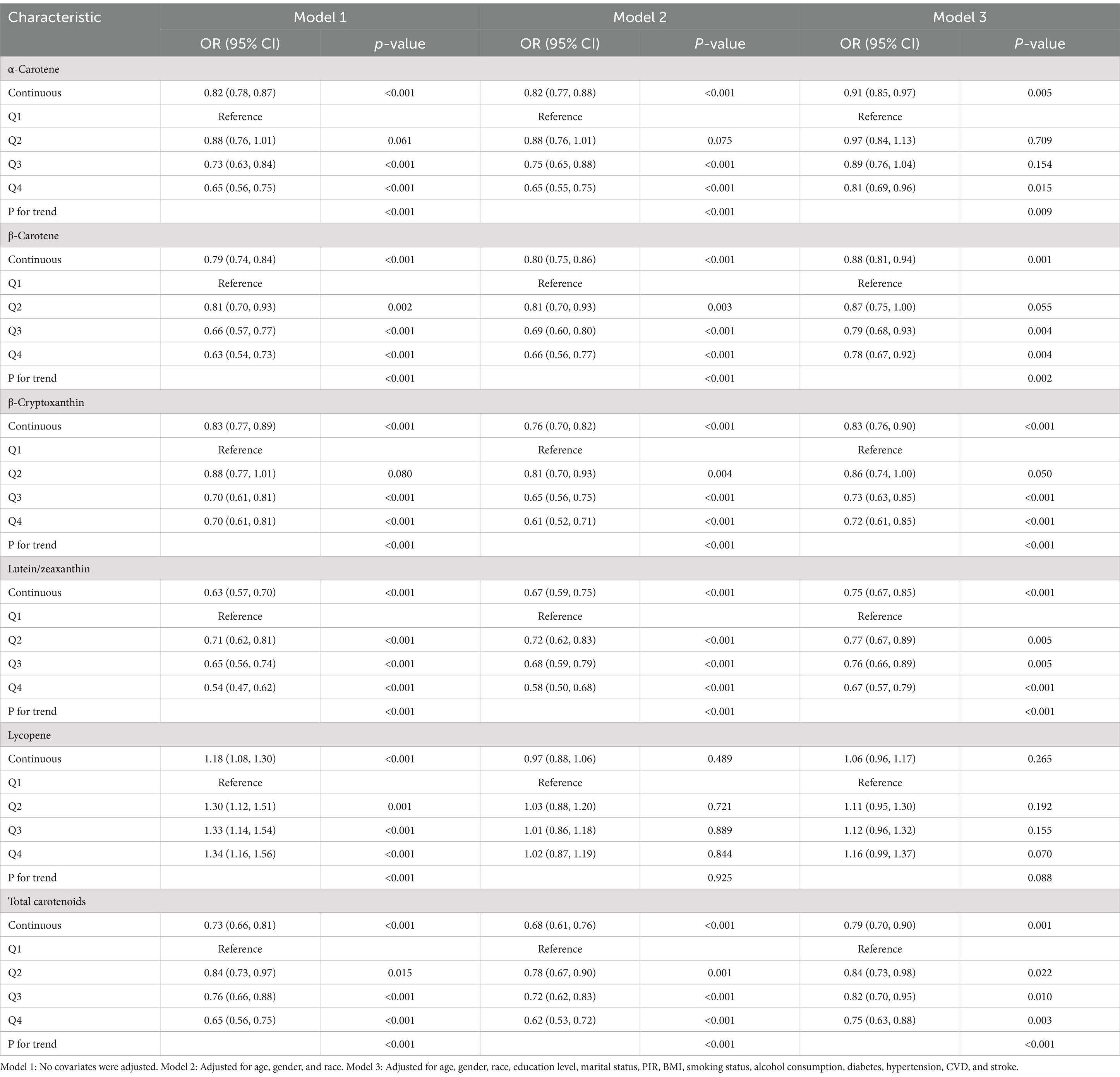
The multivariate logistic regression analysis revealed an inverse relationship between migraine and serum carotenoids. After adjusting for all covariates, α-carotene (OR = 0.91, 95% CI: 0.85–0.97), β-carotene (OR = 0.88, 95% CI: 0.81–0.94), β-cryptoxanthin (OR = 0.83, 95% CI: 0.76–0.90), lutein/zeaxanthin (OR = 0.75, 95% CI: 0.67–0.85), and total carotenoids (OR = 0.79, 95% CI: 0.70–0.90) were significantly inversely associated with migraine. In contrast, lycopene showed no significant association with migraine (p > 0.05). Furthermore, when carotenoids were changed from being represented as continuous variables to categorical variables (quartiles), there was a notable decrease in the likelihood of migraine for participants in the highest quartile compared to those in the lowest quartile (Q1). (p for trend <0.05) (Table 2). Additionally, RCS analysis revealed no nonlinear association between α-carotene, β-carotene, lutein/zeaxanthin, total carotenoids, and migraine status (nonlinearity p > 0.05). Notably, β-cryptoxanthin demonstrated an L-shaped nonlinear relationship with migraine at a threshold of approximately 9.392 μg/dL (nonlinearity p < 0.001) (Figure 2).
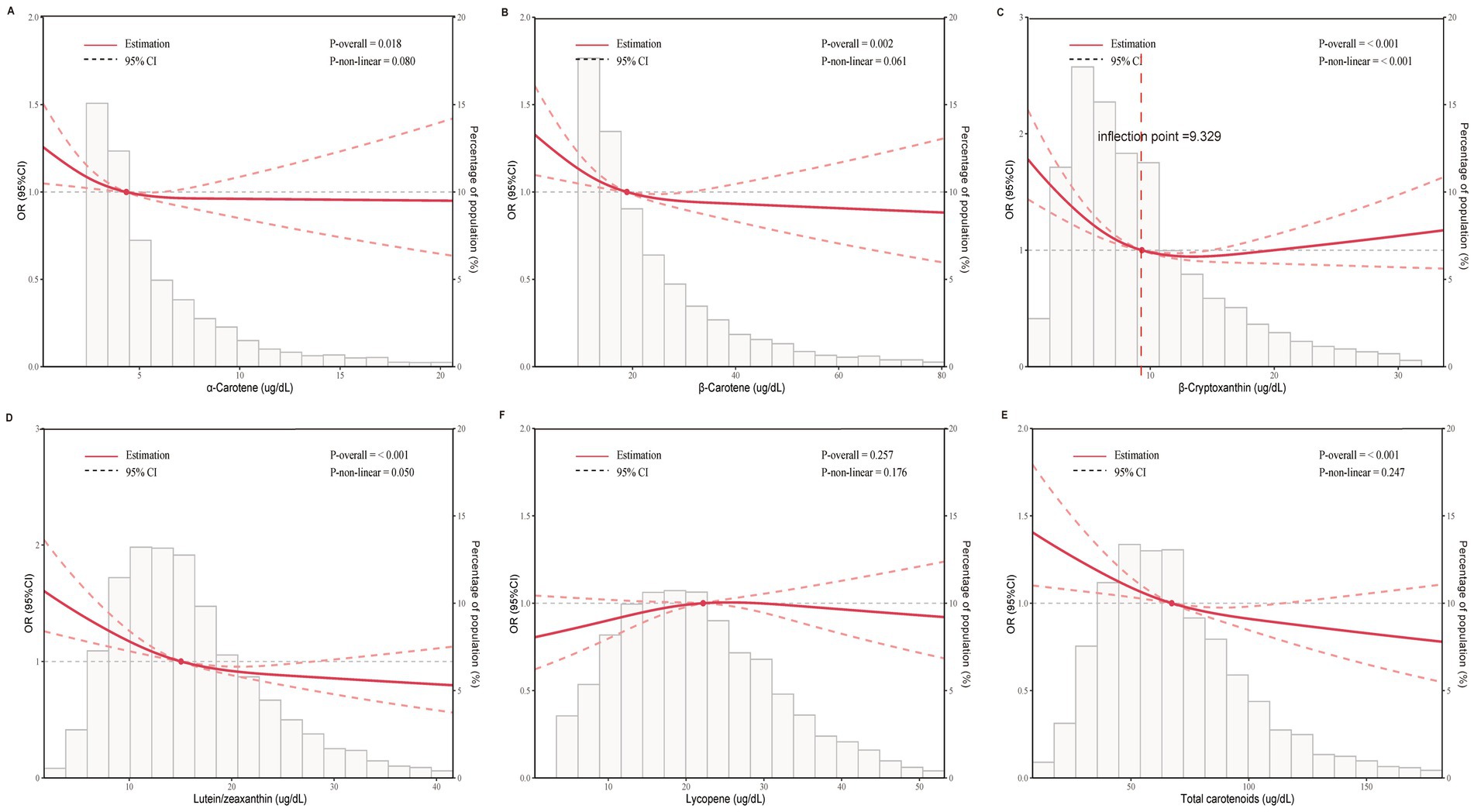
Figure 2. Dose-effect relationship between serum carotenoids and migraine. Age, gender, race, education level, marital status, PIR, BMI, smoking status, alcohol consumption, diabetes, hypertension, CVD, and stroke were adjusted. (A) α-Carotene and migraine; (B) β-Carotene and migraine; (C) β-Cryptoxanthin and migraine; (D) lutein/zeaxanthin and migraine; (E) lycopene and migraine; (F) total carotenoids and migraine. The solid red line represents the estimated serum carotenoid intake and the dotted line represents the 95% confidence interval. OR, odds ratio; 95% CI, 95% confidence interval.
3.3 Subgroup analyses
The study results showed a negative correlation between total serum carotenoid levels and migraine prevalence across all subgroups. Moreover, across the various subgroups, there was a constant correlation between serum carotenoid levels and migraine prevalence (P for interaction >0.05) (Figure 3).
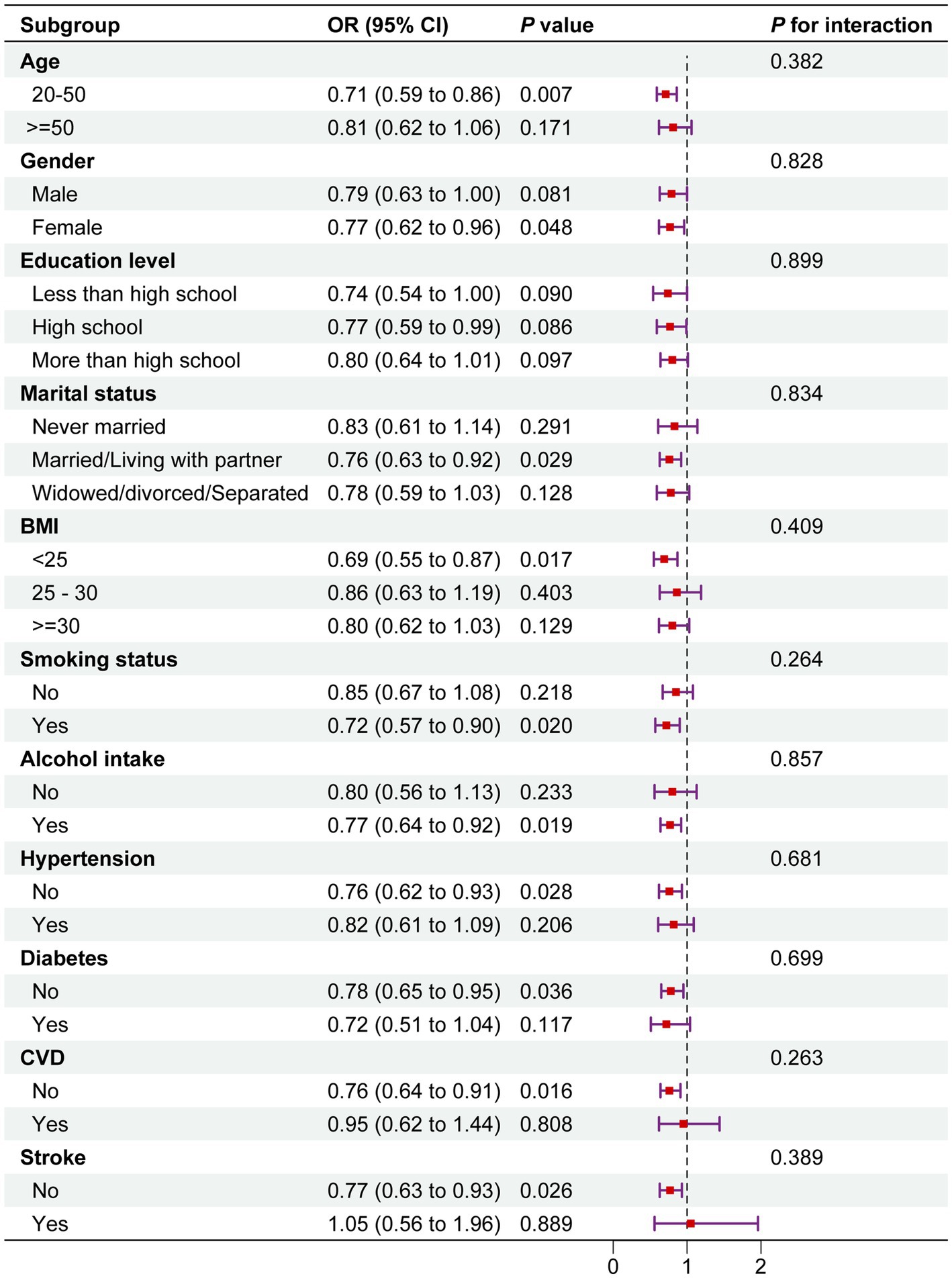
Figure 3. Subgroup analysis between total serum carotenoids and migraine. The above model was adjusted for gender, age, race, education level, marital status, PIR, BMI, smoking status, alcohol consumption, diabetes, hypertension, cardiovascular disease (CVD), and stroke. In each case, the model was not adjusted for the stratification variable. OR, odds ratio; CI, confidence interval.
4 Discussion
The research indicated a significant inverse relationship between the prevalence of migraines and serum levels of α-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin, and total carotenoids. Among the carotenoids studied, β-cryptoxanthin exhibited a unique L-shaped association, suggesting that its protective effect may plateau after reaching a certain concentration. In contrast, lycopene did not show a significant association with migraines, indicating that different carotenoids may have varying effects. Subgroup analyses confirmed the robustness of the study’s findings.
This suggests that individuals with higher serum concentrations of these carotenoids are less likely to experience migraines. The protective effect of these carotenoids is primarily attributed to their strong antioxidant activity. Reactive oxygen species (ROS) production is elevated in migraines due to oxidative stress, leading to neural tissue damage and impaired vascular function, which are known contributors to the pathophysiology of this condition (24, 25). Carotenoids, as effective scavengers of ROS, may help neutralize these free radicals, thereby protecting the brain and vasculature from oxidative damage (7). Additionally, carotenoids possess anti-inflammatory properties, which may further explain their protective role in migraine prevention (26). Frequently linked to neuroinflammation, migraines are triggered by inflammatory cytokines including tumor necrosis factor-α and interleukin-6 (27, 28). Carotenoids, particularly lutein and β-carotene, have been shown to downregulate these inflammatory mediators, potentially reducing the inflammatory processes that lead to migraine attacks (29). The observed L-shaped relationship between β-cryptoxanthin and migraines suggests that while increasing β-cryptoxanthin levels initially provides a protective effect, this effect may reach a plateau beyond a certain point. This may indicate a threshold for the bioavailability or metabolism of carotenoids, where the body can only utilize a limited amount of β-cryptoxanthin for its antioxidant and anti-inflammatory effects. Beyond this threshold, further increases in serum levels do not provide additional protection, highlighting the need for future research to determine the optimal carotenoid levels for migraine prevention.
Studies have shown that oxidative stress markers are elevated in migraine patients, while levels of endogenous antioxidants, such as glutathione, are decreased, indicating an impaired ability to neutralize ROS in these patients. Similarly, some studies have reported that individuals whose diets are rich in carotenoids, particularly β-carotene and lutein, have a lower risk of neurological disorders, including migraines (30). For instance, adherence to the Mediterranean diet—which is high in fruits and vegetables that contain carotenoid—was linked to a lower incidence of migraines, according to a study by Bakırhan et al. (31). This complements the current findings, as the measurement of serum carotenoid levels provides an objective biomarker of dietary intake and antioxidant status, further supporting the protective role of these compounds in migraine prevention. The non-linear L-shaped relationship observed in this study between β-cryptoxanthin and migraine risk is similar to findings in other health conditions, such as cardiovascular disease. Huang et al. found a similar L-shaped association between β-cryptoxanthin levels and cardiovascular disease risk, where moderate levels provided the greatest protection, but higher levels did not offer additional benefits (17). This suggests that the protective effect of β-cryptoxanthin may be dose-dependent, with an optimal range for its beneficial effects. Results from a study in the future indicated that being exposed to numerous serum carotenoids was linked to a decreased likelihood of mortality from any cause, cardiovascular disease (CVD), and cancer. These results imply that consuming a diverse range of carotenoids in one’s diet may provide extensive health advantages (17, 19). Research by Wang et al. indicated an inverse correlation between serum carotenoid concentrations and the prevalence of CVD, with the protective effect being more pronounced for heart disease and stroke (32). Higher dietary antioxidant index scores have been associated with a lower prevalence of severe headaches or migraines (33). Additionally, Research has indicated a negative correlation between the prevalence of chronic obstructive pulmonary disease and carotenoid levels, with lutein, zeaxanthin, and α-cryptoxanthin being the main contributors to this inverse association (34). Research conducted by Heqing Zheng and colleagues indicated that the potential link between zinc consumption and migraines could be attributed to the antioxidant and anti-inflammatory characteristics of zinc. Furthermore, this negative association was more pronounced in participants aged 20–50 compared to those over 50 (35). In this study, there was no significant link between lycopene and migraines. This difference may be due to the way carotenoids accumulate and function in different tissues. While lycopene effectively protects against oxidative stress in the prostate and cardiovascular system, it may not have the same protective effect in the brain, where other carotenoids, like lutein and β-carotene, are more prevalent (36, 37).
The presence of serum carotenoids may be linked to the prevalence of migraines. Carotenoids are known for their capacity to counteract reactive oxygen species (ROS) and decrease oxidative stress, which is a significant factor in the development of migraines. Migraines are frequently connected with heightened oxidative damage, particularly in neural tissue, which is highly vulnerable to oxidative stress because of its high metabolic requirements (24, 25). By mitigating oxidative damage, carotenoids may help in preventing or reducing the frequency and severity of migraine attacks.
5 Strengths and limitations
The study has multiple notable strengths. To begin with, it utilizes a sizable, nationally representative sample from the NHANES dataset, enhancing the generalizability of the findings to a broader U.S. population. The utilization of NHANES ensures high-quality data collection, including serum carotenoid level measurements conducted using advanced laboratory techniques, thereby providing accurate and objective biomarkers of antioxidant status. This research emphasizes serum carotenoids rather than self-reported dietary intake, mitigating biases associated with misreporting or inaccuracies common in dietary surveys. Furthermore, this research is among the initial inquiries into the correlation between various specific carotenoids and the frequency of migraines. Previous studies have primarily concentrated on overall dietary patterns or general antioxidant intake. By examining individual carotenoids, this research offers deeper insights into how specific antioxidants may influence migraine risk. The L-shaped relationship observed between β-cryptoxanthin and migraine risk contributes novel information to the existing literature on antioxidants and migraines, suggesting that the carotenoids exhibit dose-dependent protective effects.
Despite these strengths, this study also has several limitations. Firstly, the cross-sectional design of the NHANES dataset constrains our ability to establish causality. This study remains uncertain whether higher carotenoid levels reduce migraine risk or if individuals with fewer migraines exhibit different dietary habits or lifestyles that contribute to elevated carotenoid levels. Longitudinal studies are necessary to more accurately assess the directionality of this relationship. Secondly, the self-reported nature of migraine diagnosis may introduce recall bias or misclassification. Finally, although this study examined multiple carotenoids, it did not investigate potential interactions between carotenoids and other nutrients, which may play synergistic roles in mitigating oxidative stress and inflammation. Future research should explore how combinations of antioxidants influence migraine risk and whether specific carotenoids are more effective when consumed alongside other nutrients.
6 Conclusion
The results of this research indicate that there is a negative correlation between serum levels of α-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin, and total carotenoids and the probability of experiencing migraines. Notably, an L-shaped relationship exists between β-cryptoxanthin and migraine risk. Increasing the intake of carotenoid-rich foods may represent a straightforward and feasible strategy for enhancing antioxidant defenses and alleviating the burden of migraines.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The portions of this study involving human participants, human materials, or human data were conducted in accordance with the Declaration of Helsinki and were approved by the National Center for Health Statistics (NCHS) Ethics Review Board. The patients/participants provided their written informed consent to participate in this study. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
TH: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. YC: Formal analysis, Funding acquisition, Writing – original draft. SC: Investigation, Methodology, Software, Writing – original draft. RX: Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Key Research and Development Program of Hubei Provincial Department of Science and Technology (Grant No. 2022BCE007).
Acknowledgments
We would like to thank all participants in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
NHANES, National Health and Nutrition Examination Survey; NCHS, National Center for Health Statistics; BMI, body mass index; RCS, restricted cubic spline; PIR, income to poverty ratio; CVD, cardiovascular disease; ROS, reactive oxygen species.
References
1. Ashina, M, Buse, DC, Ashina, H, Pozo-Rosich, P, Peres, MFP, Lee, MJ, et al. Migraine: integrated approaches to clinical management and emerging treatments. Lancet. (2021) 397:1505–18. doi: 10.1016/s0140-6736(20)32342-4
2. Katsarava, Z, Mania, M, Lampl, C, Herberhold, J, and Steiner, TJ. Poor medical care for people with migraine in Europe - evidence from the Eurolight study. J Headache Pain. (2018) 19:10. doi: 10.1186/s10194-018-0839-1
3. Flynn, O, Fullen, BM, and Blake, C. Migraine in university students: a systematic review and meta-analysis. Eur J Pain. (2023) 27:14–43. doi: 10.1002/ejp.2047
4. Yalın, O, Uluduz, D, Özge, A, Sungur, MA, Selekler, M, and Siva, A. Phenotypic features of chronic migraine. J Headache Pain. (2016) 17:26. doi: 10.1186/s10194-016-0616-y
5. Kim, SJ, Lee, HJ, Lee, SH, Cho, S, Kim, KM, and Chu, MK. Most bothersome symptom in migraine and probable migraine: a population-based study. PLoS One. (2023) 18:e0289729. doi: 10.1371/journal.pone.0289729
7. Jiménez-Jiménez, FJ, Alonso-Navarro, H, García-Martín, E, Espada-Rubio, S, and Agúndez, JAG. Oxidative stress and migraine. Mol Neurobiol. (2024) 61:8344–60. doi: 10.1007/s12035-024-04114-7
8. Mawet, J, Kurth, T, and Ayata, C. Migraine and stroke: in search of shared mechanisms. Cephalalgi. (2015) 35:165–81. doi: 10.1177/0333102414550106
9. Shen, Y, Li, Z, Wang, J, and Qiu, Z. Study on the comprehensive treatment of migraine with traditional Chinese medicine based on the new pathophysiological mechanism: a review. Medicine. (2024) 103:e39487. doi: 10.1097/md.0000000000039487
10. Gross, EC, Lisicki, M, Fischer, D, Sándor, PS, and Schoenen, J. The metabolic face of migraine - from pathophysiology to treatment. Nat Rev Neurol. (2019) 15:627–43. doi: 10.1038/s41582-019-0255-4
11. Borkum, JM. Migraine triggers and oxidative stress: a narrative review and synthesis. Headache. (2016) 56:12–35. doi: 10.1111/head.12725
12. El-Agamey, A, and McGarvey, DJ. The reactivity of carotenoid radicals with oxygen. Free Radic Res. (2007) 41:295–302. doi: 10.1080/10715760601087558
13. el-Agamey, A, Lowe, G, McGarvey, D, Mortensen, A, Phillip, D, Truscott, TG, et al. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch Biochem Biophys. (2004) 430:37–48. doi: 10.1016/j.abb.2004.03.007
14. Mathews-Roth, MM. Carotenoid functions in photoprotection and cancer prevention. J Environ Patho Toxicol Oncol. (1990) 10:181–92.
15. Leermakers, ET, Darweesh, SKL, Baena, CP, Moreira, EM, Melo van Lent, D, Tielemans, MJ, et al. The effects of lutein on cardiometabolic health across the life course: a systematic review and meta-analysis. Am J Clin Nutr. (2016) 103:481–94. doi: 10.3945/ajcn.115.120931
16. Liang, J, Wang, Y, and Chen, M. The association between dietary carotenoid intake and risk of depression among patients with Cardiometabolic disease. Int Heart J. (2023) 64:223–9. doi: 10.1536/ihj.22-453
17. Zhu, X, Cheang, I, Tang, Y, Shi, M, Zhu, Q, Gao, R, et al. Associations of serum carotenoids with risk of all-cause and cardiovascular mortality in hypertensive adults. J Am Heart Assoc. (2023) 12:e027568. doi: 10.1161/jaha.122.027568
18. Udensi, J, Loskutova, E, Loughman, J, and Byrne, HJ. Quantitative Raman analysis of carotenoid protein complexes in aqueous solution. Molecules. (2022) 27:27. doi: 10.3390/molecules27154724
19. He, Q, Yuan, C, Liu, Z, and Wei, X. Associations of multiple carotenoid co-exposure with all-cause and cause-specific mortality in US adults: a prospective cohort study. Front Nutr. (2024) 11:1415537. doi: 10.3389/fnut.2024.1415537
20. Yan, S, Chen, S, Liu, Y, Liang, H, Zhang, X, Zhang, Q, et al. Associations of serum carotenoids with visceral adiposity index and lipid accumulation product: a cross-sectional study based on NHANES 2001-2006. Lipids Health Dis. (2023) 22:209. doi: 10.1186/s12944-023-01945-6
21. Huang, H, and He, K. The association between dietary fiber intake and severe headaches or migraine in US adults. Front Nutr. (2022) 9:1044066. doi: 10.3389/fnut.2022.1044066
22. Tarleton, EK, Kennedy, AG, Rose, GL, and Littenberg, B. Relationship between magnesium intake and chronic pain in U.S. Adults Nutrients. (2020) 12:2104. doi: 10.3390/nu12072104
23. Tian, S, Cheng, Z, Zheng, H, Zhong, X, Yu, X, Zhang, J, et al. Interaction between diabetes and body mass index on severe headache or migraine in adults: a cross-sectional study. BMC Geriatr. (2024) 24:76. doi: 10.1186/s12877-024-04657-3
24. Geyik, S, Altunısık, E, Neyal, AM, and Taysi, S. Oxidative stress and DNA damage in patients with migraine. J Headache Pain. (2016) 17:10. doi: 10.1186/s10194-016-0606-0
25. Chaturvedi, P, Khan, R, Sahu, P, Ludhiadch, A, Singh, G, and Munshi, A. Role of omics in migraine research and management: a narrative review. Mol Neurobiol. (2022) 59:5809–34. doi: 10.1007/s12035-022-02930-3
26. Zerres, S, and Stahl, W. Carotenoids in human skin. Biochim Biophys Acta Mol Cell Biol Lipids. (2020) 1865:158588. doi: 10.1016/j.bbalip.2019.158588
27. Koçer, A, Memişoğullari, R, Domaç, FM, İlhan, A, Koçer, E, Okuyucu, Ş, et al. IL-6 levels in migraine patients receiving topiramate. Pain Pract. (2009) 9:375–9. doi: 10.1111/j.1533-2500.2009.00301.x
28. Arzani, M, Jahromi, SR, Ghorbani, Z, Vahabizad, F, Martelletti, P, Ghaemi, A, et al. Gut-brain Axis and migraine headache: a comprehensive review. J Headache Pain. (2020) 21:15. doi: 10.1186/s10194-020-1078-9
29. Eggersdorfer, M, and Wyss, A. Carotenoids in human nutrition and health. Arch Biochem Biophys. (2018) 652:18–26. doi: 10.1016/j.abb.2018.06.001
30. Olivito, I, Simona, F, Tarsitano, A, Pagliuso, M, Tarantino, C, de Lorenzo, A, et al. Mediterranean ketogenic diet accounts for reduced pain frequency and intensity in patients with chronic migraine: a pilot study. Clin Nutr. (2024) 43:1781–7. doi: 10.1016/j.clnu.2024.06.015
31. Bakırhan, H, Yıldıran, H, and Uyar Cankay, T. Associations between diet quality, DASH and Mediterranean dietary patterns and migraine characteristics. Nutr Neurosci. (2022) 25:2324–34. doi: 10.1080/1028415x.2021.1963065
32. Wang, M, Tang, R, Zhou, R, Qian, Y, and Di, D. The protective effect of serum carotenoids on cardiovascular disease: a cross-sectional study from the general US adult population. Front Nutr. (2023) 10:1154239. doi: 10.3389/fnut.2023.1154239
33. Zhang, Z, Chen, X, Fang, H, Ye, J, Tang, X, and Huang, R. Association between the composite dietary antioxidant index and severe headache or migraine: results from the National Health and nutrition examination survey. Front Neurol. (2024) 15:1407243. doi: 10.3389/fneur.2024.1407243
34. Huang, Q, Peng, Z, Li, S, Nan, W, and He, B. Association between carotenoids and the prevalence of chronic obstructive pulmonary disease in the United States. Heart Lung. (2024) 65:93–100. doi: 10.1016/j.hrtlng.2024.02.010
35. Zheng, H, Tian, S, Wu, L, Zhong, X, Liu, M, Yu, X, et al. Dietary zinc intake in relation to migraine among adults: a cross sectional study of NHANES 1999-2004. Nutr Neurosci. (2024) 27:667–76. doi: 10.1080/1028415x.2023.2243678
36. Jian, L, Du, CJ, Lee, AH, and Binns, CW. Do dietary lycopene and other carotenoids protect against prostate cancer? Int J Cancer. (2005) 113:1010–4. doi: 10.1002/ijc.20667
37. Wang, S, He, W, Li, W, Zhou, JR, and Du, Z. Combination of lycopene and curcumin synergistically alleviates testosterone-propionate-induced benign prostatic hyperplasia in Sprague Dawley rats via modulating inflammation and proliferation. Molecules. (2023) 28:4900. doi: 10.3390/molecules28134900
Keywords: migraine, carotenoid, NHANES, cross-sectional study, α-carotene
Citation: Hu T, Chen Y, Chen S and Xue R (2025) Association between serum carotenoids levels and severe headache or migraine in adults: a cross-sectional study from NHANES. Front. Nutr. 11:1507503. doi: 10.3389/fnut.2024.1507503
Edited by:
Malgorzata Rozanowska, Cardiff University, United KingdomReviewed by:
Johra Khan, Majmaah University, Saudi ArabiaGiacomo Di Matteo, Sapienza University of Rome, Italy
Copyright © 2025 Hu, Chen, Chen and Xue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui Xue, eHVlcnVpMTEwNkAxMjYuY29t
 Tian Hu
Tian Hu Yufei Chen
Yufei Chen