- 1Department of Neurosurgery, Chongqing General Hospital, Chongqing University, Chongqing, China
- 2Department of Neurosurgery, Nanchong Central Hospital, The Second Clinical Medical College, North Sichuan Medical College, Nanchong, China
Background: Research on the association between glioma risk and coffee and tea consumption remains inconclusive. This study seeks to present a meta-analysis of the relationship between coffee and tea intake and glioma risk.
Method: Relevant cohort studies that collected coffee and tea exposure prospectively were identified through searches of the PubMed, Embase, and Scopus databases. Eligible studies included those providing adjusted relative risk estimates or hazard ratios (HRs) with 95% confidence intervals (CIs), or data sufficient for such calculations. Study quality was evaluated using the Newcastle-Ottawa Scale, while the GRADE system assessed the quality of evidence. The analysis explored glioma risk concerning the highest versus lowest levels of coffee and tea intake, supplemented by a dose–response evaluation using a one-stage robust error meta-regression model.
Results: A total of nine studies, published between 2004 and 2020, were included. In a model comparing the highest and lowest levels of coffee and tea consumption, 3,896 glioma cases were identified among 2,648,468 participants. Correspondingly, the pooled HRs with 95% CIs were 0.98 (0.87–1.09) for coffee and 0.95 (0.86–1.06) for tea, respectively. Furthermore, no evidence of publication bias was detected for either beverage. The dose–response analysis indicated a near “L”-shaped relationship between tea consumption and glioma risk, with the most notable risk reduction observed in individuals consuming more than 2.5 cups of tea per day. However, additional tea intake beyond this threshold did not confer evident risk reduction. According to Grade scoring system, the quality of meta-evidence was classified as “very low” for coffee and “low” for tea.
Conclusion: This meta-analysis provides evidence suggesting a potential inverse association between tea consumption and glioma risk, while no such association was observed for coffee consumption. Given that the evidence for coffee was classified as “very low” and for tea as “low,” cautious interpretation of the findings is warranted, and further research is needed to validate these results.
Introduction
Gliomas represent a heterogeneous group of central nervous system (CNS) tumors, characterized by varying degrees of invasiveness and prognosis. According to the 2021 WHO classification of CNS tumors, which organizes gliomas based on histopathological features and distinct molecular biomarkers, these tumors are classified into five primary clusters: pediatric-type diffuse low-grade gliomas, pediatric-type diffuse high-grade gliomas, adult-type diffuse gliomas, circumscribed astrocytic gliomas, and ependymal tumors (1, 2). Gliomas account for 80 to 85% of malignant CNS tumors in adults, making them the most common primary CNS tumors in this population, with an age-adjusted incidence rate of 5.6 per 100,000 person-years in the United States (3, 4).
As with other tumors, research into the etiology and susceptibility factors of gliomas is ongoing, with limited understanding thus far. Advanced age is currently recognized as a significant risk factor (4). In addition, glioma incidence exhibits notable variability across sex, race or ethnicity (1, 5). The highest incidence is observed among Non-Hispanic White people, with a male predominance (1, 5). Beyond exposure to ionizing radiation, which remains the only well-established environmental risk factor, a history of allergies or atopic disease has been consistently associated with a reduced risk of glioma, albeit with limited supporting evidence (6).
Coffee and tea are enjoyed across diverse cultures and regions with an estimated consumption of 2.25 billion cups of coffee (7) and approximately 3 billion cups of tea each day (8). Both drinks are rich in dietary sources of bioactive compounds, such as caffeine, polyphenol compounds, amino acids, minerals, and vitamins (9, 10). These compounds are known for their antioxidant properties, anti-cancer potential, and neuroprotective benefits in vitro and experimental studies, as summarized in several reviews (9–12). Given the extensive global consumption of coffee and tea, as well as their range of biological effects, there is growing interest in exploring the link between these beverages and health outcomes (13, 14). Currently, epidemiological evidence for the association between glioma risk and coffee and tea consumption is inconsistent (15–30). This discrepancy may be associated with different study methodologies, such as sample size, recall bias, and residual confounders. Several meta-analyses or systematic reviews have tried to settle this issue (31–37). Notably, previous meta-analyses included some retrospective case–control studies (31, 34, 36, 37), unadjusted risk estimates (33, 34, 36, 37), multiple reports from the same cohort (37), and missed earlier prospective studies in their analysis (35–37). To overcome these limitations, we performed an updated meta-analysis of cohort studies which collected coffee and tea exposure prospectively to quantitatively summarize the relationship.
Method
Report guideline
This meta-analysis was designed, conducted, and documented in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (38). There was no registered protocol.
Literature search
We carried out a comprehensive search across the PubMed, Embase, and Scopus databases, covering the period from their inception to November 17, 2024. The following keywords were utilized: coffee, tea, diet, beverages, drinking, glioma, brain tumors, brain cancer, brain neoplasms, cerebral cancer, cerebral tumors, cerebral neoplasms, intracranial cancer, central nervous system cancer, intracranial neoplasms, intracranial tumors, central nervous system tumors, and central nervous system neoplasms. Detailed search strategies are outlined in Supplementary Table 1. We also conducted a meticulous manual review of the reference lists of pertinent articles, reviews, and meta-analyses to identify any studies that may have been missed by the initial search terms.
Study selection
The criteria for inclusion were defined as follows: (1) cohort studies that examined coffee or tea consumption as the primary exposure variable and glioma as the outcome, and (2) studies that reported adjusted relative risk estimates or hazard ratios (HRs) along with 95% confidence intervals (CIs) or provided data necessary for such calculations. Exclusion criteria comprised: (1) letters and conference abstracts lacking original data, (2) studies that addressed total brain tumors, (3) retrospective case–control studies, and (4) studies presenting only crude risk estimates with 95% CIs. In our study, we also included those with a retrospective cohort design in a population cohort which collected coffee and tea exposure prospectively. The study selection was performed by JP and checked by CS.
Data extraction
Two investigators (JP and CS) independently retrieved the following information: first author’s last name, year of publication, country in which the research was conducted, name of the cohort, enrollment period, number of cases, total population size, age range of participants, time of follow-up, exposure categories, and the associated risk estimates with 95% confidence intervals (CIs), adjusted for the maximum number of potential confounding variables. Any discrepancies were resolved by discussion.
Statistical analysis
All statistical analyses were executed utilizing Stata software version 14.0 (StataCorp, College Station, Texas, USA). To evaluate the association between coffee and tea consumption and glioma risk, both categorical meta-analyses comparing the highest and lowest intake levels and dose–response analyses were performed. Pooled effects were calculated utilizing random-effects model when significant heterogeneity was found; otherwise, fixed-effects model was used. The I2 statistic was employed to evaluate heterogeneity and an I2 value greater than 50% is considered to indicate substantial heterogeneity (39). The possibility of publication bias was evaluated by visually inspecting funnel plots and utilizing the Egger’s test (40). Additionally, sensitivity analyses were performed by systematically omitting individual studies to determine the robustness of the results.
A one-stage robust error meta-regression model (REMR), as outlined by Xu and Doi, was employed to explore the dose–response relationship (41). For the analysis, all consumption measures were normalized to cups per day, with a single cup equating to four ounces of coffee. The REMR approach required at least two exposure levels, each accompanied by their respective relative risks and 95% CIs. In cases where coffee or tea intake was provided as a range, the median or average was utilized to estimate the exposure level. If these values were not available, the midpoint of the range was utilized instead. For the highest open-ended category, the width was assumed to match the interval of the preceding category, while the lower boundary for the lowest open-ended category was set to zero.
Kuan et al. conducted a pooled analysis that encompassed three larger cohorts: the UK Million Women Study, the National Institutes of Health–AARP Diet and Health study (NIH-AARP), and the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial (28). However, this study did not provide detailed exposure levels for coffee and tea (28). In contrast, the studies by Dubrow et al. (24) and Hashibe et al. (26), which utilized data from the NIH-AARP Study and the PLCO study, respectively, offered comprehensive exposure level data. To optimize data utilization and prevent redundant data usage, we incorporated Kuan et al.’s research (28) into the comparative analysis between the highest and lowest exposure groups. Additionally, the studies by Dubrow et al. (24) and Hashibe et al. (26) were included in the dose–response evaluation (24, 26).
Study quality
For study quality assessment, our study utilized the Newcastle-Ottawa Scale (NOS: available at https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp, accessed on November 18, 2024), which evaluates studies based on three domains: selection (four items, one star per item), comparability (one item, up to two stars), and outcome (three items, one star per item). In our study, we made the following definitions: Considering that confounding factors are one of the biggest concerns in observational studies, for the “Comparability” item, a study can be awarded a maximum of one star under this sub-item if it adjusts for age or birth age in the analysis. A study with follow-up (greater than the median or mean follow-up of 5 years, or with a maximum follow-up period of 10 years or more) was awarded one star. If a study collected exposure data using questionnaires with validated reliability, it was awarded one star. Since universally accepted formal criteria for high quality have not yet been defined, the thresholds for converting the Newcastle-Ottawa scales to Agency for Healthcare Research and Quality (AHRQ) standards classify studies as good quality if they score 3–4 stars in the selection domain, 1–2 stars in the comparability domain, and 2–3 stars in the outcome domain; fair quality if they score 2 stars in the selection domain, 1–2 stars in the comparability domain, and 2–3 stars in the outcome domain; and poor quality if they score 0–1 star in the selection domain, 0 stars in the comparability domain, or 0–1 star in the outcome domain.
Grading quality of evidence
The Grade scoring system was utilized to evaluate the quality of meta-evidence in this study (42). Evidence from cohort studies is initially classified as “Low.” The quality of evidence may be upgraded based on factors such as a substantial magnitude of effect, the presence of plausible residual confounding unlikely to diminish the effect size, and the existence of a dose–response gradient. Conversely, it may be downgraded due to inconsistency, indirectness, imprecision, or publication bias. Ultimately, the evidence quality is categorized into four levels: high, moderate, low, and very low.
Results
Basic characteristic
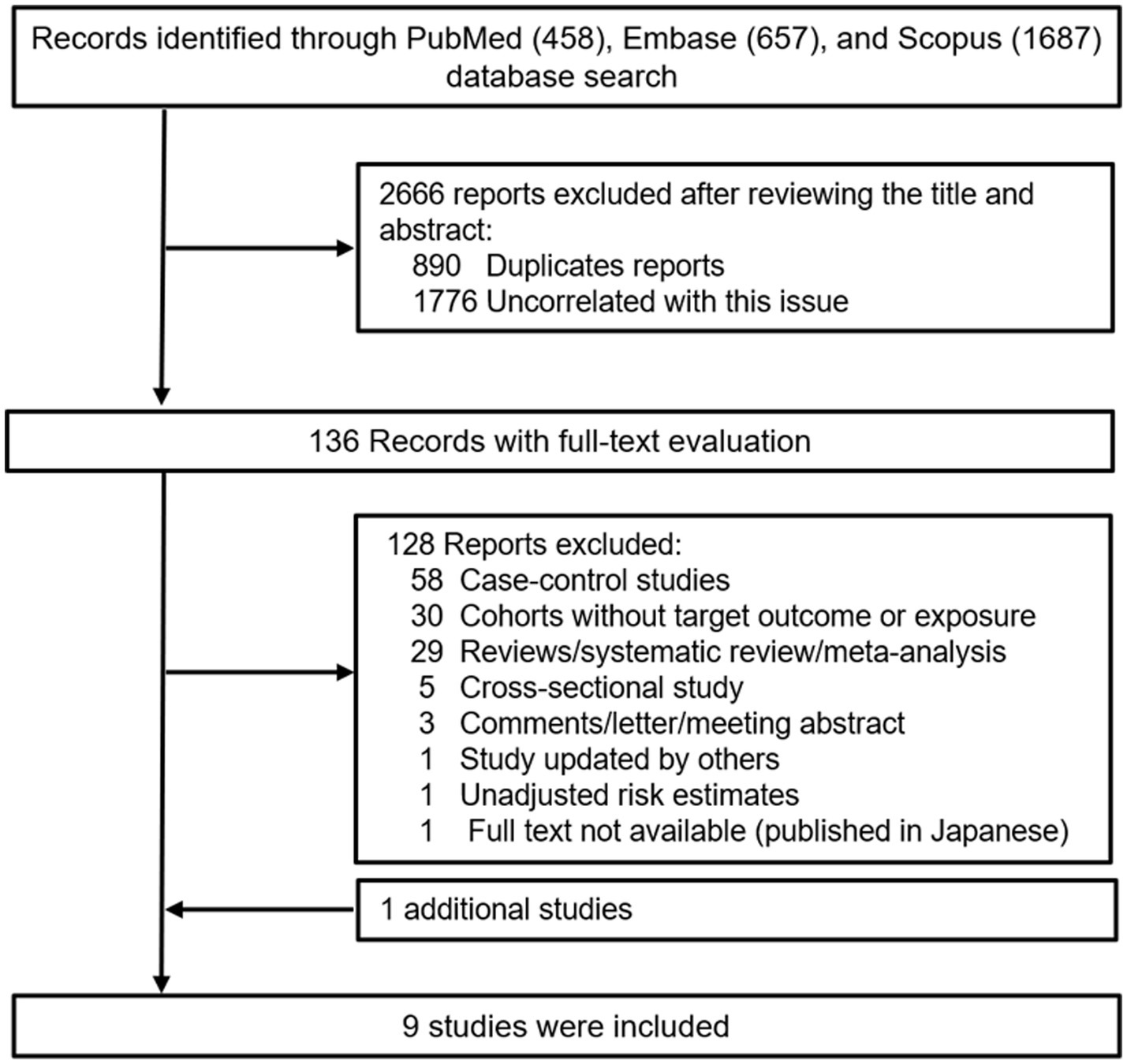
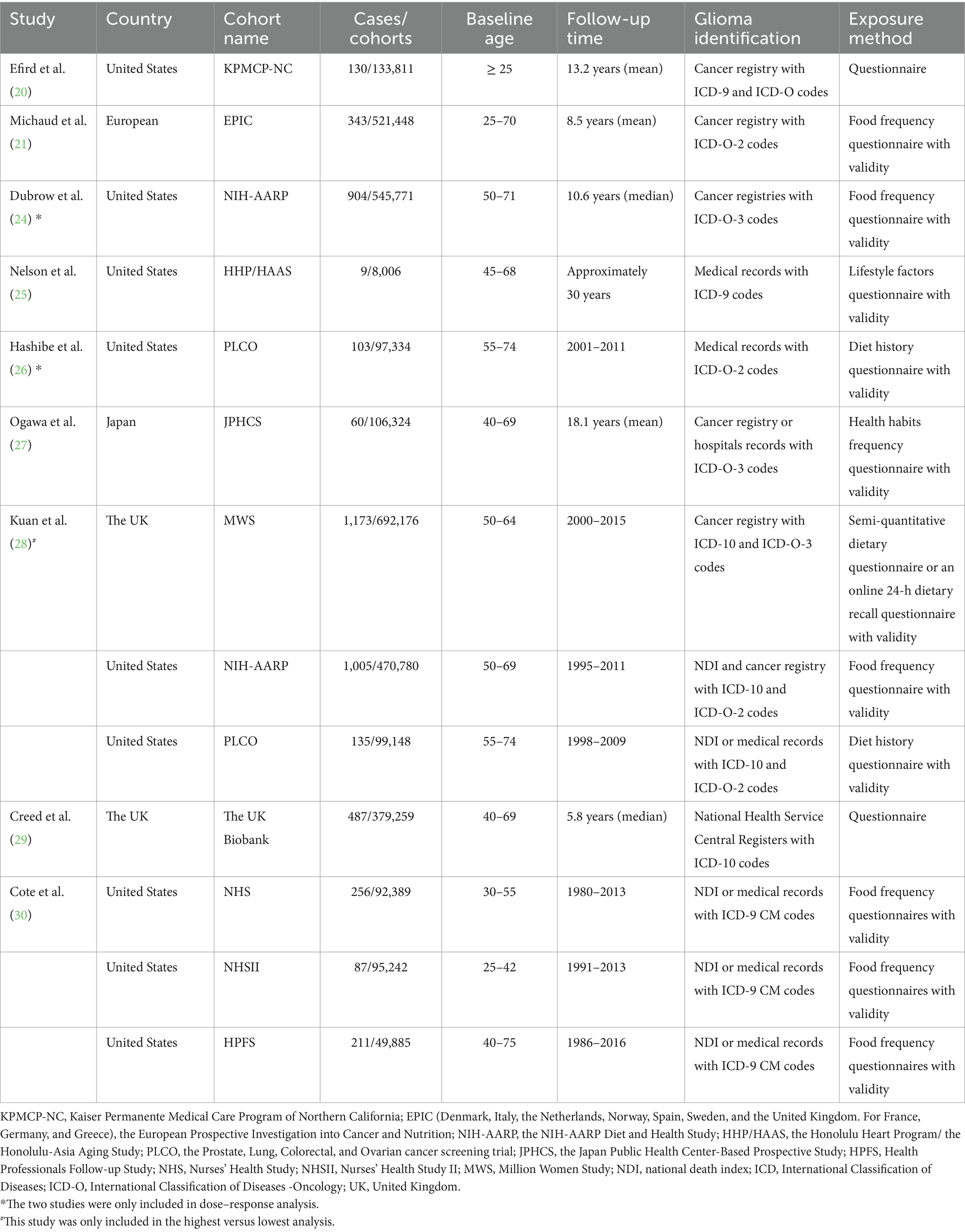
Figure 1 outlines the process for the identification and selection of literature. The initial database search yielded 458 records from PubMed, 657 records from Embase, and 1,687 records from Scopus. Following a title and/or abstract screening, 890 duplicates and 1776 records uncorrelated this issue were excluded. Upon full-text review, 128 of the remaining 136 reports were excluded, leaving eight studies that met the inclusion and exclusion criteria. Additionally, one was located through manual reference list reviews (25). Ultimately, nine studies were incorporated into the meta-analysis (20, 21, 24–30). Table 1 and Supplementary Table 2 presents the primary characteristics of the studies. These studies were published between 2004 and 2020, with the majority conducted in the United States (20, 24–26, 30), alongside one in Japan (27), one in the UK (26), and one across several European countries (21). The age of participants at the time of cohort enrollment was ≥25 years. Case identification relied on cancer registries, national death indices, hospital records, the National Health Service Central Registers, or unspecified medical records, with all cases diagnosed using the International Classification of Diseases ninth or tenth revision (ICD-9/10) and/or the International Classification of Diseases for Oncology (ICD-O) codes. Coffee and tea consumption was assessed through a variety of questionnaires. Several studies have validated dietary data using various methods, including 24-h dietary recalls (21, 25), 7-day dietary records (25, 28), two 24-h recalls (24, 28), and 14- or 28-day dietary records (27), as well as two 1-week diet records (30). In the PLCO study, a valid questionnaire was used, although no detailed methodology was reported (26). In the remaining two studies, the reliability of the questionnaires could not be confirmed from the original reports (20, 29).
Coffee consumption and glioma risk
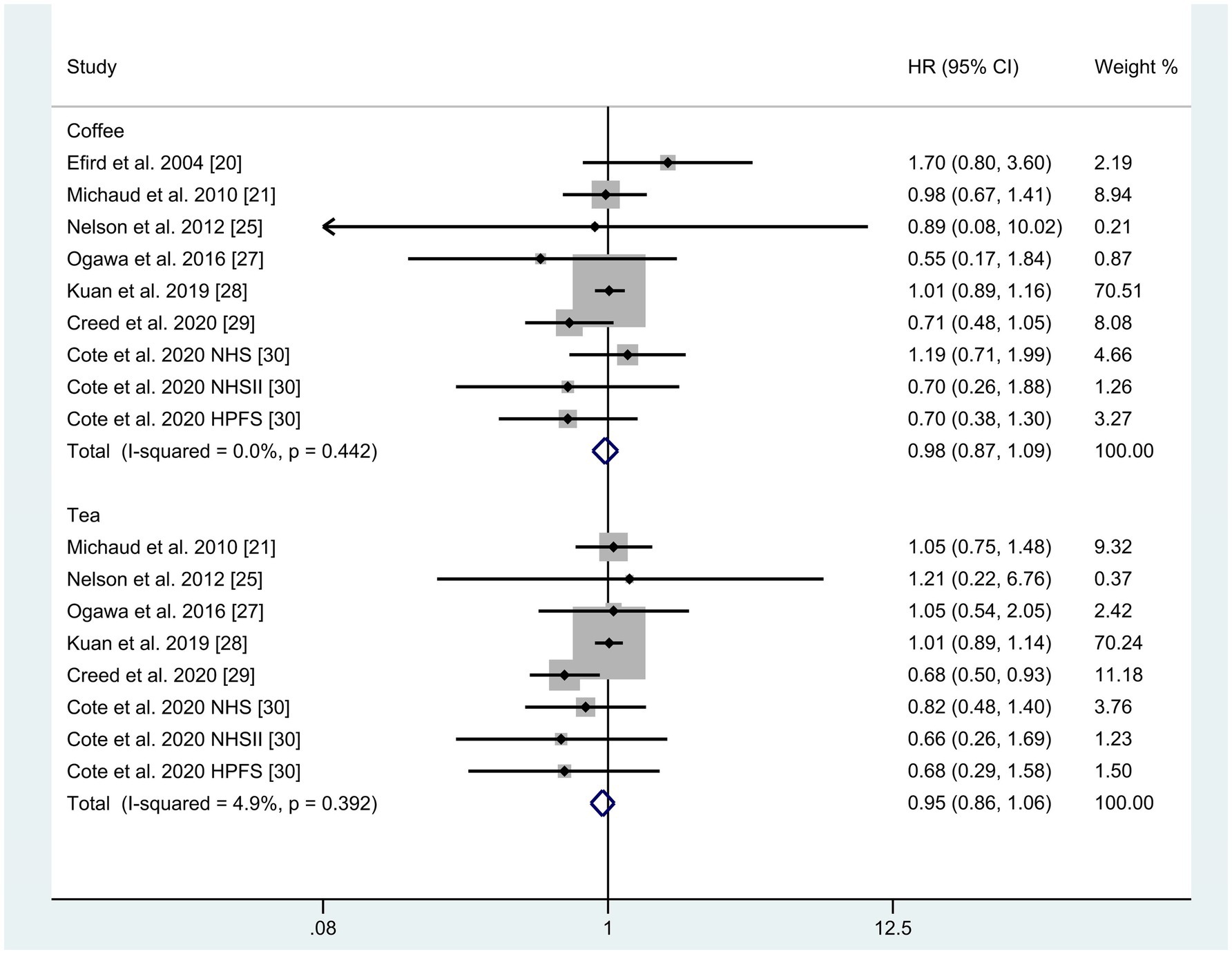
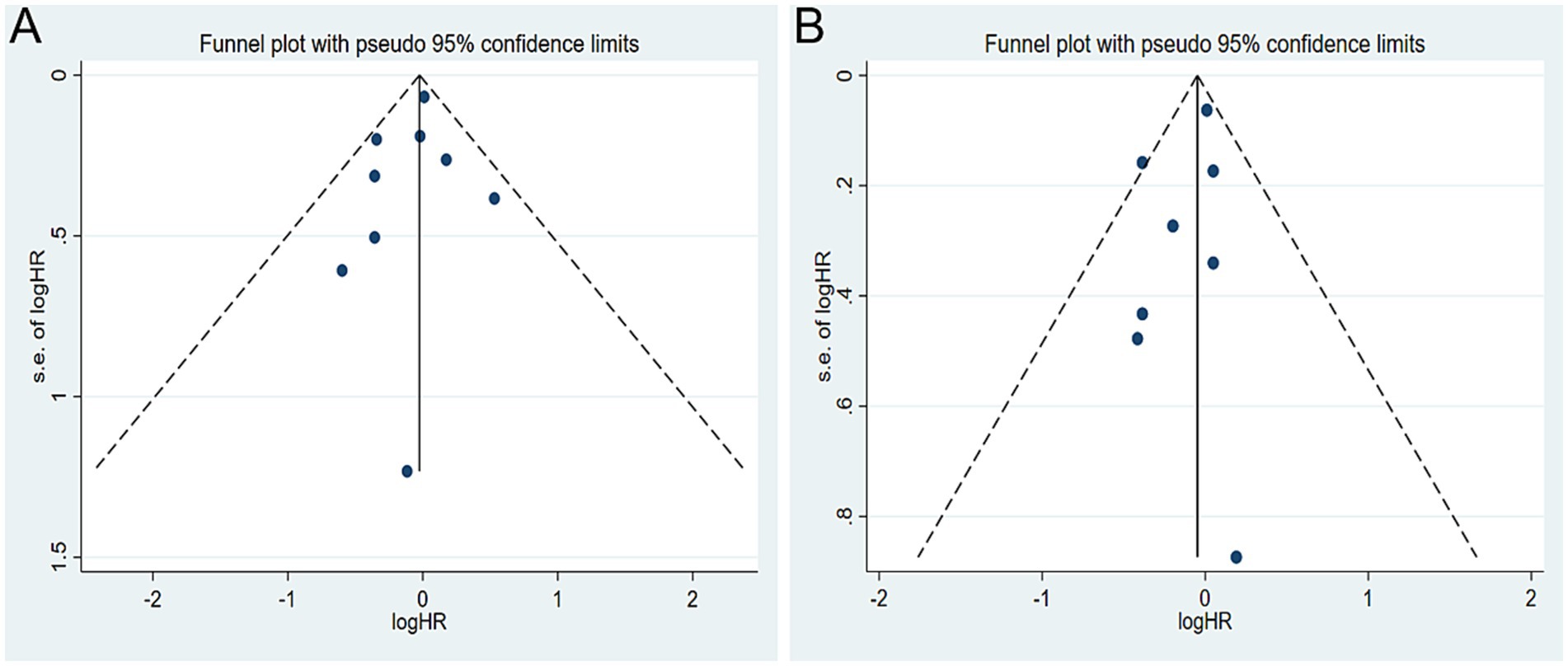
A total of seven studies were identified for the comparison between the highest and lowest levels of coffee consumption (20, 21, 25, 27–30). Figure 2 illustrates the HRs and 95% CIs for each individual study, along with the overall analysis. Heterogeneity test suggested that no significant heterogeneity was detected across the studies (I2 = 0.0%, p = 0.442) and fixed-effects model was used. The aggregated HR was found to be 0.98 (95% CI 0.87, 1.09). Sensitivity analyses confirmed that no single study had a considerable impact on the overall results (Supplementary Table 3), with the pooled HR ranging from 0.90 (95% CI 0.73, 1.10) to 1.00 (95% CI 0.89, 1.13). The symmetry of the funnel plots suggests the absence of publication bias (Figure 3A), a finding further supported by formal statistical testing (p for Egger’s test = 0.463). In a dose–response analysis, eight studies met the inclusion criteria (20, 24–27, 29, 30). A linear association between coffee intake and glioma risk was identified (Figure 4A, p = 0.656). We also perform a sensitivity analysis limited to those studies which reported adjusted risk estimates for smoking. Correspondingly, the pooled HR was 0.98 (95% CI 0.87, 1.09) in model comparing the highest and lowest levels of coffee consumption. The p for dose–response analysis was 0.457 (Supplementary Figure S1A).
Tea consumption and glioma risk
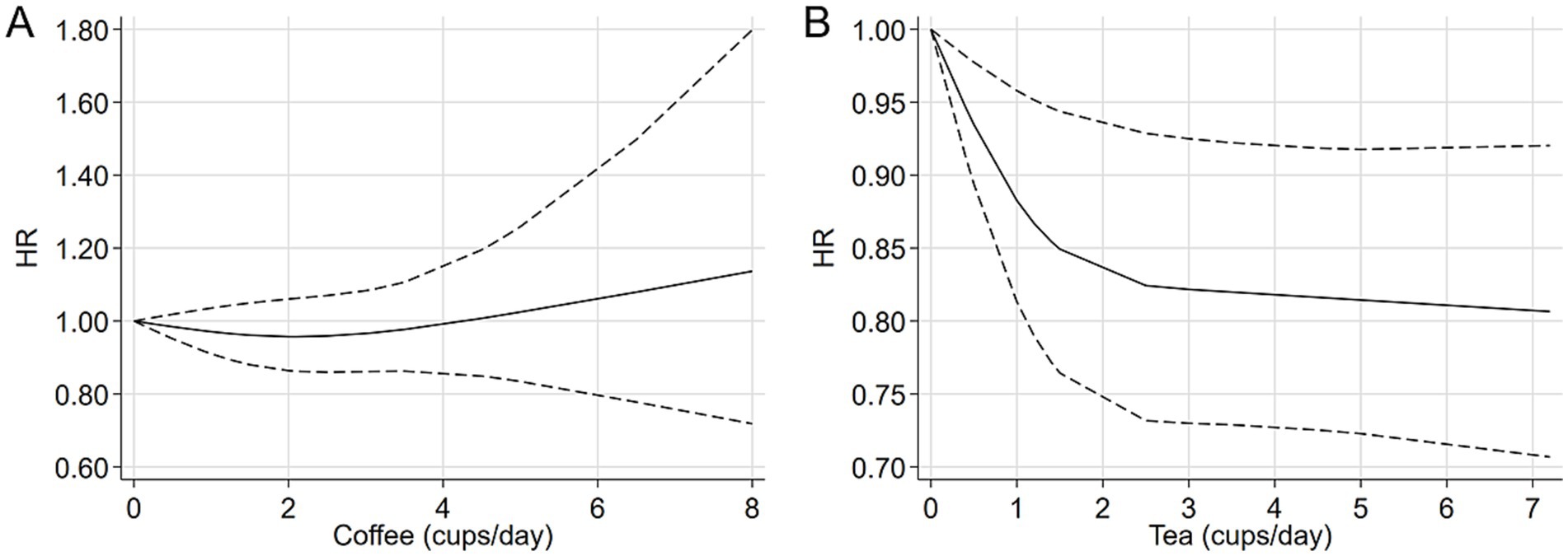
A total of six studies were included in the highest versus lowest comparison of tea consumption (21, 25, 27–30). Figure 2 illustrates the study-specific HRs with their 95% CIs, as well as the overall result. There was no statistically significant heterogeneity detected among the studies (I2 = 4.9%, p = 0.392) and fixed-effects model was used. The pooled HR was calculated as 0.95 (95% CI 0.86, 1.06). Sensitivity analyses indicated a marginal association between tea consumption and glioma risk (HR 0.83, 95% CI 0.69, 1.01, I2 = 0.0%, p = 0.605, Supplementary Table 4) when a pooled analysis of three large cohorts was excluded (28). Both the funnel plots (Figure 3B) and Egger’s test (p = 0.284) did not indicate publication bias. Six studies contained adequate data for conducting a dose–response analysis between tea consumption and glioma risk (24–27, 29, 30). The analysis demonstrated a nonlinear relationship (Figure 4B, p = 0.034), with a pronounced inverse association observed for individuals consuming more than 2.5 cups of tea per day. However, no evident reduction in glioma risk was observed with further increases in tea consumption beyond this level. Similar to our analysis of coffee, we performed an additional sensitivity analysis for tea, focusing exclusively on studies that reported adjusted risk estimates for smoking. The result was still not significant (HR 0.95, 95% CI 0.86, 1.06) in model comparing the highest and lowest levels of tea consumption. Moreover, a nonlinear relationship was found (Supplementary Figure S1B, p = 0.002).
Study quality and grading quality of evidence
According to the NOS for cohort study quality assessment, all included studies were consider to be good quality (Supplementary Table 5). Furthermore, the quality of meta-evidence was classified as “very low” for coffee and “low” for tea (Supplementary Table 6).
Discussion
Coffee and tea consumption has been examined in relation to various health outcomes, including glioma (13–30). Despite these efforts, the findings have been inconsistent. To address this, we conducted a meta-analysis of cohort studies to consolidate the available evidence and draw more definitive conclusions regarding the impact of coffee and tea consumption on glioma risk. In this comprehensive meta-analysis, no significant associations were observed between coffee or tea consumption and glioma risk when comparing the highest versus lowest intake categories. Additionally, dose–response analysis indicated a nonlinear association between tea consumption and glioma risk.
Several studies have addressed the specific type of coffee (24, 26, 30). In a large-scale, prospective NIH-AARP Diet and Health Study, which encompassed 5,268,995 person-years of follow-up, no statistically significant relationship was found between caffeinated, decaffeinated, or overall coffee intake and glioma risk (24). These results were corroborated by the PLSO trial (26), as well as a pooled analysis from the Health Professionals Follow-up Study (HPFS), the Nurses’ Health Study (NHS), and NHS II (30). Additionally, three separate investigations examined the link between caffeine intake and glioma risk (24, 26, 30). Both the NIH-AARP Study (24) and the PLCO study (26) reported no significant HRs when comparing the highest and lowest quintiles of caffeine consumption, nor were there significant trends showing a reduction in glioma risk with increasing caffeine intake. However, a borderline association was observed in the combined cohorts of NHS, NHS II, and HPFS (30). To the best of the authors’ knowledge, most studies failed to distinguish between different types of tea, such as green and black tea, with the exception of a study conducted in Japan that focused on green tea (27). The varying degrees of fermentation between green and black tea may result in differing health effects (43). Non-fermented green tea is high in catechins and contains minimal theaflavins, whereas fully fermented black tea has low catechin content but is rich in theaflavins (43, 44). Additionally, the method of coffee preparation can influence its chemical composition (45). For instance, the diterpene levels in filtered coffee are minimal in comparison to those found in boiled or French press coffee (45). Therefore, the types of coffee and tea, along with their respective brewing methods, require further investigation.
The link between coffee and tea consumption and a reduced risk of developing glioma is biologically feasible. Both tea and coffee are rich in a variety of biologically active compounds, such as caffeine, polyphenols, and flavonoids (9, 10). In vitro research has shown that caffeine may suppress the proliferation of glioma cells via the PKA/GSK3β pathways (46) and inhibit cellular migration through the ROCK-FAK pathway (47). Resveratrol, a non-flavonoid polyphenol compound, has been found to suppress glioma cell growth by modulating oncogenic microRNAs as well as the NF-κB and PI3K/AKT/mTOR pathways (48). In animal models of glioma, resveratrol has demonstrated the ability to slow tumor progression (49). Additionally, flavonoids have been shown to delay glioma growth and to enhance the efficacy of chemotherapeutic drugs in combating glioblastoma in a synergistic manner (50, 51). Collectively, these mechanisms suggest that the consumption of tea and coffee may confer protective effects against glioma.
Various meta-analyses have extensively evaluated this topic (31, 32, 34–37). For coffee consumption, almost all prior meta-analyses observed a non-significant inverse association when comparing the highest to lowest consumption levels (31, 36, 37), except an earlier study (34). Regarding tea consumption, earlier meta-analyses published before 2018 reported a non-significant association with glioma risk (31, 32, 34), while more recent meta-analyses identified a significant inverse association (35–37), including a linear association found in 2022 studies (36, 37). Although our findings partially align with those of prior meta-analyses, there are notable differences between our current study and previous research (Supplementary Table 7). Except for the four cohort studies with extended follow-up time updated by others (22, 24), our investigation exclusively incorporated cohort studies where exposure data were prospectively collected. Moreover, a dose–response analysis was conducted using the REMR method (41). Earlier meta-analyses employed the Greenland and Longnecker approach (52), which required the original studies to report the distribution of cases and person-years (non-cases) for at least three exposure levels. In contrast, using the REMR method does not need the distribution of exposure participates and necessitates a minimum of two exposure categories with corresponding relative risks and 95% CIs, thereby allowing for the inclusion of more studies in the dose–response analysis and enabling a more precise estimation of the dose–response curve. Notably, our analysis revealed a near “L”-shaped association between tea consumption and glioma risk, with the pronounced inverse association observed in individuals consuming 2.5 cups of tea per day. Beyond this level, no evident reduction in glioma risk was detected. Finally, we give a judge of the meta-evidence. Based on the current studies, the quality of meta-evidence was classified as “very low” for coffee and “low” for tea.
Our research is subject to several limitations. Firstly, the possibility of residual confounding factors from the original studies cannot be entirely ruled out. Secondly, the potential for misclassification bias warrants consideration. While all the included studies measured coffee intake based on the number of cups consumed per day, a universally accepted standard for coffee cup size does not exist. Furthermore, the variation in exposure levels, particularly between the highest and lowest reference categories, could potentially weaken the observed associations rather than enhance them, as highlighted by Poole et al. (53). Lastly, as with other meta-analyses, concerns regarding potential publication bias may affect the robustness of our findings, although no direct evidence of such bias was detected.
In conclusion, this meta-analysis suggests a potential association between tea consumption and a reduced risk of glioma, while no significant correlation was found between coffee consumption and glioma incidence. However, it is important to note that the evidence for coffee consumption was classified as “very low,” and for tea consumption, it was classified as “low.” Therefore, these findings should be interpreted with caution. Further studies with more robust evidence are warranted to confirm these associations and provide more definitive conclusions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
JP: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. CS: Conceptualization, Data curation, Formal analysis, Methodology, Software, Writing – original draft, Writing – review & editing. HT: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. NW: Conceptualization, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. We applied AI solely for grammar and style corrections without contributing any original content.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1506847/full#supplementary-material
References
1. Weller, M, Wen, PY, Chang, SM, Dirven, L, Lim, M, Monje, M, et al. Glioma. Nat Rev Dis Primers. (2024) 10:33. doi: 10.1038/s41572-024-00516-y
2. Louis, DN, Perry, A, Wesseling, P, Brat, DJ, Cree, IA, Figarella-Branger, D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro-Oncology. (2021) 23:1231–51. doi: 10.1093/neuonc/noab106
3. Schaff, LR, and Mellinghoff, IK. Glioblastoma and other primary brain malignancies in adults: a review. JAMA. (2023) 329:574–87. doi: 10.1001/jama.2023.0023
4. Lin, D, Wang, M, Chen, Y, Gong, J, Chen, L, Shi, X, et al. Trends in intracranial glioma incidence and mortality in the United States, 1975-2018. Front Oncol. (2021) 11:748061. doi: 10.3389/fonc.2021.748061
5. Ostrom, QT, Cote, DJ, Ascha, M, Kruchko, C, and Barnholtz-Sloan, JS. Adult glioma incidence and survival by race or ethnicity in the United States from 2000 to 2014. JAMA Oncol. (2018) 4:1254–62. doi: 10.1001/jamaoncol.2018.1789
6. Chen, C, Xu, T, Chen, J, Zhou, J, Yan, Y, Lu, Y, et al. Allergy and risk of glioma: a meta-analysis. Eur J Neurol. (2011) 18:387–95. doi: 10.1111/j.1468-1331.2010.03187.x
7. Gunter, MJ, Murphy, N, Cross, AJ, Dossus, L, Dartois, L, Fagherazzi, G, et al. Coffee drinking and mortality in 10 European countries: a multinational cohort study. Ann Intern Med. (2017) 167:236–47. doi: 10.7326/M16-2945
8. Chen, ML. Tea and health–an overview In: Y Zhen, Z Chen, S Cheng, and M Chen, editors. Tea: Bioactivity and therapeutic potential. London: Taylor and Francis (2002). 1–16.
9. Trevisanato, SI, and Kim, YI. Tea and health. Nutr Rev. (2000) 58:1–10. doi: 10.1111/j.1753-4887.2000.tb01818.x
10. Ludwig, IA, Clifford, MN, Lean, ME, Ashihara, H, and Crozier, A. Coffee: biochemistry and potential impact on health. Food Funct. (2014) 5:1695–717. doi: 10.1039/C4FO00042K
11. Socała, K, Szopa, A, Serefko, A, Poleszak, E, and Wlaź, P. Neuroprotective effects of coffee bioactive compounds: a review. Int J Mol Sci. (2020) 22:107. doi: 10.3390/ijms22010107
12. Klepacka, J, Tońska, E, Rafałowski, R, Czarnowska-Kujawska, M, and Opara, B. Tea as a source of biologically active compounds in the human diet. Molecules. (2021) 26:1487. doi: 10.3390/molecules26051487
13. Grosso, G, Godos, J, Galvano, F, and Giovannucci, EL. Coffee, caffeine, and health outcomes: an umbrella review. Annu Rev Nutr. (2017) 37:131–56. doi: 10.1146/annurev-nutr-071816-064941
14. Chung, M, Zhao, N, Wang, D, Shams-White, M, Karlsen, M, Cassidy, A, et al. Dose-response relation between Tea consumption and risk of cardiovascular disease and all-cause mortality: a systematic review and Meta-analysis of population-based studies. Adv Nutr. (2020) 11:790–814. doi: 10.1093/advances/nmaa010
15. Burch, JD, Craib, KJ, Choi, BC, Miller, AB, Risch, HA, and Howe, GR. An exploratory case-control study of brain tumors in adults. J Natl Cancer Inst. (1987) 78:601–9.
16. Hochberg, F, Toniolo, P, Cole, P, and Salcman, M. Nonoccupational risk indicators of glioblastoma in adults. J Neuro-Oncol. (1990) 8:55–60.
17. Giles, GG, McNeil, JJ, Donnan, G, Webley, C, Staples, MP, Ireland, PD, et al. Dietary factors and the risk of glioma in adults: results of a case-control study in Melbourne, Australia. Int J Cancer. (1994) 59:357–62. doi: 10.1002/ijc.2910590311
18. Blowers, L, Preston-Martin, S, and Mack, WJ. Dietary and other lifestyle factors of women with brain gliomas in Los Angeles County (California, USA). Cancer Causes Control. (1997) 8:5–12. doi: 10.1023/A:1018437031987
19. Malmir, H, Shayanfar, M, Mohammad-Shirazi, M, Tabibi, H, Sharifi, G, and Esmaillzadeh, A. Tea and coffee consumption in relation to glioma: a case-control study. Eur J Nutr. (2019) 58:103–11. doi: 10.1007/s00394-017-1575-z
20. Efird, JT, Friedman, GD, Sidney, S, Klatsky, A, Habel, LA, Udaltsova, NV, et al. The risk for malignant primary adult-onset glioma in a large, multiethnic, managed-care cohort: cigarette smoking and other lifestyle behaviors. J Neuro-Oncol. (2004) 68:57–69. doi: 10.1023/B:NEON.0000024746.87666.ed
21. Michaud, DS, Gallo, V, Schlehofer, B, Tjønneland, A, Olsen, A, Overvad, K, et al. Coffee and tea intake and risk of brain tumors in the European prospective investigation into Cancer and nutrition (EPIC) cohort study. Am J Clin Nutr. (2010) 92:1145–50. doi: 10.3945/ajcn.2010.29876
22. Holick, CN, Smith, SG, Giovannucci, E, and Michaud, DS. Coffee, tea, caffeine intake, and risk of adult glioma in three prospective cohort studies. Cancer Epidemiol Biomarkers Prev. (2010) 19:39–47. doi: 10.1158/1055-9965.EPI-09-0732
23. Baglietto, L, Giles, GG, English, DR, Karahalios, A, Hopper, JL, and Severi, G. Alcohol consumption and risk of glioblastoma; evidence from the Melbourne collaborative cohort study. Int J Cancer. (2011) 128:1929–34. doi: 10.1002/ijc.25770
24. Dubrow, R, Darefsky, AS, Freedman, ND, Hollenbeck, AR, and Sinha, R. Coffee, tea, soda, and caffeine intake in relation to risk of adult glioma in the NIH-AARP diet and health study. Cancer Causes Control. (2012) 23:757–68. doi: 10.1007/s10552-012-9945-6
25. Nelson, JS, Burchfiel, CM, Fekedulegn, D, and Andrew, ME. Potential risk factors for incident glioblastoma multiforme: the Honolulu heart program and Honolulu-Asia aging study. J Neuro-Oncol. (2012) 109:315–21. doi: 10.1007/s11060-012-0895-3
26. Hashibe, M, Galeone, C, Buys, SS, Gren, L, Boffetta, P, Zhang, ZF, et al. Coffee, tea, caffeine intake, and the risk of cancer in the PLCO cohort. Br J Cancer. (2015) 113:809–16. doi: 10.1038/bjc.2015.276
27. Ogawa, T, Sawada, N, Iwasaki, M, Budhathoki, S, Hidaka, A, Yamaji, T, et al. Japan public health center-based prospective study group. Coffee and green tea consumption in relation to brain tumor risk in a Japanese population. Int J Cancer. (2016) 139:2714–21. doi: 10.1002/ijc.30405
28. Kuan, AS, Green, J, Kitahara, CM, Berrington De González, A, Key, TK, Reeves, G, et al. Diet and risk of glioma: combined analysis of 3 large prospective studies in the UK and USA. Neuro-Oncology. (2019) 21:944–52. doi: 10.1093/neuonc/noz013
29. Creed, JH, Smith-Warner, SA, Gerke, TA, and Egan, KM. A prospective study of coffee and tea consumption and the risk of glioma in the UK biobank. Eur J Cancer. (2020) 129:123–31. doi: 10.1016/j.ejca.2020.01.012
30. Cote, DJ, Bever, AM, Wilson, KM, Smith, TR, Smith-Warner, SA, and Stampfer, MJ. A prospective study of tea and coffee intake and risk of glioma. Int J Cancer. (2020) 146:2442–9. doi: 10.1002/ijc.32574
31. Malerba, S, Galeone, C, Pelucchi, C, Turati, F, Hashibe, M, La Vecchia, C, et al. A meta-analysis of coffee and tea consumption and the risk of glioma in adults. Cancer Causes Control. (2013) 24:267–76. doi: 10.1007/s10552-012-0126-4
32. Zhang, YF, Xu, Q, Lu, J, Wang, P, Zhang, HW, Zhou, L, et al. Tea consumption and the incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Eur J Cancer Prev. (2015) 24:353–62. doi: 10.1097/CEJ.0000000000000094
33. Malmir, H, and Esmaillzadeh, A. The relationship between Tea and coffee consumption and glioma: a systematic review. J Babol Univ Med Sci. (2017) 19:69–75.
34. Song, Y, Wang, Z, Jin, Y, and Guo, J. Association between tea and coffee consumption and brain cancer risk: an updated meta-analysis. World J Surg Oncol. (2019) 17:51. doi: 10.1186/s12957-019-1591-y
35. Zhao, LG, Li, ZY, Feng, GS, Ji, XW, Tan, YT, Li, HL, et al. Tea drinking and risk of Cancer incidence: a Meta-analysis of prospective cohort studies and evidence evaluation. Adv Nutr. (2021) 12:402–12. doi: 10.1093/advances/nmaa117
36. Pranata, R, Feraldho, A, Lim, MA, Henrina, J, Vania, R, Golden, N, et al. Coffee and tea consumption and the risk of glioma: a systematic review and dose-response meta-analysis. Br J Nutr. (2022) 127:78–86. doi: 10.1017/S0007114521000830
37. Zhang, W, Jiang, J, Li, X, He, Y, Chen, F, and Li, W. Dietary factors and risk of glioma in adults: a systematic review and dose-response Meta-analysis of observational studies. Front Nutr. (2022) 9:834258. doi: 10.3389/fnut.2022.834258
38. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
39. Higgins, JP, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
40. Egger, M, Davey Smith, G, Schneider, M, and Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
41. Xu, C, and Doi, SAR. The robust error meta-regression method for dose-response meta-analysis. Int J Evid Based Healthc. (2018) 16:138–44. doi: 10.1097/XEB.0000000000000132
42. Atkins, D, Best, D, Briss, PA, Eccles, M, Falck-Ytter, Y, Flottorp, S, et al. Grading quality of evidence and strength of recommendations. BMJ. (2004) 328:1490. doi: 10.1136/bmj.328.7454.1490
43. Yang, CS, Li, G, Yang, Z, Guan, F, Chen, A, and Ju, J. Cancer prevention by tocopherols and tea polyphenols. Cancer Lett. (2013) 334:79–85. doi: 10.1016/j.canlet.2013.01.051
44. Khan, N, and Mukhtar, H. Tea polyphenols in promotion of human health. Nutrients. (2018) 11:39. doi: 10.3390/nu11010039
45. Nilsson, LM, Johansson, I, Lenner, P, Lindahl, B, and Guelpen, V. Consumption of filtered and boiled coffee and the risk of incident cancer: a prospective cohort study. Cancer Causes Control. (2010) 21:1533–44. doi: 10.1007/s10552-010-9582-x
46. Ku, BM, Lee, YK, Jeong, JY, Ryu, J, Choi, J, Kim, JS, et al. Caffeine inhibits cell proliferation and regulates PKA/GSK3β pathways in U87MG human glioma cells. Mol Cell. (2011) 31:275–9. doi: 10.1007/s10059-011-0027-5
47. Chen, Y, Chou, WC, Ding, YM, and Wu, YC. Caffeine inhibits migration in glioma cells through the ROCK-FAK pathway. Cell Physiol Biochem. (2014) 33:1888–98. doi: 10.1159/000362966
48. Wang, G, Dai, F, Yu, K, Jia, Z, Zhang, A, Huang, Q, et al. Resveratrol inhibits glioma cell growth via targeting oncogenic microRNAs and multiple signaling pathways. Int J Oncol. (2015) 46:1739–47. doi: 10.3892/ijo.2015.2863
49. Luís, Â, Marcelino, H, Domingues, F, Pereira, L, and Cascalheira, JF. Therapeutic potential of resveratrol for glioma: a systematic review and Meta-analysis of animal model studies. Int J Mol Sci. (2023) 24:16597. doi: 10.3390/ijms242316597
50. Parajuli, P, Joshee, N, Chinni, SR, Rimando, AM, Mittal, S, Sethi, S, et al. Delayed growth of glioma by Scutellaria flavonoids involve inhibition of Akt, GSK-3 and NF-κB signaling. J Neuro-Oncol. (2011) 101:15–24. doi: 10.1007/s11060-010-0221-x
51. Zhai, K, Mazurakova, A, Koklesova, L, Kubatka, P, and Büsselberg, D. Flavonoids synergistically enhance the anti-glioblastoma effects of chemotherapeutic drugs. Biomol Ther. (2021) 11:1841. doi: 10.3390/biom11121841
52. Greenland, S, and Longnecker, MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. (1992) 135:1301–9. doi: 10.1093/oxfordjournals.aje.a116237
Keywords: glioma, coffee, tea, cohort, risk factors, meta-analysis
Citation: Pan J, Shao C, Tang H and Wu N (2024) Coffee and tea consumption and glioma risk: a meta-analysis of cohort studies. Front. Nutr. 11:1506847. doi: 10.3389/fnut.2024.1506847
Edited by:
Macarena Lozano-Lorca, University of Granada, SpainReviewed by:
Eduard Baladia, Academia Española de Nutrición y Dietética, SpainElli Polemiti, Charité University Medicine Berlin, Germany
Copyright © 2024 Pan, Shao, Tang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Tang, aHdlaXRhbmdAZ21haWwuY29t; Nan Wu, d3VuYW5AY3F1LmVkdS5jbg==
 Jinyu Pan1
Jinyu Pan1 Chuan Shao
Chuan Shao Hui Tang
Hui Tang Nan Wu
Nan Wu