- 1Department of Nephrology, Beijing Shijitan Hospital, Capital Medical University, Beijing, China
- 2Department of Medical Oncology, Beijing Chest Hospital, Capital Medical University and Beijing Tuberculosis and Tumor Research Institute, Beijing, China
Background: As a state of metabolic and nutritional derangements, protein-energy wasting (PEW) is highly prevalent and associated with increased morbidity and mortality in hemodialysis patients. Fibroblast growth factor-23 (FGF-23) and Klotho have been proven to contribute to chronic kidney disease-mineral and bone disorder (CKD-MBD) in patients undergoing hemodialysis. Previous evidence suggested that FGF-23 and Klotho may also contribute to the malnutritional status among these patients; however, the inter-relationship between the FGF-23–Klotho axis and PEW remains unclear. Therefore, we conducted this cross-sectional study to evaluate the association between plasma FGF-23 and Klotho levels and PEW in hemodialysis patients and to explore whether these markers could predict the presence of PEW.
Methods: Plasma concentrations of FGF-23 and Klotho were measured, and their associations with PEW were assessed. PEW was evaluated based on body weight, muscle mass, biochemical data, and protein and energy intake, according to the 2008 criteria from the International Society of Renal Nutrition and Metabolism (ISRNM).
Results: In this study, 147 hemodialysis patients (mean age 61.05 ± 13.32 years) were enrolled, of whom 66 (44.90%) had PEW. PEW was significant positively correlated with FGF-23 (r = 0.403, p < 0.001), age (r = 0.225, p = 0.006), C-reactive protein (r = 0.236, p = 0.004), intact parathyroid hormone (r = 0.237, p = 0.004), and single-pool Kt/V (r = 0.170, p = 0.040), while it was negatively correlated with Klotho (r = −0.361, p < 0.001), hemoglobin (r = −0.215, p = 0.009), and serum creatinine (r = −0.278, p = 0.001). Logistic regression analyses showed that plasma FGF-23 and Klotho were independently associated with PEW, even after adjusting for covariables. The area under the ROC curve (AUC) of FGF-23 and Klotho in predicting PEW was 0.734 and 0.710 (p < 0.001), respectively. When the combination of FGF-23 and Klotho was used to predict PEW, its sensitivity was 81.8%, specificity was 60.5%, and the AUC was 0.746.
Conclusion: Plasma levels of FGF-23 and Klotho are associated with PEW in hemodialysis patients. Higher plasma FGF-23 levels and lower Klotho levels may serve as valuable predictors of PEW in these patients.
1 Introduction
As a state of metabolic and nutritional derangements, protein-energy wasting (PEW) in patients with chronic kidney disease (CKD) has received great attention in recent years, not only because it has high prevalence among these patients but also because the presence and severity of PEW are closely related to morbidity and mortality among patients with all stages of CKD (1, 2). Previous studies suggested that PEW symptoms were observed in almost 20% of stage 3–5 non-dialysis CKD patients, and the prevalence sharply increased to more than 55% once the patients initiated maintenance dialysis (3). Facing such a serious challenge, it is crucial to identify PEW as early as possible in patients undergoing hemodialysis; however, the current applied diagnostic criteria created by the International Society of Renal Nutrition and Metabolism (ISRNM) require a number of variables to be known and physical examinations to be performed; the complexity of this criteria limits its practical application in clinical settings. So finding more concise predictors of PEW in CKD patients seems to be necessary (4).
The fibroblast growth factor-23 (FGF-23)–Klotho axis plays a major role in chronic kidney disease-mineral and bone disorder (CKD-MBD) in patients with CKD (5, 6). FGF-23 is secreted mainly by osteocytes, and its plasma levels increase 100- to 1,000-fold higher in the end stage of CKD (7, 8). The etiology of plasma-elevated FGF-23 in CKD is mostly because of decreased renal clearance and a feedback response to elevated serum phosphate, and FGF-23 has a feedback relationship with its co-receptor Klotho (9, 10). Studies have shown that plasma Klotho levels decrease during the early stage of CKD (11). Klotho protein exerts diverse effects on the physiological regulation of serum calcium, phosphate, and energy metabolism by influencing the endocrine activities of the FGF-23 (12, 13). In addition to these events, accumulating evidence has shown that plasma FGF-23 and Klotho are associated with vascular calcification, infection, anemia, systemic inflammation, and oxidative stress in hemodialysis patients, which are commonly observed and closely associated with the presence and severity of PEW in hemodialysis patients (14–17). However, the role of plasma FGF-23 and Klotho in metabolic and nutritional status remains unclear, and literature on the relationship between circulating FGF-23–Klotho levels and PEW in hemodialysis patients is scarce (3).
Therefore, we conducted a cross-sectional study to evaluate the relationship between plasma FGF-23 and Klotho and PEW in hemodialysis patients and to explore whether plasma FGF-23 and Klotho could predict the presence of PEW in these patients.
2 Methods
2.1 Study design and population
We conducted a cross-sectional study on patients who underwent hemodialysis at the Dialysis Center of Beijing Shijitan Hospital, Capital Medical University from June 2023 to January 2024. The inclusion criteria were as follows: (1) patients with end-stage renal disease (ESRD) undergoing hemodialysis for at least three consecutive months and (2) age ≥ 18 years. The exclusion criteria were: (1) acute kidney injury, (2) merge malignant tumors and chronic wasting diseases, (3) severe infections, heart failure, and gastrointestinal bleeding in the past three months, (4) long-term use of glucocorticoids and immunosuppressants, and (5) unable to cooperate with the examiner for various reasons. All patients were prescribed routine hemodialysis three times a week for 4–5 h each time, with a blood flow rate of 200–250 mL/min and a dialysate flow rate of 500 mL/min.
The study was approved by the Ethics Committee of Beijing Shijitan Hospital [sjtky11-1x-2022(079)]. The protocol complied with the Declaration of Helsinki; all participants provided written consent.
2.2 Data collection and laboratory measurements
We collected participants’ data, including gender, age, etiology of ESRD, comorbidities, medications used, dialysis access, and dialysis vintage (months). Blood samples were obtained before and after the first dialysis session of the week, following a two-day interval. All blood samples were centrifuged and stored at −80°C. The hemoglobin, urea nitrogen, creatinine, albumin, prealbumin, cholesterol, triglyceride, calcium, phosphate, intact parathyroid hormone (iPTH), and C-reactive protein (CRP) levels were measured using standard laboratory techniques with an auto-analyzer. The plasma C-terminal FGF-23 and Klotho levels were measured using enzyme-linked immunoassays (human FGF-23 ELISA kit: Immutopics, San Clemente, CA, USA, human Klotho ELISA kit: Cusabio, China). Urea clearance index single-pool Kt/V (sp. Kt/V) was calculated from the pre- and post-dialysis serum urea nitrogen levels using the Daugirdas formula (18).
2.3 Dietary intake
The lead research dietitian guided participants in filling out their dietary records. Averages were then computed from these three-day records for total energy (kcal and kcal/kg) and total protein (g and g/kg).
2.4 Physical measurements
Body mass index (BMI, kg/m2) was calculated by dividing the dry weight (kg) by the square of the height (m). Mid-upper arm circumference (MUAC) reflects a patient’s muscle and fat mass. Measurements were conducted using a plastic tape at the midpoint of the arm, avoiding vascular access. The midpoint of the arm was located halfway between the shoulder tip and the elbow tip. Triceps skinfold (TSF) was measured with a skinfold caliper with the arm hanging relaxed at the side. The measurements were repeated twice, and the average value was used for analysis. The mid-upper arm muscle circumference (MUAMC) was calculated by the formula: MUAMC (cm) = MUAC (cm) – 3.14 × TSF (cm). These values were compared to the reference values provided by Frisancho (19).
2.5 Handgrip strength measurement
The patients underwent three tests while seated, with the arm positioned alongside the trunk, hips, and knees flexed at 90°, and the wrist pronated. Hand grip strength (HGS) was defined as the average measurement and assessed in the arm opposite the arteriovenous fistula.
2.6 Protein-energy wasting evaluation
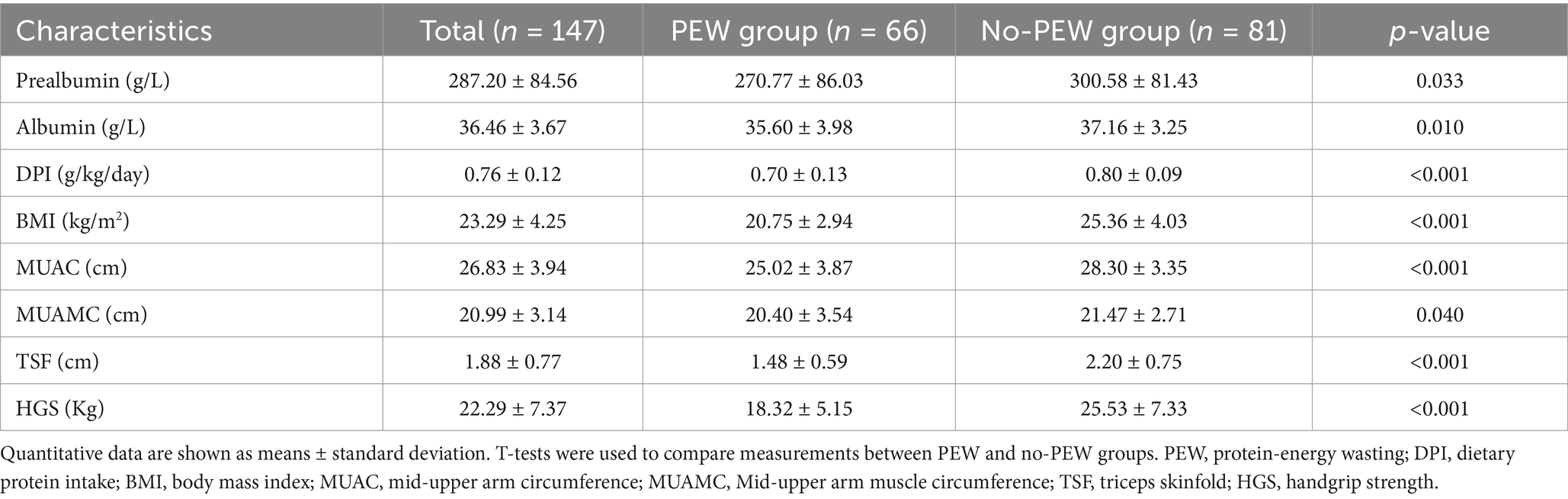
We evaluated the presence of PEW for each patient based on the criteria outlined by ISRNM in 2008 (20). PEW was considered present if at least three of the following four criteria were met: (1) serum albumin <3.8 g/100 mL or serum prealbumin <30 mg/100 mL, (2) BMI < 23 kg/m2, (3) reduced MUAMC (> 10% compared with the 50th percentile of the reference population), (4) dietary protein intake <0.8 g/kg/day.
2.7 Statistical analysis
Descriptive data were presented as frequency counts and proportions for categorical variables, means ± standard deviations (SDs) for normally distributed continuous variables, and medians with interquartile ranges (IQRs) for skewed variables. The comparison of variables between the two PEW groups used chi-squared tests, T-tests, or Mann–Whitney U tests, as they were appropriate. The correlation between PEW and clinical variables was examined using Spearman rank correlation analysis. Univariable and multivariable logistic regression analyses were conducted to assess the influence of the plasma FGF-23 and Klotho levels on the risk of PEW, with PEW as the dependent variable, plasma FGF-23 or Klotho as the independent variables entered into the multivariate logistic regression models (model 0: unadjusted; model 1: adjusted for age, gender; model 2: adjusted for age, gender, and all covariates with a p value of less than 0.05 on the univariable analysis). To further evaluate the predictive power of the plasma FGF-23 and Klotho for PEW against the ISRNM standards, a receiver operating characteristic (ROC) curve analysis was performed. The area under the curve, sensitivity, and specificity of the plasma FGF-23 and Klotho at various cutoffs were calculated. Optimal cut-off points were determined using the maximum value of Youden’s index (calculated by sensitivity + specificity – 1). The predictive value of plasma FGF-23 and Klotho concentrations on PEW was evaluated by calculating the area under the ROC curve (AUC). All the analyses were performed with SPSS version 29.0 statistical software (SPSS Inc., Chicago, IL, USA). Statistical significance was set at a value of p < 0.05.
3 Results
3.1 Characteristics of participants
We included 147 hemodialysis patients (including 48 females) with an age of 61.05 ± 13.32 years (21–85 years) and the median dialysis vintage of 61 (37, 115) months. The etiologies of the included patients were as follows: diabetes (37.41%), glomerulonephritis (35.37%), hypertension (13.61%), polycystic kidney disease (4.76%), and others (8.84%).
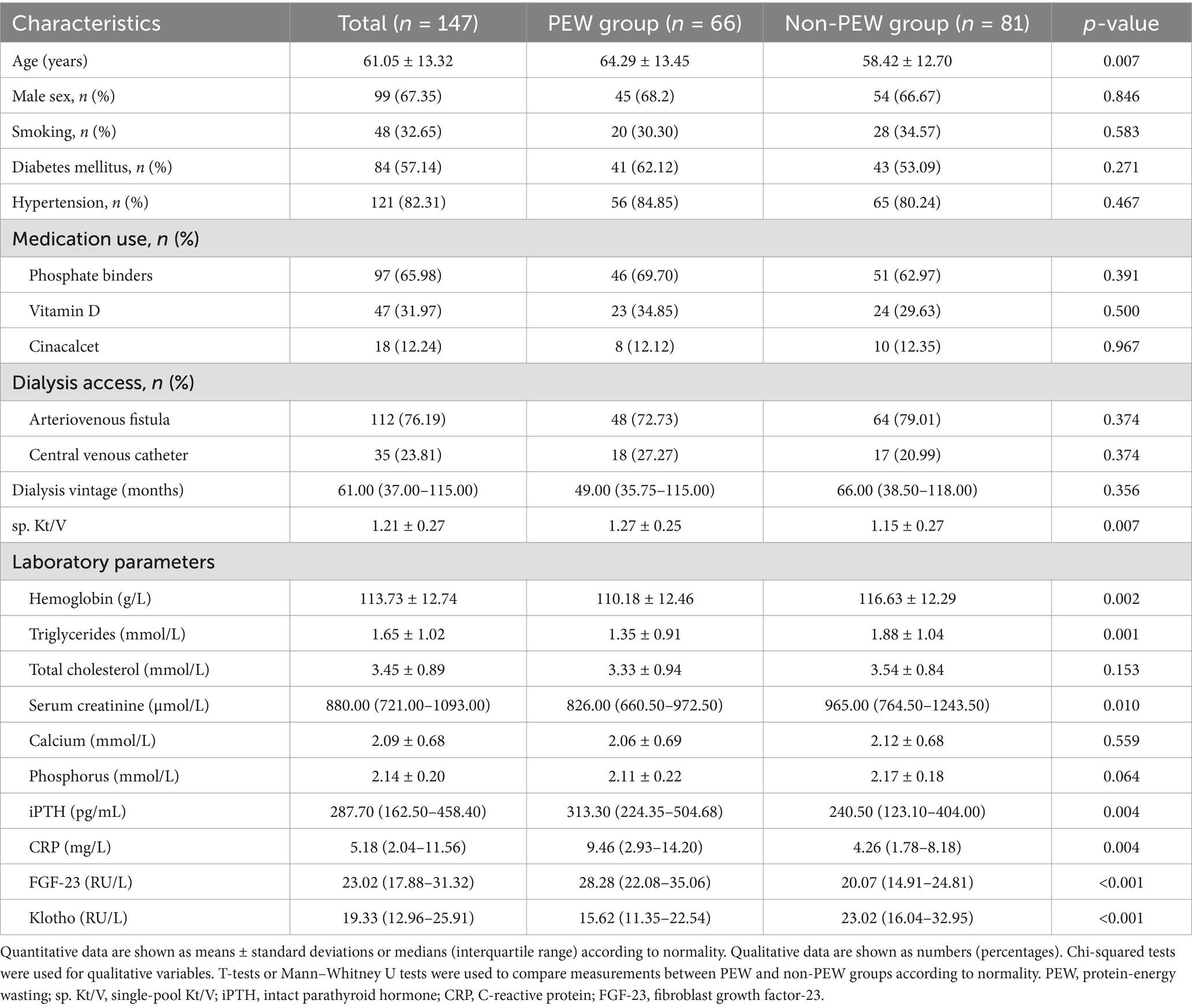
Among them, there were 66 patients (44.90%) with PEW. PEW subjects tend to be older, and with higher levels of iPTH, CRP, FGF-23, and sp. Kt/V, and lower levels of hemoglobin, triglycerides, serum creatinine, and Klotho (all p < 0.05) (Table 1). The characteristics of the components of PEW are shown in Table 2.
3.2 Correlations between PEW and clinical parameters
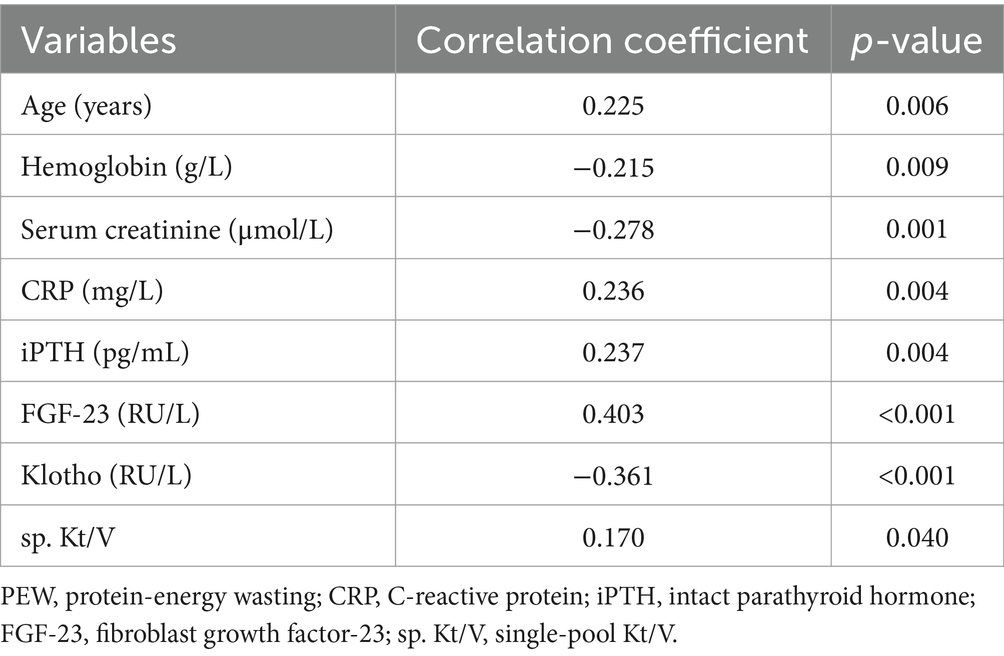
Significant positive correlations were observed between PEW and FGF-23, age, CRP, iPTH, and sp. Kt/V. Conversely, PEW was negatively correlated with Klotho, hemoglobin, and serum creatinine (Table 3).
3.3 Associations between the levels of plasma FGF-23 and Klotho and PEW
The associations between the levels of plasma FGF-23 and Klotho with PEW are presented in Table 4. The level of plasma FGF-23 (OR: 1.087; 95% CI: 1.044–1.132) and Klotho (OR: 0.951; 95% CI: 0.919–0.983) were significantly associated with PEW in unadjusted analyses. After further multivariate adjustment (Model 1 and 2), these associations remained statistically significant.
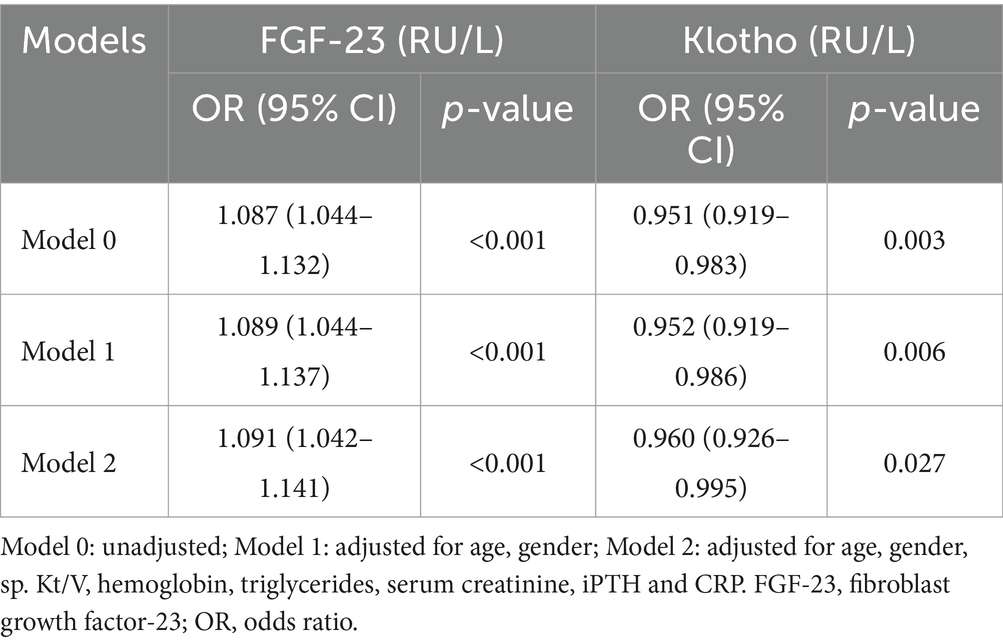
Table 4. Logistic regression analyses for the association between the levels of plasma FGF-23 and Klotho and PEW.
3.4 ROC curve analysis
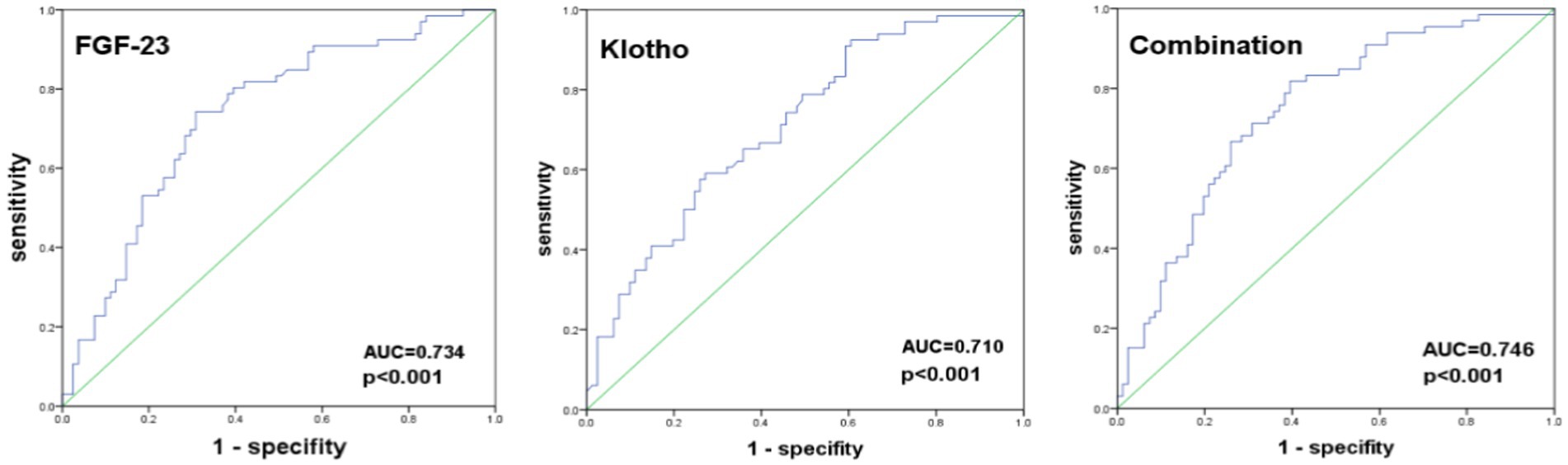
The ROC analysis identified an optimal cut-off for FGF-23 at 22.97 RU/L, with a sensitivity of 74.2%, a specificity of 69.1%, and an area under the curve (AUC) of 0.734 (95% CI: 0.652–0.815); an optimal cut-off for Klotho at 17.61 RU/L, with a sensitivity of 59.1%, a specificity of 72.8%, and an area under the curve (AUC) of 0.710 (95% CI: 0.627–0.792). When the combination of FGF-23 and Klotho was used to predict PEW, its sensitivity was 81.8%, specificity was 60.5%, and area under the curve (AUC) was 0.746 (95% CI: 0.667–0.826) (Figure 1; Table 5).
4 Discussion
In this cross-sectional study, we found that 44.9% of hemodialysis patients experienced PEW. Significant factors such as increased age, sp. Kt/V, CRP, iPTH, and decreased hemoglobin and serum creatinine were associated with PEW in these patients. Additionally, higher plasma FGF-23 and lower plasma Klotho concentrations were independently associated with PEW, even after adjusting for confounding factors. ROC analysis indicated that plasma FGF-23 and Klotho levels may serve as useful predictors for identifying PEW in hemodialysis patients.
PEW is a common metabolic and nutritional disorder in patients undergoing hemodialysis (21, 22). Depending on the 2008 ISRNM diagnostic criteria, the prevalence of PEW ranged from 30 to 75% in hemodialysis patients (23). In the present study, nearly half of the hemodialysis patients were affected by PEW. Previous studies have shown that PEW in CKD is quite different from that in the general population, encompassing a broader scope of pathologies related to poor nutritional status in the uremic milieu, persistent inflammation, increased oxidative stress, and other CKD-related complications, such as CKD-MBD and acidemia (24). These factors can decrease anabolism or increase catabolism, which may increase resting energy expenditure and cause hypermetabolism, eventually leading to progressive muscle wasting and frailty (25). In our study, the risk factors that reflect the above-mentioned pathological status in hemodialysis patients were also validated. Furthermore, multiple studies have indicated that PEW is closely associated with major adverse clinical outcomes, including increased hospitalization and mortality (26). Thus, early identification of PEW is quite important. However, the currently recommended criteria by ISRNM are complex and involve assessing multiple variables and subjecting the patient to physical examinations, impeding its practical application in clinical settings. Finding sensitive predictors that can be applied more easily in clinical practice is therefore necessary. FGF-23 and Klotho are well-known for their important role in CKD-MBD. Recently, increasing evidence suggests that FGF-23 and Klotho are associated with systemic inflammation and oxidative stress in hemodialysis patients. These results gave us a hint that FGF-23 and Klotho might have a potential relationship with PEW. Investigation of the association between the FGF-23–Klotho axis and PEW may provide new insights into early identification and prevention of PEW.
Several previous studies have focused on the correlation between plasma FGF-23 levels and the metabolic and nutritional status of hemodialysis patients. Montford et al. assessed the associations of plasma FGF23 concentrations with BMI, total cholesterol, low-density lipoprotein-cholesterol, high-density lipoprotein-cholesterol, and triglycerides (27). These results indicated that higher plasma FGF23 levels are associated with lower BMI and blood lipid levels in dialysis patients. Decreased BMI is one of the PEW diagnostic criteria, and has been shown to correlate with poor outcomes in patients requiring chronic dialysis. Montford et al.’s study seems consistent with our study, suggesting a link between FGF-23 and the nutritional status of hemodialysis patients. In another study by Fukasawa et al., the authors assessed the relationship between the FGF-23–Klotho system and muscle mass and found that FGF-23 was an independent predictor of muscle mass (28). These results seem somewhat different from our study. This discrepancy may be due to differences in study populations and nutritional assessment indicators. Fukasawa et al. used creatinine production rate and abdominal muscle area as muscle mass indicators. Creatinine production rate may be influenced by residual renal function and dialysis dose, while abdominal muscle area was related to gender and measurement method.
The FGF23–Klotho axis plays an important role in CKD-MBD and is involved in mineral metabolism. In the present study, we controlled for phosphorus, calcium, and parathyroid hormone, but FGF-23 and Klotho were still independently associated with PEW. There may be some interconnections between FGF23–Klotho and known mechanisms of PEW in the setting of CKD. A cross-sectional study of patients receiving maintenance hemodialysis demonstrated that patients in the highest tertile group stratified by serum FGF23 levels had a higher odds ratio for increased serum inflammatory markers (29). Other previous studies have shown that FGF-23 can directly stimulate the production of proinflammatory cytokines (via FGFR4 binding and activation on hepatocytes) and amplify systemic inflammation (14, 30) while interleukin-6 and tumor necrosis factor-α have been verified to promote muscle protein degradation and muscle wasting (31). In addition, previous studies have shown a positive association between FGF-23 and insulin resistance, which can decrease insulin-stimulated protein synthesis and increase protein degradation in skeletal muscle, and may play a key role in PEW (32, 33). On the other hand, Klotho has been shown to have anti-inflammatory and anti-oxidative stress effects (34, 35). Several in vivo studies suggest that Klotho may play a role in carbohydrate metabolism (36). Collectively, these data suggest the interplay between FGF23–Klotho and energy metabolism, although the mechanism remains to be elucidated.
Given the complexity of the ISRNM diagnostic criteria for PEW, we also explored the predictive value of plasma FGF-23 and Klotho using ROC curve analysis. Our findings indicated that plasma FGF-23 and Klotho levels could serve as concise predictors of PEW in hemodialysis patients. However, these results still need to be validated in future prospective clinical studies.
Although we have provided data on the association between FGF-23–Klotho and PEW, as well as identifying their predictive value of PEW, some limitations must be mentioned. First, due to the cross-sectional study design, a longitudinal causal relationship cannot be established between the changes in the plasma FGF-23–Klotho levels and alterations in PEW. Second, as a single-center study, selection bias may have been introduced; therefore, caution must be exercised before extrapolating our results to other hemodialysis patients. Larger sample sizes and validation among different cohorts should be needed to confirm our results. Third, the cut-off values used in our study should be carefully extrapolated to other patients, given the differences in research cohorts and detection methods. Fourth, we did not measure vitamin D levels in this study population, although one of the primary physiological actions of FGF-23 is vitamin D metabolism, and the association between vitamin D levels and muscle mass has been reported.
5 Conclusion
The present study found that plasma FGF-23 and Klotho levels are associated with PEW in hemodialysis patients. Higher plasma FGF-23 and lower Klotho levels may be valuable predictors of PEW in hemodialysis patients. Thus, regular assessment of plasma FGF-23 and Klotho in patients on hemodialysis may help predict the existence of PEW, and this could facilitate timely intervention of this pathological status.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Beijing Shijitan Hospital, Capital Medical. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XZ: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. YL: Funding acquisition, Resources, Supervision, Writing – review & editing. YG: Data curation, Methodology, Writing – review & editing. MJ: Investigation, Methodology, Writing – review & editing. CZ: Investigation, Methodology, Resources, Writing – review & editing. ZS: Investigation, Methodology, Writing – review & editing. YD: Project administration, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This project was supported by a grant from the Beijing Municipal Science and Technology Commission (No. Z161100002616005), the Capital’s Funds for Health Improvement and Research (2022-2-2081), and Beijing Shijitan Hospital Youth Fund (2020-q15). The funders had no role in the study design, data collection, data analysis, decision to publish, and preparation of the manuscript.
Acknowledgments
We acknowledge the staff of Beijing Shijitan Hospital Blood Purification Center. We thank all participants included in our present study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kittiskulnam, P, Chuengsaman, P, Kanjanabuch, T, Katesomboon, S, Tungsanga, S, Tiskajornsiri, K, et al. Protein-energy wasting and mortality risk prediction among peritoneal Dialysis patients. J Ren Nutr. (2021) 31:679–86. doi: 10.1053/j.jrn.2020.11.007
2. Mahmoud, M, Nagy, E, AbdAlBary, M, El-Kannishy, G, and Sayed-Ahmed, N. Relation of protein energy wasting to carotid intima media thickness in hemodialysis patients. J Hum Hypertens. (2021) 35:598–603. doi: 10.1038/s41371-020-0376-7
3. Elsurer Afsar, R, Afsar, B, and Ikizler, TA. Fibroblast growth factor 23 and muscle wasting: a metabolic point of view. Kidney Int Rep. (2023) 8:1301–14. doi: 10.1016/j.ekir.2023.04.027
4. Koppe, L, and Mak, RH. Is there a need to “modernize” and “simplify” the diagnostic criteria of protein-energy wasting? Semin Nephrol. (2023) 43:151403. doi: 10.1016/j.semnephrol.2023.151403
5. Muñoz-Castañeda, JR, Rodelo-Haad, C, Pendon-Ruiz de Mier, MV, Martin-Malo, A, Santamaria, R, and Rodriguez, M. Klotho/FGF23 and Wnt signaling as important players in the comorbidities associated with chronic kidney disease. Toxins. (2020) 12:185. doi: 10.3390/toxins12030185
6. Erben, RG. Physiological actions of fibroblast growth Factor-23. Front Endocrinol. (2018) 9:267. doi: 10.3389/fendo.2018.00267
7. Sprague, SM, Martin, KJ, and Coyne, DW. Phosphate balance and CKD-mineral bone disease. Kidney Int Rep. (2021) 6:2049–58. doi: 10.1016/j.ekir.2021.05.012
8. Kurpas, A, Supeł, K, Idzikowska, K, and Zielińska, M. FGF23: a review of its role in mineral metabolism and renal and cardiovascular disease. Dis Markers. (2021) 2021:8821292–12. doi: 10.1155/2021/8821292
9. Zou, D, Wu, W, He, Y, Ma, S, and Gao, J. The role of Klotho in chronic kidney disease. BMC Nephrol. (2018) 19:285. doi: 10.1186/s12882-018-1094-z
10. Lunyera, J, and Scialla, JJ. Update on chronic kidney disease mineral and bone disorder in cardiovascular disease. Semin Nephrol. (2018) 38:542–58. doi: 10.1016/j.semnephrol.2018.08.001
11. Li, SS, Sheng, MJ, Sun, ZY, Liang, Y, Yu, LX, and Liu, QF. Upstream and downstream regulators of Klotho expression in chronic kidney disease. Metabolism. (2023) 142:155530. doi: 10.1016/j.metabol.2023.155530
12. Buchanan, S, Combet, E, Stenvinkel, P, and Shiels, PG. Klotho, aging, and the failing kidney. Front Endocrinol. (2020) 11:560. doi: 10.3389/fendo.2020.00560
13. Mace, ML, Olgaard, K, and Lewin, E. New aspects of the kidney in the regulation of fibroblast growth factor 23 (FGF23) and mineral homeostasis. Int J Mol Sci. (2020) 21:8810. doi: 10.3390/ijms21228810
14. Babitt, JL, and Sitara, D. Crosstalk between fibroblast growth factor 23, iron, erythropoietin, and inflammation in kidney disease. Curr Opin Nephrol Hypertens. (2019) 28:304–10. doi: 10.1097/MNH.0000000000000514
15. Czaya, B, and Faul, C. The role of fibroblast growth factor 23 in inflammation and Anemia. Int J Mol Sci. (2019) 20:4195. doi: 10.3390/ijms20174195
16. Singh, S, Grabner, A, Yanucil, C, Schramm, K, Czaya, B, Krick, S, et al. Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int. (2016) 90:985–96. doi: 10.1016/j.kint.2016.05.019
17. Typiak, M, and Piwkowska, A. Antiinflammatory actions of Klotho: implications for therapy of diabetic nephropathy. Int J Mol Sci. (2021) 22:956. doi: 10.3390/ijms22020956
18. Daugirdas, JT. Second generation logarithmic estimates of single-pool variable volume Kt/V: an analysis of error. J Am Soc Nephrol. (1993) 4:1205–13. doi: 10.1681/ASN.V451205
19. Frisancho, AR. New norms of upper limb fat and muscle areas for assessment of nutritional status. Am J Clin Nutr. (1981) 34:2540–5. doi: 10.1093/ajcn/34.11.2540
20. Fouque, D, Kalantar-Zadeh, K, Kopple, J, Cano, N, Chauveau, P, Cuppari, L, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. (2008) 73:391–8. doi: 10.1038/sj.ki.5002585
21. Carrero, JJ, Stenvinkel, P, Cuppari, L, Ikizler, TA, Kalantar-Zadeh, K, Kaysen, G, et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J Ren Nutr. (2013) 23:77–90. doi: 10.1053/j.jrn.2013.01.001
22. Carrero, JJ, Thomas, F, Nagy, K, Arogundade, F, Avesani, CM, Chan, M, et al. Global prevalence of protein-energy wasting in kidney disease: a Meta-analysis of contemporary observational studies from the International Society of Renal Nutrition and Metabolism. J Ren Nutr. (2018) 28:380–92. doi: 10.1053/j.jrn.2018.08.006
23. Ho, LC, Wang, HK, Chiu, LT, Wang, HH, Lee, YC, Hung, SY, et al. Protein energy wasting-based nutritional assessment predicts outcomes of acute ischemic stroke and solves the epidemiologic paradox. Nutrition. (2022) 93:111431. doi: 10.1016/j.nut.2021.111431
24. Yamada, S, Tsuruya, K, Kitazono, T, and Nakano, T. Emerging cross-talks between chronic kidney disease-mineral and bone disorder (CKD-MBD) and malnutrition-inflammation complex syndrome (MICS) in patients receiving dialysis. Clin Exp Nephrol. (2022) 26:613–29. doi: 10.1007/s10157-022-02216-x
25. Hanna, RM, Ghobry, L, Wassef, O, Rhee, CM, and Kalantar-Zadeh, K. A practical approach to nutrition, protein-energy wasting, sarcopenia, and Cachexia in patients with chronic kidney disease. Blood Purif. (2020) 49:202–11. doi: 10.1159/000504240
26. Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis. (2000) 35:S17–S104. doi: 10.1053/ajkd.2000.v35.aajkd03517
27. Montford, JR, Chonchol, M, Cheung, AK, Kaufman, JS, Greene, T, Roberts, WL, et al. Low body mass index and dyslipidemia in dialysis patients linked to elevated plasma fibroblast growth factor 23. Am J Nephrol. (2013) 37:183–90. doi: 10.1159/000346941
28. Fukasawa, H, Ishigaki, S, Kinoshita-Katahashi, N, Niwa, H, Yasuda, H, Kumagai, H, et al. Plasma levels of fibroblast growth factor-23 are associated with muscle mass in haemodialysis patients. Nephrology. (2014) 19:784–90. doi: 10.1111/nep.12333
29. Munoz Mendoza, J, Isakova, T, Ricardo, AC, Xie, H, Navaneethan, SD, Anderson, AH, et al. Fibroblast growth factor 23 and inflammation in CKD. Clin J Am Soc Nephrol. (2012) 7:1155–62. doi: 10.2215/CJN.13281211
30. Tinti, F, Lai, S, Noce, A, Rotondi, S, Marrone, G, Mazzaferro, S, et al. Chronic kidney disease as a systemic inflammatory syndrome: update on mechanisms involved and potential treatment. Life. (2021) 11:419. doi: 10.3390/life11050419
31. Sartori, R, Romanello, V, and Sandri, M. Mechanisms of muscle atrophy and hypertrophy: implications in health and disease. Nat Commun. (2021) 12:330. doi: 10.1038/s41467-020-20123-1
32. Siew, ED, and Ikizler, TA. Determinants of insulin resistance and its effects on protein metabolism in patients with advanced chronic kidney disease. Contrib Nephrol. (2008) 161:138–44. doi: 10.1159/000130659
33. Hesse, M, Fröhlich, LF, Zeitz, U, Lanske, B, and Erben, RG. Ablation of vitamin D signaling rescues bone, mineral, and glucose homeostasis in Fgf-23 deficient mice. Matrix Biol. (2007) 26:75–84. doi: 10.1016/j.matbio.2006.10.003
34. Kuro-O, M. The Klotho proteins in health and disease. Nat Rev Nephrol. (2019) 15:27–44. doi: 10.1038/s41581-018-0078-3
35. Neyra, JA, and Hu, MC. Potential application of Klotho in human chronic kidney disease. Bone. (2017) 100:41–9. doi: 10.1016/j.bone.2017.01.017
Keywords: hemodialysis, protein-energy wasting, fibroblast growth factor-23, Klotho, malnutrition
Citation: Zhou X, Luo Y, Guo Y, Jia M, Zhang C, Shi Z and Du Y (2025) Predictive value of circulating fibroblast growth factor-23 and Klotho on protein-energy wasting in patients undergoing hemodialysis. Front. Nutr. 11:1497869. doi: 10.3389/fnut.2024.1497869
Edited by:
Barbara Perez Vogt, Federal University of Uberlandia, BrazilReviewed by:
Vasiliki Karava, Aristotle University of Thessaloniki, GreeceSatish Balan, Kerala Institute of Medical Sciences (KIMS), India
Gopalakrishnan Natarajan, Madras Medical College, India
Copyright © 2025 Zhou, Luo, Guo, Jia, Zhang, Shi and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ye Du, ZHV5ZTExMTdAc2luYS5jb20=
 Xiaoling Zhou
Xiaoling Zhou Yang Luo
Yang Luo Yidan Guo
Yidan Guo Meng Jia1
Meng Jia1