- Department of Cardiology, Huzhou Central Hospital, Fifth School of Clinical Medicine of Zhejiang Chinese Medical University, Huzhou, Zhejiang, China
Objective: This study aims to shed light on the correlation between Healthy Eating Index-2020 (HEI-2020) and heart failure (HF) in American adults aged 50 or above.
Methods: Data were from the National Health and Nutrition Examination Survey 2005–2020, encompassing 13,105 participants with an age of 50 or above. HEI-2020 score was utilized for rating the dietary quality. The link of HEI-2020 to HF was assessed via logistic regression, restricted cubic splines (RCS), generalized additive models (GAM), weighted quantile sum (WQS) regression, as well as quantile g-computation (Qgcomp) models.
Results: A negative association between HEI-2020 and HF risk was uncovered in middle-aged and older Americans (OR = 0.99, 95% CI: 0.98–1.00, p = 0.006). The highest quartile (Q4) exhibited a markedly lower HF risk than the lowest quartile (Q1) (OR = 0.70, 95% CI: 0.55–0.89, p = 0.004). RCS and GAM analyses demonstrated a linear dose–response relationship between HEI-2020 and HF. Finally, WQS regression and Qgcomp models revealed a beneficial combined influence of 13 dietary components on HF risk, with dairy and whole fruits emerging as the most influential.
Conclusion: Elevated HEI-2020 scores are linked to decreased HF risks among Americans aged 50 or above, suggesting that adherence to the Dietary Guidelines for Americans can mitigate HF risk.
1 Introduction
Heart failure (HF), a prevalent and intricate illness, often arises from weakened left ventricular myocardial function. It manifests as dyspnea, fatigue, less exercise tolerance, fluid retention, pulmonary edema, and peripheral edema (1). It is estimated that over 60 million individuals globally are afflicted with HF, and its prevalence will continue rising as global people age (2, 3). Furthermore, the mortality attributed to HF is on the rise, imposing a heavy social and economic burden on society (4).
In recent years, drug, surgical, and other interventions have markedly improved HF patients’ survival and well-being (5–8). Medications including angiotensin-converting enzyme and angiotensin receptor-neprilysin inhibitors, β-blockers, and mineralocorticoid receptor antagonists, could significantly lower all-cause and cardiovascular mortality, as well as all-cause and HF-related hospitalization rates among HF sufferers (5). Despite advancements in drug therapy, the mortality remains high among patients with advanced HF. Established treatments for advance HF include mechanical circulatory support, valve repair or replacement, coronary artery bypass grafting, and heart transplantation (9, 10). Additionally, non-pharmacological treatments, such as exercise (6), acupuncture (7), and massage therapy (8) can ameliorate the quality of life for HF patients. Overweight and obesity contribute to increased HF risk through mechanisms like neurohormonal activation, adipose tissue effects, hemodynamic changes, ectopic fat deposition, and fat toxicity (11). Kehagias et al. (12) demonstrated that sleeve gastrectomy promotes sustained weight loss and improves cardiovascular conditions, such as hypertension, diabetes, and dyslipidemia. While these therapies are important in HF management, the effects of diet on HF prevention and treatment warrants careful consideration (13, 14). Wickman et al. (13) proved that the Dietary Approaches to Stop Hypertension (DASH) diet exerts positive influence on HF patients. Additionally, Ibsen DB et al. revealed that long-term DASH diet or substitution of DASH-related foods can guard against the progression of HF (15). Recently, more studies have delved into the connection of diet with chronic conditions, such as cognitive, metabolic, pulmonary, and cardiovascular diseases (16–18). However, few have examined the specific interplay between diet and HF. Healthy Eating Index (HEI), a quantitative indicator for evaluating the dietary quality of American population, was developed by the National Cancer Institute (NCI) under the U.S. Department of Health and Human Services, in collaboration with the Center for Nutrition Policy and Promotion under the U.S. Department of Agriculture (USDA) (19–21). It is updated with the Dietary Guidelines for Americans (DGA) and has undergone revisions in 2005, 2010, 2015, and 2020. The 2020 version is the latest and currently under study (22).
We seek to clarify the possible relationship between HEI-2020 and HF in Americans aged 50 or above based on 2005–2020 data of the National Health and Nutrition Examination Survey (NHANES). In view of relatively high prevalence and mortality of HF in the U.S., combined with the significant effects of diet on health, this research holds substantial significance for public health.
2 Methods
2.1 Study sample
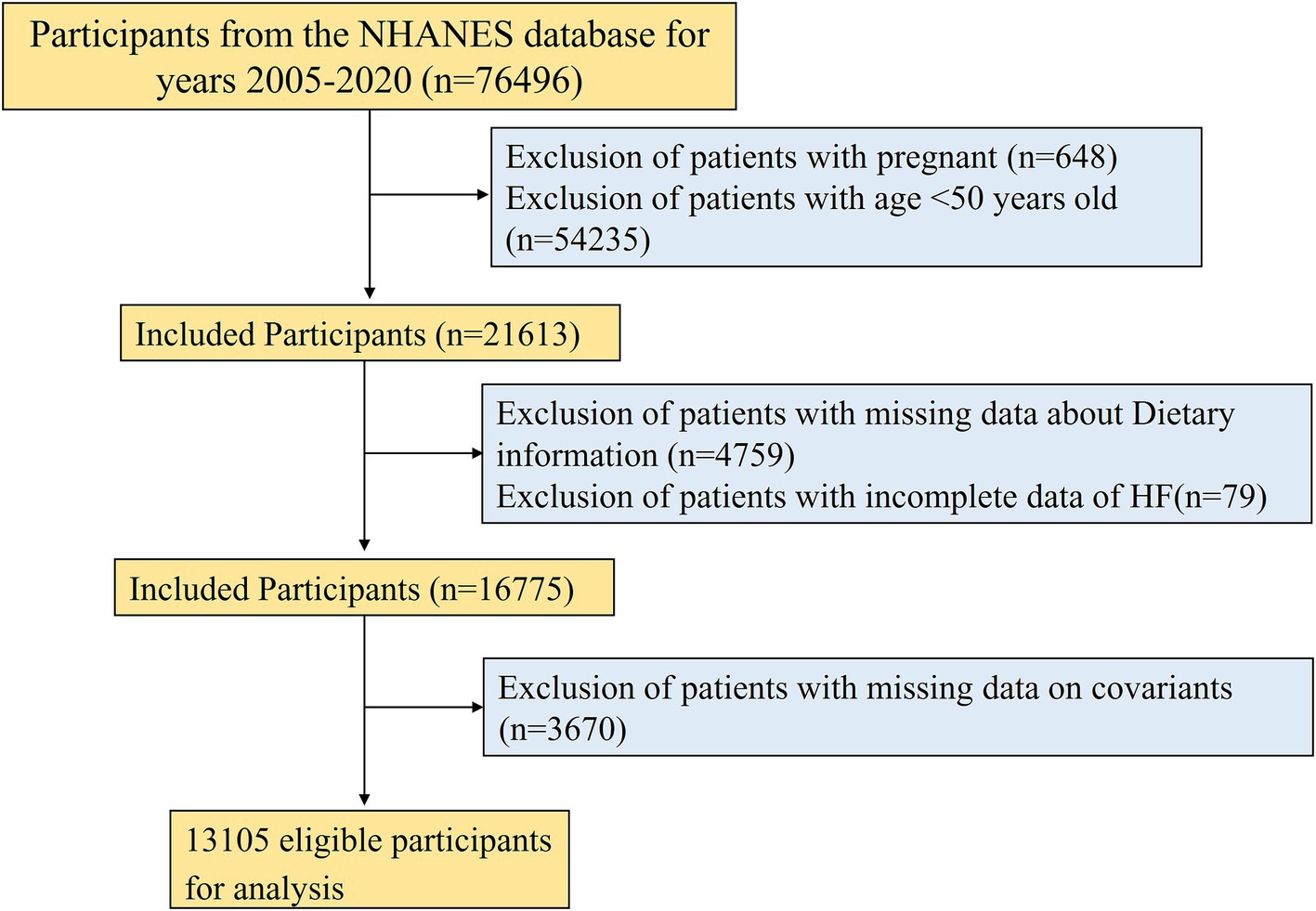
NHANES, a large-scale cross-sectional study carried out biennially by the National Center for Health Statistics (NCHS) of the U.S. Centers for Disease Control and Prevention, is accessible at https://www.cdc.gov/nchs/nhanes/index.htm (23). Its primary aim is monitoring the nutritional intake and health condition of non-institutionalized American residents and serves as a reference for public health policies. To ensure national representativeness, it employs a sophisticated, multistage sampling design. A total of 76,496 participants were included from seven consecutive cycles of NHANES data from 2005 to 2020. To guarantee that our results are complete and reliable, specific exclusion criteria were applied: (1) pregnant women (n = 648); (2) participants aged <50 years (n = 54,235); (3) participants without HEI-2020 index (n = 4,759); (4) participants with missing HF assessment (n = 79); (5) participants without incomplete covariate records (n = 3,670). Ultimately, 13,105 participants were included. A flowchart for the screening process is presented in Figure 1. The baseline characteristics of both the excluded and included participants were presented in Supplementary Table S1.
2.2 Dietary quality
HEI-2020, a dietary quality index established by the USDA Center for Nutrition Policy and Promotion in adherence to the 2020–2025 DGA with factors and scoring criteria identical to those in HEI-2015, comprises 13 different components: total vegetables, greens and beans, total fruits, whole fruits, whole grains, dairy, total protein foods, seafood and plant proteins, fatty acids, sodium, refined grains, saturated fats, and added sugars (22). HEI-2020 score is from 0 to 100, with a higher score reflecting a healthier diet (24). The scores were classified into Q1 (reference group), Q2, Q3, and Q4 (25). During review from 2005 to 2020, the dietary assessment methods used by the NHANES were relatively consistent. Participants were asked to recall and record all foods and beverages they had in the past 24 h, providing detailed information such as food descriptions, quantities, and timing. Compared to other diet evaluation approaches, this 24-h dietary recall method has several advantages: (1) It captures comprehensive details about participants’ daily diets, including various foods and dietary consumption patterns; (2) It can be adapted for use with other health assessment indicators; (3) The use of the 24-h recall method yields more scientifically rigorous results and is widely employed. However, it also has drawbacks, including recall bias resulting from inaccuracies in memory or omissions, and its inability to capture long-term dietary patterns, as it only provides short-term dietary information.
2.3 HF diagnosis
The HF was determined based on answers to a household interview question (“Have you been diagnosed with congestive heart failure?”). A positive response was deemed indicative of HF (26, 27).
2.4 Covariates
To minimize the impact of confounders and draw sound conclusions, we selected demographic characteristics, behaviors, and chronic diseases as covariates based on relevant literature (18, 28). The demographic covariates encompassed age, gender (male/female), race (Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, Other Race), education level (less than high school, high school or equivalent, college or above), marital status (married/living with partner, widowed/divorced/separated, never married), and poverty-to-income ratio (PIR) (low income [<1], medium income [1–3], high income [≥3]). Behavioral covariates included smoking (never/former/current smoker), drinking (never/former/mild/moderate/heavy drinker), and body mass index (BMI). Chronic diseases included diabetes, hypertension, hyperlipidemia, and coronary heart disease.
2.5 Statistical analysis
As per NHANES analysis guidelines, the calculations were adjusted for unequal selection probabilities, subgroup oversampling, and non-response in the analyses with sampling weights, strata, and primary sampling units. Categorical variables were expressed as counts and percentages, continuous variables in normal distribution as mean ± standard deviation (SD), and those with non-normal distribution as median and interquartile range (IQR). Baseline characteristics were compared across groups via t-test, chi-square (X2) and Mann–Whitney U tests. Next, a weighted logistic regression model assisted in assessing the link of HEI-2020 scores to HF in adults during their middle and later life stages with results in odds ratios (OR) and 95% confidence intervals (CI). HEI-2020 scores were stratified into four quartiles, and the correlation of HEI-2020 scores with HF among the middle-aged and older individuals were elucidated through logistic regression. Restricted cubic splines (RCS) were employed for exploring the dose–response relationship between HEI-2020 scores and HF, with the findings further validated through the generalized additive model (GAM) regression (29). Finally, through a weighted quantile sum (WQS) regression model (30), the combined exposure effects of 13 dietary components in HEI-2020 and health contribution ratio of each component were investigated. The combined and independent effects of the scores on HF were evaluated, and the WQS results were verified via a quantile g-computation (Qgcomp) model (27).
Every statistical test was two-sided, and p < 0.05 signified statistical significance. R 4.3.31 was employed for statistical analysis.
3 Results
3.1 Baseline characteristics of the study population
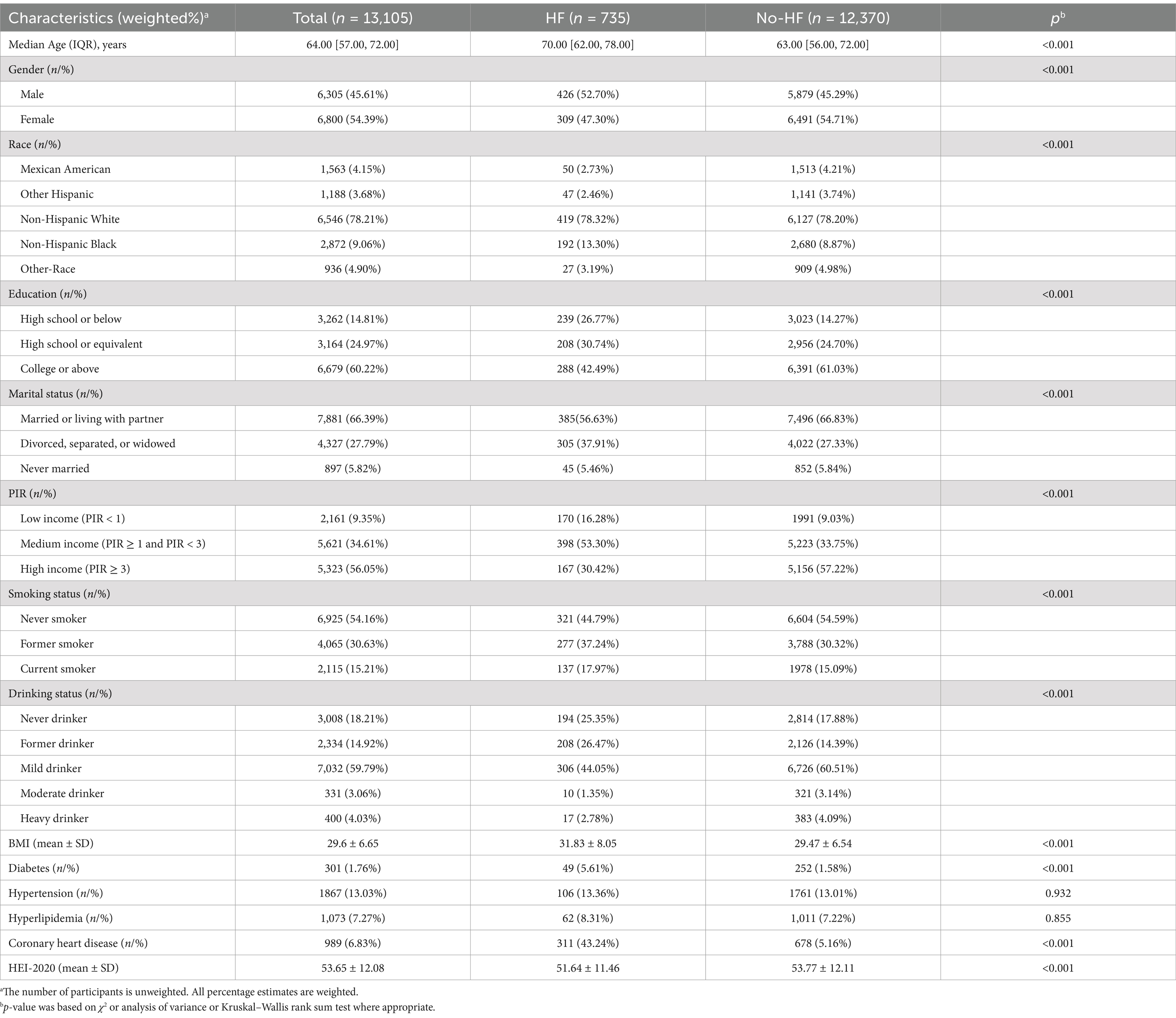
After sample screening, 13,105 participants were included, comprising 735 HF patients and 12,370 non-HF individuals. The mean HEI-2020 score was 53.65 ± 12.08, as detailed in Table 1. In contrast to the non-HF cohort, HF patients tended to be older, male, former smokers or drinkers, divorced, separated, or widowed, and have a higher BMI, lower education levels (high school or below), lower income, and a higher diabetes and coronary heart disease prevalence. HEI-2020 scores of HF patients were notably decreased compared with non-HF participants (p < 0.001), with a larger proportion in Q1 (29.70%), as depicted in Figure 2A. We also stratified the population into Q1 (<44.86), Q2 (≥44.86 and <52.97), Q3 (≥52.97 and <61.73), and Q4 (≥61.73) based on HEI-2020 scores, as presented in Supplementary Table S2. Compared with those in lower HEI-2020 quartiles, individuals in the higher quartile (Q4) tended to be older, female, non-Hispanic White, married or living with partner, never smokers, moderate drinkers, and have a college education or above, and more income. The incidence of HF decreased progressively across Q2, Q3, and Q4 in comparison to Q1, with significant differences observed (p < 0.001), as indicated in Figure 2B.
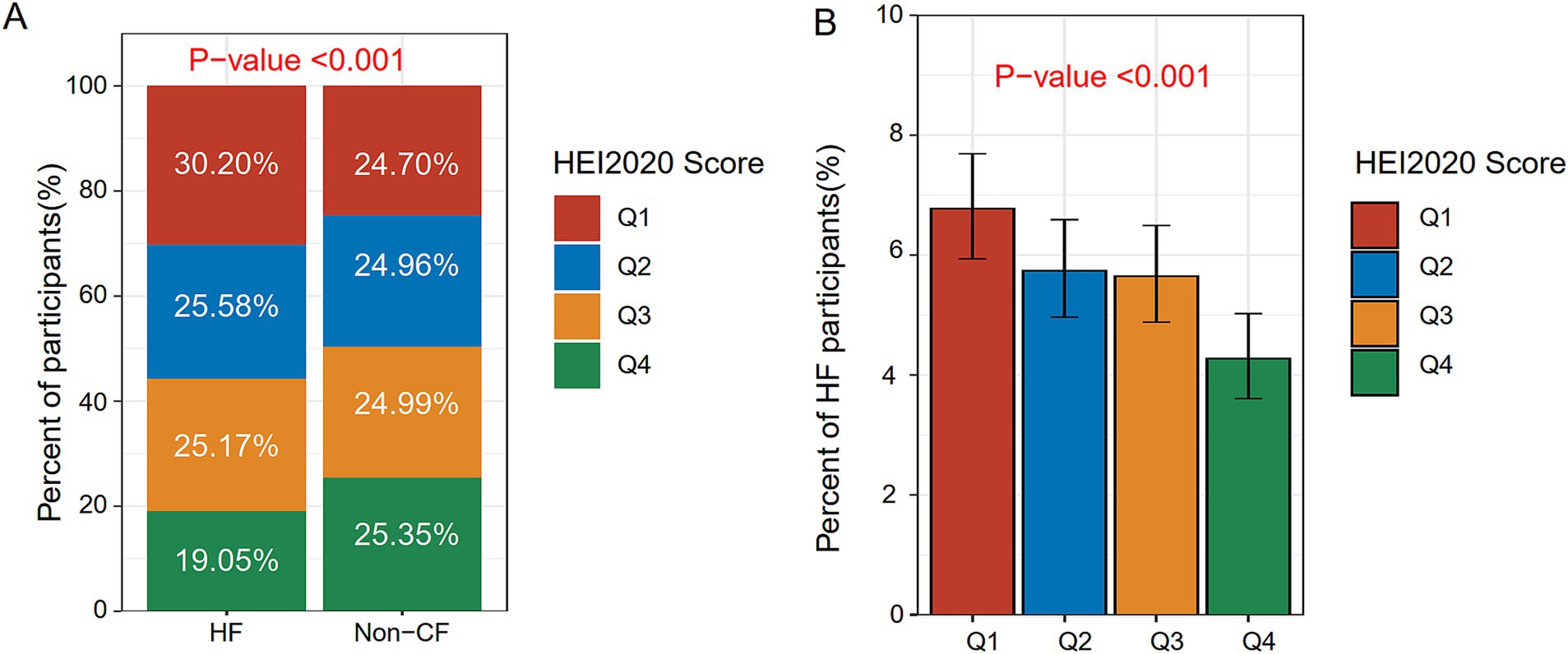
Figure 2. Distribution of HEI-2020 scores and HF across different populations. (A) Distribution of HEI-2020 scores between the non-HF group and the HF group. (B) Prevalence of HF across different HEI-2020 quartile groups.
3.2 Association between HEI-2020 and HF
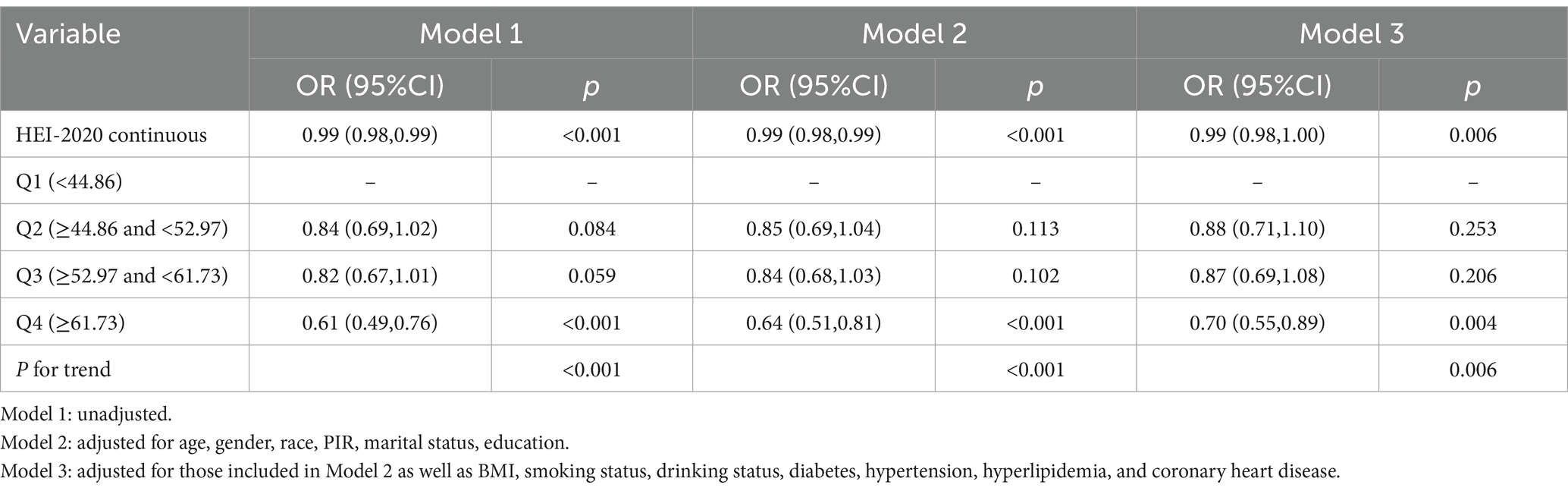
The correlation of HEI-2020 scores with HF risk in adults during their middle and later stages of life (aged 50 or above) was analyzed through logistic regression models, with the results detailed in Table 2. In Model 3, an adverse link was identified between the continuous HEI-2020 variable and HF risk in this cohort (OR = 0.99, 95% CI: 0.98–1.00, p = 0.006). Moreover, participants in the highest HEI-2020 quartile (Q4) exhibited an obviously lower risk of developing HF than those in the lowest HEI-2020 quartile (Q1) (OR = 0.70, 95% CI: 0.55–0.89, p = 0.006).
3.3 Subgroup analysis and interaction of the association between HEI-2020 and HF
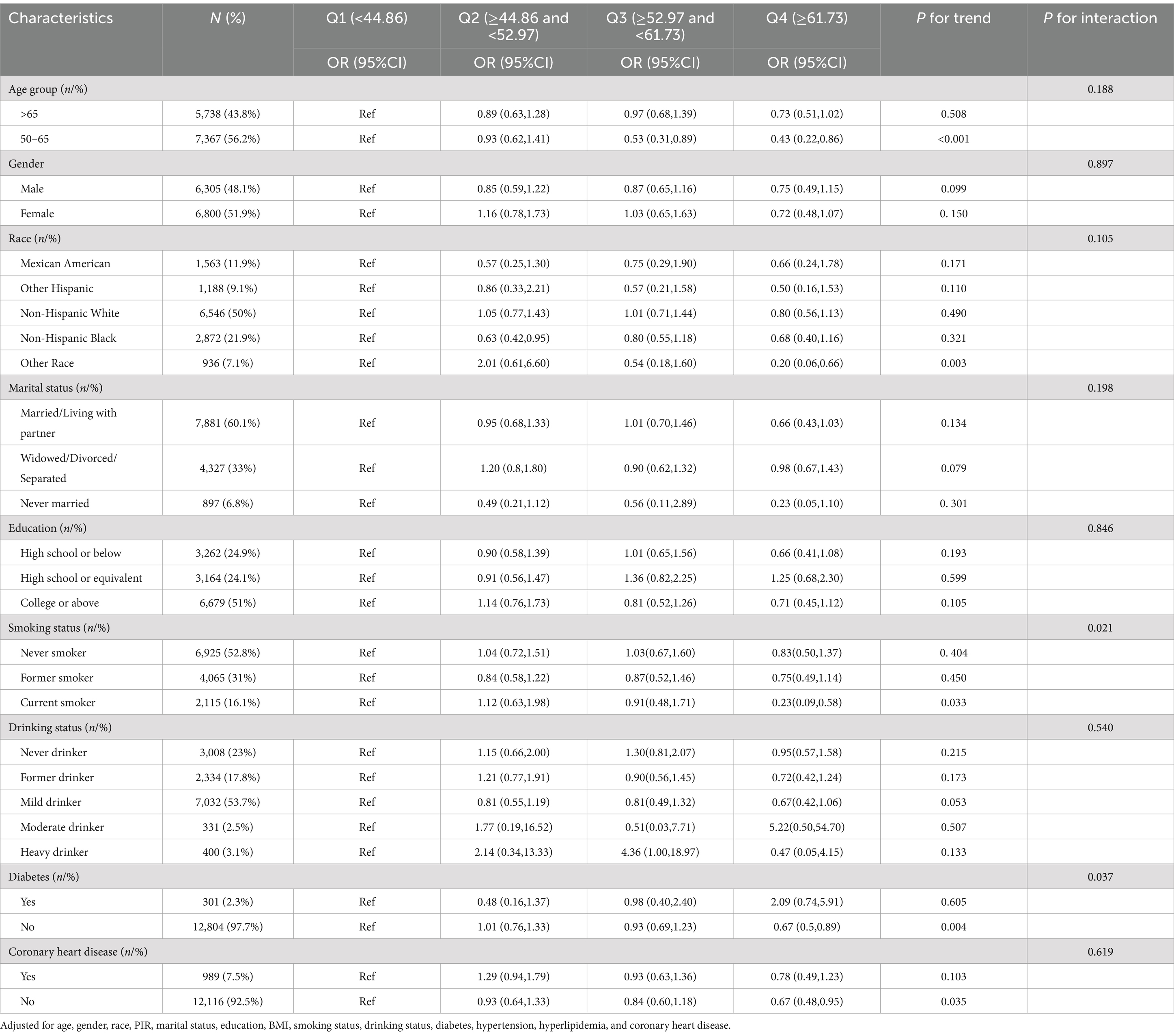
We performed subgroup analyses and interaction assessments based on age, gender, race, marital status, education, smoking, drinking, diabetes, and coronary heart disease, with the results shown in Table 3. HEI-2020 score demonstrated a negative association with HF in middle-aged and older individuals, with the trend primarily observed in specific subgroups, including those aged 50–65, individuals of other races, current smokers, and those without diabetes or coronary heart disease. However, this association was not observed in other subgroups. Moreover, evident interaction effects were found for smoking status (P for interaction = 0.021) and diabetes (P for interaction = 0.037), indicating that the adverse connection of HEI-2020 with HF was deemed significant only for the current smokers and non-diabetic subgroups.
3.4 Dose–response relationship between HEI-2020 and HF risk
The RCS assisted in examining the dose–response relationship between HEI and HF, with results validated through the GAM. HEI-2020 scores in this study followed a normal distribution, with a mean and SD of 53.65 and 12.08, respectively, as shown in Figure 3A. A linear association was found between HEI-2020 and HF risk (p-non-linear = 0.453), as depicted in Figure 3B. The GAM results also demonstrated the same trend, as presented in Figure 3C.
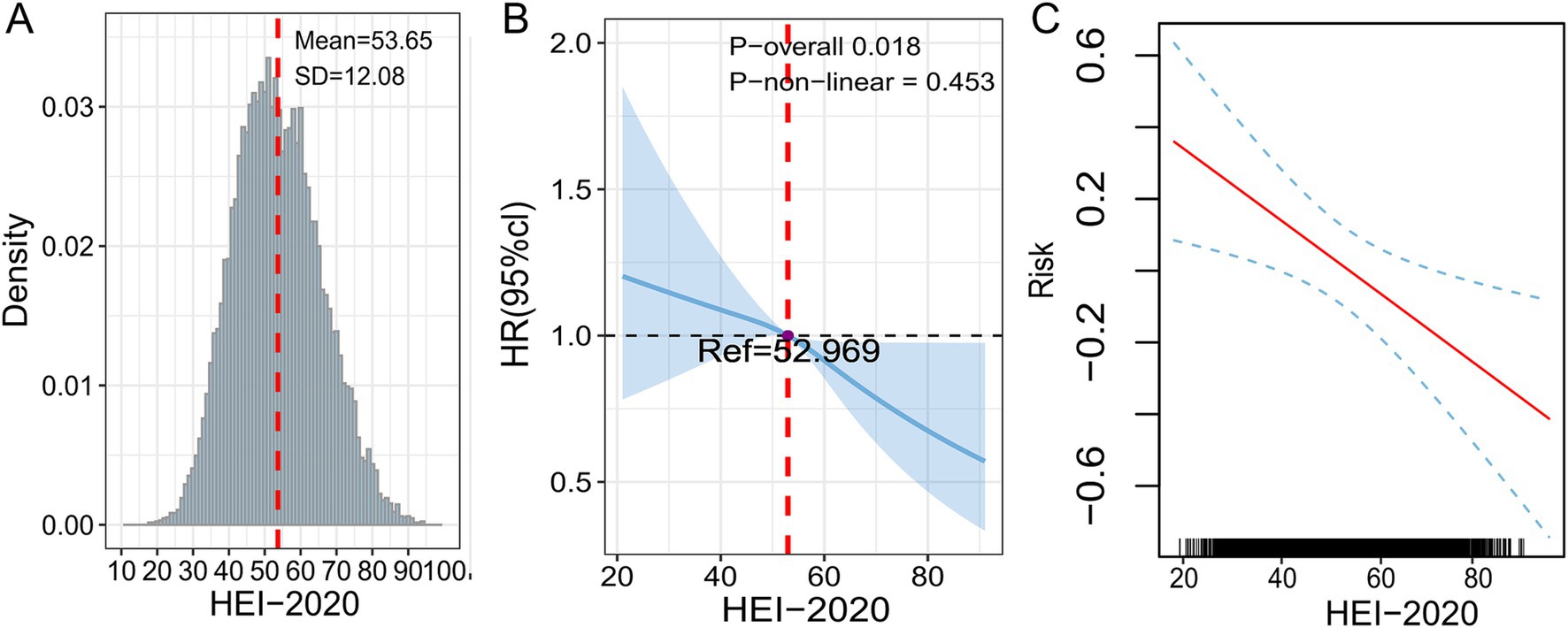
Figure 3. Dose–response relationship between HEI-2020 (in continuous form) and HF analyzed using RCS and GAM. (A) Density distribution of HEI-2020 scores. (B) RCS model. (C) GAM model. All models were adjusted for age, gender, race, PIR, marital status, education, BMI, smoking status, drinking status, diabetes, hypertension, hyperlipidemia, and coronary heart disease.
3.5 Combined effects of 13 dietary components on HF risk
We employed WQS regression model for assessing effects of 13 dietary components on HF risk mitigation, as displayed in Figures 4A,B. In Model 3, the WQS index of HEI-2020 (OR = 0.94, 95%CI: 0.92–0.97) revealed a notable relation to a lowered HF risk. Specifically, dairy (28.04%), whole fruits (26.04%), and total fruits (13.60%) were found to be the most influential components, suggesting that they contributed the most to the reduction in HF risk. Finally, the WQS results was verified through the Qgcomp model. In Model 3, the findings from the Qgcomp model aligned with those from the WQS index (OR = 0.97, 95%CI: 0.94–0.99). The dietary components that contributed most to HF risk reduction were whole fruits, dairy, and refined grains, as shown in Figures 4C,D.
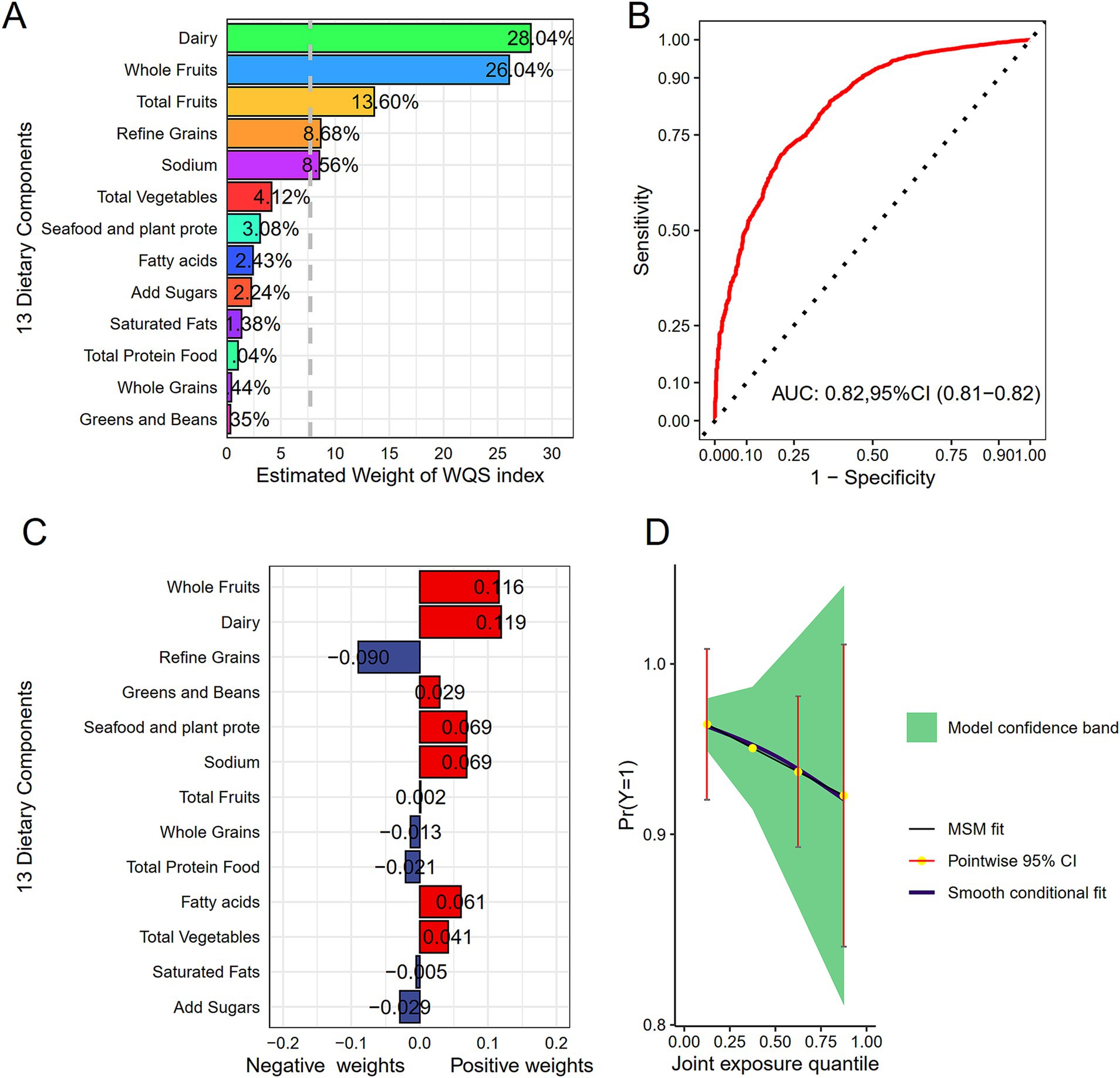
Figure 4. Display of combined effects. (A) WQS model for HF. (B) Contribution weights of dietary components in the WQS model for HF as indicated by AUC. (C) Contribution weights of dietary components in the Qgcomp model. (D) Visualization of trends in the Qgcomp model. All models included adjustment for age, gender, race, PIR, marital status, education, BMI, smoking status, drinking status, diabetes, hypertension, hyperlipidemia, as well as coronary heart disease.
4 Discussion
This study, involving seven cycles of NHANES (2005–2020) and 13,105 Americans aged 50 or above, elucidated the link of dietary quality evaluated through HEI-2020 to HF. Weighted logistic regression revealed a negative association between dietary quality and HF among individuals aged 50 or above. Subgroup analyses further confirmed this association across various subgroups, with particularly evident effects observed in current smokers and non-diabetic participants. Furthermore, weighted RCS demonstrated a linear connection between HEI-2020 and HF risk, indicating that the risk of HF decreased linearly as HEI-2020 scores increased within this age group. The GAM results corroborated the reliability of the weighted RCS findings. Finally, the contributions of 13 dietary components in HEI-2020 to mitigating HF risk were investigated through WQS and Qgcomp models. Whole fruits and dairy were proved to be the most contributory components, suggesting that these specific dietary components may have health benefits in reducing HF risk.
Previous research on dietary patterns has already proven the efficacy of nutritional strategies in preventing and treating cardiovascular diseases. A cohort study demonstrated that the DASH diet or substituting foods related to the DASH diet could offer protective effects against HF (15). Unlike the Western diet, the DASH diet features reduced sodium intake and increased consumption of foods abundant in vitamins, minerals, amino acids, and other bioactive substances in human cells (31). The DASH diet helps protect against HF via a combination of physiological mechanisms, including antioxidant, anti-inflammatory, antihypertensive, and anticoagulant effects (32, 33). Additionally, a prospective cohort study based on Swedish adults revealed benefits of the Mediterranean diet in lowering HF likelihood (OR = 0.79, 95% CI: 0.68–0.93, p = 0.004) (34), showing a reduced HF risk among participants with higher adherence to this diet pattern compared with those with lower adherence (35). These results align with the findings of the present study. Furthermore, HEI is suitable for evaluating the dietary quality of Americans.
This study employed weighted RCS and GAM models to investigate the dose–response relation of HEI-2020 to HF risk in middle-aged and older individuals. The results demonstrated an adverse linear association between HEI and HF, which is consistent with the general understanding of health benefits of a healthy diet. Furthermore, this study replaced HEI-2020 scores with the combined effects of 13 dietary components to uncover the correlation of dietary quality with HF among older individuals, and the contribution of different components to reducing HF risk. In WQS and Qgcomp models, these 13 components positively influenced HF in older adults jointly. Increasing the intake of whole fruits and dairy appropriately was found to be particularly effective in mitigating the risk of HF in this population.
Fruit and dairy consumption is strongly linked to a decreased occurrence of cardiovascular diseases like atherosclerosis, hypertension, and HF (36, 37). Djoussé et al. (38) pointed out that healthy lifestyle factors, including maintaining a normal weight, abstaining from smoking, exercising frequently, consuming moderate amounts of alcohol, and eating fruits, vegetables, and cereals at breakfast, are strongly linked to a decreased lifetime risk of HF. Kakutani et al. (39) proved that more daily citrus fruit intake was linked to a reduced incidence of depression in chronic HF individuals. Vitamins C, carotenoids, and antioxidants in fruits contribute to cardiovascular health, which may indirectly mitigate the risk of HF (40, 41). Additionally, Zemel et al. (42) demonstrated that dairy consumption is beneficial for weight loss and provides vitamins and minerals that affect blood circulation. High levels of dairy intake may counteract HF-related brain damage by reducing weight, boosting blood flow, and ensuring sufficient oxygen delivery to the brain (43). Furthermore, lactose, probiotics, and lactic acid bacteria in dairy products stimulate intestinal microbial activity, promoting the production of butyrate. Moreover, fermented dairy products, such as butter, naturally contain small amounts of butyrate, which, upon absorption through the portal vein, interacts with various organs, demonstrating anti-inflammatory, anti-obesity, anti-angiogenic, and antioxidant functions. The foregoing biological activities suggest that butyrate may be therapeutic in cardiovascular disease prevention and treatment (44). Overall, these studies reflect the health benefits of fruit and dairy intake in cardiovascular diseases.
While the exact mechanisms linking a healthy diet to HF remain incompletely understood, several hypotheses have been proposed. First, regarding the anti-inflammatory/antioxidant effect, diets with high HEI-2020 scores typically include fruits, whole grains, vegetables, and nuts, which are foods with abundant antioxidants and anti-inflammatory components like vitamin C, polyphenols, and fiber. These components may slow the onset and progression of HF by lowering oxidative stress and inflammatory reactions (41). Second, in terms of improving blood glucose and lipid profiles, diets with high HEI-2020 scores often include dairy products rich in unsaturated fats, which help enhance cardiac function and myocardial metabolism, control blood glucose, and improve lipid profiles, thus lowering HF risk (37, 43). Lastly, considering the antihypertensive effect, healthy diets often involve abundant fruits, vegetables, grains, and dairy products, which all contain rich minerals like calcium, magnesium, phosphorus, and potassium. These nutrients are conducive to preventing HF by controlling blood pressure (45). Additionally, low sodium intake contributes to blood pressure reduction, as excessive sodium intake leads to fluid retention, increased blood pressure, and greater burden on heart, ultimately promoting the development of HF. In summary, HEI is associated with HF through multiple biological pathways, including anti-inflammatory/antioxidant effects, improved lipid/glucose profiles, and blood pressure reduction.
This study has several strengths. First, the utilization of a large, nationally representative NHANES dataset renders our conclusions applicable and representative. The results from subgroup analysis and interaction effects further support the stability and reliability of the findings. Additionally, the application of weighted RCS and GAM models demonstrated a linear association between HEI-2020 and HF. The WQS and Qgcomp models offered a visual representation of the contributions of 13 dietary components. However, several limitations must be acknowledged. First, HF and chronic disease covariates, including diabetes, hypertension, hyperlipidemia, and coronary heart disease, were obtained based on self-report questionnaires, which may lead to recall or reporting bias, potentially impacting the reliability of conclusions. Second, since NHANES was not specifically designed to focus on HF patients, it lacks data on the different causes of HF. Future studies should incorporate data on the specific causes of HF for clarifying the link of diet habits to HF subtypes. Third, given that the NHANES data used in this study spans the 2005–2020 survey period, there were differences in the collection of physical activity, which could influence the results. Fourth, this study is based on Americans, so the conclusions may not be directly applicable to populations in other countries or regions. Fifth, while we controlled for several potential confounding factors, it is impossible to eliminate the influence of all possible confounding factors. Finally, while a statistically significant difference was noted in HEI-2020 between HF and non-HF cohorts (51.64 vs. 53.77, p < 0.001), this difference merely reflects the overall difference in dietary patterns between the two groups. A single dietary factor cannot predict the occurrence of HF, as it is influenced by multiple factors, including genetic predisposition, metabolism, behavioral habits, and medical history. Additionally, this study was cross-sectional, and the causality between HEI and HF could not be validated. In summary, although we found a connection of HEI-2020 score with HF, the risk of HF cannot be predicted. Future longitudinal research, including prospective studies or randomized controlled trials, should elucidate the dynamic relation of HF to changes in HEI-2020 scores as well as to investigate the causal relationship between the two.
5 Conclusion
This study demonstrated a negative and linear dose–response relationship between dietary quality rated via HEI-2020 and HF among individuals aged 50 or above. The stability and reliability of this linear relationship were confirmed through subgroup analyses, and the WQS and Qgcomp models suggested the health benefits of whole fruits and dairy in mitigating HF risk. By highlighting this association, our study emphasizes the importance of adhering to the DGA in lowering HF risk in middle-aged and older individuals.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
FG: Conceptualization, Formal analysis, Methodology, Writing – original draft. WY: Data curation, Investigation, Software, Visualization, Writing – review & editing. TS: Data curation, Investigation, Software, Visualization, Writing – review & editing. YZ: Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Zhejiang Medical and Health Science and Technology Planning Project (grant number 2023KY316).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1496379/full#supplementary-material
Footnotes
References
1. Malik, A, Brito, D, Vaqar, S, Chhabra, L, and Doerr, C. Congestive Heart Failure (Nursing). Statpearls Publishing (2024)
2. Heidenreich, P. Heart failure management guidelines: new recommendations and implementation. J Cardiol. (2024) 83:67–73. doi: 10.1016/j.jjcc.2023.10.009
3. Disease, GBD, Injury, I, and Prevalence, C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of Disease study 2017. Lancet. (2018) 392:1789–858. doi: 10.1016/S0140-6736(19)31047-5
4. Heidenreich, PA, Bozkurt, B, Aguilar, D, Allen, LA, Byun, JJ, Colvin, MM, et al. 2022 AHA/ACC/HFSA guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. (2022) 145:e895–e1032. doi: 10.1161/CIR.0000000000001063
5. Komajda, M, Bohm, M, Borer, JS, Ford, I, Tavazzi, L, Pannaux, M, et al. Incremental benefit of drug therapies for chronic heart failure with reduced ejection fraction: a network meta-analysis. Eur J Heart Fail. (2018) 20:1315–22. doi: 10.1002/ejhf.1234
6. Sebastian, SA, Padda, I, and Johal, G. Supervised exercise training in heart failure with preserved ejection fraction: a systematic review and meta-analysis of randomized controlled trials. Curr Probl Cardiol. (2024) 49:102426. doi: 10.1016/j.cpcardiol.2024.102426
7. Ganglani, V, and Geng, YJ. The efficacy of acupuncture therapy in the Management of Dyspnea and Other Symptoms Associated with heart failure: a consolidated review of trial data. Cells Tissues Organs. (2024) 31:1–24. doi: 10.1159/000539593
8. Gulbahar Eren, M, and Gok Metin, Z. Classical massage and relaxation exercise on symptom status and quality of life in advanced stage patients with heart failure: a randomized controlled trial. Holist Nurs Pract. (2022) 36:E1–E11. doi: 10.1097/HNP.0000000000000514
9. Hullin, R, Meyer, P, Yerly, P, and Kirsch, M. Cardiac surgery in advanced heart failure. J Clin Med. (2022) 11:773. doi: 10.3390/jcm11030773
10. Ahmed, R, Botezatu, B, Nanthakumar, M, Kaloti, T, and Harky, A. Surgery for heart failure: treatment options and implications. J Card Surg. (2021) 36:1511–9. doi: 10.1111/jocs.15384
11. Aryee, EK, Ozkan, B, and Ndumele, CE. Heart failure and obesity: the latest pandemic. Prog Cardiovasc Dis. (2023) 78:43–8. doi: 10.1016/j.pcad.2023.05.003
12. Kehagias, I, Bellou, A, Kehagias, D, Markopoulos, G, Amanatidis, T, Alexandrou, A, et al.Long-term (11 + years) efficacy of sleeve gastrectomy as a stand-alone bariatric procedure: a single-center retrospective observational study. Langenbeck’s Arch Surg. (2022) 29:408. doi: 10.1007/s00423-022-02734-y
13. Wickman, BE, Enkhmaa, B, Ridberg, R, Romero, E, Cadeiras, M, Meyers, F, et al. Dietary management of heart failure: DASH diet and precision nutrition perspectives. Nutrients. (2021) 13:4424. doi: 10.3390/nu13124424
14. Aggarwal, M, Bozkurt, B, Panjrath, G, Aggarwal, B, Ostfeld, RJ, Barnard, ND, et al. Lifestyle modifications for preventing and treating heart failure. J Am Coll Cardiol. (2018) 72:2391–405. doi: 10.1016/j.jacc.2018.08.2160
15. Ibsen, DB, Levitan, EB, Akesson, A, Gigante, B, and Wolk, A. The DASH diet is associated with a lower risk of heart failure: a cohort study. Eur J Prev Cardiol. (2022) 29:1114–23. doi: 10.1093/eurjpc/zwac003
16. Wang, K, Zhao, Y, Nie, J, Xu, H, Yu, C, and Wang, S. Higher HEI-2015 score is associated with reduced risk of depression: result from NHANES 2005-2016. Nutrients. (2021) 13:348. doi: 10.3390/nu13020348
17. Tian, T, Zhang, J, Xie, W, Ni, Y, Fang, X, Liu, M, et al. Dietary quality and relationships with metabolic dysfunction-associated fatty liver Disease (MAFLD) among United States adults, results from NHANES 2017-2018. Nutrients. (2022) 14:4505. doi: 10.3390/nu14214505
18. Zhiyi, L, Shuhan, Z, Libing, Z, Jiaqi, L, Xin, D, Lingxi, Q, et al. Association of the Healthy Dietary Index 2020 and its components with chronic respiratory disease among U.S. adults. Front Nutr. (2024) 11:1402635. doi: 10.3389/fnut.2024.1402635
19. Guenther, PM, Casavale, KO, Reedy, J, Kirkpatrick, SI, Hiza, HA, Kuczynski, KJ, et al. Update of the healthy eating index: HEI-2010. J Acad Nutr Diet. (2013) 113:569–80. doi: 10.1016/j.jand.2012.12.016
20. Krebs-Smith, SM, Pannucci, TE, Subar, AF, Kirkpatrick, SI, Lerman, JL, Tooze, JA, et al. Update of the healthy eating index: HEI-2015. J Acad Nutr Diet. (2018) 118:1591–602. doi: 10.1016/j.jand.2018.05.021
21. Lerman, JL, Herrick, KA, Pannucci, TE, Shams-White, MM, Kahle, LL, Zimmer, M, et al. Evaluation of the healthy eating index-Toddlers-2020. J Acad Nutr Diet. (2023) 123:1307–19. doi: 10.1016/j.jand.2023.05.014
22. Shams-White, MM, Pannucci, TE, Lerman, JL, Herrick, KA, Zimmer, M, Meyers Mathieu, K, et al. Healthy eating Index-2020: review and update process to reflect the dietary guidelines for Americans, 2020-2025. J Acad Nutr Diet. (2023) 123:1280–8. doi: 10.1016/j.jand.2023.05.015
24. Jayanama, K, Theou, O, Godin, J, Cahill, L, Shivappa, N, Hebert, JR, et al. Relationship between diet quality scores and the risk of frailty and mortality in adults across a wide age spectrum. BMC Med. (2021) 19:64. doi: 10.1186/s12916-021-01918-5
25. Gui, J, Wang, L, Han, Z, Ding, R, Yang, X, Yang, J, et al. Association between the healthy eating Index-2015 and developmental disabilities in children: a cross-sectional analysis. Brain Sci. (2023) 13:1353. doi: 10.3390/brainsci13091353
26. Yan, Y, Mao, M, Li, YQ, Chen, YJ, Yu, HD, Xie, WZ, et al. Periodontitis is associated with heart failure: a population-based study (NHANES III). Front Physiol. (2022) 13:854606. doi: 10.3389/fphys.2022.854606
27. Fan, T, and Su, D. Interaction effects between sleep disorders and depression on heart failure. BMC Cardiovasc Disord. (2023) 23:132. doi: 10.1186/s12872-023-03147-5
28. Ma, Y, Liu, J, Sun, J, Cui, Y, Wu, P, Wei, F, et al. Composite dietary antioxidant index and the risk of heart failure: a cross-sectional study from NHANES. Clin Cardiol. (2023) 46:1538–43. doi: 10.1002/clc.24144
29. Golub, JS, Brickman, AM, Ciarleglio, AJ, Schupf, N, and Luchsinger, JA. Association of Subclinical Hearing Loss with Cognitive Performance. JAMA Otolaryngol Head Neck Surg. (2020) 146:57–67. doi: 10.1001/jamaoto.2019.3375
30. Huang, Q, Wan, J, Nan, W, Li, S, He, B, and Peng, Z. Association between manganese exposure in heavy metals mixtures and the prevalence of sarcopenia in US adults from NHANES 2011-2018. J Hazard Mater. (2024) 464:133005. doi: 10.1016/j.jhazmat.2023.133005
31. Lin, PH, Allen, JD, Li, YJ, Yu, M, Lien, LF, and Svetkey, LP. Blood pressure-lowering mechanisms of the DASH dietary pattern. J Nutr Metab. (2012) 2012:472396:1–10. doi: 10.1155/2012/472396
32. Al-Solaiman, Y, Jesri, A, Zhao, Y, Morrow, JD, and Egan, BM. Low-sodium DASH reduces oxidative stress and improves vascular function in salt-sensitive humans. J Hum Hypertens. (2009) 23:826–35. doi: 10.1038/jhh.2009.32
33. Azadbakht, L, Surkan, PJ, Esmaillzadeh, A, and Willett, WC. The dietary approaches to stop hypertension eating plan affects C-reactive protein, coagulation abnormalities, and hepatic function tests among type 2 diabetic patients. J Nutr. (2011) 141:1083–8. doi: 10.3945/jn.110.136739
34. Tektonidis, TG, Akesson, A, Gigante, B, Wolk, A, and Larsson, SC. A Mediterranean diet and risk of myocardial infarction, heart failure and stroke: a population-based cohort study. Atherosclerosis. (2015) 243:93–8. doi: 10.1016/j.atherosclerosis.2015.08.039
35. Sanches Machado d'Almeida, K, Ronchi Spillere, S, Zuchinali, P, and Correa Souza, G. Mediterranean diet and other dietary patterns in primary prevention of heart failure and changes in cardiac function markers: a systematic review. Nutrients. (2018) 10:58. doi: 10.3390/nu10010058
36. Malekmohammad, K, Sewell, RDE, and Rafieian-Kopaei, M. Antioxidants and atherosclerosis: mechanistic aspects. Biomol Ther. (2019) 9:8. doi: 10.3390/biom9080301
37. Rice, BH. Dairy and cardiovascular Disease: a review of recent observational research. Curr Nutr Rep. (2014) 3:130–8. doi: 10.1007/s13668-014-0076-4
38. Djoussé, L, Driver, JA, and Gaziano, JM. Relation between modifiable lifestyle factors and lifetime risk of heart failure. JAMA. (2009) 302:394–400. doi: 10.1001/jama.2009.1062
39. Kakutani, N, Yokota, T, Fukushima, A, Obata, Y, Ono, T, Sota, T, et al. Impact of citrus fruit intake on the mental health of patients with chronic heart failure. J Cardiol. (2022) 79:719–26. doi: 10.1016/j.jjcc.2021.12.004
40. Carr, AC, and Maggini, S. Vitamin C and immune function. Nutrients. (2017) 9:11. doi: 10.3390/nu9111211
41. Bechthold, A, Boeing, H, Schwedhelm, C, Hoffmann, G, Knuppel, S, Iqbal, K, et al. Food groups and risk of coronary heart disease, stroke and heart failure: a systematic review and dose-response meta-analysis of prospective studies. Crit Rev Food Sci Nutr. (2019) 59:1071–90. doi: 10.1080/10408398.2017.1392288
42. Zemel, MB, Thompson, W, Milstead, A, Morris, K, and Campbell, P. Calcium and dairy acceleration of weight and fat loss during energy restriction in obese adults. Obes Res. (2004) 12:582–90. doi: 10.1038/oby.2004.67
43. Haug, A, Hostmark, AT, and Harstad, OM. Bovine milk in human nutrition – a review. Lipids Health Dis. (2007) 6:25. doi: 10.1186/1476-511X-6-25
44. Amiri, P, Hosseini, SA, Ghaffari, S, Tutunchi, H, Ghaffari, S, Mosharkesh, E, et al. Role of butyrate, a gut microbiota derived metabolite, in cardiovascular diseases: a comprehensive narrative review. Front Pharmacol. (2021) 12:837509. doi: 10.3389/fphar.2021.837509
Keywords: HEI-2020, HF, middle-aged and older adults, NHANES, cross-sectional study
Citation: Gu F, Yu W, Shu T and Zhu Y (2025) Association between the healthy eating index 2020 and heart failure among the U.S. middle-aged and older adults from NHANES 2005–2020: a cross-sectional study. Front. Nutr. 11:1496379. doi: 10.3389/fnut.2024.1496379
Edited by:
Gordana Kenđel Jovanović, Teaching Institute of Public Health of Primorsko-Goranska County, CroatiaReviewed by:
Wiktoria Staśkiewicz-Bartecka, Medical University of Silesia, PolandDimitrios Kehagias, University of Patras, Greece
Copyright © 2025 Gu, Yu, Shu and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingwei Zhu, aHp6aHV5aW53ZWlAMTYzLmNvbQ==
 Fangfang Gu
Fangfang Gu Yingwei Zhu
Yingwei Zhu