- Department of Hepatobiliary Surgery, Hunan Provincial People’s Hospital, The First Affiliated Hospital of Hunan Normal University, Changsha, China
Purpose: To investigate the application value of the neutrophil to lymphocyte count ratio (NLR) in the prognostic analysis of intrahepatic cholangiocarcinoma (ICC) after radical resection, and to offer guidance for the individualized perioperative diagnosis and treatment of ICC.
Methods: The clinical data of 360 patients diagnosed with ICC following radical surgery were retrospectively analyzed. The cut-off value of NLR was calculated using the minimum p-value method, and then divided into High-NLR (H-NLR) group and Low-NLR (L-NLR) group according to the NLR cut-off value. The prognostic value of NLR in ICC was analyzed. Subsequently, the patients were divided into the hepatolithiasis-related intrahepatic cholangiocarcinoma (HICC) group and the non-hepatolithiasis-related intrahepatic cholangiocarcinoma (NHICC) group based on whether they combined with hepatolithiasis. Multiple regression models were constructed based on NLR and clinicopathological indicators to verify the application value of prognostic models in the survival and recurrence of ICC patients after radical surgery.
Results: The cut-off value of NLR was 2.36, and the survival analysis disclosed that overall ICC patients with NLR ≥ 2.36 manifested a poor 5-year survival rate and a higher tumor recurrence rate (p < 0.001). In the HICC group, patients with H-NLR presented a poor 5-year survival rate and a higher tumor recurrence rate compared with L-NLR (p < 0.001). The NLR-based survival/recurrence prediction models in the HICC group demonstrated excellent predictive capacity (H-L test: 0.359/0.680, AUC: 0.764/0.791). In the NHICC group, patients with H-NLR exhibited a poor 5-year survival rate compared with L-NLR (p < 0.001), yet there was no significant difference in tumor recurrence between the two groups (p = 0.071). The NLR-based survival prediction model in the NHICC group demonstrated acceptable predictive ability (H-L test: 0.268, AUC: 0.729), while the NLR-based recurrence prediction model did not show an effective predictive ability (H-L test: 0.01, AUC: 0.649).
Conclusion: NLR is an independent risk factor influencing postoperative survival and recurrence in ICC patients, particularly in HICC patients. Preoperative NLR ≥ 2.36 suggests that patients might have a poor prognosis. The survival and recurrence prediction model constructed based on NLR and other clinical indicators demonstrates good prediction accuracy and can effectively predict the risk of postoperative adverse prognosis in patients with HICC. This study offers a novel idea for the clinical treatment of HICC patients.
Introduction
Intrahepatic cholangiocarcinoma (ICC) is a lethal malignancy and ranks as the second most common primary liver cancer, comprising 20% of all liver malignancies and 3% of all gastrointestinal malignancies (1, 2). Over the past four decades, the incidence of ICC has surged by over 140% (3). Despite current studies revealing an inadequate understanding of its etiology, risk factors with varying degrees of susceptibility are increasingly being identified. The most closely correlated risk factors to ICC include bile duct stones, choledochal cysts, cirrhosis cholangitis, chronic biliary tract diseases, viral hepatitis (specifically hepatitis B virus and hepatitis C virus), liver fluke infestations such as Clonorchis sinensis (4–6).
Surgical resection stands out as the most effective treatment method for ICC (7). Nonetheless, only 35% of patients are eligible for surgical resection at diagnosis; furthermore, the outcomes following surgical intervention remain suboptimal. The five-year survival rate post-R0 resection hovers around 30–40% (8). A multitude of factors impact ICC prognosis; key ones include tumor number and size, major vascular invasion, presence of extrahepatic disease, morphological type and histological grade of tumors, presence of lymph node metastasis,final resection margin residual free from any microscopic or macroscopic tumor thrombus/extension along insular structures left behind after any attempted excision within patient’s own factor like age gender performance score used in routine medical care assessment based on the ability to walk independently overall health status, surgical approach, molecular characteristics including specific gene mutations, and growth factor receptor expression levels (9–11). It is therefore imperative that we accurately evaluate the prognosis pertaining to ICC patients while developing corresponding treatment strategies (12). By considering individual differences among patients, we can adopt a multidisciplinary team-based personalized approach in order to optimize both prognosis and quality-of-life outcomes for individuals afflicted with this condition.
Previous research has demonstrated the significant role of serum inflammatory factors in ICC (13, 14). The analysis of preoperative inflammatory factors can provide valuable insights into the prognosis and progression of ICC, offering new perspectives and strategies for its diagnosis and treatment. Preoperative assessment of serum neutrophil/lymphocyte ratio (NLR), lymphocyte/monocyte ratio (LMR), platelet *NLR (systemic immuno-inflammation index, SII) is commonly utilized to evaluate the overall inflammatory status and immune function of tumor patients, and has been linked to tumor prognosis and treatment response in various malignancies such as digestive tract tumors (e.g., stomach cancer, colorectal cancer, pancreatic cancer), breast cancer, thyroid cancer, among others (15–17). Studies have also investigated the role of serum inflammatory factors in intrahepatic cholangiocarcinoma, revealing their significant involvement in the pathogenesis and progression of this disease (18–20). Understanding these factors can enhance our ability to manage ICC more effectively and offer specific directions for its treatment.
However, there have been limited studies on the role of serum inflammatory factors in ICC combined with hepatolithiasis, being a prevalent benign biliary condition in Asian countries (21). Prolonged stone stimulation and obstruction can lead to hepatolithiasis-related liver cirrhosis, which, if left untreated, may progress to biliary carcinogenesis. In light of this situation, we aim to categorize ICC into hepatolithiasis-related intrahepatic cholangiocarcinoma (HICC) and non-hepatolithiasis-related intrahepatic cholangiocarcinoma (NHICC) in order to investigate the role of inflammatory factors in these conditions. Our objective is to comprehend the prognostic significance of preoperative serum NLR, LMR, and SII in the resection of ICC and subsequently explore the impact of these inflammatory indicators on its prognosis based on our findings.
Patients and methods
Study population
This retrospective study collected patients from the Department of Hepatobiliary Surgery at Hunan Provincial People’s Hospital (the First Affiliated Hospital of Hunan Normal University) between January 2015 and December 2021. The patients included in the study had been pathologically diagnosed with ICC and had undergone radical surgery. A total of 525 patients with ICC were screened for inclusion in the study. The research was conducted in compliance with the protocol approved by the Ethics Committee of Hunan Provincial People’s Hospital (Approval No.: [2024]-121), following the principles outlined in the Declaration of Helsinki.
All the patients encompassed in the current study fulfilled the subsequent criteria: (1) underwent R0 resection, with postoperative pathology confirming ICC; comprehensive clinical case data and follow-up data were accessible; (2) did not undergo tumor-targeted treatments such as transcatheter arterial chemoembolization (TACE), chemotherapy, radiotherapy, targeted therapy, or immunotherapy, etc.; (3) preoperative Child-Pugh score of A/B; (4) had no history of previous malignant tumors. Patients with the following attributes were excluded: (1) R1/2 excision or distant metastasis (M1); (2) postoperative pathology indicating primary liver cancer such as hepatocellular carcinoma, mixed hepatocellular carcinoma, or other types of biliary malignancy; (3) patients with infectious diseases prior to operation; (4) taking hormones, aspirin, clopidogrel, and other drugs influencing peripheral blood cell indicators before surgery; (5) combined with blood diseases or immune system disorders; (6) postoperative perioperative mortality; (7) incomplete clinical data. After screening based on the inclusion and exclusion criteria, a total of 165 patients were excluded as they did not meet the inclusion criteria, and 360 patients with ICC were selected for inclusion in this study.
Analysis of indicators
The preoperative NLR (ratio of neutrophils to lymphocytes), LMP (ratio of lymphocytes to monocytes), and SII (platelet * NLR) were all derived from the preoperative case data of patients. The cut-off values of LMR, SII and NLR were, respectively, 3.71, 730.14 and 2.36, employing the maximum Youden index method. As depicted in Figure 1, ROC curves were constructed to assess the correlation between three preoperative serum inflammatory indicators (LMR, SII, NLR) and the postoperative survival of patients, and the AUC values were 0.557, 0.567, and 0.786, respectively. The AUCs of both LMR and SII were less than 0.6, indicating that there was no significant correlation between them and the prognosis of ICC patients. Hence, they are not important indices in this study. The AUC of NLR is 0.786, and the cut-off value is 2.36, which is included in the significant indices of this study. The preoperative maximum tumor diameter (MTD), hepatolithiasis, vascular invasion, local invasion, and nerve invasion were all obtained from the preoperative CT/MR Imaging data. The TNM staging was in accordance with the AJCC eighth edition installment (22). Postoperative complications were graded in accordance with the Clavien-Dindo complication criteria (23).
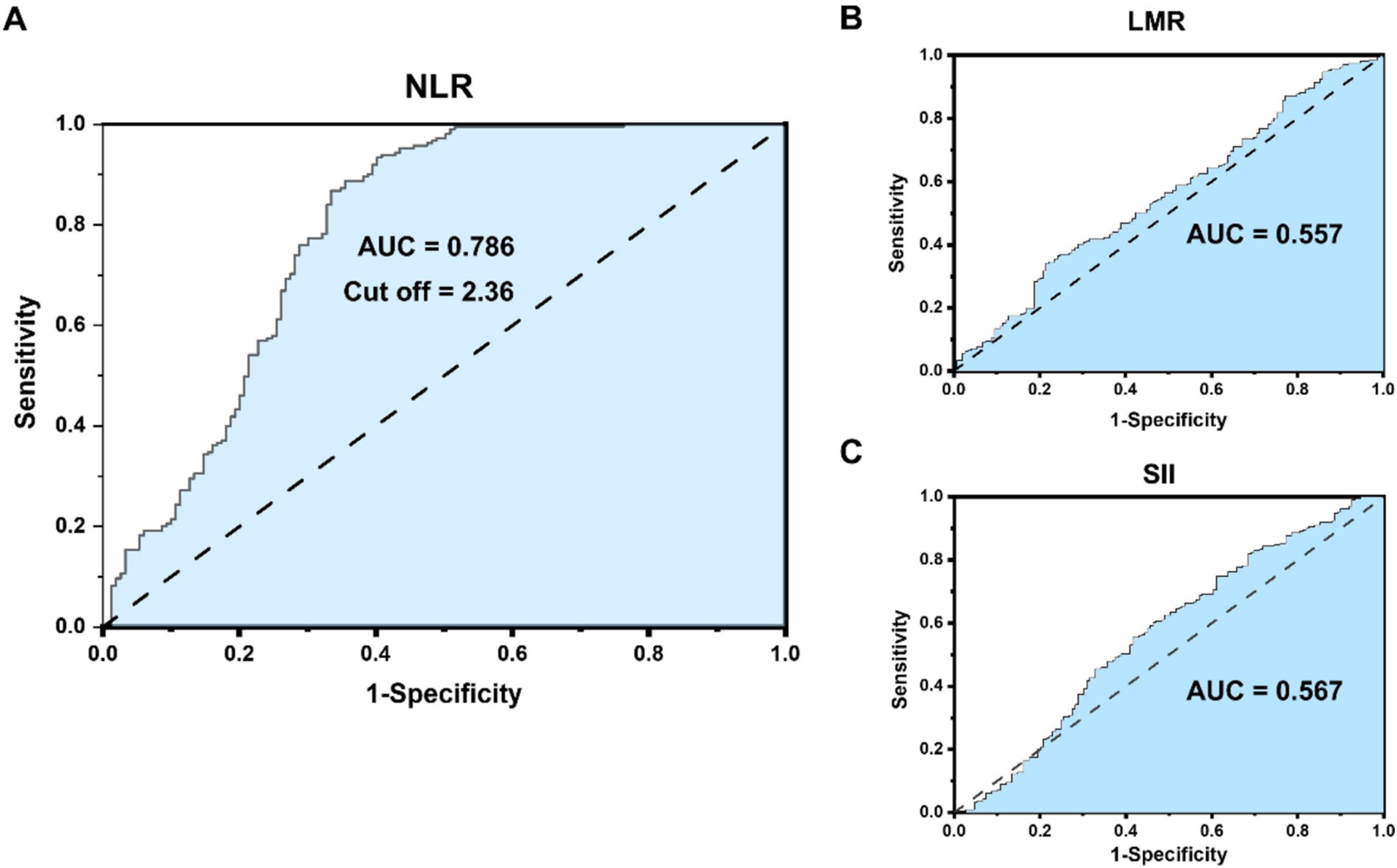
Figure 1. (A) NLR receiver operating characteristic (ROC) curve correlation and cutoff value with the prognosis of intrahepatic cholangiocarcinoma (ICC) patients. (B,C) LMR and SII ROC curve correlation with the prognosis of ICC patients.
Follow-up assessments
In this study, outpatient review and telephone contact were employed to carry out follow-up and follow-up investigation. The follow-up period extended to June 2024. The principal contents of follow-up observation encompassed follow-up treatment, recurrence time, and survival time of patients. Regular postoperative follow-up should be conducted, specifically every 3 to 6 months. Postoperative follow-up should incorporate: imaging examinations, such as CT, MRI, etc., to assess the local control of the tumor after surgery and detect possible recurrence or metastasis; Tumor markers, such as AFP, CEA, CA19-9, etc., to evaluate tumor activity status and potential risk of recurrence. Overall survival (OS) was defined as from the day of surgery until the time of death or the time of the last follow-up, and patients who remained alive at the last follow-up were recorded as censored data.
Statistical analysis
The primary endpoints of this study encompassed the overall survival (OS) and recurrence rates at 1, 3, and 5 years subsequent to surgery. The postoperative survival time pertains to the duration from the date of surgery to the patient’s decease or the last follow-up, while the postoperative recurrence time refers to the period from the date of surgery to the emergence of distant metastases in other bodily regions or the last follow-up. All measurement data were presented as mean ± standard deviation, and the disparities of measurement data were compared by means of independent sample t test or Mann–Whitney U test. The count data were expressed as n (%), and the count data were compared via Chi-square test or Fisher exact test. The difference in tumor survival and recurrence was compared through log-rank test. All binary variables were classified by referring to international authoritative literature classification, clinical normal limit and cut-off values based on the best Youden index. The survival and recurrence curves were delineated by the Kaplan–Meier curve method. A single factor logistic regression model was utilized to estimate the probability ratio and 95% confidence interval for various variables, and variables with statistical differences in the single factor logistic regression model were incorporated into multivariate analysis. Multivariate analysis was used to get the prediction model, and Hosmer-Lemeshow test was used to check the fit degree of the model. SPSS 25.0 statistical software was employed for analysis, and p value less than 0.05 was regarded as statistically significant.
Results
Study design
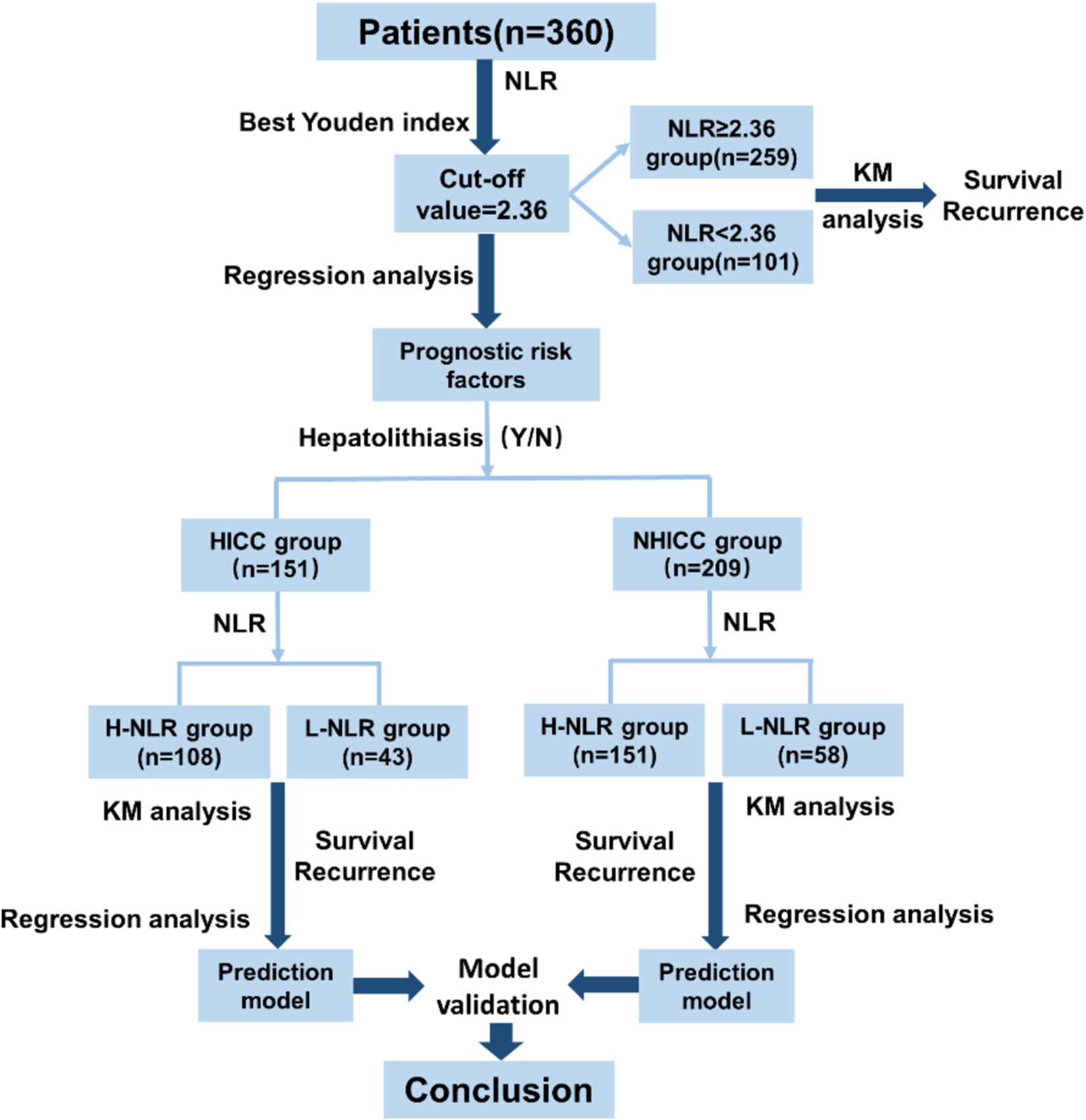
The flowchart of the manuscript design is presented in Figure 2. In this study, a total of 360 ICC patients were recruited. Based on the best Youden index of NLR, all ICC patients were categorized into the NLR < 2.36 group and the NLR ≥ 2.36 group, and subsequently, the prognostic analysis of the two groups was compared. Then, all ICC patients were divided into the HICC group and the NHICC group depending on whether they combined with hepatolithiasis. According to the value of NLR = 2.36, the two groups were further classified into the low-NLR group and the high-NLR group, and the prognosis in each group was compared. Subsequently, the prognosis prediction model based on NLR in each group was constructed.
Patients characteristics
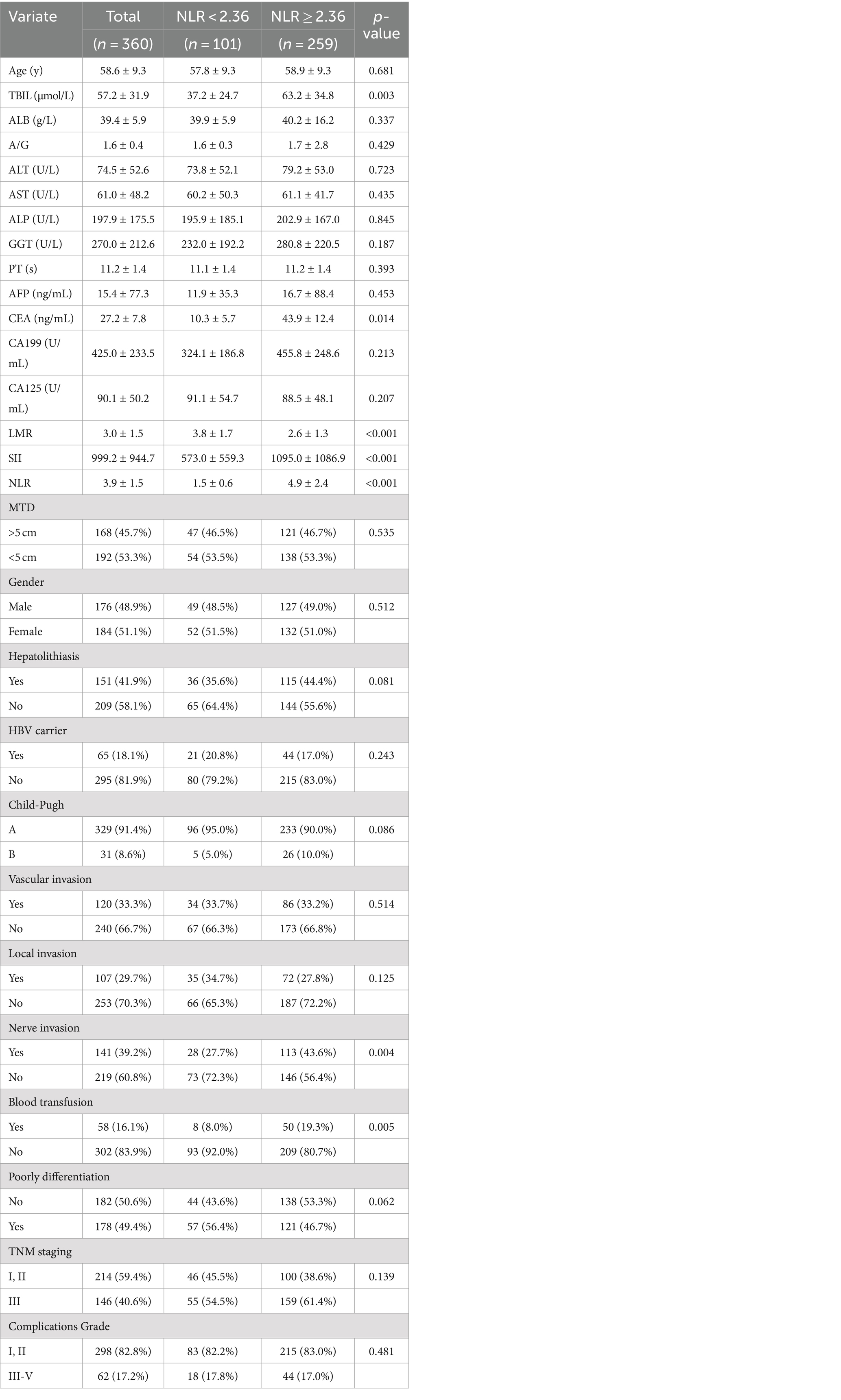
The clinical characteristics of ICC patients in the NLR < 2.36 group and the NLR ≥ 2.36 group are presented in Table 1. Among the two cohorts, factors such as age, ALB, A/G, ALT, AST, ALP, GGT, PT, AFP, CA199, CA125, MTD, gender, hepatolithiasis, HBV carrier, Child-Pugh (A/B), vascular invasion, local invasion, poorly differentiation (PD), TNM staging, and complications grade between the two groups demonstrated no significant difference (p > 0.05), while factors including TBIL, CEA, LMR, SII, NLR, nerve invasion, and blood transfusion between the two groups showed a statistically significant difference (p < 0.05).
Comparison of overall survival and recurrence of all ICC patients
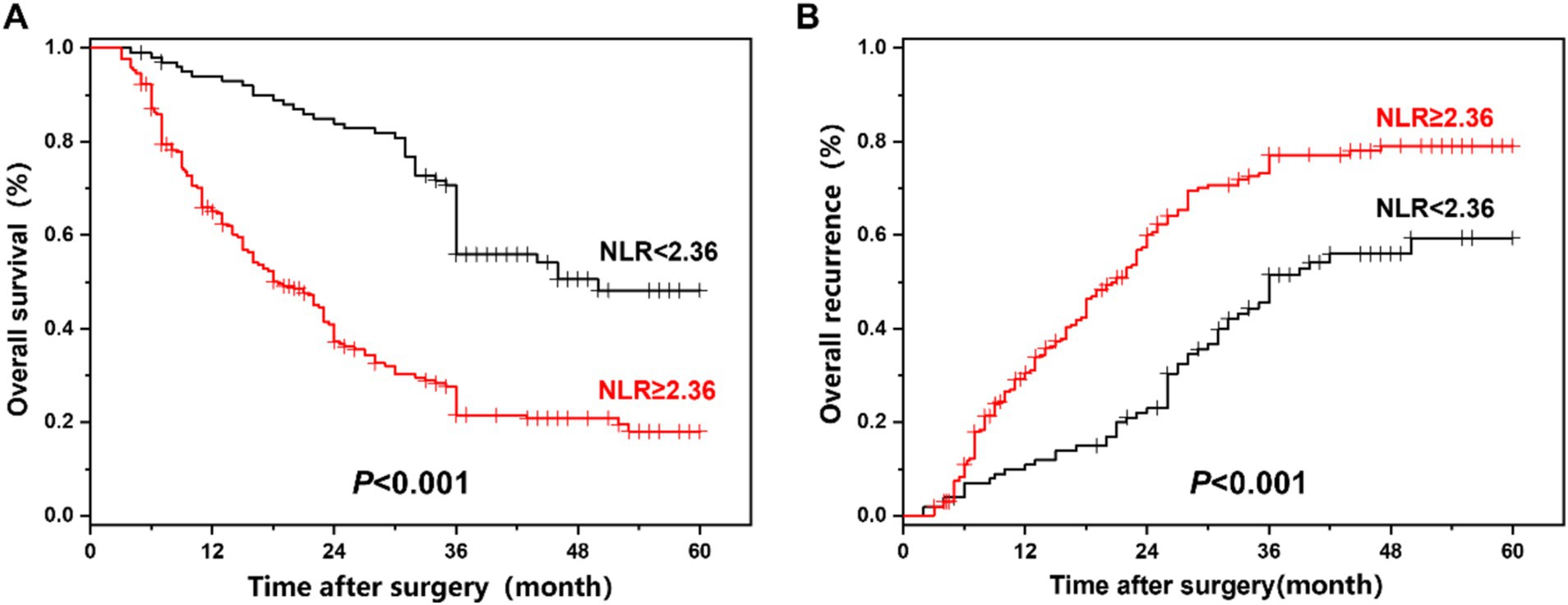
The comparison of overall survival and recurrence in all ICC patients between the NLR < 2.36 group and the NLR ≥ 2.36 group is presented in Figure 3. The median overall survival time of 360 ICC patients was 27 months, and the 1-year, 3-year, and 5-year overall survival rates were 74.0, 41.3, and 27.4%, respectively. Among ICC patients with NLR < 2.36, the 1-year, 3-year, and 5-year survival rates were 92.9, 70.7, and 48.2%, respectively; while among ICC patients with NLR ≥ 2.36, the 1-year, 3-year, and 5-year survival rates were 65.1, 27.7, and 18.0%, respectively, showing a statistically significant difference (p < 0.001). The median overall recurrence time was 24 months, and the overall recurrence rates at 1, 3, and 5 years were 24.8, 68.8, and 72.5%, respectively. Among ICC patients with NLR < 2.36, the recurrence rates at 1, 3, and 5 years were 11.1, 45.6, and 59.4%, respectively; while among ICC patients with NLR ≥ 2.36, the recurrence rates at 1, 3, and 5 years were 30.6, 73.3, and 79.1%, respectively, also showing a statistically significant difference (p < 0.001).
Analyses of prognostic factors for OS and recurrence in all ICC patients
The univariable and multivariate regression analyses of the risk factors for mortality within 5 years after surgery in all ICC patients are presented in Table 2. Univariate analysis indicated that the variables related to the overall survival (OS) of ICC patients are as follows: TBIL >17.1 μmol/L, PT > 13 s, CEA > 10 ng/mL, LMR > 3.71, NLR ≥ 2.36, combined with hepatolithiasis, vascular invasion, local invasion, nerve invasion, blood transfusion, PD, and TNM stage-III. Multivariate analysis suggested that the variables associated with the OS of ICC patients are as follows: CEA > 10 ng/mL, NLR ≥ 2.36, local invasion, PD, and TNM stage-III.
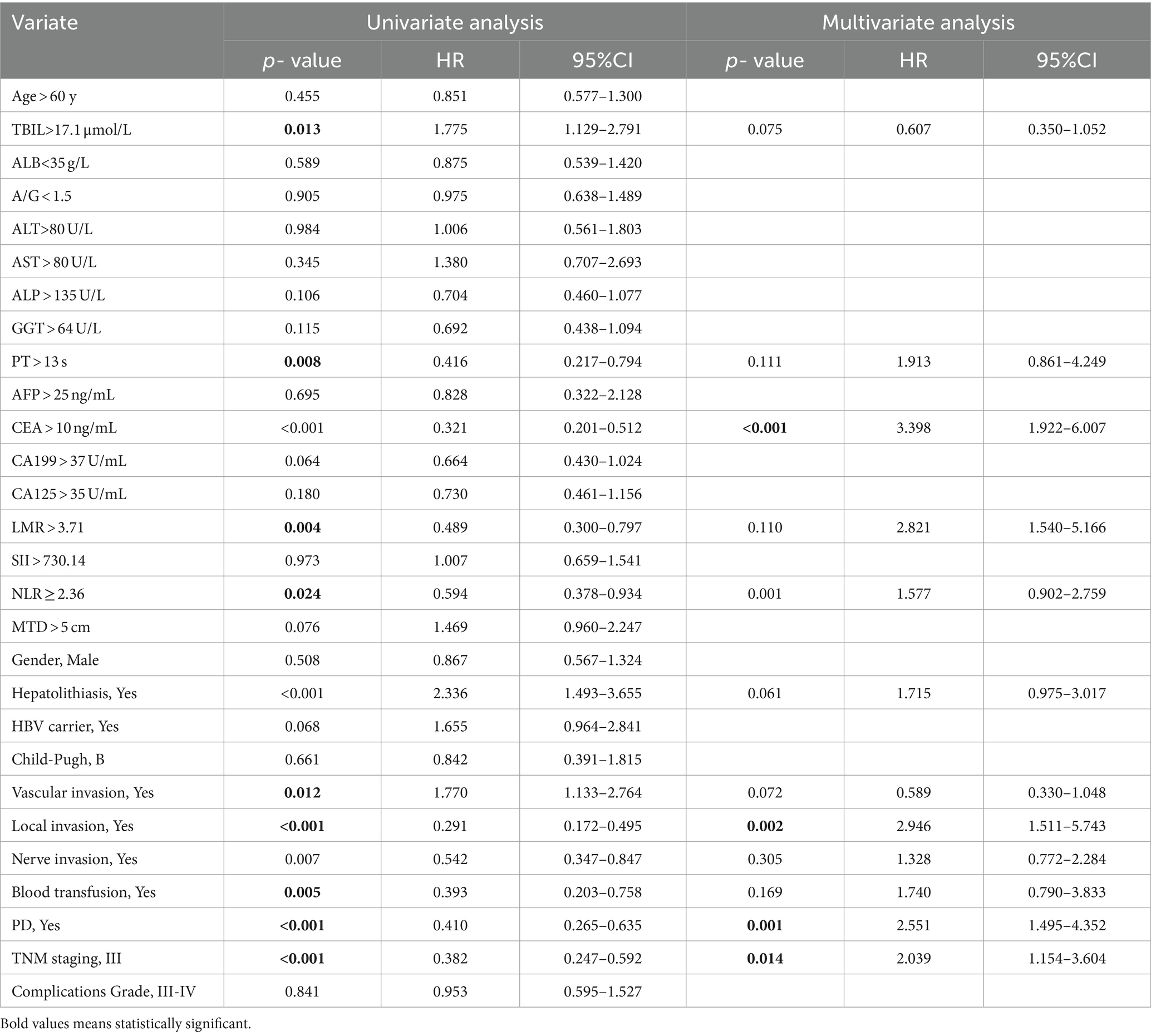
Table 2. Univariable and multivariate regression analyses of risk factors for mortality within 5 years after surgery in ICC patients.
The univariable and multivariate regression analyses of the risk factors for neoplasm recurrence within 5 years after surgery in all ICC patients are shown in Table 3. Univariate analysis indicated that the variables related to the recurrence of ICC patients are as follows: ALP > 135 U/L, PT > 13 s, CEA > 10 ng/mL, CA199 > 37 U/mL, LMR > 3.71, NLR ≥ 2.36, MTD > 5 cm, combined with hepatolithiasis, vascular invasion, nerve infiltration, blood transfusion, and TNM stage-III. Multivariate analysis suggested that the variables associated with the recurrence of ICC patients are as follows: ALP > 135 U/L, PT > 13 s, CEA > 10 ng/mL, LMR > 3.71, NLR ≥ 2.36, MTD > 5 cm, vascular invasion, blood transfusion, and TNM stage-III.
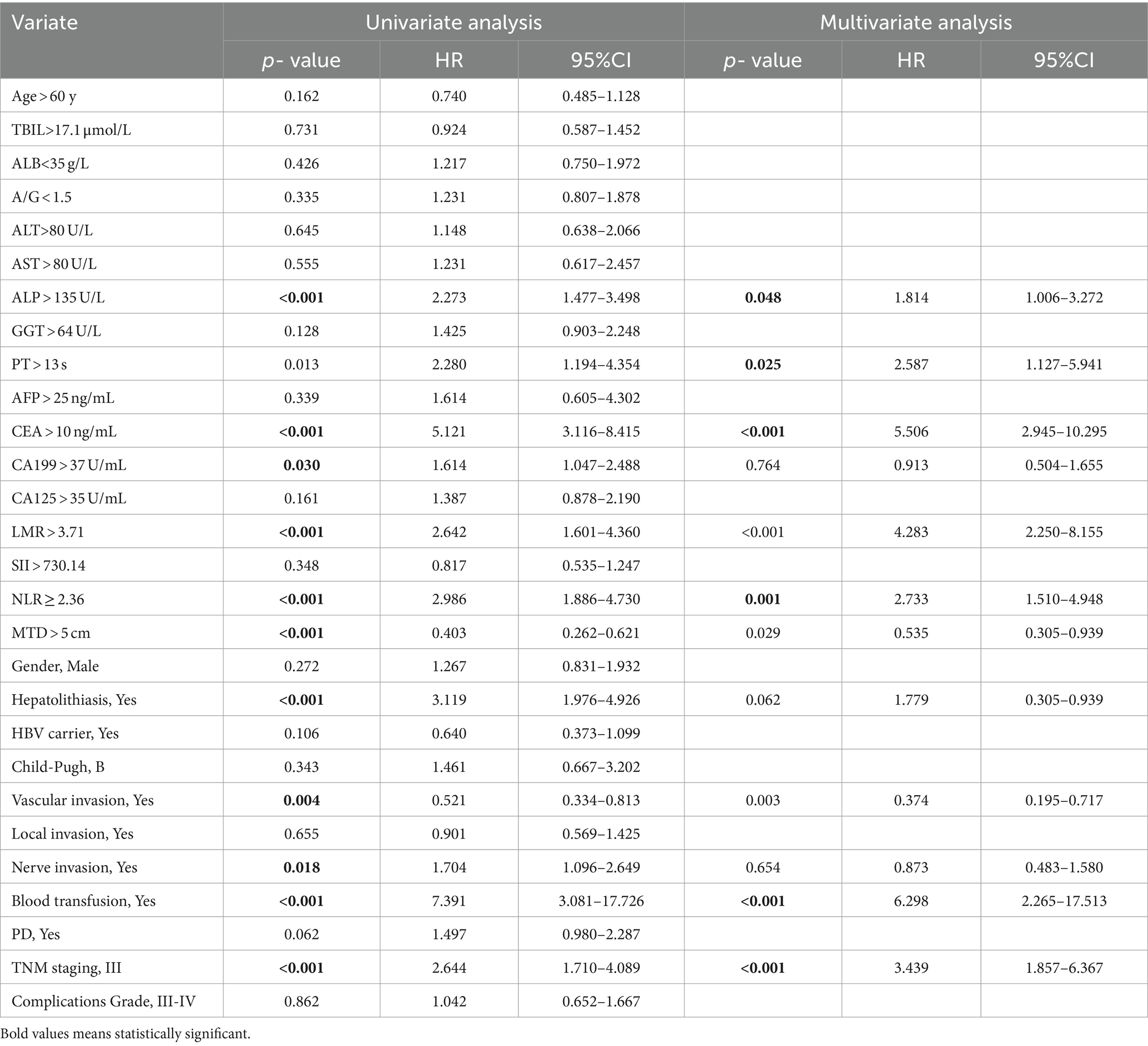
Table 3. Univariable and multivariate regression analyses of risk factors for neoplasm recurrence within 5 years after surgery in ICC patients.
Comparison of overall survival and recurrence in HICC and NHICC groups
The comparison of clinical characteristics in the NHICC group and the HICC group is presented in Supplementary Table S1 in the Supporting Information. Among the two cohorts, factors such as ALB, A/G, ALP, GGT, PT, CA199, NLR, MTD, gender, vascular invasion, local invasion, nerve invasion, and blood transfusion between the two groups exhibited a statistically significant difference (p < 0.05). Similarly, the ICC patients in the NHICC group and the HICC group were classified into the H-NLR group and the L-NLR group based on the NLR cut-off value of 2.36 for further comparative analysis.
The comparison of overall survival and recurrence in the HICC and NHICC groups between the L-NLR group and the H-NLR group is presented in Figure 4. In the HICC group, the median survival time of 151 HICC patients was 27 months, and the 1-, 3-, and 5-year survival rates were 75.6, 37.9, and 29.4%, respectively. The patients in the L-NLR group had a lower 5-year survival rate than those in the H-NLR group (49.3% vs. 12.9%, P<0.001). The median recurrence time was 25 months, and the recurrence rates at 1, 3, and 5 years were 28.1, 65.4, and 71.9%, respectively. The patients in the L-NLR group had a higher 5-year recurrence rate than those in the H-NLR group (46.8% vs. 86.3%, p < 0.001). In the NHICC group, the median survival time of 209 NHICC patients was 26 months, and the 1-, 3-, and 5-year survival rates were 74.0, 32.5, and 25.3%, respectively. The patients in the L-NLR group had a lower 5-year survival rate than those in the H-NLR group (40.8% vs. 21.4%, p < 0.001). The median recurrence time was 24.5 months, and the recurrence rates at 1, 3, and 5 years were 24.8, 68.8, and 72.5%, respectively. There was no significant difference between the two groups (65.8% vs. 67.6%, p = 0.071).

Figure 4. (A,B) The survival and recurrence comparison in hepatolithiasis-related intrahepatic cholangiocarcinoma (HICC) patients between the NLR. (C,D) The survival and recurrence comparison in non-hepatolithiasis-related intrahepatic cholangiocarcinoma (NHICC) patients between the NLR<2.36 group and the NLR≥2.36 group.
Analyses of prognostic factors for OS and recurrence in HICC and NHICC groups
The univariable and multivariate regression analyses of the risk factors for OS and recurrence within 5 years after surgery in HICC patients are presented in Tables 4, 5. Univariate and multivariate analyses indicated that the variables associated with the OS of HICC patients are as follows: PT > 13 s, CEA > 10 ng/mL, LMR > 3.71, NLR ≥ 2.36, blood transfusion, and PD; and the variables related to the recurrence of HICC patients are as follows: PT > 13 s, LMR > 3.71, NLR ≥ 2.36, blood transfusion, and PD.
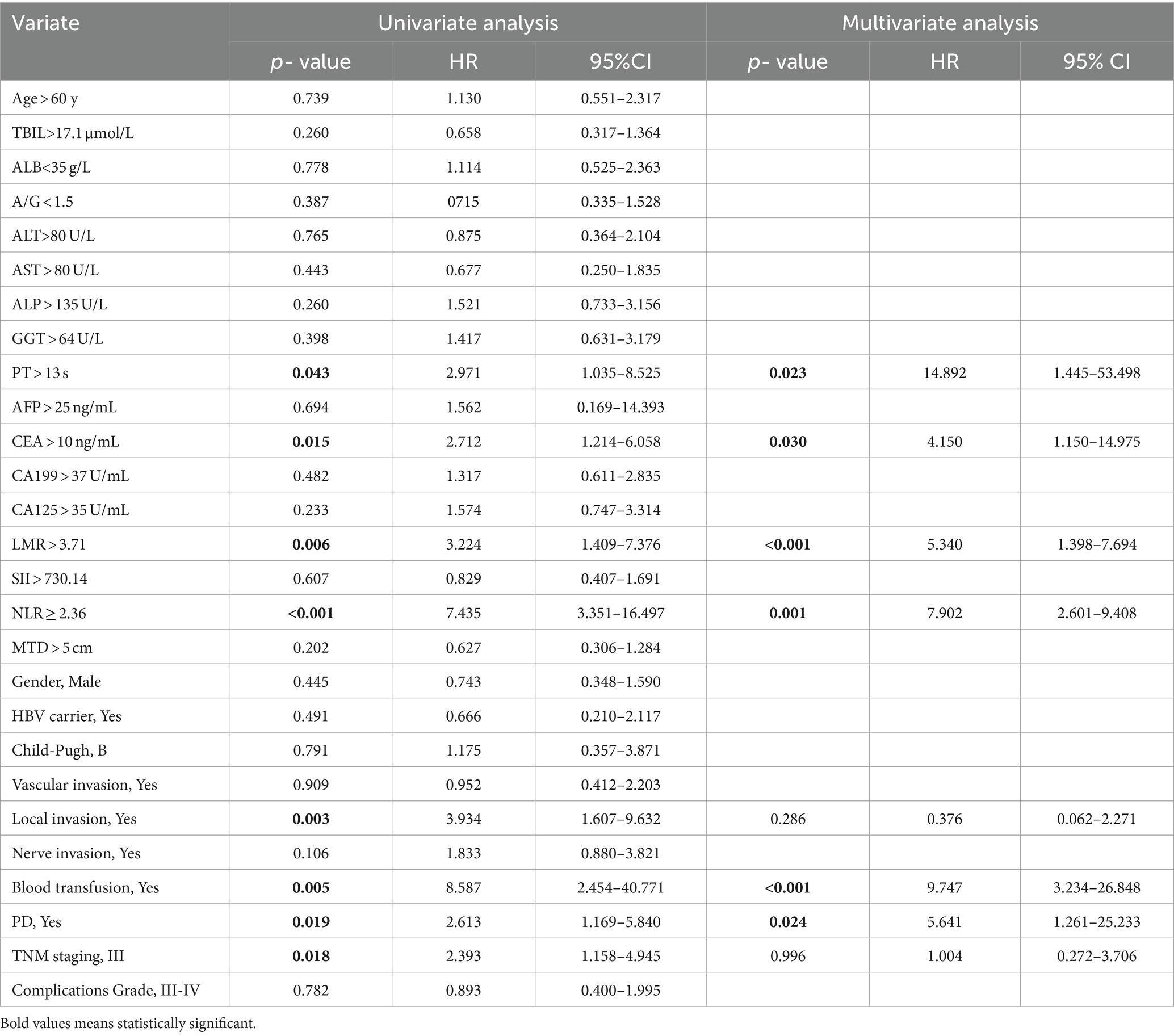
Table 4. Univariable and multivariate regression analyses of risk factors for mortality within 5 years after surgery in HICC patients.
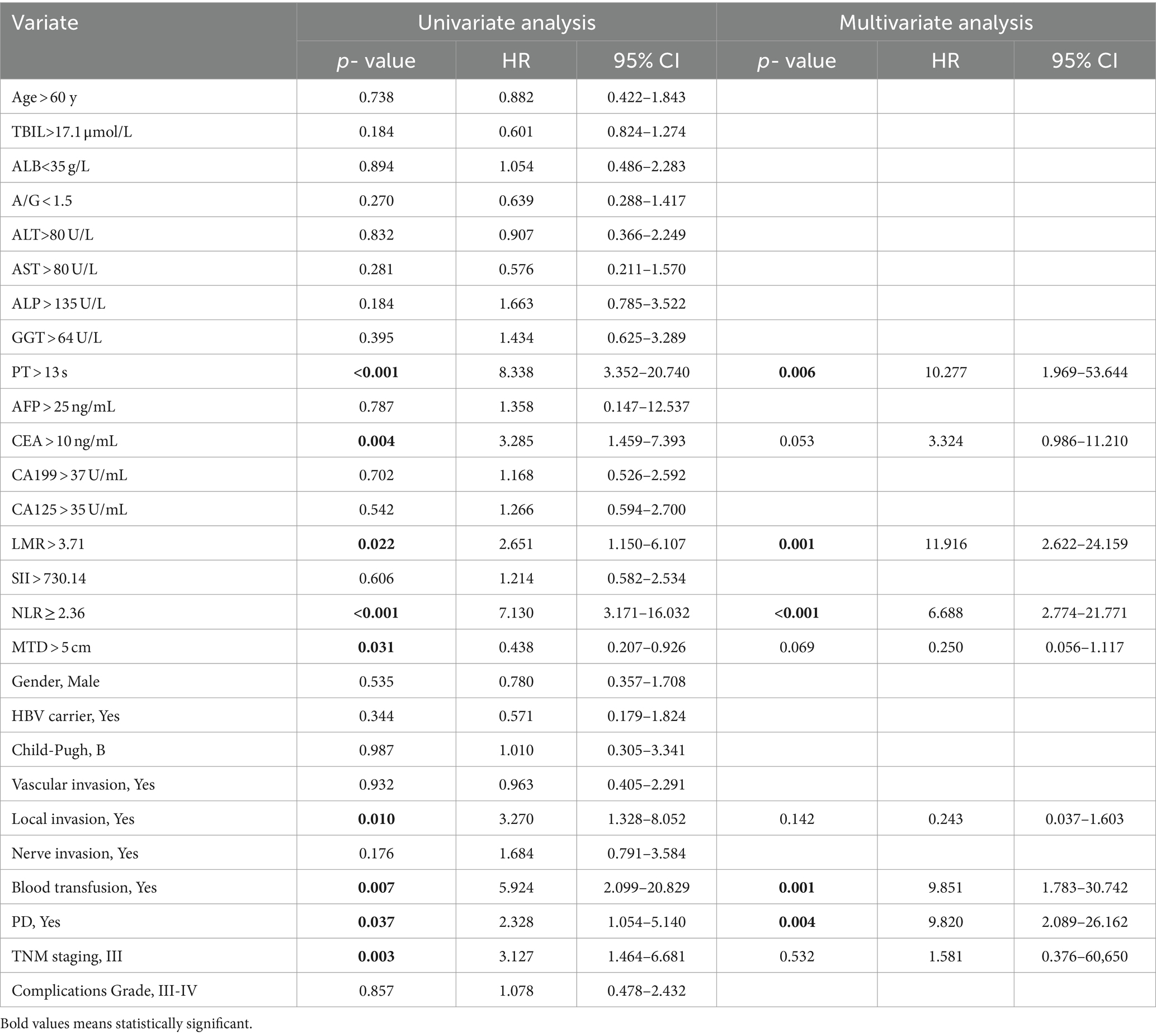
Table 5. Univariable and multivariate regression analyses of risk factors for neoplasm recurrence within 5 years after surgery in HICC patients.
The univariable and multivariate regression analyses of the risk factors for OS and recurrence within 5 years after surgery in NHICC patients are presented in Tables 6, 7. Univariate and multivariate analyses suggested that the variables associated with the OS of HICC patients are as follows: CEA > 10 ng/mL, NLR ≥ 2.36, PD, and TNM stage-III; and the variables related to the recurrence of NHICC patients are as follows: CEA > 10 ng/mL, vascular invasion, and TNM stage-III.
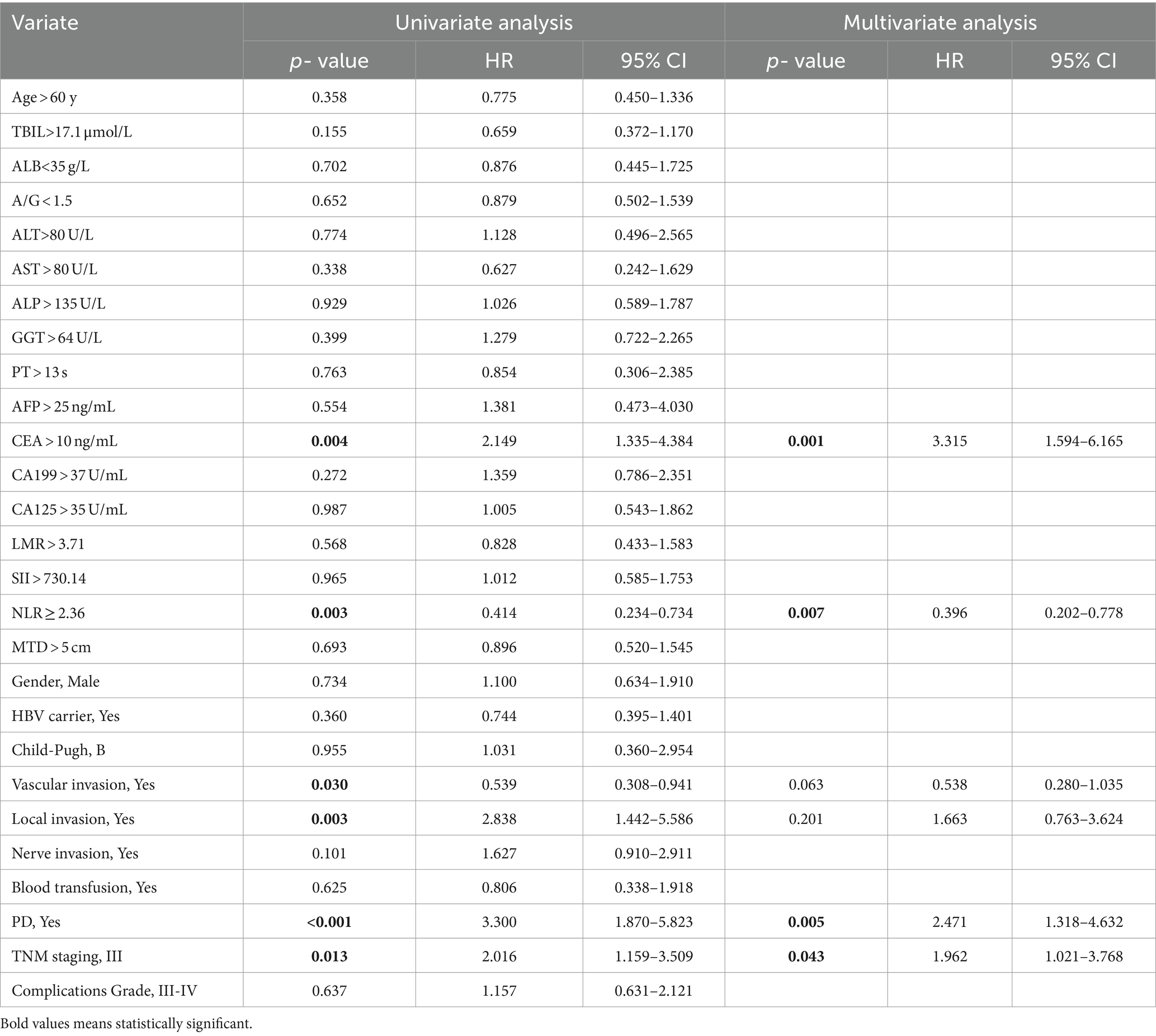
Table 6. Univariable and multivariate regression analyses of risk factors for mortality within 5 years after surgery in NHICC patients.
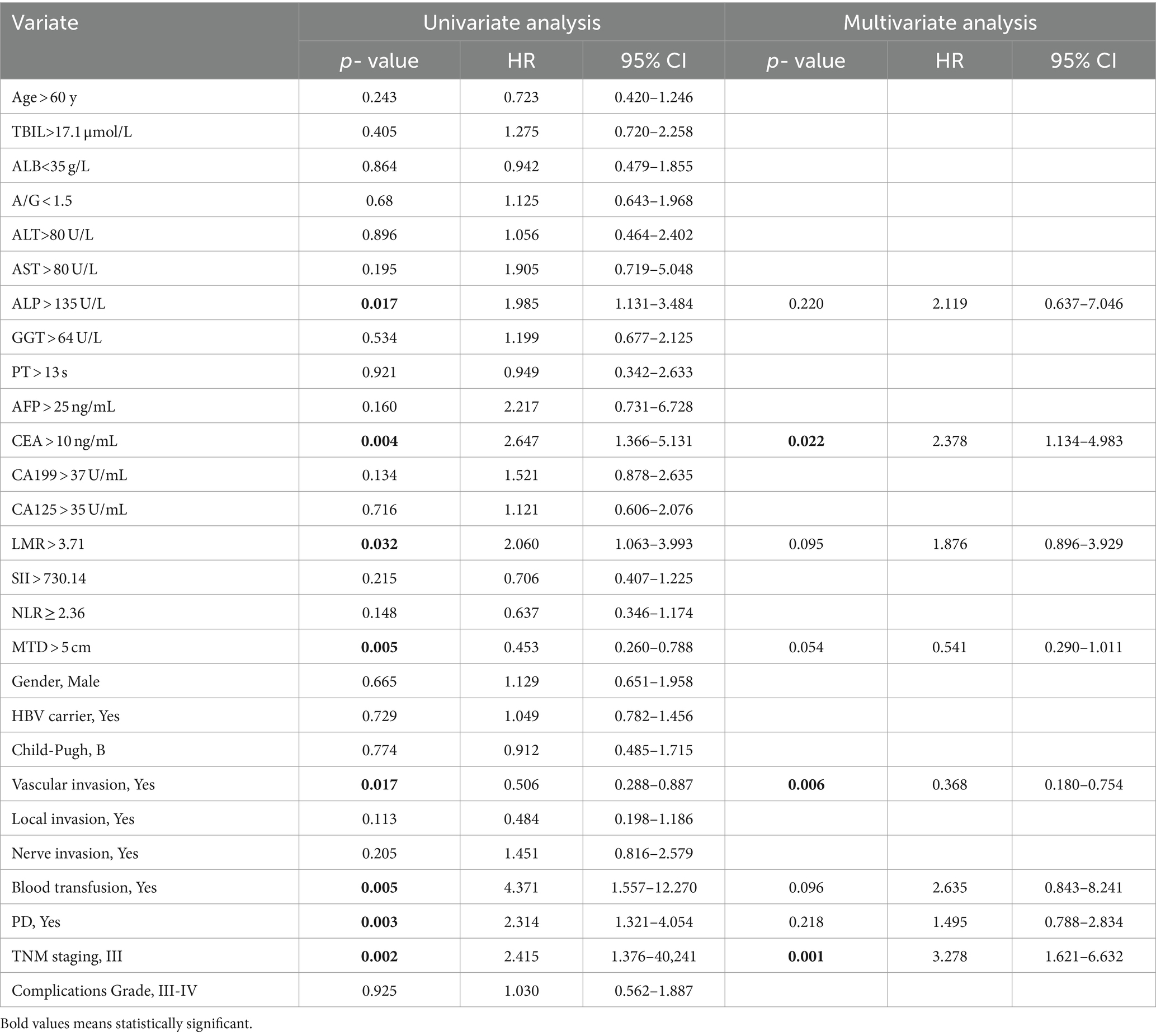
Table 7. Univariable and multivariate regression analyses of risk factors for neoplasm recurrence within 5 years after surgery in NHICC patients.
Model built and validation in HICC group
Multivariate analysis and risk score of postoperative survival and neoplasm recurrence in HICC patients 5 years after surgery are presented in Supplementary Tables S2, S3. Multivariate analysis indicated that the variables associated with the OS of HICC patients are as follows: NLR ≥ 2.36 (HR: 4.791, 95%CI: 3.452–10.002), CEA > 10 ng/mL (HR: 3.356, 95%CI: 2.352–9.683), and blood transfusion (HR: 1.332, 95%CI: 0.731–5.017). The prognosis prediction model based on NLR was obtained by adding the total number of points scored in each of the three independent risk factors. The model was: 5-year mortality risk of HICC = 2.120 * NLR + 2.123 * CEA + 3.305 * blood transfusion - 5.292. Multivariate analysis indicated that the variables associated with the neoplasm recurrence of HICC patients are as follows: NLR ≥ 2.36 (HR: 3.321, 95%CI: 1.343–5.174), PT > 13 s (HR: 1.103, 95%CI: 0.231–3.744), blood transfusion (HR: 1.842, 95%CI: 0.892–3.194), and PD (HR: 2.635, 95%CI: 0.877–7.922), and the model was: 5-year recurrence risk of HICC = 3.378 * NLR + 0.072 * PT + 2.472 * blood transfusion +0.969 *PD - 4.630.
An NLR-based survival/recurrence predictive nomogram is established for HICC patients following curative resection, and its area under the curve (AUC) in the HICC group is shown in Figure 5. It can be seen that NLR shows a higher score in predicting the incidence of 5-year mortality risk of HICC patients, followed by CEA and blood transfusion, and the AUC of the prediction model based on NLR in the HICC group was 0.764, H-L test indicated that p value was 0.359 > 0.05 (Figures 5A,B). The NLR also shows a higher score in predicting the incidence of year recurrence risk of HICC patients, followed by blood transfusion, PD, and PT, and the AUC of the prediction model based on NLR in the HICC group was 0.791, H-L test indicated that p value was 0.680 > 0.05 (Figures 5C,D).
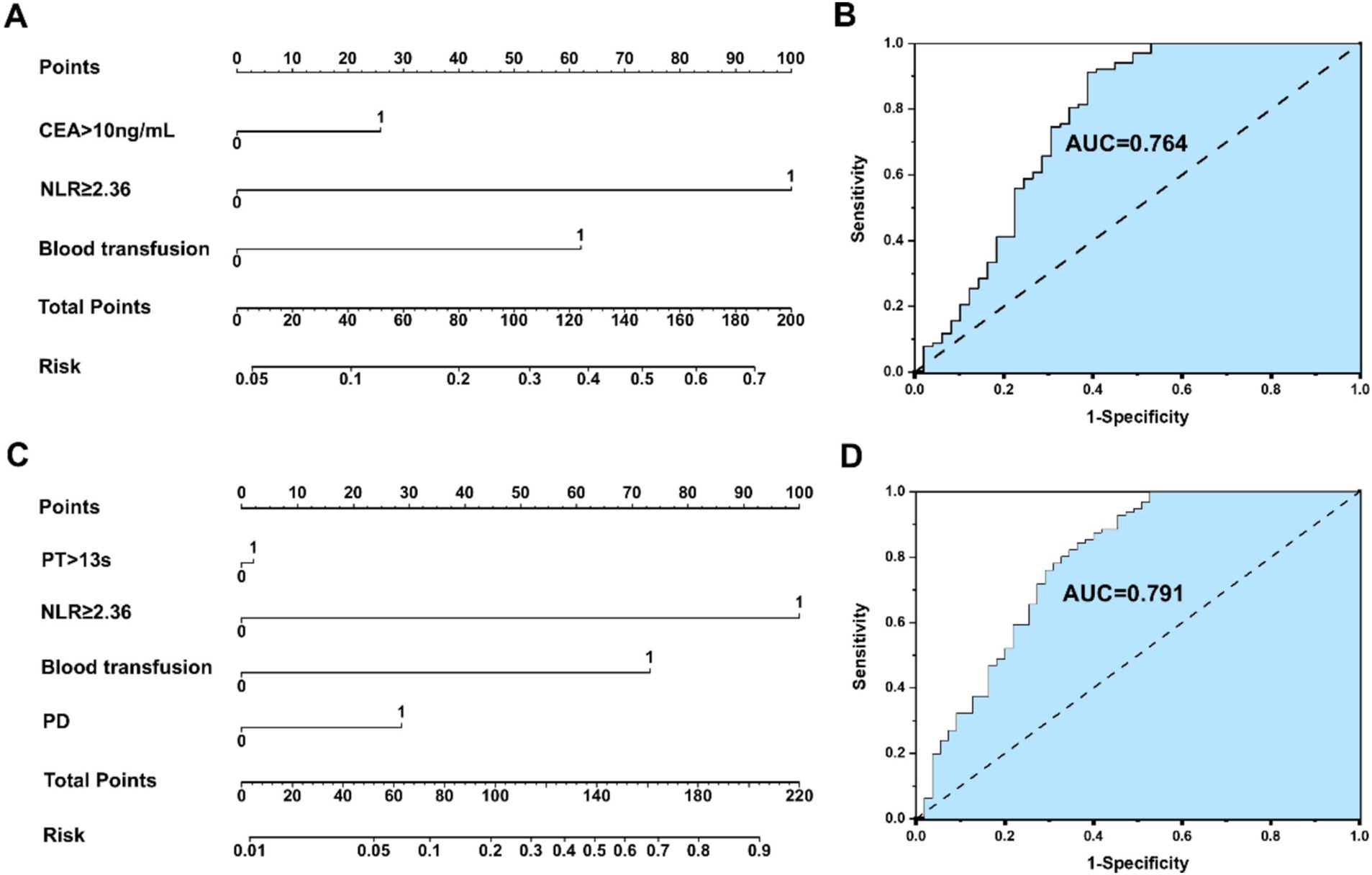
Figure 5. (A,B) An NLR-based survival predictive nomogram is established for HICC patients following curative resection and its area under the curve (AUC) in the HICC group. (C,D) An NLRbased recurrence predictive nomogram is established for HICC patients following curative resection and its area under the curve (AUC) in the HICC group.
Model built and validation in NHICC group
Multivariate analysis and risk score of postoperative survival and neoplasm recurrence in NHICC patients 5 years after surgery are presented in Supplementary Tables S4, S5. Multivariate analysis indicated that the variables associated with the OS of NHICC patients are as follows: NLR ≥ 2.36 (HR: 0.339, 95%CI: 0.177–0.649), CEA > 10 ng/mL (HR: 3.170, 95%CI: 1.617–6.217), and PD (HR: 2.936, 95%CI: 1.063–5.378), and the model was: 5-year mortality risk of NHICC = 1.082*NLR + 1.154*CEA + 1.077*PD - 1.205. Multivariate analysis indicated that the variables associated with the neoplasm recurrence of HICC patients are as follows: NLR ≥ 2.36 (HR: 1.285, 95%CI: 0.665–2.481), CEA > 10 ng/mL (HR: 6.338, 95%CI: 3.151–12.748), vascular invasion (HR: 0.310, 95%CI: 0.155–0.619), and TNM stage-III (HR: 3.214, 95%CI: 1.633–6.326), and the model based on NLR was: 5-year recurrence risk of NHICC = 0.251*NLR + 1.847*CEA + 1.172* vascular invasion +1.168* TNM stage - 1.102.
An NLR-based survival/recurrence predictive nomogram is also established for NHICC patients following curative resection, and its area under the curve (AUC) in the NHICC group is shown in Figure 6. It can be seen that CEA shows a higher score in predicting the incidence of 5-year mortality risk of NHICC patients, followed by NLR and PD, and the AUC of the prediction model based on NLR in the NHICC group was 0.729, H-L test indicated that p value was 0.268 > 0.05 (Figures 6A,B). The CEA also shows a higher score in predicting the incidence of year recurrence risk of NHICC patients, followed by vascular invasion and TNM stage, while the NLR did not present a good score in predicting. The AUC of the prediction model based on NLR in the NHICC group was 0.649, H-L test indicated that p value was 0.01 < 0.05 (Figures 6C,D).
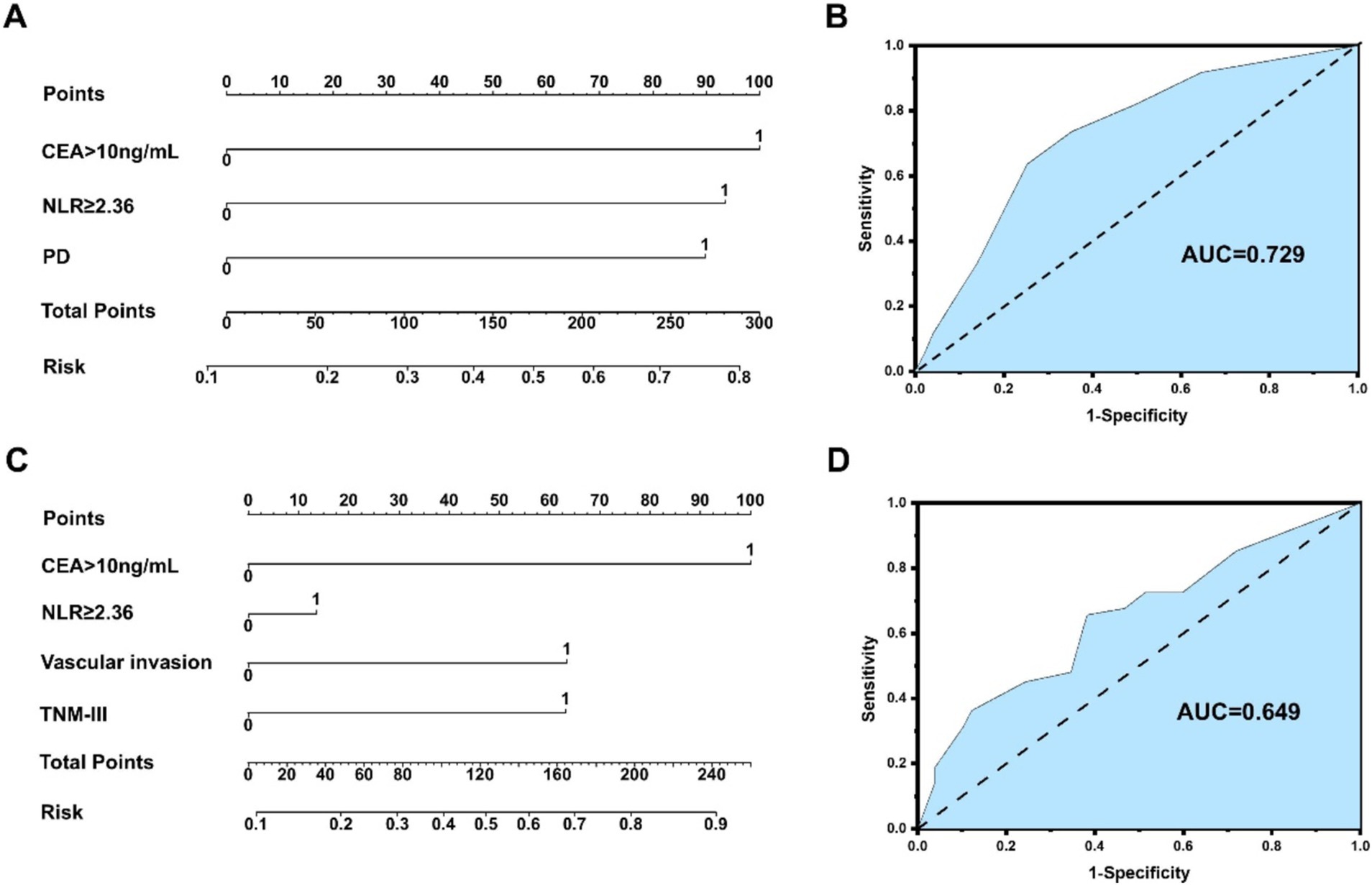
Figure 6. (A,B) An NLR-based survival predictive nomogram is established for NHICC patients 34 following curative resection and its area under the curve (AUC) in the NHICC group. (C,D) An NLR-based recurrence predictive nomogram is established for NHICC patients following curative resection and its area under the curve (AUC) in the NHICC group.
Discussion
Surgical resection constitutes an essential approach for the treatment of ICC (24). Surgical indications typically encompass patients presenting with localized lesions, no distant metastasis, and having liver function adequate to withstand surgery (25, 26). Through the collaboration of a comprehensive multidisciplinary team, it has gradually evolved and achieved certain advancements; however, it still confronts challenges, such as the accuracy of preoperative diagnosis, determination of the extent of surgical resection, and the postoperative recurrence rate (27, 28). Postoperative recurrence represents a major clinical issue and is also the focus and difficulty of current treatment. Hence, exploring the high-risk factors of postoperative recurrence and actively adopting corresponding treatments is an important measure to enhance the prognosis of ICC patients.
Serum inflammatory indicators are associated with tumors, and the diagnosis and treatment of ICC is also a research focus of concern, particularly for HICC patients, who are often accompanied by inflammation (29). Thus, serum inflammatory indicators can be utilized to assess the disease status, prognosis, and treatment response of ICC patients. Some studies have reported that certain commonly used serum inflammatory indicators, such as C-reactive protein (CRP), neutrophil/lymphocyte ratio (NLR), systemic immunoinflammatory index (SII), etc. (19, 30, 31), can serve as indicators for tumor diagnosis, prognosis evaluation, and treatment monitoring. However, reports on serum inflammatory indicators of HICC are scarce. There has been no risk factor analysis based on NLR and other clinical indicators in the prognosis of HICC. In this study, three inflammatory indicators, namely LMR, SII, and NLR, were included in the prognostic analysis, and the cut-off values were 3.71, 730.14, and 2.36, respectively. Meanwhile, the ROC curve correlation analysis only indicated that NLR was an independent risk factor influencing the prognosis of ICC patients. Therefore, in this study, NLR was employed as an indicator of serum inflammation in the study of the predictive value of ICC patients after radical surgery.
Studies have shown that a high NLR is associated with a poor prognosis of hepatobiliary tumors (such as liver cancer, gallbladder cancer, etc.), and a high NLR is related to tumor size, invasiveness, and metastatic propensity, indicating a more severe condition of hepatobiliary tumors (32, 33). Some scholars have suggested that NLR can serve as a crucial indicator for the prognosis assessment of hepatobiliary tumors, and the integration of NLR into the prognosis assessment model of hepatobiliary tumors can improve the prediction accuracy (34, 35). In this study, the NLR cut-off value of 2.36 calculated based on the data of this group of samples is more appropriate for the analysis of this sample. According to previous studies, the NLR truncation value typically ranges around 2–3, which is not significantly different from the NLR truncation value set in this study (29). Simultaneously, all patients were divided into NLR < 2.36 and NLR ≥ 2.36 groups. A comparative analysis of clinical data between the groups indicated that the high NLR group had a higher CEA value, more nerve invasion, and a poorer TNM stage. Prognostic analysis suggested that compared with NLR < 2.36, patients in the NLR ≥ 2.36 group had higher 1-, 3-, and 5-year postoperative survival rates and lower 1-, 3-, and 5-year postoperative recurrence rates. Multivariate analysis suggested that the variables associated with the overall survival (OS) of ICC patients are as follows: CEA > 10 ng/mL, NLR ≥ 2.36, local invasion, PD, and TNM stage-III; the variables associated with the recurrence of ICC patients are as follows: ALP > 135 U/L, PT > 13 s, CEA > 10 ng/mL, LMR > 3.71, NLR ≥ 2.36, MTD > 5 cm, vascular invasion, blood transfusion, and TNM stage-III, suggesting that NLR might be correlated with tumor invasion and metastasis.
Studies have indicated that individuals suffering from chronic cholangitis diseases, such as bile duct stones, cholecystitis, cholangitis, etc., have an elevated risk of ICC (36–38). Long-term bile duct disorders may cause chronic inflammation and damage to bile duct epithelial cells, thereby increasing the risk of cancer (39, 40). Some studies have proposed that the relatively favorable prognosis of patients with HICC might be associated with early detection and treatment, as well as the low proportion of HICC in patients with cirrhosis. In contrast, patients with NHICC typically present at a late stage and have a higher postoperative recurrence rate (41, 42). In this study, within the HICC group, the survival rates at 1, 3, and 5 years after surgery in the H-NLR group were higher than those in the L-NLR group. Among patients with NHICC, the survival rates at 1, 3, and 5 years after surgery in the H-NLR group were higher than those in the L-NLR group, and there was no significant difference in the recurrence rate between the two groups. It is suggested that NLR has a poorer ability to predict the prognosis of patients with NHICC compared to those with HICC. Therefore, NLR can serve as a serum inflammatory marker for predicting the prognosis of patients with HICC, and NLR is an important risk factor influencing postoperative survival and recurrence of HICC.
The application of NLR-based tumor prognosis prediction models constitutes an important research domain. The combination of NLR with imaging characteristics, tumor traits, clinicopathological factors, etc., can establish a prognosis prediction model of ICC based on NLR, which enables physicians to assess the prognosis of patients more precisely, thereby formulating individualized treatment plans and follow-up strategies (30, 43, 44). In this study, multivariate regression analyses of multiple variables in the HICC group and the NHICC group were, respectively, conducted, and it was affirmed that the risk factors related to postoperative survival of HICC encompassed: CEA > 10 ng/mL, NLR ≥ 2.36, and blood transfusion; factors associated with postoperative recurrence of HICC: PT > 13 s, NLR ≥ 2.36, blood transfusion, and PD. Risk factors associated with postoperative survival of NHICC included CEA > 10 ng/mL, NLR ≥ 2.36, and PD; factors associated with postoperative recurrence of NHICC were CEA > 10 ng/mL, vascular invasion, and TNM stage-III. The NLR-based survival/recurrence prediction models in the HICC group exhibited excellent predictive capacity (H-L test: 0.359/0.680, AUC: 0.764/0.791); the NLR-based survival prediction model in the NHICC group demonstrated acceptable predictive ability (H-L test: 0.268, AUC: 0.729), while the NLR-based recurrence prediction model did not display an effective predictive ability (H-L test: 0.01, AUC: 0.649), suggesting that the survival and recurrence prediction model constructed based on NLR shows good prediction accuracy and can effectively predict the risk of postoperative adverse prognosis in patients with HICC; however, its predictive value for patients with NHICC is limited.
This study analyzed the prognosis of ICC based on the serum inflammatory index NLR and confirmed that NLR is an independent risk factor influencing the prognosis of HICC. Subsequently, a prognostic model for survival and recurrence after HICC was constructed based on NLR, relevant clinical indicators, pathology, and other factors. This model can predict and analyze the prognosis of HICC patients to a certain extent and contribute to the decision-making and implementation of perioperative comprehensive treatment. However, this study also has certain limitations. As a single-center retrospective study, it lacks multi-center large sample data and has shortcomings such as a small sample size and significant individual differences. In the future, we will undertake a multi-center collaborative research project to jointly conduct a study on the correlation between NLR and the prognosis of ICC patients in multiple regions and centers, thereby increasing the sample size, enhancing the reliability of the research results, and obtaining a more comprehensive understanding of the correlation between NLR and the prognosis of ICC, so as to provide more reliable clinical guidance for the diagnosis, treatment, and prognosis evaluation of this disease.
Conclusion
NLR is an independent risk factor influencing postoperative survival and recurrence in ICC patients, particularly in HICC patients. Preoperative NLR ≥ 2.36 suggests that patients might have a poor prognosis. For patients with NHICC, the predictive value of CEA may be superior to NLR. The survival and recurrence prediction model constructed based on NLR and other clinical indicators demonstrates good prediction accuracy and can effectively predict the risk of postoperative adverse prognosis in patients with HICC. However, its predictive value for patients with NHICC is limited. This study offers a novel idea for the clinical treatment of HICC patients.
Data availability statement
The datasets presented in this article are not readily available because All the patients encompassed in the current study fulfilled the subsequent criteria: (1) underwent R0 resection, with postoperative pathology confirming ICC; comprehensive clinical case data and follow-up data were accessible; (2) did not undergo tumor-targeted treatments such as transcatheter arterial chemoembolization (TACE), chemotherapy, radiotherapy, targeted therapy, or immunotherapy, etc.; (3) preoperative Child-Pugh score of A/B; (4) had no history of previous malignant tumors. Patients with the following attributes were excluded: (1) R1/2 excision or distant metastasis (M1); (2) postoperative pathology indicating primary liver cancer such as hepatocellular carcinoma, mixed hepatocellular carcinoma, or other types of biliary malignancy; (3) patients with infectious diseases prior to operation; (4) taking hormones, aspirin, clopidogrel, and other drugs influencing peripheral blood cell indicators before surgery; (5) combined with blood diseases or immune system disorders; (6) postoperative perioperative mortality; (7) incomplete clinical data. Requests to access the datasets should be directed to SQ, cWlzaHVvQGh1bm51LmVkdS5jbi5iZQ==.
Ethics statement
Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
SQ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. ZM: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. LS: Data curation, Methodology, Writing – original draft. JW: Investigation, Software, Supervision, Writing – original draft. LZ: Formal analysis, Project administration, Validation, Writing – original draft. BT: Conceptualization, Data curation, Supervision, Writing – original draft. CL: Data curation, Investigation, Methodology, Software, Writing – original draft. KC: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. WC: Conceptualization, Data curation, Formal analysis, Investigation, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (no. 82404102), Scientific Research Project of Hunan Education Department (no. 22B0421), Hunan Provincial Health High-Level Talent Scientific Research Project (no. R2023096), Health Research Project of Hunan Provincial Health Commission (nos. W20243022 and 202204014556), Scientific Research General Project of Hunan Education Department (no. 23C0025), Natural Science Foundation of Hunan Province (no. 2022JJ40400).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1492358/full#supplementary-material
Abbreviations
ICC, Intrahepatic cholangiocarcinoma; NLR, Neutrophil/lymphocyte ratio; SII, Systemic immune-inflammation index; LMR, Lymphocyte/monocyte ratio; HICC, Hepatolithiasis-related intrahepatic cholangiocarcinoma; NHICC, Non-hepatolithiasis-related intrahepatic cholangiocarcinoma; MTD, Maximum tumor diameter; PD, Poorly differentiation.
References
1. Siegel, RL, Miller, KD, Wagle, NS, and Jemal, A (2023). Cancer statistics, 2023. CA Cancer J Clin 73:17–48. doi: 10.3322/caac.21763
2. Brindley, PJ, Bachini, M, Ilyas, SI, Khan, SA, Loukas, A, Sirica, AE, et al. (2021). Cholangiocarcinoma. Nat Rev Dis Primers 7:65. doi: 10.1038/s41572-021-00300-2
3. Sapisochin, G, Ivanics, T, and Heimbach, J (2022). Liver transplantation for intrahepatic Cholangiocarcinoma: ready for prime time? Hepatology 75:455–72. doi: 10.1002/hep.32258
4. Spencer, K, Pappas, L, Baiev, I, Maurer, J, Bocobo, AG, Zhang, K, et al. (2023). Molecular profiling and treatment pattern differences between intrahepatic and extrahepatic cholangiocarcinoma. J Natl Cancer Inst 115:870–80. doi: 10.1093/jnci/djad046
5. La Vecchia, C, Lotti, M, and Colombo, M (2020). Asbestos exposure and increased risk of intrahepatic Cholangiocarcinoma: enough to infer causality? Gastroenterology 159:794–5. doi: 10.1053/j.gastro.2020.06.037
6. Zhou, SL, Xin, HY, Sun, RQ, Zhou, ZJ, Hu, ZQ, Luo, CB, et al. (2022). Association of KRAS variant subtypes WithSurvival and recurrence in patients with surgically treated intrahepatic Cholangiocarcinoma. JAMA Surg 157:59–65. doi: 10.1001/jamasurg.2021.5679
7. Bressan, AK, Isherwood, S, Bathe, OF, Dixon, E, Sutherland, FR, and Ball, CG (2022). Preoperative single-dose methylprednisolone prevents surgical site infections after major liver resection: a randomized controlled trial. Ann Surg 275:281–7. doi: 10.1097/SLA.0000000000004720
8. Huang, G, Xi, P, Yao, Z, Zhao, C, Li, X, Chen, Z, et al. (2024). The clinical association between the inflammation-nutritional condition and prognosis of locally advanced intrahepatic Cholangiocarcinoma after R0 resection: evidence from competing risk and propensity matching analysis. J Inflamm Res 17:2787–99. doi: 10.2147/JIR.S460103
9. Moris, D, Palta, M, Kim, C, Allen, PJ, Morse, MA, and Lidsky, ME (2023). Advances in the treatment of intrahepatic cholangiocarcinoma: An overview of the current and future therapeutic landscape for clinicians. CA Cancer J Clin 73:198–222. doi: 10.3322/caac.21759
10. Edeline, J, Touchefeu, Y, Guiu, B, Farge, O, Tougeron, D, Baumgaertner, I, et al. (2020). Radioembolization plus chemotherapy for first-line treatment of locally advanced intrahepatic Cholangiocarcinoma: a phase 2 clinical trial. JAMA Oncol 6:51–9. doi: 10.1001/jamaoncol.2019.3702
11. Cho, SY, Hwang, H, Kim, YH, Yoo, BC, Han, N, Kong, SY, et al. (2023). Refining classification of Cholangiocarcinoma subtypes via Proteogenomic integration reveals new therapeutic prospects. Gastroenterology 164:1293–309. doi: 10.1053/j.gastro.2023.02.045
12. Kawamura, E, Matsubara, T, and Kawada, N (2023). New era of immune-based therapy in intrahepatic Cholangiocarcinoma. Cancers (Basel) 15:3993. doi: 10.3390/cancers15153993
13. Yoh, T, Hatano, E, Kasai, Y, Fuji, H, Nishi, K, Toriguchi, K, et al. (2019). Serum Nardilysin, a surrogate marker for epithelial-mesenchymal transition, predicts prognosis of intrahepatic Cholangiocarcinoma after surgical resection. Clin Cancer Res 25:619–28. doi: 10.1158/1078-0432.CCR-18-0124
14. Bo, Z, Chen, B, Yang, Y, Yao, F, Mao, Y, Yao, J, et al. (2023). Machine learning radiomics to predict the early recurrence of intrahepatic cholangiocarcinoma after curative resection: a multicentre cohort study. Eur J Nucl Med Mol Imaging 50:2501–13. doi: 10.1007/s00259-023-06184-6
15. Sui, Q, Zhang, X, Chen, C, Tang, J, Yu, J, Li, W, et al. (2022). Inflammation promotes resistance to immune checkpoint inhibitors in high microsatellite instability colorectal cancer. Nat Commun 13:7316. doi: 10.1038/s41467-022-35096-6
16. Neumann, C, Schneider, F, Hilfenhaus, G, Vecchione, L, Felsenstein, M, Ihlow, J, et al. (2023). Inflammation-based prognostic scores in pancreatic Cancer patients-a single-center analysis of 1294 patients within the last decade. Cancers (Basel) 15:2367. doi: 10.3390/cancers15082367
17. Domblides, C, Crampton, S, Liu, H, Bartleson, JM, Nguyen, A, Champagne, C, et al. (2024). Human NLRC4 expression promotes cancer survival and associates with type I interferon signaling and immune infiltration. J Clin Invest 134:85. doi: 10.1172/JCI166085
18. Zhu, J, Wang, D, Liu, C, Huang, R, Gao, F, Feng, X, et al. (2023). Development and validation of a new prognostic immune-inflammatory-nutritional score for predicting outcomes after curative resection for intrahepatic cholangiocarcinoma: a multicenter study. Front Immunol 14:1165510. doi: 10.3389/fimmu.2023.1165510
19. Yang, Z, Zhang, D, Zeng, H, Fu, Y, Hu, Z, Pan, Y, et al. (2022). Inflammation-based scores predict responses to PD-1 inhibitor treatment in intrahepatic Cholangiocarcinoma. J Inflamm Res 15:5721–31. doi: 10.2147/JIR.S385921
20. Prigent, K, Lasnon, C, Ezine, E, Janson, M, Coudrais, N, Joly, E, et al. (2021). Assessing immune organs on (18)F-FDG PET/CT imaging for therapy monitoring of immune checkpoint inhibitors: inter-observer variability, prognostic value and evolution during the treatment course of melanoma patients. Eur J Nucl Med Mol Imaging 48:2573–85. doi: 10.1007/s00259-020-05103-3
21. Wu, J, Yang, S, Xu, K, Ding, C, Zhou, Y, Fu, X, et al. (2018). Patterns and trends of liver Cancer incidence rates in eastern and southeastern Asian countries (1983-2007) and predictions to 2030. Gastroenterology 154:1719–1728.e5. doi: 10.1053/j.gastro.2018.01.033
22. Wei, T, Zhang, XF, He, J, Popescu, I, Marques, HP, Aldrighetti, L, et al. (2022). Prognostic impact of perineural invasion in intrahepatic cholangiocarcinoma: multicentre study. Br J Surg 109:610–6. doi: 10.1093/bjs/znac098
23. Ivy, ML, Baison, G, Griffin, C, Welch, AC, White, PT, Farivar, AS, et al. (2024). Thirty-and 90-day morbidity and mortality by Clavien-Dindo 30 and 90 days after surgery for Antireflux and hiatal hernia. J Am Coll Surg 239:323–32. doi: 10.1097/XCS.0000000000001114
24. Gravely, AK, Vibert, E, and Sapisochin, G (2022). Surgical treatment of intrahepatic cholangiocarcinoma. J Hepatol 77:865–7. doi: 10.1016/j.jhep.2022.01.004
25. O’Rourke, CJ, Salati, M, Rae, C, Carpino, G, Leslie, H, Pea, A, et al. (2024). Molecular portraits of patients with intrahepatic cholangiocarcinoma who diverge as rapid progressors or long survivors on chemotherapy. Gut 73:496–508. doi: 10.1136/gutjnl-2023-330748
26. Ramouz, A, Ali-Hasan-Al-Saegh, S, Shafiei, S, Fakour, S, Khajeh, E, Majlesara, A, et al. (2022). Repeat liver resection for recurrent intrahepatic cholangiocarcinoma: meta-analysis. Br J Surg 109:580–7. doi: 10.1093/bjs/znac075
27. Tsilimigras, DI, Sahara, K, Wu, L, Moris, D, Bagante, F, Guglielmi, A, et al. (2020). Very early recurrence after liver resection for intrahepatic Cholangiocarcinoma: considering alternative treatment approaches. JAMA Surg 155:823–31. doi: 10.1001/jamasurg.2020.1973
28. Wada, Y, Shimada, M, Yamamura, K, Toshima, T, Banwait, JK, Morine, Y, et al. (2021). A transcriptomic signature for risk-stratification and recurrence prediction in intrahepatic Cholangiocarcinoma. Hepatology 74:1371–83. doi: 10.1002/hep.31803
29. Alaimo, L, Moazzam, Z, Endo, Y, Lima, HA, Ruzzenente, A, Guglielmi, A, et al. (2023). Long-term recurrence-free and overall survival differ based on common, proliferative, and inflammatory subtypes after resection of intrahepatic Cholangiocarcinoma. Ann Surg Oncol 30:1392–403. doi: 10.1245/s10434-022-12795-4
30. Zhang, D, Zeng, H, Pan, Y, Zhao, Y, Wang, X, Chen, J, et al. (2022). Liver tumor markers, HALP score, and NLR: simple, cost-effective, easily accessible indexes for predicting prognosis in ICC patients after surgery. J Pers Med. 12:2041. doi: 10.3390/jpm12122041
31. Moazzam, Z, Alaimo, L, Endo, Y, Lima, HA, Ruzzenente, A, Guglielmi, A, et al. (2023). Combined tumor burden score and carbohydrate antigen 19-9 grading system to predict outcomes among patients with intrahepatic Cholangiocarcinoma. J Am Coll Surg 236:804–13. doi: 10.1097/XCS.0000000000000557
32. Dinorcia, J, Florman, SS, Haydel, B, Tabrizian, P, Ruiz, RM, Klintmalm, GB, et al. (2020). Pathologic response to Pretransplant Locoregional therapy is predictive of patient outcome after liver transplantation for hepatocellular carcinoma: analysis from the US multicenter HCC transplant consortium. Ann Surg 271:616–24. doi: 10.1097/SLA.0000000000003253
33. Cotter, G, Beal, EW, Poultsides, GA, Idrees, K, Fields, RC, Weber, SM, et al. (2022). Using machine learning to preoperatively stratify prognosis among patients with gallbladder cancer: a multi-institutional analysis. HPB (Oxford) 24:1980–8. doi: 10.1016/j.hpb.2022.06.008
34. Gavriilidis, P, and Pawlik, TM (2024). Inflammatory indicators such as systemic immune inflammation index (SIII), systemic inflammatory response index (SIRI), neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic factors of curative hepatic resections for hepatocellular carcinoma. Hepatobiliary Surg Nutr. 13:509–11. doi: 10.21037/hbsn-23-631
35. Johnson, PJ, Dhanaraj, S, Berhane, S, Bonnett, L, and Ma, YT (2021). The prognostic and diagnostic significance of the neutrophil-to-lymphocyte ratio in hepatocellular carcinoma: a prospective controlled study. Br J Cancer 125:714–6. doi: 10.1038/s41416-021-01445-3
36. Tsilimigras, DI, Mehta, R, Aldrighetti, L, Poultsides, GA, Maithel, SK, Martel, G, et al. (2020). Development and validation of a laboratory risk score (LabScore) to predict outcomes after resection for intrahepatic Cholangiocarcinoma. J Am Coll Surg 230:381–391e2. doi: 10.1016/j.jamcollsurg.2019.12.025
37. Xiong, J, Wang, Y, Xu, W, Liu, Z, Wang, H, Zhang, Z, et al. (2020). Proton pump inhibitors and odds of cholangiocarcinoma: a retrospective case-control study. Liver Int 40:2848–57. doi: 10.1111/liv.14663
38. Shen, H, Zhang, S, Xia, Y, Chen, C, Huo, L, Gan, L, et al. (2021). A nomogram in predicting risks of intrahepatic Cholangiocarcinoma after partial hepatectomy for Hepatolithiasis. J Gastrointest Surg 25:2258–67. doi: 10.1007/s11605-021-04947-w
39. Matsumori, T, Kodama, Y, Takai, A, Shiokawa, M, Nishikawa, Y, Matsumoto, T, et al. (2020). Hes1 is essential in proliferating ductal cell-mediated development of intrahepatic Cholangiocarcinoma. Cancer Res 80:5305–16. doi: 10.1158/0008-5472.CAN-20-1161
40. Job, S, Rapoud, D, dos Santos, A, Gonzalez, P, Desterke, C, Pascal, G, et al. (2020). Identification of four immune subtypes characterized by distinct composition and functions of tumor microenvironment in intrahepatic Cholangiocarcinoma. Hepatology 72:965–81. doi: 10.1002/hep.31092
41. Pascale, A, Rosmorduc, O, and Duclos-Vallee, JC (2023). New epidemiologic trends in cholangiocarcinoma. Clin Res Hepatol Gastroenterol 47:102223. doi: 10.1016/j.clinre.2023.102223
42. An, L, Zheng, R, Zhang, S, Chen, R, Wang, S, Sun, K, et al. (2023). Hepatocellular carcinoma and intrahepatic cholangiocarcinoma incidence between 2006 and 2015 in China: estimates based on data from 188 population-based cancer registries. Hepatobiliary Surg Nutr 12:45–55. doi: 10.21037/hbsn-21-75
43. Tsilimigras, DI, Hyer, JM, Paredes, AZ, Diaz, A, Moris, D, Guglielmi, A, et al. (2020). A novel classification of intrahepatic Cholangiocarcinoma phenotypes using machine learning techniques: An international multi-institutional analysis. Ann Surg Oncol 27:5224–32. doi: 10.1245/s10434-020-08696-z
Keywords: intrahepatic cholangiocarcinoma, lymphocyte count ratio, radical surgery, prognostic model, prediction
Citation: Qi S, Ma Z, Shen L, Wang J, Zhou L, Tian B, Liu C, Chen K and Cheng W (2024) Application of preoperative NLR-based prognostic model in predicting prognosis of intrahepatic cholangiocarcinoma following radical surgery. Front. Nutr. 11:1492358. doi: 10.3389/fnut.2024.1492358
Edited by:
Lucilla Crudele, University of Bari Aldo Moro, ItalyReviewed by:
Xin Long, University of Texas Southwestern Medical Center, United StatesYi Han, University of Texas Southwestern Medical Center, United States
Copyright © 2024 Qi, Ma, Shen, Wang, Zhou, Tian, Liu, Chen and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Cheng, Y2hlbmd3ZWlAaHVubnUuZWR1LmNu; Kang Chen, Y2hlbmthbmdAaHVubnUuZWR1LmNu; Changjun Liu, bGl1Y2hhbmdqdW5AaHVubnUuZWR1LmNu
 Shuo Qi
Shuo Qi Zhongzhi Ma
Zhongzhi Ma Kang Chen
Kang Chen Wei Cheng
Wei Cheng