- Department of Hepatobiliary Surgery, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China
Background: The relationship between dietary antioxidants and fatty liver disease remains a topic of debate. This study aimed to examine the association between the Composite Dietary Antioxidant Index (CDAI) and nonalcoholic fatty liver disease (NAFLD)/metabolic-associated fatty liver disease (MAFLD).
Methods: The study analyzed data from the 2003–2018 cycles of the National Health and Nutrition Examination Survey. The study included 16,321 individuals aged 20–85 years. Food and nutrient intake data were based on the 24-h recall method. Multivariate logistic regression models were employed to examine the relationship between CDAI and NAFLD/MAFLD.
Results: In the fully adjusted multivariate logistic regression model, CDAI demonstrated a significant negative correlation with NAFLD and MAFLD. Mediation analysis showed that inflammatory factors partially mediated the relationship between CDAI and NAFLD/MAFLD prevalence. The combination of high CDAI levels with effective physical activity was associated with a greater reduction in NAFLD/MAFLD prevalence than high CDAI levels alone.
Conclusion: Our study highlighted a negative association between CDAI and NAFLD/MAFLD, mediated by inflammatory factors. Additionally, participants with characteristics of active physical activity and high levels of CDAI were more strongly correlated with the reduced prevalence of NAFLD/MAFLD. Further research in clinical cohorts should be conducted to comprehensively investigate the impact of CDAI on NAFLD/MAFLD prevalence.
1 Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most common liver disease worldwide, with a global prevalence within the limits of 25–35% (1, 2), and is the first cause of chronic liver disease (3). The progression of NAFLD culminates in nonalcoholic steatohepatitis, which, in turn, can progress to fibrosis, cirrhosis, and ultimately hepatocellular carcinoma (4). In early 2020, several researchers proposed changing the term NAFLD to metabolic-associated fatty liver disease (MAFLD) to reflect a strong pathophysiological connection between steatosis and metabolic dysfunction (5). Despite the rising tide of the global burden of NAFLD/MAFLD, effective pharmacological agents are limited (6). Resmetirom is a selective thyroid hormone receptor-beta agonist that is orally active and targeted toward the liver, which is specifically developed to improve hepatic fat metabolism and reduce lipotoxicity (7). Of the more than 400 ongoing clinical trials, only resmetirom has been approved by FDA (8, 9).
NAFLD results from a multifaceted interplay of molecular factors, including lipid metabolism, inflammation, oxidative stress, and mitochondrial dysfunction (10). The current disease management plan depends mainly on lifestyle interventions (11). The holistic approach involving a balanced lifestyle encompassing diet, physical activity (PA), sleep, and other factors is recommended to prevent NAFLD/MAFLD (12, 13). Several different diet patterns, including the Mediterranean diet, ketogenic diet, low-calorie and low-fat diet have been considered as potential strategies for preventing and treating NAFLD/MAFLD (14–16). In these diet patterns, some antioxidants such as monounsaturated oils, fibers, and phytosterols have been found to help reduce liver steatosis (17). Relevant studies have confirmed that antioxidants such as vitamin E, and vitamin C in the diet are related to a decreased risk of NAFLD/MAFLD (18, 19).
Antioxidants play a crucial role in neutralizing the harmful impacts of reactive oxygen species (ROS) generated during normal cellular activities (20). Oxidative stress centrally contributes to hepatic steatosis, insufficient antioxidant intake may destroy the redox balance (21, 22). A case–control study in Iran indicated that a higher dietary total antioxidant capacity (DTAC) was associated with a lower risk of NAFLD (23). Similarly, Vahid et al. have found the dietary antioxidant index (DAI) calculated based on vitamins A, C, E, selenium, manganese, and zinc, is negatively related to the incidence of NAFLD (24). However, DTAC can be influenced by various factors such as food preparation and cooking methods, and DAI ignored some non-nutritive antioxidants such as carotenoids.
The Composite Dietary Antioxidant Index (CDAI) is a scoring system that represents the comprehensive profile of dietary antioxidant intake (including vitamins A, C, E, selenium, zinc, and carotenoids) developed by Wright et al. (25). It has been proposed to be associated with many human diseases, such as depression, hypertension, and osteoporosis (26–28). Two cross-sectional studies included similar participants from 2 cycles of the National Health and Nutrition Examination Survey (NHANES) database, have found CDAI is negatively associated with metabolic dysfunction-associated steatotic liver disease (MASLD) (29, 30). However, the dose–response relationship was not exactly consistent, moreover, the association between CDAI and NAFLD/MAFLD was not explored. Therefore, we carried out an observational study analyzing the NHANES database to delve into the potential association between CDAI and NAFLD/MAFLD.
2 Materials and methods
2.1 Study population
The NHANES is a cross-sectional survey evaluating the health and nutritional status of both adults and children in the US initiated by the Centers for Disease Control and Prevention. It has been updated every 2 years since 1999 and was approved by the National Center for Health Statistics Research Ethics Review Board. All participants provided informed consent (31). This study used eight NHANES 2-year cycles (2003–2018). This study follows the Strengthening the Reporting of Observational Studies in Epidemiology reporting guideline.
The exclusion criteria were as follows: (1) missing information about dietary data on the first day; (2) liver disease due to other causes (liver cancer, autoimmune hepatitis, hepatitis B, hepatitis C); (3) missing information about other variables such as age, sex, race, body mass index (BMI), education level, and smoking status; (4) participants with extreme energy intake (less than 800 kcal or more than 4,000 kcal for male, less than 500 kcal or more than 3,500 kcal for female); (5) participants under 20 years of age. Supplementary Figure 1 shows the details of the inclusion and exclusion processes used in this study.
2.2 Calculation of CDAI
The food and nutrient intake data of the NHANES participants were acquired through a 24-h dietary recall interview. The initial dietary recall occurred in person at a mobile inspection center, and the second was conducted 3–10 days later via telephone. The Food and Nutrient Database for Dietary Studies of the United States Department of Agriculture was used to calculate the nutritional components in foods (32). The CDAI composed of six vitamins and minerals (vitamins A, C, E, carotenoids, selenium, and zinc), was calculated as follows:
In this formula, xi is the daily intake of antioxidants, μi is the mean of xi, and Si is the standard deviation of μi.
2.3 Assessment of NAFLD and MAFLD
NAFLD is a pathological syndrome characterized by abnormal fat accumulation in the liver, defined as any degree of steatosis excluding patients resulting from excessive alcohol consumption or other liver diseases such as viral hepatitis, autoimmune hepatitis, and liver cancer (33). Excessive alcohol consumption was defined as an average of >20 g/day for males and >10 g/day for females (34). MAFLD was defined based on hepatic steatosis and any of the three criteria listed below: overweight/obese (BMI ≥ 25 kg/m2), type 2 diabetes, or metabolic dysregulation. Metabolic dysregulation is defined as the presence of at least two of the following: (1) waist circumference ≥102 cm for male and ≥88 cm for female; (2) blood pressure ≥130/85 mmHg or on anti-hypertensive therapy; (3) plasma triglycerides ≥1.70 mmol/L or on lipid-lowering therapy; (4) serum high-density lipoprotein cholesterol <1.0 mmol/L for male and <1.3 mmol/L for female or specific drug treatment; (5) diabetes or pre-diabetes (fasting glucose level 5.6–6.9 mmol/L, or 2-h post-load glucose level 7.8–11.0 mmol or HbA1c 5.7–6.4%); (6) serum hypersensitivity C-reactive protein level >2 mg/L; (7) homeostasis model assessment insulin resistance score ≥2.5 (35).
Serological-based noninvasive tests, such as the Fatty Liver Index (FLI) and United States Fatty Liver Index (USFLI), are widely embraced in clinical settings as surrogate markers for stratifying the risks associated with hepatic steatosis (9, 36). The FLI and USFLI were calculated as follows:
In this formula, “Non-Hispanic Black” and “Mexican American” have a value of 1 if the participant is of that ethnicity and 0 if not of that ethnicity (37). Subjects were defined as having hepatic steatosis if their FLI score ≥ 60 or their USFLI score ≥ 30 (38).
2.4 Measurement of inflammatory biomarkers
In this study, the inflammatory biomarkers of the subjects participating in the examination were white blood cell (WBC) count, neutrophils count, lymphocytes count, and C-reactive protein. According to the NHANES 2003–2018 cycle protocol, the Beckman Coulter method of counting and sizing, in combination with an automatic diluting and mixing device for sample processing, was applied to count blood cells from the peripheral blood samples obtained from the NHANES Mobile Examination Center. For the processing of C-reactive protein, latex-enhanced nephelometry with particle-enhanced assays was used for quantitation.
2.5 Covariates
The demographic variables included age (years); sex (male/female); race (Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, or other races, including Multiracial); education level (<high school, ≥high school); and poverty income ratio (low level, middle level, high level). The questionnaire data included recreational activity level (vigorous activities, moderate activities, and no activities. Vigorous activities were defined as activities that cause large increases in breathing or heart rate like running or basketball for at least 10 min continuously. Moderate activities were defined as activities that cause a small increase in breathing or heart rate such as brisk walking, bicycling, swimming, or volleyball for at least 10 min continuously), and active PA was defined as vigorous or moderate activities. Smoking status (never smoker, former smoker, current smoker).
2.6 Statistical analysis
To minimize the potential biases, after excluding data with missing covariates, baseline demographic characteristics between included and excluded groups were compared using standardized differences (differences <10% were regarded as negligible) (39) (Supplementary Table 1). The baseline characteristics of all included participants were described by median values and interquartile range (IQR) (continuous variables; expressed as the median [IQR]) or proportions (categorical variables; expressed as N [%]). Baseline characteristics were compared using an independent sample t-test for continuous variables and the χ2 test for categorical variables. Considering the intricate probability cluster design of the NHANES, all the statistical analyses in this study incorporated weights. Weighted multivariable-adjusted logistic regression was employed to investigate the association between CDAI and NAFLD/MAFLD, with results presented as odds ratios (OR) and 95% confidence intervals (CI). As recommended, covariates were selected for multivariable analyses based on our causal understanding of the existing literature rather than the statistical criteria (40). Model 1 lacked confounder adjustments; Model 2 was adjusted for demographic covariates (age, gender and race); Model 3 was further adjusted for family income, education level, smoking status, activity level, and energy intake, as these covariates have been regarded as the risk factors for hepatic steatosis (41–43). The results were validated by these different models to ensure the robustness of the findings.
Multivariate-adjusted restricted cubic spline (RCS) analysis and two-piecewise linear regression for threshold effect analysis were utilized to explore the nonlinear relationship. RCS is a flexible nonlinear model that accurately represents interactions and varying rates of change, then threshold effect analysis was conducted to identify potential cut-off points or thresholds. It is noted that the number of knots was selected based on the Akaike’s information criterion (AIC), with the lowest AIC values indicating the best-fitted model. Likelihood ratio test was used to test the overall significance of the two-piecewise linear regression model. Mediation analysis was conducted to assess whether inflammatory factors mediated the association between CDAI and NAFLD/MAFLD prevalence, providing statistical support for mechanistic analysis. Stratified analyses were conducted to assess potential moderating effects of age, sex, race, family income, education level, smoking status, and activity level. Weighted multivariable-adjusted logistic regression assessed joint associations of CDAI and PA with NAFLD/MAFLD. Then, the population attributable fraction (PAF) was used to estimate the proportion of NAFLD/MAFLD which could be avoided if exposure (CDAI and/or PA) were eliminated. The PAF was widely used to measure the disease burden attributable to a given risk factor, and was calculated by the “AF” package in R software (44). All the data analyses were performed with R software (version 4.2.0). p < 0.05 indicated statistical significance.
3 Results
3.1 General characteristics of NHANES
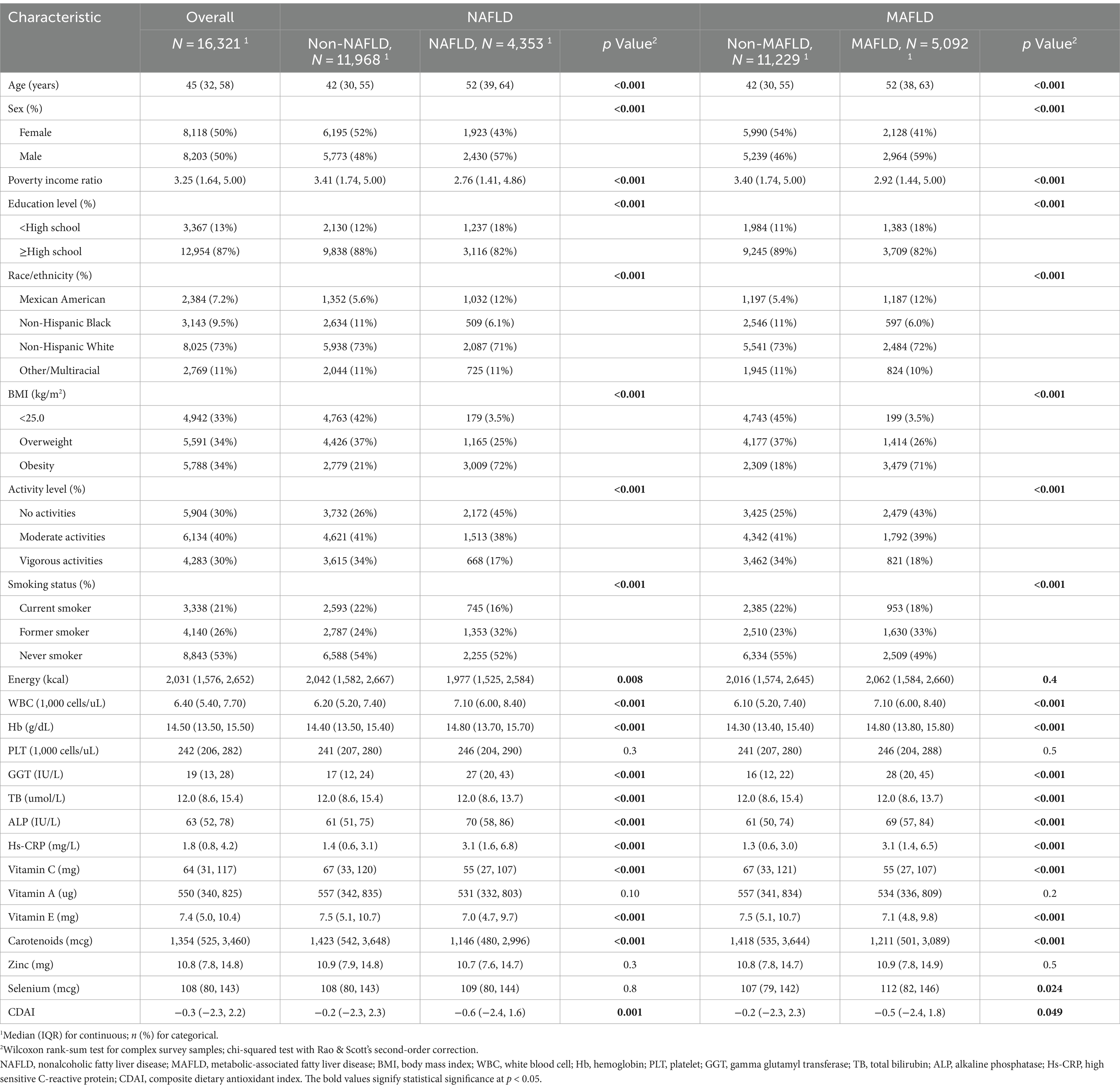
Among 16,321 eligible adults with complete data on CDAI and NAFLD/MAFLD, the mean (IQR) age was 45 (32, 45) years, and 8,118 (50%) were female; 2,384 (7.2%) of participants were Mexican American, 3,143 (9.5%) of participants were Non-Hispanic Black, 8,025 (73%) of participants were Non-Hispanic White, and 2,769 (11%) of participants identified as other race or ethnicity; 5,591 (34%) were defined as overweight and 5,788 (34%) were defined as obesity. Among the included participants, 4,353 (27%) were defined as NAFLD, and 5,092 (31%) were defined as MAFLD. The baseline characteristics compared by NAFLD/MAFLD are shown in Table 1. Participants with NAFLD/MAFLD were more likely to be older, male, had lower income, less educated, more likely to smoke, engaged in less activity, and had lower CDAI levels. The characteristics of study population according to quartiles of CDAI were shown in Supplementary Table 2.
3.2 Association of CDAI and its components with the risk of NAFLD/MAFLD
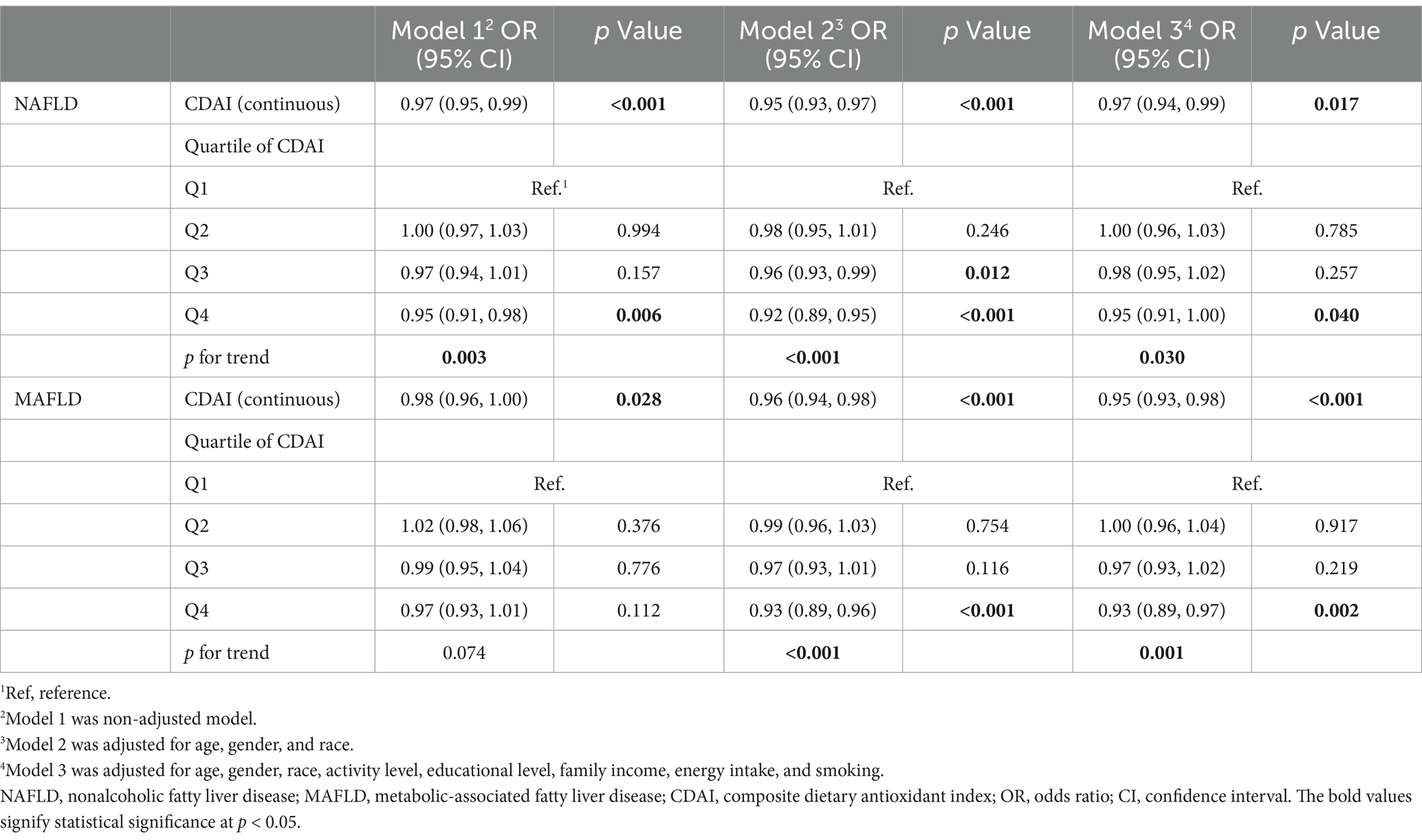
The weighted multivariable logistic regression models were performed to investigate the association between CDAI with NAFLD/MAFLD. As shown in Table 2, after adjusting for multiple variables in model 3, the OR for NAFLD was 0.95 (95% CI, 0.91–1.00, p = 0.040) in the fourth quartile of CDAI compared to the reference group (p for trend = 0.030). Similarly, compared to the reference group, the fourth quartile of CDAI (OR, 0.93; 95% CI, 0.89–0.97, p = 0.002) was significantly related to the decreased prevalence of MAFLD. These results were robust across all models.
Additional analyses were carried out to explore the association between six components of CDAI and NAFLD/MAFLD. Supplementary Table 3 reveals a negative relationship between log vitamin E (OR, 0.55; 95% CI, 0.38–0.79, p = 0.001) and log carotenoids (OR, 0.76; 95% CI, 0.65–0.88, p < 0.001) with NAFLD. Similarly, log vitamin C (OR, 0.81; 95% CI, 0.69–0.95, p = 0.012) and log vitamin E (OR, 0.46; 95% CI, 0.33–0.64, p < 0.001) were negatively associated with MAFLD (Supplementary Table 4). Multivariate adjusted RCS demonstrated a decreased risk of NAFLD/MAFLD with the intake of log vitamin C, log vitamin E, log carotenoids, and log zinc (Supplementary Figures 2, 3).
3.3 RCS curve plotting and threshold effect analysis
Figure 1 exhibits the dose–response relationships between CDAI and NAFLD/MAFLD. After multivariable adjustment, significant nonlinear associations were observed between CDAI and MAFLD (p for nonlinear = 0.002) using restricted cubic splines (p for overall <0.001). However, there was no significant nonlinear relationship between CDAI and NAFLD (p for nonlinear = 0.136; p for overall <0.001). A threshold effect analysis of CDAI on NAFLD/MAFLD was further performed by the two-piecewise linear regression. As shown in Supplementary Table 5, for MAFLD, the inflection point of CDAI was 2.792. Each unit increase of CDAI correlated with a 3% decrease of the risk of MAFLD below 2.792, with no statistically significant relationship above this threshold. However, the threshold effect for NAFLD was not significant (P for log-likelihood ratio > 0.05).
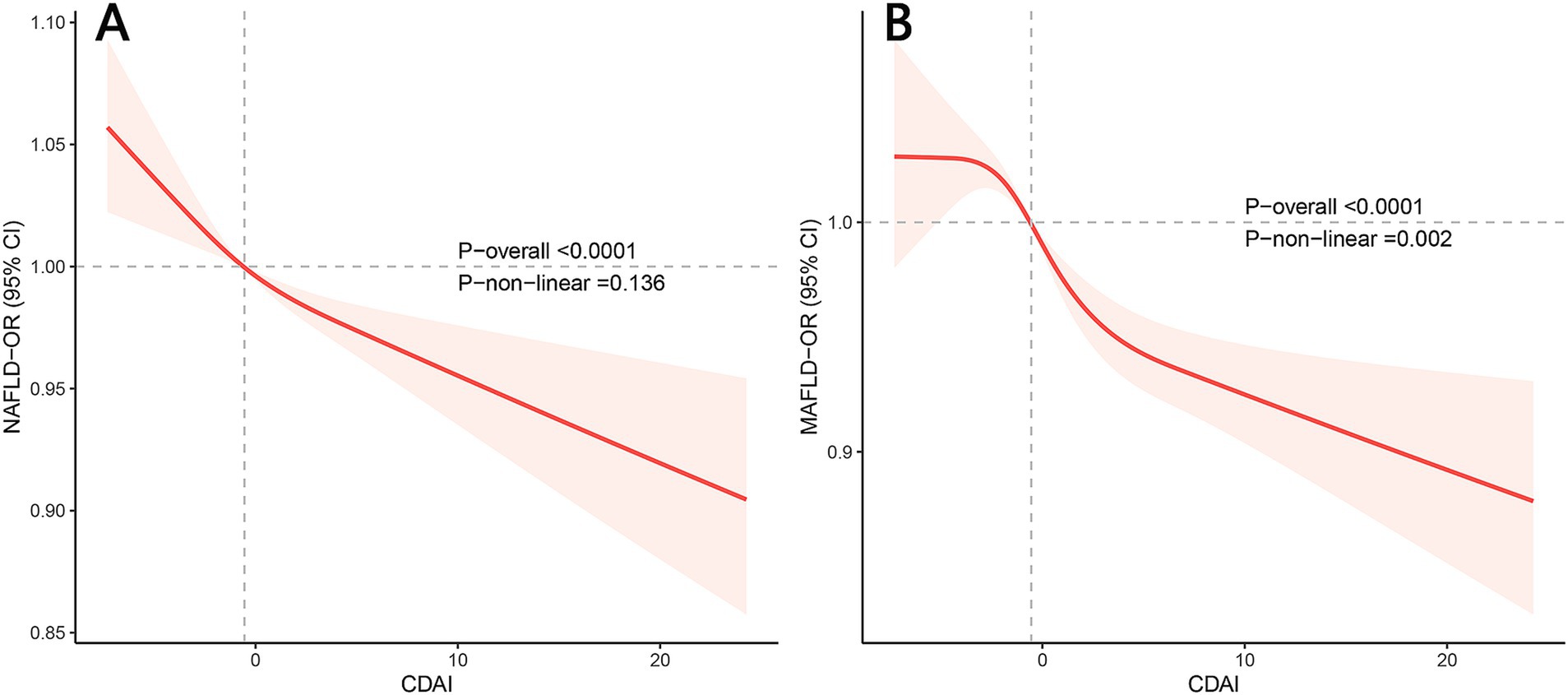
Figure 1. Restricted cubic spline plots of the association between CDAI and NAFLD/MAFLD prevalence. (A) NAFLD; (B) MAFLD. Adjusted for age, gender, race, activity level, educational level, family income, energy intake, and smoking. OR, odds ratio; CI, confidence interval; NAFLD, nonalcoholic fatty liver disease; MAFLD, metabolic-associated fatty liver disease; CDAI, composite dietary antioxidant index.
3.4 Mediation by inflammatory biomarkers
A significant negative relationship between CDAI and inflammatory biomarkers was found by the weighted multiple linear regression model (Supplementary Table 6). Mediation analyses were conducted to explore the mediating effect of inflammatory biomarkers. As shown in Figure 2, all four inflammatory biomarkers significantly mediated the association between CDAI and NAFLD. Specifically, CDAI reduced the prevalence of NAFLD by lowering the levels of these inflammatory factors, with WBC, neutrophils, lymphocytes, and C-reactive protein accounted for 20.9, 12.8, 11.5, and 10.2% of the association, respectively (all p for mediation <0.05). A similar pattern was observed for MAFLD, with WBC, neutrophils, lymphocytes, and C-reactive protein explaining 22.6, 13.5, 13.2, and 10.2% of the association, respectively (all p for mediation <0.05) (Figure 3).
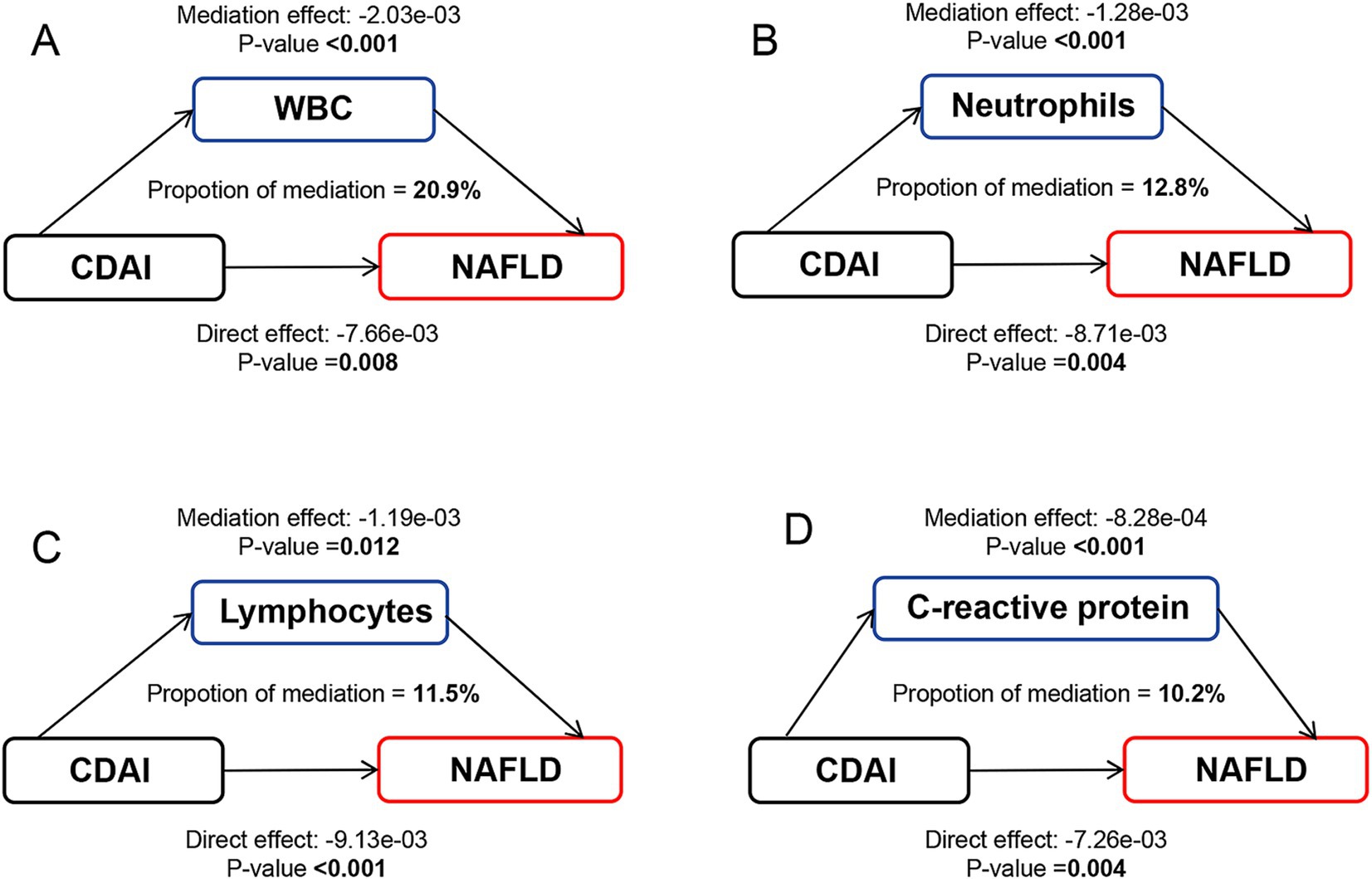
Figure 2. Path diagram of the mediation analysis of inflammatory biomarkers on the relationship between CDAI and NAFLD. The graphs in panel (A–D) represented the mediating role of WBC, neutrophils, lymphocytes, and C-reactive protein, respectively. NAFLD, nonalcoholic fatty liver disease; CDAI, composite dietary antioxidant index; WBC, white blood cell.
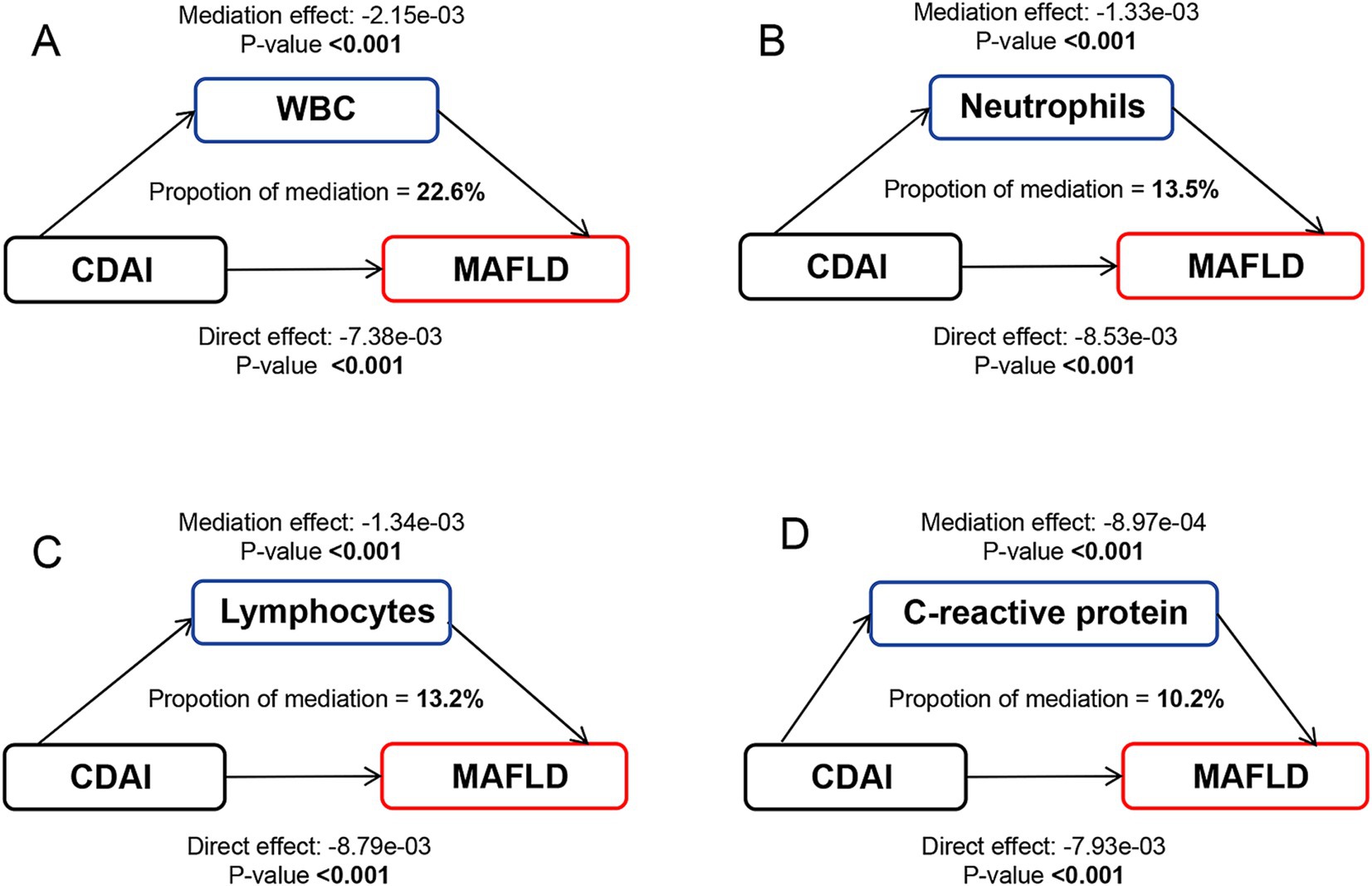
Figure 3. Path diagram of the mediation analysis of inflammatory biomarkers on the relationship between CDAI and MAFLD. The graphs in panel (A–D) represented the mediating role of WBC, neutrophils, lymphocytes, and C-reactive protein, respectively. MAFLD, metabolic-associated fatty liver disease; CDAI, composite dietary antioxidant index; WBC, white blood cell.
3.5 Stratified analysis
Stratified analysis by age, sex, race, family income, education level, smoking status, and activity level is shown in Figure 4. The results showed a stable negative association between CDAI and the prevalence of NAFLD/MAFLD in most populations (p for interaction >0.05). However, we found a significant interaction between CDAI and activity level with the risk of NAFLD/MAFLD (p for interaction <0.05), suggesting that these associations were more pronounced among vigorous activity participants.
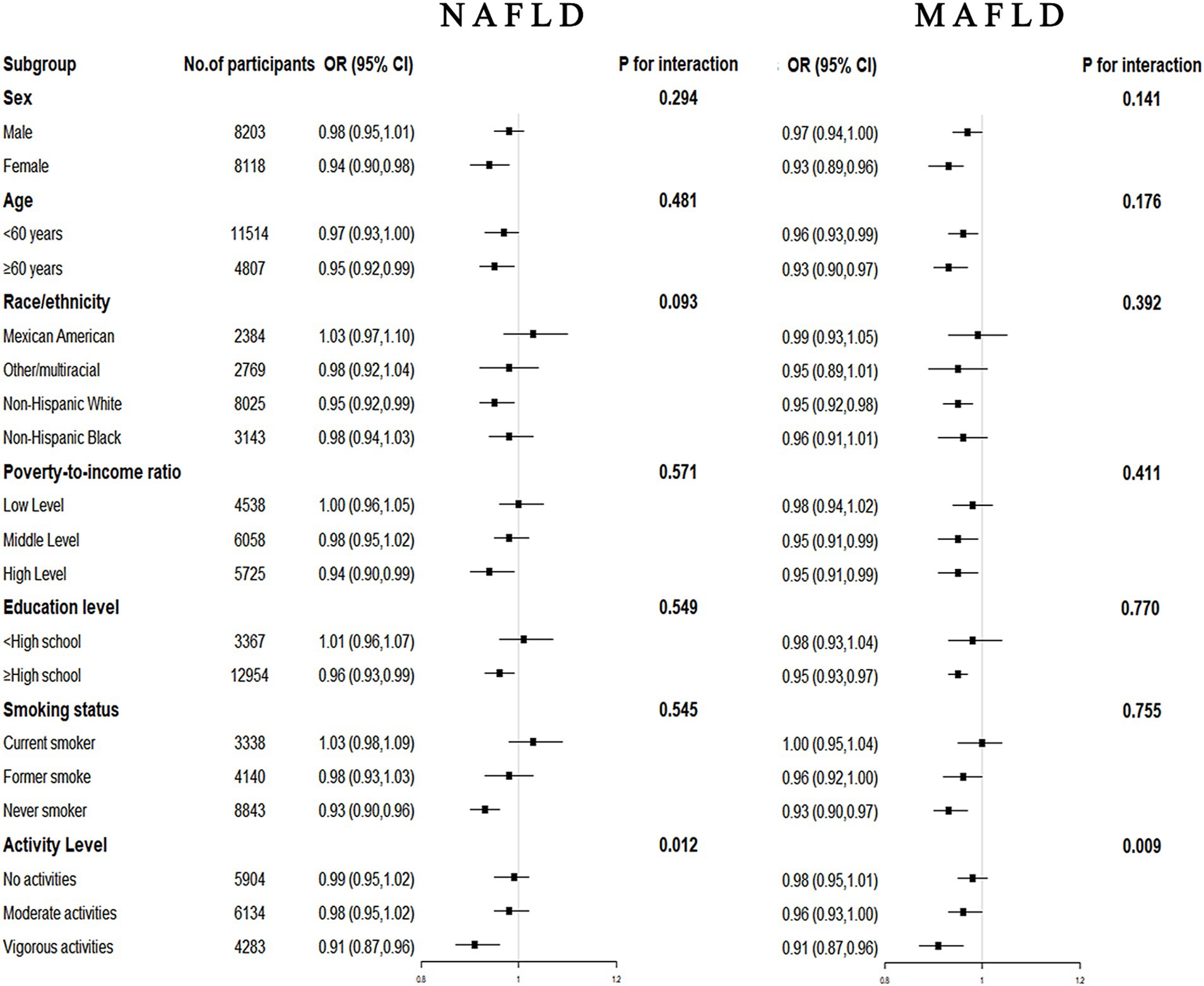
Figure 4. Forest plots for subgroup analysis. Subgroup analysis was stratified by age, sex, race, family income, education level, smoking status, and activity level. OR, odds ratio; CI, confidence interval; NAFLD, nonalcoholic fatty liver disease; MAFLD, metabolic-associated fatty liver disease.
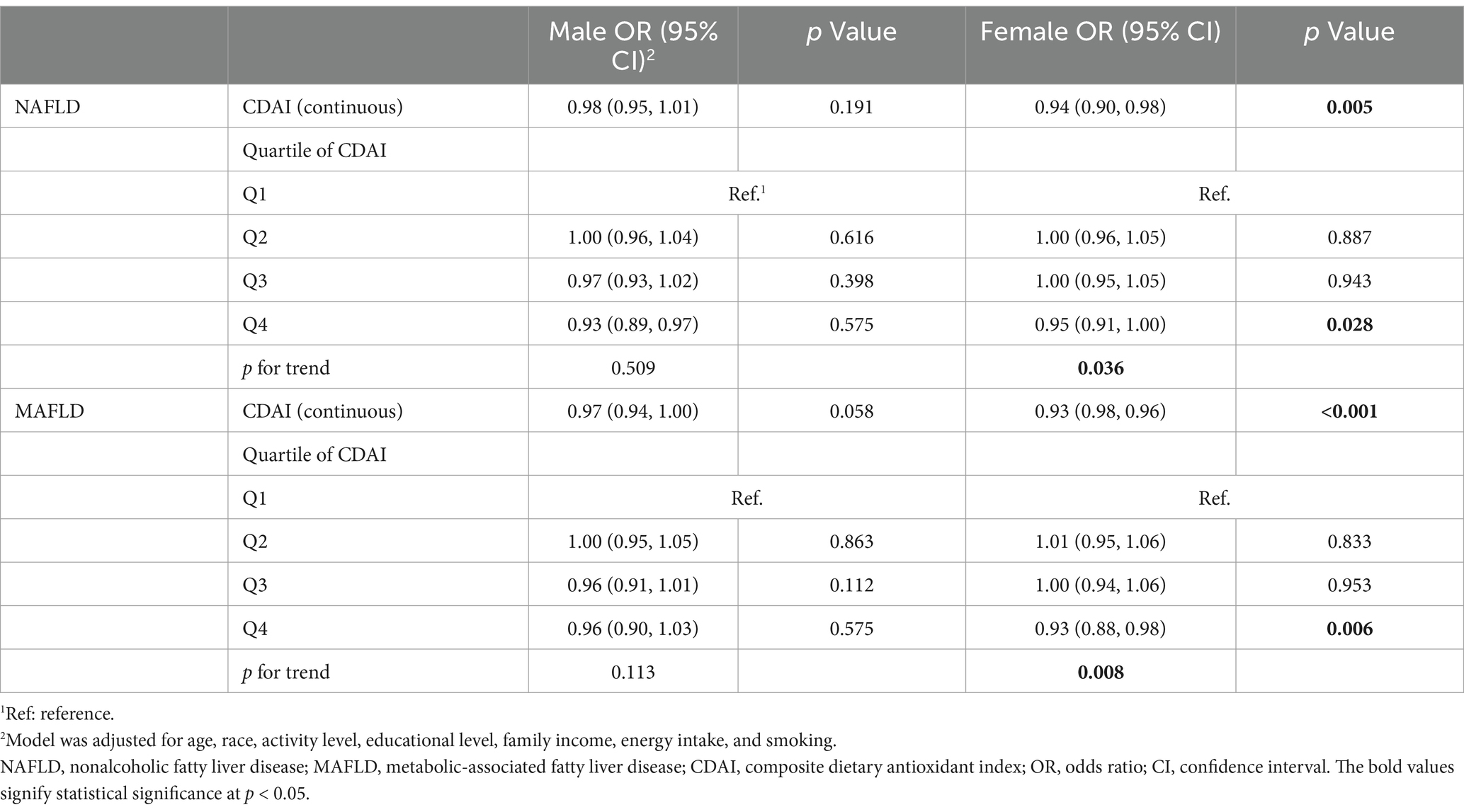
The total participants were further divided into two groups in each sex and performed weighted multivariable-adjusted logistic regression. As shown in Table 3, in females, CDAI was associated with the risk of NAFLD (OR, 0.94; 95% CI, 0.90–0.98, p = 0.005) and MAFLD (OR, 0.93; 95% CI, 0.98–0.96, p < 0.001) after adjusting for age, race, activity level, educational level, family income, energy intake, and smoking. However, the similar relationship was not significant in males.
3.6 Joint association and PAF of CDAI and PA status with NAFLD/MAFLD
Since the interaction between CDAI and activity level with the risk of NAFLD/MAFLD was discovered, joint analysis was carried out. As shown in Figure 5 and Supplementary Table 7, participants with low levels of CDAI and inadequate PA had the highest risk of NAFLD/MAFLD in the fully adjusted model. Compared to the combination of low levels of CDAI and inadequate PA, the OR for NAFLD and MAFLD in the groups of low levels of CDAI and adequate PA were both 0.90 (95% CI, 0.87–0.93). Meanwhile, when compared with other groups, participants with high CDAI and adequate PA had the lowest risks of NAFLD (OR, 0.86; 95% CI, 0.83–0.89) and MAFLD (OR, 0.83; 95% CI, 0.80–0.86).
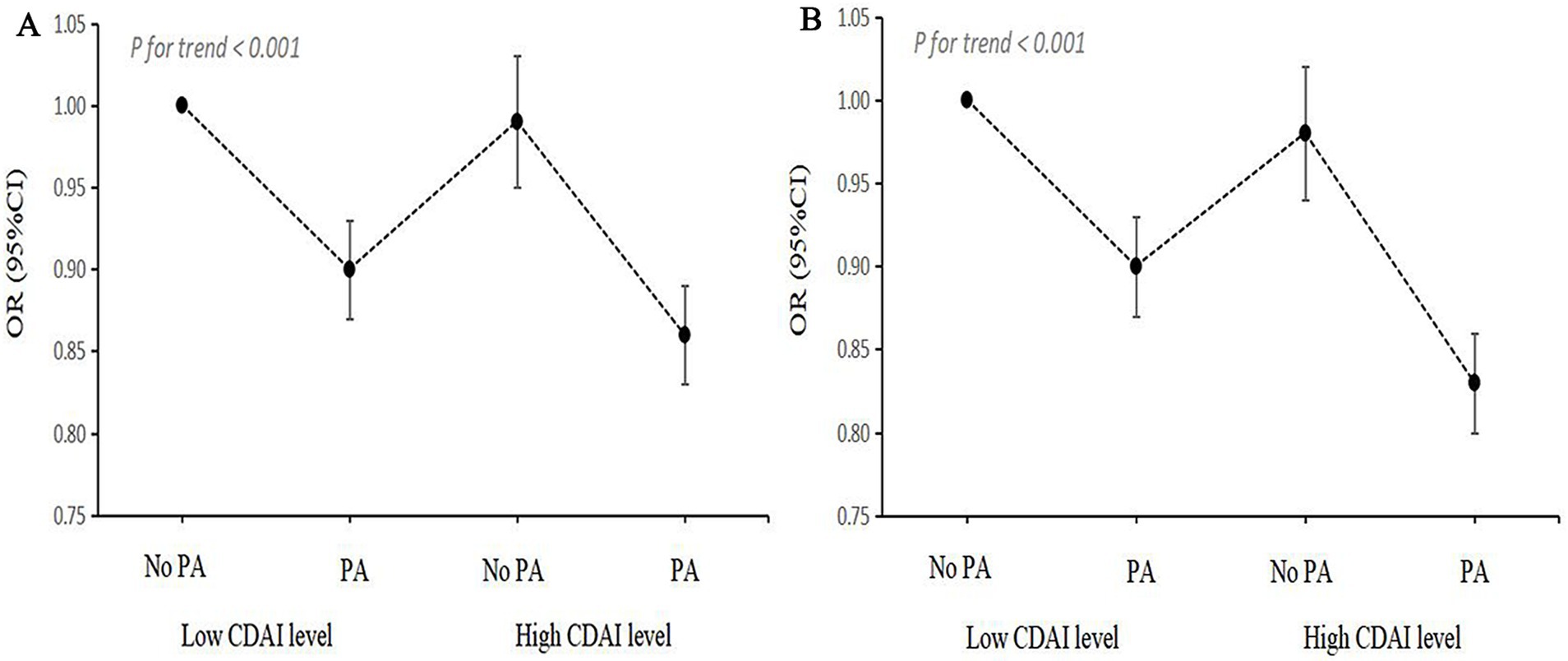
Figure 5. Joint association of CDAI levels and PA status with NAFLD/MAFLD. (A) NAFLD; (B) MAFLD. OR, odds ratio; CI, confidence interval; NAFLD, nonalcoholic fatty liver disease; MAFLD, metabolic-associated fatty liver disease; CDAI, composite dietary antioxidant index; PA, physical activity.
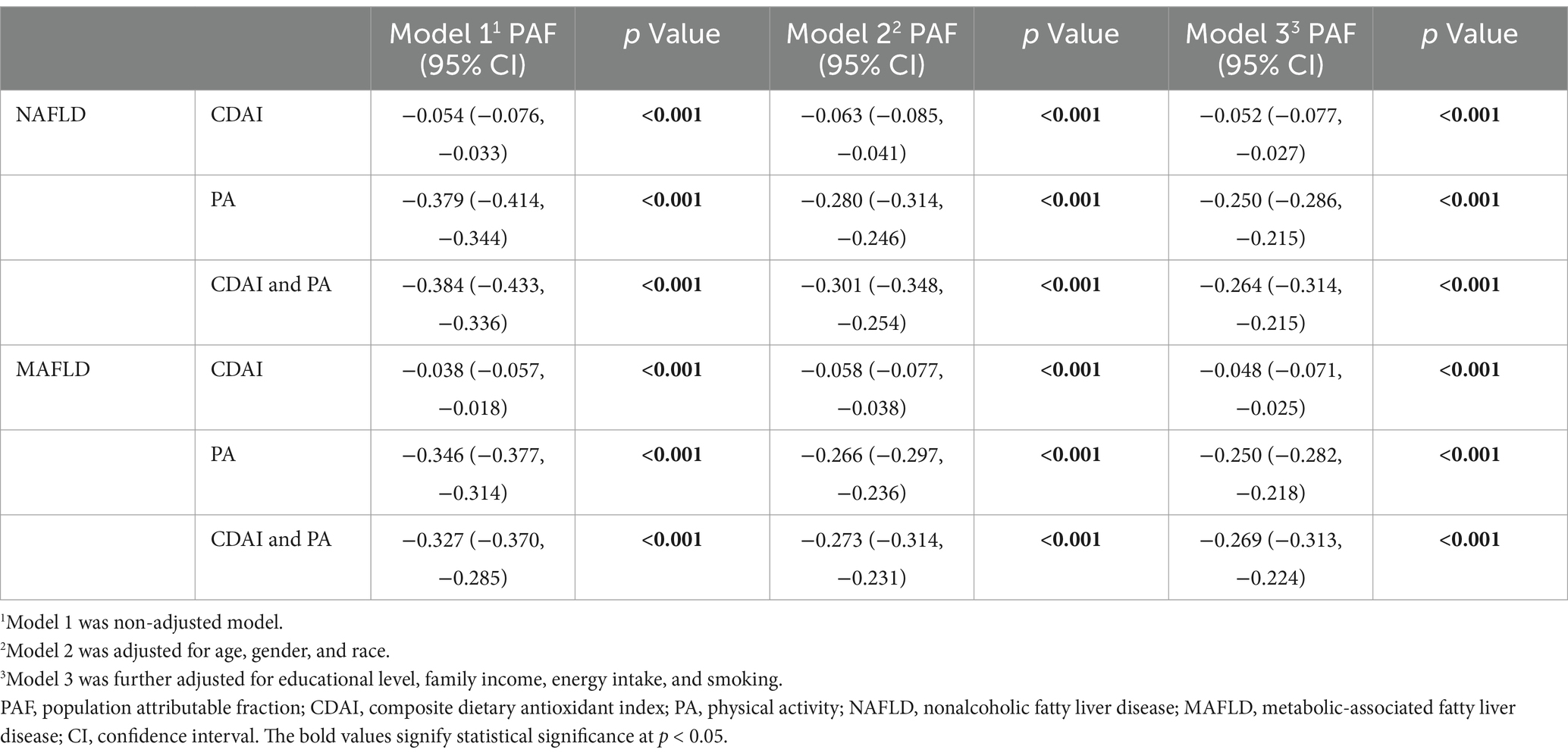
PAF analysis was performed to estimate the proportion of NAFLD/MAFLD risk among participants that could be mitigated by eliminating low CDAI and/or PA deficiency. As shown in Table 4, 5.2% of the reduction in NAFLD was attributed to high CDAI levels, while 25.0% was linked to adequate PA. Additionally, 26.4% of the reduction in NAFLD was associated with both high CDAI levels and adequate PA. For MAFLD, 4.8% of the reduction was attributed to high CDAI levels, 25.0% to adequate PA, and 26.9% to both high CDAI levels and adequate PA.
4 Discussion
In this study, we utilized the nationally representative NHANES 2003–2018 dataset to investigate the relationship between CDAI and NAFLD/MAFLD. Our findings indicate that high CDAI levels are significantly associated with NAFLD/MAFLD prevalence, as evidenced by a 0.97-fold and a 0.95-fold decrease in the odds of NAFLD/MAFLD, respectively. Additionally, some inflammatory biomarkers, such as WBC, neutrophils, lymphocytes, and C-reactive protein, mediate this relationship. The relationship between CDAI and NAFLD/MAFLD is complex, influenced by numerous factors including age, sex, race, family income, PA, educational level, and smoking. Our findings reveal that combining effective PA with high CDAI levels is more effective in reducing the prevalence of NAFLD/MAFLD than high CDAI levels alone. These findings underscore the complex effects of dietary antioxidants on NAFLD/MAFLD and could guide future research that may inform subsequent clinical guidelines.
Dietary constituents play pivotal roles in NAFLD/MAFLD development. Prior studies on the effect of dietary antioxidants on NAFLD/MAFLD have been conducted, but significant controversy remains. Several studies have shown that vitamin A has a significant protective effect on the development of NAFLD (46, 47). A similar inverse association was also observed among people who had higher dietary vitamin C levels (48). The potential mechanism may be that higher vitamin C intake can restore gut-liver functions and antioxidant status (49). However, a retrospective study from Italy showed that the ingestion of vitamins A and C was greater in patients with nonalcoholic steatohepatitis than in controls (50). For vitamin E, controversies also persist across various studies. Some studies have suggested that vitamin E may improve the progression of NAFLD (47, 51). However, others contend that it has no significant impact on this disease (52). Minerals are also crucial in NAFLD/MAFLD development. A case–control study reported significantly lower zinc intake in NAFLD patients compared to controls (53), while this relationship was considered to be non-significant in a meta-analysis (54). Similarly, the impact of selenium on NAFLD remains inconclusive (55, 56). Some researches thought the selenoprotein produced by the liver may play a role in hepatic steatosis (57). Our analysis shows a negative association between vitamin E levels and NAFLD/MAFLD prevalence, while selenium levels are positively associated with the risk of NAFLD/MAFLD.
The dietary composition of the diet is multifaceted, and focusing solely on a single vitamin or mineral intake may not yield substantial benefits. CDAI is a comprehensive assessment of the overall impact of dietary antioxidants, but related research remains limited. Previous studies have investigated the effects of CDAI on the prevalence of MASLD with three-year sample sizes, whereas our study involved 16-year participants (29, 30). Both studies reported a negative relationship. However, Zhang et al. found that a high intake of CDAI was beneficial for decreasing prevalence in MASLD in a linear manner, while Yang et al. found a non-linear relationship. One reason for this difference may be the important role of PA in this relationship, PA was included as a covariate by Zhang et al., while it was not considered in another study. It is noteworthy that regular active PA has been shown to have beneficial effects on NAFLD development (58). Notably, A “triple-hit behavioral phenotype” has been introduced recently, highlighting the joint effects of sedentary behavior, low PA, and poor diet on NAFLD (45). Our findings on the joint association of CDAI and PA consolidate this phenotype, that compared to high CDAI levels alone, combining effective PA is significantly associated with more reduction in NAFLD/MAFLD prevalence. Our findings carry out a new conception to the research on the association between diet and NAFLD/MALD, emphasizing the necessity for further exploration into the joint association of diet and PA.
The etiology of NAFLD involves both genetic and environmental factors, and early studies suggested that the “double-hit” hypothesis can explain the development of this disease. Insulin resistance-induced hepatic steatosis occurs as the “first hit,” followed by oxidative stress-induced hepatocellular injury as the “second hit” (59). The “multi-hit” hypothesis, currently considered a more accurate explanation for the pathogenesis of NAFLD/MAFLD, encompasses lipotoxicity, mitochondrial dysfunction, oxidative stress, endoplasmic reticulum stress, and inflammatory cytokines (60). Oxidative stress is defined as an imbalance between cellular antioxidants and pro-oxidants, including ROS and reactive nitrogen species, resulting in cellular damage and leading to cell death (61). A strong association existed between NAFLD/MAFLD and oxidative stress levels. Upon oxidative stress, excessive ROS are produced, subsequently damaging the respiratory chain and causing oxidative harm to the mitochondrial genome in hepatocytes, which also leads to the production of additional ROS, thus resulting in a vicious cycle (62). Some studies have demonstrated that numerous natural products, which possess antioxidant and anti-inflammatory properties, may potentially provide preventive effects against hepatic steatosis in NAFLD (63). The association noted with dietary antioxidants and NAFLD in our study is also consistent with prior studies, importantly, the present study revealed a mediate effect of inflammatory biomarkers such as WBC, neutrophils, lymphocytes, and C-reactive protein. These results reinforce the evidence suggesting that oxidative stress and inflammatory play as crucial role in the pathogenesis of NAFLD/MAFLD. However, while inflammatory markers were found to partially mediate the association between CDAI and the disease, the specific biological mechanisms still require further in-depth investigation.
In addition, the subgroup analysis indicated a significant different effect between CDAI and NAFLD/MAFLD, suggesting that the protective effect of higher levels of CDAI concentration on NAFLD/MAFLD only in females and not males. Similarly, Yang et al. found that the relationship between CDAI and MASLD particularly significant in females (29). This may be attributed to the fact that sex hormones are influencing factors of NAFLD (64). Estrogen significantly influences the mitochondrial respiratory chain by regulating mitochondrial gene transcription. Meanwhile, excessive accumulation of estrogen can lead to increased generation of reactive oxygen species, exacerbating mitochondrial DNA mutations and damaging mitochondrial proteins (65). These findings support our findings regarding gender grouping and highlight the importance of increasing dietary antioxidant intake.
There are several strengths to our study. First, we ensured a sufficiently large sample size based on the NHANES database, using a complex multi-stage probability sampling methodology, ensuring the accurate representation of the non-institutionalized population and enhancing the generalizability of our findings. Secondly, we elucidated the mediating role of inflammatory biomarkers. More importantly, we observed a combined effect of CDAI and PA with NAFLD/MAFLD, underscoring the significance of both diet and PA in NAFLD/MAFLD managing. However, there are several limitations. First, self-reported recall bias concerning the CDAI might be present in observational studies. Second, there may also be confounding factors such as comorbidities not included in the study, which may have important potential impacts on the conclusions. Finally, as the data are limited to the US population, the results may exhibit bias and cannot be extrapolated to other demographic factors. Given the inherent constraints of cross-sectional studies, further validation from evidence-based research such as randomized controlled trials and longitudinal studies is warranted. Meanwhile, as the different effect of CDAI according to gender, further researches are needed to investigate the potential importance of estrogen in this relationship.
5 Conclusion
In summary, the current findings demonstrate that CDAI is negatively associated with NAFLD/MAFLD, with inflammation identified as a mediating factor. In addition, participants with characteristics of adequate PA and high levels of CDAI were more strongly correlated with the reduced prevalence of NAFLD/MAFLD. Therefore, promoting a dietary pattern rich in antioxidants combined with adequate physical activity may be an appropriate strategy for healthcare professionals to alleviate the burden and prevalence of NAFLD/MAFLD. Our study underscores the significance of both CDAI and PA in NAFLD/MAFLD managing, thereby offering valuable insight into this field. Additional studies in clinical cohorts are needed to confirm these findings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the National Center for Health Statistics Research Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LG: Conceptualization, Methodology, Visualization, Writing – original draft. HF: Data curation, Formal analysis, Methodology, Writing – original draft. ZZ: Conceptualization, Methodology, Writing – original draft. WL: Formal analysis, Methodology, Software, Writing – original draft. JiG: Supervision, Validation, Writing – review & editing. JuG: Conceptualization, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors thank the participants and investigators of the NHANES databases.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1486700/full#supplementary-material
Abbreviations
NAFLD, Nonalcoholic fatty liver disease; MAFLD, Metabolic-associated fatty liver disease; PA, physical activity; ROS, Reactive oxygen species; DTAC, dietary total antioxidant capacity; DAI, dietary antioxidant index; CDAI, Composite Dietary Antioxidant Index; NHANES, National Health and Nutrition Examination Survey; MASLD, metabolic dysfunction-associated steatotic liver disease; BMI, Body mass index; FLI, Fatty Liver Index; USFLI, United States Fatty Liver Index; WBC, white blood cell; IQR, interquartile range; OR, Odds ratios; CI, Confidence intervals; RCS, restricted cubic spline; AIC, Akaike’s information criterion; PAF, population attributable fraction.
References
1. Younossi, Z, Anstee, QM, Marietti, M, Hardy, T, Henry, L, Eslam, M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. (2018) 15:11–20. doi: 10.1038/nrgastro.2017.109
2. Osorio-Conles, Ó, Vega-Beyhart, A, Ibarzabal, A, Balibrea, JM, Graupera, I, Rimola, J, et al. A distinctive NAFLD signature in adipose tissue from women with severe obesity. Int J Mol Sci. (2021) 22:10541. doi: 10.3390/ijms221910541
3. Torres-Peña, JD, Arenas-de Larriva, AP, Alcala-Diaz, JF, Lopez-Miranda, J, and Delgado-Lista, J. Different dietary approaches, non-alcoholic fatty liver disease and cardiovascular disease: a literature review. Nutrients. (2023) 15:1483. doi: 10.3390/nu15061483
4. Calabrese, FM, Disciglio, V, Franco, I, Sorino, P, Bonfiglio, C, Bianco, A, et al. A low glycemic index Mediterranean diet combined with aerobic physical activity rearranges the gut microbiota signature in NAFLD patients. Nutrients. (2022) 14:1773. doi: 10.3390/nu14091773
5. Eslam, M, Newsome, PN, Sarin, SK, Anstee, QM, Targher, G, Romero-Gomez, M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. (2020) 73:202–9. doi: 10.1016/j.jhep.2020.03.039
6. Kosmalski, M, Frankowski, R, Ziółkowska, S, Różycka-Kosmalska, M, and Pietras, T. What’s new in the treatment of non-alcoholic fatty liver disease (NAFLD). J Clin Med. (2023) 12:1852. doi: 10.3390/jcm12051852
7. Harrison, SA, Bashir, MR, Guy, CD, Zhou, R, Moylan, CA, Frias, JP, et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet (London, England). (2019) 394:2012–24. doi: 10.1016/S0140-6736(19)32517-6
8. Harrison, SA, Bedossa, P, Guy, CD, Schattenberg, JM, Loomba, R, Taub, R, et al. A phase 3, randomized, controlled trial of Resmetirom in NASH with liver fibrosis. N Engl J Med. (2024) 390:497–509. doi: 10.1056/NEJMoa2309000
9. Meffert, PJ, Baumeister, SE, Lerch, MM, Mayerle, J, Kratzer, W, and Völzke, H. Development, external validation, and comparative assessment of a new diagnostic score for hepatic steatosis. Am J Gastroenterol. (2014) 109:1404–14. doi: 10.1038/ajg.2014.155
10. Nie, Z, Xiao, C, Wang, Y, Li, R, and Zhao, F. Heat shock proteins (HSPs) in non-alcoholic fatty liver disease (NAFLD): from molecular mechanisms to therapeutic avenues. Biomark Res. (2024) 12:120. doi: 10.1186/s40364-024-00664-z
11. Osaka, T, Hashimoto, Y, Hamaguchi, M, Kojima, T, Obora, A, and Fukui, M. Nonalcoholic fatty liver disease remission in men through regular exercise. J Clin Biochem Nutr. (2018) 62:242–6. doi: 10.3164/jcbn.17-115
12. Charatcharoenwitthaya, P, Kuljiratitikal, K, Aksornchanya, O, Chaiyasoot, K, Bandidniyamanon, W, and Charatcharoenwitthaya, N. Moderate-intensity aerobic vs resistance exercise and dietary modification in patients with nonalcoholic fatty liver disease: a randomized clinical trial. Clin Transl Gastroenterol. (2021) 12:e00316. doi: 10.14309/ctg.0000000000000316
13. Mascaró, CM, Bouzas, C, Montemayor, S, Casares, M, Llompart, I, Ugarriza, L, et al. Effect of a six-month lifestyle intervention on the physical activity and fitness status of adults with NAFLD and metabolic syndrome. Nutrients. (2022) 14:1813. doi: 10.3390/nu14091813
14. Hassani Zadeh, S, Mansoori, A, and Hosseinzadeh, M. Relationship between dietary patterns and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Gastroenterol Hepatol. (2021) 36:1470–8. doi: 10.1111/jgh.15363
15. Watanabe, M, Tozzi, R, Risi, R, Tuccinardi, D, Mariani, S, Basciani, S, et al. Beneficial effects of the ketogenic diet on nonalcoholic fatty liver disease: a comprehensive review of the literature. Obes Rev. (2020) 21:e13024. doi: 10.1111/obr.13024
16. Crabtree, CD, Kackley, ML, Buga, A, Fell, B, LaFountain, RA, Hyde, PN, et al. Comparison of ketogenic diets with and without ketone salts versus a low-fat diet: liver fat responses in overweight adults. Nutrients. (2021) 13:966. doi: 10.3390/nu13030966
17. Berná, G, and Romero-Gomez, M. The role of nutrition in non-alcoholic fatty liver disease: pathophysiology and management. Liver Int. (2020) 40:102–8. doi: 10.1111/liv.14360
18. Petermann-Rocha, F, Wirth, MD, Boonpor, J, Parra-Soto, S, Zhou, Z, Mathers, JC, et al. Associations between an inflammatory diet index and severe non-alcoholic fatty liver disease: a prospective study of 171,544 UK biobank participants. BMC Med. (2023) 21:123. doi: 10.1186/s12916-023-02793-y
19. Jiang, Y, Cao, H, Chen, X, Yu, G, Song, C, Duan, H, et al. Associations of serum folate and vitamin C levels with metabolic dysfunction-associated fatty liver disease in US adults: a nationwide cross-sectional study. Front Public Health. (2022) 10:1022928. doi: 10.3389/fpubh.2022.1022928
20. Blokhina, O, Virolainen, E, and Fagerstedt, KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot. (2003) 91:179–94. doi: 10.1093/aob/mcf118
21. Sutti, S, and Albano, E. Adaptive immunity: an emerging player in the progression of NAFLD. Nat Rev Gastroenterol Hepatol. (2020) 17:81–92. doi: 10.1038/s41575-019-0210-2
22. Yu, YC, Paragomi, P, Wang, R, Jin, A, Schoen, RE, Sheng, LT, et al. Composite dietary antioxidant index and the risk of colorectal cancer: findings from the Singapore Chinese health study. Int J Cancer. (2022) 150:1599–608. doi: 10.1002/ijc.33925
23. Sohouli, MH, Fatahi, S, Sayyari, A, Olang, B, and Shidfar, F. Associations between dietary total antioxidant capacity and odds of non-alcoholic fatty liver disease (NAFLD) in adults: a case-control study. J Nutr Sci. (2020) 9:e48. doi: 10.1017/jns.2020.39
24. Vahid, F, Rahmani, D, and Hekmatdoost, A. The association between dietary antioxidant index (DAI) and nonalcoholic fatty liver disease (NAFLD) onset; new findings from an incident case-control study. Clin Nutr ESPEN. (2021) 41:360–4. doi: 10.1016/j.clnesp.2020.10.020
25. Wright, ME, Mayne, ST, Stolzenberg-Solomon, RZ, Li, Z, Pietinen, P, Taylor, PR, et al. Development of a comprehensive dietary antioxidant index and application to lung cancer risk in a cohort of male smokers. Am J Epidemiol. (2004) 160:68–76. doi: 10.1093/aje/kwh173
26. Zhao, L, Sun, Y, Cao, R, Wu, X, Huang, T, and Peng, W. Non-linear association between composite dietary antioxidant index and depression. Front Public Health. (2022) 10:988727. doi: 10.3389/fpubh.2022.988727
27. Wu, M, Si, J, Liu, Y, Kang, L, and Xu, B. Association between composite dietary antioxidant index and hypertension: insights from NHANES. Clin Exp Hypertens. (2023) 45:2233712. doi: 10.1080/10641963.2023.2233712
28. Chen, Y, Tang, W, Li, H, Lv, J, Chang, L, and Chen, S. Composite dietary antioxidant index negatively correlates with osteoporosis among middle-aged and older US populations. Am J Transl Res. (2023) 15:1300–8.
29. Yang, Z, Song, S, Li, L, Yuan, Z, and Li, Y. Association between the composite dietary antioxidant index and metabolic dysfunction-associated steatotic liver disease in adults: a cross-sectional study from NHANES 2017-2020. Sci Rep. (2024) 14:13801. doi: 10.1038/s41598-024-63965-1
30. Zhang, Z, Wang, H, and Chen, Y. Association between composite dietary antioxidant index and metabolic dysfunction associated steatotic liver disease: result from NHANES, 2017-2020. Front Nutr. (2024) 11:1412516. doi: 10.3389/fnut.2024.1412516
31. Zipf, G, Chiappa, M, Porter, KS, Ostchega, Y, Lewis, BG, and Dostal, J. National health and nutrition examination survey: plan and operations, 1999-2010. Vital Health Stat Ser 1. (2013) 56:1–37.
32. Ahuja, JK, Moshfegh, AJ, Holden, JM, and Harris, E. USDA food and nutrient databases provide the infrastructure for food and nutrition research, policy, and practice. J Nutr. (2013) 143:241s–9s. doi: 10.3945/jn.112.170043
33. Yu, C, Gao, J, Ge, X, Wang, X, Ding, Y, Tian, T, et al. Healthy lifestyle is associated with reduced mortality in patients with non-alcoholic fatty liver disease. Nutrients. (2022) 14:3785. doi: 10.3390/nu14183785
34. Chalasani, N, Younossi, Z, Lavine, JE, Diehl, AM, Brunt, EM, Cusi, K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. (2012) 142:1592–609. doi: 10.1053/j.gastro.2012.04.001
35. Tian, T, Zhang, J, Xie, W, Ni, Y, Fang, X, Liu, M, et al. Dietary quality and relationships with metabolic dysfunction-associated fatty liver disease (MAFLD) among United States adults, results from NHANES 2017-2018. Nutrients. (2022) 14:4505. doi: 10.3390/nu14214505
36. Bedogni, G, Bellentani, S, Miglioli, L, Masutti, F, Passalacqua, M, Castiglione, A, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. (2006) 6:33. doi: 10.1186/1471-230X-6-33
37. Ruhl, CE, and Everhart, JE. Fatty liver indices in the multiethnic United States national health and nutrition examination survey. Aliment Pharmacol Ther. (2015) 41:65–76. doi: 10.1111/apt.13012
38. Li, L, Huang, Q, Yang, L, Zhang, R, Gao, L, Han, X, et al. The association between non-alcoholic fatty liver disease (NAFLD) and advanced fibrosis with serological vitamin B12 markers: results from the NHANES 1999-2004. Nutrients. (2022) 14:1224. doi: 10.3390/nu14061224
39. Muanda, FT, Weir, MA, Bathini, L, Blake, PG, Chauvin, K, Dixon, SN, et al. Association of baclofen with encephalopathy in patients with chronic kidney disease. JAMA. (2019) 322:1987–95. doi: 10.1001/jama.2019.17725
40. Hernán, MA, Hernández-Díaz, S, Werler, MM, and Mitchell, AA. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol. (2002) 155:176–84. doi: 10.1093/aje/155.2.176
41. Wang, Y, Kong, L, Ye, C, Dou, C, Zheng, J, Xu, M, et al. Causal impacts of educational attainment on chronic liver diseases and the mediating pathways: Mendelian randomization study. Liver Int. (2023) 43:2379–92. doi: 10.1111/liv.15669
42. Franco, I, Bianco, A, Bonfiglio, C, Curci, R, Campanella, A, and Osella, AR. Leisure-time physical activity, time spent sitting and risk of non-alcoholic fatty liver disease: a cross-sectional study in Puglia. J Gen Intern Med. (2024). doi: 10.1007/s11606-024-08804-9
43. Marjot, T, Tomlinson, JW, Hodson, L, and Ray, DW. Timing of energy intake and the therapeutic potential of intermittent fasting and time-restricted eating in NAFLD. Gut. (2023) 72:1607–19. doi: 10.1136/gutjnl-2023-329998
44. Liu, C, Hua, L, and Xin, Z. Synergistic impact of 25-hydroxyvitamin D concentrations and physical activity on delaying aging. Redox Biol. (2024) 73:103188. doi: 10.1016/j.redox.2024.103188
45. Romero-Gómez, M, Zelber-Sagi, S, and Trenell, M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. (2017) 67:829–46. doi: 10.1016/j.jhep.2017.05.016
46. Lotfi, A, Saneei, P, Hekmatdost, A, Salehisahlabadi, A, Shiranian, A, and Ghiasvand, R. The relationship between dietary antioxidant intake and physical activity rate with nonalcoholic fatty liver disease (NAFLD): a case - control study. Clin Nutr ESPEN. (2019) 34:45–9. doi: 10.1016/j.clnesp.2019.09.004
47. Coelho, JM, Cansanção, K, Perez, RM, Leite, NC, Padilha, P, Ramalho, A, et al. Association between serum and dietary antioxidant micronutrients and advanced liver fibrosis in non-alcoholic fatty liver disease: an observational study. PeerJ. (2020) 8:e9838. doi: 10.7717/peerj.9838
48. Luo, X, Zhang, W, He, Z, Yang, H, Gao, J, Wu, P, et al. Dietary vitamin C intake is associated with improved liver function and glucose metabolism in Chinese adults. Front Nutr. (2021) 8:779912. doi: 10.3389/fnut.2021.779912
49. Traber, MG, Buettner, GR, and Bruno, RS. The relationship between vitamin C status, the gut-liver axis, and metabolic syndrome. Redox Biol. (2019) 21:101091. doi: 10.1016/j.redox.2018.101091
50. Federico, A, Dallio, M, Caprio, GG, Gravina, AG, Picascia, D, Masarone, M, et al. Qualitative and quantitative evaluation of dietary intake in patients with non-alcoholic steatohepatitis. Nutrients. (2017) 9:1074. doi: 10.3390/nu9101074
51. Ivancovsky-Wajcman, D, Fliss-Isakov, N, Salomone, F, Webb, M, Shibolet, O, Kariv, R, et al. Dietary vitamin E and C intake is inversely associated with the severity of nonalcoholic fatty liver disease. Dig Liver Dis. (2019) 51:1698–705. doi: 10.1016/j.dld.2019.06.005
52. Lavine, JE, Schwimmer, JB, Van Natta, ML, Molleston, JP, Murray, KF, Rosenthal, P, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. (2011) 305:1659–68. doi: 10.1001/jama.2011.520
53. Lim, HS, Choi, J, Lee, B, Kim, SG, Kim, YS, and Yoo, JJ. Association between inflammatory biomarkers and nutritional status in fatty liver. Clin Nutr Res. (2020) 9:182–94. doi: 10.7762/cnr.2020.9.3.182
54. Akdas, S, and Yazihan, N. Serum zinc level and dietary zinc intake status in non-alcoholic fatty liver disease: a meta-analysis and systematic review. Hepatol Forum. (2020) 1:59–67. doi: 10.14744/hf.2020.2020.0006
55. Aktary, ML, Eller, LK, Nicolucci, AC, and Reimer, RA. Cross-sectional analysis of the health profile and dietary intake of a sample of Canadian adults diagnosed with non-alcoholic fatty liver disease. Food Nutr Res. (2020) 64:64. doi: 10.29219/fnr.v64.4548
56. Shojaei Zarghani, S, Rahimi Kashkooli, N, Bagheri, Z, Tabatabaei, M, Fattahi, MR, and Safarpour, AR. Dietary selenium intake in relation to non-alcoholic fatty liver disease assessed by fatty liver index and hepatic steatosis index; a cross-sectional study on the baseline data of prospective PERSIAN Kavar cohort study. BMC Endocr Disord. (2023) 23:51. doi: 10.1186/s12902-023-01307-4
57. Steinbrenner, H, Duntas, LH, and Rayman, MP. The role of selenium in type-2 diabetes mellitus and its metabolic comorbidities. Redox Biol. (2022) 50:102236. doi: 10.1016/j.redox.2022.102236
58. de Brito, JN, McDonough, DJ, Mathew, M, VanWagner, LB, Schreiner, PJ, Gabriel, KP, et al. Young adult physical activity trajectories and midlife nonalcoholic fatty liver disease. JAMA Netw Open. (2023) 6:e2338952. doi: 10.1001/jamanetworkopen.2023.38952
59. Lewis, JR, and Mohanty, SR. Nonalcoholic fatty liver disease: a review and update. Dig Dis Sci. (2010) 55:560–78. doi: 10.1007/s10620-009-1081-0
60. Noureddin, M, and Sanyal, AJ. Pathogenesis of NASH: the impact of multiple pathways. Curr Hepatol Rep. (2018) 17:350–60. doi: 10.1007/s11901-018-0425-7
61. Rives, C, Fougerat, A, Ellero-Simatos, S, Loiseau, N, Guillou, H, Gamet-Payrastre, L, et al. Oxidative stress in NAFLD: role of nutrients and food contaminants. Biomol Ther. (2020) 10:1702. doi: 10.3390/biom10121702
62. Serviddio, G, Sastre, J, Bellanti, F, Viña, J, Vendemiale, G, and Altomare, E. Mitochondrial involvement in non-alcoholic steatohepatitis. Mol Asp Med. (2008) 29:22–35. doi: 10.1016/j.mam.2007.09.014
63. Zhang, CY, Liu, S, and Yang, M. Antioxidant and anti-inflammatory agents in chronic liver diseases: molecular mechanisms and therapy. World J Hepatol. (2023) 15:180–200. doi: 10.4254/wjh.v15.i2.180
64. Ballestri, S, Nascimbeni, F, Baldelli, E, Marrazzo, A, Romagnoli, D, and Lonardo, A. NAFLD as a sexual dimorphic disease: role of gender and reproductive status in the development and progression of nonalcoholic fatty liver disease and inherent cardiovascular risk. Adv Ther. (2017) 34:1291–326. doi: 10.1007/s12325-017-0556-1
Keywords: Composite Dietary Antioxidant Index, nonalcoholic fatty liver disease, metabolic-associated fatty liver disease, NHANES, inflammatory biomarkers, physical activity
Citation: Gao L, Fang H, Zhao Z, Luo W, Gong J and Gong J (2024) Synergistic impact of Composite Dietary Antioxidant Index and physical activity on fatty liver disease. Front. Nutr. 11:1486700. doi: 10.3389/fnut.2024.1486700
Edited by:
Eric Gumpricht, Isagenix International, LLC, United StatesReviewed by:
Ewelina Książek, Wroclaw University of Economics, PolandJie Jia, Guangzhou University of Chinese Medicine, China
Copyright © 2024 Gao, Fang, Zhao, Luo, Gong and Gong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junhua Gong, Z29uZ2p1bmh1YUBob3NwaXRhbC5jcW11LmVkdS5jbg==
 Linxiao Gao
Linxiao Gao Haoyu Fang
Haoyu Fang Zhibo Zhao
Zhibo Zhao Wen Luo
Wen Luo Jianping Gong
Jianping Gong Junhua Gong
Junhua Gong