- 1Department of Cardiovascular Medicine, The Second Affiliated Hospital of Nanchang University, Nanchang, China
- 2Department of Gastroenterology, The Second Affiliated Hospital of Nanchang University, Nanchang, China
Background: With the increasing prevalence of prediabetes and diabetes, exploring dietary factors associated with prediabetes and diabetes has become a global health research priority. This study aimed to assess the relationship between dietary decanoic acid (DDA) intake and the risk of diabetes and prediabetes.
Methods: Data from the National Health and Nutrition Examination Survey (NHANES) 2005–2016 included 11,477 adult participants. DDA intake was assessed through two 24-h dietary recalls and participants were grouped according to the diagnostic criteria for diabetes and prediabetes. Multivariate regression models were applied to analyze the relationship between DDA intake and diabetes and prediabetes, with subgroup analyses conducted to explore potential interactions.
Results: Dietary decanoic acid intake was significantly negatively associated with the risk of diabetes. In the fully adjusted model, each 1 g/day increase in DDA intake was associated with a 19% reduction in the odds of developing diabetes from prediabetes (OR = 0.81, 95% CI: 0.68–0.96, p = 0.015) and this negative association was more pronounced in individuals with higher education level (P for interaction = 0.006). Compared with the DDA intake ≤0.18 g/day, DDA intake >0.58 g/day is related to reduced risk of progression to diabetes in prediabetic patients. However, the relationship between DDA intake and the risk of prediabetes was not statistically significant in the fully adjusted model (OR = 0.95, 95% CI: 0.84–1.07, p = 0.404).
Conclusion: This study found that higher DDA intake may be associated with lower prevalence of diabetes among prediabetic population, and high education level strengthen this relationship.
Introduction
Diabetes has become a significant public health concern worldwide, with the incidence of diabetes continuing to rise globally (1–4). As of 2019, the number of people living with diabetes globally is estimated at 463 million, and is projected to increase to 578 million by 2030 and 700 million by 2045 (5). Recognized as the fifth highest cause of mortality globally (6), diabetes may lead to various chronic complications including cancer (7), depression (8), diabetic retinopathy (9), diabetic neuropathy (10) and cardiovascular diseases (11). These diseases greatly reduce the enjoyment of quality of an individual’s life with diabetes and even increase the risk of death, greatly contributing to the medical and economic stress on society (12). Prediabetes refers to the transitional phase before the onset of diabetes, which is an intermediate hyperglycemic state between normoglycemia and diabetes, featuring impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT) (13, 14). The number of prediabetic adults has been increasing in recent years and is projected to grow to 418 million by 2025 (15). A study by the American Diabetes Association (ADA) shows that nearly 70% of prediabetes eventually develops into diabetes. Some studies have demonstrated that the pathogenesis of diabetes and prediabetes is multifactorial (13, 16–18), involving genetic predisposition, lifestyle choice and environmental factors, among which diet habits stands out as a key modifiable risk factor (19, 20).
In more recent times, there has been increasing emphasis on the contribution of nutritional fatty acids in development and progression of diabetes mellitus (21, 22). Decanoic Acid is one of medium chain fatty acids (MCFAs) containing 10 carbon atoms, which natural sources are limited, typically found in milk fat, coconut oil and palm kernel oil (23, 24). Multiple studies suggest that decanoic acid may control the incidence of coronary artery disease and epilepsy (25–27). In comparison to long-chain fatty acids (LCFAs), decanoic acid has unique metabolic characteristics that may affect blood glucose homeostasis and insulin sensitivity (28). The biological mechanisms of the metabolic effects of decanoic acid are well known, but there are no relevant studies based large-scale population examining the association between dietary decanoic acid (DDA) intake and diabetes or prediabetes (29).
In this research, we sourced data on DDA, as well as individuals with diabetes and prediabetes, from the database named National Health and Nutrition Examination Survey (NHANES) 2005 to 2016. The objective was to investigate the potential link between DDA intake and the risk of developing prediabetes or diabetes among adult Americans.
Methods
Study design and population
Measurement of dietary decanoic acid
NHANES is a cross-sectional survey program conducted by the Centers for Disease Control and Prevention (CDC), aimed at evaluating to the health and nutritional condition of adults and children across the United States. The dietary panel of NHANES focuses on collecting information about participants’ diets, including an assessment of dietary intake through a 24-h dietary recall record. The US Department of Agriculture’s the Food and Nutrient Database for Dietary Studies (FNDDS) records the content of 64 nutrients/food ingredients in all foods/beverages, including decanoic acid. Therefore, based on the food and beverages consumed by participants within 24 h and the amount of decanoic acid they contain, DDA intake within 24 h can be calculated. Since 2002, NHANES has collected 24 h of dietary recall data over 2 days using the U.S. In our study, DDA intake was estimated as the average of two 24-h recall cycles of decanoic acid in food.
Diabetes and prediabetes
According to the diagnostic criteria of ADA, diabetes was defined as fasting blood sugar (FBG) ≥ 126 mg/dL or glycosylated hemoglobin (HbA1c) level ≥ 6.5%, or a “Yes” answer to any of the following questions: “Have doctors ever told you that you have diabetes?,” “Are you currently using insulin?,” and “Are you currently using oral hypoglycemic medications?.” Prediabetes was defined as having an FBG of 100 mg/dL-125 mg/dL or a HbA1c of 5.7–6.4%, or answering “Yes” to the question, “Have you ever been told you have prediabetes?.” Those who are not diagnosed with diabetes or prediabetes are defined as normal.
Potential covariates
Covariates considered included some of the following demographic characteristics, laboratory tests and questionnaires: age (years), gender (male and female), race (Mexican American, non-Hispanic White, Non-Hispanic Black, Other Hispanic, other race), education level (Below high school, High school, and College or above), poverty income ratio (PIR, low level: PIR < 1.30; middle level: 1.30 ≤ PIR < 3.50; and high level: ≥3.50), body mass index (BMI, Kg/m2), waist (cm), and systolic blood pressure (SBP, mmHg) and diastolic blood pressure (DBP, mmHg), FBG (mg/dL), HbA1c (%), alanine aminotransferase (ALT, U/L), aspartate aminotransferase (AST, U/L), serum creatinine (SCR, μmol/L), triglyceride (TG, mmol/L), total cholesterol (TC, mmol/L), high density lipoprotein cholesterol (HDL-C, mmol/L), low density lipoprotein cholesterol (LDL-C, mmol/L). According to the results of the questionnaire, smoking status can be categorized into never (smoked less than 100 cigarettes in life), former (smoked more than 100 cigarettes in life but has now quit.) and current smokers (smoked more than 100 cigarettes in life and now still smoking). Drinking status based on gender and alcohol consumption was categorized into mild drinker (consuming <2 drinks/day for females, <3 drinks/day for males), moderate drinker (consuming ≥2 drinks/day for females, ≥3 drinks/day for males), heavy drinker (consuming ≥3 drinks/day for females, ≥4 drinks/day for males), and non-drinker. The diagnosis of hypertension relies on blood pressure measurements and medical history inquiry: SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg, or “being told by doctors that you have hypertension” and “currently taking antihypertensive medication.” A person is considered to be suffering from cardiovascular disease if he or she answers “Yes” to any of the following questions: “Ever been told you have congestive heart failure?,” “Ever been told you have coronary heart disease?,” “Ever been told you have angina pectoris?,” “Ever been told you have myocardial infarction?” and “Ever been told you have a stroke?”.
Statistical analysis
In the baseline data analysis, we described the data according to the diagnostic criteria by dividing them into normal, prediabetic and diabetic groups. The continuous variables were expressed as median and quartiles, and the categorical variables were expressed as number and percentage. Kruskal–Wallis H and Mann–Whitney U tests were used for multiple inter- and intra-group comparisons of continuous variables, while chi-square tests were used for comparisons between categorical variables. In order to further explore the role of DDA in people with different levels of abnormal glucose metabolism, we will, respectively, conduct multivariate regression analysis of DDA intake in diabetes and prediabetes, diabetes and normal people, as well as prediabetes and normal people. Three regression models adjusted for different covariates were constructed for each group in two-by-two comparisons and were used to analyze within-group differences in the role of DDA in each group. Model 1 adjusts for no variables. Model 2 was adjusted for gender, age, ALT, SCR, TG, TC, HDL-C, LDL-C. Model 3 was adjusted for gender, age, education level, PIR, BMI, waist, smoking status, drink status, hypertension, cardiovascular, ALT, SCR, TG, TC, HDL-C, LDL-C. The adjustment of models in each group is completely consistent for ensuring maximum reduction of bias. Restricted cubic spline (RCS) analysis was used to intuitively display the non-linear associations between DDA and diabetes or prediabetes. Stratified analysis and interaction tests were performed to evaluate the factors that might influence the correlation between DDA and diabetes or prediabetes. These tests considered several variables, including gender (male and female), age (<60 years and ≥60 years), race (non-Hispanic white and other race), educational levels (High school or below and College or above), PIR (<3.0 and ≥3.0), BMI (<28 kg/m2 and ≥28 kg/m2), smoking status (Never and Smoker), drinking status (Never and Drinker), hypertension (Yes and No), cardiovascular (Yes and No). The findings of this study are articulated through the use of odds ratios (OR) and their corresponding 95% confidence intervals (95%CI). The statistical analyses were conducted within the R programming language environment (version 4.3.2). In this context, a p-value of less than 0.05 for both tails of the distribution, was adopted as the threshold for statistical significance.
Results
Selection of study population
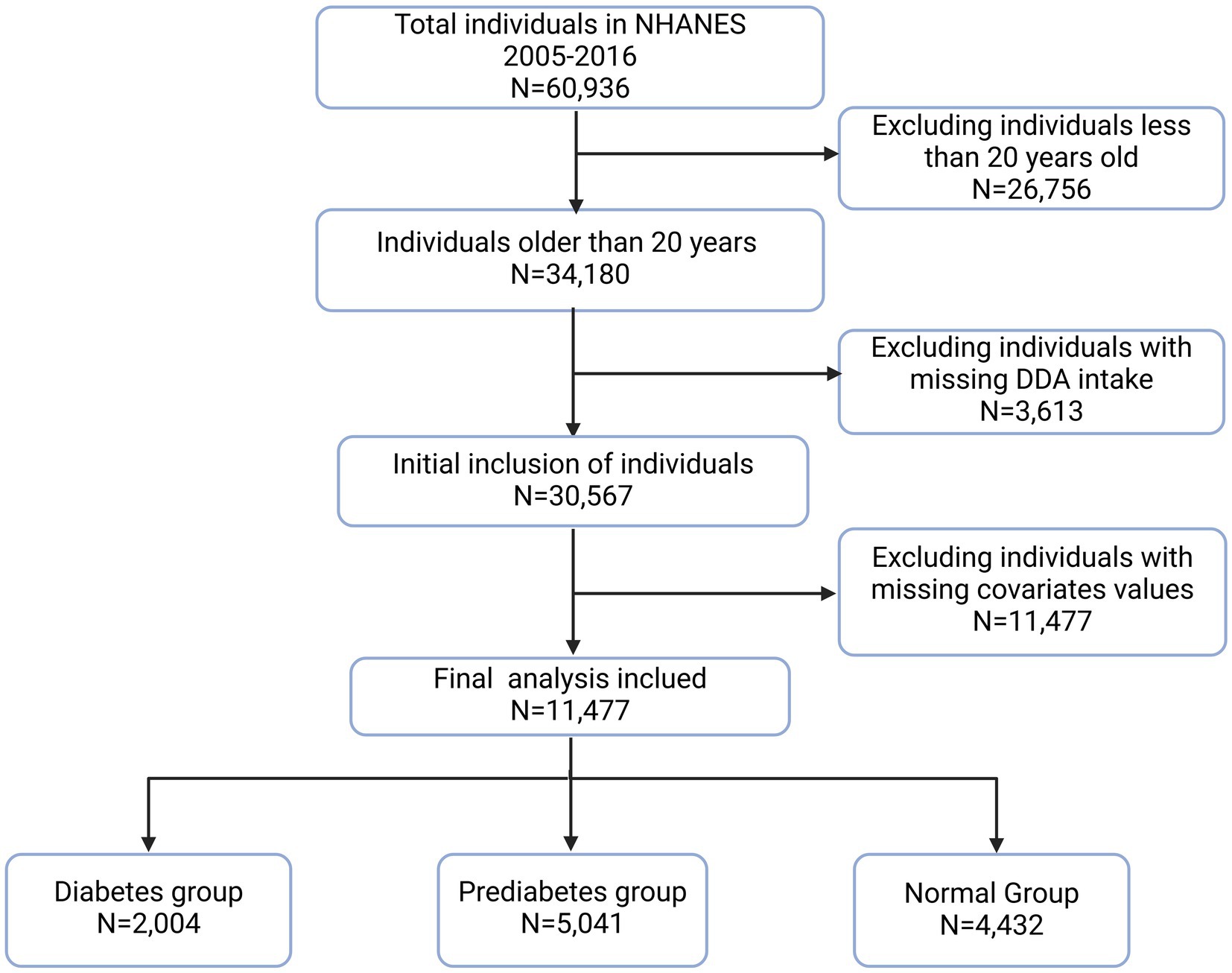
This study examined the relationship between DDA and diabetes or prediabetes using NHANES 2005–2016 data six survey cycles in total. These cycles recruited a total of 60,936 participants. Eligible participants are selected for analysis based on the following exclusion criteria: age < 20 years old (N = 26,756), missing DDA data (N = 3,613), and missing important covariates data (N = 19,090). Consequently, the final study population comprised 11,477 participants (Figure 1).
Baseline characteristics of the study population
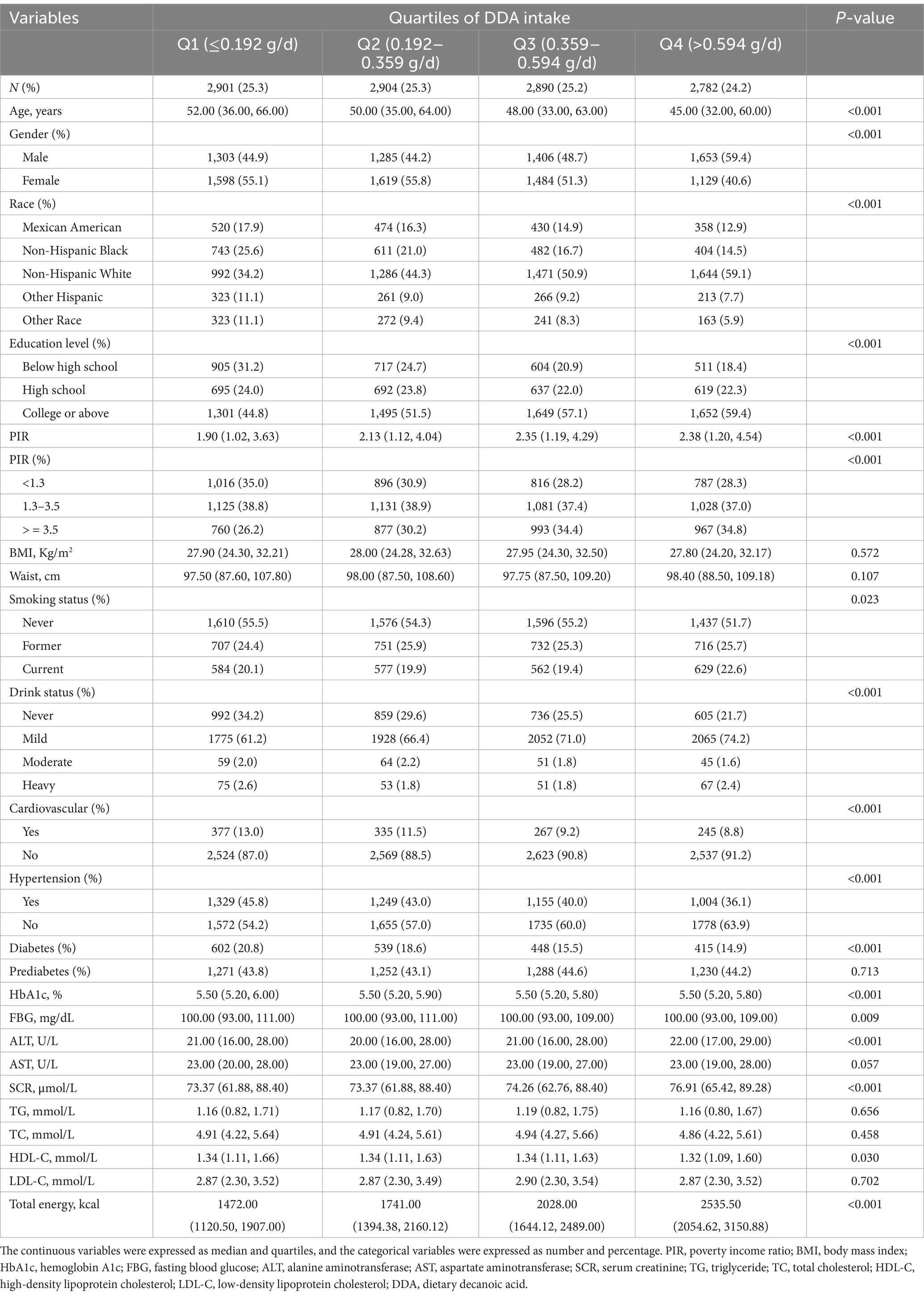
Table 1 showed the baseline characteristics of participants based on diabetes, prediabetes and normal population. This study included a total of eligible 11,477 individuals, among them, 2,004 (17.46%) suffered from diabetes, and 5,041 (43.92%) were in prediabetes state. In contrast to the normal population, individuals with diabetes or prediabetes tend to exhibit certain characteristics: they are often older, predominantly male, low-income, lower educated, smokers, non-drinkers, and non-Hispanic black ethnicity, and are more likely to experience cardiovascular and hypertension. Additionally, they have higher levels of BMI, waist, HbA1c, FBG, ALT, AST, SCR, TG, TC, LDL-C; and have lower HDL-C, DDA, total energy intake. Table 2 shows the baseline of participants based on quartiles of DDA intake. Contrasted with those in the lowest quartile, individuals in the top quartile of DDA intake are younger, male, non-Hispanic white, highly educated, high-income, non-hypertensive, non-cardiovascular, and non-diabetic. In addition, they have higher ALT and SCR levels and have lower HbA1c, FBG, and HDL levels.
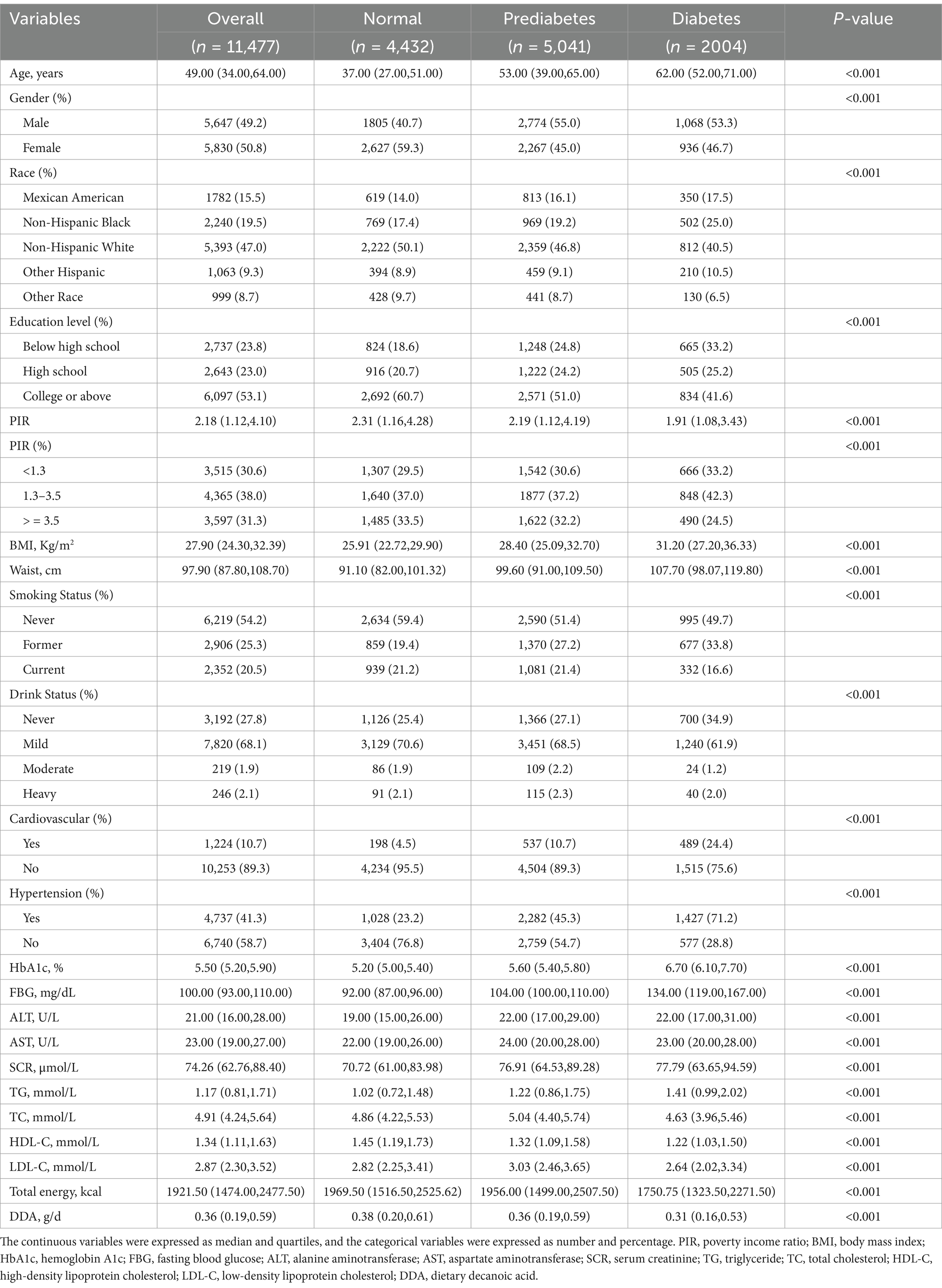
Table 1. Weighted characteristics of participants with diabetes, prediabetes and normal from NHANES 2005 to 2016.
Association between DDA and diabetes or prediabetes
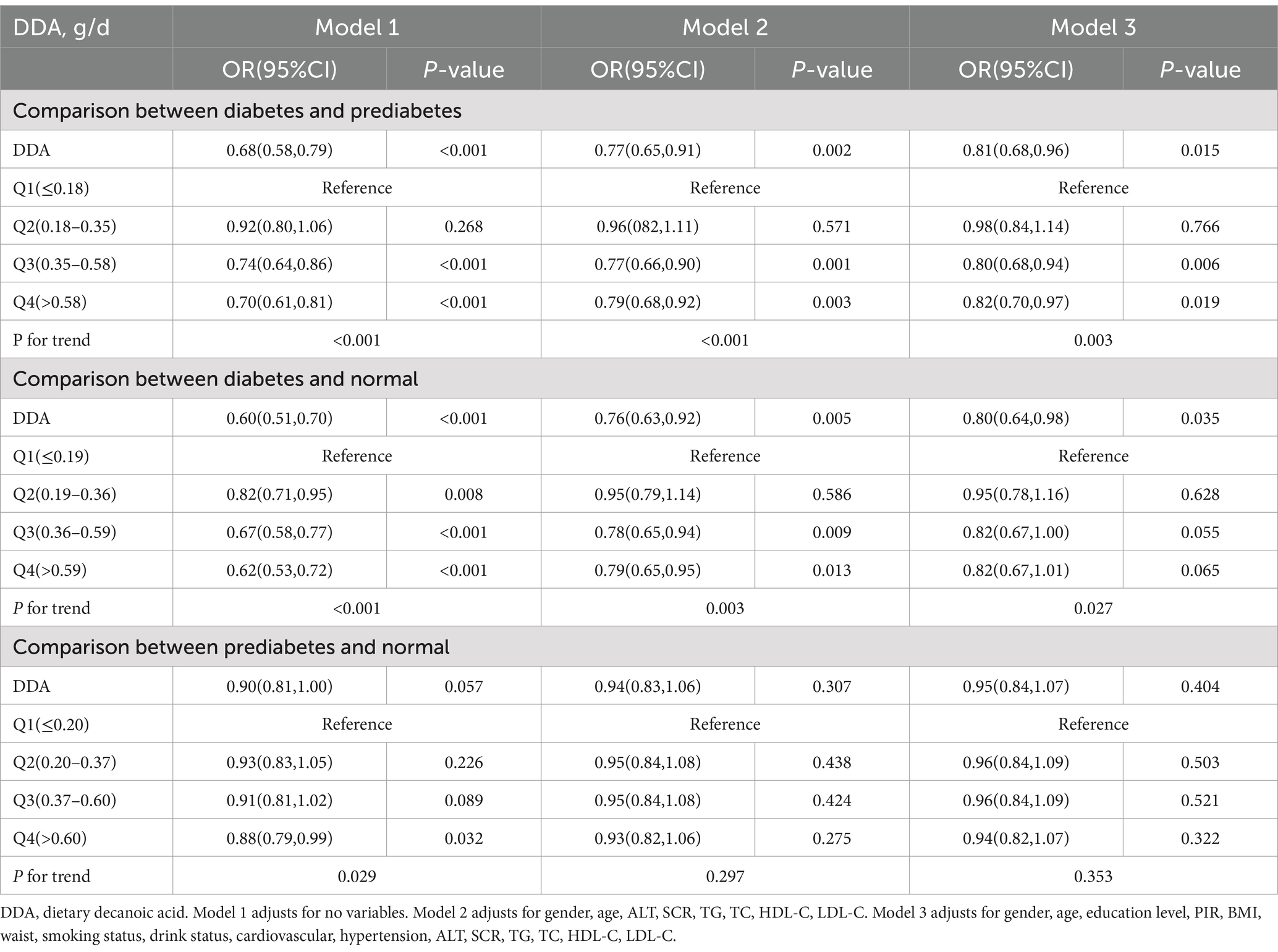
Table 3 presents a summary of the findings from the multiple logistic regression analysis.
Comparison between diabetes and prediabetes, there is a negative association between DDA consumption and the prevalence of diabetes among prediabetic patients. In the unadjusted Model 1, for every additional 1 g/d of DDA intake, the OR for diabetes prevalence was reduced by 32% (OR = 0.68, 95%CI: 0.58–0.79, p < 0.001). This association persisted strongly even after accounting for potential confounding variables in the adjusted Model 2 (OR = 0.77, 95%CI: 0.65–0.91, p = 0.002) and Model 3 (OR = 0.81, 95%CI: 0.68–0.96, p = 0.015).
Comparison between diabetes and normal, DDA intake is also negatively correlated with the diabetes prevalence in normal people. In the Model 1, the OR for prevalence of diabetes was lowered by 40% with each 1 g/d increase in DDA intake (OR = 0.60, 95%CI: 0.51–0.70, p < 0.001). This trend continued to hold in the adjusted Model 2 (OR = 0.76, 95%CI: 0.63–0.92, p = 0.005) and Model 3 (OR = 0.80, 95%CI: 0.64–0.98, p = 0.035).
Comparison between prediabetes and normal, Model 1 indicated no significant relationship between DDA intake and prediabetes in the normal population (OR = 0.90, 95% CI: 0.81–1.00, p = 0.057). Despite adjustments for confounding factors in Model 2 (OR = 0.94, 95% CI: 0.83–1.06, p = 0.307) and Model 3 (OR = 0.95, 95% CI: 0.84–1.07, p = 0.404), the correlation did not reach statistical significance.
Multiple logistic regression analysis showed that DDA intake was significantly negatively correlated with the prevalence of diabetes whether in the normal or prediabetes people, but there was no correlation between DDA intake and the prevalence of prediabetes in normal people. These results suggest that DDA might primarily function to impede the transition from a prediabetic state to diabetes. Consequently, our subsequent research will concentrate on examining the impact of DDA intake on this progression from prediabetes to diabetes. In trend testing for DDA intake quartiles and diabetes among prediabetic patients in Table 3, comparing the second, third, and fourth quartiles of DDA intake to the lowest quartile, the OR and 95% CI for diabetes in the fully adjusted Model 3 were, respectively, 0.98 (0.84–1.14), 0.80 (0.68–0.94), and 0.82 (0.70–0.97), with a significant trend (P for trend = 0.003).
After multivariable adjustment in Model 3, a non-linear association was observed between DDA intake and the risk of diabetes among prediabetic population, as depicted in the RCS curve in Figure 2 (P for overall = 0.006; P for non-linear = 0.004). The RCS curves showing other relationships, including DDA intake with prediabetes and DDA intake with diabetes in normal population, can be found in the Supplementary material.
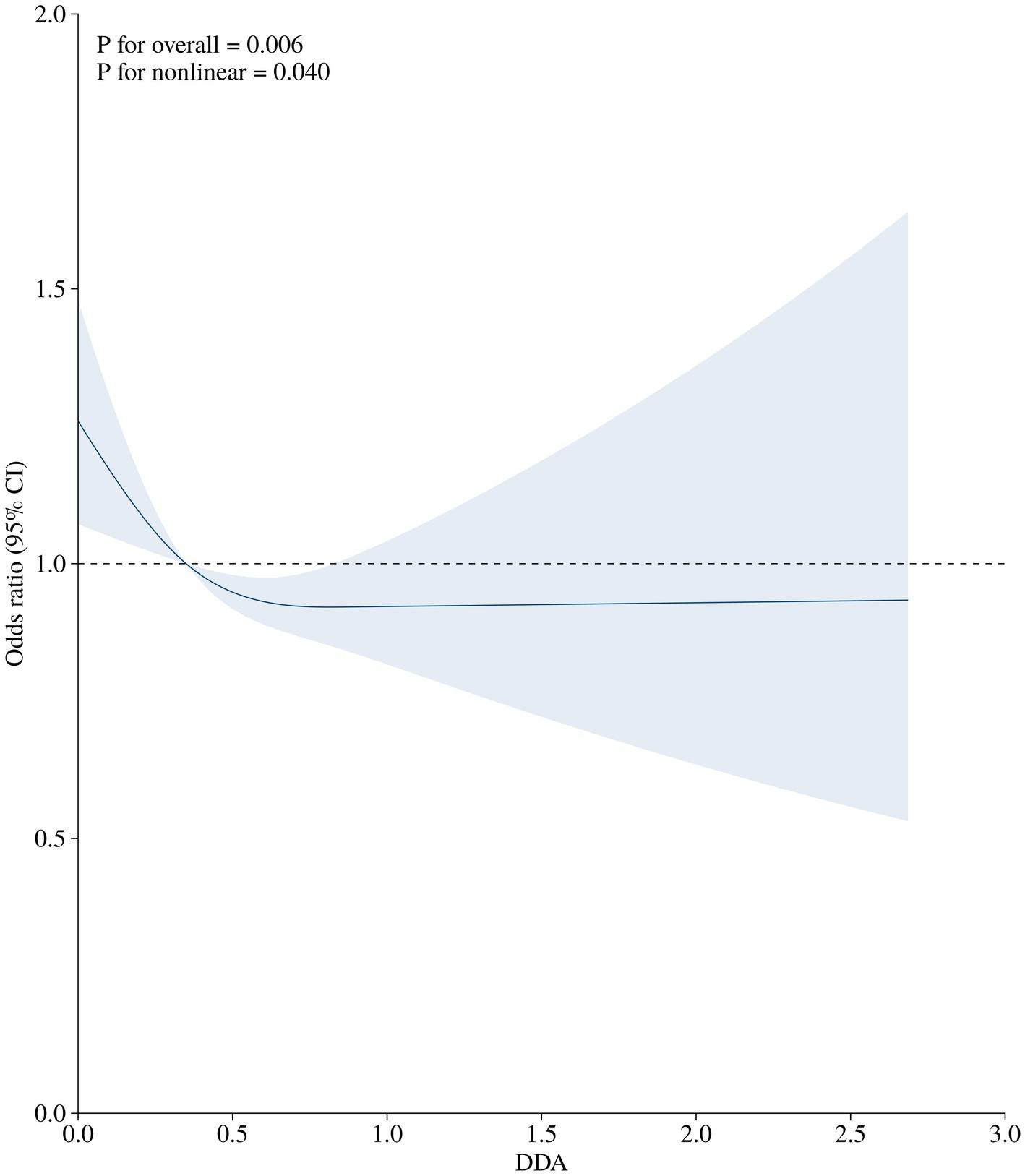
Figure 2. The association of DDA intake (g/d) with the prevalence of diabetes among prediabetic population. The OR (solid lines) and 95%CI (shaded areas) in the RCS was adjusted for gender, age, education level, PIR, BMI, waist, smoking status, drink status, cardiovascular, hypertension, ALT, SCR, TG, TC, HDL-C, LDL-C.
Subgroup analyses
In subgroup analysis, among female individuals, people with education level of college or above, BMI ≥ 28 kg/m2, and hypertension, DDA has a more significantly negative correlation with the prevalence of diabetes in prediabetes patients. Notably, a significant interaction effect was identified between DDA intake and educational level in relation to the risk of diabetes in prediabetic individuals (P for interaction = 0.006) (Figure 3). This suggests that the impact of DDA intake on diabetic risk in prediabetes may vary depending on the education level.
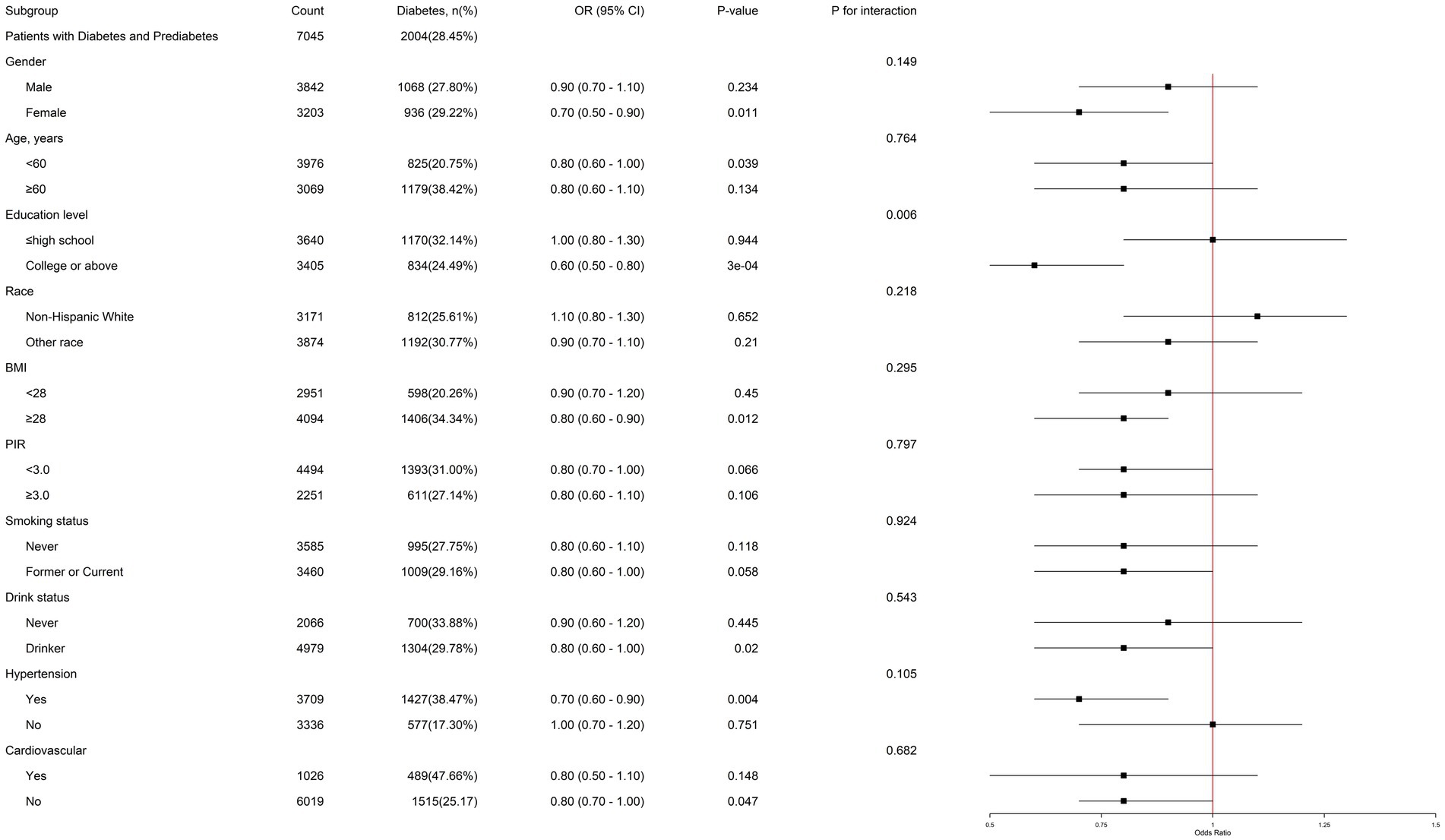
Figure 3. Subgroup analysis of the association of DDA intake and the risk of diabetes among prediabetic population. Each subgroup analysis was adjusted for gender, age, education level, PIR, BMI, waist, smoking status, drink status, cardiovascular, hypertension, ALT, SCR, TG, TC, HDL-C, and LDL-C. The strata variable was not included when stratifying by itself.
Discussion
Our study confirmed the negative correlation between DDA and diabetes for the first time based on large-scale population surveys.
As we all know, the development of diabetes is usually a gradual process, which generally goes through three stages: from normal blood sugar level to prediabetes, and finally to diabetes (14). As revealed by the multiple regression analysis and the RCS curve, the findings from this study demonstrate a significant negative relationship between DDA intake and the risk of diabetes among both prediabetic and normal individuals. However, no significant correlation was detected between DDA intake and the risk of prediabetes in the normal population. These inconsistent correlations implied that DDA potentially exerts a protective influence against the advancement of prediabetes rather than the onset of prediabetes. The results suggest that the role of DDA in lowering the prevalence of diabetes is primarily through its effect on slowing the transition from a prediabetic state to diabetes, rather than from a normal level to prediabetes. Compared with the DDA intake ≤0.18 g/day, DDA intake >0.58 g/day is related to reduced risk of progression to diabetes in prediabetic patients. Nevertheless, given prediabetic pivotal position in the progression to diabetes, the overall impact of DDA extends to reducing the risk of diabetes among normal individuals as well. This is because the preventive effect of DDA on the transition from prediabetes to diabetes indirectly lowers the overall prevalence of diabetes in the general population.
Prediabetes is the earlier stage before diabetes and eventually it contributed to the development of diabetes without effective treatment and control. About 5–10% of prediabetes patients become diabetes patients every year (30). Some studies show that 37% of prediabetes patients may develop diabetes within 4 years without timely treatment (31). Therefore, it is urgent to treat diabetes and prediabetes. Encouragingly, some studies have shown that the condition in the prediabetes stage is reversible, which provides a potential way for fighting against diabetes. Lifestyle intervention, pharmacological intervention and bariatric surgery are all important measures to prevent developing from prediabetes stage into diabetes (13, 32, 33). Lifestyle interventions stand out as the more rational and safer approach when juxtaposed with other two methods (32). Extensive longitudinal research has substantiated that adopting lifestyle modifications can notably extend the timeline before the transition from a prediabetic state to diabetes, with benefits observed over a decade (31). Regular and nutritious diet is also an important segment of lifestyle intervention. A plethora of research indicates that adhering to a diet rich in nutritious elements, particularly those with a low glycemic index like cereal fiber, whole grains, and bran, can lead to a decrease in diabetes risk by 18–40% (34). Additionally, the intakes of sugary drink has been shown to have a substantial impact on diabetes. Specifically, the risk of diabetes development escalates by 26% for individuals who regularly consume over one cup of sugar-sweetened drinks per day, in contrast to those who partake in less than one cup monthly (35, 36). These all highlights the significance of dietary habits in mitigating the risk of diabetes.
For over a decade, the role of MCFAs as a dietary component of ketogenic diet in regulating glucose and lipid metabolism has gradually become known (28). Ketogenic diet is a high-fat, moderate protein, and low carbohydrate diet that simulates the metabolic pattern of the body in a state of hunger by promoting the metabolism of fat in the body to produce ketone bodies (KBs) (37). The ingested medium chain triglycerides (MCT) are broken down into glycerol and MCFAs in the stomach and duodenum. Relying on the hydrophilicity and short carbon chain of MCFAs, they are allowed for a direct transportation via the portal vein to the liver and enter directly the mitochondria no need for the carnitine system (23). This enables a swift β-oxidation process to rapidly produce energy and MCFAs are converted into KBs, which may save consumption of muscle glycogen and liver glycogen, and reduce insulin demand (38). Under low-carbon or sugar free conditions, KBs produced by decanoic acid metabolism can serve as an energy source for the brain and other tissues, especially when glucose supply is limited (39).
The results in our study showed that an increased intake of DDA is linked to a reduced incidence of diabetes among the studied population. However, the underlying mechanism this correlation remains to be fully elucidated. It is likely that because decanoic acid plays a crucial role in alleviating insulin resistance (IR) and inflammatory response, which are key factors in the onset and progress of diabetes (18, 40). IR is the common pathway of prediabetes and diabetes, usually occurring in the years before diabetes or even prediabetes, and existing in the whole process from prediabetes to late diabetes (14, 18). An animal study based on male mice found that decanoic acid intake can effectively prevent obesity and promote glucagon-like peptide-1 (GLP-1) secretion through the MCFA receptor GPR84 to enhance glucose tolerance and improve insulin resistance (41). Abe et al. (42) found that decanoic acid enhances fatty acid oxidation capacity in mice without suppressing glycolysis in skeletal muscle. This effect is achieved by activating the peroxisome proliferator-activated receptor-δ (PPAR-δ), which in turn upregulates the expression of uncoupling protein 3 (UCP3) in skeletal muscle. Notably, this process does not interfere with the insulin-mediated phosphorylation of Akt, thus maintaining insulin signaling integrity.
Inflammation is a critical factor in the development of both diabetes and prediabetes (18, 43). The findings of a prospective study lasting 177 months in Rotterdam shown, after adjusting for multiple confounding factors, interleukin 13 (IL-13), extracellular newly identified receptor for advanced glycation end-products binding protein (EN-RAGE) and C-reactive protein (CRP) are still associated with prediabetes. In addition, certain inflammatory biomarkers, including adiponectin, CRP, and interleukin 6 (IL-6), have been correlated with the transition from a prediabetic state to diabetes (44). IL-6 and TNF-α, both pro-inflammatory cytokines involved in inflammation and immune responses, have some effects on regulating metabolism. IL-6 has been shown to increase IR and induce fasting hyperglycemia by stimulating glucagon release (45). TNF-α may increase IR through the promotion of phosphorylation of insulin receptor substrate-1 (IRS-1) (46). A study conducted both in vitro and in vivo using a acne murine model have demonstrated that capric acid (C10), the decanoic acid counterpart, exerts anti-inflammatory properties. This effect is achieved by curbing the phosphorylation of mitogen-activated protein kinase (MAPK) and the activation of nuclear factor kappa-B (NF-κB), thereby reducing the secretion of IL-6, IL-8 and TNF-α (47). In an animal experiment, it was observed that the supplementation of decanoic acid in the diet for miniature pigs led to a decrease in the levels of TNF-α and IL-6, attributed to the suppression of inflammatory gene expression (48). Peroxisome proliferator-activated receptor-γ (PPAR-γ) is a transcription factor that has anti-inflammatory properties and regulates mitochondrial function. The genetic variation and change of its gene expression may lead to mitochondrial dysfunction, which plays a role in the pathogenesis of IR (49, 50). As a direct ligand of PPAR-γ, decanoic acid can bind and partially activate PPAR-γ, stimulating mitochondrial biogenesis and increasing mitochondrial complex I activity to enhance mitochondrial function and improve glucose sensitivity (50–52). Although higher DDA intake can reduce the prevalence of diabetes and not prediabetes, we still recommend that they all need to increase the intake of decanoic acid, whether in people with normal blood glucose level or prediabetes. Because most prediabetes patients do not know their current status, these people can also slow down the progress to diabetes by supplementing appropriate decanoic acid.
As we discussed, decanoic acid, as one of MCFAs, has shown a positive effect in reducing the risk of diabetes. Other fatty acids also play different roles in the management of diabetes. The impact of fatty acids on metabolism varies with the length and saturation of the carbon chain. LCFAs have longer carbon chains compared to MCFAs. LCFAs such as palmitic acid may trigger insulin resistance in pancreatic beta cells by activating c-Jun N-terminal kinase expression (53). Excessive intake of LCFAs can cause lipid accumulation in the body, leading to lipotoxicity and insulin resistance (54). Long chain unsaturated fatty acids may promote the secretion of GLP-1 by activating the expression of G protein coupled receptors 120 (GPR120), thereby increasing circulating insulin (55).
The results of stratified analysis showed that among people with education level of college or above, DDA was still negatively correlated with the prevalence of diabetes in prediabetes patients, and there was interaction. Education level is an unchangeable social risk factor (56). A survey of educational differences among people with diabetes from 232 Latin American cities showed that there was a negative dose–response relationship between educational level and diabetes prevalence (57). In addition, genetic evidence suggests that higher levels of genetic decision education are associated with lower risk of type 2 diabetes (58). Individuals with higher levels of education are usually able to effectively access and understand the latest health research information, and tend to adopt healthy lifestyles, such as regular exercise and a healthy diet, which helps maintain a healthy weight and insulin sensitivity. They may have a deeper understanding of healthy diet and dietary fatty acids, including knowledge of different types of fatty acids in their diet. They may be more inclined to choose a diet containing healthy fats, which may indirectly affect the intake and metabolism of decanoic acid and reduce the risk of diabetes.
This study has some obvious advantages. Firstly, this study used data from NHANES 2005–2016, covering 6 cycles for a total of 12 years. This is a nationally representative large-scale sample database that ensures the accuracy of research results. Secondly, we established multiple models and adjusted for various potential confounding factors in order to reduce interference from other factors. In addition, we studied the relationship between DDA and people with different blood glucose status to ensure the reliability of the study. However, there are also some limitations that cannot be ignored. In the study, the diagnosis of patients with diabetes and prediabetes partly depends on the patient’s self-report, and dietary data was obtained through two 24-h dietary recalls, which may introduce memory bias to affect accuracy. This study adopts a cross-sectional design. We found that there is a negative correlation between DDA and the increased risk of diabetes, but it is difficult to determine the chronological order or causal relationship between them. Although the study has adjusted for multiple potential confounding factors, there may still be other unknown confounding factors that may affect the accurate assessment of true correlations. This study is based on American adults, and the conclusions drawn may not be applicable to populations in other countries.
Conclusion
Our study found that higher DDA intake was associated with lower prevalence of diabetes among prediabetic patients, suggesting that DDA is a protective factor for diabetes. In the population with high education level, this relationship still holds and there is interaction.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found: https://www.cdc.gov/nchs/nhanes/index.htm.
Ethics statement
The studies involving humans were approved by NCHS Ethics Review Board (ERB). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
HZ: Conceptualization, Data curation, Formal analysis, Writing – original draft. QF: Conceptualization, Writing – original draft. RC: Supervision, Validation, Data curation, Writing – original draft. LL: Formal analysis, Methodology, Writing – original draft. MY: Data curation, Writing – review & editing. YZ: Investigation, Software, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by Natural Science Foundation of Jiangxi Province-Youth Fund Project (Nos. 20212BAB216045 and 20224BAB216013) and the National Natural Science Foundation of China-Regional Program (No. 82360083).
Acknowledgments
We sincerely thank all the participants and staff of the NHANES project. We also acknowledge the BioRender.com to provide the image materials.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1483045/full#supplementary-material
References
1. Tinajero, MG, and Malik, VS. An update on the epidemiology of type 2 diabetes: a global perspective. Endocrinol Metab Clin N Am. (2021) 50:337–55. doi: 10.1016/j.ecl.2021.05.013
2. Harding, JL, Pavkov, ME, Magliano, DJ, Shaw, JE, and Gregg, EW. Global trends in diabetes complications: a review of current evidence. Diabetologia. (2019) 62:3–16. doi: 10.1007/s00125-018-4711-2
3. Fu, Q, Hu, L, Xu, Y, Yi, Y, and Jiang, L. High lipoprotein(a) concentrations are associated with lower type 2 diabetes risk in the Chinese Han population: a large retrospective cohort study. Lipids Health Dis. (2021) 20:76. doi: 10.1186/s12944-021-01504-x
4. Lovic, D, Piperidou, A, Zografou, I, Grassos, H, Pittaras, A, and Manolis, A. The growing epidemic of diabetes mellitus. Curr Vasc Pharmacol. (2020) 18:104–9. doi: 10.2174/1570161117666190405165911
5. Saeedi, P, Petersohn, I, Salpea, P, Malanda, B, Karuranga, S, Unwin, N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas. Diabetes Res Clin Pract. (2019) 157:107843. doi: 10.1016/j.diabres.2019.107843
6. Roglic, G, Unwin, N, Bennett, PH, Mathers, C, Tuomilehto, J, Nag, S, et al. The burden of mortality attributable to diabetes: realistic estimates for the year 2000. Diabetes Care. (2005) 28:2130–5. doi: 10.2337/diacare.28.9.2130
7. Biadgo, B, and Abebe, M. Type 2 diabetes mellitus and its association with the risk of pancreatic carcinogenesis: a review. Korean J Gastroenterol. (2016) 67:168–77. doi: 10.4166/kjg.2016.67.4.168
8. Anderson, RJ, Freedland, KE, Clouse, RE, and Lustman, PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. (2001) 24:1069–78. doi: 10.2337/diacare.24.6.1069
9. Marshall, SM, and Flyvbjerg, A. Prevention and early detection of vascular complications of diabetes. BMJ. (2006) 333:475–80. doi: 10.1136/bmj.38922.650521.80
10. Juster-Switlyk, K, and Smith, AG. Updates in diabetic peripheral neuropathy. F1000Res. (2016) 5:5. doi: 10.12688/f1000research.7898.1
11. Dal Canto, E, Ceriello, A, Rydén, L, Ferrini, M, Hansen, TB, Schnell, O, et al. Diabetes as a cardiovascular risk factor: an overview of global trends of macro and micro vascular complications. Eur J Prev Cardiol. (2019) 26:25–32. doi: 10.1177/2047487319878371
12. Williams, R, Karuranga, S, Malanda, B, Saeedi, P, Basit, A, Besançon, S, et al. Global and regional estimates and projections of diabetes-related health expenditure: results from the international diabetes federation diabetes atlas. Diabetes Res Clin Pract. (2020) 162:108072. doi: 10.1016/j.diabres.2020.108072
13. Khan, RMM, Chua, ZJY, Tan, JC, Yang, Y, Liao, Z, and Zhao, Y. From pre-diabetes to diabetes: diagnosis, treatments and translational research. Medicina. (2019) 55:546. doi: 10.3390/medicina55090546
14. Tabák, AG, Herder, C, Rathmann, W, Brunner, EJ, and Kivimäki, M. Prediabetes: a high-risk state for diabetes development. Lancet. (2012) 379:2279–90. doi: 10.1016/S0140-6736(12)60283-9
15. Gossain, VV, and Aldasouqi, S. The challenge of undiagnosed pre-diabetes, diabetes and associated cardiovascular disease. Int J Diabetes Mellit. (2010) 2:43–6. doi: 10.1016/j.ijdm.2009.10.004
16. DeFronzo, RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. (2004) 88:787–835. doi: 10.1016/j.mcna.2004.04.013
17. Stumvoll, M, Goldstein, BJ, and van Haeften, TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. (2005) 365:1333–46. doi: 10.1016/S0140-6736(05)61032-X
18. Donath, MY, and Shoelson, SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. (2011) 11:98–107. doi: 10.1038/nri2925
19. McMacken, M, and Shah, S. A plant-based diet for the prevention and treatment of type 2 diabetes. J Geriatr Cardiol. (2017) 14:342–54. doi: 10.11909/j.issn.1671-5411.2017.05.009
20. Cea-Soriano, L, Pulido, J, Franch-Nadal, J, Santos, JM, Mata-Cases, M, Díez-Espino, J, et al. Mediterranean diet and diabetes risk in a cohort study of individuals with prediabetes: propensity score analyses. Diabet Med. (2022) 39:e14768. doi: 10.1111/dme.14768
21. Calder, PC. Functional roles of fatty acids and their effects on human health. JPEN J Parenter Enteral Nutr. (2015) 39:18s–32s. doi: 10.1177/0148607115595980
22. Bhat, S, Sarkar, S, Zaffar, D, Dandona, P, and Kalyani, RR. Omega-3 fatty acids in cardiovascular disease and diabetes: a review of recent evidence. Curr Cardiol Rep. (2023) 25:51–65. doi: 10.1007/s11886-022-01831-0
23. Jadhav, HB, and Annapure, US. Triglycerides of medium-chain fatty acids: a concise review. J Food Sci Technol. (2023) 60:2143–52. doi: 10.1007/s13197-022-05499-w
24. Jensen, RG. The composition of bovine milk lipids: January 1995 to December 2000. J Dairy Sci. (2002) 85:295–350. doi: 10.3168/jds.S0022-0302(02)74079-4
25. Wu, Z, Yang, W, Li, M, Li, F, Gong, R, and Wu, Y. Relationship between dietary Decanoic acid and coronary artery disease: a population-based Cross-sectional study. Nutrients. (2023) 15:4308. doi: 10.3390/nu15204308
26. Chang, P, Augustin, K, Boddum, K, Williams, S, Sun, M, Terschak, JA, et al. Seizure control by decanoic acid through direct AMPA receptor inhibition. Brain. (2016) 139:431–43. doi: 10.1093/brain/awv325
27. Han, FY, Conboy-Schmidt, L, Rybachuk, G, Volk, HA, Zanghi, B, Pan, Y, et al. Dietary medium chain triglycerides for management of epilepsy: new data from human, dog, and rodent studies. Epilepsia. (2021) 62:1790–806. doi: 10.1111/epi.16972
28. Augustin, K, Khabbush, A, Williams, S, Eaton, S, Orford, M, Cross, JH, et al. Mechanisms of action for the medium-chain triglyceride ketogenic diet in neurological and metabolic disorders. Lancet Neurol. (2018) 17:84–93. doi: 10.1016/S1474-4422(17)30408-8
29. Huang, L, Gao, L, and Chen, C. Role of medium-chain fatty acids in healthy metabolism: a clinical perspective. Trends Endocrinol Metab. (2021) 32:351–66. doi: 10.1016/j.tem.2021.03.002
30. Forouhi, NG, Luan, J, Hennings, S, and Wareham, NJ. Incidence of type 2 diabetes in England and its association with baseline impaired fasting glucose: the Ely study 1990-2000. Diabet Med. (2007) 24:200–7. doi: 10.1111/j.1464-5491.2007.02068.x
31. Tuso, P. Prediabetes and lifestyle modification: time to prevent a preventable disease. Perm J. (2014) 18:88–93. doi: 10.7812/TPP/14-002
32. Glechner, A, Keuchel, L, Affengruber, L, Titscher, V, Sommer, I, Matyas, N, et al. Effects of lifestyle changes on adults with prediabetes: a systematic review and meta-analysis. Prim Care Diabetes. (2018) 12:393–408. doi: 10.1016/j.pcd.2018.07.003
33. Echouffo-Tcheugui, JB, Perreault, L, Ji, L, and Dagogo-Jack, S. Diagnosis and Management of Prediabetes: a review. JAMA. (2023) 329:1206–16. doi: 10.1001/jama.2023.4063
34. Cho, SS, Qi, L, Fahey, GC Jr, and Klurfeld, DM. Consumption of cereal fiber, mixtures of whole grains and bran, and whole grains and risk reduction in type 2 diabetes, obesity, and cardiovascular disease. Am J Clin Nutr. (2013) 98:594–619. doi: 10.3945/ajcn.113.067629
35. Galaviz, KI, Narayan, KMV, Lobelo, F, and Weber, MB. Lifestyle and the prevention of type 2 diabetes: a status report. Am J Lifestyle Med. (2018) 12:4–20. doi: 10.1177/1559827615619159
36. Malik, VS, Popkin, BM, Bray, GA, Després, JP, Willett, WC, and Hu, FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care. (2010) 33:2477–83. doi: 10.2337/dc10-1079
37. Zhu, H, Bi, D, Zhang, Y, Kong, C, du, J, Wu, X, et al. Ketogenic diet for human diseases: the underlying mechanisms and potential for clinical implementations. Signal Transduct Target Ther. (2022) 7:11. doi: 10.1038/s41392-021-00831-w
38. Papamandjaris, AA, MacDougall, DE, and Jones, PJ. Medium chain fatty acid metabolism and energy expenditure: obesity treatment implications. Life Sci. (1998) 62:1203–15. doi: 10.1016/S0024-3205(97)01143-0
39. Dyńka, D, Kowalcze, K, and Paziewska, A. The role of ketogenic diet in the treatment of neurological diseases. Nutrients. (2022) 14:5003. doi: 10.3390/nu14235003
40. DeFronzo, RA, and Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. (2009) 32:S157–63. doi: 10.2337/dc09-S302
41. Nonaka, H, Ohue-Kitano, R, Masujima, Y, Igarashi, M, and Kimura, I. Dietary medium-chain triglyceride Decanoate affects glucose homeostasis through GPR84-mediated GLP-1 secretion in mice. Front Nutr. (2022) 9:848450. doi: 10.3389/fnut.2022.848450
42. Abe, T, Hirasaka, K, Kohno, S, Tomida, C, Haruna, M, Uchida, T, et al. Capric acid up-regulates UCP3 expression without PDK4 induction in mouse C2C12 Myotubes. J Nutr Sci Vitaminol. (2016) 62:32–9. doi: 10.3177/jnsv.62.32
43. Luc, K, Schramm-Luc, A, Guzik, TJ, and Mikolajczyk, TP. Oxidative stress and inflammatory markers in prediabetes and diabetes. J Physiol Pharmacol. (2019) 70. doi: 10.26402/jpp.2019.6.01
44. Brahimaj, A, Ligthart, S, Ghanbari, M, Ikram, MA, Hofman, A, Franco, OH, et al. Novel inflammatory markers for incident pre-diabetes and type 2 diabetes: the Rotterdam study. Eur J Epidemiol. (2017) 32:217–26. doi: 10.1007/s10654-017-0236-0
45. Tsigos, C, Papanicolaou, DA, Kyrou, I, Defensor, R, Mitsiadis, CS, and Chrousos, GP. Dose-dependent effects of recombinant human interleukin-6 on glucose regulation. J Clin Endocrinol Metab. (1997) 82:4167–70. doi: 10.1210/jcem.82.12.4422
46. Stagakis, I, Bertsias, G, Karvounaris, S, Kavousanaki, M, Virla, D, Raptopoulou, A, et al. Anti-tumor necrosis factor therapy improves insulin resistance, beta cell function and insulin signaling in active rheumatoid arthritis patients with high insulin resistance. Arthritis Res Ther. (2012) 14:R141. doi: 10.1186/ar3874
47. Huang, WC, Tsai, TH, Chuang, LT, Li, YY, Zouboulis, CC, and Tsai, PJ. Anti-bacterial and anti-inflammatory properties of capric acid against Propionibacterium acnes: a comparative study with lauric acid. J Dermatol Sci. (2014) 73:232–40. doi: 10.1016/j.jdermsci.2013.10.010
48. Lee, SI, and Kang, KS. Function of capric acid in cyclophosphamide-induced intestinal inflammation, oxidative stress, and barrier function in pigs. Sci Rep. (2017) 7:16530. doi: 10.1038/s41598-017-16561-5
49. Mootha, VK, Lindgren, CM, Eriksson, KF, Subramanian, A, Sihag, S, Lehar, J, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. (2003) 34:267–73. doi: 10.1038/ng1180
50. Hughes, SD, Kanabus, M, Anderson, G, Hargreaves, IP, Rutherford, T, Donnell, MO, et al. The ketogenic diet component decanoic acid increases mitochondrial citrate synthase and complex I activity in neuronal cells. J Neurochem. (2014) 129:426–33. doi: 10.1111/jnc.12646
51. Malapaka, RRV, Khoo, S, Zhang, J, Choi, JH, Zhou, XE, Xu, Y, et al. Identification and mechanism of 10-carbon fatty acid as modulating ligand of peroxisome proliferator-activated receptors. J Biol Chem. (2012) 287:183–95. doi: 10.1074/jbc.M111.294785
52. Simeone, TA, Matthews, SA, Samson, KK, and Simeone, KA. Regulation of brain PPARgamma2 contributes to ketogenic diet anti-seizure efficacy. Exp Neurol. (2017) 287:54–64. doi: 10.1016/j.expneurol.2016.08.006
53. Zhu, X, Chen, L, Lin, J, Ba, M, Liao, J, Zhang, P, et al. Association between fatty acids and the risk of impaired glucose tolerance and type 2 diabetes mellitus in American adults: NHANES 2005-2016. Nutr Diabetes. (2023) 13:8. doi: 10.1038/s41387-023-00236-4
54. Jiang, LP, and Sun, HZ. Long-chain saturated fatty acids and its interaction with insulin resistance and the risk of nonalcoholic fatty liver disease in type 2 diabetes in Chinese. Front Endocrinol. (2022) 13:1051807. doi: 10.3389/fendo.2022.1051807
55. Hirasawa, A, Tsumaya, K, Awaji, T, Katsuma, S, Adachi, T, Yamada, M, et al. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. (2005) 11:90–4. doi: 10.1038/nm1168
56. Salzberg, L. Risk factors and lifestyle interventions. Prim Care. (2022) 49:201–12. doi: 10.1016/j.pop.2021.11.001
57. Braverman-Bronstein, A, Hessel, P, González-Uribe, C, Kroker, MF, Diez-Canseco, F, Langellier, B, et al. Association of education level with diabetes prevalence in Latin American cities and its modification by city social environment. J Epidemiol Community Health. (2021) 75:874–80. doi: 10.1136/jech-2020-216116
Keywords: decanoic acid, diabetes, prediabetes, NHANES, dietary fatty acids
Citation: Zhu H, Fu Q, Chen R, Luo L, Yu M and Zhou Y (2025) Association of dietary decanoic acid intake with diabetes or prediabetes: an analysis from NHANES 2005–2016. Front. Nutr. 11:1483045. doi: 10.3389/fnut.2024.1483045
Edited by:
Luciana Neri Nobre, Universidade Federal dos Vales do Jequitinhonha e Mucuri (UFVJM), BrazilReviewed by:
Kulvinder Kochar Kaur, Kulvinder Kaur Centre for Human Reproduction, IndiaJerzy Beltowski, Medical University of Lublin, Poland
Copyright © 2025 Zhu, Fu, Chen, Luo, Yu and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Miao Yu, aGFwcHlfeXVtaWFvQDE2My5jb20=; Yue Zhou, emhvdXl1ZTIwMjRAeWVhaC5uZXQ=
†These authors have contributed equally to this work
 Huangxin Zhu
Huangxin Zhu Qingan Fu
Qingan Fu Ruxin Chen
Ruxin Chen Linfei Luo2
Linfei Luo2 Yue Zhou
Yue Zhou