- 1College of Medicine and Health Sciences, Wollo University, Dessie, Ethiopia
- 2Food Science and Nutrition, Addis Ababa University, Addis Ababa, Ethiopia
Background: Antioxidant supplements are widely used during cancer treatment to prevent oxidative stress, reduce treatment toxicities, and improve patient outcomes. However, current literature reveals significant gaps suggesting that antioxidants may protect both healthy and tumor cells from oxidative damage, thereby reducing treatment efficacy. It is for this reason that antioxidant supplements have become a source of therapeutic controversy.
Objective: To review therapeutic controversies over the use of antioxidant supplements during cancer treatment.
Methods: Scoping review of the international published articles following the Arksey and O’Malley framework, cross-sectional studies, clinical and pre-clinical studies, systematic and umbrella reviews and grey literatures published from 2014 to 2024 with all age patient populations were included. A structured literature search was conducted of CINAHL, EMBASE, MEDLINE, Google Scholar, using key medical subject heading words and Cochrane Collaboration and Joanna Briggs Institute databases. All included studies were reviewed independently by two investigators. Data were extracted, collated by type of antioxidants, summarized in tables and synthesized for analysis.
Result: A total of 1, 550 articles were identified. After reviewing all literatures, twenty-one (21) were full-text articles, grey literatures (2), and systematic reviews (42) and umbrella reviews (3), met the criteria for inclusion. In this review, the use of antioxidant supplements can benefit cancer cells in the same way as they do for normal cells during cancer treatment. In addition, not all antioxidants were effective in inhibiting oxidative stress, reduce treatment toxicities, and improve patient outcomes.
Conclusion and recommendations: According to this review, the use of antioxidant supplements can benefit tumor cells in the same manner as they do for normal cells. Therefore, oncologists should advise not to take antioxidant supplements during chemotherapy and/or radiotherapy. Future research including potential clinical and preclinical trials, mechanistic studies, and exploration of different vitamin and mineral supplement studies are required to uncover the complete potential of antioxidant supplements for cancer treatment or determine their safety and effectiveness when used alongside standard cancer treatments. Furthermore, the results of this review could be used for future systematic review of therapeutic controversies over use of antioxidant supplements during cancer treatment.
1 Introduction
Antioxidants are substances that prevent, delay, or remove the oxidative damage caused by reactive oxygen species (ROS) (1). These reactive oxygen species are produced by living cells as a metabolic byproduct and play a significant role as signaling molecules throughout the entire cell death process (2).
An excess of ROS can destroy organelles’ structure and biomolecules, leading to inflammatory responses and mutagenesis, and this is a known mechanism for the development of cancer (2, 3).
The term “cancer” refers “uncontrolled growth of abnormal cells in the body (4). After cardiovascular disease, cancer was the second-leading cause of death worldwide in 2018, with 9.6 million fatalities (5).
Cancer is believed to be the cause of 13.4% of fatalities globally (6).
By 2030, there will be 23.6 million new cancer cases globally each year, which is 68% more cases than present if existing patterns in the prevalence of major cancers and population growth are seen worldwide (7).
However, when cancer has developed, the death of malignant cells by treatment with chemotherapeutic drugs and radiation that produce free radicals in the cells, such as hydrogen peroxide and superoxide, mostly depends on their oxidative damage (8).
Chemotherapy and radiation therapy can cause the production of reactive oxygen species, which can damage DNA or interfere with DNA replication machinery through direct and indirect effects on malignant cells. This can lead to aberrations in various cellular signaling pathways, which can result in cellular toxicity and death from chemotherapy or radiation therapy (9, 10).
This being said, one potential remedy might involve use of antioxidants, which are mostly taken as supplements, together with radiation therapy and/or chemotherapy that causes cellular oxidative damage (11, 12). The primary antioxidants that help in preventing free radicals inside the human body are the vitamins (A, C, D, E, beta-carotene, and coenzyme Q10) and minerals (selenium and zinc) (13) (125). These supplements have the potential to shield normal cells from the oxidative stress secondary to some chemotherapy and radiation treatments. However, the same process may protect tumor cells and possibly minimize the effect of cancer therapies (11).
Despite nearly two decades of research, there is still no consensus on whether antioxidant supplementation is beneficial or harmful during cancer treatment. Limited systematic reviews have highlighted the potential risks associated with antioxidant use, suggesting that they may protect both healthy and tumor cells from oxidative damage, thereby reducing treatment efficacy. A comprehensive review would synthesize findings from various studies, including Systematic reviews, umbrella reviews, randomized controlled trials and observational studies, to provide clearer guidance for clinicians and patients alike (13, 14).
In addition, current literature reveals significant gaps regarding the interaction between antioxidant supplements and cancer therapies. Many systematic reviews have focused primarily on the reduction of side effects rather than on therapeutic efficacy. There is a pressing need to systematically assess how these supplements influence treatment outcomes, including safety, efficacy, and recurrence risks. A detailed review could help identify these gaps and propose directions for future research [Risks and benefits of antioxidant supplement use during cancer treatment (12)].
Furthermore, the landscape of cancer treatment is continuously evolving, with new therapies and regimens emerging regularly. Since previous reviews were published, there have been significant changes in both chemotherapy protocols and the types of antioxidant supplements available. An updated review would reflect these advancements and ensure that healthcare providers are equipped with the latest information (12).
Therefore, the objective of the present review was to systematically map out the body of existing literature on therapeutic controversies over the use of antioxidant supplements during cancer treatment in order to identify type of antioxidant supplements that have been commonly given for patients, treatment outcomes reported, summarize the safety and effectiveness of these supplements during cancer treatment in the various clinical settings and opportunities for further research.
2 Methods
The review followed established methodological approaches for scoping reviews using the framework presented by Arskey and O’Malley for the conduct of scoping reviews (15). Scoping reviews aim to identify and describe the breadth of literature on a topic when it is either highly complex, involves a broad array of study designs, or when a comprehensive review is being completed for the first time; all of these factors apply to the present review. Scoping reviews aim to map key concepts in a field of study and the available types of evidence. The review is completed in a way that is systematic, highly rigorous, and transparent in order to minimize bias. Briefly, the sequential stages of the process were: identifying the research question, identifying the relevant literature, selecting the literature, charting the data and, collating, summarizing and reporting results.
2.1 Identifying the research questions
This review was guided by the following inter-related queries:
What type of antioxidant supplements have been commonly given for patients during cancer treatment in the various clinical settings?
What treatment outcomes have been reported in the published literature?
Based on the reported outcomes, are antioxidant supplements effective for patients during cancer treatment?
2.2 Identifying relevant literature
A two-tiered search strategy was used. First, CINAHL, EMBASE and MEDLINE databases were searched for relevant published articles. Hand-searching of reference lists on key papers and web-based search of the grey literature, such as Google Scholar and professional and government websites were performed with the same terms used for the published articles. Systematic reviews were retrieved from the Cochrane Library and the Joanna Briggs Institute EBP database. Second, references from the retrieved systematic reviews were screened to ensure that all relevant primary studies were included in this scoping review. Articles published in English, between January 2014 and December 2024 were retrieved. This time period was selected to enable identification and inclusion of recent literatures. Search terms and linked terms included: antioxidant OR supplement OR vitamin A OR vitamin C OR vitamin D OR vitamin E OR selenium OR zinc OR coenzyme Q10 OR cancer OR treatment OR therapy OR chemotherapy OR radiotherapy OR immunotherapy OR controversies OR oncology OR patients. This search yielded 1, 158 articles. The second-tier search involved examining the bibliographies of retrieved key articles to identify additional relevant studies and seminal articles from the literature; this process identified an additional 392 articles for a total of 1, 550 articles.
2.3 Selecting the literature (inclusion and exclusion criteria)
Articles included in this scoping review met specified inclusion criteria: (i) cross-sectional studies, pre-clinical studies, randomized controlled trial design, systematic and umbrella reviews, grey literatures published from 2014 to 2024 and (ii) all age patient populations.
Articles excluded in this scoping review were publications other than English language and those who were in Beall’s list are excluded from this review.
2.4 Charting the data
Following initial screening, two reviewers (MWS, AT) extracted three specific components using a standardized form: (i) key elements of the antioxidant supplements, (ii) the most frequently reported outcome measures used to assess therapeutic controversies, and (iii) data evaluating safety and effectiveness of the supplements.
2.5 Collating, summarizing, and reporting the literature
We provided a brief history and rationale for the reviewing the topic, a summary of the antioxidant supplements as well as outcome measures and trend of safety and effectiveness of the antioxidant supplements. For each study, outcomes were summarized and reported as better, equivalent or worse than not taking the supplements. In cases where multiple outcome measures were evaluated within the same category, the outcome was reported using majority rule.
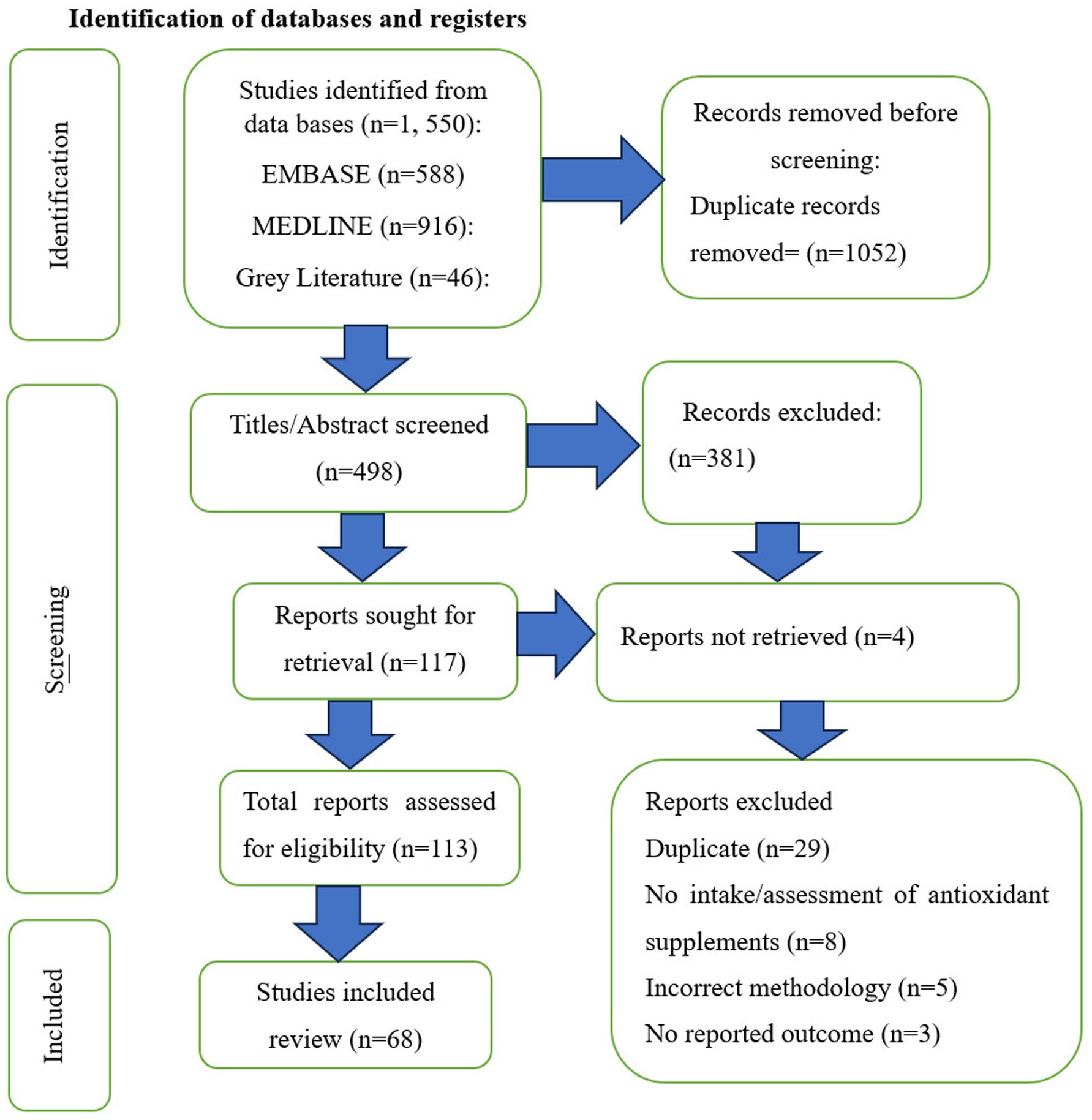
2.5.1 Identification of databases and registers
See Figure 1 for PRISMA flow diagram.
3 Results and discussion
To the best of our knowledge, this scoping review is the first highlighting the studies that reported the therapeutic controversies of anti-oxidant supplements during cancer treatment. The aim of cancer treatment should be the complete removal of malignant cells and the reduction of therapy-induced cellular toxicity in healthy tissues. Another aim should be the detoxification of detrimental effects after treatment (16). Hence, this review highlights the therapeutic controversies over the use of antioxidant supplements in cancer treatment.
3.1 Therapeutic controversies over use of vitamin A supplement during cancer treatment
During cancer treatment, giving patients vitamin A supplements can help improve the overall health and enhance survival rates. It also reduces adverse effects and damage to healthy tissues (17).
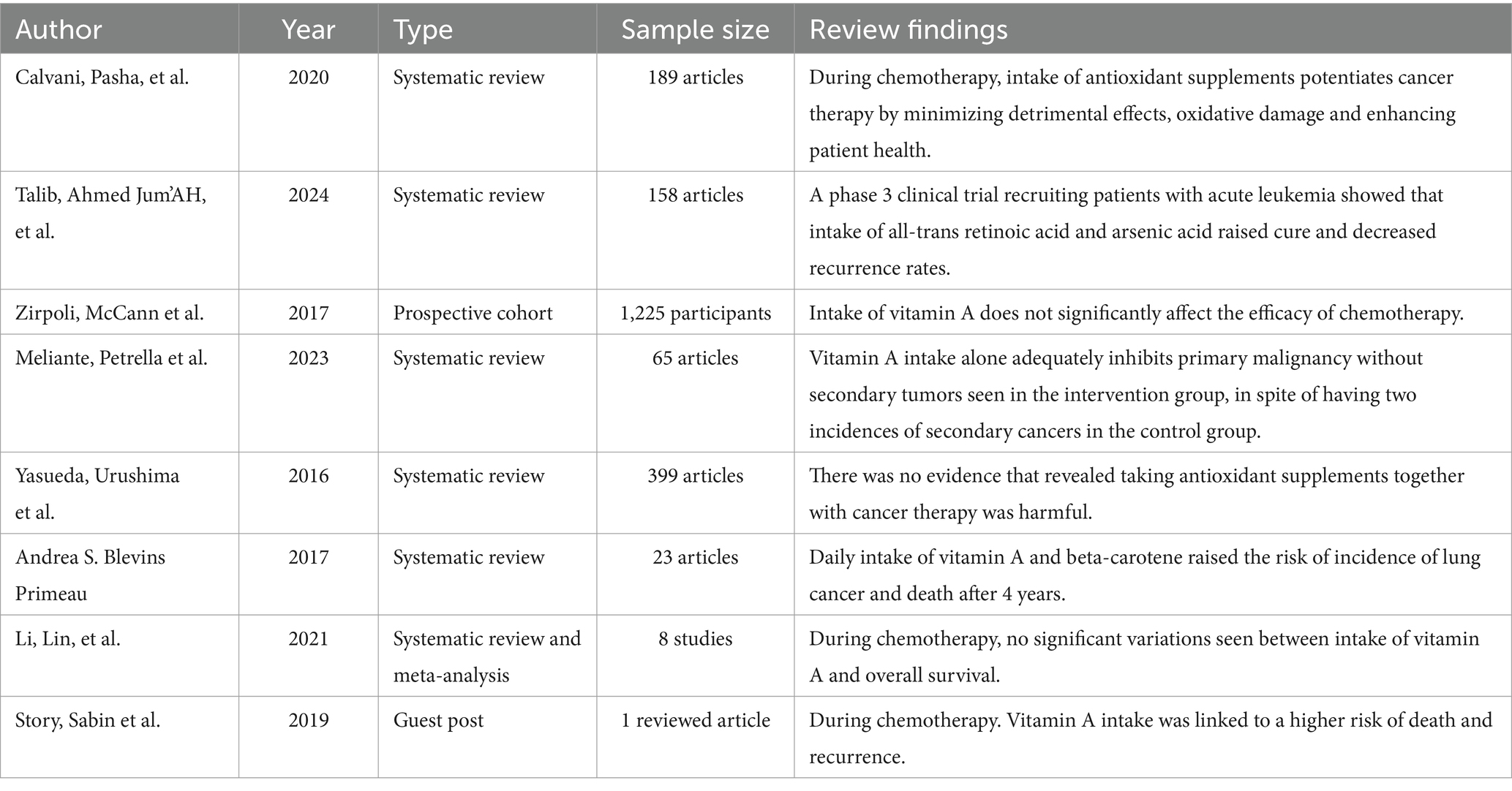
In a study with 235 patients with acute promyelocytic disease, they received a combination of arsenic acid and all-trans retinoic acid (45 mg/m2). The results showed a lower chance of cancer recurrence and a higher chance of complete cure (18).
A similar study showed that vitamin A supplementation seems safe and does not impede with chemotherapy effectiveness in majority of patients (74).
Another study revealed that giving vitamin A supplementation (8,000 IU/8 h) along with neoadjuvant chemotherapy for advanced cervical carcinoma. This approach showed a significantly improved therapeutic response in these patients (18).
In addition, when treating low-risk gestational trophoblastic neoplasia with methotrexate, oral vitamin A supplements (6,000 IU/day) were found to decrease tumor markers and chemoresistance in these patients (18). Research by Zirpoli et al. (19) found that chemotherapy efficiency was not significantly affected by taking the antioxidant vitamin A.
The EUROSCAN study also reported that retinyl palmitate alone helped prevent primary cancers when given regularly throughout the year. Participants who received vitamin A did not develop secondary cancers during the study period (20).
According to a 2015 review study, there was no evidence to suggest that taking vitamin A supplements alongside cancer therapy is detrimental (13).
On the contrary, meta-analysis research showed that no significant difference in overall survival between individuals who took antioxidant vitamin A during chemotherapy and those who did not (21).
Additionally, taking high doses of vitamin A coupled with beta-carotene daily was associated with an increased risk of lung cancer mortality after 4 years in the CARET trial. There was also an extended risk of death for up to 6 years following supplementation (74).
Moreover, vitamin A supplementation during chemotherapy was linked to a significantly higher risk of death and recurrence in cancer patients (22).
Both radiation and chemotherapy aim to damage malignant cells or increase oxidative stress around them to induce cell death efficiently (23). Meanwhile, antioxidants like vitamin A help repair cell damage by fighting oxidative damage. This function might counteract the expected effects of chemotherapy and radiotherapy by inadvertently protecting cancer cells as well. This discrepancy in goal could significantly increase the risks associated with vitamin A supplement during cancer treatment (75) (Table 1).
3.2 Therapeutic controversies over use of beta-carotene supplement during cancer treatment
Certain studies showed that ß-carotene can provide protection to the body. It achieves this by boosting the immune system through the activation of T-and B-cells, increasing the number of T-helper cells, and enhancing the activity of natural killer cells (24).
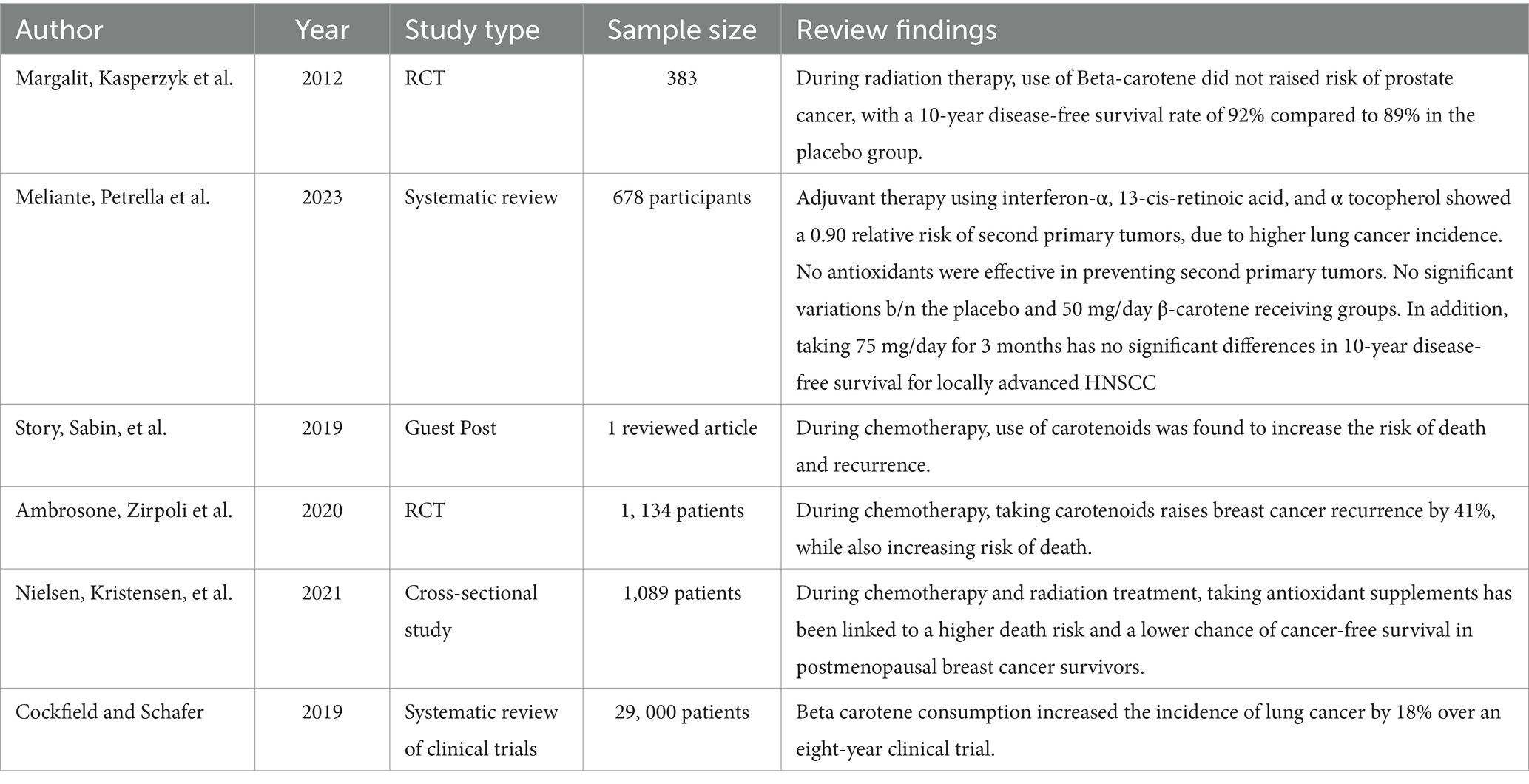
In the context of prostate cancer metastases or death, the consumption of beta-carotene during radiation therapy does not appear to increase the risk. Over a period of 10 years, the group that received the placebo had an 89% survival rate without the disease, while the group that received beta-carotene had a 92% survival rate (25).
A randomized controlled trial involving 264 patients on cancer treatment were assigned into a placebo, while the other group took 50 mg/day of beta-carotene. The investigators followed the patients for 51 months. Finally, no observed differences were seen between the two groups (20).
In addition, similar study also examined 214 patients who took 75 mg/day of beta-carotene regularly for 3 years. After a follow-up period of 59 months, they observed no significant variations in disease-free survival rates or the development of secondary neoplasms (20).
Contrary to this, giving carotenoids with chemotherapy treatment was linked to a higher chance of death and recurrence (22). Similarly, it was also reported that individuals who took carotenoids while undergoing chemotherapy had an increased chance of breast cancer recurrence (26).
Taking antioxidant supplements while undergoing chemotherapy and radiation therapy could potentially result in increased mortality rates and reduced likelihood of remaining cancer-free in breast cancer survivors (27).
In one trial, researchers combined interferon-α with additional medications to treat advanced head and neck cancer that had spread to nearby tissues. The likelihood of developing a second primary tumor was found to be low, but there was a higher occurrence of lung cancers in the experimental group (20).
A long-term clinical trial, spanning 8 years and involving nearly twenty-nine thousand male smokers, aimed to investigate the potential anti-cancer effects of beta-carotene. Astonishingly, male smokers who consumed beta-carotene experienced a 20% higher risk of developing lung cancer (28).
The American Cancer Society states that beta-carotene intake can enhance the strength of white blood cells in our immune system by preventing the harmful effects of free radicals that can harm cells (29).
Radiation therapy and numerous chemotherapy drugs function by inducing tumor cell death through their pro-oxidant effects on DNA. Beta-carotene supplements could potentially disrupt this process, diminishing the effectiveness of the treatment (25) (Table 2).
3.3 Therapeutic controversies over use of vitamin C supplement during cancer treatment
Vitamin C supplements are often taken as immune system boosters, especially during cancer treatment (30). Vitamin C supplements use varying formats. Data is conflicted about this, with some studies showing benefits and others potential risks (31).
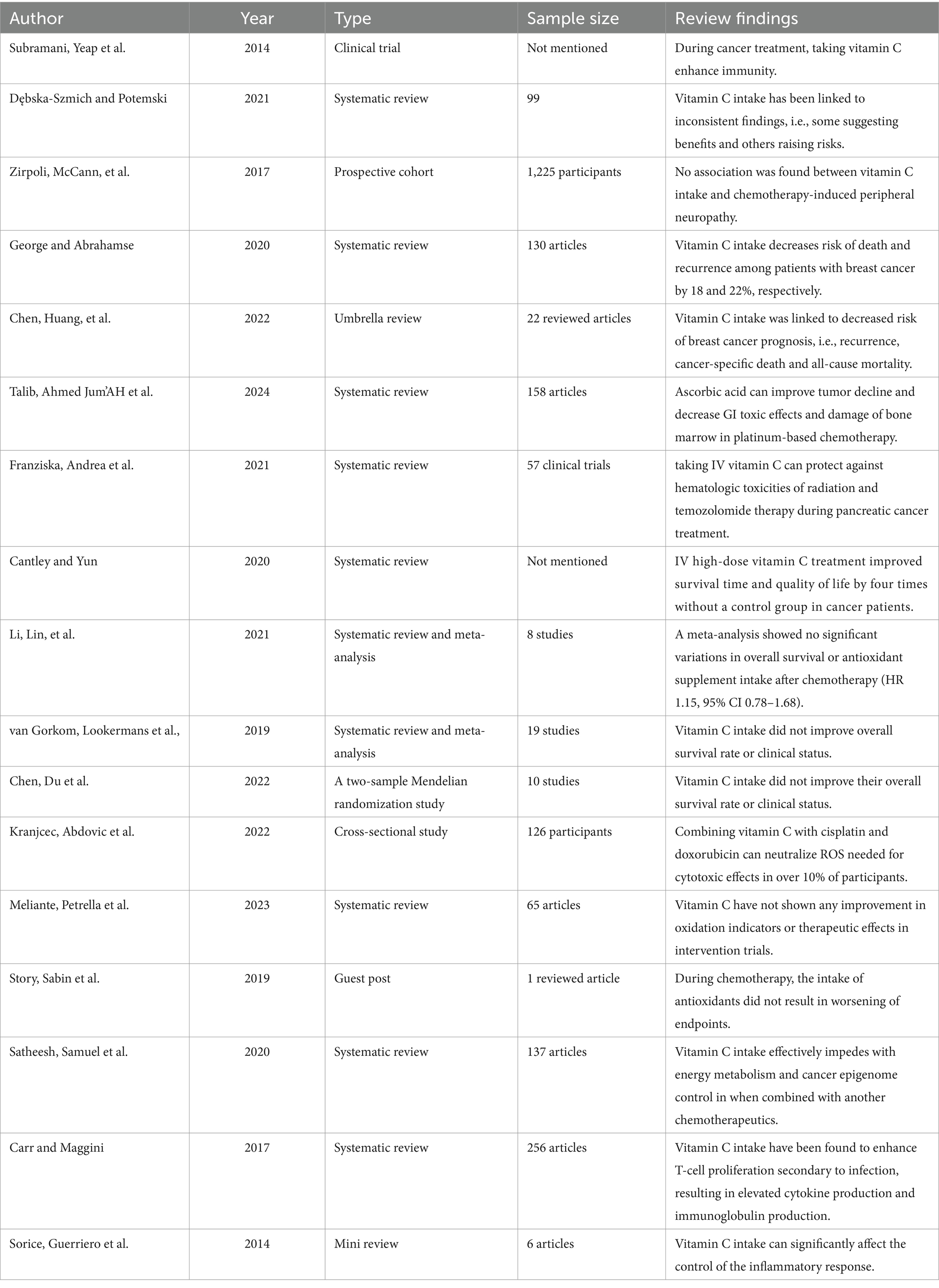
Among breast cancer patients, there wasn’t an association between use of vitamin C supplements during treatment and chemotherapy-induced peripheral neuropathy (IPN) (19).
In a similar study, people who use vitamin C as an antioxidant had a hazard ratio (HR) of 0.82, with a 95% confidence interval (CI) of 0.65–1.02. This means an 18% lower risk of death and a 22% lower chance of cancer recurrence (HR = 0.78, 95% CI: 0.63–0.95) (32). Another study discovered that using antioxidant vitamin C could lower the risk of breast cancer coming back again (11).
Additionally, taking vitamin C supplements has also been tied to a lesser chance of dying from anything, death due to cancer specifically, or having breast cancer return again (33).
A clinical test also found that combining pharmacological ascorbate with platinum-based chemotherapy helped with tumor shrinkage in patients with advanced non-small cell lung cancer. The objective response rate was 34.2%, and the disease-control rate was 84.2% (18). Similarly, a study showed that adding pharmacological ascorbic acid along with mFOLFOX6 or FOLFIRI lowered gastrointestinal side effects significantly and reduced bone marrow problems in patients of various grades (18).
According to Franziska et al. (34), giving intravenous vitamin C may have shielded against blood-related issues when treating patients with pancreatic cancer rather than using radiation therapy plus temozolomide therapy for glioblastoma alone in one study group’s research results. Another study found that high doses of intravenous vitamin C given to cancer patients displayed better quality of life and four times longer survival periods on average. However, this study did not have a group given a placebo for comparison purposes (35).
Nonetheless, meta-analyses showed no clear difference in general survival or antioxidant use post-chemo treatment [HR1.15; CI: 0.78–1.68; (21)]. Similar studies found that taking vitamin C did not improve the overall survival or health conditions of cancer patients across 19 clinical trials (33, 36).
Similarly, it was suggested in another study that the co-administration of the antioxidant vitamin C with chemotherapy drugs such as cisplatin and doxorubicin could decrease ROS necessary for medication-mediated cell death in more than 10% of subjects (37).
Further to this finding, being involved in trials with vitamin C did not show any significant changes in bio-oxidative indicators or pharmacodynamics endpoints (20). Additionally, contrastive worst endpoints were observed in trial use of anti-oxidants concurrent with chemotherapy (22).
Soluble vitamins such as Vitamin C are especially supportive of resisting the damage wrought upon our non-stop oxidation and inflammation by free radicals (18).
When treating cancer stem cells, vitamin C use impacts energy processes, cancer controls change positively, and intake could work alongside other chemotherapy therapy medications (38).
Reports have suggested that by taking vitamin C supplements, our T-cells can grow when dealing with infections, followed by more cytokine production and antibody creation. The way our body regulates inflammation may be largely influenced by vitamin C (39, 73).
However, taking Vitamin C during chemotherapy might reduce how well the treatment works because it shields out lipid peroxidation linked to chemo drugs like Tamoxifen.
Vitamin C can also cause mitochondrial potential levels to drop within cancer cells (40) (Table 3).
3.4 Therapeutic controversies over use of vitamin D supplement during cancer treatment
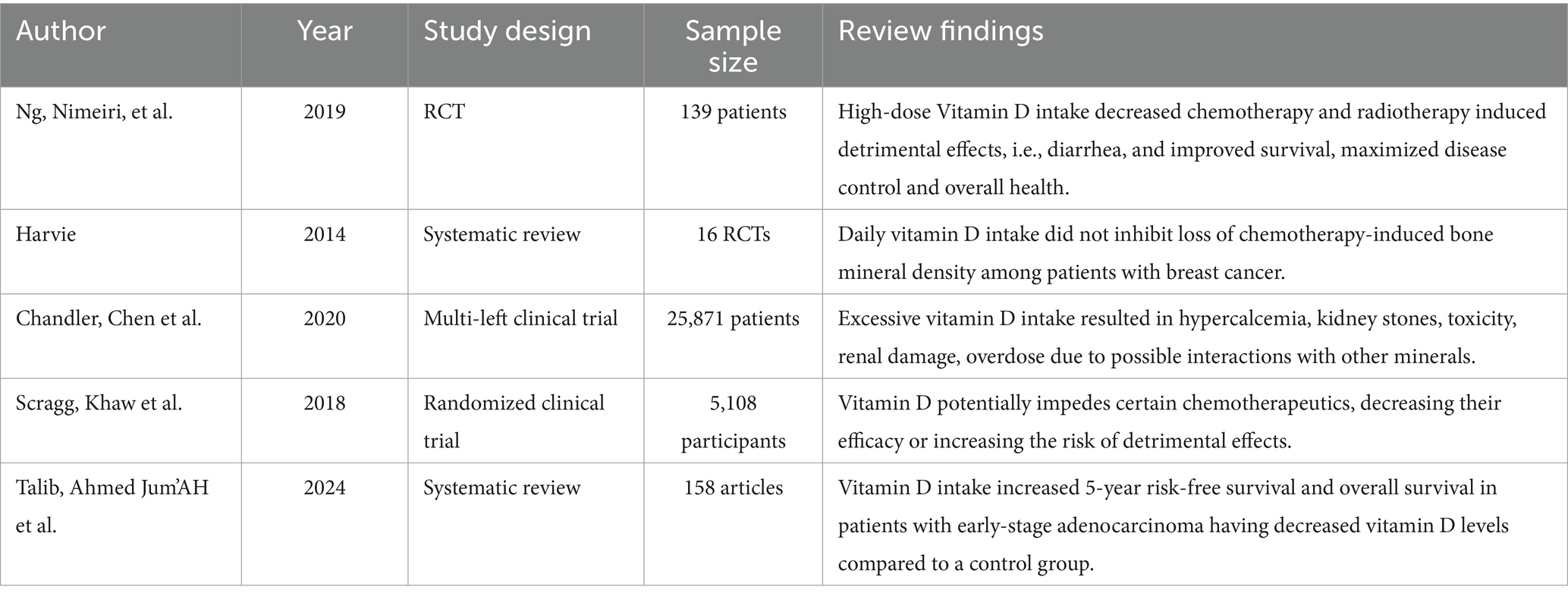
Vitamin D can prevent oxidative and cellular damage, which can reduce the detrimental effects of chemotherapy and radiotherapy. For example, high-dose vitamin D supplementation (8,000 IU/day vs. 400 IU/day) enhances survival without deterioration in colorectal cancer patients, decreases diarrhea (grades 3 and 4) and toxic effects, and maximizes the ability to control illness rates, which are 96% in the high-dose group and 84% in the low-dose group (41). The same authors also reported that having normal vitamin D levels was linked to better outcomes for many cancer patients, improved quality of life, and overall health (41).
Among patients with early-stage adenocarcinoma with low levels of vitamin D, a study concluded that vitamin D supplementation significantly outscored the placebo group for five-year survival (86% vs. 50%, p = 0.04) and overall survival (91% vs. 48%, p = 0.02) (18).
On the other hand, a recent analysis of 16 studies including breast cancer patients found that taking 5 to 25 g of vitamin D daily did not control the chemotherapy-induced loss of bone mineral density (42).
In addition, excessive vitamin D intake can result in hypercalcemia, toxicity, renal damage, and overdose, which can all be fatal. Additionally, these dosages can increase the risk of kidney stones, particularly in those with renal impairments. Likewise, there is a chance that vitamin D will interact negatively with other minerals, including phosphorus, magnesium, and calcium, leading to imbalances (43).
Furthermore, there is a chance that vitamin D will interfere with some chemotherapy medications, reducing their effectiveness or increasing the likelyhood of adverse effects. For instance, vitamin D can intensify the adverse effects of some chemotherapy drugs, such as doxorubicin (44).
Similarly, when vitamin D was used together with other supplements or drugs, high dosages of vitamin D cannot prevent the development of cancer; it can significantly increase cardiovascular disease, risk of osteoporosis, exhaustion, muscle weakness, and painful bones (44).
Vitamin D supplements play significant roles in controlling the tumorigenesis pathway via numerous cellular behaviors such as increased maturation, differentiation, proliferation, and apoptosis, epithelial-mesenchymal transition (EMT), and autophagy along with modulating interactions between cells and their microenvironment (18). However, the use of antioxidant vitamin D supplements may reduce the efficacy of chemotherapy and radiotherapy by decreasing the amount of reactive oxygen species and apoptosis (42) (Table 4).
3.5 Therapeutic controversies over use of vitamin E supplement during cancer treatment
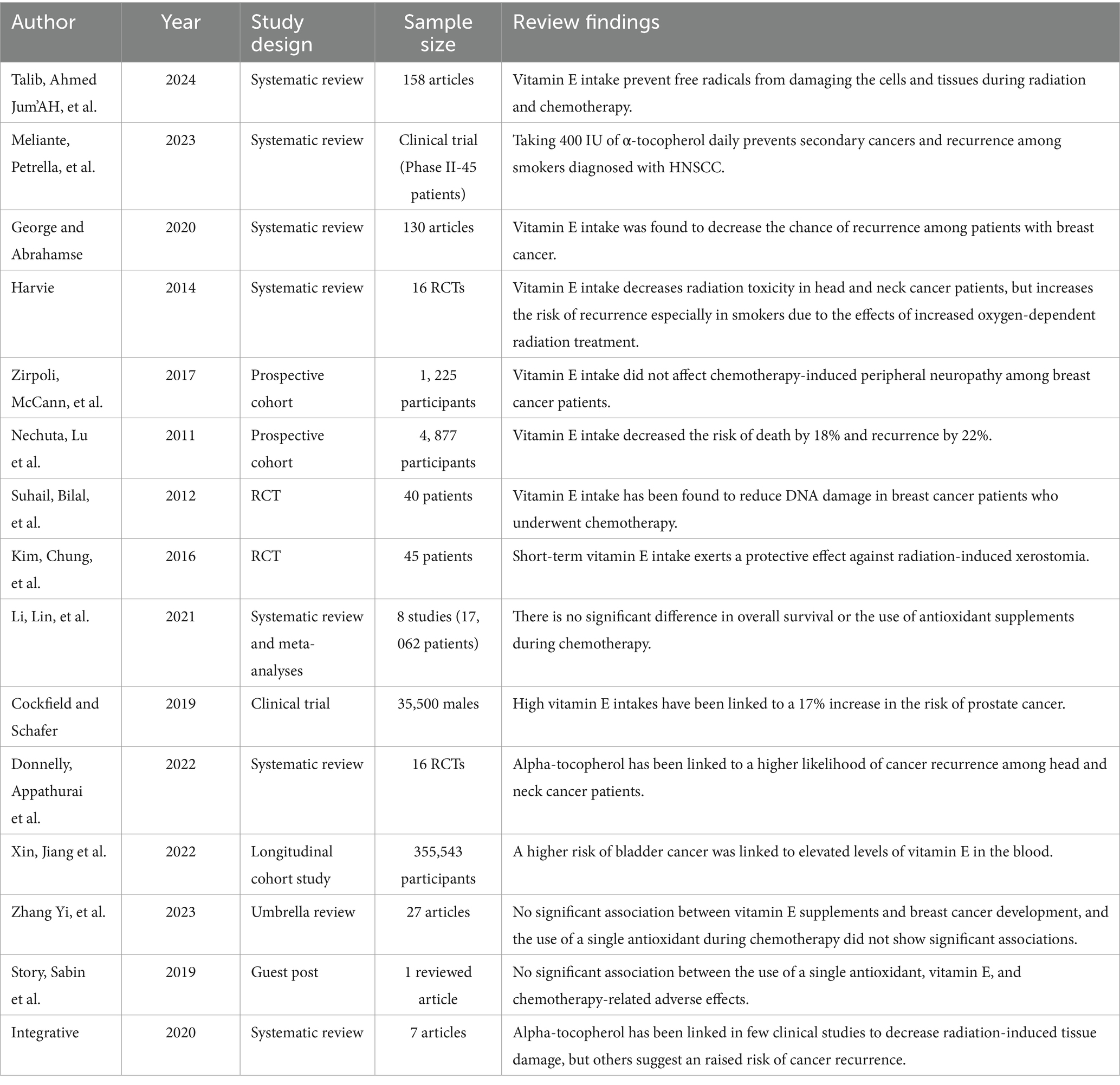
The effects of vitamin E supplements on cancer patients have been studied, especially those who are receiving radiation and chemotherapy. Because of its strong antioxidant effect, vitamin E is important for the immune system and endothelial functioning as well as protection of cells and tissues from free radicals by inhibiting fat oxidation (18).
Based on a phase 2 trial including 45 HNSCC patients, a combined regimen consisting of isotretinoin, interferon α-2a, and vitamin E prevents secondary cancers and relapse, which is an effective prevention (20).
Similarly, vitamin E intake has been shown to decrease the likelihood of breast cancer recurrence (11). Likewise, a recent RCT found minimal noninvasive bladder cancer recurrence with vitamin E supplementation (42). Furthermore, a cohort of patients with breast cancer who received vitamin E supplements during treatment had no significant association with chemotherapy-induced peripheral neuropathy (19).
Another study reported that vitamin E supplement users had a decreased risk of death by 18% (hazard ratio (HR) = 0.82, 95% CI: 0.65–1.02) and a lowered risk of recurrence by 22% (HR = 0.78, 95% CI: 0.63–0.95) (32). Furthermore, vitamin E supplementation decreased damage of DNA in breast cancer patients receiving chemotherapy (45).
A prospective, double-blinded, randomized, placebo-controlled study showed that short-term supplementation with vitamin E has a protective effect against radiation therapy-induced dry-mouth (Kim (46),).
Likewise, with the suspension of supplements, the experimental group showed a reduced incidence of secondary neoplasms (39/1000 versus 69/1000, HR = 0.57, 95% CI = 0.31 to 1.0), and it was significantly lower when α-tocopherol was the only supplement (HR = 0.41, 95% CI = 0.16 to 1.03) (20). The same authors examined if the link between smoking and supplements had an effect on secondary tumors and examined the smoking habits of the study participants.
When radiation therapy began, 343 out of 540 patients (60% of the total) smoked; this percentage dropped to 33%. Merely smokers were shown to have a higher frequency of outcomes linked to supplementation: HR 2.41 (95% CI: 1.25–4.64) for recurrence, HR 2.26 (95% CI: 1.25–4.64) for all-cause mortality, and HR 5 3.38 (95% CI: 1.11–10.34) for deaths associated with initial detection of head and neck squamous cell carcinoma (20).
However, meta-analysis study found no apparent disparity in overall survival or the usage of vitamin E supplements during chemotherapy (HR 1.15, 95% CI 0.78–1.68) (21). In addition, a study from a clinical trial that included over 35,500 males over 50 years of age discovered that high vitamin E dosages raised the risk of prostate cancer by 17% (28).
Moreover, a multicentric, double-blind, placebo-controlled, randomized trial that used the same methodology found that in patients who are suffering from stage I or II HNSCC and on radiation therapy while taking 400 IU of α-tocopherol daily had a significantly higher incidence of subsequent primary malignancies (60/1000 person-years) compared to those on placebo (25/1000 person-years); HR = 2.42 (95% CI = 1.45 to 4.04) particularly with only delivery of α-tocopherol (HR = 2.88, 95% CI = 1.56 to5.31) (20). Another study by Donnelly et al. (47), which reviewed RCTs done on alpha-tocopherol usage among head and neck cancer patients showed an increase in cancer recurrence rates.
Another Mendelian Randomization study also indicated that a higher danger of bladder cancer was connected with greater levels of vitamin E in blood (48). In addition, no evidence had been found by Zhang et al. (49) that vitamin E intake is significantly correlated with incidence of breast cancer. Similarly, one antioxidant used for chemotherapy, which is vitamin E did not present any statistically significant relations (22).
Some clinical researches have shown that alpha-tocopherol may decrease the amount of radiation-induced tissue damage while other clinical trials have reported an increased risk of recurrent cancers (50).
Similarly, vitamin E has been demonstrated to reduce radiation damage among patients suffering from head and neck cancer but on the other hand it has been associated with higher risks of recurrence especially among smokers since smoking elevates tissue hypoxia and blood carboxyhemoglobin levels thereby reducing oxygen dependent radiotherapy action (42). Moreover, vitamin e protects against chemotherapy caused injury through inhibiting lipid peroxidation and triggering death of neoplastic cells in tumors (51) (Table 5).
3.6 Therapeutic controversies over use of coenzyme Q10 supplement during cancer treatment
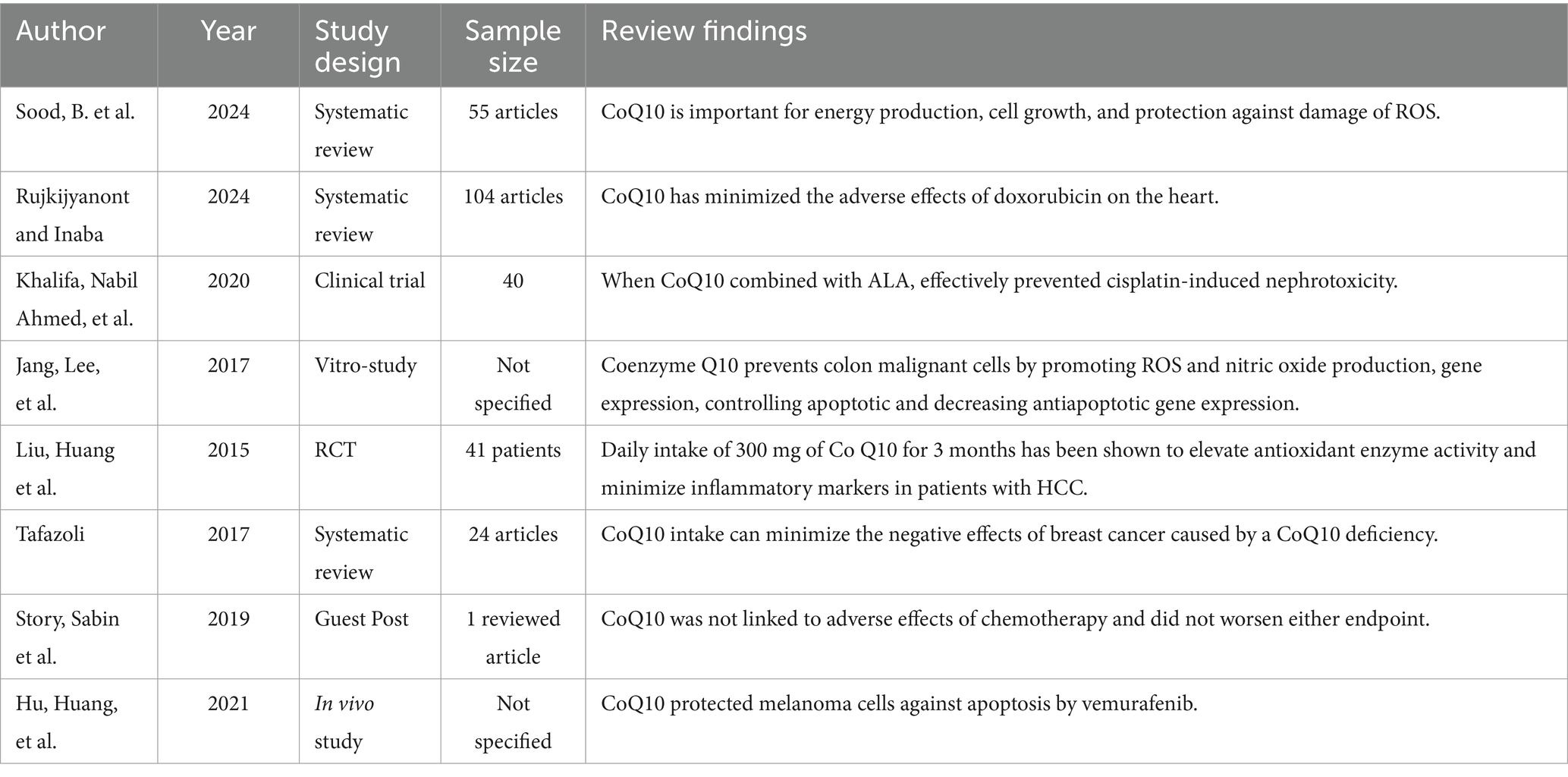
Coenzyme Q10 is important for cellular growth, energy production, and inhibition oxidative damage. It enhances the synthesis of antioxidants, decreases oxidative stress, and maintains vascular health (52).
A study conducted by Rujkijyanont and Inaba (53) investigated whether CoQ10 could keep the hearts of children with acute lymphoblastic leukemia from damage caused by doxorubicin. The researchers found that Coenzyme Q10 can reduce the drawbacks of doxorubicin on the heart.
According to Khalifa et al. (54), the supplementation of CoQ10 and α-lipoic acid inhibited cisplatin-induced nephrotoxicity. In addition, CoQ10 prevented malignant cells of the colon by enhancing the production of ROS and nitric oxide while reducing the expression of apoptotic genes and decreasing the expression of anti-apoptotic genes (55).
Likewise, daily intake (300 mg) of CoQ10 for 3 months has been shown to enhance the work of antioxidant enzymes and decrease inflammatory markers in hepatocellular carcinoma patients underwent surgery (56).
Meanwhile, it has been reported that supplementing CoQ10 decreased the adverse effects of breast cancer caused by CoQ10 deficiency (57). Furthermore, CoQ10 was not linked with side effects during chemotherapy, and did not worsen either endpoint (22).
In spite of the antioxidant effects of CoQ10 and its use in cancer treatment, there are issues that CoQ10 may decrease the efficacy of these treatments.
The American Cancer Society has warned that CoQ10 counteracts with chemotherapy and radiation therapy, making cancer physicians not to recommend during conventional cancer treatment (58).
The antioxidant CoQ10 can increase the chance of disseminating cancer cells by protecting the malignant cells from oxidative stress, preventing apoptosis, and increasing cancer cell growth. This raises questions on the use of CoQ10 in cancer patients having treatments that depend on the production of free radicals to eliminate cancer cells (59) (Table 6).
3.7 Therapeutic controversies over use of selenium supplement during cancer treatment
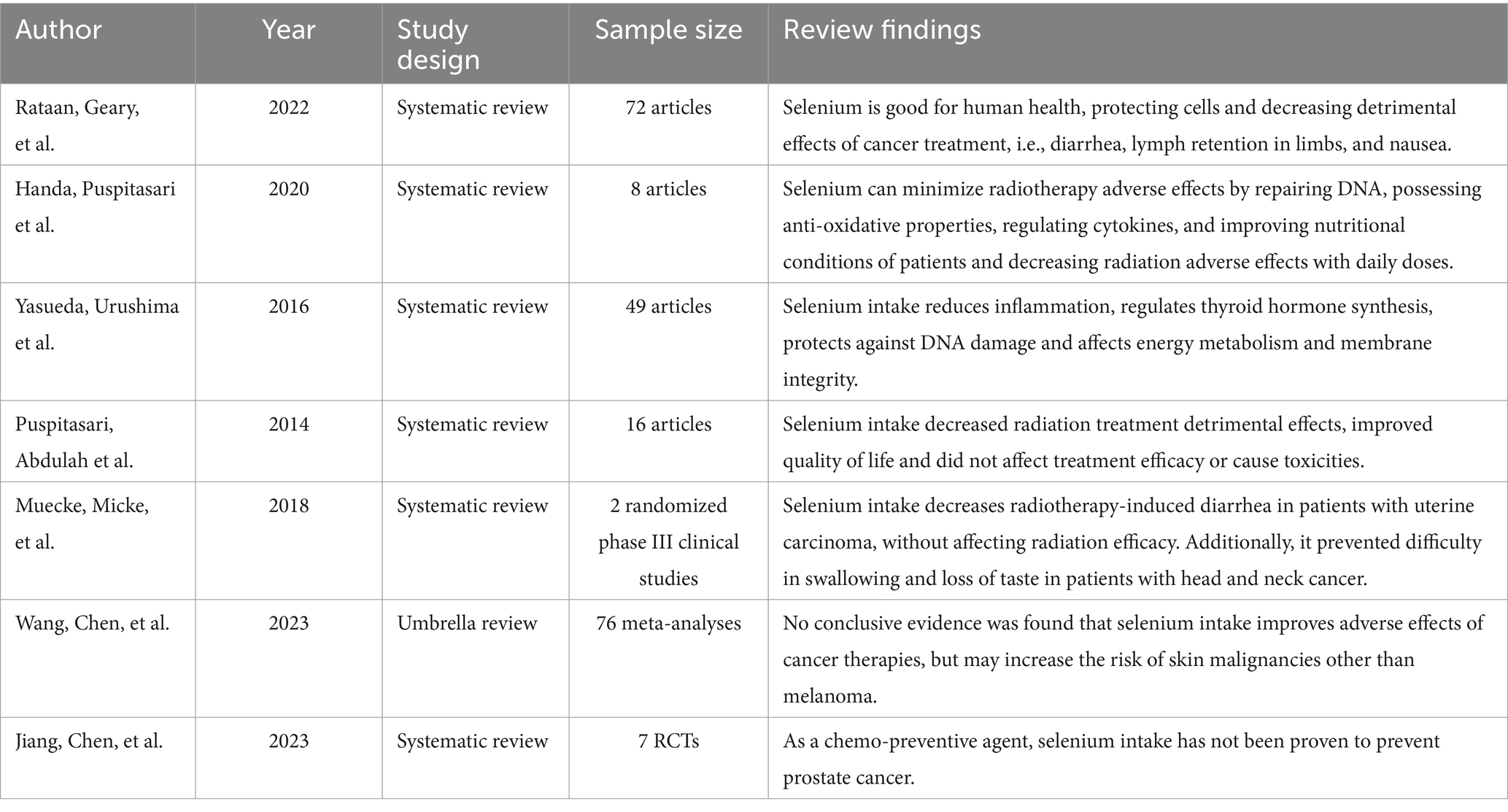
Selenium decreases inflammation, prevents DNA damage, controls the production of active thyroid hormone, and regulates a wide range of biological functions, including cell membrane and integrity energy metabolism (13).
In addition, selenium helps to decrease nausea, diarrhea, and lymph retention in extremities that are caused by the adverse effects of cancer treatment (60).
Selenium can reduce the detrimental effects of radiotherapy, as selenoproteins have a function of cytokine regulation, DNA repair, and anti-oxidative characteristics against radiation-induced free radicals (61).
A systematic review showed that daily dosages of 300–500 μg/day of selenium over 10 days to 6 months improved patient conditions and reduced radiation adverse effects. The investigators showed that there were no reported toxicities, and supplementing with selenium did not decrease the efficacy of radiation treatment (61).
A similar study found that selenium supplementation decreased the adverse effects of radiation treatment, improved the patient’s overall health and quality of life. The trials found that supplementing with selenium at the 200–500 μg/day level did not affect the efficiency of radiation treatment, and there were no observed toxicities (62).
In addition, a randomized study of radiation patients with uterine carcinomas found that selenium supplementation effectively reduced radiotherapy-induced diarrhea (63). Furthermore, selenium supplementation did not interfere the effecacy of radiation (63). A second randomized study including patients with head and neck cancer found some beneficial benefits of supplementary selenium in the prevention of swallowing difficulty and loss of taste brought on by radiation therapy (63).
In contrast, an umbrella review found no conclusive evidence that supplementing cancer patients with selenium improves the adverse effects of cancer therapy. The same study revealed that a higher risk of skin malignancies other than melanoma may be linked to selenium use (64).
Another study also reported that selenium supplementation has not been found to prevent prostate cancer as a chemo-preventive agent in randomized controlled trials (HR: 0.95; 95% CI: 0.80–1.13) (65).
Although few studies have reported that selenium can reduce the adverse effects of cancer treatments without affecting their efficacy, they lack consistent findings yet over the type and dosage of selenium for these patients, with different responses seen between normal and malignant cells (66).
The possible reason for the inconsistent findings can be explained by various factors including cancer type, drug dosage, synergism, bioavailability, patient’s lifestyle, tendency to take supplements, and the duration of the studies with other variables incorporated (67) (Table 7).
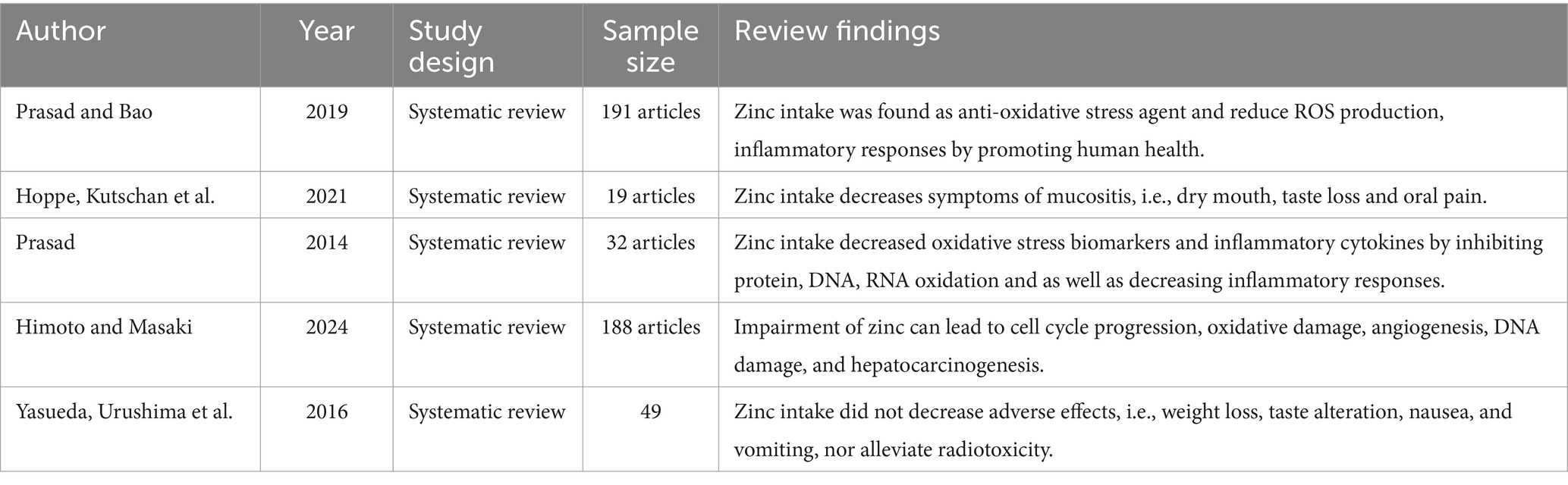
3.8 Therapeutic controversies over use of zinc supplement during cancer treatment
Zinc has anti-oxidative characteristics that lead to a reduction of free radicals and improved human health (68).
There have been scientific reports that supplementation of zinc decreases mucositis, i.e., decreased dry mouth, minimal oral pain, reduced loss of taste caused by radiation toxicities (69).
In addition, supplementation of zinc decreases oxidative stress biomarkers and inflammatory cytokines by preventing protein, DNA and RNA oxidation, as well as blocking inflammatory responses (68, 70).
Furthermore, unregulated status of zinc leads to oxidative stress, cell cycle progression, damage of DNA, and angiogenesis, finally resulting in hepatocarcinogenesis (71). Similarly, the findings of a systematic review found no significant side effects linked to zinc supplementation (13).
To the contrary, zinc supplementation did not alleviate mucositis or improve quality of life or survival rates after chemotherapy (69).
A systematic review also showed that supplementation of zinc did not significantly decrease symptoms of radiotoxicity such as weight loss, changes in taste, nausea, and vomiting (13).
It is important to bear in mind that the efficacy of zinc supplementation depends on factors like individual study characteristics, publication bias, sample size, interactions with other nutrients, differences in methodology, and timing of supplementation. This difference could contribute to inconsistent outcomes (72) (Table 8).
4 Conclusion and recommendations
4.1 Conclusion
According to this review, the use of antioxidant supplements can benefit tumor cells in the same manner as they do for normal cells. In addition, how chemotherapeutic drugs and radiation destroy cancer cells varies greatly, with distinct adverse effects, being stressful to different organs, and being toxic to humans over longer periods. Furthermore, not all antioxidants are believed to be effective. Therefore, as only a few clinical studies with small sample size, limited populations, cancer types, stages, dosages etc. have demonstrated the use of antioxidant supplements in cancer treatment, physicians should advise their patients not to take antioxidant supplements during chemotherapy or radiotherapy.
4.2 Recommendations
Based on the review findings, it is recommended that physicians need to stay up-to-date on the latest research in this fast-moving field of research so they can advise their patients about potential benefits and risks. Likewise, patients should receive personalized supplemental advice from a credible source given by their cancer physician. In addition, future research including potential clinical and preclinical trials, mechanistic studies, and exploration of different vitamin and mineral supplement studies are required to uncover the complete potential of antioxidant supplements for cancer treatment or determine their safety and effectiveness when used alongside standard cancer treatments. Furthermore, the results of this review could be used for future systematic review of therapeutic controversies over use of antioxidant supplements during cancer treatment.
4.3 Strengths and limitations
Strengths of the present review include an extremely rigorous search strategy intended to capture the full range of publications presenting data on this topic. A priori inclusion criteria and duplicate screening decreased the risk of bias. The very large scope of this review was both a strength and limitation. Due to the very large volume of articles included in the review, in-depth analysis of individual articles was not possible. In addition, the reviewer gathered English-language studies on antioxidant supplements’ therapeutic controversies during cancer treatment, primarily from North and South America, Europe, and Asia, without considering cultural and socioeconomic disparities, requiring caution in application.
Another limitation that impacts the ability to draw clear conclusions from the present data is the enormous complexity of studying all the available antioxidant supplements in medical and nutritional science.
Author contributions
MW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AT: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to thank the Addis Ababa University, College of Natural and Computational Sciences, left for Food Science and Nutrition.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hecht, F, Zocchi, M, Alimohammadi, F, and Harris, IS. Regulation of antioxidants in cancer. Mol Cell. (2024) 84:23–33. doi: 10.1016/j.molcel.2023.11.001
2. He, L, He, T, Farrar, S, Ji, L, Liu, T, and Ma, X. Antioxidants maintain cellular redox homeostasis by the elimination of reactive oxygen species. Cell Physiol Biochem. (2017) 44:532–53. doi: 10.1159/000485089
3. Lawi, ZKK, Merza, FA, Banoon, SR, Jabber Al-Saady, MAA, and Al-Abboodi, A. Mechanisms of antioxidant actions and their role in many human diseases: A review. J Chem Health Risks. (2021) 11
4. Krejbich, P, and Birringer, M. The self-administered use of complementary and alternative medicine (CAM) supplements and antioxidants in cancer therapy and the critical role of Nrf-2—a systematic review. Antioxidants. (2022) 11:2149. doi: 10.3390/antiox11112149
5. Oh, CM, Lee, D, Kong, HJ, Lee, S, Won, YJ, Jung, KW, et al. Causes of death among cancer patients in the era of cancer survivorship in Korea: attention to suicide and cardiovascular mortality. Cancer Med. (2020) 9:1741–52. doi: 10.1002/cam4.2813
6. Muchtaridi, M, Az-Zahra, F, Wongso, H, Setyawati, LU, Novitasari, D, and Ikram, EHK. Molecular mechanism of natural food antioxidants to regulate ROS in treating Cancer: a review. Antioxidants. (2024) 13:207. doi: 10.3390/antiox13020207
7. Workie, M. S., Jalali, R., and Cheng, Z. (2023). Universal health coverage and Global Health in Oncology, 17.
8. Shrivastava, A, Aggarwal, LM, Mishra, SP, Khanna, HD, Shahi, UP, and Pradhan, S. Free radicals and antioxidants in normal versus cancerous cells—an overview. Indian J Biochem Biophys. (2019) 56:7–19.
9. Oun, R, Moussa, YE, and White, NJ. Correction: the side effects of platinum-based chemotherapy drugs: a review for chemists. Dalton Trans. (2018) 47:7848–8. doi: 10.1039/C8DT90088D
10. Thyagarajan, A, and Sahu, RP. Potential contributions of antioxidants to cancer therapy: immunomodulation and radiosensitization. Integr Cancer Ther. (2018) 17:210–6. doi: 10.1177/1534735416681639
11. George, S, and Abrahamse, H. Redox potential of antioxidants in Cancer progression and prevention. Antioxidants. (2020) 9:1156. doi: 10.3390/antiox9111156
12. Wieland, LS, Moffet, I, Shade, S, Emadi, A, Knott, C, Gorman, EF, et al. Risks and benefits of antioxidant dietary supplement use during cancer treatment: protocol for a scoping review. BMJ Open. (2021) 11:e047200. doi: 10.1136/bmjopen-2020-047200
13. Yasueda, A, Urushima, H, and Ito, T. Efficacy and interaction of antioxidant supplements as adjuvant therapy in cancer treatment: a systematic review. Integr Cancer Ther. (2016) 15:17–39. doi: 10.1177/1534735415610427
14. Lawenda, BD, Kelly, KM, Ladas, EJ, Sagar, SM, Vickers, A, and Blumberg, JB. Should supplemental antioxidant administration be avoided during chemotherapy and radiation therapy? J Natl Cancer Inst. (2008) 100:773–83. doi: 10.1093/jnci/djn148
15. Arksey, H, and O'Malley, L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. (2005) 8:19–32. doi: 10.1080/1364557032000119616
16. Ferdous, UT, and Yusof, ZNB. Medicinal prospects of antioxidants from algal sources in cancer therapy. Front Pharmacol. (2021) 12:593116. doi: 10.3389/fphar.2021.593116
17. Calvani, M, Pasha, A, and Favre, C. Nutraceutical boom in cancer: inside the labyrinth of reactive oxygen species. Int J Mol Sci. (2020) 21:1936. doi: 10.3390/ijms21061936
18. Talib, WH, Ahmed Jum’AH, DA, Attallah, ZS, Jallad, MS, Al Kury, LT, Hadi, RW, et al. Role of vitamins A, C, D, E in cancer prevention and therapy: therapeutic potentials and mechanisms of action. Front Nutr. (2024) 10:1281879. doi: 10.3389/fnut.2023.1281879
19. Zirpoli, GR, McCann, SE, Sucheston-Campbell, LE, Hershman, DL, Ciupak, G, Davis, W, et al. Supplement use and chemotherapy-induced peripheral neuropathy in a cooperative group trial (S0221): the DELCaP study. JNCI J Natl Cancer Inst. (2017) 109:djx098.
20. Meliante, PG, Petrella, C, Fiore, M, Minni, A, and Barbato, C. Antioxidant use after diagnosis of head and neck squamous cell carcinoma (HNSCC): a systematic review of application during radiotherapy and in second primary Cancer prevention. Antioxidants. (2023) 12:1753. doi: 10.3390/antiox12091753
21. Li, Y, Lin, Q, Lu, X, and Li, W. Post-diagnosis use of antioxidant vitamin supplements and breast cancer prognosis: a systematic review and meta-analysis. Clin Breast Cancer. (2021) 21:477–85. doi: 10.1016/j.clbc.2021.09.001
22. Story, M., Sabin, G., and Lemanne, D. (2019). A clinician’s perspective on antioxidant use during chemotherapy for breast cancer: exploring the evidence.
23. Li, J, Lim, JYS, Eu, JQ, Chan, AKMH, Goh, BC, Wang, L, et al. Reactive oxygen species modulation in the current landscape of anticancer therapies. Antioxid Redox Signal. (2024) 41:322–41. doi: 10.1089/ars.2023.0445
24. Paul, SS, and Dey, A. Nutrition in health and immune function of ruminants. Indian J Anim Sci. (2015) 85:103–12. doi: 10.56093/ijans.v85i2.46557
25. Margalit, DN, Kasperzyk, JL, Martin, NE, Sesso, HD, Gaziano, JM, Ma, J, et al. Beta-carotene antioxidant use during radiation therapy and prostate cancer outcome in the Physicians' health study. Int J Radiat Oncol Biol Phys. (2012) 83:28–32. doi: 10.1016/j.ijrobp.2011.05.032
26. Ambrosone, CB, Zirpoli, GR, Hutson, AD, McCann, WE, McCann, SE, Barlow, WE, et al. Dietary supplement use during chemotherapy and survival outcomes of patients with breast cancer enrolled in a cooperative group clinical trial (SWOG S0221). J Clin Oncol. (2020) 38:804–14. doi: 10.1200/JCO.19.01203
27. Nielsen, AWM, Kristensen, MH, Offersen, BV, Alsner, J, Zachariae, R, and Nielsen, HM. Patient-reported outcomes in postmenopausal breast cancer survivors–comparisons with normative data. Acta Oncol. (2021) 60:78–86. doi: 10.1080/0284186X.2020.1834143
28. Cockfield, JA, and Schafer, ZT. Antioxidant defenses: a context-specific vulnerability of cancer cells. Cancer. (2019) 11:1208. doi: 10.3390/cancers11081208
29. Rock, CL, Thomson, CA, Sullivan, KR, Howe, CL, Kushi, LH, Caan, BJ, et al. American Cancer Society nutrition and physical activity guideline for cancer survivors. CA Cancer J Clin. (2022) 72:230–62. doi: 10.3322/caac.21719
30. Subramani, T, Yeap, SK, Ho, WY, Ho, CL, Omar, AR, Aziz, SA, et al. Vitamin C suppresses cell death in MCF-7 human breast cancer cells induced by tamoxifen. J Cell Mol Med. (2014) 18:305–13. doi: 10.1111/jcmm.12188
31. Dębska-Szmich, S, and Potemski, P. Vitamin C and cancer risk and treatment. Postępy Higieny i Medycyny Doświadczalnej. (2021) 75:987–1004. doi: 10.2478/ahem-2021-0031
32. Nechuta, S, Lu, W, Chen, Z, Zheng, Y, Gu, K, Cai, H, et al. Vitamin supplement use during breast cancer treatment and survival: a prospective cohort study. Cancer Epidemiol Biomarkers Prev. (2011) 20:262–71. doi: 10.1158/1055-9965.EPI-10-1072
33. Chen, Z, Huang, Y, Cao, D, Qiu, S, Chen, B, Li, J, et al. Vitamin C intake and cancers: an umbrella review. Front Nutr. (2022) 8:812394. doi: 10.3389/fnut.2021.812394
34. Franziska, B, Andrea, VM, and Loraine, C. High-dose intravenous vitamin C, a promising multi-targeting agent in the treatment of cancer. J Exp Clin Cancer Res. (2021) 40:1–44.
35. Cantley, L., and Yun, J. (2020). Intravenous high-dose vitamin C in cancer therapy-National Cancer Institute.
36. van Gorkom, GN, Lookermans, EL, Van Elssen, CH, and Bos, GM. The effect of vitamin C (ascorbic acid) in the treatment of patients with cancer: a systematic review. Nutrients. (2019) 11:977. doi: 10.3390/nu11050977
37. Kranjcec, I, Abdovic, S, Buljan, D, Matijasic, N, Slukan, M, and Stepan, J. Complementary medicine practice and use of dietary supplements in pediatric cancer patients in Croatia. Cureus. (2022) 14:e30246. doi: 10.7759/cureus.30246
38. Satheesh, NJ, Samuel, SM, and Büsselberg, D. Combination therapy with vitamin C could eradicate cancer stem cells. Biomol Ther. (2020) 10:79. doi: 10.3390/biom10010079
39. Carr, AC, and Maggini, S. Vitamin C and immune function. Nutrients. (2017) 9:1211. doi: 10.3390/nu9111211
40. Ahmed, NS, Samec, M, Liskova, A, Kubatka, P, and Saso, L. Tamoxifen and oxidative stress: an overlooked connection. Discov Oncol. (2021) 12:17. doi: 10.1007/s12672-021-00411-y
41. Ng, K, Nimeiri, HS, McCleary, NJ, Abrams, TA, Yurgelun, MB, Cleary, JM, et al. Effect of high-dose vs standard-dose vitamin D3 supplementation on progression-free survival among patients with advanced or metastatic colorectal cancer: the SUNSHINE randomized clinical trial. JAMA. (2019) 321:1370–9. doi: 10.1001/jama.2019.2402
42. Harvie, M. Nutritional supplements and cancer: potential benefits and proven harms. Am Soc Clin Oncol Educ Book. (2014) 34:e478–86.
43. Chandler, PD, Chen, WY, Ajala, ON, Hazra, A, Cook, N, Bubes, V, et al. Effect of vitamin D3 supplements on development of advanced cancer: a secondary analysis of the VITAL randomized clinical trial. JAMA Netw Open. (2020) 3:e2025850–15. doi: 10.1001/jamanetworkopen.2020.25850
44. Scragg, R, Khaw, KT, Toop, L, Sluyter, J, Lawes, CM, Waayer, D, et al. Monthly high-dose vitamin D supplementation and cancer risk: a post hoc analysis of the vitamin D assessment randomized clinical trial. JAMA Oncol. (2018) 4:e182178–8. doi: 10.1001/jamaoncol.2018.2178
45. Suhail, N, Bilal, N, Khan, HY, Hasan, S, Sharma, S, Khan, F, et al. Effect of vitamins C and E on antioxidant status of breast cancer patients undergoing chemotherapy. J Clin Pharm Ther. (2012) 37:22–6. doi: 10.1111/j.1365-2710.2010.01237.x
46. Chung, MK, Kim, DH, Ahn, YC, Choi, JY, Kim, EH, and Son, YI. Randomized trial of vitamin C/E complex for prevention of radiation-induced xerostomia in patients with head and neck cancer. Otolaryngol Head Neck Surg. (2016) 155:423–30. doi: 10.1177/0194599816642418
47. Donnelly, J, Appathurai, A, Yeoh, HL, Driscoll, K, and Faisal, W. Vitamin E in Cancer treatment: a review of clinical applications in randomized control trials. Nutrients. (2022) 14:4329. doi: 10.3390/nu14204329
48. Xin, J, Jiang, X, Ben, S, Yuan, Q, Su, L, Zhang, Z, et al. Correction: association between circulating vitamin E and ten common cancers: evidence from large-scale Mendelian randomization analysis and a longitudinal cohort study. BMC Med. (2022) 20:281. doi: 10.1186/s12916-022-02493-z
49. Zhang, T, Yi, X, Li, J, Zheng, X, Xu, H, Liao, D, et al. Vitamin E intake and multiple health outcomes: an umbrella review. Front Public Health. (2023) 11:1035674. doi: 10.3389/fpubh.2023.1035674
50. Integrative, P. D. Q. (2020). Cancer therapy interactions with foods and dietary supplements (PDQ®). In PDQ Cancer information summaries [internet]. National Cancer Institute (US).
51. Yang, CS, Luo, P, Zeng, Z, Wang, H, Malafa, M, and Suh, N. Vitamin E and cancer prevention: studies with different forms of tocopherols and tocotrienols. Mol Carcinog. (2020) 59:365–89. doi: 10.1002/mc.23160
52. Sood, B, Patel, P, and Keenaghan, M. (2024). Coenzyme Q10. In: StatPearls [internet]. Treasure Island (FL): StatPearls Publishing. Available at: https://www.ncbi.nlm.nih.gov/books/NBK531491/ (Accessed January 30, 2024).
53. Rujkijyanont, P, and Inaba, H. Diagnostic and treatment strategies for pediatric acute lymphoblastic leukemia in low-and middle-income countries. Leukemia. (2024) 38:1649–62. doi: 10.1038/s41375-024-02277-9
54. Khalifa, EA, Nabil Ahmed, A, Hashem, KS, and Allah, AG. Therapeutic effects of the combination of alpha-lipoic acid (ala) and coenzyme Q10 (CoQ10) on cisplatin-induced nephrotoxicity. Int J Inflamm. (2020) 2020:1–11. doi: 10.1155/2020/5369797
55. Jang, S, Lee, J, Ryu, SM, Lee, H, Park, JR, Kim, H, et al. Effect of coenzyme Q10 via nitric oxide production and growth arrest of human colon cancer HCT116 cells. J Prev Vet Med. (2017) 41:59–65. doi: 10.13041/jpvm.2017.41.2.59
56. Liu, HT, Huang, YC, Cheng, SB, Huang, YT, and Lin, PT. Effects of coenzyme Q10 supplementation on antioxidant capacity and inflammation in hepatocellular carcinoma patients after surgery: a randomized, placebo-controlled trial. Nutr J. (2015) 15:1–9. doi: 10.1186/s12937-016-0205-6
57. Tafazoli, A. Coenzyme Q10 in breast cancer care. Future Oncol. (2017) 13:1035–41. doi: 10.2217/fon-2016-0547
58. Hu, C, Huang, Y, Luo, P, and Yang, Y. Effect of antioxidants coenzyme Q10 and β-carotene on the cytotoxicity of vemurafenib against human malignant melanoma. Oncol Lett. (2021) 21:1. doi: 10.3892/ol.2021.12469
59. Dabaghi, GG, Rad, MR, Mohammad-Zamani, M, Sheridan, AK, Bahrami-Samani, F, and Heshmat-Ghahdarijani, K. The role of coenzyme Q10 as a preventive and therapeutic agent for the treatment of cancers. Curr Probl Cancer. (2024) 48:101063. doi: 10.1016/j.currproblcancer.2024.101063
60. Rataan, AO, Geary, SM, Zakharia, Y, Rustum, YM, and Salem, AK. Potential role of selenium in the treatment of cancer and viral infections. Int J Mol Sci. (2022) 23:2215. doi: 10.3390/ijms23042215
61. Handa, E, Puspitasari, IM, Abdulah, R, Yamazaki, C, Kameo, S, Nakano, T, et al. Recent advances in clinical studies of selenium supplementation in radiotherapy. J Trace Elem Med Biol. (2020) 62:126653. doi: 10.1016/j.jtemb.2020.126653
62. Puspitasari, IM, Abdulah, R, Yamazaki, C, Kameo, S, Nakano, T, and Koyama, H. Updates on clinical studies of selenium supplementation in radiotherapy. Radiat Oncol. (2014) 9:1–9. doi: 10.1186/1748-717X-9-125
63. Muecke, R, Micke, O, Schomburg, L, Buentzel, J, Kisters, K, Adamietz, IA, et al. Selenium in radiation oncology—15 years of experience in Germany. Nutrients. (2018) 10:483. doi: 10.3390/nu10040483
64. Wang, P, Chen, B, Huang, Y, Li, J, Cao, D, Chen, Z, et al. Selenium intake and multiple health-related outcomes: an umbrella review of meta-analyses. Front Nutr. (2023) 10:1263853. doi: 10.3389/fnut.2023.1263853
65. Jiang, J, Chen, B, Tang, B, and Wei, Q. Selenium in prostate Cancer: prevention, progression, and treatment. Pharmaceuticals. (2023) 16:1250. doi: 10.3390/ph16091250
66. Lobb, RJ, Jacobson, GM, Cursons, RT, and Jameson, MB. The interaction of selenium with chemotherapy and radiation on normal and malignant human mononuclear blood cells. Int J Mol Sci. (2018) 19:3167. doi: 10.3390/ijms19103167
67. Muecke, R, Micke, O, Schomburg, L, Kisters, K, Buentzel, J, Huebner, J, et al. Selenium supplementation in radiotherapy patients: do we need to measure selenium levels in serum or blood regularly before radiotherapy? Radiat Oncol. (2014) 9:1–2. doi: 10.1186/s13014-014-0289-0
68. Prasad, AS, and Bao, B. Molecular mechanisms of zinc as a pro-antioxidant mediator: clinical therapeutic implications. Antioxidants. (2019) 8:164. doi: 10.3390/antiox8060164
69. Hoppe, C, Kutschan, S, Dörfler, J, Büntzel, J, Büntzel, J, and Huebner, J. Zinc as a complementary treatment for cancer patients: a systematic review. Clin Exp Med. (2021) 21:297–313. doi: 10.1007/s10238-020-00677-6
70. Prasad, AS. Zinc is an antioxidant and anti-inflammatory agent: and its role in human health. Front Nutr. (2014) 1:14. doi: 10.3389/fnut.2014.00014
71. Himoto, T, and Masaki, T. Current trends on the involvement of zinc, copper, and selenium in the process of Hepatocarcinogenesis. Nutrients. (2024) 16:472. doi: 10.3390/nu16040472
72. Jung, AY, Cai, X, Thoene, K, Obi, N, Jaskulski, S, Behrens, S, et al. Antioxidant supplementation and breast cancer prognosis in postmenopausal women undergoing chemotherapy and radiation therapy. Am J Clin Nutr. (2019) 109:69–78. doi: 10.1093/ajcn/nqy223
73. Sorice, A, Guerriero, E, Capone, F, Colonna, G, Castello, G, and Costantini, S. Ascorbic acid: its role in the immune system and chronic inflammation diseases. Mini-Rev Med Chem. (2014) 14:444–52. doi: 10.2174/1389557514666140428112602
74. Kordiak, J, Bielec, F, Jabłoński, S, and Pastuszak-Lewandoska, D. Role of beta-carotene in lung cancer primary chemoprevention: a systematic review with meta-analysis and meta-regression. Nutrients. (2022) 14:1361.
75. Singh, K, Bhori, M, Kasu, YA, Bhat, G, and Marar, T. Antioxidants as precision weapons in war against cancer chemotherapy induced toxicity–Exploring the armory of obscurity. Saudi Pharm. J. (2018) 26, 177–190.
Glossary
Keywords: antioxidant, cancer, therapeutic controversy, treatment, supplements, use
Citation: Woldeselassie M and Tamene A (2024) Therapeutic controversies over use of antioxidant supplements during cancer treatment: a scoping review. Front. Nutr. 11:1480780. doi: 10.3389/fnut.2024.1480780
Edited by:
Jean-Marc A. Lobaccaro, Université Clermont Auvergne, FranceReviewed by:
Pooja Shivappa, RAK Medical and Health Sciences University, United Arab EmiratesQuan Wang, Air Force Military Medical University, China
Copyright © 2024 Woldeselassie and Tamene. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mulugeta Woldeselassie, YWx6dW5mYUB5YWhvby5jb20=
†Present address: Mulugeta Woldeselassie, Addis Ababa University, College of Natural and Computational Sciences, Center for Food Science and Nutrition, Addis Ababa, Ethiopia
 Mulugeta Woldeselassie
Mulugeta Woldeselassie Aynadis Tamene1,2
Aynadis Tamene1,2