- 1Guangdong Provincial Hospital of Chinese Medicine, Guangzhou, China
- 2The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
- 3School of Second Clinical Medicine, Guangzhou University of Chinese Medicine, Guangzhou, China
- 4Sun Yat-sen University, Guangzhou, China
Background and objectives: Metabolic disease has become a global health concern, and insulin resistance (IR) is a crucial underlying mechanism in various metabolic diseases. This study aims to compare the ability of seven anthropometric indicators in predicting IR in the Chinese population, and to find more sensitive and simple anthropometric indicator for early identification of IR.
Methods: This prospective cross-sectional study obtained participants’ medical history, anthropometric indicators, and serum samples from three hospitals in China. Various anthropometric indicators were calculated, including body mass index (BMI), Waist-to-hip ratio (WHR), waist-to-height ratio (WtHR), conicity index (CI), A Body Shape Index (ABSI), body roundness index (BRI), abdominal volume index (AVI). The evaluation of IR is performed using the homeostasis model assessment-insulin resistance (HOMA-IR). Logistic regression analysis examined the relationship between indicators and HOMA-IR. The ability of the anthropometric indicators to predict IR was analyzed using the receiver operating characteristic (ROC) curve. Additionally, a stratified analysis was performed to evaluate the ability of the indicators in different age and gender groups.
Results: The study included 1,592 adult subjects, with 531 in the non-IR group and 1,061 in the IR group. After adjusting for confounding factors, the anthropometric indicators showed a positive correlation with IR in the general population and across different genders and age groups (OR > 1, p < 0.05), except for ABSI. In the ROC curve analysis, WtHR and BRI had the highest AUC values of 0.711 for detecting IR. The optimal cut-off value for WtHR to diagnose IR was 0.53, while for BRI, it was 4.00. In the gender-stratified and age-stratified analysis, BMI, WtHR, BRI, and AVI all had AUC values >0.700 in females and individuals below 60.
Conclusion: WtHR and BRI demonstrated a better ability to predict IR in the overall study population, making them preferred indicators for screening IR, and gender and age are important considerations. In the stratified analysis of different genders or age, BMI, WtHR, BRI, and AVI are also suitable for detecting IR in women or individuals under 60 years old in this study.
Clinical trial registration: www.chictr.org.cn, ChiCTR2100054654.
1 Introduction
Metabolic diseases, including obesity, diabetes, hypertension, and dyslipidemia, have become global health concerns, with a notable upward trend in China due to lifestyle changes and an aging population (1–3). According to the latest data, the prevalence of obesity/overweight among Chinese adults is 50.7% (4), the prevalence of diabetes 11.2% (5), the prevalence of metabolic syndrome 33.9% (6). The prevalence of metabolic diseases will be an important factor in the incidence of cardiovascular diseases (7, 8). At the core of these metabolic disorders lies insulin resistance (IR), a condition characterized by decreased responsiveness of tissues such as the liver, skeletal muscle, and adipose tissue to the hormone insulin (9–11). Early detection and evaluation of IR have emerged as crucial strategies for preventing and managing these metabolic diseases as well as cardiovascular diseases.
Currently, the homeostasis model assessment-insulin resistance (HOMA-IR) is the most widely used method for evaluating IR in clinical practice (12). However, this method requires fasting blood glucose and insulin measurements, which can be challenging and resource-intensive, particularly in primary healthcare settings. Therefore, there is an urgent need to explore alternative, more accessible methods for identifying individuals at high risk of IR.
Anthropometric indicators have garnered significant attention as potential predictors of metabolic disorders. Body Mass Index (BMI), Waist-to-Hip Ratio (WHR), and Waist-to-Height Ratio (WtHR) are widely applied in clinical practice in China (13–15). More recently, researchers have developed novel indicators including the Conicity Index (CI) (16), A Body Shape Index (ABSI) (17), Body Roundness Index (BRI) (18), and Abdominal Volume Index (AVI) (19, 20), that have not yet been widely applied in clinical practice in China. These indicators, primarily calculated based on basic measurements of height, weight, waist circumference, and hip circumference, offer potential advantages in terms of accessibility and non-invasiveness, particularly in primary healthcare and epidemiological research settings (21–23). These anthropometric indicators will be beneficial for low-cost universal screening of IR and potential metabolic diseases (24, 25).
While recent studies have explored the utility of these anthropometric indicators in predicting various metabolic disorders such as diabetes (26), hypertension (27), cardiovascular disease (28–30), and metabolic syndrome (31), their relationship with IR in the Chinese population remains unclear. Moreover, the relative performance of these indicators in predicting IR across different age groups and genders has not been comprehensively evaluated.
Therefore, this study aims to compare the seven anthropometric indicators to find the most sensitive ones for early detection of IR in a Chinese population. Additionally, the study will establish optimal cut-off values for the most effective indicator(s) and assess differences in their predictive power across various age groups and genders. By addressing these objectives, it hopes to provide valuable insights for improving IR detection strategies, particularly in primary healthcare settings and epidemiological research in China. The findings could potentially inform the development of simple, cost-effective screening tools for identifying individuals at high risk of IR and associated metabolic disorders.
2 Methods
2.1 Study design and participants
This prospective cross-sectional study recruited participants from three hospitals in China: Guangdong Province Traditional Chinese Medical Hospital in southern China, and Jiangsu Provincial Hospital of Chinese Medicine in northern China, The Xinjiang Uygur Autonomous Region Institute of Traditional Chinese Medicine in northwest China between August 1, 2020, and December 31, 2021. The research recruited participants through poster advertisements in the outpatient and inpatient departments of three hospitals, and participants were enrolled a non-randomized continuous sampling method. In a quiet and private consultation room, the researcher collected questionnaire data from the participants through face-to-face interviews. The study was conducted following ethical standards and was approved by the Guangdong Province Traditional Chinese Medical Hospital (Approval No. BE2021-156-01). All participants provided written informed consent. The study was registered with the China Clinical Trial Registration Center (Registration Number: ChiCTR2100054654).
Inclusion criteria were: (1) age 18–90 years; (2) ability to provide informed consent. Exclusion criteria included: (1) hyperthyroidism or hypothyroidism; (2) secondary hyperlipidemia, hyperglycemia, or hypertension; (3) type 1 diabetes; (4) use of medications affecting weight (e.g., glucocorticoids, contraceptives, diet pills); (5) pregnancy or lactation; (6) acute infection; (7) severe heart, liver, or kidney insufficiency; (8) malignant tumors.
2.2 Anthropometric measurements and calculations
Trained researchers used standardized testing devices to collect anthropometric data. Height and weight were measured using the RGZ-120-RT integrated height and weight measuring tool (Xiheng brand). Waist and hip circumferences were measured using a non-shrinking elastic measuring tape. Body fat percentage (BFP) and visceral fat index (VFI) were measured using the HUAWEI TruFitTM (3 Pro).
Seven anthropometric indices were calculated using the following formulas:
(1) Body mass index (32) = weight (kg)/height2 (m);
(2) Waist-to-hip ratio (33) = waist circumference (cm)/hip circumference (cm);
(3) Waist-to-height ratio (34)=waist circumference (cm)/height (cm);
(4) Conicity index (16) =0.109−1 waist circumference (m)*(weight (kg)/height (m))-1/2;
(5) A body shape index (17) =waist circumference (m)/(BMI2/3 (kg/m2) *height 1/2 (m));
(6) Body Roundness Index (18) =364.2–365.5 (1-π−2 Waist 2 (m)*Height−2 (m))1/2;
(7) Abdominal volume index (35) = (2 (waist circumference)2 (cm) + 0.7 (waist circumference (cm)-hip circumference (cm))2)/1000.
2.3 Biochemical examination
Blood samples were collected after an 8-h fast and analyzed using the Cobas 8,000 biochemical analyzer for fasting blood glucose (FBG), lipid profile, and uric acid. Glycated hemoglobin was measured using the Sebia CAP instrument, and insulin levels were measured using the ATELLICO instrument.
2.4 Definition of IR and metabolic diseases
IR was evaluated using the HOMA-IR, calculated as: HOMA-IR = (FPG mmol/L) * (FINS mIU/L)/22.5. IR was defined as HOMA-IR ≥ 1.7, non-IR was defined as HOMA-IR < 1.7 (36, 37).
Metabolic syndrome (38): Three or more of the following criteria needed to be met: (1) abdominal obesity: waist circumference ≥ 90 cm for men and ≥ 85 cm for women; (2) hyperglycemia: fasting blood glucose ≥6.1 mmoL/L or 2 h postprandial blood glucose ≥7.8 mmoL/L and/or a diagnosis of diabetes with treatment; (3) hypertension: blood pressure ≥ 130/85 mmHg and/or confirmed hypertension on treatment; (4) fasting TG ≥ 1.70 mmoL/L; (5) fasting HDL-C < 1.04 mmoL/L. Hypertension (39): At least twice in the morning, blood pressure ≥ 140/90 mmHg and/or confirmed hypertension on treatment. Obesity (40): BMI ≥ 24.0 kg/m2 or waist circumference ≥ 90 cm for men and ≥ 85 cm for women. Hyperlipidemia (41): one the following criteria needed to be met: (1) fasting TG ≥ 2.3 mmoL/L; (2) fasting TC ≥ 6.2 mmoL/L; (3) fasting LDL-C ≥ 4.1 mmoL/L; (4) fasting HDL-C < 1.00 mmoL/L; (5) and/or confirmed hyperlipidemia on treatment. Abnormal blood glucose (38): fasting blood glucose ≥6.1 mmoL/L or two hour postprandial blood glucose ≥7.8 mmoL/L and/or a diagnosis of diabetes with treatment.
2.5 Statistical analysis
Sample Size Calculation: Assuming a type I error of 0.05, a power of 0.90, a AUC0 of 0.50, and an anticipated area under the ROC curve (AUC) of 0.60 to 0.7, we calculated a required sample size of 314 to1410 participants, and chosen the maximum sample size by using the pROC package in R. Assuming a response rate and efficient rate of 94%, therefore, the minimum sample calculation for this study is recalculated as 1,500.
All statistical analyses were performed using SPSS 22.0 (SPSS, Inc., Chicago, IL, USA). The normality of continuous variables was assessed using the Kolmogorov–Smirnov test, and homogeneity of variance was tested using Levene’s test. Normally distributed continuous variables were presented as mean ± standard deviation (SD) and compared using Student’s t-test. Non-normally distributed variables were presented as median (interquartile range) and compared using the Wilcoxon rank-sum test. Categorical variables were presented as numbers (percentages) and compared using the chi-square test.
Univariate and multivariate binary logistic regression analyses were performed to examine the association between anthropometric indicators and HOMA-IR. In the multivariate analysis, it adjusted the confounding variables including age, sex, smoking status, and alcohol consumption. Results were presented as odds ratios (ORs) with 95% confidence intervals (CIs).
The predictive ability of each anthropometric indicator for IR was evaluated using receiver operating characteristic (ROC) curve analysis. The area under the ROC curve (AUC) was calculated to assess the overall discriminative power of each indicator (42). An AUC > 0.700 was considered acceptable (43). The optimal cut-off value for each indicator was determined using the Youden index (sensitivity + specificity – 1) (43). Sensitivity, specificity, and Youden index were calculated for each cut-off value.
To examine potential differences in the predictive power of anthropometric indicators across different subgroups, we performed stratified analyses by gender (male vs. female) and age (<60 years vs. ≥60 years). For each subgroup, we conducted separate logistic regression and ROC curve analyses following the procedures described above. All statistical tests were two-tailed, and a p-value <0.05 was considered statistically significant.
3 Results
3.1 Clinical characteristics
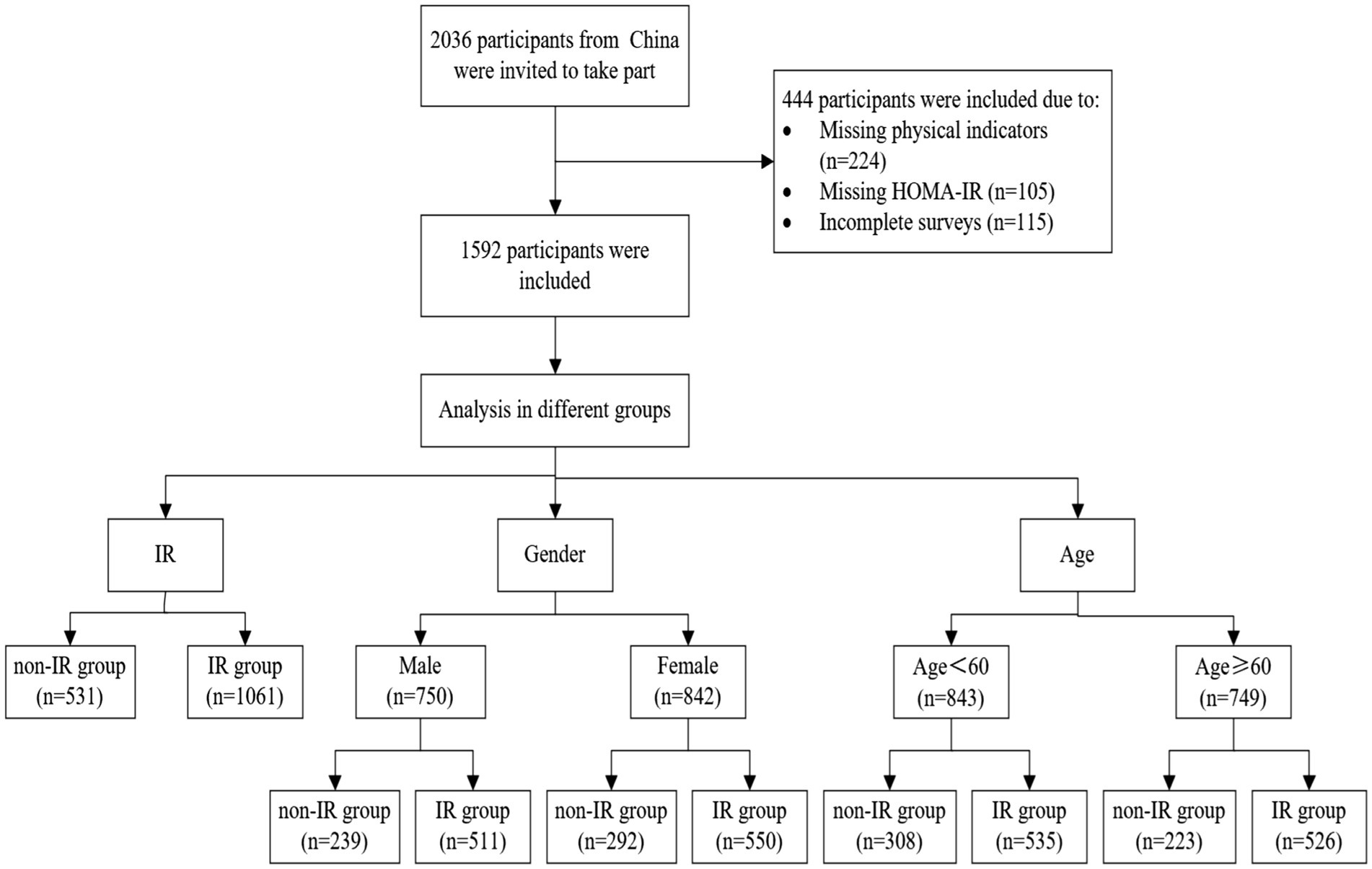
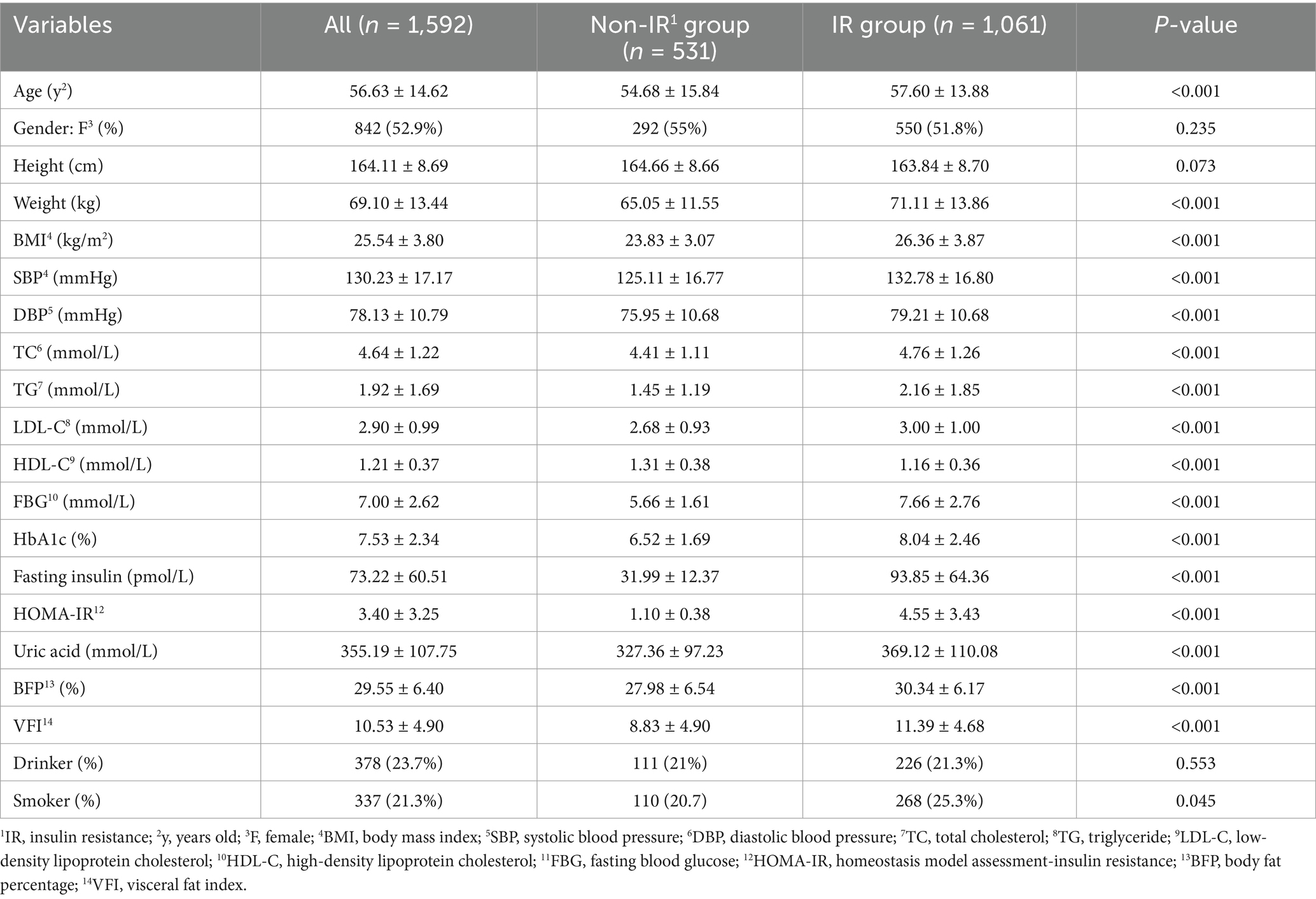
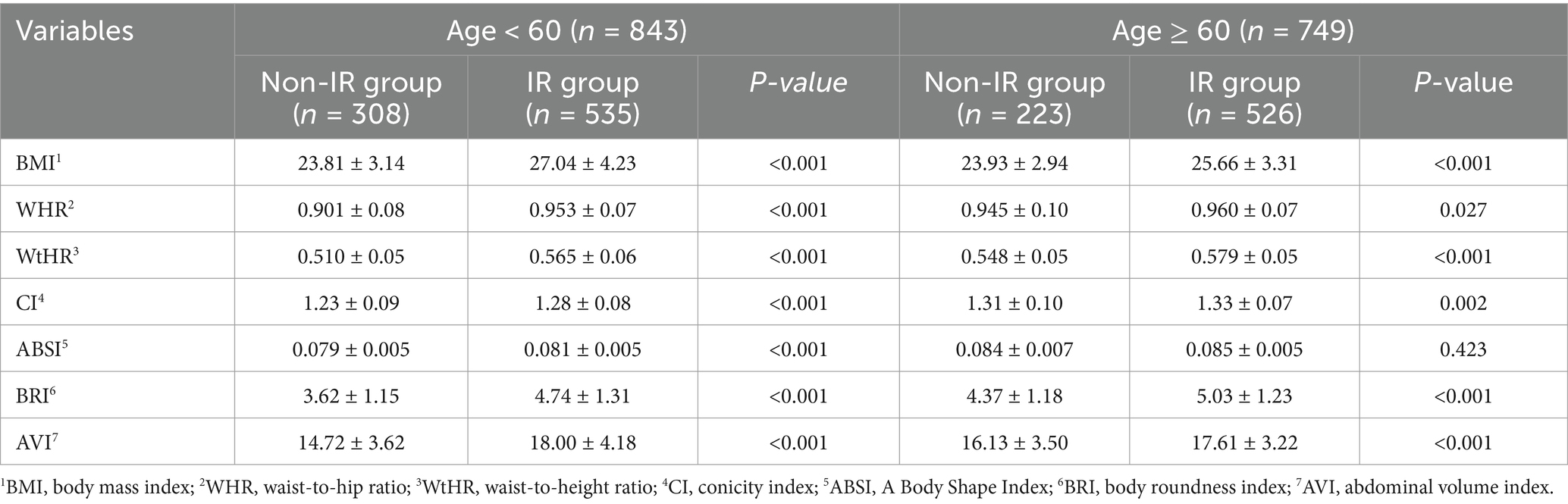
A total of 2036 participants were involved in this study. Among them, 444 participants were excluded due to missing anthropometric measurements or HOMA-IR data, or incomplete surveys. Ultimately, 1952 participants (842 females and 750 males) were successfully included for the study and data analysis, of which 531 were categorized in the non-IR group and 1,061 in the IR group (shown in Figure 1). The weight, SBP, DBP, TC, TG, LDL-C, FBG, HbA1c, HOMA-IR, uric acid, BFP, and VFI in the IR group were all higher than those in the non-IR group (p < 0.001). Additionally, the values of BMI, WHR, WtHR, CI, ABSI, BRI, and AVI in the IR group were higher than those in the non-IR group (p < 0.001). The proportions of metabolic syndrome, Hypertension, Obesity, Hyperlipidemia, and Abnormal blood glucose were higher in the IR group compared to the non-IR group (p < 0.001). The primary characteristics of the overall study population, non-IR group, and IR group are detailed in Tables 1, 2.
3.2 Comparison of anthropometric indicators between 2 groups in different gender and age participants
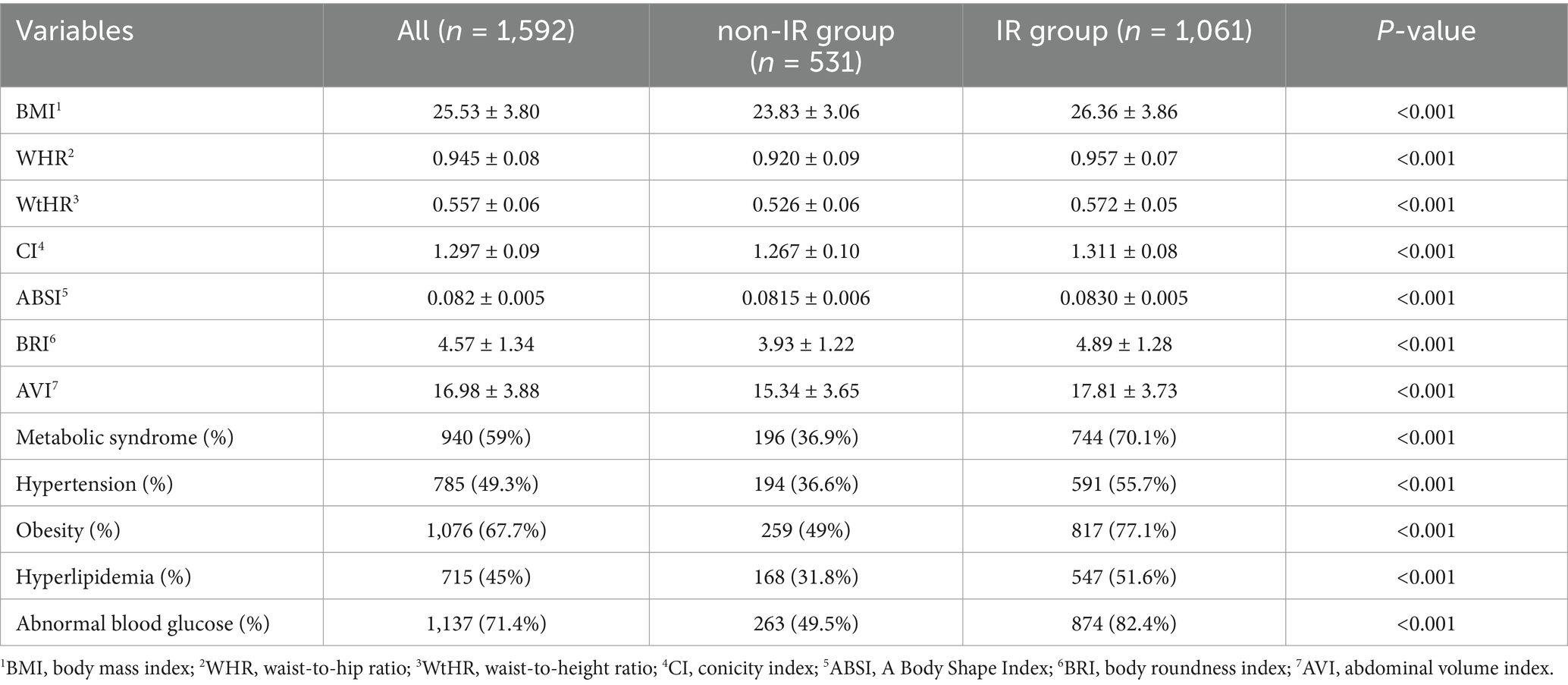
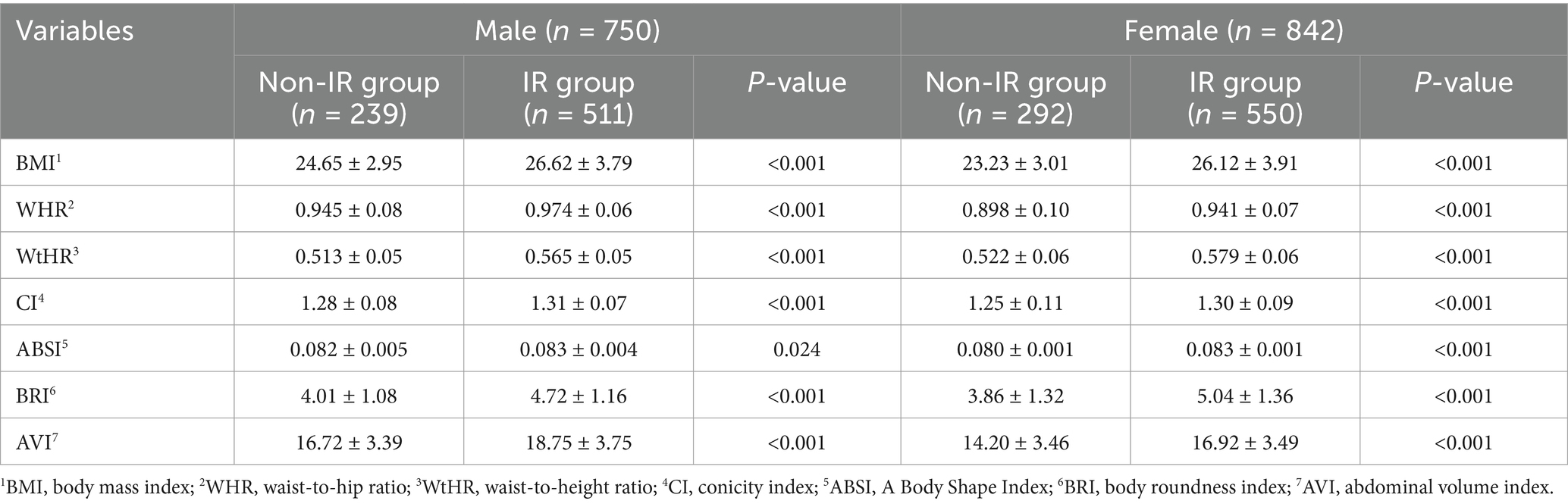
In the analysis of different gender and age participants, the anthropometric indicators also showed differences in the non-IR and IR groups, displayed in Tables 3, 4 for details. Specifically, when comparing male and female subjects, the mean values of BMI, WHR, WtHR, CI, ABSI, BRI, and AVI were significantly higher in the IR group than in the non-IR group (P < 0.05). Researchers also examined the differences in these indicators based on age groups (subjects aged over 60 or less). Similar to the findings in gender comparison, except for ABSI, the mean values of BMI, WHR, WtHR, CI, BRI, and AVI in the IR group were higher than those in the non-IR group (p < 0.05), regardless of age.
3.3 Logistic regression analysis of anthropometric indicators and IR
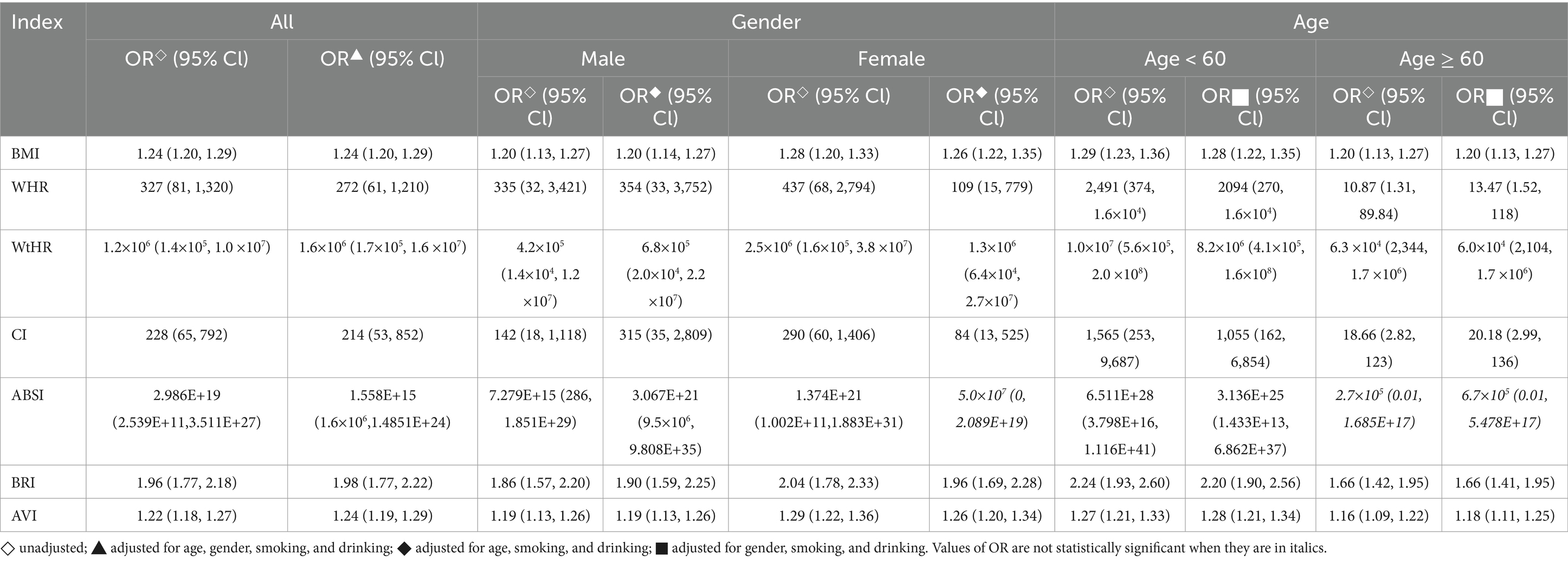
Logistic regression analysis was performed to assess the relationship between the anthropometric indicators and IR. It was found that in the general population, both individually and after controlling for confounding factors such as age, gender, smoking, and drinking, IR was positively correlated with all anthropometric indicators (OR > 1, p < 0.05). When the analysis was performed separately for male and female subjects, after adjusting for confounding factors (age, smoking, drinking), IR was still found to be correlated with all the indicators except for ABSI (p < 0.05). It indicates that in both genders, these anthropometric indicators were associated with an increased risk of IR. Furthermore, the study also revealed that regardless of age and confounding factors (gender, smoking, drinking), IR was significantly correlated with BMI, WHR, WtHR, CI, BRI, and AVI (OR > 1, p < 0.05) in subjects of different age groups. These anthropometric indicators can serve as predictors for the presence of IR, independent of age and other potential confounders. Table 5 provides detailed information on these correlations.
3.4 Analysis of the AUC value of all anthropometric indicators in predicting IR
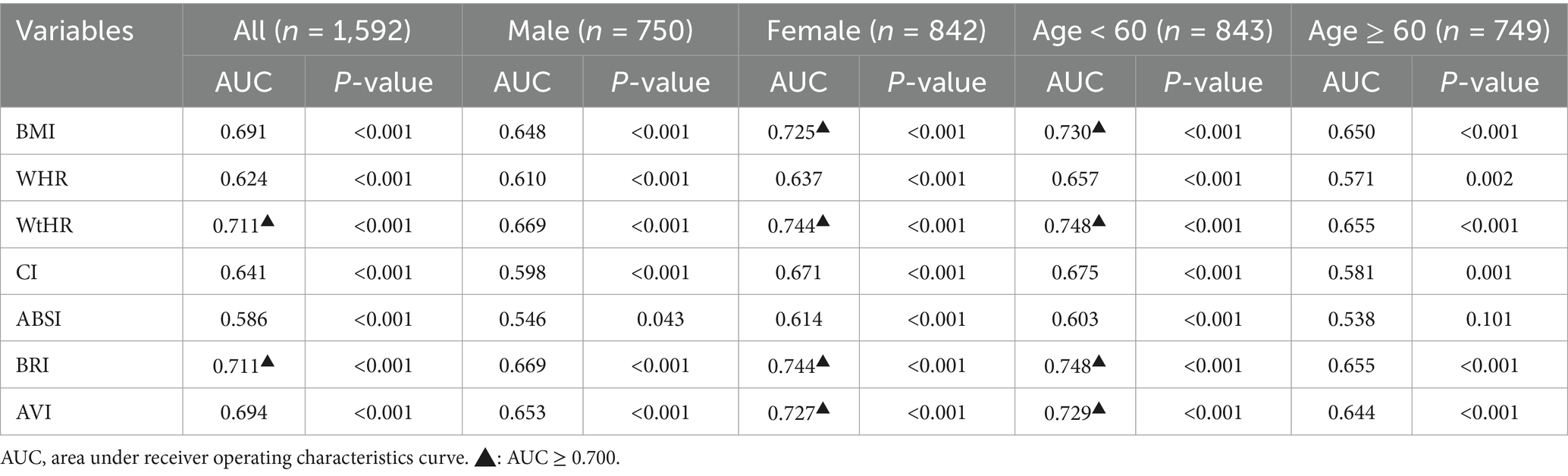
Based on the results, the study found that the AUC values, which indicate the accuracy of the anthropometric indicators in predicting IR, varied from 0.538 to 0.748 overall (Table 6). The analysis also revealed that gender and age were significant factors in stratifying the results. Among the general population, the anthropometric indicators WtHR and BRI had the highest AUC values of 0.711 (p < 0.05) (Table 6). The sensitivity of WtHR in predicting IR was 77.0%, and the specificity was 55.0%. The Youden index of WtHR, which combines sensitivity and specificity, was 0.32. The best cut-off value for diagnosing IR using WtHR was 0.53. Similarly, the sensitivity of BRI in predicting IR was 77.4%, the specificity was 53.8%, and the Youden index was 0.31. The optimal cut-off value for diagnosing IR using BRI was 4.00 (Table 7).
In the stratified analysis of different genders, the AUC values of all anthropometric indicators were generally higher in women than in men. For men, the AUC values ranged from 0.546 to 0.669 (Table 6), while for women, they ranged from 0.614 to 0.744 (Table 6). Among women, the AUC values of WtHR, BRI, AVI, and BMI were above 0.700, indicating a high accuracy in predicting IR. Specifically, the AUC values for these indicators were 0.744, 0.744, 0.727, and 0.725, respectively (Table 6).
When analyzing different age groups, the AUC values of anthropometric indicators among individuals aged < 60 years old ranged from 0.603 to 0.748 (Table 6), while for those aged ≥60 years old, the values ranged from 0.538 to 0.655 (Table 6). In the younger age group, the AUC values of WtHR, BRI, BMI, and AVI were more outstanding than 0.700, specifically, which were 0.748, 0.748, 0.730, and 0.729, respectively (Table 6). The specific values for each anthropometric indicator’s sensitivity, specificity, Youden index, and best cut-off value can be found in Table 7 if the AUC value is above 0.700.
4 Discussion
In the 1988 Banting Lecture, Reaven posited that insulin resistance (IR) increases not only the risk of diabetes but also constitutes a core feature of metabolic syndrome (44, 45). The excessive accumulation of body fat is a major cause of IR and is closely related to body shape (46–48). Due to their simplicity and ease of acquisition, anthropometric indicators are increasingly studied in relation to metabolic diseases. However, the correlation between these indicators and IR in the Chinese population still warrants further exploration.
This study included seven anthropometric indicators. WtHR, BMI, WHR are relatively traditional anthropometric indicators already applied in Chinese clinical practice (28, 49, 50). CI, ABSI, BRI, AVI are more recently developed indices with fewer studies and applications in the Chinese population (51–53). Although all seven indicators have been shown to be associated with metabolic syndrome, diabetes, fatty liver, and cardiovascular diseases, their correlations with different diseases vary to different extents, and it seems that each indicator has its own brilliance (28, 54).
Logistic regression analysis in this study demonstrated that the odds ratios (OR) for the seven anthropometric indicators and HOMA-IR were all greater than 1 (p < 0.05). This indicates a correlation between IR and these indicators, with the risk of IR increasing as these indicators rise. In predicting IR, WtHR and BRI stood out among the indicators, outperforming BMI, WHR, CI, ABSI, and AVI. The AUC value for WtHR was 0.711, with a critical value of 0.530 and a Youden index of 0.32. The AUC value for BRI was 0.711, with a critical value of 4.00 and a Youden index of 0.31. In females or individuals under 60 years old, the AUC values for BMI, WtHR, BRI, and AVI were all greater than 0.700, indicating good predictive value.
WtHR uses waist circumference and height to assess body fat distribution and is increasingly valued in studies for its importance in predicting metabolic diseases, often showing superiority over other traditional anthropometric indicators such as BMI and WHR (55). In a study on the association between obesity and insulin resistance markers in the United Arab Emirates, WtHR remained positively correlated with insulin resistance even after controlling for BMI (50). In another study predicting hypertension-diabetes risk in the Chinese population, WtHR similarly showed an advantage, outperforming BMI and WC (55). In this study, WtHR outperformed other traditional anthropometric indicators in predicting IR. A possible explanation is that WHtR can better reflect the accumulation of abdominal or ectopic fat in individuals with IR. Compared with general obesity indices, abdominal obesity is a more important risk factor for metabolic diseases (50, 56). Adipose tissue secretes factors that may impair glucose tolerance, cause chronic inflammation in adipose tissue, interfere with insulin signaling pathways, and lead to IR (56, 73). Studies suggest that WHtR, as a phenotypic marker of total fat and regional obesity, can identify individuals with lower body weight but increased ectopic fat accumulation (57). These could be the reasons why WHtR can sensitively identify IR. Related studies have shown that the risk of metabolic diseases is higher when WtHR is greater than 0.500 (34, 58). This study suggests that the threshold for predicting IR is 0.540, the same for females and individuals under 60 years old, which is 0.540. This indicates that thresholds need to be adjusted for different populations.
BRI, created by Thomas (59) et al., is a relatively new anthropometric method that, also considers waist circumference, providing a comprehensive reflection of visceral fat distribution. In this study, BRI’s predictive ability for IR was stronger compared to other indices (except WtHR). Some studies found that BRI outperformed other anthropometric indicators in estimating the risk of various clinical endpoints, including cardiometabolic diseases, kidney diseases, and cancers (60–62). Additionally, longitudinal studies have shown that high BRI is significantly associated with an increased risk of all-cause mortality and cardiovascular disease-specific mortality (63, 64). Considering numerous research results, it is reasonable to speculate that BRI is an excellent anthropometric method for predicting IR and metabolic diseases. In this study, the critical value for predicting IR using BRI was 4.00, with the critical value for females being 4.20 and for individuals under 60 years old being 4.15.
This study also conducted gender and age stratification analyses. In different gender and age stratification analyses, except for ABSI, the other six indicators also showed significant correlations with IR. In terms of diagnosis, BMI, WtHR, BRI, and AVI showed better diagnostic ability in females or individuals aged <60. This study found no significant difference in IR between males and females, but it did observe that specific parameters such as WtHR, BRI, BMI, and AVI were better predictors of IR in females than in males. This difference in predictive accuracy may be due to the higher fat content in females compared to males. Numerous studies have shown that even within the same weight range, females have significantly higher fat content than males (65, 66). This study also found that females had a significantly higher body fat percentage than males through body measurements (as shown in Supplementary Table S1). Additionally, this study observed a significantly higher proportion of IR patients in the ≥60 age group compared to the <60 age group. Age-related chronic inflammation may lead to increased IR, possibly caused by lipid accumulation, adipose tissue or mitochondrial dysfunction, and endoplasmic reticulum stress (67). However, some studies (68) suggest that in males, insulin sensitivity seems to depend more on body fat rather than age, indicating that obesity has a more pronounced impact on IR compared to physiological factors related to age.
In this study, the correlations of the WtHR and BRI with IR were significant, possibly because these two indices better reflect fat distribution, particularly visceral fat. Visceral fat is more metabolically active than subcutaneous fat. Visceral adipocytes secrete inflammatory cytokines and adipokines such as adiponectin and leptin, which can block or interfere with insulin signaling pathways through various mechanisms (69). Additionally, visceral adipocytes tend to secrete more free fatty acids (FFA). FFAs can enter the liver directly, causing hepatic fat accumulation and promoting IR (69, 70). Besides being associated with IR, visceral fat is also a high-risk factor for diabetes, hypertension, and cardiovascular diseases (71, 72).
Both WtHR and BRI are easy to obtain and simple to calculate, making them sensitive tools for identifying IR. They are particularly useful in detecting hidden IR in individuals with lower body weight but increased ectopic fat accumulation. By establishing diagnostic thresholds suitable for the Chinese population, these indices can be applied in health check-ups and clinical practice for predicting IR risk and assessing its severity. In the current routine physical examinations in China, blood tests include venous blood glucose but do not include insulin, making it impossible to calculate HOMA-IR. This provides an opportunity for the application of anthropometric indices. At the same time, the physical examination includes height, weight, waist circumference, and hip circumference, which makes the application of anthropometric indices extremely convenient without adding extra costs or labor. The application of anthropometric indices is also important in the population screening for metabolic disease risk. According to this study, the application of excellent anthropometric indices still needs to consider gender and age factors. Furthermore, calculation apps can be developed to popularize WtHR and BRI among the public, enabling regular self-monitoring and assessment. Based on monitored risks, lifestyle intervention and measures for the prevention and control of metabolic diseases can be guided. This will enhance the general public’s understanding of IR and promote the prevention of metabolic diseases.
5 Conclusion
To further elaborate on the conclusion, this study utilized a large sample size to thoroughly analyze the relationship between anthropometric indicators and IR. By doing so, the study successfully identified WtHR and BRI possessed exceptional predictive abilities for IR across the entire study population, particularly among women and individuals under 60. This means that when using WtHR and BRI to predict IR, it is necessary to consider gender and age factors and adopt different diagnostic values.
6 Deficiency
In addition to notable findings, it is also crucial to acknowledge the limitations of this study. Firstly, other potential confounding factors, such as dietary habits and levels of physical activity, disease history, family history, were not considered. This study used a non-random consecutive sampling method for enrollment, and compared to the random stratified sampling method, the possibility of bias cannot be ruled out. Finally, the impact of COVID-19 on case collection was not taken into account. Based on the preliminary findings and identified limitations, it is hoped that future research can conduct cohort studies with larger sample sizes in the Chinese population to validate and further apply these research results.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The study obtained approval from an independent ethics committee (Approved No. of ethics committee: BE2021-156-01). All the participants provided written informed consent before voluntary participation. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AL: Writing – original draft. YunwL: Data curation, Writing – original draft. QiL: Writing – original draft. YP: Formal analysis, Writing – original draft. QinL: Investigation, Writing – original draft. YT: Investigation, Writing – original draft. YunyL: Data curation, Writing – original draft. CC: Data curation, Writing – original draft. QR: Investigation, Writing – original draft. YZ: Investigation, Writing – original draft. JL: Investigation, Writing – original draft. GF: Writing – review & editing. QiyL: Writing – review & editing. ZL: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Specific Research Fund for TCM Science and Technology of the Guangdong Provincial Hospital of Chinese Medicine (No. YN2020QN12, YN2023MS07), the Zhaoyang Talent Project of the Guangdong Provincial Hospital of Chinese Medicine (No. ZY2022YL20), and Noncommunicable Chronic Diseases-National Science and Technology Major Project (No.2023ZD0509400).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1480707/full#supplementary-material
Abbreviations
IR, Insulin resistance; BMI, Body mass index; WC, Waist circumference; WtHR, Waist-to-height ratio; CI, Conicity index; ABSI, A body shape index; BRI, Body roundness index; AVI, Abdominal volume index; ROC curve, Receiver operating characteristic curve; AUC, Area under receiver operating characteristics curve; HOMA-IR, Homeostasis model assessment-insulin resistance; FBG, Fasting blood glucose; TC, Total cholesterol; TG, Triglyceride; HDL-C, High-density lipoprotein cholesterol; LDL-C, Low-density lipoprotein cholesterol; MetS, Metabolic syndrome.
References
1. Lemieux, I, and Després, J. Metabolic syndrome: past, present and future. Nutrients. (2020) 12:3501. doi: 10.3390/nu12113501
2. Chen, B, Zeng, J, Qin, M, Xu, W, Zhang, Z, Li, X, et al. The association between plant-based diet indices and obesity and metabolic diseases in Chinese adults: longitudinal analyses from the China health and nutrition survey. Front Nutr. (2022) 9:881901. doi: 10.3389/fnut.2022.881901
3. Zhang, P, Sun, X, Jin, H, Zhang, FL, Guo, ZN, and Yang, Y. Association between obesity type and common vascular and metabolic diseases: a cross-sectional study. Front Endocrinol. (2019) 10:900. doi: 10.3389/fendo.2019.00900
4. Chinese, SOE. Guideline for chronic weight management and clinical practice of anti-obesity medications (2024 version). Chinese J Endocrinol Metabol. (2024) 4:545–64. doi: 10.3760/cma.j.cn311282-20240412-00149
5. Chinese, SD. Guideline for the prevention and treatment of type 2 diabetes mellitus in China (2020 edition). Chinese J Diabet. (2021) 4:315–409. doi: 10.3760/cma.j.cn115791-20210221-00095
6. Yao, F, Bo, Y, Zhao, L, Li, Y, Ju, L, Fang, H, et al. Prevalence and influencing factors of metabolic syndrome among adults in China from 2015 to 2017. Nutrients. (2021) 13:4475. doi: 10.3390/nu13124475
7. Kuwabara, M, Kodama, T, Ae, R, Kanbay, M, Andres-Hernando, A, Borghi, C, et al. Update in uric acid, hypertension, and cardiovascular diseases. Hypertens Res. (2023) 46:1714–26. doi: 10.1038/s41440-023-01273-3
8. Singh, R, Barrios, A, Dirakvand, G, and Pervin, S. Human Brown adipose tissue and metabolic health: potential for therapeutic avenues. Cells. (2021) 10:3030. doi: 10.3390/cells10113030
9. Hill, MA, Yang, Y, Zhang, L, Sun, Z, Jia, G, Parrish, AR, et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism. (2021) 119:154766. doi: 10.1016/j.metabol.2021.154766
10. Muzurović, E, Mikhailidis, DP, and Mantzoros, C. Non-alcoholic fatty liver disease, insulin resistance, metabolic syndrome and their association with vascular risk. Metabolism. (2021) 119:154770. doi: 10.1016/j.metabol.2021.154770
11. Laakso, M, and Kuusisto, J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nature reviews. Endocrinology. (2014) 10:293–302. doi: 10.1038/nrendo.2014.29
12. Tahapary, DL, Pratisthita, LB, Fitri, NA, Marcella, C, Wafa, S, Kurniawan, F, et al. Challenges in the diagnosis of insulin resistance: focusing on the role of HOMA-IR and Tryglyceride/glucose index. Diabetes Metab Syndr. (2022) 16:102581. doi: 10.1016/j.dsx.2022.102581
13. Gu, Z, Li, D, He, H, Wang, J, Hu, X, Zhang, P, et al. Body mass index, waist circumference, and waist-to-height ratio for prediction of multiple metabolic risk factors in Chinese elderly population. Sci Rep. (2018) 8:385. doi: 10.1038/s41598-017-18854-1
14. Zhang, FL, Ren, JX, Zhang, P, Jin, H, Qu, Y, Yu, Y, et al. Strong Association of Waist Circumference (WC), body mass index (BMI), waist-to-height ratio (WHtR), and waist-to-hip ratio (WHR) with diabetes: a population-based cross-sectional study in Jilin Province, China. J Diabetes Res. (2021) 2021:1–9. doi: 10.1155/2021/8812431
15. Guan, X, Sun, G, Zheng, L, Hu, W, Li, W, and Sun, Y. Associations between metabolic risk factors and body mass index, waist circumference, waist-to-height ratio and waist-to-hip ratio in a Chinese rural population. J Diabetes Investig. (2016) 7:601–6. doi: 10.1111/jdi.12442
16. Correa-Rodríguez, M, Ramírez-Vélez, R, Correa-Bautista, J, Castellanos-Vega, R, Arias-Coronel, F, González-Ruíz, K, et al. Association of Muscular Fitness and Body Fatness with Cardiometabolic risk factors: the FUPRECOL study. Nutrients. (2018) 10:1742. doi: 10.3390/nu10111742
17. Valdez, R. A simple model-based index of abdominal adiposity. J Clin Epidemiol. (1991) 44:955–6. doi: 10.1016/0895-4356(91)90059-I
18. Nir, YK, and Jesse, CK. A new body shape index predicts mortality hazard independently of body mass index. PLoS One. (2018) 7. doi: 10.1371/journal.pone.0039504
19. Huang, SH, Chen, SC, Geng, JH, Wu, DW, and Li, CH. Metabolic syndrome and high-obesity-related indices are associated with poor cognitive function in a large Taiwanese population study older than 60 years. Nutrients. (2022) 14:1535. doi: 10.3390/nu14081535
20. Takhttavous, A, Saberi-Karimian, M, Hafezi, SG, Esmaily, H, Hosseini, M, Ferns, GA, et al. Predicting the 10-year incidence of dyslipidemia based on novel anthropometric indices, using data mining. Lipids Health Dis. (2024) 23:33. doi: 10.1186/s12944-024-02006-2
21. Svedberg, P. How many people are malnourished? Annu Rev Nutr. (2011) 31:263–83. doi: 10.1146/annurev-nutr-081810-160805
22. Piqueras, P, Ballester, A, Durá-Gil, J, Martinez-Hervas, S, Redón, J, and Real, J. Anthropometric indicators as a tool for diagnosis of obesity and other health risk factors: a literature review. Front Psychol. (2021) 12:631179. doi: 10.3389/fpsyg.2021.631179
23. Fan, YJ, Feng, YJ, Meng, Y, Su, ZZ, and Wang, PX. The relationship between anthropometric indicators and health-related quality of life in a community-based adult population: a cross-sectional study in southern China. Front Public Health. (2022) 10:955615. doi: 10.3389/fpubh.2022.955615
24. Matos, LN, Giorelli, GV, and Dias, CB. Correlation of anthropometric indicators for identifying insulin sensitivity and resistance. Sao Paulo Med J. (2011) 129:30–5. doi: 10.1590/S1516-31802011000100006
25. Polymeris, A, and Papapetrou, PD. Anthropometric indicators of insulin resistance. Hormones (Athens). (2022) 21:51–2. doi: 10.1007/s42000-021-00296-0
26. Liu, Y, Liu, X, Zhang, S, Zhu, Q, Fu, X, Chen, H, et al. Association of Anthropometric Indices with the development of diabetes among hypertensive patients in China: a cohort study. Front Endocrinol. (2021) 12:736077. doi: 10.3389/fendo.2021.736077
27. Tang, J, Zhao, S, Yu, S, Chi, C, Ji, H, Xiong, J, et al. Association between hypertension-mediated organ damage and obesity defined by novel anthropometric indices in community-dwelling elderly individuals. Clin Nutr. (2021) 40:4473–80. doi: 10.1016/j.clnu.2020.12.035
28. Ramírez-Vélez, R, Pérez-Sousa, M, Izquierdo, M, Cano-Gutierrez, C, González-Jiménez, E, Schmidt-RioValle, J, et al. Validation of surrogate anthropometric indices in older adults: what is the best Indicator of high Cardiometabolic risk factor clustering? Nutrients. (2019) 11:1701. doi: 10.3390/nu11081701
29. Li, G, Yao, T, Wu, XW, Cao, Z, Tu, YC, Ma, Y, et al. Novel and traditional anthropometric indices for identifying arterial stiffness in overweight and obese adults. Clin Nutr. (2020) 39:893–900. doi: 10.1016/j.clnu.2019.03.029
30. Liu, J, Tse, LA, Liu, Z, Rangarajan, S, Hu, B, Yin, L, et al. Predictive values of anthropometric measurements for Cardiometabolic risk factors and cardiovascular diseases among 44 048 Chinese. J Am Heart Assoc. (2019) 8:e010870. doi: 10.1161/JAHA.118.010870
31. Wu, L, Zhu, W, Qiao, Q, Huang, L, Li, Y, and Chen, L. Novel and traditional anthropometric indices for identifying metabolic syndrome in non-overweight/obese adults. Nutr Metabol. (2021) 18:3. doi: 10.1186/s12986-020-00536-x
32. Muscogiuri, G, Verde, L, and Colao, A. Body mass index (BMI): still be used? Eur J Intern Med. (2023) 117:50–1. doi: 10.1016/j.ejim.2023.09.002
33. Gerber, LM, Madhavan, S, and Alderman, MH. Waist-to-hip ratio as an index of risk for hyperglycemia among hypertensive patients. Am J Prev Med. (1987) 3:64–8. doi: 10.1016/S0749-3797(18)31276-5
34. Ashwell, M, and Hsieh, SD. Six reasons why the waist-to-height ratio is a rapid and effective global indicator for health risks of obesity and how its use could simplify the international public health message on obesity. Int J Food Sci Nutr. (2005) 56:303–7. doi: 10.1080/09637480500195066
35. Fernando, G, and Martha, R. Abdominal volume index. An anthropometry-based index for estimation of obesity is strongly related to impaired glucose tolerance and type 2 diabetes mellitus. Arch Med Res. (2003) 34:428–32. doi: 10.1016/S0188-4409(03)00073-0
36. Lin, SY, Li, WC, Yang, TA, Chen, YC, Yu, W, Huang, HY, et al. Optimal threshold of homeostasis model assessment of insulin resistance to identify metabolic syndrome in a Chinese population aged 45 years or younger. Front Endocrinol. (2022) 12:746747. doi: 10.3389/fendo.2021.746747
37. Nakai, Y, Fukushima, M, Nakaishi, S, Kishimoto, H, Seino, Y, Nagasaka, S, et al. The threshold value for insulin resistance on homeostasis model assessment of insulin sensitivity. Diabet Med. (2002) 19:346–7. doi: 10.1046/j.1464-5491.2002.00712_3.x
38. Chinese, SD. Chinese guideline for the prevention and treatment of type 2 diabetes mellitus (2017 edition). Chinese J Diabet. (2018) 10:4–67. doi: 10.3760/cma.j.issn.1674-5809.2018.01.003
39. Chinese, SOC. 2018 Chinese guidelines for the management of hypertension. Chinese J Cardiovasc Dis. (2019) 24:24–56. doi: 10.3969/j.issn.1007-5410.2019.01.002
40. Chinese, SOHM. Expert consensus & standard on weight management for overweight or obese people. Chinese J Health Manag. (2018) 12:200–7. doi: 10.3760/cma.j.issn.1674-0815.2018.03.003
41. Joint, CICG. 2016 Chinese guideline for the management of dyslipidemia in adults. Chinese J Health Manag. (2017) 11:7–28. doi: 10.3760/cma.j.issn.0253-3758.2016.10.005
42. Hanley, JA, and Mcneil, BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. (1982) 143:29–36. doi: 10.1148/radiology.143.1.7063747
43. Akobeng, AK. Understanding diagnostic tests 3: receiver operating characteristic curves. Acta Paediatr. (2007) 96:644–7. doi: 10.1111/j.1651-2227.2006.00178.x
44. Reazven, GM. Role of insulin resistance in human disease. Diabetes. (1988) 37:1595–607. doi: 10.2337/diab.37.12.1595
45. Defronzo, RA, and Ferrannini, E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. (1991) 14:173–94. doi: 10.2337/diacare.14.3.173
46. Neeland, I, Ross, R, Després, JP, Matsuzawa, Y, Yamashita, S, Shai, I, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabet Endocrinol. (2019) 7:715–25. doi: 10.1016/S2213-8587(19)30084-1
47. Després, J. Body fat distribution and risk of cardiovascular disease: an update. Circulation. (2012) 126:1301–13. doi: 10.1161/CIRCULATIONAHA.111.067264
48. Shah, R, Murthy, V, Abbasi, S, Blankstein, R, Kwong, R, Goldfine, A, et al. Visceral adiposity and the risk of metabolic syndrome across body mass index. JACC Cardiovasc Imaging. (2014) 7:1221–35. doi: 10.1016/j.jcmg.2014.07.017
49. Stefan, N, Haring, HU, Hu, FB, and Schulze, MB. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. (2013) 1:152–62. doi: 10.1016/S2213-8587(13)70062-7
50. Ali, AM, Karavetian, M, Moubareck, CA, Wazz, G, Mahdy, T, and Venema, K. The association between peptide hormones with obesity and insulin resistance markers in lean and obese individuals in the United Arab Emirates. Nutrients. (2022) 14:1271. doi: 10.3390/nu14061271
51. Zhang, Y, Zeng, Q, Li, X, Zhu, P, and Huang, F. Application of conicity index adjusted total body fat in young adults-a novel method to assess metabolic diseases risk. Sci Rep. (2018) 8:10093. doi: 10.1038/s41598-018-28463-1
52. Nagayama, D, Fujishiro, K, Tsuda, S, Watanabe, Y, Yamaguchi, T, Suzuki, K, et al. Enhanced prediction of renal function decline by replacing waist circumference with “a body shape index (ABSI)” in diagnosing metabolic syndrome: a retrospective cohort study in Japan. Int J Obes. (2022) 46:564–73. doi: 10.1038/s41366-021-01026-7
53. Perona, JS, Schmidt-RioValle, J, Fernández-Aparicio, Á, Correa-Rodríguez, M, Ramírez-Vélez, R, and González-Jiménez, E. Waist circumference and abdominal volume index can predict metabolic syndrome in adolescents, but only when the criteria of the international diabetes federation are employed for the diagnosis. Nutrients. (2019) 11:1370. doi: 10.3390/nu11061370
54. Wu, LD, Kong, CH, Shi, Y, Zhang, JX, and Chen, SL. Associations between novel anthropometric measures and the prevalence of hypertension among 45,853 adults: a cross-sectional study. Front Cardiovasc Med. (2022) 9:1050654. doi: 10.3389/fcvm.2022.1050654
55. Li, H, Shi, Z, Chen, X, Wang, J, Ding, J, Geng, S, et al. Relationship between obesity indicators and hypertension-diabetes comorbidity in an elderly population: a retrospective cohort study. BMC Geriatr. (2023) 23:789. doi: 10.1186/s12877-023-04510-z
56. Zou, H, Ma, X, Zhang, F, and Xie, Y. Comparison of the diagnostic performance of twelve noninvasive scores of metabolic dysfunction-associated fatty liver disease. Lipids Health Dis. (2023) 22:145. doi: 10.1186/s12944-023-01902-3
57. Ashwell, M, Gunn, P, and Gibson, S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes Rev. (2012) 13:275–86. doi: 10.1111/j.1467-789X.2011.00952.x
58. Browning, LM, Hsieh, SD, and Ashwell, M. A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0.5 could be a suitable global boundary value. Nutr Res Rev. (2010) 23:247–69. doi: 10.1017/S0954422410000144
59. Thomas, DM, Bredlau, C, Bosy-Westphal, A, Mueller, M, Shen, W, Gallagher, D, et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity. (2013) 21:2264–71. doi: 10.1002/oby.20408
60. Rico-Martín, S, Calderón-García, J, Sánchez-Rey, P, Franco-Antonio, C, Martínez, M, and Sánchez, J. Effectiveness of body roundness index in predicting metabolic syndrome: a systematic review and meta-analysis. Obes Rev. (2020) 21:e13023. doi: 10.1111/obr.13023
61. Zhang, N, Chang, Y, Guo, X, Chen, Y, Ye, N, and Sun, Y. A body shape index and body roundness index: two new body indices for detecting association between obesity and hyperuricemia in rural area of China. Eur J Intern Med. (2016) 29:32–6. doi: 10.1016/j.ejim.2016.01.019
62. Gao, W, Jin, L, Li, D, Zhang, Y, Zhao, W, Zhao, Y, et al. The association between the body roundness index and the risk of colorectal cancer: a cross-sectional study. Lipids Health Dis. (2023) 22:53. doi: 10.1186/s12944-023-01814-2
63. Zhang, X, Ma, N, Lin, Q, Chen, K, Zheng, F, Wu, J, et al. Body roundness index and all-cause mortality among US adults. JAMA Netw Open. (2024) 7:e2415051. doi: 10.1001/jamanetworkopen.2024.15051
64. Wu, M, Yu, X, Xu, L, Wu, S, and Tian, Y. Associations of longitudinal trajectories in body roundness index with mortality and cardiovascular outcomes: a cohort study. Am J Clin Nutr. (2022) 115:671–8. doi: 10.1093/ajcn/nqab412
65. Strack, C, Behrens, G, Sag, S, Mohr, M, Zeller, J, Lahmann, C, et al. Gender differences in cardiometabolic health and disease in a cross-sectional observational obesity study. Biol Sex Differ. (2022) 13:8. doi: 10.1186/s13293-022-00416-4
66. Moran, A, Jacobs, DJ, Steinberger, J, Steffen, L, Pankow, J, Hong, CP, et al. Changes in insulin resistance and cardiovascular risk during adolescence: establishment of differential risk in males and females. Circulation. (2008) 117:2361–8. doi: 10.1161/CIRCULATIONAHA.107.704569
67. Park, MH, Kim, DH, Lee, EK, Kim, ND, Im, DS, Lee, J, et al. Age-related inflammation and insulin resistance: a review of their intricate interdependency. Arch Pharm Res. (2014) 37:1507–14. doi: 10.1007/s12272-014-0474-6
68. Boden, G, Chen, X, DeSantis, R, and Kendrick, Z. Effects of age and body fat on insulin resistance in healthy men. Diabetes Care. (1993) 16:728–33. doi: 10.2337/diacare.16.5.728
69. Ouchi, N, Parker, JL, Lugus, JJ, and Walsh, K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. (2011) 11:85–97. doi: 10.1038/nri2921
70. Zraika, S, Dunlop, M, Proietto, J, and Andrikopoulos, S. Effects of free fatty acids on insulin secretion in obesity. Obes Rev. (2002) 3:103–12. doi: 10.1046/j.1467-789X.2002.00062.x
71. Finelli, C, Sommella, L, Gioia, S, La Sala, N, and Tarantino, G. Should visceral fat be reduced to increase longevity? Ageing Res Rev. (2013) 12:996–1004. doi: 10.1016/j.arr.2013.05.007
72. Klaver, M, van Velzen, D, de Blok, C, Nota, N, Wiepjes, C, Defreyne, J, et al. Change in visceral fat and Total body fat and the effect on Cardiometabolic risk factors during transgender hormone therapy. J Clin Endocrinol Metab. (2022) 107:e153–64. doi: 10.1210/clinem/dgab616
Keywords: anthropometric indicators, insulin resistance, obesity, risk prediction, waist-to-height ratio, body roundness index
Citation: Li A, Liu Y, Liu Q, Peng Y, Liang Q, Tao Y, Liu Y, Cui C, Ren Q, Zhou Y, Long J, Fan G, Lu Q and Liu Z (2024) Waist-to-height ratio and body roundness index: superior predictors of insulin resistance in Chinese adults and take gender and age into consideration. Front. Nutr. 11:1480707. doi: 10.3389/fnut.2024.1480707
Edited by:
Maria Morgan-Bathke, Viterbo University, United StatesReviewed by:
Hana Alkhalidy, Jordan University of Science and Technology, JordanLiying Xing, CDC of Liaoning Province, China
Copyright © 2024 Li, Liu, Liu, Peng, Liang, Tao, Liu, Cui, Ren, Zhou, Long, Fan, Lu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guanjie Fan, ZmdqaWVAZ3p1Y20uZWR1LmNu; Qiyun Lu, MTIxODgzNDE2QHFxLmNvbQ==; Zhenjie Liu, Z3psemppZUBnenVjbS5lZHUuY24=
†These authors have contributed equally to this work
‡ORCID: Zhenjie Liu, https://orcid.org/0000-0003-1008-0043
 Anxiang Li1,2,3†
Anxiang Li1,2,3† Yunwei Liu
Yunwei Liu Chongsong Cui
Chongsong Cui Qiqi Ren
Qiqi Ren Zhenjie Liu
Zhenjie Liu