- 1Division of Dermatology, Department of Medicine, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
- 2Department of Clinical Epidemiology and Biostatistics, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
Background: Numerous studies have linked vitamin D deficiency (VDD) to the pathogenesis of various alopecia disorders.
Objective: This study aimed to investigate whether patients with alopecia are more likely to have VDD or lower vitamin D levels than controls, and the prevalence of VDD among patients with certain alopecia disorders.
Methods: Electronic searches were conducted using PubMed, Embase, Scopus, and Cochrane Library databases from the dates of their inception until September 2024. Studies that reported data allowing for the calculation of odds ratios, mean differences, or correlation coefficients related to vitamin D levels and alopecia were included, while studies without a confirmed diagnosis of alopecia or those involving patients taking vitamin D supplements were excluded.
Results: It was found that 51.94% of patients with alopecia areata (AA), 50.38% of patients with female pattern hair loss (FPHL), 47.38% of patients with male androgenic alopecia (MAGA), 53.51% of patients with telogen effluvium (TE), and 38.85% of patients with primary scarring alopecia had VDD. Compared to controls, AA patients had a pooled odds ratio (OR) of VDD of 2.84 (95% confidence interval: 1.89–4.26, I2 = 84.29%, p < 0.01) and a pooled unstandardized mean difference (UMD) of vitamin D levels of −8.20 (−10.28 – −6.12, I2 = 74.25%, p < 0.01) ng/mL. For FPHL patients, a pooled OR of VDD of 5.24 (1.50–18.33, I2 = 81.65%, p < 0.01) and a pooled UMD of vitamin D levels of −15.67 (−24.55 – −6.79, I2 = 91.60%, p < 0.01) ng/mL were found. However, for MAGA, a pooled VDD OR of 4.42 (0.53–36.61, I2 = 88.40%, p < 0.01), and a pooled UMD of vitamin D levels of −2.19 ng/mL (−4.07 – −0.31 ng/mL, I2 = 7.64%, p = 0.37) were found. For TE patients, pooled UMD of vitamin D levels of −5.71 (−10.10 – −1.32) ng/mL were found.
Conclusion: People with alopecia frequently have VDD; however, only in patients with AA or FPHL was the association of VDD and decreased vitamin D levels statistically significant compared to control. The findings indicate screening for vitamin D could benefit patients with AA or FPHL, potentially addressing vitamin D deficiency. Further study on vitamin D supplementation as a treatment for alopecia is recommended.
1 Introduction
Vitamin D is a lipophilic hormone widely recognized as essential for bone development and calcium homeostasis, and it exerts its effect through the nuclear hormone receptor vitamin D receptor (VDR). Vitamin D is produced in the skin when exposed to sunlight and can also be obtained through diet. The liver synthesizes the primary form of vitamin D, 25-hydroxyvitamin D or 25(OH)D3, which is then activated in the kidneys by 1a-hydroxylase to produce its biologically active form, 1,25-dihydroxyvitamin D or 1,25(OH)2D3 (1, 2).
VDR is expressed by T and B lymphocytes, dendritic cells, and macrophages, and 1,25(OH)2D3 is known to modulate both innate and adaptive immune systems (3). Vitamin D deficiency (VDD) is believed to be an environmental trigger for the onset of autoimmunity, and many studies have found a link between VDD and autoimmune diseases (2–4). The VDR plays a crucial role in hair follicle cycling by regulating hair growth phases, particularly the transition from the anagen phase to the catagen phase (5). Additionally, VDR modulates the immune response in alopecia by interacting with key immune cells, such as T and B lymphocytes, macrophages, and dendritic cells, which are involved in the pathogenesis of autoimmune disorders (6). Non-immune-mediated alopecias (e.g., androgenetic alopecia [AGA] and telogen effluvium [TE]) and immune-mediated hair disorders (e.g., alopecia areata [AA] and primary cicatricial alopecia [PCA] such as frontal fibrosing alopecia [FFA], central centrifugal cicatricial alopecia [CCCA], and lichen planopilaris [LPP]) may therefore be associated with VDD (7, 8).
Alopecia can be classified into non-scarring and scarring types. Non-scarring alopecias are characterized by hair loss without permanent damage to hair follicles, while scarring alopecias result in permanent destruction of hair follicles due to inflammation and fibrosis (9). Alopecia is a well-known clinical sign of hereditary vitamin D resistant rickets (HVDRR), a rare disease caused by mutations in VDR. Growing evidence indicates that VDR plays a crucial role in normal hair cycling (10, 11). However, the relationship between blood vitamin D levels, tissue vitamin D concentrations, and VDR function remains to be researched since the impact of vitamin D levels on VDR function is complex and may depend on receptor sensitivity, co-regulators, and target gene expression (12). Although it is unknown whether or not deficient vitamin D levels in the blood would lead to deficient vitamin D in the tissue and whether or not this would lead to VDR dysfunction, numerous studies have linked VDD to the pathogenesis of various alopecia disorders, with a focus on AA, male androgenetic alopecia (MAGA), and female pattern hair loss (FPHL). While these studies demonstrate varying degrees of association between VDD and alopecia, there is still debate over the causality and consistency of findings (13–16). We aimed to conduct a systematic review and meta-analysis to determine the prevalence of VDD among various alopecia disorders, namely AA, AGA, TE, and PCA, the odds of VDD and differences in vitamin D levels of patients with various alopecia disorders compared to controls, and whether vitamin D levels are correlated with the severity of alopecia.
2 Materials and methods
2.1 Study design
The protocol was registered in PROSPERO (International Prospective Register of Systematic Reviews; CRD42023387901, https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=387901). The systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines (Supplementary Document 1) (17). Electronic searches were conducted from the database’s inception to September 2024 using the PubMed, Embase, Scopus, and Cochrane Library databases. The search strategy was designed to retrieve all studies on vitamin D, VDD, and alopecia using keywords and a controlled vocabulary. There were no restrictions on the language or publication period of the searches. Conference abstracts were excluded. The search included a combination of terms: ‘vitamin D,’ ‘vitamin D deficiency’, ‘alopecia,’ and ‘hair loss;’ with synonyms, related terms, and subject headings also used. Boolean operators (AND, OR) were used to combine terms. Grey literature and unpublished data were not considered. Supplementary Table S1 provides details about the search strategy.
2.2 Study selection
Two reviewers (TY and KaT) independently evaluated each article at both the full-text and title/abstract levels. Disagreements between the two reviewers regarding the studies’ eligibility were resolved via discussion with a third reviewer (PS). We included randomized controlled trials, cohort studies, and case–control studies that provided data for the calculation of the odds ratio (OR) of VDD, the mean difference in vitamin D level between cases and controls, the prevalence of VDD among patients with certain alopecia disorders, or the correlation coefficient (CC) between vitamin D level and alopecia severity. Studies involving patients without a confirmed diagnosis of alopecia or those taking vitamin D supplements were excluded to ensure consistency in the data. Our review questions in the format of PICO (population, intervention, comparator, and outcomes) are provided in Supplementary Table S2. We excluded studies that did not provide a specific type diagnosis of alopecia as well as those that included patients taking vitamin D supplements.
2.3 Data extraction
Data were extracted from the included studies using a standardized form. The following data were collected: bibliographic data (authors, year of publication), study characteristics (type of study, single or multicenter, study duration, country), alopecia group characteristics (number, age, gender, Fitzpatrick skin type (FST), ethnicity, body mass index (BMI), comorbidity, smoking status, alcohol consumption status, diet, sun-exposure, sunscreen usage, disease duration, disease severity score or grading (e.g., mean Severity of Alopecia Tool (SALT) score), alopecia pattern (e.g., % single patch, % multiple patches, % patchy type, % ophiasis type, % alopecia totalis (AT), % alopecia universalis (AU), % body site involvement), family history of alopecia, % nail involvement, % first episode, % recurrent episode, % stable/gradual disease, % active disease, treatment information, whether the diagnosis and severity assessment were done by dermatologist), control group characteristics (number, age, gender, FST, ethnicity, BMI, comorbidity, smoking status, alcohol consumption status, diet, sun exposure, sunscreen usage, whether controls were matched for any relevant factors), vitamin D results data (frequency data of VDD, vitamin D level, correlation coefficient, and relevant descriptive data), vitamin D measurement (VDD definitions, if any conversion was done, measurement methods), and other (exclusion criteria, if any conversion or data retrieval was done). Vitamin D levels were reported in various units across studies (ng/mL and nmol/L). To maintain consistency, all vitamin D values were converted to ng/mL using standard conversion methods. This ensured comparability of results across different vitamin D assays used in the included studies.
Because 1,25(OH)2D3 has a half-life of less than 4 h and the levels may remain normal in VDD, whereas 25(OH)D has a half-life of approximately 2 weeks, 25(OH)D is a stable indicator of vitamin D status and is routinely measured (18). In this review, the vitamin D level is therefore referred to as the 25(OH)D level.
Corresponding investigators were contacted via email if there was missing data. Two independent reviewers (TY and KaT) extracted data, and discrepancies were resolved with the assistance of a third reviewer (PS).
2.4 Quality assessment
TY and KaT independently assessed the quality of descriptive and case–control studies using the adapted version of the Newcastle-Ottawa Scale (NOS) (19). The NOS is a scoring tool comprised of seven items with nine scores that assess how well the investigators selected their participants (score ranges from 0 to 4), the comparability of their results (score ranges from 0 to 2), and the applicability of the outcomes (score ranges from 0 to 3). The higher the score, the higher the study’s quality and the lower the likelihood of bias. Therefore, we classified studies as having high quality if they received a total score of 7 or more, fair quality if they received a score of 4–6, and low quality if they received a score of less than 4. Any discrepancies between reviewers regarding the risk of bias in specific studies were resolved through discussion with a third reviewer (PS). The modified NOS used in our review is shown in Supplementary Table S3.
2.5 Statistical analysis
A meta-analysis was performed to pool the effect sizes, including the OR of a certain alopecia disorder and VDD, the unstandardized mean difference (UMD) of serum vitamin D level between subjects with a certain alopecia disorder and those without, the CC between vitamin D level and the SALT score. Additionally, the “metaprop” command with the Freeman-Tukey double arcsine transformation to stabilize the variances was used in Stata to pool the prevalence of VDD among various alopecia disorders (20). Each alopecia disorder was analyzed separately, and data from adult and pediatric populations was pooled independently. However, as a limited number of studies of scarring alopecia were expected, primary scarring alopecia diseases were planned to be analyzed based on their etiology, such as lymphocytic, neutrophilic, and mixed cell scarring alopecia (9).
Heterogeneity was assessed and considered present if a Cochrane Q test p-value was <0.1 or Higgins I2 ≥ 25% (21). Subgroup analyses were further performed to explore potential sources of heterogeneity. Effect sizes were pooled using the DerSimonian and Laird method if they were heterogeneous; otherwise, the inverse-variance method was used (21). The sources of heterogeneity were explored by fitting each covariate (e.g., age, female gender, disease duration, BMI, active disease, relapse disease, severe AA, and SALT score) at a time in a meta-regression model. If the τ2 was decreased by ≥50% or statistically significant β was revealed, a subgroup analysis was performed based on that covariate (22). In addition, certain pre-planned subgroup analyses (country of research origin, age group, and alopecia severity) were also performed. Severe AA is defined as AT, AU, or extensive AA, and an AA cohort is considered severe AA if it has a mean SALT score ≥ 50% or ≥ 20% severe AA. We also conducted sensitivity analyses including only studies with high quality according to the NOS (studies receiving a total NOS score of 7 or more).
To evaluate publication bias, Deeks funnel plots of the primary outcomes were generated. The Egger linear regression test was applied when a funnel plot suggested possible asymmetry (23). If Egger’s test for a regression intercept gave a p-value <0.05, a contour-enhanced funnel plot was used to determine the cause of the asymmetry (23). STATA 16.0 (StataCorp LLC, College Station, TX, United States) was used for all statistical analysis.
3 Results
3.1 Study characteristics
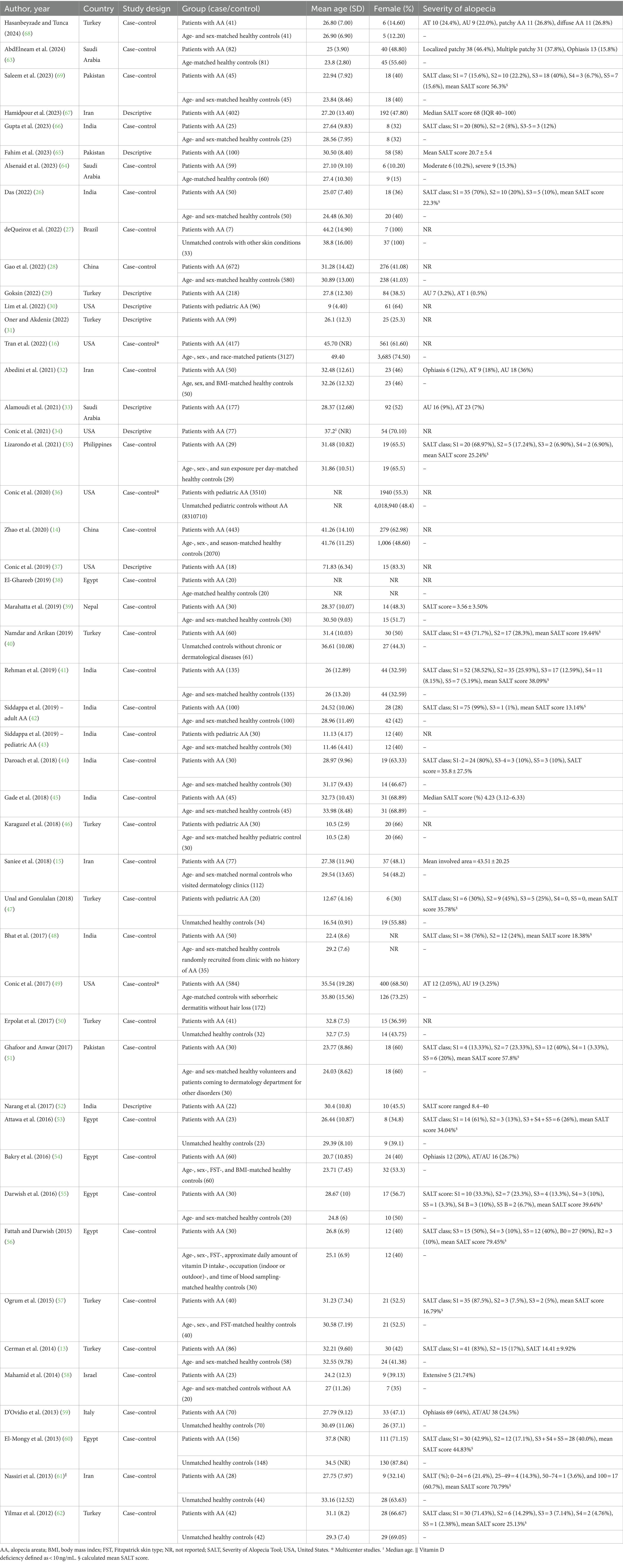
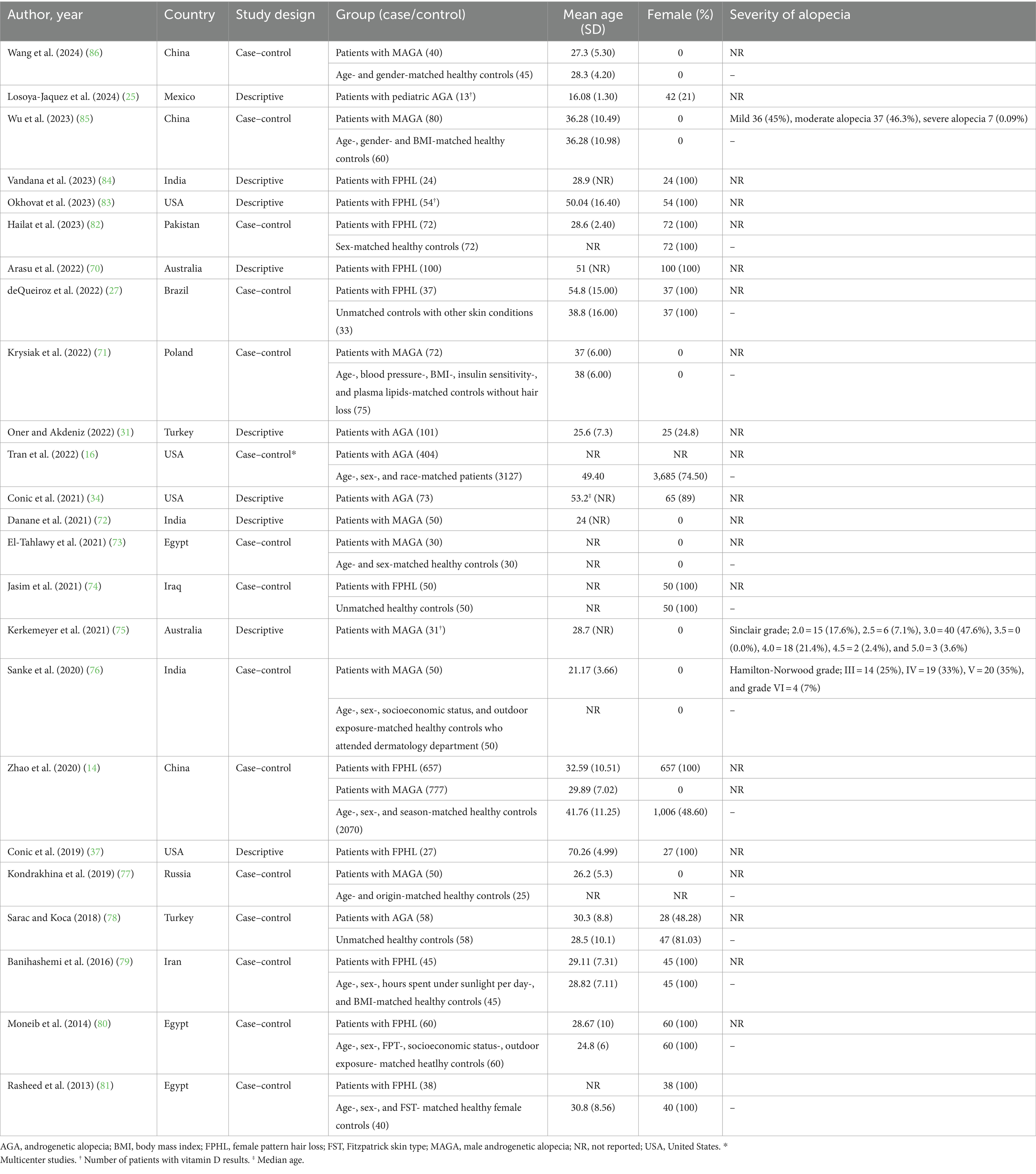
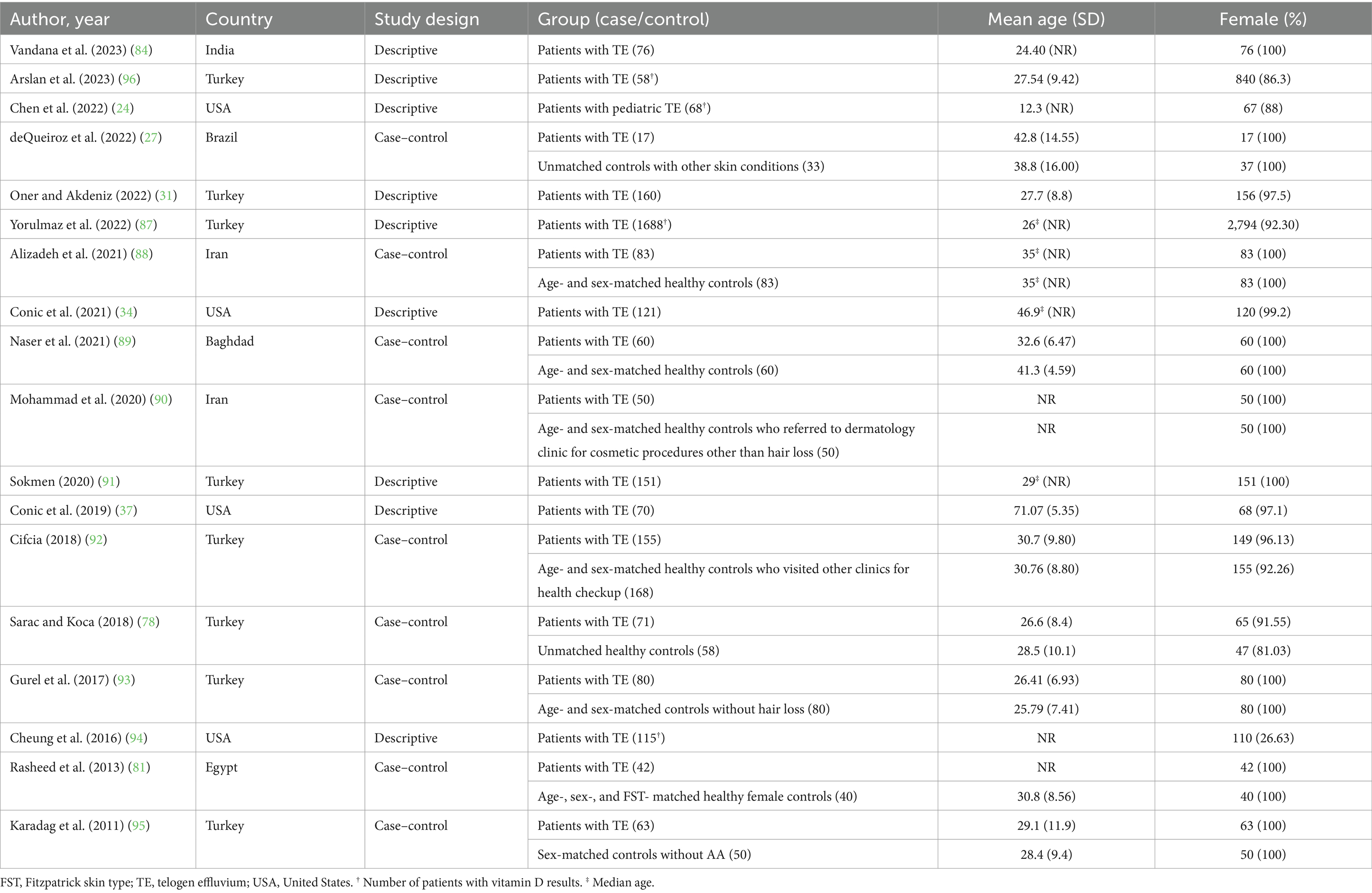
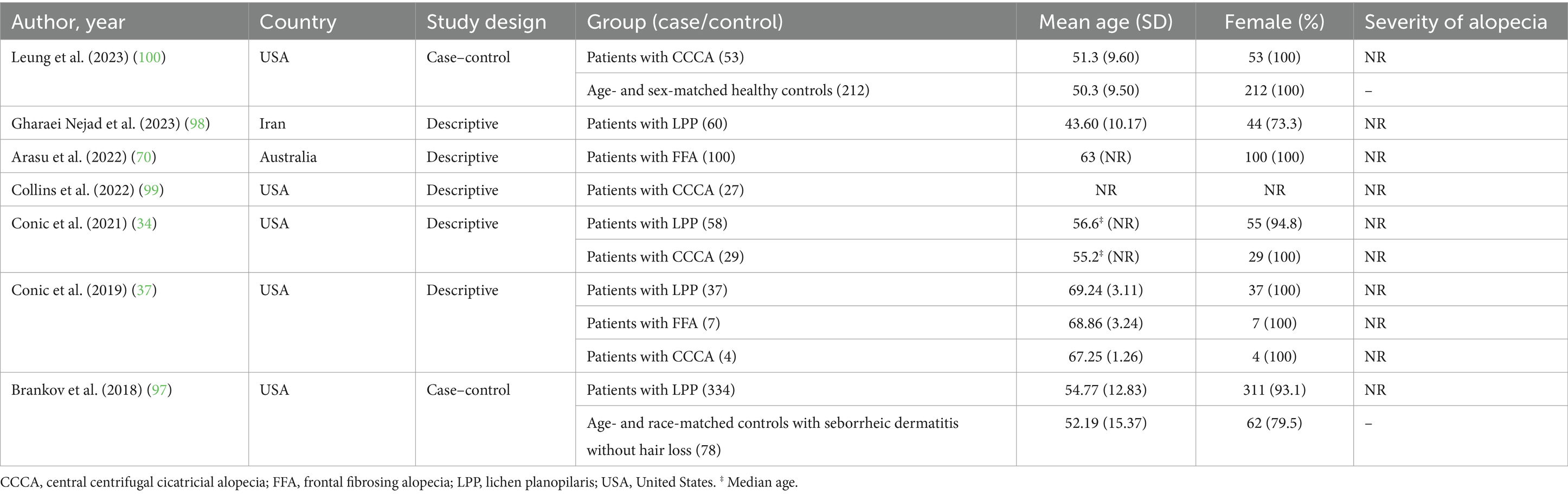
After removing duplicates, 2010 references were screened by title/abstract. At the full-text stage, 153 full articles met our predefined selection criteria and were sought. We further excluded 83 references for the following reasons: conference abstracts (n = 29), not outcome of interest (i.e., no documented vitamin D deficiency prevalence or vitamin D level of the patients, n = 30), not population of interest (i.e., non-specific alopecia diagnosis and specialized hair loss disorders [e.g., drug-induced alopecia, hair loss associated congenital disorders, n = 15), review articles (n = 5), editorial or commentary (n = 3), and case report (n = 1)] (Figure 1). Twelve additional studies were identified by manually searching reference lists of included studies and relevant review articles, and 1 study was removed due to insufficient data. The review included 81 studies (79 studies were included in the quantitative analysis; 2 studies of pediatric non-scarring alopecia (24, 25) were excluded from the quantitative analysis), enrolling a total of 15,339 patients with alopecia [8,639 AA patients (13–16, 26–69), 2,943 AGA patients (14, 16, 27, 31, 34, 37, 70–86), 3,048 TE patients (27, 31, 34, 37, 78, 81, 84, 87–96), 489 LPP patients (34, 37, 97, 98), 107 FFA patients (37, 70), and 113 CCCA patients (34, 37, 99, 100)] between 2011 and 2024, were included in the review. Characteristic features of the included studies are provided in Tables 1–4 and Supplementary Table S4.
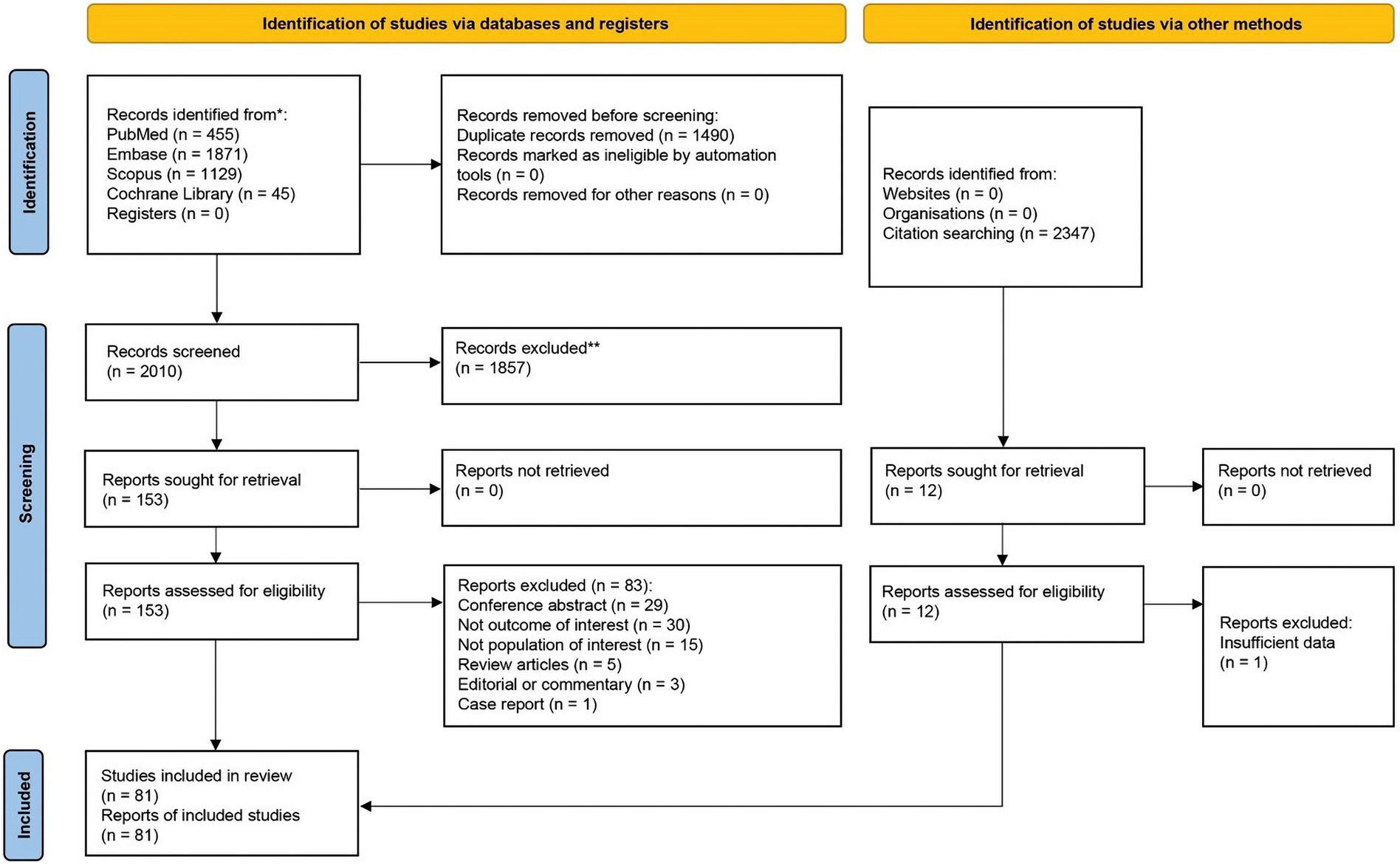
Figure 1. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram of search strategy and included studies.
3.2 Alopecia areata
Patients with AA were found to have a pooled prevalence of VDD of 51.94% (95% confidence interval: 41.54–62.25%, I2 = 97.48%, p < 0.01), a pooled OR VDD of 2.84 (1.89–4.26, I2 = 84.29%, p < 0.01), a pooled UMD of −8.20 ng/mL (−10.28 – −6.12 ng/mL, I2 = 74.25%, p < 0.01), and a pooled CC of vitamin D level and a SALT score of −0.42 (−0.59 – −0.25, I2 = 85.00%, p < 0.01), indicating a significantly higher likelihood of VDD in AA patients compared to controls.
Meta-regression analysis revealed that disease duration and relapse may account for heterogeneity in pooled OR analyses, while the female gender may account for heterogeneity in pooled UMD analyses. Subsequent subgroup analyses revealed that a disease duration of 12 months or more had a pooled OR of 2 (1.04–3.83, I2 = 73.87%, p < 0.01), whereas a disease duration of less than 12 months had a pooled OR of 11.53 (5.55–23.96, I2 = 46.31%, p = 0.13). The pooled OR for cohorts with relapse AA of 50% or more was 8.19 (1.92–35.01, I2 = 71.52%, p = 0.03), while the pooled OR for cohorts with relapse AA of less than 50% was 3.62 (1.74–7.55, I2 = 76.96%, p < 0.01), which was statistically significant (p < 0.01). Cohorts with less than 50% female had a pooled UMD of −8.44 ng/mL (−10.49 – −6.39 ng/mL, I2 = 46.78%, p = 0.01), whereas cohorts with more than 50% female had a pooled UMD of −6.94 ng/mL (−10.78–3.11 ng/mL, I2 = 81.01%, p < 0.01). Figure 2 demonstrates forest plots for the pooled prevalence of VDD (Figure 2A), pooled odds ratio of VDD (Figure 2B), pooled UMD of vitamin D levels (Figure 2C), and pooled CC of vitamin D level and SALT score (Figure 2D) in adult AA. Supplementary Figure S1 shows subgroup analyses based on disease duration, proportion of relapsed AA, and proportion of females in adult AA.
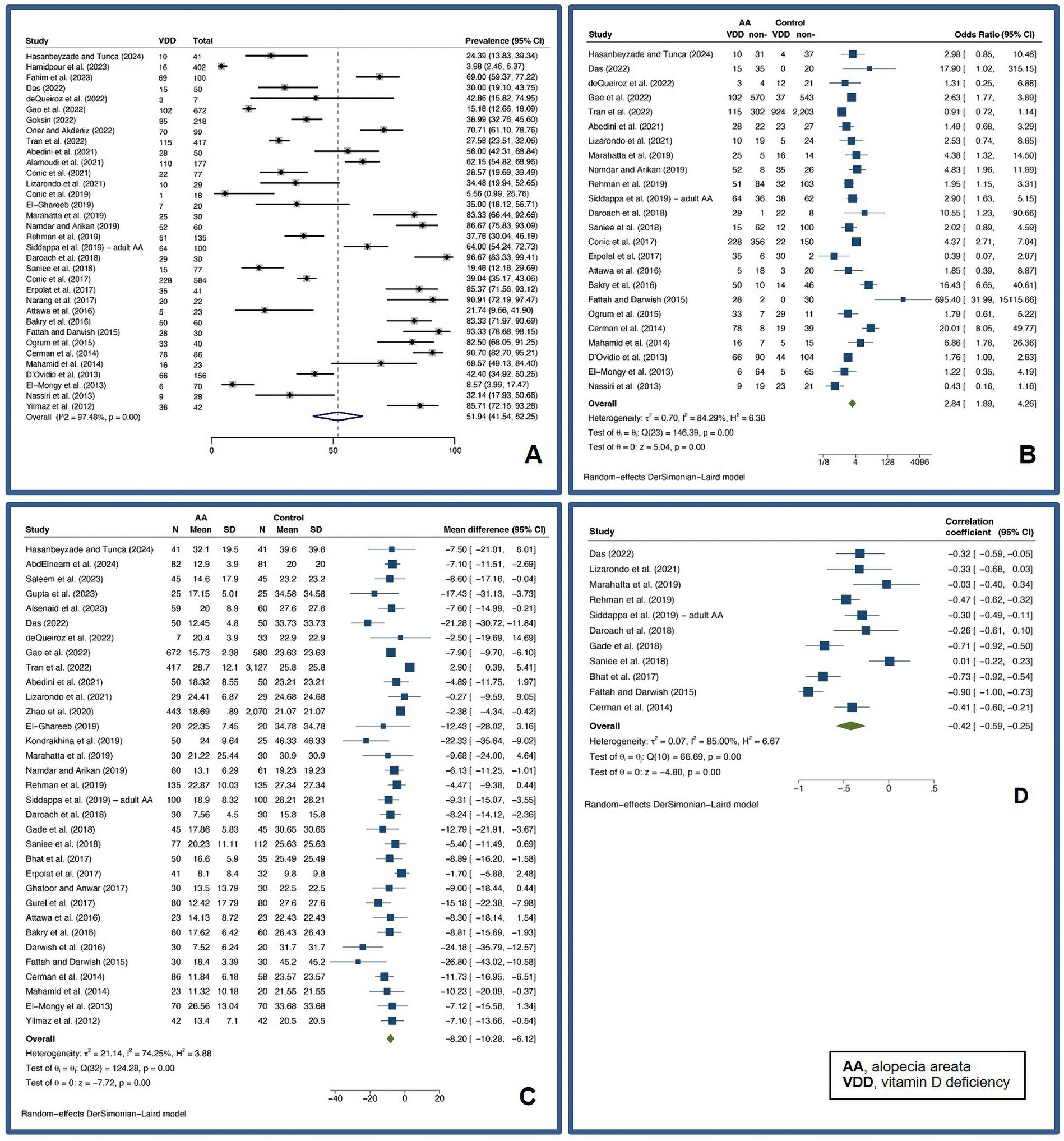
Figure 2. Forest plots for the pooled prevalence of vitamin D deficiency (A), pooled odds ratio of vitamin D deficiency (B), pooled unstandardized mean difference of vitamin D levels (C), and pooled correlation coefficient of vitamin D level and Severity of Alopecia Tool score (D) in adult alopecia areata.
3.3 Pediatric alopecia areata
A pooled VDD prevalence and a pooled VDD OR of 38.25% (7.32–75.68%, I2 = 98.35%, p < 0.01) and 3.50 (0.59–20.89, I2 = 94.02%, p < 0.01) were found, respectively. Figure 3 displays forest plots for the pooled prevalence (Figure 3A) and pooled odds ratio of VDD (Figure 3B) in pediatric AA.
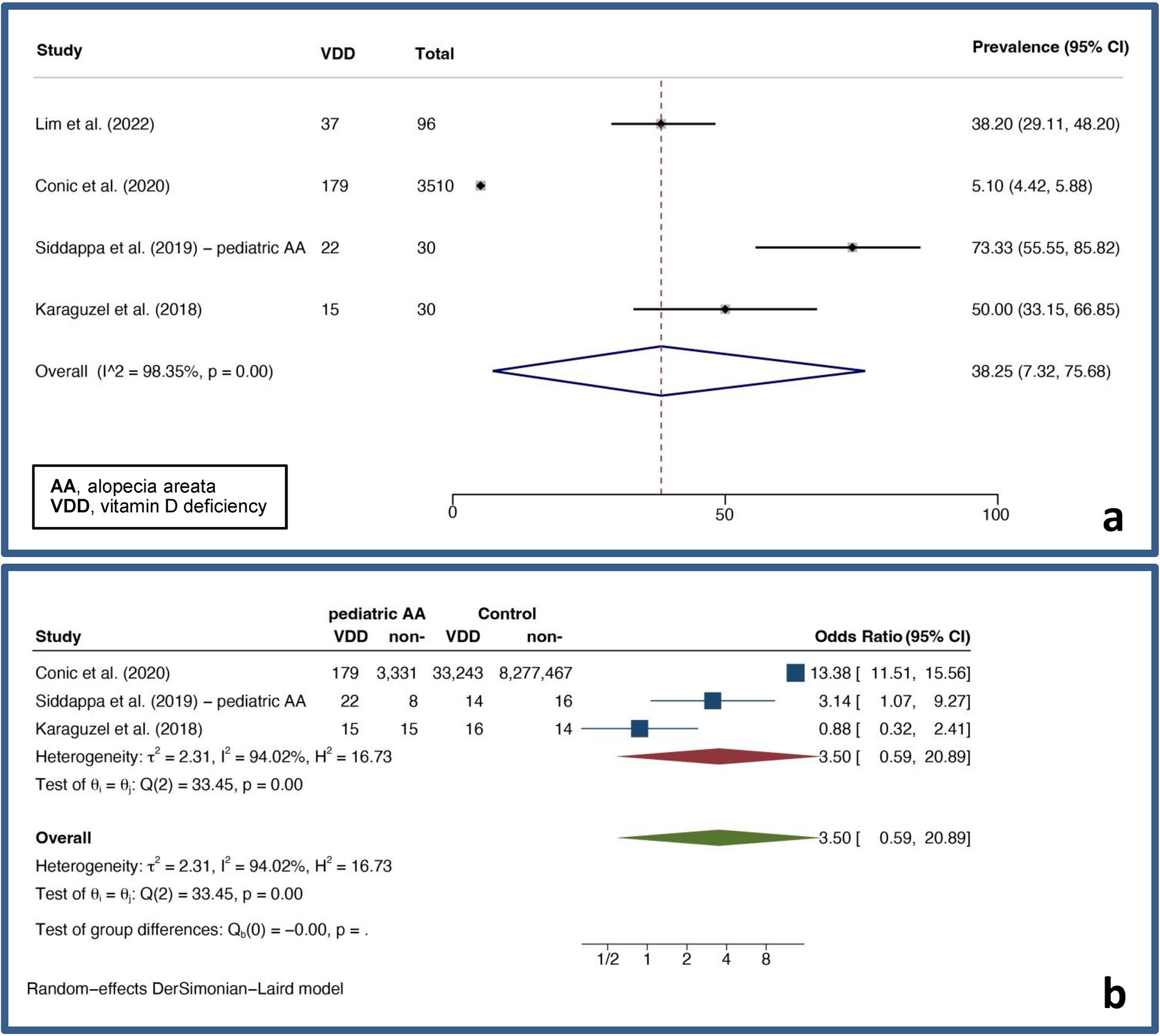
Figure 3. Forest plots for the pooled prevalence of vitamin D deficiency (A) and pooled odds ratio of vitamin D deficiency (B) in pediatric alopecia areata.
3.4 Androgenetic alopecia
A pooled VDD prevalence of 47.27% (32.49–62.29%, I2 = 96.06%, p < 0.01) was found for AGA, while a pooled VDD OR of 3.43 (0.95–12.35, I2 = 94.29%, p < 0.01) and a pooled UMD of vitamin D levels of −6.39 ng/mL (−9.81 – −2.97 ng/mL, I2 = 88.56%, p < 0.01) were found for AGA compared to controls. For FPHL, a pooled VDD prevalence of 50.38% (31.56–69.14%, I2 = 93.60%, p < 0.01) was found. Also, a pooled VDD OR of 5.24 (1.50–18.33, I2 = 81.65%, p < 0.01) and a pooled UMD of vitamin D levels of −15.67 ng/mL (−24.55 – −6.79 ng/mL, I2 = 91.60%, p < 0.01) were found compared to controls, showing a strong association between VDD and FPHL. For MAGA, a pooled VDD prevalence of 47.38% (20.41–75.17%, I2 = 94.16%, p < 0.01) was found. For MAGA, a pooled VDD OR of 4.42 (0.53–36.61, I2 = 88.40%, p < 0.01), and a pooled UMD of vitamin D levels of −2.19 ng/mL (−4.07 – −0.31 ng/mL, I2 = 7.64%, p < 0.37) were found compared to controls. Figure 4 depicts forest plots for the pooled prevalence of VDD (Figure 4A), pooled odds ratio of VDD (Figure 4B), and pooled UMD of vitamin D levels (Figure 4C) in AGA.
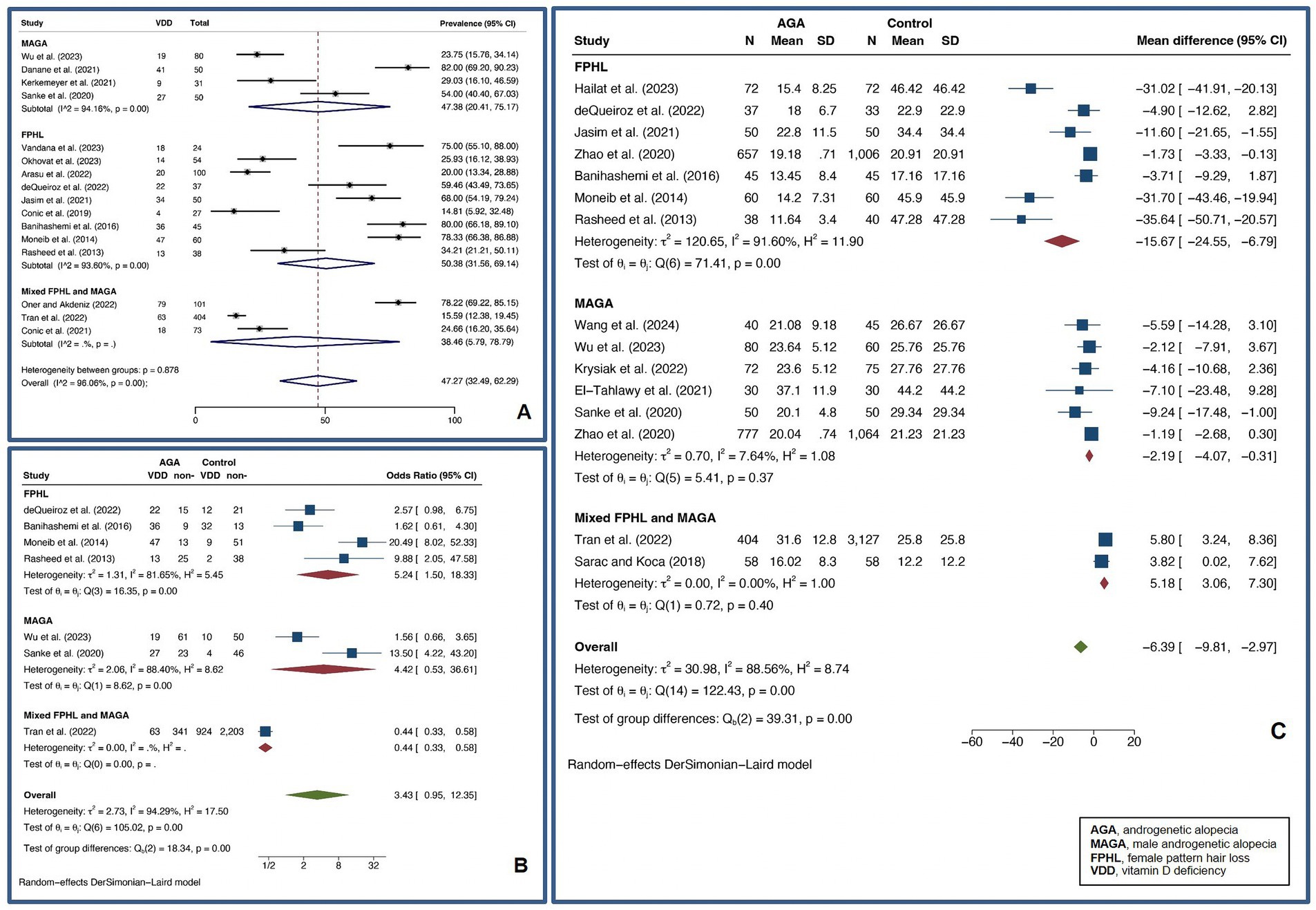
Figure 4. Forest plots for the pooled prevalence of vitamin D deficiency (A), pooled odds ratio of vitamin D deficiency (B), and pooled unstandardized mean difference of vitamin D levels (C) in androgenetic alopecia.
3.5 Telogen effluvium
A pooled VDD prevalence of 53.51% (37.33–69.33%, I2 = 97.99%, p < 0.01) was found for TE patients. A pooled VDD OR of 1.14 (0.65–1.98, I2 = 48.09%, p = 0.10) and a pooled UMD of vitamin D levels of −5.71 ng/mL (−10.10 – −1.32 ng/mL, I2 = 92.46%, p < 0.01) were found compared to controls. Figure 5 shows forest plots for the pooled prevalence of VDD (Figure 5A), pooled odds ratio of VDD (Figure 5B), and pooled UMD of vitamin D levels (Figure 5C) in TE.
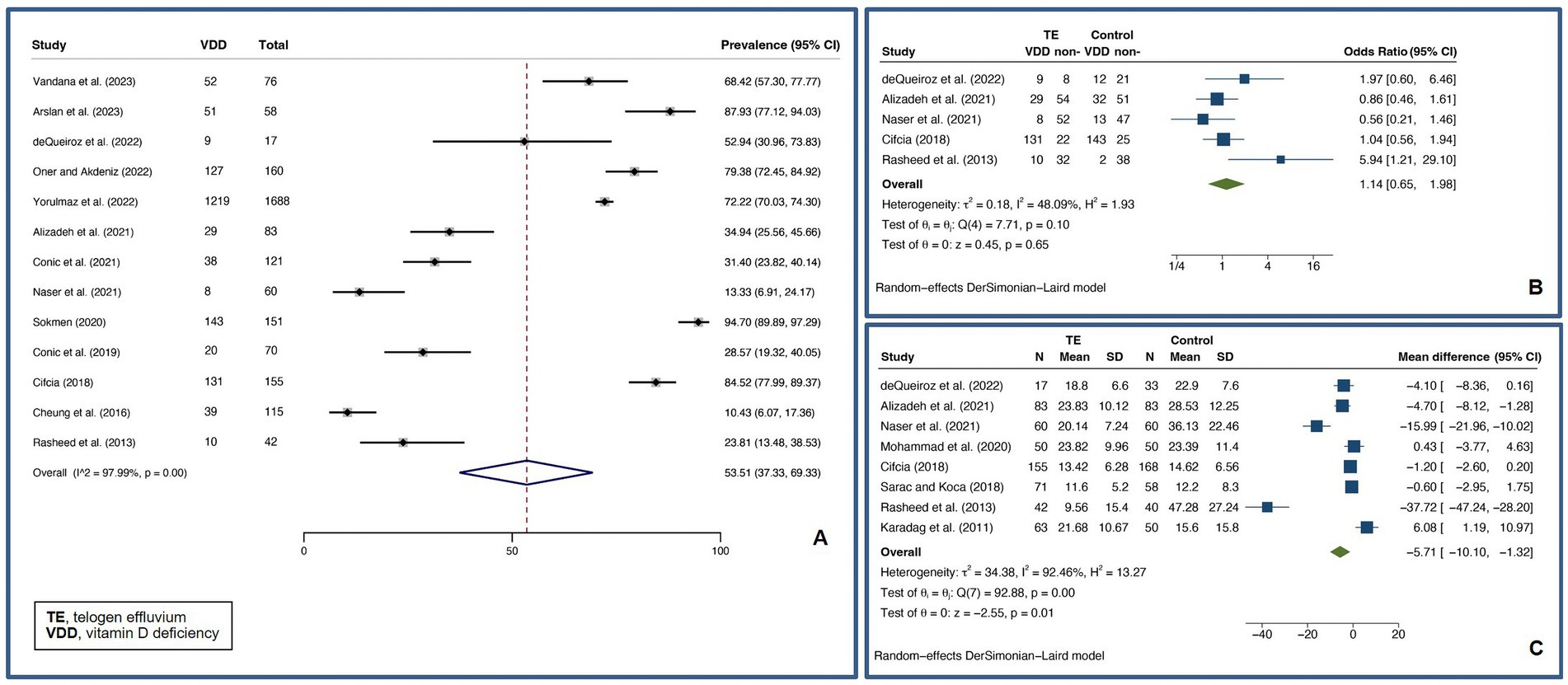
Figure 5. Forest plots for the pooled prevalence of vitamin D deficiency (A), pooled odds ratio of vitamin D deficiency (B), and pooled unstandardized mean difference of vitamin D levels (C) in telogen effluvium.
3.6 Primary scarring alopecia
A pooled VDD prevalence of 38.85% (24.29–54.40%, I2 = 91.73%, p < 0.01) was found for PCA. Subgroup analysis of specific PCA disorders was performed, and pooled VDD prevalences of 18.00% (10.57–26.61%), 56.70% (10.23–97.15%), and 37.04% (25.47–49.39%) were found for FFA, CCCA, and LPP, respectively. Figure 6 illustrates forest plot for pooled prevalence of VDD in PCA.
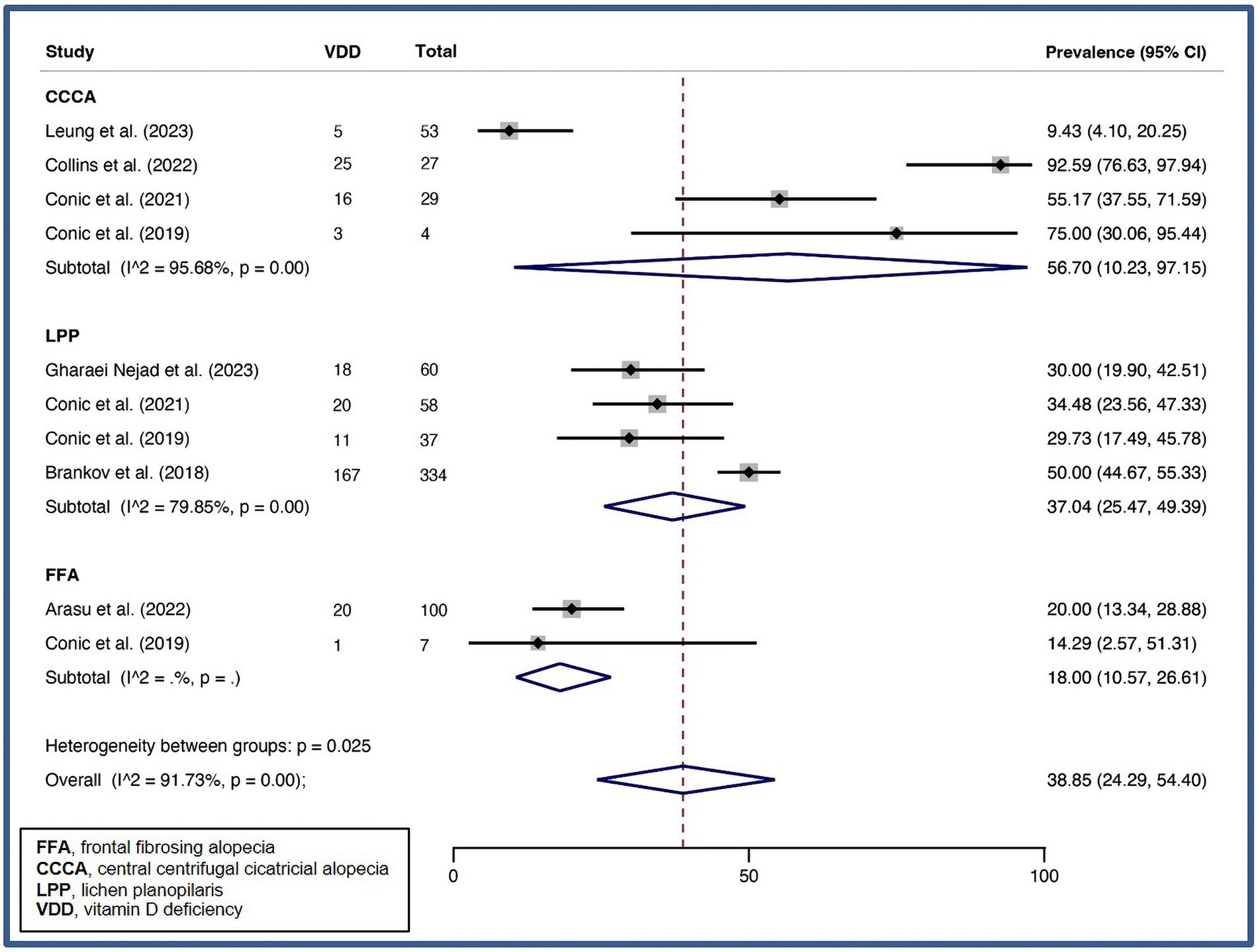
Figure 6. Forest plot for the pooled prevalence of vitamin D deficiency in primary lymphocytic scarring alopecia.
3.7 Country of research origin subgroup analysis
Pooled VDD prevalences of AA, AGA, and TE in eastern countries of 56.70% (43.04–69.87%, I2 = 97.88%, p < 0.01), 64.31% (48.41–78.81%, I2 = 92.10%, p < 0.01), and 64.42% (49.17–78.33%, I2 = 97.10%, p < 0.01), were found, respectively. Whereas pooled VDD prevalences of 31.36% (23.51–39.74%, I2 = 82.73%, p < 0.01), 25.54% (16.80–35.34%, I2 = 82.88%, p < 0.01), and 27.56% (13.59–44.07%, I2 = 88.11%, p < 0.01) were found for AA, AGA, and TE in western countries.
Pooled VDD ORs of AA, AGA, and TE in eastern countries of 3.18 (2.04–4.97, I2 = 75.74%, p < 0.01), 5.65 (1.75–18.18, I2 = 79.96%, p < 0.01), and 1.04 (0.56–1.94, I2 = 53.62%, p = 0.09) were found, respectively. While pooled VDD ORs of 1.78 (0.77–4.16, I2 = 91.62%, p < 0.01), 1.00 (0.18–5.59, I2 = 91.52%, p < 0.01), and 1.97 (0.60–6.46) were found for AA, AGA, and TE in western countries.
Pooled UMDs of vitamin D levels of AA, AGA, and TE in eastern countries of −8.24 ng/mL (−10.02 – −6.46 ng/mL, I2 = 58.11%, p < 0.01), −8.13 ng/mL (−11.97 – −4.29 ng/mL, I2 = 87.64%, p < 0.01), and − 6.07 ng/mL (−11.02 – −1.13 ng/mL, I2 = 93.47%, p < 0.01) were found, respectively. However, pooled UMDs of vitamin D levels of −6.67 ng/mL (−23.12–9.77 ng/mL, I2 = 85.29%, p < 0.01), and − 0.52 ng/mL (−8.58–7.54 ng/mL, I2 = 84.55%, p < 0.01) were found for AA and AGA in western countries. Table 5 summarizes subgroup analyses based on the country of research origin.

Table 5. Pre-planned subgroup analyses based on country of research origin, age group, and alopecia severity.
3.8 Age group subgroup analysis
AA and AGA cohorts with mean ages 18–25 years were found to have pooled VDD prevalences of 47.01% (1.32–96.78%) and 70.69% (51.32–86.96%), respectively, while AA and AGA cohorts with mean ages 25–60 years were found to have pooled VDD prevalences of 54.67% (44.56–64.60%, I2 = 96.79, p < 0.01) and 42.07% (25.49–59.60%, I2 = 96.42, p < 0.01), respectively.
AA cohorts with mean age 18–25 years were found to have a pooled VDD OR of 6.65 (1.22–36.35, I2 = 90.06, p < 0.01), while AA cohorts with mean age 25–60 years were found to have a pooled VDD OR of 2.57 (1.70–3.88, I2 = 82.36%, p < 0.01). AA cohorts with mean age 18–25 years were found to have a pooled UMD of vitamin D levels of −8.98 ng/mL (−12.23 – −5.73 ng/mL, I2 = 0.00, p = 1.00); however, AA cohorts with mean age 25–60 years were found to have a pooled UMD of vitamin D levels of −8.07 ng/mL (−10.44 – −5.70 ng/mL, I2 = 78.12, p < 0.01). Table 5 presents subgroup analyses based on age group.
3.9 Alopecia severity subgroup analysis
Severe AA cohorts were found to have a VDD prevalence of 44.36% (19.70–70.54%, I2 = 98.01%, p < 0.01), VDD OR of 3.29 (1.30–8.34, I2 = 85.53%, p < 0.01), a UMD of vitamin D levels of −10.65 ng/mL (−15.23 – −6.39 ng/mL, I2 = 44.83%, p = 0.08), while non-severe AA cohorts were found to have a VDD prevalence of 63.71% (47.43–78.58%, I2 = 95.25%, p < 0.01), VDD OR of 3.58 (2.20–5.82, I2 = 60.92%, p < 0.01), and a UMD of vitamin D levels of −8.17 ng/mL (−9.97 – −6.37 ng/mL, I2 = 15.12%, p = 0.28). Due to insufficient information, subgroup analyses based on other alopecia disorders were not conducted. Table 5 shows subgroup analyses based on alopecia severity.
3.10 Quality assessment
Supplementary Table S3 provides a summary of the quality assessment scores for comparative and descriptive studies included in the review. The average quality assessment score was 7.22 (range: 5–9), with 59 high-quality and 22 fair-quality studies. Sensitivity analyses based on study quality were performed to assess the robustness of the findings. The results were consistent with the primary analyses, suggesting that potential biases did not significantly influence the pooled estimates (Supplementary Document 2).
3.11 Publication bias
Some funnel plots were slightly asymmetric when assessing publication bias for each primary analysis (Supplementary Figure S2). As a result, Egger’s tests were conducted, and it was discovered that some analyses exhibited possible asymmetry; consequently, we performed additional contour-enhanced funnel plots. We discovered that the asymmetry in the VDD OR analyses for AA and AGA, UMD of vitamin D levels analysis in TE and AA, and CC of vitamin D level and a SALT score were likely due to heterogeneity. In the UMD of vitamin D levels analysis in AGA, however, publication bias is highly likely. Funnel plots and contour-enhanced funnel plots are shown in Supplementary Figure S2.
4 Discussion
Our analysis revealed that VDD was prevalent among patients with various alopecia disorders, including AA, FPHL, MAGA, TE, and PCA. Statistically significant associations were observed in AA and FPHL patients, who demonstrated a higher likelihood of VDD and lower vitamin D levels compared to controls, although MAGA and TE patients also exhibited lower vitamin D levels compared to controls. Geographical factors exerted an influence, as the prevalence of VDD and the reduction in vitamin D levels were more pronounced in studies conducted in Eastern countries than in Western countries. Furthermore, younger patients aged 18 to 25 years exhibited a higher prevalence of VDD and more severe reductions in vitamin D levels compared to older patients. The study also determined that patients with severe AA exhibited greater reductions in vitamin D levels compared to controls, though both severe and non-severe AA patients had comparable VDD prevalence. Overall, the findings suggest a significant relationship between vitamin D deficiency and certain types of alopecia, particularly AA and FPHL, underscoring the necessity for further research into the role of vitamin D in these conditions.
The role of vitamin D as an immunomodulator is particularly relevant in AA, in which autoimmune mechanisms are hypothesized to play a pivotal role. Vitamin D modulates the activity of cytotoxic T cells, regulatory T cells, and dendritic cells, all of which are involved in AA pathogenesis. Insufficient vitamin D levels may contribute to dysregulation of the immune response in AA, potentially resulting in an autoimmune attack on hair follicles (101).
Previously, there have been a few meta-analyses on VDD and AA. Similar to previous studies, we found that VDD is prevalent among AA, and compared to controls, AA had significantly higher odds of VDD and significantly lower vitamin D levels (102–104). In addition to updating the previous systematic review, we also pooled CC of AA disease severity and found a significant negative correlation between SALT scores and vitamin D levels, and severe AA was found to have a greater reduction of vitamin D level compared to control (vs non-severe AA). However, both severe and non-severe AA cohorts have similar odds of VDD. Currently, no cohort study has investigated the causal relationship between AA and VDD. Therefore, it is unknown whether VDD initiates AA pathogenesis or exacerbates AA conditions or whether AA causes VDD. A longitudinal study that investigates the connection between AA and VDD would provide evidence and strengthen the recommendation to screen AA patients for VDD.
A significantly stronger association between VDD and AA was observed in cohorts with AA patients with a disease duration of less than 1 year, suggesting that vitamin D status may play a crucial role in the early stages of AA development. Additionally, studies with age group of 18-to-25 years showed a higher risk of VDD, indicating that young adults with AA might be especially vulnerable to vitamin D deficiency. Interestingly, cohorts with a higher proportion of relapsed AA were found to have a higher risk of VDD, which aligns with the understanding of AA as an autoimmune disease (7). The relapsed state may represent compromised immune regulation, potentially exacerbated by low vitamin D levels (3, 7, 105–107). In contrast, cohorts with a lower proportion of females had lower vitamin D levels, hinting at possible gender-specific differences in vitamin D metabolism or AA pathogenesis. These findings underscore the complex interplay between vitamin D status and AA, highlighting the need for further research to elucidate the specific role of age, gender, and disease duration in this relationship. Such investigations could provide valuable insights into the pathophysiology of AA and potentially inform more targeted therapeutic approaches.
As for pediatric AA, current evidence indicates that the prevalence and likelihood of VDD are lower than in the adult population, with non-statistically significant odds of having VDD compared to pediatric controls. Current evidence is relatively limited, and additional well-controlled studies are required to clarify the significance of VDD in pediatric AA.
To our knowledge, this is the first systematic review and meta-analysis to investigate the association between VDD and non-AA hair loss disorders, specifically AGA, TE, and scarring alopecia. Both TE and AGA were found to have a VDD prevalence of approximately 50% and significantly lower vitamin D levels than controls but did not have an increased risk of VDD compared to controls. However, the likelihood of VDD in FPHL patients is statistically significantly higher than in controls. Vitamin D levels in FPHL cohorts are significantly lower than in controls compared to AGA in general. Although MAGA data is limited, we hypothesize that gender plays a significant role in VDD and AGA (108, 109).
Our study found that in Eastern countries, the prevalence of vitamin D deficiency, the likelihood of VDD, and the degree of lower vitamin D levels among AA, AGA, and TE were significantly higher than in Western nations. European Caucasians have a lower prevalence of VDD than non-white individuals (110). In addition to differences in skin pigmentation, the use of sunscreen and latitude are also significant factors that could cause VDD by reducing vitamin D synthesis (111–113). Melanin in darker skin tones can interfere with vitamin D synthesis, while widespread sunscreen use and higher latitudes with less direct sunlight can reduce vitamin D production (114). Moreover, because these factors are rarely matched but could significantly influence the results, additional studies matching sunscreen use and FST are required.
Due to the limited number of case–control studies, the relationship between VDD and scarring alopecia remains poorly understood. However, our analysis suggests that the prevalence of VDD may vary among different types of PCA. Based on available data, certain PCA diseases may have a higher VDD prevalence than others. For instance, CCCA has a VDD prevalence of 57%, whereas FFA has a VDD prevalence of 18%. However, the higher prevalence of VDD among CCCA patients may not be due to the disease itself but rather their skin pigmentation, as it almost exclusively affects females with FST V-VI, which are known to be associated with a higher risk of VDD due to reduced vitamin D synthesis in darker skin (111, 115). To establish a more definitive association between scarring alopecia and VDD, and to better understand the role of confounding factors such as skin type, additional well-designed controlled studies are required.
The results of our study should be interpreted with caution due to the highly heterogeneous study population and setting of the included studies, as well as the fact that serum vitamin D levels can be affected by a variety of factors, such as geographic characteristics, ethnicity, and skin tone (111). Subgroup analyses were conducted to explore potential sources of heterogeneity; however, for most alopecia types, significant sources of heterogeneity were not identified, suggesting that residual heterogeneity may be attributed to unmeasured variables or other contextual factors not captured in the included studies. Future research should focus on more detailed reporting and examination of factors contributing to heterogeneity. Also, publication bias exists in the analyses of the pooled UMD in vitamin D levels between AGA and controls, which necessitates caution when interpreting our results.
The study has several strengths and limitations. One of the strengths is its comprehensive approach, utilizing a systematic review and meta-analysis to pool data from a wide range of studies. The study also follows rigorous methodological standards, including the registration of the protocol in PROSPERO, adherence to PRISMA guidelines, and the use of validated tools such as the NOS for quality assessment, which contributes to the robustness of the conclusions. However, the study has some limitations. The heterogeneity of the included studies suggests considerable variability in study populations, settings, and methodologies, which could affect the reliability of the pooled estimates. Moreover, the study’s reliance on observational data limits the ability to infer causality. Finally, the limited number of studies on scarring alopecia and pediatric populations restricts the generalizability of the findings to these subgroups.
5 Conclusion
Even though VDD is prevalent among alopecia patients, the likelihood of VDD and decreased vitamin D levels compared to the control population was statistically significant only in adult AA and FPHL. Adult AA disease severity was found to be significantly negatively correlated with vitamin D levels, with severe AA cohorts showing a higher reduction of vitamin D levels compared to controls; however, severe and non-severe AA cohorts appear to have comparable VDD prevalence and VDD likelihood compared to controls. Cohorts with less than one year of AA duration and a higher proportion of relapsed AA were found to have a higher risk of VDD, while cohorts with a lower proportion of females had lower vitamin D levels. Studies conducted in Eastern nations appear to report a higher VDD prevalence, VDD likelihood, and vitamin D reduction than studies conducted in Western nations. Evidence is still lacking for MAGA, scarring alopecia, and pediatric AA, highlighting the need for further research in these areas. It is important to acknowledge the limitations of this study, including the heterogeneity of the included studies. Given these results, clinicians should consider routine screening for VDD in patients with severe AA or FPHL, particularly in Eastern countries or in patients with recent onset or relapsed AA. Early detection and potential correction of vitamin D deficiency could play a role in managing the severity and progression of alopecia. Future studies should focus on addressing the gaps in our understanding of the role of vitamin D in alopecia, particularly MAGA, scarring alopecia, and pediatric AA, as well as investigating the potential benefits of vitamin D supplementation in alopecia management.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
TY: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft. KaT: Conceptualization, Data curation, Investigation, Methodology, Validation, Writing – review & editing. KuT: Conceptualization, Formal analysis, Methodology, Supervision, Validation, Writing – review & editing. PS: Conceptualization, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1479337/full#supplementary-material
References
1. Mora, JR, Iwata, M, and von Andrian, UH. Vitamin effects on the immune system: vitamins a and D take Centre stage. Nat Rev Immunol. (2008) 8:685–98. doi: 10.1038/nri2378
2. Ersoy-Evans, S . Commentary: Vitamin D and autoimmunity: is there an association? J Am Acad Dermatol. (2010) 62:942–4. doi: 10.1016/j.jaad.2010.02.009
3. Hewison, M . An update on Vitamin D and human immunity. Clin Endocrinol. (2012) 76:315–25. doi: 10.1111/j.1365-2265.2011.04261.x
4. Arnson, Y, Amital, H, and Shoenfeld, Y. Vitamin D and autoimmunity: new Aetiological and therapeutic considerations. Ann Rheum Dis. (2007) 66:1137–42. doi: 10.1136/ard.2007.069831
5. Doche, I, Romiti, R, Hordinsky, MK, and Valente, NS. “Normal-appearing” scalp areas are also affected in lichen Planopilaris and frontal Fibrosing alopecia: an observational histopathologic study of 40 patients. Exp Dermatol. (2020) 29:278–81. doi: 10.1111/exd.13834
6. Dankers, W, Colin, EM, van Hamburg, JP, and Lubberts, E. Vitamin D in autoimmunity: molecular mechanisms and therapeutic potential. Front Immunol. (2017) 7:7. doi: 10.3389/fimmu.2016.00697
7. Suchonwanit, P, Kositkuljorn, C, and Pomsoong, C. Alopecia Areata: an autoimmune disease of multiple players. Immunotargets Ther. (2021) 10:299–312. doi: 10.2147/itt.S266409
8. Harries, MJ, and Paus, R. The pathogenesis of primary Cicatricial Alopecias. Am J Pathol. (2010) 177:2152–62. doi: 10.2353/ajpath.2010.100454
9. Harnchoowong, S, and Suchonwanit, P. Ppar-Γ agonists and their role in primary Cicatricial alopecia. PPAR Res. (2017) 2017:2501248. doi: 10.1155/2017/2501248
10. Malloy, PJ, and Feldman, D. The role of Vitamin D receptor mutations in the development of alopecia. Mol Cell Endocrinol. (2011) 347:90–6. doi: 10.1016/j.mce.2011.05.045
11. Bikle, DD, and Vitamin, D. Metabolism and function in the skin. Mol Cell Endocrinol. (2011) 347:80–9. doi: 10.1016/j.mce.2011.05.017
12. Solomon, JD, Heitzer, MD, Liu, TT, Beumer, JH, Parise, RA, Normolle, DP, et al. Vdr activity is differentially affected by Hic-5 in prostate Cancer and stromal cells. Mol Cancer Res. (2014) 12:1166–80. doi: 10.1158/1541-7786.Mcr-13-0395
13. Aksu Cerman, A, Sarikaya Solak, S, and Kivanc, AI. Vitamin D deficiency in alopecia Areata. Br J Dermatol. (2014) 170:1299–304. doi: 10.1111/bjd.12980
14. Zhao, J, Sheng, Y, Dai, C, Qi, S, Hu, R, Rui, W, et al. Serum 25 Hydroxyvitamin D levels in alopecia Areata, female pattern hair loss, and male androgenetic alopecia in a Chinese population. J Cosmet Dermatol. (2020) 19:3115–21. doi: 10.1111/jocd.13396
15. Saniee, S, Zare, AG, and Radmehr, A. Evaluating the serum zinc and Vitamin D levels in alopecia Areata. Iranian J Dermatol. (2018) 21:77–80. doi: 10.22034/ijd.2018.98360
16. Tran, PT, Chen, A, Yi, L, and Goh, C. Vitamin D levels in alopecia Areata and other Alopecias: a retrospective case- control study at a single institution. Int J Trichology. (2022) 14:175–7. doi: 10.4103/ijt.ijt_131_20
17. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DG. Preferred reporting items for systematic reviews and Meta-analyses: the Prisma statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
18. Holick, MF . Sunlight and Vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. (2004) 80:1678s–88s. doi: 10.1093/ajcn/80.6.1678S
19. Wells, G, Shea, B, O'Connell, D, Peterson, J, Welch, V, Losos, M, et al. The Newcastle–Ottawa scale (Nos) for assessing the quality of non-randomized studies in Meta-analysis. (2000). Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (Accessed 2024 September 26).
20. Nyaga, VN, Arbyn, M, and Aerts, M. Metaprop: a Stata command to perform Meta-analysis of binomial data. Arch Public Health. (2014) 72:39. doi: 10.1186/2049-3258-72-39
21. Deeks, JJ, Altman, DG, and Bradburn, MJ. Statistical methods for examining heterogeneity and combining results from several studies in Meta-analysis. Syst Rev Health Care. (2001):285–312. doi: 10.1002/9780470693926.ch15
22. Thompson, SG . Why sources of heterogeneity in Meta-analysis should be investigated. BMJ. (1994) 309:1351–5. doi: 10.1136/bmj.309.6965.1351
23. Sterne, JA, Egger, M, and Smith, GD. Systematic reviews in health care: investigating and dealing with publication and other biases in Meta-analysis. BMJ. (2001) 323:101–5. doi: 10.1136/bmj.323.7304.101
24. Chen, V, Strazzulla, L, Asbeck, SM, and Bellodi, SF. Etiology, management, and outcomes of pediatric Telogen effluvium: a single-center study in the United States. Pediatr Dermatol. (2022) 40:120–4. doi: 10.1111/pde.15154
25. Losoya-Jaquez, MR, Lopez Yañez-Blanco, A, Armendariz-Barragan, Y, Aguilar-Figueroa, NG, Rudnicka, L, and Sanchez-Dueñas, LE. Androgenetic alopecia in children and adolescents: from Trichoscopy to therapy. Skin Appendage Disord. (2024) 10:123–8. doi: 10.1159/000534844
26. Das, AK . A hospital-based observational evaluation of serum 25-Hydroxy Vitamin D levels in alopecia Areata of scalp. Int J Toxicol Pharmacol Res. (2022) 12:241–7.
27. de Queiroz, M, Vaske, TM, and Boza, JC. Serum ferritin and Vitamin D levels in women with non-scarring alopecia. J Cosmet Dermatol. (2022) 21:2688–90. doi: 10.1111/jocd.14472
28. Gao, Y, Huo, S, Sun, M, Zhang, C, Wang, J, Gao, J, et al. Evaluation of several immune and inflammatory indicators and their association with alopecia Areata. J Cosmet Dermatol. (2022) 21:2995–3001. doi: 10.1111/jocd.14504
29. Goksin, S . Retrospective evaluation of clinical profile and comorbidities in patients with alopecia Areata. North Clin Istanb. (2022) 9:451–8. doi: 10.14744/nci.2022.78790
30. Lim, RK, Castelo-Soccio, L, Putterman, E, Qureshi, AA, and Cho, E. Predictors of Vitamin D insufficiency in children and adolescents with alopecia Areata. Cureus. (2022) 14:e22934. doi: 10.7759/cureus.22934
31. Öner, Ü, and Akdeniz, N. Nonscarring scalp alopecia: which laboratory analysis should we perform on whom? Turkish J Med Sci. (2022) 52:188–94. doi: 10.3906/sag-2106-28
32. Abedini, R, Shakiba, S, Ghandi, N, Yazdaniamjad, F, Haddadi, NS, and Nasimi, M. Study of Vitamin D deficiency in patients with alopecia Areata attending a dermatology Center in Iran. Iranian J Dermatol. (2021) 24:97–101. doi: 10.22034/ijd.2021.132456
33. Alamoudi, SM, Marghalani, SM, Alajmi, RS, Aljefri, YE, and Alafif, AF. Association between Vitamin D and zinc levels with alopecia Areata phenotypes at a tertiary care center. Cureus. (2021) 13:e14738. doi: 10.7759/cureus.14738
34. Conic, RRZ, Piliang, M, Bergfeld, W, and Atanaskova-Mesinkovska, N. Vitamin D status in scarring and nonscarring alopecia. J Am Acad Dermatol. (2021) 85:478–80. doi: 10.1016/j.jaad.2018.04.032
35. Lizarondo, FPJ, Gervasio, MKR, Chamberlin, CVS, Gnilo, CMS, and Silva, CY. Determination of serum 25-Hydroxyvitamin D levels in patients with alopecia Areata and their comparison with levels in healthy controls: a cross-sectional study. JAAD Int. (2021) 5:78–84. doi: 10.1016/j.jdin.2021.07.008
36. Conic, RR, Tamashunas, NL, Damiani, G, Fabbrocini, G, Cantelli, M, and Bergfeld, WF. Comorbidities in pediatric alopecia Areata. J Europ Acad Dermatol Venereol. (2020) 34:2898–901. doi: 10.1111/jdv.16727
37. Conic, RRZ, Juhasz, M, Rambhia, P, Damiani, G, Atanaskova-Mesinkovska, N, Piliang, M, et al. Characterizing hair loss in the elderly: an observational study of 163 patients. J Eur Acad Dermatol Venereol. (2019) 33:e226–8. doi: 10.1111/jdv.15462
38. El-Ghareeb, M . Deficient and/or insufficient serum 25 Hydroxy Vitamin D in patients with alopecia Areata: is it a fact or a fiction? Egyptian J Dermatol Venerol. (2019) 39:21. doi: 10.4103/ejdv.ejdv_25_18
39. Marahatta, S, Agrawal, S, and Khan, S. Study on serum Vitamin D in alopecia Areata patients. J Nepal Health Res Counc. (2019) 17:21–5. doi: 10.33314/jnhrc.v17i01.1475
40. Namdar, ND, and Arikan, I. The relationship between Vitamin D levels and the quality of life in patients with alopecia Areata and vitiligo. Turk Osteoporoz Dergisi. (2019) 25:35–9. doi: 10.4274/TOD.GALENOS.2019.19327
41. Rehman, F, Dogra, N, and Wani, M. Serum Vitamin D levels and alopecia Areata- a hospital based case-control study from North-India. Int J Trichology. (2019) 11:49–57. doi: 10.4103/ijt.ijt_3_19
42. Siddappa, H, Kumar, YHK, and Vivekananda, N. Evaluation of Association of Vitamin D in alopecia Areata: a case-control study of 100 patients in a tertiary rural Hospital of Southern India. Indian Dermatol Online J. (2019) 10:45–9. doi: 10.4103/idoj.IDOJ_84_18
43. Siddappa, H, Yadalla, H, and Neladimmanahally, V. Evaluation of Vitamin D in pediatric alopecia Areata: a case–control study of thirty patients in a tertiary care hospital. Indian J Paediatr Dermatol. (2019) 20:32. doi: 10.4103/ijpd.IJPD_83_18
44. Daroach, M, Narang, T, Saikia, UN, Sachdeva, N, and Sendhil, KM. Correlation of Vitamin D and Vitamin D receptor expression in patients with alopecia Areata: a clinical paradigm. Int J Dermatol. (2018) 57:217–22. doi: 10.1111/ijd.13851
45. Gade, VKV, Mony, A, Munisamy, M, Chandrashekar, L, and Rajappa, M. An investigation of Vitamin D status in alopecia Areata. Clin Exp Med. (2018) 18:577–84. doi: 10.1007/s10238-018-0511-8
46. Karaguzel, G, Sakarya, NP, Bahadir, S, Beyhun, E, and Yaman, S. Vitamin D Status and the Effect of Oral Vitamin D Treatment in Children with Alopecia Areata. J Steroids Horm Sci. (2018) 9:189. doi: 10.4172/2157-7536.1000189
47. Unal, M, and Gonulalan, G. Serum Vitamin D level is related to disease severity in pediatric alopecia Areata. J Cosmet Dermatol. (2018) 17:101–4. doi: 10.1111/jocd.12352
48. Bhat, YJ, Latif, I, Malik, R, Hassan, I, Sheikh, G, Lone, KS, et al. Vitamin D Level in Alopecia Areata. Indian J Dermatol. (2017) 62:407–10. doi: 10.4103/ijd.IJD_677_16
49. Conic, RZ, Miller, R, Piliang, M, Bergfeld, W, and Atanaskova, MN. Comorbidities in patients with alopecia Areata. J Am Acad Dermatol. (2017) 76:755–7. doi: 10.1016/j.jaad.2016.12.007
50. Erpolat, S, Sarifakioglu, E, and Ayyildiz, A. 25-Hydroxyvitamin D status in patients with alopecia Areata. Postepy Dermatol Alergol. (2017) 34:248–52. doi: 10.5114/ada.2017.67847
51. Ghafoor, R, and Anwar, MI. Vitamin D deficiency in alopecia Areata. J College Phys Surg Pakistan. (2017) 27:200–2.
52. Narang, T, Daroach, M, and Kumaran, MS. Efficacy and safety of topical Calcipotriol in Management of Alopecia Areata: a pilot study. Dermatol Ther. (2017) 30:e12464. doi: 10.1111/dth.12464
53. Attawa, EMKA, Elbalaat, W, and Samy, AM. Assessment of Vitamin D level in patients of alopecia Areata. J Clin Investigat Dermatol. (2016) 4:4. doi: 10.13188/2373-1044.1000030
54. Bakry, OA, El Farargy, SM, El Shafiee, MK, and Soliman, A. Serum Vitamin D in patients with alopecia Areata. Indian Dermatol Online J. (2016) 7:371–7. doi: 10.4103/2229-5178.190504
55. Darwish, N, Marzok, H, Gaballah, M, and Abdellatif, H. Serum level of Vitamin D in patients with alopecia Areata. Egyptian J Basic Appl Sci. (2016) 4:9–14. doi: 10.1016/j.ejbas.2016.12.001
56. Fattah, NSAA, and Darwish, YW. Assessment of serum 25-Hydroxyvitamin D levels in patients with extensive/recalcitrant alopecia Areata before and after Puva and Nb-Uvb therapy. J Egyp Women's Dermatol Soc. (2015) 12:19–23. doi: 10.1097/01.EWX.0000450679.92939.42
57. Oʇrum, A, Boyraz, N, Toʇral, AK, Karasati, S, and Ekşioʇlu, HM. Evaluation of 25 Hydroxy Vitamin D3 levels in patients with alopecia Areata. Turkderm Deri Hastaliklari ve Frengi Arsivi. (2015) 49:50–3. doi: 10.4274/turkderm.07888
58. Mahamid, M, Abu-Elhija, O, Samamra, M, Mahamid, A, and Nseir, W. Association between Vitamin D levels and alopecia Areata. Israel Med Ass J. (2014) 16:367–70.
59. D'Ovidio, R, Vessio, M, and D'Ovidio, FD. Reduced level of 25-Hydroxyvitamin D in chronic/relapsing alopecia Areata. Dermatoendocrinol. (2013) 5:271–3. doi: 10.4161/derm.24411
60. El-Mongy, NN, El-Nabarawy, E, Hassaan, SA, Younis, ER, and Shaker, O. Serum 25-Hydroxy Vitamin D3 level in Egyptian patients with alopecia Areata. J Egyp Women’s Dermatol Soc. (2013) 10:37–41. doi: 10.1097/01.EWX.0000419612.74665.2b
61. Nassiri, S, Saffarian, Z, and Younespour, S. Association of Vitamin D Level with alopecia Areata. Iranian J Dermatol. (2013) 16:1–5.
62. Yilmaz, N, Serarslan, G, and Gokce, C. Vitamin D concentrations are decreased in patients with alopecia Areata. Vitamins & Trace. Elements. (2012) 1:01. doi: 10.4172/2167-0390.1000105
63. AbdElneam, AI, Al-Dhubaibi, MS, Bahaj, SS, Mohammed, GF, Alantry, AK, and Atef, LM. C-reactive protein as a novel biomarker for Vitamin D deficiency in alopecia Areata. Skin Res Technol. (2024) 30:e13657. doi: 10.1111/srt.13657
64. Alsenaid, A, Al-Dhubaibi, MS, Alhetheli, G, and AbdElneam, AI. Trichoscopy pattern and evaluation of serum Vitamin D status in alopecia Areata. Photodiagn Photodyn Ther. (2023) 42:103510. doi: 10.1016/j.pdpdt.2023.103510
65. Fahim, M, Khoso, H, Hussain, A, and Bakhtiar, R. Deficiency in alopecia Areata and responsiveness to Vitamin D analogues: a prospective trial. J Pak Assoc Dermatol. (2023) 33:1242–8.
66. Gupta, SS, Mahendra, A, Gupta, S, and Singla, R. Serum Vitamin D levels in alopecia Areata: a case-control study. Iranian J Dermatol. (2023) 26:1–5. doi: 10.22034/ijd.2023.169888
67. Hamidpour, E, Shakoei, S, Nasimi, M, and Ghandi, N. Effects of age and sex on the comorbidities of alopecia Areata: a cross-sectional hospital-based study. Health Sci Rep. (2023) 6:e1444. doi: 10.1002/hsr2.1444
68. Hasanbeyzade, S, and Tunca, M. Vitamin D levels and their relationship with the type of involvement in alopecia Areata patients. J Pak Assoc Dermatol. (2024) 34:200–7.
69. Saleem, S, Erfan, M, Bacha, MF, Khan, I, Jabbar, A, and Akram, S. Prevalence of Vitamin-D deficiency among individuals diagnosed with alopecia Areata. Med Forum Month. (2023) 34:66–9. doi: 10.60110/medforum.341216
70. Arasu, A, Meah, N, Eisman, S, Wall, D, and Sinclair, R. Vitamin D status in patients with frontal Fibrosing alopecia: a retrospective study. JAAD Int. (2022) 7:129–30. doi: 10.1016/j.jdin.2022.03.008
71. Krysiak, R, Kowalcze, K, and Okopień, B. Impaired metabolic effects of metformin in men with early-onset androgenic alopecia. Pharmacol Rep. (2022) 74:216–28. doi: 10.1007/s43440-021-00347-8
72. Danane, AMG, and Agrawal, S. Study of Vitamin D levels in men with premature androgenetic alopecia. Paripex Indian J Res. (2021) 10:41–2. doi: 10.36106/paripex/1503642
73. Tahlawy, S, Alkhayat, M, and Samhoud, E. Serum Vitamin D and serum ferritin levels in male pattern hair loss: is there a role? Fayoum Univ Med J. (2021) 8:1–8. doi: 10.21608/fumj.2021.184699
74. Jasim, KI, Khidhair, ASMA, and Hussain, GA. Assessment of serum Vit D and serum ferritin in female pattern androgenic alopecia. Indian J Forensic Med Toxicol. (2021) 15:3343–51. doi: 10.37506/ijfmt.v15i3.15818
75. Kerkemeyer, KL, de Carvalho, LT, Jerjen, R, John, J, Sinclair, RD, Pinczewski, J, et al. Female pattern hair loss in men: a distinct clinical variant of androgenetic alopecia. J Am Acad Dermatol. (2021) 85:260–2. doi: 10.1016/j.jaad.2020.09.042
76. Sanke, S, Samudrala, S, Yadav, A, Chander, R, and Goyal, R. Study of serum Vitamin D levels in men with premature androgenetic alopecia. Int J Dermatol. (2020) 59:1113–6. doi: 10.1111/ijd.14982
77. Kondrakhina, IN, Verbenko, DA, Zatevalov, AM, Kubanov, AA, and Deryabin, DG. The value of genetic and non-genetic factors in the emergence and in the development of androgenetic alopecia in men: multifactor analysis. Vestnik Rossiiskoi Akademii Meditsinskikh Nauk. (2019) 74:167–75. doi: 10.15690/vramn1141
78. Saraç, GKT . The importance of Vitamin-D in androgenic alopecia and Telogen effluvium. J Clin Med Kazakhstan. (2018) 4:26–9. doi: 10.23950/1812-2892-JCMK-00601
79. Banihashemi, M, Nahidi, Y, Meibodi, NT, Jarahi, L, and Dolatkhah, M. Serum Vitamin D3 level in patients with female pattern hair loss. Int J Trichology. (2016) 8:116–20. doi: 10.4103/0974-7753.188965
80. Moneib, H, Fathy, G, and Ouda, A. Possible Association of Female-Pattern Hair Loss with alteration in serum 25-Hydroxyvitamin D levels. Egypt J Dermatol Venerol. (2014) 34:15–20. doi: 10.4103/1110-6530.137254
81. Rasheed, H, Mahgoub, D, Hegazy, R, El-Komy, M, Abdel Hay, R, Hamid, MA, et al. Serum ferritin and Vitamin D in female hair loss: do they play a role? Skin Pharmacol Physiol. (2013) 26:101–7. doi: 10.1159/000346698
82. Hailat, R, Mughal, H, Malik, T, Sagheer, A, Javed, A, and Memon, Q. Possible Association of Female-Pattern Hair Loss with alteration in serum 25-Hydroxyvitamin D levels. Pakistan J Med Health Sci. (2023) 17:623–5. doi: 10.53350/pjmhs2023172623
83. Okhovat, JP, Marks, DH, Manatis-Lornell, A, Hagigeorges, D, and Senna, MM. Utility of laboratory testing in patients with female pattern hair loss. J Am Acad Dermatol. (2023) 88:153–5. doi: 10.1016/j.jaad.2019.07.015
84. Vandana, D, Gudi, SD, Raghuveer, C, and Pattar, LY. Biochemical correlates of diffuse non scarring alopecia in women. J Cardiovasc Dis Res. (2023) 14:553–9. doi: 10.48047/jcdr.2023.14.07.56
85. Wu, Y, Hui, Y, Liu, F, Chen, H, Liu, K, Chen, Q, et al. The Association of Serum Adipokines, insulin resistance and Vitamin D status in male patients with androgenetic alopecia. Clin Cosmet Investig Dermatol. (2023) 16:419–27. doi: 10.2147/ccid.S396697
86. Wang, S, Xu, S, Wang, S, Fang, W, and Shi, W. Risk factors and lipid metabolism characteristics of early-onset male androgenetic alopecia: a pilot study. J Cosmet Dermatol. (2024) 23:3038–44. doi: 10.1111/jocd.16371
87. Yorulmaz, A, Hayran, Y, Ozdemir, AK, Sen, O, Genc, I, Gur Aksoy, G, et al. Telogen effluvium in daily practice: patient characteristics, laboratory parameters, and treatment modalities of 3028 patients with Telogen effluvium. J Cosmet Dermatol. (2022) 21:2610–7. doi: 10.1111/jocd.14413
88. Alizadeh, N, Rafiei, R, Darjani, A, Eftekhari, H, Nejad, K, Rafiei, E, et al. Chronic Telogen effluvium in women: role of micronutrients, a case-control study in north of Iran. J Egypt Women's Dermatol Soc. (2021) 18:205–9. doi: 10.4103/jewd.jewd_34_21
89. Naser, RT, Fadheel, QJ, and Hafedh, AH. The significance of serum ferritin and Vitamin D levels in females patients with chronic Telogen effluvium. Indian J Forensic Med Toxicol. (2021) 15:3992–4000. doi: 10.37506/ijfmt.v15i3.15920
90. Mohammad, AP, Baba, A, and Ghassemi, M. Comparison between serum levels of Vitamin D and zinc in women with diffuse non-scarring hair loss (Telogen effluvium) and healthy women. Pakistan J Med Health Sci. (2020) 14:1400–4.
91. Sökmen, F . The etiological role of thyroid dysfunction and Vitamin deficiency in patients with Telogen effluvium. Osmangazi J Med. (2020) 42:705–9. doi: 10.20515/otd.775603
92. Çifcia, N . Evaluation of Vitamin D levels in chronic Telogen effluvium patients. Turkiye Klinikleri Dermatoloji. (2018) 28:51–5. doi: 10.5336/dermato.2018-61736
93. Gürel, G, Karadöl, M, and Çölgeçen, E. The role of ferritin and Vitamin D levels in Telogen effluvium. Turkiye Klinikleri Dermatoloji. (2017) 27:113–6. doi: 10.5336/dermato.2017-56970
94. Cheung, EJ, Sink, JR, and English Iii, JC. Vitamin and mineral deficiencies in patients with Telogen effluvium: a retrospective cross-sectional study. J Drugs Dermatol. (2016) 15:1235–7.
95. Karadaǧ, AS, Ertuǧrul, DT, Tutal, E, and Akin, KO. The role of Anemia and Vitamin D levels in acute and chronic Telogen effluvium. Turkish J Med Sci. (2011) 41:827–33. doi: 10.3906/sag-1005-853
96. Arslan, H, and Gündüz, Ö. Micronutrient deficiencies and digital computerized Phototrichogram analysis in Telogen effluvium: a retrospective correlation study in a tertiary medical center. Dermatol Pract Concept. (2023) 13:e2023202. doi: 10.5826/dpc.1303a202
97. Brankov, N, Conic, RZ, Atanaskova-Mesinkovska, N, Piliang, M, and Bergfeld, WF. Comorbid conditions in lichen Planopilaris: a retrospective data analysis of 334 patients. Int J Women's Dermatol. (2018) 4:180–4. doi: 10.1016/j.ijwd.2018.04.001
98. Gharaei Nejad, K, Ghadarjani, R, Eftekhari, H, and Sheykholeslami, S. Most frequent comorbidities in patients with lichen Planopilaris: a cross-sectional study. Int J Dermatol Venereol. (2023) 6:229–32. doi: 10.1097/JD9.0000000000000306
99. Collins, MS, Ali, S, Wiss, IP, and Senna, MM. Increased risk of Vitamin D deficiency and insufficiency in black patients with central centrifugal Cicatricial alopecia. J Am Acad Dermatol. (2022) 87:689–91. doi: 10.1016/j.jaad.2022.02.018
100. Leung, B, Lindley, L, Reisch, J, Glass, DA, and Ayoade, K. Comorbidities in patients with central centrifugal Cicatricial alopecia: a retrospective chart review of 53 patients. J Am Acad Dermatol. (2023) 88:461–3. doi: 10.1016/j.jaad.2022.06.013
101. Zeng, Y, Yang, S, Liu, Y, Tang, Z, Zong, X, Li, X, et al. The role of Vd/Vdr signaling pathway in autoimmune skin diseases. Mini Rev Med Chem. (2023) 23:652–61. doi: 10.2174/1389557523666221124123206
102. Lee, S, Kim, BJ, Lee, CH, and Lee, WS. Increased prevalence of Vitamin D deficiency in patients with alopecia Areata: a systematic review and Meta-analysis. J Eur Acad Dermatol Venereol. (2018) 32:1214–21. doi: 10.1111/jdv.14987
103. Lee, S, Lee, H, Lee, CH, and Lee, WS. Comorbidities in alopecia Areata: a systematic review and Meta-analysis. J Am Acad Dermatol. (2019) 80:466–77.e16. doi: 10.1016/j.jaad.2018.07.013
104. Liu, Y, Li, J, Liang, G, Cheng, C, Li, Y, and Wu, X. Association of Alopecia Areata with Vitamin d and calcium levels: a systematic review and Meta-analysis. Dermatol Ther (Heidelb). (2020) 10:967–83. doi: 10.1007/s13555-020-00433-4
105. Chanprapaph, K, Mahasaksiri, T, Kositkuljorn, C, Leerunyakul, K, and Suchonwanit, P. Prevalence and risk factors associated with the occurrence of autoimmune diseases in patients with alopecia Areata. J Inflamm Res. (2021) 14:4881–91. doi: 10.2147/jir.S331579
106. Suchonwanit, P, Kositkuljorn, C, Mahasaksiri, T, and Leerunyakul, K. A comparison of the efficacy and tolerability of three corticosteroid treatment regimens in patients with alopecia Areata. J Dermatolog Treat. (2022) 33:756–61. Epub 2020/05/23. doi: 10.1080/09546634.2020.1773384
107. Chanprapaph, K, Pomsoong, C, Kositkuljorn, C, and Suchonwanit, P. Intramuscular Corticosteroid Therapy in the treatment of alopecia Areata: a time-to-event analysis. Drug Des Devel Ther. (2022) 16:107–16. doi: 10.2147/dddt.S342179
108. Suchonwanit, P, Triyangkulsri, K, Ploydaeng, M, and Leerunyakul, K. Assessing biophysical and physiological profiles of scalp seborrheic dermatitis in the Thai population. Biomed Res Int. (2019) 2019:5128376:1–6. doi: 10.1155/2019/5128376
109. Chanprapaph, K, Sutharaphan, T, and Suchonwanit, P. Scalp biophysical characteristics in males with androgenetic alopecia: a comparative study with healthy controls. Clin Interv Aging. (2021) 16:781–7. doi: 10.2147/cia.S310178
110. Amrein, K, Scherkl, M, Hoffmann, M, Neuwersch-Sommeregger, S, Köstenberger, M, Tmava Berisha, A, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. (2020) 74:1498–513. doi: 10.1038/s41430-020-0558-y
111. Holick, MF, and Vitamin, D. Deficiency. N Engl J Med. (2007) 357:266–81. doi: 10.1056/NEJMra070553
112. Mahasaksiri, T, Kositkuljorn, C, Anuntrangsee, T, and Suchonwanit, P. Application of topical immunotherapy in the treatment of alopecia Areata: a review and update. Drug Des Devel Ther. (2021) 15:1285–98. doi: 10.2147/dddt.S297858
113. Rattanakaemakorn, P, and Suchonwanit, P. Scalp pruritus: review of the pathogenesis, diagnosis, and management. Biomed Res Int. (2019) 2019:1268430–11. doi: 10.1155/2019/1268430
114. Guo, J, Lovegrove, JA, and Givens, DI. A narrative review of the role of foods as dietary sources of Vitamin D of ethnic minority populations with darker skin: the underestimated challenge. Nutrients. (2019) 11:81. doi: 10.3390/nu11010081
Keywords: vitamin D insufficiency, vitamin D level, hair loss, non-cicatricial alopecia, cicatricial alopecia
Citation: Yongpisarn T, Tejapira K, Thadanipon K and Suchonwanit P (2024) Vitamin D deficiency in non-scarring and scarring alopecias: a systematic review and meta-analysis. Front. Nutr. 11:1479337. doi: 10.3389/fnut.2024.1479337
Edited by:
Cristina Vassalle, Gabriele Monasterio Tuscany Foundation (CNR), ItalyReviewed by:
Amir Hossein Behnoush, Tehran University of Medical Sciences, IranSina Bazmi, Fasa University of Medical Sciences, Iran
Copyright © 2024 Yongpisarn, Tejapira, Thadanipon and Suchonwanit. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Poonkiat Suchonwanit, cG9vbmtpYXRAaG90bWFpbC5jb20=
†ORCID: Tanat Yongpisarn, http://orcid.org/0000-0002-1300-9624
Kasama Tejapira, http://orcid.org/0000-0001-6763-978X
Kunlawat Thadanipon, http://orcid.org/0000-0001-6324-2312
Poonkiat Suchonwanit, http://orcid.org/0000-0001-9723-0563
 Tanat Yongpisarn
Tanat Yongpisarn Kasama Tejapira
Kasama Tejapira Kunlawat Thadanipon
Kunlawat Thadanipon Poonkiat Suchonwanit
Poonkiat Suchonwanit