- 1Department of Spine Osteopathic, The First Affiliated Hospital of Guangxi Medical University, Nanning, China
- 2Wuming Hospital of Guangxi Medical University, Nanning, China
Purpose: To investigate the association between dietary vitamin D intake and low muscle mass (LMM) in a representative adult population, accounting for total energy intake and other potential confounders.
Materials and methods: This cross-sectional study utilized data from the National Health and Nutrition Examination Survey (NHANES) involving 8,443 participants. Dietary vitamin D intake was assessed using 24-h dietary recalls, and LMM was defined based on appendicular lean mass (ALM) adjusted for body mass index (BMI). Multivariable logistic regression models were used to examine the association between quartiles of dietary vitamin D intake and the odds of LMM, adjusting for age, gender, race/ethnicity, BMI, total energy intake, and additional covariates.
Results: In Model 1, after adjusting for age, gender, race/ethnicity, BMI, and poverty-to-income ratio, participants in the highest quartile of vitamin D intake had an odds ratio (OR) of 0.54 (95% CI: 0.37–0.79) compared to the lowest quartile, with a p for trend <0.001. In Model 2, after further adjustment for total energy intake and several covariates, the association was attenuated but remained borderline significant (p for trend = 0.051). In Model 3, after adjusting for additional health-related factors, the OR for the highest quartile was 0.70 (95% CI: 0.47–1.05), with a significant p for trend of 0.029.
Conclusion: This study suggests that higher dietary vitamin D intake may be associated with a reduced risk of LMM. Further longitudinal research is needed to confirm these findings and explore potential interactions between vitamin D and other dietary factors in muscle mass preservation.
1 Introduction
Low muscle mass (LMM) is increasingly recognized as a critical component of age-related musculoskeletal decline, contributing to frailty, reduced mobility, and higher risk of morbidity and mortality, particularly in older populations (1, 2). While LMM is traditionally linked with aging, emerging evidence suggests that nutritional factors, including vitamin D intake, may play a key role in its development and progression (3–5).
Vitamin D is well-known for its role in calcium homeostasis and bone health, but its importance for muscle function and mass is gaining attention (6, 7). Mechanistically, vitamin D is thought to influence muscle mass by promoting protein synthesis, enhancing mitochondrial function, and reducing inflammation (8). Observational studies have found associations between low serum vitamin D levels and increased risk of muscle weakness and sarcopenia, a syndrome that includes both muscle mass and function loss (9). However, the role of dietary vitamin D intake, as opposed to serum levels, in mitigating the risk of LMM remains less explored, particularly in population-based studies.
Despite the recognized role of vitamin D in muscle health, there is limited research on the relationship between dietary vitamin D intake and LMM. This study aims to explore the association between dietary vitamin D intake and LMM in a representative adult population. By using data from the National Health and Nutrition Examination Survey (NHANES), we seek to assess whether higher vitamin D intake is associated with lower odds of LMM after adjusting for relevant confounders, such as age, sex, BMI, and total energy intake. Understanding this association may help inform public health strategies aimed at preventing LMM through diet and nutrition interventions.
2 Materials and methods
2.1 Study population
This study was conducted using NHANES data from 2011 to 2018, which included Dual-energy X-ray Absorptiometry (DXA) measures of body composition. NHANES was designed to represent the non-institutionalized U.S. civilian population using a complex, multistage probability sampling method, including oversampling of certain subgroups to produce reliable statistics. All procedural manuals and survey content are indexed and publicly accessible online, ensuring transparency and accessibility for researchers.1 For this study, participants aged 20–60 years with complete and reliable DXA measurements were considered eligible for the analysis of LMM. We excluded participants with missing data on key variables, such as sample weight, vitamin D intake, DXA results, BMI, physical activity and other relevant covariates. After applying these criteria, a total of 8,443 participants were included in the final analysis. The participant selection process is illustrated in Figure 1.
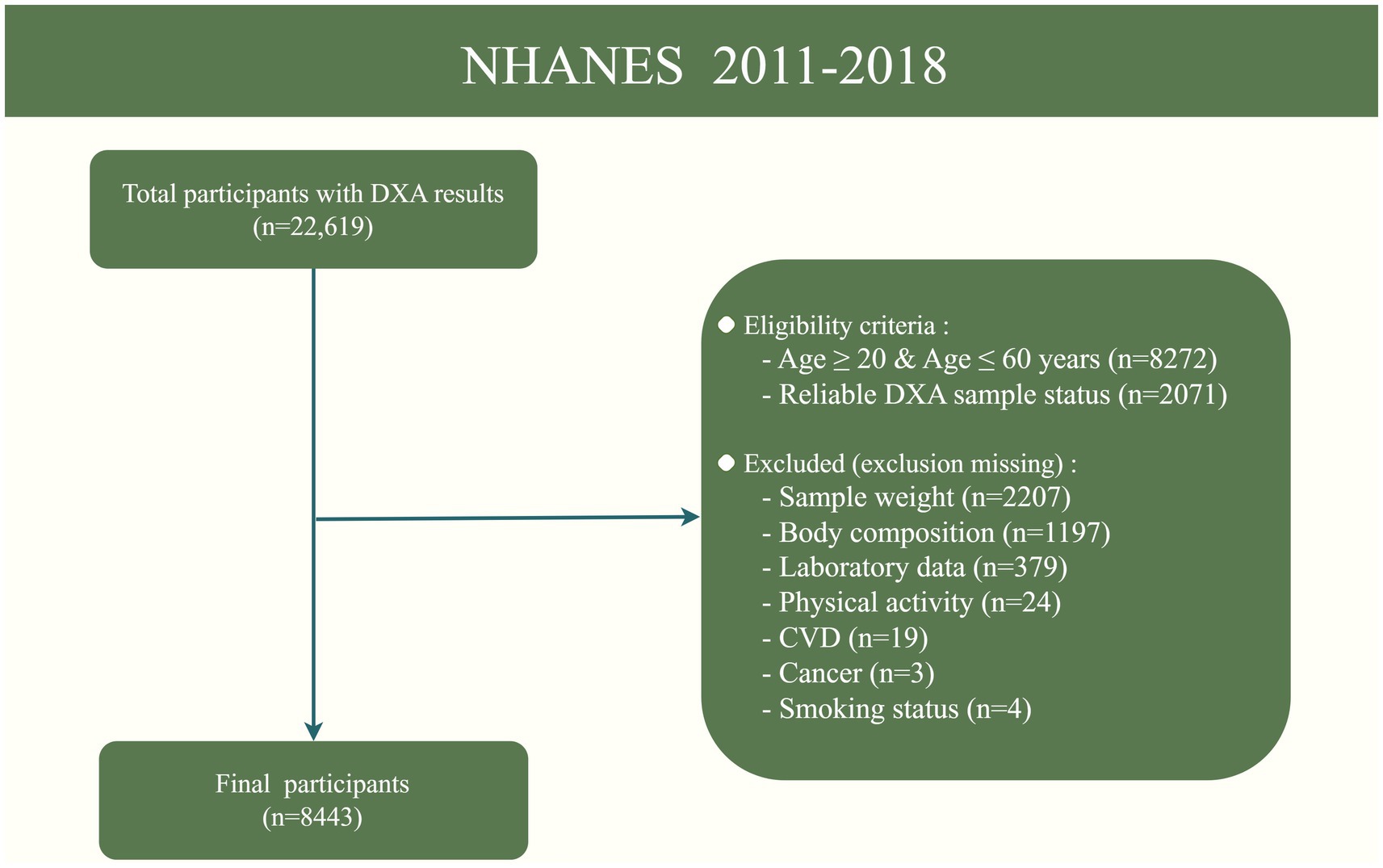
Figure 1. Flowchart of the sample selection from NHANES 2011–2018. DXA, Dual-energy X-ray Absorptiometry; CVD, Cardiovascular disease.
2.2 Vitamin D intake
Dietary intake interviews were conducted in person at the NHANES mobile examination clinics. Survey participants recalled the specific types and amounts of foods consumed the previous day. Dietary intake was evaluated using two 24-h dietary recalls for each participant. The first 24-h recall was collected in person, and the second was conducted by phone 3 to 10 days later. Vitamin D intake was calculated by averaging the values from the two 24-h recalls. If the second 24-h intake data was missing, the intake from the first 24-h recall was used instead. The dietary intake data were assessed using the automated multiple pass method (10), a structured interview technique consisting of five steps: quick list, forgotten foods, time and occasion, detail cycle, and final probe. After the dietary interview, the caloric and nutrient contents of each reported food and beverage item were coded using the U.S. Department of Agriculture (USDA) Food and Nutrient Database for Dietary Studies (FNDDS).2 Information on the consumption of specific food groups was extracted from the Food Patterns Equivalents Database (FPED), with each NHANES data cycle analyzed using its corresponding version of FPED.
2.3 Low muscle mass
LMM was defined using the criteria recommended by the Foundation for the National Institutes of Health (FNIH) Sarcopenia Project (11). Specifically, LMM was determined based on ALM adjusted for BMI. Participants were classified as having LMM if their ALM/BMI ratio was less than 0.789 for men and less than 0.512 for women. This definition has been widely used in epidemiological studies and allows for a standardized assessment of muscle mass across different population groups, accounting for variations in body size. The whole-body DXA scans were performed using a Hologic QDR-4500A fan-beam densitometer (Hologic, Inc., Bedford, MA, United States) to assess body composition. Participants were excluded from the DXA examination for several reasons, including pregnancy, self-reported weight over 450 lb. (204 kg), height over 6 ft., 5 in. (195 cm) due to DXA table limitations, and the use of radiographic contrast material (barium) in the past 7 days. Appendicular lean mass (ALM) was calculated as the sum of lean mass for the four limbs.
2.4 Covariates
The covariates included in our analysis were age, gender, race/ethnicity (Mexican American, Non-Hispanic White, Non-Hispanic Black, Other race), family poverty-to-income ratio (PIR) (<1, 1–3, >3), body mass index (BMI), total energy intake, diabetes, cardiovascular disease (CVD) status, cancer, smoking status, and drinking status. Physical activity was measured using Metabolic Equivalent (MET) values. Total MET-minutes were estimated by summing the MET-minutes for each activity based on its type and intensity. Participants reporting no regular physical activity in the past 30 days were classified as sedentary. After excluding these sedentary individuals, the remaining participants were divided into gender-specific tertiles according to their physical activity levels, resulting in four categories: sedentary, low, moderate, and high (12). Smoking status was categorized as nonsmoker (having smoked fewer than 100 cigarettes in a lifetime), former smoker (smoked more than 100 cigarettes but not currently smoking), and current smoker (smoked more than 100 cigarettes and currently smoking). Diabetes status was defined using self-reported diagnosis, HbA1c levels ≥6.5%, or fasting plasma glucose (FPG) levels ≥126 mg/dL. Drinking status was classified as never drinker, abstainer, or current drinker based on self-reported alcohol consumption patterns. The presence of CVD and cancer was determined through self-reported physician diagnoses obtained during structured interviews. Furthermore, laboratory measures included serum vitamin D, serum total cholesterol, serum albumin, and serum calcium. Serum vitamin D concentration (Serum 25-hydroxyvitamin D) was classified into four categories: severe deficiency (<25 nmol/L), deficiency (25–49.9 nmol/L), insufficiency (50–74.9 nmol/L), and sufficiency (≥75 nmol/L) (13).
2.5 Statistical analysis
Due to the complex sampling design used in NHANES, all analyses were conducted using sample weights. Baseline characteristics were presented unweighted to provide an accurate description of the study cohort. Continuous variables were shown as mean ± standard deviation (SD), while categorical variables were displayed as numbers and percentages (%). The Student’s t-test or Wilcoxon rank-sum test was used to compare continuous variables, and chi-square tests were employed to compare categorical variables. The association between vitamin D intake and sarcopenia was evaluated using multivariable logistic regression (MLR). Vitamin D intake was categorized into quartiles, with the lowest quartile (Q1) serving as the reference group. Model 1 was adjusted for age, gender, race, BMI, and PIR. Model 2 included additional adjustments for total energy intake, serum total cholesterol, serum vitamin D, serum albumin, and serum calcium. Finally, Model 3 was further adjusted for diabetes, CVD, cancer, smoking status, drinking status, and physical activity. To examine the independent effect of vitamin D intake, total energy intake was introduced as a covariate in Models 2 and 3. Before its inclusion, we conducted a Pearson correlation analysis between total energy intake and vitamin D intake, yielding an R value of 0.305 (p < 0.001, Supplementary Figure S1), indicating a moderate positive relationship. Additionally, the variance inflation factor (VIF) was calculated as 1.075, demonstrating minimal multicollinearity. Thus, total energy intake was appropriately included in these models to account for potential confounding effects. Results were reported as odds ratios (OR) with 95% confidence intervals (CI). To evaluate the trend between vitamin D intake and LMM, a p-value for trend was calculated by treating categorical exposure variables as ordinal in the MLR. All analyses were performed using R software (version 4.4.0), with statistical significance set at p < 0.05.
3 Results
3.1 Baseline characteristics
The baseline characteristics of the study population (n = 8,443) are summarized (Table 1), highlighting significant differences between participants with LMM (7.3%) and those without (93%). Participants with LMM were older (43.6 ± 12.0 vs. 39.1 ± 11.8 years, p < 0.001), had a higher BMI (35.4 ± 7.6 vs. 28.2 ± 6.2 kg/m2, p < 0.001), and a greater prevalence of diabetes (21.5% vs. 6.8%, p < 0.001) and CVD (9.3% vs. 2.7%, p < 0.001). The LMM group also showed a higher proportion of Mexican Americans (26.9% vs. 9.8%, p < 0.001). Additionally, participants with LMM had higher total energy intake, lower serum vitamin D, calcium, and albumin levels (all p < 0.001), and were more likely to be sedentary (27.5% vs. 14.9%, p < 0.001), while current alcohol consumption was lower in this group (59.4% vs. 74.3%, p < 0.001). These differences underscore the association between LMM and adverse health and lifestyle factors.
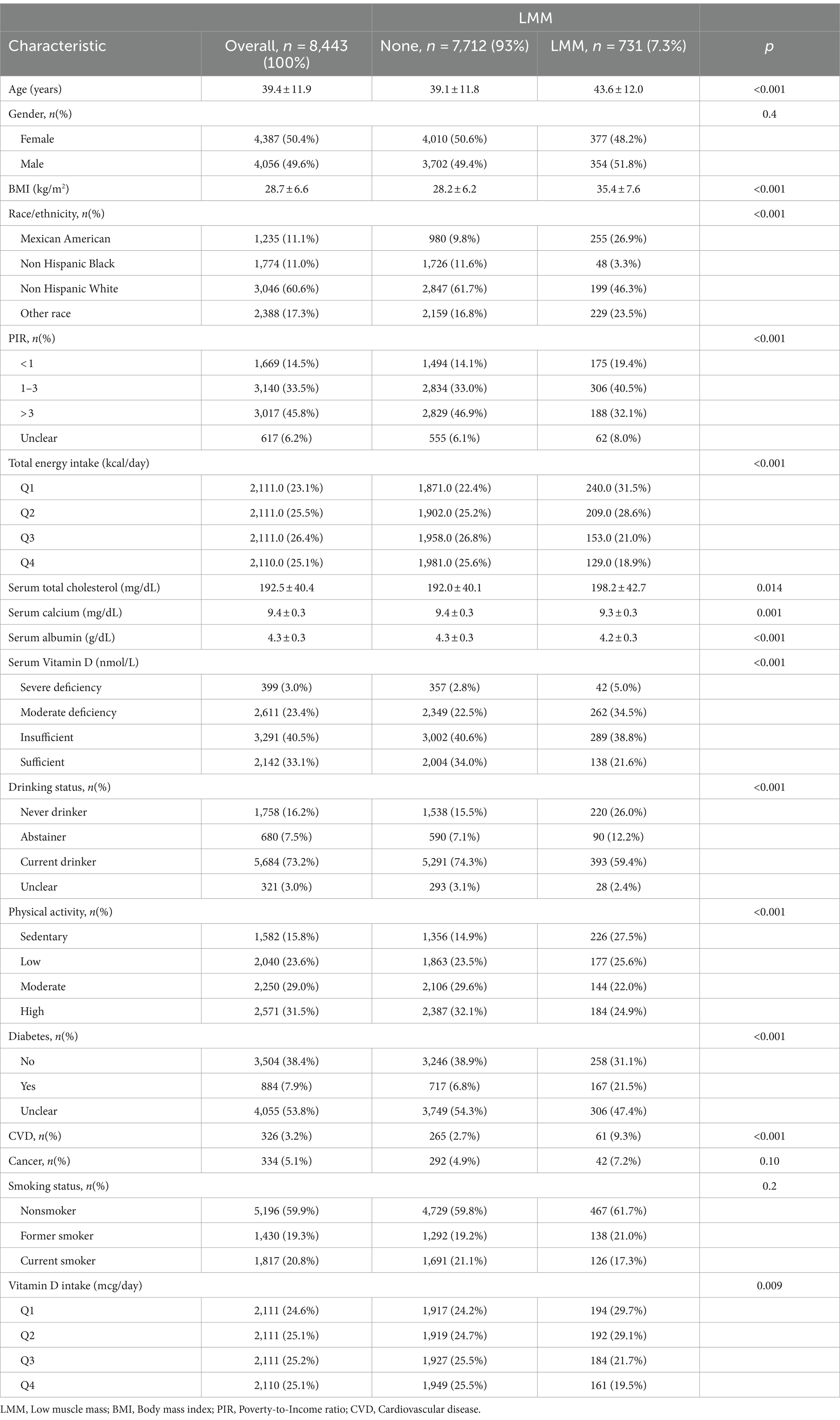
Table 1. Baseline characteristics of study participants stratified by the presence or absence of LMM.
3.2 Association between vitamin D intake and LMM
In the multivariable logistic regression models, we assessed the association between dietary vitamin D intake and the risk of LMM, adjusting for various covariates in three models (Table 2). In Model 1 (Supplementary Table S1), participants in the higher quartiles of vitamin D intake (Q3 and Q4) had significantly lower odds of LMM compared to the lowest quartile (Q1), with odds ratios (OR) of 0.57 (95% CI: 0.41–0.79) for Q3 and 0.54 (95% CI: 0.37–0.79) for Q4 (p for trend <0.001). In Model 2 (Supplementary Table S2), after adjusting for total energy intake and additional covariates, the association between higher dietary vitamin D intake and lower risk of LMM was attenuated and did not reach statistical significance (Q3: OR = 0.71, 95% CI: 0.50–1.01; Q4: OR = 0.74, 95% CI: 0.50–1.11). The trend across quartiles was borderline significant (p for trend = 0.051), suggesting a potential relationship that may warrant further investigation. In Model 3 (Supplementary Table S3; Figure 2), after further adjusting for additional health-related factors, the association between vitamin D intake and LMM reached statistical significance (p = 0.044). Participants in Q3 had an OR of 0.69 (95% CI: 0.49–0.97), and those in Q4 had an OR of 0.70 (95% CI: 0.47–1.05), with a significant trend across quartiles (p for trend = 0.029).
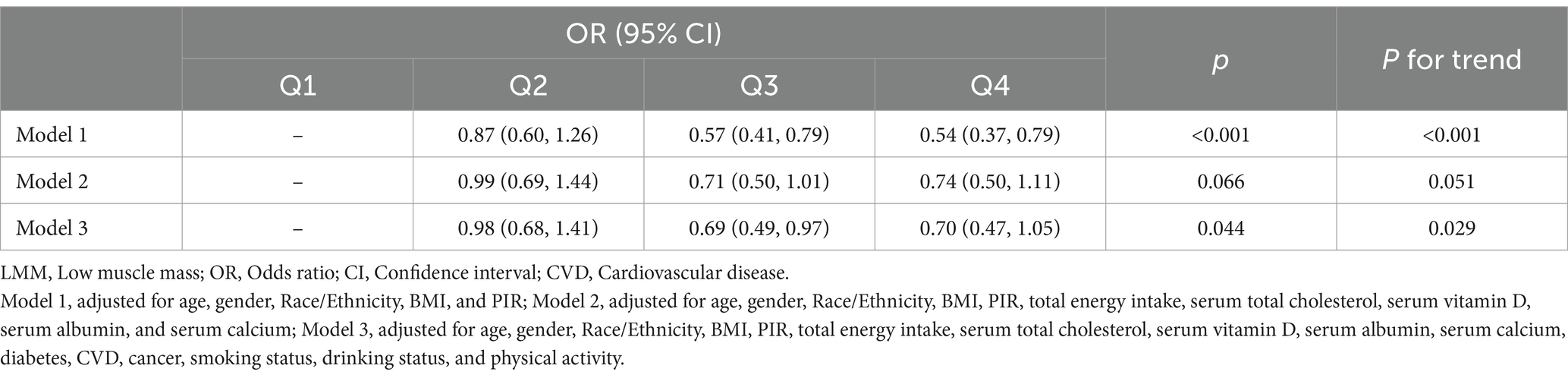
Table 2. Summary of multivariable logistic regression models assessing the association between dietary vitamin D intake and LMM risk.
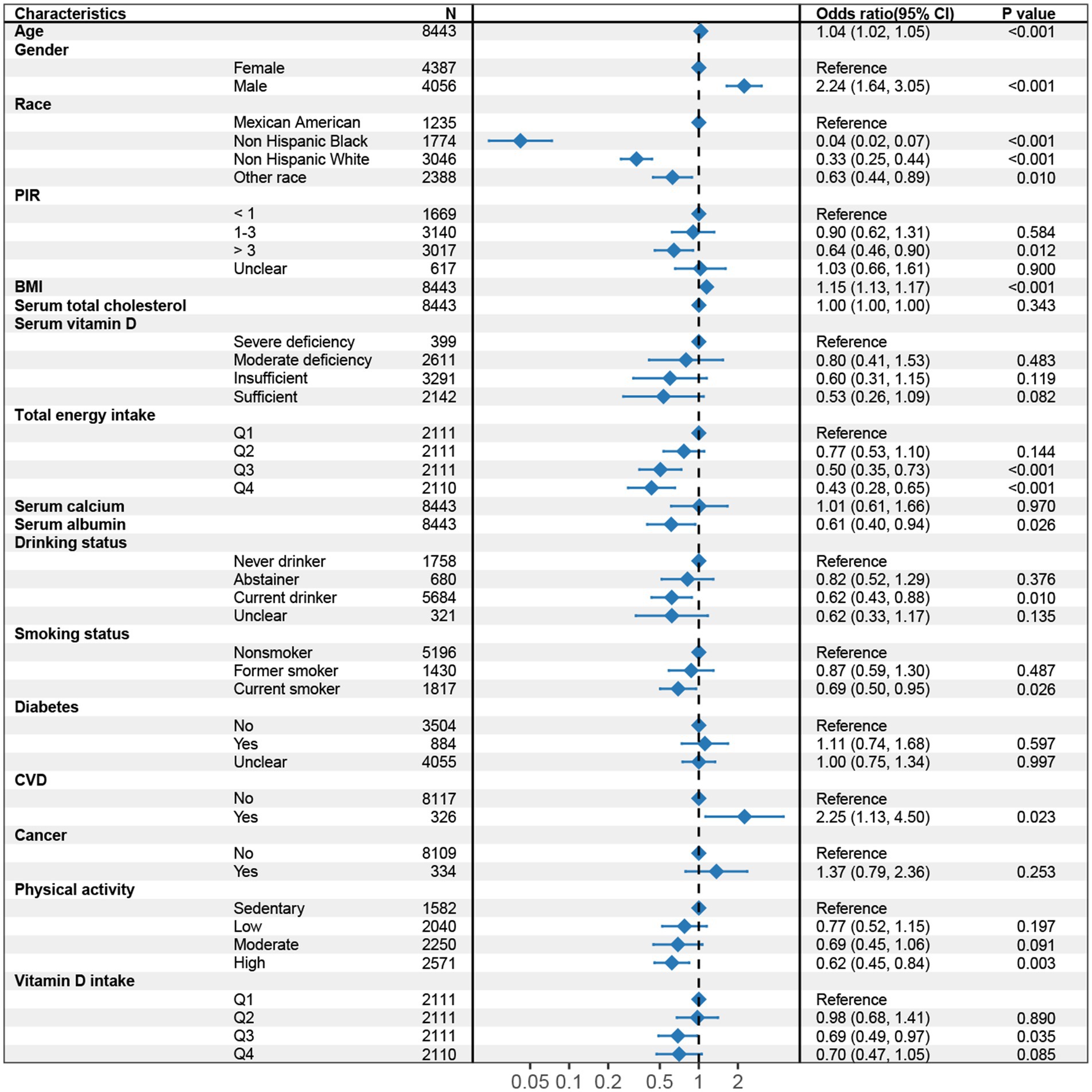
Figure 2. Forest plot of multivariable logistic regression analysis for the association between dietary vitamin D intake and sarcopenia (Model 3, adjusted for age, gender, Race/Ethnicity, BMI, PIR, total energy intake, serum total cholesterol, serum vitamin D, serum albumin, serum calcium, diabetes, CVD, cancer, smoking status, drinking status, and physical activity). CI, Confidence interval; BMI, Body mass index; PIR, Poverty-to-Income ratio; CVD, Cardiovascular disease.
4 Discussion
This study examined the association between dietary vitamin D intake and low muscle mass (LMM) in a large, representative sample, adjusting for a range of potential confounders. Our findings demonstrate that higher dietary vitamin D intake is associated with a reduced risk of LMM, even after accounting for total energy intake and other relevant covariates. These results align with existing evidence supporting the role of vitamin D in muscle health, but also highlight the complexities of interpreting nutrient-disease relationships, particularly when adjusting for factors such as energy intake.
Vitamin D is known to influence muscle function and structure through several mechanisms. It enhances calcium absorption and influences muscle protein synthesis, both of which are critical for maintaining muscle mass (14). Additionally, vitamin D has been shown to modulate inflammatory pathways, which could further support muscle preservation, particularly in aging populations where inflammation-related muscle degradation is a concern (15). Our findings suggest that dietary vitamin D intake could contribute to the maintenance of muscle mass, consistent with previous studies showing that vitamin D deficiency is associated with muscle weakness and sarcopenia (16–18). While our results indicate an association between higher dietary vitamin D intake and a reduced risk of LMM, these findings should be interpreted with caution. In Model 2, where total energy intake and additional covariates were included, the association was attenuated and did not reach statistical significance (p = 0.066). However, the overall trend across quartiles remained borderline significant (p for trend = 0.051), suggesting a possible dose–response relationship. In Model 3, after further adjustment for health-related factors, the association regained statistical significance (p = 0.044), but the effect size was modest. The overall trend across quartiles remained significant (p for trend = 0.029), indicating that higher vitamin D intake may be associated with a lower risk of LMM, though the strength of this association was not robust. The reduction in the strength of the association after adjusting for total energy intake highlights the complexity of disentangling the independent effects of specific nutrients like vitamin D from broader dietary patterns. Total energy intake influences overall dietary patterns, which could confound the relationship between specific nutrients like vitamin D and muscle mass (19). In particular, individuals with higher energy intake may have more diverse diets that include other nutrients beneficial for muscle mass, such as protein and other micronutrients (20). Therefore, while vitamin D may play a role in muscle health, its effect may be part of a more complex, multifactorial dietary environment. Further research, particularly in the form of longitudinal studies or randomized controlled trials, is needed to clarify the role of dietary vitamin D intake in the prevention of LMM and to explore whether certain subgroups may benefit more from increased vitamin D intake.
5 Conclusion
In this study, we observed a significant association between higher dietary vitamin D intake and a reduced risk of LMM. These findings add to the growing body of evidence supporting the role of vitamin D in muscle health. However, this association was attenuated after adjusting for total energy intake and other relevant covariates, suggesting that the relationship between vitamin D intake and muscle mass may be influenced by broader dietary patterns or other lifestyle factors. Given the widespread prevalence of vitamin D deficiency and its potential impact on muscle health, increasing dietary vitamin D intake through food fortification or supplementation may represent a valuable public health strategy for preventing muscle mass decline.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YeT: Conceptualization, Data curation, Investigation, Software, Visualization, Writing – original draft, Writing – review & editing. YiT: Conceptualization, Data curation, Formal analysis, Validation, Writing – review & editing. XP: Methodology, Resources, Writing – review & editing. BW: Data curation, Formal analysis, Resources, Software, Writing – review & editing. SZ: Conceptualization, Funding acquisition, Methodology, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The study was funded by the National Natural Science Foundation of China (grant no. 82060399).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1471641/full#supplementary-material
Footnotes
References
1. Prado, CM, Purcell, SA, and Laviano, A. Nutrition interventions to treat low muscle mass in cancer. J Cachexia Sarcopenia Muscle. (2020) 11:366–80. doi: 10.1002/jcsm.12525
2. Murdock, DJ, Wu, N, Grimsby, JS, Calle, RA, Donahue, S, Glass, DJ, et al. The prevalence of low muscle mass associated with obesity in the USA. Skelet Muscle. (2022) 12:26. doi: 10.1186/s13395-022-00309-5
3. Larsson, L, Degens, H, Li, M, Salviati, L, Lee, YI, Thompson, W, et al. Sarcopenia: aging-related loss of muscle mass and function. Physiol Rev. (2019) 99:427–511. doi: 10.1152/physrev.00061.2017
4. Kim, Y, Chang, Y, Ryu, S, Cho, IY, Kwon, M-J, Wild, SH, et al. Serum 25-hydroxy vitamin D and the risk of low muscle mass in young and middle-aged Korean adults. Eur J Endocrinol. (2022) 186:477–87. doi: 10.1530/EJE-21-1229
5. Aspell, N, Laird, E, Healy, M, Lawlor, B, and O’Sullivan, M. Vitamin D deficiency is associated with impaired muscle strength and physical performance in community-dwelling older adults: findings from the English longitudinal study of ageing. Clin Interv Aging. (2019) 14:1751–61. doi: 10.2147/CIA.S222143
6. Yao, P, Bennett, D, Mafham, M, Lin, X, Chen, Z, Armitage, J, et al. Vitamin D and calcium for the prevention of fracture: a systematic review and meta-analysis. JAMA Netw Open. (2019) 2:e1917789. doi: 10.1001/jamanetworkopen.2019.17789
7. Carlberg, C, Raczyk, M, and Zawrotna, N. Vitamin D: a master example of nutrigenomics. Redox Biol. (2023) 62:102695. doi: 10.1016/j.redox.2023.102695
8. Rebelos, E, Tentolouris, N, and Jude, E. The role of vitamin D in health and disease: a narrative review on the mechanisms linking vitamin D with disease and the effects of supplementation. Drugs. (2023) 83:665–85. doi: 10.1007/s40265-023-01875-8
9. Jia, S, Zhao, W, Hu, F, Zhao, Y, Ge, M, Xia, X, et al. Sex differences in the association of physical activity levels and vitamin D with obesity, sarcopenia, and sarcopenic obesity: a cross-sectional study. BMC Geriatr. (2022) 22:898. doi: 10.1186/s12877-022-03577-4
10. Moshfegh, AJ, Rhodes, DG, Baer, DJ, Murayi, T, Clemens, JC, Rumpler, WV, et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr. (2008) 88:324–32. doi: 10.1093/ajcn/88.2.324
11. Cruz-Jentoft, AJ, Bahat, G, Bauer, J, Boirie, Y, Bruyère, O, Cederholm, T, et al. et al, Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
12. Tucker, LA. Physical activity and telomere length in U.S. men and women: an NHANES investigation. Prev Med. (2017) 100:145–51. doi: 10.1016/j.ypmed.2017.04.027
13. Ross, AC, Manson, JE, Abrams, SA, Aloia, JF, Brannon, PM, Clinton, SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. (2011) 96:53–8. doi: 10.1210/jc.2010-2704
14. Montenegro, KR, Cruzat, V, Carlessi, R, and Newsholme, P. Mechanisms of vitamin D action in skeletal muscle. Nutr Res Rev. (2019) 32:192–204. doi: 10.1017/S0954422419000064
15. Granic, A, Hill, T, Davies, K, Jagger, C, Adamson, A, Siervo, M, et al. Vitamin D status, muscle strength and physical performance decline in very old adults: a prospective study. Nutrients. (2017) 9:379. doi: 10.3390/nu9040379
16. Latham, CM, Brightwell, CR, Keeble, AR, Munson, BD, Thomas, NT, Zagzoog, AM, et al. Vitamin D promotes skeletal muscle regeneration and mitochondrial health. Front Physiol. (2021) 12:660498. doi: 10.3389/fphys.2021.660498
17. Nasimi, N, Sohrabi, Z, Nunes, EA, Sadeghi, E, Jamshidi, S, Gholami, Z, et al. Whey protein supplementation with or without vitamin D on sarcopenia-related measures: a systematic review and meta-analysis. Adv Nutr. (2023) 14:762–73. doi: 10.1016/j.advnut.2023.05.011
18. Knechtle, B, and Nikolaidis, PT. Vitamin D and sport performance. Nutrients. (2020) 12:841. doi: 10.3390/nu12030841
19. Weikert, C, Trefflich, I, Menzel, J, Obeid, R, Longree, A, Dierkes, J, et al. Vitamin and mineral status in a vegan diet. Dtsch Ärztebl Int. (2020) 117:575–82. doi: 10.3238/arztebl.2020.0575
Keywords: NHANES, low muscle mass, vitamin D, dietary vitamin D intake, nutrition
Citation: Tong Y, Teng Y, Peng X, Wan B and Zong S (2024) Association between dietary vitamin D intake and low muscle mass in US adults: results from NHANES 2011–2018. Front. Nutr. 11:1471641. doi: 10.3389/fnut.2024.1471641
Edited by:
Michele Barone, University of Bari Aldo Moro, ItalyReviewed by:
Rengfei Shi, Shanghai University of Sport, ChinaSara Verde, State University of Ceará, Brazil
Copyright © 2024 Tong, Teng, Peng, Wan and Zong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaohui Zong, c2hhb2h1aV9neG11QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Ye Tong
Ye Tong Yilin Teng1†
Yilin Teng1†