- 1Health Science Center, Ningbo University, Ningbo, Zhejiang, China
- 2Department of Infectious Diseases, Shangyu People's Hospital of Shaoxing, Shaoxing, Zhejiang, China
- 3Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Ningbo University, Ningbo, Zhejiang, China
- 4Department of Infectious Diseases, The First Affiliated Hospital of Ningbo University, Ningbo, Zhejiang, China
- 5Department of Hepatology, The First Affiliated Hospital of Ningbo University, Ningbo, Zhejiang, China
Background: Previous studies have demonstrated a significant association between serum vitamin A concentration and non-alcoholic fatty liver disease (NAFLD) development. However, the long-term prognostic implications of serum vitamin A in patients with NAFLD remain underexplored. This study aims to investigate whether there exists a correlation between serum vitamin A concentrations and overall mortality among subjects diagnosed with NAFLD.
Methods: To investigate the association between serum vitamin A concentrations and NAFLD outcomes, we conducted prospective cohort studies using data from the 1999–2006 and 2017–2018 National Health and Nutrition Examination Survey (NHANES). We utilized a multivariate Cox regression model to explore the relationship between serum vitamin A levels and all-cause mortality. Survival curves related to serum vitamin A were constructed using the Kaplan–Meier method. Additionally, the restricted cubic splines (RCS) method was applied to examine potential nonlinear relationships between serum vitamin A concentrations and all-cause mortality of NAFLD.
Results: Over a median follow-up period of 10.3 years, a total of 1,399 all-cause deaths were recorded. The weighted average concentration of serum vitamin A was 61.48 ± 0.37 μg/dL. After adjusting for potential confounders, a significant U-shaped relationship was identified between serum vitamin A concentrations and the risk of all-cause mortality in NAFLD patients. This relationship was particularly pronounced in men and elderly individuals aged 60 to 85.
Conclusion: Our study reveals a significant non-linear relationship between serum vitamin A concentrations and the risk of all-cause mortality in patients with NAFLD. These findings underscore the importance of monitoring and maintaining optimal serum vitamin A levels to potentially improve survival outcomes in NAFLD patients.
1 Introduction
Non-alcoholic fatty liver disease (NAFLD) affects approximately 30% of the global population and represents a significant global public health concern due to its increasing prevalence (1, 2). It is defined by fat accumulation in hepatocytes without secondary hepatic steatosis causes, such as excessive alcohol consumption, viral hepatitis, or genetic disorders (3). NAFLD encompasses a spectrum of hepatic damage, ranging from simple steatosis to more severe conditions such as non-alcoholic steatohepatitis (NASH), with or without fibrosis, cirrhosis, and hepatocellular carcinoma (4). Despite its global prevalence, the precise mechanisms underlying the onset and progression of NAFLD remain poorly understood. The multiple parallel hit hypothesis states that NAFLD develops through complex interactions involving insulin resistance, adipokine secretion, oxidative stress, lipid peroxidation, mitochondrial damage, endoplasmic reticulum stress, intestinal microbiota, innate immunity, genetics, and epigenetic mechanisms (4). Oxidative stress and inflammation are believed to play critical roles in the transition from steatosis to NASH (5–7).
Patatin-like Phospholipase Domain Containing 3 (PNPLA3) is a multifunctional enzyme that acts as a triglyceride hydrolase, retinyl esterase, and acetyl-CoA-independent transacylase and promotes the release of retinol from lipid droplets (8–10). Pirazzi et al. reported that PNPLA3 can specifically hydrolyze retinyl palmitate in human hepatic stellate cells (HSCs), with this enzymatic activity significantly reduced in the PNPLA3-I148M variant (11). Other studies indicate that the PNPLA3-I148M variant may lead to lower serum retinol levels in patients with NAFLD, accompanied by hepatic accumulation of retinyl esters and triglycerides (4). Recent genetic studies have demonstrated that the PNPLA3-I148M variant is an independent risk factor for the development and severity of liver fibrosis, regulating the activity of HSCs and leading to a pro-inflammatory and pro-fibrotic phenotype (12). Vitamin A, a vital fat-soluble vitamin essential for human physiology, plays a crucial role in several physiological processes such as vision, cell proliferation, and differentiation, immune regulation, embryogenesis, glucose, and lipid metabolism. Approximately 60–95% of the body’s vitamin A is stored in the form of retinyl esters in HSCs (9, 10). Previous studies have suggested that vitamin A and its metabolites may have therapeutic potential for liver diseases (9, 13). Therefore, we hypothesized that there might be a connection between serum vitamin A levels and NAFLD. Lotfi et al. found that higher vitamin A intake was associated with a lower risk of developing NAFLD (14). Mazidi et al. observed that a higher quartile of serum retinol was associated with a reduced risk of NAFLD (15). Furthermore, several studies have indicated a positive correlation between serum vitamin A levels and the severity of NAFLD (16, 17). However, the relationship between serum vitamin A levels and the long-term prognosis of patients with NAFLD remains insufficiently explored. Based on these findings, we investigated the relationship between serum vitamin A concentrations and all-cause mortality in a nationally representative sample of American NAFLD patients.
2 Materials and methods
2.1 Study design and subjects
The data utilized in this study were obtained publicly from the National Health and Nutrition Examination Survey (NHANES) database. NHANES is a nationwide survey and examination program conducted by the National Center for Health Statistics (NCHS) under the Centers for Disease Control and Prevention (CDC) in the United States since 1999. All data were collected through household interviews, mobile examinations, and laboratory tests. All participants provided written informed consent. NHANES interviews gather data on demographic characteristics, dietary intake, physical examinations, and laboratory tests to assess disease prevalence, risk factors, and nutritional status among the non-institutionalized civilian population of the United States. For more information on NHANES, please refer to the relevant website.1
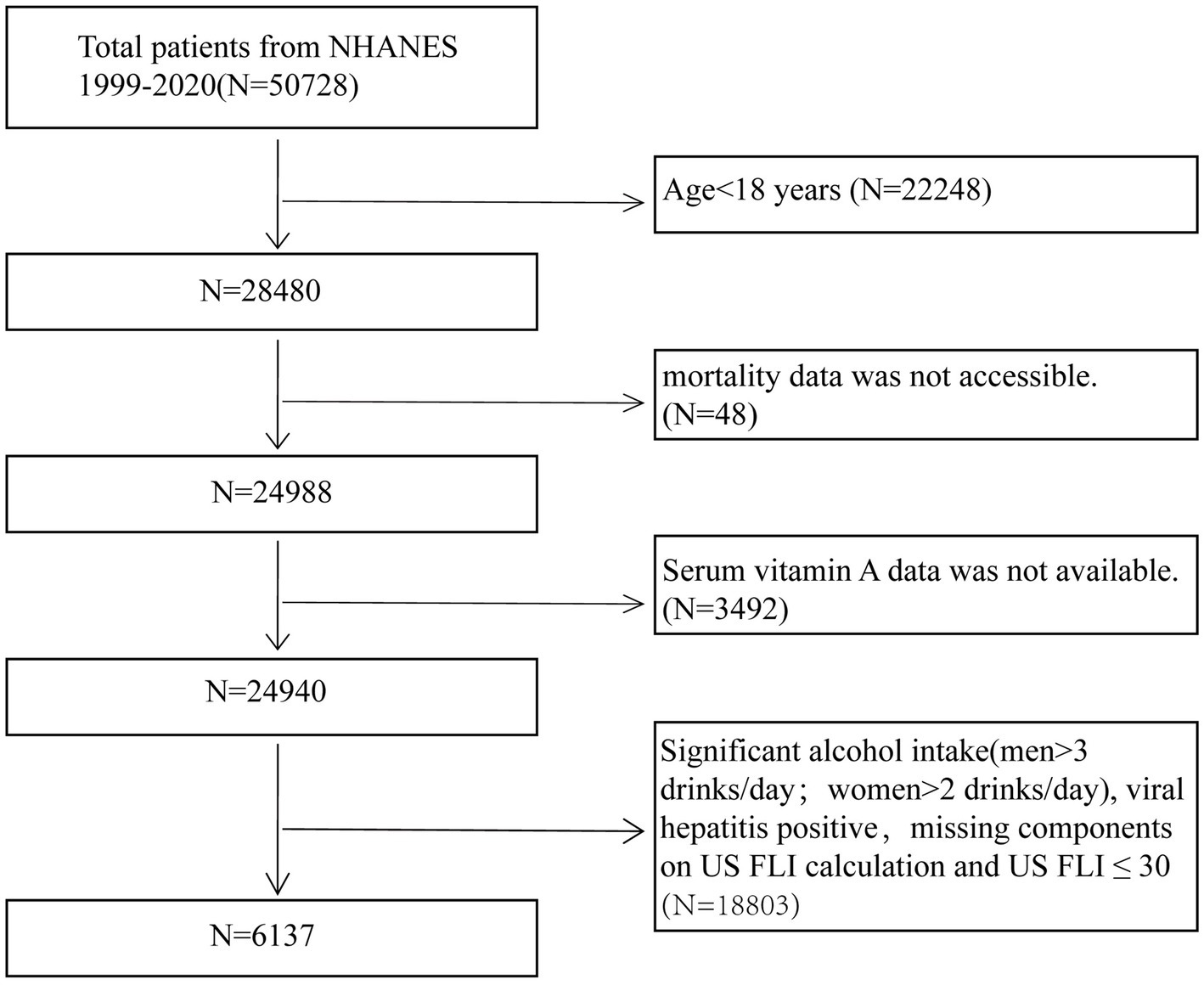
Data for this study were obtained from the NHANES conducted during 1999–2006 and 2017–2018. Due to the absence of abdominal ultrasound data in the NHANES database, the United States Fatty Liver Index (US FLI) was employed to diagnose NAFLD (18). To ensure the reliability of the study, participants were excluded based on the following criteria: (1) individuals under 18 years of age (N = 22,248); (2) those with missing serum vitamin A data (N = 3,492); (3) individuals with missing mortality rate data (N = 48), and (4) individuals meeting criteria such as excessive alcohol consumption (men >3 drinks/day, women >2 drinks/day), positive hepatitis B or C status, missing US FLI components, or US FLI ≤30 (N = 18,803) (18, 19). After applying these exclusion criteria, the final study population comprised 6,137 NAFLD participants. Figure 1 outlines the detailed flowchart illustrating the participant selection process.
2.2 Serum vitamin A
Serum samples in this study were collected, processed, and stored according to standardized protocols. Comprehensive details of all assay procedures can be accessed on the official NHANES website. Serum vitamin A concentrations were quantified using high-performance liquid chromatography and photodiode array detection. To explore the association between various serum vitamin A concentrations and all-cause mortality rates among NAFLD patients, the concentrations were divided into four groups by the quartile values: Q1 [0.7, 46.1], Q2 (46.1, 56.8], Q3 (56.8, 69.2], and Q4 (69.2, 185] μg/dL.
2.3 Non-alcoholic fatty liver disease
Liver biopsy is recognized as the gold standard for diagnosing NAFLD; however, its use is restricted due to its invasiveness and high cost. Consequently, the improved US FLI and the Fibrosis4 (FIB4) scores were employed to assess NHANES in this study. The US FLI has demonstrated predictive capabilities for hepatic steatosis. US FLI >30 is used to define NAFLD (18). The Fibrosis-4 score (FIB4 score) is employed to evaluate the risk of advanced fibrosis, with a threshold set at 2.67 (20).
The formulas are as follows:
US FLI = (e−0.8073 × non-Hispanic black + 0.3458 × Mexican American + 0.0093 × age + 0.6151 × ln (GGT) + 0.0249 × waist circumference + 1.1792 × ln (insulin) + 0.8242 × ln (glucose) − 14.7812)/(1 + e−0.8073 × non-Hispanic black + 0.3458 × Mexican American + 0.0093 × age + 0.6151 × ln (GGT) + 0.0249 × waist circumference + 1.1792 × ln (insulin) + 0.8242 × ln (glucose) − 14.7812) × 100 (18, 21).
FIB4 score = Age (year) × AST (IU/L)/(platelet count (109/L) × square-root of ALT (IU/L)) (22).
2.4 The mortality data
The mortality data utilized in this study were linked to the National Death Index (NDI), a comprehensive database maintained by the NCHS that covers all deaths in the United States. Each participant’s follow-up time was from the survey date until their date of death or until December 31, 2019. Detailed mortality data in this study can be accessed through the NHANES Public-Use Linked Mortality Files, available at the following web address: https://www.cdc.gov/nchs/data-linkage/mortality-public.htm.
2.5 Covariates
Covariates associated with NAFLD include age, sex, race, glycated hemoglobin A1c (HbA1c), C-reactive protein (CRP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), GGT, uric acid, high-density lipoprotein (HDL), low-density lipoprotein (LDL), alkaline phosphatase (ALP), serum cholesterol, serum triglycerides, and energy intake. Race was classified into four groups: non-Hispanic White, non-Hispanic Black, Mexican American, and other races. Body mass index (BMI) was categorized into 3 groups: <25 kg/m2 (normal), 25–30 kg/m2 (overweight), and ≥ 30 kg/m2 (obesity) (23, 24). Smoking behavior was classified as current, former, and never smokers. Drinking behavior was categorized into five groups: (1) never drinkers, (2) mild drinkers (<2 drinks/day for females, <3drinks/day for males), (3) moderate drinkers (≥2 drinks/day for females, ≥3drinks/day for males, or binge drinking ≥2 days/month), (4) heavy drinkers (≥3 drinks/day for females, ≥4 drinks/day for males, or ≥ 4 drinks on a single occasion for females, ≥5 drinks for males), and (5) those with unavailable drinking data (25). Participants’ physical activity levels were categorized into four groups according to the 2018 Physical Activity Guidelines for Americans: low (<500 metabolic equivalent (MET) – minutes per week), moderate (≥500 to <1,000 MET-minutes per week), high (≥1,000 to <1,500 MET-minutes per week), and very high (≥1,500 MET-minutes per week) (26). Diabetes was diagnosed using predefined criteria, including self-report, current use of anti-diabetic medications, HbA1c levels≥6.5%, or fasting blood glucose (FPG) ≥ 126 mg/dL (7 mmol/L) (27). Hypertension was defined as the existence of one of the following conditions: (1) self-reported hypertension, (2) current use of antihypertensive medications, or (3) systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg (28). Hyperlipidemia was diagnosed if participants met any of the following conditions: (1) triglycerides (TG) ≥150 mg/dL, (2) total cholesterol (TC) ≥ 200 mg/dL, (3) low-density lipoprotein cholesterol (LDL-C) ≥ 130 mg/dL, (4) high-density lipoprotein cholesterol (HDL-C) < 40 mg/dL for males and < 50 mg/dL for females, or (5) receipt of lipid-lowering medication (29).
2.6 Statistical analysis
This study’s analyses adhered to the NHANES guidelines and utilized a non-random, stratified sampling design. Continuous variables were presented as weighted means ± standard error (SE) and were examined using weighted linear regression models. Categorical variables were reported as percentages ± SE and were analyzed using weighted Rao-Scott chi-square tests. The multivariate Cox regression analysis was conducted to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for all-cause mortality in NAFLD patients based on serum vitamin A levels. Three models were developed, each adjusting for different potential confounders: Model 1 without adjustments, Model 2 adjusting for sex, age, and race, and Model 3 further adjusting for BMI, smoking behavior and drinking behavior, physical activity level, energy intake, CRP, diabetes status, and hypertension status. Stratified analyses and interaction tests were performed based on various factors including age groups (18–39, 40–59, 60–85 years), sex (male/female), BMI (<25 kg/m2 or ≥ 25 kg/m2, <30 kg/m2 or ≥ 30 kg/m2), diabetes status, hypertension status, and advanced fibrosis status. The association between serum vitamin A levels and survival was illustrated using Kaplan–Meier curves, with comparisons conducted using the log-rank test. Restricted cubic spline (RCS) curves with four nodes (5th, 35th, 65th, and 95th percentiles) were utilized to display the potential non-linear relationships between serum vitamin A levels and all-cause mortality in NAFLD patients. p-values ≤ 0.05 were considered statistically significant. Statistical analyses were conducted using the R software (version 4.2.0).
3 Results
3.1 Basic characteristics of study participants
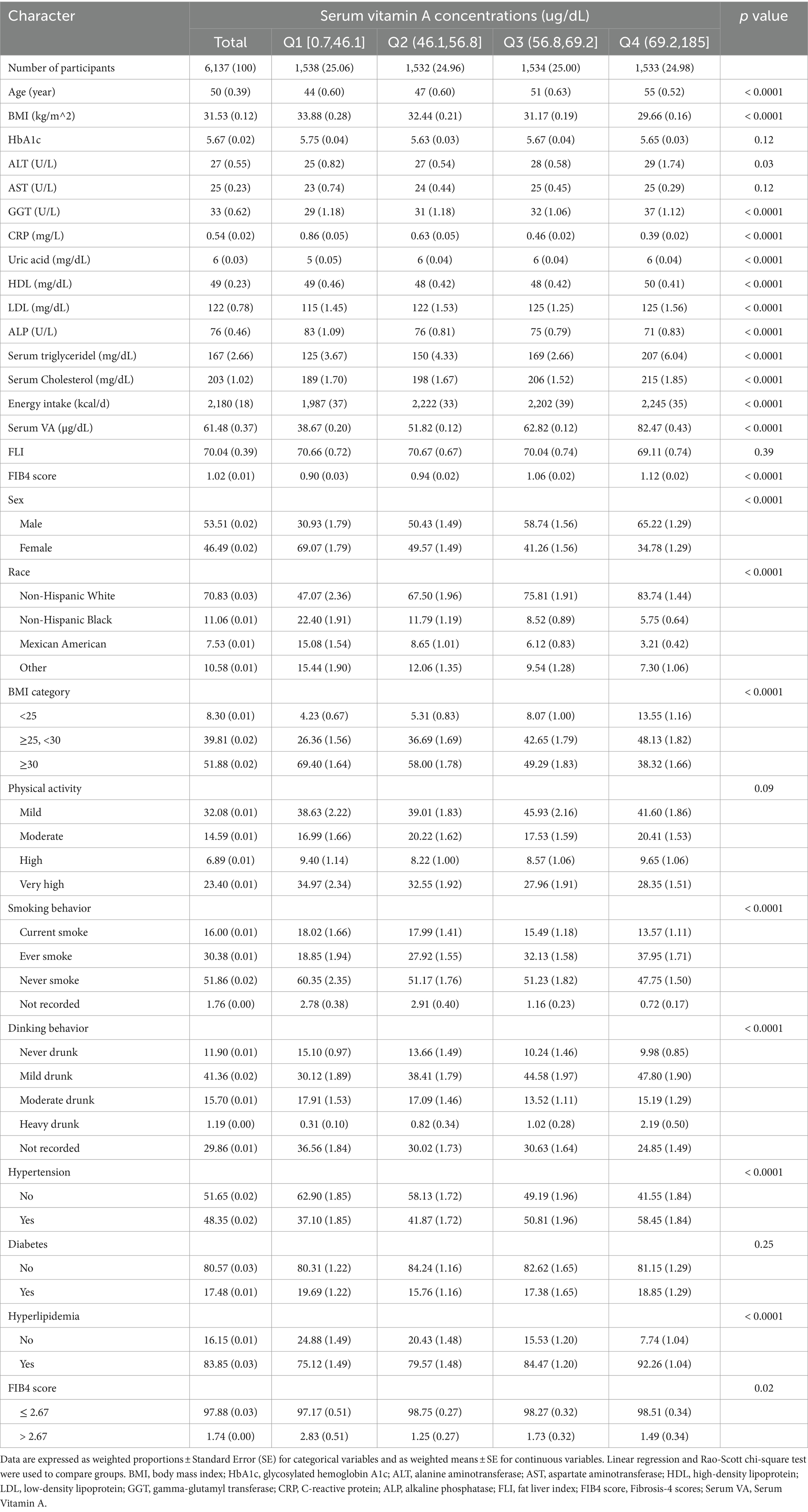
Table 1 presents the baseline characteristics of the entire study population. The mean age of participants was 50 ± 0.39 years old. The average follow-up period was 10.3 years, culminating in 1399 cases of all-cause mortality. The weighted mean concentration of serum vitamin A was 61.48 ± 0.37 μg/dL. Participants with NAFLD were predominantly obese men, of non-Hispanic white race, with a history of non-smoking or former smoking and mild drinking behavior. Baseline data distribution varied significantly among groups. Compared to those with low serum vitamin A levels, participants with higher levels were more likely to be male, overweight (25–30 kg/m2), non-Hispanic White, and have a history of smoking or mild alcohol consumption. Higher serum vitamin A levels were also associated with a greater incidence of hypertension and hyperlipidemia. Additionally, this group showed other metabolic disturbances, including elevated serum ALT, GGT, uric acid, LDL, cholesterol, and triglycerides, while observing an opposing trend for ALP and CRP levels.
Subsequently, we examined whether there were differences in the severity of liver fibrosis among the four groups, determined by a FIB-4 score. We categorized liver fibrosis into two classifications: non-advanced (≤ 2.67) and advanced (> 2.67). The majority of patients with NAFLD had non-advanced liver fibrosis. The highest proportion of advanced liver fibrosis occurred in patients with low serum vitamin A levels (p < 0.05).
3.2 Association between serum vitamin A level and all-cause mortality
The study utilized three Cox regression models to explore the independent effect of serum vitamin A levels on all-cause mortality in patients with NAFLD. As illustrated in Table 2, Model 1 revealed a significant association between serum vitamin A levels and an increased risk of all-cause mortality. Specifically, NAFLD patients in the highest serum vitamin A quartile (Q4) exhibited a greater risk of all-cause mortality compared to those in the lowest quartile (Q1). However, after adjusting for relevant variables (Models 2 and 3), serum vitamin A levels were significantly linked to a decreased risk of all-cause mortality among NAFLD patients. Notably, in Model 3, the group with moderate serum vitamin A levels (Q2) had the lowest mortality risk compared to Q1 (HR = 0.633, 95% CI = 0.456–0.880). The groups with higher serum vitamin A levels (Q3 and Q4) also had lower mortality risks than Q1, with Q3 showing HR = 0.727, 95% CI = 0.541–0.976, and Q4 showing HR = 0.663, 95% CI = 0.499–0.880. However, the trend test was insignificant (p for trend = 0.077), suggesting a potential nonlinear relationship between serum vitamin A levels and all-cause mortality.
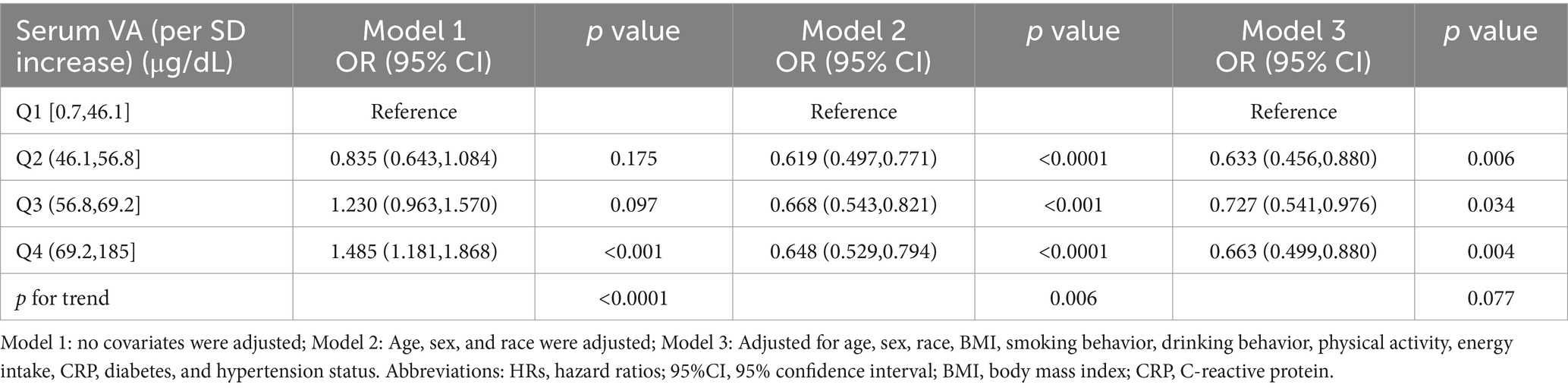
Table 2. HRs (95% CIs) for all-cause mortality according to serum vitamin A concentrations among participants.
3.3 Subgroup analysis
To further elucidate the complex relationship between serum vitamin A levels and all-cause mortality in NAFLD patients, stratified analyses and interaction tests were performed based on sex, age, BMI, diabetes status, hypertension status, and advanced fibrosis status. Details are presented in Table 3. This study demonstrated consistent results when stratified by BMI, diabetes, hypertension, and advanced fibrosis (p for interaction >0.05). However, significant interactions were observed when stratified by sex and age (p for interaction <0.05), indicating a more pronounced correlation between serum vitamin A levels and all-cause mortality in male and elderly NAFLD patients. Consequently, a thorough examination of the relationship between serum vitamin A and all-cause mortality was conducted across different sex and age categories. Supplementary Tables S1, S2 demonstrate that these associations remain generally consistent among the elderly (60–85 years) and male populations. Additionally, we conducted a Kaplan–Meier analysis on elderly (aged 60–85) and male NAFLD patients, revealing that those in the Q2 group had the lowest risk of all-cause mortality (Log-rank p < 0.05), consistent with the Cox regression results (Figure 2).
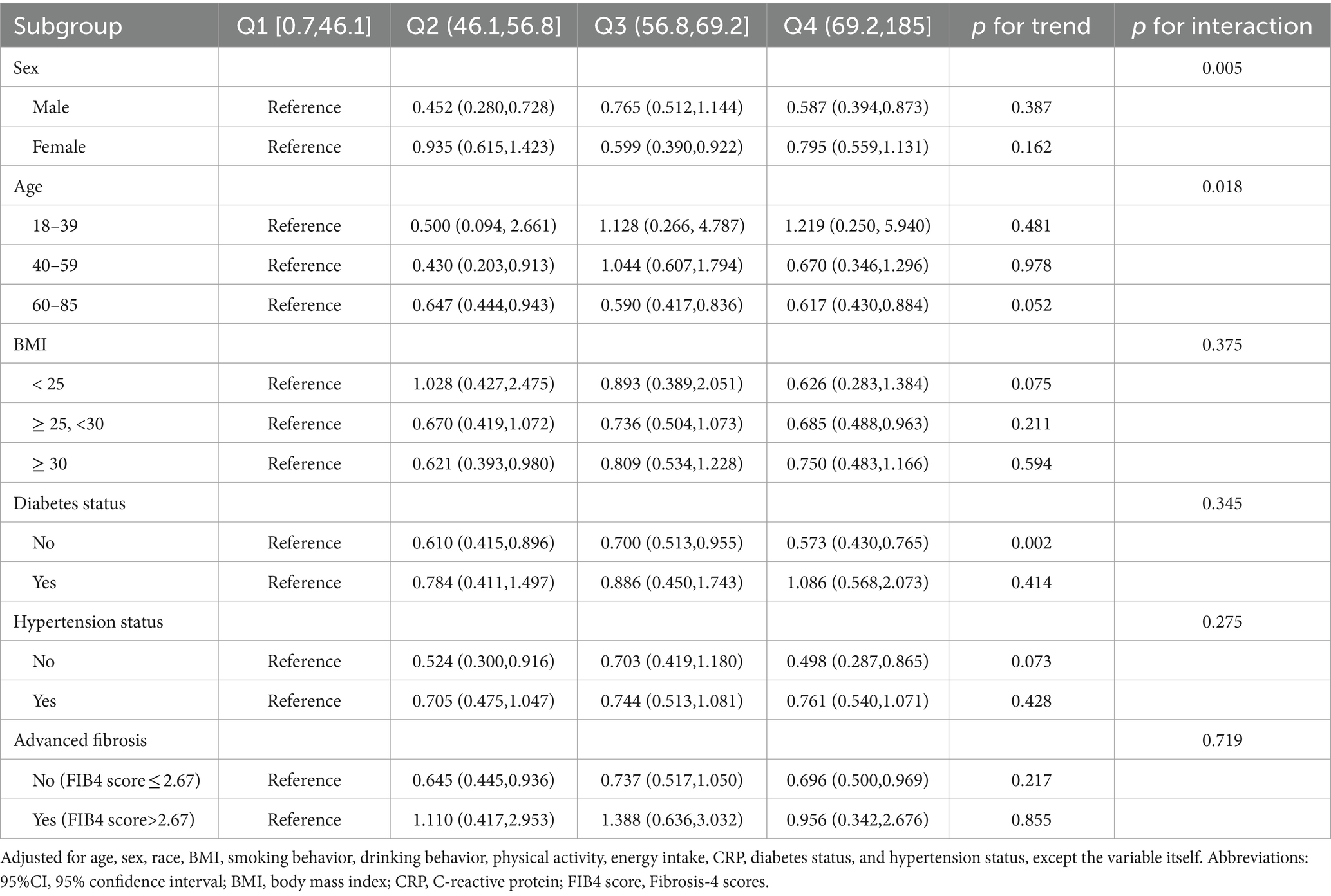
Table 3. Associations between serum vitamin A and all-cause mortality in NAFLD participants, stratified by age, sex, BMI, diabetes status, hypertension status, and advanced fibrosis status.
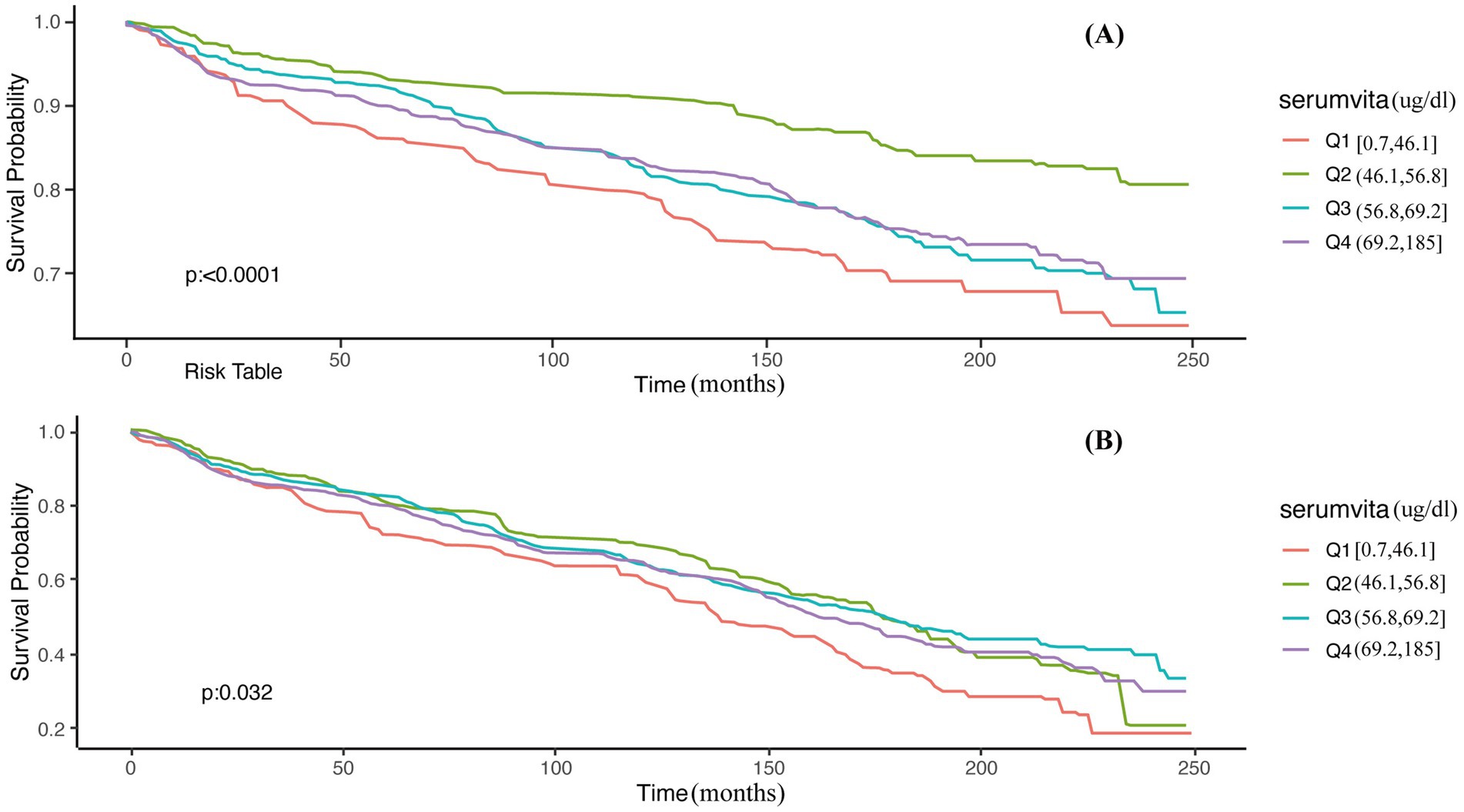
Figure 2. Unadjusted Kaplan–Meier survival curves for all-cause mortality among serum vitamin A. (A) Males; (B) 60–85 years.
3.4 Dose–response relationship between serum vitamin A levels and all-cause mortality in NAFLD patients
Figure 3 vividly illustrates the dose–response relationship between serum vitamin A levels and all-cause mortality in NAFLD patients. A notable U-shaped association was identified by applying the RCS model with comprehensive adjustment for all variables (p for non-linearity <0.001, p for overall <0.001), with a crucial threshold identified at 64.5 μg/dL.
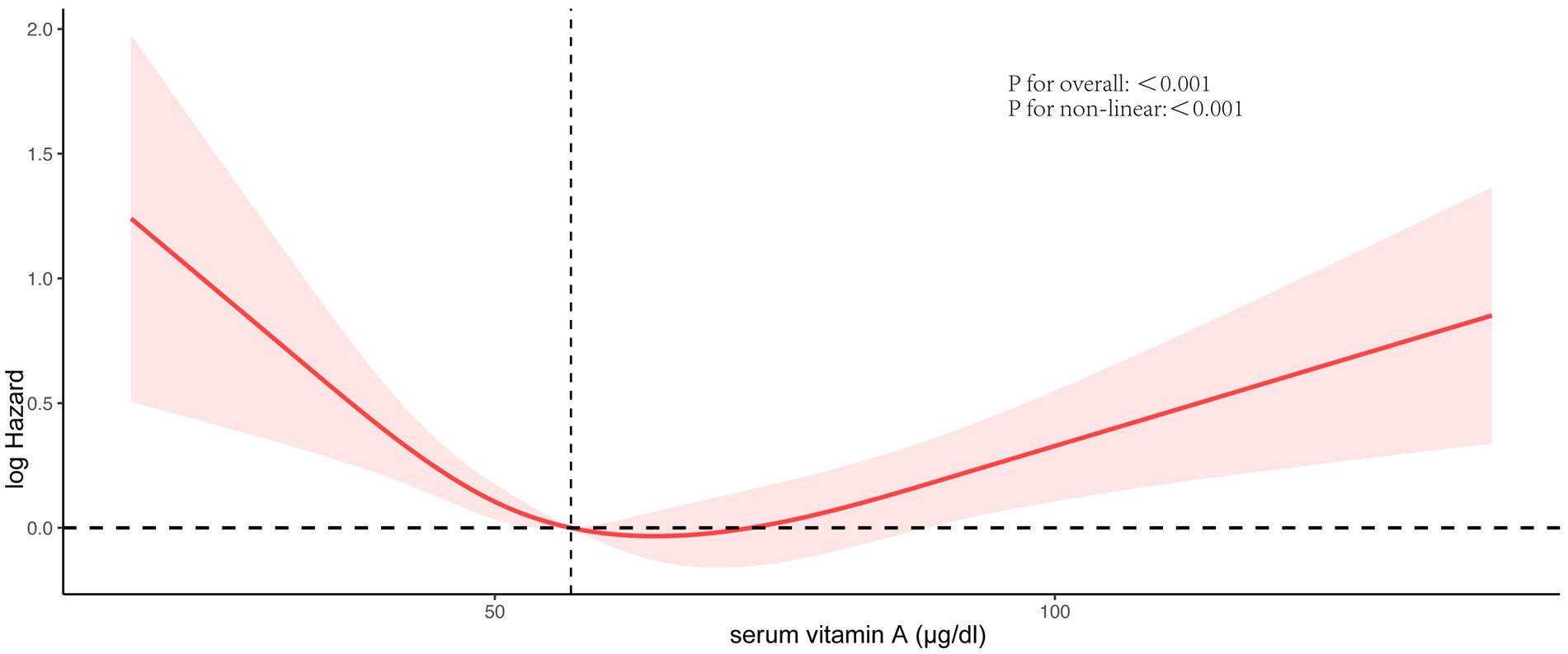
Figure 3. Association between serum vitamin A concentrations and all-cause mortality. Red solid lines and red dotted lines, respectively, represent restricted cubic spline models and 95%CI. Models were adjusted by age, race, BMI, smoking behavior, drinking behavior, physical activity, energy intake, C-reactive protein, diabetes status, and hypertension status.
4 Discussion
This study employed a prospective cohort design to investigate the relationship between serum vitamin A levels and all-cause mortality in NAFLD patients. The results revealed a U-shaped association between serum vitamin A concentrations and all-cause mortality, indicating that excessively low and high vitamin A levels increase mortality risk. This relationship was particularly pronounced among elderly individuals (aged 60–85) and males. To our knowledge, this is the first study to examine the association between serum vitamin A levels and all-cause mortality in the NAFLD population.
Current research on serum vitamin A levels and mortality rates largely focuses on pediatric populations, with less emphasis on studies involving adults. Abhishek Goyal et al. employed Cox regression analysis to reveal a substantial correlation between serum vitamin A levels and all-cause mortality in the overall population. Their findings indicate a noticeable reduction in mortality risk from Q2 to Q4 in comparison to the initial quintile Q1, while Q5 demonstrates a relative escalation in mortality risk (30). However, they did not conduct an in-depth analysis. An additional investigation discovered a strong correlation between reduced serum retinol levels and heightened occurrences of liver fibrosis and liver-related mortality within a cohort of American adults. For individuals with chronic liver disease (CLD), those in the lowest retinol category exhibited a significantly increased HR for liver-related mortality, reaching 7.76 (95% CI, 1.19–50.5) compared to the highest retinol group. However, no significant difference was observed in all-cause mortality (31). To date, there are no reported clinical studies on the relationship between serum vitamin A levels and all-cause mortality in the NAFLD population. Our study is the first to identify a U-shaped association between serum vitamin A levels and all-cause mortality in individuals with NAFLD.
We conducted stratified analyses to further identify subgroups of NAFLD patients for whom serum vitamin A levels are most strongly associated with all-cause mortality. The results revealed that sex and age are significant influencing factors. Specifically, the association between serum vitamin A levels and the risk of all-cause mortality is more pronounced in elderly individuals (aged 60–85) and males. Previous studies have suggested that NAFLD may be more severe in older populations. For instance, Mazen Noureddin et al. found a significant increase in the prevalence and severity of NAFLD among participants aged 60 or older (32). Frith et al. also observed higher rates of fibrosis and cirrhosis in elderly NAFLD patients (33). Pegah et al.’s study showed a common occurrence of NAFLD in older adults, associated with increased mortality risk in individuals aged 60–74 with NAFLD (29). Furthermore, Sun Q et al. noted that lower serum retinol levels (<50 μg/dL) were linked to increased mortality among participants aged 60 years and older with prediabetes and diabetes, potentially attributed to the increased susceptibility to malnutrition in older age, underscoring the importance of adequate vitamin A intake for nutritional enhancement (34). However, the specific relationship between serum vitamin A levels and all-cause mortality risk in older adults requires further investigation. Additionally, male predominance in NAFLD prevalence over females is believed to be influenced by the protective effects of estrogen in premenopausal women (2, 5). Several studies have underscored estrogen’s significant roles in antioxidative, anti-inflammatory, anti-apoptotic, and potential anti-fibrotic processes (5, 35–37). However, there have been no definitive reports on the relationship between serum vitamin A levels and all-cause mortality rates among different sexes. Although the exact mechanisms of these results remain unclear, clinical health management should pay particular attention to serum vitamin A levels in older adults (aged 60–85 years) and male populations.
The potential mechanisms underlying the relationship between serum vitamin A levels and all-cause mortality rates in NAFLD patients remain unclear. Oxidative stress, characterized by an imbalance between the generation of reactive oxygen species (ROS) and the clearance capacity of antioxidant systems such as superoxide dismutase and catalase, is believed to play a crucial role (4). Vitamin A exerts significant antioxidant effects in liver diseases and plays a critical role in controlling cell growth and differentiation (4, 16). It can inhibit the production of pro-inflammatory cytokines in macrophages, reduce inflammatory responses, suppress hepatocyte transformation, and inhibit liver cancer cell proliferation (38–41). Our findings showed that advanced liver fibrosis was most prevalent among patients with low serum vitamin A levels. Similarly, Song J et al. found that individuals with CLD who had the lowest retinol levels were significantly more likely to develop fibrosis and liver-related mortality compared to those with higher levels (31). A possible explanation for this is that the depletion of vitamin A may lead to oxidative stress-mediated damage observed in advanced liver disease. After liver injury, HSCs become activated and transform from vitamin A-rich, quiescent cells into proliferative and fibrogenic myofibroblasts. These activated cells produce excessive extracellular matrix, leading to liver fibrosis. Concurrently, there is a loss of characteristic perinuclear lipid droplets containing retinol (vitamin A), possibly leading to a loss of the ability of HSCs to store vitamin A (7, 11). However, our study revealed a U-shaped relationship between serum vitamin A levels and all-cause mortality in NAFLD patients. This may be due to excessive vitamin A metabolism, which could lead to an over-release of retinol-binding protein (RBP)/retinol complexes, thereby increasing lipid accumulation in liver cells and contributing to NAFLD progression. Moreover, excessive antioxidants might inhibit the induction of antioxidant defenses and the necessary pro-oxidative signals for tissue adaptation (16), potentially explaining why higher serum vitamin A levels are linked to increased all-cause mortality in NAFLD patients. Therefore, determining the most appropriate serum vitamin A levels is crucial.
Nevertheless, this study is subject to several limitations. Firstly, all measurements were conducted at baseline, and participants’ lifestyles and dietary habits may have changed during the long-term follow-up period, potentially affecting unmeasured variables that could influence the study outcomes. Secondly, we utilized the US FLI to assess hepatic steatosis and the FIB-4 score to evaluate hepatic fibrosis, which is not considered the gold standard for diagnosing NAFLD. Furthermore, despite adjusting for relevant covariates that could influence all-cause mortality rates, we cannot exclude the possibility of residual or unmeasured confounding factors affecting the study results. Lastly, there is a possibility that recall bias influenced self-reported data.
5 Conclusion
This study systematically investigated the association between serum vitamin A levels and all-cause mortality among NAFLD patients for the first time. The findings reveal a U-shaped correlation between serum vitamin A concentration and the risk of all-cause mortality among NAFLD patients in the United States. This finding offers new insights into the health management of patients with NAFLD, indicating that monitoring serum vitamin A levels may be important in clinical practice, particularly for men and older adults aged 60 and above, to reduce the risk of all-cause mortality.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found at: https://www.cdc.gov/nchs/nhanes/index.htm.
Ethics statement
The studies involving humans were approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HL: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft. JY: Writing – original draft, Data curation. YD: Writing – original draft, Conceptualization, Investigation. WK: Data curation, Writing – original draft. GQ: Supervision, Writing – review & editing. YX: Supervision, Writing – review & editing, Formal analysis, Funding acquisition, Software.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Second Batch of the Young Technical Backbone Talents Project of the Ningbo Municipal Health Commission (rc2022008).
Acknowledgments
The CDC sponsored the data collection for the NHANES.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1467659/full#supplementary-material
Footnotes
References
1. Younossi, ZM, Golabi, P, Paik, JM, Henry, A, Van Dongen, C, and Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. (2023) 77:1335–47. doi: 10.1097/HEP.0000000000000004
2. Riazi, K, Azhari, H, Charette, JH, Underwood, FE, King, JA, Afshar, EE, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. (2022) 7:851–61. doi: 10.1016/S2468-1253(22)00165-0
3. Brunt, EM, Wong, VW, Nobili, V, Day, CP, Sookoian, S, Maher, JJ, et al. Nonalcoholic fatty liver disease. Nat Rev Dis Primers. (2015) 1:15080. doi: 10.1038/nrdp.2015.80
4. Abe, RAM, Masroor, A, Khorochkov, A, Prieto, J, Singh, KB, Nnadozie, MC, et al. The role of vitamins in non-alcoholic fatty liver disease: a systematic review. Cureus. (2021) 13:e16855. doi: 10.7759/cureus.16855
5. Marchisello, S, di Pino, A, Scicali, R, Urbano, F, Piro, S, Purrello, F, et al. Pathophysiological, molecular and therapeutic issues of nonalcoholic fatty liver disease: an overview. Int J Mol Sci. (2019) 20:1948. doi: 10.3390/ijms20081948
6. Fujii, H, and Kawada, N. Inflammation and fibrogenesis in steatohepatitis. J Gastroenterol. (2012) 47:215–25. doi: 10.1007/s00535-012-0527-x
7. Niu, X, Liu, J, and Liu, K. Association of nonalcoholic fatty liver disease and liver fibrosis detected by transient elastography with serum retinol in American adults. Front Nutr. (2023) 10:1094161. doi: 10.3389/fnut.2023.1094161
8. Sookoian, S, and Pirola, CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. (2011) 53:1883–94. doi: 10.1002/hep.24283
9. Saeed, A, Dullaart, RPF, Schreuder, TCMA, Blokzijl, H, and Faber, KN. Disturbed vitamin A metabolism in non-alcoholic fatty liver disease (NAFLD). Nutrients. (2017) 10:29. doi: 10.3390/nu10010029
10. Raza, S, Tewari, A, Rajak, S, and Sinha, RA. Vitamins and non-alcoholic fatty liver disease: a molecular insight⋆. Liver Res. (2021) 5:62–71. doi: 10.1016/j.livres.2021.03.004
11. Pirazzi, C, Valenti, L, Motta, BM, Pingitore, P, Hedfalk, K, Mancina, RM, et al. PNPLA3 has retinyl-palmitate lipase activity in human hepatic stellate cells. Hum Mol Genet. (2014) 23:4077–85. doi: 10.1093/hmg/ddu121
12. Bruschi, FV, Claudel, T, Tardelli, M, Caligiuri, A, Stulnig, TM, Marra, F, et al. The PNPLA3 I148M variant modulates the fibrogenic phenotype of human hepatic stellate cells. Hepatology. (2017) 65:1875–90. doi: 10.1002/hep.29041
13. Ziouzenkova, O, Orasanu, G, Sharlach, M, Akiyama, TE, Berger, JP, Viereck, J, et al. Retinaldehyde represses adipogenesis and diet-induced obesity. Nat Med. (2007) 13:695–702. doi: 10.1038/nm1587
14. Lotfi, A, Saneei, P, Hekmatdost, A, Salehisahlabadi, A, Shiranian, A, and Ghiasvand, R. The relationship between dietary antioxidant intake and physical activity rate with nonalcoholic fatty liver disease (NAFLD): a case - control study. Clin Nutr ESPEN. (2019) 34:45–9. doi: 10.1016/j.clnesp.2019.09.004
15. Mazidi, M, Huybrechts, I, and Kengne, AP. Associations between serum lipophilic antioxidants levels and non-alcoholic fatty liver disease are moderated by adiposity. Eur J Clin Nutr. (2019) 73:1088–90. doi: 10.1038/s41430-019-0413-1
16. Zhang, K, Nulali, J, Zhang, C, Chen, Y, Cheng, J, Shi, X, et al. The association between serum vitamin A and NAFLD among US adults varied in different BMI groups: a cross-sectional study. Food Funct. (2023) 14:836–44. doi: 10.1039/d2fo02204d
17. Xiao, ML, Zhong, HL, Lin, HR, Liu, CY, Yan, Y, Ke, YB, et al. Higher serum vitamin A is associated with a worsened progression of non-alcoholic fatty liver disease in adults: a prospective study. Food Funct. (2022) 13:970–7. doi: 10.1039/d1fo03119h
18. Ruhl, CE, and Everhart, JE. Fatty liver indices in the multiethnic United States National Health and nutrition examination survey. Aliment Pharmacol Ther. (2015) 41:65–76. doi: 10.1111/apt.13012
19. Ratziu, V, Bellentani, S, Cortez-Pinto, H, Day, C, and Marchesini, G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. (2010) 53:372–84. doi: 10.1016/j.jhep.2010.04.008
20. Henry, A, Paik, JM, Austin, P, Eberly, KE, Golabi, P, Younossi, I, et al. Vigorous physical activity provides protection against all-cause deaths among adults patients with nonalcoholic fatty liver disease (NAFLD). Aliment Pharmacol Ther. (2023) 57:709–22. doi: 10.1111/apt.17308
21. Chen, C, Zhou, Q, Yang, R, Wu, Z, Yuan, H, Zhang, N, et al. Copper exposure association with prevalence of non-alcoholic fatty liver disease and insulin resistance among US adults (NHANES 2011-2014). Ecotoxicol Environ Saf. (2021) 218:112295. doi: 10.1016/j.ecoenv.2021.112295
22. Tsou, P, and Wu, CJ. Serum vitamin E levels of adults with nonalcoholic fatty liver disease: an inverse relationship with all-cause mortality in non-diabetic but not in pre-diabetic or diabetic subjects. J Clin Med. (2019) 8:1057. doi: 10.3390/jcm8071057
23. Liu, X, Shen, H, Chen, M, and Shao, J. Clinical relevance of vitamins and carotenoids with liver steatosis and fibrosis detected by transient Elastography in adults. Front Nutr. (2021) 8:760985. doi: 10.3389/fnut.2021.760985
24. Chen, X, Zhou, M, Yan, H, Chen, J, Wang, Y, and Mo, X. Association of serum total 25-hydroxy-vitamin D concentration and risk of all-cause, cardiovascular and malignancies-specific mortality in patients with hyperlipidemia in the United States. Front Nutr. (2022) 9:971720. doi: 10.3389/fnut.2022.971720
25. Rattan, P, Penrice, DD, Ahn, JC, Ferrer, A, Patnaik, M, Shah, VH, et al. Inverse Association of Telomere Length with Liver Disease and Mortality in the US population. Hepatol Commun. (2022) 6:399–410. doi: 10.1002/hep4.1803
26. Fowler, JR, Tucker, LA, Bailey, BW, and LeCheminant, JD. Physical activity and insulin resistance in 6,500 NHANES adults: the role of abdominal obesity. J Obes. (2020) 2020:3848256–10. doi: 10.1155/2020/3848256
27. Wang, L, Li, X, Wang, Z, Bancks, MP, Carnethon, MR, Greenland, P, et al. Trends in prevalence of diabetes and control of risk factors in diabetes among US adults, 1999-2018. JAMA. (2021) 326:1–13. doi: 10.1001/jama.2021.9883
28. Kim, D, Konyn, P, Cholankeril, G, and Ahmed, A. Physical activity is associated with nonalcoholic fatty liver disease and significant fibrosis measured by FibroScan. Clin Gastroenterol Hepatol. (2022) 20:e1438–55. doi: 10.1016/j.cgh.2021.06.029
29. Golabi, P, Paik, J, Reddy, R, Bugianesi, E, Trimble, G, and Younossi, ZM. Prevalence and long-term outcomes of non-alcoholic fatty liver disease among elderly individuals from the United States. BMC Gastroenterol. (2019) 19:56. doi: 10.1186/s12876-019-0972-6
30. Goyal, A, Terry, MB, and Siegel, AB. Serum antioxidant nutrients, vitamin A, and mortality in U.S. Adults Cancer. Epidemiol Biomarkers Prev. (2013) 22:2202–11. doi: 10.1158/1055-9965.EPI-13-0381
31. Song, J, and Jiang, ZG. Low vitamin A levels are associated with liver-related mortality: a nationally representative cohort study. Hepatol Commun. (2023) 7:e0124. doi: 10.1097/HC9.0000000000000124
32. Noureddin, M, Yates, KP, Vaughn, IA, Neuschwander-Tetri, BA, Sanyal, AJ, McCullough, A, et al. Clinical and histological determinants of nonalcoholic steatohepatitis and advanced fibrosis in elderly patients. Hepatology. (2013) 58:1644–54. doi: 10.1002/hep.26465
33. Frith, J, Day, CP, Henderson, E, Burt, AD, and Newton, JL. Non-alcoholic fatty liver disease in older people. Gerontology. (2009) 55:607–13. doi: 10.1159/000235677
34. Sun, Q, and Guo, J. Associations between serum retinol and all-cause mortality among adults with prediabetes and diabetes: a cohort study. PLoS One. (2024) 19:e0297552. doi: 10.1371/journal.pone.0297552
35. Lu, G, Shimizu, I, Cui, X, Itonaga, M, Tamaki, K, Fukuno, H, et al. Antioxidant and antiapoptotic activities of idoxifene and estradiol in hepatic fibrosis in rats. Life Sci. (2004) 74:897–907. doi: 10.1016/j.lfs.2003.08.004
36. Yasuda, M, Shimizu, I, Shiba, M, and Ito, S. Suppressive effects of estradiol on dimethylnitrosamine-induced fibrosis of the liver in rats. Hepatology. (1999) 29:719–27. doi: 10.1002/hep.510290307
37. Ballestri, S, Nascimbeni, F, Baldelli, E, Marrazzo, A, Romagnoli, D, and Lonardo, A. NAFLD as a sexual dimorphic disease: role of gender and reproductive status in the development and progression of nonalcoholic fatty liver disease and inherent cardiovascular risk. Adv Ther. (2017) 34:1291–326. doi: 10.1007/s12325-017-0556-1
38. Botella-Carretero, JI, Balsa, JA, Vázquez, C, Peromingo, R, Díaz-Enriquez, M, and Escobar-Morreale, HF. Retinol and alpha-tocopherol in morbid obesity and nonalcoholic fatty liver disease. Obes Surg. (2010) 20:69–76. doi: 10.1007/s11695-008-9686-5
39. Reifen, R. Vitamin a as an anti-inflammatory agent. Proc Nutr Soc. (2002) 61:397–400. doi: 10.1079/PNS2002172
40. Chi, X, Anselmi, K, Watkins, S, and Gandhi, CR. Prevention of cultured rat stellate cell transformation and endothelin-B receptor upregulation by retinoic acid. Br J Pharmacol. (2003) 139:765–74. doi: 10.1038/sj.bjp.0705303
41. Piao, YF, Shi, Y, and Gao, PJ. Inhibitory effect of all-trans retinoic acid on human hepatocellular carcinoma cell proliferation. World J Gastroenterol. (2003) 9:2117–20. doi: 10.3748/wjg.v9.i9.2117
Glossary
Keywords: serum vitamin A, all-cause mortality, NAFLD, NHANES, nonlinear
Citation: Li H, Ye J, Dong Y, Kong W, Qian G and Xie Y (2024) U-shaped association of serum vitamin A concentrations with all-cause mortality in patients with NAFLD: results from the NHANES database prospective cohort study. Front. Nutr. 11:1467659. doi: 10.3389/fnut.2024.1467659
Edited by:
Lubomir Skladany, Slovak Medical University, SlovakiaReviewed by:
Michał Kukla, Jagiellonian University Medical College, PolandPetrana Martinekova, Semmelweis University, Hungary
Copyright © 2024 Li, Ye, Dong, Kong, Qian and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiliang Kong, bGl2ZWtvbmdAaG90bWFpbC5jb20=; Guoqing Qian, YmlsbC5xaWFuQG91dGxvb2suY29t; Yilian Xie, c3VnZXI4NDIwMDNAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Hui Li
Hui Li Jiayuan Ye
Jiayuan Ye Yitian Dong
Yitian Dong Weiliang Kong
Weiliang Kong Guoqing Qian
Guoqing Qian Yilian Xie
Yilian Xie