- 1Department of Gastroenterology, The Affiliated People's Hospital of Fujian University of Traditional Chinese Medicine, Fuzhou, China
- 2Studies of Hospital of Traditional Chinese Medicine of Ningde City, Fujian University of Traditional Chinese Medicine, Fuzhou, China
- 3Department of Orthopaedics, Shengli Clinical Medical College of Fujian Medical University, Fuzhou, China
- 4Department of Orthopaedics, Fujian Provincial Hospital, Fuzhou, China
Introduction: This research utilized data from the NHANES 2011–2018 study to investigate the connection between the Oxidative Balance Score (OBS) and muscular dystrophies.
Methods: This study is a cross-sectional, observational, secondary analysis utilizing data from the NHANES 2011-2018. Spearman’s correlation, chi-square tests, logistic regression, and restricted cubic spline plots were employed for statistical analyses.
Results: This association remained significant after adjustment for various demographic and medical history factors (For continuous OBS: crude model, odds ratio [OR], 0.95, 95% confidence interval [CI:] 0.94, 0.97, p < 0.001; Model 1, OR, 0.94, 95% CI: 0.92, 0.96, p < 0.001; Model 2, OR, 0.95, 95% CI: 0.93, 0.97, p < 0.001; Model 3, OR, 0.95, 95% CI: 0.93, 0.97, p < 0.001; In quartile Q4 vs. Q1: Crude model, OR, 0. 42, 95% CI: 0.26, 0.66, p < 0.001; Model 1, OR, 0.33, 95% CI: 0.21, 0.52, p < 0.001; Model 2, OR, 0.37, 95% CI: 0.23, 0.58, p < 0.001; Model 3, OR, 0.38, 95% CI: 0.23, 0.60, p < 0.001). Restricted cubic spline (RCS) analysis further supported this inverse relationship, suggesting that OBS values above 10 may confer protection against muscular dystrophies (p for overall <0.001, p for non-linear = 0.536). However, the relationship between OBS and muscular dystrophies was not statistically significant in the subgroups with education level below high school, presence of cancer, or diabetes (p = 0.735, p = 0.574, p = 0.409, respectively).
Conclusion: The study found a significant inverse correlation between the OBS and muscular dystrophies, suggesting that individuals with higher oxidative balance had a lower risk of developing muscular dystrophies. The study highlights the potential role of oxidative balance in muscular dystrophies prevention and management.
1 Introduction
Muscular dystrophies, a condition characterized by age-related loss of skeletal muscle mass, strength, and function, has garnered significant attention as a public health concern worldwide (1, 2). In the United States, the prevalence of muscular dystrophies is estimated to be between 5 and 13% in individuals aged 60 years and older, with a greater burden in racial and ethnic minority groups (3, 4). As the global population ages, the prevalence of muscular dystrophies are expected to rise, posing a substantial burden on healthcare systems, and reducing the quality of life for affected individuals (5, 6). The loss of muscle mass not only impairs physical function but also increases the risk of adverse health outcomes, such as disability, falls, and mortality (7, 8). The consequences of muscular dystrophies extend beyond physical limitations, as it is closely linked to metabolic disorders, such as insulin resistance and type 2 diabetes (9, 10). Furthermore, muscular dystrophy is associated with an increased risk of hospitalization and mortality, highlighting its impact on public health (11).
Several factors contribute to the development of muscular dystrophy, including age-related changes in muscle biology, hormonal alterations, nutritional deficiencies, physical inactivity, and chronic diseases (12–14). Oxidative stress, characterized by an imbalance between the production of reactive oxygen species and the body’s antioxidant defenses, has been proposed as a potential mechanism linking these factors to muscular dystrophy (15, 16). The oxidative balance score (OBS), a measure of the overall oxidative stress status in the body, has been shown to be associated with muscle mass and function in previous studies (17). Xu et al. (18) reported a negative association between OBS and sarcopenia (odd ratio = 0.94, 95%CI 0.92 to 0.96, p < 0.001), Cai et al. (19) and Zhao et al. (20) indicated similarly. Therefore, investigating the relationship between oxidative balance score and sarcopenia may provide valuable insights into the pathogenesis of this condition and identify potential targets for intervention.
In this study, we utilize data from the National Health and Nutrition Examination Survey (NHANES) 2011–2018 dataset to examine the association between oxidative balance score and muscular dystrophies. By analyzing this comprehensive dataset, we aim to provide a more accurate understanding of the burden of muscular dystrophies in the United States and its potential links to oxidative stress. The findings of this study may inform future public health interventions and policies aimed at preventing and managing muscular dystrophies, ultimately improving the health and well-being of people with muscular dystrophies.
2 Materials and methods
2.1 Study design and population
This study is a cross-sectional, observational, secondary analysis utilizing data from the NHANES 2011–2018. NHANES is a continuous, nationwide survey conducted by the National Center for Health Statistics that assesses the health and nutritional status of a representative sample of the non-institutionalized U.S. population. The survey employs a complex, stratified, multistage probability sampling design to ensure that the data collected are generalizable to the U.S. population. The dataset is available online at https://www.cdc.gov/nchs/nhanes/index.htm.
The study population included all individuals aged from 8 to 59 years who participated in the NHANES survey during 2011–2018. The inclusion criterion was based on the availability of complete whole-body dual-energy X-ray absorptiometry (DEXA) data. Participants with missing data on DEXA, body mass index (BMI), OBS, demographic information [race, gender, education level, marital status, and poverty income ratio (PIR)], and medical history (hypertension, high cholesterol, diabetes, and cancer) were excluded from the analysis (Figure 1).
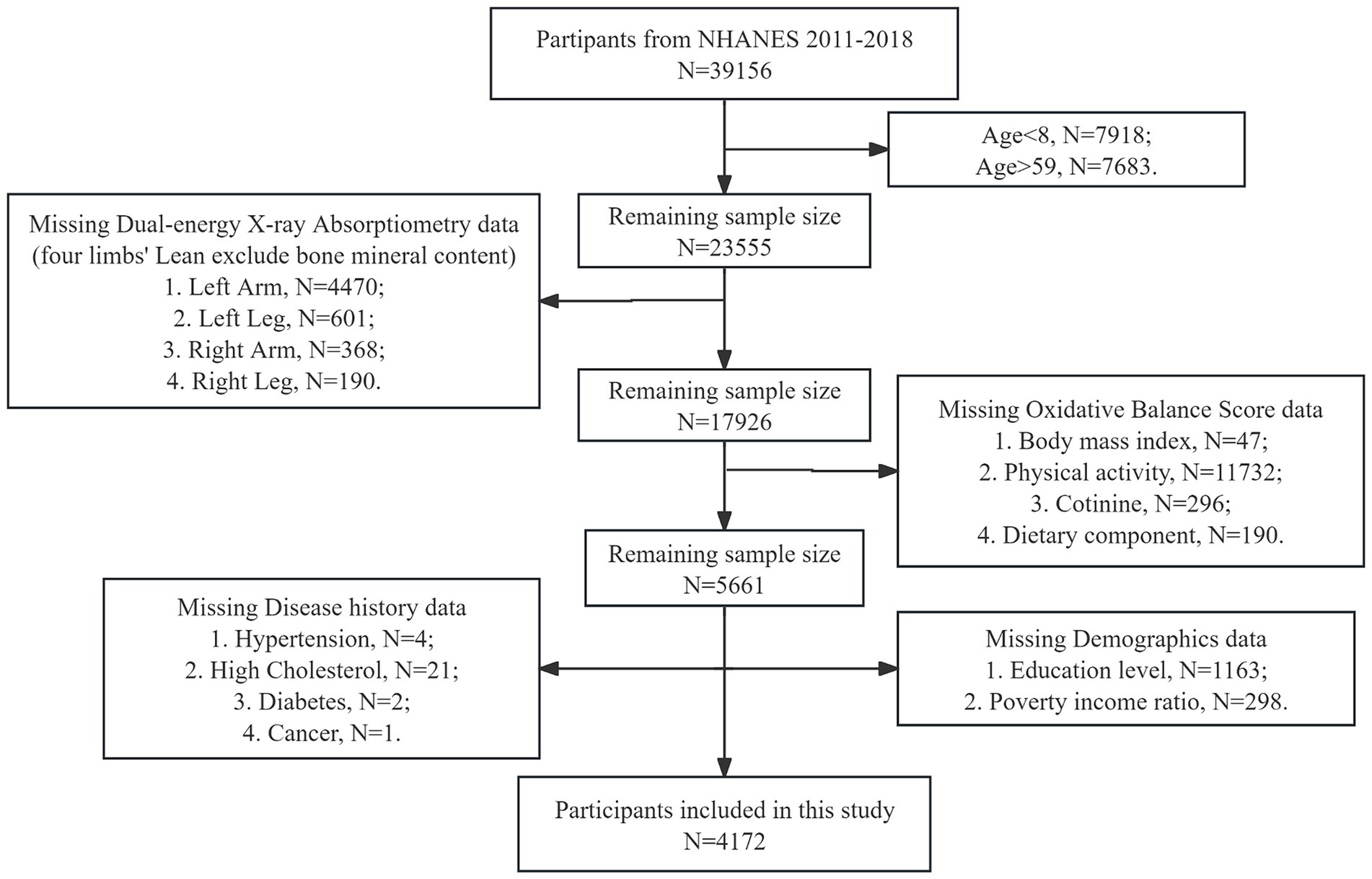
Figure 1. Flow chart of participants selection. NHANES, National Health and Nutrition Examination Survey.
2.2 Outcome definitions
The primary outcome of this study was the presence of muscular dystrophies, which was assessed using DEXA whole-body files from the examination data. Prior to analysis, participants were excluded if they were taller than 192.5 cm, weighed more than 136.4 kg, or were pregnant. Appendicular lean mass (ALM) is the sum of the mineral content of the lean body mass of the left and right arms and legs (in grams). Muscular dystrophies were defined based on ALM:BMI <0.789 for men and <0.512 for women, according to criteria established in previous research (21).
2.3 Exposure definitions
The exposure indicator in this study was the previously defined OBS (22, 23). The OBS is a composite score that captures the balance of antioxidant and pro-oxidant factors in an individual’s diet and lifestyle. The dietary components were obtained from the Dietary Interview – First Day Total Nutrient Intakes dataset and included dietary fiber, carotene, riboflavin, niacin, total folate, vitamin B12, vitamin C, vitamin E, calcium, magnesium, zinc, copper, selenium, total fat, and iron. Researchers obtained the data by interviewing participants regarding the types and amounts of food they intake during the 24-h period prior to the interview and then calculated the values using the Food and Nutrient Database for Dietary Studies (FNDDS) provided by the U.S. Department of Agriculture. Lifestyle components included physical activity, alcohol, BMI, and cotinine. These components were classified as antioxidants, with the exception of total fat, iron, alcohol, BMI, and cotinine, which were classified as prooxidants. To account for the different scales of the variables, the OBS data were categorized into three tertiles for males and females, respectively (Supplementary Table S1). For the antioxidant components, a score from 0 to 2 was assigned based on the level of intake, with higher values indicating greater antioxidant capacity. Conversely, for the pro-oxidant components, a score ranging from 2 to 0 (alcohol: 1 to 0) was assigned, with higher values indicating a greater pro-oxidant effect. The scores for each component were then summed to obtain the OBS score.
2.4 Covariates
The covariates used in this study were categorized into two broad categories: demographic characteristics and medical history. These covariates were selected to account for potential confounding factors that could influence the association between oxidative balance score and muscular dystrophies. Demographic characteristics included age, gender, race, education level, marital status, and PIR. Age was measured as a continuous variable, while gender was categorized as male or female. Race was classified into four categories: Non-Hispanic White, Non-Hispanic Black, Mexican American, and Other Race. Education level (only individuals aged 20 and above) was divided into two groups: below high school and high school or above. Marital status was simplified into married or unmarried. PIR, a measure of economic status, was considered a continuous variable. Medical history was captured through self-reported questionnaire data, assessing the presence of hypertension, high blood cholesterol level, cancer, or diabetes. These conditions were individually categorized as a history of the disease (yes or no) based on the participant’s response to the questionnaire (“have you ever been told by a doctor or other health professional that you had the disease”).
2.5 Statistical analysis
Baseline characteristics of the study population were described using muscular dystrophies status (presence or absence) and OBS quartiles. Continuous variables were presented as means and standard deviations, and Spearman’s correlation coefficients were used to assess differences between groups. Categorical variables were described with frequencies and percentages, and chi-square tests were performed to compare group differences. Logistic regression was employed to examine the association between OBS and muscular dystrophies, with adjustment for potential confounding factors. Model 1 was adjusted for age, gender, and race. Model 2 included additional adjustments for education level, marital status, and PIR. Model 3 further adjusted for hypertension, high cholesterol, cancer, and diabetes. Restricted cubic spline (RCS) plots were used to examine the non-linear relationship between muscular dystrophies and OBS. Subgroup analyses were conducted to evaluate interactions between muscular dystrophies and OBS in different populations. All data were weighted using NHANES standard weights to ensure representativeness of the results. Statistical analyses were performed using R software version 4.3.2. Statistical significance was defined as a two-tailed p-value <0.05.
3 Results
3.1 Baseline characteristics
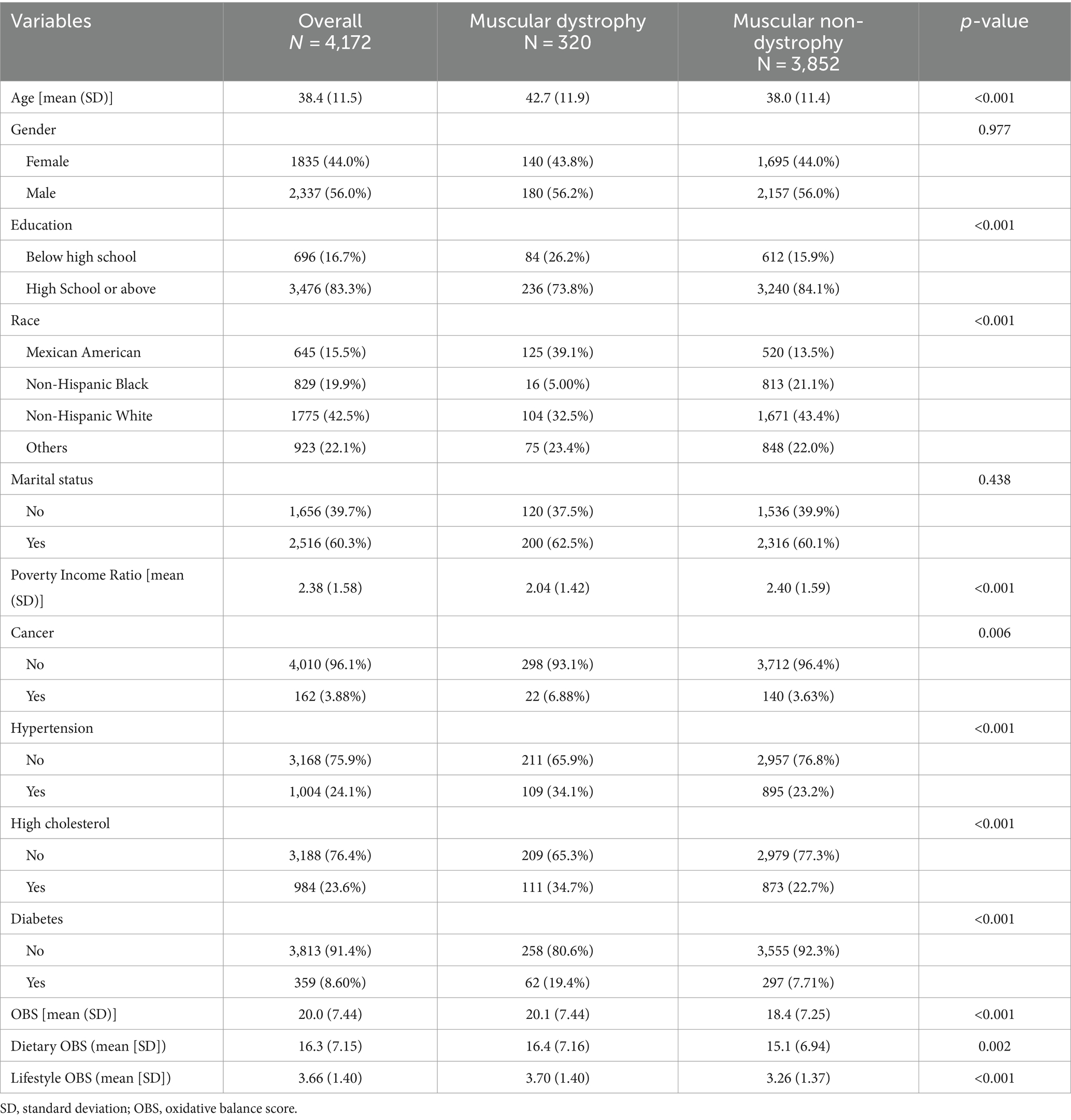
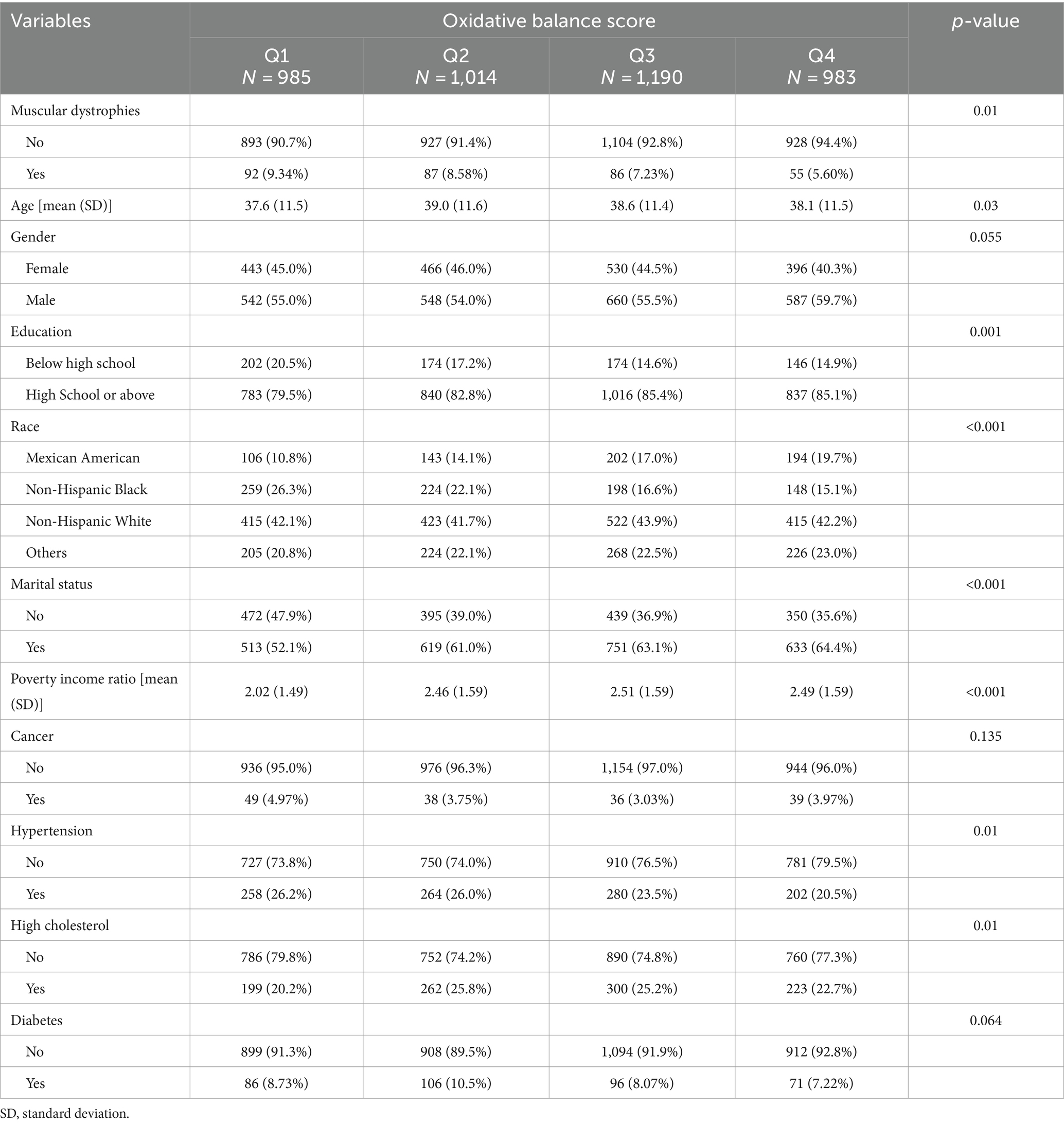
A total of 4,172 participants were included in the study, of whom 320 (7.7%) met the criteria for muscular dystrophies and 3,852 (92.3%) were muscular non-dystrophies (Table 1). The mean age of the participants was 38.4 years, with those diagnosed with muscular dystrophies being older (42.7 years) than those without muscular dystrophies (38.0 years, p < 0.001). There were more males (56.0%) and a higher proportion of individuals with a high school education or higher (83.3%) in the muscular non-dystrophies group (p = 0.977, p < 0.001, respectively). In addition, the Mexican American (15.5%) and non-Hispanic Black (19.9%) races were less prevalent in the muscular non-dystrophies group (p < 0.001). Furthermore, participants with muscular dystrophies had a lower PIR (2.04) compared to those without (2.40, p < 0.001). The prevalence of cancer, hypertension, high cholesterol, and diabetes was higher in the muscular non-dystrophies group (p = 0.006, p < 0.001, p < 0.001, p < 0.001, respectively). OBS (mean: total OBS, 20.0; dietary OBS, 16.3; lifestyle OBS, 3.66) was significantly lower in the muscular dystrophies group compared to the muscular non-dystrophies group (total OBS, 20.1 vs. 18.4, p < 0.001; dietary OBS, 16.4 vs. 15.1, p = 0.002; lifestyle OBS, 3.70 vs. 3.26, p < 0.001). Table 2 shows the baseline characteristics of all participants grouped by quartile of OBS. Participants in the Q4 group had a decreasing trend in the prevalence of muscular dystrophies (5.6%) compared to those in the Q3 group (7.23%), Q2 group (8.58%), Q1 group (9.34%). The age distribution was slightly higher in Q3 and Q4 groups compared to Q1 and Q2 groups. Regarding gender, there was a higher percentage of males in the Q4 group compared to the Q1 group. Educational attainment was higher in the Q4 group, with fewer individuals having less than a high school education. The PIR was highest in the Q4 group, indicating a higher economic status. The prevalence of cancer, hypertension, high cholesterol, and diabetes was lower in the Q4 group than in the Q1 group. Baseline characteristics based on the quartile dietary and lifestyle OBS are summarized in Supplementary Tables S2, S3, respectively.
3.2 Relationship between OBS and muscular dystrophies
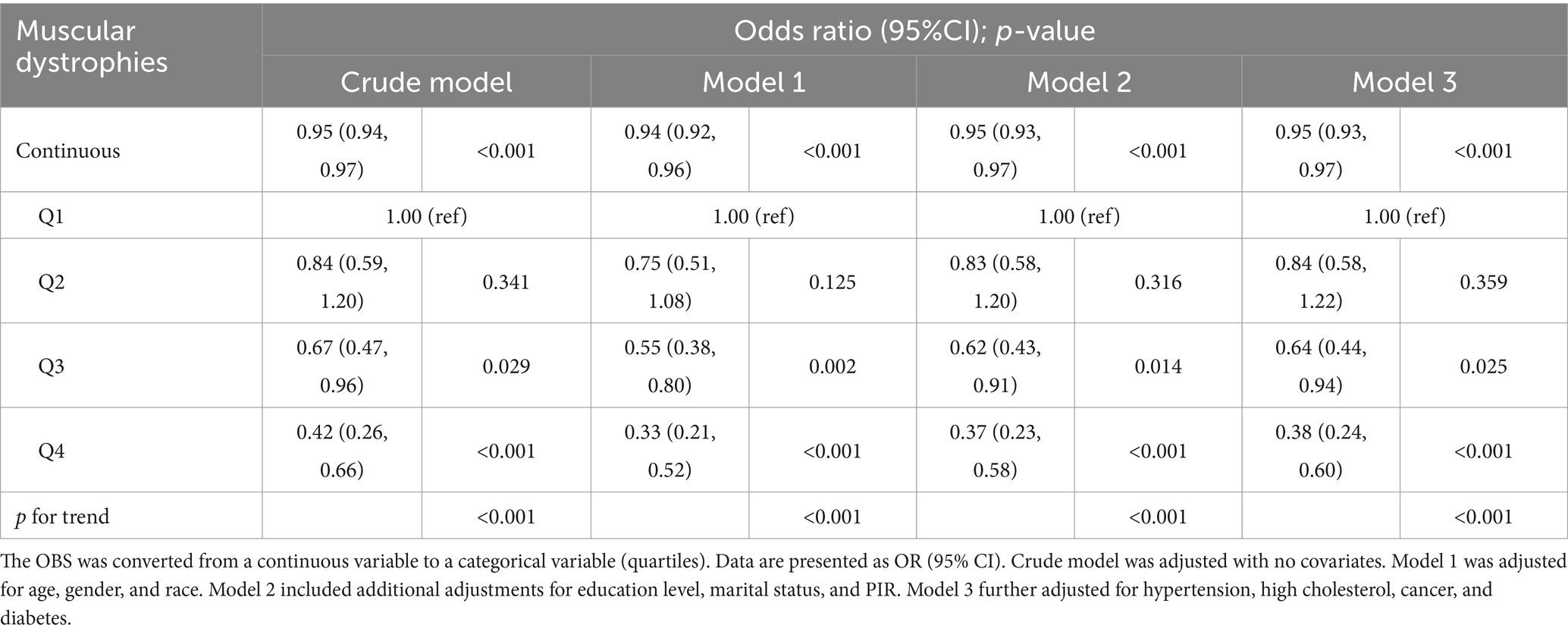
The results of multivariate logistic regression suggested a significant correlation between the dependent variable muscular dystrophies and the independent variable OBS (Table 3). The dietary and lifestyle OBS likewise demonstrated a significant association with muscular dystrophies (Supplementary Tables S4, S5, respectively). In the crude model, a significant inverse association was observed with an odds ratio (OR) of 0.95 (95% confidence interval [CI]: 0.94, 0.97), indicating that higher OBS was associated with a lower risk of muscular dystrophies (p < 0.001). After adjusting for age, gender, and race in model 1, the association remained significant with an OR of 0.94 (95% CI: 0.92, 0.96) (p < 0.001). Further adjustment for education level, marital status, and PIR in model 2 resulted in an OR of 0.95 (95% CI: 0.93, 0.97) (p < 0.001). Model 3, which included additional adjustments for hypertension, high cholesterol, cancer, and diabetes, also showed a significant inverse association with an OR of 0.95 (95% CI: 0.93, 0.97) (p < 0.001). In quartile Q4, the OR for muscular dystrophies was 0.42 (95% CI: 0.26, 0.66) in the crude model, 0.33 (95% CI: 0.21, 0.52) in model 1, 0.37 (95% CI: 0.23, 0.58) in model 2, and 0.38 (95% CI: 0.24, 0.60) in model 3, with p-values for trend <0.001 in all models.
3.3 RCS analysis
The RCS analysis was conducted to assess the relationship between OBS and muscular dystrophies, adjusting for covariates in model 3. The analysis was stratified by the overall population as well as male and female groups to explore the potential non-linear associations. The RCS curve shown in Figure 2 indicated a significant association between OBS and muscular dystrophies in the overall population (p-overall < 0.001). However, there was no evidence of a non-linear association (p for non-linear = 0.536). This suggests that with adjustment for covariates in model 3, as OBS increases, the odds of muscular dystrophies decrease linearly. The inflection point at OBS = 10.0 indicates that an OBS greater than 10 may have a protective effect on the occurrence of muscular dystrophies. In the male population, RCS analysis indicated a linear relationship between OBS and muscular dystrophies (p-overall = 0.002, p for non-linear = 0.194, Supplementary Figure S1). Similar results were observed in the female population (p-overall < 0.001, p for non-linear = 0.500, Supplementary Figure S2).
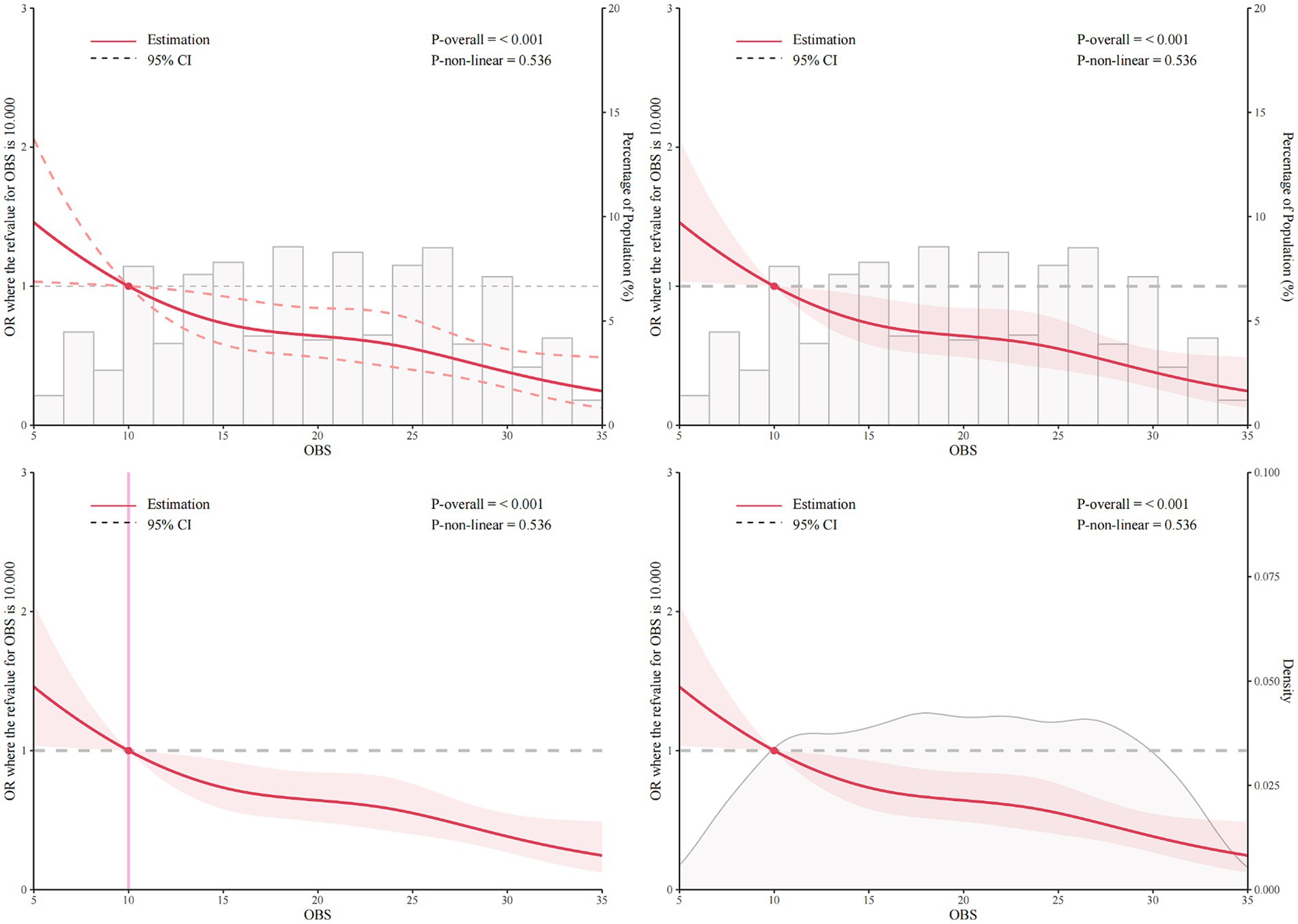
Figure 2. Restricted cubic spline (RCS) analysis of the association between OBS and muscular dystrophies with number, bar chart of proportion, and density. The association was adjusted for age, gender, race, education level, marital status, poverty income ratio, hypertension, high cholesterol, cancer, and diabetes. The median OBS was chosen as the reference. RCS, restricted cubic spline; OBS, oxidative balance score.
3.4 Subgroup analysis
Subgroup analysis was performed to evaluate the potential interaction between OBS and muscular dystrophies in several subgroups, including gender, race, education level, marital status, hypertension, high cholesterol, cancer, and diabetes (Figure 3). Overall, there was no statistically significant interaction between OBS and muscular dystrophies in any of the subgroups (all p-values for interaction >0.05). However, the association between OBS and muscular dystrophies was not statistically significant in the subgroups with education level below high school, presence of cancer, or diabetes (p = 0.735, p = 0.574, p = 0.409, respectively).
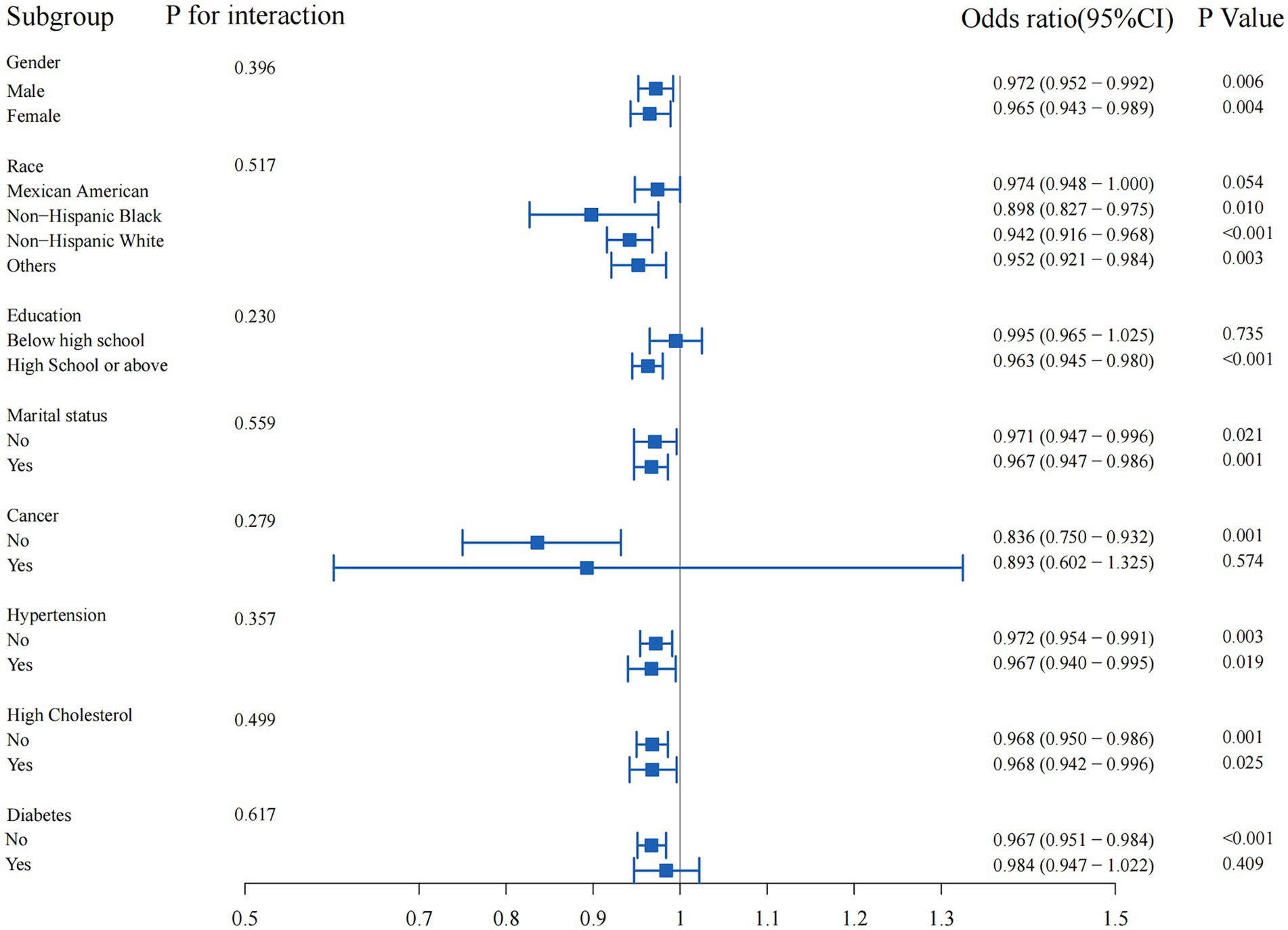
Figure 3. Subgroup analyses of the association between oxidative balance score and muscular dystrophies.
4 Discussion
This study sought to explore the association between the OBS and muscular dystrophies among a sample of participants from the NHANES 2011–2018. The findings revealed a significant inverse relationship between OBS and muscular dystrophies in the US population, indicating that higher oxidative balance was associated with a lower risk of muscular dystrophies. This relationship remained significant after adjusting for several covariates, suggesting that oxidative balance may play a crucial role in the development and progression of muscular dystrophies (19).
The observed inverse relationship between OBS and muscular dystrophies is consistent with previous findings linking oxidative stress to age-related muscle loss (18–20, 24). Oxidative stress, characterized by an imbalance between the production of reactive oxygen species and the body’s antioxidant defenses, has been proposed as a potential mechanism linking various factors, such as age-related changes in muscle biology, hormonal changes, nutritional deficiencies, physical inactivity, and chronic diseases, to muscular dystrophies (12, 15, 25, 26). OBS, a measure of indirect oxidative stress status in the body, has been shown in previous studies to be associated with muscle mass and function (27, 28). Therefore, the inverse association observed in this study underscores the potential role of oxidative balance in the pathogenesis of muscular dystrophies and highlights the importance of interventions targeting oxidative balance to prevent and treat muscular dystrophies. In addition, the association of dietary OBS and lifestyle OBS with muscular dystrophies are also noteworthy. Dietary OBS, which captures the balance of antioxidant and pro-oxidant factors in an individual’s diet, has been shown in previous studies to be associated with muscle mass and function (29, 30). Similarly, Lifestyle OBS, which reflects the balance of antioxidant and pro-oxidant factors in an individual’s lifestyle, has also been associated with muscle health (31). In this study, both Dietary OBS and Lifestyle OBS showed significant associations with muscular dystrophies, further supporting the role of oxidative balance in the development of muscular dystrophies.
Furthermore, evidence from previous research supports the notion that antioxidant factors can play a protective role against muscular dystrophies. For instance, dietary antioxidants such as vitamin C, vitamin E, and carotenoids have been shown to have a beneficial effect on muscle mass and strength (25, 32). These antioxidants can reduce oxidative stress and protect muscle fibers from damage, thereby potentially preventing the development of sarcopenia (32–34). Additionally, physical activity, which is classified as an antioxidant factor in the OBS, has been linked to increased muscle mass and strength, probably through its anti-inflammatory and antioxidant effects (35, 36). On the other hand, pro-oxidant factors, such as certain types of dietary fat, alcohol, and tobacco, have been associated with an increased risk of sarcopenia (37, 38). These factors can exacerbate oxidative stress and lead to muscle damage and loss (39). Oxidative stress could lead to an imbalance in protein synthesis and degradation, thereby increasing muscle catabolism and protein breakdown (18). Furthermore, oxidative stress may disrupt calcium homeostasis within muscle cells, impairing muscle contraction and relaxation functions, and ultimately contributing to the development of muscular dystrophies (29). Therefore, maintaining a balanced diet and lifestyle, rich in antioxidants and low in pro-oxidants, may be an effective strategy for preventing muscular dystrophies.
The RCS analysis further supported the inverse relationship between OBS and muscular dystrophies, indicating a linear decrease in the odds of muscular dystrophies as OBS increased. This finding suggests that higher OBS values may have a protective exogenous effect against the occurrence of muscular dystrophies. The inflection point of the RCS plots further underscores the potential protective effect of an OBS greater than 10 in reducing the risk of muscular dystrophies. This finding is particularly meaningful as it provides a potential target for interventions aimed at improving oxidative balance to reduce the risk of muscular dystrophies.
Subgroup analysis revealed no statistically significant interaction between OBS and muscular dystrophies in various demographic and medical history subgroups. However, the association between OBS and muscular dystrophies were not statistically significant in the subgroups with education level below high school, presence of cancer, or diabetes. This could be due to the potential influence of the categorical nature of these variables on the association between OBS and muscular dystrophies. First, the potential influence of socioeconomic status on dietary and lifestyle choices may have obscured the relationship between OBS and muscular dystrophies (18). Lower socioeconomic status is associated with less access to nutritious foods and health-promoting activities, which may affect oxidative balance and muscle health (40). In addition, individuals with lower levels of education may have less awareness or access to health information and resources, further complicating the association between OBS and muscular dystrophies (18). In the subgroup with a history of cancer, the potential effects of cancer and its treatment on muscle health and oxidative balance may have confounded the observed association between OBS and muscular dystrophies (8). Cancer and cancer therapy can lead to significant changes in muscle mass and function, as well as changes in antioxidant status, which may explain the lack of a significant association in this subgroup (41, 42). In the subgroup with diabetes, the complex interplay of metabolic factors associated with diabetes, such as insulin resistance and inflammation, may have influenced the relationship between OBS and muscular dystrophies (9, 43). Furthermore, it has been reported that ischemic heart disease and frailty may be associated with the body’s antioxidant function (44, 45). These metabolic changes may independently affect muscle health and oxidative balance, potentially masking the impact of OBS on muscular dystrophies (9). This nuanced finding highlights the complexity of the relationship between oxidative balance and muscular dystrophies and underscores the need for further investigation of potential interactions in specific subgroups and different levels of molecular organization.
Limitations of this study include reliance on self-reported data, potential unmeasured confounders (such as nutritional supplements), and the cross-sectional nature of the analysis, which limits causal inference. In future research, longitudinal studies and intervention trials may provide further insight into the causal relationship between oxidative balance and muscular dystrophies. In addition, exploring the mechanistic pathways underlying the observed associations and investigating the impact of lifestyle interventions on oxidative balance and muscular dystrophies could improve our understanding of these complex interactions and inform targeted interventions to optimize muscle health in at-risk populations.
5 Conclusion
In conclusion, this study provides evidence of a significant inverse association between OBS and muscular dystrophies in the US population, being significant in the group with a better oxidative balance. The findings highlight the importance of interventions targeting oxidative balance, such as dietary and lifestyle modifications, to promote muscle health and reduce the risk of muscular dystrophies. Future longitudinal studies and intervention trials are needed to further explore the causal relationship between oxidative balance and muscular dystrophies and to inform the development of effective public health strategies for the prevention and treatment of muscular dystrophies.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.cdc.gov/nchs/nhanes/index.htm.
Ethics statement
The studies involving humans were approved by National Center for Health Statistics Ethics Review Board Approval. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
DT: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Validation, Writing – original draft. LL: Data curation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft. YZ: Conceptualization, Funding acquisition, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing, Investigation, Methodology, Project administration, Software.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was financially supported by the Fujian Provincial Health Technology Project (Grant no. 2021QNA007), the Startup Fund for Scientific Research at Fujian Medical University (Grant no. 2020QH1185), and a Scientific Research Project from the Education Department of Fujian Province (Grant no. JAT220127).
Acknowledgments
The National Health and Nutrition Examination Program of the US is gratefully acknowledged for providing data support for this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1465486/full#supplementary-material
References
1. Cruz-Jentoft, AJ, and Sayer, AA. Sarcopenia. Lancet. (2019) 393:2636–46. doi: 10.1016/S0140-6736(19)31138-9
3. Jung, HN, Jung, CH, and Hwang, YC. Sarcopenia in youth. Metabolism. (2023) 144:155557. doi: 10.1016/j.metabol.2023.155557
4. Sayer, AA, and Cruz-Jentoft, A. Sarcopenia definition, diagnosis and treatment: consensus is growing. Age Ageing. (2022) 51:afac220. doi: 10.1093/ageing/afac220
5. Coletta, G, and Phillips, SM. An elusive consensus definition of sarcopenia impedes research and clinical treatment: a narrative review. Ageing Res Rev. (2023) 86:101883. doi: 10.1016/j.arr.2023.101883
6. Shu, X, Lin, T, Wang, H, Zhao, Y, Jiang, T, Peng, X, et al. Diagnosis, prevalence, and mortality of sarcopenia in dialysis patients: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. (2022) 13:145–58. doi: 10.1002/jcsm.12890
7. Gielen, E, Dupont, J, Dejaeger, M, and Laurent, MR. Sarcopenia, osteoporosis and frailty. Metabolism. (2023) 145:155638. doi: 10.1016/j.metabol.2023.155638
8. Williams, GR, Dunne, RF, Giri, S, Shachar, SS, and Caan, BJ. Sarcopenia in the older adult with Cancer. J Clin Oncol. (2021) 39:2068–78. doi: 10.1200/JCO.21.00102
9. Kwon, YJ, Park, HM, and Lee, JH. Inverse association between oxidative balance score and incident type 2 diabetes mellitus. Nutrients. (2023) 15:497. doi: 10.3390/nu15112497
10. Park, HM, Han, TH, Kwon, YJ, and Lee, JH. Oxidative balance score inversely associated with the prevalence and incidence of metabolic syndrome: analysis of two studies of the Korean population. Front Nutr. (2023) 10:1226107. doi: 10.3389/fnut.2023.1226107
11. González-Blanco, L, Bermúdez, M, Bermejo-Millo, JC, Gutiérrez-Rodríguez, J, Solano, JJ, Antuña, E, et al. Cell interactome in sarcopenia during aging. J Cachexia Sarcopenia Muscle. (2022) 13:919–31. doi: 10.1002/jcsm.12937
12. Axelrod, CL, Dantas, WS, and Kirwan, JP. Sarcopenic obesity: emerging mechanisms and therapeutic potential. Metabolism. (2023) 146:155639. doi: 10.1016/j.metabol.2023.155639
13. Damluji, AA, Alfaraidhy, M, AlHajri, N, Rohant, NN, Kumar, M, al Malouf, C, et al. Sarcopenia and cardiovascular diseases. Circulation. (2023) 147:1534–53. doi: 10.1161/CIRCULATIONAHA.123.064071
14. Kalinkovich, A, and Livshits, G. Sarcopenic obesity or obese sarcopenia: a cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev. (2017) 35:200–21. doi: 10.1016/j.arr.2016.09.008
15. Brioche, T, and Lemoine-Morel, S. Oxidative stress, sarcopenia, antioxidant strategies and exercise: molecular aspects. Curr Pharm Des. (2016) 22:2664–78. doi: 10.2174/1381612822666160219120531
16. Migliavacca, E, Tay, SKH, Patel, HP, Sonntag, T, Civiletto, G, McFarlane, C, et al. Mitochondrial oxidative capacity and NAD(+) biosynthesis are reduced in human sarcopenia across ethnicities. Nat Commun. (2019) 10:5808. doi: 10.1038/s41467-019-13694-1
17. Talavera-Rodriguez, I, Fernandez-Lazaro, CI, Hernández-Ruiz, Á, Hershey, MS, Galarregui, C, Sotos-Prieto, M, et al. Association between an oxidative balance score and mortality: a prospective analysis in the SUN cohort. Eur J Nutr. (2023) 62:1667–80. doi: 10.1007/s00394-023-03099-8
18. Xu, W, Mu, D, Wang, Y, Wang, Y, Wang, C, and Zhang, X. Association between oxidative balance score and sarcopenia in US adults: NHANES 2011-2018. Front Nutr. (2024) 11:1342113. doi: 10.3389/fnut.2024.1342113
19. Cai, Z, and Dong, D. Association of the oxidative balance score with sarcopenia among young and middle-aged adults: findings from NHANES 2011-2018. Front Nutr. (2024) 11:1397429. doi: 10.3389/fnut.2024.1397429
20. Zhao, X, Hua, L, Jin, K, Sun, Q, and Wang, R. Association between oxidative balance score and skeletal muscle mass and strength: NHANES from 2011 to 2018. Front Nutr. (2024) 11:1414161. doi: 10.3389/fnut.2024.1414161
21. Studenski, SA, Peters, KW, Alley, DE, Cawthon, PM, McLean, RR, Harris, TB, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. (2014) 69:547–58. doi: 10.1093/gerona/glu010
22. Chen, X, Wang, C, Dong, Z, Luo, H, Ye, C, Li, L, et al. Interplay of sleep patterns and oxidative balance score on total cardiovascular disease risk: insights from the National Health and nutrition examination survey 2005-2018. J Glob Health. (2023) 13:04170. doi: 10.7189/jogh.14.04170
23. Zhang, W, Peng, SF, Chen, L, Chen, HM, Cheng, XE, and Tang, YH. Association between the oxidative balance score and telomere length from the National Health and nutrition examination survey 1999-2002. Oxidative Med Cell Longev. (2022) 2022:1–11. doi: 10.1155/2022/1345071
24. Ticinesi, A, Nouvenne, A, Cerundolo, N, Catania, P, Prati, B, Tana, C, et al. Gut microbiota, muscle mass and function in aging: a focus on physical frailty and sarcopenia. Nutrients. (2019) 11:1633. doi: 10.3390/nu11071633
25. Barazzoni, R, Bischoff, SC, Boirie, Y, Busetto, L, Cederholm, T, Dicker, D, et al. Sarcopenic obesity: time to meet the challenge. Clin Nutr. (2018) 37:1787–93. doi: 10.1016/j.clnu.2018.04.018
26. Meng, SJ, and Yu, LJ. Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci. (2010) 11:1509–26. doi: 10.3390/ijms11041509
27. Cannataro, R, Carbone, L, Petro, JL, Cione, E, Vargas, S, Angulo, H, et al. Sarcopenia: etiology, nutritional approaches, and miRNAs. Int J Mol Sci. (2021) 22:724. doi: 10.3390/ijms22189724
28. Zhang, H, Qi, G, Wang, K, Yang, J, Shen, Y, Yang, X, et al. Oxidative stress: roles in skeletal muscle atrophy. Biochem Pharmacol. (2023) 214:115664. doi: 10.1016/j.bcp.2023.115664
29. Yang, YF, Yang, W, Liao, ZY, Wu, YX, Fan, Z, Guo, A, et al. MICU3 regulates mitochondrial ca(2+)-dependent antioxidant response in skeletal muscle aging. Cell Death Dis. (2021) 12:1115. doi: 10.1038/s41419-021-04400-5
30. Yoshiko, A, Shiozawa, K, Niwa, S, Takahashi, H, Koike, T, Watanabe, K, et al. Association of skeletal muscle oxidative capacity with muscle function, sarcopenia-related exercise performance, and intramuscular adipose tissue in older adults. Geroscience. (2024) 46:2715–27. doi: 10.1007/s11357-023-01043-6
31. Angulo, J, El Assar, M, Álvarez-Bustos, A, and Rodríguez-Mañas, L. Physical activity and exercise: strategies to manage frailty. Redox Biol. (2020) 35:101513. doi: 10.1016/j.redox.2020.101513
32. Cesare, MM, Felice, F, Santini, V, and di Stefano, R. Antioxidants in sport sarcopenia. Nutrients. (2020) 12:869. doi: 10.3390/nu12092869
33. Bagherniya, M, Mahdavi, A, Shokri-Mashhadi, N, Banach, M, von Haehling, S, Johnston, TP, et al. The beneficial therapeutic effects of plant-derived natural products for the treatment of sarcopenia. J Cachexia Sarcopenia Muscle. (2022) 13:2772–90. doi: 10.1002/jcsm.13057
34. Zhang, X, Zhao, Y, Chen, S, and Shao, H. Anti-diabetic drugs and sarcopenia: emerging links, mechanistic insights, and clinical implications. J Cachexia Sarcopenia Muscle. (2021) 12:1368–79. doi: 10.1002/jcsm.12838
35. Cho, AR, Kwon, YJ, and Lee, JH. Oxidative balance score is inversely associated with the incidence of non-alcoholic fatty liver disease. Clin Nutr. (2023) 42:1292–300. doi: 10.1016/j.clnu.2023.06.015
36. Saud Gany, SL, Chin, KY, Tan, JK, Aminuddin, A, and Makpol, S. Curcumin as a therapeutic agent for sarcopenia. Nutrients. (2023) 15:526. doi: 10.3390/nu15112526
37. Batsis, JA, Mackenzie, TA, Jones, JD, Lopez-Jimenez, F, and Bartels, SJ. Sarcopenia, sarcopenic obesity and inflammation: results from the 1999-2004 National Health and nutrition examination survey. Clin Nutr. (2016) 35:1472–83. doi: 10.1016/j.clnu.2016.03.028
38. Xie, WQ, He, M, Yu, DJ, Wu, YX, Wang, XH, Lv, S, et al. Mouse models of sarcopenia: classification and evaluation. J Cachexia Sarcopenia Muscle. (2021) 12:538–54. doi: 10.1002/jcsm.12709
39. Jackson, MJ. Reactive oxygen species in sarcopenia: should we focus on excess oxidative damage or defective redox signalling? Mol Asp Med. (2016) 50:33–40. doi: 10.1016/j.mam.2016.05.002
40. Smith, C, Woessner, MN, Sim, M, and Levinger, I. Sarcopenia definition: does it really matter? Implications for resistance training. Ageing Res Rev. (2022) 78:101617. doi: 10.1016/j.arr.2022.101617
41. Park, YM, Shivappa, N, Petimar, J, Hodgson, M, Nichols, H, Steck, S, et al. Dietary inflammatory potential, oxidative balance score, and risk of breast cancer: findings from the sister study. Int J Cancer. (2021) 149:615–26. doi: 10.1002/ijc.33581
42. Kim, EY, Kim, K, Kim, YS, Ahn, HK, Jeong, YM, Kim, JH, et al. Prevalence of and factors associated with sarcopenia in Korean Cancer survivors: based on data obtained by the Korea National Health and nutrition examination survey (KNHANES) 2008-2011. Nutr Cancer. (2017) 69:394–401. doi: 10.1080/01635581.2017.1267776
43. Hashimoto, Y, Takahashi, F, Okamura, T, Hamaguchi, M, and Fukui, M. Diet, exercise, and pharmacotherapy for sarcopenia in people with diabetes. Metabolism. (2023) 144:155585. doi: 10.1016/j.metabol.2023.155585
44. Wang, R, Gao, K, Wang, L, Gong, X, Wu, Y, Zheng, L, et al. A cross-sectional study exploring the relationship between oxidative balance score and 10-year atherosclerotic cardiovascular disease risk based on the National Health and nutrition examination survey (2011-2020). Diab Vasc Dis Res. (2024) 21:14791641241244658. doi: 10.1177/14791641241244658
Keywords: muscular dystrophies, oxidative balance score, antioxidants, cross-sectional study, NHANES
Citation: Tang D, Lin L and Zheng Y (2024) The association between oxidative balance score and muscular dystrophies. Front. Nutr. 11:1465486. doi: 10.3389/fnut.2024.1465486
Edited by:
Olivia Di Vincenzo, University of Naples Federico II, ItalyReviewed by:
Eduardo Antuña, University of Oviedo, SpainSilvia Giovannini, Catholic University of the Sacred Heart, Rome, Italy
Copyright © 2024 Tang, Lin and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yixin Zheng, eWl4aW56aGVuZ0Bmam11LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Dupeng Tang
Dupeng Tang Lanqing Lin
Lanqing Lin Yixin Zheng
Yixin Zheng