- 1Department of Physical Education and Sports Science, Faculty of Humanities, University of Kashan, Kashan, Iran
- 2Department of Sport Physiology, Dehdasht Branch, Islamic Azad University, Dehdasht, Iran
- 3Department of Exercise Physiology, University of Isfahan, Isfahan, Iran
- 4School of Medicine, Federal University of Uberlandia (UFU), Uberlandia, Minas Gerais, Brazil
- 5Université Clermont Auvergne, CNRS, LaPSCo, Physiological and Psychosocial Stress, CHU Clermont-Ferrand, University Hospital of Clermont-Ferrand, Preventive and Occupational Medicine, Witty Fit, Clermont-Ferrand, France
Background: The effects of exercise training combined with plant-based diets (PBD) on leptin and adiponectin levels have been studied. However, little is known regarding the impact of exercise training combined with PBD on leptin and adiponectin levels in adults with or without chronic diseases.
Methods: PubMed, Web of Science, and Scopus were searched to identify original articles, published until May 2024, to assess the effects of exercise training combined with PBD on leptin and adiponectin levels in adults with or without chronic diseases. Standardized mean differences (SMD) and 95% confidence intervals were calculated using random models.
Results: Nine studies comprising 960 participants with overweight and obesity were included in the current meta-analysis. Exercise training combined with PBD reduced leptin [SMD = -0.33 (95% CI: −0.62 to −0.04); p = 0.025] while increasing adiponectin [SMD = 0.93 (95% CI: 0.12 to 1.74); p = 0.024] levels.
Conclusion: Exercise training combined with PBD is suggested as a non-invasive intervention for reducing leptin while increasing adiponectin levels to control body mass and other disorders related to obesity in adults.
1 Introduction
Obesity is a global phenomenon that has reached epidemic proportions, affecting both developed and developing countries (1). The worldwide prevalence of obesity is escalating swiftly, with a substantial surge in the past years (2). Obesity is associated with several metabolic disorders and chronic diseases, including type 2 diabetes mellitus (T2DM), cardiovascular diseases (CVD), and certain types of cancer (3, 4). Adipose tissue plays a crucial role in the progression of obesity and its associated health risks (4). Leptin and adiponectin are adipokines that modulate energy balance, cardiometabolism, and low-grade inflammation (5). Leptin levels are elevated in individuals with obesity and are associated with leptin resistance, which is related to various metabolic diseases (6). Leptin affects body mass by reducing food intake and increasing energy intake (7). It also plays a role in the reproductive processes, angiogenesis, blood pressure, glucose and lipid metabolism, bone formation, and immune responses (8–12). Leptin levels are positively correlated with fat mass, resulting in elevated levels in individuals with obesity (13). On the other hand, adiponectin regulates energy expenditure, stimulates appetite, and its secretion escalates in tandem with enhanced insulin sensitivity (6). Adiponectin, the adipokine most abundantly secreted by adipose tissue (7), exhibits a decline in serum concentrations with obesity and is also associated with an elevated risk of T2DM and CVD (14, 15).
Drug and surgical interventions (e.g., bariatric surgery or intragastric balloon) for obesity, despite demonstrating effectiveness, frequently entail a multitude of adverse effects and the recurrence of body mass (16–18). Consequently, efforts must be directed toward dietary and lifestyle modifications, especially those that are abundant in plant-based diets (PBD), since those consuming PBD lower their risk for obesity, CVD, T2DM, and other health conditions (19, 20). PBD are capable of potentially exerting an influence on the quantities of leptin and adiponectin in the human body (21, 22). As per the investigation conducted by Lederer et al. (23), plasma adiponectin concentrations were noticeably higher in females who transitioned from an omnivorous to PBD when compared to those following a diet rich in meat; nevertheless, this study did not observe any significant alterations in plasma leptin concentrations. Another study conducted by Menzel et al. (24) failed to identify any notable variances in inflammatory markers, including adiponectin, between vegans and individuals who consume both plant-and animal-based foods.
While vegetarian and vegan diets have the potential to influence leptin and adiponectin levels, further research is needed to fully understand the impacts of PBD. This includes exploring the benefits of reducing animal food consumption rather than completely eliminating it. PBD focus on foods primarily from plants; however, they are not the same as vegetarian or vegan diets since PBD may include meat and dairy, although proportionately predominating in plant sources such as fruits, vegetables, nuts, seeds, oils, whole grains, legumes, and beans (25). However, it is widely acknowledged that the Mediterranean diet and other vegetarian diets (e.g., semi-vegetarian, flexitarian, pescatarian, lacto-ovo vegetarian, and vegan) are often classified as PBD (26).
Many investigations have examined the correlation between physical activity and leptin and adiponectin levels. A narrative review reported that physical activity (only daily physical activity was measured but not any specific physical exercises) elevated adiponectin and decreased leptin levels while simultaneously lowering body mass index (BMI) and body fat percentage (BFP) (27). Physical activity, either on its own or in conjunction with changes in diet or lifestyle, can reduce the levels of leptin in individuals with prediabetes (19) and obesity (28). In a meta-analysis of seven studies by Jadhav et al. (29), physical activity promotion, with or without dietary or lifestyle modification, reduced leptin levels but had no remarkable effects on adiponectin levels in individuals with prediabetes. Seemingly, there is a weak to moderate relationship between aerobic exercise with increased adiponectin levels and decreased leptin levels (30), but it cannot be neglected and ought to be further examined in conjunction with diet, mainly PBD as a cost-effective, low-risk intervention that may improve obesity and cardiometabolic parameters (31, 32). In terms of different forms of physical activity, it was shown that only aerobic exercise but not other forms led to a rise in adiponectin levels and a decrease in leptin levels. To summarize, engaging in physical activity, particularly aerobic exercise, results in increased adiponectin levels and decreased leptin levels in individuals with prediabetes and diabetes (30).
The combination of both exercise training and PBD would be anticipated to produce an additive effect, which may lead to improved health outcomes after the intervention (33–35). The previous study suggests that a combination of exercise training with the Mediterranean diet is more effective than a normal diet for reducing leptin in overweight/obese adults with metabolic syndrome (36). Nevertheless, these results are not consistently reliable. For example, one study did not suggest further increases in adiponectin with exercise training in combination with Mediterranean diet as compared with non-vegetarian diets (37). However, the combined impact of exercise training combined with PBD has not been elucidated in previous meta-analyses. Therefore, the aim of this study was to determine the impact of exercise training combined with PBD on leptin and adiponectin in adults with or without chronic diseases using a systematic review and meta-analysis method.
2 Methods
2.1 Study registration
The current systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines (38) and the Cochrane Handbook of Systematic Reviews of Interventions. The systematic review and meta-analysis was registered prospectively with the International Prospective Register of Systematic Reviews (PROSPERO) under the registration number: CRD42023457202.
2.2 Search strategy
PubMed, Scopus, and Web of Science were the primary databases searched to recognize original research articles, published up until May 2024, using a combination of exercise training and PBD terms and the outcomes (leptin and adiponectin). In addition, reference lists of all included studies and previous relevant meta-analyses (39, 40) were searched to detect records not found during the initial electronic search. The search was limited to articles written in the English language and human studies. There was no limit on publication dates. Studies were limited only based on the age of the participants. Adults were included in the present study. Studies performing PBD combined with exercise training interventions were searched by using the following keywords: “vegetarian diet,” “diet,” “Mediterranean diet,” “vegetarian diet,” “plant-based diet,” “paleolithic diet,” “dietary pattern,” “DASH,” “vegan diet,” “lacto-ovo-vegetarian diet,” and “exercise,” “training,” “exercise training,” “physical activity,” and “adipokine,” “adipocytokine,” “adiponectin,” “leptin.” The search strategy for each database is reported in Supplementary Table S1.
2.3 Eligibility criteria
Studies were eligible for inclusion if they met the following PICO (population, intervention, comparison, and outcome) criteria: (1) For population, adults with or without chronic diseases. (2) For the intervention, studies with exercise training combined with PBD were included. (3) For comparison, the pre- and post-test comparisons for the combined effects of exercise training combined with PBD were required. (4) For outcomes, studies that reported leptin and adiponectin measured using a fully validated method were included.
2.4 Study selection
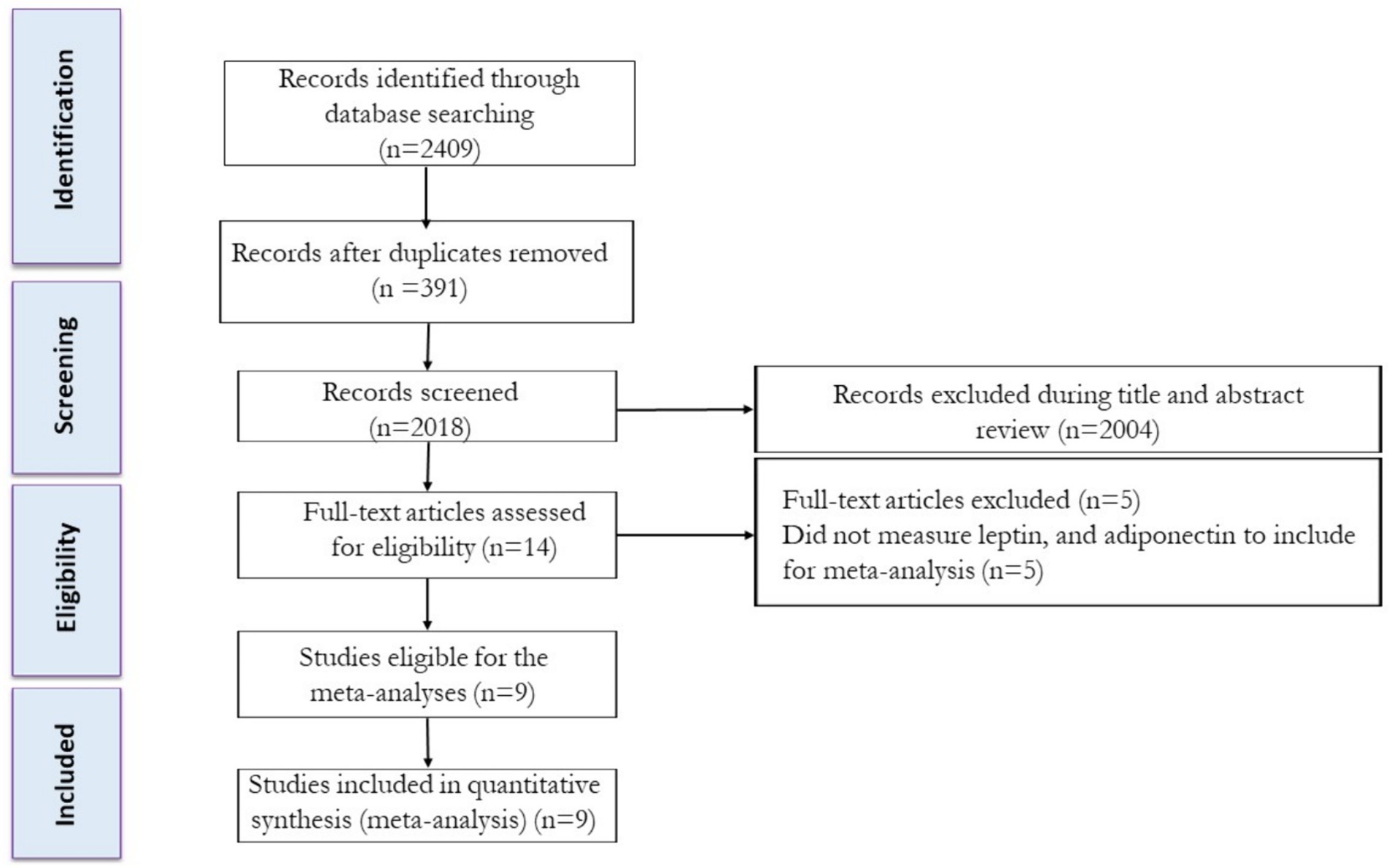
The study selection process is shown in Figure 1. Following the removal of duplicate studies, titles and abstracts of articles were independently assessed, and then the full texts of potentially eligible studies were reviewed by two reviewers to determine eligibility. Any disagreements were resolved through discussion with another author.
Studies that met the following criteria were included in this systematic review and meta-analysis: (1) randomized controlled trials (RCTs), prospective cohort studies, non-randomized controlled trials (non-RCTs); (2) the types of diets included vegetarian, lacto-ovo vegetarian, lacto-vegetarian, ovo-vegetarian diets, Mediterranean diets, Apulian diet (aphypoD); (3) exercise training types were not limited; (4) studies that examined the effect of exercise training combined with PBD on serum or plasma leptin and adiponectin; and (5) studies that presented outcome data of interest as the mean and standard deviations (SD), mean and standard error of the mean (SEM), or quartile and 95% confidence interval (CI) in the intervention group. The exclusion criteria were as follows: (1) vegetarian participants; (2) other interventions along with exercise training and diet have been applied; (3) studies that were animal studies, narrative reviews, commentaries, letters, books, case reports, case studies, pilot studies, and conferences; and (4) studies that were not published in English language.
2.5 Data extraction
Data extraction was completed independently by 2 researchers, and disagreements were resolved through discussion with a third review author. The following study characteristics were extracted: (A) participant information, including age, biological sex, BMI, health status, and sample size; (B) study design; and (C) exercise training characteristics (type, duration, and frequency); (D) diet characteristics and duration of intervention; and I outcome analysis. For each outcome (leptin, and adiponectin), pre-and post-intervention (mean and SD), or mean differences and associated standard deviations of the experimental group were entered into the meta-analyses to generate forest plots. If the means and standard deviations (SDs) were not reported, the SDs were calculated from standard errors of means (SEM), medians and interquartile ranges (IQRs), or means and IQRs (41–43).
2.6 Quality assessment
The risk of bias was evaluated using the Physiotherapy Evidence Database (PEDro) scale. Two items were omitted from the initial 11-item scale due to factors such as similarity of groups at baseline, absence of blinding for participants and intervention providers, and the inability to obscure the allocated dietary conditions during the studies for both participants and intervention providers. The scale used for the current study consisted of nine items: (1) specified eligibility criteria, (2) randomized participant allocation, (3) concealed allocation, (4) blinding of all assessors, (5) evaluated outcomes in 85% of participants, (6) intention-to-treat (ITT) analysis, (7) reporting of statistical comparisons between groups, (8) and point measures and measures of variability (Supplementary Table S2).
2.7 Statistical analysis
Meta-analyses were performed using the Comprehensive Meta-analysis (CMA) software (version 2.0, United States) to compute standardized mean differences (SMD) and 95% CIs for outcomes using random-effects models. Effect sizes were calculated to compare the impact of exercise training combined with PBD on leptin and adiponectin in adults. Heterogeneity was evaluated by using the I2 statistic, and significance was set at p < 0.05. According to Cochrane guidelines, I2 statistics were interpreted as follows: 25% as low, 50% as moderate, and 75% as high heterogeneity. Publication bias was detected through the interpretation of funnel plots. If publication bias was present, Egger’s test was used as a secondary test. Significant publication bias was deemed apparent if p < 0.1 (44). Furthermore, the trim and fill method was used to correct the potential effects of publication bias when visual interpretation of funnel plots demonstrated publication bias.
3 Results
3.1 Included studies
Based on our original search technique, we discovered a combined total of 808 articles in PubMed, 136 articles in Scopus, and 1,465 articles in Web of Science. After excluding duplicate records and evaluating the titles and abstracts, 16 studies were determined to be relevant and necessitated a comprehensive assessment of their complete texts. After conducting a detailed assessment of the full texts, five studies were excluded for the following reasons: did not measure leptin and adiponectin (n = 5) levels. In this systematic review and meta-analysis, a total of nine studies were evaluated, which comprised intervention groups involving the combination of exercise training and PBD. The flow diagram of the systematic literature search is shown in Figure 1.
3.2 Participant characteristics
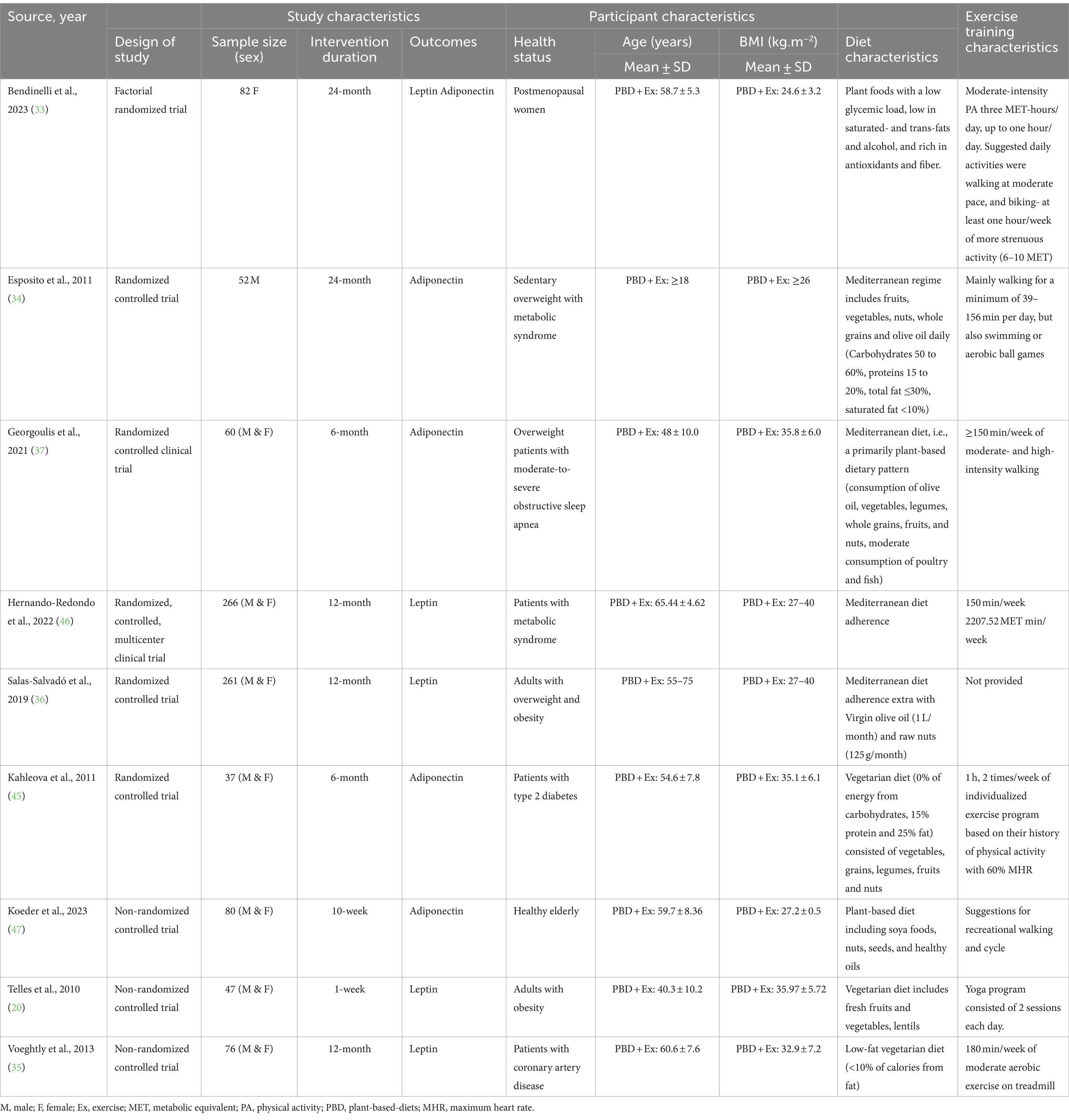
A combined group of 960 adults with different diseases such as metabolic syndrome, obesity, postmenopausal women, obstructive sleep apnea, T2DM, and coronary artery disease were included, with sample sizes ranging from 37 (45) to 266 (46). The average age ranged from 18 years (34) to 75 years (36), while the BMI ranged from 24.6 kg.m−2 (33) to 40 kg.m−2 (36, 46). A total of nine studies were encompassed in the analysis. Among them, seven studies had both males and females (20, 35–37, 45–47), whereas one study only included males (34) and one study only included females (33). The participants’ health status varied across the studies, including healthy elderly participants (47), metabolic syndrome (34, 46), overweight and obesity (20, 36), postmenopausal women (33), obstructive sleep apnea (37), T2DM (45), and coronary artery disease (35). Table 1 provides a more comprehensive breakdown of the characteristics of the participants.
3.3 Intervention characteristics
A combination of exercise training and PBD methodologies was implemented across the included studies in the analysis. Some studies used plant foods with a low glycemic load, low in saturated fats and alcohol, and rich in antioxidants and fiber (33), PBD including soya foods, nuts, seeds, and healthy oils (47), and others used Mediterranean diet including fruits, vegetables, nuts, whole grains, and olive oil daily (34, 36, 37, 46). Additionally, the length of the interventions varied, ranging from 1 week (20) to 24 months (33–37, 45–47). The studies incorporated various forms of exercise training interventions, including moderate-intensity physical activity, moderate- and high-intensity walking, yoga programs, moderate aerobic exercise on a treadmill, swimming or aerobic ball games, or cycling. In most studies, participants exercised 150 min/week. Detailed information about the intervention characteristics can be found in Table 1.
3.4 Meta-analysis
3.4.1 The effects of PBD combined with exercise training on leptin in adults
Based on five intervention arms, PBD combined with exercise training had significantly lower serum concentrations of leptin [SMD = -0.33 (95% CI: −0.62 to −0.04) and p = 0.025] (Figure 2). The studies included in the analysis demonstrated high heterogeneity (I2 = 92.19%, p = 0.001). The absence of publication bias was supported by the results of funnel plots and Egger’s test (p = 0.90). Given that the result of the funnel plots and Egger’s test was not significant, the trim and fill method was not performed.
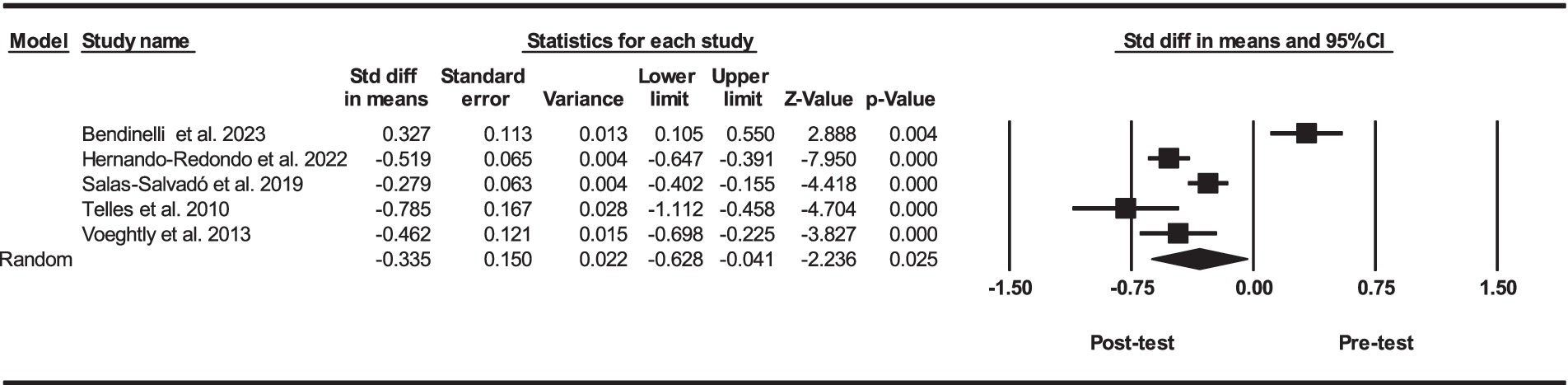
Figure 2. Forest plot of the effects of exercise training combined with PBD on serum leptin. Data are reported as SMD (95% confidence limits). SMD, standardized mean differences.
3.4.2 The effects of PBD combined with exercise training on adiponectin in adults
Based on five intervention arms, PBD combined with exercise training had significantly higher serum concentrations of adiponectin [SMD = 0.93 (95% CI: 0.12 to 1.74) and p = 0.024] (Figure 3). The studies included in the analysis demonstrated high heterogeneity (I2 = 96.84%, p = 0.001). The existence of publication bias was supported by the results of funnel plots and Egger’s test (p = 0.003). In addition, the trim and fill results showed that the overall changes were as follows: adiponectin [SMD = 0.93 (95% CI: 0.12 to 1.74)]. The results of adiponectin did not change [SMD = 0.93 (95% CI: 0.12 to 1.74)]. Since no bias is present in these simulations all “missing” studies detected are because of chance funnel plot asymmetry.
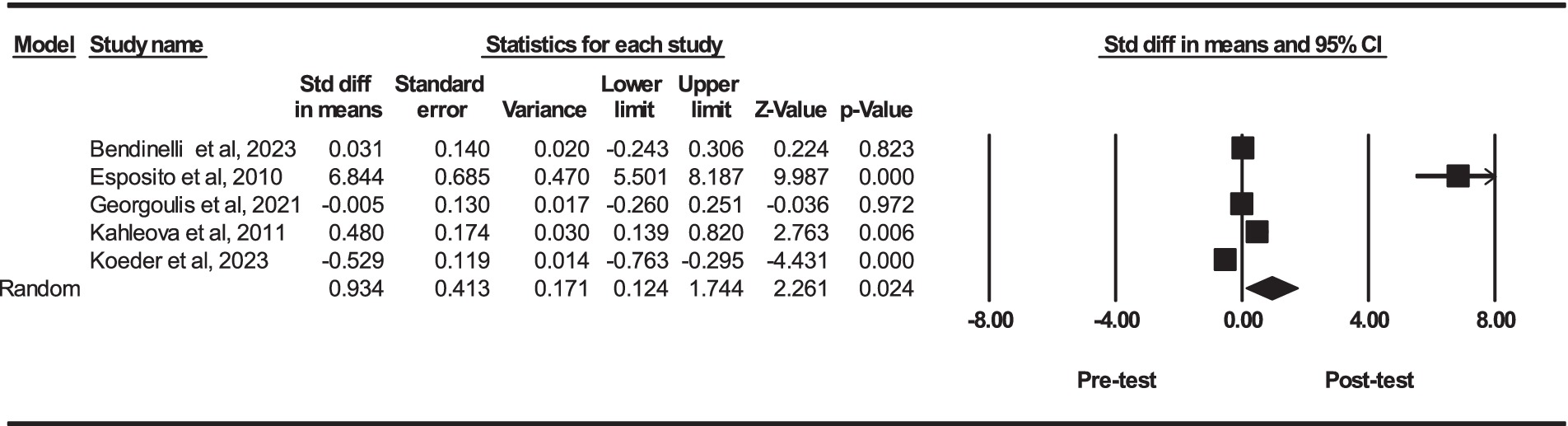
Figure 3. Forest plot of the effects of exercise training combined with PBD on serum adiponectin. Data are reported as SMD (95% confidence limits). SMD, standardized mean differences.
3.5 Quality assessment
The PEDro tool was used to assess the methodological quality of each individual study, and their scores varied between 3 and 9 out of a possible maximum of 9 points. One study had a score of 9 (37), one study had a score of 8 (33), two studies had scores of 6 (36, 45), four studies had 5 (20, 34, 35, 46), and one study had a score of 3 (47). Most of the PEDro scores were lowered due to three items (concealed allocation, blinding of all assessors, and intention-to-treat analysis). The details of the quality analysis are shown in Supplementary Table S2.
4 Discussion
The aim of this meta-analysis was to investigate the effect of PBD combined with exercise training on leptin and adiponectin levels in adults with or without chronic diseases. The main findings of the study showed that exercise training combined with PBD caused a significant decrease in leptin while a significant increase in the levels of adiponectin in adults. Many studies support these findings (20, 33, 35, 36, 46). Our findings have important implications for understanding the effects of lifestyle modification on leptin regulation and obesity. Leptin is a hormone secreted by adipose tissue and acts centrally in the hypothalamus to regulate energy balance and appetite (48). Higher levels of leptin indicate that the hypothalamus has sufficient fat stores to suppress appetite and increase energy expenditure. Individuals with obesity typically have chronically elevated leptin levels, indicating leptin resistance, in which increased leptin signaling does not translate into appropriate appetite suppressant effects (49). The reduction in leptin observed with dietary and exercise training interventions may contribute to sensitizing the brain to the effects of leptin and better body mass control.
Several mechanisms may underlie the leptin-lowering effects of exercise training. For instance, exercise training has been shown to increase catecholamines such as epinephrine and norepinephrine, which inhibit the production and secretion of leptin in adipose tissue (50). Exercise-induced activation of the sympathetic nervous system can also directly inhibit transcription (51). In addition, the anti-inflammatory effects of exercise training may reduce leptin production, as inflammatory cytokines stimulate leptin secretion (52).
PBD are lower in fat and higher in fiber than omnivorous diets. Inadequate dietary fat consumption significantly contributes to the reduction of circulating leptin, given that acute increases in adipose triglycerides and plasma fatty acids modulate leptin secretion (53). Fiber has also been shown to bind leptin in the small intestine and inhibit its absorption. Additionally, lower amounts of protein in PBD may affect leptin levels, as protein strongly stimulates leptin secretion (22, 54).
The synergistic effect of physical activity and PBD on leptin reduction may be explained by the reduction of fat cell size and total body fat mass (39). Exercise training and negative energy balance activate triglycerides stored in fat cells and reduce fat diameter. The mass of leptin-producing adipose tissue also decreases with body mass loss caused by diet and exercise training. These morphological changes in fat probably underlie the concomitant decrease in plasma leptin levels (55).
These findings have clinical implications for the treatment of obesity. Increased leptin levels are associated with an increased risk of metabolic diseases, cardiovascular events, and some cancers (56). The leptin-lowering effects of aerobic exercise and PBD demonstrated in this meta-analysis may contribute to the reduction in morbidity and mortality observed with these lifestyle changes. For adults with obesity, combining regular aerobic exercise with a Mediterranean diet may be an effective strategy not only for body mass loss but also for improving leptin sensitivity and metabolic health (46).
The results of the current meta-analyses also showed that a combination of PBD with exercise training led to remarkable increases in adiponectin levels in adults. Other studies also support these findings (33, 34, 37, 45, 47). Several mechanisms may contribute to this upregulation of adiponectin by exercise training and PBD. First, exercise training is a potent stimulus for increased adiponectin production and release from adipose tissue. Skeletal muscle contractions during aerobic exercise, such as walking, running, or cycling, stimulate the release of adiponectin into the circulation (57). This may be mediated by the activation of p38 mitogen-activated protein kinase (MAPK) signaling in adipocytes in response to muscle contraction (58). In addition, exercise training reduces inflammation within visceral fat, which may reduce transcriptional and translational repression of adiponectin (59).
Second, PBD are rich in nutrients and bioactive compounds that have been shown to increase adiponectin levels. Specifically, high fiber intake from whole grains, fruits, vegetables, and legumes regulates adiponectin by increasing insulin sensitivity and suppressing inflammatory signals (60). PBD also consist of adequate vitamin C, magnesium, and omega-3 fatty acids from plant sources, all of which are associated with increased adiponectin production (22). Eliminating meat in Mediterranean diets may help by removing pro-inflammatory factors and improving oxidative balance (46).
Together, these synergistic mechanisms induced by aerobic exercise and PBD significantly increase adiponectin levels. The large effect size shown in this meta-analysis is clinically significant, as higher circulating adiponectin is independently associated with a variety of health benefits. Prospective studies show that increased adiponectin reduces the risk of obesity, T2DM, CVD, and some cancers (61). Adiponectin exerts these protective effects through its insulin-sensitizing, anti-inflammatory, and anti-atherogenic properties (62).
Given the increasing prevalence of hypoadiponectinemia in many populations, our findings have important implications. Adiponectin levels have declined significantly over the past few decades, likely due to increased levels of physical inactivity and unhealthy diets high in saturated fat and processed foods (63). Low adiponectin is now thought to be an independent risk factor for insulin resistance, metabolic syndrome, and CVD (64). Therefore, lifestyle modification aimed at increasing adiponectin may provide a powerful therapeutic strategy for the prevention and management of several interrelated chronic diseases.
In summary, the present meta-analysis study showed that a combination of exercise training and PBD decreased circulating leptin levels while increasing adiponectin. The decrease in leptin and increase in adiponectin may be attributed to the decrease in fat mass, as well as the direct effect of exercise training and diet composition on leptin production and secretion. Increasing chronic adiponectin and leptin levels through lifestyle modification may improve leptin sensitivity and obesity-related diseases. Further research is needed to identify long-term clinical effects and determine definitive strategies.
Overall, findings are different regarding the effect of PBD and physical activity on leptin and adiponectin levels. One study showed that PBD had no effect on leptin and adiponectin levels (21), while the other study showed that a short-term intervention with a vegetarian diet resulted in improved plasma concentrations of adiponectin and leptin (23). Certain dietary components that are lower in PBD diets, such as energy intake, saturated fat, heme iron, and red and processed meat, may influence the risk of metabolic syndrome. In addition, PBD contain more fruits, vegetables, and fiber, which protect against metabolic syndrome. Additionally, intensive lifestyle changes, including PBD diet, have been associated with a reduced risk of metabolic syndrome (65).
This meta-analysis has several strengths that lend credence to the results. To the best of our knowledge, the current study is the most comprehensive meta-analysis addressing the effects of PBD combined with exercise training on leptin and adiponectin levels in adults with or without chronic diseases. We minimized the potential for bias in the review process by executing a comprehensive search of the literature and also conducting a systematic review and reporting the meta-analytic results according to the PRISMA guidelines. Nevertheless, some potential limitations should be considered when interpreting these findings. First, diet and exercise programs varied somewhat across trials, which may introduce heterogeneity. Additional standardized intervention studies in different populations could further substantiate the effects on adiponectin. Also, some studies were conducted on healthy people and some on adults with overweight and obesity, which may distort the homogeneity of the results to some extent. One of the reasons for the high heterogeneity can be attributed to the different health characteristics of participants, such as menopause, metabolic syndrome, obstructive sleep apnea, obesity, or coronary artery disease, and the duration of the interventions. Conducting meta-regressions could potentially offer more robust findings in this case. However, the limited number of studies that provided data on BFP and BMI precluded us from conducting meta-regression or subgroup analyses to examine the relationship between leptin and adiponectin with BFP or BMI. Also, due to the small number of studies, it was not possible to perform subgroup analysis based on the type, duration, and intensity of exercise.
5 Conclusion
The findings of the present systematic review and meta-analysis show the important role of PBD combined with exercise training in improving leptin and adiponectin. Exercise training combined with PBD are suggested as non-invasive interventions for reducing leptin and increasing adiponectin levels to control body mass and other disorders related to obesity in adults with or without chronic diseases.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
FK: Conceptualization, Data curation, Investigation, Methodology, Project administration, Software, Supervision, Writing – original draft, Writing – review & editing. RF: Conceptualization, Data curation, Investigation, Writing – original draft. RB: Writing – review & editing, Writing – original draft. HS: Writing – review & editing. FD: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1465378/full#supplementary-material
References
1. Caballero, B. The global epidemic of obesity: an overview. Epidemiol Rev. (2007) 29:1–5. doi: 10.1093/epirev/mxm012
2. Hruby, A, and Hu, FB. The epidemiology of obesity: a big picture. PharmacoEconomics. (2015) 33:673–89. doi: 10.1007/s40273-014-0243-x
3. Tremblay, A, Clinchamps, M, Pereira, B, Courteix, D, Lesourd, B, Chapier, R, et al. Dietary fibres and the management of obesity and metabolic syndrome: the RESOLVE study. Nutrients. (2020) 12:2911. doi: 10.3390/nu12102911
4. Khanna, D, Khanna, S, Khanna, P, Kahar, P, and Patel, BM. Obesity: a chronic low-grade inflammation and its markers. Cureus. (2022):14. doi: 10.7759/cureus.22711
5. Clemente-Suárez, VJ, Redondo-Flórez, L, Beltrán-Velasco, AI, Martín-Rodríguez, A, Martínez-Guardado, I, Navarro-Jiménez, E, et al. The role of Adipokines in health and disease. Biomedicines. (2023) 11:1290. doi: 10.3390/biomedicines11051290
6. Nikolettos, K, Nikolettos, N, Vlahos, N, Pagonopoulou, O, and Asimakopoulos, B. Role of leptin, adiponectin, and kisspeptin in polycystic ovarian syndrome pathogenesis. Minerva Obstet Gynecol. (2023) 75:460–7. doi: 10.23736/S2724-606X.22.05139-9
7. Li, Y, Zheng, H, Yang, J, Zhang, B, Xing, X, Zhang, Z, et al. Association of genetic variants in leptin, leptin receptor and adiponectin with hypertension risk and circulating leptin/adiponectin changes. Gene. (2023) 853:147080. doi: 10.1016/j.gene.2022.147080
8. Anagnostoulis, S, Karayiannakis, AJ, Lambropoulou, M, Efthimiadou, A, Polychronidis, A, and Simopoulos, C. Human leptin induces angiogenesis in vivo. Cytokine. (2008) 42:353–7. doi: 10.1016/j.cyto.2008.03.009
9. Beltowski, J. Role of leptin in blood pressure regulation and arterial hypertension. J Hypertens. (2006) 24:789–801. doi: 10.1097/01.hjh.0000222743.06584.66
10. Minokoshi, Y, Toda, C, and Okamoto, S. Regulatory role of leptin in glucose and lipid metabolism in skeletal muscle. Indian J Endocrinol Metab. (2012) 16:S562–S8. doi: 10.4103/2230-8210.105573
11. Turner, RT, Kalra, SP, Wong, CP, Philbrick, KA, Lindenmaier, LB, Boghossian, S, et al. Peripheral leptin regulates bone formation. J Bone Miner Res. (2013) 28:22–34. doi: 10.1002/jbmr.1734
12. Bernotiene, E, Palmer, G, and Gabay, C. The role of leptin in innate and adaptive immune responses. Arthritis Res. (2006) 8:217–10. doi: 10.1186/ar2004
13. Ye, Y, Wu, P, Wang, Y, Yang, X, Ye, Y, Yuan, J, et al. Adiponectin, leptin, and leptin/adiponectin ratio with risk of gestational diabetes mellitus: a prospective nested case-control study among Chinese women. Diabetes Res Clin Pract. (2022) 191:110039. doi: 10.1016/j.diabres.2022.110039
14. Mohammadi, S, Arefhosseini, SR, Ebrahimi-Mamaeghani, M, Fallah, P, and Bazi, Z. Adiponectin as a potential biomarker of vascular disease. Vasc Health Risk Manag. (2015) 11:55–70. doi: 10.2147/VHRM.S48753
15. Spranger, J, Kroke, A, Möhlig, M, Bergmann, MM, Ristow, M, Boeing, H, et al. Adiponectin and protection against type 2 diabetes mellitus. Lancet. (2003) 361:226–8. doi: 10.1016/S0140-6736(03)12255-6
16. Tate, CM, and Geliebter, A. Intragastric balloon treatment for obesity: review of recent studies. Adv Ther. (2017) 34:1859–75. doi: 10.1007/s12325-017-0562-3
17. Wolfe, BM, Kvach, E, and Eckel, RH. Treatment of obesity: weight loss and bariatric surgery. Circ Res. (2016) 118:1844–55. doi: 10.1161/CIRCRESAHA.116.307591
18. Haghighat, N, Ashtary-Larky, D, Bagheri, R, Aghakhani, L, Asbaghi, O, Amini, M, et al. Preservation of fat-free mass in the first year after bariatric surgery: a systematic review and meta-analysis of 122 studies and 10,758 participants. Surg Obes Relat Dis. (2022) 18:964–82. doi: 10.1016/j.soard.2022.02.022
19. Chang, S-L, Nfor, ON, Ho, C-C, Lee, K-J, Lu, W-Y, Lung, C-C, et al. Combination of exercise and vegetarian diet: relationship with high density-lipoprotein cholesterol in Taiwanese adults based on MTHFR rs1801133 polymorphism. Nutrients. (2020) 12:1564. doi: 10.3390/nu12061564
20. Telles, S, Naveen, VK, Balkrishna, A, and Kumar, S. Short term health impact of a yoga and diet change program on obesity. Med Sci Monit. (2010) 16:Cr35–40.
21. Eichelmann, F, Schwingshackl, L, Fedirko, V, and Aleksandrova, K. Effect of plant-based diets on obesity-related inflammatory profiles: a systematic review and meta-analysis of intervention trials. Obes Rev. (2016) 17:1067–79. doi: 10.1111/obr.12439
22. Gogga, P, Janczy, A, Szupryczyńska, N, Śliwińska, A, Kochan, Z, and Malgorzewicz, S. Plant-based diets contribute to lower circulating leptin in healthy subjects independently of BMI. Acta Biochim Pol. (2022) 69:879–82. doi: 10.18388/abp.2020_6388
23. Lederer, A-K, Storz, MA, Huber, R, Hannibal, L, and Neumann, E. Plasma leptin and adiponectin after a 4-week vegan diet: a randomized-controlled pilot trial in healthy participants. Int J Environ Res Public Health. (2022) 19:11370. doi: 10.3390/ijerph191811370
24. Menzel, J, Biemann, R, Longree, A, Isermann, B, Mai, K, Schulze, MB, et al. Associations of a vegan diet with inflammatory biomarkers. Sci Rep. (2020) 10:1933. doi: 10.1038/s41598-020-58875-x
25. Hargreaves, SM, Rosenfeld, DL, Moreira, AVB, and Zandonadi, RP. Plant-based and vegetarian diets: an overview and definition of these dietary patterns. Eur J Nutr. (2023) 62:1109–21. doi: 10.1007/s00394-023-03086-z
26. Craig, WJ, Mangels, AR, Fresán, U, Marsh, K, Miles, FL, Saunders, AV, et al. The safe and effective use of plant-based diets with guidelines for health professionals. Nutrients. (2021) 13:4144. doi: 10.3390/nu13114144
27. Nurnazahiah, A, Lua, PL, and Shahril, MR. Adiponectin, leptin and objectively measured physical activity in adults: a narrative review. Malays J Med Sci. (2016) 23:7–24. doi: 10.21315/mjms2016.23.6.2
28. Eskandari, M, Hooshmand Moghadam, B, Bagheri, R, Ashtary-Larky, D, Eskandari, E, Nordvall, M, et al. Effects of interval jump rope exercise combined with dark chocolate supplementation on inflammatory adipokine, cytokine concentrations, and body composition in obese adolescent boys. Nutrients. (2020) 12:3011. doi: 10.3390/nu12103011
29. Jadhav, RA, Maiya, GA, Hombali, A, Umakanth, S, and Shivashankar, K. Effect of physical activity promotion on adiponectin, leptin and other inflammatory markers in prediabetes: a systematic review and meta-analysis of randomized controlled trials. Acta Diabetol. (2021) 58:419–29. doi: 10.1007/s00592-020-01626-1
30. Becic, T, Studenik, C, and Hoffmann, G. Exercise increases adiponectin and reduces leptin levels in prediabetic and diabetic individuals: systematic review and meta-analysis of randomized controlled trials. Med Sci. (2018) 6:97. doi: 10.3390/medsci6040097
31. Tuso, PJ, Ismail, MH, Ha, BP, and Bartolotto, C. Nutritional update for physicians: plant-based diets. Perm J. (2013) 17:61–6. doi: 10.7812/TPP/12-085
32. Li, H, Zeng, X, Wang, Y, Zhang, Z, Zhu, Y, Li, X, et al. A prospective study of healthful and unhealthful plant-based diet and risk of overall and cause-specific mortality. Eur J Nutr. (2022) 61:387–98. doi: 10.1007/s00394-021-02660-7
33. Bendinelli, B, Masala, G, Bella, CD, Assedi, M, Benagiano, M, Pratesi, S, et al. Adipocytokine plasma level changes in a 24-month dietary and physical activity randomised intervention trial in postmenopausal women. Eur J Nutr. (2023) 62:1185–94. doi: 10.1007/s00394-022-03055-y
34. Esposito, K, di Palo, C, Maiorino, MI, Petrizzo, M, Bellastella, G, Siniscalchi, I, et al. Long-term effect of Mediterranean-style diet and calorie restriction on biomarkers of longevity and oxidative stress in overweight men. Cardiol Res Pract. (2011) 2011:293916:1–5. doi: 10.4061/2011/293916
35. Voeghtly, LM, Neatrour, DM, Decewicz, DJ, Burke, A, Haberkorn, MJ, Lechak, F, et al. Cardiometabolic risk reduction in an intensive cardiovascular health program. Nutr Metab Cardiovasc Dis. (2013) 23:662–9. doi: 10.1016/j.numecd.2012.01.012
36. Salas-Salvadó, J, Díaz-López, A, Ruiz-Canela, M, Basora, J, Fitó, M, Corella, D, et al. Effect of a lifestyle intervention program with energy-restricted Mediterranean diet and exercise on weight loss and cardiovascular risk factors: one-year results of the PREDIMED-plus trial. Diabetes Care. (2019) 42:777–88. doi: 10.2337/dc18-0836
37. Georgoulis, M, Yiannakouris, N, Tenta, R, Fragopoulou, E, Kechribari, I, Lamprou, K, et al. A weight-loss Mediterranean diet/lifestyle intervention ameliorates inflammation and oxidative stress in patients with obstructive sleep apnea: results of the "MIMOSA" randomized clinical trial. Eur J Nutr. (2021) 60:3799–810. doi: 10.1007/s00394-021-02552-w
38. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DGGroup* P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. (2009) 151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135
39. Long, Y, Ye, H, Yang, J, Tao, X, Xie, H, Zhang, J, et al. Effects of a vegetarian diet combined with aerobic exercise on glycemic control, insulin resistance, and body composition: a systematic review and meta-analysis. Eat Weight Disord. (2023) 28:9. doi: 10.1007/s40519-023-01536-5
40. Niu, Y, Cao, H, Zhou, H, Cao, J, and Wang, Z. Effects of a vegetarian diet combined with exercise on lipid profiles and blood pressure: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. (2022) 64:2289–2303. doi: 10.1080/10408398.2022.2122923
41. Wan, X, Wang, W, Liu, J, and Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:1–13. doi: 10.1186/1471-2288-14-135
42. Higgins, JP. Cochrane handbook for systematic reviews of interventions version 5.0. 1 The Cochrane Collaboration (2008).
43. Hozo, SP, Djulbegovic, B, and Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. (2005) 5:1–10. doi: 10.1186/1471-2288-5-13
44. Egger, M, Smith, GD, Schneider, M, and Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
45. Kahleova, H, Matoulek, M, Malinska, H, Oliyarnik, O, Kazdova, L, Neskudla, T, et al. Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with type 2 diabetes. Diabet Med. (2011) 28:549–59. doi: 10.1111/j.1464-5491.2010.03209.x
46. Hernando-Redondo, J, Toloba, A, Benaiges, D, Salas-Salvadó, J, Martínez-Gonzalez, MA, Corella, D, et al. Mid- and long-term changes in satiety-related hormones, lipid and glucose metabolism, and inflammation after a Mediterranean diet intervention with the goal of losing weight: a randomized, clinical trial. Front Nutr. (2022) 9:950900. doi: 10.3389/fnut.2022.950900
47. Koeder, C, Anand, C, Husain, S, Kranz, RM, Schoch, N, Alzughayyar, D, et al. Exploratory analysis of the effect of a controlled lifestyle intervention on inflammatory markers – the healthy lifestyle community Programme (cohort 2). BMC Nutr. (2023) 9:25. doi: 10.1186/s40795-023-00684-2
48. Friedman, JM, and Halaas, JL. Leptin and the regulation of body weight in mammals. Nature. (1998) 395:763–70. doi: 10.1038/27376
49. Myers, MG Jr, Leibel, RL, Seeley, RJ, and Schwartz, MW. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab. (2010) 21:643–51. doi: 10.1016/j.tem.2010.08.002
50. Essig, DA, Alderson, NL, Ferguson, MA, Bartoli, WP, and Durstine, JL. Delayed effects of exercise on the plasma leptin concentration. Metabolism. (2000) 49:395–9. doi: 10.1016/S0026-0495(00)90396-2
51. Daniela, M, Catalina, L, Ilie, O, Paula, M, Daniel-Andrei, I, and Ioana, B. Effects of exercise training on the autonomic nervous system with a focus on anti-inflammatory and antioxidants effects. Antioxidants (Basel). (2022) 11:350. doi: 10.3390/antiox11020350
52. Pérez-Pérez, A, Sánchez-Jiménez, F, Vilariño-García, T, and Sánchez-Margalet, V. Role of leptin in inflammation and vice versa. Int J Mol Sci. (2020) 21:5887. doi: 10.3390/ijms21165887
53. Izadi, V, Saraf-Bank, S, and Azadbakht, L. Dietary intakes and leptin concentrations. ARYA Atheroscler. (2014) 10:266–72.
54. Kim, MH, and Kim, H. Role of leptin in the digestive system. Front Pharmacol. (2021) 12:660040. doi: 10.3389/fphar.2021.660040
55. Lin, D, Sturgeon, KM, Gordon, BR, Brown, JC, Sears, DD, Sarwer, DB, et al. WISER survivor trial: combined effect of exercise and weight loss interventions on adiponectin and leptin levels in breast Cancer survivors with overweight or obesity. Nutrients. (2023) 15:3453. doi: 10.3390/nu15153453
56. Khanbabaei, N, Mozafar Saadati, H, Valizadeh Shahbazloo, S, Hoseinpoor, R, Naderi, SH, Taghvamanesh, R, et al. Association of serum leptin with angiographically proven cardiovascular disease and with components of the metabolic syndrome: a cross-sectional study in East Azerbaijan. Cardiovasc Endocrinol Metab. (2021) 10:45–50. doi: 10.1097/XCE.0000000000000227
57. Simpson, KA, and Singh, MAF. Effects of exercise on adiponectin: a systematic review. Obesity. (2008) 16:241–56. doi: 10.1038/oby.2007.53
58. Qiao, L, Kinney, B, Yoo, H s, Lee, B, Schaack, J, and Shao, J. Adiponectin increases skeletal muscle mitochondrial biogenesis by suppressing mitogen-activated protein kinase phosphatase-1. Diabetes. (2012) 61:1463–70. doi: 10.2337/db11-1475
59. You, T, Berman, DM, Ryan, AS, and Nicklas, BJ. Effects of hypocaloric diet and exercise training on inflammation and adipocyte lipolysis in obese postmenopausal women. J Clin Endocrinol Metab. (2004) 89:1739–46. doi: 10.1210/jc.2003-031310
60. Neyrinck, AM, Van Hee, VF, Piront, N, De Backer, F, Toussaint, O, Cani, PD, et al. Wheat-derived arabinoxylan oligosaccharides with prebiotic effect increase satietogenic gut peptides and reduce metabolic endotoxemia in diet-induced obese mice. Nutr. Diabetes. (2012) 2:e28. doi: 10.1038/nutd.2011.24
61. Khoramipour, K, Chamari, K, Hekmatikar, AA, Ziyaiyan, A, Taherkhani, S, Elguindy, NM, et al. Adiponectin: structure, physiological functions, role in diseases, and effects of nutrition. Nutrients. (2021) 13:1180. doi: 10.3390/nu13041180
62. Oh, DK, Ciaraldi, T, and Henry, RR. Adiponectin in health and disease. Diabetes Obes Metab. (2007) 9:282–9. doi: 10.1111/j.1463-1326.2006.00610.x
63. Janiszewska, J, Ostrowska, J, and Szostak-Węgierek, D. The influence of nutrition on adiponectin-a narrative review. Nutrients. (2021) 13:1394. doi: 10.3390/nu13051394
64. Muratsu, J, Kamide, K, Fujimoto, T, Takeya, Y, Sugimoto, K, Taniyama, Y, et al. The combination of high levels of adiponectin and insulin resistance are affected by aging in non-obese old peoples. Front Endocrinol. (2022) 12:805244. doi: 10.3389/fendo.2021.805244
Keywords: metabolic syndrome, adipokines, leptin, metabolic health, obesity
Citation: Kazeminasab F, Fatemi R, Bagheri R, Santos HO and Dutheil F (2024) Effects of plant-based diets combined with exercise training on leptin and adiponectin levels in adults with or without chronic diseases: a systematic review and meta-analysis of clinical studies. Front. Nutr. 11:1465378. doi: 10.3389/fnut.2024.1465378
Edited by:
Mark Elisabeth Willems, University of Chichester, United KingdomReviewed by:
Claire Joanne Stocker, Aston University, United KingdomWeibing Ye, Zhejiang Normal University, China
Sajjad Moradi, Isfahan University of Medical Sciences, Iran
Copyright © 2024 Kazeminasab, Fatemi, Bagheri, Santos and Dutheil. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fatemeh Kazeminasab, ZmthemVtaW5hc2FiQGthc2hhbnUuYWMuaXI=
 Fatemeh Kazeminasab
Fatemeh Kazeminasab Rouholah Fatemi2
Rouholah Fatemi2 Reza Bagheri
Reza Bagheri Heitor O. Santos
Heitor O. Santos Fred Dutheil
Fred Dutheil