- 1Department of Oncology Surgery, Fuzhou Hospital of Traditional Chinese Medicine Affiliated to Fujian University of Traditional Chinese Medicine, Fuzhou, China
- 2Department of Physiology, College of Medicine, Chosun University, Gwangju, Republic of Korea
- 3College of Acupuncture and Orthopedics, Hubei University of Chinese Medicine, Wuhan, China
Background: While prior research has established a correlation between dietary choline intake and bone density in the elderly, the relationship in adolescents remains ambiguous. This study seeks to examine the association between dietary choline intake and bone density in American adolescents.
Methods: Data from the National Health and Nutrition Examination Survey (NHANES) for 2005 to 2018 were used in this study, encompassing participants aged 12–19 years. The relationship between dietary choline intake and bone density was assessed using multivariate linear regression models and restricted cubic spline (RCS) models. Subgroup analyses were also performed to investigate differences across various subgroups.
Results: 3,800 participants with an average age of 15 years were included in this study. After adjusting for relevant confounding factors, a positive correlation was observed between dietary choline intake and total bone density in adolescents (95% CI: 0.03–0.17, p = 0.010). Gender-specific analysis indicated a significant positive correlation between dietary choline intake and total bone density in males (95% CI: 0.07–0.23, p < 0.001), while no significant correlation was found in females (95% CI: −0.19 to 0.09, p = 0.500). The stratified analysis revealed that the positive association was more pronounced in males and non-Hispanic whites (interaction p < 0.05). The restricted cubic spline model demonstrated a linear positive correlation between dietary choline intake and total bone density.
Conclusion: This study demonstrates that dietary choline intake levels are positively correlated with bone density in adolescents, with this association being specific to males.
1 Introduction
Osteoporosis is a systemic bone disease caused by multiple factors, characterized by reduced bone density and quality, impaired bone microstructure, increased bone fragility, and a higher risk of fractures (1, 2). Epidemiological studies from China indicate that approximately 60.2 million people nationwide suffer from osteoporosis. Among those aged 50 and above, the prevalence is 6.46% in men and 29.13% in women, with these rates continuing to rise as the population ages (3, 4). The high fracture risk associated with osteoporosis significantly threatens individual quality of life and socioeconomic development (5), highlighting the critical importance of enhanced prevention and treatment efforts.
Adolescence is a crucial period for bone growth, with more than half of peak bone mass being formed during this time (6, 7). Research indicates that the peak bone mass achieved during adolescence is a key determinant of fracture and osteoporosis risk in later life (8, 9). Interventions during this period can effectively prevent osteoporosis and fractures in adulthood. While genetics play a vital role in determining peak bone mass, dietary nutrition can modify the genetic potential for bone growth and is essential for the skeletal development of adolescents (10–12).
Mojgan and colleagues have shown that nutrients such as calcium, vitamin D and dietary antioxidants play an important role in preventing chronic diseases such as blood pressure and bone disorders (13, 14). Choline, an essential nutrient, participates in numerous physiological functions in the human body, such as acetylcholine synthesis, neurotransmission, cell membrane stability, and lipid metabolism, all crucial for maintaining health and well-being (15, 16). Existing research has established correlations between dietary choline levels and the risk of osteoporosis in older adults (17). However, studies examining the relationship between dietary choline and bone density are still limited, particularly in adolescent populations.
Therefore, this study utilized the National Health and Nutrition Examination Survey (NHANES) database to investigate the relationship between dietary choline intake and bone density in American adolescents.
2 Methods
The National Health and Nutrition Examination Survey (NHANES) is a nationwide health survey conducted jointly by the Centers for Disease Control and Prevention (CDC) and the National Center for Health Statistics (NCHS). It employs a complex sampling design to select participants and collects data through questionnaires, physical examinations, and laboratory tests. NHANES aims to evaluate the health and nutritional status of the non-institutionalized U.S. population. Both the research data and survey methods of NHANES are openly accessible on its website. The research protocol has received ethical approval from a review board, and all participants have provided written informed consent.
2.1 Study design and population
This study utilized data collected from the National Health and Nutrition Examination Survey (NHANES) spanning from 2005 to 2018, excluding periods with missing bone density data in 2011–2012 and 2015–2016. Initially, a total of 50,463 participants were considered. Following the exclusion of individuals outside the 12–19 age range (N = 42,972), those with missing dietary choline data (N = 1,040), and those lacking total bone density data (N = 2,651), the final analysis included 3,800 participants (Figure 1).
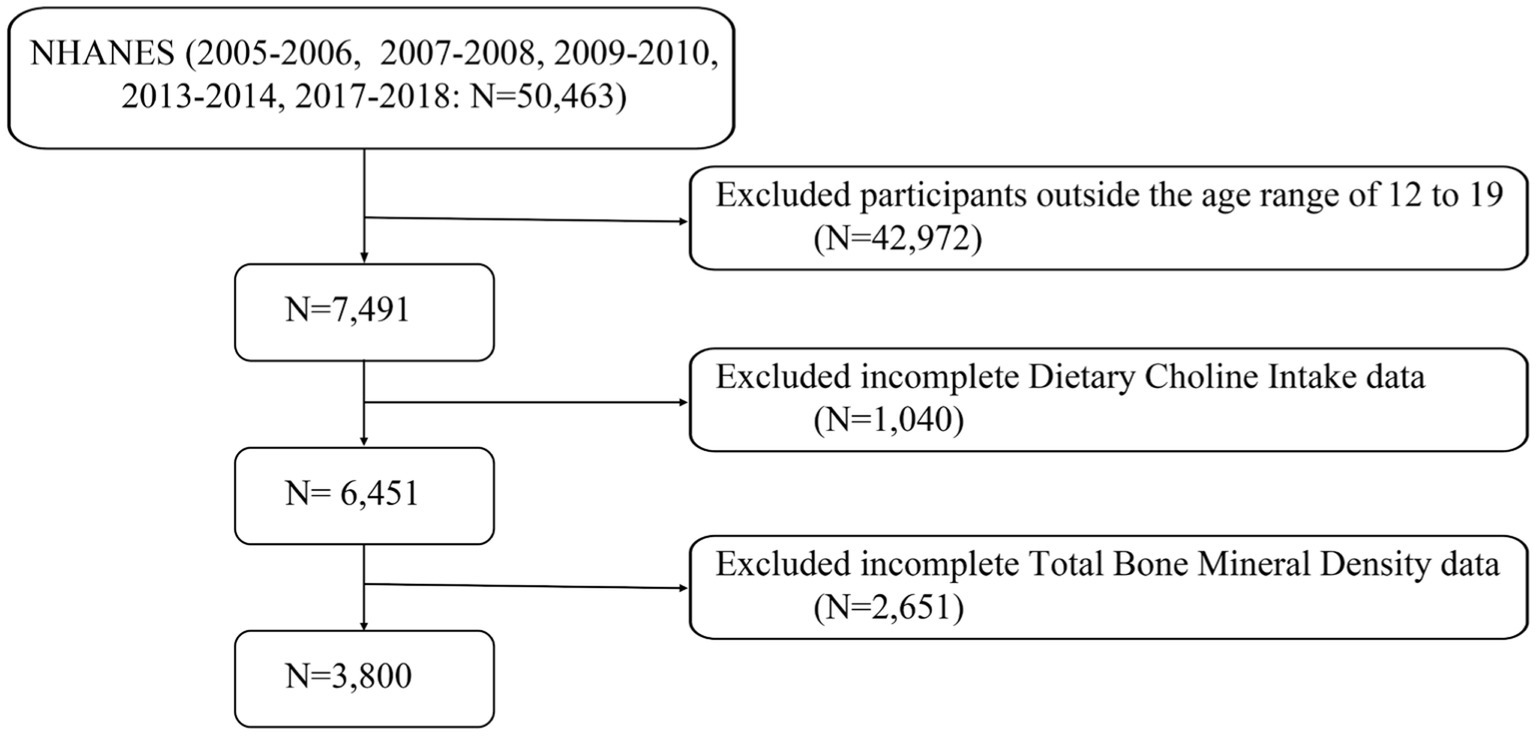
Figure 1. A flow diagram of eligible participant selection in the National Health and Nutrition Examination Survey.
2.2 Assessment of dietary choline intake
Dietary choline intake was assessed using data from two 24-h dietary recall interviews. The first interview occurred at a mobile examination center, followed by a second interview conducted via phone several days later. Nutrient and micronutrient intake was calculated using the United States Department of Agriculture (USDA) database, with the analysis based on the average intake from the two recall days.
2.3 Evaluation of total bone mineral density
Dual-energy X-ray absorptiometry (DXA) is regarded as the primary method for measuring bone density in humans due to its advantages of speed, simplicity, and minimal radiation exposure. Total bone density was measured using a Hologic QDR 4500A fan-beam densitometer by trained radiologic technologists (18). Detailed measurement procedures are available on the NHANES website.
2.4 Covariates
NHANES has gathered extensive data on demographic and dietary factors, encompassing age, sex, race, family income-to-poverty ratio, obesity, physical activity level, daily energy intake, dietary carbohydrates, fiber, fat, calcium, and phosphorus intake. Definitions of covariates can be found in Supplementary Table S1.
2.5 Statistical analysis
Given the complex, multi-stage, probability sampling design of the NHANES database, sample weights were applied to combine data across multiple survey cycles to ensure that results better represent the characteristics of the entire U.S. population. In this study, we utilized the WTDRD2 weight (weight for participants who completed the second 24-h dietary recall interview).
Initially, descriptive statistics were used to analyze population characteristics, with continuous variables presented as means ± standard deviations and categorical variables as frequencies and percentages. Weighted t-tests were employed for comparing continuous variables, while weighted chi-square tests were used for categorical variables (19). Multiple linear regression models were used to analyze the correlation between dietary choline intake and adolescent bone density. Further analyses were conducted separately for males and females to validate these relationships. Three regression models were employed to control for confounding factors: Model 1 was unadjusted, Model 2 adjusted for age, sex, race, and family income-to-poverty ratio, and Model 3 additionally adjusted for obesity, physical activity, daily energy intake, dietary carbohydrates, fiber, fat, calcium, and phosphorus intake.
Restricted cubic spline (RCS) models were employed to analyze whether there was a non-linear relationship between choline intake and adolescent bone density. Subgroup analyses based on key covariates (e.g., gender, race, household income, obesity, and physical activity) were conducted to examine possible interactions between dietary choline intake and different stratification factors. All statistical analyses were performed using R version 4.2.2 software, with statistical significance set at p < 0.05.
3 Results
3.1 Characteristics of the participants
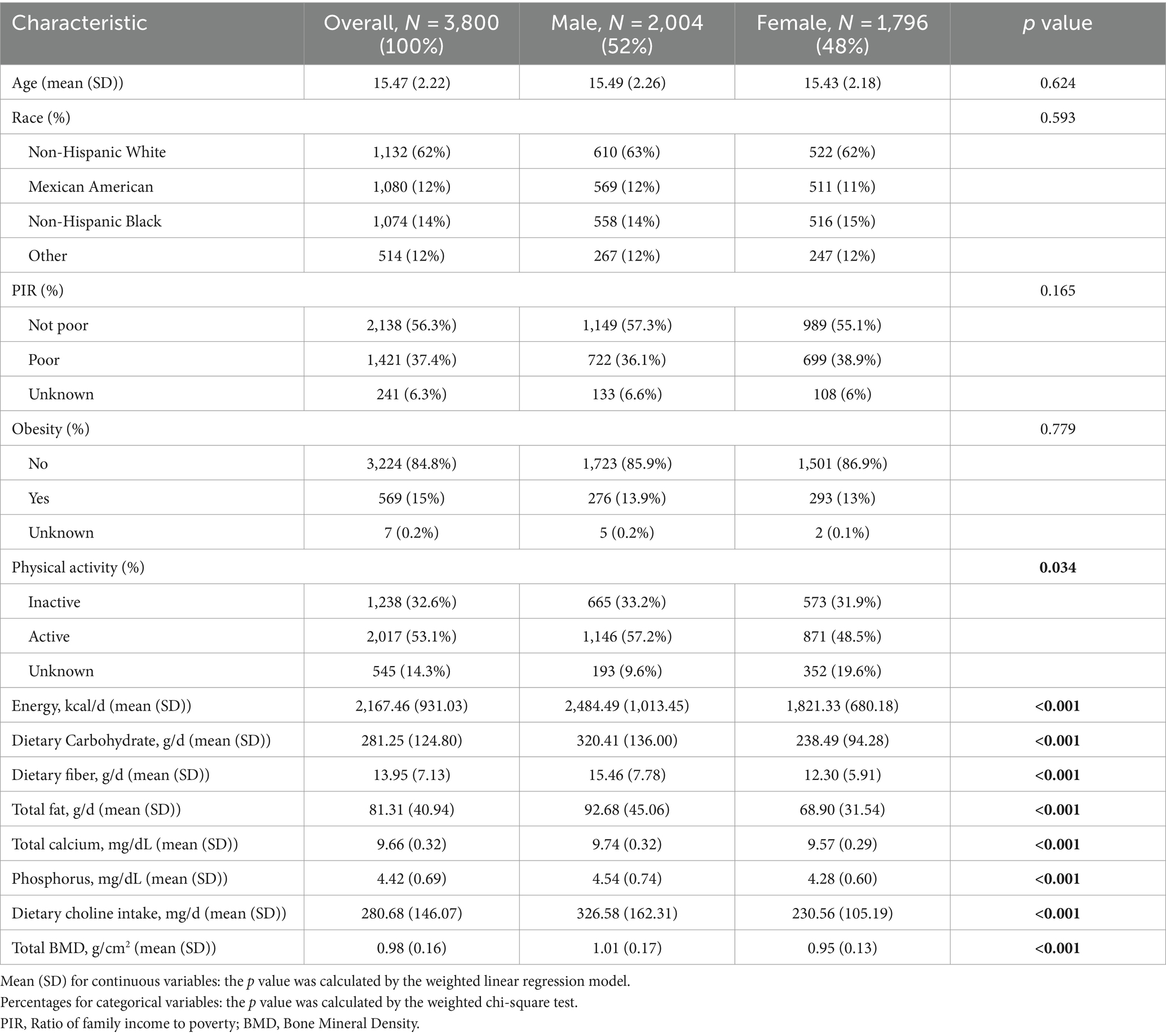
Table 1 outlines the baseline characteristics of participants, encompassing a total of 3,800 subjects included in our study. The average age of the study cohort was 15.47 ± 2.22 years, with a predominant representation of non-Hispanic White individuals (1,132 cases, 62%). Compared to females, males exhibited a higher proportion engaged in vigorous physical activity (p < 0.05). Moreover, male participants demonstrated higher daily energy intake, dietary choline, carbohydrates, fiber, fat, calcium, and phosphorus intake compared to females (p < 0.001). Regarding bone density, males also exhibited significantly higher total bone density than females (p < 0.001).
3.2 Association between dietary choline intake and total BMD
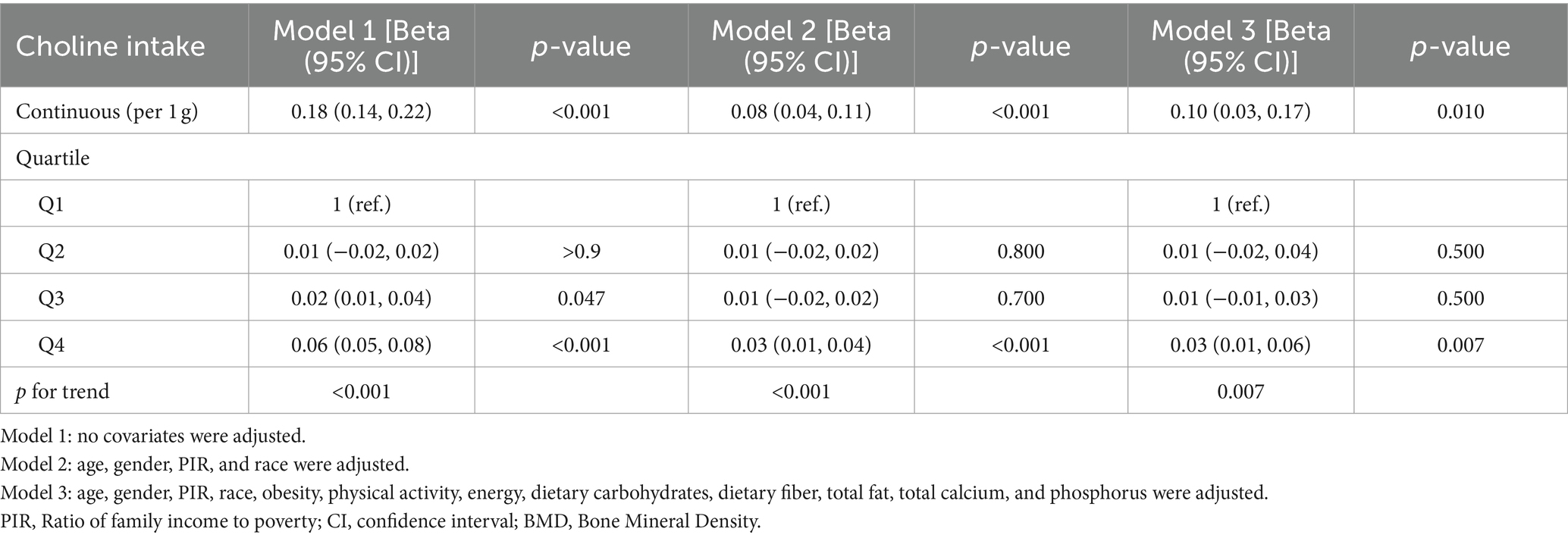
We employed three linear regression models to investigate the independent relationship between dietary choline intake and adolescent bone density (Table 2). In Model 1, choline intake exhibited a significant positive association with adolescent bone density (β: 0.18, 95% CI: 0.14–0.22), which remained significant in the minimally adjusted Model 2 (β: 0.08, 95% CI: 0.04–0.11) and fully adjusted Model 3 (β: 0.10, 95% CI: 0.03–0.17). Stratifying choline intake into quartiles yielded consistent findings.
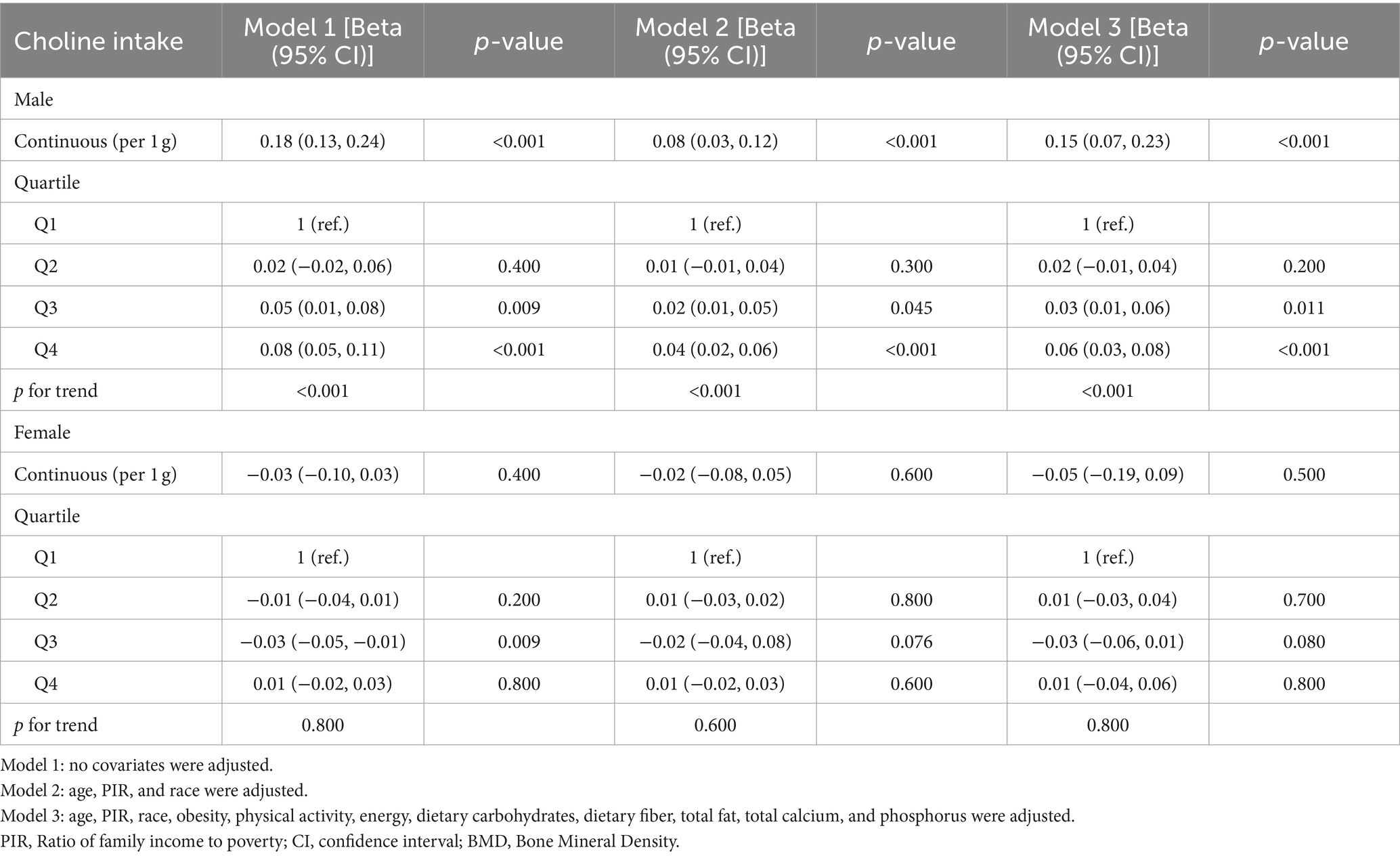
Upon performing separate regression analyses for males and females (Table 3), we observed that the positive correlation between choline intake and bone density was exclusively significant among male participants (p < 0.001), whereas no significant correlation was found among females (p > 0.05). Quartile analysis further supported these results. Restricted cubic spline (RCS) models demonstrated a linear positive association between dietary choline intake and adolescent bone density, which was statistically significant solely in male participants (p < 0.001) (Figure 2).
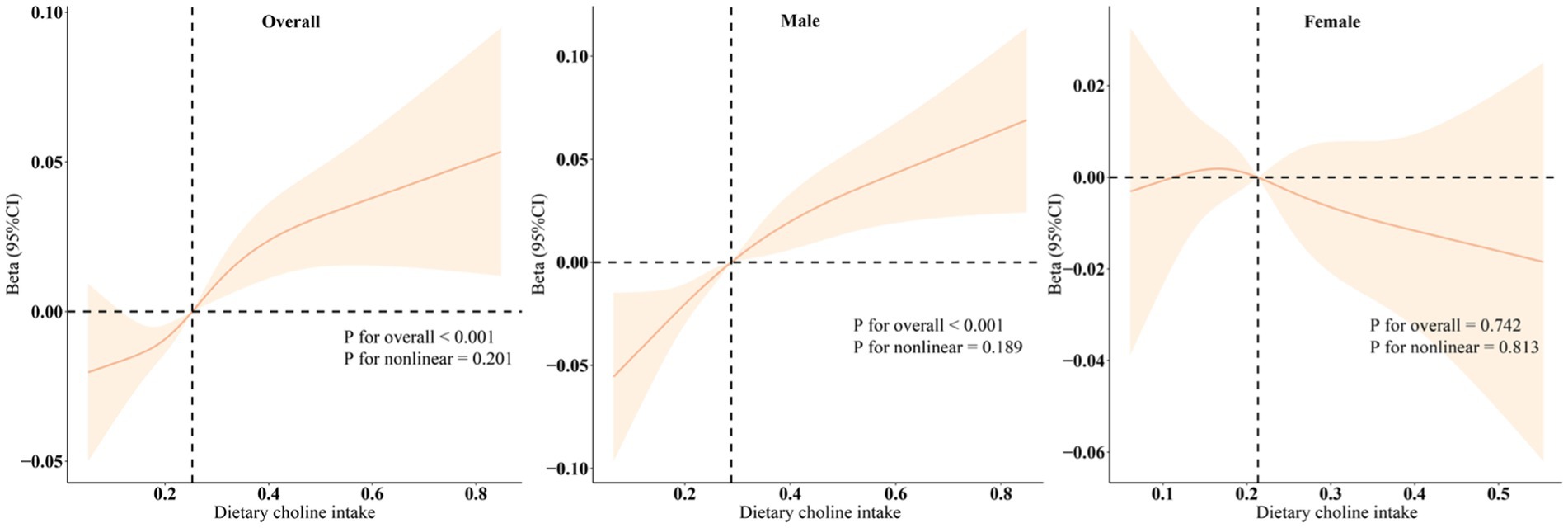
Figure 2. Dose–response relationships between dietary choline intake and total BMD. Beta (solid lines) and 95% confidence levels (shaded areas) were adjusted for age, gender, PIR, race, obesity, physical activity, energy, dietary carbohydrates, dietary fiber, total fat, total calcium, and phosphorus.
3.3 Subgroup analysis
Subgroup analysis revealed a positive correlation between dietary choline intake and adolescent bone density across most subgroups (Figure 3). This correlation was notably stronger among males and non-Hispanic White individuals, aligning consistently with the results of our regression analysis.
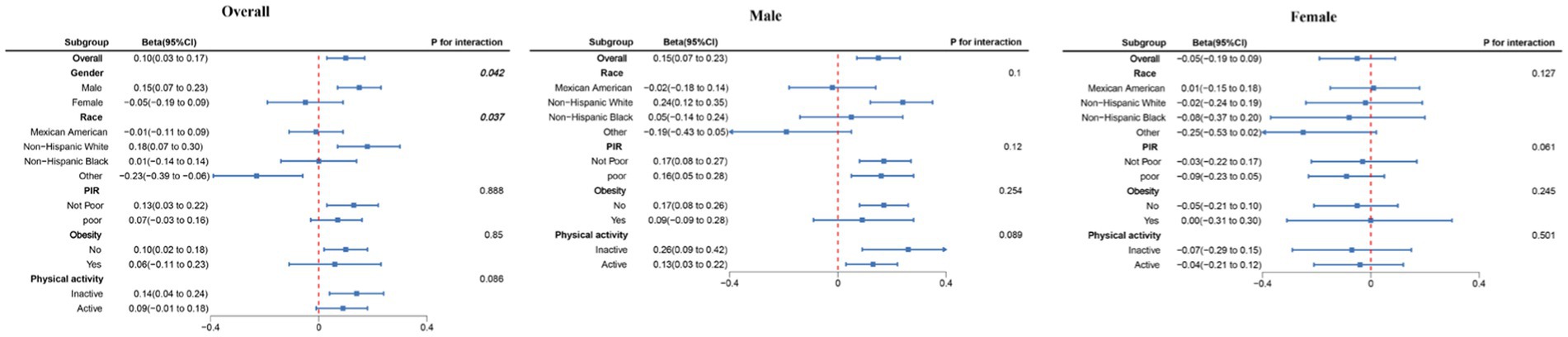
Figure 3. Subgroup analysis between dietary choline intake and total BMD. Beta was calculated as per 1 g increase in PHDI. Analyses were adjusted for age, gender, PIR, race, obesity, physical activity, energy, dietary carbohydrates, dietary fiber, total fat, total calcium, and phosphorus.
4 Discussion
This study explored the relationship between dietary choline intake and bone density among American adolescents. We observed a significant positive correlation between dietary choline intake and adolescent bone density. In fully adjusted linear models, higher choline intake was consistently associated with higher bone density, primarily observed in male participants. Subsequent subgroup analyses further highlighted that this association was notably stronger among males and non-Hispanic White individuals.
As the population ages, the prevalence of osteoporosis continues to rise, accompanied by a heightened risk of fractures, imposing significant burdens on both households and socio-economic development (20, 21). Peak bone mass (PBM) is recognized as a determinant of osteoporosis and brittle bone fractures later in life (22), with the majority of bone mass accumulating during adolescence. Intervening effectively during this period to promote bone growth may hold crucial implications for preventing osteoporosis in old age (23–25). Dietary intake plays a critical role in bone growth among adolescents, as adequate consumption of key nutrients facilitates healthy skeletal development and maximizes bone density, thereby reducing the risks of osteoporosis and fractures.
Choline is an essential nutrient in the human body, serving as a crucial component of phosphatidylcholine and a precursor to the important neurotransmitter acetylcholine. It plays a significant role in maintaining cellular membrane structure and function, neurotransmission, lipid metabolism, regulation of intestinal microbiota, fetal development, and methylation processes (15, 26, 27). Recent studies have explored the relationship between choline and bone density. A health study involving 4,632 participants in the Hordaland region found a positive correlation between dietary choline intake and bone density, particularly strong among elderly men (17). Similarly, analysis of NHANES data from 2005 to 2010 revealed that increased daily dietary choline intake effectively reduces osteoporosis risk in elderly individuals (28). However, research on the relationship between dietary choline and bone density remains limited, primarily focusing on elderly populations. Currently, there is a lack of clinical reports on the impact of dietary choline on skeletal health in adolescents.
Our research has found a positive correlation between dietary choline intake and bone density in adolescents, although the specific mechanisms remain unclear. Based on existing research, we hypothesize several potential mechanisms. Firstly, dietary choline serves as a crucial precursor for acetylcholine synthesis. Acetylcholine, as the primary neurotransmitter of the parasympathetic nervous system (PSNS), activates the neuro-skeletal axis. This regulation through the cholinergic system promotes osteoblast proliferation and osteoclast apoptosis, thereby maintaining bone homeostasis (29–31). Additionally, acetylcholine has been shown to stimulate growth hormone release by activating α2-adrenergic receptors in the hypothalamus or nicotinic cholinergic receptors, further promoting skeletal health (32). Secondly, choline can be oxidized to betaine, which serves as a methyl donor for DNA epigenetic regulation (33). Research by Jing et al. (34) suggests that betaine induces osteogenic differentiation of bone marrow mesenchymal stem cells in vitro and inhibits adipogenic differentiation. Moreover, betaine has been found to enhance bone strength by reducing plasma homocysteine levels (35). Furthermore, choline’s regulation of lipid metabolism may also promote bone growth. Phosphatidylcholine, produced from choline, is essential for chylomicron formation in the intestine and serves as a preferred substrate for probiotic lactobacilli (36, 37). Through modulation of gut microbiota, it affects lipid metabolism, exerting dual regulatory effects on bone growth: increasing bone mass by adjusting lipid levels and mitigating bone loss caused by inflammation and oxidative damage due to lipid imbalance (38–40). However, these mechanisms require further investigation for clarification.
It is noteworthy that our regression analyses conducted separately for male and female participants revealed a positive correlation between dietary choline intake and bone density exclusively among adolescent males, a finding consistent across subgroup analyses. We speculate this outcome may be linked to estrogen secretion. Endogenous choline synthesis in the body primarily relies on phosphatidylethanolamine N-methyltransferase (PEMT) activity in the liver, which estrogen can enhance (41, 42). Increased estrogen secretion during puberty in females stimulates PEMT production, thereby elevating endogenous choline biosynthesis rates. This may explain why adolescent females are less susceptible to the effects of choline deficiency.
This study utilized nationally representative large-scale follow-up data to analyze the relationship between dietary choline intake and bone density in a cohort of American adolescents. We adjusted for demographic, clinical, and laboratory covariates to explore the impact of gender differences on the association between dietary choline and bone density. Overall, our findings underscore the significant role of dietary choline intake in enhancing bone density specifically among adolescent males. These results provide valuable insights for managing adolescent skeletal health. However, several limitations should be noted: (1) The observational study design precludes establishing causal relationships; (2) Dietary choline intake is estimated rather than directly measured and may not accurately reflect actual intake, and individual dietary habits may change during long-term follow-up; in addition, multivitamins from other supplements are commonly used by young people, and all of the above may have some influence on the results of this study. In future studies, we will consider controlling more fully for the effects of supplement use on study outcomes by collecting additional information on supplement use or by using a prospective design. (3) Despite extensive adjustment for relevant confounders, residual confounding factors may still exist. Therefore, in future studies, we will consider using more stringent methods, such as restriction strategies, to further mitigate the impact of these primary confounding factors and improve our findings’ interpretability and scientific validity. (4) For the age range of participants in this study, BMI classification should be based on BMI Z-scores, which can be standardized and adjusted for age and gender to more accurately reflect differences in growth and development. Therefore, this study may have some bias in classifying the obesity status of some participants. We will use BMI Z-scores to improve the accuracy of BMI classification in future studies in adolescent populations to further explore the effects of nutritional intake on bone health.
5 Conclusion
In conclusion, our study identified a positive correlation between dietary choline intake and bone density in adolescents, which was observed exclusively in males. This suggests that supplementing dietary choline may promote skeletal health development in male adolescents. Further epidemiological and laboratory research is needed to validate our findings.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
HG: Conceptualization, Data curation, Investigation, Validation, Visualization, Writing – original draft, Writing – review & editing. JJ: Conceptualization, Data curation, Investigation, Software, Validation, Writing – original draft, Writing – review & editing. SC: Data curation, Writing – original draft, Writing – review & editing. SH: Conceptualization, Funding acquisition, Methodology, Project administration, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We are very grateful to the NHANES database for all the data provided.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1459117/full#supplementary-material
References
1. Black, DM, and Rosen, CJ. Clinical Practice. Postmenopausal Osteoporosis. N Engl J Med. (2016) 374:254–62. doi: 10.1056/NEJMcp1513724
2. Lane, JM, Russell, L, and Khan, SN. Osteoporosis. Clin Orthop Relat Res. (2000) 372:139–50. doi: 10.1097/00003086-200003000-00016
3. Xiao, P-L, Cui, A-Y, Hsu, C-J, Peng, R, Jiang, N, Xu, X-H, et al. Global, regional prevalence, and risk factors of osteoporosis according to the World Health Organization diagnostic criteria: a systematic review and meta-analysis. Osteoporos Int. (2022) 33:2137–53. doi: 10.1007/s00198-022-06454-3
4. Zeng, Q, Li, N, Wang, Q, Feng, J, Sun, D, Zhang, Q, et al. The prevalence of osteoporosis in China, a Nationwide, multicenter DXA survey. J Bone Miner Res. (2019) 34:1789–97. doi: 10.1002/jbmr.3757
5. Crandall, CJ, and Ensrud, KE. Osteoporosis screening in younger postmenopausal women. JAMA. (2020) 323:367–8. doi: 10.1001/jama.2019.18343
6. Bachrach, LK. Acquisition of optimal bone mass in childhood and adolescence. Trends Endocrinol Metab. (2001) 12:22–8. doi: 10.1016/s1043-2760(00)00336-2
7. Baronio, F, and Baptista, F. Editorial: bone health and development in children and adolescents. Front Endocrinol (Lausanne). (2022) 13:1101403. doi: 10.3389/fendo.2022.1101403
8. Elhakeem, A, Frysz, M, Tilling, K, Tobias, JH, and Lawlor, DA. Association between age at puberty and bone accrual from 10 to 25 years of age. JAMA Netw Open. (2019) 2:e198918. doi: 10.1001/jamanetworkopen.2019.8918
9. Bonjour, J-P, and Chevalley, T. Pubertal timing, bone acquisition, and risk of fracture throughout life. Endocr Rev. (2014) 35:820–47. doi: 10.1210/er.2014-1007
10. Rizzoli, R, Bianchi, ML, Garabédian, M, McKay, HA, and Moreno, LA. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone. (2010) 46:294–305. doi: 10.1016/j.bone.2009.10.005
11. Rizzoli, R, Biver, E, and Brennan-Speranza, TC. Nutritional intake and bone health. Lancet Diabetes Endocrinol. (2021) 9:606–21. doi: 10.1016/S2213-8587(21)00119-4
12. Hidayat, K, Zhang, L-L, Rizzoli, R, Guo, Y-X, Zhou, Y, Shi, Y-J, et al. The effects of dairy product supplementation on bone health indices in children aged 3 to 18 years: a meta-analysis of randomized controlled trials. Adv Nutr. (2023) 14:1187–96. doi: 10.1016/j.advnut.2023.06.010
13. Amirkhizi, F, Hamedi-Shahraki, S, and Rahimlou, M. Dietary total antioxidant capacity is associated with lower disease severity and inflammatory and oxidative stress biomarkers in patients with knee osteoarthritis. J Health Popul Nutr. (2023) 42:104. doi: 10.1186/s41043-023-00450-x
14. Morvaridzadeh, M, Sepidarkish, M, Fazelian, S, Rahimlou, M, Omidi, A, Ardehali, SH, et al. Effect of calcium and vitamin D co-supplementation on blood pressure: a systematic review and meta-analysis. Clin Ther. (2020) 42:e45–63. doi: 10.1016/j.clinthera.2020.01.005
15. Wiedeman, AM, Barr, SI, Green, TJ, Xu, Z, Innis, SM, and Kitts, DD. Dietary choline intake: current state of knowledge across the life cycle. Nutrients. (2018) 10:1513. doi: 10.3390/nu10101513
16. Goh, YQ, Cheam, G, and Wang, Y. Understanding choline bioavailability and utilization: first step toward personalizing choline nutrition. J Agric Food Chem. (2021) 69:10774–89. doi: 10.1021/acs.jafc.1c03077
17. Øyen, J, Gjesdal, CG, Karlsson, T, Svingen, GF, Tell, GS, Strand, E, et al. Dietary choline intake is directly associated with bone mineral density in the Hordaland Health Study. J Nutr. (2017) 147:572–8. doi: 10.3945/jn.116.243006
18. Tian, N, Chen, S, Han, H, Jin, J, and Li, Z. Association between triglyceride glucose index and total bone mineral density: a cross-sectional study from NHANES 2011-2018. Sci Rep. (2024) 14:4208. doi: 10.1038/s41598-024-54192-9
19. Feng, G, Huang, S, Zhao, W, and Gong, H. Association between life’s essential 8 and overactive bladder. Sci Rep. (2024) 14:11842. doi: 10.1038/s41598-024-62842-1
20. Ensrud, KE, and Crandall, CJ. Osteoporosis. Ann Intern Med. (2017) 167:ITC17–32. doi: 10.7326/AITC201708010
21. The North American Menopause Society (NAMS). Management of osteoporosis in postmenopausal women: the 2021 position statement of the North American Menopause Society. Menopause. (2021) 28:973–97. doi: 10.1097/GME.0000000000001831
22. Chevalley, T, and Rizzoli, R. Acquisition of peak bone mass. Best Pract Res Clin Endocrinol Metab. (2022) 36:101616. doi: 10.1016/j.beem.2022.101616
23. Obbagy, JE, English, LK, Wong, YP, Butte, NF, Dewey, KG, Fox, MK, et al. Complementary feeding and bone health: a systematic review. Am J Clin Nutr. (2019) 109:872S–8S. doi: 10.1093/ajcn/nqy227
24. Suhett, LG, Filgueiras, MDS, de Novaes, JF, and Sukumar, D. Role of diet quality in bone health in children and adolescents: a systematic review. Nutr Rev. (2023) 82:47–59. doi: 10.1093/nutrit/nuad036
25. Zuckerman-Levin, N, Hochberg, Z, and Latzer, Y. Bone health in eating disorders. Obes Rev. (2014) 15:215–23. doi: 10.1111/obr.12117
26. Arias, N, Arboleya, S, Allison, J, Kaliszewska, A, Higarza, SG, Gueimonde, M, et al. The relationship between choline bioavailability from diet, intestinal microbiota composition, and its modulation of human diseases. Nutrients. (2020) 12:2340. doi: 10.3390/nu12082340
27. Blusztajn, JK, Slack, BE, and Mellott, TJ. Neuroprotective actions of dietary choline. Nutrients. (2017) 9:815. doi: 10.3390/nu9080815
28. Zhang, Y-W, Lu, P-P, Li, Y-J, Dai, G-C, Cao, M-M, Xie, T, et al. Low dietary choline intake is associated with the risk of osteoporosis in elderly individuals: a population-based study. Food Funct. (2021) 12:6442–51. doi: 10.1039/d1fo00825k
29. Li, J, Zhang, Z, Tang, J, Hou, Z, Li, L, and Li, B. Emerging roles of nerve-bone axis in modulating skeletal system. Med Res Rev. (2024) 44:1867–903. doi: 10.1002/med.22031
30. Spieker, J, Frieß, JL, Sperling, L, Thangaraj, G, Vogel-Höpker, A, and Layer, PG. Cholinergic control of bone development and beyond. Int Immunopharmacol. (2020) 83:106405. doi: 10.1016/j.intimp.2020.106405
31. Luo, X, Lauwers, M, Layer, PG, and Wen, C. Non-neuronal role of acetylcholinesterase in bone development and degeneration. Front Cell Dev Biol. (2020) 8:620543. doi: 10.3389/fcell.2020.620543
32. Müller, EE, Rolla, M, Ghigo, E, Belliti, D, Arvat, E, Andreoni, A, et al. Involvement of brain catecholamines and acetylcholine in growth hormone hypersecretory states. Pathophysiological, diagnostic and therapeutic implications. Drugs. (1995) 50:805–37. doi: 10.2165/00003495-199550050-00004
33. Li, Y, Jiang, W, Feng, Y, Wu, L, Jia, Y, and Zhao, R. Betaine alleviates high-fat diet-induced Disruptionof hepatic lipid and Iron homeostasis in mice. Int J Mol Sci. (2022) 23:6263. doi: 10.3390/ijms23116263
34. Jing, Y, Zhou, J, Guo, F, Yu, L, Ren, X, and Yin, X. Betaine regulates adipogenic and osteogenic differentiation of hAD-MSCs. Mol Biol Rep. (2023) 50:5081–9. doi: 10.1007/s11033-023-08404-6
35. Maidin, MBM, McCormack, HA, Wilson, PW, Caughey, SD, Whenham, N, and Dunn, IC. Dietary betaine reduces plasma homocysteine concentrations and improves bone strength in laying hens. Br Poult Sci. (2021) 62:573–8. doi: 10.1080/00071668.2021.1883550
36. Mansbach, CM. The origin of chylomicron phosphatidylcholine in the rat. J Clin Invest. (1977) 60:411–20. doi: 10.1172/JCI108790
37. da Silva, RP, Kelly, KB, Lewis, ED, Leonard, K-A, Goruk, S, Curtis, JM, et al. Choline deficiency impairs intestinal lipid metabolism in the lactating rat. J Nutr Biochem. (2015) 26:1077–83. doi: 10.1016/j.jnutbio.2015.04.015
38. Wang, X, Zhang, C, Zhao, G, Yang, K, and Tao, L. Obesity and lipid metabolism in the development of osteoporosis (review). Int J Mol Med. (2024) 54:61. doi: 10.3892/ijmm.2024.5385
39. van Gastel, N, Stegen, S, Eelen, G, Schoors, S, Carlier, A, Daniëls, VW, et al. Lipid availability determines fate of skeletal progenitor cells via SOX9. Nature. (2020) 579:111–7. doi: 10.1038/s41586-020-2050-1
40. Zhou, T, Heianza, Y, Chen, Y, Li, X, Sun, D, DiDonato, JA, et al. Circulating gut microbiota metabolite trimethylamine N-oxide (TMAO) and changes in bone density in response to weight loss diets: the POUNDS lost trial. Diabetes Care. (2019) 42:1365–71. doi: 10.2337/dc19-0134
41. Fischer, LM, daCosta, KA, Kwock, L, Stewart, PW, Lu, T-S, Stabler, SP, et al. Sex and menopausal status influence human dietary requirements for the nutrient choline. Am J Clin Nutr. (2007) 85:1275–85. doi: 10.1093/ajcn/85.5.1275
Keywords: dietary choline intake, bone mineral density, sex differences, NHANES, adolescents
Citation: Gong H, Jiang J, Choi S and Huang S (2024) Sex differences in the association between dietary choline intake and total bone mineral density among adolescents aged 12–19 in the United States. Front. Nutr. 11:1459117. doi: 10.3389/fnut.2024.1459117
Edited by:
Charlotte Cosemans, University of Hasselt, BelgiumReviewed by:
Mehran Rahimlou, Zanjan University of Medical Sciences, IranAna Inês Silva, University of Hasselt, Belgium
Deisi Maria Vargas, Regional University of Blumenau, Brazil
Copyright © 2024 Gong, Jiang, Choi and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaoqun Huang, c3FodWFuZ0BmanRjbS5lZHUuY24=
†These authors have contributed equally to this work
 Hongyang Gong
Hongyang Gong Jiecheng Jiang
Jiecheng Jiang Seok Choi2
Seok Choi2 Shaoqun Huang
Shaoqun Huang