- Department of Gastroenterology, Shengjing Hospital of China Medical University, Shenyang, China
Background: Prior research suggests polyunsaturated fatty acids (PUFA) may prevent gallstones, but evidence on saturated fatty acids (SFA) and monounsaturated fatty acids (MUFA) is limited. This study aims to explore the associations between fatty acids and gallstones using a large sample of American population and Mendelian randomization (MR) methods.
Methods: The cross-sectional study involved 6,629 participants from the National Health and Nutrition Examination Survey (NHANES) 2017–2020. Logistic regression and restricted cubic spline (RCS) analysis were conducted after stratifying by gender subgroups. Two-sample MR analysis was used to explore the causal relationship between fatty acids and gallstones without confounding factors.
Results: In females, higher SFA intake was positively associated with gallstone risk, while higher intake of n-3 and n-6 PUFA was negatively associated. No significant associations were found in males. No nonlinear correlations were found in any group by RCS analysis. MR analysis indicated that SFA, n-3, and n-6 PUFA could reduce gallstone risk.
Conclusion: The influence of dietary fatty acid composition on gallstone development differs by gender, providing insights into dietary prevention and treatment of gallstones.
1 Introduction
Gallstones are a globally prevalent digestive disease, affecting 10–20% of individuals in developed countries and often presenting without obvious symptoms in the early stages (1). However, as the disease progresses, serious and potentially life-threatening complications may occur, including acute cholangitis, cholecystitis, and biliary pancreatitis (2–4). The high incidence and complications of gallstones impose significant economic and health burdens, necessitating effective preventive and curative measures.
Gallstone formation is influenced by genetic predisposition, lipid metabolic disorders, and lifestyle habits, with genetic factors contributing only 25% to the risk, emphasizing the importance of environmental and lifestyle influences (1, 5, 6). Growing emphasis is placed on the influence of dietary fatty acids on gallstone formation. Prior research has explored the potential protective role of polyunsaturated fatty acids (PUFA) in preventing gallstones (7–10). However, the evidence on saturated fatty acids (SFA) and monounsaturated fatty acids (MUFA) is limited by small sample sizes and the influence of confounding factors.
This study conducted a comprehensive investigation using publicly available databases. The relationship between dietary fatty acids and gallstones was explored in a large population sample using National Health and Nutrition Examination Survey (NHANES) data. Mendelian randomization (MR) was then applied to investigate the causality of fatty acids on gallstones, adjusting for residual confounding factors and addressing the limitations of the cross-sectional study. This study aims to provide scientific evidence for preventing gallstones through dietary intervention.
2 Methods
2.1 Cross-sectional study
2.1.1 Study population
NHANES is a continuous research initiative by the National Center for Health Statistics (NCHS), gathering data on characteristics, dietary habits, and health conditions of American participants. The study obtained ethical approval from the NCHS review committee. The NHANES data are collected using multi-stage stratified sampling, ensuring comprehensive national representation.
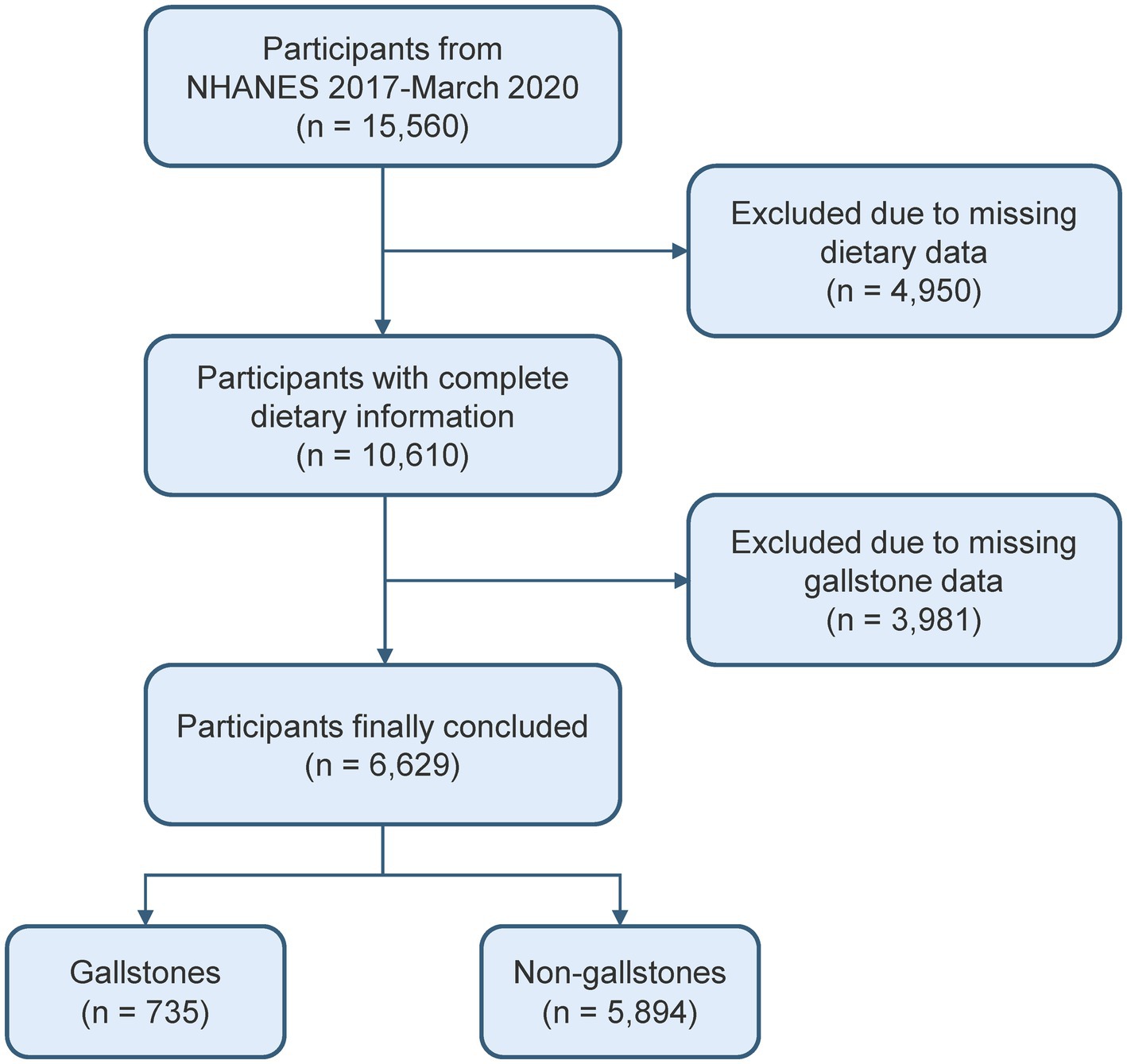
This study involved a cohort of 15,560 people from the 2017–2020 cycle, as the gallstones questionnaire was only available during that time period. Since the gallstones questionnaire was only administered to individuals over 20 years old, those under 20 were excluded from this study. After excluding missing dietary data and gallstone information, 6,629 participants were included (Figure 1).
2.1.2 Study variables
The dietary fatty acids of interest in this study include SFA, MUFA, PUFA, n-3 PUFA, and n-6 PUFA. Each participant underwent two 24-h dietary recall interviews: the first was conducted in person at the Mobile Examination Center, and the second over the phone 3–10 days later. In order to maintain precision, the analysis utilized the average of the two dietary data, with “dietary two-day sample weight” serving as weight.
Gallstone cases were identified using the inquiry “Has DR ever said you have gallstones”. Participants who responded affirmatively were classified as having gallstones.
2.1.3 Covariates
To address potential confounding variables, the study incorporated the following covariates. These covariates encompassed continuous variables such as age, body mass index (BMI), triglyceride, high-density lipoprotein-cholesterol (HDL-C), dietary energy intake, fat intake, and cholesterol intake. Additionally, categorical variables such as gender, race, education level, marital status, poverty income ratio (PIR), hypertension, diabetes, hepatic steatosis, smoking, and drinking were also considered. Detailed definitions of covariates are available in Supplementary Table S1.
2.1.4 Statistical analysis
The sample weights from the NHANES stratified multistage sampling were taken into account in the baseline characteristics and correlation analysis. The baseline characteristics of the participants were determined depending on their gallstone status. As the continuous variables were all skewed, median and interquartile range (IQR) were used to describe them, and frequency and weighted percentage were used to describe the categorical variables. Group comparisons were carried out employing the weighted χ2 test and the Wilcoxon rank-sum test.
Fully adjusted for all covariates, a weighted multivariate logistic regression model was used for analysis. We first analyzed fatty acid intake as a continuous variable for its association with gallstones. Subsequently, fatty acid intake was divided into quartiles to conduct a trend test. Given that gallstones are more prevalent in women and that there are gender disparities in fatty acid intake (11, 12), subgroup logistic regression analysis was conducted by gender. Additionally, restricted cubic splines (RCS) were employed to examine the potential nonlinear relationship in different genders.
Statistical analysis was conducted using R software (version 4.4.0) with the R packages “survey” (version 4.4-2) and “rms” (version 6.8-1). p values below 0.05 were deemed significant.
2.2 Mendelian randomization study
2.2.1 Data sources
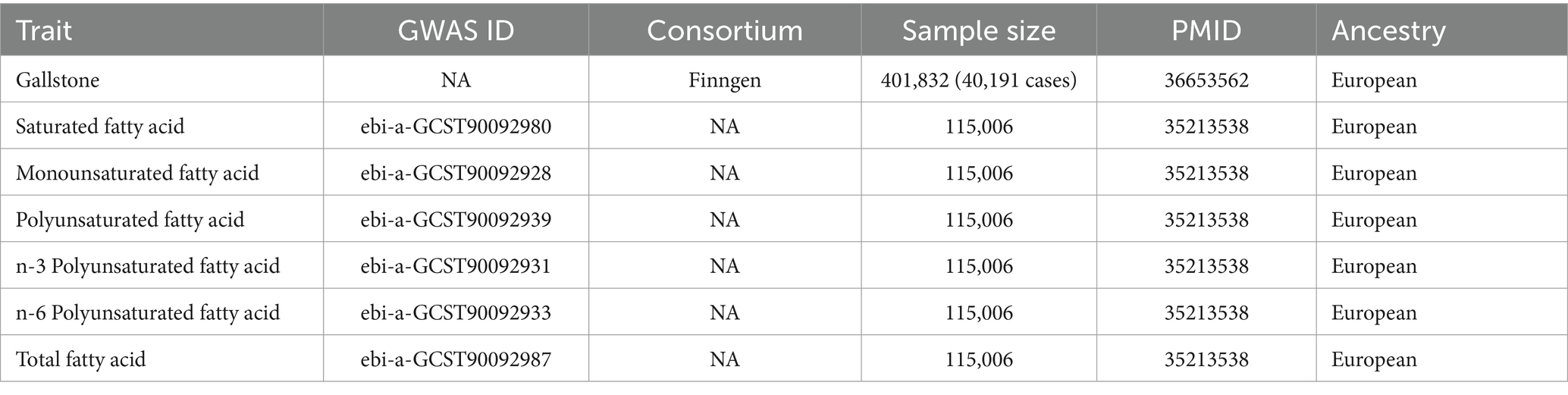
The fatty acid genome-wide association studies (GWAS) data were acquired from the IEU OpenGWAS project, which included a total of 115,006 European participants. The gallstone GWAS data were acquired from the Finngen R10 Release (13), comprising 40,191 European cases and 361,641 European controls overall. For these original GWAS studies, corresponding ethical approvals have been obtained. The population samples of exposure and outcome were from different consortia, ensuring minimal overlap. Detailed GWAS data information is presented in Table 1.
2.2.2 Instrumental variables selection
Single nucleotide polymorphisms (SNPs) strongly associated with the exposure factors were employed as unconfounded instrumental variables (IVs) for the analysis. The MR analysis was based on three fundamental assumptions: (1) IVs are substantially linked with exposure; (2) IVs do not influence outcomes via confounders; (3) IVs impact outcomes only through their effect on exposure. Based on the above criteria, the candidate SNP must reach the genome-wide significance (5 × 10−8). To guarantee the independence of candidate SNPs, the linkage disequilibrium threshold was set to be r2 = 0.001 and clumping distance = 10,000 kb. The intensity of each SNP was calculated using the formula: F statistic = Beta2/SE2 (14), and SNPs with F < 10 were discarded as weak IVs. To ensure adherence to the core assumptions of MR, we screened for and removed confounders using the GWAS Catalog (15). Detailed information on the confounding SNPs and traits is provided in Supplementary Table S2.
2.2.3 Mendelian randomization analysis
The main method for MR analysis was the inverse variance weighted (IVW) approach. It combines Wald ratios of each IV to conduct a meta-analysis. It is considered the most accurate statistical method when horizontal pleiotropy does not exist (16). Additionally, supplementary analytical methods such as weighted median, MR-Egger, and MR Robust Adjusted Profile Score (MR-RAPS) were also employed in this study (17–19). We employed MR-PRESSO and RadialMR to detect and eliminate outliers (20, 21). Horizontal pleiotropy was evaluated using the MR Egger intercept and MR-PRESSO global test, while heterogeneity was assessed with Cochran’s Q test (22). p values below 0.05 suggest the existence of pleiotropy or heterogeneity. The impact of individual outlier IVs was assessed using the funnel plot and the leave-one-out analysis. We applied STROBE-MR to design this study (Supplementary Table S3) (23). The R packages used for MR analysis were “TwoSampleMR” (version 0.6.3), “mr. raps” (version 0.2), “MRPRESSO” (version 1.0), and “RadialMR” (version 1.1).
3 Results
3.1 Cross-sectional study
3.1.1 Baseline characteristics
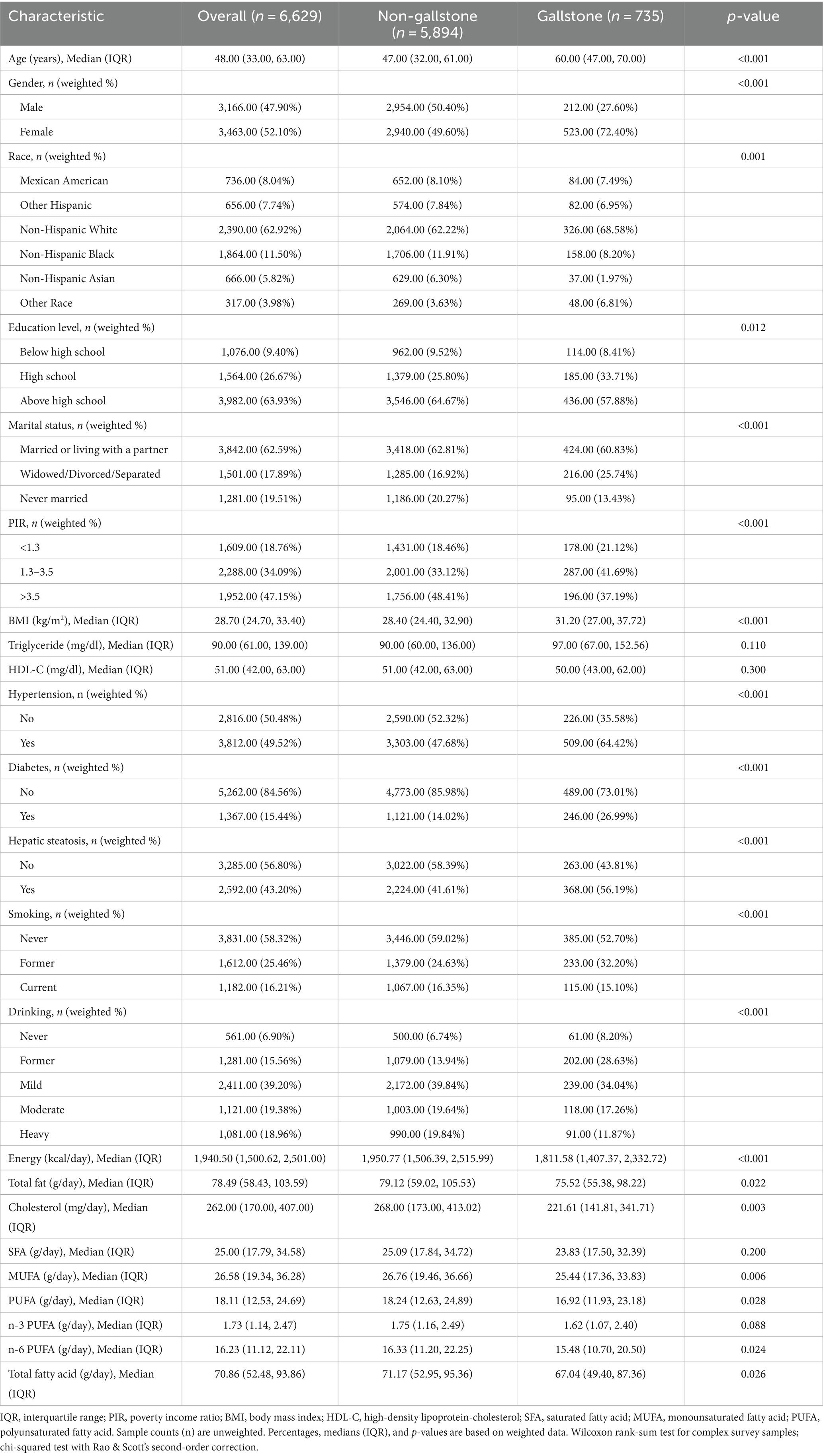
Table 2 presents the weighted baseline characteristics of the study participants grouped by gallstone status. The study involved 6,629 participants, comprising 5,894 individuals without gallstones and 735 individuals with gallstones. The analysis showed that individuals with gallstones were more likely to be older, female, obese, former smokers, and former drinkers, and exhibited a higher prevalence of hypertension, diabetes, and hepatic steatosis compared to those without gallstones (p < 0.001). Additionally, the two groups differed significantly in race, education level, marital status, and PIR. The intake of energy, fat, cholesterol, MUFA, and n-6 PUFA was found to be lower in the gallstone group.
3.1.2 Association between dietary fatty acids and gallstone
After adjusting for all covariates, the results of the weighted logistic regression analysis are presented in Table 3. The results showed that in the female subgroup, higher consumption of SFA was positively associated with an increased risk of gallstones (OR = 1.054, 95% CI: 1.003–1.107), while higher n-3 PUFA (OR = 0.647, 95% CI: 0.420–0.996) and n-6 PUFA (OR = 0.926, 95% CI: 0.871–0.984) intake was linked to a reduced risk. When analyzing fatty acid intake by quartiles, compared to Q1, Q3 and Q4 groups of n-3 PUFA and Q3 group of n-6 PUFA were inversely associated with gallstone risk among females. However, no notable associations were found in the male subgroup. Figure 2 displays the RCS study illustrating the impact of dietary fatty acids on gallstone risk according to gender. After accounting for all confounding variables, no nonlinear correlations were discovered in any of the groups (P-nonlinear >0.05).
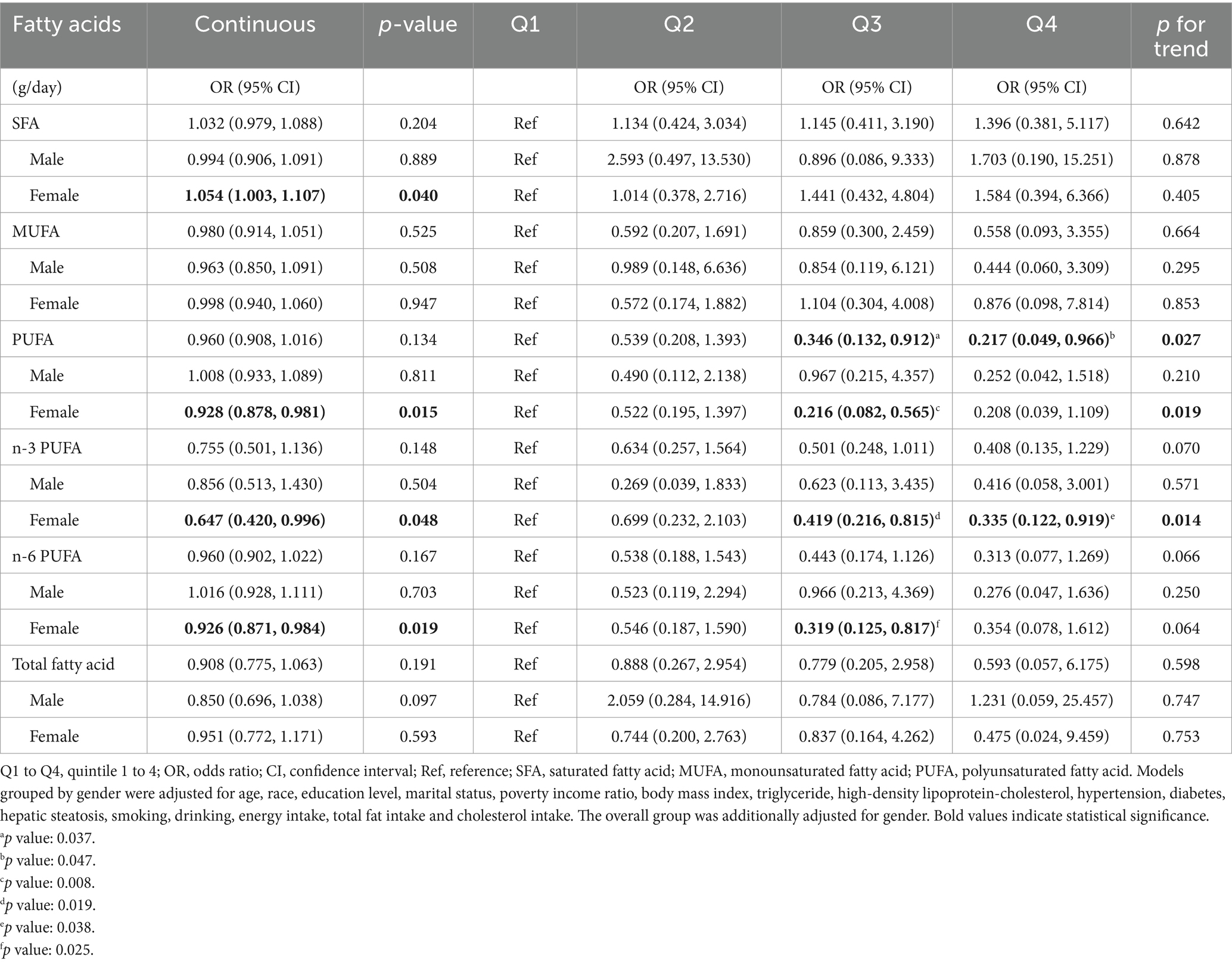
Table 3. Weighted logistic regression analysis of the association between fatty acid intake and gallstone.
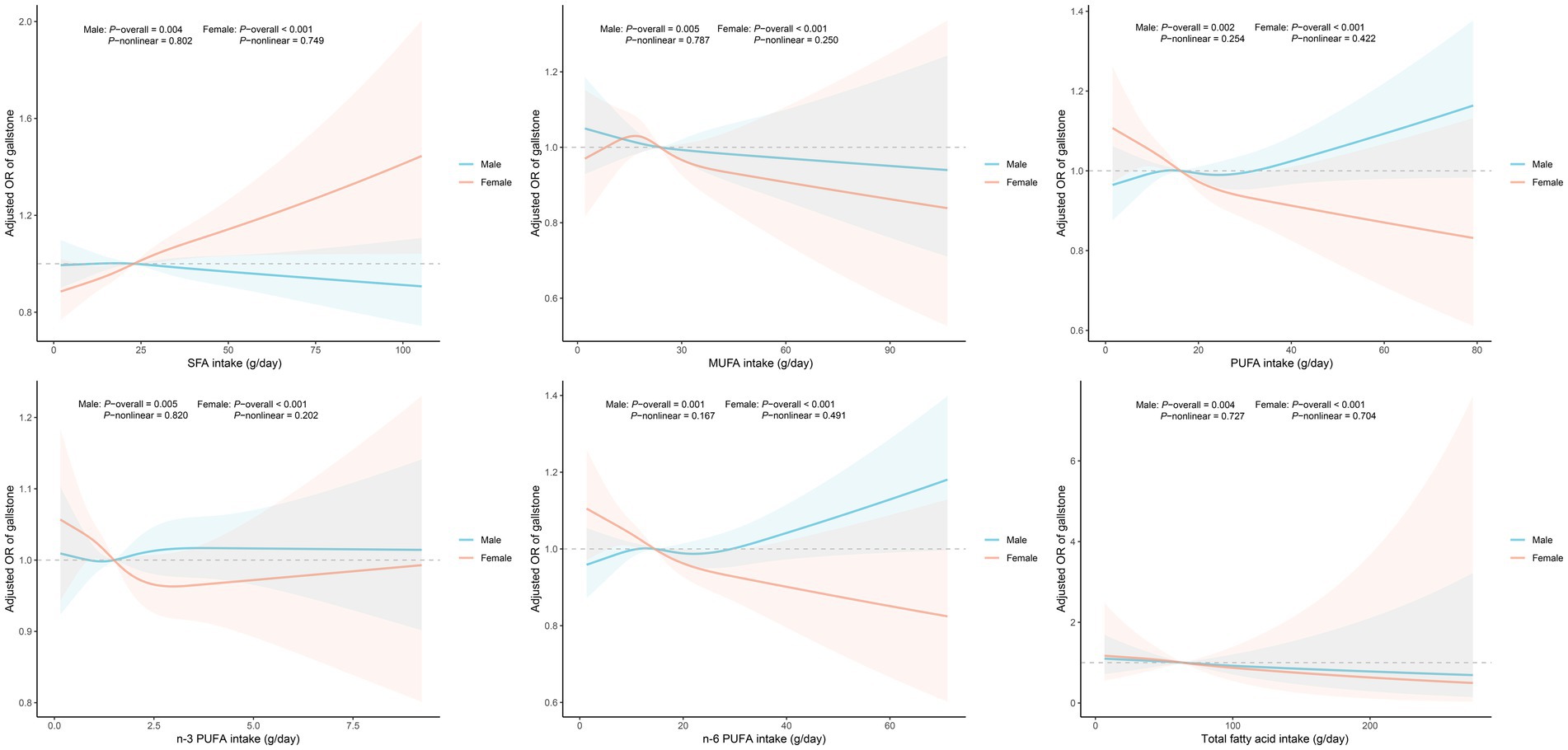
Figure 2. Dose-response analysis of fatty acids intake and gallstone risk. Models grouped by gender were adjusted for age, race, education level, marital status, poverty income ratio, body mass index, triglyceride, high-density lipoprotein-cholesterol, hypertension, diabetes, hepatic steatosis, smoking, drinking, energy intake, total fat intake and cholesterol intake. Abbreviation: OR, odds ratio; CI, confidence interval; SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid.
3.2 Mendelian randomization study
After applying the selection criteria for IVs and removing outliers, the SNPs utilized in the MR analysis are listed in Supplementary Tables S4–S9. All SNPs exhibited F-statistics over 10, indicating no weak IVs.
The IVW method showed that SFA (OR = 0.842, 95% CI: 0.781–0.908), n-3 PUFA (OR = 0.895, 95% CI: 0.841–0.952), and n-6 PUFA (OR = 0.887, 95% CI: 0.838–0.939) could reduce gallstone risk (all p < 0.001), as shown in Figure 3. All three supplementary MR methods demonstrated results consistent with the IVW method, enhancing the reliability of the findings. Table 4 shows that there is no indication of pleiotropy or heterogeneity. No potential outliers were observed that could affect the results, as shown by the leave-one-out results and funnel plots (Supplementary Figures S1, S2).
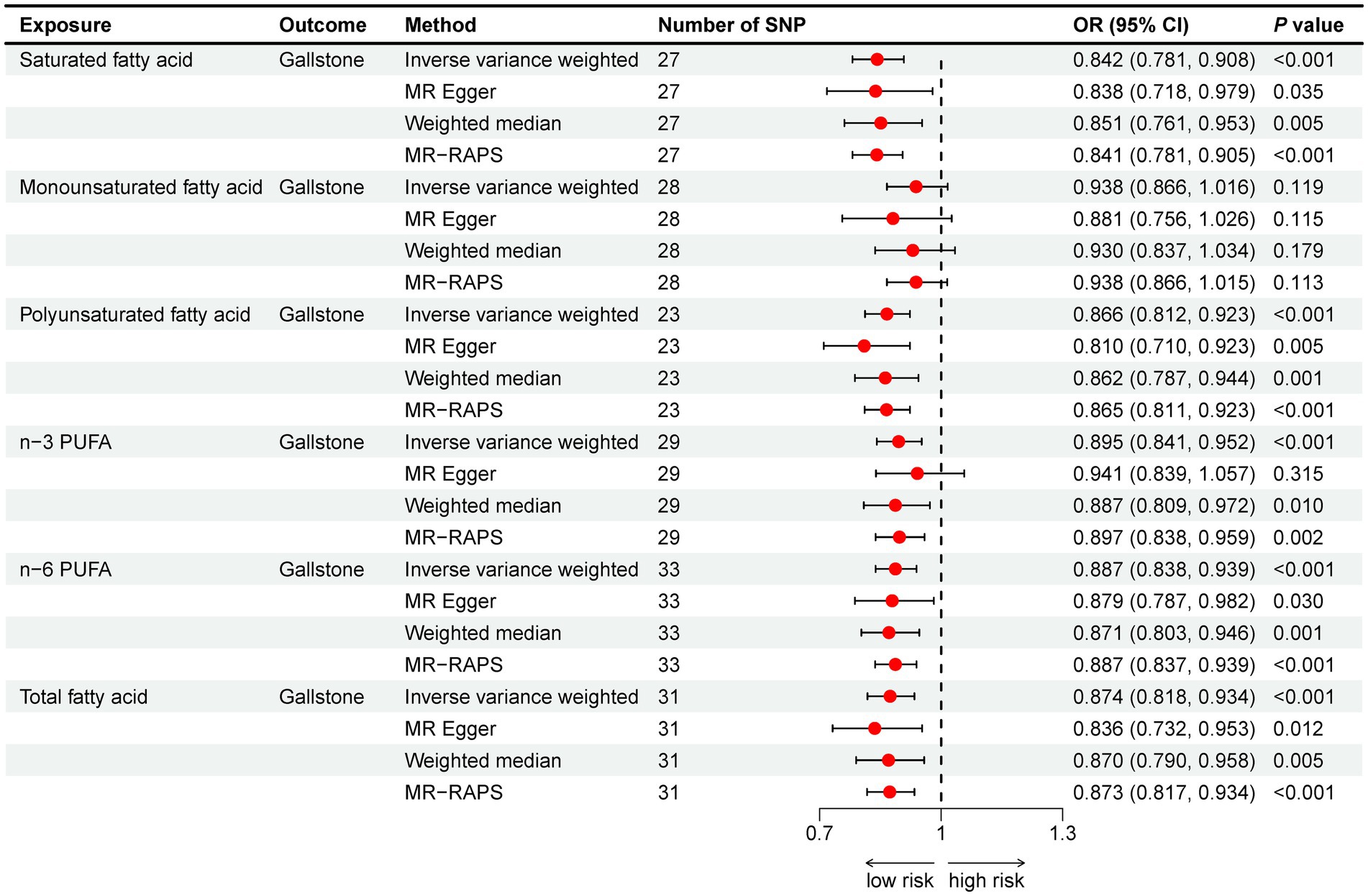
Figure 3. Mendelian randomization analysis of fatty acids and gallstone risk. Abbreviation: OR, odds ratio; CI, confidence interval; PUFA, polyunsaturated fatty acid; MR-RAPS, MR Robust adjusted profile score.
4 Discussion
This study is the first to utilize NHANES large sample data and the latest GWAS data to investigate the relationship between various dietary fatty acids and gallstones in the American population, and to explore the causal relationship without confounding factors using MR analysis. Results from the NHANES study indicated that for females, higher SFA intake was linked to higher gallstone risk, whereas higher n-3 and n-6 PUFA intake were linked to a reduced risk. No notable associations were found in the male group. MR analysis indicated that SFA, n-3 and n-6 PUFA could reduce gallstone risk.
Studies have demonstrated that the main causes contributing to the development of gallstones are supersaturation of bile components, promotion of crystal nucleation by mucin and similar substances, and reduced gallbladder motility (24). Multiple epidemiological studies have confirmed that PUFA can prevent gallstone formation (10, 25, 26). This may be attributed to the following mechanisms: firstly, the intake of fish oil, which contains high levels of n-3 PUFA, can lower cholesterol saturation in bile, inhibiting the formation of cholesterol crystals (8, 27). Secondly, n-3 PUFA can also increase the secretion of bile acids and phospholipids, inhibit the formation of biliary mucin, and improve gallbladder motility to prevent gallstone formation (9, 28, 29). Thirdly, PUFA can reduce serum and liver cholesterol and triglyceride levels, thereby lowering the risk of gallstones (9, 30–32). Additionally, since inflammation plays a significant role in gallstone formation (33, 34), PUFA may also prevent gallstones through their anti-inflammatory effects (35, 36).
Regarding SFA, two clinical studies conducted in France and Italy found a positive correlation between dietary SFA and gallstones (37, 38). An American clinical investigation found that consuming more long-chain SFA is linked to a greater likelihood of developing gallstones in men, whereas intake of medium- or short-chain SFA does not seem to affect this risk (39). The close correlation between SFA and metabolic syndrome has been highlighted (40). SFA has been found to be linked to several disorders connected to metabolic syndrome, such as cardiovascular disease (41), insulin resistance (42, 43), and cancer (44). Since several components of metabolic syndrome are risk factors for gallstones, gallstones can be considered a biliary manifestation of metabolic syndrome (45, 46). Anyway, the precise impact of MUFA remains uncertain, with some clinical studies finding a negative correlation between MUFA and gallstone risk (10, 38), while others have found the opposite result (47, 48).
Our study findings suggest that in females, consuming dietary PUFA can lower the likelihood of developing gallstones, while higher intake of SFA is associated with an increased risk. These findings align with prior research. However, no such correlation was observed in males. Differences in estrogen and lipid metabolism may lead to a higher prevalence of gallstones in females (49, 50), and this disparity may influence the effects of fatty acids on different genders. Moreover, the cross-sectional study and the MR study yielded contradictory findings regarding the role of SFA. This discrepancy can be attributed to the inherent limitations of each study design. Despite adjusting for potential covariates as thoroughly as possible, the cross-sectional study remains susceptible to residual confounding. In contrast, the MR study indicates a causal relationship between lifetime exposure and outcome with minimal confounding, but it cannot account for gender differences and cannot fully eliminate the influence of pleiotropy. However, further clinical and experimental studies are required to validate these gender differences and explore possible mechanisms.
This study utilized a nationally representative NHANES sample for weighted analysis and conducted subgroup analysis by gender, making the results generalizable and instructive. Combining observational studies with MR analysis enhanced the reliability of the findings. However, there are some limitations to this study that should not be overlooked. Firstly, the dietary and gallstone data were self-reported, which introduces the possibility of recall bias and lack of precise imaging diagnosis. Secondly, the 24-h dietary recall interview data may not accurately reflect an individual’s long-term dietary habits. Thirdly, this study only targeted adults in the United States, so caution is needed when generalizing the results to other populations.
5 Conclusion
Our study found that in females, dietary SFA was positively associated with gallstone risk, while higher intake of n-3 and n-6 PUFA was associated with a decreased risk. No significant associations were found in men. MR analysis supports that SFA, n-3 and n-6 PUFA could reduce gallstone risk. These findings provide new perspectives on dietary strategies for the prevention and treatment of gallstones. However, further prospective cohort studies and experimental research are required to validate these results and explore the underlying mechanisms.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethical review committee of the National Center for Health Statistics, UK biobank and Finngen consortium. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
MW: Conceptualization, Data curation, Formal analysis, Methodology, Software, Visualization, Writing – original draft. JG: Project administration, Supervision, Writing – review & editing. SS: Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1454648/full#supplementary-material
References
1. Lammert, F, Gurusamy, K, Ko, CW, Miquel, J-F, Méndez-Sánchez, N, Portincasa, P, et al. Gallstones. Nat Rev Dis Primers. (2016) 2:16024. doi: 10.1038/nrdp.2016.24
2. Shaheen, NJ, Hansen, RA, Morgan, DR, Gangarosa, LM, Ringel, Y, Thiny, MT, et al. The burden of gastrointestinal and liver diseases, 2006. Am J Gastroenterol. (2006) 101:2128–38. doi: 10.1111/j.1572-0241.2006.00723.x
3. Festi, D, Reggiani, MLB, Attili, AF, Loria, P, Pazzi, P, Scaioli, E, et al. Natural history of gallstone disease: expectant management or active treatment? Results from a population-based cohort study. J Gastroenterol Hepatol. (2010) 25:719–24. doi: 10.1111/j.1440-1746.2009.06146.x
4. Sandler, RS, Everhart, JE, Donowitz, M, Adams, E, Cronin, K, Goodman, C, et al. The burden of selected digestive diseases in the United States. Gastroenterology. (2002) 122:1500–11. doi: 10.1053/gast.2002.32978
5. Stokes, CS, Krawczyk, M, and Lammert, F. Gallstones: environment, lifestyle and genes. Dig Dis Basel Switz. (2011) 29:191–201. doi: 10.1159/000323885
6. Katsika, D, Grjibovski, A, Einarsson, C, Lammert, F, Lichtenstein, P, and Marschall, H-U. Genetic and environmental influences on symptomatic gallstone disease: a Swedish study of 43,141 twin pairs. Hepatol Baltim Md. (2005) 41:1138–43. doi: 10.1002/hep.20654
7. Jang, SI, Fang, S, Kim, KP, Ko, Y, Kim, H, Oh, J, et al. Combination treatment with n-3 polyunsaturated fatty acids and ursodeoxycholic acid dissolves cholesterol gallstones in mice. Sci Rep. (2019) 9:12740. doi: 10.1038/s41598-019-49095-z
8. Magnuson, TH, Lillemoe, KD, High, RC, and Pitt, HA. Dietary fish oil inhibits cholesterol monohydrate crystal nucleation and gallstone formation in the prairie dog. Surgery. (1995) 118:517–23. doi: 10.1016/S0039-6060(05)80368-X
9. Talahalli, RR, Vallikannan, B, Sambaiah, K, and Lokesh, BR. Lower efficacy in the utilization of dietary ALA as compared to preformed EPA + DHA on long chain n-3 PUFA levels in rats. Lipids. (2010) 45:799–808. doi: 10.1007/s11745-010-3464-6
10. Tsai, C-J, Leitzmann, MF, Willett, WC, and Giovannucci, EL. The effect of long-term intake of cis unsaturated fats on the risk for gallstone disease in men: a prospective cohort study. Ann Intern Med. (2004) 141:514–22. doi: 10.7326/0003-4819-141-7-200410050-00007
11. Sun, H, Warren, J, Yip, J, Ji, Y, Hao, S, Han, W, et al. Factors influencing gallstone formation: a review of the literature. Biomol Ther. (2022) 12:550. doi: 10.3390/biom12040550
12. Thompson, M, Hein, N, Hanson, C, Smith, LM, Anderson-Berry, A, Richter, CK, et al. Omega-3 fatty acid intake by age, gender, and pregnancy status in the United States: National Health and nutrition examination survey 2003−2014. Nutrients. (2019) 11:177. doi: 10.3390/nu11010177
13. Kurki, MI, Karjalainen, J, Palta, P, Sipilä, TP, Kristiansson, K, Donner, KM, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. (2023) 613:508–18. doi: 10.1038/s41586-022-05473-8
14. Xie, J, Huang, H, Liu, Z, Li, Y, Yu, C, Xu, L, et al. The associations between modifiable risk factors and nonalcoholic fatty liver disease: a comprehensive Mendelian randomization study. Hepatol Baltim Md. (2023) 77:949–64. doi: 10.1002/hep.32728
15. Sollis, E, Mosaku, A, Abid, A, Buniello, A, Cerezo, M, Gil, L, et al. The NHGRI-EBI GWAS catalog: knowledgebase and deposition resource. Nucleic Acids Res. (2023) 51:D977–85. doi: 10.1093/nar/gkac1010
16. Burgess, S, Butterworth, A, and Thompson, SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. (2013) 37:658–65. doi: 10.1002/gepi.21758
17. Bowden, J, Davey Smith, G, and Burgess, S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
18. Hartwig, FP, Davey Smith, G, and Bowden, J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. (2017) 46:1985–98. doi: 10.1093/ije/dyx102
19. Zhao, Q, Chen, Y, Wang, J, and Small, DS. Powerful three-sample genome-wide design and robust statistical inference in summary-data Mendelian randomization. Int J Epidemiol. (2019) 48:1478–92. doi: 10.1093/ije/dyz142
20. Verbanck, M, Chen, C-Y, Neale, B, and Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
21. Bowden, J, Spiller, W, Del Greco, MF, Sheehan, N, Thompson, J, Minelli, C, et al. Improving the visualization, interpretation and analysis of two-sample summary data Mendelian randomization via the radial plot and radial regression. Int J Epidemiol. (2018) 47:2100. doi: 10.1093/ije/dyy265
22. Greco, MFD, Minelli, C, Sheehan, NA, and Thompson, JR. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med. (2015) 34:2926–40. doi: 10.1002/sim.6522
23. Skrivankova, VW, Richmond, RC, Woolf, BAR, Yarmolinsky, J, Davies, NM, Swanson, SA, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR statement. JAMA. (2021) 326:1614–21. doi: 10.1001/jama.2021.18236
24. Acalovschi, M. Gallstones in patients with liver cirrhosis: incidence, etiology, clinical and therapeutical aspects. World J Gastroenterol. (2014) 20:7277–85. doi: 10.3748/wjg.v20.i23.7277
25. Méndez-Sánchez, N, González, V, Aguayo, P, Sánchez, JM, Tanimoto, MA, Elizondo, J, et al. Fish oil (n-3) polyunsaturated fatty acids beneficially affect biliary cholesterol nucleation time in obese women losing weight. J Nutr. (2001) 131:2300–3. doi: 10.1093/jn/131.9.2300
26. Lee, SY, Jang, SI, Cho, JH, Do, MY, Lee, SY, Choi, A, et al. (2024). Gallstone dissolution effects of combination therapy with n-3 polyunsaturated fatty acids and ursodeoxycholic acid: a randomized, prospective, preliminary clinical trial. Gut Liver. doi: 10.5009/gnl230494 [Epub ahead of print].
27. Smelt, AHM. Triglycerides and gallstone formation. Clin Chim Acta Int J Clin Chem. (2010) 411:1625–31. doi: 10.1016/j.cca.2010.08.003
28. Kim, JK, Cho, SM, Kang, SH, Kim, E, Yi, H, Yun, ES, et al. N-3 polyunsaturated fatty acid attenuates cholesterol gallstones by suppressing mucin production with a high cholesterol diet in mice. J Gastroenterol Hepatol. (2012) 27:1745–51. doi: 10.1111/j.1440-1746.2012.07227.x
29. Jonkers, IJ, Smelt, AHM, Ledeboer, M, Hollum, ME, Biemond, I, Kuipers, F, et al. Gall bladder dysmotility: a risk factor for gall stone formation in hypertriglyceridaemia and reversal on triglyceride lowering therapy by bezafibrate and fish oil. Gut. (2003) 52:109–15. doi: 10.1136/gut.52.1.109
30. Chen, J, Sun, B, and Zhang, D. Association of Dietary n3 and n6 fatty acids intake with hypertension: NHANES 2007-2014. Nutrients. (2019) 11:1232. doi: 10.3390/nu11061232
31. Zhang, M, Mao, M, Zhang, C, Hu, F, Cui, P, Li, G, et al. Blood lipid metabolism and the risk of gallstone disease: a multi-center study and meta-analysis. Lipids Health Dis. (2022) 21:26. doi: 10.1186/s12944-022-01635-9
32. Atamanalp, SS, Keles, MS, Atamanalp, RS, Acemoglu, H, and Laloglu, E. The effects of serum cholesterol, LDL, and HDL levels on gallstone cholesterol concentration. Pak J Med Sci. (2013) 29:187–90. doi: 10.12669/pjms.291.2798
33. Maurer, KJ, Carey, MC, and Fox, JG. Roles of infection, inflammation, and the immune system in cholesterol gallstone formation. Gastroenterology. (2009) 136:425–40. doi: 10.1053/j.gastro.2008.12.031
34. Liu, Z, Kemp, TJ, Gao, Y-T, Corbel, A, McGee, EE, Wang, B, et al. Association of circulating inflammation proteins and gallstone disease. J Gastroenterol Hepatol. (2018) 33:1920–4. doi: 10.1111/jgh.14265
35. Mozaffarian, D, and Wu, JHY. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. (2011) 58:2047–67. doi: 10.1016/j.jacc.2011.06.063
36. Seah, JYH, Gay, GMW, Su, J, Tai, E-S, Yuan, J-M, Koh, W-P, et al. Consumption of red meat, but not cooking oils high in polyunsaturated fat, is associated with higher arachidonic acid status in Singapore Chinese adults. Nutrients. (2017) 9:101. doi: 10.3390/nu9020101
37. Caroli-Bosc, FX, Deveau, C, Peten, EP, Delabre, B, Zanaldi, H, Hebuterne, X, et al. Cholelithiasis and dietary risk factors: an epidemiologic investigation in Vidauban, Southeast France. General Practitioner’s group of Vidauban. Dig Dis Sci. (1998) 43:2131–7. doi: 10.1023/A:1018879819301
38. Misciagna, G, Centonze, S, Leoci, C, Guerra, V, Cisternino, AM, Ceo, R, et al. Diet, physical activity, and gallstones—a population-based, case-control study in southern Italy. Am J Clin Nutr. (1999) 69:120–6. doi: 10.1093/ajcn/69.1.120
39. Tsai, C-J, Leitzmann, MF, Willett, WC, and Giovannucci, EL. Long-chain saturated fatty acids consumption and risk of gallstone disease among men. Ann Surg. (2008) 247:95–103. doi: 10.1097/SLA.0b013e31815792c2
40. Julibert, A, Bibiloni, MDM, and Tur, JA. Dietary fat intake and metabolic syndrome in adults: a systematic review. Nutr Metab Cardiovasc Dis. (2019) 29:887–905. doi: 10.1016/j.numecd.2019.05.055
41. Hooper, L, Martin, N, Jimoh, OF, Kirk, C, Foster, E, and Abdelhamid, AS. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst Rev. (2020) 5:CD011737. doi: 10.1002/14651858.CD011737.pub2
42. Lovejoy, JC. Dietary fatty acids and insulin resistance. Curr Atheroscler Rep. (1999) 1:215–20. doi: 10.1007/s11883-999-0035-5
43. Kennedy, A, Martinez, K, Chuang, C-C, LaPoint, K, and McIntosh, M. Saturated fatty acid-mediated inflammation and insulin resistance in adipose tissue: mechanisms of action and implications. J Nutr. (2009) 139:1–4. doi: 10.3945/jn.108.098269
44. Mei, J, Qian, M, Hou, Y, Liang, M, Chen, Y, Wang, C, et al. Association of saturated fatty acids with cancer risk: a systematic review and meta-analysis. Lipids Health Dis. (2024) 23:32. doi: 10.1186/s12944-024-02025-z
45. Chen, L-Y, Qiao, Q-H, Zhang, S-C, Chen, Y-H, Chao, G-Q, and Fang, L-Z. Metabolic syndrome and gallstone disease. World J Gastroenterol. (2012) 18:4215–20. doi: 10.3748/wjg.v18.i31.4215
46. Méndez-Sánchez, N, Chavez-Tapia, NC, Motola-Kuba, D, Sanchez-Lara, K, Ponciano-Rodríguez, G, Baptista, H, et al. Metabolic syndrome as a risk factor for gallstone disease. World J Gastroenterol. (2005) 11:1653–7. doi: 10.3748/wjg.v11.i11.1653
47. Compagnucci, AB, Perroud, HA, Batallés, SM, Villavicencio, R, Brasca, A, Berli, D, et al. A nested case-control study on dietary fat consumption and the risk for gallstone disease. J Hum Nutr Diet Off J Br Diet Assoc. (2016) 29:338–44. doi: 10.1111/jhn.12332
48. Ortega, RM, Fernández-Azuela, M, Encinas-Sotillos, A, Andrés, P, and López-Sobaler, AM. Differences in diet and food habits between patients with gallstones and controls. J Am Coll Nutr. (1997) 16:88–95. doi: 10.1080/07315724.1997.10718655
49. Maurer, KR, Everhart, JE, Knowler, WC, Shawker, TH, and Roth, HP. Risk factors for gallstone disease in the Hispanic populations of the United States. Am J Epidemiol. (1990) 131:836–44. doi: 10.1093/oxfordjournals.aje.a115574
Keywords: fatty acid, gallstone, cross-sectional study, NHANES, Mendelian randomization
Citation: Wang M, Guo J and Sun S (2024) Dietary fatty acids and gallstone risk: insights from NHANES and Mendelian randomization analysis. Front. Nutr. 11:1454648. doi: 10.3389/fnut.2024.1454648
Edited by:
Francesk Mulita, General University Hospital of Patras, GreeceReviewed by:
Andreas Antzoulas, General University Hospital of Patras, GreeceAngelis Peteinaris, University of Patras, Greece
Copyright © 2024 Wang, Guo and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Siyu Sun, c3Vuc3lAc2otaG9zcGl0YWwub3Jn
 Minghe Wang
Minghe Wang Jintao Guo
Jintao Guo Siyu Sun*
Siyu Sun*