- 1Second Clinical Medical College, Guangzhou University of Chinese Medicine, Guangzhou, China
- 2Meizhou Maternity and Child HealthCare Hospital, Meizhou, China
- 3Department of Endocrinology, Guangdong Provincial Hospital of Chinese Medicine, Guangdong, China
- 4State Key Laboratory of Dampness Syndrome of Chinese Medicine, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
Objective: This study aims to explore the correlation between patients with Hashimoto’s thyroiditis and food intolerance.
Methods: A total of 172 subjects who visited Guangdong Provincial Hospital of Traditional Chinese Medicine between January 2020 and March 2023 were selected and tested for 90 food-specific IgG antibodies. The study group composed 85 individuals diagnosed with Hashimoto’s thyroiditis, while the control group consisted of 87 healthy individuals. Data were analyzed to determine the correlation between Hashimoto’s thyroiditis and food intolerance.
Results: Among the 85 patients with Hashimoto’s thyroiditis, 97.65% exhibited food intolerance, with an average of 15.76 ± 10.61 types of food intolerances. The most common intolerances were to eggs (75.29%), bok choy (71.76%), and milk (65.88%), each exceeding a 60% intolerance rate. In the control group of 87 healthy individuals, the intolerance rate was 95.40%, with an average of 9.57 ± 8.90 types of food intolerances. The most prevalent intolerances in the control group were to bok choy (54.02%) and eggs (52.87%), each exceeding a 50% intolerance rate.
Conclusion: The findings suggest that patients with Hashimoto’s thyroiditis are more likely to develop food intolerance compared to the healthy population, which may indicate a correlation between Hashimoto’s thyroiditis and food intolerance. Different dietary patterns may affect the activity of the thyroid axis and may even be the cause of autoimmune thyroid disease. The technique of detecting food intolerance IgG antibodies has the potential to be an important reference for dietary interventions in patients with Hashimoto’s thyroiditis.
1 Introduction
Hashimoto’s thyroiditis (HT) (1), also known as chronic lymphocytic thyroiditis, is a common autoimmune disorder characterized by widespread thyroid enlargement and the presence of high levels of thyroid peroxidase antibodies (TPOAb) and thyroglobulin antibodies (TgAb) in the serum. As the disease progresses, the thyroid gland is gradually destroyed, leading to abnormal gland function, with about 20–30% (2) of patients developing hypothyroidism. Certain patients (3) display extremely high antibody titers, significant changes in thyroid texture, and persistent thyroid function abnormalities, including pronounced fluctuations in TSH levels (fluctuating between hyperthyroidism and hypothyroidism). These cases present significant clinical challenges, as conventional treatments often fail to maintain normal thyroid function and reduce thyroid-associated antibodies, highlighting the urgent need for innovative diagnostic and therapeutic approaches.
It has been found that (4, 5) the immune response is more pronounced in patients with Hashimoto’s thyroiditis compared to the normal population and is closely related to the gut flora (6). Due to the strong link between diet and gut flora, we hope to find effective interventions and treatments by modifying diet. Food intolerance (FI) (7) is an IgG-mediated immune response that can cause inflammation in multiple tissues. If dietary structures are not promptly adjusted to mitigate this immune response, it can lead to abnormal organ function. An et al. (8) conducted food-specific IgG antibody testing on 153 HT patients and found a positive rate of 64.05%. They concluded that food intolerance is common in HT patients. The aim of this study was to investigate the relationship between Hashimoto’s thyroiditis and food intolerance (specific IgG antibody test for 90 foods).
2 Materials and methods
2.1 Subjects
A total of 172 subjects who visited Guangdong Provincial Hospital of Traditional Chinese Medicine between January 2020 and March 2023 were selected and tested for 90 food-specific IgG antibodies. Among them, 85 were diagnosed with Hashimoto’s thyroiditis (HT group), and 87 were included in a healthy control group (Non-HT group).
2.1.1 Diagnostic criteria for Hashimoto’s thyroiditis
In conjunction with the 2008 Chinese Guidelines for the Diagnosis and Treatment of Thyroid Diseases-Thyroiditis (9) and the American Thyroid Association’s (10) manual on Hashimoto’s thyroiditis: Any patient with diffuse thyroid enlargement, a tougher gland texture, especially with an enlarged pyramidal lobe, should be suspected of HT. Diagnosis can be confirmed if serum TPOAb and TgAb are positive. Fine Needle Aspiration Cytology (FNAC) has diagnostic value. Clinical or subclinical hypothyroidism further supports the diagnosis.
In this study, the diagnosis of HT was mainly made by thyroid ultrasound presenting changes characteristic of HT (e.g., abundant blood flow signal, diffuse enlargement, etc.) and positive TPOAb and TgAb.
2.1.2 Criteria for assessing the healthy population
The healthy control group (Non-HT group) was defined as people whose thyroid-related antibody (TPOAb and TgAb) tests were suggestive of negativity and whose thyroid ultrasound did not show Hashimoto’s thyroiditis-specific changes. In addition, in order to minimize the interference of other autoimmune diseases with the results of this study, we excluded subjects with a clear diagnosis of other autoimmune diseases, such as type 1 diabetes mellitus, Addison’s syndrome, and systemic lupus erythematosus. Sensitized subjects with multiple allergies were also excluded (mainly to multiple IgE antibodies).
2.2 Methods
2.2.1 Collection of clinical data
We meticulously recorded data concerning the subjects’ age, gender, thyroid ultrasound findings, thyroid function test results, and levels of thyroid-related antibodies (TgAb, TPOAb).
2.2.2 Measurement of thyroid-related indicators
Blood samples were collected from all subjects, and quality control was conducted by the Laboratory Department of Guangdong Provincial Hospital of Traditional Chinese Medicine. Free triiodothyronine (FT3), free thyroxine (FT4), and thyroid-stimulating hormone (TSH) levels were measured using chemiluminescence, while TgAb and TPOAb levels were measured using electrochemiluminescence.
The normal reference ranges are as follows: FT3: 3.5–6.5 pmol/L, FT4: 11.5–22.7 pmol/L, TSH: 0.51–4.97 mIU/L, TgAb: < 60 U/mL, TPOAb: < 60 U/mL.
2.2.3 Measurement of food-specific IgG antibodies
Blood samples were collected from all subjects after fasting for 12 h and were sent to the Guangzhou JingYu Center for Clinical Laboratory. Food-specific IgG antibody test kits (HOB Biotech Group) were used to measure serum concentrations of 90 food-specific antibodies and to perform grading.
The grading standards were as follows: < 50 U/mL as negative; ≥ 50 U/mL as positive; under the positive result, the antibody titer level was graded as follows: 50–100 U/mL as level I intolerance; 100–200 U/mL as level II intolerance; ≥ 200 U/mL as level III intolerance.
The 90 food items total include: Cheddar cheese, White soft cheese, Beef, Chicken, Pork, Eggs, Lamb, Turkey, Yogurt, Chocolate, Corn, Rice, Wheat, Soybeans, Lemons, Spinach, Green beans, Millet, Almonds, Sesame seeds, Cashews, Peanuts, Dried, celery, Eggplants, Green peppers, Parsley, Cabbage, Carrots, Cauliflower, Young peas, Sunflower seeds, Black walnuts, Blueberries, Pineapple, Oranges, Peaches, Apples, Durian, Mango, Banana, Grape Grapefruit, Strawberry, Cantaloupe, Malt, Rye, Yeast, Cane sugar, Butter, Cinnamon, Mustard, Honey, Coffee, Oats, Barley, Buckwheat, Broccoli, Mixed colored peas, Garlic, Onion, Cola beans, Black tea, Milk, Crab, Shrimp, Cod, Goat’s milk, Clam, Salmon, Sardines, Cutlassfish, Scallop, Lobster, Grass carp, Oysters, Trout, Tuna, Red pepper garlic, Spring onion, Lettuce, Cucumber, Potato, Pumpkin, Sweet potato, Cilantro, Bok choy, Tomato, Mushroom, Watermelon, and Olive.
2.2.4 Statistical
Data were analyzed using IBM Statistical Package for the Social Sciences, Version 26.0. The level of significance for tests was set at α = 0.05. Count data were expressed as frequencies and percentages. Comparisons between groups were made using the Chi-square test or Fisher’s exact test, with P < 0.05 indicating statistical significance.
3 Results
3.1 General characteristics of patients with Hashimoto’s thyroiditis and healthy individuals
Among the 85 patients with Hashimoto’s thyroiditis, TgAb levels averaged (391.42 ± 528.82) U/mL, and TPOAb levels averaged (864.41 ± 518.45) U/mL. The average age was (35.94 ± 14.10) years, with 73 females and 12 males. In the Non-HT group of 87 individuals, the average age was (44.39 ± 13.47) years, with 63 females and 24 males.
3.2 Comparison of intolerance to 90 food groups
Among the 90 food items tested, the intolerance rates for cheddar cheese, white cottage cheese, eggs, yogurt, wheat, carrots, pineapple, bananas, cantaloupe, malt, butter, cinnamon, mustard, cow’s milk, crab, goat’s milk, scallops, grass carp, cucumber, pumpkin, and mushrooms showed significant differences between the two groups (see Table 1). Notably, none of the subjects in this study exhibited intolerance to turkey meat, strawberries, cola beans, or sweet potato (see Table 1).
3.3 Distribution of food intolerance in serum of HT patients
A total of 85 patients with HT were included in the study, 83 of whom exhibited elevated food-specific IgG antibodies, resulting in a positive detection rate of 97.65%. The average number of intolerances per patient was (15.76 ± 10.61), with a range from 0 to 57 types. Among the 90 food items tested, the top fourteen with the highest positive rates were: eggs (75.29%), bok choy (71.76%), milk (65.88%), pumpkin (55.29%), white soft cheese (49.41%), almonds (49.41%), cinnamon (47.06%), peanuts (45.88%), yogurt (44.71%), malt (44.71%), goat’s milk (44.71%), potatoes (44.71%), mustard (38.82%), and cheddar cheese (35.29%). The highest rates of intolerance were primarily to eggs and dairy products (including cow’s milk, yogurt, goat’s milk, soft white cheese, and cheddar cheese).
Analysis of the severity levels of intolerance for the three foods with the highest positive rates revealed the following: For eggs, 26 patients (41%) exhibited level I intolerance, 21 patients (33%) exhibited level II intolerance, and 17 patients (26%) exhibited level III intolerance. For bok choy, 50 patients (82%) exhibited level I intolerance, 10 patients (16%) exhibited level II intolerance, and 1 patient (2%) exhibited level III intolerance. For cow’s milk, 14 patients (25%) exhibited level I intolerance, 17 patients (30%) exhibited level II intolerance, and 25 patients (45%) exhibited level III intolerance (see Figure 1).

Figure 1. Proportion of severity levels for the top three foods with the highest positive intolerance rates in the HT group. (a) For eggs: 26 patients (41%) exhibited level I intolerance, 21 patients (33%) exhibited level II intolerance, and 17 patients (26%) exhibited level III intolerance. (b) For bok choy: 50 patients (82%) exhibited level I intolerance, 10 patients (16%) exhibited level II intolerance, and 1 patient (2%) exhibited level III intolerance. (c) For milk: 14 patients (25%) exhibited level I intolerance, 17 patients (30%) exhibited level II intolerance, and 25 patients (45%) exhibited level III intolerance.
3.3.1 Distribution of the top 14 foods with the highest positive rates among Hashimoto’s thyroiditis patients by gender
An analysis comparing the food intolerance rates of the top 14 items among different genders within the HT group revealed that only potatoes showed significant differences between genders (see Table 2).
3.3.2 Distribution of the top 14 foods with the highest positive rates among Hashimoto’s thyroiditis patients by age
The analysis of food intolerance rates for the top 14 items among different age groups within the HT group revealed significant variations in the positive rates for eggs, cow’s milk, soft white cheese, yogurt, goat’s milk, and cheddar cheese across different age brackets (see Table 3).
3.4 Distribution of food intolerance in serum of the healthy population
This study included 87 healthy individuals, 83 of whom showed elevated levels of food-specific IgG antibodies, resulting in a positive detection rate of 95.40%. The average number of food intolerances per individual was (9.57 ± 8.90), with a range from none to as many as 41 different types. Among the 90 foods tested, the fourteen with the highest positive rates were bok choy (54.02%), eggs (52.87%), milk (35.63%), almonds (29.89%), peanuts (29.89%), cinnamon (28.74%), malt (27.59%), pumpkin (27.59%), cashews (24.14%), soft white cheese (22.99%), millet (22.99%), potatoes (22.99%), sunflower seeds (21.84%), and onions (19.54%).
Analyzing the three foods with the highest rates of food intolerance: For bok choy, 40 individuals (85%) exhibited level I intolerance, 7 individuals (15%) exhibited level II intolerance, and none (0%) exhibited level III intolerance. For eggs, 27 individuals (59%) exhibited level I intolerance, 10 individuals (22%) exhibited level II intolerance, and 9 individuals (19%) exhibited level III intolerance. For cow’s milk, 13 individuals (42%) exhibited level I intolerance, 9 individuals (29%) exhibited level II intolerance, and 9 individuals (29%) exhibited level III intolerance (see Figure 2).
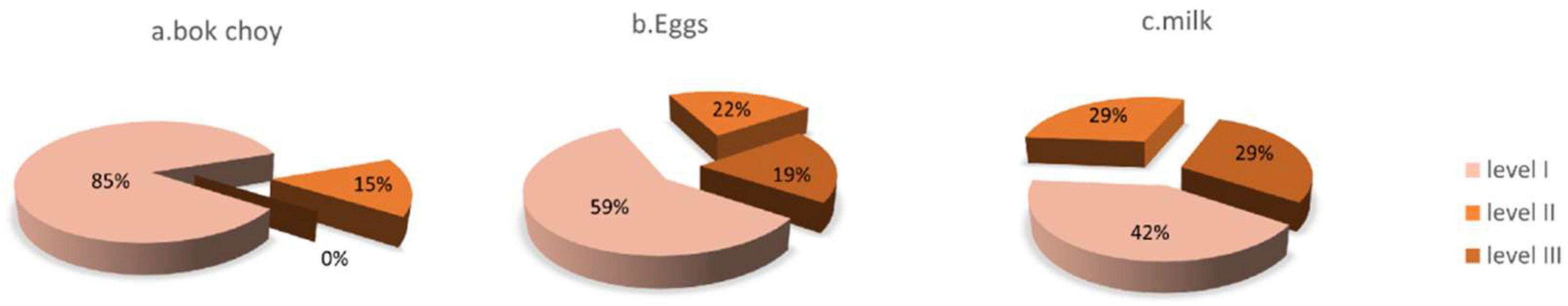
Figure 2. Proportion of severity levels for the top three foods with the highest positive intolerance rates in the non-HT group. (a) For bok choy: 40 individuals (85%) exhibited level I intolerance, 7 individuals (15%) exhibited level II intolerance, and none (0%) exhibited level III intolerance. (b) For eggs: 27 individuals(59%) exhibited level I intolerance, 10 individuals (22%) exhibited level II intolerance, and 9 individuals (19%) exhibited level III intolerance. (c) For milk: 13 individuals (42%) exhibited level I intolerance, 9 individuals (29%) exhibited level II intolerance, and 9 individuals (29%) exhibited level III intolerance.
4 Discussion
Xue (11) and Zhao et al. (12) analyzed the serum specific IgE and IgG of nearly 100 children with allergic purpura, and found that food sIgG, food allergen sIgE, inhalant allergens sIgE and tIgE were closely related to the development of the disease in the children, and concluded that avoiding contact with allergens in the clinic could play a therapeutic and preventive role in the disease. In addition, the results showed that the positive rates of food-specific IgG antibodies were higher than those of inhalant allergen IgE, which led some scholars to suggest that “food intolerance-specific IgG antibodies can be a powerful complement to the detection of allergen IgE antibodies.” The rapid onset of IgE-mediated food allergy, with onset of symptoms within minutes to hours and severe anaphylactic shock, is a far cry from the chronic onset of Hashimoto’s thyroiditis, which is not easy to detect.
Compared to IgE-mediated allergies, IgG-mediated food intolerances align more closely with the characteristics of Hashimoto’s thyroiditis. Recent research indicates that food intolerances are not limited to gastrointestinal disorders but are also associated with the development of various diseases, such as psoriasis (13), systemic lupus erythematosus (14), chronic urticaria (15), and many other immune disorders.
The mechanisms underlying food intolerance are diverse and debated. The predominant theory (16, 17) posits that food should be completely digested into low molecular weight nutrients in the gastrointestinal tract. However, intolerance can arise from factors such as the food’s matrix composition, enzyme deficiencies, or imbalances in gut microbiota, which lead to the entry of food as peptides or other large molecules into the intestines. When these large molecules are recognized as foreign by the body, they trigger an autoimmune response. Hu et al. (18) studied seventeen types of wheat to assess whether the digestibility of gluten correlates with its potential allergenicity. Their findings suggest that undigested gluten has significant immunogenicity, which diminishes as the gluten is broken down during digestion, thereby reducing its immune imprint. This research provides indirect evidence supporting the proposed mechanisms of food intolerance. Furthermore, studies by Maleki et al. (19) indicate that the Maillard reaction during peanut processing creates higher molecular weight aggregates, increasing their resistance to digestive breakdown and enhancing their allergenic potential. Consequently, the composition of the food matrix and processing techniques may also play a critical role in sensitization.
All foods have the potential to act as allergens (18, 20), with specific proteins within the foods being the primary culprits. The larger and more complex the protein, the stronger its potential for causing allergies. In this study, eggs and dairy had particularly high rates of food intolerance, with both appearing among the top 14 most intolerant foods out of the 90 tested. The high protein content in eggs and dairy, along with their stable structures that resist heat processing and enzymatic breakdown, may contribute to their high allergenic potential. Additionally, the likelihood of allergic reactions to eggs and dairy appears to diminish with age (8),possibly due to changes in consumption patterns across different life stages.
Daily diet often influences the development of diseases (21–25). Dietary management (26, 27) has been shown to improve nutritional status, quality of life, blood sugar levels, and thyroid function, while effectively reducing the incidence of acute and chronic complications. Many researchers propose the existence of a thyroid-gut axis (28), where different dietary patterns can indirectly or directly influence thyroid function (23, 25) and even be a contributing factor to autoimmune thyroid diseases. A PREDIMED trial by Ruggeri et al. (29), involving a questionnaire survey of 81 HT patients and 119 healthy individuals, revealed that HT patients consumed higher amounts of animal-based foods (especially red meat and processed products), whereas the healthy group consumed more plant-based foods, including legumes.
Modern medical research has recognized that food intolerance is widespread; Kalicanin et al. (30) conducted tests for 125 food-specific IgG antibodies on 74 HT patients and 245 healthy controls. By calculating the food intolerance rates and analyzing the correlation with thyroid function, thyroid-related antibodies, and thyroid volume, they concluded that elevated IgG antibodies have no clinical correlation with the progression of Hashimoto’s thyroiditis to hyperthyroidism or hypothyroidism. Furthermore, the increase in IgG antibodies is not related to the severity of hypothyroidism symptoms; Chen et al. (31) analyzed the correlation between seven thyroid function indicators and the results of 14 food intolerance tests in 45,764 individuals undergoing physical examinations. Their findings indicated that food intolerance is a risk factor for abnormal thyroid function. Eggs, soybeans, crabs, and pork were particularly noteworthy, as intolerance to these four foods correlated with thyroid function abnormalities. Among the study subjects, 24.77% exhibited at least one of the seven thyroid function abnormalities. The highest abnormality rate was observed in TSH levels, followed by TgAb and TPOAb, all of which were statistically significant.
Taken together with previous studies, we consider that food intolerance is associated with thyroid dysfunction in the immune state, rather than being a specific factor that leads to hyperthyroidism or hypothyroidism, so we did not clearly distinguish between combined hyperthyroidism or combined hypothyroidism in our study. Given the high cost of the test, we did not perform it on all patients with Hashimoto’s thyroiditis, but rather on those with severe symptoms, such as high antibody titers, significant changes in thyroid texture, difficult-to-correct thyroid dysfunction, and significant fluctuations in thyroid-stimulating hormone (alternating between hyperthyroidism and hypothyroidism), which may have contributed to the increased positivity rate. The use of a 90-food test in the present study, compared to previous studies that tested 7 or 14 foods, also contributed to the higher overall positive rate of intolerance observed.
This study demonstrates a close correlation between Hashimoto’s thyroiditis and food intolerance. Compared to the healthy population, patients with Hashimoto’s thyroiditis are intolerant to a broader array of foods. Among the 90 foods tested, significant differences in intolerance rates between the two groups were noted for cheddar cheese, soft white cheese, eggs, yogurt, wheat, carrots, pineapple, bananas, cantaloupe, malt, butter, cinnamon, mustard, cow’s milk, crab, goat’s milk, belt fish, grass carp, cucumber, pumpkin, and mushrooms (P < 0.05). HT patients showed particularly high intolerance rates to eggs and dairy products, predominantly at levels II and III of intolerance; interestingly, bok choy had a very high intolerance rate (HT group: 71.76%, healthy population: 54.02%), mostly at level I, but the difference between the two groups was not statistically significant (χ2 = 6.534, P = 0.061), which may be related to dietary habits in the Guangdong region.
Mei et al. (15) employed a treatment regimen combining medication with dietary restrictions (mainly including “alternate diet” “No-Eat” and “Absolute No-Eat”) based on food intolerance test results in 180 patients with chronic urticaria. Their study found that compared to the control group, the quality of life of the treatment group improved, and the duration and frequency of outbreaks decreased. Therefore, future prospective studies could enhance the clinical value of this research. Currently, there are few studies on the correlation between food intolerance and Hashimoto’s thyroiditis, and no observational studies have been conducted in China regarding dietary management changes.
Additionally, the limitations of this study include a small sample size, significant gender imbalance, and a relatively narrow range of tested foods, indicating the need for further in-depth research in the future.
Author contributions
MY: Data curation, Writing – original draft, Writing – review & editing. HaW: Data curation, Writing – original draft. KZ: Data curation, Writing – original draft. PG: Data curation, Writing – original draft. YW: Data curation, Writing – original draft. HuW: Writing – review & editing.
Funding
The authors declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the Guangdong Famous Chinese Medicine Workshop: Shusen Li Workshop Foundation Program, the Guangdong Famous Chinese Medicine Workshop: Zhizheng Lu Workshop Foundation Program (E43710) and State Key Laboratory of Dampness Syndrome of Chinese Medicine (SZ2021ZZ3203).
Acknowledgments
We express our gratitude to Mr. Shusen Li for his invaluable guidance and support. His insights into my research topic were crucial in shaping my understanding and direction.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Song Y, Bai Y, Liu C, Zhai X, Zhang L. The impact of gut microbiota on autoimmune thyroiditis and relationship with pregnancy outcomes: a review. Front Cell Infect Microbiol. (2024) 14:1361660. doi: 10.3389/fcimb.2024.1361660
2. Wu C, Guo F, Qln M, Han Y. Clinical effect of wenyang xiaoying formula on Hashimoto’s Thyroiditis and lts influence on TPOAb and TGAb titers. J Pract Traditional Chin Int Med. (2024) 38(02):31–4. doi: 10.13729/j.issn.1671-7813
3. Vassallo A, Ferrari F, Di Filippo L, Giustina A, Loli P. Transition from Hashimoto thyroiditis to Graves’s disease: an unpredictable change? Endocrine. (2024) 84(2):541–8. doi: 10.1007/s12020-023-03634-x
4. Polymeris A, Papapetrou PD, Psachna S, Ioannidis D, Lilis D, Drakou M, et al. Patients with Hashimoto’s thyroiditis present higher immune response to COVID-19 mRNA vaccine compared to normal individuals. Horm Int J Endocrino. (2024) 23(1):89–95. doi: 10.1007/s42000-023-00470-6
5. Sánchez-Gutiérrez R, Martínez-Hernández R, Serrano-Somavilla A, Sampedro-Nuñez M, Mendoza-Pérez A, de Nova J, et al. Analysis of T follicular and T peripheral helper lymphocytes in autoimmune thyroid disease. Endocrine. (2024): Online ahead of print. doi: 10.1007/s12020-024-03686-7
6. Zhu XX, Zhang C, Feng SY, He R, Zhang S. Intestinal microbiota regulates the gut-thyroid axis: the new dawn of improving Hashimoto thyroiditis. Clin Exp Med. (2024) 24(1):39. doi: 10.1007/s10238-024-01304-4
8. An C, Lu D, Kong X, Li X, Lu C, Wen L, et al. Study on the relationship between food specific lgG antibody and Hashimoto’s thyroiditis. Int J Immunol. (2021) 44:626–30. doi: 10.3760/cma.j.issn.1673-4394.2021.06.005
9. Organization of the Endocrinology Branch of the Chinese Medical Association. Chinese Society of Endocrinology, Chinese Society of Medical Sciences, Guidelines for the Diagnosis and Treatment of Thyroid Diseases in China (2008).
10. American Thyroid Association. Hashimoto’s Thyroiditis (Chronic Lymphocytic Thyroiditis or Autoimmune Thyroiditis. Virginia: American Thyroid Association
11. Xue X. The Study on the Relationship Between Food intolerance lgG and Serum Specific lgE in Abdominal type Henoch-SchönleinPurpura in children [D]. (2017). Master’s thesis. Binzhou: Binzhou Medical University.
12. Zhao C, Zhao P, Bai Y, et al. Analysis of serum specific immunoglobulin E and immunoglobulin G in children with allergic purpura. Chin J Med. (2021) 56(02):212–5. doi: 10.3969/j.issn.1008-70.2021.02.029
13. Wei C, Ying-Juan W, Xiao-Yuan W, et al. Detection and analysis of food intolerance in patients with psoriasis. Dermatol Bull. (2014) 31(04):212–4.
14. Yang Q. A Preliminary Study on the Relationship between Systemic Lupus Erythematosus and Food Intolerance. Master’s Degree. (2016). Master’s thesis. Guangzhou: Medical University of the South (MUMSouth).
15. Mei T, Han H, Jin H. Patients with chronic urticaria triggered by food intolerance were treated with a combination of drug food avoidance therapy in patients with chronic urticaria caused by food intolerance. J Qiqihar Med Univer. (2020) 41(01):61–2.
16. Chen Y. Current Status of IgG Antibody Detection of 14 Food Intolerances in Health Checkup Population and its Relationship with Related Diseases. Master’s Degree. (2014). Master’s thesis. Guangzhou: Medical University of the South (MUMSouth).
17. Wang G, Feng L. Study on the prevalence of food intolerance and related factors in health checkup population. J Preventive Med Chin Peopte’s Liberatton Army. (2020) 38(06):73–5.
18. Hu X. Digestibility and Potential Allergenicity of Gluten Proteins Inseventeen Wheat Varieties. Master’s Degree. (2021). Master’s thesis. Nanchang: Nanchang University.
19. Maleki SJ, Chung SY, Champagne ET, Raufman JP. The effects of roasting on the allergenic properties of peanut proteins. J Allergy Clin Immun. (2000) 106(4):763–8. doi: 10.1067/mai.2000.109620
20. Liu H. Identification and Quantification Study of Major Allergenic Proteins in Milk, Peanut, Egg and Soybean by Liquid Phase Tandem High Resolution Mass Spectrometry (Master’s Degree) [D]. (2022). Master’s thesis. Yantai: Yantai University.
21. Han H, Xin P, Zhao LN, Hu J, Xia Y, Yang X, et al. Excess iodine and high-fat diet combination modulates lipid profile, thyroid hormone, and hepatic LDLr expression values in mice. Biol Trace Element Res. (2012) 147(1–3):233–9. doi: 10.1007/s12011-011-9300-x
22. Gao J, Lin XY, Liu XH, Yang Q, Zhang Z, Jiang Q, et al. Effect of combined excess iodine and low-protein diet on thyroid hormones and ultrastructure in wistar rats. Biol Trace Element Res. (2013) 155(3):416–22. doi: 10.1007/s12011-013-9811-8
23. Shao SS, Zhao YF, Song YF, Xu C, Yang J, Xuan S, et al. Dietary high-fat lard intake induces thyroid dysfunction and abnormal morphology in rats. Acta Pharmacol Sin. (2014) 35(11):1411–20. doi: 10.038/aps.2014.82
24. Palkowska-Gozdzik E, Lachowicz K, Rosolowska-Huszcz D. Effects of dietary protein on thyroid axis activity. Nutrients. (2018) 10:5. doi: 10.3390/nu10010005
25. Zhang XH, Chen WB, Shao SS, Xu G, Song Y, Xu C, et al. A high-fat diet rich in saturated and mono-unsaturated fatty acids induces disturbance of thyroid lipid profile and hypothyroxinemia in male rats. Mol Nutr Food Res. (2018) 62(6):e1700599. doi: 10.1002/mnfr.201700599
26. Larsen D, Singh S, Brito M. Thyroid, diet, and alternative approaches. J Clin Endocrinol Metab. (2022) 107(11):2973–81. doi: 10.1210/clinem/dgac473
27. Zheng Y, Campbell Rice B, Melkus GD, Zweig S, Jia W, Parekh N, et al. Dietary self-management using mobile health technology for adults with type 2 diabetes: A scoping review. J Diabetes Sci Technol. (2023) 17(5):1212–25. doi: 10.177/19322968231174038
28. Knezevic J, Starchl C, Berisha AT, Amrein K. Thyroid-gut-axis: how does the microbiota influence thyroid function? Nutrients. (2020) 12(6): doi: 10.3390/nu12061769
29. Ruggeri RM, Giovinazzo S, Barbalace MC, Cristani M, Alibrandi A, Vicchio T, et al. Influence of dietary habits on oxidative stress markers in Hashimoto’s Thyroiditis. Thyroid. (2021) 31(1):96–105. doi: 10.1089/thy.2020.0299
30. Kalicanin D, Brcic L, Baric A, Zlodre S, Barbalić M, Torlak Lovrić V, et al. Evaluation of correlations between food-specific antibodies and clinical aspects of Hashimoto’s thyroiditis. J Am Coll Nutr. (2019) 38(3):259–66. doi: 10.1080/07315724.2018.1503103
Keywords: Hashimoto’s thyroiditis, food intolerance, food-specific IgG antibodies, thyroid peroxidase antibodies, thyroglobulin antibodies
Citation: Yan M, Wu H, Zhang K, Gong P, Wang Y and Wei H (2024) Analysis of the correlation between Hashimoto’s thyroiditis and food intolerance. Front. Nutr. 11:1452371. doi: 10.3389/fnut.2024.1452371
Received: 20 June 2024; Accepted: 16 September 2024;
Published: 30 September 2024.
Edited by:
Genco Görgü, Ministry of Health, TürkiyeReviewed by:
Yasemin Özkaya, Other, İzmir, TürkiyeBurak Altındağ, Bandirma Onyedi Eylül University, Türkiye
Copyright © 2024 Yan, Wu, Zhang, Gong, Wang and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua Wei, MTM4Mjk3MDExNjhAMTYzLmNvbQ==
 Manli Yan
Manli Yan Hai Wu
Hai Wu Kaiyuan Zhang
Kaiyuan Zhang Ping Gong
Ping Gong Yiting Wang3
Yiting Wang3