- 1Student Research Committee, Isfahan University of Medical Sciences, Isfahan, Iran
- 2Nutrition and Food Security Research Center, Department of Community Nutrition, School of Nutrition and Food Science, Isfahan University of Medical Sciences, Isfahan, Iran
- 3Nutrition Research Center, Department of Clinical Nutrition, School of Nutrition and Food Sciences, Shiraz University of Medical Sciences, Shiraz, Iran
Background and aim: Although the relationship between selenium and metabolic syndrome (MetS) was previously investigated, the findings were inconsistent. Therefore, we performed a systematic review and dose–response meta-analysis to summarize the association between blood selenium and MetS in adults.
Methods: A comprehensive search was conducted in Medline (PubMed), ISI Web of Science, Scopus, and motor engineering of Google Scholar up to October 1st, 2024. Observational studies which reported the risk of MetS in relation to blood selenium in adults were included. The protocol of the current analysis was registered at PROSPERO as CRD42024486035.
Results: Overall, 16,779 participants and 6,471 cases with MetS from 5 cross-sectional and 7 case–control studies were included in the current systematic review and meta-analysis. The findings showed that participants with the highest blood values of selenium (mean: 268.5 μg/L) in comparison to those with the lowest values (mean: 75.27 μg/L) had 40% higher risk of MetS. Nevertheless, this association was not significant (95%CI: 0.99–1.97). Due to a significant between-study heterogeneity (I2 = 90.4%, p < 0.001), subgroup analysis was conducted based on potential confounders. However, this association was only significant in a few subgroups with low number effect sizes. Linear dose–response analysis illustrated each 50 μg/L increment in circulating selenium was related to 7% higher risk of MetS (RR: 1.07, 95%CI: 0.99, 1.15) However, this association was not statistically significant. Additionally, non-linear dose–response analysis indicated a U-shaped association between blood selenium and risk of MetS with the lowest risk at 160 ug/L of blood selenium (p < 0.001).
Conclusion: There is a U-shaped relationship between blood selenium levels risk of MetS. However, more longitudinal studies are needed to verify the causality of findings and clarify the underlying mechanisms.
Introduction
The combination of metabolic disorders such as hypertension, hyperglycemia, hyperlipidemia, and abdominal obesity has been named metabolic syndrome (MetS) (1). MetS has a growing prevalence worldwide and patients with MetS are strongly prone to have non-communicable diseases (NCDs) such as diabetes, cardiovascular diseases (CVD), and even all-cause mortality. Environmental factors, genetics, aging, lifestyle, obesity, dietary intakes, and eating habits are leading risk factors that are involved in the MetS etiology (1–6) or incidence of its components (4, 7–12). Therefore, antioxidants and dietary intakes would have important role in controlling the metabolic health status (13–20).
Considering the role of essential metals in metabolism, evidence documented the inverse association between abnormal blood values of essential metals and NCDs (21). Selenium is one of the important essential metals that is involved in various physiological and ecological processes due to its role in the metabolism of hormones such as sexual, thyroid, and insulin (22). Additionally, selenium has a protective effect on lipid peroxidation and the structure and function of the cell membrane due to its role in glutathione peroxidase (GSH-PX), an anti-oxidant enzyme (23).
A previous meta-analysis documented that higher dietary selenium intake is protectively related to a lower risk of Mets because of its antioxidant properties (24). However, they did not investigate the association between circulating selenium and the risk of MetS. Although some previous studies illustrated a protective association between blood selenium and MetS (25), some others found a neutral or an adverse association between selenium and MetS (26–28). Furthermore, a previous systematic review investigated this subject (29); however, its findings were controversial. Moreover, due to a lack of enough eligible studies, they could not perform meta-analysis to confirm this relationship. In this regard, a systematic review and dose–response analysis were needed to exactly define the ranges of blood selenium that are protectively or adversely along with the risk of MetS. Therefore, we performed a systematic review and dose–response meta-analysis to summarize the relationship between blood selenium and MetS in adults.
Materials and methods
We provided this study based on the Preferred Reporting Items of Systematic Reviews and Meta-Analysis (PRISMA) Guideline (30). Furthermore, the protocol of the current analysis was registered at PROSPERO1 as CRD42024486035.
Search strategy
A comprehensive search was conducted in Medline (PubMed), ISI Web of Science, and Scopus, up to October 1st, 2024. Moreover, manual screening was conducted for the reference lists of eligible articles and motor engineering of Google Scholar. No limitation was considered for language or publication year. Details of mesh terms and keywords are provided in Supplementary Table 1. After including the results in Endnote software, duplicated articles were removed. Then, the title and abstract of the rest of the publications were independently screened by two investigators (ZH and SF). Any problem was dissolved through consulting by the main researcher (GA).
Inclusion criteria
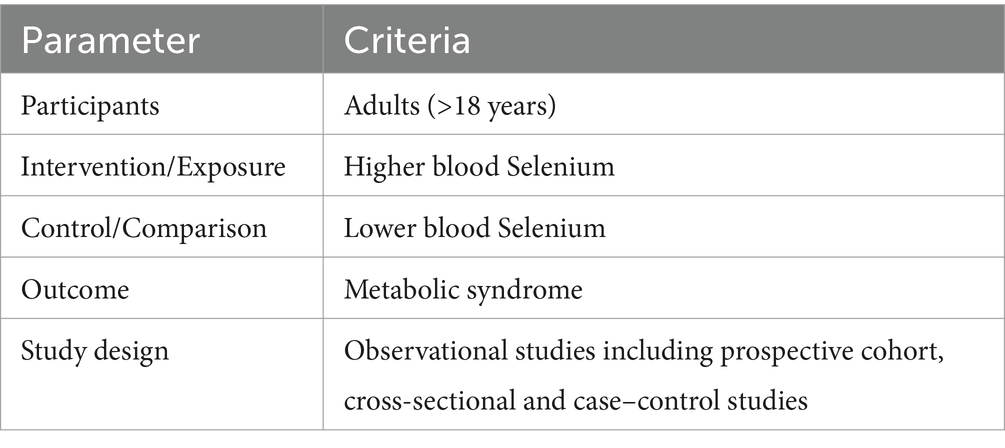
Relevant articles were included if they: (1) considered blood selenium as exposure and MetS as outcome; (2) investigated adults (≥18); and (3) reported odds ratios (ORs), relative risks (RRs), or hazard ratios (HRs) with 95% confidence intervals (CIs) for this association. Details of population, intervention/exposure, comparison/control, outcome, and study design (PICOS) criteria are provided in Table 1.
Exclusion criteria
Studies that met at least one of the following items were excluded: (1) assessed dietary selenium intakes, selenoprotein P or urine, nail, and hair selenium instead of blood selenium; (2) investigated children or adolescents; (3) reported mean ± standard deviation (SD), beta coefficient or correlation and absolute abundance instead of OR, RR, HR.
Data extraction
The necessary information including demographic characteristics (age, gender, and target population), blood selenium, method of blood selenium or MetS assessment, related statistical tests, and their results were separately extracted by two investigators (ZH and SF). The principal investigator (GA) supervised this process.
Quality assessment
The Newcastle-Ottawa Scale (NOS) was used to evaluate the quality of studies. Considering the domains of selection of participants, comparability, and evaluation of outcomes, a total of 10 scores were given to each cross-sectional study. Additionally, a maximum of 9 scores was assigned to each case–control study by evaluating the items of participant selection, comparability, and exposure. Details of quality assessment are provided in Supplementary Table 2.
Statistical analysis
The overall risk of MetS in categories of selenium was estimated using the ORs/RRs/HRs and related 95%CIs. In studies that considered the last category of selenium as the reference group, the effect sizes were converted to get the risk of MetS by comparing the highest vs. lowest category of circulating selenium. To determine the between-study heterogeneity, Cochran’s Q test, I2, and fixed-effect models were used. Due to significant between-study heterogeneity, the overall effect was estimated by the random-effect model. If studies reported separated effect sizes based on gender, their effect sizes were merged through the fixed model to achieve an overall effect size for this study. Furthermore, the source of heterogeneity was explored through the use of subgroup analysis and meta-regression. Moreover, the individual effect of each included study was examined by performing sensitivity analysis. Additionally, Begg’s test was used to explore the publication bias. One study reported a risk of MetS per-one SD increment in blood selenium (31). Therefore, to include this study in the meta-analysis, we calculated the risk of Mets for the comparison of the third versus first tertiles of blood selenium, based on the Danesh et al. method (32). In which, the log risk estimates reported for the comparison between the highest and lowest tertiles are equivalent to 2.18 times the log risk estimates for a 1-SD increase. Furthermore, dose–response analysis was conducted based on the Greenland and Longnecker (33) and Orsini et al. (34) methods. In this method, the total number of participants, cases with MetS, the mean values of blood selenium, reported RRs/ORs/HRs, and 95% CIs of each category of circulating selenium were required. Additionally, for non-linear dose–response analysis, only studies with at least 3 categories were included. If one study did not report the median or mean values of blood selenium, the mean values of the lower and upper bounds of blood selenium were calculated. Additionally, for the highest or lowest categories with open-ended intervals, we assumed the length of adjacent intervals. A 2-stage random-effects dose–response meta-analysis was used to estimate the non-linear association between blood selenium and the risk of MetS. Nonlinear dose–response analysis was estimated through the use of the modeling of blood selenium and restricted cubic splines (three knots at fixed percentiles of 10, 50, and 90% of the distribution). Based on the Orsini et al. (34) method, the generalized least-squares trend estimation method, which takes into account the correlation within each set of reported RRs was used to calculate restricted cubic spline models. Then, all study-specific estimates were combined by using the restricted maximum likelihood method in a multivariate random-effects meta-analysis (35). The non-linear association between blood selenium and risk of MetS was estimated through the null hypothesis testing in which the coefficient of the second spline was considered equal to 0. Additionally, a 2-stage generalized least-squares trend estimation method was used to assess the linear relationship between each 50 μg/L increment in blood selenium and the risk of MetS. Such that the overall average slope was calculated through estimating the study-specific slope lines and combining them using a random-effects model (34). Analyses were conducted by STATA version 14.0. p values <0.05 were considered statistically significant.
Results
Findings from the systematic search
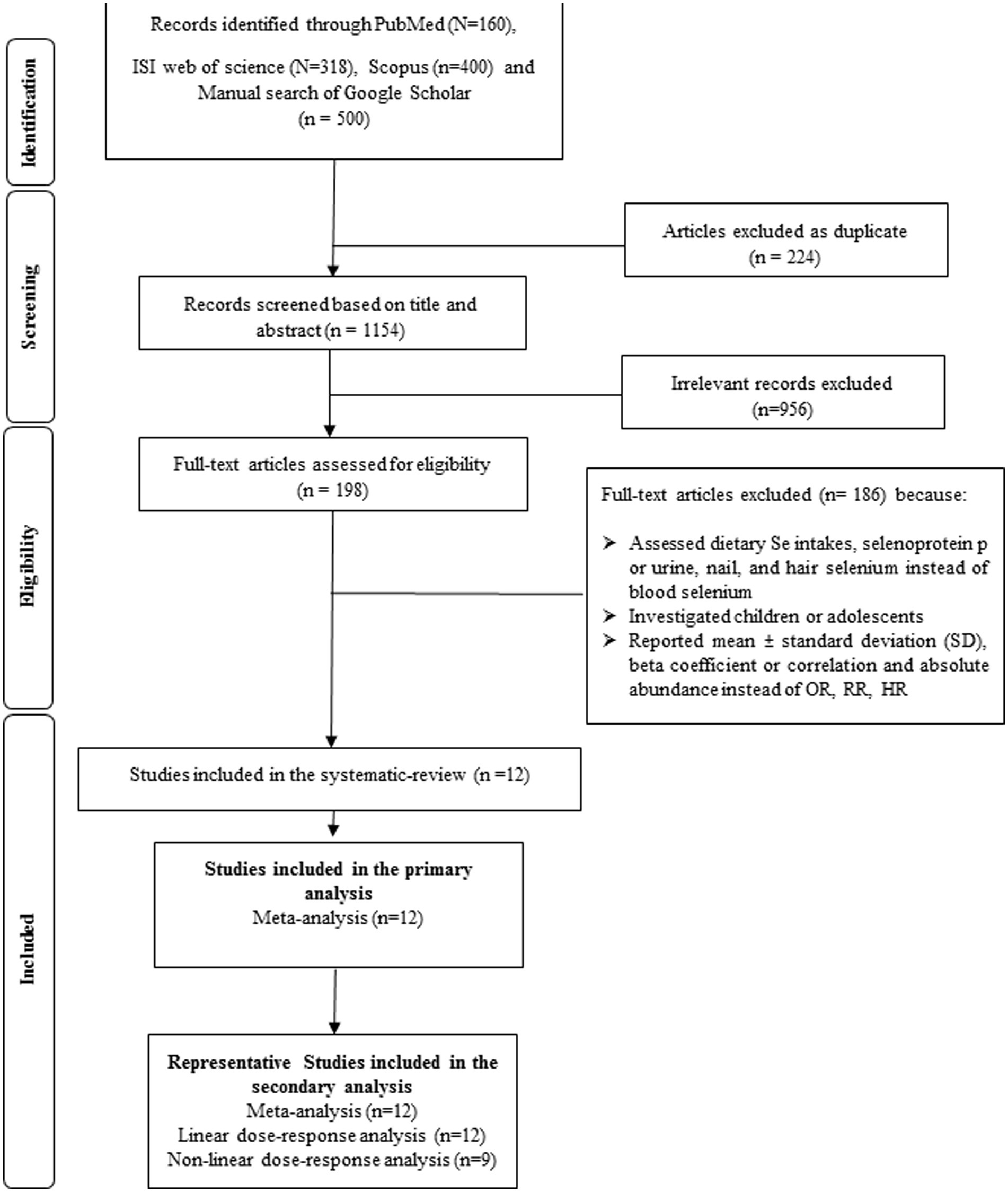
A total of 1,378 publications resulted in the systematic search which 224 of them were duplicated. The title and abstract of 1,154 investigations were screened and after that, the full text of 198 papers were exactly assessed. Finally, 12 eligible studies were included in the systematic review. Some irrelevant studies and the reasons of excluding them are shown in Supplementary Table 3. The study selection process is provided in Figure 1.
Study characteristics
Overall, 16,779 participants and 6,471 cases with MetS from 5 cross-sectional (31, 36–39) and 7 case–control studies (25–28, 40–42) were included in the current systematic review and meta-analysis (Table 2). These investigations were published in China (25–27, 36, 38–42), Italy (31), United States (37), and Taiwan (28) between 2012 and 2022. Nine studies (25–27, 36, 38–42) were conducted in Asian countries and the others were from non-Asian countries. Circulating selenium was assessed by inductively coupled plasma mass spectrometry (ICP-MS) (25–28, 36, 37, 39, 40, 42) and graphite furnace atomic absorption spectrometry (GFAAS) methods (26, 31, 41). Included studies defined MetS through the use of 4 methods including the Chinese diagnostic criterion for metabolic syndrome (CDS) (26, 38–41), joint interim statement (JIS) (27, 37, 42), international diabetes Federation (IDF) (25, 28, 31) and NCEP ATPШ (36). Most of the included studies had representative populations (25, 26, 28, 31, 36–39, 42). Nevertheless, 2 of them had a non-representative population (27, 41). All publications were rated as high quality because their quality scores were 7 or more. Although most of the publications adjusted their analyses for age, only 4 of them adjusted for BMI (25, 28, 37, 42).
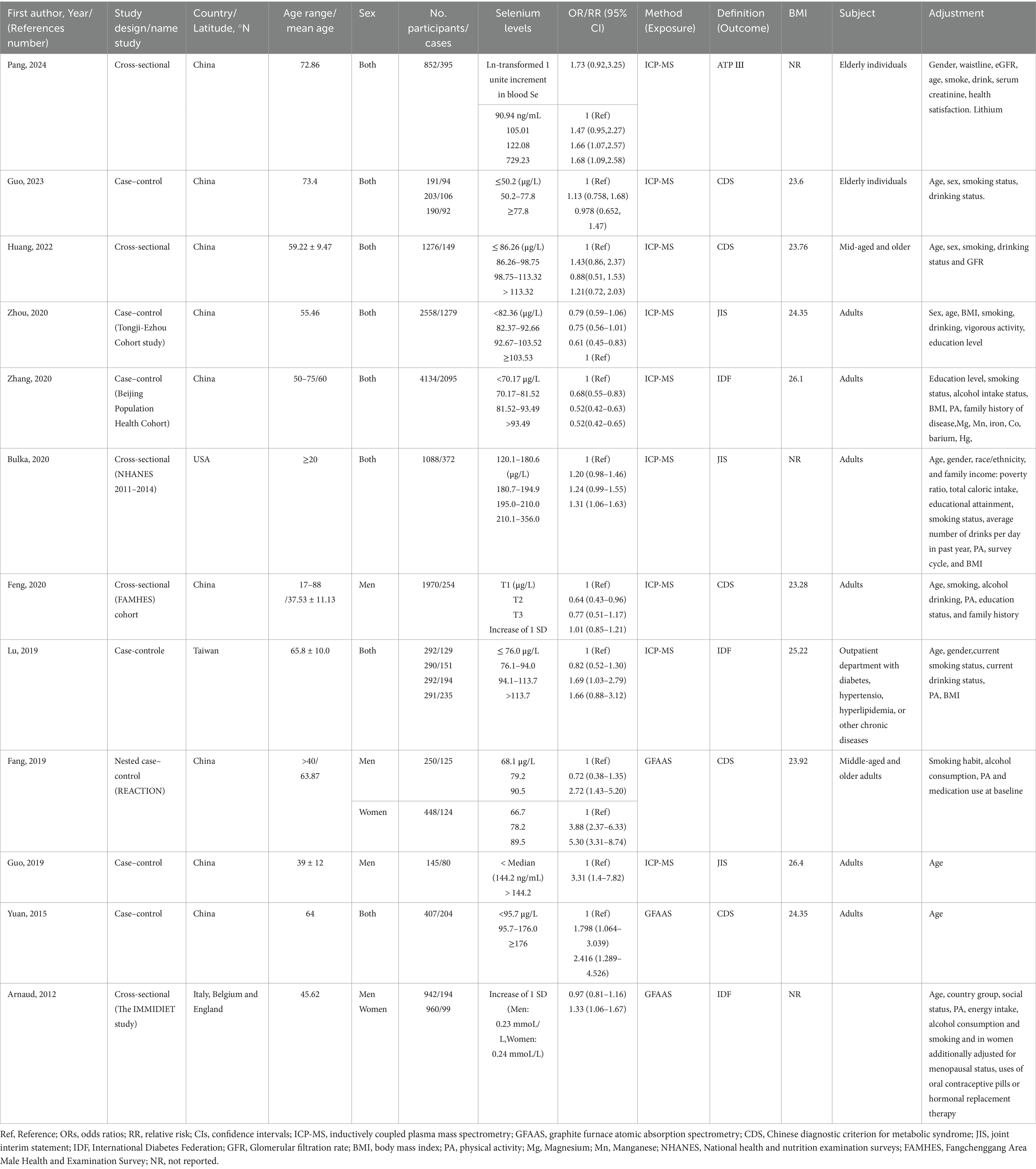
Table 2. Main characteristics of included studies examined the relation between blood selenium levels and metabolic syndrome in adults.
Finding from meta-analysis of highest versus lowest level of blood selenium in relation to MetS
Twelve effect sizes (including 16,779 participants and 6,471 cases with MetS from 12 publications) (25–28, 31, 36–42) investigated the relationship between circulating selenium and MetS in adults and were included in the meta-analysis. The findings of meta-analysis showed that participants with the highest blood values of selenium (mean: 268.5 μg/L) in comparison to those with the lowest values (mean: 75.27 μg/L) had 40% higher risk of MetS. Nevertheless, this association was not statistically significant (95%CI: 0.99–1.97), as shown in Figure 2.
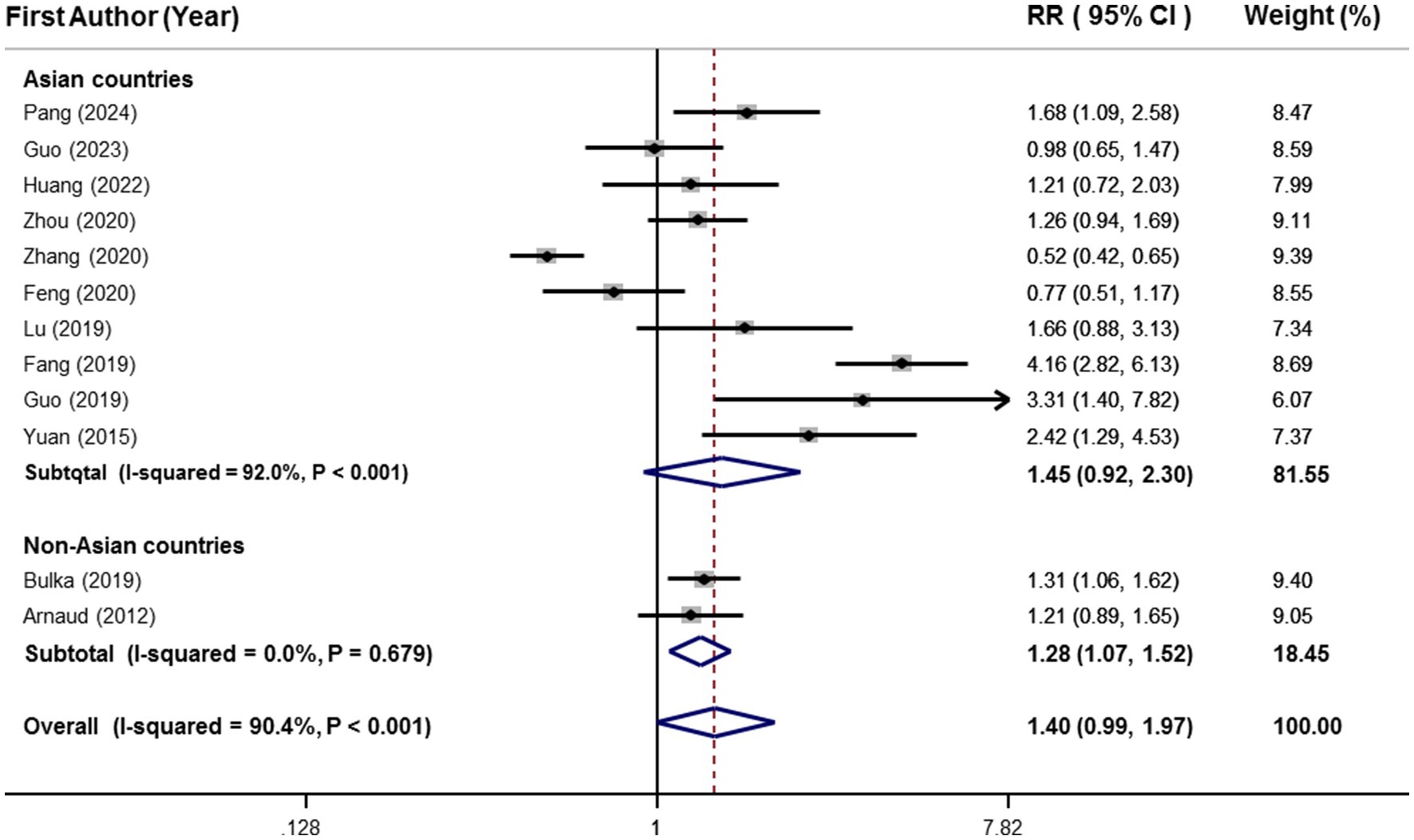
Figure 2. Forest plots of the relationship between blood selenium levels and metabolic syndrome in adults, stratified by study location.
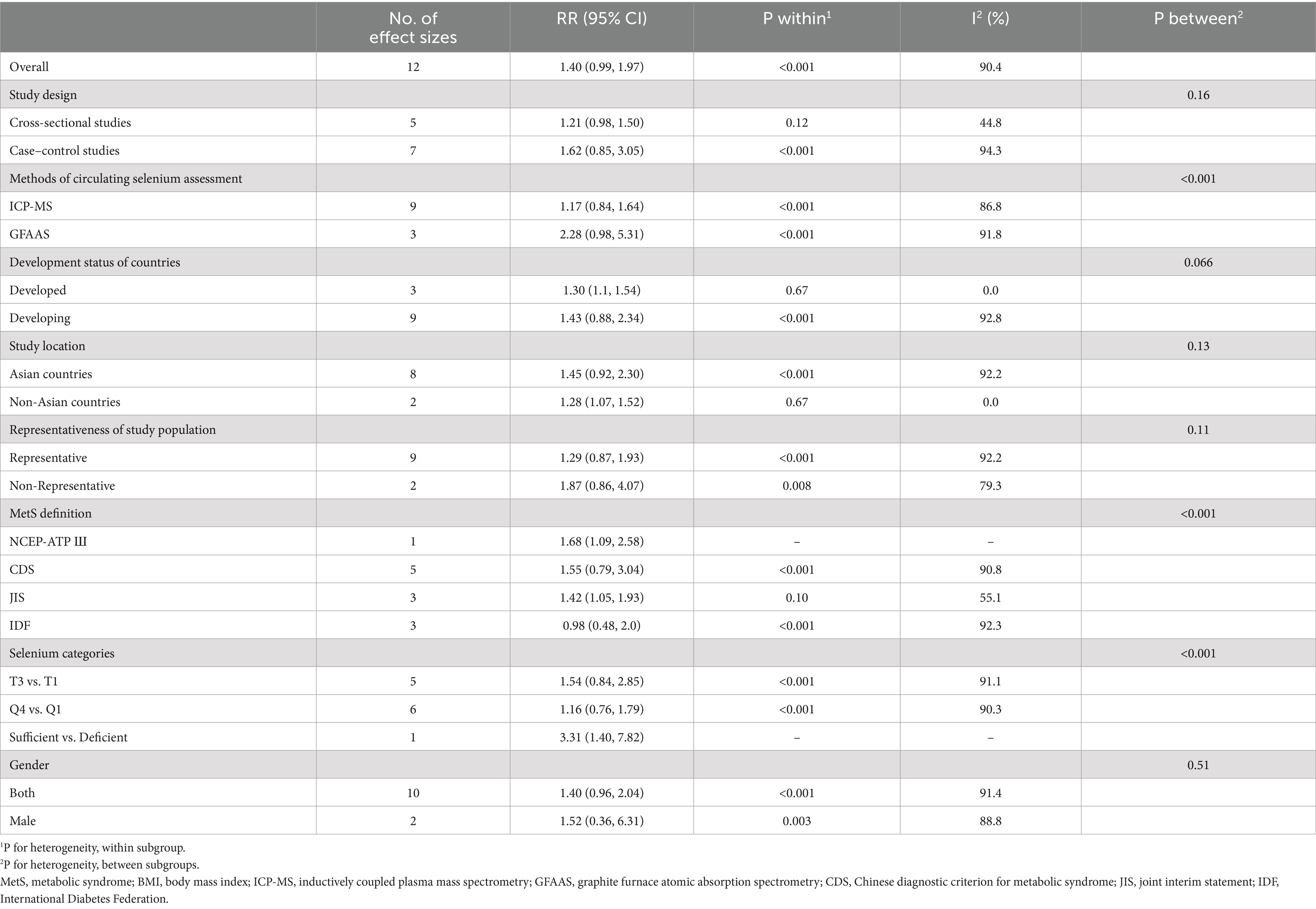
Due to a significant between-study heterogeneity (I2 = 90.4%, p < 0.001), subgroup analysis was conducted based on study location (Figure 2). Although highest vs. lowest blood selenium was significantly related to 28% higher risk of MetS (95%CI: 1.07–1.52) in the subgroup of non-Asian countries, there was not any significant association in the subgroup of Asian countries (RR:1.45; 95%CI: 0.92–2.30). Moreover, heterogeneity was completely removed in the subgroup of non-Asian countries (I2 = 0.0%, p = 0.67); however, it was kept significant in the subgroup of Asian countries (I2 = 92.0%, p < 0.001), as shown in Figure 2. Details of subgroup analysis based on other confounders are provided in Table 3. There was a significant and straight association between blood selenium and risk of MetS in the subgroups with low number of effect sizes including non-Asian countries, developed countries, NCEP-ATP Ш, JIS, and sufficient vs. deficient.
Moreover, meta-regression was conducted based on the mean age of participants (β = 0.0057, p = 0.71, I2 residual = 91.25%) and quality score of eligible investigations (β = −0.264, p = 0.15, I2 residual = 91.21%). However, none of them had a significant effect on the overall estimate. Furthermore, based on sensitivity analysis, the overall effect was not influenced by any included studies. Additionally, according to Begg’s plot and Begg’s test (p = 0.27), there was no evidence of publication bias.
Findings from dose–response analysis
Data from 16,779 participants and 6,471 cases with MetS from 12 studies were included in the linear dose–response analysis (25–28, 31, 36–42). Results showed each 50 μg/L increment in circulating selenium was related to 7% higher risk of MetS (RR: 1.07, 95%CI: 0.99, 1.15), as shown in Figure 3. However, this association was not statistically significant.
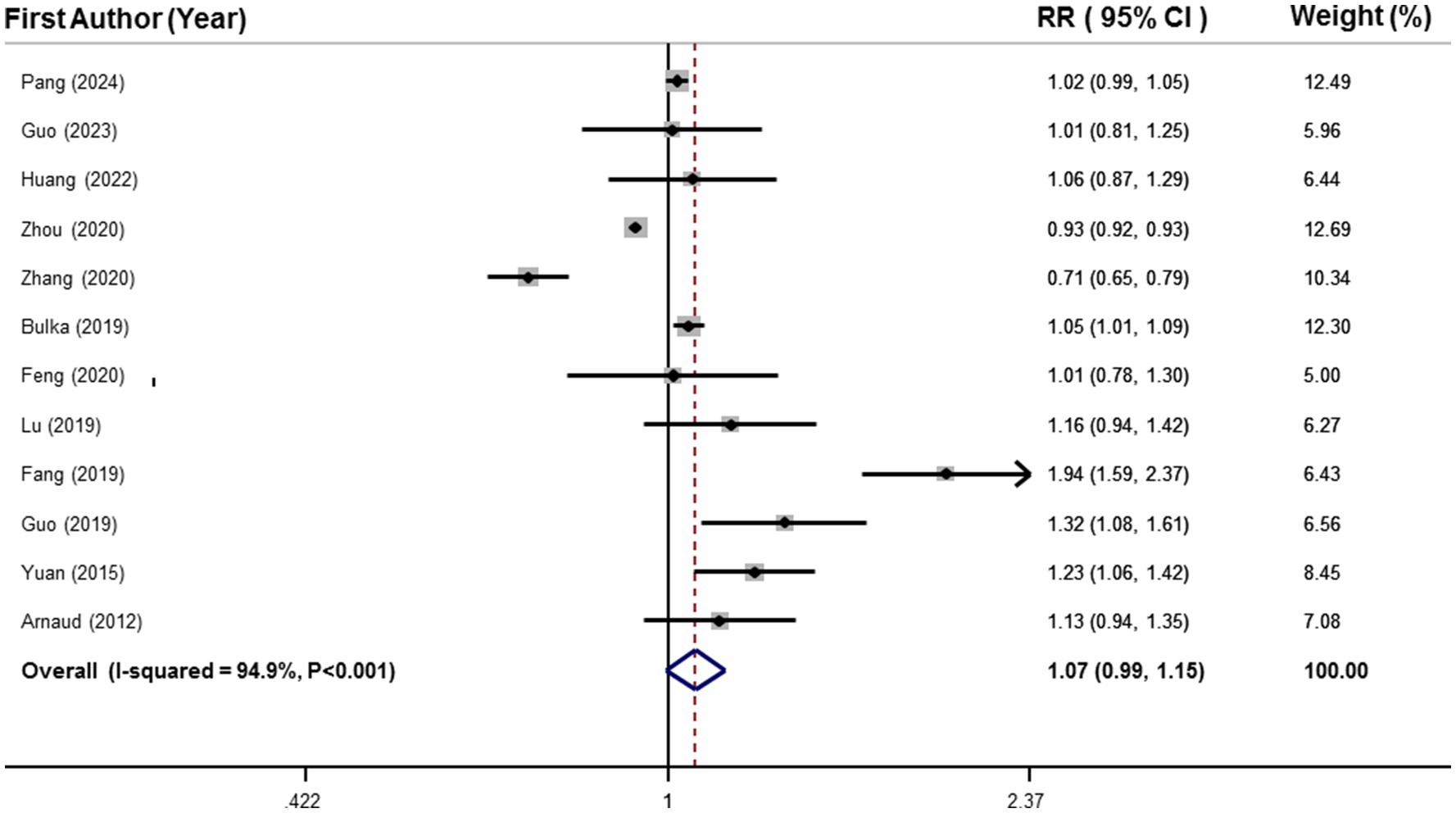
Figure 3. Forest plots of the linear relationship between blood selenium levels and metabolic syndrome.
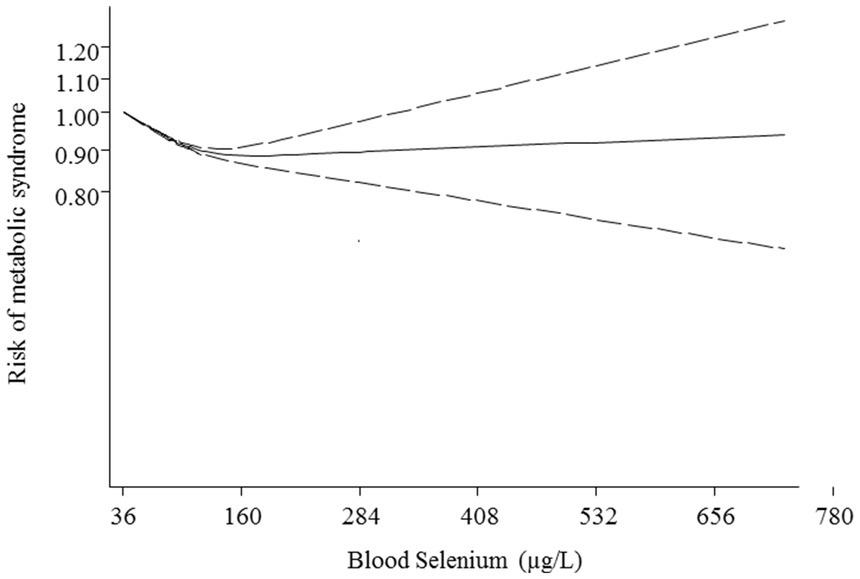
Additionally, non-linear dose–response analysis was conducted on 9 studies including 12,762 participants and 5,844 cases with MetS (25, 26, 28, 36, 37, 39–42). Findings indicated a U-shaped association between blood selenium and risk of MetS with the lowest risk at 160 μg/L of blood selenium (p < 0.001; Figure 4).
Discussion
The results of this systematic review and dose–response meta-analysis of observational studies revealed that although the highest versus lowest concentration of blood selenium was related to higher risk of MetS, this association was not statistically significant. After performing subgroup analysis, this relationship was significant only in the subgroups with low number of effect sizes. Additionally, the findings of the linear dose–response analysis indicated a direct association between each 50 μg/L increment in blood selenium and the risk of MetS. However, this relationship was not statistically significant. Furthermore, non-linear dose–response analysis illustrated a U-shaped association between blood selenium values and risk of MetS, with the lowest risk at 160 μg/L of blood selenium.
To the best of our knowledge, the current meta-analysis is the first one that investigated the association between blood selenium levels and the risk of MetS. Interestingly, the findings obtained from multiple studies support the protective role of selenium in the etiology of MetS. For instance, prior to this study, two meta-analyses of three case–control studies found that patients with MetS had significantly lower serum levels of selenoprotein P than controls (43, 44). In addition, another meta-analysis of four cross-sectional studies reported a significant inverse relationship between dietary selenium intake and the risk of MetS (24). While interpreting the findings of these three meta-analyses, we should consider the nature of the exposures used in the aforesaid meta-analyses. Selenoprotein P is a negative acute-phase reactant that its serum levels can be reduced in inflammatory states such as MetS (43, 45). Besides, selenoprotein P is produced by hepatocyte. Moreover, patients with MetS have been indicated to suffer from impaired liver function; therefore, a reduction in selenoprotein synthetic capacity is probable in MetS (43, 46, 47). Moreover, dietary selenium intake, estimated from a food frequency questionnaire or a 3-day or 24-h recall (24), is subject to a high degree of bias and cannot appropriately predict selenium status (48). In contrast to selenoprotein P and also dietary selenium intake, selenium concentration in blood (plasma, serum, or whole blood) is a reliable indicator of selenium status (48, 49).
On the other hand, the findings of some meta-analyses confirmed the adverse relationship between selenium and the risk of diseases. For instance, recent meta-analyses showed a significantly positive and roughly linear relationship between blood selenium levels and the risk of diabetes mellitus (50–52). It is worth mentioning that the prevalence of MetS is very high in the diabetic population, particularly among female patients (53). Furthermore, observational surveys indicated significant positive associations between blood selenium concentrations and the risk of MetS components including insulin resistance (54), hyperglycemia (55), dyslipidemia (55, 56), hypertension (57, 58), and central obesity (59). In addition, some meta-analyses of randomized controlled trials revealed that selenium supplementation significantly increased systolic blood pressure (60), tumor necrosis factor-alpha (an inflammatory trigger for insulin resistance) (61), and low-density lipoprotein cholesterol (60). Additionally, a study from National Health and Nutrition Examination Survey (NHANES) Ш indicated a U-shaped relationship between serum selenium and cardiovascular mortality (62). In the current study, we also revealed a U-shaped association between circulation selenium and risk of MetS with the lowest risk at 160 μg/L of blood selenium. Therefore, it seems that the relationship between blood selenium and NCDs is dose-dependent. Although the normal range of blood selenium (120–160 μg/L) is protectively related to a lower risk of MetS, the toxic values of circulating selenium might increase the risk of MetS.
The protective relationship between blood selenium and MetS may be due to the antioxidant properties of selenium. Inflammation and oxidative stress are the leading causes of MetS and its components (63). On the other hand, selenium is one of the components of glutathione peroxidase (GSH-PX) and the process of antioxidant defense. Therefore, blood values of selenium are protectively related to a lower risk of MetS due to its antioxidant features (64). However, potential biochemical mechanisms underlying the direct relationship between toxic blood selenium levels and MetS risk have not been well elucidated. As a known fact, the range of selenium status is very narrow from deficiency to sufficiency to toxicity (65). Therefore, it seems that blood selenium can easily reach excessive levels and cause detrimental effects on human health (66). In detail, excess blood levels of selenium (especially inorganic selenium) may lead to oxidative stress by increasing the production of various reactive oxygen and nitrogen species (42). It is worth noting that oxidative stress is a central pathophysiological feature of MetS (63). In addition, high blood levels of selenium can affect the expression of protein tyrosine phosphatase and result in insulin resistance, diabetes mellitus, and obesity (67, 68). Moreover, it has been shown that high blood selenium concentrations may increase protein biosynthesis, gluconeogenesis, cholesterol biosynthesis, and lipogenesis and decrease glycolysis and cholesterol hydrolysis in the liver (69). Furthermore, high blood selenium can deplete chromium (70), and chromium deficiency may elevate blood pressure by increasing renin-angiotensin system activity and reducing nitric oxide system activity (65, 71).
The results of subgroup analyses implied that there was geographical difference in the association between blood selenium concentrations and the risk of MetS. The geographical difference may be due to variations in the selenium content of soil, water, plants, and air across different countries. As an example, people living in industrially developed countries have higher inhalation exposure to selenium than others (72). Additionally, due to different lifestyles in countries, the prevalence of MetS and its components varies in different regions (73).
Strengths and limitations
This study has multiple strengths. First, to the best of our knowledge, this is the first meta-analysis investigating the relationship between blood selenium levels and the risk of MetS. Second, several subgroup analyses and meta-regressions were conducted to explore potential sources of heterogeneity between the included studies. Third, a dose–response analysis was performed to clarify the quantitative estimation of the above association.
However, there are several limitations that warrant attention. The number of included studies was relatively small. Hence, more investigations especially with prospective design are needed to confirm the causality of this association. Moreover, there was substantial heterogeneity in this meta-analysis that could not be fully resolved by subgroup analyses and meta-regressions. This may be due to within and between-subject biological variations in blood selenium (75), the presence of multiple single nucleotide polymorphisms in selenium metabolism-related genes (66), the multi-causality and complex genetic and phenotypic nature of MetS (76), and the different selenium content of foods, soul and even air and water in different countries (72). Furthermore, the design of all included studies was cross-sectional or case–control; therefore, a cause-and-effect relationship could not be established. Additionally, most of the included studies originated from Eastern countries, therefore the findings could not be generalizable to Western populations.
Implications for practice and research
The results of this meta-analysis suggested that there is a U-shaped relationship between circulating selenium and the risk of MetS. Although the increasing blood selenium from 36 up to 160 μg/L was along with a reduction risk of MetS, the values more than 160 μg/L were related to higher risk of MetS. Therefore, it is important to intake enough selenium through the diet; however, it is necessary to avoid selenium supplementation in people without confirmed deficiency. Moreover, conducting prospective cohort studies is required to confirm our findings and establish causation.
Conclusion
In a nutshell, there is a U-shaped relationship between blood selenium levels risk of adult MetS. However, more longitudinal studies are needed to verify the causality of findings and clarify the underlying mechanisms.
Data availability statement
All the included data in the analysis are provided in the paper (Table 2). Further inquiries can be directed to the corresponding author.
Author contributions
ZH: Conceptualization, Data curation, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. SF: Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MB: Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. GA: Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The financial support for conception, design, data analysis and manuscript drafting comes from Student Research Committee, Isfahan University of Medical Sciences, Isfahan, Iran (No.1402106).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1451342/full#supplementary-material
Footnotes
References
1. Bovolini, A, Garcia, J, Andrade, MA, and Duarte, JA. Metabolic syndrome pathophysiology and predisposing factors. Int J Sports Med. (2021) 42:199–214. doi: 10.1055/a-1263-0898
2. Kaur, J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. (2014) 2014:1–21. doi: 10.1155/2014/943162
3. Hajhashemy, Z, Shahdadian, F, Moslemi, E, Mirenayat, FS, and Saneei, P. Serum vitamin D levels in relation to metabolic syndrome: a systematic review and dose-response meta-analysis of epidemiologic studies. Obes Rev. (2021) 22:e13223. doi: 10.1111/obr.13223
4. Rouhani, P, Hajhashemy, Z, and Saneei, P. Circulating serum vitamin D levels in relation to metabolic syndrome in children: a systematic review and dose-response meta-analysis of epidemiologic studies. Obes Rev. (2021) 22:e13314. doi: 10.1111/obr.13314
5. Assi, MJ, Poursalehi, D, Tirani, SA, Shahdadian, F, Hajhashemy, Z, Mokhtari, E, et al. Legumes and nuts intake in relation to metabolic health status, serum brain derived neurotrophic factor and adropin levels in adults. Sci Rep. (2023) 13:16455. doi: 10.1038/s41598-023-43855-8
6. Heshmatipour, H, Hajhashemy, Z, Mirzaei, S, Asadi, A, Akhlaghi, M, and Saneei, P. Association of legumes and nuts consumption with metabolic health status in Iranian overweight and obese adolescents. Sci Rep. (2023) 13:5784. doi: 10.1038/s41598-023-32961-2
7. Bahadorpour, S, Hajhashemy, Z, and Saneei, P. Serum 25-hydroxyvitamin D levels and dyslipidemia: a systematic review and dose-response meta-analysis of epidemiologic studies. Nutr Rev. (2022) 81:1–25. doi: 10.1093/nutrit/nuac038
8. Hajhashemy, Z, Foshati, S, and Saneei, P. Relationship between abdominal obesity (based on waist circumference) and serum vitamin D levels: a systematic review and meta-analysis of epidemiologic studies. Nutr Rev. (2022) 80:1105–17. doi: 10.1093/nutrit/nuab070
9. Hajhashemy, Z, Lotfi, K, Heidari, Z, and Saneei, P. Serum vitamin D levels in relation to abdominal obesity in children and adolescents: a systematic review and dose-response Meta-analysis. Front Nutr. (2022) 9:806459. doi: 10.3389/fnut.2022.806459
10. Hajhashemy, Z, Rouhani, P, and Saneei, P. Dietary calcium intake in relation to blood lipids and lipoproteins profiles: a systematic review and meta-analysis of epidemiologic studies. Nutr Metab Cardiovasc Dis. (2022) 32:1609–26. doi: 10.1016/j.numecd.2022.03.018
11. Mohammadi, S, Hajhashemy, Z, and Saneei, P. Serum vitamin D levels in relation to type-2 diabetes and prediabetes in adults: a systematic review and dose-response meta-analysis of epidemiologic studies. Crit Rev Food Sci Nutr. (2022) 62:8178–98. doi: 10.1080/10408398.2021.1926220
12. Mokhtari, E, Hajhashemy, Z, and Saneei, P. Serum vitamin D levels in relation to hypertension and pre-hypertension in adults: a systematic review and dose-response Meta-analysis of epidemiologic studies. Front Nutr. (2022) 9:829307. doi: 10.3389/fnut.2022.829307
13. Hajhashemy, Z, Lotfi, K, Shahdadian, F, Rouhani, P, Heidari, Z, and Saneei, P. Dietary insulin index and insulin load in relation to hypertriglyceridemic waist phenotype and low brain derived neurotrophic factor in adults. Front Nutr. (2022) 9:980274. doi: 10.3389/fnut.2022.980274
14. Balali, A, Tirani, SA, Rouhani, P, Shahdadian, F, Hajhashemy, Z, Mohammadi, S, et al. Nutrient patterns in relation to metabolic health status and serum levels of brain-derived neurotrophic factor (BDNF) and adropin in adults. Sci Rep. (2024) 14:4650. doi: 10.1038/s41598-024-54913-0
15. Poursalehi, D, Lotfi, K, Shahdadian, F, Hajhashemy, Z, Rouhani, P, and Saneei, P. Dietary intake of methyl donor nutrients in relation to metabolic health status, serum levels of brain-derived neurotrophic factor and adropin. Clin Nutr. (2024) 43:1353–62. doi: 10.1016/j.clnu.2024.04.031
16. Poursalehi, D, Tirani, SA, Shahdadian, F, Hajhashemy, Z, Rouhani, P, and Saneei, P. Ultra-processed foods intake in relation to metabolic health status, serum brain-derived neurotrophic factor and adropin levels in adults. Nutr J. (2024) 23:121. doi: 10.1186/s12937-024-01024-1
17. Tirani, SA, Lotfi, K, Shahdadian, F, Hajhashemy, Z, Rouhani, P, and Saneei, P. Dietary phytochemical index in relation to metabolic health status, serum Adropin, and brain-derived neurotrophic factor levels in adults. Curr Dev Nutr. (2024) 8:102103. doi: 10.1016/j.cdnut.2024.102103
18. Hajhashemy, Z, Mirenayat, FS, Siavash, M, and Saneei, P. The effect of sumac supplementation on insulin resistance, inflammation, oxidative stress, and antioxidant capacity in adults with metabolic syndrome: a randomized crossover clinical trial. Phytother Res. (2023) 37:1319–29. doi: 10.1002/ptr.7688
19. Mirenayat, FS, Hajhashemy, Z, Siavash, M, and Saneei, P. Effects of sumac supplementation on metabolic markers in adults with metabolic syndrome: a triple-blinded randomized placebo-controlled cross-over clinical trial. Nutr J. (2023) 22:25. doi: 10.1186/s12937-023-00854-9
20. Hajhashemy, Z, Mirzaei, S, Asadi, A, Akhlaghi, M, and Saneei, P. Association of Dietary Insulin Index and Dietary Insulin Load with Metabolic Health Status in Iranian overweight and obese adolescents. Front Nutr. (2022) 9:821089. doi: 10.3389/fnut.2022.821089
21. Zoroddu, MA, Aaseth, J, Crisponi, G, Medici, S, Peana, M, and Nurchi, VM. The essential metals for humans: a brief overview. J Inorg Biochem. (2019) 195:120–9. doi: 10.1016/j.jinorgbio.2019.03.013
22. Rayman, MP. The importance of selenium to human health. Lancet. (2000) 356:233–41. doi: 10.1016/S0140-6736(00)02490-9
23. Conrad, M, and Proneth, B. Selenium: tracing another essential element of ferroptotic cell death. Cell Chem Biol. (2020) 27:409–19. doi: 10.1016/j.chembiol.2020.03.012
24. Ding, J, Liu, Q, Liu, Z, Guo, H, Liang, J, and Zhang, Y. Associations of the dietary iron, copper, and selenium level with metabolic syndrome: a meta-analysis of observational studies. Front Nutr. (2022) 8:810494. doi: 10.3389/fnut.2021.810494
25. Zhang, W, Du, J, Li, H, Yang, Y, Cai, C, Gao, Q, et al. Multiple-element exposure and metabolic syndrome in Chinese adults: a case-control study based on the Beijing population health cohort. Environ Int. (2020) 143:105959. doi: 10.1016/j.envint.2020.105959
26. Fang, C, Wu, W, Gu, X, Dai, S, Zhou, Q, Deng, H, et al. Association of serum copper, zinc and selenium levels with risk of metabolic syndrome: a nested case-control study of middle-aged and older Chinese adults. J Trace Elem Med Biol. (2019) 52:209–15. doi: 10.1016/j.jtemb.2018.12.017
27. Guo, X, Yang, Q, Zhang, W, Chen, Y, Ren, J, and Gao, A. Associations of blood levels of trace elements and heavy metals with metabolic syndrome in Chinese male adults with microRNA as mediators involved. Environ Pollut. (2019) 248:66–73. doi: 10.1016/j.envpol.2019.02.015
28. Lu, C-W, Chang, H-H, Yang, K-C, Chiang, C-H, Yao, C-A, and Huang, K-C. Gender differences with dose–response relationship between serum selenium levels and metabolic syndrome—a case-control study. Nutrients. (2019) 11:477. doi: 10.3390/nu11020477
29. Tajaddini, MH, Keikha, M, Razzazzadeh, A, and Kelishadi, R. A systematic review on the association of serum selenium and metabolic syndrome. J Res Med Sci. (2015) 20:782–9. doi: 10.4103/1735-1995.168403
30. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
31. Arnaud, J, De Lorgeril, M, Akbaraly, T, Salen, P, Arnout, J, Cappuccio, F, et al. Gender differences in copper, zinc and selenium status in diabetic-free metabolic syndrome European population–the IMMIDIET study. Nutr Metab Cardiovasc Dis. (2012) 22:517–24. doi: 10.1016/j.numecd.2010.09.005
32. Danesh, J, Collins, R, Appleby, P, and Peto, R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. (1998) 279:1477–82. doi: 10.1001/jama.279.18.1477
33. Greenland, S, and Longnecker, MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. (1992) 135:1301–9. doi: 10.1093/oxfordjournals.aje.a116237
34. Orsini, N, Bellocco, R, and Greenland, S. Generalized least squares for trend estimation of summarized dose–response data. Stata J. (2006) 6:40–57. doi: 10.1177/1536867X0600600103
35. Jackson, D, White, IR, and Thompson, SG. Extending DerSimonian and Laird's methodology to perform multivariate random effects meta-analyses. Stat Med. (2010) 29:1282–97. doi: 10.1002/sim.3602
36. Pang, Y, Wang, Y, Hao, H, Zhu, W, Zou, M, Liu, Q, et al. Associations of multiple serum metals with the risk of metabolic syndrome among the older population in China based on a community study: a mediation role of peripheral blood cells. Ecotoxicol Environ Saf. (2024) 284:116981. doi: 10.1016/j.ecoenv.2024.116981
37. Bulka, CM, Persky, VW, Daviglus, ML, Durazo-Arvizu, RA, and Argos, M. Multiple metal exposures and metabolic syndrome: a cross-sectional analysis of the National Health and nutrition examination survey 2011–2014. Environ Res. (2019) 168:397–405. doi: 10.1016/j.envres.2018.10.022
38. Feng, X, Li, L, Huang, L, Zhang, H, Mo, Z, and Yang, X. Associations between serum multiple metals exposures and metabolic syndrome: a longitudinal cohort study. Biol Trace Elem Res. (2021) 199:2444–55. doi: 10.1007/s12011-020-02371-w
39. Huang, S, Zhong, D, Lv, Z, Cheng, J, Zou, X, Wang, T, et al. Associations of multiple plasma metals with the risk of metabolic syndrome: a cross-sectional study in the mid-aged and older population of China. Ecotoxicol Environ Saf. (2022) 231:113183. doi: 10.1016/j.ecoenv.2022.113183
40. Guo, S, Hua, L, Liu, W, Liu, H, Chen, Q, Li, Y, et al. Multiple metal exposure and metabolic syndrome in elderly individuals: a case-control study in an active mining district, Northwest China. Chemosphere. (2023) 326:138494. doi: 10.1016/j.chemosphere.2023.138494
41. Yuan, Z, Xu, X, Ye, H, Jin, L, Zhang, X, and Zhu, Y. High levels of plasma selenium are associated with metabolic syndrome and elevated fasting plasma glucose in a Chinese population: a case-control study. J Trace Elem Med Biol. (2015) 32:189–94. doi: 10.1016/j.jtemb.2015.07.009
42. Zhou, L, Luo, C, Yin, J, Zhu, Y, Li, P, Chen, S, et al. Diverse associations of plasma selenium concentrations and SELENOP gene polymorphism with metabolic syndrome and its components. Oxidative Med Cell Longev. (2020) 2020:1–11. doi: 10.1155/2020/5343014
43. Yu, R, Wang, Z, Ma, M, Xu, P, Liu, L, Tinkov, AA, et al. Associations between circulating SELENOP level and disorders of glucose and lipid metabolism: a Meta-analysis. Antioxidants. (2022) 11:1263. doi: 10.3390/antiox11071263
44. Tinkov, AA, Ajsuvakova, OP, Filippini, T, Zhou, J-C, Lei, XG, Gatiatulina, ER, et al. Selenium and selenoproteins in adipose tissue physiology and obesity. Biomol Ther. (2020) 10:658. doi: 10.3390/biom10040658
45. Foshati, S, Mirjalili, F, Rezazadegan, M, Fakoorziba, F, and Amani, R. Antioxidants and clinical outcomes of patients with coronavirus disease 2019: a systematic review of observational and interventional studies. Food Sci Nutr. (2022) 10:4112–25. doi: 10.1002/fsn3.3034
46. Wang, S, Zhang, J, Zhu, L, Song, L, Meng, Z, Jia, Q, et al. Association between liver function and metabolic syndrome in Chinese men and women. Sci Rep. (2017) 7:1–9. doi: 10.1038/srep44844
47. Lim, S, Kim, J-W, and Targher, G. Links between metabolic syndrome and metabolic dysfunction-associated fatty liver disease. Trends Endocrinol Metab. (2021) 32:500–14. doi: 10.1016/j.tem.2021.04.008
48. Combs, GF, Watts, JC, Jackson, MI, Johnson, LK, Zeng, H, Scheett, AJ, et al. Determinants of selenium status in healthy adults. Nutr J. (2011) 10:1–10. doi: 10.1186/1475-2891-10-75
49. Combs, GF Jr. Biomarkers of selenium status. Nutrients. (2015) 7:2209–36. doi: 10.3390/nu7042209
50. Vinceti, M, Filippini, T, and Rothman, KJ. Selenium exposure and the risk of type 2 diabetes: a systematic review and meta-analysis. Eur J Epidemiol. (2018) 33:789–810. doi: 10.1007/s10654-018-0422-8
51. Kim, J, Chung, HS, Choi, M-K, Roh, YK, Yoo, HJ, Park, JH, et al. Association between serum selenium level and the presence of diabetes mellitus: a meta-analysis of observational studies. Diabetes Metab J. (2019) 43:447–60. doi: 10.4093/dmj.2018.0123
52. Vinceti, M, Filippini, T, Wise, LA, and Rothman, KJ. A systematic review and dose-response meta-analysis of exposure to environmental selenium and the risk of type 2 diabetes in nonexperimental studies. Environ Res. (2021) 197:111210. doi: 10.1016/j.envres.2021.111210
53. James, M, Varghese, TP, Sharma, R, and Chand, S. Association between metabolic syndrome and diabetes mellitus according to international diabetic federation and National Cholesterol Education Program Adult Treatment Panel III criteria: a cross-sectional study. J Diabetes Metab Disord. (2020) 19:437–43. doi: 10.1007/s40200-020-00523-2
54. Cardoso, BR, Braat, S, and Graham, RM. Selenium status is associated with insulin resistance markers in adults: findings from the 2013 to 2018 National Health and nutrition examination survey (NHANES). Front Nutr. (2021) 8:696024. doi: 10.3389/fnut.2021.696024
55. Liu, A, Xu, P, Gong, C, Zhu, Y, Zhang, H, Nie, W, et al. High serum concentration of selenium, but not calcium, cobalt, copper, iron, and magnesium, increased the risk of both hyperglycemia and dyslipidemia in adults: a health examination center based cross-sectional study. J Trace Elem Med Biol. (2020) 59:126470. doi: 10.1016/j.jtemb.2020.126470
56. Ju, W, Ji, M, Li, X, Li, Z, Wu, G, Fu, X, et al. Relationship between higher serum selenium level and adverse blood lipid profile. Clin Nutr. (2018) 37:1512–7. doi: 10.1016/j.clnu.2017.08.025
57. Zhang, Z, Zhao, S, Wu, H, Qin, W, Zhang, T, Wang, Y, et al. Cross-sectional study: relationship between serum trace elements and hypertension. J Trace Elem Med Biol. (2022) 69:126893. doi: 10.1016/j.jtemb.2021.126893
58. Laclaustra, M, Navas-Acien, A, Stranges, S, Ordovas, JM, and Guallar, E. Serum selenium concentrations and hypertension in the US population. Circ Cardiovasc Qual Outcomes. (2009) 2:369–76. doi: 10.1161/CIRCOUTCOMES.108.831552
59. Lu, C-W, Chang, H-H, Yang, K-C, Kuo, C-S, Lee, L-T, and Huang, K-C. High serum selenium levels are associated with increased risk for diabetes mellitus independent of central obesity and insulin resistance. BMJ Open Diabetes Res Care. (2016) 4:e000253. doi: 10.1136/bmjdrc-2016-000253
60. Kelishadi, MR, Ashtary-Larky, D, Davoodi, SH, Clark, CC, and Asbaghi, O. The effects of selenium supplementation on blood lipids and blood pressure in adults: a systematic review and dose-response meta-analysis of randomized control trials. J Trace Elem Med Biol. (2022) 74:127046. doi: 10.1016/j.jtemb.2022.127046
61. Gholizadeh, M, Khalili, A, Roodi, PB, Saeedy, SAG, Najafi, S, Mohamadian, M, et al. Selenium supplementation decreases CRP and IL-6 and increases TNF-alpha: a systematic review and meta-analysis of randomized controlled trials. J Trace Elem Med Biol. (2023) 79:127199. doi: 10.1016/j.jtemb.2023.127199
62. Bleys, J, Navas-Acien, A, and Guallar, E. Serum selenium levels and all-cause, Cancer, and cardiovascular mortality among US adults. Arch Intern Med. (2008) 168:404–10. doi: 10.1001/archinternmed.2007.74
63. Masenga, SK, Kabwe, LS, Chakulya, M, and Kirabo, A. Mechanisms of oxidative stress in metabolic syndrome. Int J Mol Sci. (2023) 24:7898. doi: 10.3390/ijms24097898
64. Brenneisen, P, Steinbrenner, H, and Sies, H. Selenium, oxidative stress, and health aspects. Mol Asp Med. (2005) 26:256–67. doi: 10.1016/j.mam.2005.07.004
65. Panchal, SK, Wanyonyi, S, and Brown, L. Selenium, vanadium, and chromium as micronutrients to improve metabolic syndrome. Curr Hypertens Rep. (2017) 19:1–11. doi: 10.1007/s11906-017-0701-x
66. Rayman, MP. Selenium intake, status, and health: a complex relationship. Hormones. (2020) 19:9–14. doi: 10.1007/s42000-019-00125-5
67. Mueller, AS, Klomann, SD, Wolf, NM, Schneider, S, Schmidt, R, Spielmann, J, et al. Redox regulation of protein tyrosine phosphatase 1B by manipulation of dietary selenium affects the triglyceride concentration in rat liver. J Nutr. (2008) 138:2328–36. doi: 10.3945/jn.108.089482
68. Abdelsalam, SS, Korashy, HM, Zeidan, A, and Agouni, A. The role of protein tyrosine phosphatase (PTP)-1B in cardiovascular disease and its interplay with insulin resistance. Biomol Ther. (2019) 9:286. doi: 10.3390/biom9070286
69. Steinbrenner, H, Duntas, LH, and Rayman, MP. The role of selenium in type-2 diabetes mellitus and its metabolic comorbidities. Redox Biol. (2022) 50:102236. doi: 10.1016/j.redox.2022.102236
70. Retondario, A, Fernandes, R, Rockenbach, G, de Almeida, AM, Bricarello, LP, de Moraes Trindade, EBS, et al. Selenium intake and metabolic syndrome: a systematic review. Clin Nutr. (2019) 38:603–14. doi: 10.1016/j.clnu.2018.02.021
71. Lari, A, Fatahi, S, Sohouli, MH, and Shidfar, F. The impact of chromium supplementation on blood pressure: a systematic review and dose–response Meta‑analysis of randomized‑controlled trials. High Blood Press Cardiovasc Prev. (2021) 28:333–42. doi: 10.1007/s40292-021-00456-8
72. Skalny, AV, Burtseva, TI, Salnikova, EV, Ajsuvakova, OP, Skalnaya, MG, Kirichuk, AA, et al. Geographic variation of environmental, food, and human hair selenium content in an industrial region of Russia. Environ Res. (2019) 171:293–301. doi: 10.1016/j.envres.2019.01.038
73. Noubiap, JJ, Nansseu, JR, Lontchi-Yimagou, E, Nkeck, JR, Nyaga, UF, Ngouo, AT, et al. Geographic distribution of metabolic syndrome and its components in the general adult population: a meta-analysis of global data from 28 million individuals. Diabetes Res Clin Pract. (2022) 188:109924. doi: 10.1016/j.diabres.2022.109924
74. Xie, J, Li, Y, Zhang, Y, Vgontzas, AN, Basta, M, Chen, B, et al. Sleep duration and metabolic syndrome: an updated systematic review and meta-analysis. Sleep Med Rev. (2021) 59:101451. doi: 10.1016/j.smrv.2021.101451
75. Coşkun, A, Aarsand, AK, Braga, F, Carobene, A, Díaz-Garzón, J, Fernandez-Calle, P, et al. Systematic review and meta-analysis of within-subject and between-subject biological variation estimates of serum zinc, copper and selenium. Clin Chem Lab Med. (2022) 60:479–82. doi: 10.1515/cclm-2021-0723
Keywords: selenium, metabolic syndrome, adults, systematic review, dose–response, meta-analysis
Citation: Hajhashemy Z, Foshati S, Bagherniya M and Askari G (2025) The association between blood selenium and metabolic syndrome in adults: a systematic review and dose–response meta-analysis of epidemiologic studies. Front. Nutr. 11:1451342. doi: 10.3389/fnut.2024.1451342
Edited by:
M. Dulce Estêvão, University of Algarve, PortugalReviewed by:
Javad Heshmati, University of Ottawa Heart Institute, CanadaNeluwa-Liyanage Indika, University of Sri Jayewardenepura, Sri Lanka
Copyright © 2025 Hajhashemy, Foshati, Bagherniya and Askari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gholamreza Askari, YXNrYXJpQG11aS5hYy5pcg==
 Zahra Hajhashemy
Zahra Hajhashemy Sahar Foshati
Sahar Foshati Mohammad Bagherniya
Mohammad Bagherniya Gholamreza Askari
Gholamreza Askari