- 1Department of Pharmacy Practice, NIMS Institute of Pharmacy, NIMS University Rajasthan, Jaipur, India
- 2School of Health Science, Faculty of Biology, Medicine and Health, University of Manchester, Manchester, Academic Health Science Center, Manchester, United Kingdom
- 3Department of Endocrinology, National Institute of Medical Science and Research Hospital, NIMS University Rajasthan, Jaipur, India
- 4Department of Clinical Studies, Fourth Hospital of Yulin (Xingyuan), Yulin, Shaanxi, China
- 5Department of Clinical Sciences, Shenmu Hospital, Shenmu, Shaanxi, China
- 6Institute of Pediatric Gastroenterology and Hepatology, NIMS University Rajasthan, Jaipur, India
Background: Vitamin D, essential hormone for endocrine, autocrine, and paracrine functions. A billion people are deficient globally which contributing to numerous health issues. This study explores the link between vitamin D levels and sleep quality, impacting mental and physical health in adults.
Methods: This prospective cross-sectional study was conducted at Nims Hospital, Jaipur, involving 484 adults’ participants. Blood samples were collected for serum 25(OH) D measurements. Data were gathered using the SF-36 and ISI questionnaires to assess health and sleep quality.
Results: Higher vitamin D levels were strongly linked to better physical health, including physical function (r = 0.642, p < 0.001), general health (r = 0.560, p < 0.001), and PCS score (r = 0.441, p < 0.001). Vitamin D also positively impacted social functioning (r = 0.096, p = 0.035) and was negatively related to ISI scores (r = −0.112, p = 0.014).
Conclusion: The study highlights a strong link between higher vitamin D levels and improved physical and mental health, with significant negative correlation to ISI scores. This underscores the importance of adequate vitamin D for overall well-being. The findings call for urgent measures to address vitamin D deficiency and further research into its health impacts.
1 Introduction
Vitamin D, often celebrated as the “sunshine vitamin,” is a pivotal hormone with broad-reaching roles in the body’s endocrine, autocrine, and paracrine systems. This essential nutrient supports bone health, enhances calcium absorption, modulates immune function, and plays a preventive role against a wide range of health conditions, including osteoporosis, autoimmune diseases, and certain cancers (1–3). Despite its importance, about a billion people globally are affected by insufficient vitamin D levels, including 7.4% of Canadians, 5.9% of Americans, and 13% of Europeans (4–6). Deficiency rates are similarly high in countries like Australia, Turkey, and across regions in Africa and South America, indicating a pressing global concern (1, 3, 7, 8). India also reflects this trend, with studies showing deficiency rates between 50% and 94% across diverse population groups (9).
The health implications of vitamin D deficiency are profound. Beyond its foundational role in calcium and phosphorus metabolism, vitamin D insufficiency is linked to an increased risk of chronic illnesses such as osteoporosis, rickets, and serious conditions including breast and colon cancer, cardiovascular disease, hypertension, and diabetes. It has even been associated with neurodegenerative conditions such as Parkinson’s disease, while also impacting mental health by contributing to mood disorders, like depression, which are exacerbated by low vitamin D levels (10). These links became especially relevant during the COVID-19 pandemic, when vitamin D’s immune-modulating properties gained attention for potentially reducing complications related to the virus (11). These extensive connections highlight how vitamin D plays an essential role not only in preventing physical diseases but also in supporting mental well-being, influencing disease susceptibility and overall health across all ages (12–20). In 2019 alone, insufficient vitamin D levels were estimated to affect the mental health of 293 million individuals worldwide (21).
Vitamin D’s influence extends to sleep, an essential aspect of daily functioning and well-being. A deficiency in vitamin D is connected to various sleep disorders, including restless legs syndrome and sleep apnea, which can further exacerbate chronic health conditions through disrupted sleep cycles and diminished sleep quality (22–26). Sleep itself is regulated by a complex interaction of circadian rhythms, neural pathways, and hormonal signals originating in the hypothalamus, which responds to environmental cues like light. Nevertheless, sleep disorders remain underdiagnosed, even within healthcare settings. Research underscores that both excessive sleep and sleep deprivation are associated with heightened risks of diabetes, hypertension, cancers, and increased mortality rates (27).
The relationship between vitamin D insufficiency and sleep disruption has thus emerged as a significant area of study. Vitamin D receptors play a role in producing melatonin, the hormone central to regulating the sleep–wake cycle, supporting mood, and sustaining an optimal quality of life (28–31). Furthermore, geographic and cultural factors, particularly in India, intensify the need to investigate this link. While India enjoys abundant sunlight, limited outdoor exposure, urban lifestyles, and dietary preferences restrict sufficient vitamin D synthesis among large segments of the population. Additionally, factors like dietary restrictions and skin pigmentation can further reduce vitamin D synthesis, elevating deficiency risks. Given the high prevalence of vitamin D insufficiency in India and its potential impact on both physical and mental health, a comprehensive study examining the connection between vitamin D levels and sleep quality is crucial. This investigation aims to explore how these factors jointly influence well-being in adults aged 18 and older, with insights that could improve health outcomes and highlight the importance of vitamin D in maintaining quality of life across diverse lifestyles and geographies.
2 Materials and methods
2.1 Study design and patient enrollment
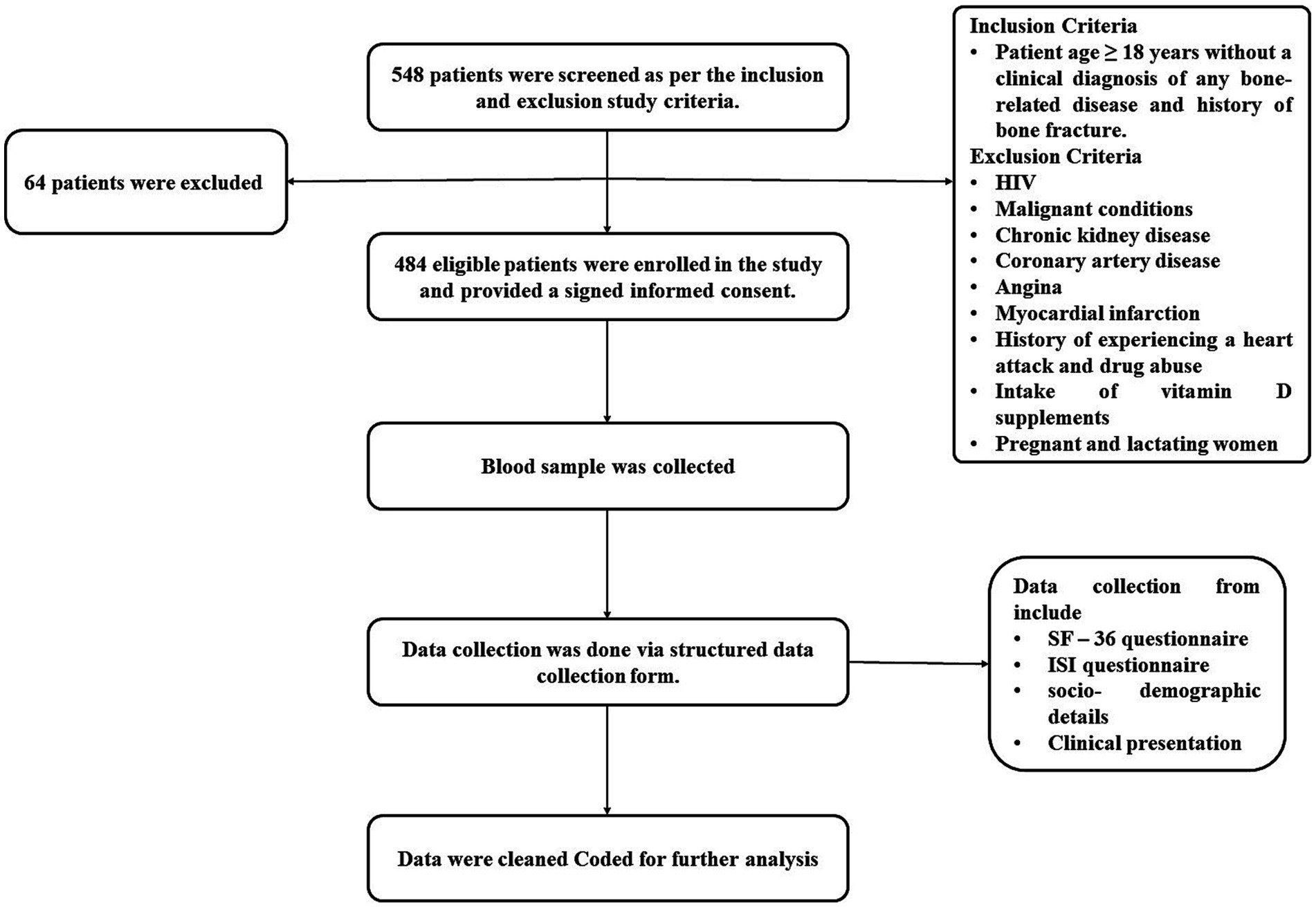
A prospective cross-sectional study was conducted at the NIMS hospital, A Tertiary Teaching Care Hospital, A unit of NIMS University Rajasthan, Jaipur, North India for a duration of 8 months (from August 2023 to March 2024). Patients aged 18 years and above visiting the outpatient (OPD) and inpatient (IPD) departments of general medicine without a clinical diagnosis of any bone-related disease and history of bone fractures were enrolled. Whereas, the patients undergoing hormone replacement therapy, diagnosed with HIV, malignant conditions, chronic kidney disease, coronary artery disease, angina, myocardial infarction, history of experiencing a heart attack and drug abuse, consumption of vitamin D supplements, women who were pregnant or breastfeeding, and the people who did not willingly participate or give their consent were excluded. The blood samples of the enrolled participants were collected for serum 25(OH) D measurements in the department of biochemistry and laboratory services at the NIMS hospital. The vitamin D and serum calcium levels were determined as per the standard guidelines of Virtus 5600 (32). Checking serum 25(OH)D levels was done with the Virtus 5600 integrated system [Model number J56001308].
2.2 Study recruitment and data collection process
548 patients were screened using the planned inclusion and exclusion criteria. Of these, 64 patients did not meet the inclusion criteria, leaving 484 patients who were eligible to join the study after giving written consent. A structured data collection form with the SF-36 questionnaire, a commonly used tool for evaluating patients’ mental and physical health, was used to collect the data. Physical health and mental health are the two-summary metrics derived from its eight scales. Physical functioning (10 components), role-physical (four items), bodily pain (two items), and overall health make up the physical health summary score (five items). Physical health (four items), social skills (two items), emotional and role-related skills (three items), and mental health make up the mental health measure (five items). These domains evaluate energy levels, social engagement, the impact of emotional issues on daily activities, and overall mental well-being. The RAND Healthcare SF-36 scoring instructions from the RAND Corporation are used to decide the scores. Along with the Insomnia Severity Index (ISI) questionnaire, domain scores range from 0 to 100, with higher scores indicating better health-related quality of life and lower scores indicating worse health. The seven-item ISI questionnaire was used to find out the insomnia’s nature, severity, and effects. Participants were asked about sleep maintenance, sleep onset, sleep dissatisfaction, early morning waking issues, daytime functioning, and anxiety related to sleep difficulties for the past month. Four levels of insomnia severity were found based on the answers: no insomnia (0–7 level), sub-threshold insomnia (8–14 score), moderate insomnia (15–21 score), and severe insomnia (22–28 score) (33–41), encompassing socio-demographic details (such as name, age, gender, address, residential area, marital status, history of smoking, alcohol and tobacco use, milk consumption, and sun exposure), clinical presentation (concerns related to bones, history of bone fractures, comorbidity, current symptoms, and present diagnosis), and all questions from the questionnaires were duly addressed (Figure 1).
2.3 Ethics
The research conducted in this study obtained approval from the Institutional Ethics Committee (IEC) at NIMS University Rajasthan. The reference number for this approval is NIMSUR/IEC/2023/677. It is important to note that throughout the entire research process, strict adherence was maintained to the ethical principles delineated in the Declaration of Helsinki, which was established in 1975.
2.4 Statistical analysis
The statistical analysis for this study was conducted using two software tools: Excel (version 2019) and IBM SPSS (version 28.0). Power analysis with a power of 0.80 with an alpha level of 0.05, and a confidence interval of 95% was used to reach the required sample size (42). Continuous variables were summarized by calculating the mean and standard deviation, while categorical variables were presented through median, frequency, and percentage measures. To compare categorical variables, the Fisher exact test was employed, whereas for comparing quantities, the t-test was utilized. This approach ensured comprehensive and accurate data interpretation in accordance with the study’s objectives.
3 Results
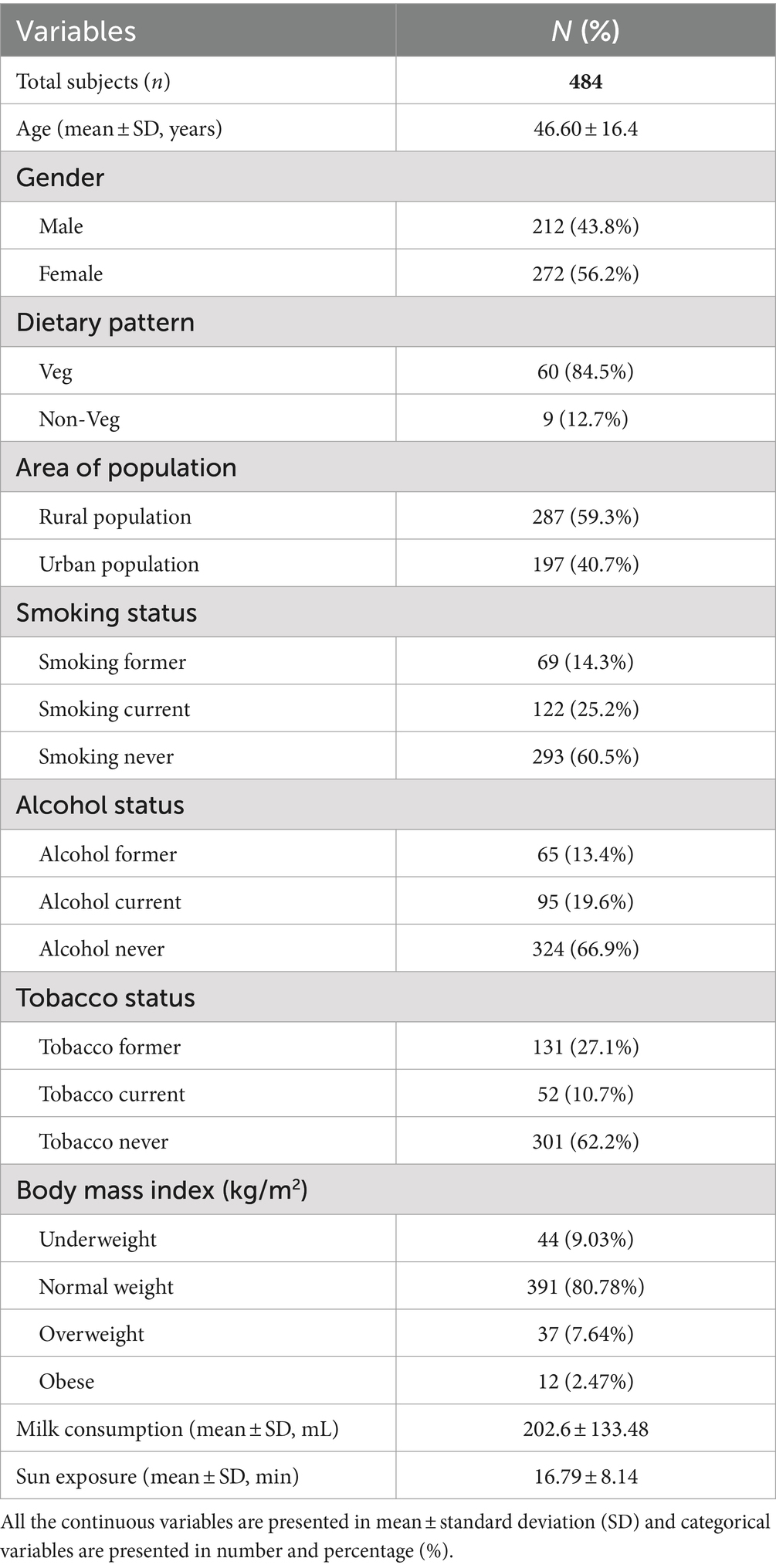
A total of 484 participants were enrolled with an average age of 46.60 ± 16.4 years, 43.8% male and 56.2% female. The majority of participants followed a vegetarian diet (84.5%), while 12.7% followed a non-vegetarian diet. There were people from rural areas (59.3% of the participants) and people from cities (40.7%). 25.2% were smokers, with 14.3% being former smokers. Additionally, 19.6% were identified as alcoholics, with 13.4% being former alcoholics. Regarding chewing tobacco, 10.7% were consumers, while 27.1% were former consumers. The average milk consumption was 202.6 ± 133.48 mL/day, while the average sun exposure received was 16.79 ± 8.14 min/day. Based on Body Mass Index (BMI) categorization, 9.03% of participants were classified as underweight, 80.78% as normal weight, 7.64% as overweight, and 2.47% as obese (Table 1).
3.1 Laboratory investigations
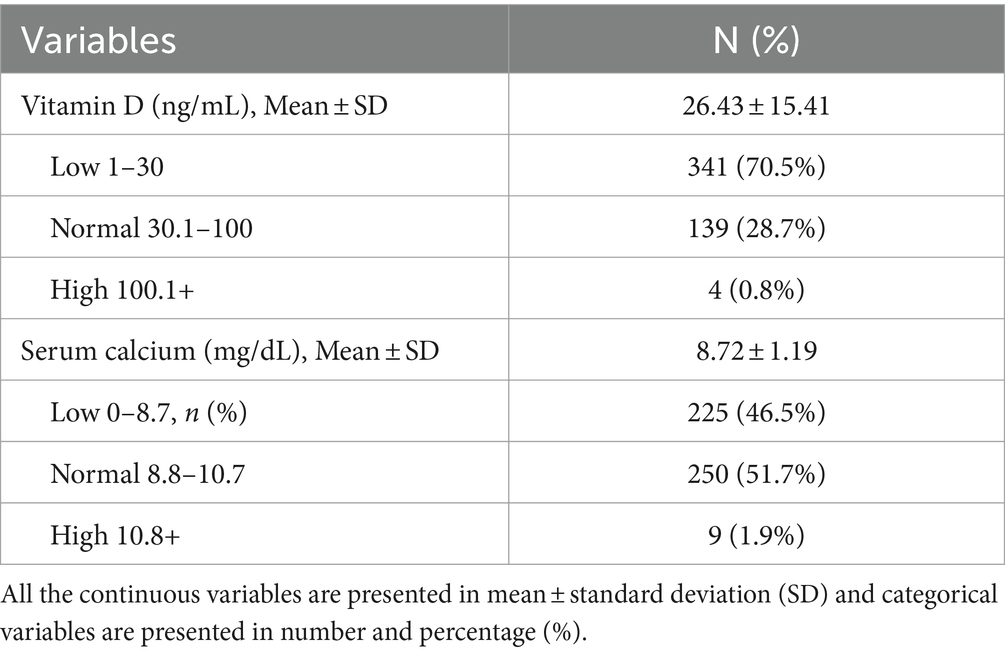
There were 70.5% subject with low (≤30 ng/mL), 28.7% normal (30.1–100 ng/mL), and 0.8% high (>100 ng/mL) vitamin D levels with an average of 26.43 ± 15.41 ng/mL. 46.5% subjects were having low (≤8.7 mg/dL), 51.7% normal (8.8–10.7 mg/dL), and 1.9% high (>10.8 mg/dL) serum calcium levels, accounting for an average of 8.72 ± 1.19 mg/dL (Table 2).
3.2 Scores of participants for the mental and physical components (SF-36)
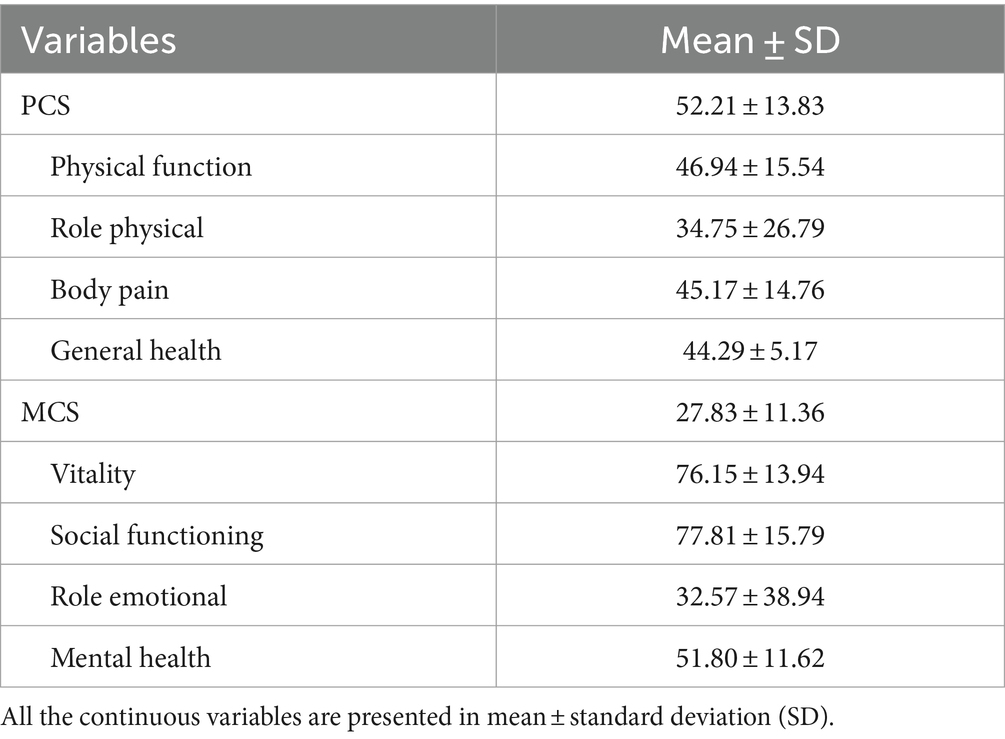
For the mental component, the overall score is 27.83 ± 11.36, and it is split into four areas: Vitality, Social Functioning, Role Emotional, and Mental Health. These areas have average scores of 76.15 ± 13.94, 77.81 ± 15.79, 32.57 ± 38.94, and 51.80 ± 11.62, respectively. Physical function, Body Pain, Role Physical and General Health each have an average score of 46.94 ± 15.54, 34.75 ± 26.79, 45.17 ± 14.76, and 44.29 ± 5.17, respectively, on the physical component summary score (52.21 ± 13.83) (Table 3).
3.3 Insomnia severity index scores of the enrolled participants
The average ISI score of participants was 18.73 ± 6.34, with 3.9% having no clinically significant insomnia, 28.1% with subthreshold insomnia, 14.7% with moderate insomnia, and 53.3% with severe clinical insomnia (Table 4).
3.4 Correlation of vitamin D and serum calcium levels with SF-36 (physical, mental health) and ISI score
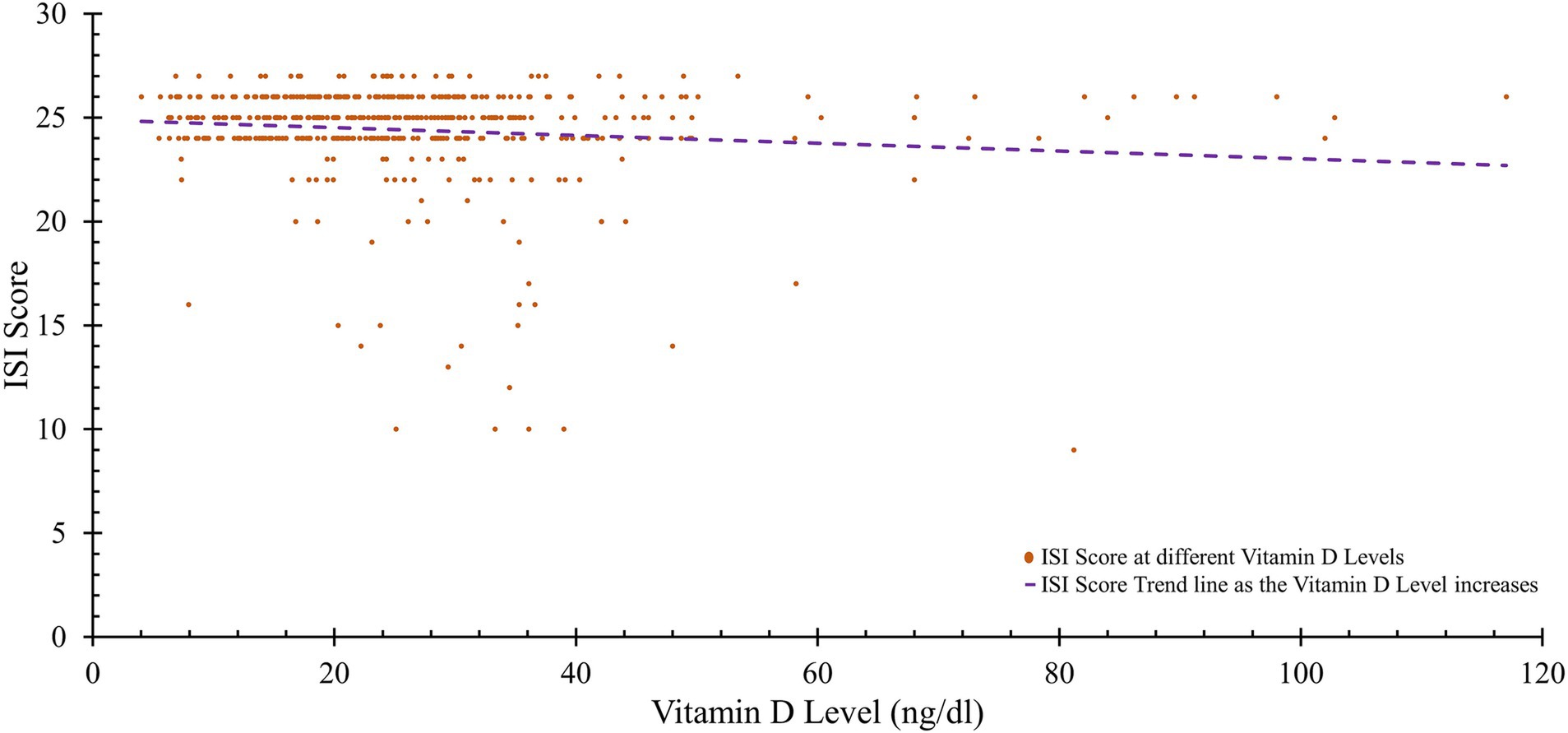
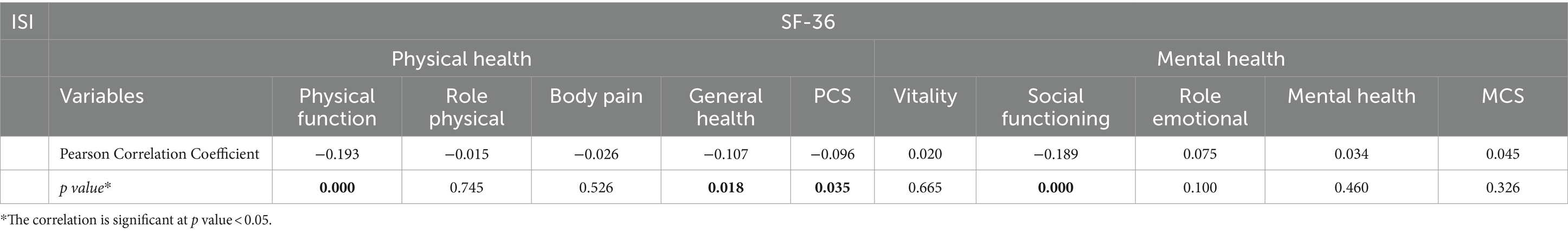
There was a strong link between higher levels of vitamin D and various aspects of physical health, including the physical function score (r = 0.642, p < 0.001), the general health score (r = 0.560, p < 0.001), and the physical component summary (PCS) score (r = 0.441, p < 0.001). Concerning mental health, there was a positive correlation between social functioning and vitamin D levels (r = 0.096, p = 0.035), while Insomnia Severity Index (ISI) scores were significantly negatively related to vitamin D levels (r = −0.112, p = 0.014). Additionally, there was a significant positive correlation between serum calcium levels and various aspects of physical health, such as general health (r = 0.185, p = 0.021) and the PCS score (r = 0.018, p = 0.047). For mental health, role physical does not show any significant correlation with serum calcium (r = 0.050, p = 0.276). However, social functioning and role emotional also exhibited no significant correlations with serum calcium (r = 0.006, p = 0.894 and r = −0.051, p = 0.267, respectively). ISI scores had a negligible association with serum calcium (r = 0.008, p < 0.001) (Table 5; Figure 2).
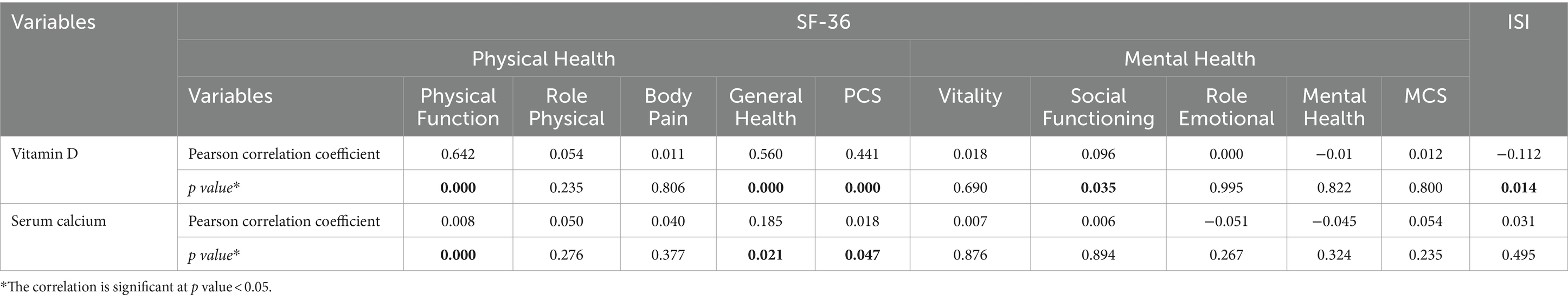
Table 5. Correlation of vitamin D and serum calcium levels with SF-36 (Physical, mental health) and ISI score.
3.5 Correlation between SF-36 (physical, mental health) and ISI score
There was a significant negative correlation between ISI score and various aspects of physical health, such as physical function (r = −0.193, p < 0.001) and general health (r = −0.107, p = 0.018), with the physical component summary (PCS) score showing a similar trend (r = −0.096, p = 0.035). Regarding mental health, social functioning was negatively correlated with ISI score (r = −0.189, p < 0.001) (Table 6).
4 Discussion
The primary objective of this research was to examine the correlation between vitamin D levels, health-related quality of life (HRQoL) as measured by the SF-36 questionnaire, and the severity of insomnia in adults aged 18 and older without bone-related diseases. Results revealed strong associations between vitamin D levels, several dimensions of HRQoL, and insomnia severity, indicating that vitamin D status significantly affects both physical and mental health outcomes.
Laboratory analyses showed a significant prevalence of vitamin D deficiency, with 70.5% of participants having serum levels below 30 ng/mL, consistent with prior studies emphasizing the high occurrence of vitamin D deficiency in regions with limited sunlight exposure or diets low in vitamin D-rich foods (43). This widespread insufficiency underscores a potential need for interventions to improve vitamin D status as a preventive health measure.
Vitamin D levels were positively correlated with several physical HRQoL dimensions, including physical functioning, general health perception, and overall physical component summary (PCS) scores. Higher vitamin D levels were associated with better physical functioning, fewer limitations in physical role activities, and more positive general health perceptions. This suggests a potential role of vitamin D in reducing physical health limitations and enhancing overall well-being. Additionally, a positive correlation between vitamin D levels and social functioning was observed, indicating that sufficient vitamin D may support better social engagement and interpersonal interactions. While vitamin D’s impact on social functioning is indirect and mediated through its effects on mental health (e.g., depression, social withdrawal), it highlights the multifactorial nature of social behavior, shaped by biological, psychological, and environmental factors (44, 45). Therefore, the complexity of these interactions results in weak but significant correlations, reflecting the nuanced role of vitamin D in social and emotional health (31, 46).
Notably, ISI scores, reflecting insomnia severity, were negatively correlated with vitamin D levels. This suggests that adequate vitamin D levels may help mitigate the severity of insomnia, likely due to the influence of the vitamin D receptor (VDR) on genes related to hormones, neurotransmitters, and circadian rhythm regulation. VDR activation promotes the synthesis of serotonin and melatonin, which are essential for sleep initiation and maintenance. Disrupted VDR signaling can impair these processes, potentially leading to poor sleep quality and disrupted sleep patterns (47). This observation reinforces the relevance of monitoring vitamin D status as part of diagnosing and managing sleep disorders, with implications for both mental and physical health.
Additionally, serum calcium levels were positively associated with physical health aspects within SF-36, particularly in general health and PCS scores, highlighting calcium’s critical role in musculoskeletal and neuromuscular functions. Adequate calcium levels contribute to better muscle function and reduced risks of muscular weakness, as reflected in improved physical function scores. However, serum calcium’s association with ISI scores was minimal, suggesting that calcium levels do not significantly impact sleep-related outcomes. This underscores vitamin D’s unique role in sleep health, distinguishing it from calcium’s primary influence on physical well-being.
Significant correlations between SF-36 physical health parameters—specifically physical functioning and general health—and ISI scores were observed, with lower physical functioning scores associated with higher ISI scores, indicating more severe insomnia in individuals experiencing greater physical limitations. This relationship underscores the interdependent nature of physical impairments and sleep disturbances, as physical limitations may exacerbate the severity of insomnia. Additionally, the significant association between general health perceptions and ISI scores suggests that individuals who perceive poorer overall health also report more severe insomnia, emphasizing how physical and mental health collectively impact sleep quality.
The correlations observed between vitamin D levels, HRQoL, and ISI scores may be partially explained by vitamin D’s involvement in hormonal and neurological regulation. Vitamin D interacts with vitamin D receptors (VDRs), influencing gene expression related to essential hormones and neurotransmitters that maintain physical and mental health. Specifically, VDR activation promotes serotonin and melatonin synthesis, both of which play critical roles in mood regulation and sleep maintenance. Low vitamin D levels can interfere with this regulatory process, potentially disrupting circadian rhythms and aggravating insomnia. Additionally, vitamin D influences melatonin production by binding to receptors in the hypothalamus, which governs circadian alignment; insufficient vitamin D can lead to circadian misalignment and exacerbate sleep disorders, including insomnia and obstructive sleep apnea syndrome (OSAS).
Vitamin D deficiency may also intensify immune and inflammatory responses by elevating pro-inflammatory cytokines, such as IL-6 and TNF-α, which are associated with disrupted sleep patterns and poor sleep quality. This inflammatory response may lead to fragmented sleep and worsen airway obstruction, as seen in OSAS (48). Furthermore, vitamin D modulates the body’s hypoxic response via its influence on HIF-1α, potentially mitigating sleep disturbances related to hypoxic episodes. These physiological pathways suggest a complex and multifactorial role of vitamin D in maintaining sleep quality, providing further insights into the observed associations between vitamin D, HRQoL, ISI scores, and overall health (49, 50).
These findings underscore the importance of vitamin D assessments in public health and clinical settings to improve both physical and mental health outcomes. Positive correlations between vitamin D, HRQoL, and insomnia severity suggest that vitamin D status is a modifiable factor with potential to enhance overall quality of life, particularly for populations with high vitamin D deficiency rates. Integrating vitamin D screening and supplementation into healthcare practices may help reduce the burden of sleep disorders and physical impairments, contributing to improved HRQoL and well-being across affected populations. Furthermore, this study contributes to the existing literature by examining vitamin D’s broader impact, not only on physical health but also on sleep quality, mental well-being, and social functioning. This study provides novel insights into vitamin D’s relationship with insomnia severity and social engagement, offering a more comprehensive understanding of vitamin D’s influence on overall HRQoL.
Additionally, the focus on an Indian population with distinct cultural and dietary practices adds valuable perspectives to the global research on vitamin D, highlighting region-specific health interventions and preventive care strategies.
4.1 Limitations of the study
The study’s 6-month duration may have limited its ability to fully capture the long-term effects of vitamin D on physical health, mental well-being, and sleep quality. Recruitment challenges due to specific selection criteria and the single-center design further restrict the generalizability of the findings to broader populations or different settings. Additionally, direct measures of sunlight exposure—a key factor in vitamin D synthesis—were not included, potentially affecting the study’s accuracy regarding vitamin D levels and related health outcomes. Future studies would benefit from incorporating direct sunlight measurements to provide a clearer understanding of its impact. Notably, the correlation coefficients for physical and mental health and insomnia were found to be close to or slightly higher than those for insomnia and vitamin D levels, suggesting that the observed effects on sleep quality may arise not only from vitamin D’s direct influence on sleep biochemistry, such as melatonin synthesis, but also through secondary effects on physical and mental health. Expanding future research to longitudinal, multi-site studies with diverse populations could further clarify these relationships, enhancing the generalizability and causal understanding of vitamin D’s role in HRQoL, insomnia severity, and overall health metrics.
5 Conclusion
The findings of this study underscore a meaningful connection between higher levels of vitamin D and improved physical and mental well-being, as indicated by better performance on the SF-36 mental health scale and the Physical Component Summary (PCS). Notably, we also observed a significant negative relationship between vitamin D levels and insomnia severity, suggesting that adequate vitamin D may play a vital role in enhancing overall health and well-being. Given these results, there is a pressing need for initiatives aimed at reducing vitamin D deficiency. Additionally, further research is essential to deepen our understanding of the specific mechanisms that link vitamin D to a diverse range of health outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Ethics Committee (IEC) at NIMS University Rajasthan. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AS: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. SK: Data curation, Formal analysis, Investigation, Writing – original draft. SM: Formal analysis, Methodology, Visualization, Writing – original draft. SR: Formal analysis, Investigation, Visualization, Writing – original draft. SD: Formal analysis, Methodology, Writing – original draft. PR: Conceptualization, Supervision, Validation, Writing – review & editing. HB: Resources, Supervision, Validation, Writing – review & editing. MS: Conceptualization, Supervision, Validation, Writing – review & editing. DN: Project administration, Resources, Software, Supervision, Writing – review & editing. BT: Resources, Software, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
All the authors extend their sincere appreciation to the staff and doctors of the Department of General Medicine at NIMS Hospital, Jaipur, for their unwavering support and guidance throughout our research on “The Effects of Vitamin D Levels on Physical, Mental Health, and Sleep Quality in Adults: A Comprehensive Investigation.” Additionally, we express our gratitude to the professors of the Biochemistry department for their consistent support and assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nair, R, and Maseeh, A. Vitamin D: the “sunshine” vitamin. J Pharmacol Pharmacother. (2012) 3:118–26. doi: 10.4103/0976-500X.95506
2. Mostafa, WZ, and Hegazy, RA. Vitamin D and the skin: focus on a complex relationship: a review. J Adv Res. (2015) 6:793–804. doi: 10.1016/j.jare.2014.01.011
3. Holick, MF, Binkley, NC, Bischoff-Ferrari, HA, Gordon, CM, Hanley, DA, Heaney, RP, et al. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J Clin Endocrinol Metab. (2012) 97:1153–8. doi: 10.1210/jc.2011-2601
4. Schleicher, RL, Sternberg, MR, Looker, AC, Yetley, EA, Lacher, DA, Sempos, CT, et al. National estimates of serum total 25-hydroxyvitamin D and metabolite concentrations measured by liquid chromatography–tandem mass spectrometry in the US population during 2007–2010. J Nutr. (2016) 146:1051–61. doi: 10.3945/jn.115.227728
5. Sarafin, K, Durazo-Arvizu, R, Tian, L, Phinney, KW, Tai, S, Camara, JE, et al. Standardizing 25-hydroxyvitamin D values from the Canadian health measures survey. Am J Clin Nutr. (2015) 102:1044–50. doi: 10.3945/ajcn.114.103689
6. Cashman, KD, Dowling, KG, Škrabáková, Z, Gonzalez-Gross, M, Valtueña, J, De Henauw, S, et al. Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr. (2016) 103:1033–44. doi: 10.3945/ajcn.115.120873
7. Amrein, K, Scherkl, M, Hoffmann, M, Neuwersch-Sommeregger, S, Köstenberger, M, Tmava Berisha, A, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. (2020) 74:1498–513. doi: 10.1038/s41430-020-0558-y
8. Lips, P, Cashman, KD, Lamberg-Allardt, C, Bischoff-Ferrari, HA, Obermayer-Pietsch, B, Bianchi, ML, et al. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European calcified tissue society. Eur J Endocrinol. (2019) 180:P23–54. doi: 10.1530/EJE-18-0736
9. Aparna, P, Muthathal, S, Nongkynrih, B, and Gupta, SK. Vitamin D deficiency in India. J Family Med Prim Care. (2018) 7:324–30. doi: 10.4103/jfmpc.jfmpc_78_18
10. Huiberts, LM, and Smolders, KC. Effects of vitamin D on mood and sleep in the healthy population: interpretations from the serotonergic pathway. Sleep Med Rev. (2021) 55:101379. doi: 10.1016/j.smrv.2020.101379
11. Ashique, S, Gupta, K, Gupta, G, Mishra, N, Singh, SK, Wadhwa, S, et al. Vitamin D—A prominent immunomodulator to prevent COVID‐19 infection. Int. J. Rheum. Dis. (2023) 26:13–30. doi: 10.1007/s10787-024-01578-w
12. Shaukat, N, Jaleel, F, Moosa, FA, and Qureshi, NA. Association between vitamin D deficiency and breast cancer. Pak J Med Sci. (2017) 33:645–9. doi: 10.12669/pjms.333.11753
13. Papandreou, D, and Hamid, ZT. The role of vitamin D in diabetes and cardiovascular disease: an updated review of the literature. Dis Markers. (2015) 2015:1–15. doi: 10.1155/2015/580474
14. Kostoglou-Athanassiou, I, Athanassiou, P, Lyraki, A, Raftakis, I, and Antoniadis, C. Vitamin D and rheumatoid arthritis. Ther Adv Endocrinol Metab. (2012) 3:181–7. doi: 10.1177/2042018812471070
15. Gombash, SE, Lee, PW, Sawdai, E, and Lovett-Racke, AE. Vitamin D as a risk factor for multiple sclerosis: immunoregulatory or neuroprotective? Front Neurol. (2022) 13:796933. doi: 10.3389/fneur.2022.796933
16. Kearns, MD, and Tangpricha, V. The role of vitamin D in tuberculosis. J Clin Transl Endocrinol. (2014) 1:167–9. doi: 10.1016/j.jcte.2014.08.002
17. Pignolo, A, Mastrilli, S, Davì, C, Arnao, V, Aridon, P, dos Santos Mendes, FA, et al. Vitamin D and Parkinson’s disease. Nutrients. (2022) 14:1220. doi: 10.3390/nu14061220
18. Wiciński, M, Adamkiewicz, D, Adamkiewicz, M, Śniegocki, M, Podhorecka, M, Szychta, P, et al. Impact of vitamin D on physical efficiency and exercise performance—a review. Nutrients. (2019) 11:2826. doi: 10.3390/nu11112826
19. Cuomo, A, Giordano, N, Goracci, A, and Fagiolini, A. Depression and vitamin D deficiency: causality, assessment, and clinical practice implications. Neuropsychiatry. (2017) 7:606–14. doi: 10.4172/Neuropsychiatry.1000255
20. Karadeniz, Y, Özpamuk-Karadeniz, F, Ahbab, S, Ataoğlu, E, and Can, G. Vitamin D deficiency is a potential risk for blood pressure elevation and the development of hypertension. Medicina. (2021) 57:1297. doi: 10.3390/medicina57121297
21. Kieling, C, Buchweitz, C, Caye, A, Silvani, J, Ameis, SH, Brunoni, AR, et al. Worldwide prevalence and disability from mental disorders across childhood and adolescence: evidence from the global burden of disease study. JAMA Psychiatry. (2024) 81:347–56. doi: 10.1001/jamapsychiatry.2023.5051
22. Wali, S, Alsafadi, S, Abaalkhail, B, Ramadan, I, Abulhamail, B, Kousa, M, et al. The association between vitamin D level and restless legs syndrome: a population-based case-control study. J Clin Sleep Med. (2018) 14:557–64. doi: 10.5664/jcsm.7044
23. Ayas, NT, White, DP, Manson, JE, Stampfer, MJ, Speizer, FE, Malhotra, A, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. (2003) 163:205–9. doi: 10.1001/archinte.163.2.205
24. Janszky, I, and Ljung, R. Shifts to and from daylight saving time and incidence of myocardial infarction. N Engl J Med. (2008) 359:1966–8. doi: 10.1056/NEJMc0807104
25. Schlafer, O, Wenzel, V, and Högl, B. Sleep disorders among physicians on shift work. Anaesthesist. (2014) 63:844–51. doi: 10.1007/s00101-014-2374-z
26. Romano, F, Muscogiuri, G, Di Benedetto, E, Zhukouskaya, VV, Barrea, L, Savastano, S, et al. Vitamin D and sleep regulation: is there a role for vitamin D? Curr Pharm Des. (2020) 26:2492–6. doi: 10.2174/1381612826666200310145935
27. Gao, Q, Kou, T, Zhuang, B, Ren, Y, Dong, X, and Wang, Q. The association between vitamin D deficiency and sleep disorders: a systematic review and meta-analysis. Nutrients. (2018) 10:1395. doi: 10.3390/nu10101395
28. Geng, C, Yang, Z, Kong, X, Xu, P, and Zhang, H. Correlation between vitamin D and poor sleep status in restless legs syndrome. Front Endocrinol. (2022) 13:994545. doi: 10.3389/fendo.2022.994545
29. Chattu, VK, Manzar, MD, Kumary, S, Burman, D, Spence, DW, and Pandi-Perumal, SR. The global problem of insufficient sleep and its serious public health implications. InHealthcare. (2018) 7:1. doi: 10.3390/healthcare7010001
30. Prono, F, Bernardi, K, Ferri, R, and Bruni, O. The role of vitamin D in sleep disorders of children and adolescents: a systematic review. Int J Mol Sci. (2022) 23:1430. doi: 10.3390/ijms23031430
31. Menezes-Júnior, LA, Sabião, TD, Moura, SS, Batista, AP, Menezes, MC, Carraro, JC, et al. The role of interaction between vitamin D and VDR FokI gene polymorphism (rs2228570) in sleep quality of adults. Sci Rep. (2024) 14:8141. doi: 10.1038/s41598-024-58561-2
32. Vitros Immunodiagnostic. Version 6.0. Pub. No. GEM1361_US_EN. 11 of 14. Page 12. 17. CLSI. Method Comparison and Bias Estimation Using Patient … Pub. No. GEM1361_US_EN. Version 6.0. (2013). Available online at: https://imgcdn.mckesson.com/CumulusWeb/Click_and_learn/Vitros_25OH_Vitamin_D_Total_Reagent_Pack_IFU.pdf
33. Brazier, JE, Harper, R, Jones, NM, O'Cathain, A, Thomas, KJ, Usherwood, T, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. Br Med J. (1992) 305:160–4. doi: 10.1136/bmj.305.6846.160
34. Sinha, R, van den Heuvel, WJ, and Arokiasamy, P. Validity and reliability of MOS short form health survey (SF-36) for use in India. Ind J Commun Med. (2013) 38:22–6. doi: 10.4103/0970-0218.106623
35. RAND (1992). 36-Item Short Form Survey (SF-36) Scoring Instructions. Available online at: https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form/scoring.html
36. Ware, JE Jr, and Sherbourne, CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. (1992) 30:473–83. doi: 10.1097/00005650-199206000-00002
37. Hays, RD, and Shapiro, MF. An overview of generic health-related quality of life measures for HIV research. Qual Life Res. (1992) 1:91–7. doi: 10.1007/BF00439716
38. Steward, AL, Sherbourne, C, Hayes, RD, et al. Summary and discussion of MOS measures In: AL Stewart and JE Ware, editors. Measuring Functioning and Well-Being: The Medical Outcome Study Approach. Durham, NC: Duke University Press (1992). 345–71.
39. Manoy, P, Yuktanandana, P, Tanavalee, A, Anomasiri, W, Ngarmukos, S, Tanpowpong, T, et al. Vitamin D supplementation improves quality of life and physical performance in osteoarthritis patients. Nutrients. (2017) 9:799. doi: 10.3390/nu9080799
40. Bastien, CH, Vallières, A, and Morin, CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. (2001) 2:297–307. doi: 10.1016/S1389-9457(00)00065-4
41. Blais, FC, Gendron, L, Mimeault, V, and Morin, CM. Assessment of insomnia: validation of three questionnaires. Encephale Rev Psychiatr Clin Biol Therap. (1997) 23:447–53.
42. Cohen, J. Statistical Power Analysis for the Behavioral Sciences. Lawrence Erlbaum Associates, Publishers (2013).
43. de Souza de Santana, KV, Oliver, SL, Mendes, MM, Lanham-New, S, Charlton, KE, and Ribeiro, H. Association between vitamin D status and lifestyle factors in Brazilian women: implications of sun exposure levels, diet, and health. EClinicalMedicine. (2022) 47:101400. doi: 10.1016/j.eclinm.2022.101400
44. Alegría, M, NeMoyer, A, Falgàs Bagué, I, Wang, Y, and Alvarez, K. Social determinants of mental health: where we are and where we need to go. Curr Psychiatry Rep. (2018) 20:1–3. doi: 10.1007/s11920-018-0969-9
45. da Silva, ST, Alves de Menezes-Júnior, LA, Batista, AP, Silva de Moura, S, Meireles, AL, Carvalho de Menezes, M, et al. Interaction between Fokl polymorphism and vitamin D deficiency in the symptoms of mental disorders in adults: a population-based study. Sci Rep. (2024) 14:6–8. doi: 10.1038/s41598-024-57558-1
46. Anglin, RE, Samaan, Z, Walter, SD, and McDonald, SD. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. (2013) 202:100–7. doi: 10.1192/bjp.bp.111.106666
47. Eyles, DW, Liu, PY, Josh, P, and Cui, X. Intracellular distribution of the vitamin D receptor in the brain: comparison with classic target tissues and redistribution with development. Neuroscience. (2014) 268:1–9. doi: 10.1016/j.neuroscience.2014.02.042
48. Archontogeorgis, K, Nena, E, Papanas, N, Zissimopoulos, A, Voulgaris, A, Xanthoudaki, M, et al. Vitamin D levels in middle-aged patients with obstructive sleep apnoea syndrome. Curr Vasc Pharmacol. (2018) 16:289–97. doi: 10.2174/1570161115666170529085708
49. Archontogeorgis, K, Nena, E, Papanas, N, and Steiropoulos, P. The role of vitamin D in obstructive sleep apnoea syndrome. Breathe. (2018) 14:206–15. doi: 10.1183/20734735.000618
Keywords: vitamin D, SF-36, ISI, mental health, physical health, sleep
Citation: Singh AK, Kumar S, Mishra S, Rajotiya S, Debnath S, Raj P, Bareth H, Singh M, Nathiya D and Tomar BS (2024) The effects of vitamin D levels on physical, mental health, and sleep quality in adults: a comprehensive investigation. Front. Nutr. 11:1451037. doi: 10.3389/fnut.2024.1451037
Edited by:
Cristina Vassalle, Gabriele Monasterio Tuscany Foundation (CNR), ItalyReviewed by:
Denis Baranenko, ITMO University, RussiaEmre Batuhan Kenger, Istanbul Bilgi University, Türkiye
Copyright © 2024 Singh, Kumar, Mishra, Rajotiya, Debnath, Raj, Bareth, Singh, Nathiya and Tomar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shivang Mishra, c2hpdmFuZy5taXNocmEyM0BnbWFpbC5jb20=; Sumit Rajotiya, c3VtaXRyYWpvdGl5YTE5OUBnbWFpbC5jb20=; Sourav Debnath, c2RuYXRobWVAZ21haWwuY29t
†ORCID: Sumit Rajotiya, orcid.org/0009-0001-2080-9953
 Anurag Kumar Singh
Anurag Kumar Singh Sachin Kumar
Sachin Kumar Shivang Mishra
Shivang Mishra Sumit Rajotiya
Sumit Rajotiya Sourav Debnath
Sourav Debnath Preeti Raj
Preeti Raj Hemant Bareth
Hemant Bareth Mahaveer Singh
Mahaveer Singh Deepak Nathiya
Deepak Nathiya Balvir Singh Tomar4,5,6
Balvir Singh Tomar4,5,6