- 1Department of Endocrinology, The Second Medical Center and National Clinical Research Center for Geriatric Diseases, Chinese PLA General Hospital, Beijing, China
- 2Clinics of Cadre, Department of Outpatient, The First Medical Center, Chinese PLA General Hospital, Beijing, China
- 3Department of Hematology, The Second Medical Center, Chinese PLA General Hospital, Beijing, China
Introduction: The aim of this study is to investigate the impact of vitamin D supplementation on the muscle strength of the elderly.
Methods: This retrospective, propensity score-matched study included 160 middle-aged and elderly individuals from a community in Beijing, China. The control group (n=110) received health education and lifestyle guidance, while the intervention group (n=50) was given oral vitamin D supplementation in addition to health education and lifestyle guidance. All participants underwent laboratory tests, muscle function, and physical function at baseline and follow-up.
Results: In the propensity score-matched cohort of 41 patients per group, the levels of serum calcium and 25-hydroxyvitamin D in both groups were improved significantly by the end of the study (p<0.05), with the intervention group showing a more significant improvement. The muscle strength of the left lower limb in the intervention group significantly increased after the intervention (p<0.05). The results also showed that the grip strength and pinch strength of the patients in both groups increased after the intervention, and the difference between the two groups was statistically significant (p<0.05).
Discussion: The findings of this study suggest that vitamin D supplementation, in conjunction with lifestyle guidance and health education, is beneficial for enhancing the upper and lower limb strength of patients.
1 Introduction
Vitamin D is a fat-soluble vitamin with multiple physiological functions, and its most critical role is promoting the absorption of calcium, magnesium, and phosphorus for normal skeletal development and maintenance (1). Vitamin D is synthesized in the skin, primarily through exposure to sunlight radiation. However, humans can also obtain a small amount of vitamin D through dietary intake or exogenous nutritional supplementation. Insufficient or deficient levels of vitamin D have been positively associated with various health risks, including myopathy, osteoporosis, as well as conditions such as rickets in adolescents and osteomalacia in adults (2). Numerous cross-sectional studies have reported that individuals with vitamin D deficiency commonly experience muscle pain, reduced muscle strength, decreased physical performance, an increased risk of falls, and alterations in muscle morphology (3–5).
Epidemiological investigations have revealed that a low vitamin D status is emerging as a very common condition in the global range, with the prevalence of vitamin D deficiency or insufficiency ranging from 30 to 60%, affecting over 1 billion individuals (6–8). Researchers found that globally, 15.7, 47.9, and 76.6% of people had serum 25-hydroxyvitamin D levels below 30, 50, and 75 nmol/L, respectively (9). Vitamin D is recognized as a pivotal nutritional factor in bone formation, garnering extensive attention for its integral role in bone metabolism. Insufficient vitamin D levels during adolescence may lead to delayed or aberrant skeletal development, and in severe cases, result in the occurrence of rickets. In middle-aged and elderly populations, vitamin D deficiency can contribute to impaired bone mineralization and osteoporosis, significantly elevating the risk of fractures and falls (10, 11). Moreover, due to hormonal fluctuations and alterations in lifestyle habits that accompany the aging process, a considerable number of middle-aged and elderly individuals present with abnormally low levels of serum 25-hydroxyvitamin D [25(OH)D] concentrations. Consequently, the issue of vitamin D deficiency in this particular demographic necessitates heightened scrutiny and consideration (12).
Bislev et al. conducted a systematic review and meta-analysis on the supplementation of vitamin D and its impact on muscle health. The results indicated that the current evidence does not support the beneficial effects of vitamin D supplementation on muscle health and may even suggest potential adverse effects (13). Increased muscle strength is regarded as a key factor in the prevention of falls and fractures, and improving the status of vitamin D may represent a straightforward approach to achieving these critical objectives (14). However, further clinical data is still required to establish the correlation between vitamin D supplementation and muscle strength conclusively.
Several randomized controlled trials (RCT) studies have been conducted to investigate the effect of oral vitamin D supplementation in older patients to prevent or treat sarcopenia, but the results are still controversial. Previously, a random control trial was conducted by the same team of this study to investigate the effects of stratified vitamin D supplementation in middle-aged and elderly individuals with vitamin D insufficiency in Beijing (15). It concluded that stratified vitamin D supplementation effectively increased the blood 25(OH)D level, as well as the number of cases with corrected vitamin D insufficiency or deficiency. In the RCT study, 247 observation variables have been collected and recorded in a research and development (R&D) database. Owing to the strict study design and case follow-up, the quality of this database is high and its data is more reliable than other databases such as electronic medical records (EMRs). Except the previous results, a number of other research directions could be explored by utilizing the same database of the RCT. This would save cost and time of the related research, and provide more evidence of vitamin D’s effectiveness and safety.
Based on real-world data with propensity score matching (PSM), we explored the relationship between vitamin D supplementation and muscle strength.
2 Methods
2.1 Study design
A total of 160 middle-aged and elderly people from a community in Beijing, China surveyed between March to April 2014 were retrospectively reviewed. Inclusion criteria comprised as follows: (a) According to the international universal assessment system for vitamin D status (16), the serum 25(OH)D level was used as an indicator. Vitamin D insufficiency was defined as 25(OH)D < 30 ng/mL. All participants had serum 25(OH)D levels below 30 ng/mL. (b) Participants were long-term residents of Beijing and aged 40–69 years old. Exclusion criteria: (a) hyperparathyroidism, chronic hepatic and renal insufficiency, malignant tumor; (b) use of medications (e.g., glucocorticoids) within 6 months; (c) use of vitamin D supplements, calcium supplements, thyrocalcitonin, bisphosphonates, anticonvulsants, anticoagulants, estrogen and estrogen receptor modulators that affect bone metabolism within 3 months; (d) poor glycemic control in diabetic patients; (e) Women preparing for pregnancy, pregnant women and lactating women. All included subjects voluntarily participated in this study and provided informed consent. Throughout the study, participants were given the option to withdraw from the study, and those who experienced serious adverse events during vitamin D supplementation were asked to discontinue participation in the study.
2.2 Grouping and intervention measures
A total of 160 subjects were included and divided into control group and intervention group. The control group (n = 110) received health education and lifestyle guidance. The intervention group (n = 50) received oral vitamin D supplementation in addition to health education and lifestyle guidance. Oral vitamin D3 supplements were provided by General Nutrition Corporation (GNC). The dose was stratified as follows: for vitamin D insufficiency [20 ng/mL ≤ 25(OH)D < 30 ng/ ml], oral vitamin D3 supplement was given at 5,000 IU/w; for mild vitamin D deficiency [12 ng/mL ≤ 25(OH)D < 20 ng/mL], oral vitamin D3 supplement was given at 10,000 IU/w; for severe vitamin D deficiency [25(OH)D < 12 ng/mL], oral vitamin D3 supplement was given at 15,000 IU/w. The Ethics Committee of Chinese PLA General Hospital approved this study.
2.3 Data collection
A questionnaire survey was conducted on all participants by specially trained staff to collect baseline information, encompassing general conditions, lifestyle, past medical history, and medication history. During the research, a follow-up was conducted every 2 weeks on all participants by the trained staff and adverse events were inquired. The adverse events following oral vitamin D administration were defined as toxicity symptoms caused by an overdose of vitamin D, with early manifestations including nausea, bone and joint pain, skin-itching, hypercalcemia, and abnormalities in kidney and liver function. All biochemical indicators were reassessed for safety evaluation 3 months after the intervention. The intervention period lasted for a total of 9 months, ending from March to April 2015. To avoid the influence of seasonal fluctuations of 25(OH)D on the test results, the determination of the terminal level of biochemical indicators was conducted in the same month as the baseline level.
2.4 Physical examination
Body height and weight (exactly 0.5 cm and 0.5 kg, respectively) were measured uniformly and strictly by trained staff using calibrated and qualified equipment. The participants were requested to be in a fasted state, remove their shoes, and wear light clothing. BMI was calculated as follows: weight (kg)/ height (m2). According to the Seventh Report of the Joint National Committee (JNC), systolic and diastolic blood pressure were measured. The upper right brachial arterial pressure was measured once every 2 min with a mercury column sphygmomanometer, and the average value was recorded for 3 times in total.
2.5 Sample collection and laboratory tests
Fasting venous blood samples were collected at 6:00–8:00 h the next day after an 8–10 h fast. Biochemical measurements were completed within 1 week after sampling. Roche automatic biochemical analyzer (Cobas E601, Roche) was used to detect the indicators including calcium (Ca), phosphorus (P), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), etc. Serum 25 (OH) D levels were measured by electrochemiluminescence (Roche Cobas C6000, Roch Diagnostics GmbH).
2.6 Muscle strength and physical function measures
Grip strength of the participants’ handedness was measured using the Jamar grip device (Sammons Preston, United States). The participants were explained how to use the handgrip device, and the formal measurement was started after one exercise. Pinch strength of the participants’ handedness was measured using the Jamar pinch device (Sammons Preston, United States). During the process, the participants used the thumb and index finger to press the pinch device relative to each other in a sit-to-stand position, and continued to hold the device with maximum force for a few seconds before release. Rest for at least 3 min between each test to avoid muscle fatigue. Both the grip and pinch strength of the dominant hand was measured three times in a row, and the average of the three measurement results was taken in Kilogram (Kg).
The lower limb muscle strength of the participants was measured using a portable hand-held muscle strength dynamometer (Hoggan Health Industries, Inc., United States). The quadriceps muscle strength was used to represent the lower limb muscle strength. Participants were seated with a chair height of 46 cm and joint and knee flexion of 90°. The dynamometer was placed on the lower leg near the ankle joint to measure bilateral lower limb muscle strength. Each side was measured three times, and the average of the three measurements was taken in Newton (N).
The timed up and go test (TUG) and timed chair stands (TCS) were used to assess lower physical function (16). TUG measures the amount of time it takes to get up from a chair, walk 3 m, and return to a sitting position without any physical help. Two trials of the TUG were performed by each participant. The TCS test can be used as a simple alternative to measure the strength of lower limbs, mainly to measure the strength of quadriceps femoris. A seat with a height of about 46 cm was used for measurement. Participants were seated with their arms folded across their chests and asked to complete 5 sit-to-stand movements without stopping or using their arms as fast as possible. Increasing time reflect worse performance and a decline in function.
2.7 Statistical analysis
Data collection was blinded. Data collected by two trained researchers using Epidata software for entry. Statistical analysis was performed using the software package SPSS Statistics version 26.0 (IBM Corp., Armonk, N.Y., United States), with a significance level set at p < 0.05. Quantitative characteristics were expressed as mean ± standard deviation (SD), and intergroup comparison was performed using. The Kolmogorov–Smirnov test was applied to test normal distribution. Non-parametric Wilcoxon test was used for data that did not conform to a normal distribution. A p-value < 0.05 indicated significant difference.
This study was retrospective and data collection was not strictly randomized, which inevitably led to confounding factors. It was not possible to determine directly whether the changes in muscle strength among the participants were caused by vitamin D supplementation. Therefore, to ensure comparability between the experimental and control groups and to minimize the influence of confounding factors, the two groups were propensity score matched in terms of sex, drinking history, body mass index (BMI), and other parameters. We used propensity score-matching to compare selected baseline variables with variables with similar characteristics and expected risk in the two groups.
3 Results
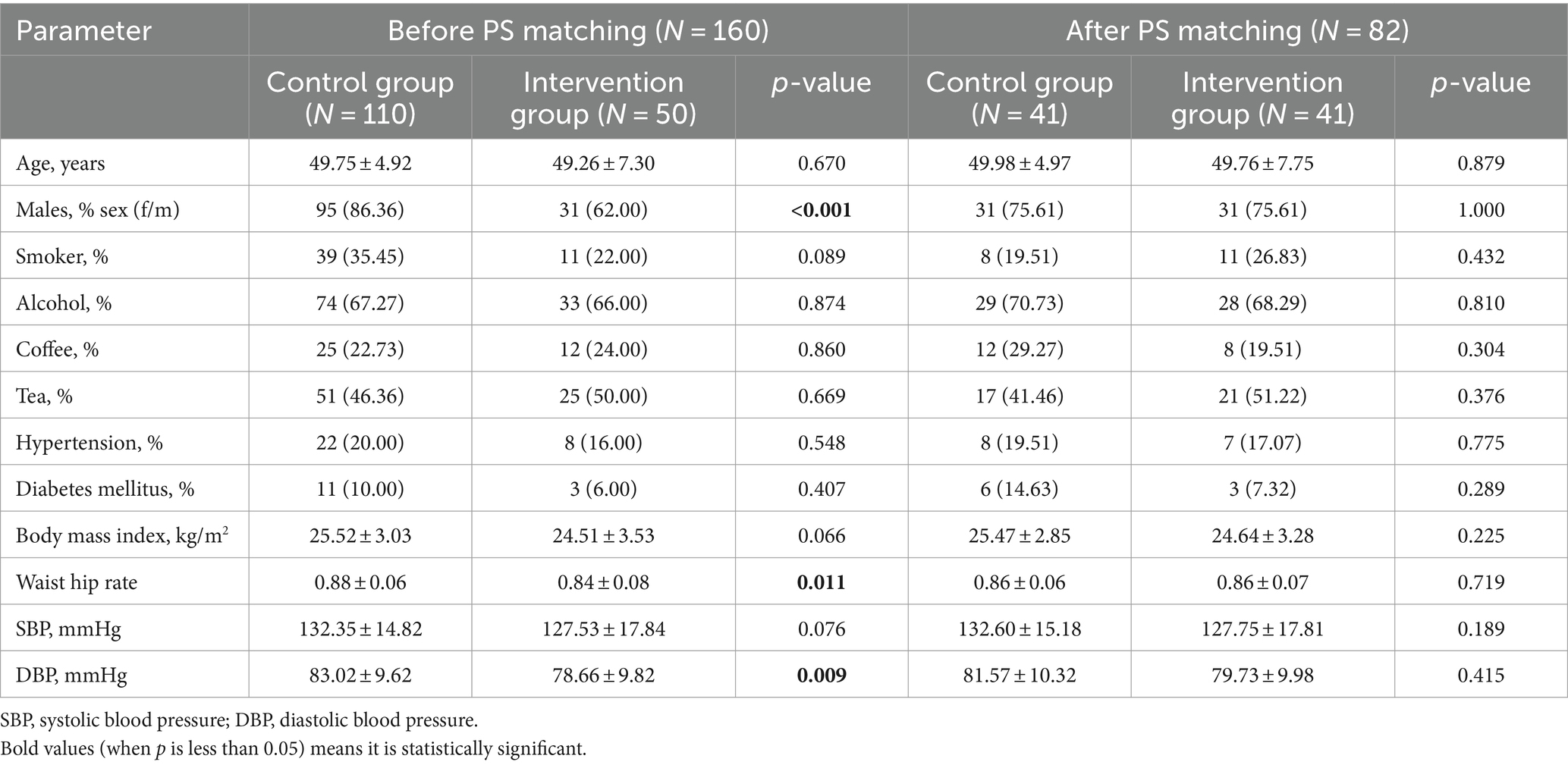
An overview of the baseline characteristics of the study population before and after the propensity score-matching is presented in Table 1. Among the 160 middle-aged and elderly people included, 50 (31.25%) received vitamin D supplementation, and the remaining 110 (68.78%) were in the control group before the propensity score matching. There were significant differences in gender, waist-hip ratio (WHR) and diastolic blood pressure (DBP) between the two groups (p < 0.05). With the group as the dependent variable and gender, age, smoking history, drinking history, hypertension, glucose metabolism, BMI, and WHR as the independent variables, the nearest neighbor matching method was used to conduct a 1:1 propensity score-matching. Logistic regression was used to calculate the propensity score, and the matching tolerance was 0.02. After propensity score-matching, 41 pairs of fuzzy matching cases were found with no exact matching cases. A total of 41 pairs were successfully matched, and 9 cases failed to find an effective matching population. There was no significant difference in general data between the two groups (p < 0.05).
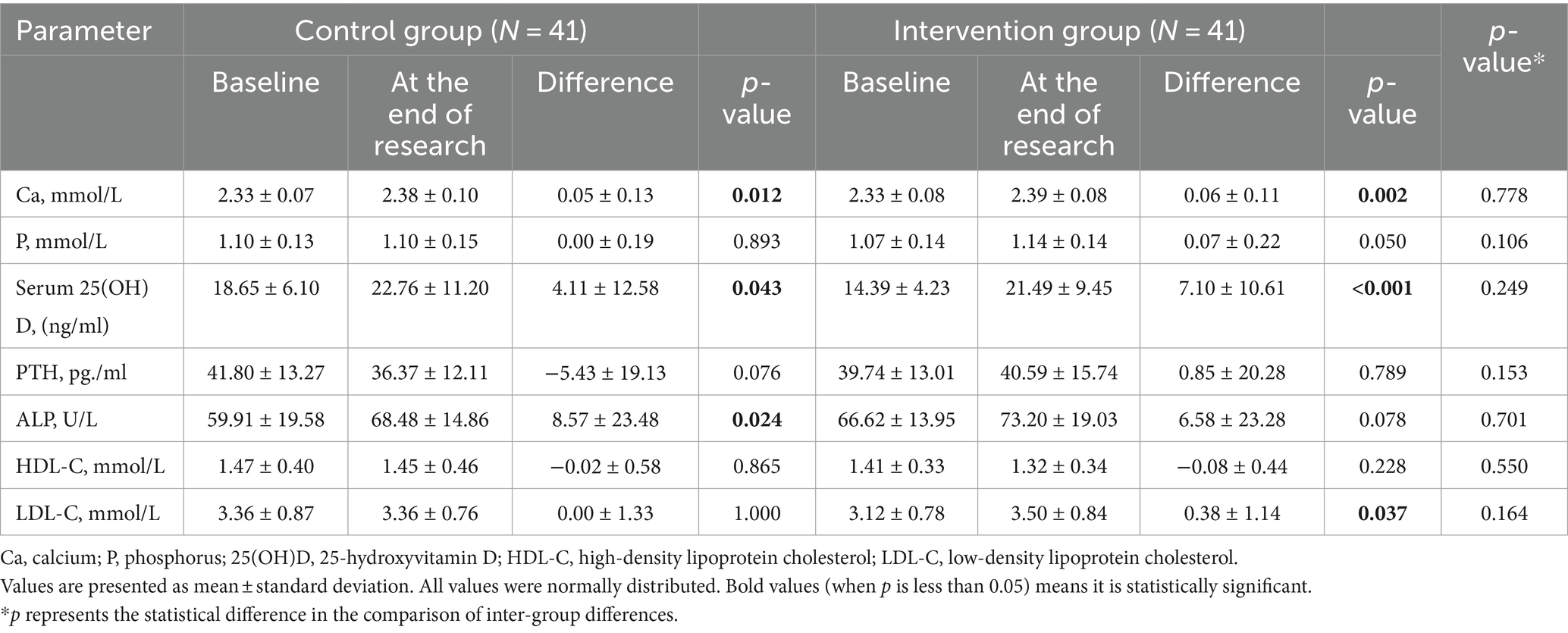
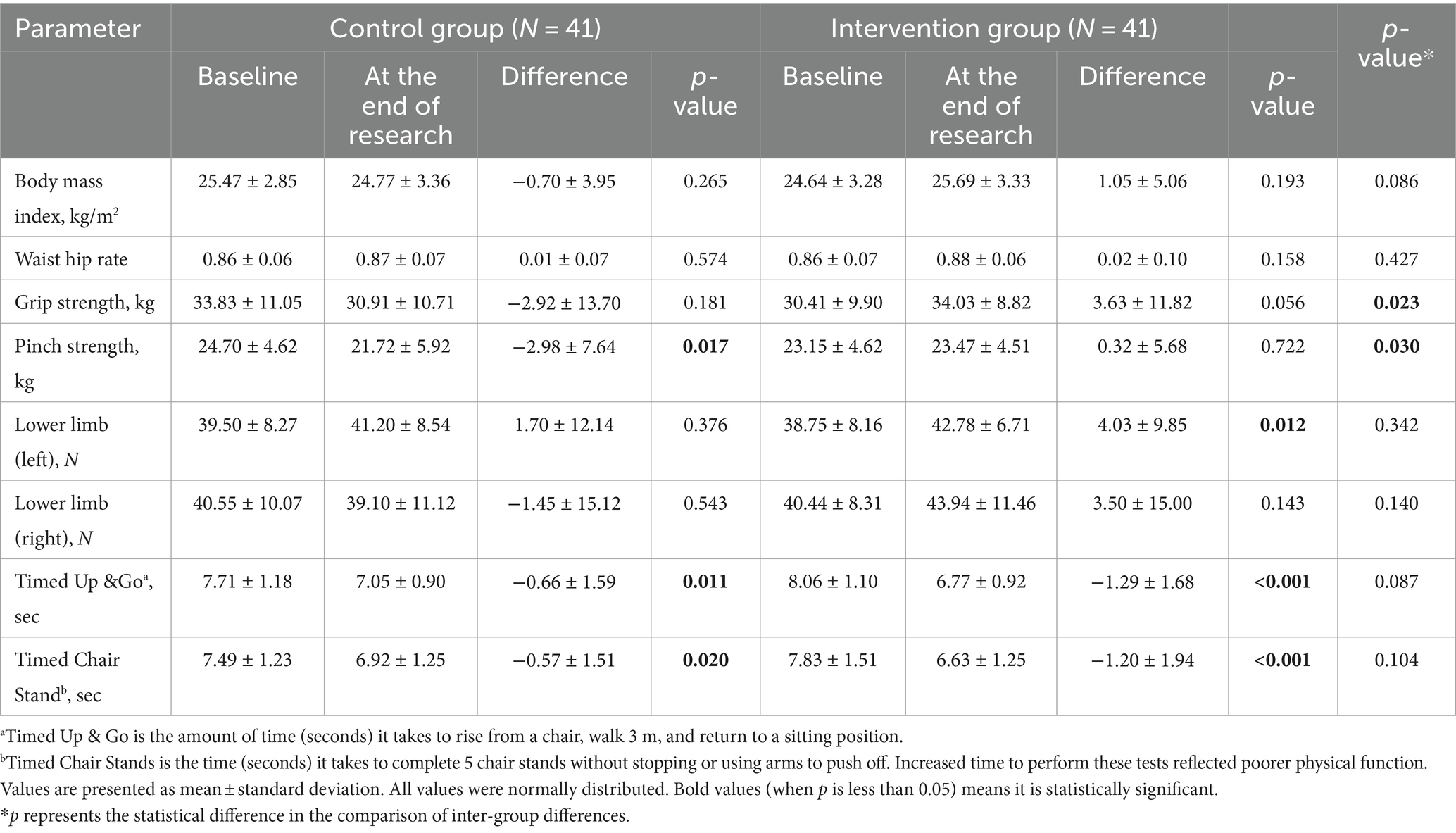
After propensity score matching, the comparison of serum 25(OH)D levels between the two groups showed a statistically significant difference (p < 0.05), while other indicators did not show significant differences in baseline data (Table 1). The BMI, waist-to-hip ratio, biochemical indicators, muscle strength, and physical function of the participants in both groups were compared before and after the intervention. The results showed that at the end of the study, the levels of serum calcium and 25(OH)D in both groups were improved (p < 0.05), with the intervention group showing a more significant improvement (Table 2). The muscle strength of the left lower limb in the intervention group significantly increased after the intervention (p < 0.05). Although there were significant differences in the TUG and TCS test results of the patients in both groups before and after the intervention, there was no improvement reflected. The study also showed that the grip strength and pinch strength of the patients in both groups increased after the intervention, and the difference between the two groups was statistically significant (p < 0.05) (Table 3).
4 Discussion
In recent years, increasing evidence has shown that vitamin D status can modulate musculoskeletal effects on muscle health, especially on muscle mass and muscle function in the elderly with vitamin D deficiency and in patients with chronic diseases (17, 18). However, the physical effects of vitamin D supplementation remain unclear because many factors may affect the improvement in physical function, including physical performance level, physical activity level, race, vitamin D status, and type of exercise. Currently, there is still a lack of clear consensus on the association between vitamin D status and muscle mass or strength. Previous studies have yielded conflicting conclusions about whether vitamin D supplementation improves muscle strength (19). In 2017, Iolascon et al. conducted a study of 113 postmenopausal women with osteoporosis or vitamin D deficiency and found that 6 months of active vitamin D supplementation significantly improved muscle strength in their upper and lower limbs and reduced the incidence of falls (20). However, a randomized, double-blind, placebo-controlled study showed no benefit of vitamin D supplementation for muscle strength in postmenopausal women (21). In a randomized, double-blind, placebo-controlled trial in a deficient dialysis population, vitamin D3 supplementation had no effect on muscle strength (22). Aschauer et al. found that vitamin D3 supplementation had no effect on regulating or improving muscle strength endurance, functional ability, or aerobic capacity (23). In our study, although there was no statistically significant difference in the change of upper limb strength between the control and intervention groups, both groups showed an increase in grip and pinch strength after the intervention, with the difference between the two groups being statistically significant. Moreover, the intervention group exhibited a significant improvement in the strength of the left lower limb (p < 0.05), indicating that vitamin D3 supplementation is beneficial for the maintenance and enhancement of both upper and lower limb strength.
Handgrip strength tests are widely used as a measure of muscle strength and have become an outcome measure of nutritional status. However, in previous studies, the results were inconsistent regarding the role of vitamin D in grip strength. Studies have shown that malnutrition and vitamin D deficiency are associated with weaker grip strength. In this study, the upper limb muscle strength of participants was assessed by measuring grip strength (24–26). This study found that after propensity score matching, vitamin D3 supplementation improved upper grip strength, as well as hand muscle pinch strength in middle-aged and elderly participants. In this study, the baseline serum 25(OH)D levels in the intervention group were lower than those in the control group. Furthermore, at the conclusion of the follow-up period, the difference in the change of 25(OH)D levels in the intervention group was greater than that of the control group. At the end of the follow-up, there was a significant increase in serum 25(OH)D concentrations in both groups (p < 0.05), with the intervention group showing a greater increase in serum 25(OH)D concentration than the control group. This is consistent with the results of muscle strength measurements. However, the TUG and TCS test results showed no improvement, which we speculate may be related to factors such as the age of the subjects, compliance, baseline 25(OH)D levels, body absorption, and lifestyle. However, the specific reasons and correlations require further investigation. The muscle strength measurement results in this study indicate that vitamin D supplementation can enhance upper limb strength. We have reported this phenomenon in our study and plan to delve deeper into the mechanism in future research.
Oral active vitamin D (calcifediol) has a higher intestinal absorption rate than regular vitamins, which may have important advantages in situations where intestinal absorption capacity is reduced due to various diseases. Cholecalciferol or vitamin D3 is the most commonly used form of supplement today. Relevant literature reports that the cost of active vitamin D treatment is about 3.6 times that of regular vitamin treatment (27). Although the treatment cost of active vitamin D is significantly higher than that of ordinary vitamin D, a meta-analysis study showed that there was no significant difference in efficacy and safety between the two groups in studies with the number of new vertebral and non-vertebral fractures as the primary outcome (28). For muscle strength comparison, in a double-blind RCT study, active vitamin supplementation was 2.8 times more effective than vitamin D3 supplementation in improving lower-extremity muscle strength in healthy white postmenopausal women (29).
Feng et al. (30) evaluated the effects of regular vitamin D and active vitamin D interventions on physical function, muscle strength, and fall risk in elderly patients with osteoporosis. The results show that active vitamin D can improve the physical ability of patients, reduce the risk of falls, and effectively improve the bone mass of the lumbar spine in patients with osteoporosis, while the improvement of the bone mass of the lumbar spine and hip of patients with ordinary vitamin D is not obvious. The results of the study found that the effect of vitamin D supplementation on muscle strength was relatively improved after 6 and 12 months of intervention, but there was no significant difference between the effects of the two vitamin D supplements.
Vitamin D deficiency has been shown to impair muscle function, but the association of vitamin D deficiency with cardiopulmonary endurance is uncertain. A 19-week controlled intervention study in India found that vitamin D3 supplementation did not improve muscle strength and cardiopulmonary endurance in healthy young Indian men with vitamin D deficiency (31). A meta-analysis published in 2017 only included RCT studies that used grip strength and TUAG as indicators of muscle function (15 RCTS, 2408 people) and did not find an improvement effect of vitamin D3 supplementation, with significant heterogeneity among studies (32).
Vitamin D supplementation plays an important role in the treatment of osteoporosis. Although the guidelines include vitamin D supplementation as a recommendation, there is still a lack of high-quality evidence to confirm the difference and priority of choice between active vitamin D and regular vitamin D. In 2019, Xiao et al. (28) conducted a meta-analysis to explore the difference between regular vitamin D and active vitamin D in reducing the risk of fractures in patients with osteoporosis, and no difference was found between the two. Calcifediol supplementation is more effective and faster at increasing 25 (OH) D serum levels than cholecalciferol and is associated with greater improvements in muscle function.
In terms of statistical methods, this study is retrospective, and potential limitations of the database in terms of data completeness, and limitations in the availability of historical medical data are unavoidable. In addition, due to the non-intervention study design and the inherent limitations of observational research, the results of this study are susceptible to bias, such as selection bias and limited access to historical medical data. After propensity score matching, there was no difference in demographic data between the control group and the intervention group, which excluded confounding factors to a certain extent.
In this study, residual confounding cannot be ruled out, given that the treatments in the two groups will be assigned without randomization. Although this study will use a propensity score approach to derive a comparison group that is more balanced in terms of measured baseline characteristics and known confounders, achieving comparability and eliminating possible selection bias, residual confounding is still possible. Due to the limited number of patients and the retrospective nature of the data collection, the results should be interpreted with caution. However, further studies with larger sample size and longer follow-up time are needed to confirm the results.
5 Conclusion
In this study, we found that vitamin D3 supplementation was associated with improvement of upper and lower limb strength, delayed muscle strength decline, and was beneficial for maintaining muscle strength. These findings provide evidence that warrants confirmation in future intervention studies with a larger sample size and longer follow-up.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Committee on Ethics of PLA General Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
PQ: Conceptualization, Formal analysis, Methodology, Writing – original draft. XF: Investigation, Methodology, Writing – original draft. DZ: Formal analysis, Investigation, Writing – original draft. CL: Conceptualization, Supervision, Writing – review & editing. YL: Investigation, Supervision, Writing – review & editing. NL: Conceptualization, Methodology, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sizar, O, Khare, S, Goyal, A, and Givler, A. Vitamin D deficiency In: StatPearls. Treasure Island, FL: StatPearls Publishing LLC (2023)
2. Uchitomi, R, Oyabu, M, and Kamei, Y. Vitamin D and sarcopenia: potential of vitamin D supplementation in sarcopenia prevention and treatment. Nutrients. (2020) 12:3189. doi: 10.3390/nu12103189
3. Girgis, CM, Clifton-Bligh, RJ, Hamrick, MW, Holick, MF, and Gunton, JE. The roles of vitamin D in skeletal muscle: form, function, and metabolism. Endocr Rev. (2013) 34:33–83. doi: 10.1210/er.2012-1012
4. Tanner, SB, and Harwell, SA. More than healthy bones: a review of vitamin D in muscle health. Ther Adv Musculoskelet Dis. (2015) 7:152–9. doi: 10.1177/1759720x15588521
5. Yu, F, and Xia, W. Effects of vitamin D on muscle function. Chin J Osteoporos Bone Miner Res. (2018) 11:597–607. doi: 10.3969/j.issn.1674-2591.2018.06.013
6. Amrein, K, Scherkl, M, Hoffmann, M, Neuwersch-Sommeregger, S, Köstenberger, M, Tmava Berisha, A, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. (2020) 74:1498–513. doi: 10.1038/s41430-020-0558-y
7. Holick, MF. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. (2017) 18:153–65. doi: 10.1007/s11154-017-9424-1
8. Hossein-nezhad, A, and Holick, MF. Vitamin D for health: a global perspective. Mayo Clin Proc. (2013) 88:720–55. doi: 10.1016/j.mayocp.2013.05.011
9. Cui, A, Zhang, T, Xiao, P, Fan, Z, Wang, H, and Zhuang, Y. Global and regional prevalence of vitamin D deficiency in population-based studies from 2000 to 2022: a pooled analysis of 7.9 million participants. Front Nutr. (2023) 10:1070808. doi: 10.3389/fnut.2023.1070808
10. Haq, A, Svobodová, J, Imran, S, Stanford, C, and Razzaque, MS. Vitamin D deficiency: a single Centre analysis of patients from 136 countries. J Steroid Biochem Mol Biol. (2016) 164:209–13. doi: 10.1016/j.jsbmb.2016.02.007
12. Holick, MF, Siris, ES, Binkley, N, Beard, MK, Khan, A, Katzer, JT, et al. Prevalence of vitamin D inadequacy among postmenopausal north American women receiving osteoporosis therapy. J Clin Endocrinol Metab. (2005) 90:3215–24. doi: 10.1210/jc.2004-2364
13. Bislev, LS, Grove-Laugesen, D, and Rejnmark, L. Vitamin D and muscle health: a systematic review and Meta-analysis of randomized placebo-controlled trials. J Bone Miner Res. (2021) 36:1651–60. doi: 10.1002/jbmr.4412
14. Bislev, LS, Wamberg, L, Rolighed, L, Grove-Laugesen, D, and Rejnmark, L. Effect of daily vitamin D3 supplementation on muscle health: an individual participant Meta-analysis. J Clin Endocrinol Metab. (2022) 107:1317–27. doi: 10.1210/clinem/dgac004
15. Lu, Y, Fu, X, Zhang, L, Liu, M, Cheng, X, Yan, S, et al. Effects of stratified vitamin D supplementation in middle-aged and elderly individuals with vitamin D insufficiency. Horm Metab Res. (2018) 50:747–53. doi: 10.1055/a-0746-5031
16. Dam, TT, von Mühlen, D, and Barrett-Connor, EL. Sex-specific association of serum vitamin D levels with physical function in older adults. Osteoporos Int. (2009) 20:751–60. doi: 10.1007/s00198-008-0749-1
17. Byers, AW, Connolly, G, and Campbell, WW. Vitamin D status and supplementation impacts on skeletal muscle function: comparisons between young athletes and older adults. Curr Opin Clin Nutr Metab Care. (2020) 23:421–7. doi: 10.1097/mco.0000000000000692
18. Montenegro, KR, Cruzat, V, Carlessi, R, and Newsholme, P. Mechanisms of vitamin D action in skeletal muscle. Nutr Res Rev. (2019) 32:192–204. doi: 10.1017/s0954422419000064
19. El Hajj, C, Fares, S, Chardigny, JM, Boirie, Y, and Walrand, S. Vitamin D supplementation and muscle strength in pre-sarcopenic elderly Lebanese people: a randomized controlled trial. Arch Osteoporos. (2018) 14:4. doi: 10.1007/s11657-018-0553-2
20. Iolascon, G, Moretti, A, de Sire, A, Calafiore, D, and Gimigliano, F. Effectiveness of Calcifediol in improving muscle function in post-menopausal women: a prospective cohort study. Adv Ther. (2017) 34:744–52. doi: 10.1007/s12325-017-0492-0
21. Haghighi, AH, Shojaee, M, Askari, R, Abbasian, S, and Gentil, P. The effects of 12 weeks resistance training and vitamin D administration on neuromuscular joint, muscle strength and power in postmenopausal women. Physiol Behav. (2024) 274:114419. doi: 10.1016/j.physbeh.2023.114419
22. Singer, R, Chacko, B, Talaulikar, G, Karpe, K, and Walters, G. Placebo-controlled, randomized clinical trial of high-dose cholecalciferol in renal dialysis patients: effect on muscle strength and quality of life. Clin Kidney J. (2019) 12:281–7. doi: 10.1093/ckj/sfy039
23. Aschauer, R, Unterberger, S, Zöhrer, PA, Draxler, A, Franzke, B, Strasser, EM, et al. Effects of vitamin D3 supplementation and resistance training on 25-Hydroxyvitamin D status and functional performance of older adults: a randomized placebo-controlled trial. Nutrients. (2021) 14:86. doi: 10.3390/nu14010086
24. Kalliokoski, P, Rodhe, N, Bergqvist, Y, and Löfvander, M. Long-term adherence and effects on grip strength and upper leg performance of prescribed supplemental vitamin D in pregnant and recently pregnant women of Somali and Swedish birth with 25-hydroxyvitamin D deficiency: a before-and-after treatment study. BMC Pregnancy Childbirth. (2016) 16:353. doi: 10.1186/s12884-016-1117-3
25. Norman, K, Kirchner, H, Freudenreich, M, Ockenga, J, Lochs, H, and Pirlich, M. Three month intervention with protein and energy rich supplements improve muscle function and quality of life in malnourished patients with non-neoplastic gastrointestinal disease--a randomized controlled trial. Clin Nutr. (2008) 27:48–56. doi: 10.1016/j.clnu.2007.08.011
26. Norman, K, Stobäus, N, Gonzalez, MC, Schulzke, JD, and Pirlich, M. Hand grip strength: outcome predictor and marker of nutritional status. Clin Nutr. (2011) 30:135–42. doi: 10.1016/j.clnu.2010.09.010
27. Uenishi, K, Tokiwa, M, Kato, S, and Shiraki, M. Stimulation of intestinal calcium absorption by orally administrated vitamin D3 compounds: a prospective open-label randomized trial in osteoporosis. Osteoporos Int. (2018) 29:723–32. doi: 10.1007/s00198-017-4351-2
28. Xiao, Y, Zhou, Q, He, J, Yu, L, Sun, X, Zhang, J, et al. Comparison of the effects of active vitamin D and plain vitamin D on fracture risk in patients with osteoporosis: meta analysis. Chin J Osteoporos Bone Miner Res. (2019) 12:377–87. doi: 10.3969/j.issn.1674-2591.2019.04.008
29. Bischoff-Ferrari, HA, Dawson-Hughes, B, Stöcklin, E, Sidelnikov, E, Willett, WC, Edel, JO, et al. Oral supplementation with 25(OH)D3 versus vitamin D3: effects on 25(OH)D levels, lower extremity function, blood pressure, and markers of innate immunity. J Bone Miner Res. (2012) 27:160–9. doi: 10.1002/jbmr.551
30. Feng, F, Chen, H, Zhang, Z, Jia, P, Bao, L, Yang, H, et al. Effects of vitamin D on physical performance and fall risk in elderly osteoporotic patients. Chin J Osteoporos Bone Miner Res. (2017) 10:313–9. doi: 10.3969/j.issn.1674-2591.2017.04.002
31. Menon, AS, Anayath, S, Garg, MK, Ravi, K, and Pisharody, I. The effect of vitamin D supplementation on cardiorespiratory fitness and muscle strength in male adults undergoing basic military training. Med J Armed Forces India. (2020) 76:71–6. doi: 10.1016/j.mjafi.2018.12.004
Keywords: vitamin D, supplementation, muscle strength, retrospective studies, propensity score
Citation: Qi P, Fu X, Zhao D, Li C, Lu Y and Li N (2024) Effects of vitamin D supplementation on muscle strength in middle-aged and elderly individuals: a retrospective, propensity score-matched study. Front. Nutr. 11:1450265. doi: 10.3389/fnut.2024.1450265
Edited by:
Antoni Sureda, University of the Balearic Islands, SpainReviewed by:
Giuseppe Annunziata, University of Campania Luigi Vanvitelli, ItalyConnie M. Weaver, San Diego State University, United States
Copyright © 2024 Qi, Fu, Zhao, Li, Lu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanhui Lu, Y25iamx1bHVAMTYzLmNvbQ==; Nan Li, d3Nsbl9tYXkwN0AxNjMuY29t
 Peiyao Qi
Peiyao Qi Xiaomin Fu
Xiaomin Fu Dan Zhao3
Dan Zhao3