- 1Department of Oncology, Guang’anmen Hospital of the Chinese Academy of Traditional Chinese Medicine, Beijing, China
- 2Department of Surgery, Shanghai University of Traditional Chinese Medicine Affiliated Putuo Central Hospital, Shanghai, China
- 3Beijing University of Chinese Medicine, Beijing, China
Objective: This study aims to analyze the association between the weight-adjusted waist index (WWI) and the risk of gynecologic cancers, using data collected from the National Health and Nutrition Examination Survey (NHANES) from 2011 to 2016.
Methods: We employed multiple logistic regression analysis to investigate the relationship between WWI and risk of gynecologic cancers. Subsequent subgroup analyses were performed on specific populations of interest. A restricted cubic spline model was used to explore potential non-linear relationships. Additionally, the effectiveness of WWI in predicting sarcopenia was assessed through Receiver Operating Characteristic (ROC) curve analysis. K-fold cross-validation was applied for model assessment.
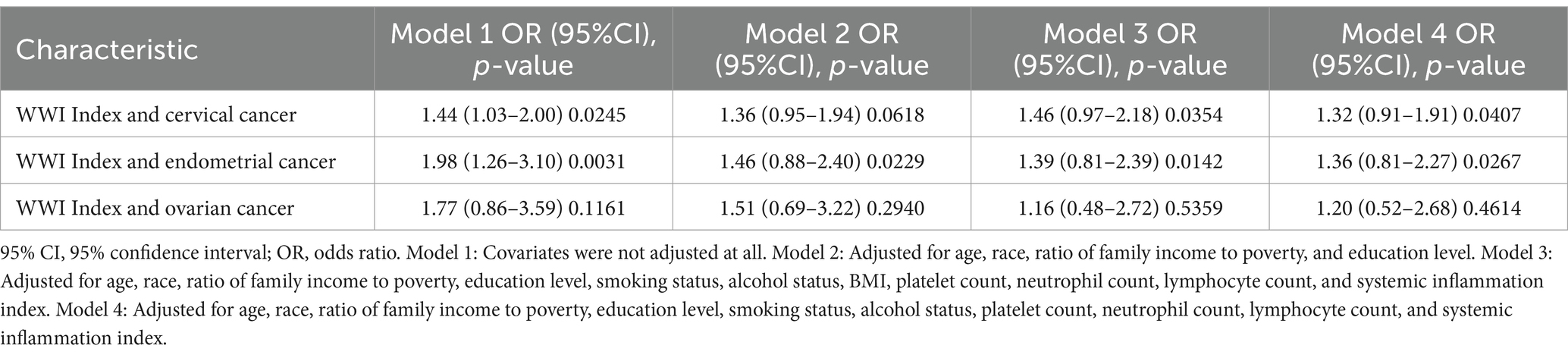
Results: Among the 4,144 participants, 98 self-reported having gynecologic cancers. In the fully adjusted model, WWI was significantly associated with the prevalence of gynecologic cancers (OR = 1.38, 95% CI: 1.02–1.88, p = 0.0344). Our findings indicate a linear positive association between WWI and the risk of gynecologic cancers. Subgroup analysis revealed that WWI had the strongest association with cervical cancer (OR = 1.46, 95% CI: 0.97–2.18, p = 0.0354) and endometrial cancer (OR = 1.39, 95% CI: 0.81–2.39, p = 0.0142). No significant association was found between WWI and the risk of ovarian cancer (OR = 1.16, 95% CI: 0.48–2.72, p = 0.5359). Restricted cubic spline analysis confirmed a linear relationship between WWI and the risk of cervical, endometrial, and ovarian cancers. ROC curve analysis demonstrated that WWI had superior predictive capability for gynecologic cancers.
Conclusion: Elevated levels of WWI were significantly associated with an increased risk of gynecologic cancers in American women, displaying a stronger association than other obesity markers. Therefore, WWI may serve as a distinct and valuable biomarker for assessing the risk of gynecologic cancers, particularly cervical and endometrial cancers.
1 Introduction
Malignant tumors have become the second leading cause of death worldwide, creating a substantial health burden that includes diminished quality of life, strained healthcare systems, and significant economic impacts (1, 2). According to 2022 statistics from the International Agency for Research on Cancer, millions of women are diagnosed annually with ovarian, cervical, and endometrial cancers, posing severe threats to both their physical and mental health. In developed countries, the incidence rates of ovarian and cervical cancers have stabilized (3, 4); however, the incidence of endometrial cancer continues to rise (5). Studies indicate that BRCA1 mutations can increase the risk of ovarian cancer by up to 40% (6), while approximately 5–10% of endometrial cancer cases are driven by POLE mutations (7). Environmental factors, including exogenous hormones, radiation exposure, and heavy metal ions, are known to elevate the risk of gynecological cancers (8). In addition to genetic predispositions and environmental exposures, lifestyle factors such as smoking, obesity, and physical inactivity significantly increase the risk of developing gynecological malignancies (9). Globally, the prevalence of overweight and obesity is increasing, with obesity rates in adult women significantly surpassing those in men across all age groups (10). By 2030, obesity rates in certain regions are projected to exceed 50% (11) Traditional measures such as body mass index (BMI) and waist circumference (WC) have been used as indicators of obesity, but they have limitations, notably their inability to distinguish the distribution of adipose tissue (12).
The Weight-Adjusted Waist Index is a novel and straightforward anthropometric measure of obesity, gaining significant attention for its efficacy in assessing obesity and related health risks (13). Compared to BMI and WC, WWI differentiates more effectively between fat and muscle mass. It provides a more accurate measure of central obesity and the health implications of visceral fat (14). Studies have demonstrated that elevated WWI is associated with an increased risk of several conditions, including depression (15), hypertension (16), diabetic nephropathy (17), and secondary infertility (18). However, the relationship between WWI and the prevalence of gynecological cancers remains unexplored, highlighting the necessity for further investigation. This study utilizes data from NHANES collected between 2011 and 2016. Multivariate logistic regression and restricted cubic spline analyses are employed to investigate the association between WWI and gynecological cancers, providing robust and flexible modeling to capture complex relationships. Additionally, subgroup analyses are conducted to explore the association between WWI and gynecological cancers across different demographic and clinical subgroups, offering a comprehensive understanding of potential variations in risk.
2 Methods
2.1 Study population
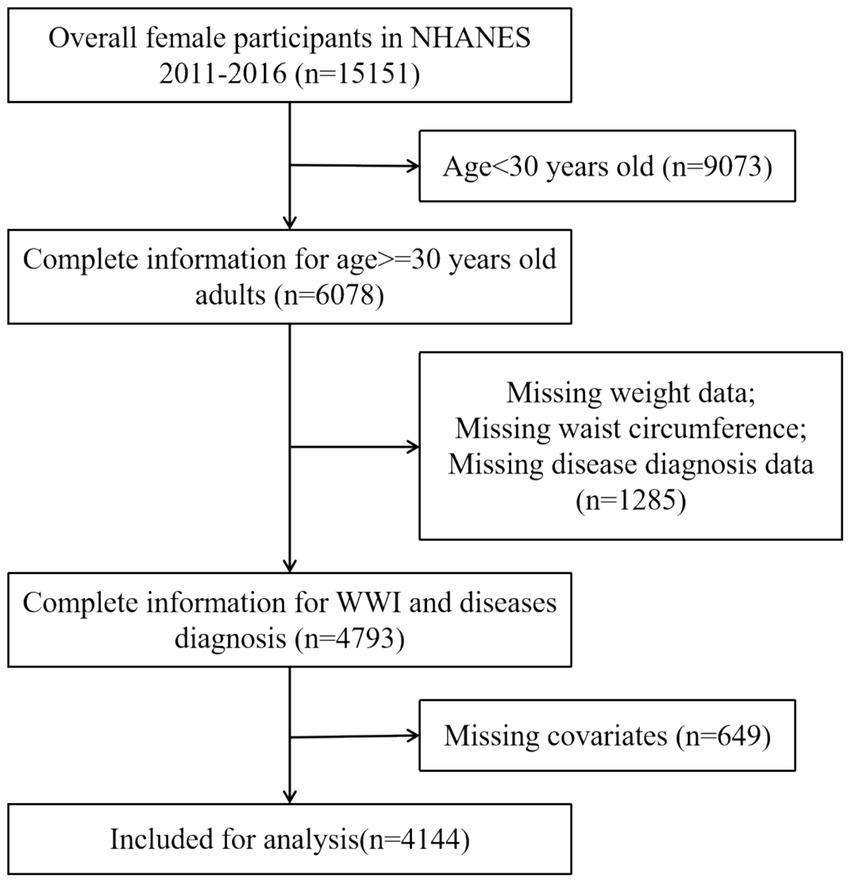
The National Health and Nutrition Examination Survey (NHANES), conducted by the National Center for Health Statistics (NCHS), employs a stratified multistage probability sampling method to obtain a representative sample of the civilian, non-institutionalized U.S. population. This methodology involves a structured selection process that includes counties, blocks, households, and individuals within those households. Since 1999, NHANES has conducted cross-sectional surveys, releasing new data every 2 years. To maintain the rigor and accuracy of the study, specific exclusion criteria were implemented, particularly the exclusion of participants under 30 years of age (19). This decision was informed by the widespread uptake of the HPV vaccine and heightened self-care awareness in this demographic, both of which have contributed to a lower incidence of gynecological tumors in individuals younger than 30. The detailed inclusion and exclusion criteria are presented in Figure 1. In summary, the study encompassed 4,144 participants. Among these participants, 98 self-reported a history of gynecologic cancers, including 12 with ovarian cancer, 56 with cervical cancer, and 30 with endometrial cancer. The NHANES protocol received approval from the NCHS Research Ethics Review Board, and informed consent was obtained from all participants prior to their inclusion in the study.
2.2 Calculation of WWI
The WWI was the primary exposure factor in this study. WWI was calculated for each participant by dividing the waist circumference (in centimeters) by the square root of the body weight (in kilograms). Anthropometric measurements were meticulously recorded by trained medical personnel and specialized recorders to ensure data accuracy. Body weight was measured using a digital scale. Participants were dressed in examination clothing, stood barefoot on the scale, held their arms close to their bodies, and fixed their gaze straight ahead, as previously outlined. WC was measured with a tape measure positioned at the intersection of the midaxillary line and a horizontal line just above the outermost upper point of the right kneecap.
2.3 Diagnosis of cancer
Data on cancer diagnoses were obtained from a structured questionnaire. Participants were asked if a doctor or other health professional had ever informed them of a cancer or malignancy diagnosis (MCQ-220). Participants who answered affirmatively were identified as cancer patients and were subsequently prompted to answer MCQ-230A. In MCQ-230A, code 15 indicates cervical cancer, code 28 indicates ovarian cancer, and code 38 indicates endometrial cancer.
2.4 Covariates
Based on previous research findings (20, 21), our study also accounted for additional variables, including age (years), the ratio of family income to poverty (PIR), race/ethnicity (Mexican American/Non-Hispanic White/Non-Hispanic Black/Other Race), education level (less than high school/high school graduate/more than high school), smoking status (ever smoked at least 100 cigarettes: yes/no), alcohol consumption (at least 12 drinks per year: yes/no), as well as platelet count, neutrophil count, lymphocyte count, and systemic inflammation index. PIR was characterized into three categories (22): low-income (PIR ≤ 1.3), middle-income (PIR > 1.3–3.5), and high-income (PIR > 3.5).
2.5 Statistical analysis
In accordance with the Centers for Disease Control and Prevention (CDC) recommendations on statistical analysis of complex survey data, all statistical analyses were carried out using the proper NHANES sampling weights and took into consideration intricate multistage cluster surveys. Continuous variables were presented as mean ± standard deviation, whereas categorical variables were represented as percentages. Differences between groups were evaluated using a weighted Student’s t-test (for continuous variables) or a weighted chi-square test (for categorical variables). Logistic regression was employed to examine the association between WWI and gynecological cancers, utilizing the adjusted odds ratio (OR) and the corresponding 95% confidence intervals (CI) to delineate the relationships. Following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (23), three multivariate regression models were constructed. In model 1, no covariates were adjusted. In model 2, age, race, ratio of family income to poverty, and education level were adjusted. Model 3 was adjusted for age, race, ratio of family income to poverty, education level, smoking status, alcohol status, BMI, platelet count, neutrophil count, lymphocyte count, and systemic inflammation index. Model 4, which included all variables from Model 3 except for BMI. To assess its robustness, the continuous variable WWI was categorized into tertiles for sensitivity analysis. We performed subgroup analyses and interactions for age, education level, smoking, alcohol status, and the ratio of family income to poverty in fully adjusted models. The predictive capacity of WWI for gynecological cancers was assessed using ROC curve analysis, obtaining area under the curve (AUC), sensitivity, and specificity values (24). In general, an AUC value of 0.5 indicates a lack of discrimination, while a range of 0.7–0.8 is deemed acceptable. As part of the model validation process, we employed k-fold cross-validation (k = 10) to rigorously assess the predictive performance of our model (25). In the fully adjusted model, we employed the restricted cubic spline (RCS) method to investigate the non-linear association between the WWI, serving as the exposure variable, and gynecological cancers outcome variable. Statistical analyses were conducted using R version 4.2.3 (http://www.R-project.org, The R Foundation). In the context of statistical analysis, a p-value less than 0.05 is considered statistically significant.
3 Results
3.1 Participant characteristics
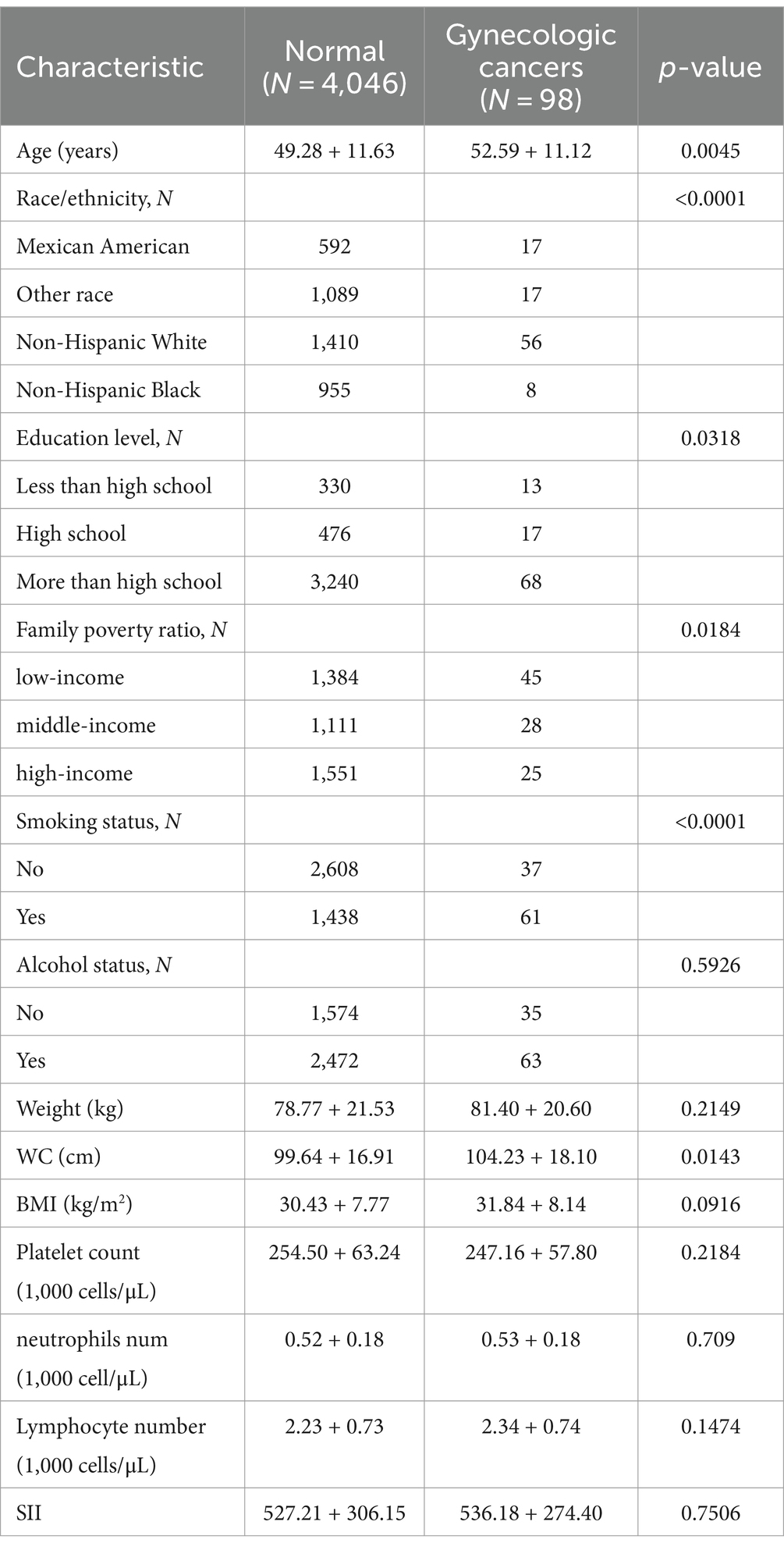
This study encompassed a total of 4,144 participants, whose detailed characteristics are presented in Table 1. Compared to the normal group, patients diagnosed with gynecological tumors were generally older, had higher educational attainment, better economic status, a history of smoking, and greater waist circumferences.
3.2 The association between WWI and gynecologic cancers
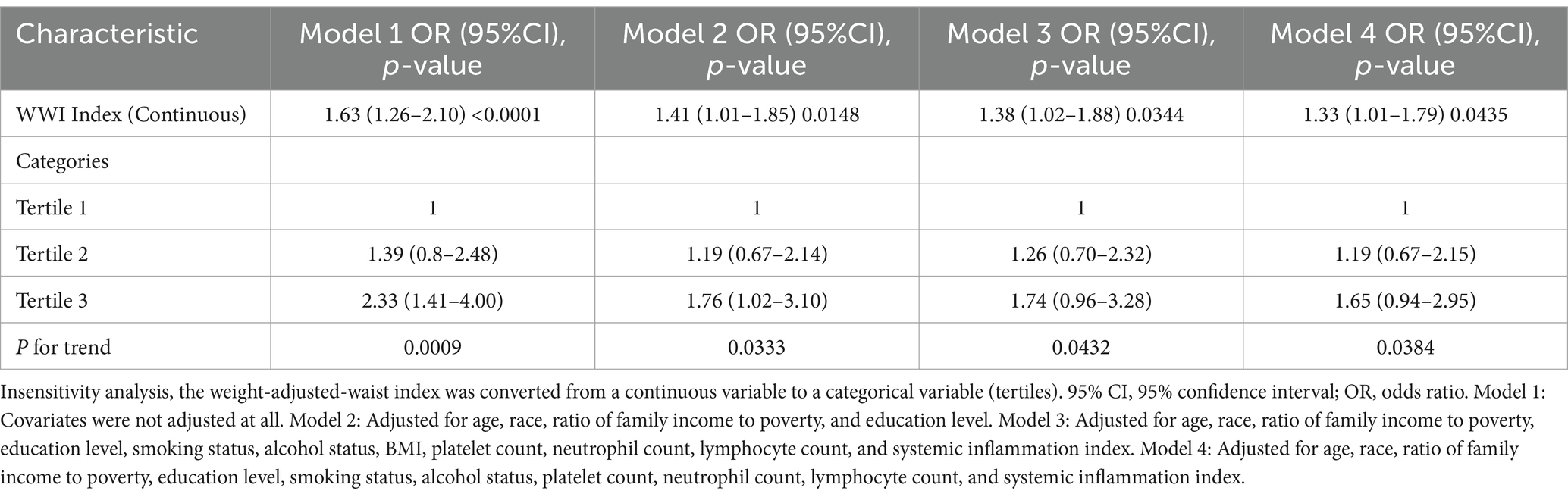
Multivariate regression analysis demonstrated that, in the fully adjusted continuous model, each unit increase in WWI corresponded to a 38% higher risk of gynecological tumors (OR = 1.38 95%CI: 1.02–1.88 p = 0.0344). In the fully adjusted categorical model, the risk of gynecological tumors increased by 26 and 74% for the tertiles 2 and tertiles 3, respectively, compared to the tertile 1(P for trend = 0.0432) (Table 2). In Model 4, even when BMI is excluded, WWI still demonstrates a significant association with the risk of gynecologic cancers. Moreover, restricted cubic spline analysis indicated a positive linear trend (P for nonlinear = 0.946) (Figure 2).
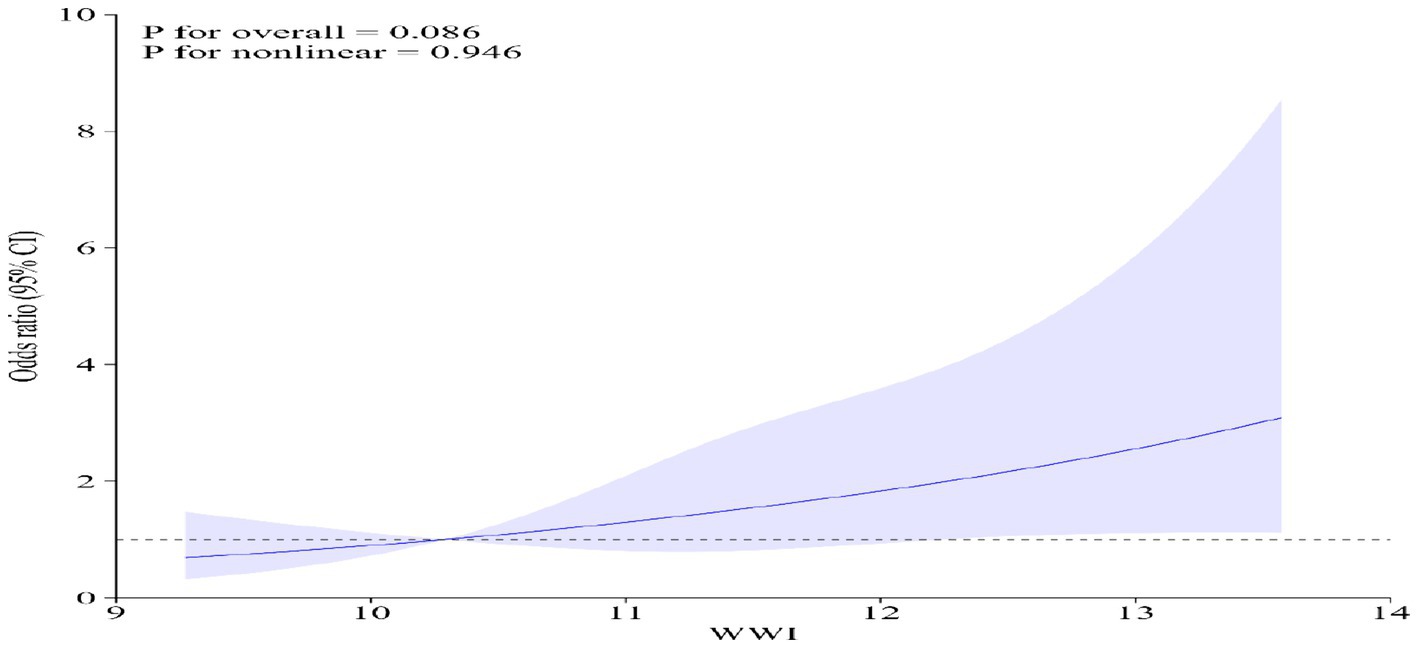
Figure 2. Restricted cubic spline analysis of WWI and gynecological tumors. Adjusted for age, race, ratio of family income to poverty, education level, smoking status, alcohol status, BMI, platelet count, neutrophil count, lymphocyte count, and systemic inflammation index.
3.3 Subgroup analysis
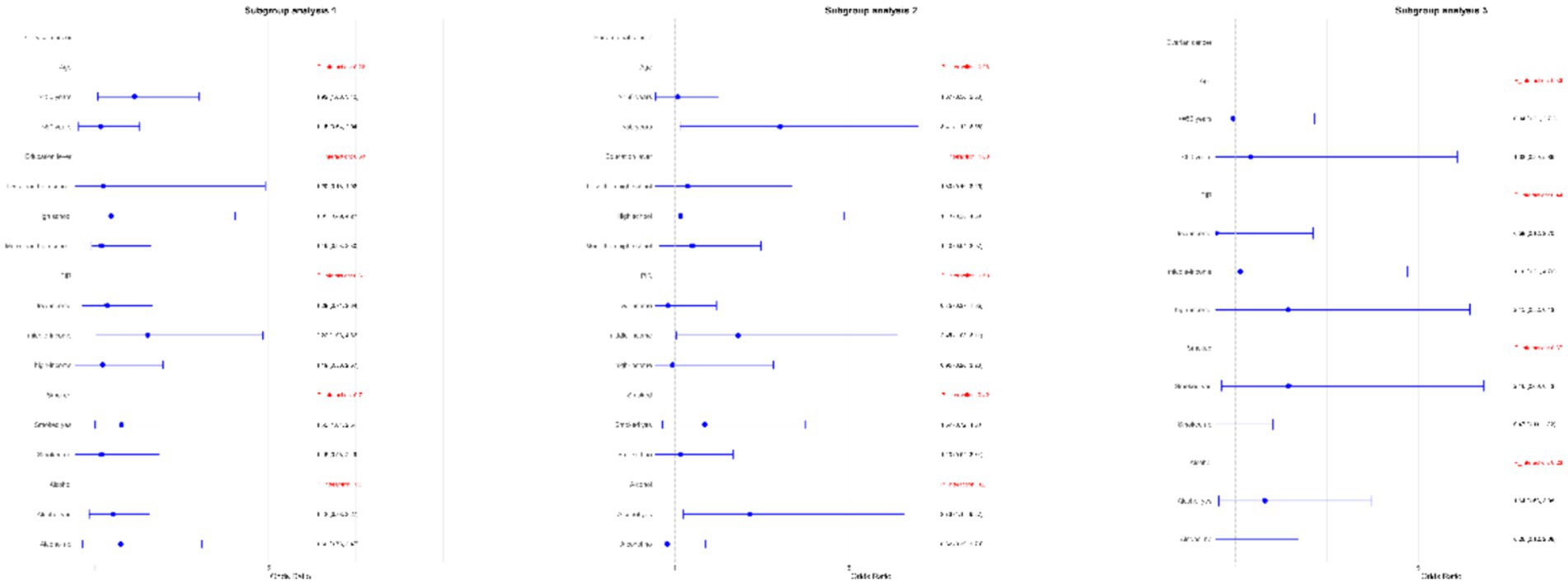
Subgroup analyses were performed to assess the relationship between WWI and the risk of ovarian, cervical, and endometrial cancers. The results indicated that, in the fully adjusted continuous model, each unit increase in WWI was associated with a 46% increase in the risk of cervical cancer (OR = 1.46 95%CI: 0.97–2.18 p = 0.0354) and a 39% increase in the risk of endometrial cancer (OR = 1.39 95%CI: 0.81–2.39 p = 0.0142). However, no statistically significant association was found between increased WWI and ovarian cancer risk (OR = 1.16 95%CI: 0.48–2.72 p = 0.5359) (Table 3). Restricted cubic spline analysis revealed a linear relationship between WWI and the risks of cervical, endometrial, and ovarian cancer (Figure 3). When WWI reaches 11, there is a marked increase in the risk of cervical and endometrial cancers, while the impact on ovarian cancer risk appears to be less pronounced. Although these curves demonstrate an upward trend, the associations observed do not achieve statistical significance. In addition, the subgroup analysis revealed that although variations in odds ratios were observed across different demographic and behavioral subgroups, the interactions between WWI and these factors—age, educational level, poverty status, smoking, and alcohol consumption—did not reach statistical significance. Specifically, while certain subgroups, such as individuals with middle income or those over 50 years old, exhibited higher odds ratios for specific cancers, the interaction p-values indicated no statistically significant differences (Figure 4). ROC curve analysis demonstrated that WWI had superior predictive capability compared to other obesity indicators, such as BMI and WC (Figure 5).

Figure 3. Restricted cubic spline analysis of WWI and the cervical, endometrial and ovarian cancer. Adjusted for age, race, ratio of family income to poverty, education level, smoking status, alcohol status, BMI, platelet count, neutrophil count, lymphocyte count, and systemic inflammation index.
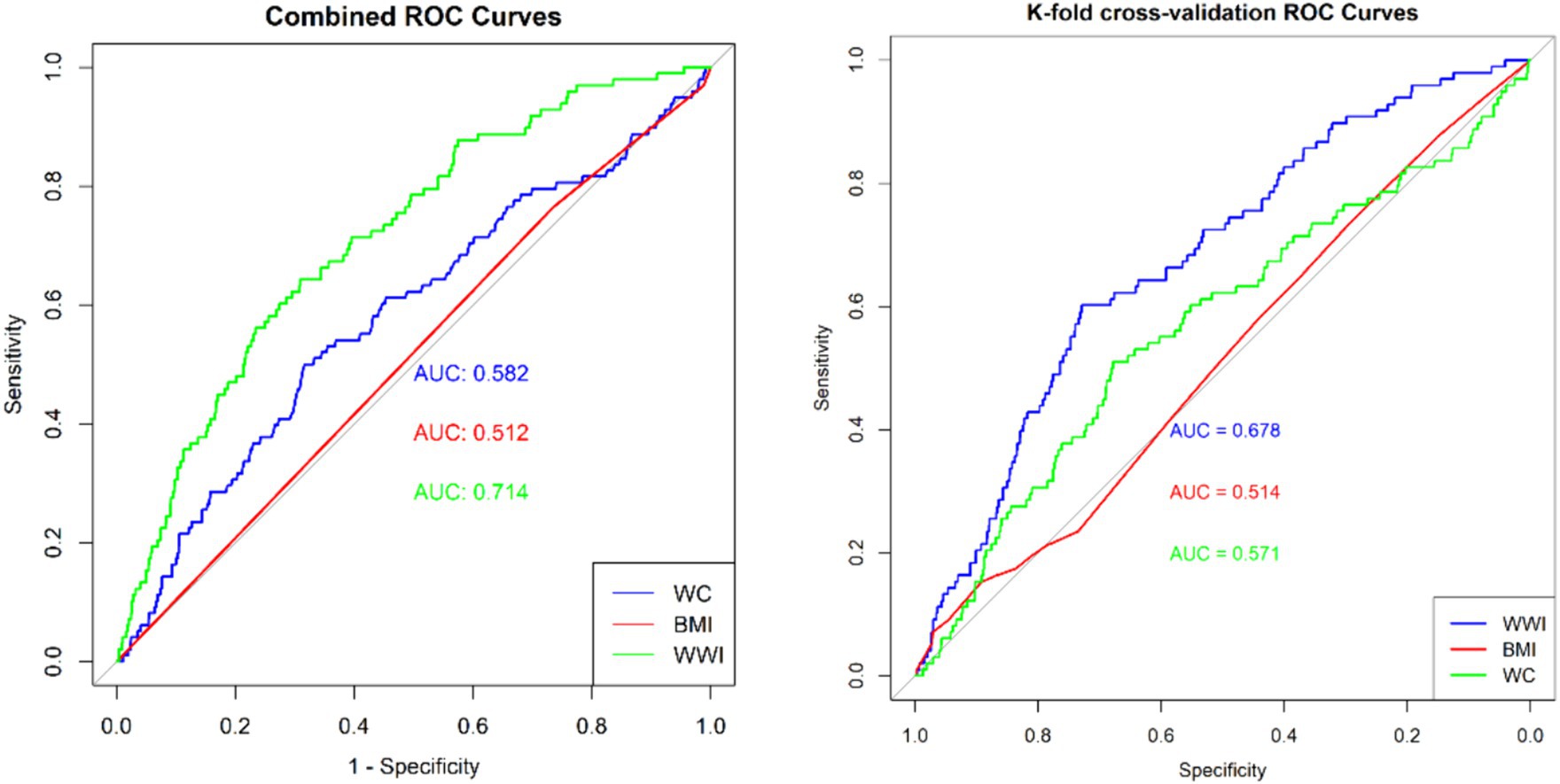
Figure 5. Area under the receiver operating characteristic curve of WWI, BMI and WC for predicting the gynecological tumors.
4 Discussion
In this cross-sectional study, we investigate the association between WWI and the incidence of gynecological cancers. Our findings indicate that an elevated WWI is linked to a high risk of gynecological malignancies, particularly cervical and endometrial cancers. The results of the subgroup analysis indicate that the association between WWI and gynecological cancers is not confounded by factors such as age, smoking, or alcohol consumption. This finding further underscores the importance of considering WWI as an independent risk factor for gynecological malignancies. Moreover, our research indicate that WWI may serve as a valuable independent predictor of gynecological cancer risk, even in the absence of BMI as a covariate.
Although obesity is widely recognized as a risk factor for cancer, the relationship between traditional measures of obesity, such as BMI and WC, and the risk of gynecological cancers remains unclear. Recent Mendelian randomization studies indicate that genetically predicted increases in BMI are linked to a higher risk of endometrial cancer (26). A cohort study involving 5 million individuals shows that for every 5 kg/m2 increase in BMI, the risks of endometrial and cervical cancers rise by 62 and 10%, respectively (27). Additionally, a large retrospective cohort study from Israel demonstrates an inverse association between higher adolescent BMI and the risk of cervical cancer in middle age (28). A meta-analysis reveals that being overweight or obese significantly increases the risk of ovarian cancer, with a 6% increase in risk for every 10 cm increase in WC (29, 30). Pathological analysis of ovarian cancer subtypes indicates that a higher BMI increases the risk of non-high-grade serous ovarian cancer but does not affect the risk of the more aggressive high-grade serous ovarian cancer (31). A cohort study finds no association between WC and BMI and the risk of epithelial ovarian cancer in premenopausal or postmenopausal women (32). Similarly, Mendelian randomization studies from Japanese and European populations show no association between higher BMI and ovarian cancer risk (33). BMI and WC are limited by their inability to distinguish between fat and muscle mass (34), often leading to the “obesity paradox.” Evidence suggests that during aging, the interplay between fat, muscle, and bone tissues results in changes in body composition, such as increased fat mass and decreased muscle and bone mass (35). A growing number of experts argue that BMI and WC are inadequate measures of obesity because they cannot differentiate between lean body mass and fat mass and are influenced by age, sex, and racial differences (36).
To the best of our knowledge, this is the first study to utilize the WWI to evaluate its relationship with the risk of gynecological cancers. Our findings demonstrate that a higher WWI is associated with an increased risk of gynecological malignancies. Compared to BMI and WC, WWI is a recently developed obesity assessment metric that accurately reflects the total body fat ratio and has been extensively studied across various fields. Liu et al. observed that WWI had a stronger association with depression compared to traditional indices like BMI and WC (15). Wang et al. found that in American adults over 60 years old, each unit increase in WWI was associated with a 32% increase in the prevalence of hypertension (16). This association persisted even after adjusting for age, sex, race, and adverse lifestyle factors. Xie et al. discovered that WWI was superior to BMI and WC in predicting severe abdominal aortic calcification (AAC). Higher WWI was significantly associated with severe AAC scores (37). Wen et al. found that among 3,526 participants, higher WWI values were linked to an increased incidence of infertility. Compared to other obesity metrics, including WC and BMI, WWI showed a stronger association with infertility risk (38).
The WWI is an essential metric for assessing abdominal fat, providing a more nuanced understanding of obesity. In gynecological cancers, adipose tissue primarily facilitates carcinogenesis through hormonal, inflammatory, and metabolic mechanisms (39). Adipose tissue, functioning as an active endocrine organ, secretes glucocorticoids and estrogens, among other hormones. Glucocorticoids facilitate the conversion of androgens to estrogens. Elevated estrogen levels, in turn, activate downstream mitotic signaling pathways, promoting cancer cell proliferation (40). Obesity often involves chronic low-grade inflammation, significantly altering metabolism and tissue homeostasis, and thereby leading to tumorigenesis (41). Chronic inflammation activates intracellular signaling pathways involving nuclear factor-κB, which regulates interleukin-6 (IL-6). IL-6, via its receptor and an intracellular cascade mediated by Janus kinase proteins, activates the signal transducer and activator of transcription 3. This activation leads to the expression of genes, including cyclins, which induce cell proliferation (42). Additionally, excessive accumulation of adipose tissue can result in metabolic disturbances, characterized by reduced glycolysis and oxidative phosphorylation. These changes impair the function of natural killer cells, CD8 T cells, and CD4 T cells, reducing their cytotoxicity and consequently increasing cancer risk (43, 44).
This study presents several strengths. Firstly, it uses NHANES data, ensuring the objectivity of the information. Secondly, we meticulously adjusted for confounding variables, which enhances the reliability of our findings and broadens their applicability. Thirdly, the study introduces and validates the WWI as a superior predictor of gynecological cancer risk compared to traditional obesity measures such as BMI and WC. In low-resource settings, where access to advanced diagnostic tools may be restricted, the WWI offers a valuable alternative for identifying individuals at high risk for gynecological cancers. The simplicity of WWI calculation, which relies on basic anthropometric measurements, makes it a cost-effective and easily implementable tool for early cancer detection. By incorporating WWI into routine screenings, healthcare providers can more effectively allocate resources and prioritize further diagnostic evaluations for those identified as high-risk.
However, this study has certain limitations. As a cross-sectional study, it cannot fully establish the relationship between WWI and the risk of gynecological cancers. Moreover, given that our data analysis is based on an American population, the generalizability of these findings to other populations remains uncertain. Future studies should validate the predictive power of WWI across cohorts with diverse ethnicities, regions, and age groups to enhance its generalizability and clinical utility. Furthermore, we acknowledge that the imbalance in sample size may affect the robustness of the statistical model. Future research should consider replicating our analyses in larger and more balanced cohorts to further validate the robustness of our findings. Self-reported data is a common practice in epidemiological studies, though its accuracy may be limited by recall bias or respondent misinterpretation. These limitations can result in inaccuracies in cancer diagnosis information, potentially impacting the reliability of the study’s findings.
5 Conclusion
The research indicates that elevated WWI levels are associated with an increased risk of gynecological cancers, particularly cervical and endometrial cancers. Compared to BMI and WC, WWI exhibits superior predictive capabilities and may serve as a valuable anthropometric indicator for assessing gynecological cancer risk.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the NHANES protocol received approval from the NCHS Research Ethics Review Board, and informed consent was obtained from all participants prior to their inclusion in the study. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
LF: Formal analysis, Methodology, Writing – original draft. XL: Investigation, Validation, Writing – original draft. YF: Conceptualization, Investigation, Methodology, Writing – review & editing. YW: Methodology, Writing – review & editing. RW: Writing – review & editing. YX: Conceptualization, Writing – review & editing. YZ: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by Inheritance and Innovation Team of the National Administration of Traditional Chinese Medicine (grant number ZYYCXTD-C-202205) and Science and Technology Innovation Project of the Chinese Academy of Traditional Chinese Medicine (grant number CI2022C002-03).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray, F, Laversanne, M, Sung, H, Ferlay, J, Siegel, RL, Soerjomataram, I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Chen, S, Cao, Z, Prettner, K, Kuhn, M, Yang, J, Jiao, L, et al. Estimates and projections of the global economic cost of 29 cancers in 204 countries and territories from 2020 to 2050. JAMA Oncol. (2023) 9:465–72. doi: 10.1001/jamaoncol.2022.7826
3. Webb, PM, and Jordan, SJ. Global epidemiology of epithelial ovarian cancer. Nat Rev Clin Oncol. (2024) 21:389–400. doi: 10.1038/s41571-024-00881-3
4. Singh, D, Vignat, J, Lorenzoni, V, Eslahi, M, Ginsburg, O, Lauby-Secretan, B, et al. Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO global cervical Cancer elimination initiative. Lancet Glob Health. (2023) 11:e197–206. doi: 10.1016/S2214-109X(22)00501-0
5. Bruchim, I, Capasso, I, Polonsky, A, Meisel, S, Salutari, V, Werner, H, et al. New therapeutic targets for endometrial cancer: a glimpse into the preclinical sphere. Expert Opin Ther Targets. (2024) 28:29–43. doi: 10.1080/14728222.2024.2316739
6. Li, S, Silvestri, V, Leslie, G, Rebbeck, TR, Neuhausen, SL, Hopper, JL, et al. Cancer risks associated with BRCA1 and BRCA2 pathogenic variants. J Clin Oncol. (2022) 40:1529–41. doi: 10.1200/JCO.21.02112
7. Zheng, S, Donnelly, ED, and Strauss, JB. Race, prevalence of POLE and POLD1 alterations, and survival among patients with endometrial Cancer. JAMA Netw Open. (2024) 7:e2351906. doi: 10.1001/jamanetworkopen.2023.51906
8. Kiljańczyk, A, Matuszczak, M, Marciniak, W, Derkacz, R, Stempa, K, Baszuk, P, et al. Blood lead level as marker of increased risk of ovarian Cancer in BRCA1 carriers. Nutrients. (2024) 16:1370. doi: 10.3390/nu16091370
9. Went, M, Sud, A, Mills, C, Hyde, A, Culliford, R, Law, P, et al. Phenome-wide Mendelian randomisation analysis of 378,142 cases reveals risk factors for eight common cancers. Nat Commun. (2024) 15:2637. doi: 10.1038/s41467-024-46927-z
10. GBD 2015 Obesity CollaboratorsAfshin, A, Forouzanfar, MH, Reitsma, MB, Sur, P, Estep, K, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. (2017) 377:13–27. doi: 10.1056/NEJMoa1614362
11. Ward, ZJ, Bleich, SN, Cradock, AL, Barrett, JL, Giles, CM, Flax, C, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. (2019) 381:2440–50. doi: 10.1056/NEJMsa1909301
12. Bosy-Westphal, A, and Müller, MJ. Diagnosis of obesity based on body composition-associated health risks-time for a change in paradigm. Obes Rev. (2021) 22:e13190. doi: 10.1111/obr.13190
13. Park, Y, Kim, N, Kwon, T, and Kim, SG. A novel adiposity index as an integrated predictor of cardiometabolic disease morbidity and mortality. Sci Rep. (2018) 8:16753. doi: 10.1038/s41598-018-35073-4
14. Kim, KJ, Son, S, Kim, KJ, Kim, SG, and Kim, NH. Weight-adjusted waist as an integrated index for fat, muscle and bone health in adults. J Cachexia Sarcopenia Muscle. (2023) 14:2196–203. doi: 10.1002/jcsm.13302
15. Liu, H, Zhi, J, Zhang, C, Huang, S, Ma, Y, Luo, D, et al. Association between weight-adjusted waist index and depressive symptoms: a nationally representative cross-sectional study from NHANES 2005 to 2018. J Affect Disord. (2024) 350:49–57. doi: 10.1016/j.jad.2024.01.104
16. Wang, J, Yang, QY, Chai, DJ, Su, Y, Jin, QZ, and Wang, JH. The relationship between obesity associated weight-adjusted waist index and the prevalence of hypertension in US adults aged ≥60 years: a brief report. Front Public Health. (2023) 11:1210669. doi: 10.3389/fpubh.2023.1210669
17. Wang, Z, Shao, X, Xu, W, Xue, B, Zhong, S, and Yang, Q. The relationship between weight-adjusted-waist index and diabetic kidney disease in patients with type 2 diabetes mellitus. Front Endocrinol. (2024) 15:1345411. doi: 10.3389/fendo.2024.1345411
18. Sun, F, Liu, M, Hu, S, Xie, R, Chen, H, Sun, Z, et al. Associations of weight-adjusted-waist index and depression with secondary infertility. Front Endocrinol. (2024) 15:1330206. doi: 10.3389/fendo.2024.1330206
19. Siegel, RL, Giaquinto, AN, and Jemal, A. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
20. Gui, Z, Yu, L, Chen, Y, Zhang, M, He, J, and Hao, Y. Study from the United States: increased prevalence of kidney stones in patients with high weight-adjusted waist index. Front Nutr. (2024) 10:1171775. doi: 10.3389/fnut.2023.1171775
21. Xie, H, Wei, L, Zhang, H, Ruan, G, Liu, X, Lin, S, et al. Association of systemic inflammation with the obesity paradox in cancer: results from multi-cohort studies. Inflamm Res. (2024) 73:243–52. doi: 10.1007/s00011-023-01832-x
22. Gangrade, N, Figueroa, J, and Leak, TM. Socioeconomic disparities in foods/beverages and nutrients consumed by U.S. adolescents when snacking: National Health and nutrition examination survey 2005-2018. Nutrients. (2021) 13:2530. doi: 10.3390/nu13082530
23. von Elm, E, Altman, DG, Egger, M, Pocock, SJ, Gøtzsche, PC, Vandenbroucke, JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. (2007) 370:1453–7. doi: 10.1016/S0140-6736(07)61602-X
24. Zhou, H, Su, H, Gong, Y, Chen, L, Xu, L, Chen, G, et al. The association between weight-adjusted-waist index and sarcopenia in adults: a population-based study. Sci Rep. (2024) 14:10943. doi: 10.1038/s41598-024-61928-0
25. Mao, S, Qian, G, Xiao, K, Xu, H, Zhou, H, and Guo, X. Study on the relationship between body mass index and blood pressure indices in children aged 7-17 during COVID-19. Front Public Health. (2024) 12:1409214. doi: 10.3389/fpubh.2024.1409214
26. Hazelwood, E, Sanderson, E, Tan, VY, Ruth, KS, Frayling, TM, Dimou, N, et al. Identifying molecular mediators of the relationship between body mass index and endometrial cancer risk: a Mendelian randomization analysis. BMC Med. (2022) 20:125. doi: 10.1186/s12916-022-02322-3
27. Bhaskaran, K, Douglas, I, Forbes, H, Dos-Santos-Silva, I, Leon, DA, and Smeeth, L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5·24 million UK adults. Lancet. (2014) 384:755–65. doi: 10.1016/S0140-6736(14)60892-8
28. Furer, A, Afek, A, Sommer, A, Keinan-Boker, L, Derazne, E, Levi, Z, et al. Adolescent obesity and midlife cancer risk: a population-based cohort study of 2.3 million adolescents in Israel. Lancet Diabetes Endocrinol. (2020) 8:216–25. doi: 10.1016/S2213-8587(20)30019-X
29. Choi, EK, Park, HB, Lee, KH, Park, JH, Eisenhut, M, van der Vliet, HJ, et al. Body mass index and 20 specific cancers: re-analyses of dose-response meta-analyses of observational studies. Ann Oncol. (2018) 29:749–57. doi: 10.1093/annonc/mdx819
30. Aune, D, Navarro Rosenblatt, DA, Chan, DS, Abar, L, Vingeliene, S, Vieira, AR, et al. Anthropometric factors and ovarian cancer risk: a systematic review and nonlinear dose-response meta-analysis of prospective studies. Int J Cancer. (2015) 136:1888–98. doi: 10.1002/ijc.29207
31. Dixon, SC, Nagle, CM, Thrift, AP, Pharoah, PD, Pearce, CL, Zheng, W, et al. Adult body mass index and risk of ovarian cancer by subtype: a Mendelian randomization study. Int J Epidemiol. (2016) 45:884–95. doi: 10.1093/ije/dyw158
32. Kotsopoulos, J, Baer, HJ, and Tworoger, SS. Anthropometric measures and risk of epithelial ovarian cancer: results from the nurses' health study. Obesity. (2010) 18:1625–31. doi: 10.1038/oby.2009.461
33. Masuda, T, Ogawa, K, Kamatani, Y, Murakami, Y, Kimura, T, and Okada, Y. A Mendelian randomization study identified obesity as a causal risk factor of uterine endometrial cancer in Japanese. Cancer Sci. (2020) 111:4646–51. doi: 10.1111/cas.14667
34. Romero-Corral, A, Somers, VK, Sierra-Johnson, J, Thomas, RJ, Collazo-Clavell, ML, Korinek, J, et al. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes. (2008) 32:959–66. doi: 10.1038/ijo.2008.11
35. Rule, AD, Grossardt, BR, Weston, AD, Garner, HW, Kline, TL, Chamberlain, AM, et al. Older tissue age derived from abdominal computed tomography biomarkers of muscle, fat, and bone is associated with chronic conditions and higher mortality. Mayo Clin Proc. (2024) 99:878–90. doi: 10.1016/j.mayocp.2023.09.021
36. Lam, BC, Koh, GC, Chen, C, Wong, MT, and Fallows, SJ. Comparison of body mass index (BMI), body adiposity index (BAI), waist circumference (WC), waist-to-hip ratio (WHR) and waist-to-height ratio (WHtR) as predictors of cardiovascular disease risk factors in an adult population in Singapore. PLoS One. (2015) 10:e0122985. doi: 10.1371/journal.pone.0122985
37. Xie, F, Xiao, Y, Li, X, and Wu, Y. Association between the weight-adjusted-waist index and abdominal aortic calcification in United States adults: results from the national health and nutrition examination survey 2013–2014. Front Cardiovasc Med. (2022) 9:948194. doi: 10.3389/fcvm.2022.948194
38. Wen, Z, and Li, X. Association between weight-adjusted-waist index and female infertility: a population-based study. Front Endocrinol. (2023) 14:1175394. doi: 10.3389/fendo.2023.1175394
39. Wichmann, IA, and Cuello, MA. Obesity and gynecological cancers: a toxic relationship. Int J Gynaecol Obstet. (2021) 155:123–34. doi: 10.1002/ijgo.13870
40. Laforest, S, Pelletier, M, Denver, N, Poirier, B, Nguyen, S, Walker, BR, et al. Simultaneous quantification of estrogens and glucocorticoids in human adipose tissue by liquid-chromatography-tandem mass spectrometry. J Steroid Biochem Mol Biol. (2019) 195:105476. doi: 10.1016/j.jsbmb.2019.105476
41. Lee, YS, and Olefsky, J. Chronic tissue inflammation and metabolic disease. Genes Dev. (2021) 35:307–28. doi: 10.1101/gad.346312.120
42. Johnson, DE, O’Keefe, RA, and Grandis, JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. (2018) 15:234–48. doi: 10.1038/nrclinonc.2018.8
43. Michelet, X, Dyck, L, Hogan, A, Loftus, RM, Duquette, D, Wei, K, et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat Immunol. (2018) 19:1330–40. doi: 10.1038/s41590-018-0251-7
Keywords: weight-adjusted-waist index, obesity, non-linear, NHANES, gynecologic cancers
Citation: Fang L, Li X, Fang Y, Wang Y, Wang R, Xie Y and Zhang Y (2024) Association between weight-adjusted-waist index and gynecologic cancers: a population-based study. Front. Nutr. 11:1449643. doi: 10.3389/fnut.2024.1449643
Edited by:
Ozra Tabatabaei-Malazy, Tehran University of Medical Sciences, IranReviewed by:
Ali Golestani, Tehran University of Medical Sciences, IranShirin Djalalinia, Ministry of Health and Medical Education, Iran
Sepehr Khosravi, Tehran University of Medical Sciences, Iran
Copyright © 2024 Fang, Li, Fang, Wang, Wang, Xie and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Zhang, enlsenk1MDFAMTYzLmNvbQ==
 Liyuan Fang
Liyuan Fang Xiaotong Li2
Xiaotong Li2 Ying Zhang
Ying Zhang