- 1Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences, Beijing, China
- 2Institute of Traditional Chinese Medicine Health Industry, China Academy of Chinese Medical Sciences, Nanchang, China
Introduction: Kaempferol, a flavonoid found in numerous foods and medicinal plants, offers a range of health benefits such as anti-inflammatory, antioxidant, antiviral, anticancer, cardioprotective, and neuroprotective effects.
Methods: Herein, a bibliometric and visual analysis of global publications on kaempferol was performed to map the evolution of frontiers and hotspots in the field. Using the search string TS = kaempferol, bibliometric data for this analysis was extracted from the Web of Science Core Collection database and analyzed using the VOSviewer, CiteSpace, and Scimago Graphica software.
Results: As a result, by February 26, 2024, 11,214 publications were identified, comprising articles (n = 10,746, 96%) and review articles (n = 468, 4%). Globally, the annual number of kaempferol publications surpassed 100 per year since 2000, exceeded 500 per year since 2018, and further crossed the threshold of 1,000 per year starting in 2022. The major contributing countries were China, the United States of America, and India, while the top three institutes of the citations of kaempferol were the Chinese Academy of Sciences, Consejo Superio de Investigaciones Cientficas, and Uniersidade do Porto. These publications were mainly published in agricultural and food chemistry journals, food chemistry, and phytochemistry.
Discussion: The keywords frequently mentioned include phenolic compounds, antioxidant activity, flavonoids, NF-kappa B, inflammation, bioactive compounds, etc. Anti-inflammation, anti-oxidation, and anti-cancer have consistently been the focus of kaempferol research, while cardiovascular protection, neuroprotection, antiviral, and anti-bacterial effects have emerged as recent highlights. The field of kaempferol research is thriving.
1 Introduction
Generally, the daily intake of natural plant substances has been highlighted by scholarly research for their numerous health benefits and their potential bioactivity in preventing various diseases (1–4). Among these natural plant substances, flavonoids have been isolated from plants and proven to have multiple preventive and therapeutic effects on diseases (5, 6). Especially, kaempferol, a bioactive flavonoid monomer extracted from different plants, has garnered extensive global research and scholarly attention due to its properties, including anti-inflammatory, anti-tumor, anti-oxidative stress anti-allergy, anti-viral, and cardiac protection effects (7–12). Kaempferol stands as a flavonoid that has been isolated and purified through modern scientific methods. It is widely found in medicinal plants such as Galangal, Rhizoma Drynariae, Semen Cuscutae, Ginkgo Biloba, Leaf of Eucommla Ulmoides, etc. (8, 13). Kaempferol is a tetrahydroxy flavone that presents as yellow needle-shaped crystals with four hydroxy groups positioned at the 3, 5, 7, and 4′ positions (14). Researchers have been summarizing the presence of kaempferol in various parts of plants, including seeds, leaves, fruits, and flowers. Besides, kaempferol is also widely distributed in natural sources of medical plants. It can be prepared and purified using microporous adsorption resin column chromatography, silica gel column chromatography, and other chromatographic techniques (15). Currently, kaempferol represents more than 5.7 billion US dollars in the global consumer market of medicine and food (16). As a medicine and health compound, kaempferol has broad worldwide research and development potential.
Kaempferol has the molecular formula C15H10O6, with a relative molecular weight 286.23. Its chemical name is 3,5,7,4′-tetrahydroxyflavone. It is slightly soluble in water but in ether, alkali, and hot ethanol (8, 17). Like most flavonoids, kaempferol often exists in various natural plants, fruits, and vegetables in different glycosides. An aglycone produced by hydrolysis of an acidic substance can improve the total extraction rate of kaempferol (13). In addition, kaempferol, in the form of glycosides, can directly enter the bloodstream and exhibit various biological activities within the human body (18). The antioxidant stress activity of kaempferol has been confirmed. Kaempferol can promote the expression of heme oxygenase-1, inhibit the production of nitric oxide and inducible nitric oxide synthase, and reduce the damage of lipopolysaccharide on RAW264.7 macrophages (8, 19, 20). Furthermore, kaempferol plays a neuroprotective role by inhibiting oxidative stress. Previous studies have also revealed that kaempferol has notable anti-inflammatory effects, including regulating the expression of inflammation-related genes and the activity of pro-inflammatory enzymes and inhibiting the expression of matrix metalloproteinases, adhesion molecules, and transcription factors (8, 21). Besides, considerable studies have shown kaempferol’s efficacy in accelerating cancer cell apoptosis. For instance, in vitro experiments have indicated that kaempferol can inhibit the proliferation of malignant tumor cells such as breast, colon, lung, bladder, and liver (9, 22). In addition, studies have demonstrated that kaempferol can increase the number of endothelial progenitor cells in the lumen after treatment with advanced glycosylation end-products in diabetic patients who have suffered a myocardial infarction. This finding implies that kaempferol can improve microvascular complications associated with diabetes (23–25). This kind of valuable research into the therapeutic potential for myocardial protection also bolsters kaempferol’s clinical medicinal research value (26). Meanwhile, kaempferol also exerts potential therapeutic effects in treating cataracts, coughs, fertility inhibition, anti-ulcer, anti-epilepsy, etc. (27, 28). However, a holistic analysis of the scientific literature on kaempferol is missing. Thus, performing a thorough analysis and offering a guide to current research trends is essential for informing ongoing scholarly work.
Bibliometrics focuses on the literature system and the characteristics of bibliometric studies as its subject matter. It employs mathematical and statistical measurement methods to primarily investigate the structure, quantitative relationships, change patterns, and quantitative management of the distribution of literature and information. Furthermore, it delves into the structures, characteristics, and laws of science and technology (29, 30). Bibliometrics mainly takes literature as the research object and analyzes the quantitative attributes of existing literature. The CiteSpace software, developed by Dr. Chen Chaomei and his team, serves as a citation visualization analysis software that has been gradually developed against the background of bibliometrics and data visualization. It is currently the most widely used software in bibliometrics (31, 32). The CiteSpace or VOSviewer software can extract and analyze the semantic contents of the titles, abstracts, and keywords of publications. It performs well in relating them to the citation count data and generating a bubble map to visualize the results (33, 34). These bibliometrics and visualization results assist researchers in grasping the research’s frontier and hotspot. Besides, Scimago Graphica software can aesthetically visualize the results of bibliometric analyzes conducted with tools like CiteSpace or VOSviewer (35). Furthermore, Scimago Graphica software can generate a national geographic map where each node signifies a distinct country. The node’s size is directly proportional to the volume of literature published by that country (36). The connection between nodes represents the cooperation between nations, while the connection’s thickness indicates the cooperation’s closeness (37). Besides, in the co-occurrence analysis graph of VOSviewer software, node and font size represent the frequency of occurrence, the lines between nodes indicate cooperation, and the thickness indicates closeness (38). In addition, in the co-occurrence and cluster analysis results of CiteSpace software, different colors indicate different years. In the spectrum of the cluster analysis graph, the value of the cluster module (Q value) and the mean value of the cluster profile (S value) can be observed (39). The former represents the association of the cluster nodes, and the latter represents the association of the cluster topics. In the outburst map, one should look for the bright red areas, which signify the time range of the outburst (40). Briefly, bibliometrics presents the knowledge structure and frontier trends of a research field utilizing modern techniques such as CiteSpace, VOSviewer, Scimago Graphica, etc., to visualize countries, institutions, authors, journals, documents, and keywords intuitively. To this end, a bibliometric and visual analysis was performed regarding global publications on kaempferol in the Web of Science (WoS) Core Collection database. The aim was to provide total-scale analysis results, track research hotspots, and offer insights for future kaempferol research.
2 Materials and methods
2.1 Data collection
Literature on kaempferol was retrieved from the Web of Science (WoS) Core Collection database, focusing on journal articles. The MeSH1 confirmed the term “kaempferol” with no synonyms found (41). Subsequently, the search term and strategy used for the WoS database was “TS = kaempferol.” Articles from the databases’ inception until February 26, 2024, were searched. The search was limited to English-language publications and included articles and review document types. The data search and export were finalized on February 26, 2024, to prevent any data deviation that subsequent database updates might cause. Ultimately, 11,214 documents were obtained, and the data results were exported in the “txt” format, including “Full Record and Cited References” for subsequent analysis.
2.2 Data selection
The inclusion criteria for literature were as follows: (1) the research content of the literature revolved around kaempferol; (2) the literature involved kaempferol-related pharmaceutical experiments, animal experiments, cell experiments, clinical trials, and other studies; and (3) both research papers and review papers could be included. Otherwise, the exclusion criteria for literature were as follows: (1) literature with incomplete information such as title, keywords, journal, and author; (2) literature of news reports, scientific and technological achievements, conference notices, conference papers, call for papers, patents, and other types; and (3) only one duplicate publication was retained.
All 11,214 documents were imported into Endnote X9 literature management software, and two researchers screened them independently to remove duplicate ones and retain their uniqueness. Then, the data was extracted and cross-checked. Any discrepancies were discussed among the other research members to decide on inclusion. In the end, it was confirmed that there were no duplicate documents among the 11,214 documents.
2.3 Rationale of the bibliometric tools
The CiteSpace software, developed using Java, is an information visualization tool that employs co-citation analysis theory and the pathFinder algorithm to assess literature in specific fields (42). Consequently, it effectively reveals the research field’s hotspots, development trends, and novel advancements. Besides, VOSviewer is grounded in the principles and methodologies of bibliometrics, employing techniques such as data mining, information processing, and knowledge measurement to represent intricate literature as knowledge maps visually. It uses processes including standardization of similarity, clustering networks based on keywords, and visual representation to generate visual keyword clusters, density maps, and multi-window time-series graphs of keywords, thereby unveiling the latest trends and advancements in a research domain (43). Furthermore, Scimago Graphica is a drag-and-drop data visualization software that simplifies the process of data analysis and chart creation. Users do not need to write complex codes or formulas. They can quickly import data into the software by dragging, dropping, and selecting the appropriate chart type and settings to generate visual charts (44).
2.4 Data analysis
Microsoft Excel was used to compile statistics and create visualizations of the annual and cumulative publications (44). Subsequently, the VOSviewer 1.6.20 software facilitated a bibliometric analysis encompassing countries, institutions, authors, co-cited authors, journals, and references per established research methodologies. The method parameters of the VOSviewer were set as Linlog/modularity, and the weight parameter was set according to the number of documents (37, 45). Following that, the analysis results from VOSviewer were exported and saved in the format of “gml.” for the network visualization of Scimago Graphica.
Scimago Graphica 1.0.30 Software was used to display the analysis results from VOSviewer visually (46). In Scimago Graphica’s network visualizations, nodes symbolize entities such as countries/regions, institutes, authors, co-cited authors, journals, and references. Node size corresponds to publication volume; lines denote cooperative ties, line thickness reflects the strength of collaboration, and node colors differentiate clusters (47, 48).
The CiteSpace 6.2 R6 software was utilized to conduct a co-occurrence analysis of the keywords from global publications on kaempferol. The parameters of CiteSpace were set as follows: method: LLR; time slicing: from January 1975 to February 2024; years per slice: 5; term source: all selection; and node type: one at a time (41). Besides, the silhouette value represents the cluster network homogeneity, with a silhouette value greater than 0.7 indicating the high reliability of the results (41, 49). Furthermore, the Q value of the cluster network signifies its modularity, with a Q value greater than 0.5 indicating a significant cluster structure of the network (39, 41). Finally, the timeline plot and the top keywords with the most robust citation bursts of kaempferol’s global publications were visually displayed and exported using CiteSpace.
3 Results
3.1 Annual publication statistics and analysis results
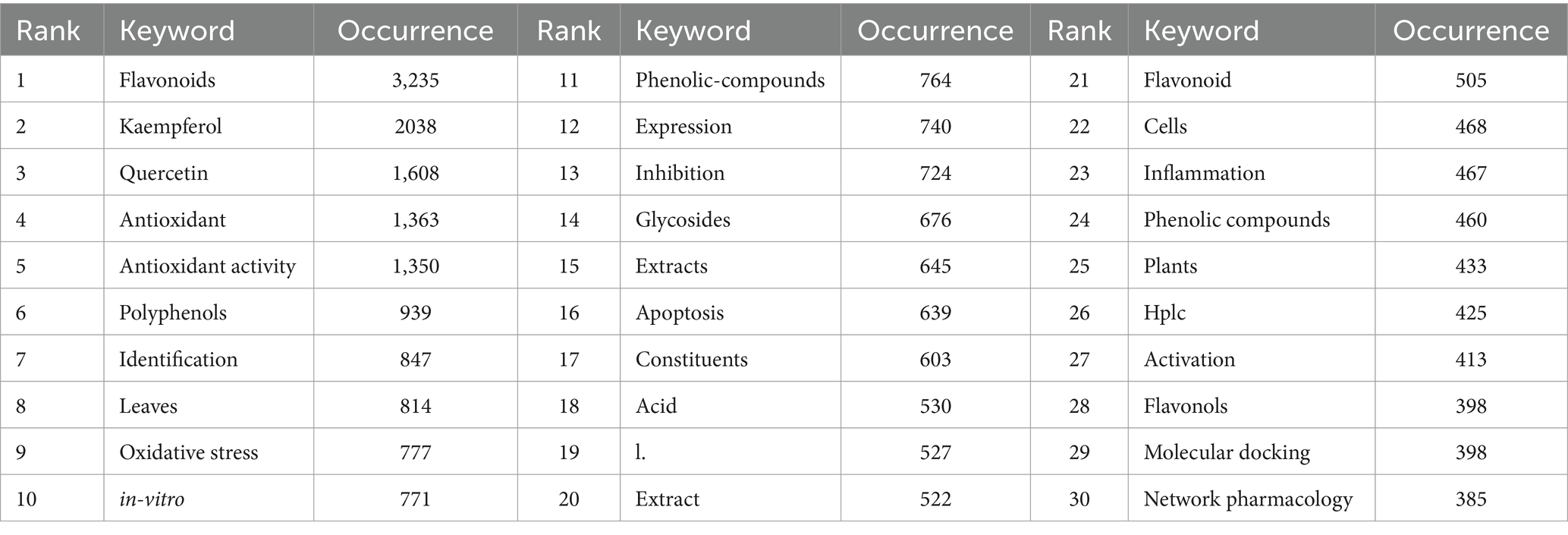
As shown in Figure 1, since 1975, research results of kaempferol have been published worldwide. The publication count experienced a gradual increase from 1990 to 2015, but it surged dramatically from 2016 to 2023, peaking with the highest number of publications in 2023 (n = 1,234). Besides, since the retrieval time of this study concluded on February 26, 2024, all relevant literature in 2024 could not be included, resulting in a decline in the number of papers published in 2024.
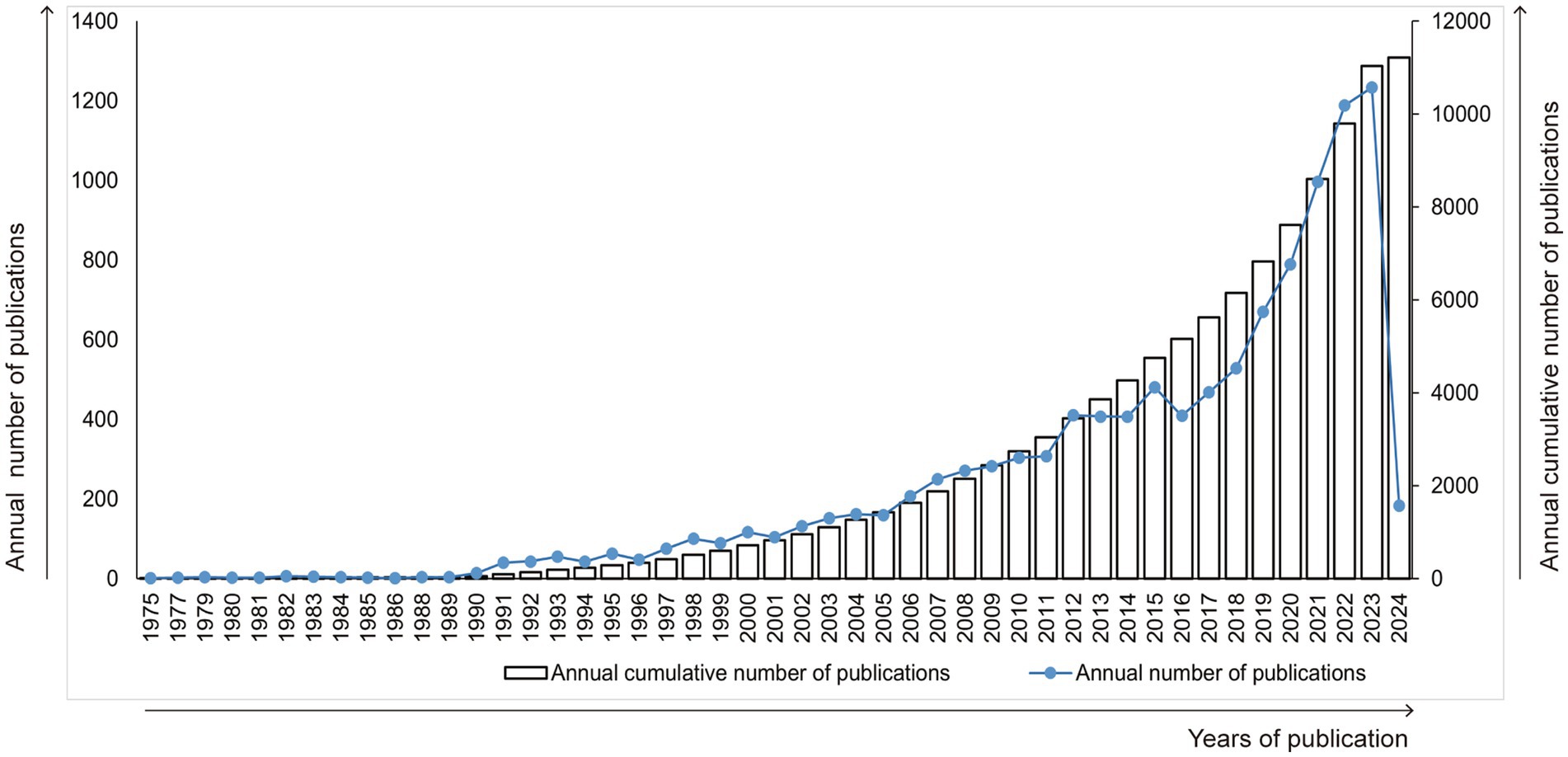
Figure 1. Trend graph of the growth of annual and cumulative annual number of kaempferol’s publications.
3.2 Contribution of countries/regions
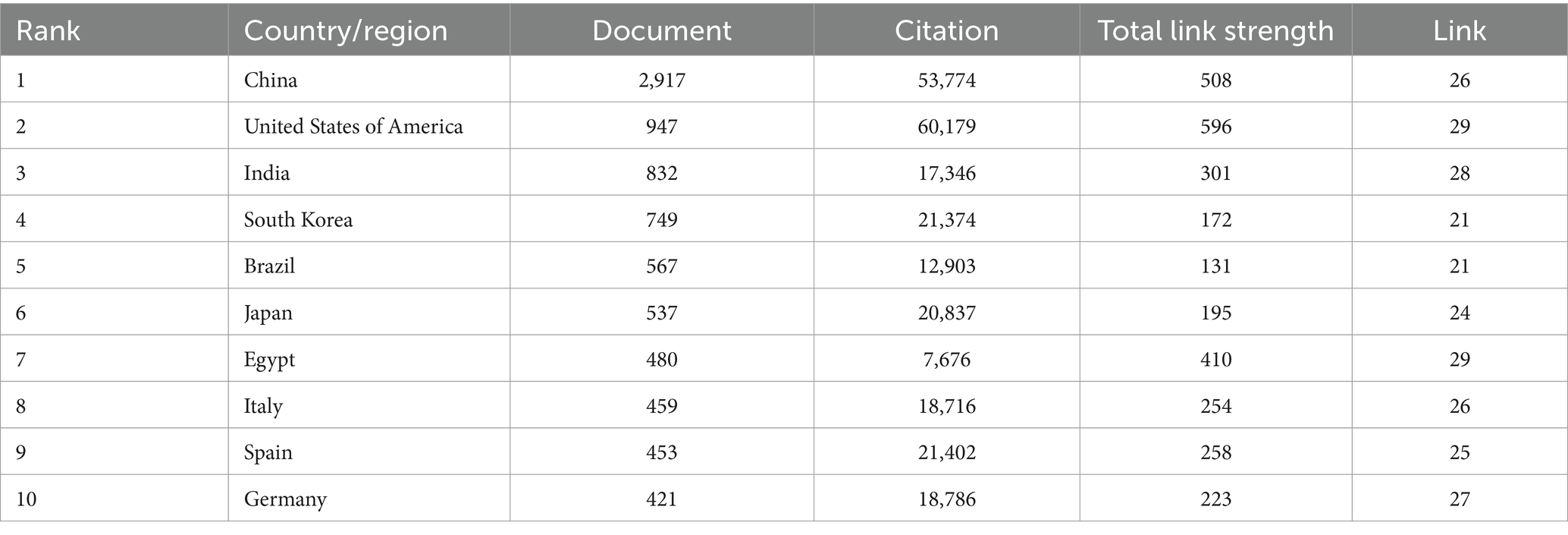
A total of 147 countries/regions participated in the research on kaempferol. Figure 2A presents the spatial distribution of the top 30 countries/regions, while Figure 2B shows a visualization analysis of 30 countries/regions with the publication volume exceeding 100 documents. The size of the nodes corresponds to the document counts, the lines suggest the connections between countries/regions, and the thickness of the lines indicates the strength of the connection between countries/regions. The countries/regions were divided into four clusters based on the degree of cooperation represented by different colors. As clearly shown in Figure 2, China had the most published documents and the closest collaboration with the United States on the research of kaempferol. Besides, Table 1 exhibits detailed information on the document counts, citation counts, total link strength, and link counts of the top 30 countries/regions. According to the results in Table 1, China (n = 2,917), the United States of America (n = 947), and India (n = 832) were the three leading contributors to global kaempferol research publications, while the United States of America (n = 60,179), China (n = 53,774), and Spain (n = 21,402) were the top three countries/regions contributing the most citations of kaempferol publications. The connections between countries/regions were mainly focused on the cooperation between the United States of America (the total link strength was 596) and other countries, including China (the total link strength was 508), Egypt (the total link strength was 410), and India (the total link strength was 301).
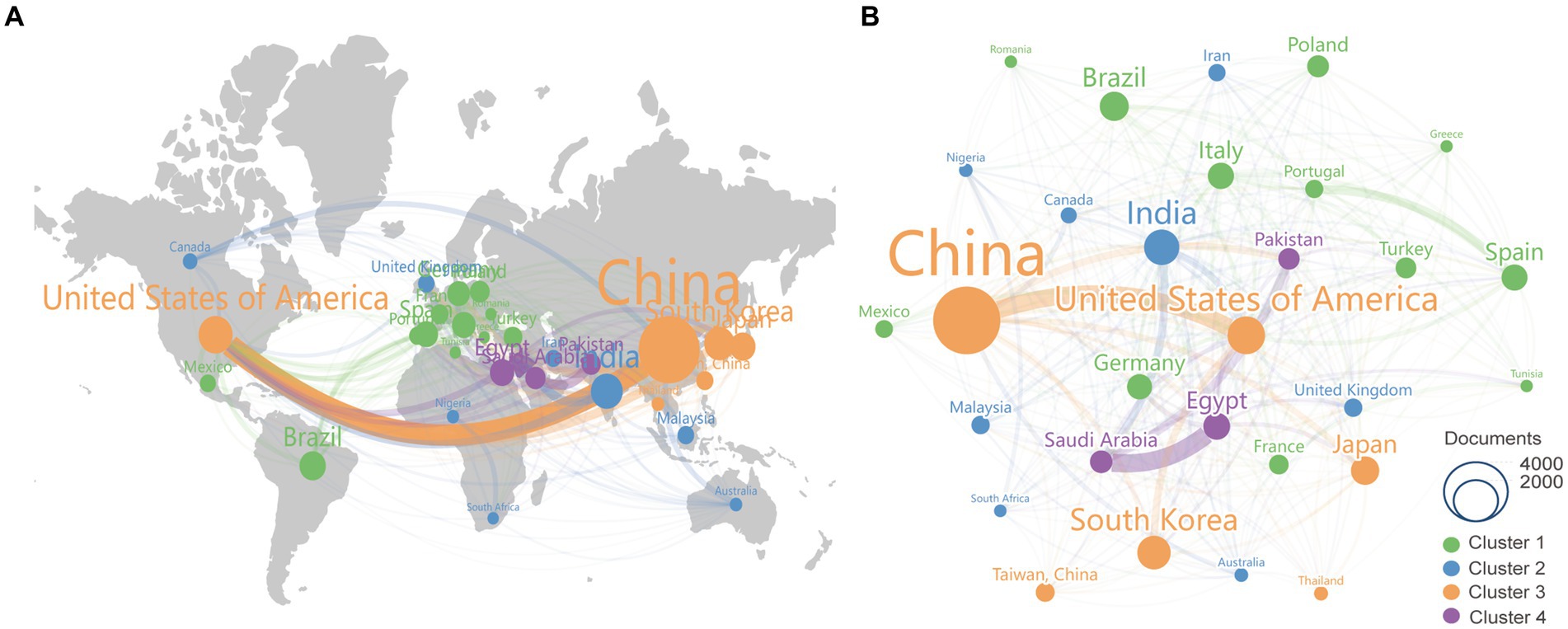
Figure 2. The most productive countries/regions of publications on kaempferol. (A) Spatial distribution of kaempferol’s global publications’ top 30 countries/regions. (B) Visual display of the top 30 countries/areas of kaempferol’s global publications. VOSviewer was used for analysis; the method was ling/modularity, weight documented, the minimum number of documents of a country was set as 100, and results were visually displayed by Scimago Graphics software. The thickness of the lines indicates the strength of the relationship. The different colors of the circle represent the various clusters of countries/regions. The larger the circle and label, the more kaempferol’s global publications the country has published.
3.3 Contribution of institutes, co-cited journals, authors, and co-cited authors
3.3.1 Contribution of institutes
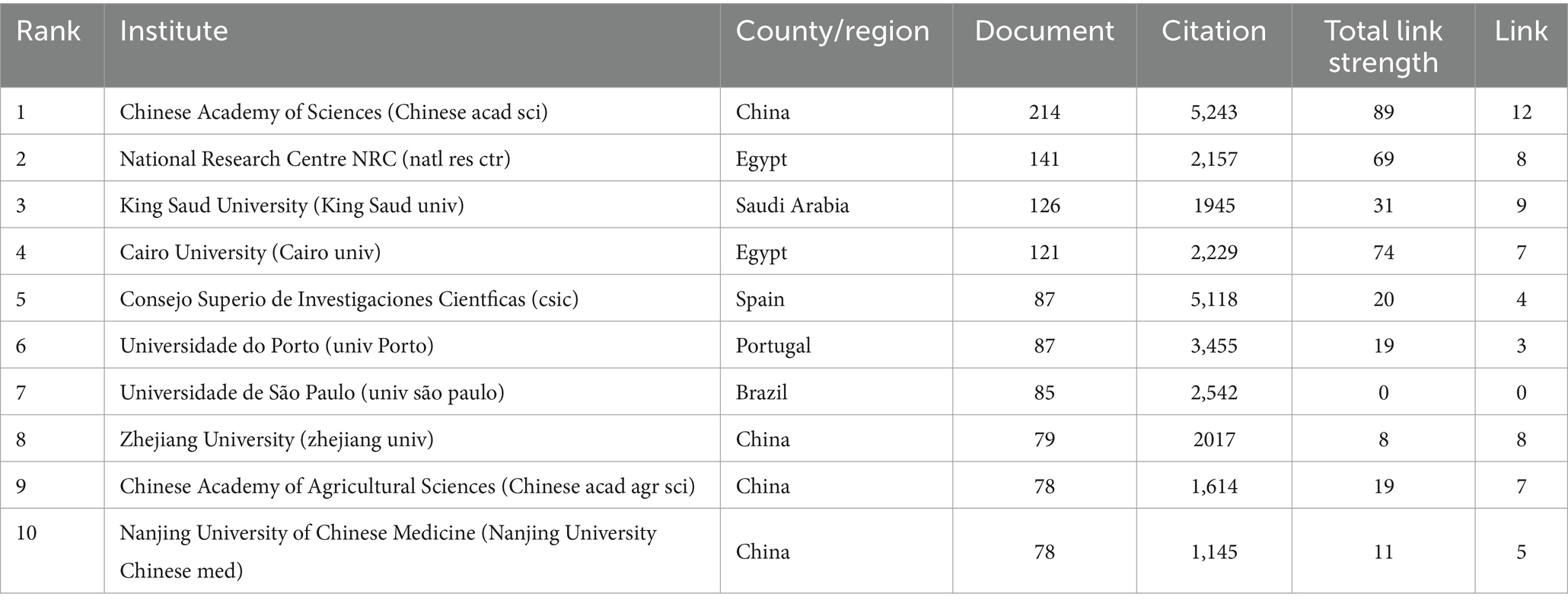
A total of 698 institutes participated in the research of kaempferol. According to VOSviewer analysis results, there were 31 institutions with more than 50 global publications related to kaempferol, and the visual network diagram is shown in Figure 3A (the minimum number of documents of an organization was 50). The size of the nodes corresponds to the document counts; the lines suggest the connections between institutes, and the thickness of the lines indicates the strength of the connection between institutes. The institutes were divided into six clusters based on the degree of cooperation represented by different colors. As shown in Figure 3A, the Chinese Academy of Sciences had the most published documents and the closest collaboration with the Chinese Academy of Agricultural Sciences on the research of kaempferol. Table 2 presents detailed information on the top 10 most productive institutes for global publications on kaempferol. According to the results, the Chinese Academy of Sciences (n = 214), National Research Centre NRC (n = 141), and King Saud University (n = 126) ranked as the top three institutes contributing to the global body of kaempferol publications. Besides, the top three institutes in terms of citation counts for international publications on kaempferol were: the Chinese Academy of Sciences (n = 5,243), Consejo Superio de Investigaciones Cientficas (n = 5,118), and Uniersidade do Porto (n = 3,455). The top three in total link strength, indicating prominent collaborative ties, were primarily centered around the Chinese Academy of Sciences (the total link strength was 89), Cairo University (the total link strength was 74), and the National Research Centre NRC (the total link strength was 69).
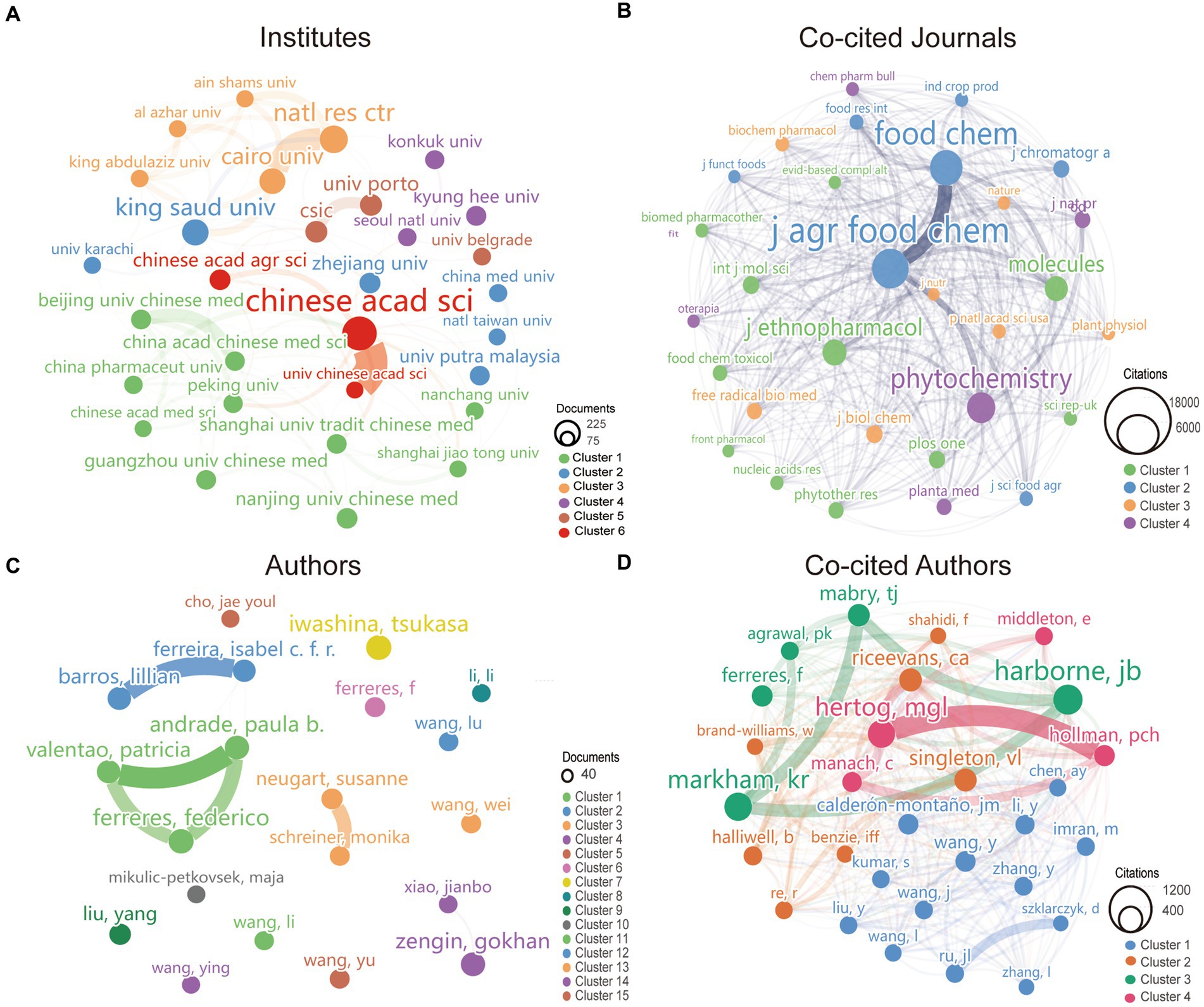
Figure 3. The most productive institutes, co-cited journals, authors, and co-cited authors of publications on kaempferol. (A) Visual display of the top 31 institutes of kaempferol’s global publications. The minimum number of documents required by an institute was set at 50. (B) Visual display of the top 26 co-cited journals of kaempferol’s global publications. The minimum number of citations of a co-cited journal was set as 2000. (C) Visual display of the top 20 authors of kaempferol’s global publications. The minimum number of documents an author has was set at 19. (D) Visual display of the top 29 co-cited authors of kaempferol’s global publications. The minimum number of citations for a co-cited author was set at 260. VOSviewer was used for analysis; the method was ling/modularity, weight was documented, and results were displayed visually by Scimago Graphica software. The thickness of the lines indicates the strength of the relationship. The different colors of the circle represented the various clusters of institutes, co-cited journals, authors, or co-cited authors. The larger the circle and label, the more kaempferol’s global publications the institutes, co-cited journals, authors, or authors have published.
3.3.2 Distribution of co-cited journals
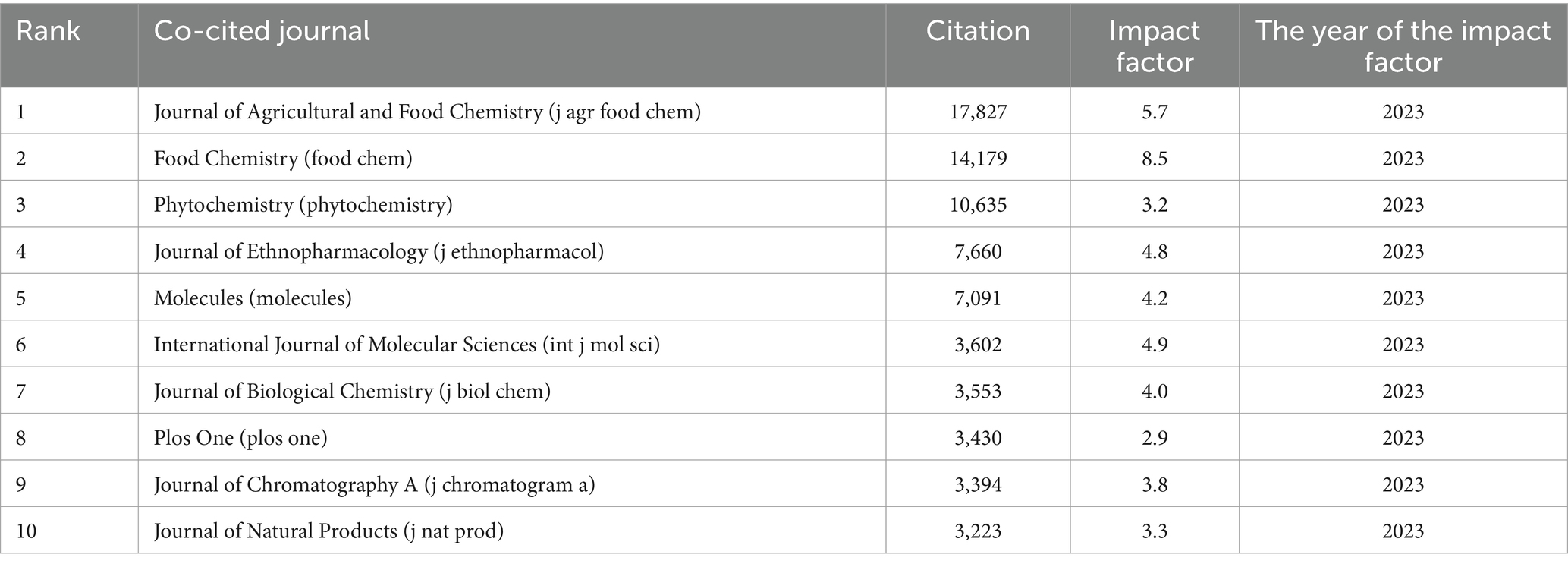
A total of 1,621 co-cited journals were found in the research on kaempferol. According to VOSviewer analysis results, there were 26 co-cited journals with more than 2,000 global publications on kaempferol, and the visual network diagram is shown in Figure 3B (the minimum number of citations of a co-cited journal was 2,000). The co-cited journals were divided into four clusters based on the degree of cooperation represented by different colors. A co-citation relationship was identified between journals if the same document cited two or more journals. The size of the nodes represents the total frequency of co-citation. A larger node indicates a higher frequency of co-citations, which implies a more significant influence of the journal within the research field of kaempferol. Therefore, as shown in Figure 3B, the Journal of Agricultural and Food Chemistry had the most citations, indicating that more researchers would publish their studies results of kaempferol in this journal. Table 3 presents detailed information on the top 10 most cited journals in the global publications on kaempferol. According to the results, the Journal of Agricultural and Food Chemistry (n = 17,827), Food Chemistry (n = 14,179), and Phytochemistry (n = 10,635) were identified as the top three most cited journals in the global publications on kaempferol.
3.3.3 Contribution of authors and co-cited authors
A total of 741 authors performed research on kaempferol. According to VOSviewer analysis results, there were 20 authors with more than 19 global publications on kaempferol, and the visual network diagram is shown in Figure 3C (the minimum number of documents of an author was 19). The size of the nodes corresponds to the document counts; the lines suggest the connections between authors, and the thickness of the lines indicates the strength of the connection between authors. The authors were divided into 15 clusters based on the degree of cooperation represented by different colors. As shown in Figure 3C, Iwashina and Tsukasa from Japan had the most published documents on kaempferol research. Clusters signified close collaboration and guided researchers in identifying potential research partners. In kaempferol research, the closest cooperative relationships were observed between Andrade, Paula Branquinho, Valentao, Patricia, and Ferreres, Federico. Besides, there was also a notable collaboration between Barros, Lillian, Ferreira, Isabel C. F. R., Neugart, Susanne, and Schreiner, Monika.
A total of 47,853 co-cited authors performed research on kaempferol. Among them, 29 co-cited authors with more than 260 citations were selected for visualization analysis, as shown in Figure 3D. A co-citation relationship existed between authors if the same document cited two or more authors. The size of the node represents the total frequency of co-citation. A larger node indicates a higher frequency of co-citations, which implies a more significant influence of the author within the research field of kaempferol. As noted in the analysis results, Harborne JB from the United Kingdom was the most cited author, making significant contributions to and having a far-reaching influence on kaempferol research. Moreover, Harborne JB, Markham KR, and Mabry TJ had a close cooperative relationship.
Table 4 presents detailed information on the top 10 most productive authors and most cited authors of kaempferol publications. According to the results, the top three authors in terms of publications on kaempferol globally were Iwashina, Tsukasa (n = 37), National Museum of Nature and Science, Japan; Ferreres, Federico (n = 36), CSIC – Centro de Edafologia y Biologia Aplicada del Segura, Spain; and Andrade, Paula Branquinho (n = 34), Universidade do Porto, Portugal. Besides, the top three co-cited authors in the global citations of kaempferol publications were Harborne JB (n = 939), University of Reading, United Kingdom; Markham KR (n = 833), Callaghan Innovation DSIR CHEM DIV, New Zealand, and Rice-Evans CA (n = 557), King’s College London, United Kingdom.
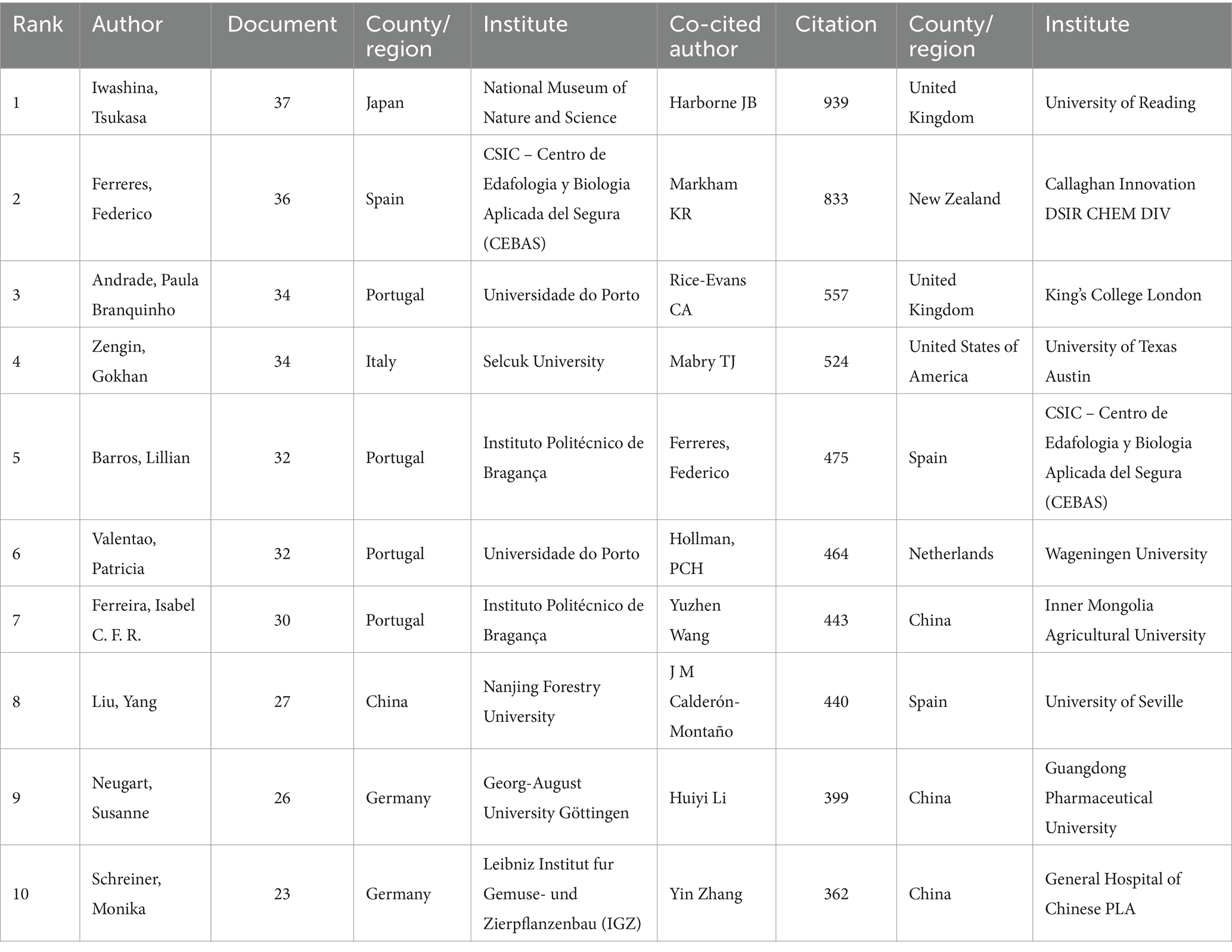
Table 4. Top 10 the most productive authors and co-cited authors of kaempferol’s global publications.
3.4 Highly co-cited references
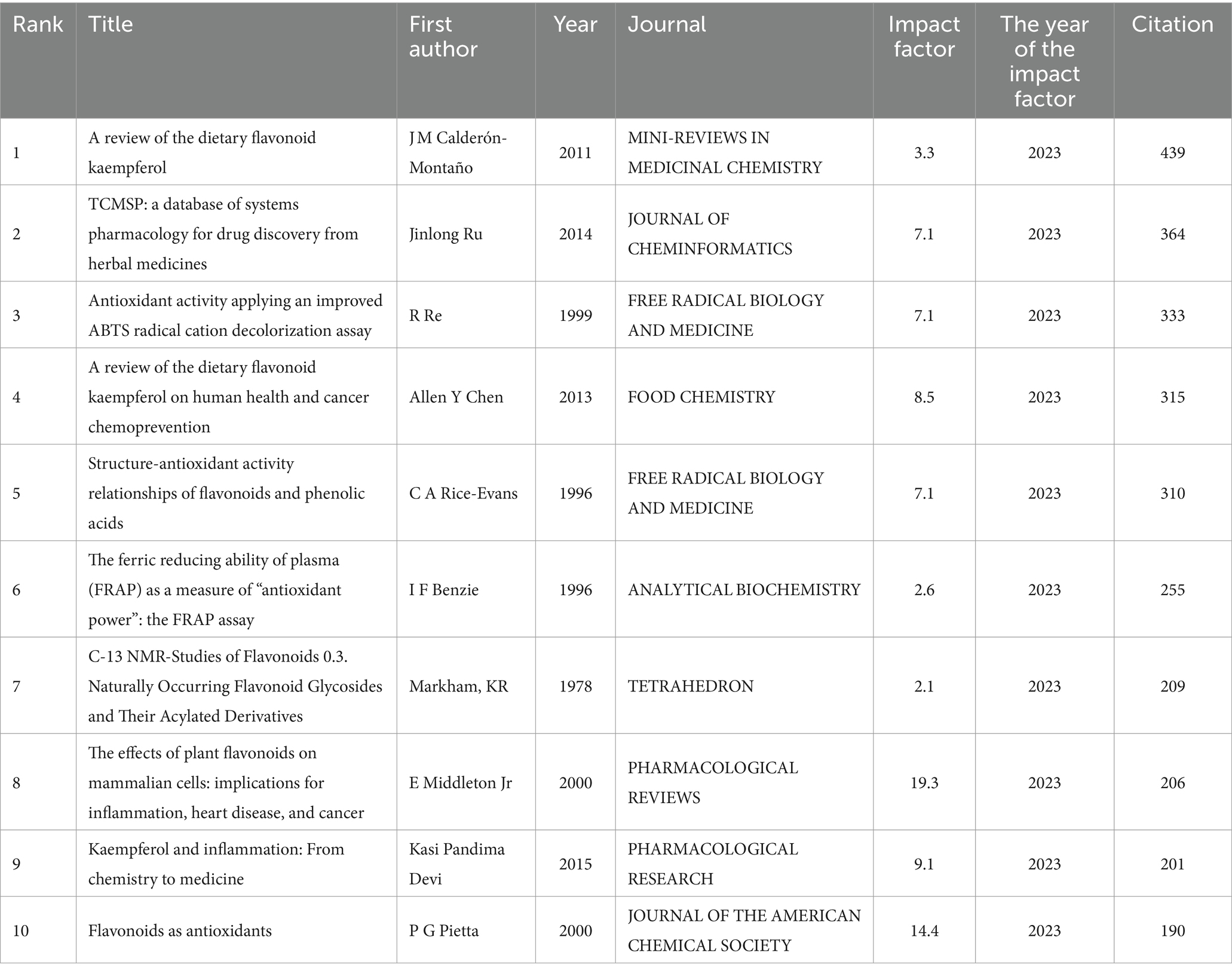
Co-cited references were used to study the internal connections between literature and depict scientific development’s dynamic structure. The results revealed that 1,621 co-cited references were closely related to the research topic of kaempferol. According to the citation analysis of documents, which reflected the citations of documents, the top 10 high citations are listed in Table 5. The work titled “A review on the dietary flavonoid kaempferol” by J M Calderón-Montaño had the most citations (439), followed by that titled “TCMSP: a database of systems pharmacology for drug discovery from herbal medicines” by Jinlong Ru (n = 364); “Antioxidant activity applying an improved ABTS radical cation decolorization assay” by R Re (n = 333); “A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention” by Allen Y Chen (n = 315), and “Structure-antioxidant activity relationships of flavonoids and phenolic acids” by C A Rice-Evans (n = 310).
3.5 Research hotspots analysis results
A co-occurrence analysis of keywords from global publications on kaempferol was conducted using CiteSpace Software. Keywords encapsulate the core topics of a publication and are instrumental for identifying research hotspots and directions in the field of kaempferol. Herein, the analysis yielded a total of 1,080 distinct keywords in the field of kaempferol research. Figure 4A presents a timeline plot of keyword co-occurrence clustering for global publications on kaempferol, as generated by CiteSpace. The results indicated that research hotspots and directions of kaempferol were mainly focused on “phenolic compounds,” “antioxidant activity,” “flavonoids,” etc. Besides, the keywords that suddenly increased included “NF-kappa B,” “inflammation,” “bioactive compounds,” “molecule docking,” “network pharmacology,” etc. Furthermore, CiteSpace was used to find clusters, and there were 31 clusters in total in the analysis results. The clusters were mainly identified in the research field related to keywords including “phenolic compounds,” “antioxidants capacity,” “breast cancer,” “flavonoid glycosides,” “response surface methodology,” “green tea,” “abiotic stress,” “sars-cov-2,” “coronary heart disease,” “antimicrobial activity,” “ionic liquids,” “network pharmacology,” “free radicals,” “antioxidant activity,” “kaempferol glycosides,” “comet assay,” “Alzheimer’s disease,” “activation,” “quercetin,” “in vitro,” “ginkgo biloba,” “inflammation,” “lipid peroxidation,” “flavonol glycosides,” “botanical origin,” “phenolic acids,” “ulcerative colitis,” “nitric oxide synthase,” “Dennstaedtiaceae,” “cultured myocardial cell,” “wgcna,” and “bacterial biofilms.” This cluster information provided potential directions and hotspots for further study regarding kaempferol.
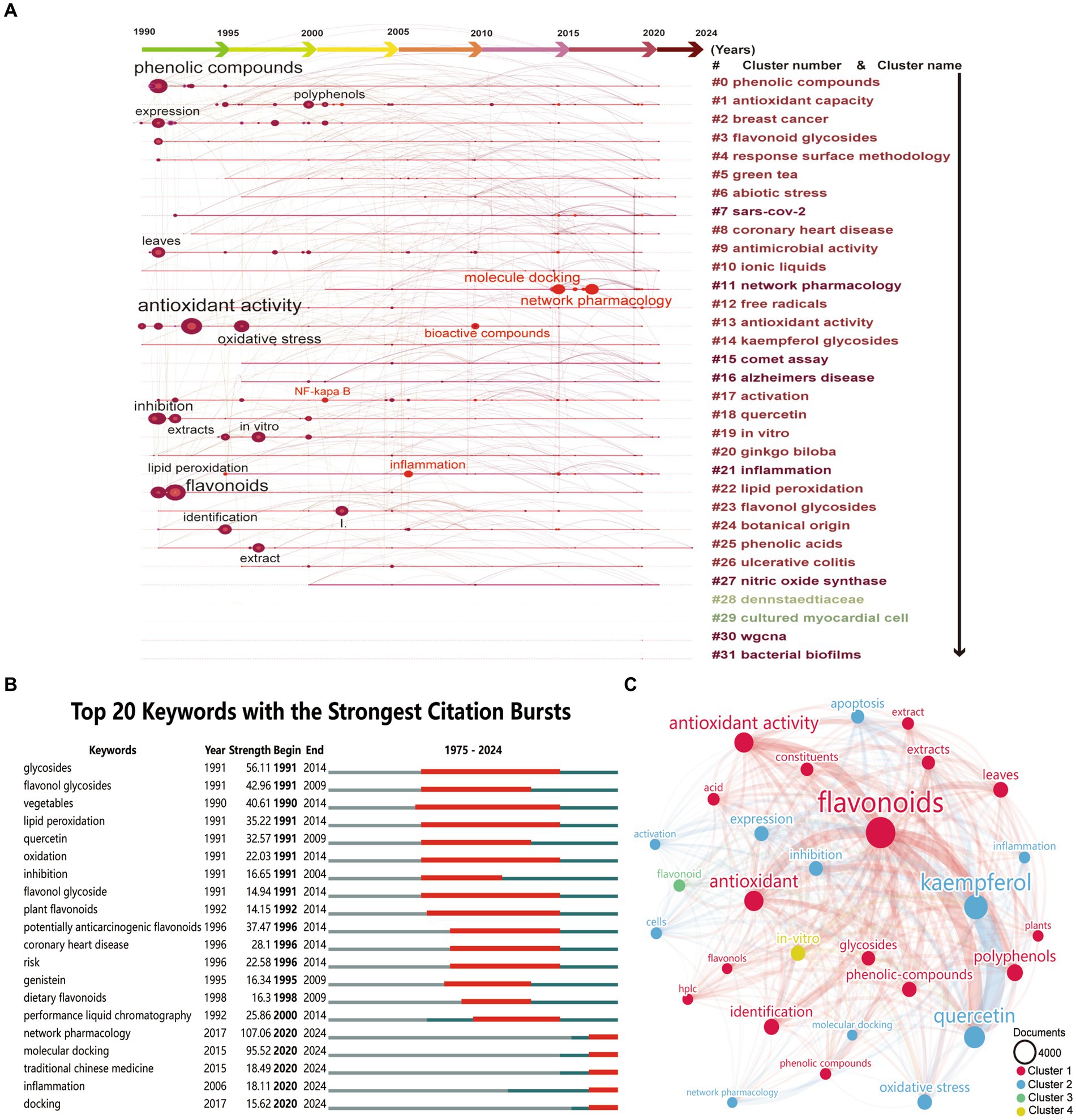
Figure 4. Co-occurrence analysis of keywords of kaempferol’s global publications. (A) Timeline plot of keywords co-occurrence clustering of kaempferol’s global publications by CiteSpace. The graph shows the time order from left to right, the results of keyword clustering from top to bottom, and the circles represent keywords. The larger the circle, the more times the keyword appears, and the bright red circle indicates a sudden increase in the keywords. (B) Top 20 keywords with the strongest citation bursts of kaempferol’s global publications by CiteSpace. ɣ: 1.0; minimum duration: 2. (C) Visual display of the top 30 keywords of kaempferol’s global publications. VOSviewer was used for analysis, the method was linlog/modularity, weight was documents, minimum number of occurrences of a keyword was set as 380, and visual display by Scimago Graphica software. The thickness of the lines indicates the strength of the relationship. The different colors of circle represented the difference clusters of keywords. The larger the circle and label, the more kaempferol’s global publications the keywords have occurred.
Figure 4B highlights the top 20 keywords with the most significant citation bursts(a great change of publications in a short period), with “network pharmacology” and “molecule docking” leading from 2020 to 2024 at 107.06 and 95.52, respectively. This indicated a growing scholarly focus on utilizing network pharmacology and molecular docking techniques to study kaempferol. Besides, the strongest citation bursts were observed in the following order: “glycosides,” 56.11 (from 1991 to 2014), “flavonol glycosides,” 42.96 (from 1991 to 2009), “vegetables,” 40.61 (from 1990 to 2014), “potentially anticarcinogenic flavonoids,” 37.47 (from 1996 to 2014), and “lipid peroxidation,” 35.22 (from 1991 to 2014), respectively. The sustained strong citation bursts of the top 20 keywords up to 2024 suggested that the field of kaempferol research would continue to attract a significant number of scholars.
VOSviewer was used for “all keywords” analysis. Figure 4C provides a visual representation of the top 30 keywords from global publications on kaempferol after establishing a minimum occurrence threshold of 380 for a keyword to be included. The size of the nodes corresponds to the occurrences; the lines suggest the connections between keywords, and the thickness of the lines indicates the strength of the connection between keywords. The institutes were roughly divided into four clusters based on the degree of occurrences, represented by different colors. Table 6 offers detailed information on the top 30 keywords from the global publications concerning kaempferol. These keywords could be divided into four different directions as follows: (1) keywords related to flavonoids, antioxidant, antioxidant activity, polyphenols, identification, leaves, phenolic-compounds, glycosides, extracts, constituents, acid, phenolic compounds, plants, HPLC, and flavonols; (2) keywords related to kaempferol, quercetin, oxidative stress, expression, inhibition, apoptosis, cells, inflammation, activation, molecular docking, and network pharmacology; (3) keywords related to flavonoid research; and (4) keywords related to in-vitro research.
4 Discussion
4.1 Distribution of kaempferol research
In this study, through the bibliometric and visual analysis of the literature knowledge map in the field of global research related to kaempferol, this study interpreted the research hotspot, research depth, and research frontier in the field of kaempferol, and explored the future development direction of kaempferol. In general, the introduction and application of new scientific and technological methods have enabled numerous renowned research teams to achieve significant advancements in various aspects of kaempferol, including its physical and chemical properties, extraction and preparation processes, and pharmacological mechanisms. In terms of publications, a total 11,214 documents related to the topic of kaempferol were obtained from the Web of Science Core Collection database until February 26, 2024, including articles (n = 10,746, 96%) and review articles (n = 468, 4%). The global annual number of kaempferol publications was more than 100 documents per year since 2000, and over 500 documents per year since 2018, which further exceeded 1,000 per year since 2022. This indicated that research on kaempferol remained a major focus in the scientific research. Among them, the total number of papers published in China was more than 3,054, accounting for the most documents of the total publication output. Especially since 2014, the number of publications had been rapidly increasing, indicating that research on kaempferol had received increasing attention from the Chinese in the past decade. Among the top 10 most co-cited authors, 4 were from China. However, there was still a problem of insufficient influence globally of China in the research field of kaempferol. As one of the earliest institutions to begin research on kaempferol, the United States of America was another country with more than 947 publications, presenting the highest centrality. Regarding cooperative relationships, frequent collaborations existed between countries/regions and institutions. For instance, Chinese academic institutions represented by Chinese Academy of Sciences, and Egyptian academic institutions represented by National Research Centre NRC, all maintained frequent collaborative relationships with other institutions. In addition, according to this bibliometric study, kaempferol papers mainly contributed to the categories of Food Science Technology, Pharmacology Pharmacy, Biochemistry Molecular Biology, Chemistry Medicinal, Plant Sciences, Chemistry Applied, Chemistry Multidisciplinary, Nutrition Dietetics, Chemistry Analytica, Integrative Complementary Medicine, etc., with at least 600 documents published per category. Our study revealed the hotspots, depth, and frontiers of kaempferol research in the world through bibliometrics and visualization analysis. It pointed out that although China has made significant progress in research quantity and cooperation, its international influence needs to be improved. At the same time, it pointed out that kaempferol research has made in-depth development in many fields such as food, pharmacology, and biochemistry.
4.2 Dietary benefits and pharmacological effects of kaempferol
The interest in medicinal plants for their therapeutic potential is expanding worldwide, with a significant portion of their health benefits being attributed to phenolic compounds, notably flavonoids (7). Reports have shown that kaempferol has a broad spectrum of preventative properties, including antioxidant, anticancer, and anti-inflammatory effects, making it beneficial in a daily human diet (50). The inhibition kinetics assay provides evidence that kaempferol can inhibit xanthine oxidase activity reversibly in a competitive manner, which is beneficial to prevent and treat hyperuricemia (51, 52). Besides, some studies have suggested that dietary kaempferol may exert the potential cardiovascular protection effects on human body (53). Kaempferol is also regarded as a dietary anti-inflammatory agent in preventing inflammation risks (54). In addition, studies have shown that supplementation of kaempferol in the diet of obese mice can increase lipid metabolism by down-regulating PPARγ and SREBP1c, reduce lipid accumulation in adipose tissue, and improve antioxidant defense abilities (55). Kaempferol serves as an excellent raw material for the processing of green health foods. Leveraging its bactericidal properties, it can be utilized as a preservative and freshness-retaining agent in the food industry (50). As health awareness increases, nutrition, safety, and health in diet have become key focuses in food development, with functional natural active ingredients gaining global attention. Kaempferol, being abundant in plants and recognized as safe and non-toxic, exhibits a range of biological activities and holds promising potential in the dietary sector.
Kaempferol, a naturally occurring polyphenolic compound, exhibits a wide range of significant pharmacological effects. Regarding the nervous system, kaempferol elicits neuroprotective effects by mitigating inflammation and oxidative stress triggered by microglial cell activation (56, 57). In the digestive realm, kaempferol boasts pronounced anti-inflammatory properties, inhibiting the synthesis and release of diverse inflammatory mediators, thereby diminishing digestive system inflammation and alleviating gastritis, enteritis, and other related symptoms (58, 59). Within the respiratory system, kaempferol showcases antiviral capabilities, inhibiting the proliferation of multiple viruses and contributing to the prevention and treatment of respiratory diseases, including viral colds and viral pneumonia (60, 61). In the circulatory system, kaempferol offers cardiovascular protection by employing mechanisms such as inhibiting apoptosis of vascular smooth muscle cells and modulating blood pressure and lipid profiles (62, 63). For the endocrine system, kaempferol possesses a therapeutic potential in diabetes management, attributed to its ability to regulate insulin secretion and improve insulin sensitivity (64). In the urinary system, kaempferol contributes to the prevention and treatment of urinary disorders by mitigating inflammatory responses and oxidative damage in organs like the kidneys (65). Within the immune system, kaempferol modulates the activity and functionality of immune cells, bolstering the body’s immune defenses and enhancing its resilience against diseases (66, 67). Furthermore, kaempferol demonstrates a broad spectrum of antitumor activity in the reproductive system, combating ovarian cancer, prostate cancer, and other malignancies through mechanisms that include inducing tumor cell apoptosis and inhibiting tumor angiogenesis (68–71). In conclusion, kaempferol’s extensive biological activities underscore its significant pharmacological effects in anti-inflammatory, antioxidant, antiviral, anti-cancer, cardiovascular protection, and neuroprotective protection firmly establishing a theoretical foundation for its utilization in health foods and pharmaceutical products.
4.3 Anti-inflammation and antioxidant activity of kaempferol
Recently, natural-sourced drugs are considered the therapeutic strategy for the treatment of inflammatory diseases (8, 72), including rheumatic arthritis, inflammatory bowel disease, periodontitis, etc., which are common diseases seriously harming people’s health. With the progress of modern medical technology, more and more researchers pay attention to the application of kaempferol in the treatment of inflammatory diseases (12). The pharmacological effects of kaempferol in inflammatory diseases are primarily realized through the inhibition of inflammatory factors, anti-oxidative actions, and the prevention of inflammatory cell infiltration (73). The inhibitory effect on inflammatory factors is the key to the anti-inflammatory effect of kaempferol. Besides, kaempferol also exerts anti-inflammatory effects by directly inhibiting the activity of epoxidase and shifting the metabolic pathway of arachidonic acid, thus inhibiting the production of inflammatory response (74, 75). Evidence has demonstrated that kaempferol achieves its anti-inflammatory effects by diminishing the attraction of inflammatory cells and their accumulation during inflammatory responses. It also reduces the expression of inflammation-related mediators such as IL-6, IL-1β, TNF-α, and MIP-2, and exerts inhibitory regulation on pattern recognition receptor Dectin-1 and the pro-inflammatory pathway p38 MAPK (76, 77). Furthermore, another major effect of kaempferol is its antioxidant effect. By directly eliminating the generation of free radicals, kaempferol protects cells from oxidative damage, thus inhibiting the inflammatory response and the further spread of cell damage (19). Generally, reactive oxygen species (ROS) are harmful to cells because their increased level promotes cascade reactions and causes oxidative damage. Kaempferol plays an antioxidant role by aiding the body in clearing the abnormal increased levels of ROS (78). The above researches suggested that kaempferol has the potential in treating inflammatory diseases such as rheumatoid arthritis and inflammatory bowel disease by inhibiting inflammatory factors, antioxidation, and preventing inflammatory cell infiltration.
4.4 Antiviral and antimicrobial activity of kaempferol
Currently, viral infection is the most serious health issue that causes an unexpected higher rate of death worldwide (79). The antiviral medicinal plants and the isolated bioactive compounds including kaempferol, apigenin, luteolin, etc., have been considered for further advanced investigations with the aim to develop effective and affordable antiviral drugs (61, 80, 81). Furthermore, kaempferol can also inhibit neuraminidase activities, and has a high inhibitory ability against influenza H1N1 and H9N2 viruses (82, 83). Evidence has demonstrated that kaempferol is effective against DNA viruses such as hepatitis B virus, African swine fever virus, and pseudorabies virus. Besides, kaempferol also performs well in fighting against RNA viruses, namely feline SARS coronavirus, dengue fever virus, etc. (84). Kaempferol is one of the most well-known flavonoids, and it can be found in a variety of herbal and plant families. Kaempferol and its related compounds have antibacterial and antifungal activities in addition to anti-inflammatory effects (85, 86). Meanwhile, kaempferol is also the most effective flavonoid for cell membrane destruction in E. coli tested in a study that objectified the findings by showing bacterial proteins leaking into the extracellular environment (87). Kaempferol can significantly inhibit the expression of IL-1β, IL-6, IL-10, TGF-β, TNF-α and other inflammatory factors in the intestinal tract of salmonella-infected chicks, and significantly enhance the expression levels of intestinal mucosal immune factors such as MUC1 and MUC2 (88, 89). Therefore, kaempferol has a certain protective effect on intestinal damage caused by salmonella enteritidis infection (90, 91). In addition, oral kaempferol significantly improves the survival rate of mice infected with klebsiella pneumoniae (92, 93). Kaempferol may serve as a significant antiviral and antimicrobial agent, holding substantial promise for the prevention and treatment of infectious diseases and thus possessing important research value. As a multifunctional flavonoid, kaempferol has shown great potential in the field of infectious disease prevention and treatment due to its multiple activities such as antiviral, antibacterial, antifungal, and immune regulation. In particular, as an effective and economical therapeutic drug candidate for viral and bacterial infections, it is worthy of further research and development.
4.5 Anticancer potential of kaempferol
Evidence has confirmed the anticancer effects of kaempferol in breast cancer, skin cancer, liver cancer, prostate cancer, colon cancer, among others (94). In recent years, an increasing body of evidence has suggested that kaempferol possesses anti-tumor effects through multiple mechanisms, such as interfering with the pericellular phase, inducing the death of the cell through various ways, inhibiting the formation of the tumor-blood vessel, stimulating the immune system of the live host, and acting as the superoxide dismutase in the cell (95, 96). Therefore, kaempferol holds a promising clinical application prospect in the prevention and treatment of tumors. Studies have also shown that kaempferol can inhibit the proliferation and induce apoptosis of breast cancer SK-BR-3 cells in vitro, which may be related to the regulation of Notch1 and Cleaved caspase-3 protein expression (96–99). Meanwhile, kaempferol can inhibit the proliferation of SGC-7901 cells and induce cell apoptosis by the apoptosis mechanisms of increasing the release level of intracellular ROS (100–102). Besides, kaempferol also performs well in inhibiting the maturation of dendritic cells induced by peripheral blood mononuclear cells in gastric cancer patients (103, 104). Furthermore, kaempferol may play an anti-colon cancer role by regulating the expression of MMP1, MMP2, and MMP9 protein, thereby increasing the expression level of miR-339-5p to mediate PKM alternative splicing reversing aerobic glycolysis in colon cancer cells (105–109). Moreover, kaempferol markedly suppresses HepG2 cell proliferation and combats liver cancer by increasing BAX and JUN protein expression and decreasing CDK1 protein levels (110, 111). In addition, kaempferol plays an anti-prostate role by inducing cycle arrest of prostate cancer cells, inducing apoptosis of prostate cancer cells, and inhibiting the proliferation of prostate cancer cells (71, 98). Briefly, kaempferol exhibits significant inhibitory effects on various cancers through multiple anticancer mechanisms, including interfering with the cell cycle, inducing apoptosis, inhibiting tumor angiogenesis, activating the immune system, and regulating gene expression. This suggests a broad clinical application prospect in cancer prevention and treatment, but more clinical studies are needed to confirm its efficacy.
4.6 Cardiovascular protection of kaempferol
Heart failure is a complex clinical syndrome characterized by the heart’s inability to pump enough blood into the body through structural and/or functional cardiac abnormalities (112). Studies have suggested that kaempferol has effects of anti-cardiomyocyte hypertrophy and interstitial fibrosis (113, 114). Kaempferol can reduce LPS-induced apoptosis of H9c2 cells in rat myocardium, possibly through the TLR4/NF-κB pathway (115). Cardiomyocytes injured by local anoxia/reoxygenation (A/R) can be treated with kaempferol. It has been observed that kaempferol can significantly enhance the expression of Bcl-2 via the SIRT1-mediated mitochondrial pathway, thereby exerting protective effects against apoptosis in cardiomyocytes induced by A/R injury (116, 117). Kaempferol can reduce the activity of CK, LDH and MDA, and increase the activity of SOD to protect cardiomyocytes from hypoxic injury (25, 113, 118). Research has indicated that by regulating the PI3K/AKT signaling pathway, kaempferol may attenuate atherosclerosis (119). Besides, kaempferol can significantly reduce blood pressure, inhibit Scr and BUN levels, improve renal function, alleviate renal fibrosis damage, inhibit intrarenal inflammation and oxidative stress, and possibly prevent hypertension and its target organ damage by inhibiting the TGF-β1/β-arrestin1 pathway (120). Kaempferol has also been found to have a protective effect on cardiovascular diseases through mechanisms such as inhibition of TNF-α production and activation of NF-κB; activation of Ca2+ − activated K+ channels; enhancement of NOS activity of endothelial cells by stimulating arterial dilation; and mitigation of oxidative stress (121, 122). Heart failure is a complex clinical syndrome, the core of which is the insufficient blood pumping capacity of the heart. Kaempferol exhibits significant protective effects on heart failure and related cardiovascular pathological processes through multiple pathways, such as anti-myocardial cell hypertrophy, fibrosis, and apoptosis. These findings not only reveal the extensive role of kaempferol in cardiovascular protection, but also provide scientific evidence for its potential as a low-risk treatment method, emphasizing its great prospects in clinical application. Despite the need for further clinical research to confirm kaempferol’s role in cardiovascular protection, it is still considered a potentially low-risk treatment and highlights the substance’s promising potential for clinical use.
4.7 Neuroprotective protection of kaempferol
Numerous studies have indicated that kaempferol possesses therapeutic effects for neurological diseases such as Alzheimer’s disease, Parkinson disease, major depressive disorder, anxiety disorders, neuropathic pain, etc. (123, 124). Besides, previous research has demonstrated kaempferol as a neuroprotective protection agent for secondary spinal cord injury by reducing oxidative stress and inflammatory response through the down-regulation of ROS dependent MAPKs- NF-κB and the pyroptosis signaling pathway (125). Meanwhile, studies have also suggested that kaempferol has the neuroprotective effect in reducing neuropathic pain by regulating the TLR4/NF-kappa B signaling pathway (126). In the oxygen–glucose deprivation/reperfusion (OGD/R)-induced neuronal injury mouse model, kaempferol provides neuronal protection from OGD/R-induced ferroptosis partly by activating the Nrf2/SLC7A11/GPX4 signaling pathway (127). Moreover, kaempferol has also been found to be a potent mitophagy modulator in stimulating neuronal health and brain homeostasis (128). Furthermore, kaempferol can protect the nervous system damage caused by oxidative stress by inhibiting oxidative stress. For example, kaempferol has been shown to significantly protect SH-SY5Y neuronal cells, mitigating damage to major neuron cells by reducing protease cleavage and nuclear apoptosis. Additionally, it significantly lowers levels of ROS and mitochondrial hydroxyl radicals (129–131). Collectively, evidence supports that kaempferol offers neuroprotective capabilities, positioning it as a candidate for development into a neuroprotective therapeutic agent. In summary, kaempferol exhibits significant therapeutic effects on various neurological diseases by reducing oxidative stress, regulating signaling pathways, and promoting mitochondrial health through multiple mechanisms, strengthening its scientific basis as a candidate for neuroprotective therapeutic agents.
4.8 Other biological activities of kaempferol
Kaempferol is also used to treat numerous acute or chronic diseases, including intervertebral disc degeneration, colitis, post-menopausal bone loss, acute lung injury, liver injury, obesity, diabetes, hypertrophic scar, among others (132, 133). Research has demonstrated that kaempferol may interact with some amino acid residues within the active site of alpha-glucosidase, thereby achieving the purpose of treating diabetes (134). Furthermore, kaempferol can improve insulin resistance in db/db mice by inhibiting NLPR3 inflammatory-mediated adipose tissue inflammation (135). In addition, as network pharmacology and molecular docking technologies develop and are applied, an increasing number of mechanisms by which Traditional Chinese Medicine (TCM) compounds treat diseases are being uncovered. Kaempferol is frequently identified as a key component in the material basis of these TCM formulae (136). A study explored the therapeutic effect of an anticancer traditional Chinese medicine (TCM) formula containing kaempferol on non-small cell lung cancer (NSCLC) based on the methodology of network pharmacology (137). Researchers first predicted the potential targets of kaempferol through network pharmacology databases and then constructed a compound-target-disease network by integrating relevant targets of NSCLC. Subsequently, the impact of kaempferol and its formula on the proliferation, migration, invasion, and apoptosis of NSCLC cells was verified through in vitro cellular experiments. Furthermore, scholars utilized network pharmacology to predict the potential targets of a kaempferol-containing TCM formula for the prevention and treatment of high-altitude pulmonary edema (HAPE), and the preventive and therapeutic effects were validated through animal experiments (138). Therefore, Through the network pharmacology method, researchers can systematically analyze the pharmacological mechanism of kaempferol in Chinese medicine compounds, and provide scientific basis for its optimization and expansion in clinical application. Evidence has also indicated that kaempferol can improve the oxidative and inflammatory damage of knee osteoarthritis rat chondrocytes by inhibiting the ROS/TXNIP pathway, thereby regulating the levels of oxidative markers and inflammatory factors (139). Furthermore, kaempferol can also increase the vitality and proliferation of rat preosteoclasts, and promote osteogenesis through the Wnt/β-catenin signaling pathway, which may be the mechanism of osteoporosis prevention and treatment (140, 141). Briefly, kaempferol not only shows potential in the treatment of various acute and chronic diseases, but also affects various physiological and pathological processes through complex molecular mechanisms. Its multi-pathway and multi-target characteristics play an important role in traditional Chinese medicine compound and modern pharmacological research, indicating a broad prospect for further exploration of kaempferol and its related applications in the future.
5 Conclusion
Kaempferol, a flavonoid compound, is widely present in natural plants such as fruits, vegetables, and traditional Chinese medicinal herbs, exhibiting diverse biological activities. Herein, information visualization techniques were employed to elucidate the research progress, hot topics, and frontiers in kaempferol. While the annual publication output in China has far exceeded that in the United States of America in recent years, its academic influence is still left far behind. In addition, scholars, institutions, and representative literature playing important roles in this field were hereby identified. Keyword analysis demonstrated the anti-inflammatory and antioxidant activity of kaempferol and its potential dietary benefits as the main research direction, with network pharmacology and molecular docking identified as the latest hot technical means. Given its prominent anti-inflammatory and antioxidant properties, research on kaempferol’s bioactivity has progressively uncovered and expanded into areas such as anti-viral capabilities (against influenza, novel coronavirus), cardiovascular protection (preventing myocardial ischemic injury, atherosclerosis, and hypertension, etc.), neuroprotection (against neuritis, Alzheimer’s disease, myelitis, etc.), and anti-diabetic effects. Kaempferol significantly reduces the risk of cancer, particularly in pancreatic cancer and colorectal adenoma, indicating its potential application value in cancer prevention and treatment, and providing crucial clues for the future development of novel anticancer drugs. Furthermore, its anti-inflammatory and antibacterial properties make kaempferol a promising candidate for the treatment of inflammatory and infectious diseases, potentially helping to mitigate overuse of antibiotics and issues related to antibiotic resistance. Kaempferol’s antioxidant and antiviral characteristics aid in protecting cells from oxidative damage and viral infections, thereby playing a significant role in the prevention and treatment of related diseases. Additionally, kaempferol regulates blood sugar and lipid levels, contributing to the prevention and treatment of metabolic diseases like diabetes and atherosclerosis. These discoveries offer new perspectives for the development of novel hypoglycemic and hypolipidemic drugs, thus improving the global prevention and control of metabolic diseases. Consequently, future research directions for kaempferol can be explored from the following aspects: (1) Further elucidating the specific mechanisms of kaempferol in different types of cancer, particularly its synergistic effects with other anticancer drugs, to provide a scientific basis for the development of novel anticancer drugs. (2) Investigating the potential applications of kaempferol in other fields such as inflammatory diseases, infectious diseases, and metabolic disorders, thereby expanding its clinical application scope. (3) Conducting a comprehensive toxicity assessment of kaempferol to ensure its safety in clinical use. Simultaneously, studying its metabolic differences and pharmacokinetic characteristics across different populations will provide a basis for individualized medication. To this end, the dietary advantages and multiple pharmacological effects of kaempferol underscore its clinical significance and highlight its crucial role in disease prevention and treatment.
6 Limitation
Despite the findings, the present bibliometric analysis only included publications in the Web of Science Core Collection (WoSCC) database, overlooking other databases such as PubMed, Cochrane library, and Google Scholar. However, it should be noted that WoSCC is widely recognized as one of the most authoritative scientific literature search platforms, covering the vast majority of research on kaempferol and maintaining a certain degree of representativeness.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
RT: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing, Data curation, Formal analysis. LL: Methodology, Writing – review & editing, Data curation. YL: Methodology, Writing – review & editing, Formal analysis. HL: Writing – review & editing, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The Fundamental Research Funds for the Central Public Welfare Research Institutes (No. ZXKT24006 and No. ZZ17-XRZ-089).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Liu, RH. Health-promoting components of fruits and vegetables in the diet. Adv Nutr. (2013) 4:384s–92s. doi: 10.3945/an.112.003517
2. Shen, N, Wang, T, Gan, Q, Liu, S, Wang, L, and Jin, B. Plant flavonoids: classification, distribution, biosynthesis, and antioxidant activity. Food Chem. (2022) 383:132531. doi: 10.1016/j.foodchem.2022.132531
3. Masilamani, M, Wei, J, and Sampson, HA. Regulation of the immune response by soybean isoflavones. Immunol Res. (2012) 54:95–110. doi: 10.1007/s12026-012-8331-5
4. Mathew, B, Parambi, DGT, Sivasankarapillai, VS, Uddin, MS, Suresh, J, Mathew, GE, et al. Perspective Design of Chalcones for the management of CNS disorders: a Mini-review. CNS Neurol Disord Drug Targets. (2019) 18:432–45. doi: 10.2174/1871527318666190610111246
5. Dias, MC, Pinto, D, and Silva, AMS. Plant flavonoids: chemical characteristics and biological activity. Molecules. (2021) 26:5377. doi: 10.3390/molecules26175377
6. Wen, K, Fang, X, Yang, J, Yao, Y, Nandakumar, KS, Salem, ML, et al. Recent research on flavonoids and their biomedical applications. Curr Med Chem. (2021) 28:1042–66. doi: 10.2174/0929867327666200713184138
7. Bangar, SP, Chaudhary, V, Sharma, N, Bansal, V, Ozogul, F, and Lorenzo, JM. Kaempferol: a flavonoid with wider biological activities and its applications. Crit Rev Food Sci Nutr. (2023) 63:9580–604. doi: 10.1080/10408398.2022.2067121
8. Devi, KP, Malar, DS, Nabavi, SF, Sureda, A, Xiao, J, Nabavi, SM, et al. Kaempferol and inflammation: from chemistry to medicine. Pharmacol Res. (2015) 99:1–10. doi: 10.1016/j.phrs.2015.05.002
9. Felice, MR, Maugeri, A, De Sarro, G, Navarra, M, and Barreca, D. Molecular pathways involved in the anti-Cancer activity of Flavonols: a focus on Myricetin and Kaempferol. Int J Mol Sci. (2022) 23:4411. doi: 10.3390/ijms23084411
10. Kooti, W, and Daraei, N. A review of the antioxidant activity of celery (Apium graveolens L). J Evid Based Complementary Altern Med. (2017) 22:1029–34. doi: 10.1177/2156587217717415
11. Rahul,, and Siddique, YH. Neurodegenerative diseases and flavonoids: special reference to Kaempferol. CNS Neurol Disord Drug Targets. (2021) 20:327–42. doi: 10.2174/1871527320666210129122033
12. Rajendran, P, Rengarajan, T, Nandakumar, N, Palaniswami, R, Nishigaki, Y, and Nishigaki, I. Kaempferol, a potential cytostatic and cure for inflammatory disorders. Eur J Med Chem. (2014) 86:103–12. doi: 10.1016/j.ejmech.2014.08.011
13. Cid-Ortega, S, and Monroy-Rivera, JA. Extraction of Kaempferol and its glycosides using supercritical fluids from plant sources: a review. Food Technol Biotechnol. (2018) 56:480–93. doi: 10.17113/ftb.56.04.18.5870
14. Syed, DN, Adhami, VM, Khan, MI, and Mukhtar, H. Inhibition of Akt/mTOR signaling by the dietary flavonoid fisetin. Anti Cancer Agents Med Chem. (2013) 13:995–1001. doi: 10.2174/18715206113139990129
15. Orhan, I, Küpeli, E, Terzioğlu, S, and Yesilada, E. Bioassay-guided isolation of kaempferol-3-O-beta-D-galactoside with anti-inflammatory and antinociceptive activity from the aerial part of Calluna vulgaris L. J Ethnopharmacol. (2007) 114:32–7. doi: 10.1016/j.jep.2007.06.017
16. Tucak, M, Horvat, D, Cupic, T, Krizmanic, G, Tomas, V, Ravlic, M, et al. Forage legumes as sources of bioactive phytoestrogens for use in pharmaceutics: a review. Curr Pharm Biotechnol. (2018) 19:537–44. doi: 10.2174/1389201019666180730165917
17. Jin, S, Zhang, L, and Wang, L. Kaempferol, a potential neuroprotective agent in neurodegenerative diseases: from chemistry to medicine. Biomed Pharmacother. (2023) 165:115215. doi: 10.1016/j.biopha.2023.115215
18. Jeyaraj, EJ, Lim, YY, and Choo, WS. Extraction methods of butterfly pea (Clitoria ternatea) flower and biological activities of its phytochemicals. J Food Sci Technol. (2021) 58:2054–67. doi: 10.1007/s13197-020-04745-3
19. Chagas, M, Behrens, MD, Moragas-Tellis, CJ, Penedo, GXM, Silva, AR, and Gonçalves-de-Albuquerque, CF. Flavonols and flavones as potential anti-inflammatory, antioxidant, and antibacterial compounds. Oxidative Med Cell Longev. (2022) 2022:9966750. doi: 10.1155/2022/9966750
20. Devkota, HP, Paudel, KR, Khanal, S, Baral, A, Panth, N, Adhikari-Devkota, A, et al. Stinging nettle (Urtica dioica L.): nutritional composition, bioactive compounds, and food functional properties. Molecules. (2022) 27:5219. doi: 10.3390/molecules27165219
21. Almatroudi, A, Allemailem, KS, Alwanian, WM, Alharbi, BF, Alrumaihi, F, Khan, AA, et al. Effects and mechanisms of Kaempferol in the Management of Cancers through modulation of inflammation and signal transduction pathways. Int J Mol Sci. (2023) 24:8630. doi: 10.3390/ijms24108630
22. Amjad, E, Sokouti, B, and Asnaashari, S. A systematic review of anti-cancer roles and mechanisms of kaempferol as a natural compound. Cancer Cell Int. (2022) 22:260. doi: 10.1186/s12935-022-02673-0
23. Alshehri, AS, El-Kott, AF, Eleawa, SM, El-Gerbed, MSA, Khalifa, HS, El-Kenawy, AE, et al. Kaempferol protects against streptozotocin-induced diabetic cardiomyopathy in rats by a hypoglycemic effect and upregulating SIRT1. J Physiol Pharmacol. (2021) 339:355. doi: 10.26402/jpp.2021.3.04
24. Sheng, H, Zhang, D, Zhang, J, Zhang, Y, Lu, Z, Mao, W, et al. Kaempferol attenuated diabetic nephropathy by reducing apoptosis and promoting autophagy through AMPK/mTOR pathways. Front Med. (2022) 9:986825. doi: 10.3389/fmed.2022.986825
25. Vishwakarma, A, Singh, TU, Rungsung, S, Kumar, T, Kandasamy, A, Parida, S, et al. Effect of Kaempferol pretreatment on myocardial injury in rats. Cardiovasc Toxicol. (2018) 18:312–28. doi: 10.1007/s12012-018-9443-5
26. Wu, X, Li, X, Yang, C, and Diao, Y. Target characterization of Kaempferol against myocardial infarction using novel in silico docking and DARTS prediction strategy. Int J Mol Sci. (2021) 22:12908. doi: 10.3390/ijms222312908
27. Knekt, P, Kumpulainen, J, Järvinen, R, Rissanen, H, Heliövaara, M, Reunanen, A, et al. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr. (2002) 76:560–8. doi: 10.1093/ajcn/76.3.560
28. Firoz, A, and Talwar, P. COVID-19 and retinal degenerative diseases: promising link "Kaempferol". Curr Opin Pharmacol. (2022) 64:102231. doi: 10.1016/j.coph.2022.102231
29. Ge, Y, Chao, T, Sun, J, Liu, W, Chen, Y, and Wang, C. Frontiers and hotspots evolution in psycho-cardiology: a bibliometric analysis from 2004 to 2022. Curr Probl Cardiol. (2022) 47:101361. doi: 10.1016/j.cpcardiol.2022.101361
30. Park, E, and Kim, WH. A retrospective literature review of eating disorder research (1990–2021): application of Bibliometrics and topical trends. Int J Environ Res Public Health. (2022) 19:7710. doi: 10.3390/ijerph19137710
31. Chen, C. A glimpse of the first eight months of the COVID-19 literature on Microsoft academic graph: themes, citation contexts, and uncertainties. Front Res Metr Anal. (2020) 5:607286. doi: 10.3389/frma.2020.607286
32. Synnestvedt, MB, Chen, C, and Holmes, JH. CiteSpace II: visualization and knowledge discovery in bibliographic databases. AMIA Annu Symp Proc. (2005) 2005:724–8.
33. Lin, J, Ling, F, Huang, P, Chen, M, Song, M, Lu, K, et al. The development of GABAergic network in depression in recent 17 years: a visual analysis based on CiteSpace and VOSviewer. Front Psych. (2022) 13:874137. doi: 10.3389/fpsyt.2022.874137
34. Liu, M, Li, W, Qiao, W, Liang, L, and Wang, Z. Knowledge domain and emerging trends in HIV-MTB co-infection from 2017 to 2022: a scientometric analysis based on VOSviewer and CiteSpace. Front Public Health. (2023) 11:1044426. doi: 10.3389/fpubh.2023.1044426
35. Zhang, Y, Yan, L, Wang, Z, Li, F, Lv, J, Liu, J, et al. Bibliometric analysis of global research on tumor dormancy. Cancers. (2023) 15:5691. doi: 10.3390/cancers15235691
36. Wang, M, Xie, X, Zhao, S, Han, W, and Zhang, Y. Global research trends and hotspots of fecal microbiota transplantation: a bibliometric and visualization study. Front Microbiol. (2022) 13:990800. doi: 10.3389/fmicb.2022.990800
37. Arruda, H, Silva, ER, Lessa, M, Proença, D Jr, and Bartholo, R. VOSviewer and Bibliometrix. J Med Libr Assoc. (2022) 110:392–5. doi: 10.5195/jmla.2022.1434
38. Fu, R, Xu, H, Lai, Y, Sun, X, Zhu, Z, Zang, H, et al. A VOSviewer-based bibliometric analysis of prescription refills. Front Med. (2022) 9:856420. doi: 10.3389/fmed.2022.856420
39. Zhong, D, Li, Y, Huang, Y, Hong, X, Li, J, and Jin, R. Molecular mechanisms of exercise on Cancer: a Bibliometrics study and visualization analysis via CiteSpace. Front Mol Biosci. (2021) 8:797902. doi: 10.3389/fmolb.2021.797902
40. Liu, S, Sun, YP, Gao, XL, and Sui, Y. Knowledge domain and emerging trends in Alzheimer's disease: a scientometric review based on CiteSpace analysis. Neural Regen Res. (2019) 14:1643–50. doi: 10.4103/1673-5374.255995
41. Chen, Y, Li, Y, Guo, L, Hong, J, Zhao, W, Hu, X, et al. Bibliometric analysis of the Inflammasome and Pyroptosis in brain. Front Pharmacol. (2020) 11:626502. doi: 10.3389/fphar.2020.608247
42. Zhang, K, Liu, F, Zhang, H, Duan, Y, Luo, J, Sun, X, et al. Trends in phytoremediation of heavy metals-contaminated soils: a web of science and CiteSpace bibliometric analysis. Chemosphere. (2024) 352:141293. doi: 10.1016/j.chemosphere.2024.141293
43. Liu, X, Yuan, J, Feng, Y, Zhang, Z, Tang, L, and Chen, H. Knowledge graph and development hotspots of biochar as an emerging aquatic antibiotic remediator: a scientometric exploration based on VOSviewer and CiteSpace. J Environ Manag. (2024) 360:121165. doi: 10.1016/j.jenvman.2024.121165
44. Yuan, WC, Zhang, JX, Chen, HB, Yuan, Y, Zhuang, YP, Zhou, HL, et al. A bibliometric and visual analysis of cancer-associated fibroblasts. Front Immunol. (2023) 14:1323115. doi: 10.3389/fimmu.2023.1323115
45. van Eck, NJ, and Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. (2010) 84:523–38. doi: 10.1007/s11192-009-0146-3
46. Li, XH, Li, Y, Jia, ZX, Li, JL, Liu, YX, and Yang, JG. Frontiers of monkeypox research: An analysis from the top 100 most influential articles in the field. Heliyon. (2023) 9:e20566. doi: 10.1016/j.heliyon.2023.e20566
47. Wu, S, Wen, S, An, K, Xiong, L, Zeng, H, Niu, Y, et al. Bibliometric analysis of global research trends between gut microbiota and pancreatic cancer: from 2004 to 2023. Front Microbiol. (2023) 14:1281451. doi: 10.3389/fmicb.2023.1281451
48. Hassan-Montero, Y, De-Moya-Anegón, F, and Guerrero-Bote, VP. SCImago Graphica: a new tool for exploring and visually communicating data. Profesional de la Informacion. (2022) 5:e310502. doi: 10.3145/epi.2022.sep.02
49. Chen, C, Hu, Z, Liu, S, and Tseng, H. Emerging trends in regenerative medicine: a scientometric analysis in CiteSpace. Expert Opin Biol Ther. (2012) 12:593–608. doi: 10.1517/14712598.2012.674507
50. Imran, M, Rauf, A, Shah, ZA, Saeed, F, Imran, A, Arshad, MU, et al. Chemo-preventive and therapeutic effect of the dietary flavonoid kaempferol: a comprehensive review. Phytother Res. (2019) 33:263–75. doi: 10.1002/ptr.6227
51. Wang, YJ, Zhang, GW, Pan, JH, and Gong, DM. Novel insights into the inhibitory mechanism of Kaempferol on xanthine oxidase. J Agric Food Chem. (2015) 63:526–34. doi: 10.1021/jf505584m
52. Li, J, Gong, YH, Li, JW, and Fan, LP. In vitro inhibitory effects of polyphenols from Tartary buckwheat on xanthine oxidase: identification, inhibitory activity, and action mechanism. Food Chem. (2022) 379:132100. doi: 10.1016/j.foodchem.2022.132100
53. Dabeek, WM, and Marra, MV. Dietary quercetin and Kaempferol: bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients. (2019) 11:2288. doi: 10.3390/nu11102288
54. Alam, W, Khan, H, Shah, MA, Cauli, O, and Saso, L. Kaempferol as a dietary anti-inflammatory agent: current therapeutic standing. Molecules. (2020) 25:4073. doi: 10.3390/molecules25184073
55. Zang, Y, Zhang, L, Igarashi, K, and Yu, C. The anti-obesity and anti-diabetic effects of kaempferol glycosides from unripe soybean leaves in high-fat-diet mice. Food Funct. (2015) 6:834–41. doi: 10.1039/C4FO00844H
56. Chen, L, and Zhuang, K. Kaempferol counteracts bupivacaine-induced neurotoxicity in mouse dorsal root ganglia neurons by regulating TRAF6-dependent NF-κB signaling. Kaohsiung J Med Sci. (2023) 39:710–7. doi: 10.1002/kjm2.12682
57. Dong, X, Zhou, S, and Nao, J. Kaempferol as a therapeutic agent in Alzheimer's disease: evidence from preclinical studies. Ageing Res Rev. (2023) 87:101910. doi: 10.1016/j.arr.2023.101910
58. Barber, E, Houghton, MJ, and Williamson, G. Flavonoids as human intestinal α-glucosidase inhibitors. Food Secur. (2021) 10:1939. doi: 10.3390/foods10081939
59. Qu, Y, Li, X, Xu, F, Zhao, S, Wu, X, Wang, Y, et al. Kaempferol alleviates murine experimental colitis by restoring gut microbiota and inhibiting the LPS-TLR4-NF-κB Axis. Front Immunol. (2021) 12:679897. doi: 10.3389/fimmu.2021.679897
60. Gao, J, Cao, C, Shi, M, Hong, S, Guo, S, Li, J, et al. Kaempferol inhibits SARS-CoV-2 invasion by impairing heptad repeats-mediated viral fusion. Phytomedicine. (2023) 118:154942. doi: 10.1016/j.phymed.2023.154942
61. Hao, K, Wang, Y, Xu, JH, Nie, C, Song, S, Yu, F, et al. Kaempferol is a novel antiviral agent against channel catfish virus infection through blocking viral attachment and penetration in vitro. Front Vet Sci. (2023) 10:1323646. doi: 10.3389/fvets.2023.1323646
62. Chen, M, Xiao, J, El-Seedi, HR, Woźniak, KS, Daglia, M, Little, PJ, et al. Kaempferol and atherosclerosis: from mechanism to medicine. Crit Rev Food Sci Nutr. (2024) 64:2157–75. doi: 10.1080/10408398.2022.2121261
63. Maneesai, P, Potue, P, Khamseekaew, J, Sangartit, W, Rattanakanokchai, S, Poasakate, A, et al. Kaempferol protects against cardiovascular abnormalities induced by nitric oxide deficiency in rats by suppressing the TNF-α pathway. Eur J Pharmacol. (2023) 960:176112. doi: 10.1016/j.ejphar.2023.176112
64. Alshehri, AS. Kaempferol attenuates diabetic nephropathy in streptozotocin-induced diabetic rats by a hypoglycaemic effect and concomitant activation of the Nrf-2/ho-1/antioxidants axis. Arch Physiol Biochem. (2023) 129:984–97. doi: 10.1080/13813455.2021.1890129
65. Yuan, P, Sun, X, Liu, X, Hutterer, G, Pummer, K, Hager, B, et al. Kaempferol alleviates calcium oxalate crystal-induced renal injury and crystal deposition via regulation of the AR/NOX2 signaling pathway. Phytomedicine. (2021) 86:153555. doi: 10.1016/j.phymed.2021.153555
66. Albalawi, FE, Alsharif, I, Moawadh, MS, Alkhoshaiban, A, Falah Alshehri, F, Albalawi, AE, et al. Immunomodulatory effects of Kaempferol on microglial and macrophage cells during the progression of diabetic retinopathy. Int Immunopharmacol. (2024) 133:112021. doi: 10.1016/j.intimp.2024.112021
67. Guan, M, Xu, W, Bai, H, Geng, Z, Yu, Z, Li, H, et al. Potential mechanisms underlying inhibition of xenograft lung cancer models by kaempferol: modulation of gut microbiota in activating immune cell function. J Cancer. (2024) 15:1314–27. doi: 10.7150/jca.88038
68. El-Kott, AF, Shati, AA, Al-Kahtani, MA, and Alharbi, SA. Kaempferol induces cell death in A2780 ovarian Cancer cells and increases their sensitivity to cisplatin by activation of cytotoxic endoplasmic reticulum-mediated autophagy and inhibition of protein kinase B. Folia Biol (Praha). (2020) 66:36–46. doi: 10.14712/fb2020066010036
69. Harrath, AH, Jalouli, M, Oueslati, MH, Farah, MA, Feriani, A, Aldahmash, W, et al. The flavonoid, kaempferol-3-O-apiofuranosyl-7-O-rhamnopyranosyl, as a potential therapeutic agent for breast cancer with a promoting effect on ovarian function. Phytother Res. (2021) 35:6170–80. doi: 10.1002/ptr.7067
70. Lee, MJ, Cho, Y, Hwang, Y, Jo, Y, Kim, YG, Lee, SH, et al. Kaempferol alleviates mitochondrial damage by reducing mitochondrial reactive oxygen species production in lipopolysaccharide-induced prostate organoids. Food Secur. (2023) 12:3836. doi: 10.3390/foods12203836
71. Zhou, Q, Fang, G, Pang, Y, and Wang, X. Combination of Kaempferol and docetaxel induces autophagy in prostate Cancer cells in vitro and in vivo. Int J Mol Sci. (2023) 24:17219. doi: 10.3390/ijms242417219
72. Tian, CL, Liu, X, Chang, Y, Wang, RX, Lv, TM, Cui, CC, et al. Investigation of the anti-inflammatory and antioxidant activities of luteolin, kaempferol, apigenin and quercetin. S Afr J Bot. (2021) 137:257–64. doi: 10.1016/j.sajb.2020.10.022
73. Zhu, L, Wang, P, Yuan, W, and Zhu, G. Kaempferol inhibited bovine herpesvirus 1 replication and LPS-induced inflammatory response. Acta Virol. (2018) 62:220–5. doi: 10.4149/av_2018_206
74. Dong, Y, Liu, Y, Tang, J, Du, J, Zhuang, X, Tan, S, et al. Zhisou powder displays therapeutic effect on chronic bronchitis through inhibiting PI3K/Akt/HIF-1α/VEGFA signaling pathway and reprograming metabolic pathway of arachidonic acid. J Ethnopharmacol. (2024) 319:117110. doi: 10.1016/j.jep.2023.117110
75. Kim, MJ, Song, YR, Kim, YE, Bae, SJ, Lee, WY, Bak, SB, et al. Kaempferol stimulation of autophagy regulates the ferroptosis under the oxidative stress as mediated with AMP-activated protein kinase. Free Radic Biol Med. (2023) 208:630–42. doi: 10.1016/j.freeradbiomed.2023.09.008
76. Yu, X, Wu, Q, Ren, Z, Chen, B, Wang, D, Yuan, T, et al. Kaempferol attenuates wear particle-induced inflammatory osteolysis via JNK and p38-MAPK signaling pathways. J Ethnopharmacol. (2024) 318:117019. doi: 10.1016/j.jep.2023.117019
77. Zhu, X, Wang, X, Ying, T, Li, X, Tang, Y, Wang, Y, et al. Kaempferol alleviates the inflammatory response and stabilizes the pulmonary vascular endothelial barrier in LPS-induced sepsis through regulating the SphK1/S1P signaling pathway. Chem Biol Interact. (2022) 368:110221. doi: 10.1016/j.cbi.2022.110221
78. Sharma, N, Biswas, S, Al-Dayan, N, Alhegaili, AS, and Sarwat, M. Antioxidant role of Kaempferol in prevention of hepatocellular carcinoma. Antioxidants (Basel). (2021) 10:1419. doi: 10.3390/antiox10091419
79. Zannella, C, Giugliano, R, Chianese, A, Buonocore, C, Vitale, GA, Sanna, G, et al. Antiviral activity of Vitis vinifera leaf extract against SARS-CoV-2 and HSV-1. Viruses. (2021) 13:1263. doi: 10.3390/v13071263
80. Bachar, SC, Mazumder, K, Bachar, R, Aktar, A, and Al Mahtab, M. A review of medicinal plants with antiviral activity available in Bangladesh and mechanistic insight into their bioactive metabolites on SARS-CoV-2, HIV and HBV. Front Pharmacol. (2021) 12:732891. doi: 10.3389/fphar.2021.732891
81. Behbahani, M, Sayedipour, S, Pourazar, A, and Shanehsazzadeh, M. In vitro anti-HIV-1 activities of kaempferol and kaempferol-7-O-glucoside isolated from Securigera securidaca. Res Pharm Sci. (2014) 9:463–9.
82. Arabyan, E, Hakobyan, A, Hakobyan, T, Grigoryan, R, Izmailyan, R, Avetisyan, A, et al. Flavonoid library screening reveals Kaempferol as a potential antiviral agent against African swine fever virus. Front Microbiol. (2021) 12:736780. doi: 10.3389/fmicb.2021.736780
83. Li, L, Wang, R, Hu, H, Chen, X, Yin, Z, Liang, X, et al. The antiviral activity of kaempferol against pseudorabies virus in mice. BMC Vet Res. (2021) 17:247. doi: 10.1186/s12917-021-02953-3
84. Periferakis, A, Periferakis, AT, Troumpata, L, Periferakis, K, Scheau, AE, Savulescu-Fiedler, I, et al. Kaempferol: a review of current evidence of its antiviral potential. Int J Mol Sci. (2023) 24:16299. doi: 10.3390/ijms242216299
85. Wu, M, Gong, DC, Yang, Q, Zhang, MQ, Mei, YZ, and Dai, CC. Activation of Naringenin and Kaempferol through pathway refactoring in the endophyte Phomopsis Liquidambaris. ACS Synth Biol. (2021) 10:2030–9. doi: 10.1021/acssynbio.1c00205
86. Yang, R, Li, J, Wang, J, Wang, Y, Ma, F, Zhai, R, et al. Kaempferol inhibits the growth of Helicobacter pylori in a manner distinct from antibiotics. J Food Biochem. (2022) 46:e14210. doi: 10.1111/jfbc.14210
87. Periferakis, A, Periferakis, K, Badarau, IA, Petran, EM, Popa, DC, Caruntu, A, et al. Kaempferol: antimicrobial properties, sources, clinical, and traditional applications. Int J Mol Sci. (2022) 23:15054. doi: 10.3390/ijms232315054
88. Elgorashi, E, van Heerden, F, and van Staden, J. Kaempferol, a mutagenic flavonol from Helichrysum simillimum. Hum Exp Toxicol. (2008) 27:845–9. doi: 10.1177/0960327108099532
89. Francis, AR, Shetty, TK, and Bhattacharya, RK. Modifying role of dietary factors on the mutagenicity of aflatoxin B1: in vitro effect of plant flavonoids. Mutat Res. (1989) 222:393–401. doi: 10.1016/0165-1218(89)90114-6
90. Sugimura, T, and Nagao, M. Mutagenic factors in cooked foods. CRC Crit Rev Toxicol. (1979) 6:189–209. doi: 10.3109/10408447909037483
91. Topcagic, A, Cavar Zeljkovic, S, Karalija, E, Galijasevic, S, and Sofic, E. Evaluation of phenolic profile, enzyme inhibitory and antimicrobial activities of Nigella sativa L. seed extracts. Bosn J Basic Med Sci. (2017) 17:286–94. doi: 10.17305/bjbms.2017.2049
92. Sun, Z, Li, Q, Hou, R, Sun, H, Tang, Q, Wang, H, et al. Kaempferol-3-O-glucorhamnoside inhibits inflammatory responses via MAPK and NF-κB pathways in vitro and in vivo. Toxicol Appl Pharmacol. (2019) 364:22–8. doi: 10.1016/j.taap.2018.12.008
93. Wang, Z, Ding, Z, Li, Z, Ding, Y, Jiang, F, and Liu, J. Antioxidant and antibacterial study of 10 flavonoids revealed rutin as a potential antibiofilm agent in Klebsiella pneumoniae strains isolated from hospitalized patients. Microb Pathog. (2021) 159:105121. doi: 10.1016/j.micpath.2021.105121
94. Imran, M, Salehi, B, Sharifi-Rad, J, Gondal, TA, Saeed, F, Imran, A, et al. Kaempferol: a key emphasis to its anticancer potential. Molecules. (2019) 24:2277. doi: 10.3390/molecules24122277
95. Kashyap, D, Sharma, A, Tuli, HS, Sak, K, Punia, S, and Mukherjee, TK. Kaempferol – a dietary anticancer molecule with multiple mechanisms of action: recent trends and advancements. J Funct Foods. (2017) 30:203–19. doi: 10.1016/j.jff.2017.01.022
96. Wang, X, Yang, Y, An, Y, and Fang, G. The mechanism of anticancer action and potential clinical use of kaempferol in the treatment of breast cancer. Biomed Pharmacother. (2019) 117:109086. doi: 10.1016/j.biopha.2019.109086
97. Nandi, SK, Chatterjee, N, Roychowdhury, T, Pradhan, A, Moiz, S, Manna, K, et al. Kaempferol with verapamil impeded panoramic chemoevasion pathways in breast cancer through ROS overproduction and disruption of lysosomal biogenesis. Phytomedicine. (2023) 113:154689. doi: 10.1016/j.phymed.2023.154689
98. Zhang, Y, Chen, J, Fang, W, Liang, K, Li, X, Zhang, F, et al. Kaempferol suppresses androgen-dependent and androgen-independent prostate cancer by regulating Ki67 expression. Mol Biol Rep. (2022) 49:4607–17. doi: 10.1007/s11033-022-07307-2
99. Zhu, Q, Han, Y, He, Y, Fu, Y, Yang, H, Chen, Y, et al. Kaempferol improves breast Cancer-related depression through the COX-2/PGE2 pathway. Front Biosci (Landmark Ed). (2023) 28:311. doi: 10.31083/j.fbl2811311
100. Song, H, Bao, J, Wei, Y, Chen, Y, Mao, X, Li, J, et al. Kaempferol inhibits gastric cancer tumor growth: An in vitro and in vivo study. Oncol Rep. (2015) 33:868–74. doi: 10.3892/or.2014.3662
101. Zhang, F, and Ma, C. Kaempferol suppresses human gastric cancer SNU-216 cell proliferation, promotes cell autophagy, but has no influence on cell apoptosis. Braz J Med Biol Res. (2019) 52:e7843. doi: 10.1590/1414-431X20187843
102. Adebayo, AH, Tan, NH, Akindahunsi, AA, Zeng, GZ, and Zhang, YM. Anticancer and antiradical scavenging activity of Ageratum conyzoides L. (Asteraceae). Pharmacogn Mag. (2010) 6:62–6. doi: 10.4103/0973-1296.59968
103. Kim, TW, Lee, SY, Kim, M, Cheon, C, and Ko, SG. Kaempferol induces autophagic cell death via IRE1-JNK-CHOP pathway and inhibition of G9a in gastric cancer cells. Cell Death Dis. (2018) 9:875. doi: 10.1038/s41419-018-0930-1
104. Radziejewska, I, Supruniuk, K, Tomczyk, M, Izdebska, W, Borzym-Kluczyk, M, Bielawska, A, et al. P-Coumaric acid, Kaempferol, astragalin and Tiliroside influence the expression of Glycoforms in AGS gastric Cancer cells. Int J Mol Sci. (2022) 23:8602. doi: 10.3390/ijms23158602
105. Budisan, L, Gulei, D, Jurj, A, Braicu, C, Zanoaga, O, Cojocneanu, R, et al. Inhibitory effect of CAPE and Kaempferol in Colon Cancer cell lines-possible implications in new therapeutic strategies. Int J Mol Sci. (2019) 20:1199. doi: 10.3390/ijms20051199
106. Gutierrez-Uribe, JA, Salinas-Santander, M, Serna-Guerrero, D, Serna-Saldivar, SRO, Rivas-Estilla, AM, and Rios-Ibarra, CP. Inhibition of miR31 and miR92a as oncological biomarkers in RKO Colon Cancer cells treated with Kaempferol-3-O-glycoside isolated from black bean. J Med Food. (2020) 23:50–5. doi: 10.1089/jmf.2019.0059
107. Park, J, Lee, GE, An, HJ, Lee, CJ, Cho, ES, Kang, HC, et al. Kaempferol sensitizes cell proliferation inhibition in oxaliplatin-resistant colon cancer cells. Arch Pharm Res. (2021) 44:1091–108. doi: 10.1007/s12272-021-01358-y
108. Wu, H, Cui, M, Li, C, Li, H, Dai, Y, Cui, K, et al. Kaempferol reverses aerobic glycolysis via miR-339-5p-mediated PKM alternative splicing in Colon Cancer cells. J Agric Food Chem. (2021) 69:3060–8. doi: 10.1021/acs.jafc.0c07640
109. Zhang, X, Fan, J, Guo, F, Huang, H, Xue, J, Wu, X, et al. Effect of Kaempferol on the biological behavior of human Colon Cancer via regulating MMP1, MMP2, and MMP9. J Oncol. (2022) 2022:2841762. doi: 10.1155/2022/2841762
110. Tie, F, Ding, J, Hu, N, Dong, Q, Chen, Z, and Wang, H. Kaempferol and kaempferide attenuate oleic acid-induced lipid accumulation and oxidative stress in HepG2 cells. Int J Mol Sci. (2021) 22:8847. doi: 10.3390/ijms22168847
111. Zhang, Q, Chen, L, Gao, M, Wang, S, Meng, L, and Guo, L. Molecular docking and in vitro experiments verified that kaempferol induced apoptosis and inhibited human HepG2 cell proliferation by targeting BAX, CDK1, and JUN. Mol Cell Biochem. (2023) 478:767–80. doi: 10.1007/s11010-022-04546-6
112. Mosterd, A, and Hoes, AW. Clinical epidemiology of heart failure. Heart. (2007) 93:1137–46. doi: 10.1136/hrt.2003.025270
113. Feng, H, Cao, J, Zhang, G, and Wang, Y. Kaempferol attenuates cardiac hypertrophy via regulation of ASK1/MAPK signaling pathway and oxidative stress. Planta Med. (2017) 83:837–45. doi: 10.1055/s-0043-103415
114. Liu, Y, Gao, L, Guo, S, Liu, Y, Zhao, X, Li, R, et al. Kaempferol alleviates angiotensin II-induced cardiac dysfunction and interstitial fibrosis in mice. Cell Physiol Biochem. (2017) 43:2253–63. doi: 10.1159/000484304
115. Zhu, J, Tang, H, Zhang, Z, Zhang, Y, Qiu, C, Zhang, L, et al. Kaempferol slows intervertebral disc degeneration by modifying LPS-induced osteogenesis/adipogenesis imbalance and inflammation response in BMSCs. Int Immunopharmacol. (2017) 43:236–42. doi: 10.1016/j.intimp.2016.12.020
116. Bakhshii, S, Khezri, S, Ahangari, R, Jahedsani, A, and Salimi, A. Protection of clozapine-induced oxidative stress and mitochondrial dysfunction by kaempferol in rat cardiomyocytes. Drug Dev Res. (2021) 82:835–43. doi: 10.1002/ddr.21790
117. Guo, Z, Liao, Z, Huang, L, Liu, D, Yin, D, and He, M. Kaempferol protects cardiomyocytes against anoxia/reoxygenation injury via mitochondrial pathway mediated by SIRT1. Eur J Pharmacol. (2015) 761:245–53. doi: 10.1016/j.ejphar.2015.05.056
118. Chen, X, Qian, J, Wang, L, Li, J, Zhao, Y, Han, J, et al. Kaempferol attenuates hyperglycemia-induced cardiac injuries by inhibiting inflammatory responses and oxidative stress. Endocrine. (2018) 60:83–94. doi: 10.1007/s12020-018-1525-4
119. Feng, Z, Wang, C, Yue, J, Meng, Q, Wu, J, and Sun, H. Kaempferol-induced GPER upregulation via the PI3K/AKT/Nrf2 pathway. Pharm Biol. (2021) 59:1106–16. doi: 10.1080/13880209.2021.1961823
120. Zhang, L, Guo, Z, Wang, Y, Geng, J, and Han, S. The protective effect of kaempferol on heart via the regulation of Nrf2, NF-κβ, and PI3K/Akt/GSK-3β signaling pathways in isoproterenol-induced heart failure in diabetic rats. Drug Dev Res. (2019) 80:294–309. doi: 10.1002/ddr.21495
121. Olas, B. Honey and its phenolic compounds as an effective natural medicine for cardiovascular diseases in humans? Nutrients. (2020) 12:283. doi: 10.3390/nu12020283
122. Hosseini, A, Alipour, A, Baradaran Rahimi, V, and Askari, VR. A comprehensive and mechanistic review on protective effects of kaempferol against natural and chemical toxins: role of NF-κB inhibition and Nrf2 activation. Biofactors. (2023) 49:322–50. doi: 10.1002/biof.1923
123. dos Santos, JS, Cirino, JPG, Carvalho, PD, and Ortega, MM. The pharmacological action of Kaempferol in central nervous system diseases: a review. Front Pharmacol. (2021) 11:565700. doi: 10.3389/fphar.2020.565700
124. Holland, TM, Agarwal, P, Wang, YM, Dhana, K, Leurgans, SE, Shea, K, et al. Association of Dietary Intake of Flavonols with changes in global cognition and several cognitive abilities. Neurology. (2023) 100:E694–702. doi: 10.1212/WNL.0000000000201541
125. Liu, ZY, Yao, XQ, Sun, BH, Jiang, WS, Liao, CR, Dai, XH, et al. Pretreatment with kaempferol attenuates microglia-mediate neuroinflammation by inhibiting MAPKs?NF??B signaling pathway and pyroptosis after secondary spinal cord injury. Free Radic Biol Med. (2021) 168:142–54. doi: 10.1016/j.freeradbiomed.2021.03.037
126. Chang, SQ, Li, X, Zheng, YC, Shi, HM, Zhang, D, Jing, B, et al. Kaempferol exerts a neuroprotective effect to reduce neuropathic pain through TLR4/NF-kappa B signaling pathway. Phytother Res. (2022) 36:1678–91. doi: 10.1002/ptr.7396
127. Yuan, Y, Zhai, YY, Chen, JJ, Xu, XF, and Wang, HM. Kaempferol ameliorates oxygen-glucose deprivation/Reoxygenation-induced neuronal Ferroptosis by activating Nrf2/SLC7A11/GPX4 Axis. Biomol Ther. (2021) 11:923. doi: 10.3390/biom11070923
128. Xie, CL, Zhuang, XX, Niu, ZM, Ai, RX, Lautrup, S, Zheng, SJ, et al. Amelioration of Alzheimer's disease pathology by mitophagy inducers identified via machine learning and a cross-species workflow. Nature. Biomed Eng. (2022) 6:76:93. doi: 10.1038/s41551-021-00819-5
129. Filomeni, G, Graziani, I, De Zio, D, Dini, L, Centonze, D, Rotilio, G, et al. Neuroprotection of kaempferol by autophagy in models of rotenone-mediated acute toxicity: possible implications for Parkinson's disease. Neurobiol Aging. (2012) 33:767–85. doi: 10.1016/j.neurobiolaging.2010.05.021
130. Jeong, DW, Cho, CH, Lee, JS, Lee, SH, Kim, T, and Kim, DO. Deastringent Peel extracts of persimmon (Diospyros kaki Thunb. cv. Cheongdo-Bansi) protect neuronal PC-12 and SH-SY5Y cells against oxidative stress. J Microbiol Biotechnol. (2018) 28:1094–104. doi: 10.4014/jmb.1801.01013
131. Zheng, L, Bakker, W, Estruch, IM, Widjaja, F, and Rietjens, I. Comparison of the methylglyoxal scavenging effects of kaempferol and glutathione and the consequences for the toxicity of methylglyoxal in SH-SY5Y cells. Food Chem X. (2023) 20:100920. doi: 10.1016/j.fochx.2023.100920
132. Ren, J, Lu, YF, Qian, YH, Chen, BZ, Wu, T, and Ji, G. Recent progress regarding kaempferol for the treatment of various diseases. Exp Ther Med. (2019) 18:2759–76. doi: 10.3892/etm.2019.7886
133. Jan, R, Khan, M, Asaf, S, Lubna, SA, and Kim, KM. Bioactivity and therapeutic potential of Kaempferol and quercetin: new insights for plant and human health. Plants (Basel). (2022) 11:3584. doi: 10.3390/plants11243584
134. Peng, X, Zhang, GW, Liao, YJ, and Gong, DM. Inhibitory kinetics and mechanism of kaempferol on α-glucosidase. Food Chem. (2016) 190:207–15. doi: 10.1016/j.foodchem.2015.05.088
135. Li, H, Ji, HS, Kang, JH, Shin, DH, Park, HY, Choi, MS, et al. Soy leaf extract containing Kaempferol glycosides and Pheophorbides improves glucose homeostasis by enhancing pancreatic β-cell function and suppressing hepatic lipid accumulation in db/db mice. J Agric Food Chem. (2015) 63:7198–210. doi: 10.1021/acs.jafc.5b01639
136. Li, X, Wei, SZ, Niu, SQ, Ma, X, Li, HT, Jing, MY, et al. Network pharmacology prediction and molecular docking-based strategy to explore the potential mechanism of Huanglian Jiedu decoction against sepsis. Comput Biol Med. (2022) 144:105389. doi: 10.1016/j.compbiomed.2022.105389
137. Liu, Z, Zhang, J, Liu, J, Guo, L, Chen, G, Fang, Y, et al. Combining network pharmacology, molecular docking and preliminary experiments to explore the mechanism of action of FZKA formula on non-small cell lung Cancer. Protein Pept Lett. (2023) 30:1038–47. doi: 10.2174/0109298665268153231024111622
138. Li, N, Cheng, Y, Jin, T, Cao, L, Zha, J, Zhu, X, et al. Kaempferol and ginsenoside Rg1 ameliorate acute hypobaric hypoxia induced lung injury based on network pharmacology analysis. Toxicol Appl Pharmacol. (2023) 480:116742. doi: 10.1016/j.taap.2023.116742
139. Estakhri, F, Panjehshahin, MR, Tanideh, N, Gheisari, R, Mahmoodzadeh, A, Azarpira, N, et al. The effect of kaempferol and apigenin on allogenic synovial membrane-derived stem cells therapy in knee osteoarthritic male rats. Knee. (2020) 27:817–32. doi: 10.1016/j.knee.2020.03.005
140. Nie, F, Zhang, W, Cui, Q, Fu, Y, Li, H, and Zhang, J. Kaempferol promotes proliferation and osteogenic differentiation of periodontal ligament stem cells via Wnt/β-catenin signaling pathway. Life Sci. (2020) 258:118143. doi: 10.1016/j.lfs.2020.118143
Keywords: kaempferol, bibliometrics, CiteSpace, VOSviewer, Scimago Graphica, Web of Science, pharmacology
Citation: Tang R, Lin L, Liu Y and Li H (2024) Bibliometric and visual analysis of global publications on kaempferol. Front. Nutr. 11:1442574. doi: 10.3389/fnut.2024.1442574
Edited by:
Dunja Miletić, University of Belgrade, SerbiaReviewed by:
Dong Xu, Air Force Medical University, ChinaReviewed by:
Franklin Chamorro, University of Vigo, SpainDong Xu, Air Force Medical University, China
Shaohui Wang, Chengdu University of Traditional Chinese Medicine, China
Copyright © 2024 Tang, Lin, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Li, bGlodWl6eXNAMTI2LmNvbQ==
 Ruying Tang
Ruying Tang Longfei Lin1
Longfei Lin1 Hui Li
Hui Li