- 1Department of Hepatobiliary Surgery, Second Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang, China
- 2Department of Hepatobiliary Surgery, First Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang, China
Background: Gallstones represent a prevalent health issue globally, resulting in significant annual healthcare costs. While tobacco exposure is recognized for its association with numerous diseases, its correlation with gallstones remains contentious. Serum cotinine, a metabolite of nicotine, serves as a widely utilized indicator for assessing tobacco exposure. Crucially, no research has yet examined the association between serum cotinine levels and the gallstones.
Methods: This study is designed as a cross-sectional analysis, utilizing data from the NHANES public database. The relationship between serum cotinine levels and gallstones was analyzed using multinomial logistic regression models and smooth curve fitting. Subgroup analyses and interaction tests were performed to examine the potential contributions of different populations and covariates to the findings.
Results: A total of 5,856 participants were included in this study. After adjusting for relevant covariates, the multiple logistic regression model results indicated that for each unit increase in serum cotinine concentration above 0.29 ng/mL, there was a 29% increase in the prevalence of gallstones. Furthermore, smooth curve fitting analysis revealed a positive correlation between these variables. These findings underscore the impact of tobacco exposure on gallstone prevalence.
Conclusion: This study demonstrates a positive correlation between tobacco exposure, as measured by serum cotinine levels, and the prevalence of gallstones, thus adding to the body of existing research on this relationship.
1 Introduction
Gallstones are one of the most prevalent hepatobiliary disorders, affecting 10 to 15% of adults in Western countries and placing a considerable burden on healthcare systems. Despite their prevalence, the etiology and pathogenesis of gallstones are not fully understood, and there is a notable lack of effective non-surgical treatment options (1, 2). Previous studies have identified several primary risk factors for gallstones, including genetic predisposition, elevated cholesterol secretion, and bile supersaturation. Additional risk factors encompass obesity, female gender, advancing age, type 2 diabetes, and physical inactivity (3, 4). Gallstones commonly originate within the wall of the gallbladder or the biliary tract and can manifest either as asymptomatic or symptomatic conditions (4). They are categorized based on their primary components into pure cholesterol stones, pure pigment stones, or mixed stones (5, 6), pure cholesterol stones predominantly comprise 90% cholesterol, whereas bile pigment stones, containing 90% bilirubin, are further distinguished as either brown or black pigment stones (7, 8). Gallstones predominantly consist of either cholesterol or melanin, with brown pigment stones being the most common type among bile duct stones (9). Moreover, besides the pain associated with gallstone disease itself, complications such as biliary pancreatitis can arise, potentially leading to life-threatening situations. Worldwide, gallstones stand as the primary etiology of acute pancreatitis, with an augmented risk correlated to the escalating quantity and dwindling size of stones (10, 11).
Cotinine, a major metabolite of nicotine, serves as a widely utilized biomarker for assessing population tobacco exposure, with its concentration in blood, urine, and saliva commonly measured (12, 13). Studies have demonstrated that serum cotinine concentrations offer a more reliable indicator of tobacco exposure compared to self-reported smoking numbers (14, 15). Furthermore, serum cotinine concentration is also employed as an effective measure of smoking status (16).
Tobacco exposure has emerged as the most significant preventable public health issue globally, with various toxic substances produced by tobacco combustion contributing to a multitude of diseases and fatalities (17). However, the association between tobacco exposure and gallstones remains contentious (18), while some studies have reported a heightened prevalence of gallstones among smokers (19–21), others contend that no such relationship exists (22–25) Therefore, this study aimed to delve deeper into the correlation between tobacco exposure and gallstones, utilizing serum cotinine concentration as an indicator of tobacco exposure.
2 Methods and materials
2.1 Study population and design
NHANES is a large cross-sectional survey designed to represent the health and nutrition conditions of the US population, with each cycle spanning two years. The survey includes demographic, socioeconomic, nutritional, and health-related questions in interviews. Approval for NHANES research protocols was obtained from the National Center for Health Statistics (NCHS) Research Ethics Review Board, and written informed consent was provided by all participants.
This study utilized data from the NHANES database spanning the years 2017 to 2020, encompassing a survey population of 15,560 participants. Initially, exclusion criteria were applied, leading to the removal of 6,328 participants under the age of 20. Subsequently, 1,282 participants with missing data on the exposure variable (serum cotinine), 19 participants with missing outcome data (gallstones), and finally, 2075 participants with missing covariates were excluded from the analysis. This resulted in a final analysis sample of 5,856 participants. The detailed process of inclusion and exclusion criteria is illustrated in Figure 1.

Figure 1. After inclusion and exclusion criteria screening, a total of 5,856 subjects were included in this study.
2.2 Definition of gallstones
A specific questionnaire within the NHANES database contained the inquiry: “Has a doctor or other health professional ever diagnosed you with gallstones?” Respondents answering affirmatively were classified as having gallstones, while those responding negatively were categorized as gallstone-free. Subjects who declined to respond or answered “do not know” were omitted from the study.
2.3 Serum cotinine
Serum cotinine, serving as the exposure variable in this study, was quantified using an isotope-dilution high-performance liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometry (ID HPLC-APCI MS/MS) method. We stratified serum cotinine concentrations into tertiles to investigate the dose–response association between exposure and outcome.
2.4 Covariates
All covariates for this study were derived from demographic, examination, laboratory, and questionnaire data obtained from the NHANES database. Confounding factors included age, gender, race, education level, marital status, ratio of family income to poverty (PIR), body mass index (BMI), physical activity, drinking status, diabetes, hypertension, direct HDL-cholesterol (HDL), and total cholesterol (TC). Age was categorized into groups based on 40 and 60 years old, while race and marital status were classified according to NHANES classifications. Education level was divided into three groups according to completion of high school education. PIR was categorized using cut-off points of 1.3 and 3.5 as previously defined (26), BMI was used to differentiate between overweight and obesity, with thresholds set at 25 kg/m2 and 30 kg/m2, respectively. Physical activity levels were determined based on responses to questions about engaging in vigorous-intensity and moderate-intensity activities for at least 10 min continuously. Positive responses to both were categorized as engaging in vigorous or moderate activity. Regarding drinking status, participants were asked, “How often have you consumed alcoholic beverages in the past 12 months?” Those reporting consumption less than once a month were categorized as non-drinkers. Diabetes and hypertension status were determined based on responses to questions regarding prior diagnoses by a doctor or health professional. Data on HDL and TC were obtained from NHANES laboratory records. The selection of covariates in this study was guided by three key principles: identifying relevant covariates from previous studies utilizing the NHANES database for gallstone research (27–29), incorporating known risk factors from comprehensive review on gallstones (18), and ensuring that the selected covariates were available within the NHANES database for further analysis. This approach ensured that the covariates included in the analysis were both relevant and supported by existing literature and available data.
2.5 Statistical analysis
Continuous variables were reported as means ± standard errors (SEs), while categorical variables were presented as numbers and percentages. The Kruskal-Wallis Rank Test was utilized for continuous variables, and Fisher’s Exact Test was applied for categorical variables, particularly when the expected count was less than 10. To explore the relationship between serum cotinine and gallstones across different models, multinomial logistic regression models were employed. Three statistical models were established: the Non-adjusted model, with no covariate adjustments; Adjusted Model I, which included adjustments for age, gender, and race; and Adjusted Model II, incorporating adjustments for age, gender, race, education level, marital status, ratio of family income to poverty (PIR), body mass index (BMI), physical activity, drinking status, diabetes, hypertension, high-density lipoprotein (HDL), and total cholesterol (TC). Additionally, smooth curve fitting (penalized saline method) was conducted to further examine the association between gallstones and serum cotinine. Subsequently, subgroup analyses of all covariates were performed to investigate potential modifications in the association between exposure and outcome. These covariates were also considered as interaction terms, and interaction tests were conducted. In the interaction tests and subgroup analyses, HDL and TC were additionally converted from continuous variables to tertile categorical variables. All statistical analysis processes were carried out based on EmpowerStats1 and statistical significance was defined as a p value below 0.05.
3 Results
3.1 Baseline characteristics of participants
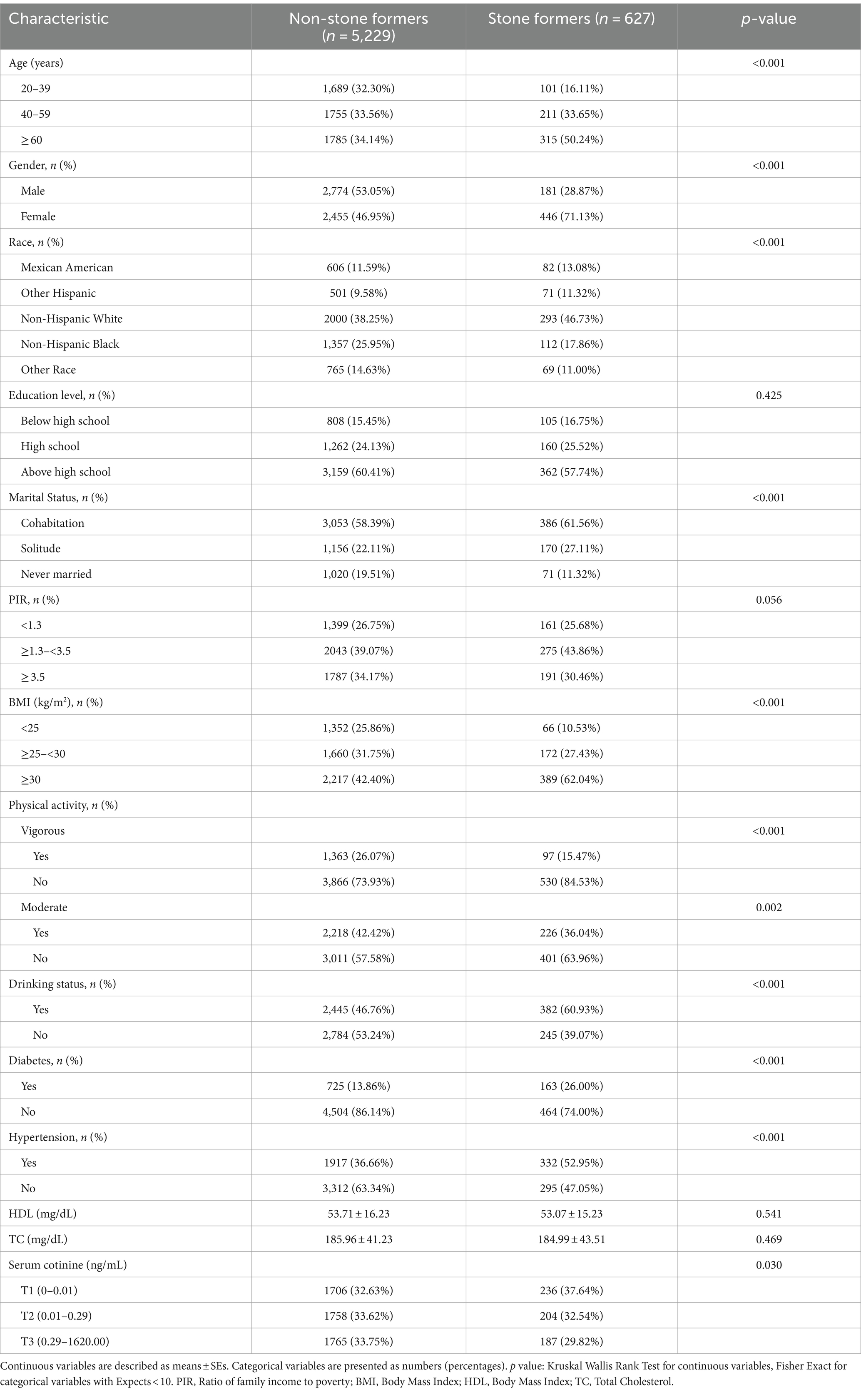
After establishing reasonable inclusion and exclusion criteria, a total of 5,856 participants aged 20 years and above were included in this study. In Table 1, there were 5,229 subjects without gallstones and 627 subjects with gallstones. We found significant differences (p < 0.05) between the two groups of participants with and without gallstones in terms of age, gender, race, marital status, BMI, physical activity level, drinking status, diabetes, hypertension, and serum cotinine grouping.
Among the participants with gallstones specifically, individuals aged 60 years and above accounted for a higher proportion (50.24%), while females had a higher percentage (71.13%) compared to males (28.27%). Regarding marital status distribution among those with gallstones versus those without them, cohabitation and solitude had a higher proportion among patients with gallstones than those without them. In terms of BMI distribution, overweight participants accounted for as much as 62.04% of patients with gallstones. Concerning physical activity levels, non-exercisers constituted a larger proportion of patients with gallstones compared to those without them. The proportions regarding drinking status showed an inversion between participants with and without stones, as drinkers represented approximately 60.93% of patients with gallstones. Participants diagnosed with diabetes or hypertension had higher proportions within the group of patients suffering from gallstones compared to those who did not have them.
3.2 Relationship between serum cotinine and gallstones
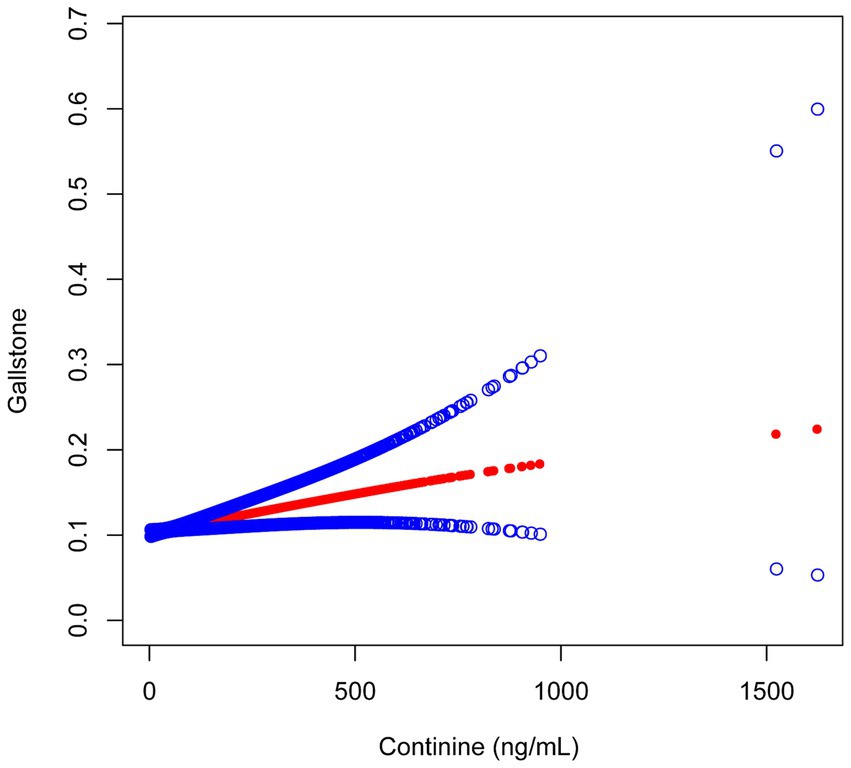
The serum cotinine concentration exhibited a positive correlation with the prevalence of gallstones. In the Adjust II model presented in Table 2, an association was observed between serum cotinine levels within the T3 range (0.29–1620.00 ng/mL) and a 29% increase in gallstone prevalence (OR = 1.29; 95% CI: 1.01–1.63; p = 0.0374). Furthermore, as the concentration of cotinine increased, this positive correlation demonstrated a gradual increment (p for trend = 0.0149). Smooth curve fitting analysis further substantiated the existence of a positive correlation between these two variables (Figure 2).
3.3 Subgroup analysis and interaction tests for the associations between serum cotinine and gallstones
In the subgroup analysis, all covariates were also treated as interaction terms and underwent interaction testing. These covariates were identical to those used in the final adjusted model (Adjusted II model), with one necessary exception: during the subgroup analysis for each specific covariate, that particular covariate was not included as an adjusted variable in its own analysis. Additionally, HDL and TC were converted from continuous variables to tertile categorical variables. In each analysis, all covariates except those under examination were adjusted. The findings indicated that none of the subgroups exhibited a significant impact on the relationship between serum cotinine concentration and gallstones (Table 3).

Table 3. Subgroups analysis and interaction tests for the associations between serum cotinine and gallstones.
4 Discussion
In this cross-sectional analysis involving 5,856 participants from the NHANES database, we identified a positive correlation between serum cotinine levels and the prevalence of gallstones. This association was further substantiated by curve fitting results. Following subgroup analysis and interaction tests, no confounding factor was identified to potentially influence this relationship. We assert that tobacco exposure, as indicated by serum cotinine concentration, warrants attention, emphasizing the importance of minimizing tobacco exposure or quitting smoking early to prevent gallstones. This finding complements prior research on the association between tobacco exposure and gallstones.
Our study represents the first attempt to utilize serum cotinine concentrations to evaluate the relationship between tobacco exposure status and gallstones. Murray et al. (30) conducted a long-term prospective study involving 46,000 oral contraceptive users in the United Kingdom over 19 years, revealing that smokers had a higher likelihood of developing symptomatic gallstones compared to non-smokers. However, this study could not definitively determine whether the occurrence of gallstones was solely attributed to oral contraceptives and did not investigate the male population. Similarly, Stampfer et al. (31) conducted an 8-year prospective study that identified smoking as an independent risk factor for gallstones after adjusting for BMI, weight change, and other risk factors. However, this study also exclusively focused on the female population, leaving the influence of gender on this association unclear. Kato et al. (32) and Sahi et al. (33) conducted prospective studies on men, both observing a positive correlation between smoking and gallstones. However, these studies faced challenges in fully excluding the influence of race and post-immigrant lifestyle, and the latter did not find a statistically significant association between smoking duration and gallstones. Misciagna et al. (34) conducted a long-term follow-up study in a small town in southern Italy, involving 3,500 participants, and found a strong association between smoking and gallstones, although no dose–response relationship was evident. Yamada et al. (35) observed a positive association between smoking and gallstones in a prospective study of a Japanese population. However, limitations such as questionnaire-derived smoking data, lack of timely information, and unavailability of data on cigarette or alcohol intake hindered the possibility of conducting a dose–response analysis. Our study addressed some of the limitations of previous research by utilizing extensive and comprehensive data from the NHANES database. Furthermore, the use of serum cotinine concentration to assess smoking status enabled the quantification of tobacco exposure levels, facilitating a more nuanced exploration of the association between tobacco exposure and gallstones through smooth curve fitting.
The observed impact of serum cotinine on gallstone prevalence in this study may be associated with the FXR-megalin/cubilin signaling pathway. Erran et al. (36) discovered that megalin and cubilin proteins are expressed in gallbladder epithelial cells but not in hepatocytes, and their expression is regulated by bile acids rather than cholesterol. This regulation is mediated by the bile acid nuclear hormone receptor farnesoid X receptor (FXR). The FXR-megalin/cubilin signaling pathway plays a role in cholesterol balance regulation and participates in gallstone formation (37). Notably, nicotine inhibits the expression of FXR and megalin in gallbladder epithelial cells (38). When the gallbladder relies on megalin protein for cholesterol transport, this mechanism becomes weakened, leading to disturbances in cholesterol metabolism within the gallbladder. Consequently, this disruption increases the likelihood of cholesterol stone formation. Another potential mechanism involves the down-regulation of megalin expression, disrupting the balance between upstream and downstream signaling pathway components. This disruption may result in an imbalance in bile acid ratios, preventing cholesterol from maintaining its micellar shape. Consequently, cholesterol precipitates as crystals, ultimately leading to gallstone formation (39, 40).
What we have done is the first study to explore the relationship between tobacco exposure and gallstones using serum cotinine concentrations. The strength of this study lies in its utilization of a large and comprehensive dataset derived from the NHANES database, encompassing numerous covariates identified in previous research. Through meticulous adjustment for these confounding factors, we aimed to attain more reliable research outcomes. Additionally, subgroup analyses and interaction tests were conducted to assess the robustness of the findings across different populations and to evaluate the potential impact of various covariates on the results. However, this study also exhibits certain limitations. Despite the consideration of numerous confounding factors, there may still be some omissions, thereby preventing the complete exclusion of potential confounding effects on the results. Furthermore, due to the nature of cross-sectional studies, we are inherently unable to establish a causal relationship between serum cotinine concentration and gallstones. Thus, further investigation is warranted to elucidate the relationship between tobacco exposure and gallstones.
5 Conclusion
In this study, it is noteworthy that when serum cotinine concentration exceeded 0.29 ng/mL, each unit increase was associated with a 29% rise in gallstone prevalence. Additionally, curve fitting analysis revealed a positive association between the two variables. These findings prompt further consideration of tobacco exposure and underscore the health risks associated with tobacco and its harmful constituents when burned. Further investigation is needed to clarify the causal relationship between these variables.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found at: https://www.cdc.gov/nchs/nhanes/index.htm.
Ethics statement
The studies involving humans were approved by National Center for Health Statistics (NCHS) Research Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XL: Methodology, Writing – original draft, Writing – review & editing, Project administration, Software. ZZ: Data curation, Writing – original draft, Software. HW: Formal analysis, Writing – original draft, Software. SF: Validation, Writing – original draft, Investigation. MH: Project administration, Writing – original draft, Validation. ST: Conceptualization, Writing – original draft, Methodology. YL: Conceptualization, Methodology, Writing – review & editing, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. We are grateful for the funding provided by the National Natural Science Foundation of China (No. 81901576).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Portincasa, P, Moschetta, A, and Palasciano, G. Cholesterol gallstone disease. Lancet. (2006) 368:230–9. doi: 10.1016/S0140-6736(06)69044-2
2. Marschall, HU, and Einarsson, C. Gallstone disease. J Intern Med. (2007) 261:529–42. doi: 10.1111/j.1365-2796.2007.01783.x
3. Di Ciaula, A, Wang, DQ, and Portincasa, P. An update on the pathogenesis of cholesterol gallstone disease. Curr Opin Gastroenterol. (2018) 34:71–80. doi: 10.1097/MOG.0000000000000423
4. Lammert, F, Gurusamy, K, Ko, CW, Miquel, JF, Méndez-Sánchez, N, Portincasa, P, et al. Gallstones. Nat Rev Dis Primers. (2016) 2:16024. doi: 10.1038/nrdp.2016.24
5. Rains, AJ. Gallstones. An introduction to research into causes and remarks on some problems in treatment. Ann R Coll Surg Engl. (1961) 29:85–101.
6. Shabanzadeh, DM. New determinants for gallstone disease? Dan Med J. (2018) 65:1239–1248. doi: 10.1080/00365521.2016.1182583
8. Sharma, B, and Sharma, SR. Evaluation of gallstone classification and their diagnosis through serum parameters as emerging tools in treatment: a narrative review. Postgrad Med. (2022) 134:644–53. doi: 10.1080/00325481.2022.2103350
9. Tazuma, S. Gallstone disease: epidemiology, pathogenesis, and classification of biliary stones (common bile duct and intrahepatic). Best Pract Res Clin Gastroenterol. (2006) 20:1075–83. doi: 10.1016/j.bpg.2006.05.009
10. Kundumadam, S, Fogel, EL, and Gromski, MA. Gallstone pancreatitis: general clinical approach and the role of endoscopic retrograde cholangiopancreatography. Korean J Intern Med. (2021) 36:25–31. doi: 10.3904/kjim.2020.537
11. Tess, A, Freedman, SD, Kent, T, and Libman, H. How would You treat this patient with gallstone pancreatitis?: grand rounds discussion from Beth Israel Deaconess Medical Center. Ann Intern Med. (2019) 170:175–81. doi: 10.7326/M18-3536
12. Benowitz, NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. (1996) 18:188–204.
13. Avila-Tang, E, Al-Delaimy, WK, Ashley, DL, Benowitz, N, Bernert, JT, Kim, S, et al. Assessing secondhand smoke using biological markers. Tob Control. (2013) 22:164–71. doi: 10.1136/tobaccocontrol-2011-050298
14. Pérez-Stable, EJ, Benowitz, NL, and Marín, G. Is serum cotinine a better measure of cigarette smoking than self-report? Prev Med. (1995) 24:171–9. doi: 10.1006/pmed.1995.1031
15. George, L, Granath, F, Johansson, AL, and Cnattingius, S. Self-reported nicotine exposure and plasma levels of cotinine in early and late pregnancy. Acta Obstet Gynecol Scand. (2006) 85:1331–7. doi: 10.1080/00016340600935433
16. Kim, S. Overview of cotinine cutoff values for smoking status classification. Int J Environ Res Public Health. (2016) 13:1236. doi: 10.3390/ijerph13121236
17. Mattes, W, Yang, X, Orr, MS, Richter, P, and Mendrick, DL. Biomarkers of tobacco smoke exposure. Adv Clin Chem. (2014) 67:1–45. doi: 10.1016/bs.acc.2014.09.001
18. Pak, M, and Lindseth, G. Risk factors for Cholelithiasis. Gastroenterol Nurs. (2016) 39:297–309. doi: 10.1097/SGA.0000000000000235
19. Aune, D, Vatten, LJ, and Boffetta, P. Tobacco smoking and the risk of gallbladder disease. Eur J Epidemiol. (2016) 31:643–53. doi: 10.1007/s10654-016-0124-z
20. Bai, Y, Zhang, M, Cui, H, Shan, X, Gu, D, Wang, Y, et al. Addictive behavior and incident gallstone disease: a dose-response meta-analysis and Mendelian randomization study. Front Nutr. (2022) 9:940689. doi: 10.3389/fnut.2022.940689
21. Larsson, SC, and Burgess, S. Appraising the causal role of smoking in multiple diseases: a systematic review and meta-analysis of Mendelian randomization studies. EBioMedicine. (2022) 82:104154. doi: 10.1016/j.ebiom.2022.104154
22. Kono, S, Eguchi, H, Honjo, S, Todoroki, I, Oda, T, Shinchi, K, et al. Cigarette smoking, alcohol use, and gallstone risk in Japanese men. Digestion. (2002) 65:177–83. doi: 10.1159/000064938
23. Shabanzadeh, DM, Sørensen, LT, and Jørgensen, T. Determinants for gallstone formation – a new data cohort study and a systematic review with meta-analysis. Scand J Gastroenterol. (2016) 51:1239–48. doi: 10.1080/00365521.2016.1182583
24. Shabanzadeh, DM, and Novovic, S. Alcohol, smoking and benign hepato-biliary disease. Best Pract Res Clin Gastroenterol. (2017) 31:519–27. doi: 10.1016/j.bpg.2017.09.005
25. Walcher, T, Haenle, MM, Mason, RA, Koenig, W, Imhof, A, and Kratzer, W. The effect of alcohol, tobacco and caffeine consumption and vegetarian diet on gallstone prevalence. Eur J Gastroenterol Hepatol. (2010) 22:1345–51. doi: 10.1097/MEG.0b013e32833efdb2
26. Bao, W, Liu, B, Simonsen, DW, and Lehmler, HJ. Association between exposure to Pyrethroid insecticides and risk of all-cause and cause-specific mortality in the general US adult population. JAMA Intern Med. (2020) 180:367–74. doi: 10.1001/jamainternmed.2019.6019
27. Li, Y, Han, H, You, K, Ma, C, and Fan, X. Investigating the association between blood cobalt and gallstones: a cross-sectional study utilizing NHANES data. Front Public Health. (2024) 12:1363815. doi: 10.3389/fpubh.2024.1363815
28. Wen, SH, Tang, X, Tang, T, and Ye, ZR. Association between weight-adjusted-waist index and gallstones: an analysis of the National Health and nutrition examination survey. BMC Gastroenterol. (2024) 24:40. doi: 10.1186/s12876-024-03127-9
29. Zhang, G, Ding, Z, Yang, J, Wang, T, Tong, L, Cheng, J, et al. Higher visceral adiposity index was associated with an elevated prevalence of gallstones and an earlier age at first gallstone surgery in US adults: the results are based on a cross-sectional study. Front Endocrinol. (2023) 14:1189553. doi: 10.3389/fendo.2023.1189553
30. Murray, FE, Logan, RF, Hannaford, PC, and Kay, CR. Cigarette smoking and parity as risk factors for the development of symptomatic gall bladder disease in women: results of the Royal College of general Practitioners’ oral contraception study. Gut. (1994) 35:107–11.
31. Stampfer, MJ, Maclure, KM, Colditz, GA, Manson, JE, and Willett, WC. Risk of symptomatic gallstones in women with severe obesity. Am J Clin Nutr. (1992) 55:652–8.
32. Kato, I, Nomura, A, Stemmermann, GN, and Chyou, PH. Prospective study of clinical gallbladder disease and its association with obesity, physical activity, and other factors. Dig Dis Sci. (1992) 37:784–90.
33. Sahi, T, Paffenbarger, RS Jr, Hsieh, CC, and Lee, IM. Body mass index, cigarette smoking, and other characteristics as predictors of self-reported, physician-diagnosed gallbladder disease in male college alumni. Am J Epidemiol. (1998) 147:644–51.
34. Misciagna, G, Leoci, C, Guerra, V, Chiloiro, M, Elba, S, Petruzzi, J, et al. Epidemiology of cholelithiasis in southern Italy. Part II: risk factors. Eur J Gastroenterol Hepatol. (1996) 8:585–93.
35. Yamada, M, Wong, FL, Fujiwara, S, Tatsukawa, Y, and Suzuki, G. Smoking and alcohol habits as risk factors for benign digestive diseases in a Japanese population: the radiation effects research foundation adult health study. Digestion. (2005) 71:231–7. doi: 10.1159/000087048
36. Erranz, B, Miquel, JF, Argraves, WS, Barth, JL, Pimentel, F, and Marzolo, MP. Megalin and cubilin expression in gallbladder epithelium and regulation by bile acids. J Lipid Res. (2004) 45:2185–98. doi: 10.1194/jlr.M400235-JLR200
37. Marzolo, MP, and Farfán, P. New insights into the roles of megalin/LRP2 and the regulation of its functional expression. Biol Res. (2011) 44:89–105. doi: 10.4067/S0716-97602011000100012
38. Gao, Q, Bi, P, Mi, Q, Guan, Y, Jiang, J, Li, X, et al. Effect of nicotine on cholesterol gallstone formation in C57BL/6J mice fed on a lithogenic diet. Exp Ther Med. (2023) 25:84. doi: 10.3892/etm.2023.11783
39. Rudling, M, Laskar, A, and Straniero, S. Gallbladder bile supersaturated with cholesterol in gallstone patients preferentially develops from shortage of bile acids. J Lipid Res. (2019) 60:498–505. doi: 10.1194/jlr.S091199
Keywords: gallstones, serum cotinine, tobacco exposure, smoking, NHANES
Citation: Liu X, Zhang Z, Wang H, Faisal S, He M, Tai S and Lin Y (2024) The link between serum cotinine levels and gallstones prevalence in adults: a cross-sectional analysis using NHANES data (2017–2020). Front. Nutr. 11:1438170. doi: 10.3389/fnut.2024.1438170
Edited by:
Mehran Rahimlou, Zanjan University of Medical Sciences, IranReviewed by:
Gabriel Lai, National Institute on Minority Health and Health Disparities (NIH), United StatesMona Mohamed Taha, National Research Centre, Egypt
Copyright © 2024 Liu, Zhang, Wang, Faisal, He, Tai and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sheng Tai, dGFpc2hlbmcxOTczQDE2My5jb20=; Yujia Lin, bGlueXVqaWFAaHJibXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Xin Liu
Xin Liu Zheng Zhang1†
Zheng Zhang1† Haoran Wang
Haoran Wang Sheng Tai
Sheng Tai