- Department of Nephrology, Institute of Kidney Diseases, West China Hospital of Sichuan University, Chengdu, China
Background: Chronic kidney disease (CKD) is a serious and steadily growing health problem worldwide. Probiotic and synbiotic supplementation are expected to improve kidney function in CKD patients by altering imbalanced intestinal flora, regulating microbiota metabolites, modulating the brain-gut axis, and reducing inflammation.
Objectives: Our aim is to report the latest and largest pooled analyses and evidence updates to explore whether probiotic and synbiotic have beneficial effects on renal function and general conditions in patients with CKD.
Methods: We conducted a systematic literature search using PubMed, Embase, Web of Science, and the Cochrane Central Register of Controlled Trials from inception until 1 December 2023. Eligible literatures were screened according to inclusion and exclusion criteria, data were extracted, and a systematic review and meta-analysis was performed. Measurements included renal function-related markers, inflammatory markers, uremic toxins, lipid metabolism-related markers and electrolytes levels.
Results: Twenty-one studies were included. The results showed that probiotic/synbiotic significantly reduced blood urea nitrogen (BUN) (standardized mean difference (SMD), −0.23, 95% confidence interval (CI) −0.41, −0.04; p = 0.02, I2 = 10%) and lowered c-reactive protein level (CRP) (SMD: −0.34; 95% CI: −0.62, −0.07; p = 0.01, I2 = 37%) in CKD patients, compared with the control group.
Conclusion: In summary, probiotic/synbiotic supplementation seems to be effective in improving renal function indices and inflammation indices in CKD patients. Subgroup analyses suggested that longer-term supplementation is more favorable for CKD patients, but there is a high degree of heterogeneity in the results of partial subgroup analyses. The efficacy of probiotic/synbiotic in treating CKD needs to be supported by more evidence from large-scale clinical studies.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42024526836, Unique identifier: CRD42024526836.
1 Introduction
CKD is characterized by abnormalities in kidney structure or function that persist for a period of at least 3 months (1). The clinical features of CKD include decreased renal function and/or increased urinary albumin excretion (proteinuria) (2). It is a disease that progresses slowly over time, and a significant number of patients eventually reach end-stage renal disease and require dialysis for treatment (3, 4). Currently, the global prevalence of CKD is about 11%, and with increasing age, the prevalence in people over 70 years old is as high as 34% (5, 6). In addition, patients with CKD have an increased risk of cardiovascular disease, hypertension, diabetes, and infections (7). Although CKD has received considerable attention from scientists and clinicians, the care and treatment of the condition are still not up to par (8). Consequently, there is a pressing need to explore new drugs or therapeutic approaches.
Previous studies have shown that ecosystem imbalances are strongly associated with a number of chronic diseases, such as chronic kidney disease, diabetes and cardiovascular disease (9, 10). There is also evidence showed that imbalances in the gut microbiota may contribute to CKD (11). In addition, worsening CKD can further exacerbate imbalances in gut flora (12). Researchers proposed the gut-kidney axis and the CKD-colon axis in 2011 (13) and 2015 (14) to characterize the interactions between the kidney and the gut. Probiotics, synbiotic supplements are then expected to slow the progression of CKD by regulating the balance of intestinal flora (15). Probiotics, consisting of active microorganisms, colonize the human intestinal tract to improve the microbiological balance and benefit human health (16). Recent studies indicated that probiotics could potentially offer advantages to individuals with chronic kidney disease (17–19). The current definition of synbiotic has been updated to “a mixture of live microorganisms and substrates selectively utilized by host microorganisms to provide health benefits to the host” (20). There have been a number of studies aimed at evaluating the role of synbiotic supplementation in patients with CKD (21–24). Currently, non-food probiotics and synbiotic supplements are becoming increasingly available in the United States (25).
The use of probiotics has shown potential as a nutritional strategy for the prevention and/or treatment of CKD. In some animal studies, Lactobacillus supplementation has been shown to slow the progression of chronic kidney disease and delay renal failure by altering short-chain fatty acid and nicotinamide metabolism (26). In addition, an exploratory clinical study found that serum levels of tumor necrosis factor-α (TNF-α), Interleukin (IL)-6, IL-18, and endotoxin were significantly reduced in patients with CKD after probiotics administration (27). Despite growing interest in the potential role of probiotics in improving chronic kidney disease, there is a lack of extensive cross-sectional studies to comprehensively assess the effect of probiotics/synbiotics on the general condition of CKD patients in the population. In addition, although there have been previous meta-analyses of the relationship between probiotics and CKD, the outcome indicators of these analyses have focused on one of many metrics, such as kidney function or metabolism (28, 29). Therefore, a systematic review and meta-analysis incorporated latest RCT studies was designed to comprehensively investigate the effects of probiotics/synbiotics supplementation on renal function, lipid metabolism, inflammation, uremic toxin levels and electrolyte levels in dialysis/non-dialysis CKD patients.
2 Methods
2.1 Search strategy
The review program was established by two investigators (LC) and (WW) prior to the start of the study and registered with the PROSPERO International Prospective Registry of Systematic Reviews (registration number CRD42024526836). This study was conducted according to the Cochrane Manual and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (30). Two independent reviewers (LC and YLT) searched PubMed, Embase, Web of Science and Cochrane Library from inception until December 2023. We searched the databases using the following terms: “probiotics,” “probiotic,” “synbiotics,” “synbiotic,” “renal insufficiency, chronic,” “chronic renal insufficiencies,” “renal insufficiencies, chronic,” “chronic renal insufficiency,” “kidney insufficiency, chronic,” “chronic kidney insufficiencies,” “kidney insufficiencies, chronic,” “chronic kidney diseases,” “chronic kidney disease” “disease, chronic kidney,” “diseases, chronic kidney,” “kidney disease, chronic,” “kidney diseases, chronic,” “chronic renal diseases,” “chronic renal disease,” “disease, chronic renal,” “diseases, chronic renal,” “renal disease, chronic,” “renal diseases, chronic” and “chronic kidney insufficiency.” Two researchers independently searched and evaluated the included studies, and any disagreements in the literature search were resolved by conferring with a third researcher (WW). Specific search strategies are shown in Supplementary Table 1.
2.2 Eligible criteria
The study met all of the following criteria (1) study design: randomized controlled study; (2) study participants: patients with a confirmed diagnosis of chronic kidney disease; (3) intervention: the intervention group should receive any dose of probiotic or synbiotic supplementation; (4) comparison regimen/control group: participants in the control group may receive a placebo or other medication and if other medications are used in the treatment group, they also control group must be used in the same way; (6) language: articles published in English.
Studies were excluded for the following reasons: (1) they were reviews, meta-analyses, case reports, conference abstracts, and guidelines; (2) the study was animal-based; (3) the study was published in a language other than English.
2.3 Research screening
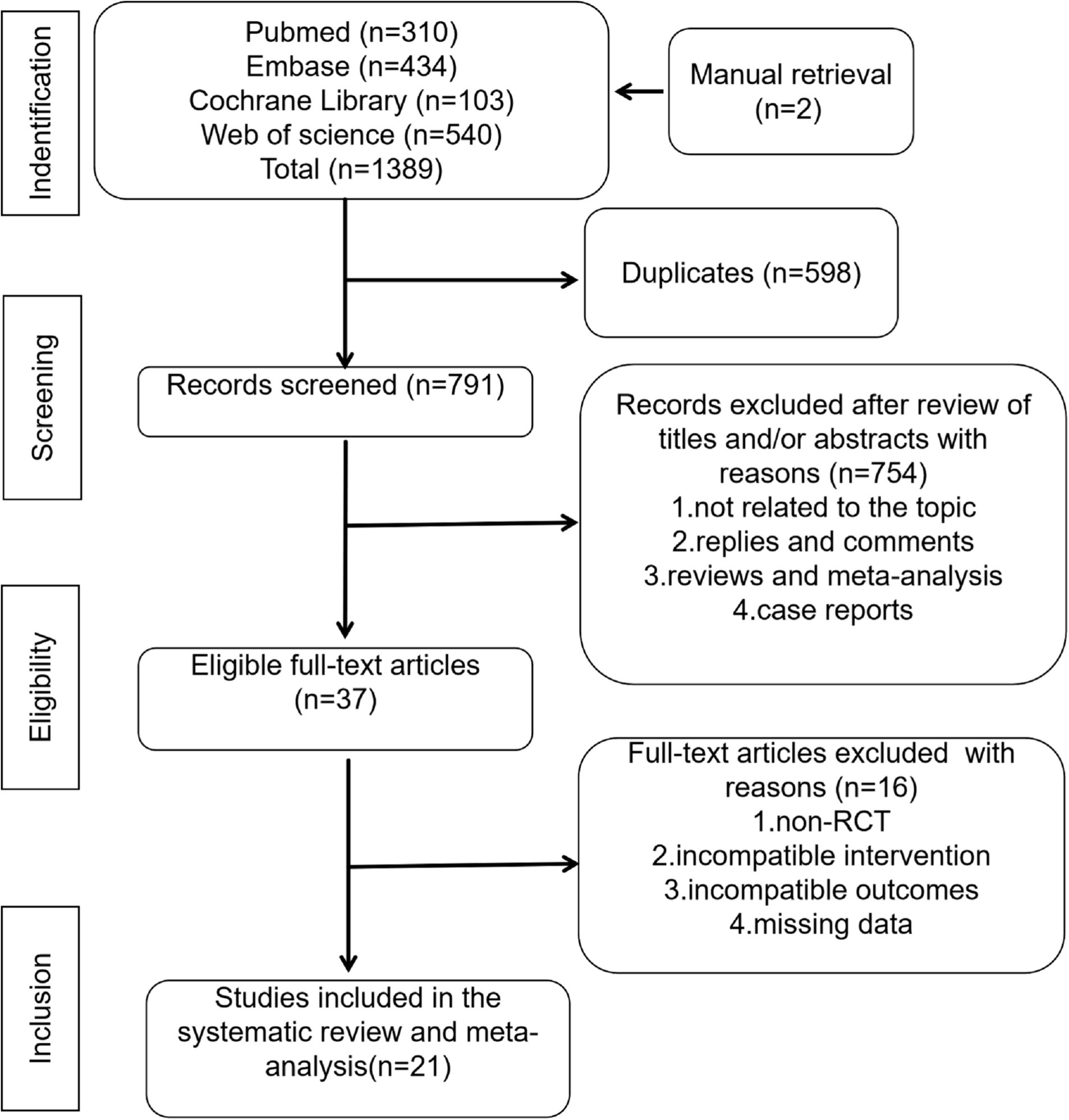
After excluding duplicate records, two researchers independently screened the titles and abstracts of all identified records to remove irrelevant documents. A full-text review was then conducted to determine eligibility for inclusion. Any disagreements regarding study selection could be resolved through discussion with a third researcher (LC, YLT, and WW). The study selection process is shown in Figure 1.
2.4 Data extraction
The following data were extracted from the included studies: (a) The basic information, including first author, publication year, region, data source, study design, and enrollment period; (b) Characteristics of the participants, including sample size, sex ratio (male), median age, median Body Mass Index (BMI) and hemodialysis time; (c) Interventions: probiotics/synbiotics types, dosage, frequency, intervention time; (d) Disease-related indicators: creatinine, BUN, eGFR, hemoglobin, uric acid, potassium, total cholesterol, HDL-cholesterol, LDL-cholesterol, indoxyl sulfate, p-cresyl sulfate, indole-3-acetic acid, CRP, IL-6, triglycerides, blood sodium, blood calcium and blood phosphorus. When continuous variables in the study were reported as median with range or interquartile range, we calculated the mean ± standard deviation through the validated mathematical method. When data were missing or not reported in the study, we contacted the corresponding authors to obtain completed data if available.
2.5 Quality assessment
Quality assessment of eligible RCTs was performed according to the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0, based on seven terms: randomized sequence generation, allocation concealment, participant and personnel blinding, blinding of outcome assessment, incomplete outcome data, selective reporting and other biases sources (31). Three outcomes were assessed for each study, including low risk, high risk, and unclear risk. Studies with more “low risk” of bias assessment were considered superior.
2.6 Statistical analysis
Evidence synthesis was performed in Review Manager 5.4 version (Cochrane Collaboration, Oxford, United Kingdom). The SMD was applied for the comparison of continuous variables. All metrics were reported with mean ± SD. The heterogeneity in studies was assessed through the inconsistency index (I2). I2 > 50% were considered as significant heterogeneity. A random-effect model was used to estimate the combined SMD when significant heterogeneity was detected (I2 > 50%). Otherwise, the fixed-effect model was applied. In addition, we performed one-way sensitivity analyses to evaluate the effect of included studies on the combined results for outcomes with significant heterogeneity. Subgroup analyses were used to explore sources of heterogeneity. The subgroup analysis has not been conducted for indicators which were few included in literatures. Because the limited number of literatures may lead to a significant discrepancy between subgroups, which could impact the accuracy of the results. Publication bias was evaluated visually by creating funnel plots via Review Manager 5.4 version (Cochrane Collaboration, Oxford, United Kingdom), as well as by conducting Egger’s regression tests using Stata 15.0 version (Stata Corp, College Station, TX, United States) for outcomes with 5 or more included studies. p-value < 0.05 was considered as statistically significant publication bias.
3 Results
3.1 Literature search
The initial search was completed on 1 December 2023. We have identified 310 potentially relevant publications from PubMed, 434 from Embase, 103 from The Cochrane library, 540 from Web of science and 2 from manual retrieval. Endnote was used to eliminate duplicate publications, resulting in 791 records for review. After excluding publications that did not meet the inclusion criteria, we included 21 studies for systematic review and meta-analysis. A flow diagram illustrating the exclusion of articles with specific reasons is shown in Figure 1 (PRISMA flowchart).
3.2 Study characteristics
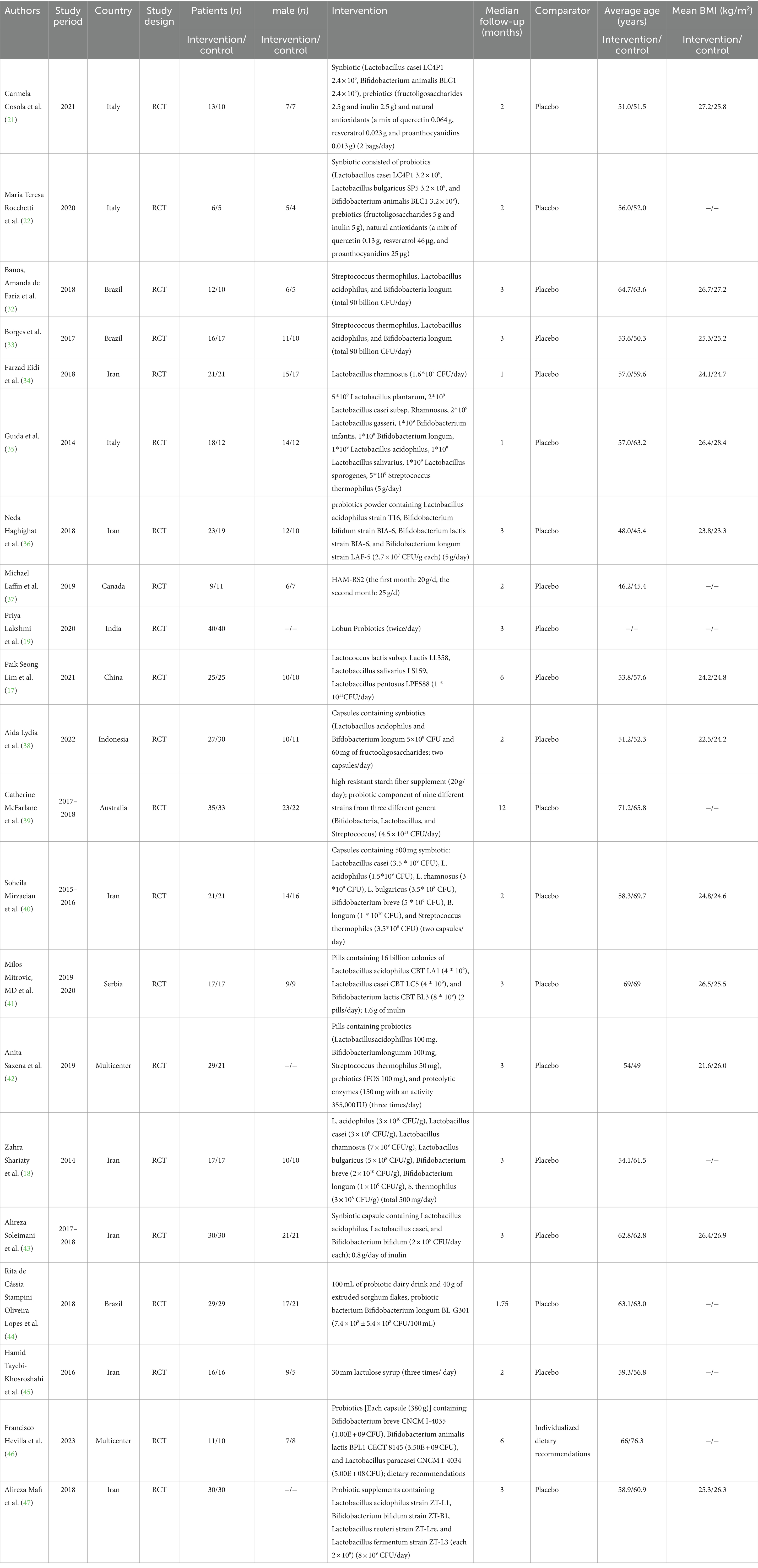
We conducted a systematic review and meta-analysis of 869 patients with chronic kidney disease involved in 21 RCT studies (17–19, 21, 22, 32–47). The sample size ranged between 11 and 80, and the mean age of the patients was recorded ranged from a minimum of 45 years old to a maximum of 76 years old. The majority of the patients were from Asia. The articles were studied from 2013 to 2023 (Table 1).
3.3 Risk of bias assessment
The risk of bias assessment is presented in Supplementary Figures S1, S2. Most of the included studies were considered to have a low or unclear risk of bias. Two papers had significant baseline imbalance (17, 36). The main source of bias in two pieces of literatures was the failure to implement double blinding (21, 44). Six literatures were at high risk because of poor completeness of data results (19, 32–34, 44, 46).
3.4 Renal function parameters
3.4.1 Change in blood urea nitrogen
Twelve studies (17, 19, 32, 33, 36, 37, 40–42, 45–47) including 527 patients (271 probiotics/synbiotics; 256 controls) were included in the evaluation of blood urea nitrogen (BUN). Pooled analysis showed that the reduction of BUN in patients treated with probiotics/synbiotics was significantly better than in the control group (SMD: −0.23; 95% CI: −0.41, −0.04; p = 0.02; Figure 2A). No evidence of significant heterogeneity (I2 = 10%, p = 0.34) and statistical (Egger’s test, p = 0.452) or visual (Figure 3A) publication bias was detected.
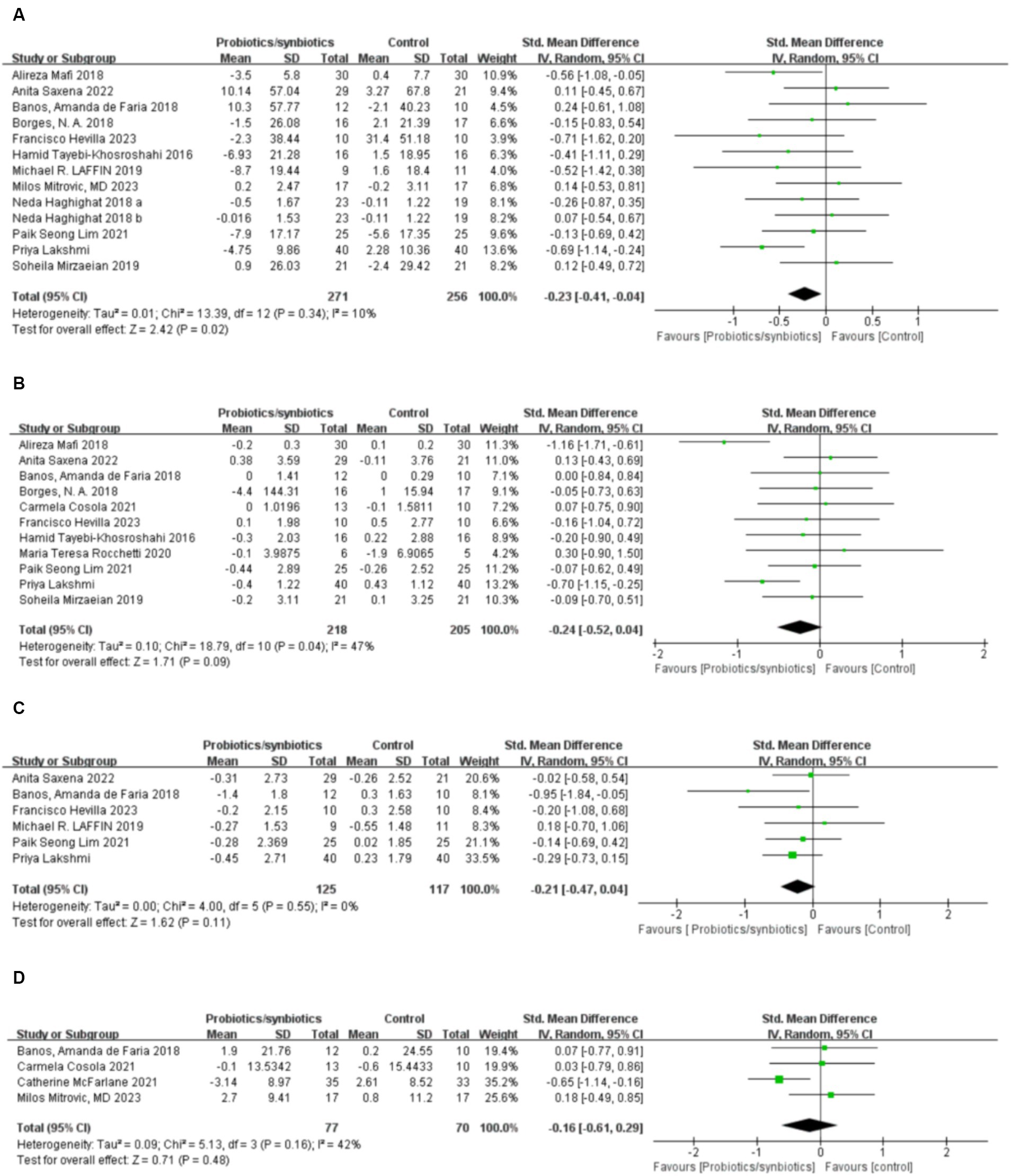
Figure 2. Forest plots of kidney function outcomes: (A) BUN, (B) serum creatinine, (C) uric acid, (D) eGFR.
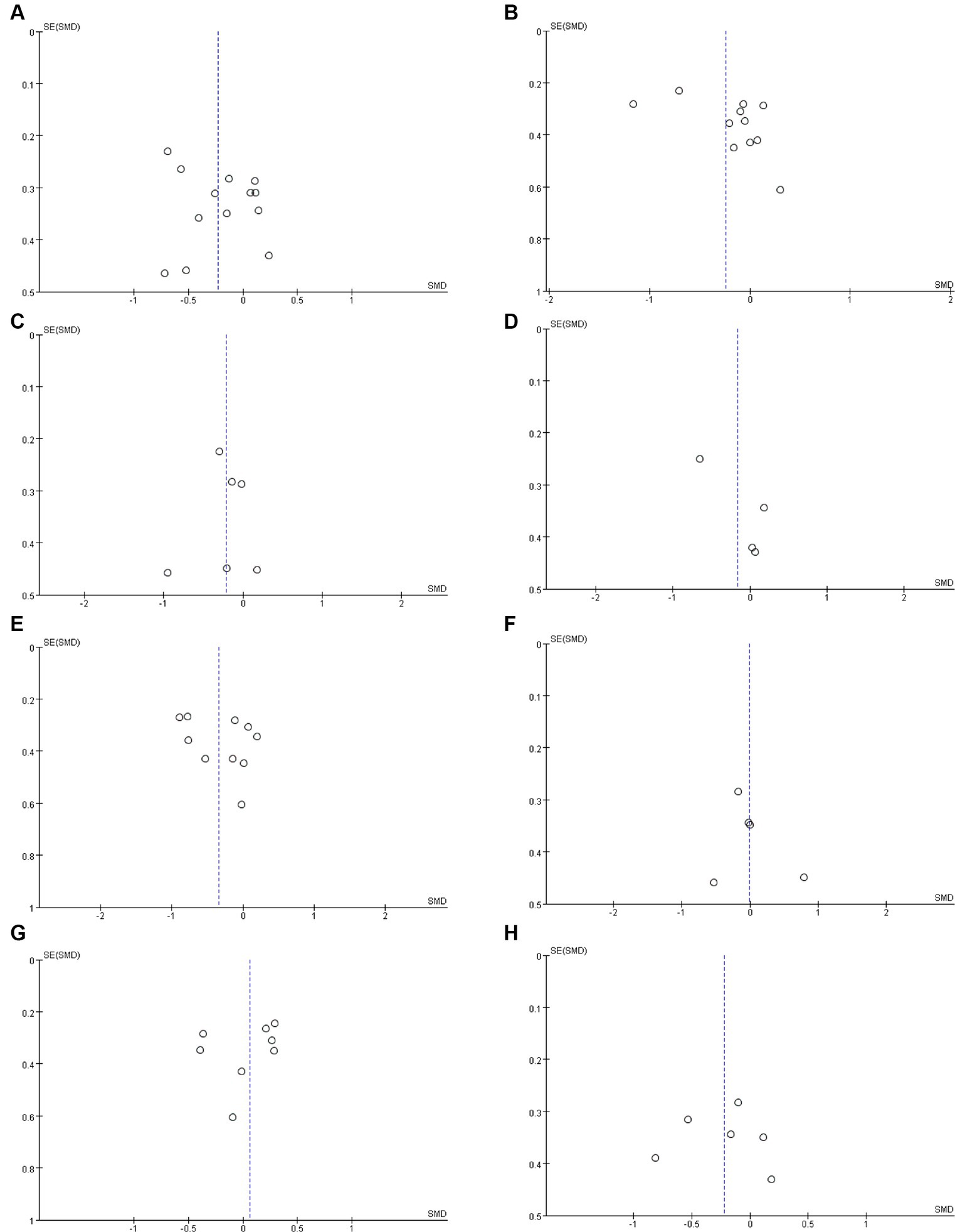
Figure 3. Funnel plots of (A) BUN, (B) serum creatinine, (C) uric acid, (D) eGFR, (E) CRP, (F) IL-6, (G) indoxyl sulfate (H) p-cresyl sulfate.
Based on a range of subgroup analyses, we did not observe an effect of probiotic/synbiotics supplementation on BUN in American patients (k = 3, SMD: −0.13, 95% CI: −0.59, 0.33, I2 = 0%, p = 0.57). However, we found a significant decrease in BUN following probiotic/synbiotics supplementation in Asian individuals (k = 8, SMD: −0.28, 95% CI: −0.48, −0.09, I2 = 22%, p = 0.004). In addition, probiotic/synbiotics supplementation significantly reduced BUN in the long-term treatment (≥3 months; k = 10, SMD: −0.23, 95% CI: −0.44, −0.01, I2 = 21%, p = 0.04), but not in the short term (<3 months; k = 3, SMD: −0.19, 95% CI: −0.60, 0.22, I2 = 0%, p = 0.35). In addition, probiotics/synbiotics did not change BUN level in the HD patients (k = 7, SMD: −0.15, 95% CI: −0.4, 0.1, I2 = 0%, p = 0.23). However, probiotics/synbiotics significantly decreased the BUN level in non-HD patients (k = 6, SMD: −0.31, 95% CI: −0.54, −0.07, I2 = 45%, p = 0.01). And probiotics/synbiotics did not change BUN level in patients ≥60 years (k = 4, SMD: 0.01, 95% CI: −0.35, 0.38, I2 = 0%, p = 0.95) and patients <6 0 y (k = 8, SMD: −0.21, 95% CI: −0.43, 0.00, I2 = 0%, p = 0.05; Table 2).
3.4.2 Change in serum creatinine
Eleven articles (17, 19, 21, 22, 32, 33, 40, 42, 45–47) were included in the analysis of serum creatinine levels involving 423 patients (218 probiotics/synbiotics; 205 controls). The evidence synthesis showed similar changes of serum creatinine in patients in the probiotic/synbiotics group and the placebo group (SMD: −0.24; 95% CI: −0.52, 0.04; p = 0.09) without significant heterogeneity (I2 = 47%, p = 0.04; Figure 2B). No publication bias was detected by the funnel plot (Figure 3B) or Egger’s test (p = 0.097).
Based on a series of subgroup analyses (Table 2), we did not observe any changes in serum creatinine following probiotic/synbiotics supplementation in individuals on hemodialysis (k = 5, SMD: −0.06, 95% CI: −0.37, 0.26, I2 = 0%, p = 0.72) and non-hemodialysis individuals (k = 6, SMD: −0.36, 95% CI: −0.8, 0.09, I 2 = 66%, p = 0.11). Based on geographical location, we observed no significant change of serum creatinine in countries located in America (k = 2; SMD: −0.03, 95% CI: −0.56, 0.5, I2 = 61%, p = 0.03), Asia (k = 6, SMD: −0.36, 95% CI: −0.76, 0.04, I2 = 67%, p = 0.07) and Europe (k = 2; SMD: 0.15, 95% CI: −0.53, 0.83, I2 = 0%, p = 0.67). In addition, probiotics/synbiotics did not change the serum creatinine in the short term (<3 months; k = 4, SMD: −0.05, 95% CI: −0.43, 0.33, I2 = 0%, p = 0.79) and the long term (≥3 months; k = 7, SMD: −0.32, 95% CI: −0.7, 0.06, I2 = 63%, p = 0.1) or in older patients ≥ 60 years (k = 3, SMD: −0.08, 95% CI: −0.51, 0.34, I2 = 0%, p = 0.7) and younger patients < 60 years (k = 7, SMD: −0.28, 95% CI: −0.64, 0.08, I2 = 60%, p = 0.13).
3.4.3 Change in uric acid
Six studies (17, 19, 32, 37, 42, 46) including 242 patients (125 probiotics/synbiotics; 117 controls) were included in the analysis of uric acid. Pooled analysis showed similar levels of alteration of uric acid in the probiotic/synbiotics group and the control group (SMD: −0.21; 95% CI: −0.47, 0.04; p = 0.11; Figure 2C). No significant heterogeneity (I2 = 0%, p = 0.55) and no evidence of statistical (Egger’s test, p = 0.799) or visual (Figure 3C) publication bias was observed.
3.4.4 Change in eGFR
Four articles (21, 32, 39, 41) reported data on eGFR levels between the two groups of 147 cases (77 probiotics/synbiotics; 70 controls). Evidence synthesis observed similar changes in eGFR levels in patients with probiotics/synbiotics and placebo (SMD: −0.16; 95% CI: −0.61, 0.29; p = 0.48), with significant heterogeneity (I2 = 42%, p = 0.16; Figure 2D). No evidence of visual publication bias was observed (Figure 3D).
3.5 Inflammation indicators and uremic toxins
3.5.1 Change in C-reactive protein
Ten studies (17, 18, 21, 22, 32, 40, 41, 43, 46, 47) with a total of 356 patients (181 probiotics/synbiotics patients; 175 control patients) were included in the analysis of CRP. Pooled analysis showed that the probiotic/synbiotics group was significantly more effective in reducing CRP than the control group (SMD: −0.34; 95% CI: −0.62, −0.07; p = 0.01; Figure 4A). No significant heterogeneity was observed (I2 = 37%, p = 0.12) and no evidence of statistical (Egger’s test, p = 0.288) or visual (Figure 3E) publication bias was observed.
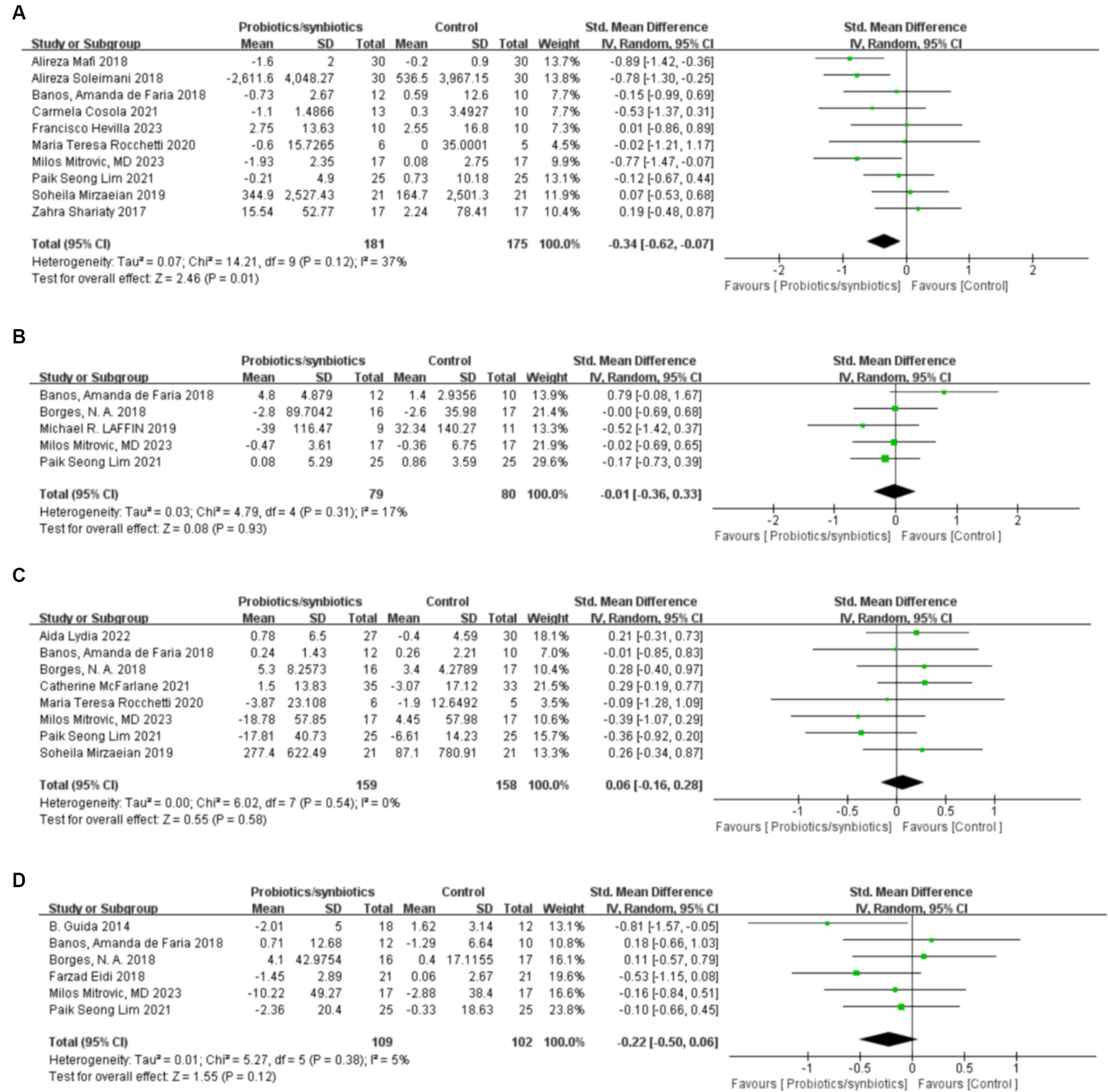
Figure 4. Forest plots of inflammation and uremic toxins outcomes: (A) CRP, (B) IL-6, (C) Indoxyl sulfate, (D) p-cresyl sulfate.
Subgroup analysis based on patient population suggested no changes in CRP following probiotics/synbiotics supplementation in individuals on hemodialysis (k = 5, SMD: −0.16, 95% CI: −0.49, 0.17, I2 = 29%, p = 0.34; Table 2). Furthermore, probiotics/synbiotics did not change CRP level in American individuals (k = 5, SMD: −0.33, 95% CI: −0.76, 0.11, I2 = 65%, p = 0.14), older individuals (k = 5, SMD: −0.36, 95% CI: −0.76,0.03, I2 = 39%, p = 0.07), younger individuals (k = 5, SMD: −0.31, 95% CI: −0.75, 0.12, I2 = 48%, p = 0.16) and individuals treated for a shorter period of time (k = 4, SMD: −0.02, 95% CI: −0.40, 0.36, I2 = 0%, p = 0.92). However, we found a significant decrease in CRP following probiotics/synbiotics supplementation in non-hemodialysis individuals (k = 5, SMD: −0.67, 95% CI: −1.02, −0.33, I2 = 0%, p = 0.0001), individuals treated for a longer period of time (k = 4, SMD: −0.51, 95% CI: −0.83, −0.19, I2 = 33%, p = 0.002) and European individuals (k = 3, SMD: −0.56, 95% CI: −1.05, −0.07, I2 = 0%, p = 0.03).
3.5.2 Change in IL-6
Five studies (17, 32, 33, 37, 41) were included in the analysis of IL-6, comprising a total of 159 patients (79 probiotics/synbiotics patients; 80 control patients). Pooled analysis suggested no statistically significant difference in the change of IL-6 levels between the two groups (SMD: −0.01; 95% CI: −0.36, 0.33; p = 0.93; Figure 4B). No significant heterogeneity was observed (I2 = 17%, p = 0.31). Nor was evidence of publication bias observed statistically Egger’s test, (p = 0.626) or visually (Figure 3F).
3.5.3 Change in indoxyl sulfate
Three hundred and seventeen patients from 8 studies (17, 22, 32, 33, 38–41) were included in the analysis of indoxyl sulfate (159 probiotics/synbiotics patients; 158 control patients). No statistically significant differences were found in the results of the pooled analysis between the two groups (SMD: 0.06; 95% CI: −0.16, 0.28; p = 0.58; Figure 4C). No significant heterogeneity was observed (I2 = 0%, p = 0.54) as well as evidence of statistical (Egger’s test, p = 0.507) or visual (Figure 3G) publication bias.
Subgroup analysis based on patient population revealed no changes in indoxyl sulfate level after evaluation with either hemodialysis patients (k = 4, SMD: 0.03, 95% CI: −0.29, 0.34, I2 = 0%, p = 0.87) or non-hemodialysis patients (k = 4, SMD: 0.10, 95% CI: −0.21, 0.42, I2 = 0%, p = 0.53; Table 2). Furthermore, probiotics did not change the indoxyl sulfate level in the short term (<3 months; k = 3, SMD: 0.20, 95% CI: −0.18, 0.58, I2 = 0%, p = 0.30) and the long term (≥3 months; k = 5, SMD: −0.02, 95% CI: −0.33, 0.29, I2 = 19%, p = 0.90) or in older patients (≥60 years; k = 4, SMD: 0.11, 95% CI: −0.20, 0.41, I2 = 10%, p = 0.50) and younger patients (<60 years; k = 4, SMD: 0.02, 95% CI: −0.31, 0.34, I2 = 0%, p = 0.93). Besides, probiotics did not change the indoxyl sulfate level in Asia individuals (k = 4, SMD: 0.03, 95% CI: −0.36, 0.42, I2 = 31%, p = 0.86), America individuals (k = 2, SMD: 0.17, 95% CI: −0.37, 0.70, I2 = 0%, p = 0.54) and European individuals (k = 2, SMD: −0.32, 95% CI: −0.91, 0.27, I2 = 0%, p = 0.29).
3.5.4 Change in p-cresyl sulfate
A total of 211 patients from 6 studies (17, 32–35, 41) were included in the analysis of p-cresyl sulfate (109 probiotics/synbiotics patients; 102 control patients). No significant statistical differences were found in the results of the pooled analyses between the probiotics/synbiotics group and the control group (SMD: −0.22; 95% CI: −0.5, 0.06; p = 0.12; Figure 4D). No significant heterogeneity (I2 = 5%, p = 0.38) was observed as well as statistical (Egger’s test, p = 0.947) or visual (Figure 3H) evidence of publication bias.
3.6 Lipid metabolism-related indicators
3.6.1 Change in total cholesterol
A total of six articles (17, 32, 39, 43, 46, 47) involving 280 patients were included to analyze total cholesterol levels (142 probiotics/synbiotics patients; 138 control patients). Pooled analysis showed that the changes in total cholesterol levels were similar in the probiotics/synbiotics group and the control group (SMD: −0.16; 95% CI: −0.39, 0.08; p = 0.19) with no significant heterogeneity (I2 = 0%, p = 0.8; Figure 5A). Neither the funnel plot (Figure 6A) nor the Egger test (p = 0.544) revealed publication bias.
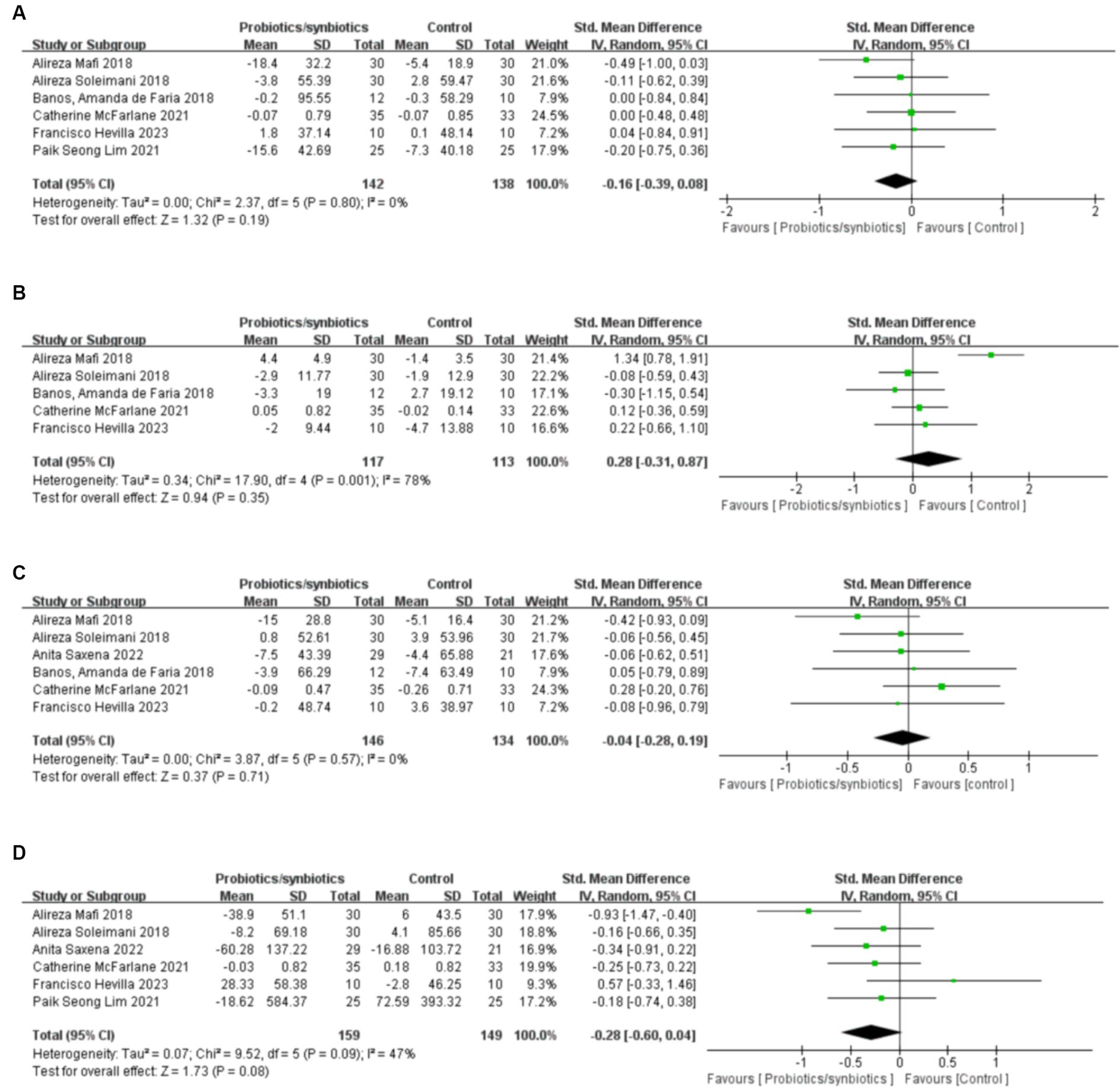
Figure 5. Forest plots of lipid metabolism evaluation outcomes: (A) total cholesterol, (B) HDL, (C) LDL, (D) triglyceride.
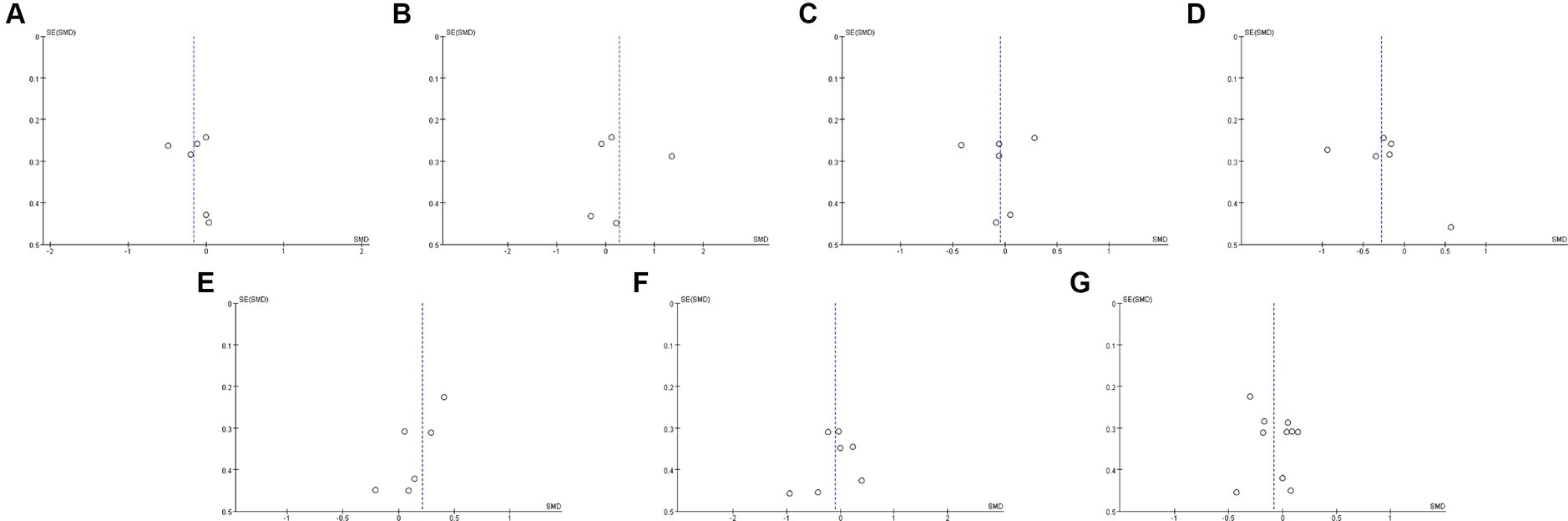
Figure 6. Funnel plots of (A) total cholesterol, (B) HDL, (C) LDL, (D) triglyceride, (E) blood calcium, (F) blood potassium, (G) blood phosphorus.
3.6.2 Change in high density lipoprotein
Five studies (32, 39, 43, 46, 47) were included in the analysis of high density lipoprotein (HDL), involving 230 patients (117 probiotics/synbiotics patients; 113 control patients). No significant statistical differences were found in the results of the pooled analyses between the probiotics/synbiotics group and the control group (SMD: 0.28; 95% CI: −0.31, 0.87; p = 0.35; Figure 5B). Significant heterogeneity (I2 = 78%, p = 0.001) was observed. There was no statistical (Egger’s test, p = 0.874) or visual (Figure 6B) evidence of publication bias.
3.6.3 Change in low density lipoprotein
Six articles (32, 39, 42, 43, 46, 47) were included in the analysis for low density lipoprotein (LDL), involving 280 patients (146 probiotics/synbiotics group patients; 134 control patients). Evidence synthesis showed that probiotics/synbiotics group had a similar change in LDL level with the control group (SMD: −0.04; 95% CI: −0.28, 0.19; p = 0.71) with no significant heterogeneity (I 2 = 0%, p = 0.57; Figure 5C). Both funnel plot (Figure 6C) and Egger’s test (p = 0.936) did not detect publication bias.
3.6.4 Change in triglyceride
Analysis of triglyceride levels in 308 patients (159 probiotic/synbiotics patients; 149 control patients) from six publications (17, 39, 42, 43, 46, 47) showed that there was no statistically significant difference (SMD: −0.28; 95% CI: −0.6, 0.04; p = 0.08) or significant heterogeneity (I 2 = 47%, p = 0.09) in the change of triglyceride levels in the probiotics/synbiotics group compared with the control group (Figure 5D). No publication bias was found after both funnel plot (Figure 6D) and Egger’s test (p = 0.26) evaluation.
3.7 Electrolytes
3.7.1 Change in blood calcium
Six publications (19, 21, 34, 37, 40, 46) involving 227 patients (114 probiotics/synbiotics patients; 113 control patients) were included in the analysis regarding blood calcium levels, and the results suggested that there were no statistically significant differences (SMD: 0.21; 95% CI: −0.05, 0.47; p = 0.11) and no significant heterogeneity (I 2 = 0%, p = 0.84) in the change of blood calcium levels in patients in the probiotics/synbiotics group compared with the control group (Figure 7A). It is worth noting that funnel plots (Figure 6E) and Egger’s test revealed significant publication bias (p = 0.049).
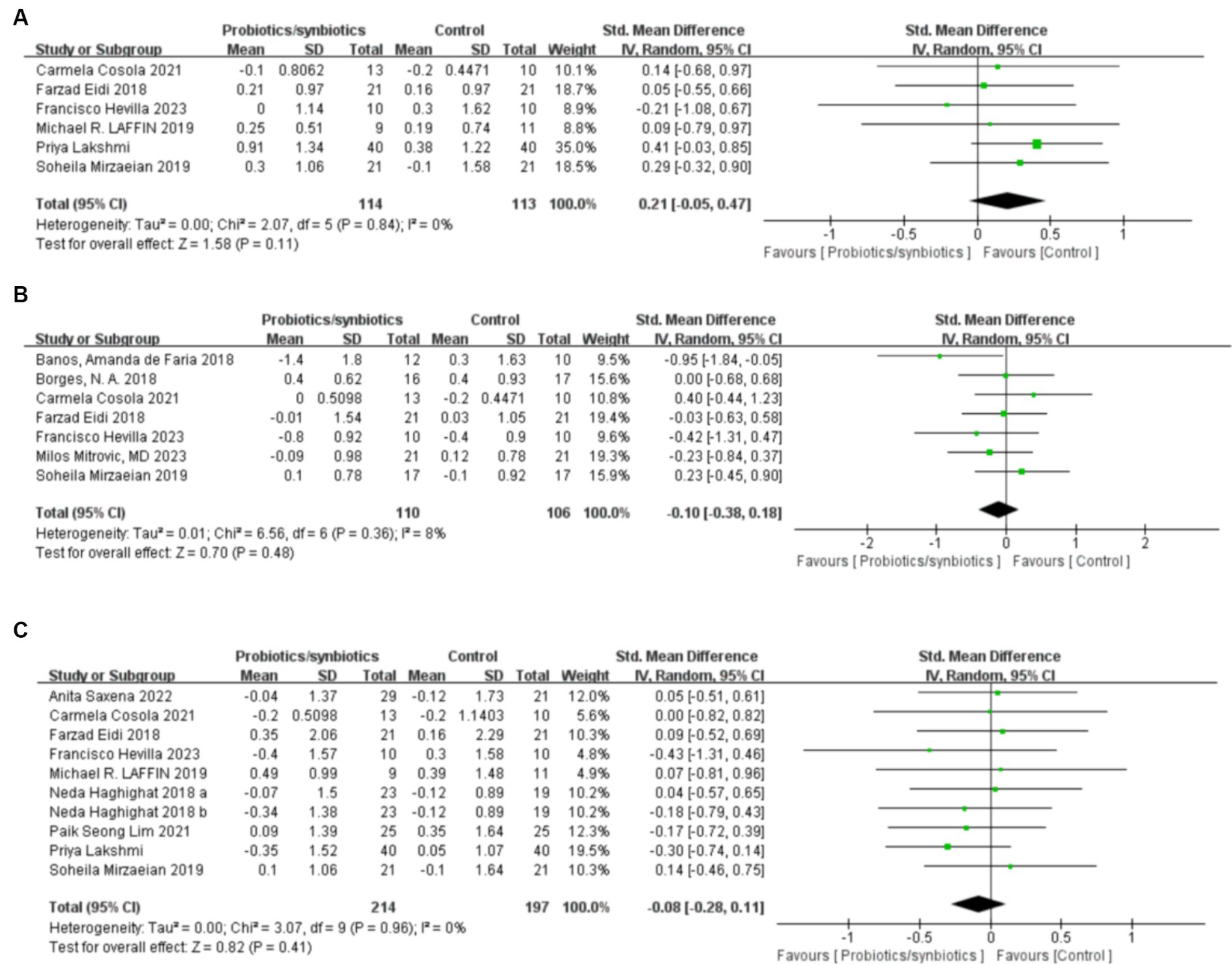
Figure 7. Forest plots of electrolytic outcomes: (A) blood calcium, (B) blood potassium, (C) blood phosphorus.
3.7.2 Change in blood potassium
Two hundred and sixteen patients (110 probiotics/synbiotics patients; 106 control patients) originating from seven publications (21, 32–34, 40, 41, 46) were included in the analysis regarding blood potassium levels. And the results suggested that there were no statistically significant differences (SMD: −0.1; 95% CI: −0.38, 0.18; p = 0.48) and no significant heterogeneity (I 2 = 8%, p = 0.36) in the change of blood potassium levels of the patients in the probiotics/synbiotics group compared with the control group (Figure 7B). Funnel plot (Figure 6F) and Egger’s test did not reveal significant publication bias (p = 0.426).
3.7.3 Change in blood phosphorus
Nine publications (17, 19, 21, 34, 36, 37, 40, 42, 46) analyzed blood phosphorus levels in 411 patients (214 probiotics/synbiotics patients; 197 control patients). After comprehensive analysis, there was no statistically significant difference (SMD: −0.08; 95% CI: −0.28, 0.11; p = 0.41) or heterogeneity (I 2 = 0%, p = 0.96) in the change of blood phosphorus levels of the patients in the probiotics/synbiotics group compared with the control group (Figure 7C). Funnel plots (Figure 6G) and Egger’s test did not reveal significant publication bias (p = 0.503).
Based on an array of subgroup analyses, we did not observe an effect of probiotics/ synbiotics supplementation on blood phosphorus in hemodialysis individuals (k = 7, SMD: −0.05, 95% CI: −0.29, 0.2, I2 = 0%, p = 0.72) or non-hemodialysis individuals (k = 3, SMD: −0.14, 95% CI: −0.46, 0.18, I2 = 0%, p = 0.38). Based on geographical location, we observed no significant change of blood phosphorus in patients from countries located in Asia (k = 7; SMD: −0.08, 95% CI: −0.29, 0.14, I2 = 0%, p = 0.48). In terms of treatment time, we observed no significant change of blood phosphorus in both long term (k = 6; SMD: −0.16, 95% CI: −0.39, 0.08, I2 = 0%, p = 0.19) and short term (k = 4, SMD: 0.09, 95% CI: −0.26, 0.44, I2 = 0%, p = 0.62). In addition, no significant changes in blood phosphorus were observed with probiotics/synbiotics supplementation in individuals above and below 60 years of age (≥60 years, k = 2, SMD: −0.04, 95% CI: −0.57, 0.48, I2 = 7%, p = 0.87), (<60 years, k = 7, SMD: −0.03, 95% CI: −0.27, 0.22, I2 = 0%, p = 0.84; Table 2).
3.8 Sensitivity analysis
Because the comprehensive analysis of HDL showed significant heterogeneity, we conducted one-way sensitivity analyses for comparison of HDL to evaluate the influence of each individual study on the combined SMD through removing the individual study one by one. Sensitivity analyses revealed that the new combined SMD remained constant after exclusion of any individual study for HDL (Figure 8).
4 Discussion
4.1 Findings from meta-analysis
The gut microbiota consists of more than 100 trillion bacteria and plays an important role in normal body functions, particularly in immune and metabolic homeostasis. There is growing evidence that alterations in the gut microbiota can affect multiple organ systems and lead to many chronic diseases such as CKD (48). CKD is a serious and steadily growing health problem worldwide. As a progressive disease, the majority of CKD patients are referred to dialysis treatment, and effective pharmacological treatments are still being explored (49). One of the promising drug candidates is the modification of dysbiotic gut flora through probiotics/synbiotics supplementation to reduce levels of gut-derived uremic toxins and reduce chronic microinflammation thereby improving renal function (50). Due to the small number of published studies, it is still highly controversial whether this probiotics/synbiotics intervention affects renal function, uremic toxicity, and inflammation levels in patients with CKD. In this study, we systematically compiled and analyzed the clinical evidence of RCT on probiotics/synbiotics for the treatment of CKD to provide better guidance for clinical practice.
Our results showed that probiotic/synbiotics supplementation of CKD patients can decreased BUN in CKD patients, and no significant heterogeneity was found in the analysis results, which to a certain extent reflects the effect of probiotic/synbiotics to improve renal function in CKD patients. However, the use of probiotic/synbiotics had no effect on eGFR and serum creatinine, indicators of renal function. And the analysis results of eGFR and serum creatinine did not show apparent heterogeneity. This result may be due to the fact that eGFR and serum creatinine is strongly influenced by ethnicity, gender and age. And the baseline could not be standardized across studies. In addition to this, the use of probiotics/synbiotics can reduce CRP expression levels and improve inflammatory status in CKD patients. However, probiotic/synbiotic supplementation did not significantly alter blood electrolyte levels and lipid metabolism-related markers in CKD patients compared with placebo. No significant publication bias was detected by Egger’s test and funnel plot for all indicators except blood calcium.
We also stratified patients according to region, duration of treatment, age, and whether or not they were receiving dialysis treatment. Subgroups were analyzed for several important indicators: urea nitrogen, serum creatinine, CRP, indoxyl sulfate, and blood phosphorus. The results of subgroup analyses suggested that probiotics/synbiotics were more effective in reducing BUN in non-hemodialysis CKD patients. This probably due to the fact that CKD in hemodialysis patients usually has progressed to the stage of end-stage renal disease, and the renal units are irreversibly damaged, which is difficult to be improved by drug treatment (51). The results of subgroup analysis also showed that probiotics/synbiotics had a better effect on CRP reduction in non-hemodialysis CKD patients, suggesting that probiotics/synbiotics had a better effect on the improvement of inflammation in non-hemodialysis patients. In CKD patients, increased inflammation is associated with several negative clinical outcomes such as increased oxidative stress, vascular dysfunction and increased risk of cardiovascular disease, as the ameliorative effect of probiotics/synbiotics on inflammation in non-hemodialysis patients is beneficial to patients (52, 53). And one of the goals of treatment for hemodialysis patients is to reduce inflammation, thus effectively improving the survival of these patients (54). Meanwhile, the results of the subgroup analysis stratified on the basis of the duration of probiotics/synbiotics treatment showed that there is a significant reduction in BUN and CRP levels in CKD patients when probiotics/synbiotics are applied for a longer period of time compared to the control group. This suggested that adherence to probiotics/synbiotics for a longer period of time is more favorable for CKD patients. Interestingly, when subgroup analyses were performed on a regional basis, we found that probiotics/synbiotics supplementation was more effective in reducing BUN in Asian patients and in reducing CRP in patients from the Europe. The number of studies that included patients from the Europe was small, so the related results need to be further verified.
4.2 Possible mechanisms
The relationship between probiotics/synbiotics and CKD has been recognized with the increasing understanding of the health effects of microbial balance on the host. Essentially, probiotics/synbiotics supplementation can modulate the imbalance of the gut microbiota for the biosynthesis of targeted compounds with bioactive properties in CKD patients (55). At the same time, probiotics improves the integrity of the intestinal epithelial barrier and reduces the production of uremic toxins to some extent (56, 57). With the fluctuation of gut bacteria, probiotics can modulate inflammation by establishing a balance between pro-inflammatory and anti-inflammatory cytokines in the body (58). In addition, metabolites from the gut microbiota also play an important role in maintaining homeostasis in the gut for the benefit of host health through fermentation of amino acids and dietary fiber, production of vitamins and neurotransmitters, and modification of bile acids (59). For example, Zhu et al. (60) showed that short-chain fatty acids (SCFAs) from a variety of bacteria reduced the expression of genes for inflammatory cytokines, chemokines, and pro-fibrotic proteins in diabetic kidneys, which, in turn, reduced proteinuria, glomerular hypertrophy, pedunculated cell injury, and interstitial fibrosis in mice with acute kidney injury and CKD. Indeed, probiotic or synbiotics supplementation may also reverse the expansion of harmful gut microbes that produce excess uremic toxins and attenuate the development of CKD (32, 61).
4.3 Strengths and limitations
4.3.1 Limitations
Firstly, most of the publications included in this meta-analysis were RCT cohort studies. The sample sizes of RCT studies are small, and potential bias from small samples is unavoidable. Secondly, the main population groups of the studies we included were from Asia, with fewer people from other states, and there may be regional selectivity bias. Whether the results can be generalized to other regions needs to be confirmed by further studies. Thirdly, the heterogeneity of the studies included in the analysis of HDL was large, which may hinder the robustness of the results. In addition, the RCTs involved in the study did not report adverse events in patients, so adverse events were not included in the study.
4.3.2 Strengths
Firstly, this study is the latest meta-analysis of probiotics/synbiotics for CKD with the largest sample size available. Secondly, the original studies included in this article were all RCTs, which were of high quality, with good study design and a balanced baseline. Thirdly, this study confirmed that probiotics/synbiotics had an ameliorative effect on renal function and inflammatory status in patients with CKD, which has been consistent with previous studies. Fourthly, compared with previous meta-analyses, our study included a wider range of outcome indicators and incorporated the most recent RCT studies, thus allowing for the most up-to-date evidence on probiotics/synbiotics supplementation in CKD treatment. What’s more, this study provides more options and guidance notes for clinical CKD treatment.
5 Conclusion
In conclusion, this is the latest systematic review and meta-analysis demonstrating that probiotic/synbiotics interventions reduced BUN and CRP in patients with CKD, although there was insufficient evidence of a positive effect of probiotics/synbiotics on lipids and blood electrolytes. Regarding BUN and CRP, the results of our meta-analysis emphasize the positive effects of probiotic/synbiotics supplementation using longer (≥3 months) treatment durations in Asian patients. This area deserves further research to elucidate the mechanism of probiotics/synbiotics for the possible treatment of CKD and to further assess the safety of different types of probiotics/synbiotics through randomized controlled trials.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
CL: Formal analysis, Writing – original draft. LY: Writing – review & editing. WW: Investigation, Writing – review & editing. PF: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. 1.3.5 project for disciplines of excellence from West China Hospital of Sichuan University (grant number ZYGD23015).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1434613/full#supplementary-material
References
1. Eckardt, K-U, Coresh, J, Devuyst, O, Johnson, RJ, Köttgen, A, Levey, AS, et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. (2013) 382:158–69. doi: 10.1016/S0140-6736(13)60439-0
2. Li, L, Astor, BC, Lewis, J, Hu, B, Appel, LJ, Lipkowitz, MS, et al. Longitudinal progression trajectory of GFR among patients with CKD. Am J Kidney Dis. (2012) 59:504–12. doi: 10.1053/j.ajkd.2011.12.009
3. Liu, J, Bankir, L, Verma, A, Waikar, SS, and Palsson, R. Association of the urine-to-plasma urea ratio with CKD progression. Am J Kidney Dis. (2023) 81:394–405. doi: 10.1053/j.ajkd.2022.09.010
4. Kula, AJ, Prince, DK, Flynn, JT, and Bansal, N. BP in young adults with CKD and associations with cardiovascular events and decline in kidney function. J Am Soc Nephrol. (2021) 32:1200–9. doi: 10.1681/ASN.2020081156
5. Mazidi, M, Kengne, AP, Siervo, M, and Kirwan, R. Association of dietary intakes and genetically determined serum concentrations of mono and poly unsaturated fatty acids on chronic kidney disease: insights from dietary analysis and Mendelian randomization. Nutrients. (2022) 14:1231. doi: 10.3390/nu14061231
6. Liu, X, Gao, W, Yang, J, Mao, G, Lu, H, and Xing, W. Association between probiotic, prebiotic, and yogurt consumption and chronic kidney disease: the NHANES 2010-2020. Front Nutr. (2022) 9:1058238. doi: 10.3389/fnut.2022.1058238
7. Zoccali, C, Vanholder, R, Massy, ZA, Ortiz, A, Sarafidis, P, Dekker, FW, et al. The systemic nature of CKD. Nat Rev Nephrol. (2017) 13:344–58. doi: 10.1038/nrneph.2017.52
8. Neuen, BL, Heerspink, HJL, Vart, P, Claggett, BL, Fletcher, RA, Arnott, C, et al. Estimated lifetime cardiovascular, kidney and mortality benefits of combination treatment with SGLT2 inhibitors, GLP-1 receptor agonists, and non-steroidal MRA compared with conventional care in patients with type 2 diabetes and albuminuria. Circulation. (2023) 149:450–62. doi: 10.1161/CIRCULATIONAHA.123.067584
9. Durack, J, and Lynch, SV. The gut microbiome: relationships with disease and opportunities for therapy. J Exp Med. (2019) 216:20–40. doi: 10.1084/jem.20180448
10. Martens, EC, Neumann, M, and Desai, MS. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat Rev Microbiol. (2018) 16:457–70. doi: 10.1038/s41579-018-0036-x
11. Krukowski, H, Valkenburg, S, Madella, A-M, Garssen, J, van Bergenhenegouwen, J, Overbeek, SA, et al. Gut microbiome studies in CKD: opportunities, pitfalls and therapeutic potential. Nat Rev Nephrol. (2023) 19:87–101. doi: 10.1038/s41581-022-00647-z
12. Glorieux, G, Nigam, SK, Vanholder, R, and Verbeke, F. Role of the microbiome in gut-heart-kidney cross talk. Circ Res. (2023) 132:1064–83. doi: 10.1161/CIRCRESAHA.123.321763
13. Meijers, BKI, and Evenepoel, P. The gut-kidney axis: indoxyl sulfate, p-cresyl sulfate and CKD progression. Nephrol Dial Transplant. (2011) 26:759–61. doi: 10.1093/ndt/gfq818
14. Pahl, MV, and Vaziri, ND. The chronic kidney disease—colonic axis. Semin Dial. (2015) 28:459–63. doi: 10.1111/sdi.12381
15. Meijers, B, Evenepoel, P, and Anders, H-J. Intestinal microbiome and fitness in kidney disease. Nat Rev Nephrol. (2019) 15:531–45. doi: 10.1038/s41581-019-0172-1
16. Li, T, Teng, D, Mao, R, Hao, Y, Wang, X, and Wang, J. A critical review of antibiotic resistance in probiotic bacteria. Food Res Int. (2020) 136:109571. doi: 10.1016/j.foodres.2020.109571
17. Lim, PS, Wang, HF, Lee, MC, Chiu, L-S, Wu, M-Y, Chang, W-C, et al. The efficacy of Lactobacillus-containing probiotic supplementation in hemodialysis patients: a randomized, double-blind, placebo-controlled trial. J Ren Nutr. (2021) 31:189–98. doi: 10.1053/j.jrn.2020.07.002
18. Shariaty, Z, Mahmoodi Shan, GR, Farajollahi, M, Amerian, M, and Behnam Pour, N. The effects of probiotic supplement on hemoglobin in chronic renal failure patients under hemodialysis: a randomized clinical trial. J Res Med Sci. (2017) 22:74. doi: 10.4103/jrms.JRMS_614_16
19. Lakshmi, P, Babu, S, Ahil, M, Raadhika, M, Susila, M, and Geetha, M. Effect of lobun probiotics in patients with chronic kidney disease stage III and IV, placebo-controlled open-labeled study. Br J Clin Pharmacol. (2020) 86:1198–9. doi: 10.1111/bcp.14266
20. Swanson, KS, Gibson, GR, Hutkins, R, Reimer, RA, Reid, G, Verbeke, K, et al. The international scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol. (2020) 17:687–701. doi: 10.1038/s41575-020-0344-2
21. Cosola, C, Rocchetti, MT, Di, B, Acquaviva, PM, Maranzano, V, Corciulo, S, et al. An innovative Synbiotic formulation decreases free serum Indoxyl sulfate, small intestine permeability and ameliorates gastrointestinal symptoms in a randomized pilot trial in stage IIIb-IV CKD patients. Toxins. (2021) 13:334. doi: 10.3390/toxins13050334
22. Rocchetti, MT, Cosola, C, di Bari, I, Magnani, S, Galleggiante, V, Scandiffio, L, et al. Efficacy of Divinylbenzenic resin in removing Indoxyl sulfate and P-cresol sulfate in hemodialysis patients: results from an in vitro study and an in vivo pilot trial (xuanro4-nature 3.2). Toxins. (2020) 12:170. doi: 10.3390/toxins12030170
23. Nakabayashi, I, Nakamura, M, Kawakami, K, Ohta, T, Kato, I, Uchida, K, et al. Effects of synbiotic treatment on serum level of p-cresol in haemodialysis patients: a preliminary study. Nephrol Dial Transplant. (2011) 26:1094–8. doi: 10.1093/ndt/gfq624
24. Vacca, M, Celano, G, Calabrese, FM, Rocchetti, MT, Iacobellis, I, Serale, N, et al. In vivo evaluation of an innovative synbiotics on stage IIIb-IV chronic kidney disease patients. Front Nutr. (2023) 10:1215836. doi: 10.3389/fnut.2023.1215836
25. Sanders, ME, Shane, AL, and Merenstein, DJ. Advancing probiotic research in humans in the United States: challenges and strategies. Gut Microbes. (2016) 7:97–100. doi: 10.1080/19490976.2016.1138198
26. Barrows, IR, Ramezani, A, and Raj, DS. Gut feeling in AKI: the long arm of short-chain fatty acids: the long arm of short-chain fatty acids. J Am Soc Nephrol. (2015) 26:1755–7. doi: 10.1681/ASN.2014111157
27. De Araújo, ÉMR, Meneses, GC, Carioca, AAF, Martins, AMC, Daher, EDF, and Silva Junior, GB. Use of probiotics in patients with chronic kidney disease on hemodialysis: a randomized clinical trial. J Bras Nefrol. (2023) 45:152–61. doi: 10.1590/2175-8239-JBN-2022-0021en
28. Chen, L, Shi, J, Ma, X, Shi, D, and Qu, H. Effects of microbiota-driven therapy on circulating Indoxyl sulfate and P-Cresyl sulfate in patients with chronic kidney disease: a systematic review and Meta-analysis of randomized controlled trials. Adv Nutr. (2022) 13:1267–78. doi: 10.1093/advances/nmab149
29. Zheng, HJ, Guo, J, Wang, Q, Wang, L, Wang, Y, Zhang, F, et al. Probiotics, prebiotics, and synbiotics for the improvement of metabolic profiles in patients with chronic kidney disease: a systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. (2021) 61:577–98. doi: 10.1080/10408398.2020.1740645
30. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. (2021) 134:178–89. doi: 10.1016/j.jclinepi.2021.03.001
31. Cumpston, M, Li, T, Page, MJ, Chandler, J, Welch, VA, Higgins, JPT, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10:ED000142. doi: 10.1002/14651858.ED000142
32. de Faria Barros, A, Borges, NA, Nakao, LS, Dolenga, CJ, Do Carmo, FL, De Carvalho Ferreira, D, et al. Effects of probiotic supplementation on inflammatory biomarkers and uremic toxins in non-dialysis chronic kidney patients: a double-blind, randomized, placebo-controlled trial. J Funct Foods. (2018) 46:378–83. doi: 10.1016/j.jff.2018.05.018
33. Borges, NA, Carmo, FL, Stockler-Pinto, MB, de Brito, JS, Dolenga, CJ, Ferreira, DC, et al. Probiotic supplementation in chronic kidney disease: a double-blind, randomized, placebo-controlled trial. J Ren Nutr. (2018) 28:28–36. doi: 10.1053/j.jrn.2017.06.010
34. Eidi, F, Poor-Reza Gholi, F, Ostadrahimi, A, Dalili, N, Samadian, F, and Barzegari, A. Effect of Lactobacillus Rhamnosus on serum uremic toxins (phenol and P-cresol) in hemodialysis patients: a double blind randomized clinical trial. Clin Nutr ESPEN. (2018) 28:158–64. doi: 10.1016/j.clnesp.2018.08.010
35. Guida, B, Germanò, R, Trio, R, Russo, D, Memoli, B, Grumetto, L, et al. Effect of short-term synbiotic treatment on plasma p-cresol levels in patients with chronic renal failure: a randomized clinical trial. Nutr Metab Cardiovasc Dis. (2014) 24:1043–9. doi: 10.1016/j.numecd.2014.04.007
36. Haghighat, N, Mohammadshahi, M, Shayanpour, S, and Haghighizadeh, MH. Effect of synbiotic and probiotic supplementation on serum levels of endothelial cell adhesion molecules in hemodialysis patients: a randomized control study. Probio Antimicrob Proteins. (2019) 11:1210–8. doi: 10.1007/s12602-018-9477-9
37. Laffin, MR, Tayebi Khosroshahi, H, Park, H, Laffin, LJ, Madsen, K, Kafil, HS, et al. Amylose resistant starch (HAM-RS2) supplementation increases the proportion of Faecalibacterium bacteria in end-stage renal disease patients: microbial analysis from a randomized placebo-controlled trial. Hemodial Int. (2019) 23:343–7. doi: 10.1111/hdi.12753
38. Aida, L, Tities Anggraeni, I, Aulia, R, and Murdani, A. The effects of synbiotics on indoxyl sulphate level, constipation, and quality of life associated with constipation in chronic haemodialysis patients: a randomized controlled trial. BMC Nephrol. (2022) 23:259. doi: 10.1186/s12882-022-02890-9
39. McFarlane, C, Krishnasamy, R, Stanton, T, Savill, E, Snelson, M, Mihala, G, et al. Synbiotics easing renal failure by improving gut microbiology II (SYNERGY II): a feasibility randomized controlled trial. Nutrients. (2021) 13:481. doi: 10.3390/nu13124481
40. Mirzaeian, S, Saraf-Bank, S., Entezari, MH, Hekmatdoost, A, Feizi, A, and Atapour, A. Effects of synbiotic supplementation on microbiota-derived protein-bound uremic toxins, systemic inflammation, and biochemical parameters in patients on hemodialysis: a double-blind, placebo-controlled, randomized clinical trial. Nutrition. (2020) 73:110713. doi: 10.1016/j.nut.2019.110713
41. Mitrović, M, Stanković-Popović, V, Tolinački, M, Golić, N, Soković Bajić, S, Veljović, K, et al. The impact of synbiotic treatment on the levels of gut-derived uremic toxins, inflammation, and gut microbiome of chronic kidney disease patients-a randomized trial. J Ren Nutr. (2023) 33:278–88. doi: 10.1053/j.jrn.2022.07.008
42. Saxena, A, Srinivasa, S, Veerappan, I, Jacob, C, Mahaldar, A, Gupta, A, et al. Enzobiotics—a novel therapy for the elimination of uremic toxins in patients with CKD (EETOX study): a multicenter double-blind randomized controlled trial. Nutrients. (2022) 14:3804. doi: 10.3390/nu14183804
43. Soleimani, A, Motamedzadeh, A, Zarrati Mojarrad, M, Bahmani, F, Amirani, E, Ostadmohammadi, V, et al. The effects of synbiotic supplementation on metabolic status in diabetic patients undergoing hemodialysis: a randomized, double-blinded, placebo-controlled trial. Probiotics Antimicrob Proteins. (2019) 11:1248–56. doi: 10.1007/s12602-018-9499-3
44. Lopes, RDCSO, Theodoro, JMV, Da Silva, BP, Queiroz, VAV, De Castro Moreira, ME, Mantovani, HC, et al. Synbiotic meal decreases uremic toxins in hemodialysis individuals: a placebo-controlled trial. Food Res Int. (2019) 116:241–8. doi: 10.1016/j.foodres.2018.08.024
45. Tayebi-Khosroshahi, H, Habibzadeh, A, Niknafs, B, Ghotaslou, R, Yeganeh Sefidan, F, Ghojazadeh, M, et al. The effect of lactulose supplementation on fecal microflora of patients with chronic kidney disease; a randomized clinical trial. J Renal Inj Prev. (2016) 5:162–7. doi: 10.15171/jrip.2016.34
46. Hevilla, F, Padial, M, Blanca, M, Barril, G, Jiménez-Salcedo, T, Ramirez-Ortiz, M, et al. Effect on nutritional status and biomarkers of inflammation and oxidation of an oral nutritional supplement (with or without probiotics) in malnourished hemodialysis patients. A multicenter randomized clinical trial “Renacare trial”. Front Nutr. (2023) 10:1107869. doi: 10.3389/fnut.2023.1107869
47. Mafi, ARCFB, Namazi, G, Soleimani, A, Bahmani, F, Aghadavod, E, and Asemi, Z. Metabolic and genetic response to probiotics supplementation in patients with diabetic nephropathy: A randomized, double-blind, placebo-controlled trial. Food Funct. (2018) 9:4763–70. doi: 10.1039/c8fo00888d
48. Relman, DA. The human microbiome and the future practice of medicine. JAMA. (2015) 314:1127–8. doi: 10.1001/jama.2015.10700
49. Canaud, B, Collins, A, and Maddux, F. The renal replacement therapy landscape in 2030: reducing the global cardiovascular burden in dialysis patients. Nephrol Dial Transplant. (2020) 35:ii51. doi: 10.1093/ndt/gfaa005
50. Huang, HW, and Chen, M-J. Exploring the preventive and therapeutic mechanisms of probiotics in chronic kidney disease through the gut–kidney axis. J Agric Food Chem. (2024) 72:8347–64. doi: 10.1021/acs.jafc.4c00263
51. Chawla, LS, and Kimmel, PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. (2012) 82:516–24. doi: 10.1038/ki.2012.208
52. Stenvinkel, P, Chertow, GM, Devarajan, P, Levin, A, Andreoli, SP, Bangalore, S, et al. Chronic inflammation in chronic kidney disease progression: role of Nrf2. Kidney Int Rep. (2021) 6:1775–87. doi: 10.1016/j.ekir.2021.04.023
53. Petreski, T, Piko, N, Ekart, R, Hojs, R, and Bevc, S. Review on inflammation markers in chronic kidney disease. Biomedicines. (2021) 9:182. doi: 10.3390/biomedicines9020182
54. Lousa, I, Belo, L, Valente, MJ, Rocha, S, Preguiça, I, Rocha-Pereira, P, et al. Inflammatory biomarkers in staging of chronic kidney disease: elevated TNFR2 levels accompanies renal function decline. Inflamm Res. (2022) 71:591–602. doi: 10.1007/s00011-022-01574-2
55. Mitrea, L, Medeleanu, M, Pop, CR, Rotar, AM, and Vodnar, DC. Biotics (pre-, pro-, post-) and uremic toxicity: implications, mechanisms, and possible therapies. Toxins. (2023) 15:548. doi: 10.3390/toxins15090548
56. Beker, BM, Colombo, I, Gonzalez-Torres, H, and Musso, CG. Decreasing microbiota-derived uremic toxins to improve CKD outcomes. Clin Kidney J. (2022) 15:2214–9. doi: 10.1093/ckj/sfac154
57. Favero, C, Giordano, L, Mihaila, SM, Masereeuw, R, Ortiz, A, and Sanchez-Niño, MD. Postbiotics and kidney disease. Toxins. (2022) 14:623. doi: 10.3390/toxins14090623
58. Wang, J, Chen, W-D, and Wang, Y-D. The relationship between gut microbiota and inflammatory diseases: the role of macrophages. Front Microbiol. (2020) 11:1065. doi: 10.3389/fmicb.2020.01065
59. Feng, W, Ao, H, Peng, C, and Yan, D. Gut microbiota, a new frontier to understand traditional Chinese medicines. Pharmacol Res. (2019) 142:176–91. doi: 10.1016/j.phrs.2019.02.024
60. Zhu, H, Cao, C, Wu, Z, Zhang, H, Sun, Z, Wang, M, et al. The probiotic L. casei Zhang slows the progression of acute and chronic kidney disease. Cell Metab. (2021) 33:1926–1942.e8. doi: 10.1016/j.cmet.2021.06.014
Keywords: probiotic, synbiotic, chronic kidney disease, renal function, metabolism
Citation: Liu C, Yang L, Wei W and Fu P (2024) Efficacy of probiotics/synbiotics supplementation in patients with chronic kidney disease: a systematic review and meta-analysis of randomized controlled trials. Front. Nutr. 11:1434613. doi: 10.3389/fnut.2024.1434613
Edited by:
Laura Mitrea, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, RomaniaReviewed by:
Maria Teresa Rocchetti, University of Foggia, ItalyIoana Corina Bocsan, University of Medicine and Pharmacy Iuliu Hatieganu, Romania
Copyright © 2024 Liu, Yang, Wei and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Fu, ZnVwaW5naHhAc2N1LmVkdS5jbg==
 Chang Liu
Chang Liu Letian Yang
Letian Yang Ping Fu
Ping Fu