- 1Laboratory of Apidology and Apitherapy, Department of Microbial Genetics, Institute of Molecular Biology, Slovak Academy of Sciences, Bratislava, Slovakia
- 2Department of Microbiology, Faculty of Medicine, Slovak Medical University, Bratislava, Slovakia
Honey is an attractive functional food that often becomes a subject of clinical studies on the treatment of diverse diseases. However, the clinical efficacy of honey is rather controversial due, at least in part, to its variable composition and botanical origin as well as thermal processing or improper storage conditions. This review addresses the importance of honey quality standards and in vitro testing of the biological properties of honey prior to performing clinical studies, which can have a great impact on clinical outcomes. It focused on recently performed meta-analyses and systematic reviews where honey was used in the management of various disorders including respiratory tract infections, and metabolic and cardiometabolic diseases, with the goal of characterising the honeys used in clinical studies. In addition, it provides recommendations for the use and storage of honey for clinical testing. The vast majority of clinical studies included in meta-analyses do not provide any information about honey quality parameters. In fact, indicators of thermal damage or prolonged storage of honey were analysed only in one clinical study. This observation highlights on the alarming status of honey quality in clinical studies. Furthermore, in vitro biological properties of the analysed honeys were assessed in two clinical studies. Therefore, this review strongly advocates the clinical use of only fully characterised honey samples of known botanical origin with proven in vitro biological functionality and no or minimal thermal processing.
1 Introduction
Natural products from plants, microorganisms and insects are traditional sources of pharmacologically active compounds, especially in the area of novel antimicrobial drug discovery to address antimicrobial resistance (1). One of the major advantages of natural products is the synergic and poly-pharmacological effects their of active ingredients; however, these are difficult to prove experimentally (2). On the other hand, the low and highly variable content of biologically active ingredients in natural products represents the major limiting factor for their therapeutic assessment. The observed variability is due to the ambient environmental conditions, pre- and post-processing of natural products and their storage conditions (3). In addition, the absence of quality parameters that are correlated with the pharmacological activity of natural products often leads to a shortage of natural product-derived medicinal products.
Functional foods, defined as industrially processed or raw natural foods having potentially positive effects on health beyond basic nutrition, represent the largest group of natural products (4). However, to claim a certain food as a functional food, respectable experimental long-term safety evidence and proven clinical efficacy are essential. Clinical testing of functional foods is challenging and can be more complicated than conventional drug testing, especially in the case of double-blind, placebo-controlled, randomised trials (5). In addition, clinical trials provide information about functional efficacy but do not give insights into the mechanism(s) underlying it. Food functionality is based on the activity of ingredients possessing certain biological properties, including antioxidant, antibacterial and anti-inflammatory activities. These properties can be easily determined by in vitro tests but do not consider the complexity of physiological and biochemical processes in the human body (6). Therefore, preliminary in vitro screening of the biological activities of functional foods should be accompanied by in vivo testing in animal model and thus characterise the metabolic pathways driven by bioactive molecules in certain diseases.
The first essential step before any subsequent pre-clinical or clinical validation of functional foods is to evaluate their in vitro biological functionalities relevant to a specific disease. Some of the bioactive molecules in functional foods, such as polyphenols and proteins/enzymes, are characterised by poor stability and heat and light sensitivity, resulting in their degradation and loss of biological activity (7). Industrial processing and prolonged storage of functional foods may have a detrimental effect on the functionality of bioactive molecules.
Honeybee products have been used as remedies in traditional/folk medicine since ancient times. Honey, the best studied and most used honeybee product, has been considered not only as a sweetener but also as an attractive functional food (8). It has been the subject of numerous clinical studies to investigate its health benefits in the prevention and treatment of various diseases (9). In a substantial proportion of pre-clinical and clinical studies, honey was applied topically to treat various types of wounds. Positive clinical outcomes from topically applied honey enabled the creation of so-called ‘medical-grade honey’. Medical-grade honeys, used as medical devices, are prepared from certain types of honey which are subjected to sterilisation by gamma irradiation (10). In the case of medical-grade honey, rigorous standards must be met and comprehensive testing conducted to guarantee its safety and quality (10). However, most honey produced globally is classified as table honey for direct consumption, which does not need to fulfil strict criteria as medical-grade honey. Currently, honey quality criteria are based on the international honey standards, which are specified in the European Honey Directive (11) and in the Codex Alimentarius Standard for honey (12). Besides the fact that honey is a functional food, current legislative parameters do not consider the health-promoting biological properties of honey (9). A recent survey among honey consumers in the Balkans and Western European countries indicated that the main reason for honey consumption is its health benefits (13). Although honey is claimed as a functional food, results from clinical studies are rather inconsistent and conflicting, and it is difficult to draw comprehensive conclusions. There are several weaknesses associated with previously conducted clinical trials, and one of the systematically overlooked factors is the quality and functionality of the tested honey (14).
This study aims to provide perspectives and scientific opinions on honey as a therapeutic agent and to stimulate discussion about the quality issue with honey used in clinical studies. Furthermore, it proposes general recommendations for honey processing and storage conditions in which the biological functionality of honey is maintained over time.
2 International qualitative parameters of honey. Do they need an update?
Composition may differ from one honey to another and is affected by several factors, such as the botanical and geographical origin of the honey, environmental conditions and bee species. Apart from these factors, its composition can also be affected by beekeeper manipulation and processing (15). In order to guarantee honey safety and quality, a set of legislative qualitative standards has been adopted by Codex Alimentarius and the European Honey Directive (12) (Table 1). Due to natural differences in honey composition, the range of current honey standards is too wide, and numerous exceptions have been identified and codified in Codex Alimentarius for honey. Many differences exist and the majority of these differences are in moisture and sugar content and HMF content (15). Some countries adopted their own national provisions where more strict regulations are applied.
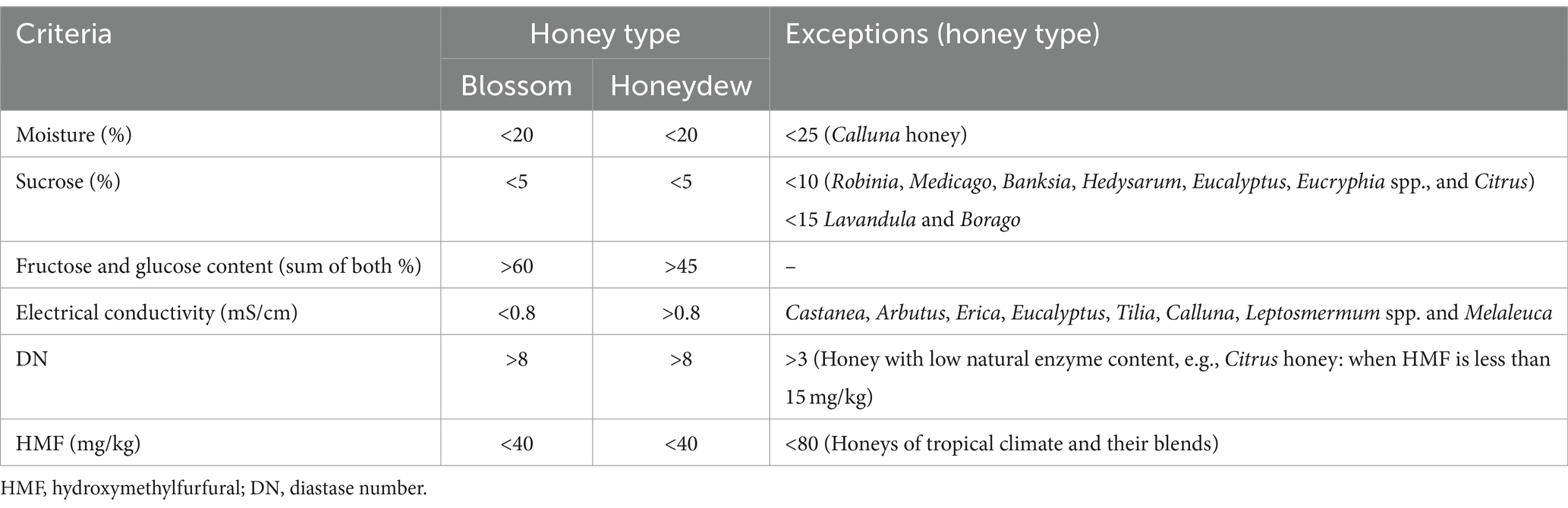
Table 1. General compositional criteria of honey with exceptions according to honey directive 2001/110 EU (11).
Two of the legislative parameters, namely hydroxymethylfurfural (HMF) and enzymatic activity of α-amylase (diastase), which are related to honey freshness and its overheating during processing, are the most important parameters, especially for samples assigned for clinical testing. Numerous biologically active compounds are heat sensitive and tend to degrade over time. However, mounting evidence suggests that neither of these qualitative standards are always reliable parameters of marketed honey samples.
The legislated qualitative upper limit for HMF is 40 mg/kg, and its content in freshly collected honey is extremely low and highly unexpected. Although thermal treatment of honey increases the HMF content in treated honey, its concentration values are within the legislative limit in various types of honey, especially in stingless bee honey samples (16–20). Pasteurisation of pot honey at 65°C for 15 and 21 min as well as tyndallisation at 80°C for 15 and 21 min caused a significant increase in HMF formation, but the obtained values did not exceed the plausible limit for HMF (20). Similarly, the HMF content in stingless bee honeys exposed to high temperatures (between 75 and 95°C) for a short period of time (between 20 and 60 s) remained stable (16). In addition, extreme heating of stingless bee honeys at 90 and 95°C for 15 and 60 s did not change the initial HMF values in honeys before heating (17). These data indicate that thermal processing of stingless honey did not change the HMF level, most likely due to the presence of specific polyphenols acting as potential inhibitors towards the formation of harmful HMF (21).
Diastase and its enzymatic activity, expressed as diastase number (DN), are the sole qualitative parameters directly related to honeybee secretions from the hypopharyngeal glands. The enzymatic activity of diastase is more sensitive to thermal treatment in comparison with heat-induced formation of HMF. However, there is also evidence that the DN value does not change after thermal treatment (20, 22, 23) due to the ability of diastase to recover its enzymatic activity. The enzymatic activity of diastase can be naturally dramatically reduced in manuka honey type to DN values bellow permissible levels (24). Methylglyoxal, a major antibacterial compound in manuka honey, and 3-phenyllactic acid were found to accelerate the loss of diastase above that normally observed with time and elevated temperature. Likewise, citrus honey with a low natural enzyme content (DN value should be less than 3) and manuka honey should be treated as exceptions.
Another important weakness of DN as a qualitative parameter is based on the evaluation of enzymatic activity but not the origin of diastase. One of the current trends in honey adulteration is adding foreign diastase to disguise the enzymatic activity of bee-derived diastase (25–28). Almost 37% of honey samples from different regions in Türkiye (74 out of 202 samples) were diagnosed as having been adulterated by direct addition of foreign diastase or addition of syrup containing foreign diastase (27). This finding is alarming and represents the growing problem with honey authenticity worldwide.
Both DN and HMF content are important quality standards of honey; however, based on their above-mentioned weaknesses, it is desirable to assess more heat-sensitive parameters, such as enzymatic activity of bee-derived enzymes invertase (29) and glucose oxidase (30).
3 What is the status of clinical evidence for honey as a functional food?
Honey is a popular remedy worldwide and is still an attractive therapeutic product, especially in countries where healthcare systems might have low capacity, and patients mostly rely on traditional medicine. On the other hand, honey is also used in a modern medicine, especially as a medical-grade honey in wound care. Honey is a nutritional product where carbohydrates (95–97% of dry weight) represent the main composition. Fructose and glucose are the most important sugars of honey and contribute to most of the nutritional effects of honey (31, 32). Besides monosaccharides, honey contains small quantities of disaccharides (e.g., sucrose, galactose), trisaccharides (e.g., melezitose) and oligosaccharides which are mainly formed during honey ripening and maturation in bee colonies (31).
In the present work, searching for systematic reviews and meta-analyses was carried out, where honey was used as a functional food, and studies were published in scientific databases (PubMed, Scopus, and Web of Science) between 2019 and 2023. In the case of similar meta-analyses or systematic reviews describing identical health targets (e.g., cough, metabolic diseases), only the most recent one was included. Meta-analyses or systematic reviews using honey as a topical agent for the treatment of wounds, burns or oral mucositis were excluded. Furthermore, studies where honey was combined with other therapeutics or was part of natural product mixtures were not evaluated.
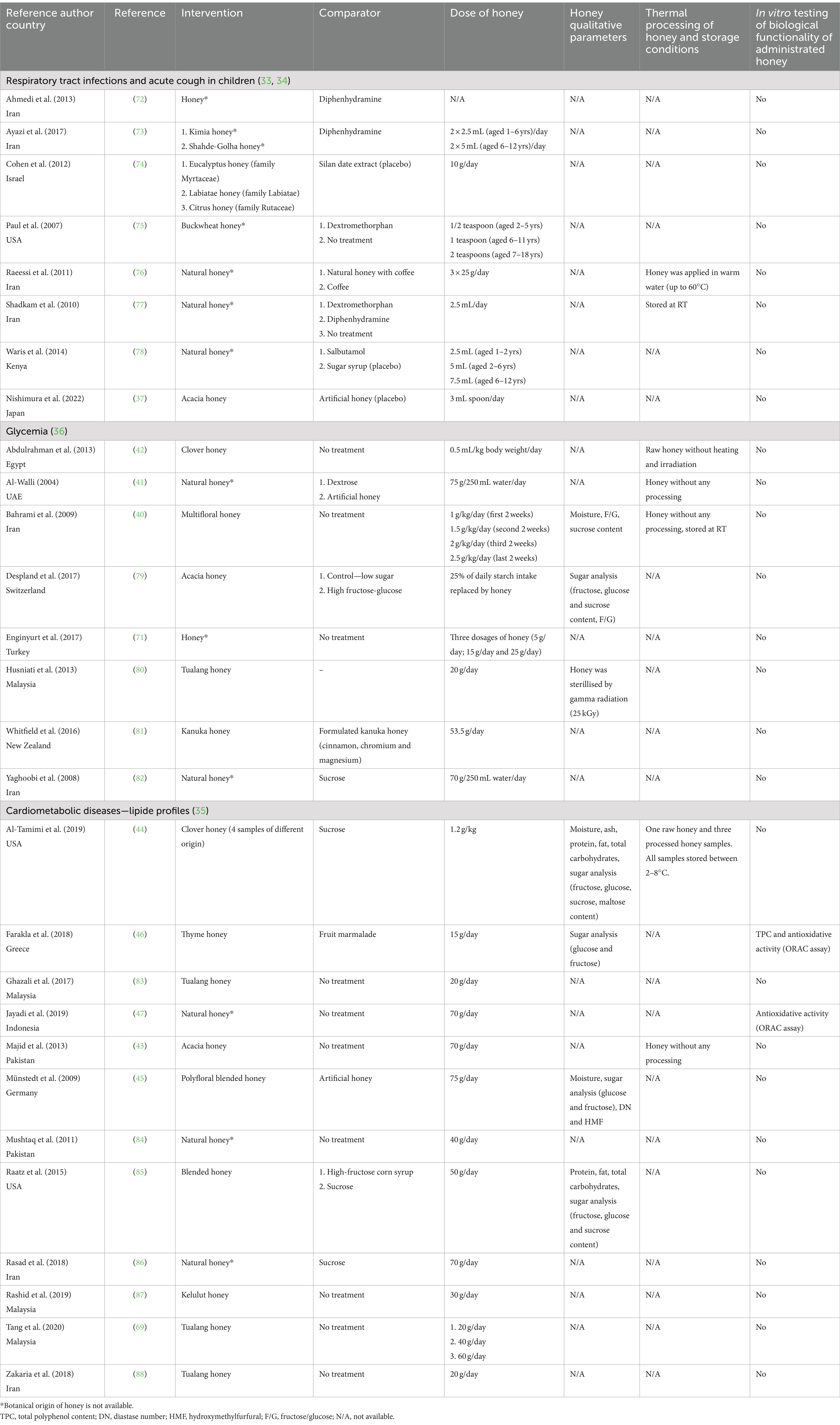
Four studies were identified where the effect of honey consumption on upper respiratory tract infections (URTIs) (33), acute cough in children (34), lipid profiles (35) and glycaemic control (36) was examined. Individual human clinical studies involved in selected meta-analyses/systematic reviews are listed in Table 2.
A recent meta-analysis of the clinical effectiveness of honey revealed that honey improves URTIs more effectively than the usual care in children, but only two of the included clinical studies were placebo controlled (33). In addition, honey was associated with a significantly greater reduction in cough frequency and severity. A very recent multicentre, placebo-controlled study conducted in paediatric patients with URTI showed a positive effect of acacia honey on nocturnal cough, but the observed effect did not differ from that of artificial honey, suggesting a role of sugar content in the antitussive effect of honey (37). The author of a recent systematic review on the efficacy of using honey to treat acute cough in children found a low quality of evidence that honey is effective in treating acute cough and improving sleep in children with acute cough (34). None of the analysed clinical studies involved in the meta-analysis (33) or systematic review (34) used honey that had been characterised in terms of qualitative legislative parameters (e.g., moisture, HMF, DN) nor in vitro biological functionality related to URTIs and cough (e.g., antimicrobial activity) (Table 1). Furthermore, in most clinical studies, no botanical and/or geographical origin was stated for the honey employed (Table 2).
Despite numerous pre-clinical studies reporting on the beneficial effects of honey on glycaemia, evidence regarding the effects of honey consumption on glycaemia in humans remains controversial, and no definite conclusion can currently be drawn (36). These controversies may be partly due to variations in study duration and in the type and dose of honey administered. The basic physicochemical characteristics (mainly the fructose and glucose contents) of administrated honey were determined in a few clinical studies but in vitro biological properties of honey related to glycemia were not analysed.
High intake of free or added sugars is one the major factors responsible for overweight, type 2 diabetes and cardiovascular disease development. In both adults and children, WHO recommends reducing the intake of free sugars, including honey, to less than 10% of total energy intake (38). However, honey has been considered as a natural remedy for diverse noncommunicable diseases and exerts numerous health benefits in pre-clinical and clinical studies. Two recently conducted systematic reviews and meta-analyses (35, 39) did not resolve the controversy surrounding the use of honey for improvement of the lipid profile, body weight and glycaemic control. Gholami et al. (39) demonstrated in the performed meta-analysis that honey consumption had no effect on serum lipids, including triglycerides, total cholesterol and low/high-density lipoprotein cholesterol. On the other hand, Ahmed et al. (35) showed in conducted meta-analysis that oral honey intake reduced fasting glucose and triglycerides, total cholesterol and low-density lipoprotein cholesterol. Interestingly, the authors demonstrated that some types of honey, such as clover and acacia honey, exhibited the beneficial effect of lowering glycaemic and cholesterols values. Likewise, raw and/or unprocessed honey showed higher efficacy than the processed variety. This observation highlights the importance of botanical origin and processing when selecting a honey type for clinical use. Raw, unprocessed honey of known botanical origin with certain proven biological properties should be used in clinical studies.
In total, 5 out of the 28 clinical studies (40–44) listed in Table 2 used raw, unprocessed honey for clinical testing. Furthermore, DN and HMF values, as indicators of honey overheating or prolonged storage, were determined only in one study (45). The biological activity, namely antioxidant activity, of honey was evaluated in two studies (46, 47) (Table 2). These findings indicate that the vast majority of clinical studies conducted ignored qualitative parameters of honey. In addition, the authors were not aware of the importance of the biological functionality of honey and the necessity of in vitro testing prior to clinical testing.
4 Qualitative and biological parameters of tailored honey for initial screening of its functionality
Although the qualitative parameters of honey are widely established worldwide, none of them consider the health-promoting effects of honey or its biological properties. Moreover, as mentioned above, cumulative scientific evidence indicates certain weaknesses of qualitative parameters, particularly in honey processing and handling.
Raw or unprocessed honey seems to have some advantages compared to thermally processed honey; however, it is necessary to minimise the risk of fermentation during storage and avoid spoilage. Honey is a natural product which may contain a great variety of bacteria, yeasts and fungi. Some bacteria and fungi encountered in honey have been associated with human diseases and although the health risk to humans is low in adults (48), it is essential to assess the microbiological quality of honey.
This review strongly advocates the adoption of basic honey qualitative parameters according to Codex Alimentarius (12) by researchers/clinicians who are planning to conduct human clinical studies where honey will be used as a potential therapeutic product. Researchers should pay particular attention to the HMF and DN values of tested honey samples. Additionally, it is essential to evaluate the biological functionality of tested honey samples before their use in a clinical study. There are several well-described biological properties of honey, such as antimicrobial/antibiofilm, antioxidant and anti-inflammatory activity, which might be suitable for in vitro honey testing. The selection of an in vitro assay depends on the subject of the clinical study to be investigated.
4.1 Antimicrobial activity of honey
Antimicrobial activity is the most studied biological property of honey, and recently it has been proposed as a suitable qualitative parameter of honey (9). The antibacterial activity of honey is determined by several different factors and molecules, such as low pH, high sugar content (osmolarity), hydrogen peroxide (H2O2), methylglyoxal and the bee antibacterial peptide defensin-1. Furthermore, certain types of phytochemicals, especially polyphenolic compounds, may elevate the antibacterial effect of honey (49–51). A detailed characterisation of the antibacterial effect of honey allows effective use of topical honey-based formulations and medical-grade honeys in wound care.
A range of testing methods are used to determine the antimicrobial activity of honey against diverse bacteria and fungi. Nowadays, it is very demanding to compare the overall antibacterial efficacy of various types of honey due to differences in the assays employed. Among testing methods, the agar diffusion and broth dilution method are the most often used worldwide (52). Since honey is a complex of diverse compounds with different molecular weights, the agar diffusion method has some limitations and can generate inaccurate outcomes. Therefore, the broth dilution method seems to be the most suitable method of determining the antibacterial potential of honey regardless of the botanical and geographical origin of analysed honey. The antibacterial activity of honey is typically expressed as the minimum inhibitory concentration (MIC) or minimum bactericidal concentration (MBC). However, serious discrepancies were found in the broth dilution method where different experimental conditions (e.g., static vs. shaking, doubling dilution vs. small increments, visual vs. optical inspection) were used. This review advocates following the methods published by the Clinical and Laboratory Standards Institute (CLSI) (53) or European Committee for Antimicrobial Susceptibility Testing (EUCAST) (54), with some modifications for honey testing. A robust, standardised, cost-effective method of determining the antibacterial potential of honey is urgently needed. The antibacterial activity of honey can be significantly reduced after thermal processing (55, 56) or improper storage (57, 58), whereas values of the traditional quality parameters HMF and DN can be within the range of permissible levels.
4.2 Antioxidant activity of honey
Investigation and characterisation of antioxidant activity in honey is an attractive research area. Honey is deemed a potential source of natural antioxidants that can counteract the effects of oxidative stress underlying the pathogenesis of many diseases. Several components of honey are responsible for antioxidant activity, including polyphenolic compounds (phenolic acids and flavonoids), vitamin C and E, enzymes (catalase and peroxidase), Maillard reaction products and trace elements (59, 60). The concentration of these bioactive compounds is highly variable and depends on the type of flora, geographical location of production, climatic conditions, seasonal factors and soil composition, as well as the production process (61).
Measurement of the antioxidative potential of natural products is not based solely on one method; rather, several different types of methods (e.g., spectrometric, electrochemical and chromatographical assays) should be employed (62). There is no official method for determination of the antioxidant activity of honey, and none of the methods used are ideal, each being designed to measure a different group of antioxidants (61). The antioxidant activity of honey is generally measured in the form of antiradical activity using the 1,1-diphenyl-2-picrylhydrazyl (DPPH) scavenging assay, 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) assay, oxygen radical absorbance capacity (ORAC) assay and ferric reducing antioxidant power (FRAP) assay (63).
The total phenolic content and antioxidative activity of honey can be greatly affected by its processing and storage. Uneven changes in the antioxidant activity of thermally processed honey have been observed. Thermal processing is able to either decrease or increase the antioxidative potential of a particular honey sample [reviewed in Faraz et al. (64)]. The changes in the antioxidant activity of honey depend on two major factors: honey composition and the temperature and duration of thermal treatment (65). On the other hand, the antioxidant capacity of honey after thermal processing also depends on the method(s) used to evaluate this activity. Considering the fact that honeys of different botanical origin are clinically tested, this review proposes that thermal processing or prolonged storage of honey be avoided, particularly in cases where oxidative stress plays a role.
4.3 Anti-inflammatory activity of honey
Inflammation is a key factor in the development of chronic diseases, including cardiovascular diseases and diabetes. Honey is considered as an anti-inflammatory agent which may suppress the production of mediators of inflammation (e.g., pro-inflammatory cytokines) and support haemostasis (32). Various in vitro models of inflammation have been used to characterise the anti-inflammatory effect of honey.
The composition of honey precludes the testing of honey itself due to its effect on the cultivation medium. Therefore, the individual components of extracted or purified honey, particularly polyphenolic compounds, are tested for anti-inflammatory activities. The cell types most often used for in vitro screening are primary cultures of human cells and human cell lines such as peripheral blood mononuclear cells, RAW 264.7 macrophages, HaCaT cells and human THP-1 monocytes (66).
Honey components act as (i) modulators of pro-oxidant enzymes (cyclooxygenase-1 and -2 inhibition, nitric oxide synthase and lipoxygenase inhibition), (ii) modulators of pro-inflammatory mediators (TNF-α, IL-1 and Il-6 production and nitrate/nitrite measurement) and (iii) modulators of nuclear factor expression (NF-kB) (p65NF-kB levels) (66).
Literature is scarce regarding characterisation of the effect of industrial processing including thermal treatment or prolonged storage on the anti-inflammatory activity of honey. Similar to the antioxidative effect, it is assumed that thermal treatment affects anti-inflammatory activity in the same way, since polyphenols are responsible for the observed in vitro anti-inflammatory effect.
5 Recommendations for honey use in clinical medicine
As stated above, honey is an attractive therapeutic agent for both topical and oral use. According to the web resource www.clinicaltrails.gov (date: January 26, 2024; terms: honey), seven human clinical trials using honey as a dietary supplement are currently active or in recruiting mode focusing on periodontal disease, postprandial glycemia, poor quality sleep, oxidative stress, xerostomia, overweight and insulin resistance.
This review recommends that only honey of known botanical/geographical origin, with fully characterised qualitative parameters and, if possible, without thermal processing should be used for clinical testing (Figure 1). In addition, it is also strongly recommended to verify its biological functionality using an in vitro assay, depending on the disease/condition to be treated.
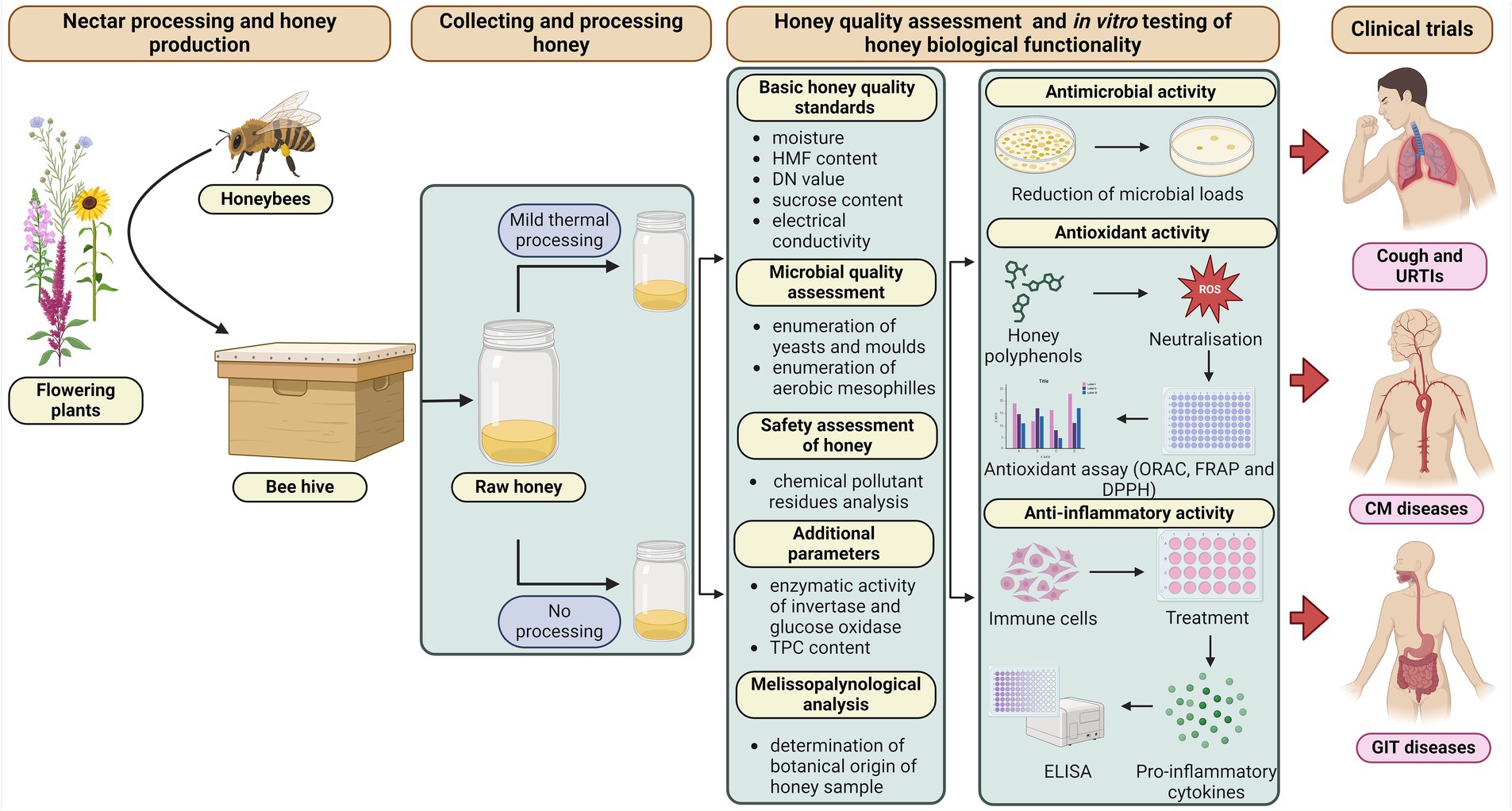
Figure 1. Ideal and recommend practises for honey processing and in vitro testing of its qualitative parameters and biological activities. Created with BioRender.com.
In order to test for a safe honey without harmful microorganisms or if reduction of the honey microbial load is needed, alternative methods of honey preservation, other than thermal treatment, is recommended (Figure 2). Nowadays, due to unfavourable side-effects of thermal pasteurisation, other approaches such as ultrasound and high-pressure processing are being tested to avoid honey spoilage and fermentation whilst maintaining health-promoting properties (67, 68).
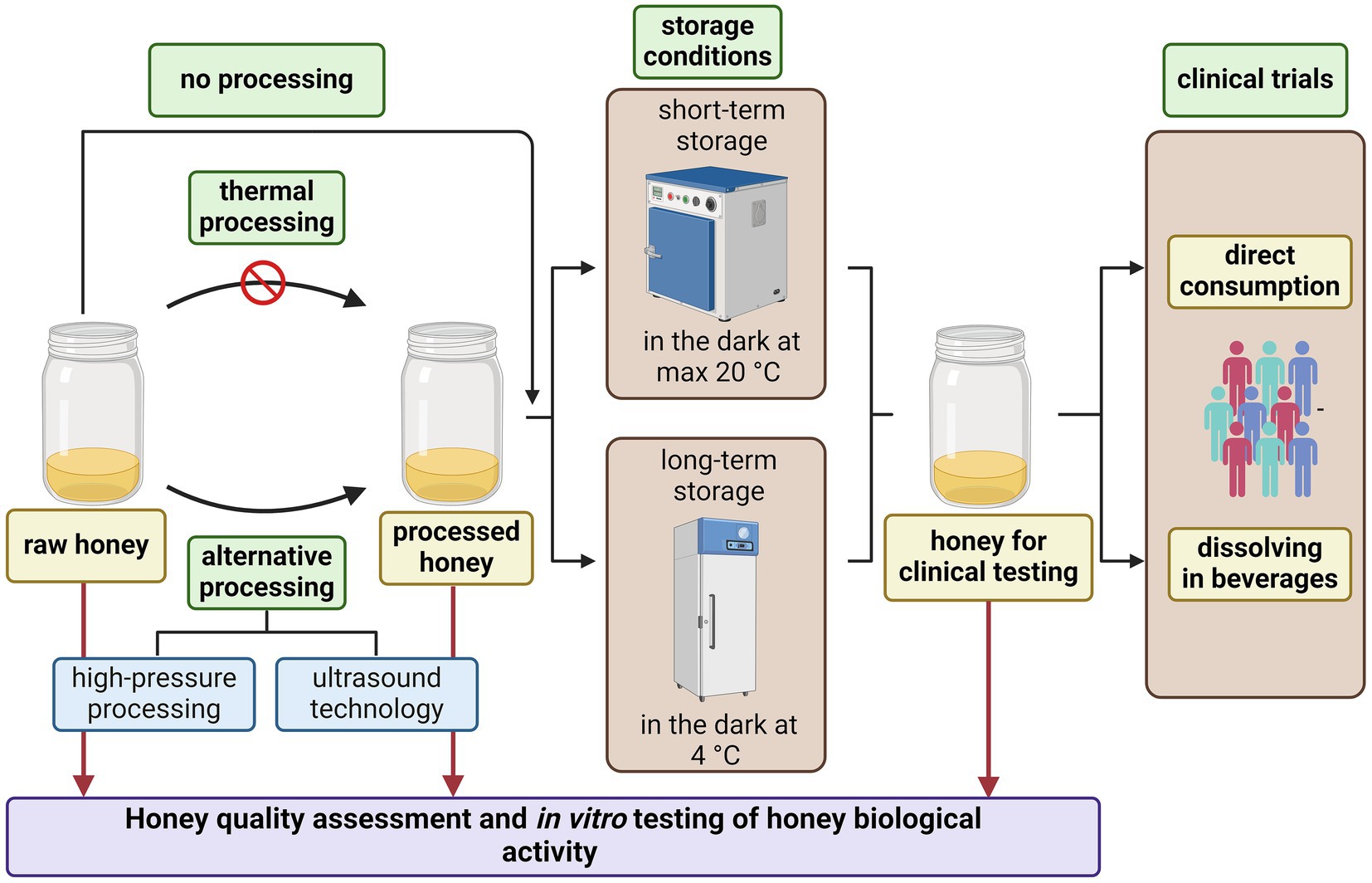
Figure 2. Recommendations for honey processing and its use as oral therapeutic agent in clinical medicine. Created with BioRender.com.
Regarding the storage of tested honey samples, it is recommended that honey samples be kept at temperatures up to 20°C (preferably at 4°C) in a dark place (Figure 2). If the study design requires dissolution of honey in water or other beverages, it is recommended that they be used at room temperature and, if possible, that warm/hot beverages be avoided. Honey or honey in beverages should be consumed on an empty stomach, thus avoiding potential unwanted interactions with other food/food supplements.
One of the most debatable issues is the optimal consumed dose of honey to use in clinical studies. There have been several studies that tested different doses of honey in the clinical setting (69–71). Although different doses of honey were tested, no significant changes in honey efficacy were found. However, the optimal dose of consumed honey for clinical studies together with the duration of administrated honey must be established. On the other hand, precautions are needed for high-dose honey supplementation, as it may cause increased blood glucose levels.
6 Conclusion
Controversial outcomes of clinical trials that used honey as a functional therapeutic agent in a broad spectrum of diseases are becoming the object of debate. Differences in honey composition have been suggested as major explanations for opposite clinical outcomes. However, it is showed here that honey quality and biological functionality are the key parameters which may significantly determine the clinical efficacy of honey in clinical trials. According to the evaluated meta-analyses and systematic reviews, both parameters are deeply underestimated by clinicians. Furthermore, storage conditions and/or thermal processing may negatively affected honey quality and biological functionality and these facts need to be taken into account before conducting clinical studies. Therefore, the review strongly advocates the clinical use of only fully characterised honey samples of known botanical origin with proven in vitro biological functionality and no or minimal thermal processing.
Author contributions
JM: Conceptualization, Formal analysis, Funding acquisition, Methodology, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author declares that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Scientific Grant Agency of the Ministry of Education of the Slovak Republic and the Slovak Academy of Sciences VEGA 2/0022/22, the Slovak Research and Development Agency under Contract No. APVV-21-0262 and the COST Action CA22105-BEekeeping products valorization and biomonitoring for the SAFEty of BEEs and HONEY (BeSafeBeeHoney).
Acknowledgments
The figures were generated with BioRender.com.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Atanasov, AG, Zotchev, SB, Dirsch, VM, and Supuran, CT. Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov. (2021) 20:200–16. doi: 10.1038/s41573-020-00114-z
2. David, B, Wolfender, JL, and Dias, DA. The pharmaceutical industry and natural products: historical status and new trends. Phytochem Rev. (2015) 14:299–315. doi: 10.1007/s11101-014-9367-z
3. Dhami, N, and Mishra, AD. Phytochemical variation: how to resolve the quality controversies of herbal medicinal products? J Herb Med. (2015) 5:118–27. doi: 10.1016/j.hermed.2015.04.002
4. Granato, D, Barba, FJ, Bursać Kovačević, D, Lorenzo, JM, Cruz, AG, and Putnik, P. Functional foods: product development, technological trends, efficacy testing, and safety. Annu Rev Food Sci Technol. (2020) 11:93–118. doi: 10.1146/annurev-food-032519-051708
5. Brown, L, Caligiuri, SP, Brown, D, and Pierce, GN. Clinical trials using functional foods provide unique challenges. J Funct Foods. (2018) 45:233–8. doi: 10.1016/j.jff.2018.01.024
6. Alongi, M, and Anese, M. Re-thinking functional food development through a holistic approach. J Funct Foods. (2021) 81:104466. doi: 10.1016/j.jff.2021.104466
7. Cao, H, Saroglu, O, Karadag, A, Diaconeasa, Z, Zoccatelli, G, Conte-Junior, CA, et al. Available technologies on improving the stability of polyphenols in food processing. Food Front. (2021) 2:109–39. doi: 10.1002/fft2.65
8. Berenbaum, MR, and Calla, B. Honey as a functional food for Apis mellifera. Annu Rev Entomol. (2021) 66:185–208. doi: 10.1146/annurev-ento-040320-074933
9. Majtan, J, Bucekova, M, Kafantaris, I, Szweda, P, Hammer, K, and Mossialos, D. Honey antibacterial activity: a neglected aspect of honey quality assurance as functional food. Trends Food Sci Technol. (2021) 118:870–86. doi: 10.1016/j.tifs.2021.11.012
10. Hermanns, R, Mateescu, C, Thrasyvoulou, A, Tananaki, C, Wagener, FADTG, and Cremers, NAJ. Defining the standards for medical grade honey. J Apic Res. (2020) 59:125–35. doi: 10.1080/00218839.2019.1693713
11. EU Directive. European Commission Council Directive 2001/110/EC of 20 December 2001 relating to honey. Official Journal of the European Communities. Brussels. (2002): 10–47.
12. Codex Alimentarius. Codex standards for honey. Codex Stan 12–1981. Rome, Italy. (2001) Rev. 1 (1987), Rev. 2 (2001).
13. Kleisiari, C, Kleftodimos, G, and Vlontzos, G. Be(e)ha(i)viour(e): assessment of honey consumption in Europe. Br Food J. (2023) 125:1374–89. doi: 10.1108/BFJ-12-2021-1300
14. Deglovic, J, Majtanova, N, and Majtan, J. Antibacterial and antibiofilm effect of honey in the prevention of dental caries: a recent perspective. Food Secur. (2022) 11:2670. doi: 10.3390/foods11172670
15. Thrasyvoulou, A, Tananaki, C, Goras, G, Karazafiris, E, Dimou, M, Liolios, V, et al. Legislation of honey criteria and standards. J Apic Res. (2018) 57:88–96. doi: 10.1080/00218839.2017.1411181
16. Biluca, FC, Della Betta, F, de Oliveira, GP, Pereira, LM, Gonzaga, LV, Costa, AC, et al. 5-HMF and carbohydrates content in stingless bee honey by CE before and after thermal treatment. Food Chem. (2014) 159:244–9. doi: 10.1016/j.foodchem.2014.03.016
17. Braghini, F, Biluca, FC, Gonzaga, LV, Kracik, AS, Vieira, CR, Vitali, L, et al. Impact of short-term thermal treatment on stingless bee honey (Meliponinae): quality, phenolic compounds and antioxidant capacity. J Food Process Preserv. (2019) 43:e13954. doi: 10.1111/jfpp.13954
18. Elamine, Y, Anjos, O, Estevinho, LM, Lyoussi, B, Aazza, S, and Miguel, MG. Effect of extreme heat processing on the Moroccan Zantaz’ honey antioxidant activities. J Food Sci Technol. (2020) 57:3323–33. doi: 10.1007/s13197-020-04365-x
19. Braghini, F, Biluca, FC, Gonzaga, LV, Vitali, L, Costa, ACO, and Fett, R. Effect thermal processing in the honey of Tetragonisca angustula: profile physicochemical, individual phenolic compounds and antioxidant capacity. J Apic Res. (2021) 60:290–6. doi: 10.1080/00218839.2020.1737362
20. Correa-Mosquera, AR, Quicazán, MC, and Zuluaga-Domínguez, CM. Shelf-life prediction of pot-honey subjected to thermal treatments based on quality attributes at accelerated storage conditions. Food Control. (2022) 142:109237. doi: 10.1016/j.foodcont.2022.109237
21. Han, Z, Zhu, M, Wan, X, Zhai, X, Ho, CT, and Zhang, L. Food polyphenols and Maillard reaction: regulation effect and chemical mechanism. Crit Rev Food Sci Nutr. (2022) 16:1–17. doi: 10.1080/10408398.2022.2146653
22. Kowalski, S, Lukasiewicz, M, Bednarz, S, and Panus, M. Diastase number changes during thermal and microwave processing of honey. Czech J Food Sci. (2012) 30:21–6. doi: 10.17221/123/2010-CJFS
23. Tosi, E, Martinet, R, Ortega, M, Lucero, H, and Re, E. Honey diastase activity modified by heating. Food Chem. (2008) 106:883–7. doi: 10.1016/j.foodchem.2007.04.025
24. Bell, AR, and Grainger, MN. Accelerated loss of diastase in mānuka honey: investigation of mānuka specific compounds. Food Chem. (2023) 426:136614. doi: 10.1016/j.foodchem.2023.136614
25. Erban, T, Shcherbachenko, E, Talacko, P, and Harant, K. A single honey proteome dataset for identifying adulteration by foreign amylases and mining various protein markers natural to honey. J Proteome. (2021) 239:104157. doi: 10.1016/j.jprot.2021.104157
26. Bucekova, M, Godocikova, J, Kohutova, L, Danchenko, M, Barath, P, and Majtan, J. Antibacterial activity and bee-derived protein content of honey as important and suitable complementary tools for the assessment of honey quality. J Food Compost Anal. (2023) 123:105610. doi: 10.1016/j.jfca.2023.105610
27. Akyıldız, İE, Erdem, Ö, Raday, S, Acar, S, Uzunöner, D, Damarlı, E, et al. Straightforward monitoring of honey with foreign diastase by leveraging the differentiation in LC-UV proteome profiles of authentic and fraudulent samples. Microchem J. (2023) 193:109039. doi: 10.1016/j.microc.2023.109039
28. Takahashi, Y, Yoshida, I, Yokozeki, T, Igarashi, T, and Fujita, K. Investigation of foreign amylase adulteration in honey distributed in Japan by rapid and improved native PAGE activity staining method. J Appl Glycosci. (2023) 70:67–73. doi: 10.5458/jag.jag.JAG-2023_0002
29. Kowalski, S, and Lukasiewicz, M. Diastase and invertase activity changes and 5-hydroxymethyl-2-furfural formation in honeys under influence of microwave irradiation. J Food Process Eng. (2017) 40:e12410. doi: 10.1111/jfpe.12410
30. Bucekova, M, Juricova, V, Monton, E, Martinotti, S, Ranzato, E, and Majtan, J. Microwave processing of honey negatively affects honey antibacterial activity by inactivation of bee-derived glucose oxidase and defensin-1. Food Chem. (2018) 240:1131–6. doi: 10.1016/j.foodchem.2017.08.054
31. Samarghandian, S, Farkhondeh, T, and Samini, F. Honey and health: a review of recent clinical research. Pharm Res. (2017) 9:121–7. doi: 10.4103/0974-8490.204647
32. Ranneh, Y, Akim, AM, Hamid, HA, Khazaai, H, Fadel, A, Zakaria, ZA, et al. Honey and its nutritional and anti-inflammatory value. BMC Complement Altern Med. (2021) 21:30. doi: 10.1186/s12906-020-03170-5
33. Abuelgasim, H, Albury, C, and Lee, J. Effectiveness of honey for symptomatic relief in upper respiratory tract infections: a systematic review and meta-analysis. BMJ Evid Based Med. (2021) 26:57–64. doi: 10.1136/bmjebm-2020-111336
34. Kuitunen, I, and Renko, M. Honey for acute cough in children – a systematic review. Eur J Pediatr. (2023) 182:3949–56. doi: 10.1007/s00431-023-05066-1
35. Ahmed, A, Tul-Noor, Z, Lee, D, Bajwah, S, Ahmed, Z, Zafar, S, et al. Effect of honey on cardiometabolic risk factors: a systematic review and meta-analysis. Nutr Rev. (2023) 81:758–74. doi: 10.1093/nutrit/nuac086
36. Zamanian, M, and Azizi-Soleiman, F. Honey and glycemic control: a systematic review. PharmaNutrition. (2020) 11:100180. doi: 10.1016/j.phanu.2020.100180
37. Nishimura, T, Muta, H, Hosaka, T, Ueda, M, and Kishida, K. Honey and coughs study Group of the Society of ambulatory and general paediatrics of Japan. Multicentre, randomised study found that honey had no pharmacological effect on nocturnal coughs and sleep quality at 1-5 years of age. Acta Paediatr. (2022) 111:2157–64. doi: 10.1111/apa.16509
38. World Health Organization. Guideline: sugars intake for adults and children. Geneva, Switzerland: WHO Press. (2015).
39. Gholami, Z, Sohrabi, Z, Zare, M, Pourrajab, B, and Nasimi, N. The effect of honey on lipid profiles: a systematic review and meta-analysis of controlled clinical trials. Br J Nutr. (2022) 127:1482–96. doi: 10.1017/S0007114521002506
40. Bahrami, M, Ataie-Jafari, A, Hosseini, S, Foruzanfar, MH, Rahmani, M, and Pajouhi, M. Effects of natural honey consumption in diabetic patients: an 8-week randomized clinical trial. Int J Food Sci Nutr. (2009) 60:618–26. doi: 10.3109/09637480801990389
41. Al-Waili, NS. Natural honey lowers plasma glucose, C-reactive protein, homocysteine, and blood lipids in healthy, diabetic, and hyperlipidemic subjects: comparison with dextrose and sucrose. J Med Food. (2004) 7:100–7. doi: 10.1089/109662004322984789
42. Abdulrhman, M, El Hefnawy, M, Ali, R, Hamid, IA, Abou El-Goud, A, and Refai, D. Effects of honey, sucrose and glucose on blood glucose and C-peptide in patients with type 1 diabetes mellitus. Complement Ther Clin Pract. (2013) 19:15–9. doi: 10.1016/j.ctcp.2012.08.002
43. Majid, M, Younis, MA, Naveed, AK, Shah, MU, Azeem, Z, and Tirmizi, SH. Effects of natural honey on blood glucose and lipid profile in young healthy Pakistani males. J Ayub Med Coll Abbottabad. (2013) 25:44–7.
44. Al-Tamimi, AM, Petrisko, M, Hong, MY, Rezende, L, Clayton, ZS, and Kern, M. Honey does not adversely impact blood lipids of adult men and women: a randomized cross-over trial. Nutr J. (2020) 74:87–95. doi: 10.1016/j.nutres.2019.11.012
45. Münstedt, K, Hoffmann, S, Hauenschild, A, Bülte, M, von Georgi, R, and Hackethal, A. Effect of honey on serum cholesterol and lipid values. J Med Food. (2009) 12:624–8. doi: 10.1089/jmf.2008.0188
46. Farakla, I, Koui, E, Arditi, J, Papageorgiou, I, Bartzeliotou, A, Papadopoulos, GE, et al. Effect of honey on glucose and insulin concentrations in obese girls. Eur J Clin Investig. (2019) 49:e13042. doi: 10.1111/eci.13042
47. Jayadi, Y, Thaha, AR, Hadju, V, Bukhari, A, and Dewi, NU. The potential of Indonesian honey to change the lipid profiles of individuals with central obesity. Pak J Nutr. (2019) 18:508–13. doi: 10.3923/pjn.2019.508.513
48. Grabowski, NT, and Klein, G. Microbiology and foodborne pathogens in honey. Crit Rev Food Sci Nutr. (2017) 57:1852–62. doi: 10.1080/10408398.2015.1029041
49. Bucekova, M, Buriova, M, Pekarik, L, Majtan, V, and Majtan, J. Phytochemicals-mediated production of hydrogen peroxide is crucial for high antibacterial activity of honeydew honey. Sci Rep. (2018) 8:9061. doi: 10.1038/s41598-018-27449-3
50. Brudzynski, K. Unexpected value of honey color for prediction of a non-enzymatic H2O2 production and honey antibacterial activity: a perspective. Meta. (2023) 13:526. doi: 10.3390/metabo13040526
51. Thierig, M, Raupbach, J, Wolf, D, Mascher, T, Subramanian, K, and Henle, T. 3-Phenyllactic acid and polyphenols are substances enhancing the antibacterial effect of methylglyoxal in manuka honey. Food Secur. (2023) 12:1098. doi: 10.3390/foods12051098
52. Osés, SM, Pascual-Maté, A, de la Fuente, D, de Pablo, A, Fernández-Muiño, MA, and Sancho, MT. Comparison of methods to determine antibacterial activity of honeys against Staphylococcus aureus. NJAS Wageningen J Life Sci. (2016) 78:29–33. doi: 10.1016/j.njas.2015.12.005
53. Clinical and Laboratory Standards Institute. Methods for dilution of antimicrobial susceptibility tests for bacteria that growth aerobically. 11th ed. CLSI document M07-A11. CLSI/NCCLS M7-A11. Wayne (PA): The Institute (2018).
54. European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin Microbiol Infect. (2003) 9:ix–xv. doi: 10.1046/j.1469-0691.2003.00790.x
55. Villacrés-Granda, I, Proaño, A, Coello, D, Debut, A, Vizuete, K, Ballesteros, I, et al. Effect of thermal liquefaction on quality, chemical composition and antibiofilm activity against multiresistant human pathogens of crystallized eucalyptus honey. Food Chem. (2021) 365:130519. doi: 10.1016/j.foodchem.2021.130519
56. Majkut, M, Kwiecinska-Pirog, J, Wszelaczynska, E, Pobereżny, J, Gospodarek-Komkowska, E, Wojtacki, K, et al. Antimicrobial activity of heat-treated polish honeys. Food Chem. (2021) 343:128561. doi: 10.1016/j.foodchem.2020.128561
57. Brudzynski, K, and Kim, L. Storage-induced chemical changes in active components of honey de-regulate its antibacterial activity. Food Chem. (2011) 126:1155–63. doi: 10.1016/j.foodchem.2010.11.151
58. Jones, ZJM, Huang, Y, Green, KJ, and Hammer, KA. Changes in antibacterial activity, colour, and hydrogen peroxide content of Western Australian Jarrah and Marri honeys after storage at different temperatures over time. J Appl Microbiol. (2023) 134:lxad164. doi: 10.1093/jambio/lxad164
59. Gheldof, N, Wang, XH, and Engeseth, NJ. Identification and quantification of antioxidant components of honeys from various floral sources. J Agric Food Chem. (2002) 50:5870–7. doi: 10.1021/jf0256135
60. Dżugan, M, Tomczyk, M, Sowa, P, and Grabek-Lejko, D. Antioxidant activity as biomarker of honey variety. Molecules. (2018) 23:2069. doi: 10.3390/molecules23082069
61. Martinello, M, and Mutinelli, F. Antioxidant activity in bee products: a review. Antioxidants. (2021) 10:71. doi: 10.3390/antiox10010071
62. Munteanu, IG, and Apetrei, C. Analytical methods used in determining antioxidant activity: a review. Int J Mol Sci. (2021) 22:3380. doi: 10.3390/ijms22073380
63. Erejuwa, OO, Sulaiman, SA, and Ab Wahab, MS. Honey: a novel antioxidant. Molecules. (2012) 17:4400–23. doi: 10.3390/molecules17044400
64. Faraz, A, Fernando, WB, Williams, M, and Jayasena, V. Effects of different processing methods on the antioxidant and antimicrobial properties of honey: a review. Int J Food Sci Technol. (2023) 58:3489–501. doi: 10.1111/ijfs.16460
65. Šarić, G, Marković, K, Vukičević, D, Lež, E, Hruškar, M, and Vahčić, N. Changes of antioxidant activity in honey after heat treatment. Czech J Food Sci. (2013) 31:601–6. doi: 10.17221/509/2012-CJFS
66. Silva, B, Biluca, FC, Gonzaga, LV, Fett, R, Dalmarco, EM, Caon, T, et al. In vitro anti-inflammatory properties of honey flavonoids: a review. Food Res Int. (2021) 141:110086. doi: 10.1016/j.foodres.2020.110086
67. Önür, İ, Misra, NN, Barba, FJ, Putnik, P, Lorenzo, JM, Gökmen, V, et al. Effects of ultrasound and high pressure on physicochemical properties and HMF formation in Turkish honey types. J Food Eng. (2018) 219:129–36. doi: 10.1016/j.jfoodeng.2017.09.019
68. Quintero-Lira, A, Ángeles Santos, A, Aguirre-Álvarez, G, Reyes-Munguía, A, Almaraz-Buendía, I, and Campos-Montiel, RG. Effects of liquefying crystallized honey by ultrasound on crystal size, 5-hydroxymethylfurfural, colour, phenolic compounds and antioxidant activity. Eur Food Res Technol. (2017) 243:619–26. doi: 10.1007/s00217-016-2775-0
69. Tang, SP, Wan Yusuf, WN, Abd Aziz, CB, Mustafa, M, and Mohamed, M. Effects of six-month Tualang honey supplementation on physiological and biochemical profiles in asymptomatic, treatment-naïve HIV-infected patients. Trop J Nat Prod Res. (2020) 4:1116–23. doi: 10.26538/tjnpr/v4i12.14
70. Ahmad, NS, Abdul Aziz, A, Kong, KW, Hamid, MSA, Cheong, JPG, and Hamzah, SH. Dose–response effect of Tualang honey on postprandial antioxidant activity and oxidative stress in female athletes: a pilot study. J Altern Complement Med. (2017) 23:989–95. doi: 10.1089/acm.2017.0129
71. Enginyurt, O, Cakir, L, Karatas, A, Cankaya, S, Kaya, Y, Tugcu, HH, et al. The role of pure honey in the treatment of diabetes mellitus. Biomed Res. (2017) 28:3305–12.
72. Ahmadi, M, Moosavi, SM, and Sh, Z. Comparison of the effect of honey and diphenhydramine on cough alleviation in 2-5-year-old children with viral upper respiratory tract infection. J Gorgan Univ Med Sci. (2013) 15:8–13.
73. Ayazi, P, Mahyar, A, Yousef-Zanjani, M, Allami, A, Esmailzadehha, N, and Beyhaghi, T. Comparison of the effect of two kinds of Iranian honey and diphenhydramine on nocturnal cough and the sleep quality in coughing children and their parents. PLoS One. (2017) 12:e0170277. doi: 10.1371/journal.pone.0170277
74. Cohen, HA, Rozen, J, Kristal, H, Laks, Y, Berkovitch, M, Uziel, Y, et al. Effect of honey on nocturnal cough and sleep quality: a double-blind, randomized, placebo-controlled study. Peadiatrics. (2012) 130:465–71. doi: 10.1542/peds.2011-3075
75. Paul, IM, Beiler, J, McMonagle, A, Shaffer, ML, Duda, L, and Berlin, CM Jr. Effect of honey, dextromethorphan, and no treatment on nocturnal cough and sleep quality for coughing children and their parents. Arch Pediatr Adolesc Med. (2007) 161:1140–6. doi: 10.1001/archpedi.161.12.1140
76. Raeessi, MA, Aslani, J, Raeessi, N, Gharaie, H, Karimi Zarchi, AA, and Raeessi, F. Honey plus coffee versus systemic steroid in the treatment of persistent post-infectious cough: a randomised controlled trial. Prim Care Respir J. (2013) 22:325–30. doi: 10.4104/pcrj.2013.00072
77. Shadkam, MN, Mozaffari-Khosravi, H, and Mozayan, MR. A comparison of the effect of honey, dextromethorphan, and diphenhydramine on nightly cough and sleep quality in children and their parents. J Altern Complement Med. (2010) 16:787–93. doi: 10.1089/acm.2009.0311
78. Waris, A, Macharia, WM, Njeru, EK, and Essajee, F. Randomised double blind study to compare effectiveness of honey, salbutamol and placebo in treatment of cough in children with common cold. East Afr Med J. (2014) 91:50–6.
79. Despland, C, Walther, B, Kast, C, Campos, V, Rey, V, Stefanoni, N, et al. A randomized-controlled clinical trial of high fructose diets from either Robinia honey or free fructose and glucose in healthy normal weight males. Clin Nutr ESPEN. (2017) 19:16–22. doi: 10.1016/j.clnesp.2017.01.009
80. Husniati, YL, Hazlina, NN, Azidah, AK, Norhayati, MN, Amrah, SS, Idiana, HI, et al. Safety of honey in postmenopausal women. Int Med J. (2013) 20:25–8.
81. Whitfield, P, Parry-Strong, A, Walsh, E, Weatherall, M, and Krebs, JD. The effect of a cinnamon-, chromium- and magnesium-formulated honey on glycaemic control, weight loss and lipid parameters in type 2 diabetes: an open-label cross-over randomised controlled trial. Eur J Nutr. (2016) 55:1123–31. doi: 10.1007/s00394-015-0926-x
82. Yaghoobi, N, Al-Waili, N, Ghayour-Mobarhan, M, Parizadeh, SMR, Abasalti, Z, Yaghoobi, Z, et al. Natural honey and cardiovascular risk factors; effects on blood glucose, cholesterol, triacylglycerole, CRP, and body weight compared with sucrose. Sci World J. (2008) 8:463–9. doi: 10.1100/tsw.2008.64
83. Ghazali, WS, Romli, AC, and Mohamed, M. Effects of honey supplementation on inflammatory markers among chronic smokers: a randomized controlled trial. BMC Complement Altern Med. (2017) 17:175. doi: 10.1186/s12906-017-1703-6
84. Mushtaq, R, Mushtaq, R, and Khan, ZT. Effects of natural honey on lipid profile and body weight in normal weight and obese adults: a randomized clinical trial. Pak J Zool. (2011) 43:161–9.
85. Raatz, SK, Johnson, LK, and Picklo, MJ. Consumption of honey, sucrose, and high-fructose corn syrup produces similar metabolic effects in glucose-tolerant and -intolerant individuals. J Nutr. (2015) 145:2265–72. doi: 10.3945/jn.115.218016
86. Rasad, H, Entezari, MH, Ghadiri, E, Mahaki, B, and Pahlavani, N. The effect of honey consumption compared with sucrose on lipid profile in young healthy subjects (randomized clinical trial). Clin Nutr ESPEN. (2018) 26:8–12. doi: 10.1016/j.clnesp.2018.04.016
87. Rashid, MR, Nor Aripin, KN, Syed Mohideen, FB, Baharom, N, Omar, K, Md Taujuddin, NMS, et al. The effect of Kelulut honey on fasting blood glucose and metabolic parameters in patients with impaired fasting glucose. J Nutr Metab. (2019) 2019:1–7. doi: 10.1155/2019/3176018
Keywords: quality standards, functional food, clinical trial, human health, natural product
Citation: Majtan J (2024) In vitro testing of honey quality and biological functionality: underestimated elements in the clinical testing of honey. Front. Nutr. 11:1433786. doi: 10.3389/fnut.2024.1433786
Edited by:
Tatiana Colombo Pimentel, Federal Institute of Education, Science and Technology of Paraná, BrazilReviewed by:
Lee Sin Chang, UCSI University, MalaysiaSandra Rayén Quilodrán-Vega, University of Concepción, Chile
Otilia Bobis, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, Romania
Copyright © 2024 Majtan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juraj Majtan, anVyYWoubWFqdGFuQHNhdmJhLnNr
 Juraj Majtan
Juraj Majtan