
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Nutr. , 17 July 2024
Sec. Nutritional Epidemiology
Volume 11 - 2024 | https://doi.org/10.3389/fnut.2024.1430140
This article is part of the Research Topic Polyphenols and Betalains in Obesity and Metabolic Syndrome View all 4 articles
Aim: The prevalence of obesity (Ob), overweight (Ow) and central obesity (CO) in children and adolescents has increased dramatically over the past decades globally. Flavanones have been recently studied as adjuvants for the treatment of obesity. This study was aimed at evaluating the association between intake of flavanones and its subclasses and the Ow/Ob and CO in children and adolescents.
Methods: This cross-sectional study extracted the data of children and adolescents with Ow/Ob and CO from the National Health and Nutrition Examination Survey (NHANES) database for 2007–2010 and 2017–2018. Ow and Ob were defined as a body mass index (BMI) ≥ 85th percentile. CO was defined as a waist circumference (WC) ≥ 90th percentile. The association between intake of flavanones and its subclasses and the Ow/Ob and CO in children and adolescents was determined by weighted univariate and multivariate Logistic regression models adjusted for potential covariates, and odds ratios (ORs) with 95% confidence intervals (CIs) was calculated. To further explore association between intake of flavanones and its subclasses and the Ow/Ob and CO in children and adolescents, subgroup analyses stratified by age, and gender.
Results: Of the total 5,970 children and adolescents, 2,463 (41.2%) developed Ow/Ob and 1,294 (21.7%) patients developed CO. High intake of flavanones, eriodictyol, hesperetin, and naringenin were associated with lower odds of Ow/Ob in children and adolescents. (OR: 0.75, 95%CI: 0.62–0.92, OR: 0.69, 95%CI: 0.55–0.87, OR: 0.69, 95%CI: 0.55–0.87, and OR: 0.76, 95%CI: 0.63–0.92, respectively). In addition, high intake of flavanones, eriodictyol, and naringenin were associated with lower odds of CO in children and adolescents (OR: 0.71, 95%CI: 0.57–0.88, OR: 0.67, 95%CI: 0.51–0.86, and OR: 0.69, 95%CI: 0.55–0.86, respectively). Subgroup analyses showed that among all the different subgroups, high intake of flavanones was associated with lower odds of Ow/Ob and CO in children and adolescents.
Conclusion: A diet loaded with high flavanones were associated with lower odds of Ow/Ob and CO in children and adolescents, and children and adolescents should be encouraged to increase their intake of flavanones.
Childhood obesity (Ob) is one of the most serious worldwide public health problems in the twenty-first century (1). According to the data of WHO in 2022, 37 million children were overweight of those aged <5 years, and 390 million of aged 5–19 years (2). Overweight (Ow) and Ob are indirect indicators based on body mass index (BMI, kg/m2), indicating excessive accumulations of fat, while central obesity (CO) is an indirect indicator based on waist circumference, indicating excessive accumulation of visceral fat around the stomach and abdomen (3). Ow, Ob and CO have been reported to be associated with adverse outcomes in many diseases, such as metabolic syndrome, type 2 diabetes, and cardiovascular disease (3–5). Being overweight in children and adolescents affects their immediate health, has adverse psychosocial consequences, and is associated with greater risk and earlier onset of various non-communicable diseases, it is crucial to adopt preventive interventions. Accumulating evidence indicates that low-quality diets were associated with an increased risk of Ow, Ob, and CO (4, 6, 7). It is essential to recommend dietary modification as the primary approach for the prevention and intervention of childhood Ob.
Flavanone, a common subclass of flavonoids, is a natural phenolic compound with diverse biological effects, mostly found in citrus fruits, mainly including hesperidin, naringenin, eriodictyol, etc. (8, 9). Mounting evidence has demonstrated that flavanones exert multiple therapeutic effects including anti-inflammation (10, 11), antioxidant (12, 13), anti-cancer (14, 15), cardioprotection (16, 17), anti-diabetic (18), and anti-obesity (19, 20). Vernarelli et al. (21) found that in U.S. adults, intake of flavonoids, including flavanones, was inversely associated with Ob and C-reactive protein levels (a marker of inflammation) in women. Studies in Korean adults have found that a high intake of flavanones may be associated with a decreased body fat percentage and CO in women (22). In addition, several animal experimental studies have found that hesperidin, naringenin, and eriodictyol can improve lipid metabolism and control body weight and Ob (10, 18, 19, 23, 24). To our knowledge, however, most of the current studies have focused on adults and animals, and no studies on the association of flavanones and its subclasses, with Ow/Ob and CO in children and adolescents.
This study is based on the NHANES database to analyze the association of flavanones and its subclasses intake, with Ow/Ob and CO in children and adolescents, and whether this association remains in patients stratified by age, and gender.
This cross-sectional study extracted the data of children and adolescents with Ow/Ob and CO from the National Health and Nutrition Examination Survey (NHANES) database for 2007–2010 and 2017–2018. The NHANES is a nationally representative survey conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC), that surveys health, nutrition, and other lifestyle factors across the adults and children in the United States (25). Complete details about the NHANES data collection and interview process are available on the NHANES website.1 NHANES is a publicly available dataset and was approved by the NCHS Ethics Review Board, and all patients/participants provided their written informed consent.
The inclusion criteria were: (1) patients aged 6–18 years old. The exclusion criteria were: (1) patients with missing data on energy and flavanones intake; (2) patients with missing data on height, weight, and waist circumference.
Covariates included age (in years), gender (female/male), race (Non-Hispanic White, Non-Hispanic Black, or others), educational level (<9th grade, or ≥ 9th grade), poverty income ratio (PIR) (<1.0, ≥1.0, or unknown), household education level (less than high school degree, or high school degree and above), mother smoked when pregnant, total energy, fiber, total fat, birth weight (<5.5, 5.5–8.9, ≥9 or unknown), tobacco exposure were defined as subjects who had responded “Yes” to the question “Does anyone smoke in the home/of people who live here smoke tobacco/of people who smoke inside this home? Physical activity level was classified as under 12 years old (days physically active at least 60 min), over 12 years old high level (any one of the vigorous work activity and vigorous recreational activities), medium level (any two of moderate work activity and walk or bicycle and moderate recreational activities).
The recommended body mass index (BMI) percentiles from CDC for children with different age and gender were used.2 Ow and Ob were defined as a BMI ≥ 85th percentile (26). Waist circumference (WC) was measured at the thinnest point of the abdomen at the end of a normal expiration. CO was defined as a WC ≥ 90th percentile (27).
The main explanatory variables were the intake of flavanones and its subclasses.
Total flavanones intake (mg/1000 kcal) = Flavanones/Total energy*1000 (mg/1000 kcal).
Total eriodictyol intake (mg/1000 kcal) = Eriodictyol/Total energy*1000 (mg/1000 kcal).
Total hesperetin intake (mg/1000 kcal) = Hesperetin /Total energy*1000 (mg/1000 kcal).
Total naringenin intake (mg/1000 kcal) = Naringenin/Total energy*1000 (mg/1000 kcal).
Flavanones, eriodictyol, hesperetin, and naringenin levels were assigned using the following ranges based on previous study (28):
Flavanones: grade 1 (level = 0 mg/1000 kcal), grade 2 (0<level ≤ 1.74 mg/1000 kcal), grade 3 (level > 1.74 mg/1000 kcal).
Eriodictyol: grade 1 (level = 0 mg/1000 kcal), grade 2 (0<level ≤ 0.09 mg/1000 kcal), grade 3 (level > 0.09 mg/1000 kcal).
Hesperetin: grade 1 (level = 0 mg/1000 kcal), grade 2 (0<level ≤ 7.17 mg/1000 kcal), grade 3 (level > 7.17 mg/1000 kcal).
Naringenin: grade 1 (level = 0 mg/1000 kcal), grade 2 (0<level ≤ 0.32 mg/1000 kcal), grade 3 (level > 0.32 mg/1000 kcal).
Continuous variables were described as the mean ± standard error [Mean(±SE)], and compared using Student’s t test. Categorical variables were represented as number (n) and percentage, and Chi-square test was used for comparison between groups.
Random forests were used to impute missing values. Sensitivity analyses were performed on the data before and after imputation (Supplementary Table S1). Weighted univariate and multivariate Logistic regression models were used for analyzing the association between the intake of flavanones and the Ow/Ob and CO in children and adolescents, and odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. Model I [a] and Model I [b] as well as Model II [a] and Model II [b] were adjusted based on the statistically significant variables in Supplementary Tables S2, S3 by step-based regression, respectively. Model I [a] and Model I [b] finally adjusted for biological factors of age, gender, and race. The Model II [a] and Model II [b] finally adjusted for all confounding factors including age, gender, race, education, PIR, household education, tobacco exposure, birth weight, and mother smoking when pregnant. Subgroup analysis was performed stratified by age (6 < age ≤ 11 years, or 12 < age ≤ 18 years), and gender (female or male).
Data cleaning, missing value imputation, and modeling were performed using Python 3.9 (Python Software Foundation, Delaware, United States). Statistical analysis and sensitivity analyses were performed using SAS 9.4 software (SAS Institute Inc., Cary, NC, United States). The p-values <0.05 was considered significant and 0.05<p-values <0.1 was considered marginal significance for all analyses.
According to the inclusion and exclusion criteria in Figure 1, 5,970 eligible children and adolescents were extracted for analysis, of which 2,463 (41.2%) patients developed Ow/Ob and 1,294 (21.7%) patients developed CO. Table 1 compares the baseline characteristics of non-Ow/Ob group and Ow/Ob group, as well as non-CO group and CO group. The proportion of eriodictyol intake of 0 was significantly higher in the Ow/Ob group than in the non-Ow/Ob group (77.98% vs. 73.71%, p = 0.037). Ow/Ob group was more likely to be non-Hispanic Black, have less than 9-grade education, PIR<1.0, and household education level less than high school degree. There were significant differences between the Ow/Ob group and non-Ow/Ob group with respect to birth weight, tobacco exposure, and mother smoking when pregnant (p < 0.05 for all). The proportion of flavanones intake of 0 and naringenin intake of 0 was significantly higher in the CO group than in the non-CO group (49.45% vs. 43.65%, p = 0.029, and 49.83% vs. 44.06%, p = 0.023). CO group was younger, more likely to be Non-Hispanic Black, have less than 9 grade education, PIR < 1.0, household education level less than high school degree. There were significant differences between the CO group and non-CO group with respect to birth weight, tobacco exposure, and mother smoking when pregnant (p < 0.05 for all).
Compared with no intake of flavanones and its subclasses, intake of flavanones (level > 1.74), eriodictyol (0<level ≤ 0.09 and level > 0.09), hesperetin (0 < level ≤ 7.17), and naringenin (level > 0.32), were associated with lower odds of Ow/Ob in children and adolescents (OR: 0.75, 95%CI: 0.62–0.92, OR: 0.70, 95%CI: 0.52–0.94, OR: 0.69, 95%CI: 0.55–0.87, OR: 0.69, 95%CI: 0.55–0.87, and OR: 0.76, 95%CI: 0.63–0.92, respectively) (Table 2). In addition, compared with no intake of flavanones and its subclasses, intake of flavanones (level > 1.74), eriodictyol (level > 0.09), hesperetin (0 < level ≤ 7.17), and naringenin (level > 0.32) were associated with lower odds of CO in children and adolescents (OR: 0.71, 95%CI: 0.57–0.88, OR: 0.67, 95%CI: 0.51–0.86, OR: 0.73, 95%CI: 0.54–0.98, and OR: 0.69, 95%CI: 0.55–0.86, respectively) (Table 2).
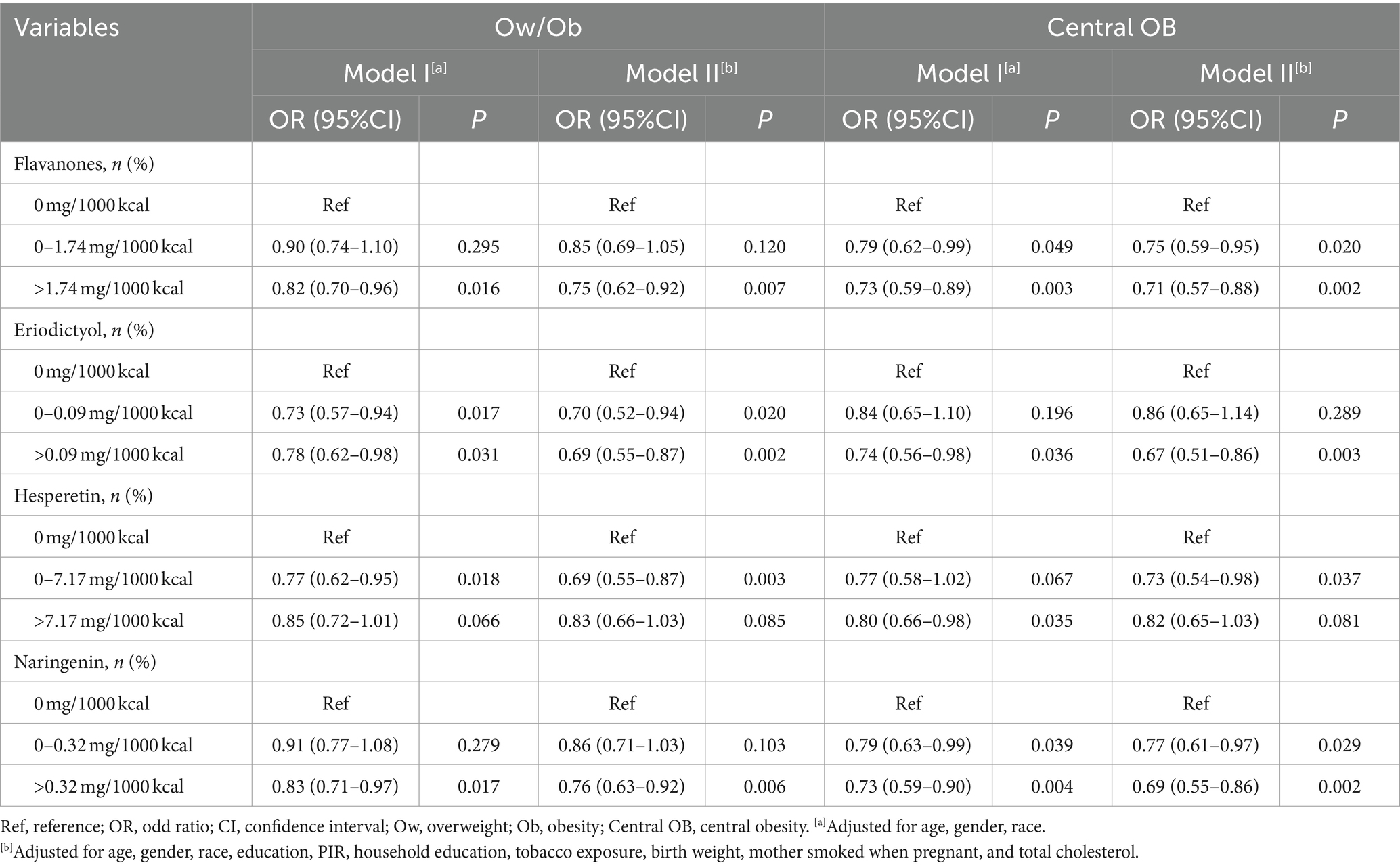
Table 2. Associations of flavanones and its subclasses intake with Ow/Ob and Central OB in children and adolescents.
To further analyze whether this association exists in children and adolescents with different status, subgroup analyses were performed (Table 3). Flavanones (level > 1.74) were associated with lower odds of Ow/Ob in children and adolescents aged 6–11 years in males (p = 0.082, p = 0.015, respectively). Eriodictyol (0<level ≤ 0.09 or/and level > 0.09) was associated with lower odds of Ow/Ob in children and adolescents aged 6–11 years in males (p = 0.034, p = 0.011, p = 0.003, respectively). Hesperetin (0<level ≤ 7.17 or/and level > 7.17) was associated with lower odds of Ow/Ob in children and adolescents aged 12–18 years in males or females (p = 0.029, p = 0.013, p = 0.030, respectively). Naringenin (level > 0.32) was associated with lower odds of Ow/Ob in children and adolescents aged 12 ~ 18 years in males (p = 0.100, p = 0.051, respectively). In addition, flavanones (level > 1.74) were associated with lower odds of CO in children and adolescents aged 12 ~ 18 years in males (p = 0.012, p = 0.004, respectively). Eriodictyol (0<level ≤ 0.09 or/and level > 0.09) was associated with lower odds of CO in children and adolescents aged 12–18 years in males (p = 0.048, p = 0.064, p = 0.061, respectively). Hesperetin (0<level ≤ 7.17 or/and level > 7.17) was associated with lower odds of CO in children and adolescents in males or females (p = 0.023, p = 0.079, respectively). Naringenin (0<level ≤ 0.32 or/and level > 0.32) was associated with lower odds of CO in children and adolescents aged 6–11 or 12 ~ 18 years in males (p = 0.076, p = 0.018, p = 0.006, respectively).
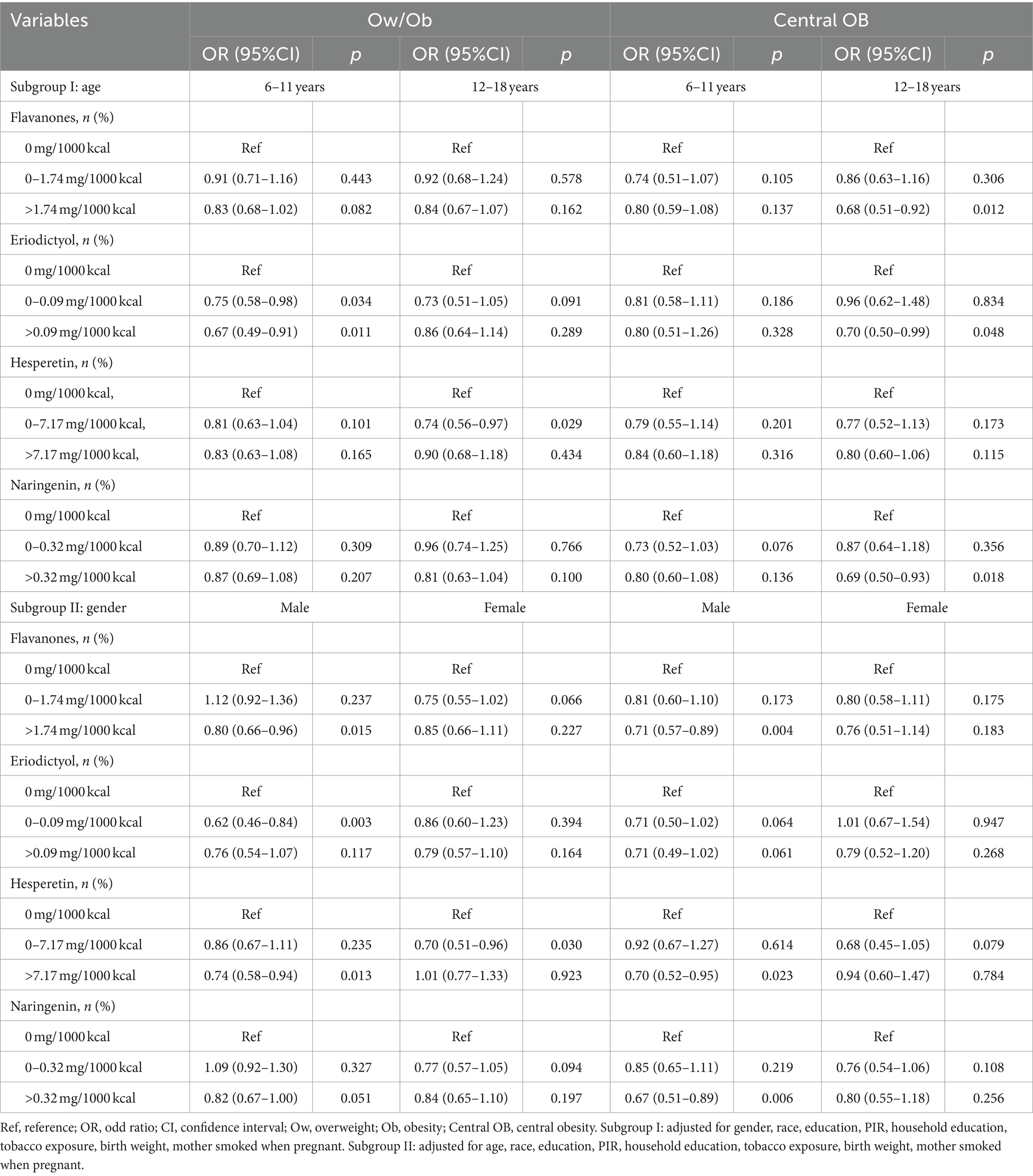
Table 3. Association between flavanones intake and Ow/Ob and Central OB in children and adolescents stratified by age, and gender.
This study aims to investigate the association of flavanones intake with Ow/Ob and CO in children and adolescents. We found that high intake of flavanones, eriodictyol, hesperetin, and naringenin were associated with lower odds of Ow/Ob in children and adolescents. In addition, high intake of flavanones, eriodictyol, and naringenin were associated with lower odds of CO in children and adolescents. Further subgroup analyses showed that among all the different subgroups, high intake of flavanones was associated with lower odds of Ow/Ob and CO in children and adolescents.
Phytochemicals including flavonoids, phenolic compounds, carotenoids, alkaloids, and organosulfur compounds (29, 30), have been reported to be associated with weight control (21, 31). For example, an analysis from a sample of Iranian school-age children elaborated that a higher load of phytochemicals in the diet was associated with a lower risk of Ow/Ob (32). In addition, habitual flavonoid intake has been reported to be inversely associated with Ow/Ob risk or changes in adiposity measures (21, 33). Flavonoids also could modulate adipokines, which were involved in obesity and inflammation (34, 35). Flavanones, also called citrus flavonoids, are an important series of flavonoids, mainly including hesperidin, naringenin, and eriodictyol (36). Studies in Korean adults have found that a high intake of flavanones may be associated with a decreased body fat percentage and CO in women (22). In addition, naringenin, eriodictyol, and hesperetin have emerged as promising therapeutic agents for the control of Ow/Ob and the treatment of metabolic disorders (24, 37). Lopez-Almada et al. (24) found that naringenin may be involved in inhibiting the onset and progression of Ob and its comorbidities through multiple pathways such as insulin resistance (IR), inflammation, OS, macrophage infiltration, dyslipidemia, and hepatic steatosis. Kwon et al. (38) found that dietary eriodictyol can prevent Ob and related metabolic diseases caused by diet, including hepatic steatosis, dyslipidemia, IR, and inflammation. Yoshida et al. demonstrated that citrus flavonoids hesperetin and naringenin could directly block TNF-alpha-stimulated FFA secretion (39). Phenolic compounds, among which isoflavones, may inhibit appetite through a modulation of the pattern of gene expression of peripheral and central peptides involved in feeding control, thus further supporting their importance as anti-obesity agents (40). Based on the above conclusions, in this study, we demonstrated that high intake of flavanones, eriodictyol, hesperetin, and naringenin were associated with lower odds of Ow/Ob in children and adolescents. In addition, high intake of flavanones, eriodictyol, and naringenin were associated with lower odds of CO in children and adolescents.
Several mechanisms have been proposed to describe the association between high intake of flavanones and the lower odds of Ow/Ob and CO in children and adolescents. Studies have shown that several transcription factors play important roles in activating lipogenesis, including peroxisome proliferator-activated receptor gamma(PPAR-γ) and CCAAT/enhancer binding protein(C/EBPs) (41). Citrus flavonoids extracts can inhibit fat accumulation and intracellular triglycerides, reducing the PPAR-γ expression (42). Tumor necrosis factor-α (TNF-α) stimulates free fatty acid (FFA) secretion through adipocyte lipolysis, and increased plasma levels of FFA promote insulin resistance (39, 43). The hesperetin and naringenin inhibit NF-κB and ERK pathways, which in turn suppress TNF-α-stimulated FFA secretion and thereby inhibiting adipocyte lipolysis (39).
This study is the first to demonstrate that a diet loaded with high flavanones was associated with a decreased risk of Ow/Ob and CO in children and adolescents. Flavanone is a citrus phytochemical with health-promoting properties associated with a lower risk of Ob. Given that the incidence rates of Ow and Ob in children and adolescents are continuing to increase globally, it is imperative that current public health strategies should include education about flavanones intake. Encourage children and adolescents to increase their intake of flavanones, such as fruits, vegetables, cereals, legumes, dark chocolate, coffee, tea, and wine, among others.
The present study has several limitations. First, because of the cross-sectional design of this study, it cannot identify a causal link between the exposure and the outcome. Second, this study may be limited by other uncollected confounders, such as genetic Ob. Third, the tobacco exposure, birth weight, and physical activity levels were collected using self-report; thus, recall bias is inevitable.
A diet loaded with high flavanones was associated with lower odds of Ow/Ob and CO in children and adolescents. Further studies particularly the prospective ones are needed to confirm these findings.
Publicly available datasets were analyzed in this study. This data can be found at: NHANES database, https://wwwn.cdc.gov/nchs/nhanes/.
The requirement of ethical approval was waived by The Sixth Affiliated Hospital of Harbin Medical University for the studies involving humans because NHANES is a publicly available dataset and was approved by the NCHS Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
YL: Conceptualization, Project administration, Supervision, Writing – original draft, Writing – review & editing. ZL: Writing – review & editing, Methodology, Investigation, Formal analysis, Data curation. NW: Writing – review & editing, Project administration, Conceptualization.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1430140/full#supplementary-material
1. The Lancet Diabetes E. Childhood obesity: a growing pandemic. Lancet Diabetes Endocrinol. (2022) 10:1. doi: 10.1016/s2213-8587(21)00314-4
2. Obesity and overweight Obesity and overweight. Available at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (Accessed June 26, 2024).
3. Jebeile, H, Kelly, AS, O'Malley, G, and Baur, LA. Obesity in children and adolescents: epidemiology, causes, assessment, and management. Lancet Diabetes Endocrinol. (2022) 10:351–65. doi: 10.1016/s2213-8587(22)00047-x
4. Janakiraman, B, Abebe, SM, Chala, MB, and Demissie, SF. Epidemiology of general, central obesity and associated cardio-metabolic risks among university employees, Ethiopia: a cross-sectional study. Diabetes Metab Syndr Obes. (2020) 13:343–53. doi: 10.2147/dmso.S235981
5. Bradwisch, SA, Smith, EM, Mooney, C, and Scaccia, D. Obesity in children and adolescents: an overview. Nursing. (2020) 50:60–6. doi: 10.1097/01.Nurse.0000718908.20119.01
6. Schutte, S, Esser, D, Siebelink, E, Michielsen, CJR, Daanje, M, Matualatupauw, JC, et al. Diverging metabolic effects of 2 energy-restricted diets differing in nutrient quality: a 12-week randomized controlled trial in subjects with abdominal obesity. Am J Clin Nutr. (2022) 116:132–50. doi: 10.1093/ajcn/nqac025
7. Rousham, EK, Goudet, S, Markey, O, Griffiths, P, Boxer, B, Carroll, C, et al. Unhealthy food and beverage consumption in children and risk of overweight and obesity: a systematic review and meta-analysis. Adv Nutr. (2022) 13:1669–96. doi: 10.1093/advances/nmac032
8. Cai, J, Wen, H, Zhou, H, Zhang, D, Lan, D, Liu, S, et al. Naringenin: a flavanone with anti-inflammatory and anti-infective properties. Biomed Pharmacother. (2023) 164:114990. doi: 10.1016/j.biopha.2023.114990
9. Fraga, LN, Milenkovic, D, Anacleto, SL, Salemi, M, Lajolo, FM, and Hassimotto, NMA. Citrus flavanone metabolites significantly modulate global proteomic profile in pancreatic Β-cells under high-glucose-induced metabolic stress. Biochim Biophys Acta Proteins Proteom. (2023) 1871:140898. doi: 10.1016/j.bbapap.2023.140898
10. Deng, Z, Hassan, S, Rafiq, M, Li, H, He, Y, Cai, Y, et al. Pharmacological activity of Eriodictyol: the major natural polyphenolic flavanone. Evid Based Complement Alternat Med. (2020) 2020:6681352–11. doi: 10.1155/2020/6681352
11. Bustos-Salgado, P, Andrade-Carrera, B, Domínguez-Villegas, V, Díaz-Garrido, N, Rodríguez-Lagunas, MJ, Badía, J, et al. Screening anti-inflammatory effects of flavanones solutions. Int J Mol Sci. (2021) 22:878. doi: 10.3390/ijms22168878
12. Santos, CMM, and Silva, AMS. The antioxidant activity of Prenylflavonoids. Molecules. (2020) 25:3. doi: 10.3390/molecules25030696
13. Singh, B, Singh, JP, Kaur, A, and Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus Peel. Food Res Int. (2020) 132:109114. doi: 10.1016/j.foodres.2020.109114
14. Motallebi, M, Bhia, M, Rajani, HF, Bhia, I, Tabarraei, H, Mohammadkhani, N, et al. Naringenin: a potential flavonoid phytochemical for cancer therapy. Life Sci. (2022) 305:120752. doi: 10.1016/j.lfs.2022.120752
15. Aiello, P, Consalvi, S, Poce, G, Raguzzini, A, Toti, E, Palmery, M, et al. Dietary flavonoids: Nano delivery and nanoparticles for cancer therapy. Semin Cancer Biol. (2021) 69:150–65. doi: 10.1016/j.semcancer.2019.08.029
16. Liu, P, Li, J, Liu, M, Zhang, M, Xue, Y, Zhang, Y, et al. Hesperetin modulates the Sirt1/Nrf2 Signaling pathway in counteracting myocardial ischemia through suppression of oxidative stress, inflammation, and apoptosis. Biomed Pharmacother. (2021) 139:111552. doi: 10.1016/j.biopha.2021.111552
17. Heidary Moghaddam, R, Samimi, Z, Moradi, SZ, Little, PJ, Xu, S, and Farzaei, MH. Naringenin and Naringin in cardiovascular disease prevention: a preclinical review. Eur J Pharmacol. (2020) 887:173535. doi: 10.1016/j.ejphar.2020.173535
18. Rajan, P, Natraj, P, Ranaweera, SS, Dayarathne, LA, Lee, YJ, and Han, CH. Anti-diabetic effect of hesperidin on palmitate (pa)-treated Hepg2 cells and high fat diet-induced obese mice. Food Res Int. (2022) 162:112059. doi: 10.1016/j.foodres.2022.112059
19. Zhang, S, Li, J, Shi, X, Tan, X, and Si, Q. Naringenin activates beige adipocyte Browning in high fat diet-fed C57bl/6 mice by shaping the gut microbiota. Food Funct. (2022) 13:9918–30. doi: 10.1039/d2fo01610a
20. Islam, A, Islam, MS, Rahman, MK, Uddin, MN, and Akanda, MR. The pharmacological and biological roles of Eriodictyol. Arch Pharm Res. (2020) 43:582–92. doi: 10.1007/s12272-020-01243-0
21. Vernarelli, JA, and Lambert, JD. Flavonoid intake is inversely associated with obesity and C-reactive protein, a marker for inflammation, in us adults. Nutr Diabetes. (2017) 7:e276. doi: 10.1038/nutd.2017.22
22. Kim, SA, Kim, J, Jun, S, Wie, GA, Shin, S, and Joung, H. Association between dietary flavonoid intake and obesity among adults in Korea. Appl Physiol Nutr Metab. (2020) 45:203–12. doi: 10.1139/apnm-2019-0211
23. Liu, S, Liu, K, Wang, Y, Wu, C, Xiao, Y, Liu, S, et al. Hesperidin methyl Chalcone ameliorates lipid metabolic disorders by activating lipase activity and increasing energy metabolism. Biochim Biophys Acta Mol basis Dis. (2023) 1869:166620. doi: 10.1016/j.bbadis.2022.166620
24. López-Almada, G, Domínguez-Avila, JA, Mejía-León, ME, Robles-Sánchez, M, González-Aguilar, GA, and Salazar-López, NJ. Could Naringenin participate as a regulator of obesity and satiety? Molecules. (2023) 28:1450. doi: 10.3390/molecules28031450
25. Curtin, LR, Mohadjer, LK, Dohrmann, SM, Kruszon-Moran, D, Mirel, LB, Carroll, MD, et al. National Health and nutrition examination survey: sample design, 2007-2010. Vital Health Stat. (2013) 2:1–23.
26. Tsoi, MF, Li, HL, Feng, Q, Cheung, CL, Cheung, TT, and Cheung, BMY. Prevalence of childhood obesity in the United States in 1999-2018: a 20-year analysis. Obes Facts. (2022) 15:560–9. doi: 10.1159/000524261
27. Fernández, JR, Redden, DT, Pietrobelli, A, and Allison, DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr. (2004) 145:439–44. doi: 10.1016/j.jpeds.2004.06.044
28. Li, J, Shi, H, Wang, L, and He, N. Effect of dietary flavonoids on circadian syndrome: a population-based cross-sectional study. Metab Syndr Relat Disord. (2024) 22:385–93. doi: 10.1089/met.2023.0245
29. Singh, VK, Arora, D, Ansari, MI, and Sharma, PK. Phytochemicals based Chemopreventive and chemotherapeutic strategies and modern technologies to overcome limitations for better clinical applications. Phytother Res. (2019) 33:3064–89. doi: 10.1002/ptr.6508
30. Sivakumar, D, Chen, L, and Sultanbawa, Y. A comprehensive review on beneficial dietary phytochemicals in common traditional southern African leafy vegetables. Food Sci Nutr. (2018) 6:714–27. doi: 10.1002/fsn3.643
31. Pourreza, S, Mirzababaei, A, Naeini, F, Naghshi, S, and Mirzaei, K. Association of Dietary Phytochemical Index with metabolically unhealthy overweight/obesity phenotype among Iranian women: a cross-sectional study. Front Nutr. (2022) 9:959341. doi: 10.3389/fnut.2022.959341
32. Eslami, O, Khoshgoo, M, and Shidfar, F. Dietary phytochemical index and overweight/obesity in children: a cross-sectional study. BMC Res Notes. (2020) 13:132. doi: 10.1186/s13104-020-04979-6
33. Kim, K, Vance, TM, and Chun, OK. Greater flavonoid intake is associated with improved Cvd risk factors in us adults. Br J Nutr. (2016) 115:1481–8. doi: 10.1017/s0007114516000519
34. Seo, MJ, Lee, YJ, Hwang, JH, Kim, KJ, and Lee, BY. The inhibitory effects of quercetin on obesity and obesity-induced inflammation by regulation of MAPK signaling. J Nutr Biochem. (2015) 26:1308–16. doi: 10.1016/j.jnutbio.2015.06.005
35. Oriquat, G, Masoud, IM, Kamel, MA, Aboudeya, HM, Bakir, MB, and Shaker, SA. The anti-obesity and anti-Steatotic effects of Chrysin in a rat model of obesity mediated through modulating the hepatic AMPK/mTOR/lipogenesis pathways. Molecules. (2023) 28:1734. doi: 10.3390/molecules28041734
36. Barreca, D, Gattuso, G, Bellocco, E, Calderaro, A, Trombetta, D, Smeriglio, A, et al. Flavanones: citrus phytochemical with health-promoting properties. Biofactors. (2017) 43:495–506. doi: 10.1002/biof.1363
37. Xiong, H, Wang, J, Ran, Q, Lou, G, Peng, C, Gan, Q, et al. Hesperidin: a therapeutic agent for obesity. Drug Des Devel Ther. (2019) 13:3855–66. doi: 10.2147/dddt.S227499
38. Kwon, EY, and Choi, MS. Dietary Eriodictyol alleviates adiposity, hepatic steatosis, insulin resistance, and inflammation in diet-induced obese mice. Int J Mol Sci. (2019) 20:1227. doi: 10.3390/ijms20051227
39. Yoshida, H, Takamura, N, Shuto, T, Ogata, K, Tokunaga, J, Kawai, K, et al. The citrus flavonoids Hesperetin and Naringenin block the Lipolytic actions of Tnf-alpha in mouse adipocytes. Biochem Biophys Res Commun. (2010) 394:728–32. doi: 10.1016/j.bbrc.2010.03.060
40. Rahman, MM, Rahaman, MS, Islam, MR, Rahman, F, Mithi, FM, Alqahtani, T, et al. Role of phenolic compounds in human disease: current knowledge and future prospects. Molecules. (2021) 27:233. doi: 10.3390/molecules27010233
41. Lim, H, Yeo, E, Song, E, Chang, YH, Han, BK, Choi, HJ, et al. Bioconversion of citrus Unshiu Peel extracts with Cytolase suppresses Adipogenic activity in 3t3-L1 cells. Nutr Res Pract. (2015) 9:599–605. doi: 10.4162/nrp.2015.9.6.599
42. Dudhia, Z, Louw, J, Muller, C, Joubert, E, de Beer, D, Kinnear, C, et al. Cyclopia Maculata and Cyclopia Subternata (Honeybush tea) inhibits Adipogenesis in 3t3-L1 pre-adipocytes. Phytomedicine. (2013) 20:401–8. doi: 10.1016/j.phymed.2012.12.002
Keywords: flavanones, children and adolescence, overweight & obesity, central obesity, NHANES
Citation: Liu Y, Liu Z and Wu N (2024) Association between intake of flavanones and the overweight/obesity and central obesity in children and adolescents: a cross-sectional study from the NHANES database. Front. Nutr. 11:1430140. doi: 10.3389/fnut.2024.1430140
Received: 09 May 2024; Accepted: 01 July 2024;
Published: 17 July 2024.
Edited by:
Victoria Ramírez, National Institute of Medical Sciences and Nutrition Salvador Zubirán, MexicoReviewed by:
Claudio Ferrante, University of Studies G. d’Annunzio Chieti and Pescara, ItalyCopyright © 2024 Liu, Liu and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nan Wu, bmFud3VfY2hpbGhhcmJpbkBob3RtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.