- Department of Emergency Medicine, Nanjing Drum Tower Hospital, Nanjing University Medical School, Nanjing, China
Sepsis is a life-threatening organ dysfunction syndrome caused by a dysregulated response to infection in the body. Effective treatment of sepsis poses a significant challenge in today’s clinical field. In recent years, enteral nutrition has garnered significant attention as an essential supportive therapeutic strategy. Serving as a means to provide ample nutritional support directly through the gastrointestinal tract, enteral nutrition not only addresses the nutritional depletion caused by the disease but also holds potential advantages in regulating immune function, maintaining intestinal mucosal barrier integrity, and promoting tissue repair. This article delves into the latest advancements of enteral nutrition in the treatment of sepsis, with a particular focus on its application effectiveness in clinical practice, potential mechanisms, and challenges faced. By examining relevant basic and clinical research, the aim is to provide a deeper understanding of nutritional therapy for sepsis patients and offer valuable insights for future research and clinical practice.
1 Introduction
Sepsis is a life-threatening syndrome of organ dysfunction caused by a dysregulated host response to infection (1). It poses a significant challenge in the current clinical field. The clinical manifestations of sepsis not only include a systemic inflammatory response but May also be accompanied by multiple organ dysfunction, which necessitates appropriate clinical treatment for the patients (2). In this context, enteral nutrition has garnered increasing attention in recent years as an important supportive treatment strategy.
The importance of the gut in both physiological and pathological processes has been widely recognized. In the development of sepsis, the gut is not only a source of infection but is also considered the “engine” of multiple organ dysfunction syndrome (3). The impairment of gut barrier function and the dysregulation of the immune system make sepsis patients more susceptible to secondary infections and exacerbate systemic inflammation (4, 5). Therefore, targeting the gut has become a breakthrough in alleviating the condition of sepsis patients.
Enteral nutrition, as a means of providing adequate nutritional support directly through the gut, not only compensates for the nutritional depletion caused by the disease but also has potential advantages in regulating immune function, maintaining the gut mucosal barrier, and promoting tissue repair (6). This article will delve into the latest advancements in enteral nutrition in the treatment of sepsis, focusing on its effectiveness in clinical practice, potential mechanisms, and challenges faced. By reviewing relevant basic research and clinical trials, we aim to provide a deeper understanding of nutritional therapy for sepsis patients and offer valuable insights for future research and clinical practice.
2 Sepsis and intestinal function
2.1 Impact of sepsis on the intestine
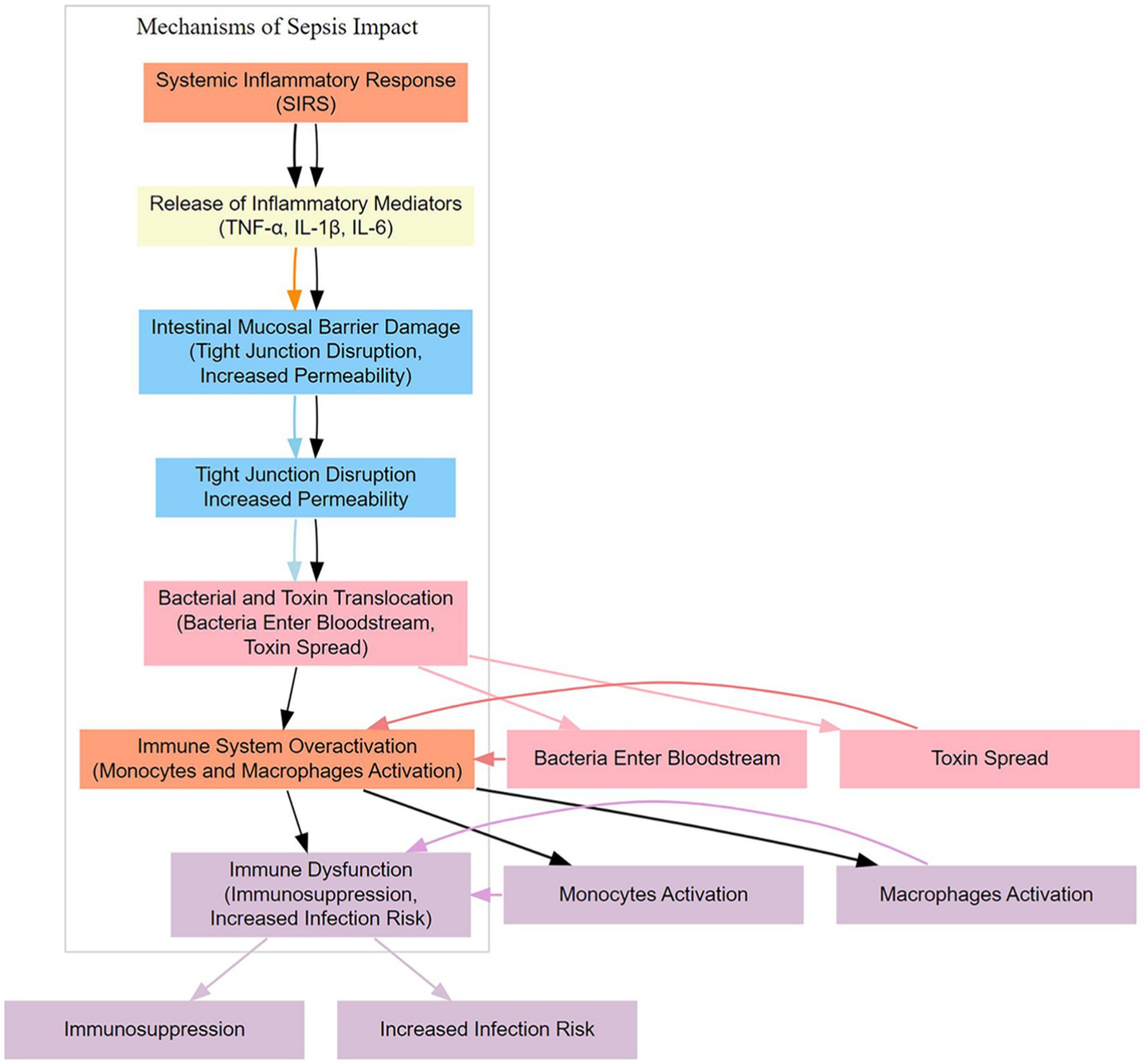
Sepsis is a severe systemic infection, with its impact on the gut primarily involving the disruption of the intestinal mucosal barrier due to inflammation. During sepsis, the immune system’s intense response to the infection can lead to the release of inflammatory mediators, which trigger a series of adverse reactions in the intestinal mucosa (2, 7). Firstly, inflammation can increase mucosal permeability, leading to a loosening of the mucosal barrier. Under normal conditions, the intestinal mucosal barrier maintains a relatively tight structure through intercellular junctions and secreted substances to prevent the translocation of harmful substances and microorganisms. However, during sepsis, inflammation-induced changes can cause the mucosal barrier to become more permeable, allowing bacteria, toxins, and other pathogens to traverse the mucosa more easily. Secondly, the inflammatory response can increase vascular permeability, resulting in the leakage of fluids and proteins from the vasculature into the surrounding tissues. These leaked substances can exacerbate damage to the intestinal mucosal barrier and negatively impact normal digestive and absorptive functions. Finally, inflammation-induced cellular damage and cell death can lead to ulceration of the intestinal mucosa. These ulcers are a direct result of mucosal barrier disruption and provide additional entry points for pathogens. Furthermore, the ulcers can cause localized bleeding, worsening tissue damage. Overall, the impact of sepsis on the gut is primarily characterized by inflammation-induced disruption of the intestinal mucosal barrier, leading to increased permeability, fluid leakage, and ulcer formation, which severely interfere with normal gut function.
2.2 Role of the immune system
The immune system plays a crucial role in the gut of sepsis patients, with its activities primarily influenced by the inflammatory response triggered by the infection source (8, 9) (Figure 1). The immune system is essential in maintaining intestinal homeostasis, preventing pathogen invasion, and protecting the integrity of the mucosal barrier.
After the onset of sepsis, the immune system is activated to respond to the pathogens and toxins released by the infection source. Inflammation is a natural response of the immune system aimed at clearing harmful substances, repairing damaged tissue, and preventing the spread of infection. However, in sepsis patients, the level of inflammation can become excessively heightened, leading to collateral damage to normal tissues by the immune system. Immune cells, such as leukocytes and macrophages, are recruited to the site of infection to clear pathogens. In the gut, this can result in the release of inflammatory mediators, including cytokines and other inflammatory agents, which, when released in excess, can negatively impact the intestinal mucosal barrier. The overactivation of the immune system may increase the permeability of the mucosal barrier, making it easier for bacteria, toxins, and other harmful substances to cross the mucosa. Simultaneously, the immune attack May damage normal tissues, contributing to the formation of ulcers. Therefore, the activity of the immune system in the gut of sepsis patients, particularly the excessive degree of inflammation, can adversely affect the integrity of the mucosal barrier, thereby worsening the condition (10). When managing sepsis, balancing the immune response to mitigate excessive inflammation and prevent damage to the gut is a critical therapeutic consideration.
3 Basic principles of enteral nutrition
3.1 Definition of enteral nutrition
Enteral nutrition refers to the delivery of nutrients into the body through oral or intestinal routes to meet the body’s nutritional requirements. This approach utilizes the absorptive and metabolic capacity of the intestines to provide essential nutrients necessary for maintaining life (11).
Key concepts of enteral nutrition include:
3.1.1 Oral route
The most common form of enteral nutrition is through oral intake. This involves the consumption of various foods, beverages, or specialized nutritional supplements via the oral cavity. The oral route not only aligns with physiological habits but also provides the body with essential energy, proteins, vitamins, and minerals through the normal gastrointestinal absorption process.
3.1.2 Intestinal route
When patients are unable to consume sufficient nutrients orally, the intestinal route serves as an alternative option. This can be achieved by delivering specially formulated nutrient solutions directly into the stomach or small intestine via a gastric or jejunal tube. This method bypasses oral intake and delivers nutrients directly, suitable for certain special circumstances such as postoperative recovery, critically ill patients, or individuals with swallowing difficulties.
3.1.3 Absorption and utilization
The intestine is the primary site for nutrient absorption, where epithelial cells of the mucosa transport nutrients into the bloodstream through active and passive absorption mechanisms. Carbohydrates, fats, proteins, vitamins, minerals, and other nutrients are broken down, absorbed, and metabolized in the intestine to provide the body with energy and essential substances for maintaining physiological functions.
Providing nutrition via the enteral route contributes to maintaining the health and integrity of the intestinal mucosa and promotes normal intestinal function. This holds significant clinical significance for patients who cannot obtain sufficient nutrition through regular dietary intake, such as those undergoing postoperative recovery, experiencing malnutrition, or suffering from intestinal dysfunction.
3.2 Maintenance of intestinal mucosal barrier
3.2.1 Provision of nutritional support
The intestine plays a crucial role in maintaining immune function and mucosal integrity. Enteral nutrition delivers various nutrients, including proteins, vitamins, and minerals, via oral or intestinal routes, providing the required energy and essential components to mucosal cells. This aids in promoting mucosal cell repair and regeneration, maintaining the normal structure and function of the intestine. Through the action of enteral nutrition, infusion of specific nutrients helps alleviate organ damage caused by sepsis (12–15).
3.2.2 Anti-inflammatory effects
Certain components of enteral nutrition, such as Omega-3 fatty acids, antioxidants, glucose, and calcitriol, are known to possess anti-inflammatory properties (16–19). They can attenuate inflammatory responses through various pathways by regulating the release of inflammatory mediators, thereby reducing mucosal barrier damage (20).
3.2.3 Support of the immune system
Enteral nutrition aids in maintaining the normal function of the immune system. Adequate nutritional support enhances the immune system’s resistance to infection and mitigates the inflammatory process. Maintaining immune system balance helps prevent excessive immune responses, thereby reducing damage to the intestinal mucosa (10, 21, 22).
3.2.4 Maintenance of intestinal microbial balance
Alterations in gut microbiota influence the progression of sepsis in patients (23, 24). Enteral nutrition helps maintain intestinal microbial balance, including the quantity and types of beneficial bacteria within the intestine. Beneficial bacteria play a critical role in maintaining the integrity of the intestinal mucosal barrier and combating harmful microorganisms. By providing nutrients such as probiotics and prebiotics, enteral nutrition supports a healthy gut microbiota, preventing the overgrowth of harmful bacteria and reducing potential inflammatory factors (25).
In summary, enteral nutrition plays a vital role in maintaining the intestinal mucosal barrier and attenuating the inflammatory process by providing the necessary nutrients for mucosal health, exerting anti-inflammatory effects, supporting the immune system, and maintaining intestinal microbial balance (6). This maintenance role holds significant clinical significance for many disease states, particularly inflammatory conditions related to intestinal health.
4 Nutritional status assessment in sepsis patients
4.1 Common nutritional issues in sepsis patients
4.1.1 Protein depletion
Sepsis patients often face significant protein depletion due to intense inflammatory responses and accelerated metabolism. Proteins are utilized in sepsis for synthesis of immune cells, tissue repair, and cell proliferation among various physiological processes. Consequently, protein depletion in sepsis May lead to muscle loss, compromised immune function, and associated nutritional problems.
4.1.2 Imbalance of trace elements
Infection and inflammatory processes may lead to imbalances in trace elements within the body. For instance, elements like zinc, iron, and selenium might be utilized extensively under inflammatory conditions, potentially depleting reserves in the body. This depletion could impair immune function and disrupt cellular metabolism.
4.1.3 Abnormal energy metabolism
Sepsis patients typically exhibit heightened metabolic activity, resulting in significantly increased energy demands. Inadequate energy supply may lead to abnormal energy metabolism, resulting in weight loss, muscle wasting, and related issues.
4.1.4 Vitamin deficiency
Chronic inflammatory states may cause metabolic abnormalities in vitamins, particularly vitamins C and D. Vitamins play crucial roles in immune function and tissue repair, hence deficiencies may further impact patient recovery.
4.1.5 Fluid and electrolyte imbalance
Sepsis patients may experience significant fluid loss and electrolyte disturbances, especially during fever, vomiting, diarrhea, and other infection-induced conditions. This may lead to reduced blood volume, impaired circulatory function, and subsequently affect normal organ function.
Therefore, maintaining an appropriate nutritional status is crucial when managing sepsis patients. Individualized nutritional support plans, including adequate protein intake, supplementation of trace elements, appropriate energy provision, and balancing of vitamins and electrolytes, are essential for promoting patient recovery and mitigating the inflammatory process.
4.2 The necessity of nutritional support
Sepsis, a severe infectious disease, typically accompanies intense immune responses and systemic inflammation. In this pathological state, patients undergo a series of complex physiological changes, including heightened metabolic activity, excessive activation of the immune system, and increased demand for tissue repair. These physiological changes pose significant challenges to patients’ nutritional requirements, rendering traditional diets insufficient to meet their body’s needs. Therefore, providing specialized nutritional support is crucial for sepsis patients. Firstly, sepsis patients often face rapid protein depletion and significant loss. Proteins are critical components for tissue repair, immune responses, and cell proliferation. Hence, sepsis patients require increased protein intake to meet these additional demands (26). Secondly, energy requirements are markedly elevated during sepsis due to excessive metabolic activity and elevated body temperature. Normal diets may not provide sufficient energy; thus, specialized high-energy nutritional support, such as high-energy oral supplements or intravenous infusion via enteral routes, may be beneficial. Moreover, the loss of trace elements, vitamins, and electrolytes is another reason sepsis patients require specialized nutritional support. These substances play crucial roles in immune function, anti-inflammatory responses, and tissue repair.
In summary, due to their unique physiological status, sepsis patients require specialized nutritional support to ensure adequate intake of protein, energy, and other essential nutrients. This support facilitates recovery, alleviates inflammation, and ultimately improves patient survival and quality of life. Therefore, individualized and comprehensive nutritional support plans are indispensable components of sepsis patient management.
5 Clinical research and advancements
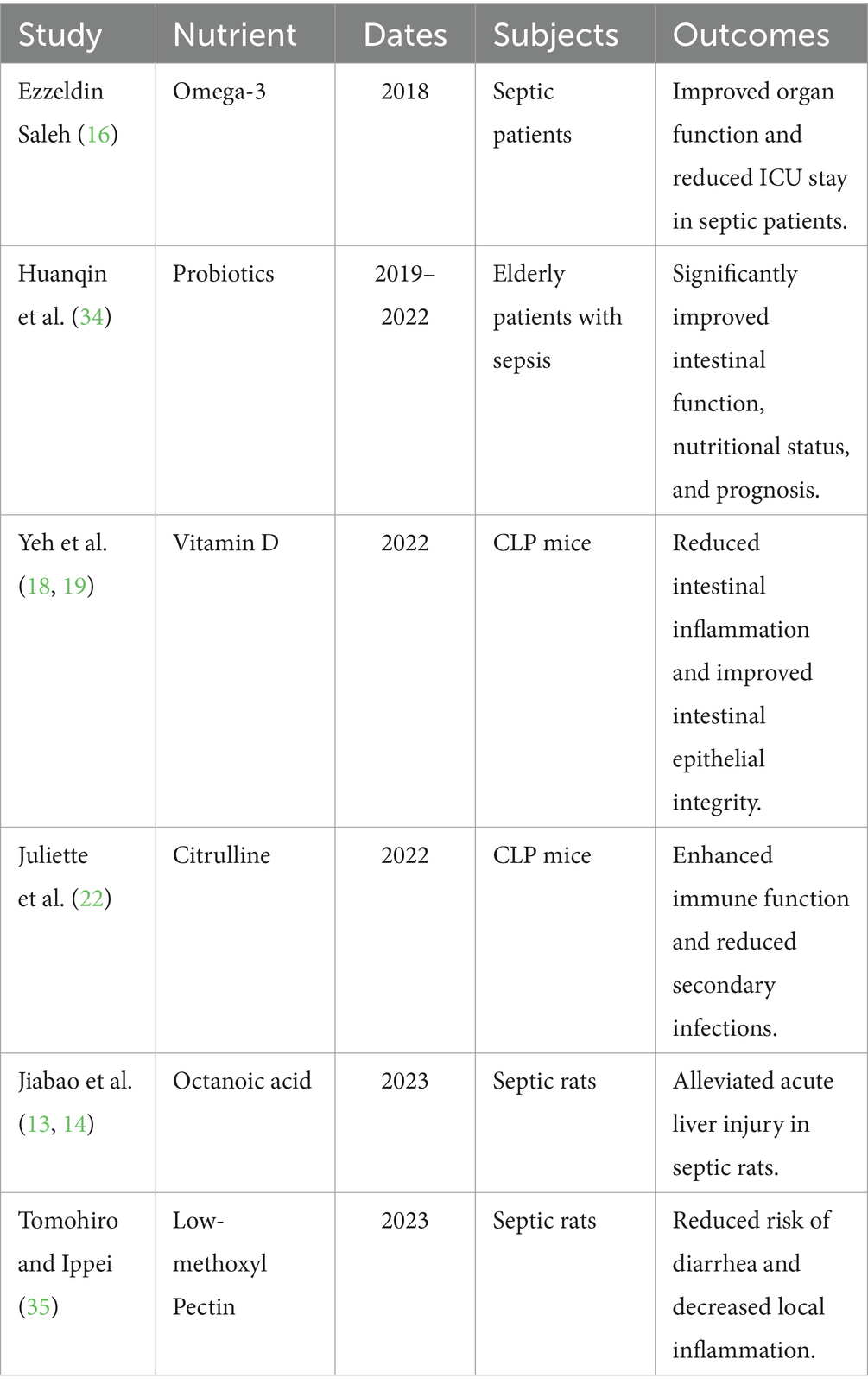
Recent research has deeply explored the crucial role of enteral nutrition in the treatment of sepsis, particularly regarding changes in the gut microbiota (27). In the realm of personalized nutrition, researchers are focusing on tailoring enteral nutrition regimens based on the patient’s pathophysiological conditions and metabolic needs. By analyzing the patient’s microbiome, they are proposing new approaches to adjust formulations, aiming to improve immune regulation and reduce inflammation to optimize treatment outcomes (Table 1).
At the molecular level, recent research has concentrated on uncovering the mechanisms by which enteral nutrition regulates immune cell activity. Studies on specific nutritional components suggest that they may modulate immune cell functions by affecting various signaling pathways and gene expression (28). For instance, enteral nutrition has been shown to significantly alleviate hypercatabolism in endotoxemic rats through the ghrelin/GHS-R1α-POMC pathway (29). Supplementation with citrulline can markedly improve B cell suppression and plasma cell differentiation in sepsis, reducing immunosuppression and secondary infections (22). Similarly, in rat models, it was found that the addition of caprylic acid to enteral nutrition, compared to enteral nutrition alone, alleviated acute liver injury and improved gut function in septic rats by activating the PPARγ/STAT-1/MyD88 pathway (13, 14). The targeted inclusion of specific nutrients in enteral nutrition therapy holds promise for modulating inflammatory responses, providing a new theoretical foundation for designing more precise immunomodulatory strategies.
Recent clinical studies have found that early enteral nutrition can improve the severity of disease in postoperative sepsis patients (30). Similarly, early enteral nutrition may offer potential benefits for sepsis patients with concomitant muscle wasting and those with circulatory shock (31, 32). It may be particularly beneficial for sepsis patients with lower lactate levels (33). For elderly sepsis patients, early administration of probiotic-enriched enteral nutrition has been shown to significantly enhance gut function, nutritional status, and prognosis (34). Dietary additions such as low-methoxy pectin can effectively reduce the risk of diarrhea and alleviate local inflammation in sepsis conditions (35). Additionally, enteral nutrition containing dietary fiber has demonstrated substantial potential in reducing sepsis-related outcomes and preventing the development of sepsis in critically ill patients (15). However, in critically ill patients requiring vasopressors, enteral nutrition should be delayed or cautiously administered if cardiac output is low and dobutamine is needed, or in cases of high SAPS II scores with multiple organ failure (36). Early moderate enteral feeding (targeting 60% of requirements) can improve gut barrier function and nutritional and inflammatory status without increasing the incidence of feeding intolerance symptoms in sepsis (37). While early enteral nutrition helps maintain gut barrier function, it may pose risks of complications such as intestinal ischemia in patients with septic shock. Recent research suggests that a “small and better” enteral nutrition strategy during the acute phase may be safer, taking into account the severity of illness, dosage of vasopressors, and nutritional needs (38).
Evaluating the effectiveness of enteral nutrition during treatment is crucial (39, 40). Concurrently, early prediction and prevention of complications related to enteral nutrition remain a clinical focus. Research has found that a higher ratio of Firmicutes to Bacteroidetes and greater microbial diversity on the first day of enteral nutrition may aid in early prediction of enteral nutrition tolerance (41). The establishment and validation of XGBoost models can be used for early prediction of enteral nutrition initiation in ICU patients (42). New predictive models based on deep learning effectively forecast enteral feeding intolerance in sepsis patients in the ICU, which can be used for stratifying the risk of enteral nutrition intolerance in sepsis patients (43). The predictive capability of norepinephrine equivalent dose (NEQ) and the mean arterial pressure (MAP)/NEQ index can distinguish earlier whether shock patients receiving vasopressors are suitable for starting enteral nutrition, thus reducing the incidence of feeding intolerance and non-occlusive mesenteric ischemia (44, 45). Studies have shown that using improved ultrasound methods to guide enteral nutrition in sepsis patients allows for faster initiation of nutritional support and better outcomes compared to traditional clinical experience (40). For septic shock patients, a comprehensive assessment based on the clinical condition is required to determine the suitability for enteral nutrition. Obese patients are more prone to enteral nutrition intolerance, and the dosage of norepinephrine is significantly associated with tolerance (46).
Recent studies indicate that early enteral nutrition may be beneficial for sepsis patients (47–52). These latest findings offer valuable insights into the role of enteral nutrition in sepsis treatment and open new avenues for personalized treatment and innovative therapies. However, further research is needed to comprehensively understand the best practices and application strategies for enteral nutrition in sepsis treatment.
6 Limitations and challenges
6.1 Potential risks
6.1.1 Risk of infection
Enteral or tube feeding may pose a risk of infection, particularly when improper procedures are followed during tube insertion or maintenance. Bacteria can enter the gastrointestinal tract through the tube, increasing the likelihood of infection. Regular monitoring of tube placement, maintaining cleanliness of the tube, and employing sterile techniques are vital measures for reducing the risk of infection.
6.1.2 Risk of hypersensitivity reactions
In certain cases, patients may develop allergies or hypersensitivity reactions to certain components of enteral nutrition. This may include adverse reactions to specific proteins, lipid emulsions, or other additives. Therefore, when devising enteral nutrition regimens, careful consideration of the patient’s allergy history and individual differences is warranted.
6.1.3 Electrolyte imbalance
Prolonged enteral nutrition can lead to electrolyte imbalances, especially in patients with intestinal absorption issues. This may involve loss or accumulation of electrolytes such as sodium, potassium, calcium, affecting the function of the cardiovascular and nervous systems.
6.1.4 Gastrointestinal complications
The insertion of gastrointestinal tubes may result in complications such as gastric perforation, tube dislocation, among others. These complications could increase patient discomfort and treatment complexity (53, 54).
6.2 Therapeutic challenges
6.2.1 Patient acceptance
Some patients may exhibit psychological or physiological aversions to enteral or tube feeding, impacting treatment adherence. Additionally, septic patients may experience symptoms like anorexia and vomiting, reducing the feasibility of oral intake or enteral nutrition.
6.2.2 Variability in nutritional requirements
The progression and treatment of sepsis can alter patients’ nutritional needs. For instance, infection and inflammation states may accelerate metabolism, increasing the demand for protein and energy. Hence, nutritional regimens need timely adjustments to meet the evolving needs of patients.
6.2.3 Impaired intestinal function
Some septic patients may suffer from impaired intestinal function, affecting the absorption of enteral nutrition. This might necessitate the selection of more easily absorbable special formulas or consideration of alternative routes of nutrition support, such as parenteral nutrition.
In conclusion, understanding and addressing the potential risks and therapeutic challenges of enteral nutrition in the treatment of sepsis are crucial for enhancing treatment efficacy. Individualized treatment plans, close monitoring, and timely adjustments are key strategies for addressing these issues.
7 Key strategies for treatment
7.1 Key strategies for enteral nutrition in sepsis patients
7.1.1 Multifaceted consideration for individualized treatment plans
When devising personalized enteral nutrition regimens, a comprehensive assessment of the patient’s physical condition, nutritional needs, metabolic status, and treatment goals is necessary. This may involve utilizing advanced nutritional assessment tools and medical techniques to ensure the accuracy and effectiveness of the plan.
7.1.2 Precision management through monitoring and adjustment
Close monitoring forms the cornerstone of successful treatment. Regular assessment of the patient’s nutritional indicators, physiological parameters, and clinical presentation aids in timely detection of issues and subsequent adjustments. This necessitates healthcare professionals to possess a high level of expertise and skills to ensure the health and safety of patients.
7.1.3 Optimization of nutritional support and immune modulation
Nutritional support should not only aim to meet energy and nutrient requirements but also focus on its role in immune function modulation. Therefore, the design of enteral nutrition regimens should consider the types, proportions, and intake levels of nutrients to maximize the normal functioning of the immune system.
7.1.4 Delicate regulation of microbial balance
The gut microbiota play a significant role in the treatment of sepsis. Hence, the formulation of enteral nutrition plans should consider the patient’s microbial characteristics and take measures to promote the growth of beneficial bacteria while inhibiting the overgrowth of pathogenic bacteria to maintain gut health.
7.1.5 Comprehensive prevention and control of complications
Various complications may accompany the treatment of sepsis, such as malnutrition, infections, and organ dysfunction. Therefore, when formulating enteral nutrition plans, it is necessary to consider various potential complication risks and implement corresponding preventive and management measures to ensure smooth treatment progress.
During the process of providing enteral nutrition to sepsis patients, interdisciplinary teamwork among healthcare professionals is crucial. Only through comprehensive and meticulous assessment and management can treatment efficacy be maximized, laying a solid foundation for patient recovery and rehabilitation.
8 Summary and outlook
In summary, enteral nutrition holds promising prospects for the treatment of sepsis, offering a novel approach to improving patient outcomes. However, further research is warranted to gain a deeper understanding of the mechanisms underlying enteral nutrition and to develop more precise therapeutic strategies. These efforts aim to maximize both the survival rates and the quality of life for septic patients.
Author contributions
FX: Writing – original draft. GL: Writing – review & editing. JW: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Evans, L, Rhodes, A, Alhazzani, W, Antonelli, M, Coopersmith, CM, French, C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. (2021) 47:1181–247. doi: 10.1007/s00134-021-06506-y
2. Patel, J, Shukla, A, and Heyland, D. Enteral nutrition in septic shock: a pathophysiologic conundrum. JPEN J Parenter Enteral Nutr. (2021) 45:74–8. doi: 10.1002/jpen.2246
3. Fay, K, Ford, M, and Coopersmith, C. The intestinal microenvironment in sepsis. Biochim Biophys Acta Mol Basis Dis. (2017) 1863:2574–83. doi: 10.1016/j.bbadis.2017.03.005
4. Yang, X, Liu, D, Ren, H, Zhang, X, Zhang, J, and Yang, X. Effects of sepsis and its treatment measures on intestinal flora structure in critical care patients. World J Gastroenterol. (2021) 27:2376–93. doi: 10.3748/wjg.v27.i19.2376
5. Adelman, M, Woodworth, M, Langelier, C, Busch, L, Kempker, J, Kraft, C, et al. The gut microbiome's role in the development, maintenance, and outcomes of sepsis. Crit Care. (2020) 24:278. doi: 10.1186/s13054-020-02989-1
6. Pu, H, Doig, G, Heighes, P, and Allingstrup, M. Early enteral nutrition reduces mortality and improves other key outcomes in patients with major burn injury: a meta-analysis of randomized controlled trials. Crit Care Med. (2018) 46:2036–42. doi: 10.1097/CCM.0000000000003445
7. Haussner, F, Chakraborty, S, Halbgebauer, R, and Huber-Lang, M. Challenge to the intestinal mucosa during Sepsis. Front Immunol. (2019) 10:891. doi: 10.3389/fimmu.2019.00891
8. Nedeva, C . Inflammation and cell death of the innate and adaptive immune system during Sepsis. Biomol Ther. (2021) 11:11. doi: 10.3390/biom11071011
9. Delano, M, and Ward, P. The immune system's role in sepsis progression, resolution, and long-term outcome. Immunol Rev. (2016) 274:330–53. doi: 10.1111/imr.12499
10. McClave, S . Can feeding strategies alter immune signaling and gut sepsis in critical illness? JPEN J Parenter Enteral Nutr. (2021) 45:66–73. doi: 10.1002/jpen.2260
11. Doganay, M, Akcay, K, Cil, T, Dag, B, Demirag, K, Demirkan, K, et al. Enteral nutrition consensus report from KEPAN: indications, choice, practical application, and follow-up. Nutrition. (2023) 118:112269. doi: 10.1016/j.nut.2023.112269
12. He, K, Cao, C, Xu, X, Ye, Z, Ma, X, Chen, W, et al. Octanoic acid-rich enteral nutrition prevented lipopolysaccharide-induced acute liver injury through c-Jun N-terminal kinase-dependent autophagy. JPEN J Parenter Enteral Nutr. (2022) 46:1353–60. doi: 10.1002/jpen.2297
13. Tang, J, Li, X, Li, W, and Cao, C. Effects of enteral nutrition supplemented with octanoic acid on lipopolysaccharide-induced intestinal injury: role of peroxisome proliferator-activated receptor γ/STAT-1/myeloid differentiation factor 88 pathway. Nutrition. (2023) 116:112216. doi: 10.1016/j.nut.2023.112216
14. Tang, J, Li, X, Li, W, and Cao, C. Octanoic acid-rich enteral nutrition alleviated acute liver injury through PPARγ/STAT-1/MyD88 pathway in endotoxemic rats. In Vivo. (2023) 37:1609–18. doi: 10.21873/invivo.13246
15. Huwiler, V, Scalise, M, Schönenberger, K, Mühlebach, S, Stanga, Z, and Balmer, M. The role of dietary fibre in enteral nutrition in Sepsis prevention and therapy: a narrative review. Nutrients. (2023) 15:489. doi: 10.3390/nu15112489
16. Ezzeldin Saleh, I . Enteral nutrition with omega-3 fatty acids in critically ill septic patients: a randomized double-blinded study. Saudi J Anaesth. (2018) 12:529. doi: 10.4103/sja.SJA_50_18
17. Shah, F, Kitsios, G, Zhang, Y, Morris, A, Yende, S, Huang, D, et al. Rationale for and Design of the Study of early enteral dextrose in sepsis: a pilot placebo-controlled randomized clinical trial. JPEN J Parenter Enteral Nutr. (2020) 44:541–7. doi: 10.1002/jpen.1608
18. Yeh, C, Wu, J, Chen, K, Wu, M, Yang, P, Lee, P, et al. Effects of different routes and forms of vitamin D administration on mesenteric lymph node CD4+ T cell polarization and intestinal injury in obese mice complicated with polymicrobial sepsis. Nutrients. (2022) 14:557. doi: 10.3390/nu14173557
19. Yeh, C, Wu, J, Yang, P, Lee, P, Chen, K, Huang, C, et al. Intravenous calcitriol administration modulates mesenteric lymph node CD4 T-cell polarization and attenuates intestinal inflammation in obese mice complicated with polymicrobial sepsis. JPEN J Parenter Enteral Nutr. (2022) 46:1371–83. doi: 10.1002/jpen.2313
20. Sun, J, Zhang, W, Chen, W, Wang, X, and Mu, X. Effects of early enteral nutrition on Th17/Treg cells and IL-23/IL-17 in septic patients. World J Gastroenterol. (2019) 25:2799–808. doi: 10.3748/wjg.v25.i22.2799
21. Liu, Y, Zhao, W, Chen, W, Shen, X, Fu, R, Zhao, Y, et al. Effects of early enteral nutrition on immune function and prognosis of patients with Sepsis on mechanical ventilation. J Intensive Care Med. (2020) 35:1053–61. doi: 10.1177/0885066618809893
22. Gauthier, J, Grégoire, M, Reizine, F, Lesouhaitier, M, Desvois, Y, Ghukasyan, G, et al. Citrulline enteral administration markedly reduces immunosuppressive extrafollicular plasma cell differentiation in a preclinical model of sepsis. Eur J Immunol. (2023) 53:e2250154. doi: 10.1002/eji.202250154
23. Zhao, J, Pan, X, Hao, D, Zhao, Y, Chen, Y, Zhou, S, et al. Causal associations of gut microbiota and metabolites on sepsis: a two-sample Mendelian randomization study. Front Immunol. (2023) 14:1190230. doi: 10.3389/fimmu.2023.1190230
24. Klingensmith, N, and Coopersmith, C. Gut microbiome in Sepsis. Surg Infect. (2023) 24:250–7. doi: 10.1089/sur.2022.420
25. Yang, X, Wang, X, Yang, M, Ren, H, Chen, H, Zhang, X, et al. Exploring choices of early nutritional support for patients with sepsis based on changes in intestinal microecology. World J Gastroenterol. (2023) 29:2034–49. doi: 10.3748/wjg.v29.i13.2034
26. Cha, J, Kim, H, Kim, E, Lee, E, Lee, J, and Song, I. Effect of early nutritional support on clinical outcomes of critically ill patients with Sepsis and septic shock: a single-center retrospective study. Nutrients. (2022) 14:318. doi: 10.3390/nu14112318
27. Schill, E, Joyce, E, Floyd, A, Udayan, S, Rusconi, B, Gaddipati, S, et al. Vancomycin-induced gut microbial dysbiosis alters enteric neuron-macrophage interactions during a critical period of postnatal development. Front Immunol. (2023) 14:1268909. doi: 10.3389/fimmu.2023.1268909
28. Reizine, F, Grégoire, M, Lesouhaitier, M, Coirier, V, Gauthier, J, Delaloy, C, et al. Beneficial effects of citrulline enteral administration on sepsis-induced T cell mitochondrial dysfunction. Proc Natl Acad Sci USA. (2022) 119:e2115139119. doi: 10.1073/pnas.2115139119
29. Cao, C, Zhang, Y, Zuo, S, Zhao, W, Wu, Y, and Ma, X. Enteral nutrition alleviated lipopolysaccharides-induced hypercatabolism through ghrelin/GHS-R1α-POMC. Biochem Biophys Res Commun. (2022) 597:122–7. doi: 10.1016/j.bbrc.2022.01.130
30. Sun, J, Yuan, S, Mu, X, Zhang, W, Liu, Y, Zou, L, et al. Effects of early enteral nutrition on T helper lymphocytes of surgical septic patients: a retrospective observational study. Medicine. (2017) 96:e7702. doi: 10.1097/MD.0000000000007702
31. Koga, Y, Fujita, M, Yagi, T, Todani, M, Nakahara, T, Kawamura, Y, et al. Early enteral nutrition is associated with reduced in-hospital mortality from sepsis in patients with sarcopenia. J Crit Care. (2018) 47:153–8. doi: 10.1016/j.jcrc.2018.06.026
32. Luis, O-R, Jayshil, JP, Xuran, J, Angel, CY, Andrew, GD, Faraaz, S, et al. Early versus delayed enteral nutrition in mechanically ventilated patients with circulatory shock: a nested cohort analysis of an international multicenter, pragmatic clinical trial. Crit Care. (2022) 26:173. doi: 10.1186/s13054-022-04047-4
33. Fuchao, X, Jianxin, X, Jinjin, M, Wenbo, X, Shuangshuang, G, Geng, L, et al. Early versus delayed enteral nutrition in ICU patients with sepsis: a propensity score-matched analysis based on the MIMIC-IV database. Front Nutr. (2024) 11:472. doi: 10.3389/fnut.2024.1370472
34. Huanqin, X, Minjie, C, and Yijun, Z. Influence of early enteral nutrition plus probiotics on intestinal function of senile patients with sepsis. Am J Transl Res. (2023) 15:445–450.
35. Tomohiro, K, and Ippei, Y. Intragastric infusion of a liquid diet with low-methoxyl pectin alleviates fecal inconsistency and local proinflammatory cytokine expression in lipopolysaccharide-septic rats. Nutrition. (2023) 118:112271. doi: 10.1016/j.nut.2023.112271
36. Piton, G, Le Gouge, A, Boisramé-Helms, J, Anguel, N, Argaud, L, Asfar, P, et al. Factors associated with acute mesenteric ischemia among critically ill ventilated patients with shock: a post hoc analysis of the NUTRIREA2 trial. Intensive Care Med. (2022) 48:458–66. doi: 10.1007/s00134-022-06637-w
37. Sun, J, Nie, S, Chen, Y, Zhou, J, Wang, X, Zhou, S, et al. Effects of permissive hypocaloric standard enteral feeding on gastrointestinal function and outcomes in sepsis. World J Gastroenterol. (2021) 27:4900–12. doi: 10.3748/wjg.v27.i29.4900
38. Jayshil, JP, Juan Carlos, L-D, Christian, S, and Stephen, AM. Enteral nutrition in septic shock: a call for a paradigm shift. Curr Opin Crit Care. (2024) 30:165–71. doi: 10.1097/MCC.0000000000001134
39. Franzosi, O, Nunes, D, Klanovicz, T, Loss, S, Batassini, É, Turra, E, et al. Hemodynamic and skin perfusion is associated with successful enteral nutrition therapy in septic shock patients. Clin. Nutr. (2020) 39:3721–9. doi: 10.1016/j.clnu.2020.03.033
40. Gao, L, Li, S, Li, H, Guo, Q, Yan, J, and Shi, Q. Effectiveness of the improved B-ultrasound method for measuring the antral section to guide enteral nutrition in patients with sepsis in a randomized controlled trial. Asia Pac J Clin Nutr. (2021) 30:224–30. doi: 10.6133/apjcn.202106_30(2).0006
41. Xu, W, Zhong, M, Pan, T, Qu, H, and Chen, E. Gut microbiota and enteral nutrition tolerance in non-abdominal infection septic ICU patients: an observational study. Nutrients. (2022) 14:342. doi: 10.3390/nu14245342
42. Wang, Y, Li, X, Zhang, L, Li, H, Liu, X, Song, W, et al. Machine learning algorithms assist early evaluation of enteral nutrition in ICU patients. Front Nutr. (2023) 10:1060398. doi: 10.3389/fnut.2023.1060398
43. Hu, K, Deng, X, Han, L, Xiang, S, Xiong, B, and Pinhu, L. Development and validation of a predictive model for feeding intolerance in intensive care unit patients with sepsis. Saudi J Gastroenterol. (2022) 28:32–8. doi: 10.4103/sjg.sjg_286_21
44. Wang, L, Yang, H, Cheng, Y, Fu, X, Yao, H, Jin, X, et al. Mean arterial pressure/norepinephrine equivalent dose index as an early measure of initiation time for enteral nutrition in patients with shock: a prospective observational study. Nutrition. (2022) 96:111586. doi: 10.1016/j.nut.2021.111586
45. Nicole, CR, Amir, YK, Bethany, RS, Martin, DR, Irina, MM-C, Scott, CB, et al. Non-occlusive mesenteric ischemia: a rare but lethal complication of enteral nutrition in critically ill patients. Nutr Clin Pract. (2021) 37:715–26. doi: 10.1002/ncp.10761
46. Qi, F, Huang, G, Li, H, Zhao, X, and Liu, J. Correlation analysis of norepinephrine dose on enteral nutrition tolerance and prognosis in patients with septic shock. BMC Infect Dis. (2023) 23:386. doi: 10.1186/s12879-023-08366-x
47. Jiang, Y, Hu, B, Zhang, S, Cai, M, Chu, X, Zheng, D, et al. Effects of early enteral nutrition on the prognosis of patients with sepsis: secondary analysis of acute gastrointestinal injury study. Ann Palliat Med. (2020) 9:3793–801. doi: 10.21037/apm-20-1650
48. Patel, J, Kozeniecki, M, Peppard, W, Peppard, S, Zellner-Jones, S, Graf, J, et al. Phase 3 pilot randomized controlled trial comparing early trophic enteral nutrition with “no enteral nutrition” in mechanically ventilated patients with septic shock. JPEN J Parenter Enteral Nutr. (2020) 44:866–73. doi: 10.1002/jpen.1706
49. Talebi, S, Zeraattalab-Motlagh, S, Vajdi, M, Nielsen, S, Talebi, A, Ghavami, A, et al. Early vs. delayed enteral nutrition or parenteral nutrition in hospitalized patients: an umbrella review of systematic reviews and meta-analyses of randomized trials. Nutr Clin Pract. (2023) 38:564–79. doi: 10.1002/ncp.10976
50. Tomino, T, Harada, N, Toshida, K, Tomiyama, T, Kosai, Y, Kurihara, T, et al. Effect of early enteral nutrition on graft Loss after living donor liver transplantation: a propensity score matching analysis. Transplant Proc. (2023) 55:2164–70. doi: 10.1016/j.transproceed.2023.07.029
51. Wang, J, Jiang, L, Ding, S, He, S, Liu, S, Lu, Z, et al. Early enteral nutrition and Sepsis-associated acute kidney injury: a propensity score matched cohort study based on the MIMIC-III database. Yonsei Med J. (2023) 64:259–68. doi: 10.3349/ymj.2022.0276
52. Carlos, FG-A, Diego, T-P, Luis, CT, Saúl, JR, María, TV-O, Carlos, HC-F, et al. Early enteral nutrition (within 48 h) for patients with Sepsis or septic shock: a systematic review and Meta-analysis. Nutrients. (2024) 16:560. doi: 10.3390/nu16111560
53. Reignier, J, Boisramé-Helms, J, Brisard, L, Lascarrou, J, Ait Hssain, A, Anguel, N, et al. Enteral versus parenteral early nutrition in ventilated adults with shock: a randomised, controlled, multicentre, open-label, parallel-group study (NUTRIREA-2). Lancet. (2018) 391:133–43. doi: 10.1016/S0140-6736(17)32146-3
Keywords: sepsis, enteral nutrition, septic shock, nutritional support, research progress
Citation: Xu F, Lu G and Wang J (2024) Enhancing sepsis therapy: the evolving role of enteral nutrition. Front. Nutr. 11:1421632. doi: 10.3389/fnut.2024.1421632
Edited by:
Sladjana Sobajic, University of Belgrade, SerbiaReviewed by:
Li Cong, Affiliated Hospital of Xuzhou Medical University, ChinaYong Jiang, Southern Medical University, China
Copyright © 2024 Xu, Lu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Wang, d2pnYW9nb3VAYWxpeXVuLmNvbQ==
 Fuchao Xu
Fuchao Xu Geng Lu
Geng Lu Jun Wang
Jun Wang