- 1Department of Pediatrics, West China Second University Hospital, Sichuan University, Chengdu, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children, Sichuan University, Ministry of Education, Chengdu, China
- 3Department of Pediatric Surgery, West China Hospital, Sichuan University, Chengdu, China
- 4Department of Plastic, Aesthetic, Reparative and Reconstructive Surgery, West China Second University Hospital, Sichuan University, Chengdu, China
We herein present a case of a ruptured giant omphalocele with congenital short small intestine. Vacuum-sealing drainage and carboxymethylcellulose silver dressing promoted wound healing after repair, avoided abdominal compartment syndrome, and reduced the risks of multiple procedures. We review the perioperative management of omphaloceles in congenital short small intestines.
1 Introduction
Omphalocele, which occurs in approximately 1–3 per 10,000 live births (1–3), is a midline abdominal wall defect of variable size arising at the root of the umbilical cord. The defect is covered by a capsule composed of an amnion, Wharton’s jelly, and peritoneum, which often contains herniated abdominal contents (4). A giant omphalocele (GO) is defined as a defect ≥5 cm in diameter, presence of an omphalocele, or a large omphalocele relative to the fetal abdomen (5). Capsular rupture can occur in 7–15% of children with GOs and can significantly increase the risk of complications and mortality (6, 7). The mortality rate of omphalocele with malformations is approximately seven times that of isolated omphalocele (8, 9).
A congenital short small intestine is a rare developmental malformation of the small intestine that is functionally or anatomically inadequate in length, resulting in severely reduced intestinal absorptive capacity, manifesting as vomiting, diarrhea, growth retardation, and intestinal malrotation (10, 11). Congenital short small intestine may be inherited in an autosomal recessive pattern, such as loss-of-function mutations in coxsackie and adenovirus receptor-like membrane protein, or X-linked type of inheritance (12, 13). Currently, only two studies have reported omphaloceles in congenital short bowel syndrome (7, 14), while most cases of short bowel syndrome are due to necrotizing enterocolitis and intestinal atresia (15).
Herein, we report a case of ruptured GO closed by the application of vacuum sealing drainage (VSD) combined with silver dressing and share the nutritional support strategy for congenital short small intestine.
2 Clinical case
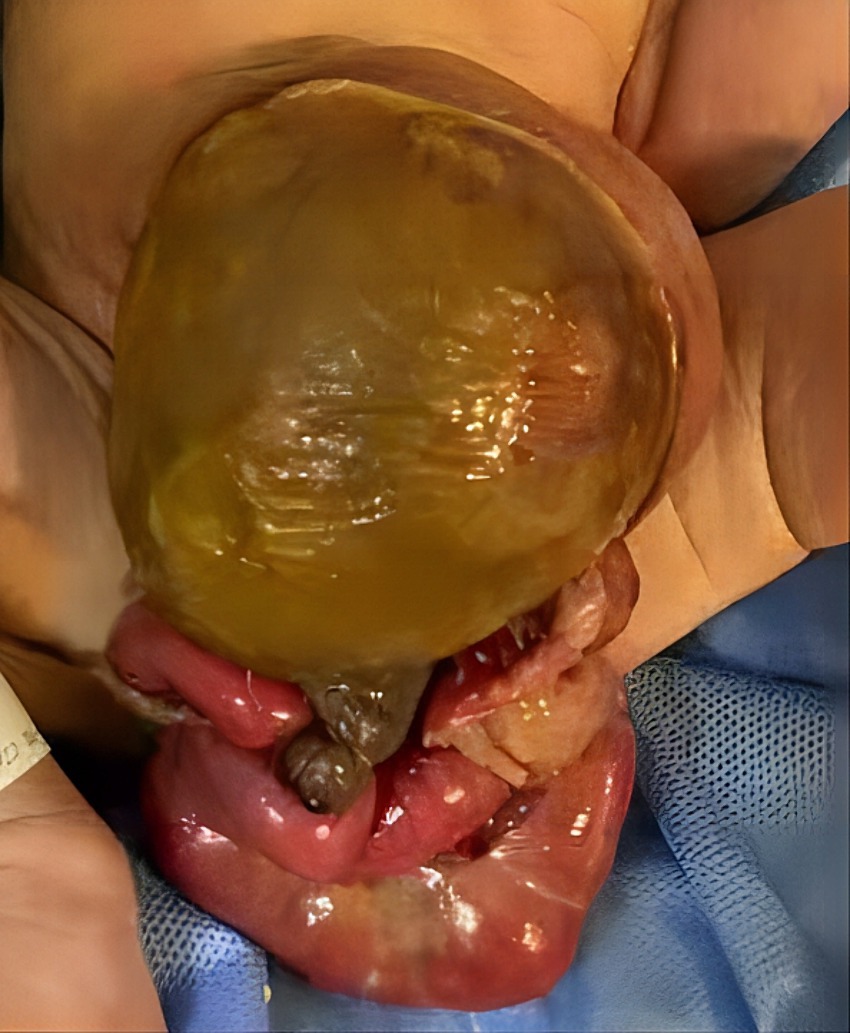
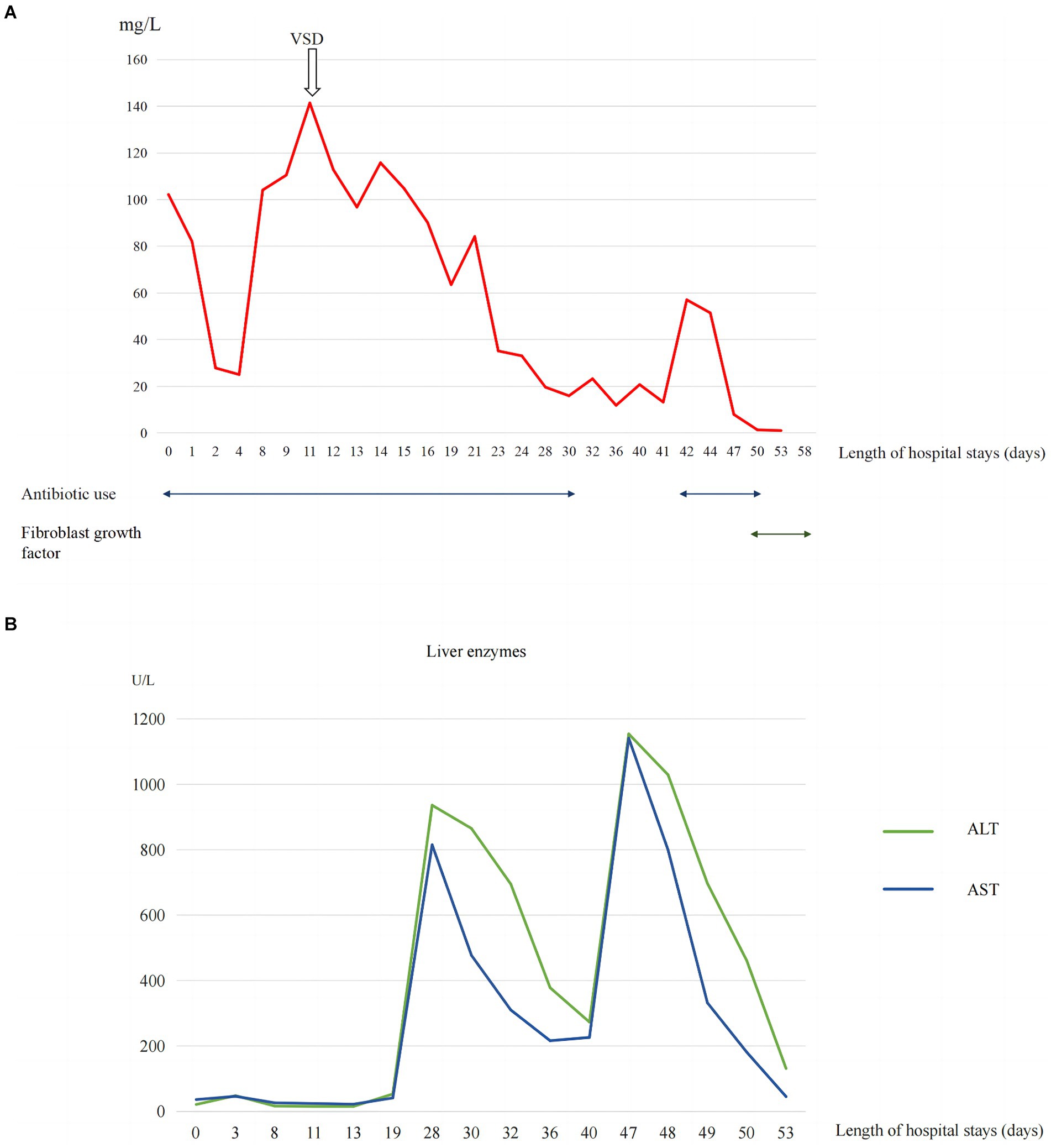
A 2-day-old girl with a 12 × 10 cm ruptured GO was transferred to our hospital on June 5, 2023. She was born vaginally with a birth weight of 3,000 g. Gestational age was 40 + 4 weeks. Apgar scores at 1 and 5 min were 10. The bowel was partially visible through the postnatally ruptured sac (Figure 1). The neonate had dyspnea and poor peripheral perfusion on examination owing to enteral fasting and evaporative fluid loss, characterized by decreased urine output, skin mottling, and prolonged capillary refill time. Radiography revealed polydactyly of the left foot and a right-sided thoracolumbar curve. After stabilization with fluid resuscitation, vasoactive drugs, antibiotics, and mechanical ventilation, pediatric surgeons placed a silo bag that night. The silo bag was anchored to the full layer of the abdominal wall with interrupted sutures so that pressures could be added post-operatively for reduction. The membrane of the sac was completely broken. There was severe adhesion and contamination on the intestines. Insensible water loss was increased from the intestines through the postnatally ruptured sac. The entire small bowel and colon, part of the stomach, and most of the liver were in omphalocele. The herniated portion of the liver was spherical. The length of the entire small intestine was 50 cm. Postoperatively, her intra-abdominal pressure was monitored, and she had a foley catheter to drain urine, thereby reducing the increase in intra-abdominal pressure caused by urinary retention. On the 10th postoperative day, the tension in the abdominal wound increased gradually, and the inferior suture ruptured with skin erythema and exudate. Additionally, she had recurrent fever with persistently elevated C-reactive protein (CRP) levels of up to 141 mg/L (Figure 2A).
Proper assessment and personalized wound repair strategy followed a multidisciplinary approach by an expert team, including neonatologists, plastic surgeons, pediatric surgeons, pediatric infectious disease specialist, enterostomal therapist, and radiologist. The protective wound material comprised three layers: the first layer was petroleum gauze, second layer was a carboxymethylcellulose silver dressing, and third layer was the closed VSD protective wound material; the VSD machine was connected. The VSD dressing was changed at the bedside 6 times over 38 days. CRP levels gradually decreased to normal without fever (Figure 2), the amount of liver exposure gradually decreased, and the wound gradually narrowed. A large amount of cellulosic exudate was observed on the surface of the antiadhesive petroleum gauze, which could be observed through the growth of granulation tissue and bled easily to touch (Figure 3), forming an intact granulation barrier. When the dressing was removed, the wound size was reduced to 2.3 × 2.6 cm; then, bovine basic fibroblast growth factor gel was applied to the wound to promote epithelialization. Finally, the abdominal wall defect was restored. On hospital days 28 and 47, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were markedly elevated (Figure 2B). Other liver function tests, including bilirubin, albumin, international normalized ratio, and coagulation function, were normal. ALT and AST returned to normal levels within 1 week after weaning off VSD. Full enteral feeding was achieved within 19 days after parenteral nutrition and early trophic feeding. After 11 days of mechanical ventilation, she was successfully extubated. Through 58 days of hospitalization, the infant grew from 2,930 to 4,670 g, with an average growth of 30 g per day. At the last follow-up, the infant was 6 months old and breastfed exclusively for 3 months with normal growth and development.
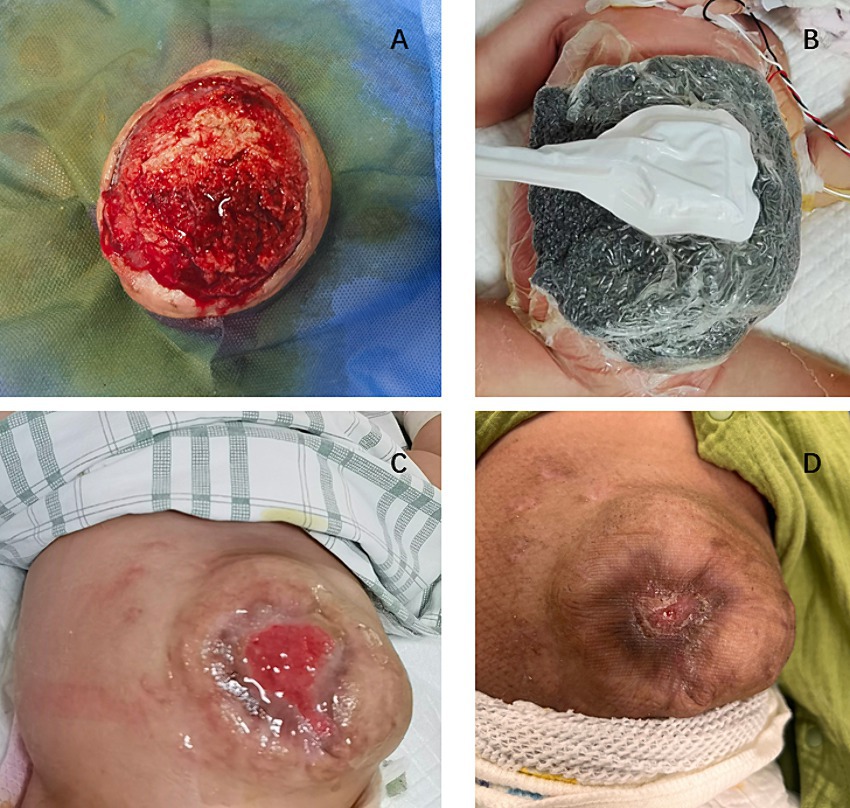
Figure 3. (A) Wound granulation tissue during replacement of VSD dressing (B) VSD and carboxymethylcellulose silver dressing used on the wound (C) Bovine basic fibroblast growth factor gel applied to the wound (D) Wound healing at outpatient follow-up.
3 Discussion
Omphaloceles are frequently associated with chromosomal abnormalities or non-chromosomal syndromes. GOs have a lower incidence of chromosomal abnormalities than small-sized omphaloceles but are more likely to cause pulmonary dysplasia and often require longer mechanical ventilation (16). The mother of the patient had no relevant risk factors for omphalocele, including low or advanced maternal age, prenatal overweight, maternal smoking, or alcohol consumption (17–19). Chromosomal microarray and whole-genome sequencing of the neonate revealed no abnormalities. Eleven days of postoperative mechanical ventilation was shorter than the mean postoperative duration of mechanical ventilation reported in previous studies in children with non-isolated omphalocele (20). This may be related to the absence of severe cardiac malformations or pulmonary hypoplasia, in addition to frequent ventilator support mode (synchronized intermittent mandatory ventilation, pressure support ventilation, and volume guarantee), fluid balance, correction of hypoalbuminemia, and intra-abdominal pressure monitoring.
Staged closure avoids acute increases in intra-abdominal pressure (21). The first part of the staging surgery included protection from intestinal perfusion, which creates conditions for the establishment of enteral feeding at an early stage. Wharton’s jelly is a component of the hernia sac’s components (4). Wharton’s jelly-derived mesenchymal stem cells and fibroblasts can promote the proliferation of epithelial cells and excretion of extracellular matrix (22). Therefore, before the application of VSD, the liver covered by the sac can accelerate the growth of granulation tissue and repair wounds. In this case, the second stage was six bedside VSD dressing changes over 38 days, and the third stage was VSD evacuation using fibroblast growth factor to promote wound epithelization and healing, which avoided infection, repeat anesthesia, and transport, and shortened the duration of VSD treatment, antibiotic course, and length of hospital stay. VSD is a non-surgical closed wound method that produces negative pressure continuously or intermittently through a pump, thereby increasing blood circulation, inhibiting bacterial growth, reducing wound edema, and accelerating wound healing (23, 24). VSD is a safe, convenient, and effective treatment for GOs, with a median treatment time of 68 days, median time of 19 days for full enteral feeding, and wound healing time with combined silver dressings varying (25–27). Carboxymethylcellulose silver dressing is a sterile, non-adhesive, and non-occlusive dressing comprising silver sulfate and a polyester textile mesh impregnated with hydrocolloid particles and petrolatum. When the dressing comes in contact with the wound fluid, silver sulfate releases silver ions and promotes wound healing (28). Abdominal wall plastic surgery is expected to be performed at 2 years of age.
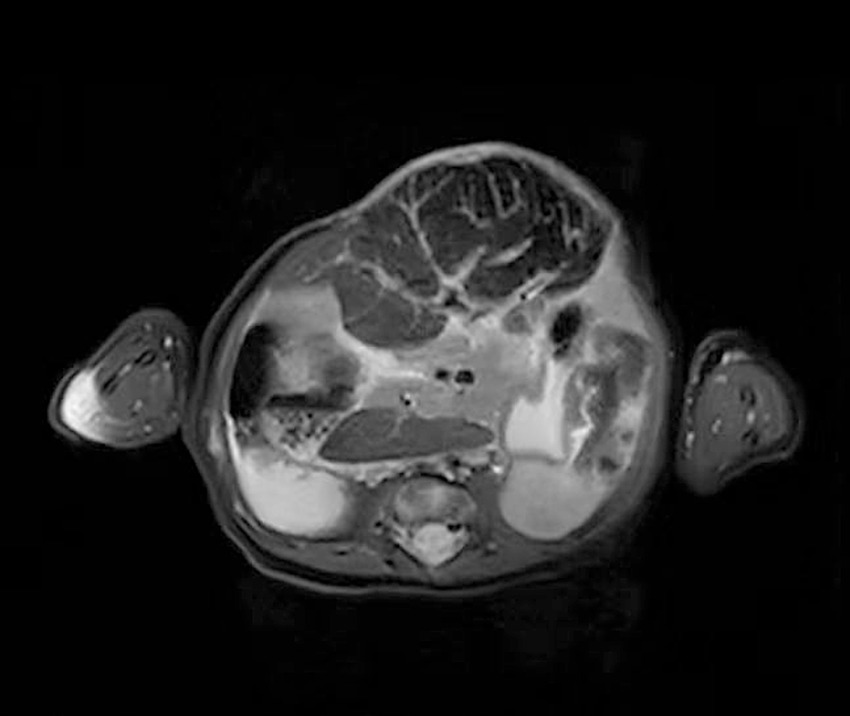
This patient developed markedly elevated serum ALT and AST levels. These suggested that the biochemical liver alterations were caused by hepatocellular injury. The etiologies of hepatocellular injury include acute viral hepatitis, ischemic hepatitis, septic shock, toxin/medication, metabolic disorders, and so on (29). This patient was tested for viruses, heavy metals toxin, and genetic metabolic disease, all of which were negative. Since the spherical shape of the liver (detected by MRI, Figure 4) was susceptible to injury by vacuum sealing drainage, the increased intra-abdominal pressure may have led to hepatic ischemia. In this case, the ALT and AST returned to normal ranges after withdrawing VSD. Therefore, we think that the elevated ALT and AST may be associated with hepatic ischemia/reperfusion injury.
The mean small bowel length in a child with congenital short bowel is 57 cm (30). The patient’s small bowel length was only 50 cm, which was consistent with a congenital short bowel. It has been reported that the early onset of clinical signs, including such as vomiting and diarrhea, in children with congenital short bowel disease suggests a poor prognosis, and delayed presentation suggests a favorable outcome (12). This patient did not develop relevant symptoms early on, and full enteral feeding was eventually successfully established. The management goals for congenital short small bowel and short bowel syndrome caused by intestinal resection postoperatively are similar. During the early intestinal maladaptation period, parenteral nutrition support is required to gradually enhance intestinal adaptation and achieve enteral autonomy, while avoiding complications such as intestinal failure-related liver disease and catheter-related bloodstream infections (15, 31). Breast milk is the first choice for enteral nutrition for children with short bowel syndrome; however, when breast milk is not available, some scholars recommend the use of hydrolyzed or amino acid milk because children with short bowel syndrome are prone to cow’s milk protein allergy (32, 33). Breast milk was unavailable; therefore, we chose extensively hydrolyzed formulas (eHF) combined with active parenteral nutrition for early trophoblastic feeding. Owing to the low osmolality of eHF, its energy density (67–81 kcal/dL) could be increased with the use of additional milk powder. Moreover, eHF is more prone to inducing immune tolerance than the amino acid formula (34). After 19 days, she successfully transitioned to full enteral feeding by eliminating parenteral nutrition without complications such as intestinal failure-related liver disease or cow’s milk protein allergy.
4 Conclusion
A cohesive, multidisciplinary approach is recommended to combine surgical management with repair for a GO as early as possible; the approach can use VSD protective material as the initial protective shield while closely monitoring the intra-abdominal pressure to avoid abdominal compartment syndrome. Moreover, early enteral trophic feeding with an extensively hydrolyzed formula could be initiated for patients with a congenitally short small intestine when human milk is not available.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
WZ: Data curation, Investigation, Validation, Visualization, Writing – original draft. YW: Conceptualization, Methodology, Writing – review & editing. CP: Formal analysis, Investigation, Methodology, Supervision, Writing – review & editing. XZ: Data curation, Formal analysis, Methodology, Writing – review & editing. HY: Conceptualization, Data curation, Formal analysis, Resources, Writing – review & editing. LZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing, Software, Validation, Visualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the grants from the Science and Technology Bureau of Sichuan Province (2022YFS0044).
Acknowledgments
We thank Dezhi Mu for his review and discussion of the manuscript. We thank Xiaowen Li (enterostomal therapist), the resident doctors, chief resident, and nurses at West China Second University Hospital, Sichuan University, for their caring the patient in NICU. In addition, we thank our patient and her parents for their support and consent.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nembhard, WN, Bergman, JEH, Politis, MD, Arteaga-Vázquez, J, Bermejo-Sánchez, E, Canfield, MA, et al. A multi-country study of prevalence and early childhood mortality among children with omphalocele. Birth Defects Res. (2020) 112:1787–801. doi: 10.1002/bdr2.1822
2. Fogelström, A, Caldeman, C, Oddsberg, J, Löf Granström, A, and Mesas, BC. Omphalocele: national current birth prevalence and survival. Pediatr Surg Int. (2021) 37:1515–20. doi: 10.1007/s00383-021-04978-z
3. Bhatt, P, Poku, FA, Umscheid, J, Ayensu, M, Parmar, N, Vasudeva, R, et al. Trends in prevalence and mortality of gastroschisis and omphalocele in the United States from 2010 to 2018. World J Pediatrics. (2022) 18:511–4. doi: 10.1007/s12519-022-00544-2
4. Khan, FA, Hashmi, A, and Islam, S. Insights into embryology and development of omphalocele. Semin Pediatr Surg. (2019) 28:80–3. doi: 10.1053/j.sempedsurg.2019.04.003
5. Bauman, B, Stephens, D, Gershone, H, Bongiorno, C, Osterholm, E, Acton, R, et al. Management of giant omphaloceles: a systematic review of methods of staged surgical vs. nonoperative delayed closure. J Pediatr Surg. (2016) 51:1725–30. doi: 10.1016/j.jpedsurg.2016.07.006
6. Gonzalez, KW, and Chandler, NM. Ruptured omphalocele: diagnosis and management. Semin Pediatr Surg. (2019) 28:101–5. doi: 10.1053/j.sempedsurg.2019.04.009
7. Saxena, AK, and Raicevic, M. Predictors of mortality in neonates with giant omphaloceles. Minerva Pediatr. (2018) 70:289–95. doi: 10.23736/S0026-4946.17.05109-X
8. Pijpers, AGH, de Beaufort, CMC, Maat, SC, Broers, CJM, Straver, B, van Heurn, E, et al. Additional anomalies in children with Gastroschisis and Omphalocele: a retrospective cohort study. Children. (2023) 10:688. doi: 10.3390/children10040688
9. Que, Y, Cai, M, Yang, F, Ji, Q, Zhang, S, Huang, W, et al. Ultrasonographic characteristics, genetic features, and maternal and fetal outcomes in fetuses with omphalocele in China: a single tertiary center study. BMC Pregnancy Childbirth. (2023) 23:679. doi: 10.1186/s12884-023-05999-3
10. Negri, E, Coletta, R, and Morabito, A. Congenital short bowel syndrome: systematic review of a rare condition. J Pediatr Surg. (2020) 55:1809–14. doi: 10.1016/j.jpedsurg.2020.03.009
11. van der Werf, CS, Halim, D, Verheij, JB, Alves, MM, and Hofstra, RM. Congenital short bowel syndrome: from clinical and genetic diagnosis to the molecular mechanisms involved in intestinal elongation. Biochim Biophys Acta. (2015) 1852:2352–61. doi: 10.1016/j.bbadis.2015.08.007
12. Rathjen, FG, and Jüttner, R. The IgSF cell adhesion protein CLMP and congenital short bowel syndrome (CSBS). Int J Mol Sci. (2023) 24:5719. doi: 10.3390/ijms24065719
13. Wang, Y, Chen, S, Yan, W, Lu, L, Tao, Y, Xiao, Y, et al. Congenital short-bowel syndrome: clinical and genetic presentation in China. JPEN J Parenter Enteral Nutr. (2021) 45:1009–15. doi: 10.1002/jpen.1974
14. Wen, CC, Kuo, TC, Lee, HC, Yeung, CY, Chan, WT, Jiang, CB, et al. Coexisting gastrointestinal and hepatobiliary tract anomalies in omphalocele and gastroschisis: a twenty-year experience in a single tertiary medical center. Pediatr Neonatol. (2022) 63:468–73. doi: 10.1016/j.pedneo.2022.03.009
15. Caporilli, C, Giannì, G, Grassi, F, and Esposito, S. An overview of short-bowel syndrome in pediatric patients: focus on clinical management and prevention of complications. Nutrients. (2023) 15:2341. doi: 10.3390/nu15102341
16. Adams, AD, Stover, S, and Rac, MW. Omphalocele-what should we tell the prospective parents? Prenat Diagn. (2021) 41:486–96. doi: 10.1002/pd.5886
17. Frolov, P, Alali, J, and Klein, MD. Clinical risk factors for gastroschisis and omphalocele in humans: a review of the literature. Pediatr Surg Int. (2010) 26:1135–48. doi: 10.1007/s00383-010-2701-7
18. Waller, DK, Shaw, GM, Rasmussen, SA, Hobbs, CA, Canfield, MA, Siega-Riz, AM, et al. Prepregnancy obesity as a risk factor for structural birth defects. Arch Pediatr Adolesc Med. (2007) 161:745–50. doi: 10.1001/archpedi.161.8.745
19. Mac Bird, T, Robbins, JM, Druschel, C, Cleves, MA, Yang, S, and Hobbs, CA. Demographic and environmental risk factors for gastroschisis and omphalocele in the National Birth Defects Prevention Study. J Pediatr Surg. (2009) 44:1546–51. doi: 10.1016/j.jpedsurg.2008.10.109
20. Roux, N, Jakubowicz, D, Salomon, L, Grangé, G, Giuseppi, A, Rousseau, V, et al. Early surgical management for giant omphalocele: results and prognostic factors. J Pediatr Surg. (2018) 53:1908–13. doi: 10.1016/j.jpedsurg.2018.04.036
21. Akinkuotu, AC, Sheikh, F, Olutoye, OO, Lee, TC, Fernandes, CJ, Welty, SE, et al. Giant omphaloceles: surgical management and perinatal outcomes. J Surg Res. (2015) 198:388–92. doi: 10.1016/j.jss.2015.03.060
22. Correa-Araujo, L, Prieto-Abello, L, Lara-Bertrand, A, Medina-Solano, M, Guerrero, L, Camacho, B, et al. Bioengineered skin constructs based on mesenchymal stromal cells and acellular dermal matrix exposed to inflammatory microenvironment releasing growth factors involved in skin repair. Stem Cell Res Ther. (2023) 14:306. doi: 10.1186/s13287-023-03535-w
23. Wei, S, Wang, W, Li, L, Meng, HY, Feng, CZ, Dong, YY, et al. Recombinant human epidermal growth factor combined with vacuum sealing drainage for wound healing in Bama pigs. Mil Med Res. (2021) 8:18. doi: 10.1186/s40779-021-00308-5
24. Xue, X, Li, N, and Ren, L. Effect of vacuum sealing drainage on healing time and inflammation-related indicators in patients with soft tissue wounds. Int Wound J. (2021) 18:639–46. doi: 10.1111/iwj.13565
25. Nakagawa, Y, Uchida, H, Hinoki, A, Shirota, C, Sumida, W, Makita, S, et al. Combined negative pressure wound therapy with irrigation and dwell time and artificial dermis prevents infection and promotes granulation formation in a ruptured giant omphalocele: a case report. BMC Pediatr. (2022) 22:680. doi: 10.1186/s12887-022-03755-8
26. Aldridge, B, Ladd, AP, Kepple, J, Wingle, T, Ring, C, and Kokoska, ER. Negative pressure wound therapy for initial management of giant omphalocele. Am J Surg. (2016) 211:605–9. doi: 10.1016/j.amjsurg.2015.11.009
27. Nissen, M, Romanova, A, Weigl, E, Petrikowski, L, Alrefai, M, and Hubertus, J. Vacuum-assisted staged omphalocele reduction: a preliminary report. Front Pediatr. (2022) 10:1053568. doi: 10.3389/fped.2022.1053568
28. Oquendo, M, Agrawal, V, Reyna, R, Patel, HI, Emran, MA, and Almond, PS. Silver-impregnated hydrofiber dressing followed by delayed surgical closure for management of infants born with giant omphaloceles. J Pediatr Surg. (2015) 50:1668–72. doi: 10.1016/j.jpedsurg.2015.06.011
29. Kwo, PY, Cohen, SM, and Lim, JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol. (2017) 112:18–35. doi: 10.1038/ajg.2016.517
30. Hijkoop, A, Peters, NCJ, Lechner, RL, van Bever, Y, van Gils-Frijters, A, Tibboel, D, et al. Omphalocele: from diagnosis to growth and development at 2 years of age. Arch Dis Child Fetal Neonatal Ed. (2019) 104:F18–f23. doi: 10.1136/archdischild-2017-314700
31. Norsa, L, Goulet, O, Alberti, D, DeKooning, B, Domellöf, M, Haiden, N, et al. Nutrition and intestinal rehabilitation of children with short bowel syndrome: a position paper of the ESPGHAN committee on nutrition. Part 1: from intestinal resection to home discharge. J Pediatr Gastroenterol Nutr. (2023) 77:281–97. doi: 10.1097/MPG.0000000000003849
32. Diamanti, A, Fiocchi, AG, Capriati, T, Panetta, F, Pucci, N, Bellucci, F, et al. Cow's milk allergy and neonatal short bowel syndrome: comorbidity or true association? Eur J Clin Nutr. (2015) 69:102–6. doi: 10.1038/ejcn.2014.156
33. Sukhotnik, I, Levi, R, and Moran-Lev, H. Impact of dietary protein on the Management of Pediatric Short Bowel Syndrome. Nutrients. (2023) 15:2826. doi: 10.3390/nu15132826
34. El-Hodhod, MA, El-Shabrawi, MHF, AlBadi, A, Hussein, A, Almehaidib, A, Nasrallah, B, et al. Consensus statement on the epidemiology, diagnosis, prevention, and management of cow's milk protein allergy in the Middle East: a modified Delphi-based study. World J Pediatrics. (2021) 17:576–89. doi: 10.1007/s12519-021-00476-3
Keywords: omphalocele, congenital short small intestine, vacuum-sealing drainage, nutrition, perioperative management
Citation: Zhang W, Wu Y, Pan C, Zhang X, Yan H and Zhang L (2024) Ruptured giant omphalocele with congenital short small intestine: a case report. Front. Nutr. 11:1421033. doi: 10.3389/fnut.2024.1421033
Edited by:
Pablo Andrés Lobos, Italian Hospital of Buenos Aires, ArgentinaReviewed by:
Luz-Ma-Adriana Balderas-Peña, Instituto Mexicano del Seguro Social, MexicoPaolo Gasparella, Medical University of Graz, Austria
Copyright © 2024 Zhang, Wu, Pan, Zhang, Yan and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Zhang, zhanglihk@126.com
 Wenjing Zhang
Wenjing Zhang Yang Wu3
Yang Wu3 Li Zhang
Li Zhang