- 1Division of Life Science and Applied Life Science (BK21 FOUR), College of Natural Sciences, Gyeongsang National University, Jinju-si, Republic of Korea
- 2Department of Psychiatry and Neuropsychology, School for Mental Health and Neuroscience (MHeNs), Maastricht University, Maastricht, Netherlands
- 3Department of Pediatrics, Maastricht University Medical Center (MUMC+), Maastricht, Netherlands
- 4Haemato-Oncology/Systems Medicine Group, Paul O’Gorman Leukaemia Research Centre, Institute of Cancer Sciences, College of Medical, Veterinary and Life Sciences (MVLS), University of Glasgow, Glasgow, United Kingdom
- 5Alz-Dementia Korea Co., Jinju, Republic of Korea
Neuroinflammation includes the activation of immune glial cells in the central nervous system, release pro-inflammatory cytokines, which disrupt normal neural function and contribute to various neurological disorders, including Alzheimer’s disease (AD), Parkinson’s disease, multiple sclerosis, and stroke. AD is characterized by various factors including amyloidogenesis, synaptic dysfunction, memory impairment and neuroinflammation. Lipopolysaccharide (LPS) constitutes a vital element of membrane of the gram-negative bacterial cell, triggering vigorous neuroinflammation and facilitating neurodegeneration. Lupeol, a naturally occurring pentacyclic triterpene, has demonstrated several pharmacological properties, notably its anti-inflammatory activity. In this study, we evaluated the anti-inflammatory and anti-Alzheimer activity of lupeol in lipopolysaccharide (LPS)-injected mice model. LPS (250ug/kg) was administered intraperitoneally to C57BL/6 N male mice for 1 week to induce neuroinflammation and cognitive impairment. For biochemical analysis, acetylcholinesterase (AChE) assay, western blotting and confocal microscopy were performed. AChE, western blot and immunofluorescence results showed that lupeol treatment (50 mg/kg) along with LPS administration significantly inhibited the LPS-induced activation of neuroinflammatory mediators and cytokines like nuclear factor (NF-κB), tumor necrosis factor (TNF-α), cyclooxygenase (COX-2) and interleukin (IL-1β). Furthermore, we found that LPS-induced systemic inflammation lead to Alzheimer’s symptoms as LPS treatment enhances level of amyloid beta (Aβ), amyloid precursor protein (APP), Beta-site APP cleaving enzyme (BACE-1) and hyperphosphorylated Tau (p-Tau). Lupeol treatment reversed the LPS-induced elevated level of Aβ, APP, BACE-1 and p-Tau in the hippocampus, showing anti-Alzheimer’s properties. It is also determined that lupeol prevented LPS-induced synaptic dysfunction via enhanced expression of pre-and post-synaptic markers like SNAP-23, synaptophysin and PSD-95. Overall, our study shows that lupeol prevents memory impairment and synaptic dysfunction via inhibition of neuroinflammatory processes. Hence, we suggest that lupeol might be a useful therapeutic agent in prevention of neuroinflammation-induced neurological disorders like AD.
1 Introduction
Neuroinflammation involves the activation of immune system of the central nervous system (CNS), which includes glial cells like microglia and astrocytes, to protect against infections, injuries, or neurological diseases and maintain neuronal homeostasis (1, 2). Evidence suggests that neuroinflammation plays a key role in Alzheimer’s disease (AD) development. Studies have shown that systemic inflammation or septic shock can lead to memory impairment and neuronal loss in animals (3, 4). Lipopolysaccharide (LPS), an endotoxin from gram-negative bacteria, triggers immune responses and inflammation. Research indicates that systemic LPS administration increases the production of inflammatory mediators such as nitric oxide synthase (NOS-2), cyclooxygenase (COX-2), and cytokines like TNF-α, IL-1, and IL-6, causing various neurobiological effects (5, 6). The major ligand for LPS is the TLR4 receptor which activates the nuclear factor (NF-κB) downstream signaling pathway (7, 8). Progressive neuroinflammation has been shown to induce neurodegeneration in the different forms of brain disorders like sepsis, AD, Parkinson’s disease and multiple sclerosis, etc. (9–11). Although the exact mechanism for LPS to induce Alzheimer’s pathogenesis is not fully elucidated, various research studies described that LPS-induced neuroinflammation impairs memory function via increased accumulation of amyloid beta and inactivation of beta (β) and gamma (γ) secretases. Other studies have shown that inflammatory mediators or cytokines like NOS-2, Prostaglandins, IL-1β, IL-6, TNF-α or transforming growth factors (TGF) can induce augmented expression of amyloid precursor protein (APP) and generation of amyloid beta (Aβ) (12, 13). Furthermore, it has also been stated that the promoter region of β-secretase (BACE) has NFκB binding site which may regulate Aβ formation (14). Likewise, other studies showed that LPS administration impairs memory performance and induces synaptic dysfunction (15, 16).
Recently, there has been extensive research into the therapeutic potential of natural products sourced from plants, along with their bioactive components, in addressing various neurodegenerative diseases such as Alzheimer’s (AD), Huntington’s (HD), and Parkinson’s (PD) (17). Lupeol is a nutritional pentacyclic triterpene found in various medicinal plants and fruits like mango, olive, and strawberry. Lupeol exhibits a wide range of biological properties as experimental studies have shown that lupeol possess strong anti-oxidant, anti-inflammatory, anti-diabetic, anti-mutagenic, antineoplastic, and hepatoprotective properties (18, 19). Moreover, in vitro studies have described the neuroprotective effect of lupeol in different types of cells like mouse hippocampal HT22 cells, hepatocytes, and glioma cells. The anti-inflammatory and anti-oxidant activity of lupeol mainly contributed to its protective capability in hepatic or gastric disturbances (20, 21). In this study, we evaluated the effect of systemic administration of LPS on proinflammatory cytokines, Aβ production, Tau hyperphosphorylation, and proteins associated with synaptic dysfunction in the hippocampus. We determined the inhibitory role of lupeol in LPS-evoked neuroinflammatory responses and associated memory dysfunction.
2 Materials and methods
2.1 Behavioral study
Behavioral study was performed to investigate the effect of lupeol on memory functions by using a Morris water maze (MWM) task and a Y-maze task.
The experimental setup of MWM test comprised a circular water tank measuring 100 cm in diameter and 40 cm in height, containing opaque water at a temperature of 23 ± 1°C to a depth of 15.5 cm (22). Within this tank, a transparent escape platform measuring 10 cm in diameter and 20 cm in height was concealed 1 cm beneath the water’s surface, positioned at the midpoint of a designated quadrant. Each mouse underwent daily training sessions for five consecutive days, each involving a single hidden platform placed in one quadrant, with three rotating starting quadrants. The time taken by each mouse to escape from the water maze, locating the submerged escape platform, was recorded for every trial. On the fifth day, probe tests were conducted to assess memory consolidation. During the probe test, the platform was removed, and each mouse was allowed to swim freely for 60 s. The duration spent by the mice in the target quadrant, where the platform was situated was then measured. The time spent in the target quadrant is considered to represent the degree of memory consolidation that has taken place after learning. All data were recorded using video-tracking software (SMART, Panlab Harvard Apparatus Bioscience Company, United States).
The Y-maze apparatus consisted of three arms made up of transparent Plexiglas (23). Each arm measuring 50 cm in length, 20 cm in height, and 10 cm in width at both the bottom and top. Each mouse was placed at the center of the apparatus and allowed to move freely through the maze for three sessions of 8-min. Spontaneous alteration was defined as the successive entry of the mice into the three arms in overlapping triplet sets. Alteration behavior (%) was calculated as follows: [successive triplet sets (entries into three different arms consecutively)/total number of arm entries-2] × 100.
2.2 Animals grouping and treatment
Wild type C57BL/6 N male mice (n = 30, 8 weeks old, approximately 25-28 g body weight) were purchased from Samtako Biolabs (Ulsan, South Korea). All mice were housed in a temperature-controlled environment and maintained on a 12 h light/dark cycle with food ad libitum. Mice were randomly divided into 3 experimental groups as: Control group (0.9% saline I/P as control mice), LPS group (250 μg/kg I/P, for 7 days), LPS + Lupeol group (50 mg/kg orally, for 7 days) as mentioned in (Figure 1). LPS was dissolved in normal saline while lupeol was dissolved in an aqueous solution containing 0.25% DMSO. All the experimental procedures were carried out in accordance with the rules established by the animal ethics committee (IACUC) (approval ID: 125, animal ethics code: GNU-200331-M0020) of the Division of Applied Life Sciences, Department of Biology, Gyeongsang National University South Korea.

Figure 1. Experimental plan of lupeol against LPS-induced neuroinflammation mediated neurodegeneration in AD-Like pathologies.
2.3 Protein extraction from mouse brain and assessment of acetylcholine activity
After behavioral analysis, using ketamine and xylazine the mice were anesthetized and sacrificed (24). The brain hippocampal tissues were dissected carefully and stored at −80°C. The hippocampus tissues were homogenized in PRO-PREP™ protein extraction solution (iNTRON Biotechnology, Inc., Sungnam, South Korea), and centrifuged at 13,000 rpm for 25 min at 4°C. The supernatants were collected and kept at −80°C for further process. Furthermore, the acetylcholinesterase (AChE) activities were also assessed in hippocampus of mice brain homogenates followed by Ellman protocol, which were standardized for the protein contents (5 mg/mL) (25–27).
2.4 Western blot analysis
Western blotting was performed as described in previous studies (28–30). Briefly a Bio-Rad assay kit (Bio-Rad Laboratories, Irvine, CA, United States) was used to measure protein concentrations. Consequently, proteins from the brain of all experimental groups of mice were run by SDS-PAGE on 4–18% gels in comparison under reducing conditions and subsequently transferred to polyvinylidene difluoride (PVDF) membranes (Immobilon-PSQ, Transfer membrane, Merck Millipore, Burlington, MA, United States). The membranes were blocked with 5% skim milk (Difco™ Skim Milk, BD, France), and incubated with primary antibodies at 4°C. After incubation, the membranes were probed with HRP-conjugated secondary antibodies. The detection was carried out using an enhanced chemiluminescent (ECL) reagent (ATTO Corporation, Tokyo, Japan), and the optical densities of the bands were quantified using ImageJ software.
2.5 Immunofluorescence assays
After anesthesia, transcardial perfusion was performed with normal saline (0.9%). The brains were carefully removed and preserved in ice-cold 4% neutral buffer paraformaldehyde at 4°C for 72 h. Subsequently, they underwent a dehydration process in 20% sucrose for another 72 h. Using a microtome (CM 3050C cryostat, Leica, Germany) on gelatin-coated slides, sections of 14 μm thickness were obtained (31). Immunofluorescence assays were conducted according to established protocols (32–34). Hippocampal tissue slides were then prepared, subjected to a 10 min wash with 0.01 M PBS, and treated with proteinase K for 5 min. Subsequent to PBS washing, sections underwent blocking with normal serum (Vector, diluted 1:20 in PBS) for 1 h. Incubation with specific antibodies (TNFα, IL-1β, Aβ, p-Tau, and PSD-95) was carried out overnight at 4°C. Following incubation, brain sections were washed with PBS and exposed to secondary antibodies that were tetramethylrhodamine isothiocyanate (TRITC) or fluorescein isothiocyanate (FITC) (anti-rabbit, anti-goat, or anti-mouse) diluted 1:50 in PBS for 90 min at room temperature. Tissue slides were then counterstained for 8 min with 4′,6-diamidino-2-phenylindole (DAPI) nucleus solution and further, the slides were prepared with mounting media by applying DPX (Distyrene Plasticizer Xylene), and protected with glass coverslips. Immunofluorescence imaging was performed using a confocal laser scanning microscope (FV 1000MPE, Olympus, Japan).
2.6 Antibodies
The primary antibodies in Table 1obtained from both Santa Cruz Biotechnology (Dallas, TX, United States) and Cell Signaling Technology.
2.7 Data analysis
Data were presented as mean ± standard error of the mean (SEM). Data were analyzed by ANOVA followed by a multi-comparison t-test. A level of (#p ≤ 0.01) and (*p ≤ 0.05) was considered to be significant. ‘#’ indicates a significant difference with the LPS-treated group, while ‘*’ indicates a significant difference with the control group.
3 Results
3.1 Lupeol inhibits LPS-induced elevated levels of p-NFκB and TNF-α
To investigate the inhibitory effect of lupeol on memory impairment via preventing neuroinflammation, we determined the protein expressions of p-NFκB and TNF-α by western blot analysis. Our results showed that systemic administration of LPS significantly elevated the protein expression level of p-NFκB and TNF-α in the hippocampus of adult mice. Treatment with lupeol reversed the effect of LPS administration and reduced the elevated levels of p-NFκB and TNF-α compared to the LPS-treated group (Figure 2A). Likewise, immunofluorescence findings for TNF-α also showed that lupeol administration along with lupeol significantly inhibited the nuclear translocation of TNF-α compared to the LPS-induced effect (Figure 2B).
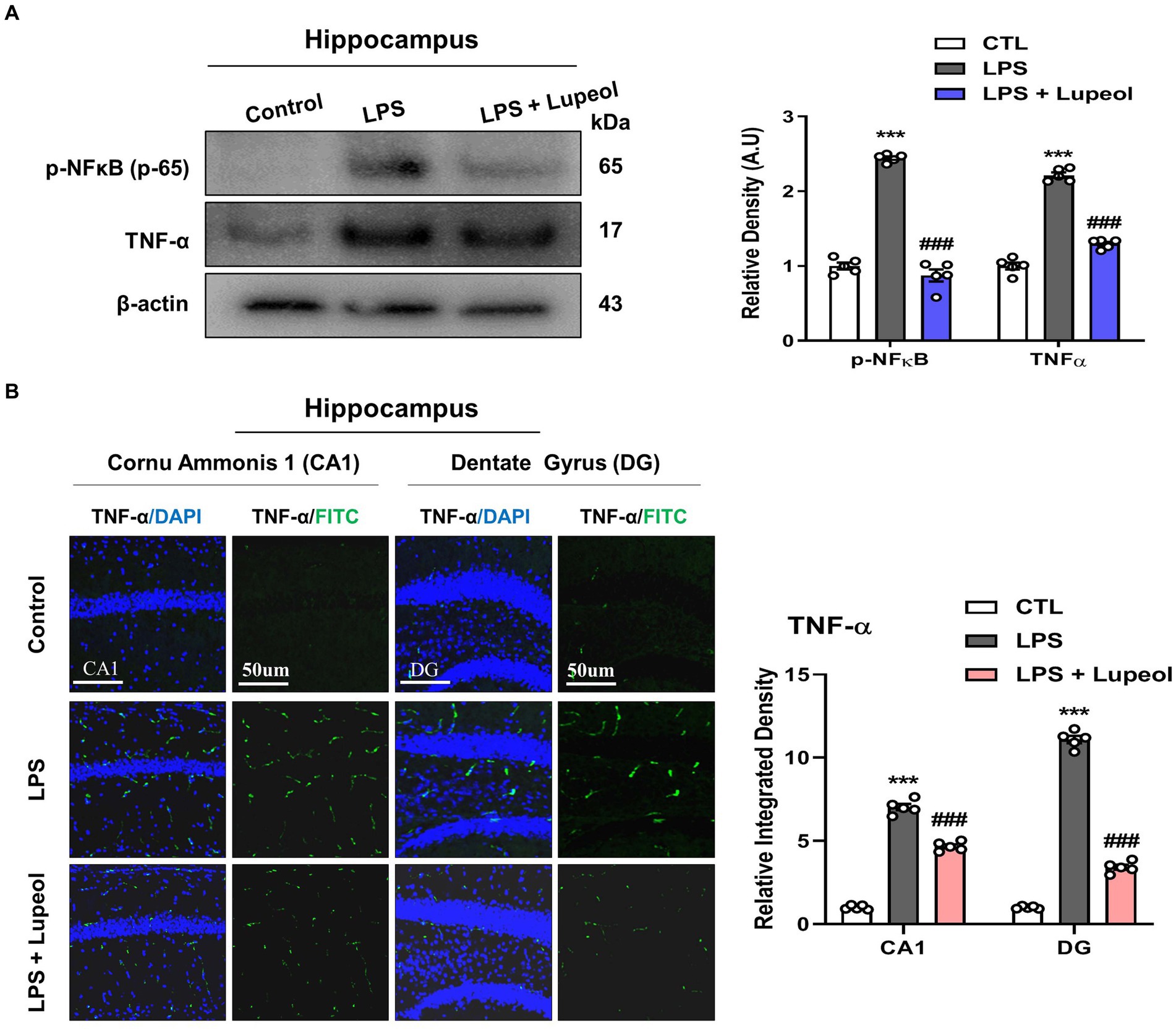
Figure 2. Lupeol inhibits LPS-induced elevated levels of p-NFκB and TNF-α. (A) Shown are representative western blots probed with antibodies of p-NFκB and TNF-α in the hippocampus of experimental mice. The protein bands were quantified using Sigma gel software. The density values are expressed in arbitrary units as the mean ± SEM for the indicated proteins (n = 5 animals per group). (B) Confocal microscopy represents immunofluorescence of TNF-α positive cells in the cornu ammonis 1 (CA1) and dentate gyrus (DG) regions of hippocampal mice brains. The symbol * showed a significant difference (*p ≤ 0.05) from control group, while symbol # represents a significant difference (#p ≤ 0.01) from the LPS-treated group.
3.2 Lupeol inhibits LPS-induced elevated levels of inflammatory markers like COX-2 and IL-1β
We studied the effect of LPS and lupeol on proinflammatory mediators and cytokines such as COX-2 and IL-1β. Western blot analysis results showed that the protein expression level of these inflammatory markers was significantly elevated in the hippocampus of LPS-injected mice compared to the vehicle-treated mice. Systemic administration of lupeol lowered the LPS-induced increased expression of COX-2 and IL-1β showing a protective effect of lupeol in neuroinflammatory conditions (Figure 3A). Additionally, we performed immunofluorescence staining to determine the inhibitory effect of lupeol on the LPS induced expressions of inflammatory proteins. Our morphological results showed that LPS administration significantly elevated the expression level of IL-1β compared to the control group while lupeol treatment along with LPS showed a decreased hippocampal expression of IL-1β compared to the LPS-treated group (Figure 3B).
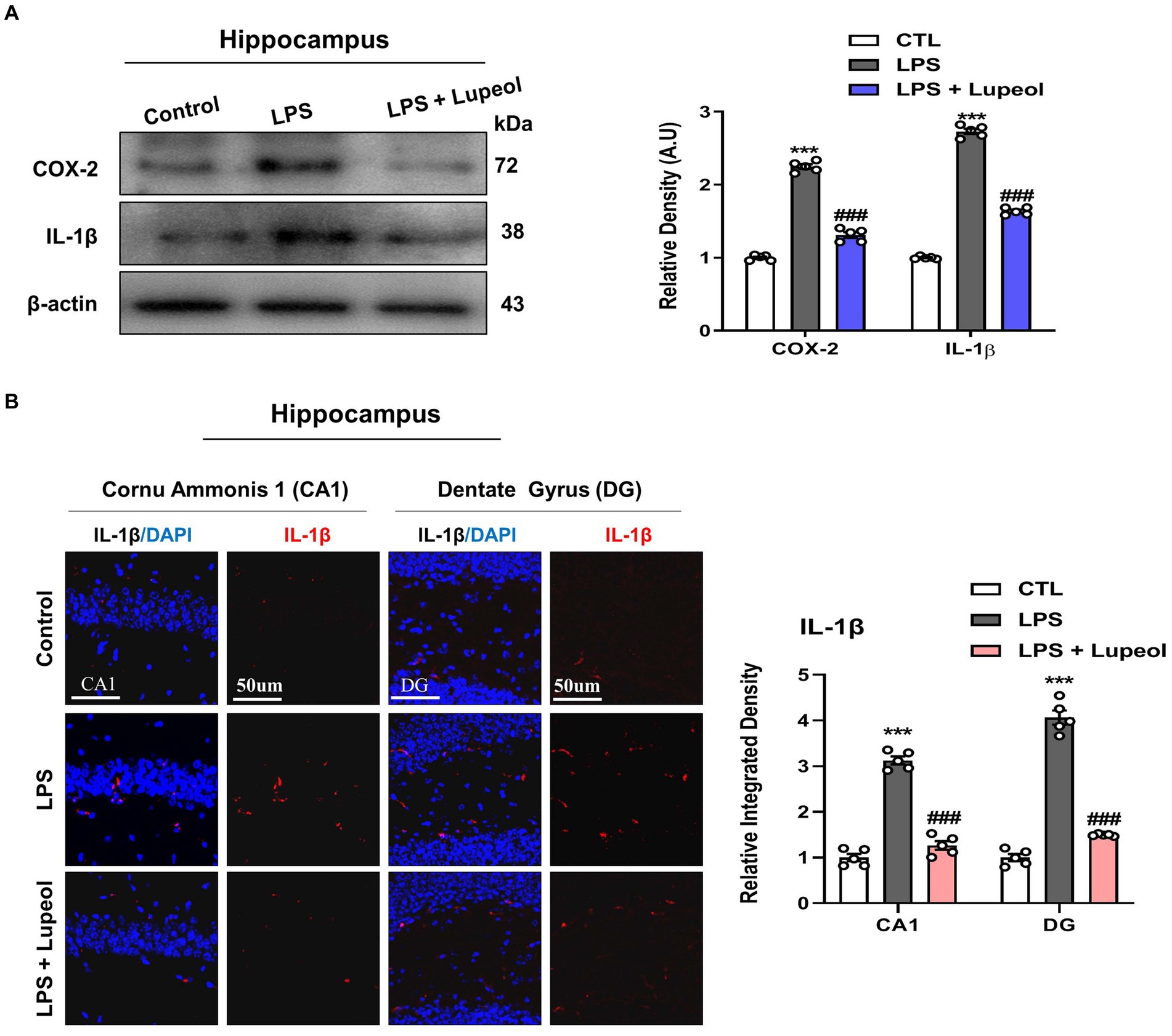
Figure 3. Lupeol inhibits LPS-induced elevated levels of COX-2 and IL-1β. (A) Shown are representative western blots probed with antibodies of COX-2 and IL-1β in the hippocampus of experimental mice. The protein bands were quantified using Sigma gel software. The density values are expressed in arbitrary units as the mean ± SEM for the indicated proteins (n = 5 animals per group). (B) Confocal microscopy shows immunoreactivity of IL-1β positive cells in the cornu ammonis 1 (CA1) and dentate gyrus (DG) regions of hippocampal mice brains. Symbol * showed a significant difference (*p ≤ 0.05) from control group, while symbol # represents a significant difference (#p ≤ 0.01) from the LPS-treated group.
3.3 Lupeol inhibits LPS-induced elevated levels of amyloid precursor protein (APP) and Aβ expressions
Next, to determine the effect of the administration of LPS and lupeol on Alzheimer-associated proteins like APP and Aβ, Western blot analysis was performed. LPS treatment of 250ug/kg for 1 week induced an Alzheimer-like effect and showed an increased protein expression level of APP and Aβ in the hippocampus as compared to the vehicle-treated group. Treatment with lupeol attenuated the LPS-induced elevated level of APP and Aβ as compared to the LPS-treated group (Figure 4A). Similarly, confocal microscopy results showed that lupeol administration significantly inhibited the LPS-induced elevated expression of Aβ as compared to the LPS-treated group (Figure 4B).
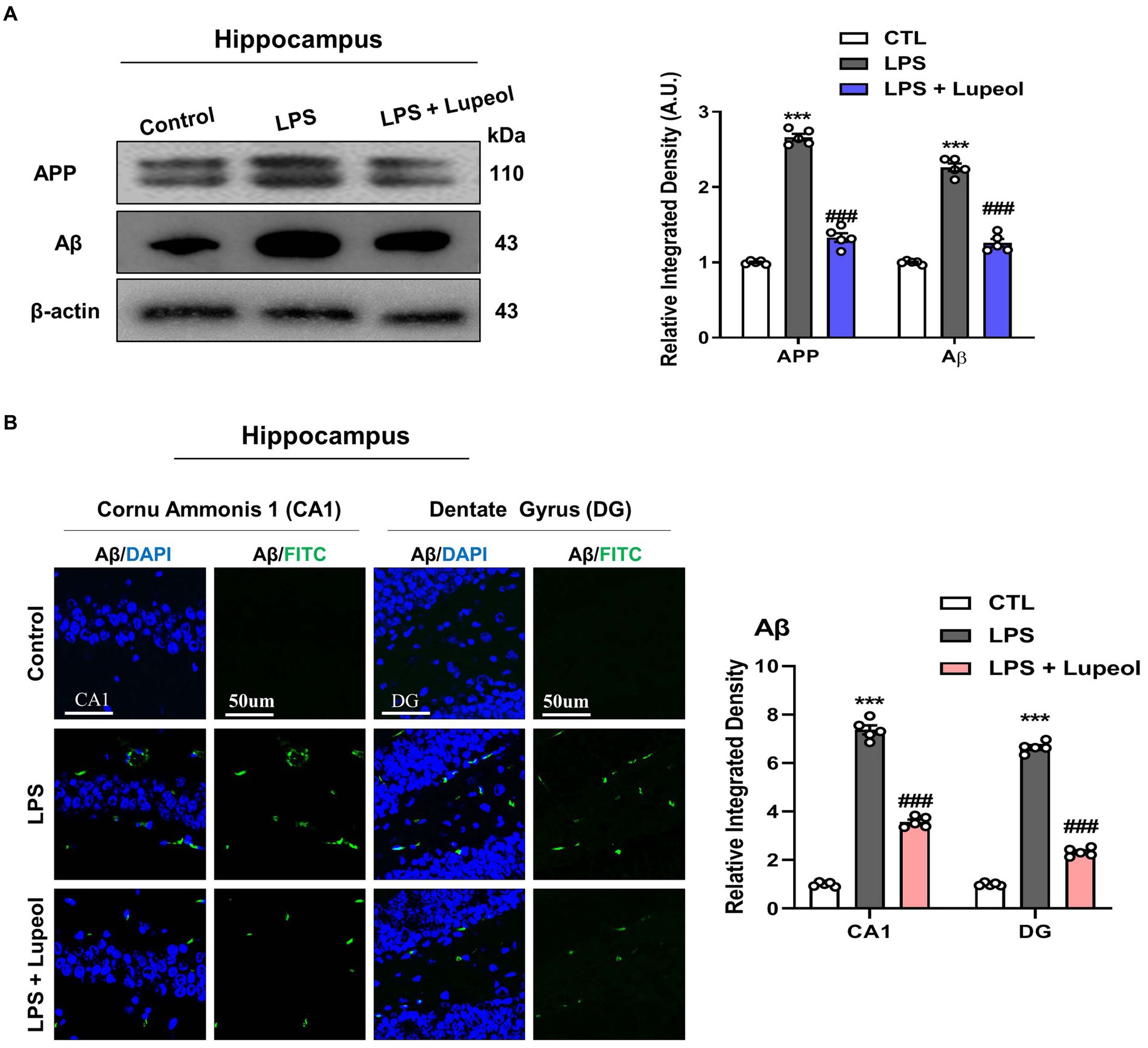
Figure 4. Lupeol inhibits LPS-induced elevated levels of APP and Aβ. (A) Shown are representative western blots probed with antibodies of APP and Aβ in the hippocampus of experimental mice. The protein bands were quantified using Sigma gel software. The density values are expressed in arbitrary units as the mean ± SEM for the indicated proteins (n = 5 animals per group). (B) Shown are representative immunofluorescence photomicrographs of Aβ positive cells in the cornu ammonis 1 (CA1) and dentate gyrus (DG) regions of hippocampal mice brains. Symbol * showed a significant difference (*p ≤ 0.05) from control group, while symbol # represents significant difference (#p ≤ 0.01) from the LPS-treated group.
3.4 Lupeol inhibits LPS-induced elevated levels of BACE-1 and p-tau
We also investigated the effect of LPS and lupeol on other Alzheimer-associated proteins like BACE-1 and hyperphosphorylated tau. Tau is a neuronal microtubule-associated protein and its major biological function is to maintain microtubule assembly. The abnormal hyperphosphorylation of tau (p-Tau) is responsible for the loss of normal physiological functions and is mainly involved in the pathophysiology of AD (35, 36).
Western blot analysis was performed and our result showed that systemic administration of LPS significantly elevated the protein expression level of BACE-1 and p-Tau in the hippocampus. Oral administration of lupeol along with LPS significantly decreased the expression level of BACE-1 and p-Tau as compared to LPS-treated (Figure 5A). Furthermore, we also performed immunofluorescence staining for p-Tau in the hippocampus of the experimental groups. The results were consistent with the Western blot result as lupeol significantly reduced the LPS-induced hyperphosphorylation of Tau protein as compared to the LPS-treated group (Figure 5B).
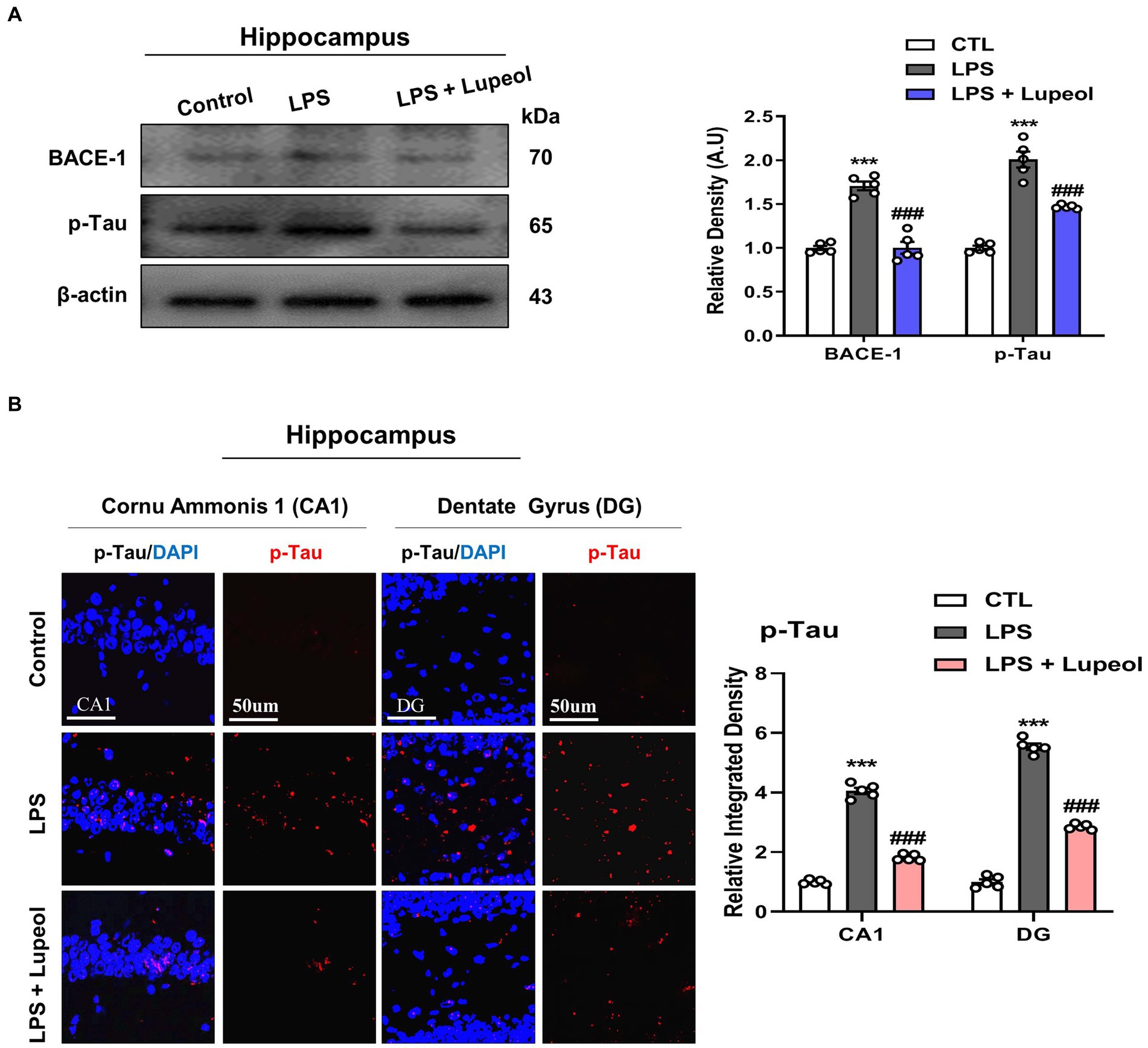
Figure 5. Lupeol inhibits LPS-induced elevated levels of BACE-1 and p-Tau. (A) Shown are representative western blots probed with antibodies of BACE-1 and p-Tau in the hippocampus of experimental mice. The protein bands were quantified using Sigma gel software. The density values are expressed in arbitrary units as the mean ± SEM for the indicated proteins (n = 5 animals per group). (B) Shown are representative immunofluorescence photomicrographs of p-Tau positive cells in the cornu ammonis 1 (CA1) and dentate gyrus (DG) regions of hippocampal mice brains. Symbol * showed a significant difference (*p ≤ 0.05) from the control group, while symbol # represents significant difference (#p ≤ 0.01) from the LPS-treated group.
3.5 Lupeol inhibits LPS-induced elevated levels of PSD-95, SNAP-23, and SYP
A number of research studies have shown that disruption of synaptic function is one of the main features of AD, which may result in cognitive dysfunction and memory impairment (37, 38).
In order to investigate the change in synaptic function by treatment with LPS and lupeol, western blot analyses were performed. The protein expression level of pre- and post-synaptic markers like PSD-95, SNAP-23, and SYP were determined. Our results showed that systemic administration of LPS significantly decreased the protein expression level of pre-synaptic markers like SNAP-23 and SYP, and post-synaptic markers like PSD-95 in the hippocampus, while administration of lupeol reverses the effect of LPS and increased expression of PSD-95, SNAP-23, and SYP (Figure 6A). In accordance with these, morphological results for PSD-95 also showed a significant decrease in immunofluorescence results of PSD-95 in LPS treated group as compared to vehicle-treated group. Treatment with lupeol showed an increased expression of PSD-95 in the hippocampus as compared to the LPS-treated group (Figure 6B).

Figure 6. Lupeol inhibits LPS-induced elevated levels of PSD-95, SYP, and SNAP-23. (A) Shown are representative western blots probed with antibodies of PSD-95, SYP, and SNAP-23 in the dentate gyrus (DG) of hippocampus of experimental mice. The protein bands were quantified using Sigma gel software. The density values are expressed in arbitrary units as the mean ± SEM for the indicated proteins (n = 5 animals per group). (B) Shown are representative immunoreactivity of PSD-95 positive cells in the hippocampal mice brain. (C) Indicates Acetylcholinesterase (AChE) in mouse brain hippocampal tissue. Symbol * showed a significant difference (*p ≤ 0.05) from control group, while symbol # represents a significant difference (#p ≤ 0.01) from LPS-treated group.
3.6 Lupeol reduced acetylcholinesterase activity in LPS-induced neuroinflammation in Alzheimer’s disease associated pathology
Acetylcholinesterase (AChE) plays a critical role in the hydrolysis of acetylcholine, a neurotransmitter essential for cognitive function. The study demonstrated that LPS-induced neuroinflammation significantly increased AChE activity in the hippocampus of LPS treated group of animals, mimicked AD-like cholinergic dysfunction. While lupeol administration at 50 mg/kg dose effectively reduced AChE activity as compared to LPS treated adult mice showing a more pronounced effect. These findings suggest that lupeol mitigates cholinergic deficits associated with neuroinflammation and highlights its potential as a therapeutic agent for Alzheimer’s disease (Figure 6C) (39, 40).
3.7 Lupeol ameliorated cognitive functions in LPS-administered mice
Natural-derived compounds such as flavonoids show great potential in improving learning and memory functions has been confirmed by various research studies (5, 41, 42). Lupeol also has a beneficial effect on memory and cognitive functions (43, 44). Nevertheless, several studies have explored that systemic LPS administration induces memory and cognitive dysfunction (45–47). Consequently, to evaluate the memory-improving effect of lupeol against LPS, we designed a dosage treatment of lupeol at a 50 mg/kg body weight dose for one week (cotreated with LPS) via orally. Other studies also mentioned lupeol of 50 mg/kg /day P.O. for a short period of time induce beneficial effects (21, 43).
We assessed the memory capabilities of the mice through MWM and Y-maze assessments. In the MWM task, all mice underwent training to locate a submerged platform, after which we analyzed the time taken to reach it. The mice injected with LPS exhibited prolonged search times compared to the control group (Figure 7A). Nonetheless, administration of lupeol countered the impact of LPS, notably enhancing memory performance, as evidenced by the reduced duration required by the subjects to locate the concealed platform in comparison to mice injected only with LPS. Additionally, the probe test revealed that lupeol also counteracted the effects of LPS, resulting in a significant increase in the number of crosses across the platform and an increase in the duration spent within the specific quadrant where the concealed platform had previously been positioned (Figures 7A–C). These results revealed that lupeol reversed the harmful effect of LPS and significantly enhanced memory functions. The findings from the Y-maze experiment show that compared to the control group, LPS caused short-term spatial memory impairment. However, treatment with lupeol significantly increased the percentage of spontaneous alteration behavior, suggesting an improvement in spatial working memory function in LPS-injected mice (Figure 7D).
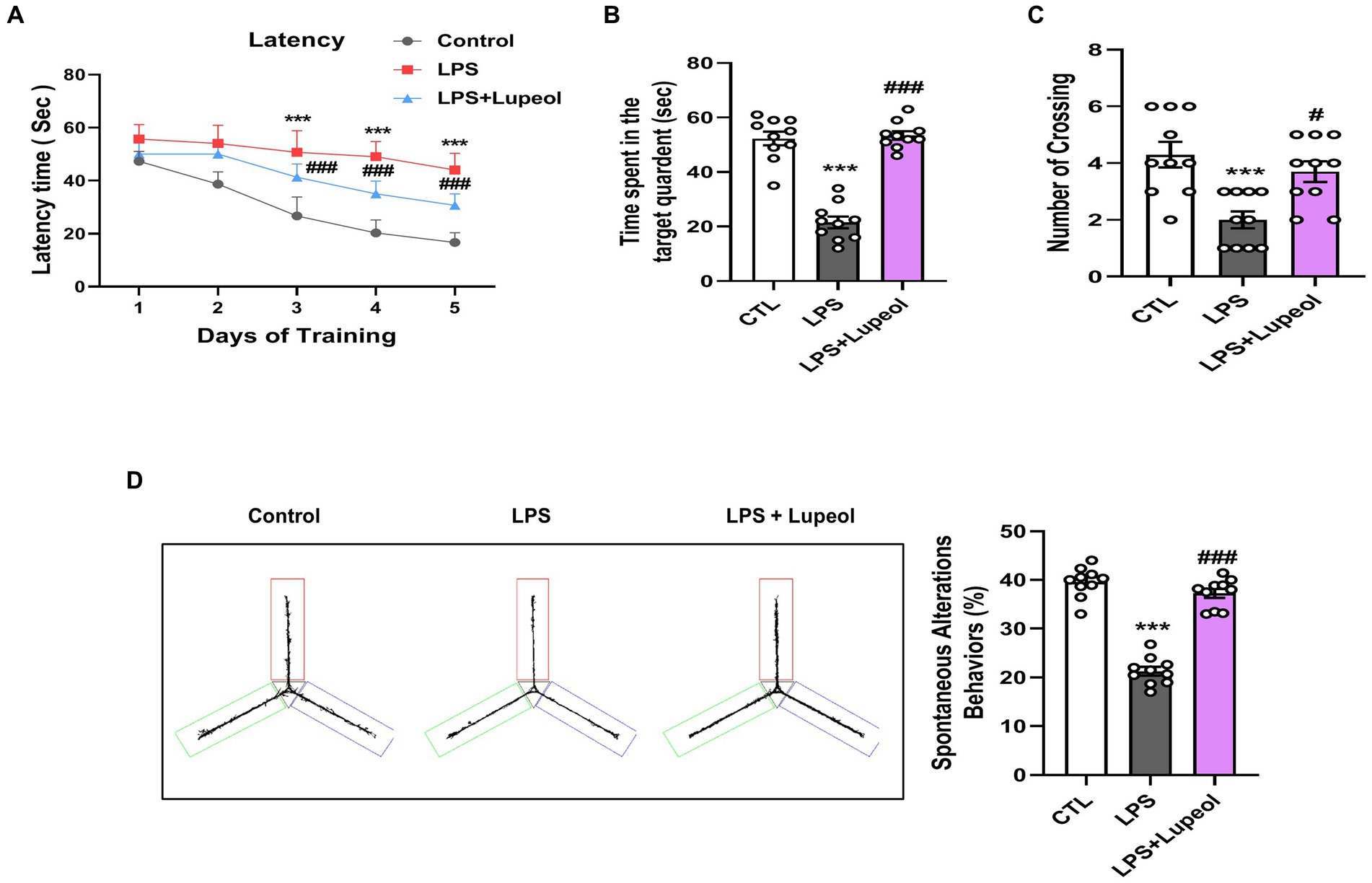
Figure 7. Lupeol enhanced memory function in mice treated with LPS. Behavioral assessments were conducted using the MWM and Y-maze tests to assess memory function in control mice, LPS and LPS + lupeol group mice. (A) Average escape latency time for experimental mice to reach the hidden platform from 1 to 4 days. (B) Time spent in the platform quadrant, where the hidden platform was placed during the trial session. (C) The average number of crossings at the hidden platform during the probe test of the MWM test. (D) Spontaneous alteration behavior % of the mice during the Y-maze test. Histograms indicate the means ± SEM for the mice (n = 15/group). ∗Significantly different from the control; # significantly different from LPS-treated group. Significance: ∗p ≤ 0.05, #p ≤ 0.01.
4 Discussion
Although extensive ongoing research is investigating various therapeutic agents for treating neurodegenerative diseases such as Alzheimer’s disease (AD), no single intervention has been found to comprehensively address the treatment of AD. It has been widely studied that neuroinflammation plays a crucial role in the progression of neurological disorders especially AD (48–50). Several lines of evidence supported that neuroinflammatory mechanisms are involved in the pathogenesis of neurodegenerative disorders like AD. Induction of inflammatory conditions by an exogenous stimulant like LPS is well studied and it has been described that LPS administration alleviates the level of pro-inflammatory mediators and other cytokines (51, 52). The release of these inflammatory cytokines like TNF-α, IL-1β, NOS-2, and prostaglandins are the intermediary mediators to induce neuronal injury and apoptosis (53, 54). In this regard, therapeutic agents having potential anti-inflammatory activity can be considered suitable candidates to target inflammation-induced neurological disorders. In the present study, we described that the anti-inflammatory activity of lupeol may be attributed to memory improvement in a mouse model of Alzheimer’s disease.
Previously, lupeol has been extensively studied both in vitro and in vivo for its anti-inflammatory and anti-carcinogenic properties (55, 56). Furthermore, we showed that lupeol inhibits LPS-induced neuroinflammation and neurodegeneration via preventing the p38/JNK pathway (57). It has been reported that both P38/JNK pathway and NFκB pathway are major inflammatory pathways involved in neuroinflammation-induced neurodegeneration (58, 59).
It is determined that LPS administration in adult mice develops neuroinflammatory conditions with enhanced release of inflammatory cytokines that ultimately leads to memory impairment and cognitive dysfunction (60, 61). Geetha et al. (56) checked the anti-inflammatory activities of lupeol in rat arthritis model, so we hypothesize that whether lupeol could reduce the inflammation in the brain. In our previous study, we showed that neuroinflammatory markers like TNF-alpha, NOS2, and IL-1β were decreased in lupeol treated LPS model. This finding was validated in our current study and further investigated neuroinflammation markers such as NF-ĸB and COX2. Also, our previous study did not examine the amyloid beta-related pathology and pre- and post-synaptic markers. Therefore, the findings from this study further increased our knowledge in the neuroprotective properties of lupeol. Likewise, other studies have described disrupted synaptic and memory function involving increased levels of Alzheimer’s markers like APP and Aβ, BACE-1, and p-Tau with LPS administration in rodents (62–64). It has been demonstrated that NFκB regulates Aβ production as the promoter region of β-secretase (BACE-1) has NFκB binding site, also stated that inflammatory cytokine-like TNF-α and IFN in combination provokes the production of Aβ peptides (65, 66). Furthermore, it has been well-studied in human and animal models that neuroinflammation, amyloid accumulation, and other phenomena of AD lead to local synaptic damage and memory deficits (67). A number of research bodies have shown that pre-synaptic markers like SYP, SNAP-25, SNAP-23, etc., and post-synaptic markers like PSD-95 are associated with synaptic plasticity and cognitive function (68). It has been demonstrated that the expression of pre-and post-synaptic markers has been reduced to a significant level in AD patients as well as in rodent’s animal models (69, 70). In line with these studies, our results showed that LPS treatment enhanced the level of inflammatory markers like NFκB, TNF-α, and IL-1β, and Alzheimer’s associated proteins like APP and Aβ, and p-Tau while lupeol administration significantly inhibited the LPS-induced effect on neuroinflammatory markers, AD markers and pre-synaptic markers like SNAP-23 and SYP, and post-synaptic markers like PSD-95. Moreover, lupeol significantly reduced AChE activity in LPS-induced neuroinflammation model of Alzheimer’s disease like pathologies. These results provide the neuroprotective role of lupeol and its therapeutic potential in regulating cholinergic dysfunctions in AD (71, 72).
In conclusion, our results showed that lupeol possess properties to inhibit LPS-induced expression of pro-inflammatory mediators, which may further lead to inhibition of inflammation-induced memory impairment and synaptic dysfunction. However, we suggest that detail experimental research need to be performed for considering it a potential treatment for neuroinflammatory diseases and inflammation-induced neurological disorders like AD.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Animal Ethics Committee (IACUC) of the Division of Applied Life Science, Gyeongsang National University, Jinju, South Korea. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
KC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. JSP: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. HYP: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. MT: Formal analysis, Methodology, Writing – review & editing. TJP: Formal analysis, Investigation, Supervision, Writing – review & editing. MOK: Formal analysis, Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Neurological Disorder Research Program of the National Research Foundation (NRF), funded by the Korean Government (MSIT) (2020M3E5D9080660).
Conflict of interest
MOK was employed by the Alz-Dementia Korea Co.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kwon, HS, and Koh, S-H. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener. (2020) 9:42. doi: 10.1186/s40035-020-00221-2
2. Qq, Y, and Jw, Z. Neuroinflammation in the central nervous system: symphony of glial cells. Glia. (2019) 67:1017–35. doi: 10.1002/glia.23571
3. Badshah, H, Ali, T, and Kim, MO. Osmotin attenuates LPS-induced neuroinflammation and memory impairments via the TLR4/NFkappaB signaling pathway. Sci Rep. (2016) 6:24493. doi: 10.1038/srep24493
4. Marques, A, Torre, C, Pinto, R, Sepodes, B, and Rocha, J. Treatment advances in sepsis and septic shock: modulating pro-and anti-inflammatory mechanisms. J Clin Med. (2023) 12:2892. doi: 10.3390/jcm12082892
5. Khan, A, Ali, T, Rehman, SU, Khan, MS, Alam, SI, Ikram, M, et al. Neuroprotective effect of quercetin against the detrimental effects of LPS in the adult mouse brain. Front Pharmacol. (2018) 9:1383. doi: 10.3389/fphar.2018.01383
6. Al-Harbi, NO, Imam, F, Al-Harbi, MM, Qamar, W, Aljerian, K, Anwer, MK, et al. Effect of Apremilast on LPS-induced immunomodulation and inflammation via activation of Nrf2/HO-1 pathways in rat lungs. Saudi Pharmaceut J. (2023) 31:1327–38. doi: 10.1016/j.jsps.2023.05.022
7. Garate, I, Garcia-Bueno, B, Madrigal, JL, Caso, JR, Alou, L, Gomez-Lus, ML, et al. Toll-like 4 receptor inhibitor TAK-242 decreases neuroinflammation in rat brain frontal cortex after stress. J Neuroinflamm. (2014) 11:8. doi: 10.1186/1742-2094-11-8
8. Senol, SP, Temiz-Resitoglu, M, Guden, DS, Sari, AN, Sahan-Firat, S, and Tunctan, B. Suppression of TLR4/MyD88/TAK1/NF-κB/COX-2 signaling pathway in the central nervous system by Bexarotene, a selective RXR agonist, prevents hyperalgesia in the lipopolysaccharide-induced pain mouse model. Neurochem Res. (2021) 46:624–37. doi: 10.1007/s11064-020-03197-7
9. Hirsch, EC, and Hunot, S. Neuroinflammation in Parkinson's disease: a target for neuroprotection? Lancet Neurol. (2009) 8:382–97. doi: 10.1016/S1474-4422(09)70062-6
10. Garmendia, JV, De Sanctis, CV, Das, V, Annadurai, N, Hajduch, M, and De Sanctis, JB. Inflammation, autoimmunity and neurodegenerative diseases, therapeutics and beyond. Curr Neuropharmacol. (2024) 22:1080–109. doi: 10.2174/1570159X22666231017141636
11. Isik, S, Yeman Kiyak, B, Akbayir, R, Seyhali, R, and Arpaci, T. Microglia mediated neuroinflammation in Parkinson’s disease. Cells. (2023) 12:1012. doi: 10.3390/cells12071012
12. Skrzypczak-Wiercioch, A, and Sałat, K. Lipopolysaccharide-induced model of neuroinflammation: mechanisms of action, research application and future directions for its use. Molecules. (2022) 27:5481. doi: 10.3390/molecules27175481
13. Wang, W-Y, Tan, M-S, Yu, J-T, and Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med. (2015) 3:136. doi: 10.3978/j.issn.2305-5839.2015.03.49
14. Sambamurti, K, Kinsey, R, Maloney, B, Ge, YW, and Lahiri, DK. Gene structure and organization of the human beta-secretase (BACE) promoter. FASEB J. (2004) 18:1034–6. doi: 10.1096/fj.03-1378fje
15. Deng, X, Li, M, Ai, W, He, L, Lu, D, Patrylo, PR, et al. Lipolysaccharide-induced Neuroinflammation is associated with Alzheimer-like Amyloidogenic axonal pathology and dendritic degeneration in rats. Adv Alzheimers Dis. (2014) 3:78–93. doi: 10.4236/aad.2014.32009
16. Zhao, J, Bi, W, Xiao, S, Lan, X, Cheng, X, Zhang, J, et al. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci Rep. (2019) 9:5790. doi: 10.1038/s41598-019-42286-8
17. Rahman, MH, Bajgai, J, Fadriquela, A, Sharma, S, Trinh, TT, Akter, R, et al. Therapeutic potential of natural products in treating neurodegenerative disorders and their future prospects and challenges. Molecules. (2021) 26:5327. doi: 10.3390/molecules26175327
18. Papi Reddy, K, Singh, AB, Puri, A, Srivastava, AK, and Narender, T. Synthesis of novel triterpenoid (lupeol) derivatives and their in vivo antihyperglycemic and antidyslipidemic activity. Bioorg Med Chem Lett. (2009) 19:4463–6. doi: 10.1016/j.bmcl.2009.05.034
19. He, Y, Liu, F, Zhang, L, Wu, Y, Hu, B, Zhang, Y, et al. Growth inhibition and apoptosis induced by lupeol, a dietary triterpene, in human hepatocellular carcinoma cells. Biol Pharm Bull. (2011) 34:517–22. doi: 10.1248/bpb.34.517
20. Kumari, A, and Kakkar, P. Lupeol protects against acetaminophen-induced oxidative stress and cell death in rat primary hepatocytes. Food Chem Toxicol. (2012) 50:1781–9. doi: 10.1016/j.fct.2012.02.042
21. Ahmad, R, Khan, A, Rehman, IU, Lee, HJ, Khan, I, and Kim, MO. Lupeol treatment attenuates activation of glial cells and oxidative-stress-mediated neuropathology in mouse model of traumatic brain injury. Int J Mol Sci. (2022) 23:6086. doi: 10.3390/ijms23116086
22. Yan, R, and Vassar, R. Targeting the β secretase BACE1 for Alzheimer's disease therapy. Lancet Neurol. (2014) 13:319–29. doi: 10.1016/S1474-4422(13)70276-X
23. Ikram, M, Muhammad, T, Rehman, SU, Khan, A, Jo, MG, Ali, T, et al. Hesperetin confers neuroprotection by regulating Nrf2/TLR4/NF-κB signaling in an Aβ mouse model. Mol Neurobiol. (2019) 56:6293–309. doi: 10.1007/s12035-019-1512-7
24. Chuang, K-A, Li, M-H, Lin, N-H, Chang, C-H, Lu, I-H, Pan, I-H, et al. Rhinacanthin C alleviates amyloid-β fibrils' toxicity on neurons and attenuates neuroinflammation triggered by LPS, amyloid-β, and interferon-γ in glial cells. Oxidative Med Cell Longev. (2017) 2017:1–18. doi: 10.1155/2017/5414297
25. Hira, S, Saleem, U, Anwar, F, Sohail, MF, Raza, Z, and Ahmad, B. β-Carotene: a natural compound improves cognitive impairment and oxidative stress in a mouse model of streptozotocin-induced Alzheimer’s disease. Biomol Ther. (2019) 9:441. doi: 10.3390/biom9090441
26. Ahmad, SI, Ali, G, Muhammad, T, Ullah, R, Umar, MN, and Hashmi, AN. Synthetic β-hydroxy ketone derivative inhibits cholinesterases, rescues oxidative stress and ameliorates cognitive deficits in 5XFAD mice model of AD. Mol Biol Rep. (2020) 47:9553–66. doi: 10.1007/s11033-020-05997-0
27. Bradford, MM . A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. (1976) 72:248–54. doi: 10.1016/0003-2697(76)90527-3
28. Ali, T, Rehman, SU, Khan, A, Badshah, H, Abid, NB, Kim, MW, et al. Adiponectin-mimetic novel nonapeptide rescues aberrant neuronal metabolic-associated memory deficits in Alzheimer's disease. Mol Neurodegener. (2021) 16:23. doi: 10.1186/s13024-021-00445-4
29. Shah, SA, Yoon, GH, Chung, SS, Abid, MN, Kim, TH, Lee, HY, et al. Novel osmotin inhibits SREBP2 via the AdipoR1/AMPK/SIRT1 pathway to improve Alzheimer's disease neuropathological deficits. Mol Psychiatry. (2017) 22:407–16. doi: 10.1038/mp.2016.23
30. Ahmad, A, Ali, T, Kim, MW, Khan, A, Jo, MH, Rehman, SU, et al. Adiponectin homolog novel osmotin protects obesity/diabetes-induced NAFLD by upregulating AdipoRs/PPARalpha signaling in Ob/Ob and db/db transgenic mouse models. Metab Clin Exp. (2019) 90:31–43. doi: 10.1016/j.metabol.2018.10.004
31. Khan, A, Park, JS, Kang, MH, Lee, HJ, Ali, J, Tahir, M, et al. Caffeic acid, a polyphenolic micronutrient rescues mice brains against Aβ-induced neurodegeneration and memory impairment. Antioxidants. (2023) 12:1284. doi: 10.3390/antiox12061284
32. Park, JS, Kim, ST, Kim, SY, Jo, MG, Choi, MJ, and Kim, MO. A novel kit for early diagnosis of Alzheimer's disease using a fluorescent nanoparticle imaging. Sci Rep. (2019) 9:13184. doi: 10.1038/s41598-019-49711-y
33. Amin, FU, Shah, SA, Badshah, H, Khan, M, and Kim, MO. Anthocyanins encapsulated by PLGA@PEG nanoparticles potentially improved its free radical scavenging capabilities via p38/JNK pathway against Abeta (1-42)-induced oxidative stress. J Nanobiotechnol. (2017) 15:12. doi: 10.1186/s12951-016-0227-4
34. Khan, A, Ikram, M, Muhammad, T, Park, J, and Kim, MO. Caffeine modulates cadmium-induced oxidative stress, Neuroinflammation, and cognitive impairments by regulating Nrf-2/HO-1 in vivo and in vitro. J Clin Med. (2019) 8:680. doi: 10.3390/jcm8050680
35. Miao, J, Shi, R, Li, L, Chen, F, Zhou, Y, Tung, YC, et al. Pathological tau from Alzheimer's brain induces site-specific hyperphosphorylation and SDS- and reducing agent-resistant aggregation of tau in vivo. Front Aging Neurosci. (2019) 11:34. doi: 10.3389/fnagi.2019.00034
36. Gong, CX, and Iqbal, K. Hyperphosphorylation of microtubule-associated protein tau: a promising therapeutic target for Alzheimer disease. Curr Med Chem. (2008) 15:2321–8. doi: 10.2174/092986708785909111
37. Marcello, E, Epis, R, Saraceno, C, and Di Luca, M. Synaptic dysfunction in Alzheimer's disease. Adv Exp Med Biol. (2012) 970:573–601. doi: 10.1007/978-3-7091-0932-8_25
38. Hemar, A, and Mulle, C. Alzheimer's disease, amyloid peptide and synaptic dysfunction. Med Sci. (2011) 27:733–6. doi: 10.1051/medsci/2011278015
39. Trang, A, and Khandhar, PB. Physiology, acetylcholinesterase. In: StatPearls. Treasure Island (FL): StatPearls Publishing (2023).
40. Amenta, F, and Tayebati, SK. Pathways of acetylcholine synthesis, transport and release as targets for treatment of adult-onset cognitive dysfunction. Curr Med Chem. (2008) 15:488–98. doi: 10.2174/092986708783503203
41. Scapagnini, G, Sonya, V, Nader, AG, Calogero, C, Zella, D, and Fabio, G. Modulation of Nrf2/ARE pathway by food polyphenols: a nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol Neurobiol. (2011) 44:192–201. doi: 10.1007/s12035-011-8181-5
42. Ali, T, Kim, T, Rehman, SU, Khan, MS, Amin, FU, Khan, M, et al. Natural dietary supplementation of anthocyanins via PI3K/Akt/Nrf2/HO-1 pathways mitigate oxidative stress, neurodegeneration, and memory impairment in a mouse model of Alzheimer’s disease. Mol Neurobiol. (2018) 55:6076–93. doi: 10.1007/s12035-017-0798-6
43. Ahmad, R, Khan, A, Lee, HJ, Ur Rehman, I, Khan, I, Alam, SI, et al. Lupeol, a plant-derived triterpenoid, protects mice brains against Aβ-induced oxidative stress and neurodegeneration. Biomedicines. (2020) 8:380. doi: 10.3390/biomedicines8100380
44. Park, JS, Rehman, IU, Choe, K, Ahmad, R, Lee, HJ, and Kim, MO. A triterpenoid lupeol as an antioxidant and anti-neuroinflammatory agent: impacts on oxidative stress in Alzheimer’s disease. Nutrients. (2023) 15:3059. doi: 10.3390/nu15133059
45. Khan, MS, Ali, T, Kim, MW, Jo, MH, Jo, MG, Badshah, H, et al. Anthocyanins protect against LPS-induced oxidative stress-mediated neuroinflammation and neurodegeneration in the adult mouse cortex. Neurochem Int. (2016) 100:1–10. doi: 10.1016/j.neuint.2016.08.005
46. Qin, L, Wu, X, Block, ML, Liu, Y, Breese, GR, Hong, JS, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. (2007) 55:453–62. doi: 10.1002/glia.20467
47. Ullah, R, Ikram, M, Park, TJ, Ahmad, R, Saeed, K, Alam, SI, et al. Vanillic acid, a bioactive phenolic compound, counteracts LPS-induced neurotoxicity by regulating c-Jun N-terminal kinase in mouse brain. Int J Mol Sci. (2020) 22:361. doi: 10.3390/ijms22010361
48. Khan, A, Park, TJ, Ikram, M, Ahmad, S, Ahmad, R, Jo, MG, et al. Antioxidative and anti-inflammatory effects of Kojic acid in Abeta-induced mouse model of Alzheimer's disease. Mol Neurobiol. (2021) 58:5127–40. doi: 10.1007/s12035-021-02460-4
49. Khan, MS, Ali, T, Abid, MN, Jo, MH, Khan, A, Kim, MW, et al. Lithium ameliorates lipopolysaccharide-induced neurotoxicity in the cortex and hippocampus of the adult rat brain. Neurochem Int. (2017) 108:343–54. doi: 10.1016/j.neuint.2017.05.008
50. Ahmad, S, Jo, MH, Ikram, M, Khan, A, and Kim, MO. Deciphering the potential neuroprotective effects of Luteolin against Abeta(1)-(42)-induced Alzheimer's disease. Int J Mol Sci. (2021) 22:9583. doi: 10.3390/ijms22179583
51. Song, X, Wang, T, Zhang, Z, Jiang, H, Wang, W, Cao, Y, et al. Leonurine exerts anti-inflammatory effect by regulating inflammatory signaling pathways and cytokines in LPS-induced mouse mastitis. Inflammation. (2015) 38:79–88. doi: 10.1007/s10753-014-0009-9
52. Ma, Q, Wei, Y, Meng, Z, Chen, Y, and Zhao, G. Effects of water extract from Artemisia argyi leaves on LPS-induced mastitis in mice. Animals. (2022) 12:907. doi: 10.3390/ani12070907
53. Rankine, EL, Hughes, PM, Botham, MS, Perry, VH, and Felton, LM. Brain cytokine synthesis induced by an intraparenchymal injection of LPS is reduced in MCP-1-deficient mice prior to leucocyte recruitment. Eur J Neurosci. (2006) 24:77–86. doi: 10.1111/j.1460-9568.2006.04891.x
54. Lynch, MA . The multifaceted profile of activated microglia. Mol Neurobiol. (2009) 40:139–56. doi: 10.1007/s12035-009-8077-9
55. Saleem, M . Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett. (2009) 285:109–15. doi: 10.1016/j.canlet.2009.04.033
56. Geetha, T, and Varalakshmi, P. Anti-inflammatory activity of lupeol and lupeol linoleate in rats. J Ethnopharmacol. (2001) 76:77–80. doi: 10.1016/S0378-8741(01)00175-1
57. Badshah, H, Ali, T, Shafiq-ur, R, Faiz-ul, A, Ullah, F, Kim, TH, et al. Protective effect of Lupeol against lipopolysaccharide-induced Neuroinflammation via the p38/c-Jun N-terminal kinase pathway in the adult mouse brain. J Neuroimmune Pharmacol. (2016) 11:48–60. doi: 10.1007/s11481-015-9623-z
58. Okun, E, Griffioen, KJ, and Mattson, MP. Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci. (2011) 34:269–81. doi: 10.1016/j.tins.2011.02.005
59. Kacimi, R, Giffard, RG, and Yenari, MA. Endotoxin-activated microglia injure brain derived endothelial cells via NF-kappaB, JAK-STAT and JNK stress kinase pathways. J Inflamm. (2011) 8:7. doi: 10.1186/1476-9255-8-7
60. Zhao, WX, Zhang, JH, Cao, JB, Wang, W, Wang, DX, Zhang, XY, et al. Acetaminophen attenuates lipopolysaccharide-induced cognitive impairment through antioxidant activity. J Neuroinflammation. (2017) 14:17. doi: 10.1186/s12974-016-0781-6
61. Catorce, MN, and Gevorkian, G. LPS-induced murine Neuroinflammation model: Main features and suitability for pre-clinical assessment of nutraceuticals. Curr Neuropharmacol. (2016) 14:155–64. doi: 10.2174/1570159X14666151204122017
62. Rosi, S, Vazdarjanova, A, Ramirez-Amaya, V, Worley, PF, Barnes, CA, and Wenk, GL. Memantine protects against LPS-induced neuroinflammation, restores behaviorally-induced gene expression and spatial learning in the rat. Neuroscience. (2006) 142:1303–15. doi: 10.1016/j.neuroscience.2006.08.017
63. Lee, B, Shim, I, and Lee, H. Gypenosides attenuate lipopolysaccharide-induced Neuroinflammation and memory impairment in rats. Evid Based Complement Alternat Med. (2018) 2018:1–10. doi: 10.1155/2018/4183670
64. Amraie, E, Pouraboli, I, and Rajaei, Z. Neuroprotective effects of Levisticum officinale on LPS-induced spatial learning and memory impairments through neurotrophic, anti-inflammatory, and antioxidant properties. Food Funct. (2020) 11:6608–21. doi: 10.1039/D0FO01030H
65. Blasko, I, Apochal, A, Boeck, G, Hartmann, T, Grubeck-Loebenstein, B, and Ransmayr, G. Ibuprofen decreases cytokine-induced amyloid beta production in neuronal cells. Neurobiol Dis. (2001) 8:1094–101. doi: 10.1006/nbdi.2001.0451
66. Chen, CH, Zhou, W, Liu, S, Deng, Y, Cai, F, Tone, M, et al. Increased NF-kappaB signalling up-regulates BACE1 expression and its therapeutic potential in Alzheimer's disease. Int J Neuropsychopharmacol. (2012) 15:77–90. doi: 10.1017/S1461145711000149
67. Spires-Jones, TL, and Hyman, BT. The intersection of amyloid beta and tau at synapses in Alzheimer's disease. Neuron. (2014) 82:756–71. doi: 10.1016/j.neuron.2014.05.004
68. Bereczki, E, Francis, PT, Howlett, D, Pereira, JB, Hoglund, K, Bogstedt, A, et al. Synaptic proteins predict cognitive decline in Alzheimer's disease and Lewy body dementia. Alzheimers Dement. (2016) 12:1149–58. doi: 10.1016/j.jalz.2016.04.005
69. Kim, MJ, Futai, K, Jo, J, Hayashi, Y, Cho, K, and Sheng, M. Synaptic accumulation of PSD-95 and synaptic function regulated by phosphorylation of serine-295 of PSD-95. Neuron. (2007) 56:488–502. doi: 10.1016/j.neuron.2007.09.007
70. Gylys, KH, Fein, JA, Yang, F, Wiley, DJ, Miller, CA, and Cole, GM. Synaptic changes in Alzheimer's disease: increased amyloid-beta and gliosis in surviving terminals is accompanied by decreased PSD-95 fluorescence. Am J Pathol. (2004) 165:1809–17. doi: 10.1016/S0002-9440(10)63436-0
71. Akıncıoğlu, H, and Gülçin, İ. Potent acetylcholinesterase inhibitors: potential drugs for Alzheimer’s disease. Mini Rev Med Chem. (2020) 20:703–15. doi: 10.2174/1389557520666200103100521
Keywords: Alzheimer’s disease, lipopolysaccharide, lupeol, amyloid-beta, neuroinflammation
Citation: Choe K, Park JS, Park HY, Tahir M, Park TJ and Kim MO (2024) Lupeol protect against LPS-induced neuroinflammation and amyloid beta in adult mouse hippocampus. Front. Nutr. 11:1414696. doi: 10.3389/fnut.2024.1414696
Edited by:
Idris Long, University of Science Malaysia (USM), MalaysiaReviewed by:
Giulia Abate, University of Brescia, ItalySandeep Kumar Singh, Indian Scientific Education and Technology Foundation, India
Copyright © 2024 Choe, Park, Park, Tahir, Park and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tae Ju Park, dC5wYXJrLjFAcmVzZWFyY2guZ2xhLmFjLnVr; Myeong Ok Kim, bW9raW1AZ251LmFjLmty
†These authors have contributed equally to this work
 Kyonghwan Choe1,2†
Kyonghwan Choe1,2† Myeong Ok Kim
Myeong Ok Kim