- 1Dehong People's Hospital, Kunming Medical University Affiliated Dehong Hospital, Mangshi, China
- 2Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 3Tianjin Orthopedic Research Institute, Tianjin Hospital, Tianjin, China
- 4Tianjin Medical University, Tianjin, China
Background: The role of vitamin C in osteoarthritis (OA) is still a subject of debate. Our aim was to combine the National Health and Nutrition Examination Survey (NHANES) and MR studies to explore the relationship between vitamin C intake and OA.
Methods: Clinical information on participants during NHANES 2003–2018 was collected and the relationship between vitamin C intake and OA risk was assessed using logistic regression modelling. In MR analyses, three methods were used to explore the causality of vitamin C intake with OA. Sensitivity analysis to verify the stability of the MR study.
Results: The cross-sectional study included a total of 31,527 participants, categorizing them into low (<30.2 mg), medium (30.2–93.0 mg) and high (>93.0 mg) level groups based on their vitamin C intake levels. Logistic regression models showed that vitamin C intake was not associated with OA risk (p > 0.05). Inverse-variance weighted (IVW) method of MR study showed no causality between vitamin C intake and OA (OR = 0.993, 95% CI: 0.901 ~ 1.095, p = 0.882). Sensitivity analysis indicated that the MR study was reliable.
Conclusion: Our cross-sectional and MR studies showed that vitamin C intake was not associated with OA risk. More researches are needed in the future to investigate the link between vitamin C and OA.
Background
Osteoarthritis (OA) is a widespread and incapacitating disease that impacts joints, marked by the progressive breakdown of articular cartilage (1). Globally, it stands as a primary contributor to pain and functional impairment, with a pronounced impact on individuals, especially among the elderly (2). Unfortunately, as the population ages and obesity becomes more prevalent, the occurrence of OA is steadily increasing each year (3). The main treatment approach for OA is symptomatic management, aiming to alleviate symptoms such as swelling and pain, however, this is temporary (4). After the failure of symptomatic treatment, invasive methods such as joint replacement and intra-articular injections are employed. While effective, these approaches come with numerous side effects (5). Therefore, it is crucial to seek a treatment method for preventing and continually improving OA.
Reactive oxygen species (ROS) can induce the death of cartilage cells, constituting a crucial risk factor for age-related degenerative OA (6). Vitamin C, recognized for its potent antioxidant properties, serves as an effective scavenger of ROS (7). Considering this information, the antioxidant attributes of vitamin C could potentially offer a protective effect against OA. Yet, current researches on the relationship between vitamin C and OA remains controversial. An animal experiment conducted by Kurz et al. indicated that supplementing with vitamin C can prevent the development of OA (8). A cohort study revealed that vitamin C intake can alleviate bone lesions in patients with OA (9). However, a longitudinal study with a 4-year follow-up found no correlation between vitamin C and OA (10). Therefore, additional researches are needed to explore the connection between vitamin C and OA.
NHANES focuses on the nutritional and health situation of the U.S. population. The substantial sample size of NHANES performs a vital role in informing the development of public health policies and strategy for implementation (11). Mendelian randomization (MR) studies explore the causal relationship between exposure and outcome from a genetic dimension, greatly mitigating the effects of reverse causation and confounding factors (12, 13). To determine the link of vitamin C intake to OA, we are combining NHANES with Mendelian MR studies, aiming to provide theoretical support for the prevention and improvement of OA.
Methods
Participant selection
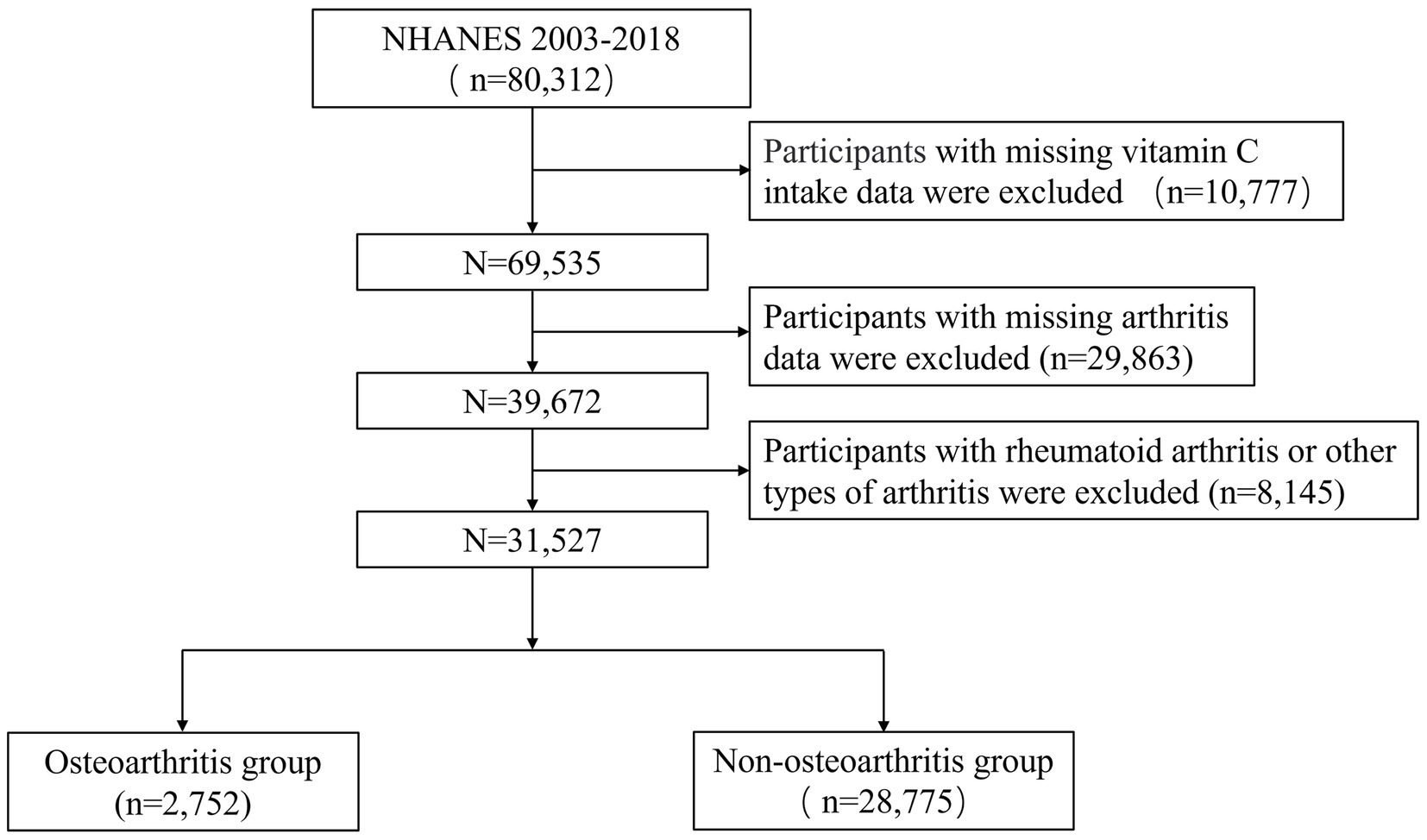
We collected information from NHANES 2003–2018 and obtained a total of 80,312 individuals. Firstly, participants with incomplete vitamin C intake data were excluded. Secondly, individuals with incomplete arthritis information were excluded. Lastly, participants with rheumatoid arthritis or other types of arthritis were excluded (Figure 1).
Variables in NHANES
Two questions on the questionnaire were used to determine whether participants had OA: “(1) Doctor ever said you had arthritis? (2) Which type of arthritis was it?” We obtained data on vitamin C intake through the Dietary Interview of Total Nutrient Intakes-First Day. Based on previous studies (14–16), the following variables are included in this study: gender, age, race, educational level, poverty-income ratio (PIR), marital status, smoking, drinking, physical activity, body mass index (BMI), diabetes, hypertension, hyperlipidemia, energy intake, protein intake and sugar intake. See Supplementary Table S1 for a full breakdown of these variables. All participants were equally divided in low, medium and high groups according to their vitamin C intake levels.
Instrumental variables selection
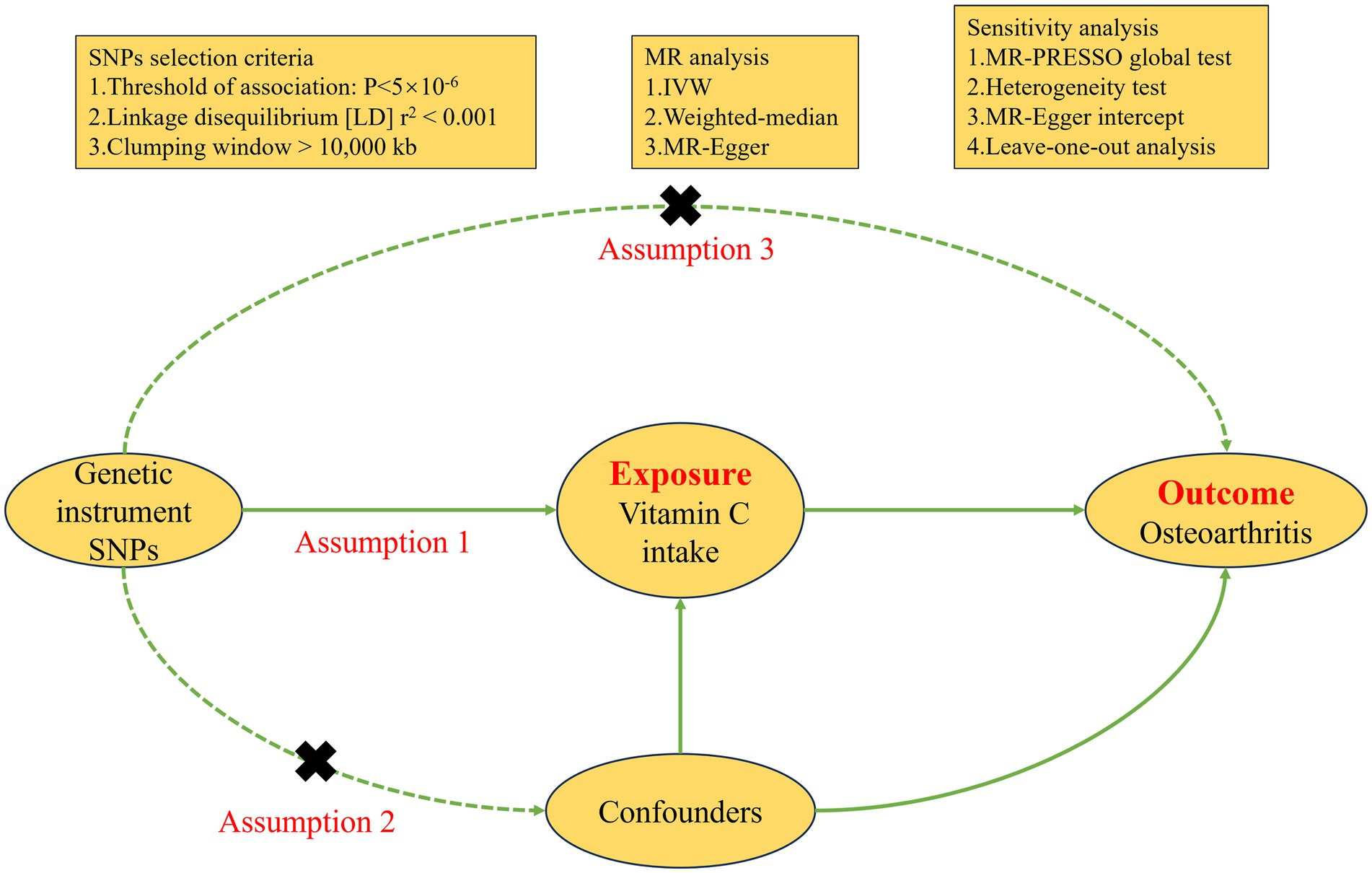
Vitamin C intake served as the exposure variable in this study, we obtained instrumental variables (IVs) from a study on a European population in UK biobank. The Genome Wide Association Study (GWAS) summary data of vitamin C intake was extracted directly from the IEU open GWAS project (ID: ukb-b-15175). The study had the sample of 460,351 cases, of which 39,880 were patients and 420,471 were control. To test the effectiveness of IVs, we used the F statistic. F > 10 indicates that IVs are effective (17). The specific calculation method and explanation of F (18) can be found in Supplementary Table S2. The choice of IVs must meet the three main MR assumptions. First, Single Nucleotide Polymorphisms (SNPs) need to be strongly correlated with exposure (p < 5 × 10−6) and have F-statistics >10. Second, after determining linkage disequilibrium (LD) with r2 < 0.001 and clumping distance = 10,000 kb, independent SNPs were retained. Last, SNPs linked to the outcome and confounding variables were not included (p < 5 × 10−6). Based on these selection criteria, we obtained 25 SNPs associated with vitamin C intake (Supplementary Table S3). We searched for other phenotypes on PhenoScanner and did not find any SNPs associated with OA and confounding factors. Figure 2 illustrates the detailed process of MR.
GWAS data summary of OA
We used OA as the outcome, which has GWAS data from a European population study by Dönertaş et al. (19). OA cases in this study were defined based on International Classification of Diseases (ICD)-10 coding (M15–M19, M47). This study contained a sample of 484,598 cases, including 39,515 patients. MR-PRESSO test did not find outlier SNPs. After harmonization, we found rs10000324 and rs12535840 as palindromic SNPs and excluded them, and finally 23 SNPs were included in the MR analysis. The impact which vitamin C intake has upon OA is shown at Supplementary Table S4.
Statistical analysis
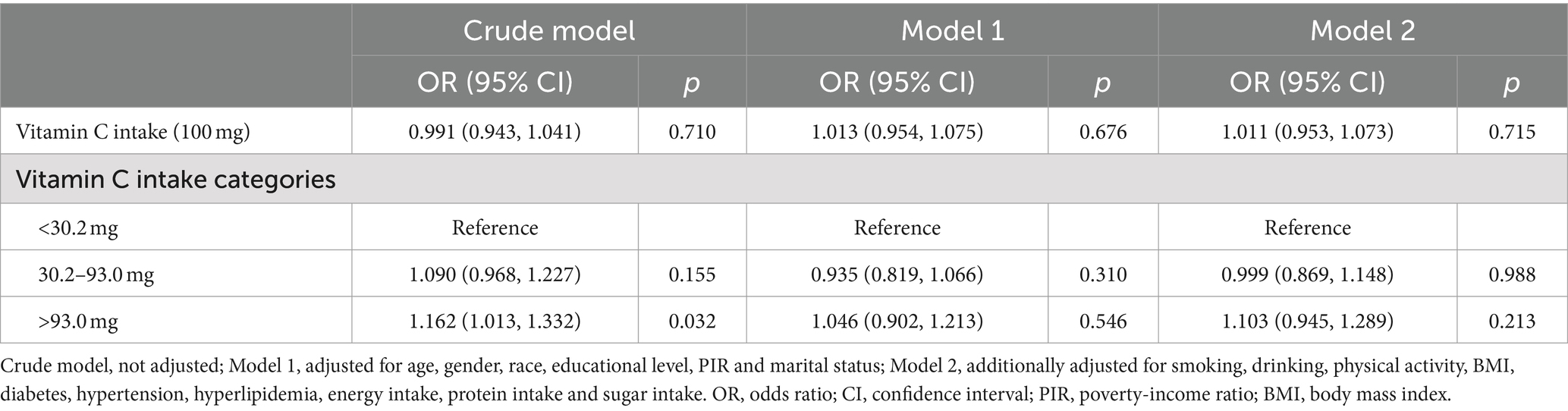
Graphing and analysis using R software (version 4.2.1). All analyses were conducted using survey weights provided by the NHANES to account for the complex, multistage probability sampling design. Categorical variables were expressed as weighted frequencies (%), and group comparisons were conducted by the weighted chi-square test. For continuous variables, data were presented as mean and standard error (SE), group comparisons were conducted using weighted linear regression models. We developed three multivariate logistic regression models for evaluating the link of vitamin C intake to OA. Crude model: not adjusted; model 1: adjusted for age, gender, race, educational level, PIR and marital status; model 2: adjusted for smoking, drinking, physical activity, BMI, diabetes, hypertension, hyperlipidemia, energy intake, protein intake and sugar intake based on model 1.
Inverse-variance weighted (IVW) served as the main method of MR analysis, with MR Egger and weighted median as supplements (20). The findings from the MR analysis were presented by odds ratios (ORs), representing the increased hazard of OA per unit rise in vitamin C intake. MR-Egger intercepts were applied for testing horizontal pleiotropy in SNPs, with an intercept close to 0 indicating the absence of pleiotropy (p > 0.05) (21). Heterogeneity of SNPs was checked by Cochran’s Q test, and no heterogeneity was required to satisfy p > 0.05 and I2 < 25%. Reliability of MR analysis assessed by Funnel plot symmetry and Leave-one-out. The R packages used in MR analysis are “MRPRESSO” and “TwoSampleMR.” p < 0.05 was considered statistically significant.
Results
Clinical features
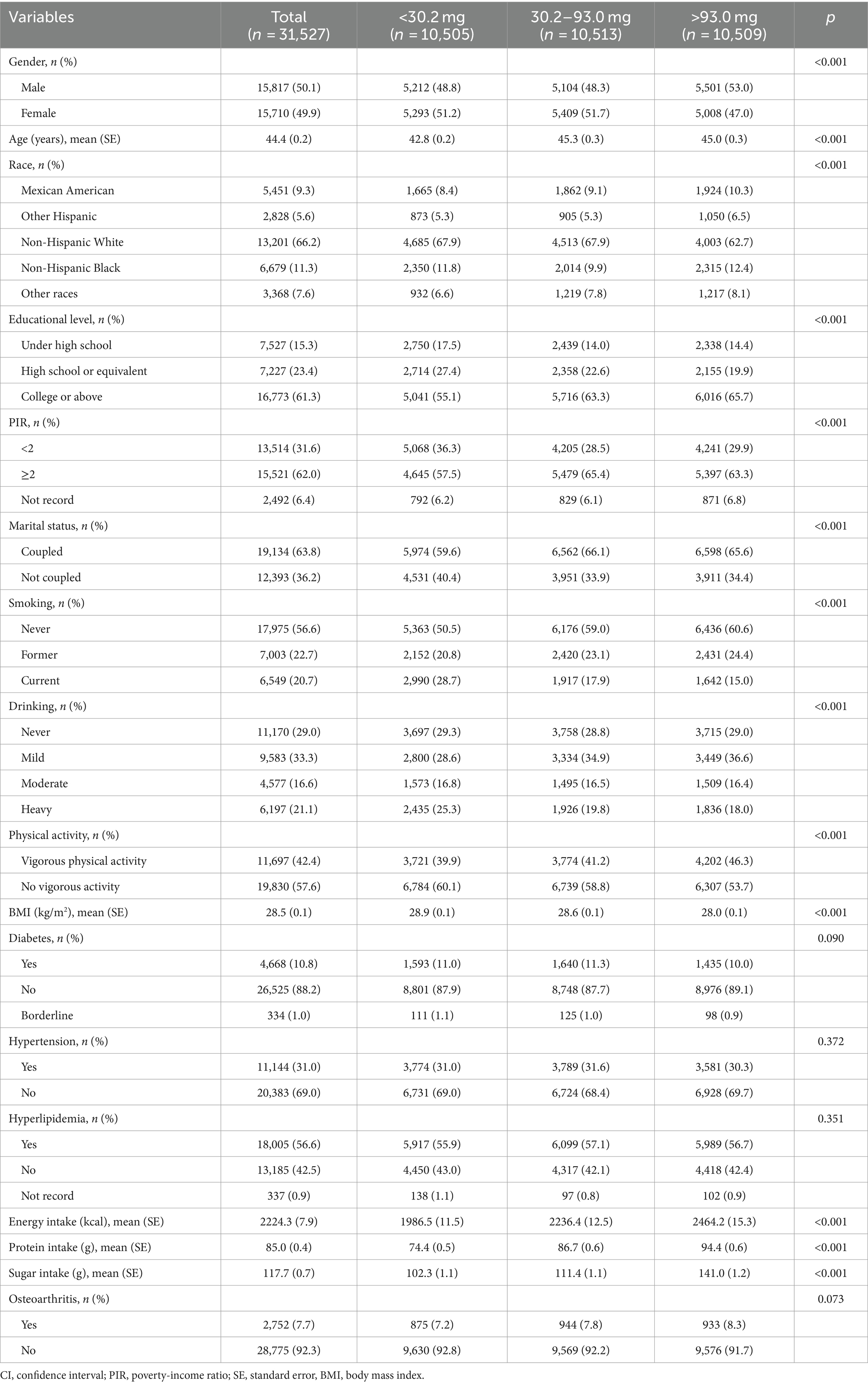
The research included 31,527 individuals, the average age of whom was 44.4 ± 0.2 years; 50.1% of the participants were male and 49.9% were female. All participants were divided into tertiles based on their vitamin C intake levels: low (<30.2 mg), medium (30.2–93.0 mg) and high (>93.0 mg) groups. In the entire study, 7.7% of individuals had OA. The incidence of OA in the three groups was 7.2, 7.8, and 8.3%, respectively; the difference was not statistically significant (p > 0.05). As shown in Table 1, race, gender, age, educational level, PIR, marital status, BMI, smoking, drinking, physical activity, energy, protein, and sugar intakes (all p < 0.05) were statistically significant compared to the three groups. Compared to the low group, participants in the high group were older, more likely to be male, Mexican American, accompanied by a partner, had higher education levels, were wealthier, exercised more, had higher energy, protein, and sugar intake, and had less smoking and drinking history, as well as lower body weight (all p < 0.05).
Correlation of vitamin C intake with OA
Weighted Logistic regression analysis with vitamin C intake level as a continuous variable showed no correlation of vitamin C intake with OA (OR = 0.991, p = 0.710). After adjusting the variables, both model 1 and 2 still do not find a correlation between them (OR = 1.013, 1.011; p = 0.676, 0.715). After categorizing the vitamin C intake level, none of the three models detected a link of vitamin C intake to OA (p > 0.05). Details are given in Table 2.
Causality and MR sensitivity analysis of vitamin C intake and OA
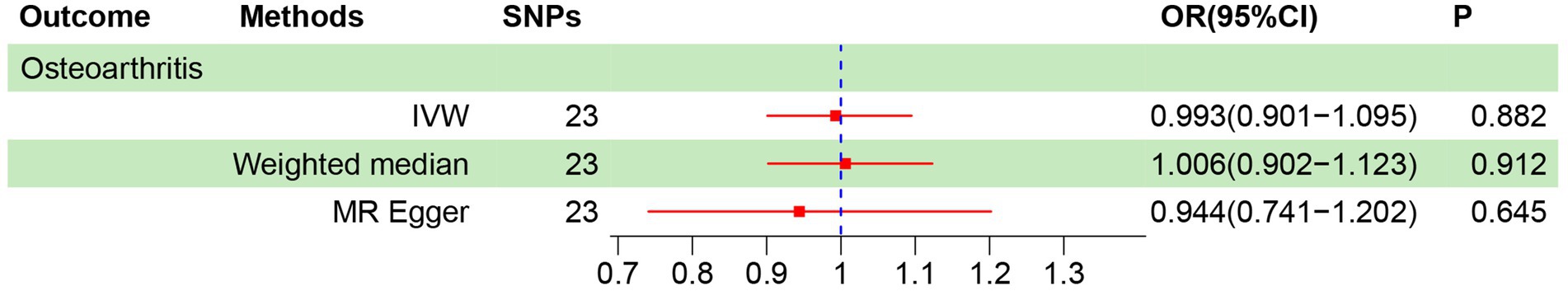
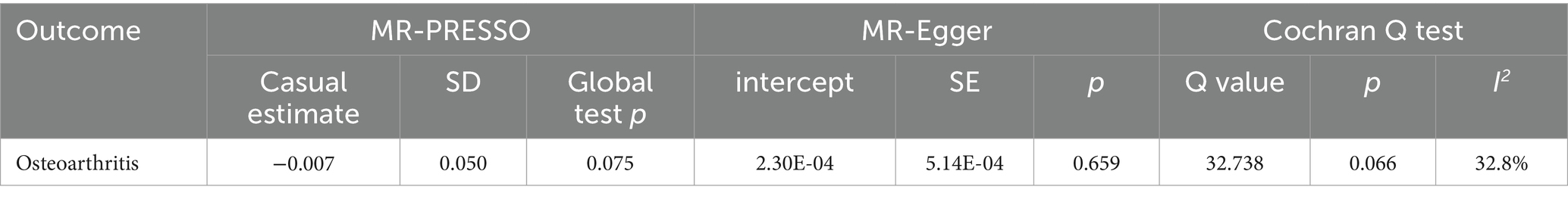
There are 23 SNPs in the MR analysis that all had F > 10 (Supplementary Table S3). As depicted in Figure 3, IVW, weighted median and MR-Egger models revealed no causality between vitamin C intake and OA (OR = 0.993, 1.006, 0.944; all p > 0.05). We also plotted the forest plot for each SNP assessing the causality of vitamin C intake and OA (Supplementary Figure S1). Mild heterogeneity existed among SNPs (p = 0.066, I2 = 32.8%) (Table 3). MR-Egger intercept analysis showed no horizontal pleiotropy (intercept = 2.30E-04, p = 0.659) (Table 3; Supplementary Figure S2). MR-PRESSO test revealed no outlier SNPs (p = 0.075) (Table 3). No single SNP highly influences the overall effect in leave-one-out analysis (Supplementary Figure S3). In addition, the symmetry of funnel plot was better, showing the pleiotropy did not exist (Supplementary Figure S4). The sensitivity analysis results above suggest the reliability on MR results.
Discussion
In NHANES, logistic regression analysis revealed no link of vitamin C intake to OA. Subsequent MR analyses also confirmed the absence of causality between the two.
OA mainly influences weight-bearing joints, with hands and knees being the most common. The main pathological manifestations include synovial inflammation and subchondral bone damage in habitual weight-bearing areas. In severe cases, osteophytes may form, leading to pain, swelling, limited mobility, crepitus, and a grinding sensation in the affected joints. The joint cavity may exhibit varying degrees of exudative inflammation, while systemic symptoms are generally less common (22–24). In OA, oxidative stress resulting from sustained ROS production can promote cartilage degradation through signaling pathways such as PI3K/AKT, leading to cartilage degeneration (25). Vitamin C can play a beneficial role in preventing cartilage damage and slow down the progression of OA by inhibiting lipid oxidation through its antioxidative capabilities (26). Antioxidant levels in joint fluid are often significantly lower in patients with severe OA compared to normal subjects (27). However, the findings from multiple investigations regarding the link of vitamin C to OA are inconsistent. Peregoy et al.’s clinical study, which included a total of 1,023 patients aged 40 and above, revealed that supplementing with vitamin C could reduce the incidence risk of OA by 11% after a 20-year follow-up (28). McAlindon et al. conducted a study with 640 participants to investigate the potential of antioxidant intake in lowering the risk of OA. The findings indicated a significant decrease in the advancement of OA and cartilage loss with elevated levels of vitamin C supplementation (29). However, other studies have concluded no link was found for vitamin C and OA, or that vitamin C can increase the OA risk. Veen et al.’s study involved 43,865 participants, with 5,976 cases in the case group, followed all participants for 19 years. After adjusting for confounding factors, the study found no link of vitamin C supplementation to OA (30). The research by Joseph et al. that included 1,785 participants also failed to find a link between vitamin C intake and OA (10). A total of 4,685 participants were included in Li et al. ‘s study that explored the connection of supplementation with antioxidants and OA, and found that vitamin C increased OA risk (31).
In the cross-sectional study from NHANES, we first divided participants into OA and non-OA groups, and logistic regression analyses after adjusting for confounders confirmed that no correlation was found among vitamin C intake and OA. We then transformed vitamin C intake level into categorical variables and still found no association. The results of our observational study are consistent with those of Veen et al. To further confirm our findings, MR analyses were performed. We used 23 SNPs strongly associated with vitamin C intake as IVs to perform MR analysis, and found that there was no causality between vitamin C intake and OA, and sensitivity analyses demonstrated that our MR analyses were reliable. The MR analyses validated the results obtained from the observational study at the genetic level. We speculate that the probable reason is that the etiology of OA is complex and many of the pathogenic mechanisms are still unclear (32). Although the vitamin C has antioxidant function, it cannot exert a decisive function on the pathogenesis of OA, and therefore its association with OA is not obvious. Additionally, individuals have varying requirements for vitamin C, making it challenging to determine the appropriate dosage when supplementing. Excessive intake of vitamin C may have negative effects on the body (33, 34). The innovation of our study is to combine NHANES and MR studies to explore the connection of vitamin C intake and OA in epidemiological and genetic perspectives, avoiding the influence of many confounding factors on the results and making them more scientific and reliable. The consistent results of the two methods also make the conclusions more convincing. Nevertheless, our study has some limitations. First, different ethnicities included in cross-sectional and MR studies may bias results. As our study only included European and US populations, our conclusions have yet to be validated in other populations. Second, we did not explore the association of vitamin C deficiency with the risk of OA. Last, we did not explore the specific underlying mechanisms by which the antioxidant effects of vitamin C may affect OA, and future studies are needed to explore them further.
Conclusion
In summary, in the study from NHANES, logistic regression analyses did not find an association between vitamin C intake and the risk of OA. Further MR analyses also showed no causality between vitamin C intake and OA, and the conclusions were consistent between the two research methods. More studies are needed to explore the relationship between vitamin C and OA in the future.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
HZ: Conceptualization, Formal analysis, Writing – original draft. XJ: Conceptualization, Writing – review & editing. LB: Data curation, Writing – review & editing. JC: Data curation, Formal analysis, Writing – review & editing. WL: Writing – original draft. JM: Data curation, Writing – review & editing. XM: Formal analysis, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Dehong Prefecture Science and Technology Plan project (ZC202318); Dehong People’s Hospital Scientific Research Fund project (2023DY009); The Central Government Guides Local Funds for Scientific and Technological Development (22ZYJDSY00110); Yunnan Province Talent Development Planent Plan (2023RC006); and Tianjin Health Science and Technology project (TJWJ2022MS025).
Acknowledgments
The authors wish to thank the study participants for their contribution to the research, as well as current and past investigators and staff.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1409578/full#supplementary-material
References
1. Bortoluzzi, A, Furini, F, and Scirè, CA. Osteoarthritis and its management – epidemiology, nutritional aspects and environmental factors. Autoimmun Rev. (2018) 17:1097–104. doi: 10.1016/j.autrev.2018.06.002
2. Cross, M, Smith, E, Hoy, D, Nolte, S, Ackerman, I, Fransen, M, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. (2014) 73:1323–30. doi: 10.1136/annrheumdis-2013-204763
3. Palazzo, C, Nguyen, C, Lefevre-Colau, MM, Rannou, F, and Poiraudeau, S. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. (2016) 59:134–8. doi: 10.1016/j.rehab.2016.01.006
4. Dantas, LO, Salvini, TF, and McAlindon, TE. Knee osteoarthritis: key treatments and implications for physical therapy. Braz J Phys Ther. (2021) 25:135–46. doi: 10.1016/j.bjpt.2020.08.004
5. Ferguson, RJ, Palmer, AJ, Taylor, A, Porter, ML, Malchau, H, and Glyn-Jones, S. Hip replacement. Lancet. (2018) 392:1662–71. doi: 10.1016/S0140-6736(18)31777-X
6. Bolduc, JA, Collins, JA, and Loeser, RF. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic Biol Med. (2019) 132:73–82. doi: 10.1016/j.freeradbiomed.2018.08.038
7. Blokhina, O, Virolainen, E, and Fagerstedt, KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot. (2003) 91:179–94. doi: 10.1093/aob/mcf118
8. Kurz, B, Jost, B, and Schünke, M. Dietary vitamins and selenium diminish the development of mechanically induced osteoarthritis and increase the expression of antioxidative enzymes in the knee joint of STR/1N mice. Osteoarthr Cartil. (2002) 10:119–26. doi: 10.1053/joca.2001.0489
9. Wang, Y, Hodge, AM, Wluka, AE, English, DR, Giles, GG, O'Sullivan, R, et al. Effect of antioxidants on knee cartilage and bone in healthy, middle-aged subjects: a cross-sectional study. Arthritis Res Ther. (2007) 9:R66. doi: 10.1186/ar2225
10. Joseph, GB, McCulloch, CE, Nevitt, MC, Neumann, J, Lynch, JA, Lane, NE, et al. Associations between vitamins C and D intake and cartilage composition and knee joint morphology over 4 years: data from the osteoarthritis initiative. Arthritis Care Res (Hoboken). (2020) 72:1239–47. doi: 10.1002/acr.24021
11. Ahluwalia, N, Dwyer, J, Terry, A, Moshfegh, A, and Johnson, C. Update on NHANES dietary data: focus on collection, release, analytical considerations, and uses to inform public policy. Adv Nutr. (2016) 7:121–34. doi: 10.3945/an.115.009258
12. Sekula, P, del Greco M, F, Pattaro, C, and Köttgen, A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol. (2016) 27:3253–65. doi: 10.1681/ASN.2016010098
13. Wang, K, Zhao, L, Luo, H, Deng, C, Gong, L, and Chen, Z. Association of serum vitamin C levels with asthma in adults: results of NHANES 2003–2006 and mendelian randomization study. BMC Pulm Med. (2024) 24:4. doi: 10.1186/s12890-023-02821-w
14. Deng, X, and Tan, Y. A national cross-sectional analysis of selenium intake and risk of osteoarthritis: NHANES 2003–2016. Front Public Health. (2023) 10:1047605. doi: 10.3389/fpubh.2022.1047605
15. Huang, G, Qian, D, Liu, Y, Qu, G, Qian, Y, and Pei, B. The association between frailty and osteoarthritis based on the NHANES and Mendelian randomization study. Arch Med Sci. (2023) 19:1545–50. doi: 10.5114/aoms/171270
16. Yu, G, Lin, Y, Dai, H, Xu, J, and Liu, J. Association between serum 25-hydroxyvitamin D and osteoarthritis: a national population-based analysis of NHANES 2001–2018. Front Nutr. (2023) 10:1016809. doi: 10.3389/fnut.2023.1016809
17. Burgess, S, and Thompson, SGCRP CHD Genetics Collaboration. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. (2011) 40:755–64. doi: 10.1093/ije/dyr036
18. Papadimitriou, N, Dimou, N, Tsilidis, KK, Banbury, B, Martin, RM, Lewis, SJ, et al. Physical activity and risks of breast and colorectal cancer: a Mendelian randomisation analysis. Nat Commun. (2020) 11:597. doi: 10.1038/s41467-020-14389-8
19. Dönertaş, HM, Fabian, DK, Fuentealba, M, Partridge, L, and Thornton, JM. Common genetic associations between age-related diseases. Nat Aging. (2021) 1:400–12. doi: 10.1038/s43587-021-00051-5
20. Hartwig, FP, Davey Smith, G, and Bowden, J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. (2017) 46:1985–98. doi: 10.1093/ije/dyx102
21. Bowden, J, Davey Smith, G, and Burgess, S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
23. Mandl, LA. Osteoarthritis year in review 2018: clinical. Osteoarthr Cartil. (2019) 27:359–64. doi: 10.1016/j.joca.2018.11.001
24. Abramoff, B, and Caldera, FE. Osteoarthritis. Med Clin North Am. (2020) 104:293–311. doi: 10.1016/j.mcna.2019.10.007
25. Li, D, Ni, S, Miao, KS, and Zhuang, C. PI3K/Akt and caspase pathways mediate oxidative stress-induced chondrocyte apoptosis. Cell Stress Chaperones. (2019) 24:195–202. doi: 10.1007/s12192-018-0956-4
26. Omata, S, Sonokawa, S, Sawae, Y, and Murakami, T. Effects of both vitamin C and mechanical stimulation on improving the mechanical characteristics of regenerated cartilage. Biochem Biophys Res Commun. (2012) 424:724–9. doi: 10.1016/j.bbrc.2012.07.019
27. Regan, EA, Bowler, RP, and Crapo, JD. Joint fluid antioxidants are decreased in osteoarthritic joints compared to joints with macroscopically intact cartilage and subacute injury. Osteoarthr Cartil. (2008) 16:515–21. doi: 10.1016/j.joca.2007.09.001
28. Peregoy, J, and Wilder, FV. The effects of vitamin C supplementation on incident and progressive knee osteoarthritis: a longitudinal study. Public Health Nutr. (2011) 14:709–15. doi: 10.1017/S1368980010001783
29. McAlindon, TE, Jacques, P, Zhang, Y, Hannan, MT, Aliabadi, P, Weissman, B, et al. Do antioxidant micronutrients protect against the development and progression of knee osteoarthritis? Arthritis Rheum. (1996) 39:648–56. doi: 10.1002/art.1780390417
30. Veen, L, Hantikainen, E, Bellocco, R, Ye, W, Serafini, M, Ponzano, M, et al. Dietary antioxidants, non-enzymatic antioxidant capacity and the risk of osteoarthritis in the Swedish National March Cohort. Eur J Nutr. (2021) 60:169–78. doi: 10.1007/s00394-020-02239-8
31. Li, H, Zeng, C, Wei, J, Yang, T, Gao, SG, Li, YS, et al. Associations between dietary antioxidants intake and radiographic knee osteoarthritis. Clin Rheumatol. (2016) 35:1585–92. doi: 10.1007/s10067-016-3177-1
32. Yao, Q, Wu, X, Tao, C, Gong, W, Chen, M, Qu, M, et al. Osteoarthritis: pathogenic signaling pathways and therapeutic targets. Signal Transduct Target Ther. (2023) 8:56. doi: 10.1038/s41392-023-01330-w
33. Spoelstra-de Man, AME, Elbers, PWG, and Oudemans-van Straaten, HM. Vitamin C: should we supplement? Curr Opin Crit Care. (2018) 24:248–55. doi: 10.1097/MCC.0000000000000510
Keywords: NHANES, vitamin C intake, osteoarthritis, Mendelian randomization, nutrition
Citation: Zhang H, Jiang X, Bai L, Chen J, Luo W, Ma J and Ma X (2024) Vitamin C intake and osteoarthritis: findings of NHANES 2003–2018 and Mendelian randomization study. Front. Nutr. 11:1409578. doi: 10.3389/fnut.2024.1409578
Edited by:
Robert Fred Clark, St. Jude Children’s Research Hospital, United StatesReviewed by:
Mayana Bsoul, Tulane University, United StatesLinshuoshuo Lyu, Vanderbilt University Medical Center, United States
Ye Li, Southern Medical University, China
Copyright © 2024 Zhang, Jiang, Bai, Chen, Luo, Ma and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinlong Ma, bWF4aW5sb25nQHRqdS5lZHUuY24=; Jianxiong Ma, bWp4OTY5QHRqdS5lZHUuY24=
†These authors have contributed equally to this work
 Hongjie Zhang
Hongjie Zhang Xuan Jiang3,4†
Xuan Jiang3,4† Jianxiong Ma
Jianxiong Ma Xinlong Ma
Xinlong Ma