- 1Department of Nephrology, Dongfang Hospital of Beijing University of Chinese Medicine, Beijing, China
- 2Graduate School of Beijing University of Chinese Medicine, Beijing, China
- 3Department of Nephrology, Tianjin Academy of Traditional Chinese Medicine Affiliated Hospital, Tianjin, China
- 4Tianjin Famous Chinese Medicine Inheritance Workshop of Mianzhi Zhang, Tianjin, China
Background: The oxidative balance score (OBS) is a comprehensive concept that includes 16 dietary components and four lifestyle factors to assess an individual's exposure to pro-oxidants and antioxidants. This study aims to explore the relationship between OBS and the risk of chronic kidney disease (CKD).
Methods: This cross-sectional study included nationally representative National Health and Nutrition Examination Survey (NHANES) participants aged 18 and above from 2005 to 2018. The OBS, a novel concept derived from multiple dietary (pro-oxidant and antioxidant nutrients) and lifestyle exposures (including smoking, alcohol consumption, obesity, and physical activity), serves as a useful tool for assessing an individual's oxidative stress status. The continuous variable OBS was converted into categorical variables by quartiles. Covariates included age, gender, race, education level, marital status, poverty-income ratio, sleep duration, depression, hypertension, diabetes, hyperlipidemia, cardiovascular disease, use of hypoglycemic medications, and use of antihypertensive medications. The relationship between OBS and CKD was explored using multiple logistic regression analysis and restricted cubic spline models. Additionally, subgroup analyses, interaction tests, and sensitivity analyses were conducted to validate the stability of the results.
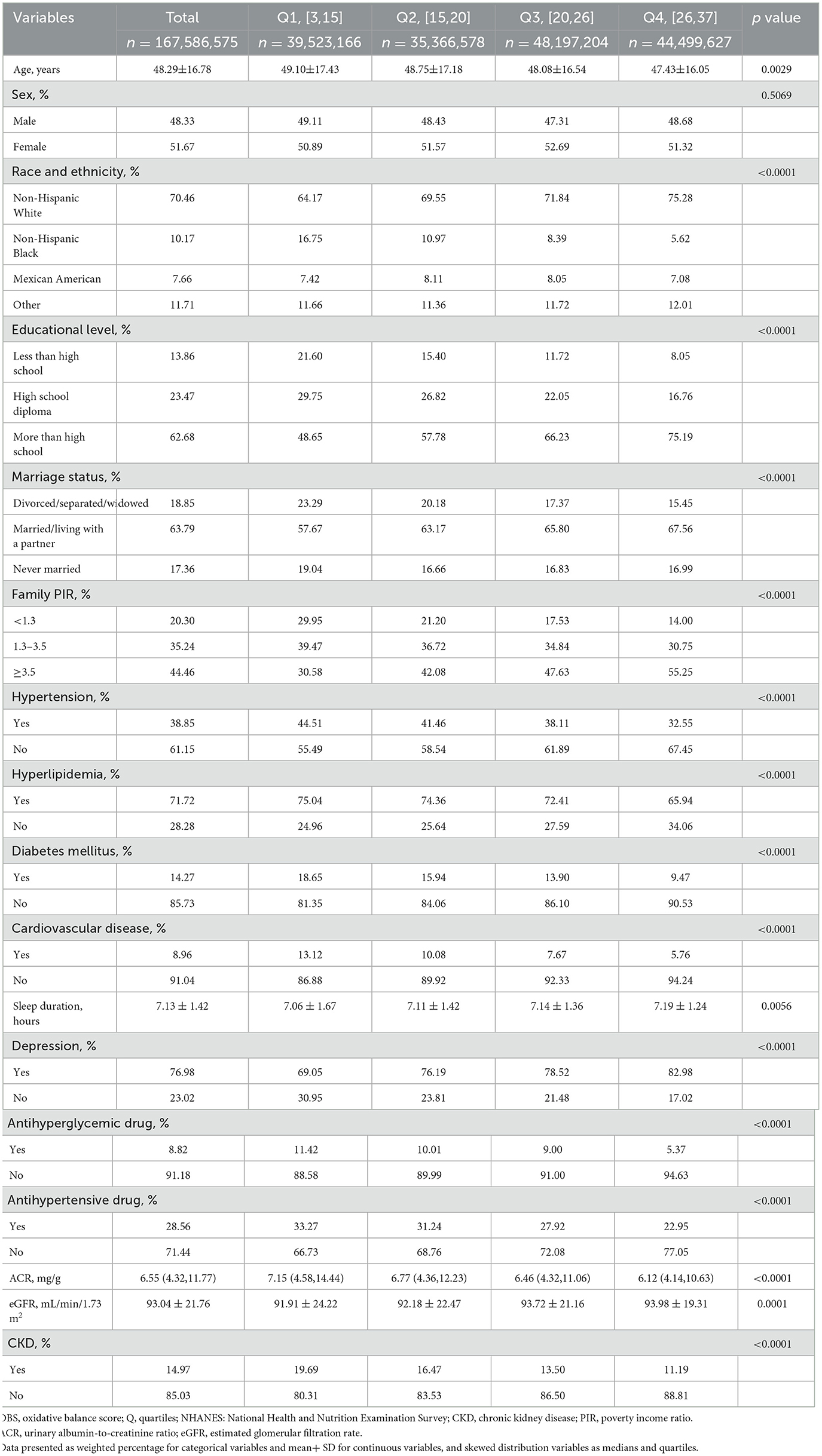
Results: A total of 25,118 NHANES participants were included in this study. The weighted prevalence of CKD was 14.97%. In the fully adjusted model, compared to the lowest OBS quartile, participants in the highest quartile had a 26% reduced risk of CKD (OR = 0.74, 95%CI: 0.63–0.87, p < 0.001). In restricted cubic spline regression, there was a linear association between OBS and CKD. The results of subgroup analysis and sensitivity analysis remain consistent. A significant interaction was found in the stratified analysis by age group (p for interaction = 0.012), suggesting that individuals older than 60 years may benefit more significantly from an increase in OBS scores compared to those aged 60 years or younger.
Conclusion: This study demonstrates that higher OBS is associated with a lower risk of CKD, particularly among the elderly population, providing innovative insights and preliminary evidence for the development of preventive strategies against CKD.
1 Introduction
Chronic kidney disease (CKD) has emerged as a significant public health issue globally, posing a threat to human health. Both early-stage CKD and complications of renal failure are highly prevalent, consuming substantial medical resources (1, 2). According to the latest Global Burden of Disease report (3), the global median prevalence of CKD was 9.5%, with the highest prevalence observed in Eastern and Central Europe. The annual median costs associated with kidney replacement therapy were: $19,380 per person for hemodialysis, $18,959 for peritoneal dialysis, and $26,903 for the first year of kidney transplantation. The escalating prevalence rates and the substantial socioeconomic burden imposed by end-stage renal disease underscore the urgency for physicians to actively adopt preventive strategies in clinical practice. However, there remains a lack of evidence-based lifestyle recommendations for primary prevention of CKD, emphasizing the need for innovative research on modifiable risk factors and preventive strategies.
Oxidative stress refers to the process of cellular oxidative damage induced by the accumulation of reactive oxygen species (ROS), which is a crucial factor in inducing renal fibrosis (4, 5). Elevated levels of oxidative stress are already present in the early stages of CKD (6, 7). As the disease progresses, the levels of pro-oxidant biomarkers gradually increase, while those of antioxidant biomarkers, such as glutathione peroxidase and carbonyl stress biomarkers, decrease (8, 9). However, measuring these biomarkers requires specialized techniques and is challenging to implement, thus limiting their clinical application. The high interconnectivity of the biological molecular network in the body also poses limitations, as it fails to comprehensively consider the intricate and interdependent interactions among biomolecules (10). Additionally, oxidative stress levels are influenced by various factors, including diet and lifestyle habits, rendering individual biomarkers insufficient to accurately reflect an individual's oxidative stress status. Thus, the oxidative balance score (OBS) (11) has been developed to evaluate an individual's exposure to pro-oxidants and antioxidants, with a higher score indicating greater antioxidant activity.
Previous studies have found that OBS is associated with a variety of diseases, including chronic obstructive pulmonary disease (12), kidney stones (13), and cardiovascular disease (CVD) (14), depression (15). A cohort study has shown that a higher OBS is associated with a lower risk of CKD development among the Korean population (16). However, two studies (17, 18) conducted in the US population have yielded inconsistent results, with Haibin Wen et al. suggesting a linear negative correlation between OBS and CKD without a clear inflection point, while Yuewei Yin et al. proposing a nonlinear negative relationship between the two. To further clarify the association between OBS and CKD and explore whether this correlation differs among populations with distinct characteristics, the present study aims to conduct a cross-sectional analysis using data from the National Health and Nutrition Examination Survey (NHANES), with the goal of providing new evidence-based insights into the potential of OBS as an innovative strategy for CKD prevention.
2 Materials and methods
2.1 Study population
This study is a nationwide cross-sectional research that draws data from the NHANES conducted during the 2005–2018 survey cycles. NHANES is administered by the National Center for Health Statistics, which employs a stratified multistage probability sampling technique to conduct household interviews, physical examinations, and laboratory tests among participants, aiming to assess the health and nutritional status of the non-institutionalized civilian population in the US. NHANES has received approval from the National Center for Health Statistics Ethics Review Committee, and all participants have provided written informed consent.
Participants in this study underwent screening based on the following criteria, individuals were excluded if (1) < 18 years; (2) incomplete data of CKD; (3) items comprising the OBS were < 16; (4) incomplete data of covariates.
2.2 Creatinine and albumin measurements and CKD
Serum and urinary creatinine were measured by the Jaffe rate method, and urinary albumin was measured by a solid-phase fluorescent immunoassay. For estimated glomerular filtration rate (eGFR) calculation, we applied the CKD-Epidemiology Collaboration (19) as follows: GFR = 141·min [Scr/κ,1]α × max [Scr/κ, 1] – 1.209 × 0.993 Age × 1.018 [if women] × 1.159 [if black]; κ was 0.7 (women) or 0.9 (men), a was −0.329 (women) or −0.411 (men), and min/max indicate the minimum/maximum of Scr/κ or 1. CKD (20) was diagnosed based on the low eGFR (< 60 mL/min/1.73 m2) or urinary albumin-to-creatinine ratio (ACR) of more than 30 mg/g.
2.3 OBS
OBS is calculated based on 16 dietary nutrients and four lifestyle factors. The dietary nutrients include: dietary fiber, carotene, riboflavin, niacin, vitamin B6, total folic acid, vitamin B12, vitamin C, vitamin E, calcium, magnesium, zinc, copper, selenium, total fat, and iron. The intake of these nutrients is obtained through 24-h dietary recall interviews, and the intake of each nutrient is calculated using the University of Texas Food Intake Analysis System and the US Department of Agriculture's Survey Nutrient Database. The four lifestyle factors include physical activity, body mass index (BMI), alcohol consumption, and smoking status, with smoking intensity represented by serum cotinine levels. Physical activity is calculated based on the total metabolic equivalent of task level of various exercises performed within a week (metabolic equivalent of each physical activity × frequency of physical activity × duration). Obesity scores are assigned based on weight status: obesity [BMI ≥ 30 kg per square meter (kg/m2)], overweight (25 ≤ BMI < 30 kg/m2), and normal weight (BMI < 25 kg/m2). Among these, pro-oxidant factors include total fat, total iron intake, smoking status, alcohol consumption, and obesity status, while the rest are antioxidant factors. The calculation of the OBS total score is as follows (Supplementary Table 1): alcohol consumption is categorized into three groups based on daily intake: non-drinkers, moderate drinkers (females 0–15 g/d, males 0–30 g/d), and heavy drinkers (females ≥ 15 g/d, males ≥ 30 g/d), with scores of 2, 1, and 0 respectively. Obesity, overweight, and normal weight are assigned scores of 0, 1, and 2 respectively. Other components are grouped into tertiles based on gender, with antioxidant factors in tertiles 1–3 assigned scores of 0–2, and pro-oxidant factors in tertiles 1–3 assigned scores of 2–0. The OBS total score is obtained by summing up the scores of all components, with a higher OBS total score indicating a higher level of antioxidant exposure.
2.4 Assessment of covariates
Based on previous studies (21–23), and in conjunction with our research objectives, potential covariates included age, gender, marital status (divorced/separated/widowed, married/living with a partner, never married), race/ethnicity (non-Hispanic White, non-Hispanic Black, Mexican American and other), education level (less than high school, high school diploma, more than high school), the family poverty income ratio [PIR(< 1.3, 1.3-3.5, >3.5)], sleep duration, depressive status (as assessed by The Patient Health Questionnaire), hypertension (taking antihypertensive medications, diagnosed by a doctor, systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥90 mmHg) (24), diabetes mellitus (using diabetes medications or insulin, diagnosed by a doctor, glycated hemoglobin HbA1c ≥6.5%, fasting glucose ≥7 mmol/L, random blood glucose ≥11.1 mmol/L, 2-h oral glucose tolerance test with blood glucose ≥11.1 mmol/L) (25), hyperlipidemia (taking cholesterol-lowering drugs, total cholesterol ≥200 mg/dL, triglyceride ≥150 mg/dL, low-density lipoprotein ≥130 mg/dL, or high-density lipoprotein ≤ 50 mg/dL for women and ≤ 40 mg/dL for men), CVD(coronary heart disease, congestive heart failure, heart attack, stroke, angina), use of hypoglycemic medications, and use of antihypertensive medications.
2.5 Statistical analyses
This study utilized R software (version 4.3.3) for all statistical analyses. Given the stratified and complex multi-stage sampling design of NHANES, we weighted the data according to the sample weighting method recommended by NHANES, combining data from 2005 to 2018, with the 14-year weight equal to 1/7 of the 2-year weight. Continuous variables are presented as mean ± sd, while categorical variables are represented as weighted proportions. A Shapiro-Wilk statistical test was employed to confirm the normal distribution of continuous variables. Variables with skewed distributions are presented as medians and quartiles. We categorized the continuous variable OBS into quartiles and used Weighted Analysis of Variance or Chi-square tests to assess differences between OBS (quantile) groups. Weighted Logistic regression models were employed to analyze the association between OBS and CKD, with results reported as odds ratios (OR) with their 95% confidence intervals (95% CI) and p-values, and trend p-values were calculated. Three models were used in this study: Model 1 was adjusted for sociodemographic characteristics including gender, age, marital status, race, education level, and family PIR; Model 2 further adjusted for comorbidities in Model 1: diabetes, hyperlipidemia, hypertension, CVD, and depression; Model 3 adjusted for all covariates. In addition to conducting subgroup analyses to explore the specific relationships between OBS and CKD in different gender, age, and status of diabetes, hypertension, hyperlipidemia, and CVD, we also performed interaction tests to determine whether the effect of OBS on CKD risk was consistent across populations with different characteristics, i.e., whether there were significant interactions. A multivariate-adjusted restricted cubic spline model with three knots was constructed to establish an OR curve, exploring the presence of a nonlinear dose-response association between OBS and CKD. Furthermore, to validate the stability of the results, after employing multiple imputation with five replications and the chained equation to handle missing data, the logistic regression results were compared between the imputed dataset and the dataset with original values. A two-sided p < 0.05 was considered statistically significant.
3 Results
3.1 Baseline characteristics
After subject screening (as shown in Figure 1), 25,118 NHANES participants were included in the study, representing 167.6 million non-institutionalized residents of the US. Table 1 presents the baseline characteristics of the study participants grouped according to OBS quartiles. The mean age of the participants was 48.29 ± 16.78 years, with 51.67% being female, and the weighted prevalence of CKD was 14.97%. Compared to the participants in the lowest OBS quartile, those in the highest OBS quartile were younger, had longer sleep duration, higher levels of education, were more likely to be married or have a partner, had higher wealth levels, and had a higher proportion of Non-Hispanic White individuals. The prevalence of hypertension, diabetes, hyperlipidemia, CVD, depression, and CKD also decreased with increasing OBS. There was no statistically significant difference in gender distribution between the different OBS groups.
3.2 Association between OBS and CKD
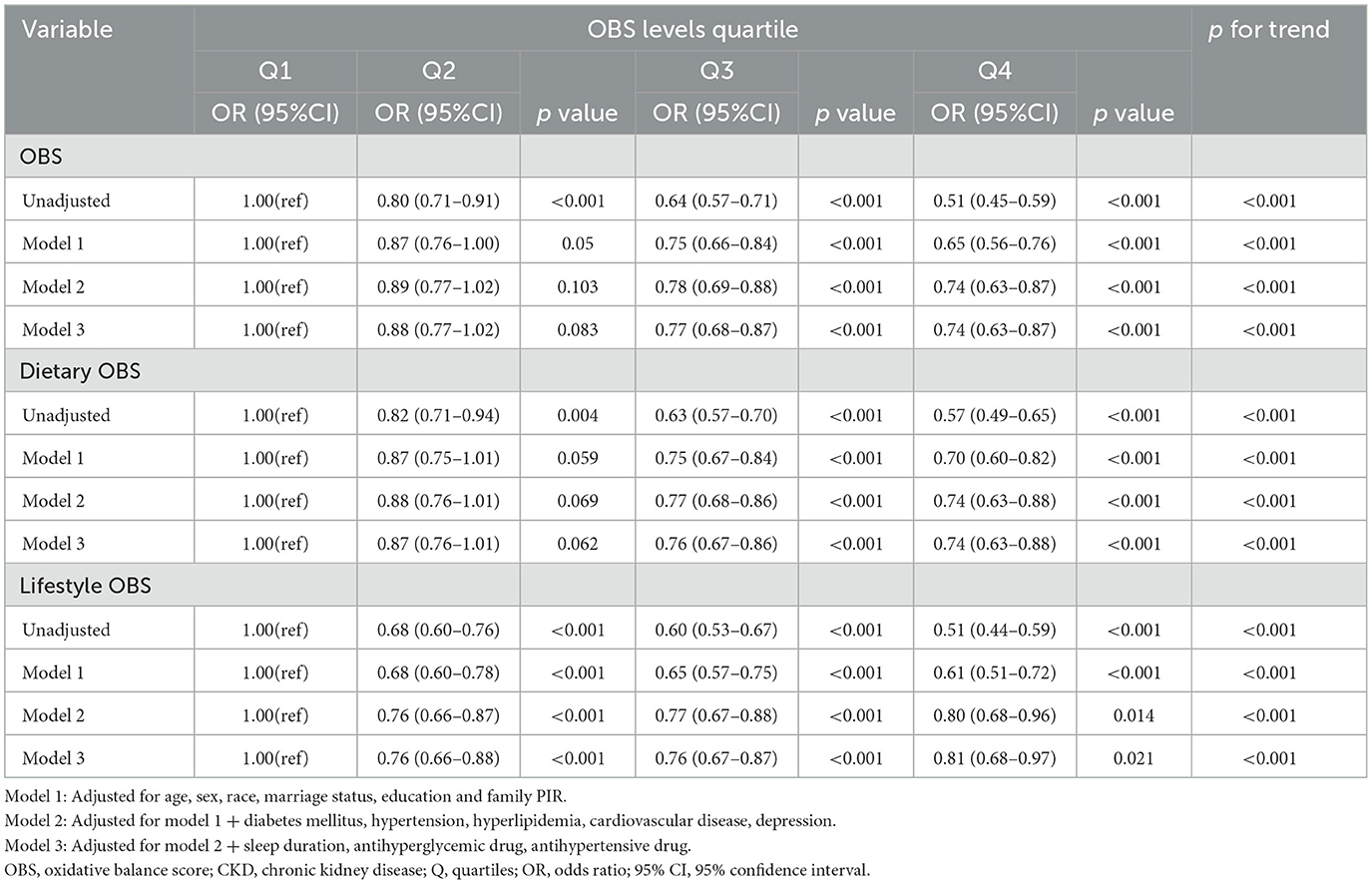
As shown in Table 2, weighted logistic regression analysis revealed a negative association between OBS and CKD. In the fully adjusted model, compared to the lowest OBS quartile, participants in the highest quartile had a 26% reduced risk of CKD (OR = 0.74, 95%CI: 0.63–0.87, p < 0.001). The trend remained relatively stable across different models. Additionally, compared to the lowest quartile, participants in the highest quartile of dietary OBS had a 26% reduced risk of CKD (OR = 0.74, 95%CI: 0.63-0.88, p < 0.001), and those in the highest quartile of lifestyle OBS had a 19% reduced risk of CKD (OR = 0.81, 95%CI: 0.68-0.97, p = 0.021).
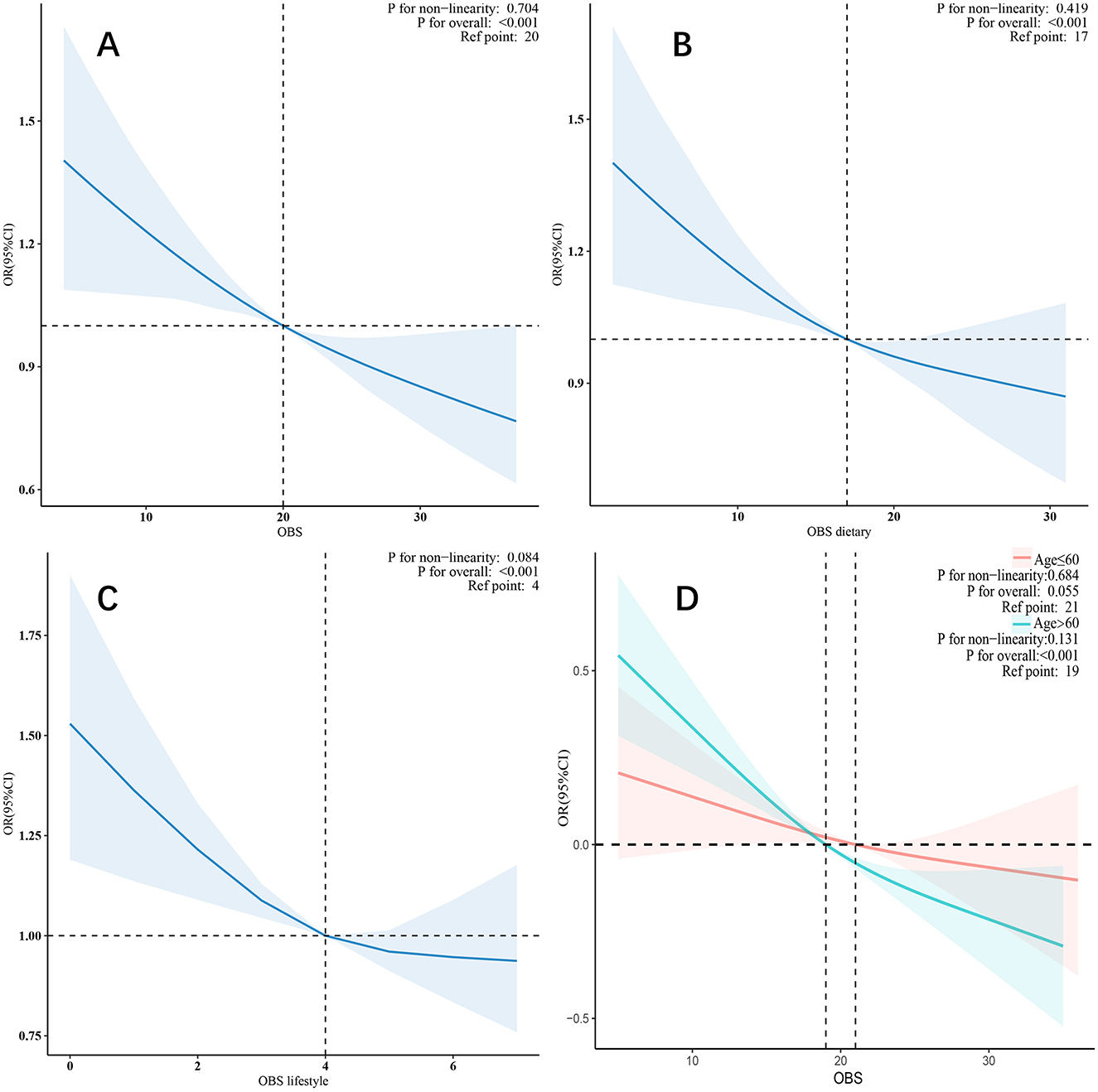
In the restricted cubic spline model, after adjusting for all covariates, a linear relationship was observed between OBS, dietary OBS, lifestyle OBS (Figures 2A–C), and CKD. As OBS increased, the risk of CKD gradually decreased.
3.3 Subgroup analyses and sensitivity analysis
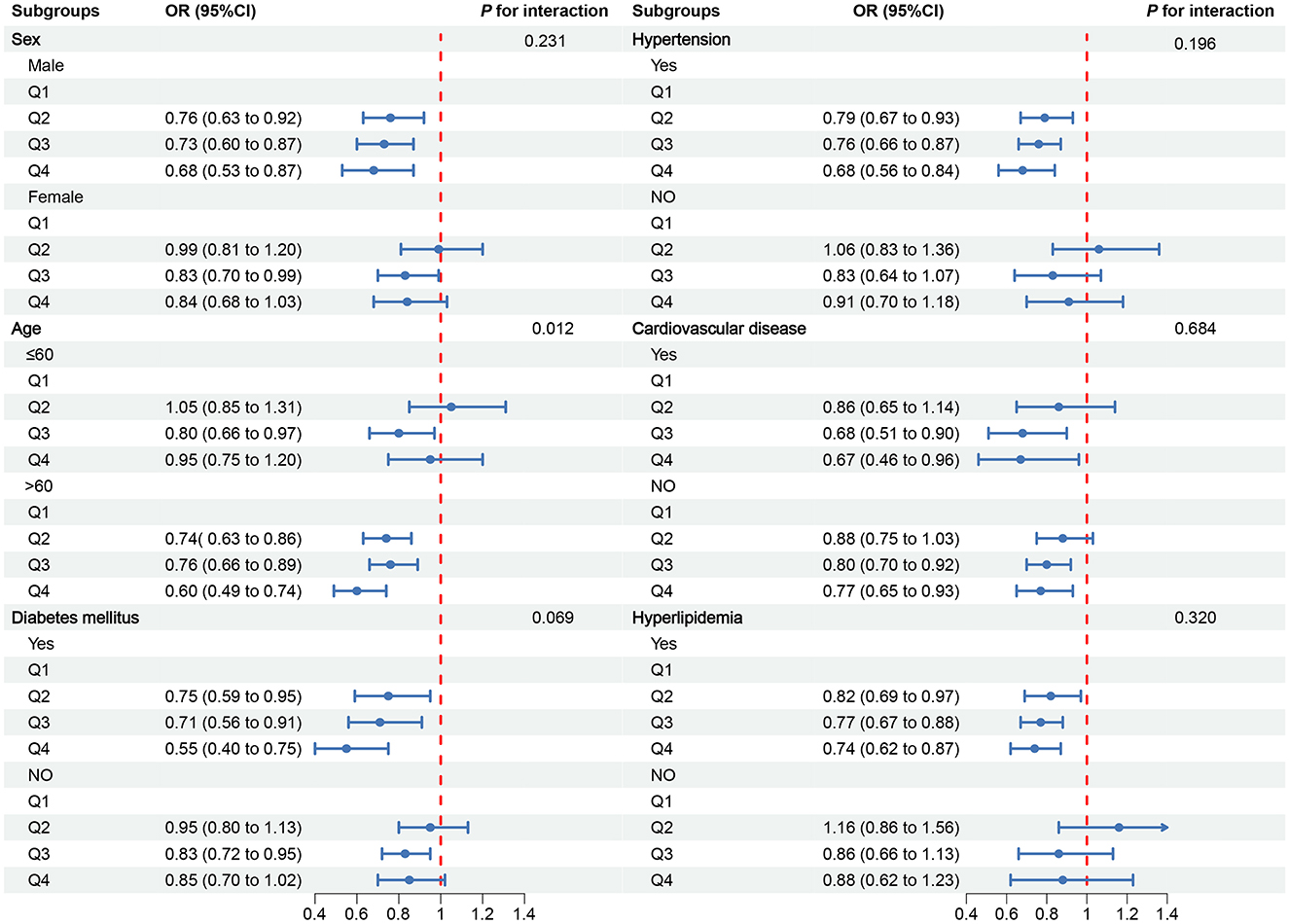
To further explore the relationship between OBS and CKD, we conducted subgroup analyses in different population contexts and tested for interactions (Figure 3). In the CVD subgroups, a negative correlation was observed between OBS and CKD. However, in other subgroups, the negative correlation was only observed in males, individuals older than 60 years, those with diabetes, hypertension, and hyperlipidemia. Notably, a significant interaction was found in the stratified analysis by age group (p for interaction = 0.012), suggesting that individuals older than 60 years may benefit more significantly from an increase in OBS compared to those aged 60 years or younger (Figure 2D).
To further validate the robustness of our findings, we applied multiple imputation methods to generate five imputed datasets. Multivariate logistic regression analyses were conducted on each of the five imputed datasets, and similar results were obtained in the fully adjusted models, confirming the negative association between OBS and the risk of CKD (Supplementary Table 2).
4 Discussion
We conducted a cross-sectional analysis involving 25,118 participants from the NHANES database. Our findings revealed a negative association between OBS and the risk of CKD, with this relationship remaining consistent across subgroup and sensitivity analyses. Furthermore, we observed a more pronounced correlation between OBS and CKD in the subgroup of participants aged over 60 years. Collectively, our study provides preliminary evidence for the exploration of the correlation between OBS and CKD, offering novel insights for future clinical and basic research endeavors.
OBS is an index that integrates 20 factors to assess pro-oxidants and antioxidants exposure. The kidney is rich in mitochondria and is highly susceptible to oxidative stress (26). Studies (9, 27, 28) have shown that patients with CKD have elevated levels of pro-oxidant biomarkers and significantly lower levels of antioxidant biomarkers. As a major source of bioactive components, diet mediates redox reactions and regulates the body's oxidative homeostatic system (29). Study (30) has reported that low molecular weight antioxidants, such as vitamin C, vitamin E, and carotenoids, have protective effects against chronic diseases, these antioxidants activate the Nrf2 antioxidant factor. Additionally, a large cohort study based on a UK sample bank showed that increased fiber intake is associated with a reduced risk of CKD. A systematic review (31) has suggested that diets rich in dietary fiber may slow the progression of CKD and its associated complications, and advocated the development of dietary plans high in dietary fiber for CKD patients. Additionally, a high-level intake of carotene may reduce the risk of death in CKD populations due to its antioxidant properties. Vitamin C, also known as ascorbic acid, is a crucial water-soluble antioxidant. Elevated serum levels of vitamin C have been shown to reduce the risk of albuminuria, low eGFR, and CKD (32). Appropriately increasing serum vitamin C levels may help protect renal function, especially in the elderly. Additionally, vitamin C may improve oxidative stress in patients with type 2 diabetes mellitus by modulating the immune response (33). Folic acid, betaine, vitamins B6 and B12 can also delay the progression of diabetes mellitus and protect the kidney by degrading homocysteine through methylation (34).
Exogenous oxidative balance is influenced by various lifestyle factors, including smoking, alcohol consumption, and physical activity in OBS (35). A meta-analysis (36) of 103 observational studies found that a diet high in potassium and low in sodium, as well as vegetables, moderate alcohol consumption, higher levels of physical activity, and cessation of smoking, all of which are components of OBS, can reduce the risk of CKD. Research (37) has demonstrated that physical activity can decrease the risk of kidney damage and rapid decline in kidney function through mechanisms such as up-regulating endothelial nitric oxide and other antioxidant enzymes (38, 39). Additionally, adopting a healthy diet and lifestyle can reduce the risk of obesity and overweight (40), which are also significant factors in the development of CKD (41). Furthermore, smoking is strongly linked to an increased risk of CKD and proteinuria. This is due to its potential to cause insulin resistance, advanced glycosylation end-products, and increased renal vascular permeability. Smoking is a risk factor for kidney damage especially in diabetic patients. Notably, multiple studies (42–44) have shown that moderate alcohol consumption is associated with a lower risk of CKD compared to no alcohol consumption or heavy drinking. However, considering the differences in the classification of alcohol intake in the world, as well as the potential risk of alcohol consumption to CVD, it is still not recommended for non-drinkers to start drinking, and the appropriate alcohol intake of the general population still needs further research to determine that once excessive alcohol consumption will lead to the production of a large amount of ROS, reduce the antioxidant activity of glutathione peroxidase (45) and cause damage to the kidneys and heart, drinking alcohol is still a risk factor that needs attention.
The subgroup analysis revealed a consistent negative correlation between OBS and CKD within the CVD subgroups. However, in other subgroups, this negative correlation was only significantly observed among males, individuals older than 60 years, and those with diabetes, hypertension, or hyperlipidemia. Notably, age-stratified analysis uncovered a significant interaction, which may be attributed to the increased accumulation of ROS and decreased antioxidant capacity with advancing age (46). Gender differences may stem from hormonal influences, as male androgen testosterone potentially exacerbates oxidative stress (47). Abnormalities in glucose metabolism, elevated blood pressure, and dyslipidemia can all diminish antioxidant enzyme activity and oxidative stress clearance capacity (48, 49). Despite controlling for covariates to minimize interference during analysis, the limited sample size remains a potential limitation, potentially contributing to the randomness and uncertainty of the results. Therefore, these conclusions should be treated with caution, and further research is warranted to validate these preliminary findings.
This study boasts several strengths. Firstly, assessing OBS through dietary and lifestyle questionnaires provides an economical and efficient means to evaluate the balance between exogenous pro-oxidants and antioxidants. Our findings of a significant correlation between OBS and CKD, particularly in individuals over 60, pave the way for future longitudinal studies to explore the mechanisms underlying OBS's impact on CKD and ascertain age as a crucial factor. Once these results are further confirmed and expanded, they may inform or refine public health policies, such as emphasizing specific antioxidant-rich diets and lifestyles in the health management of elderly populations. Secondly, NHANES's utilization of a multistage complex probability sampling method enhances the generalizability of our findings within the US context.
However, this study also acknowledges several limitations. Firstly, antioxidants exhibit a threshold effect, where excess levels may exhibit pro-oxidant properties. Yet, OBS is defined under the assumption of a linear relationship between all components and oxidative stress, and its assessment via questionnaires is prone to recall and selection biases in reporting nutrient intake. Secondly, as this study was conducted in the US population, the racial universality of our findings remains to be further validated. Thirdly, despite constructing multivariate logistic regression models and conducting subgroup and sensitivity analyses to mitigate confounding factors, residual confounding effects cannot be entirely ruled out. Lastly, as a cross-sectional study, this research cannot establish a causal relationship between OBS and CKD, highlighting the need for more prospective studies in this area.
5 Conclusions
This study demonstrates that higher OBS is associated with a lower risk of CKD, particularly among the elderly. This suggests that OBS has the potential to serve as an important reference in clinical practice for structuring dietary patterns and lifestyles, offering innovative insights and preliminary evidence for developing preventive strategies against CKD. Nevertheless, further large-scale prospective studies are warranted to validate and expand upon our findings.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.cdc.gov/nchs/nhanes/index.htm.
Ethics statement
The studies involving humans were approved by NHANES was approved by the Ethics Review Board of the NCHS, and all participants submitted informed consent forms. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CL: Data curation, Formal analysis, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. JY: Conceptualization, Data curation, Formal analysis, Methodology, Writing – review & editing. HL: Conceptualization, Methodology, Visualization, Writing – review & editing. YD: Formal analysis, Software, Visualization, Writing – review & editing. PH: Conceptualization, Methodology, Validation, Visualization, Writing – review & editing. JZ: Validation, Writing – review & editing. MZ: Data curation, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by Project of Tianjin Academy of Traditional Chinese Medicine Affiliated Hospital (No. 403245).
Acknowledgments
The authors thank the staff and the participants of the NHANES study for their valuable contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1406780/full#supplementary-material
References
1. Honeycutt AA, Segel JE, Zhuo X, Hoerger TJ, Imai K, Williams D. Medical costs of CKD in the Medicare population. J Am Soc Nephrol. (2013) 24:1478–83. doi: 10.1681/ASN.2012040392
2. Manns B, Hemmelgarn B, Tonelli M, Au F, So H, Weaver R, et al. The cost of care for people with chronic kidney disease. Can J Kidney Health Dis. (2019) 6:2047252607. doi: 10.1177/2054358119835521
3. Bello AK, Okpechi IG, Levin A, Ye F, Damster S, Arruebo S, et al. An update on the global disparities in kidney disease burden and care across world countries and regions. Lancet Glob Health. (2024) 12:e382–95. doi: 10.1016/S2214-109X(23)00570-3
4. Tucker PS, Dalbo VJ, Han T, Kingsley MI. Clinical and research markers of oxidative stress in chronic kidney disease. Biomarkers. (2013) 18:103–15. doi: 10.3109/1354750X.2012.749302
5. Oberg BP, McMenamin E, Lucas FL, McMonagle E, Morrow J, Ikizler TA, et al. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. (2004) 65:1009–16. doi: 10.1111/j.1523-1755.2004.00465.x
6. Tamma G, Valenti G. Evaluating the oxidative stress in renal diseases: what is the role for S-glutathionylation? Antioxid Redox Signal. (2016) 25:147–64. doi: 10.1089/ars.2016.6656
7. Krata N, Zagozdzon R, Foroncewicz B, Mucha K. Oxidative stress in kidney diseases: the cause or the consequence? Arch Immunol Ther Exp (Warsz). (2018) 66:211–20. doi: 10.1007/s00005-017-0496-0
8. Palathingal P, Mahendra J, Annamalai PT, Varma SS, Mahendra L, Thomas L, et al. A cross-sectional study of serum glutathione peroxidase: an antioxidative marker in chronic periodontitis and chronic kidney disease. Cureus. (2022) 14:e22016. doi: 10.7759/cureus.22016
9. Aveles PR, Criminacio CR, Goncalves S, Bignelli AT, Claro LM, Siqueira SS, et al. Association between biomarkers of carbonyl stress with increased systemic inflammatory response in different stages of chronic kidney disease and after renal transplantation. Nephron Clin Pract. (2010) 116:c294–9. doi: 10.1159/000318792
10. Andres-Blasco I, Gallego-Martinez A, Machado X, Cruz-Espinosa J, Di Lauro S, Casaroli-Marano R, et al. Oxidative stress, inflammatory, angiogenic, and apoptotic molecules in proliferative diabetic retinopathy and diabetic macular edema patients. Int J Mol Sci. (2023) 24:8227. doi: 10.3390/ijms24098227
11. Zhang W, Peng SF, Chen L, Chen HM, Cheng XE, Tang YH. Association between the oxidative balance score and telomere length from the National Health and Nutrition Examination Survey 1999-2002. Oxid Med Cell Longev. (2022) 2022:1345071. doi: 10.1155/2022/1345071
12. He X, Lin X, He B, Xu H, Suo Z, Zhang H. Association between oxidative balance score and frailty in chronic obstructive pulmonary disease. Heliyon. (2024) 10:e25750. doi: 10.1016/j.heliyon.2024.e25750
13. Chen Q, Bao W, Kong X, Zhu J, Hou S, Zhang Y, et al. Association between the oxidative balance score and kidney stones in adults. World J Urol. (2024) 42:425. doi: 10.1007/s00345-024-05144-5
14. Chen X, Wang C, Dong Z, Luo H, Ye C, Li L, et al. Interplay of sleep patterns and oxidative balance score on total cardiovascular disease risk: insights from the National Health and Nutrition Examination Survey 2005-2018. J Glob Health. (2023) 13:4170. doi: 10.7189/jogh.14.04170
15. Liu X, Liu X, Wang Y, Zeng B, Zhu B, Dai F. Association between depression and oxidative balance score: National Health and Nutrition Examination Survey (NHANES) 2005-2018. J Affect Disord. (2023) 337:57–65. doi: 10.1016/j.jad.2023.05.071
16. Son DH, Lee HS, Seol SY, Lee YJ, Lee JH. Association between the oxidative balance score and incident chronic kidney disease in adults. Antioxidants (Basel). (2023) 12:335. doi: 10.3390/antiox12020335
17. Wen H, Li X, Chen J, Li Y, Yang N, Tan N. Association of oxidative balance score with chronic kidney disease: NHANES 1999-2018. Front Endocrinol. (2024) 15:1396465. doi: 10.3389/fendo.2024.1396465
18. Yin Y, Zhao C, Niu Y, Qi J, Zhang Y, Lu B. Associations between oxidative balance score and chronic kidney disease events in US adults: a population-based study. Sci Rep. (2024) 14:13743. doi: 10.1038/s41598-024-64147-9
19. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AR, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
20. KDIGO 2021. Clinical practice guideline for the management of glomerular diseases. Kidney Int. (2021) 100: S1–276. doi: 10.1016/j.kint.2021.05.021
21. Kang H, Lee JP, Choi K. Exposure to phthalates and environmental phenols in association with chronic kidney disease (CKD) among the general US population participating in multi-cycle NHANES (2005-2016). Sci Total Environ. (2021) 791:148343. doi: 10.1016/j.scitotenv.2021.148343
22. Li J, Huang Z, Hou J, Sawyer AM, Wu Z, Cai J, et al. Sleep and CKD in Chinese adults: a cross-sectional study. Clin J Am Soc Nephrol. (2017) 12:885–92. doi: 10.2215/CJN.09270816
23. Qu S, Fang J, Zhao S, Wang Y, Gao W, Li Z, et al. Associations of dietary inflammatory index with low estimated glomerular filtration rate, albuminuria and chronic kidney disease in U.S adults: Results from the NHANES 2011-2018. Nutr Metab Cardiovasc Dis. (2024) 34:1036–45. doi: 10.1016/j.numecd.2023.11.006
24. Xiao Y, Xiao Z. Association between Serum Klotho and kidney stones in US middle-aged and older individuals with diabetes mellitus: results from 2007 to 2016 National Health and Nutrition Survey. Am J Nephrol. (2023) 54:224–33. doi: 10.1159/000531045
25. Liu H, Wang D, Wu F, Dong Z, Yu S. Association between inflammatory potential of diet and self-reported severe headache or migraine: a cross-sectional study of the National Health and Nutrition Examination Survey. Nutrition. (2023) 113:112098. doi: 10.1016/j.nut.2023.112098
26. Che R, Yuan Y, Huang S, Zhang A. Mitochondrial dysfunction in the pathophysiology of renal diseases. Am J Physiol Renal Physiol. (2014) 306:F367–78. doi: 10.1152/ajprenal.00571.2013
27. Liakopoulos V, Roumeliotis S, Gorny X, Dounousi E, Mertens PR. Oxidative stress in hemodialysis patients: a review of the literature. Oxid Med Cell Longev. (2017) 2017:3081856. doi: 10.1155/2017/3081856
28. Kuchta A, Pacanis A, Kortas-Stempak B, Cwiklinska A, Zietkiewicz M, Renke M, et al. Estimation of oxidative stress markers in chronic kidney disease. Kidney Blood Press Res. (2011) 34:12–9. doi: 10.1159/000321508
29. Goodman M, Bostick RM, Dash C, Flanders WD, Mandel JS. Hypothesis: oxidative stress score as a combined measure of pro-oxidant and antioxidant exposures. Ann Epidemiol. (2007) 17:394–9. doi: 10.1016/j.annepidem.2007.01.034
30. Jomova K, Alomar SY, Alwasel SH, Nepovimova E, Kuca K, Valko M. Several lines of antioxidant defense against oxidative stress: antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch Toxicol. (2024). doi: 10.1007/s00204-024-03696-4
31. Su G, Qin X, Yang C, Sabatino A, Kelly JT, Avesani CM, et al. Fiber intake and health in people with chronic kidney disease. Clin Kidney J. (2022) 15:213–25. doi: 10.1093/ckj/sfab169
32. Wang C, Zhao J, Zhou Q, Li J. Serum vitamin C levels and their correlation with chronic kidney disease in adults: a nationwide study. Ren Fail. (2024) 46:2298079. doi: 10.1080/0886022X.2023.2298079
33. Mahdavi A, Leclercq M, Droit A, Rudkowska I, Lebel M. Predictive model for vitamin C levels in hyperinsulinemic individuals based on age, sex, waist circumference, low-density lipoprotein, and immune-associated serum proteins. J Nutr Biochem. (2024) 125:109538. doi: 10.1016/j.jnutbio.2023.109538
34. Zhu J, Saikia G, Zhang X, Shen X, Kahe K. One-carbon metabolism nutrients, genetic variation, and diabetes mellitus. Diabetes Metab J. (2024). doi: 10.4093/dmj.2023.0272
35. Xia J, Wang L, Ma Z, Zhong L, Wang Y, Gao Y, et al. Cigarette smoking and chronic kidney disease in the general population: a systematic review and meta-analysis of prospective cohort studies. Nephrol Dial Transplant. (2017) 32:475–87. doi: 10.1093/ndt/gfw452
36. Kelly JT, Su G, Zhang L, Qin X, Marshall S, Gonzalez-Ortiz A, et al. Modifiable lifestyle factors for primary prevention of CKD: a systematic review and meta-analysis. J Am Soc Nephrol. (2021) 32:239–53. doi: 10.1681/ASN.2020030384
37. Kubota Y, Iso H, Yamagishi K, Sawada N, Tsugane S. Daily total physical activity and incident cardiovascular disease in Japanese men and women: japan public health center-based prospective study. Circulation. (2017) 135:1471–3. doi: 10.1161/CIRCULATIONAHA.116.026557
38. Chomistek AK, Cook NR, Rimm EB, Ridker PM, Buring JE, Lee IM. Physical activity and incident cardiovascular disease in women: is the relation modified by level of global cardiovascular risk? J Am Heart Assoc. (2018) 7:8234. doi: 10.1161/JAHA.117.008234
39. Kanbay M, Copur S, Yildiz AB, Tanriover C, Mallamaci F, Zoccali C. Physical exercise in kidney disease: a commonly undervalued treatment modality. Eur J Clin Invest. (2024) 54:e14105. doi: 10.1111/eci.14105
40. Mu M, Xu LF, Hu D, Wu J, Bai MJ. Dietary patterns and overweight/obesity: a review article. Iran J Public Health. (2017) 46:869–76.
41. Garofalo C, Borrelli S, Minutolo R, Chiodini P, De Nicola L, Conte G, et al. systematic review and meta-analysis suggests obesity predicts onset of chronic kidney disease in the general population. Kidney Int. (2017) 91:1224–35. doi: 10.1016/j.kint.2016.12.013
42. Fan Z, Yun J, Yu S, Yang Q, Song L. Alcohol consumption can be a “double-edged sword” for chronic kidney disease patients. Med Sci Monit. (2019) 25:7059–72. doi: 10.12659/MSM.916121
43. Dunkler D, Kohl M, Heinze G, Teo KK, Rosengren A, Pogue J, et al. Modifiable lifestyle and social factors affect chronic kidney disease in high-risk individuals with type 2 diabetes mellitus. Kidney Int. (2015) 87:784–91. doi: 10.1038/ki.2014.370
44. Dunkler D, Dehghan M, Teo KK, Heinze G, Gao P, Kohl M, et al. Diet and kidney disease in high-risk individuals with type 2 diabetes mellitus. JAMA Intern Med. (2013) 173:1682–92. doi: 10.1001/jamainternmed.2013.9051
45. Ojeda ML, Nogales F, Del CGM, Carreras O. Binge drinking during the adolescence period causes oxidative damage-induced cardiometabolic disorders: a possible ameliorative approach with selenium supplementation. Life Sci. (2022) 301:120618. doi: 10.1016/j.lfs.2022.120618
46. Hajam YA, Rani R, Ganie SY, Sheikh TA, Javaid D, Qadri SS, et al. Oxidative stress in human pathology and aging: molecular mechanisms and perspectives. Cells. (2022) 11:552. doi: 10.3390/cells11030552
47. Liu S, Navarro G, Mauvais-Jarvis F. Androgen excess produces systemic oxidative stress and predisposes to beta-cell failure in female mice. PLoS ONE. (2010) 5:e11302. doi: 10.1371/journal.pone.0011302
48. Montezano AC, Dulak-Lis M, Tsiropoulou S, Harvey A, Briones AM, Touyz RM. Oxidative stress and human hypertension: vascular mechanisms, biomarkers, and novel therapies. Can J Cardiol. (2015) 31:631–41. doi: 10.1016/j.cjca.2015.02.008
Keywords: chronic kidney disease, oxidative balance score, NHANES, a cross-sectional study, dietary components
Citation: Liu C, Yang J, Li H, Deng Y, He P, Zhang J and Zhang M (2025) Association between chronic kidney disease and oxidative balance score: National Health and Nutrition Examination Survey (NHANES) 2005–2018. Front. Nutr. 11:1406780. doi: 10.3389/fnut.2024.1406780
Received: 25 March 2024; Accepted: 04 December 2024;
Published: 03 January 2025.
Edited by:
Munkhtuya Tumurkhuu, Wake Forest Baptist Medical Center, United StatesReviewed by:
Nicolas Padilla-Raygoza, Institute of Public Health of the State of Guanajuato (ISAPEG), MexicoCuauhtemoc Sandoval Salazar, University of Guanajuato, Mexico
Copyright © 2025 Liu, Yang, Li, Deng, He, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mianzhi Zhang, emhhbmdtaWFuemhpQHZpcC5zaW5hLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Cong Liu
Cong Liu Jiju Yang
Jiju Yang Hongdian Li1
Hongdian Li1