- 1Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
- 2Tongzhou District Branch, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
Background: Psoriasis is a chronic inflammatory skin disease characterized primarily by erythema and scales, having a wide-ranging impact globally. Previous studies have suggested that dietary consumption habits may influence psoriasis. The objective of this study was to determine the causal relationship between dietary consumption habits and psoriasis using the Mendelian Randomization (MR) approach.
Methods: SNP data for 29 dietary consumption habits and psoriasis were obtained from the GWAS catalog database and the FinnGen database, respectively. The Mendelian Randomization analysis was performed using R software, with the 29 dietary consumption habits as the exposure factors and psoriasis as the outcome. Three MR analysis methods—Inverse Variance Weighted (IVW), Weighted Median Estimator (WME), and MR-Egger regression—were employed to study the causal relationship between dietary consumption habits and psoriasis.
Results: The IVW analysis indicated an OR (95%CI) of 0.065 (0.008–0.555), p = 0.012, demonstrating a negative correlation between the consumption of dried fruit and psoriasis.
Conclusion: Among the 29 dietary consumption habits analyzed, the intake of dried fruits is a protective factor against psoriasis. Therefore, it is clinically advisable to appropriately increase the intake of dried fruits among patients with psoriasis, serving as a nutritional therapy method in conjunction with pharmacological treatment.
1 Introduction
Psoriasis is a chronic inflammatory skin disease characterized mainly by erythema and scales, representing an immune-mediated condition against a genetic background. The global prevalence among adults ranges from 0.09 to 11.43%, and between 0.0 to 1.3% in children, with an average prevalence rate of 2% (1), affecting the mental and physical health of approximately 125 million people worldwide (2). The World Health Organization has recognized this disease as a global issue, significantly disrupting the normal social interactions of patients, increasing their economic burden, and reducing their quality of life.
As psoriasis gradually becomes a burdensome global medical condition (3, 4), identifying potential risk factors triggering the disease has increasingly become a focus of attention. Infections, dysbiosis of the gut microbiome, psychological stress, and drugs are common risk factors for psoriasis, usually interacting with other risk factors, including genetic predispositions (5). In recent years, there has been a growing interest in exploring the association between dietary factors and psoriasis to determine whether they act as risk or protective factors. To date, more than one study has investigated the relationship between dietary consumption habits and psoriasis. Patients with obesity and psoriasis who followed a low-calorie diet of ≤1,000 kcal/day for 8 weeks exhibited a significantly greater reduction in the average Psoriasis Area and Severity Index (PASI) score compared to the control group (84% vs. 69%) (6). Studies by others (7) have similarly found that, under the same Mediterranean diet, the intake of berries, olive oil, nuts, and eggs by psoriasis patients was significantly lower than that of the control group, with psoriasis patients consuming more dairy products and soft drinks. However, due to the susceptibility of clinical case–control studies to confounding factors, the association between dietary consumption habits and psoriasis remains not very clear.
Mendelian randomization is a research method that examines the relationship between exposure factors and outcomes through genetic variants associated with the exposure (8). Compared to randomized controlled trials and case–control studies, it offers broad applicability, reduced confounding bias, ethical compliance, and low cost (9). Previous studies have indicated that low-calorie, Mediterranean, and restricted protein/vegetarian diets may all be beneficial dietary patterns for improving psoriasis (10). This study employs the two-sample Mendelian randomization approach to test the causal relationship between 29 dietary consumption habits and psoriasis, providing a reference for developing scientific dietary strategies for the prevention and improvement of psoriasis.
2 Materials and methods
2.1 Acquisition of Instrumental Variables
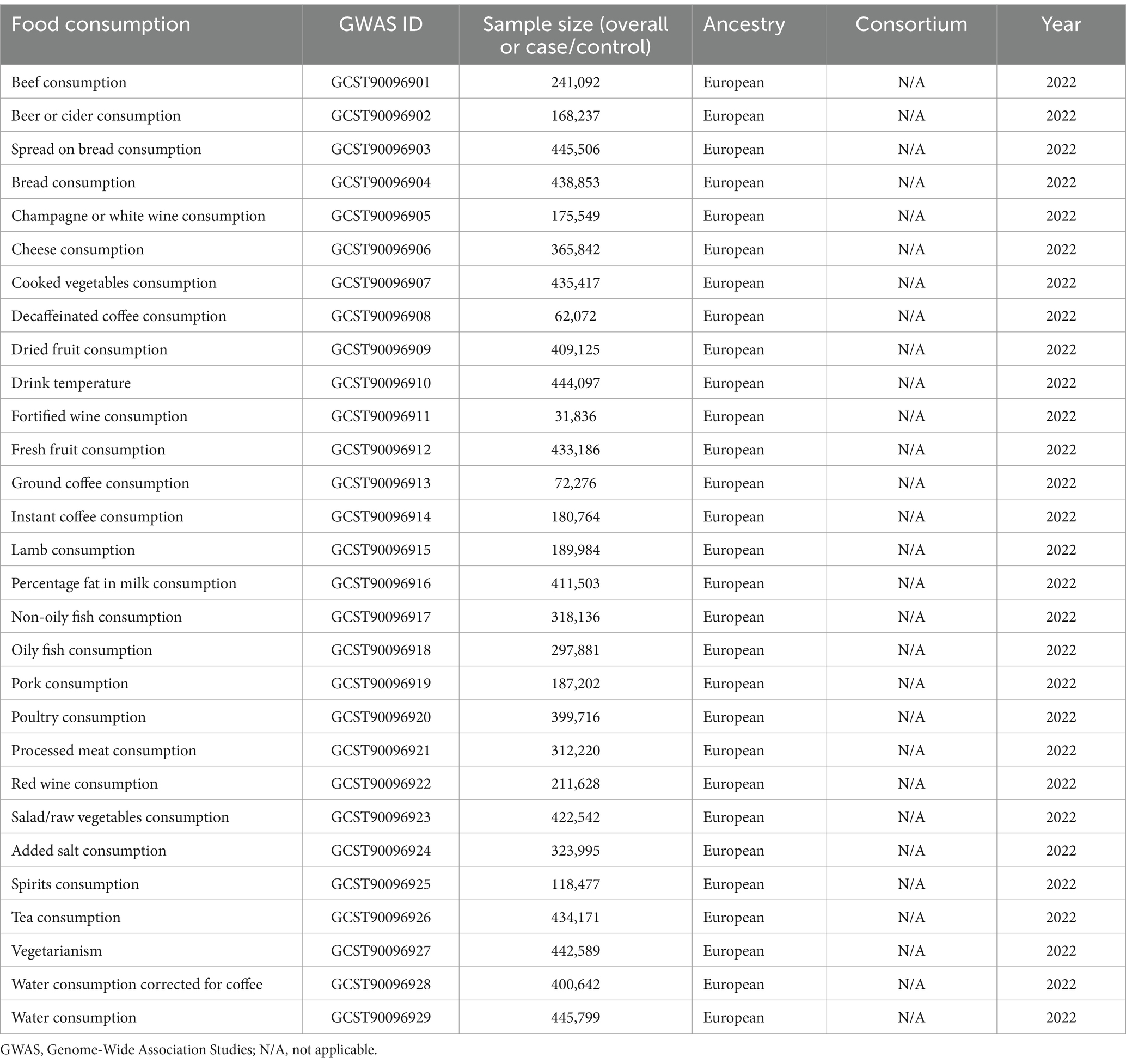
The keywords “food consumption” and “psoriasis” were used to search in the GWAS catalog database1 and FinnGen database.2 The SNP dataset for dietary consumption habits was derived from the study by Pirastu N et al. in the GWAS catalog database. The dataset is presented in Table 1 (11); the SNP dataset for psoriasis was obtained from the FinnGen database, including 407,876 samples, of which 10,312 were cases and 397,564 controls (12). All sample populations were of European ancestry to avoid potential biases due to racial factors. All data used have been published in the public domain and have obtained informed consent from participants. The research protocol was approved by local, regional, or institutional ethics committees, and no additional ethical approval was required.
2.2 Selection of instrumental variables
Since Mendelian Randomization (MR) requires that the chosen instrumental variables meet three criteria: the instrumental variables must be strongly associated with the exposure factor, independent of confounding factors, and affect the outcome variable only through the exposure factor. We first set p < 5 × 10−8 and F > 10 to select SNPs strongly associated with the exposure factor, thereby avoiding the impact of weak instrumental variables. Secondly, we set an r2 threshold of 0.001 and a base pair region range of 10,000 kb to ensure there is no linkage disequilibrium in the results. Finally, the PhenoScanner database was utilized to exclude confounding factors (Figure 1).
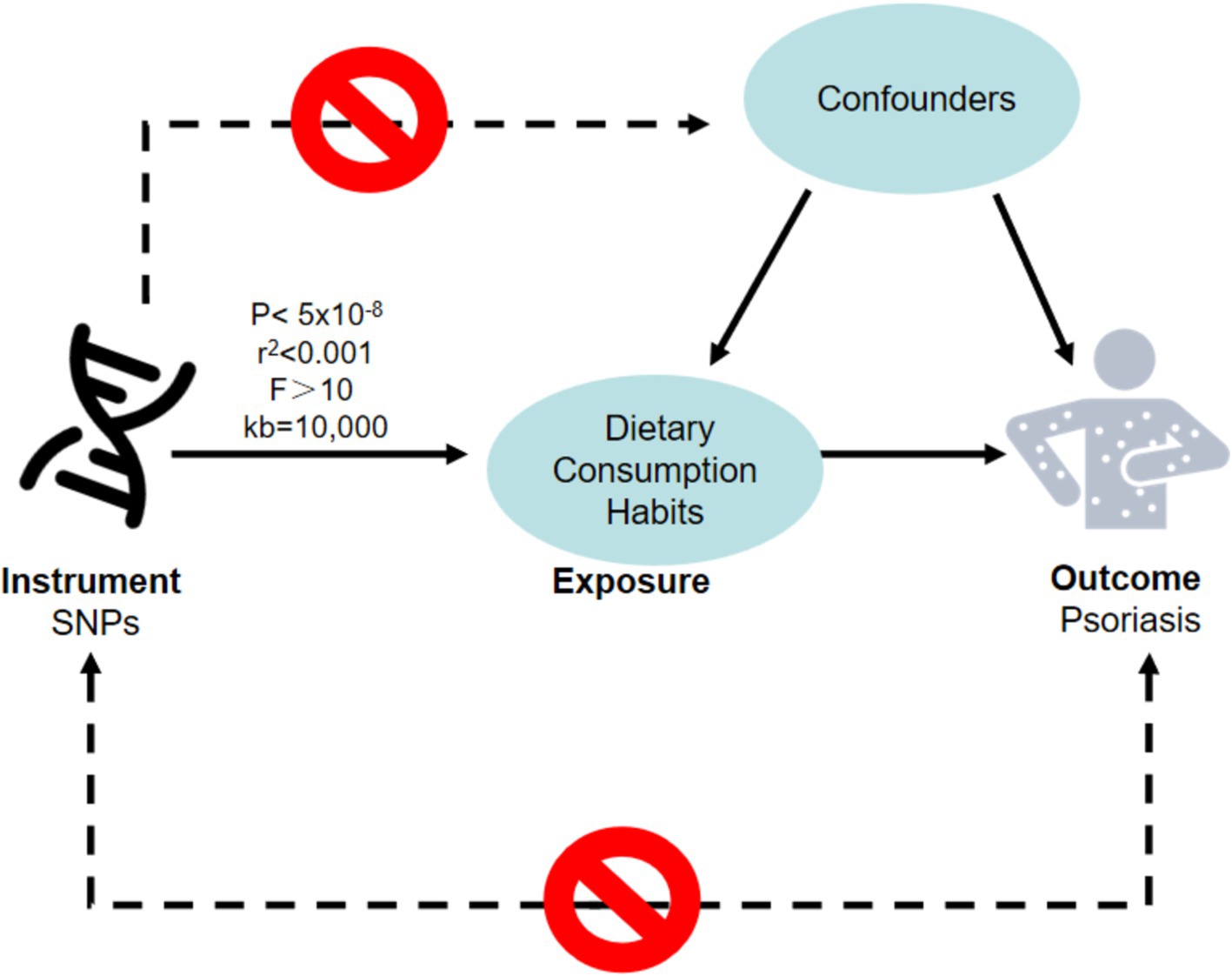
Figure 1. The hypotheses that must be met for Mendelian randomization studies. A node represents a variable, a solid line represents MR analysis processes and can only influence the outcome by exposure and a dashed line represents a causal relationship is prohibited. MR, Mendelian randomization; SNPs, single nucleotide polymorphisms.
2.3 Mendelian randomization analysis
This study employed the “TwoSampleMR” package in R 4.2.1, integrating three Mendelian Randomization analysis methods to analyze the association between 29 dietary consumption habits and psoriasis. These methods are the Inverse-Variance Weighted (IVW) method, MR-Egger regression, and the Weighted Median Estimator (WME), with the IVW results generally serving as the primary reference, while the other two methods provide secondary evidence. Heterogeneity was tested using the Inverse-Variance Weighted and MR-Egger methods combined with Cochran’s Q test. A p-value <0.05 indicates the presence of heterogeneity, which can impact the analysis results to some extent. The intercept obtained through the MR-Egger method was further analyzed to test for horizontal pleiotropy. If p < 0.05, the data exhibit pleiotropy, and the analysis results cannot meet the basic requirements of Mendelian Randomization. Finally, a leave-one-out approach was employed to sequentially exclude each SNP and analyze the remaining SNPs to identify any with a significant impact on the results. Procedures and methods as seen in Figure 2.
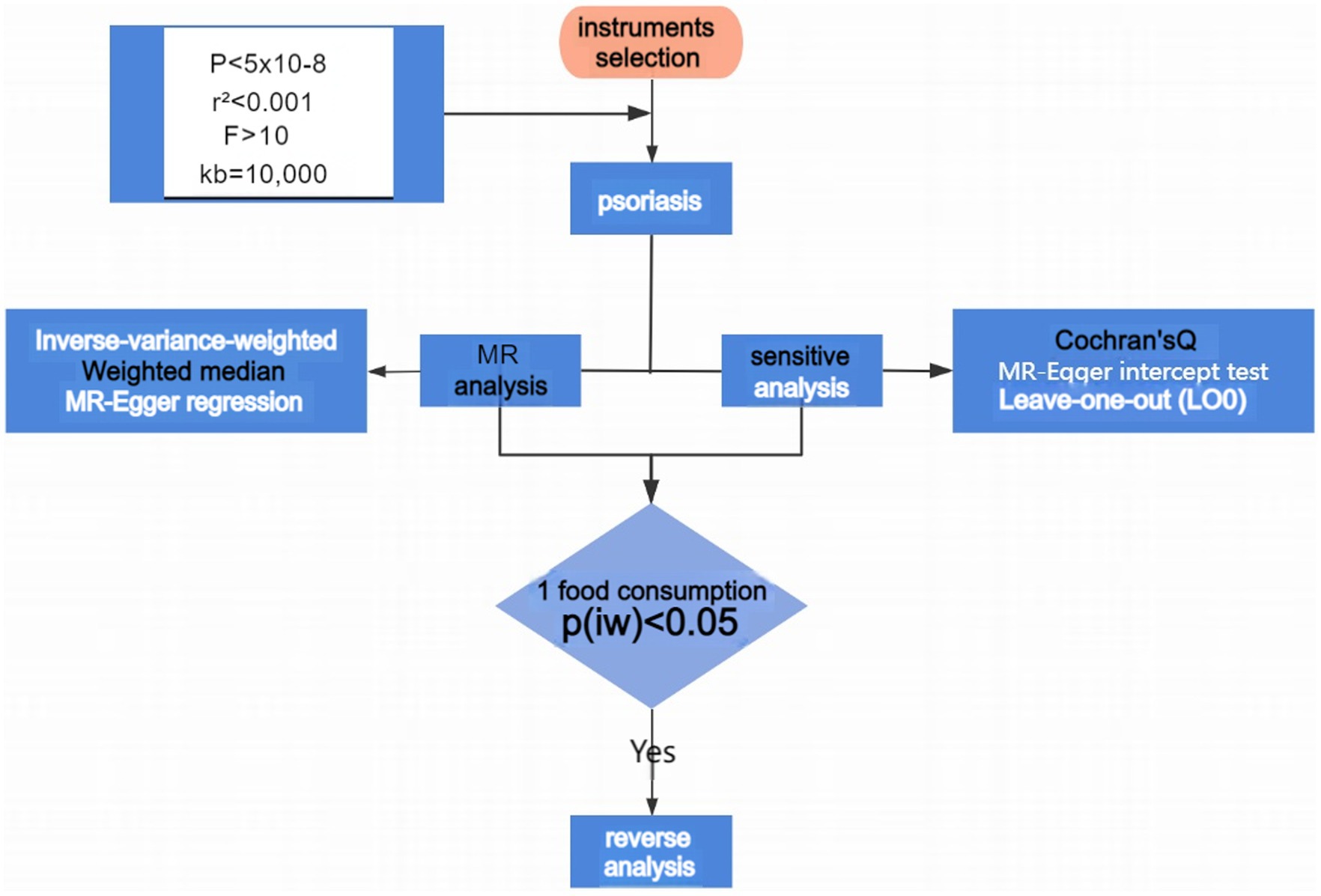
Figure 2. Flowchart of MR analyses in this study. MR, Mendelian randomization; IVW, inverse variance weighted.
3 Results
3.1 The impact of dietary consumption habits on psoriasis MR analysis
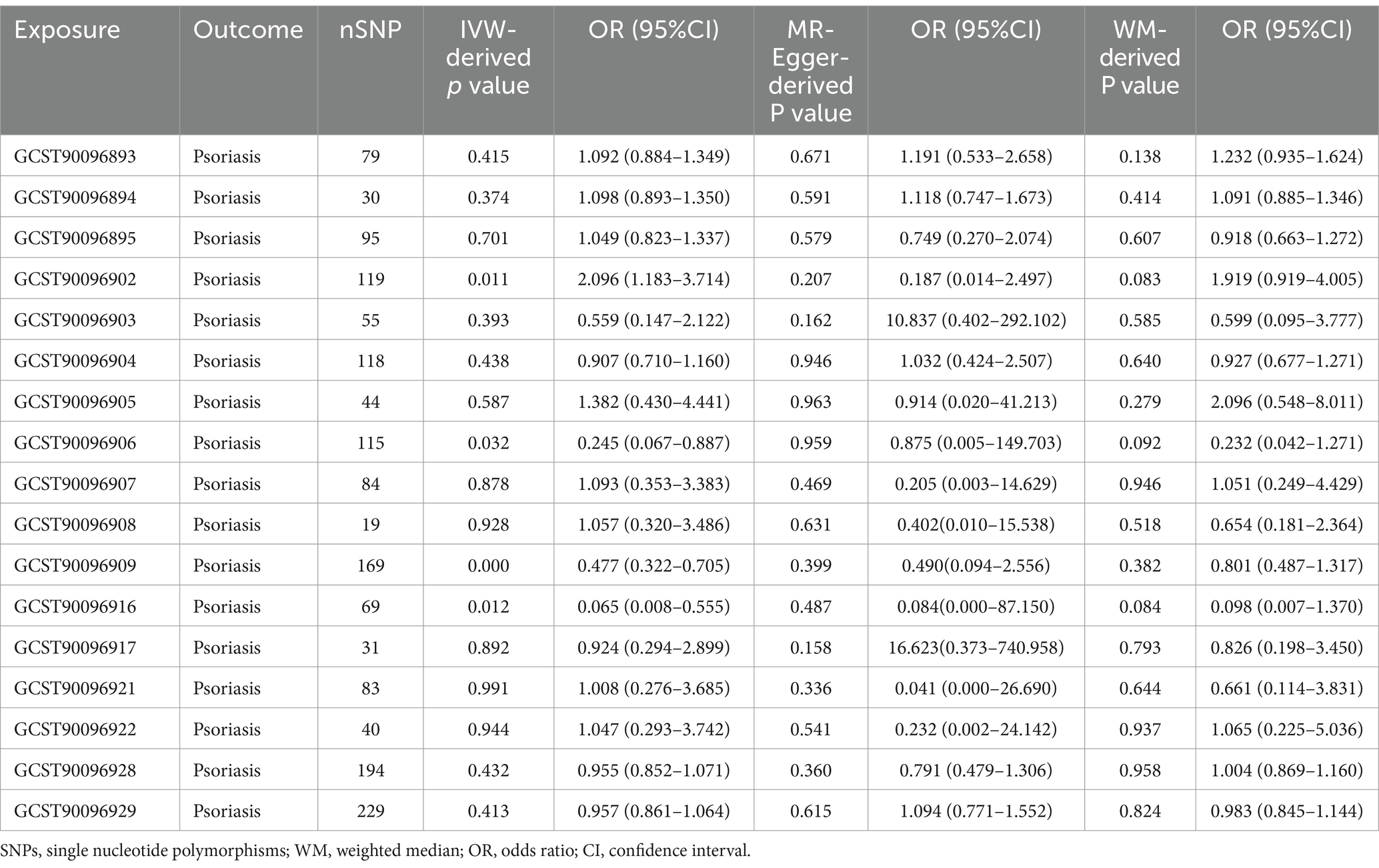
After relevant screening, 12 dietary habits with fewer than 3 SNPs were removed from the dietary consumption habits sample dataset, leaving 17 dietary habits with a larger number of SNPs (Table 2).
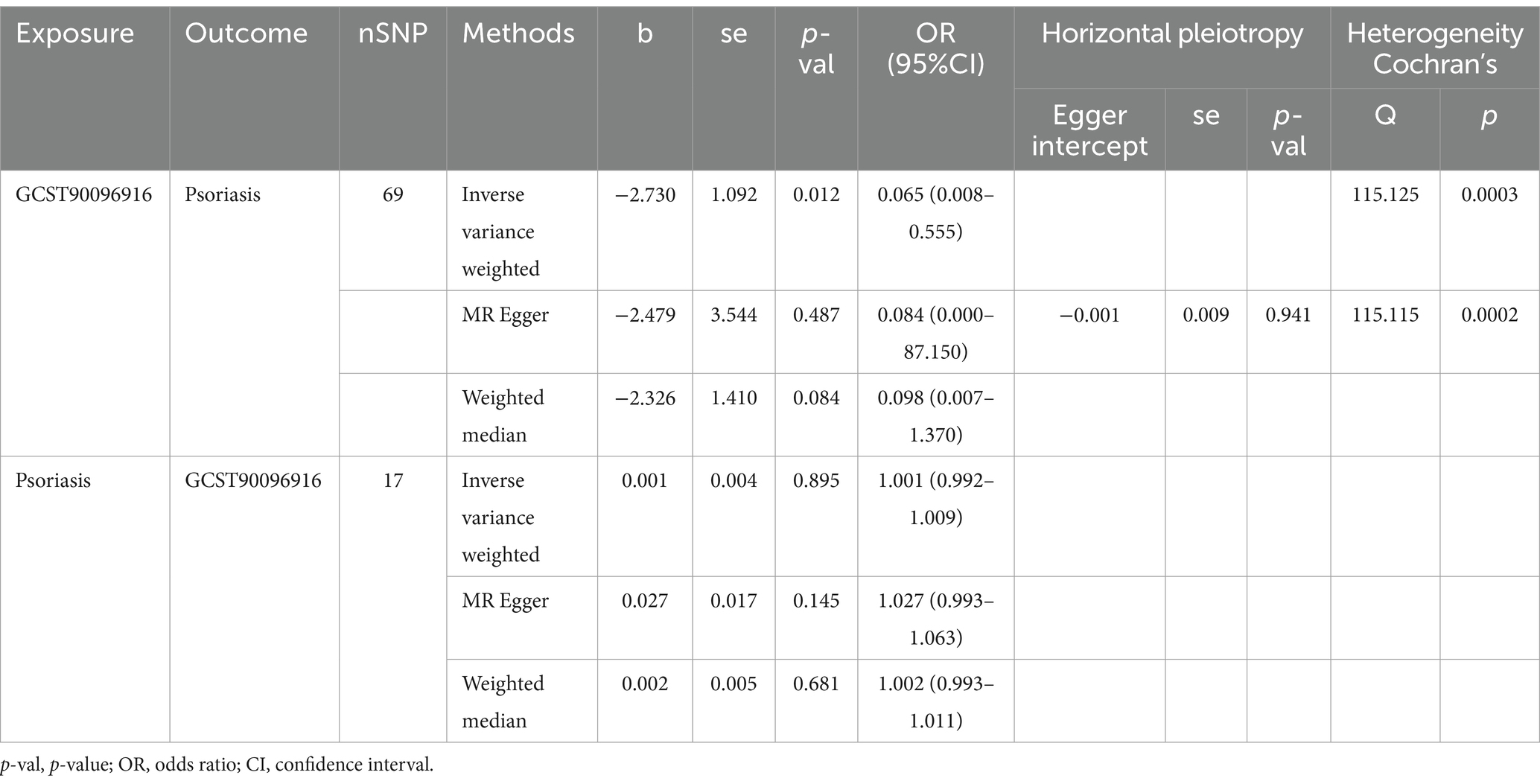
The IVW method analysis showed that the habit of consuming dried fruits (OR: 0.065, 95% CI: 0.008 ~ 0.555, p = 0.012) has a causal effect on psoriasis, indicating a negative correlation. Regular consumption of dried fruits can reduce the risk and severity of psoriasis. However, the p-values for the MR-Egger and WME method analyses were greater than 0.05, thus lacking statistical significance (Table 3). The p-values obtained from Cochran’s Q test for the IVW and MR-Egger methods were 0.0003 and 0.0002, respectively, both less than 0.05, indicating some heterogeneity in the analysis results, but with minor impact on the IVW results. The p-value for the intercept obtained using the MR-Egger method was 0.941, suggesting no horizontal pleiotropy. After sequentially excluding each SNP using the leave-one-out approach, the effect sizes of the remaining SNPs did not significantly differ from the overall effect size, indicating the absence of SNPs with a significant impact on the results, as shown in Figure 3. The forest plot, funnel plot, and scatter plot obtained from the MR analysis are shown in Figures 4–6, respectively.
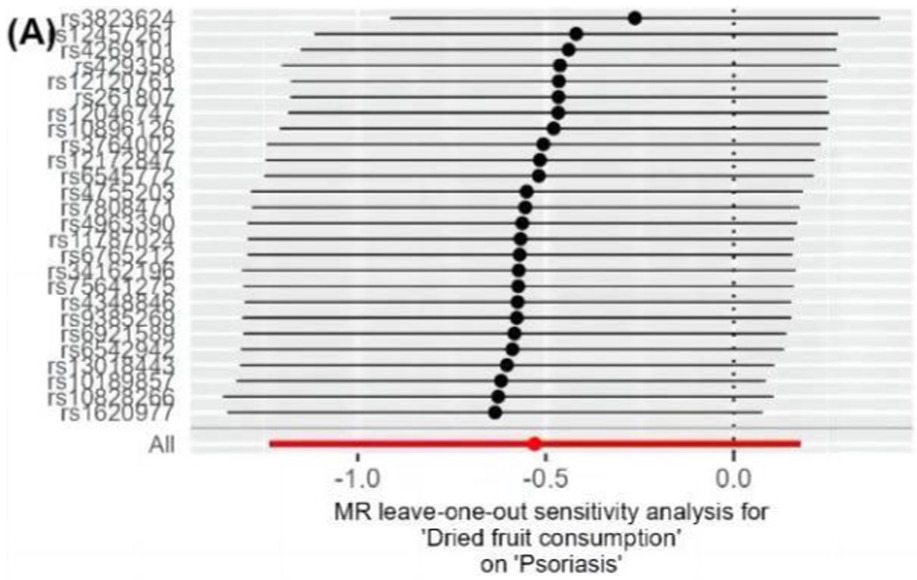
Figure 3. Leave-one-out analysis results of dietary habits on psoriasis. Forest plots of the “leave-one-out” sensitivity analyses to demonstrate the impact of individual SNPs on the results. The x-axis shows MR “leave-one-out” sensitivity analyses for dried fruit intake on psoriasis. The y-axis shows the analyses for the effect of “leave-one-out” of SNPs on psoriasis. The black point on the bottom line of each panel indicates the IVW estimate using all SNPs. MR, Mendelian randomization; SNP, single nucleotide polymorphism; IVW, inverse variance weighted.
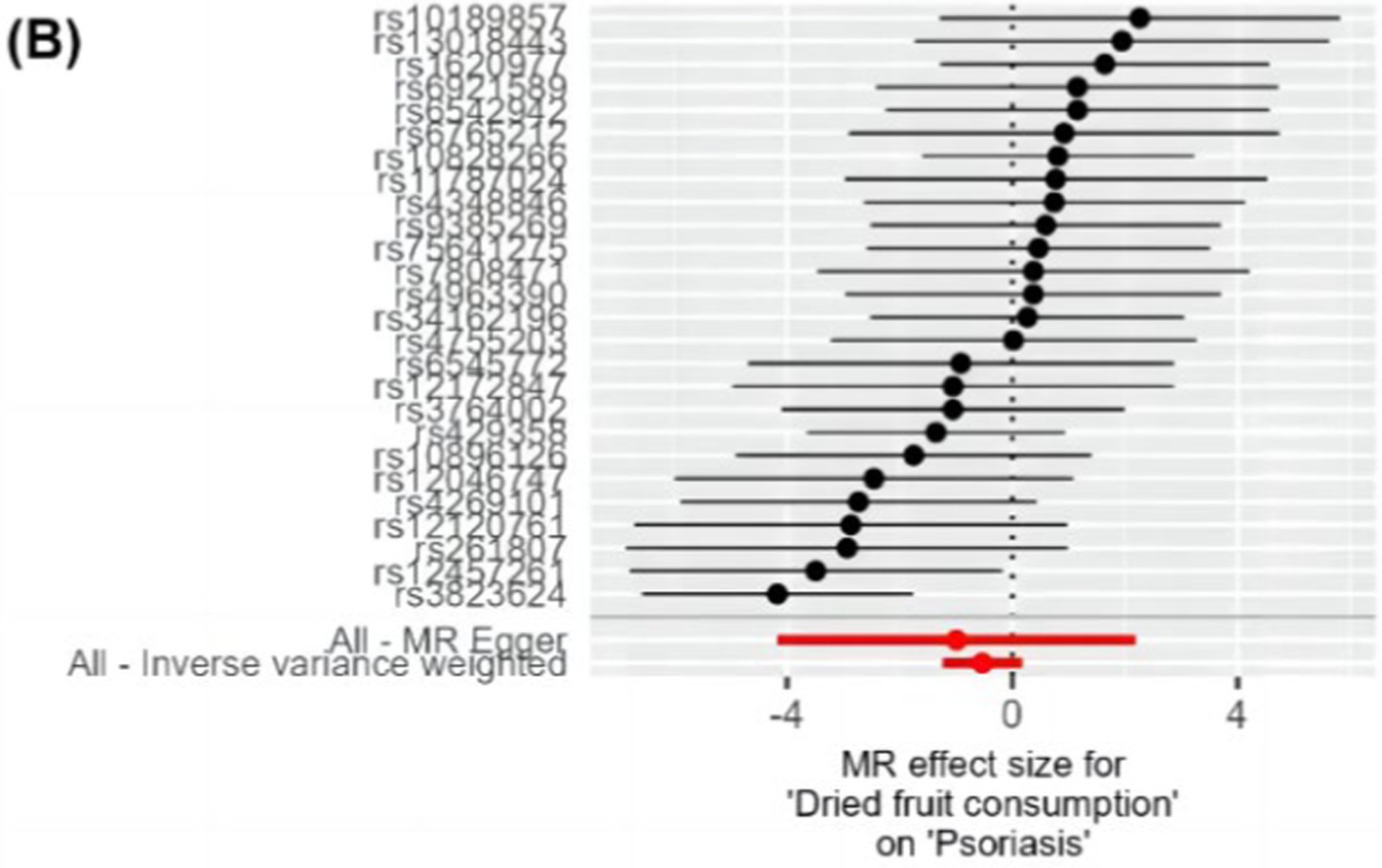
Figure 4. Forest plot of MR analysis of dietary habits on psoriasis. The vertical axis represents SNPs, and the horizontal axis represents effect values. Black dots and black horizontal lines represent the effect values and their fluctuation ranges for each SNP. Red dots and red horizontal lines represent the combined effect values and their fluctuation ranges obtained through MR Egger and IVW methods. Values greater than 0 indicate that the exposure factor is a risk factor, and values less than 0 indicate that the exposure factor is a protective factor. MR, Mendelian randomization; SNP, single nucleotide polymorphism; IVW, inverse variance weighted.

Figure 5. Funnel plot of MR analysis of dietary habits on psoriasis. Black dots represent SNPs. The MR estimate for each SNP is plotted against its minor-allele frequency corrected association with dried fruit intake. The vertical lines show the results of IVW or MR-ER using all SNPs. If the black dots on both sides of the vertical line are roughly symmetrical, it indicates that the results are homogenous. IVW, inverse variance weighted; MR, Mendelian randomization; SNP, single nucleotide polymorphism.
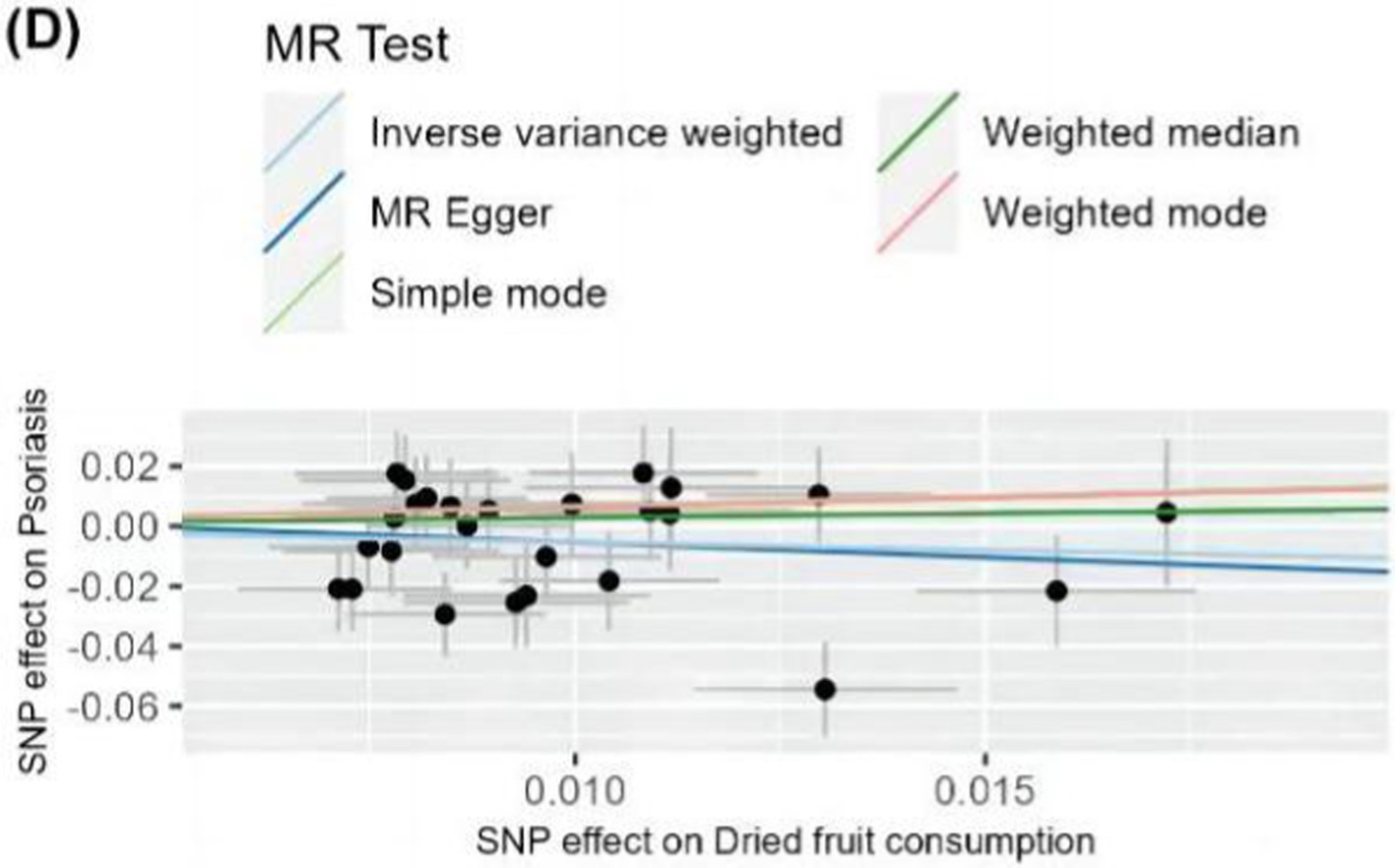
Figure 6. Scatter plot of MR analysis of dietary habits on psoriasis. Scatter plots were used to visualize the causal effect between dried fruit intake and psoriasis using five MR methods. Black dots represent SNPs, and the cross lines indicate the fluctuation range of the effect values for each SNP. The x-axis shows the SNP effect and SE on dietary factors. The y-axis shows the SNP effect and SE on psoriasis. The regression lines for the inverse-variance weighted (IVW) method, the MR-Egger regression method, the weighted median, the weighted mode, and the simple mode are shown. The slope of each straight line indicates the magnitude of the causal association. SNP, single nucleotide polymorphism; SE, standard error.
4 Discussion
Epidemiological surveys indicate that from 1990 to 2019, the global incidence of psoriasis has shown an upward trend with increasing age, with a notable rise anticipated in the next decade among the 30–49 age group (13). Identifying potential risk and protective factors, such as dietary consumption habits, is crucial in preventing the onset and progression of psoriasis against a backdrop of genetic factors. This study utilized a two-sample MR approach to analyze the GWAS database, investigating the relationship between 29 dietary consumption habits and psoriasis. Previous literature has confirmed the correlation between psoriasis and dietary factors. However, to our knowledge, this is the first study to investigate the causal relationship between dietary consumption habits and psoriasis. The results show that among the 29 dietary habits analyzed, the intake of dried fruits has a causal relationship with psoriasis, with increased consumption acting as a protective factor against the disease.
In our study, we observed a causal relationship between increased intake of dried fruits and the alleviation of psoriasis symptoms. We believe this is primarily related to the following three factors:
Firstly, an increase in dried fruit consumption can effectively reduce calorie intake while ensuring sufficient energy. Previous studies have shown that low-calorie diets and intermittent fasting are effective interventions for alleviating psoriasis. These dietary approaches can decrease levels of interleukin-6 (IL-6), vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), and low-density lipoprotein (LDL) in patients with psoriasis, thereby suppressing skin inflammation and abnormal keratinization (14, 15). A secondary analysis of the National Health and Nutrition Examination Survey indicated that dried fruit consumers had significantly lower average body mass index (BMI) (−0.8, 99% CI -1.4 to −0.2; p = 0.002) and waist circumference (−2.6 cm, 99% CI -4.2 to −0.9 cm; p < 0.001) compared to non-consumers (16, 17). With the same caloric content, dried fruits provide more satiety compared to liquids and other fresh fruits and vegetables, leading to a decrease in appetite and a reduction in energy intake, thus preventing an increase in inflammatory mediators such as secreted phosphoprotein 1 (SPP1) and IL-17 due to weight gain (18). Additionally, dried fruits have long been a crucial component of the Mediterranean diet (19). A European observational cross-sectional study demonstrated a significant inverse association between the Mediterranean diet and the risk of psoriasis (OR: 0.34, 95% CI: 0.13–0.92, p = 0.03). Psoriasis patients who adopted a Mediterranean diet showed improvements in both BMI and PASI scores (20, 21).
Secondly, dried fruits are rich in dietary fiber (22), which can modulate the gut microbiota of patients, influencing their lipid and glucose metabolism and immune homeostasis, thereby reducing the severity of psoriasis and delaying its progression (23). Tian et al. found (24) that adding dried goji berries to the diet can increase the abundance of gastrointestinal lactobacilli and bifidobacteria, both of which are major components of probiotic therapy for psoriasis. The gut microbial community, through its metabolites entering the bloodstream and accumulating in the skin, regulates levels of skin inflammation factors, thus affecting the development of psoriasis (25).
Finally, dried fruits are rich in antioxidants and bioactive substances that can mitigate oxidative stress damage. The nutritional content of dried fruits is comparable to that of fresh fruits; however, the drying and concentration process results in an increase in polyphenol content, thereby enhancing the antioxidant activity of dried fruits more than that of fresh fruits (26–28). A prospective study in Romania showed that patients with psoriasis exhibited elevated levels of reactive oxygen species and free radical test levels. After undergoing psoriasis treatment, the oxidative stress levels in patients decreased but still did not return to normal ranges (29), although this conclusion still requires experimental research for support. Additionally, the high content of vitamins A, C, and E in dried fruits can reduce the severity of psoriasis by interacting with free radicals and preventing oxidative damage to macromolecules such as low-density lipoproteins (30).
The strength of this study lies in establishing a causal relationship between the dietary habit of dried fruit consumption and psoriasis. Consuming dried fruits does not replace the intake of other fruits; rather, it increases the total fruit intake (17) due to its satiating effect and minimal impact on metabolism (such as lipids and blood sugar), aligning with the patterns of a healthy diet. Therefore, for patients with psoriasis, increasing the intake of dried fruits could be an effective strategy to simultaneously boost the intake of bioactive substances, fiber, and trace elements, reducing the severity of psoriasis. However, evidence of the underlying mechanisms is limited, and further experimental and clinical studies are needed to validate these conclusions.
This study still has limitations. All research subjects were derived from European samples and do not represent a truly random population sample, thus the generalizability to other ethnic groups remains unclear, and there is a potential for overlap within the sample population. Although various sensitivity analyses were conducted to test the assumptions of the MR study, it is challenging to completely eliminate pleiotropy in the instrumental variables. Additionally, Steiger filtering and MR-PRESSO filtering are also helpful to mitigate horizontal pleiotropy. Moreover, the study’s findings are primarily based on conclusions drawn from the IVW method, with results from MR-Egger and the WME methods not being significant, which still raises concerns about the robustness of the study results. Additional GWAS dataset are expected to perform repeated validation in order to further confirm the causal relationship between dried fruit intake and psoriasis, such as Meta-analysis, or MVMR analyses, in order to examine whether dietary consumption habits impacts psoriasis by working through other factors. Additionally, this study did not find a causal relationship between the consumption of red wine, beer, vodka, and other dietary habits and psoriasis, despite previous research indicating these as risk dietary factors for psoriasis, a more comprehensive catalog database is needed for further research. The number of available items in the dietary questionnaire in the GWAS catalog database is limited, hence only existing effective and specific food groups could be studied as exposure factors, and the focus remains on the genetic influences on food groups or dietary patterns rather than more specific foods.
Overall, this study identifies the consumption of dried fruits as a protective factor against psoriasis, providing a basis for further research to refine and identify genetic markers associated with specific foods, thereby facilitating more precise analyses of the impact of dietary factors on psoriasis.
5 Conclusion
In summary, this study finds that increasing the intake of dried fruits may act as a protective factor against psoriasis. This result could serve as a reference for further exploration of healthy dietary patterns for patients with psoriasis.
Data availability statement
Publicly available datasets were analyzed in this study. This GWAS data for dietary consumption can be found: https://ftp.ebi.ac.uk/pub/databases/gwas/summary_statistics/GCST90096001-GCST90097000/GCST90096892/ and the data for psoriasis analysis can be found: https://r10.finngen.fi/pheno/L12_PSORIASIS.
Author contributions
LM: Data curation, Methodology, Writing – original draft, Writing – review & editing. GC: Writing – original draft, Writing – review & editing. ZR: Supervision, Validation, Writing – review & editing. LJ: Formal analysis, Supervision, Validation, Writing – review & editing. YL: Writing – review & editing. CX: Conceptualization, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Samotij, D, Nedoszytko, B, Bartosińska, J, Batycka-Baran, A, Czajkowski, R, Dobrucki, I, et al. Pathogenesis of psoriasis in the “omic” era. Part I. Epidemiology, clinical manifestation, immunological and neuroendocrine disturbances. Postepy Dermatol Alergol. (2020) 37:135–53. doi: 10.5114/ada.2020.94832
2. Michalek, IM, Loring, B, and John, SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. (2017) 31:205–12. doi: 10.1111/jdv.13854
3. Kulkarni, V, Liu, D, Gamsarian, V, Grewal, T, and Sivesind, T. Risk factors associated with burden of disease of psoriasis from 1990 to 2019: epidemiological analysis. JMIR Dermatol. (2024) 7:e48749. doi: 10.2196/48749
4. Hileman, CO, Malakooti, SK, Patil, N, Singer, NG, and McComsey, GA. New-onset autoimmune disease after COVID-19. Front Immunol. (2024) 15:1337406. doi: 10.3389/fimmu.2024.1337406
5. Liu, S, He, M, Jiang, J, Duan, X, Chai, B, Zhang, J, et al. Triggers for the onset and recurrence of psoriasis: a review and update. Cell Commun Signal. (2024) 22:108. doi: 10.1186/s12964-023-01381-0
6. Zuccotti, E, Oliveri, M, Girometta, C, Ratto, D, di Iorio, C, Occhinegro, A, et al. Nutritional strategies for psoriasis: current scientific evidence in clinical trials. Eur Rev Med Pharmacol Sci. (2018) 22:8537–51. doi: 10.26355/eurrev_201812_16554
7. Ingkapairoj, K, Chularojanamontri, L, Chaiyabutr, C, Silpa-archa, N, Wongpraparut, C, and Bunyaratavej, S. Dietary habits and perceptions of psoriatic patients: Mediterranean versus Asian diets. J Dermatolog Treat. (2022) 33:2290–6. doi: 10.1080/09546634.2021.1959500
8. Burgess, S, Butterworth, A, and Thompson, SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. (2013) 37:658–65. doi: 10.1002/gepi.21758
9. Bowden, J, and Holmes, MV. Meta-analysis and Mendelian randomization: a review. Res Synth Methods. (2019) 10:486–96. doi: 10.1002/jrsm.1346
10. Musumeci, ML, Nasca, MR, Boscaglia, S, and Micali, G. The role of lifestyle and nutrition in psoriasis: current status of knowledge and interventions. Dermatol Ther. (2022) 35:e15685. doi: 10.1111/dth.15685
11. Pirastu, N, McDonnell, C, Grzeszkowiak, EJ, Mounier, N, Imamura, F, Merino, J, et al. Using genetic variation to disentangle the complex relationship between food intake and health outcomes. PLoS Genet. (2022) 18:e1010162. doi: 10.1371/journal.pgen.1010162
12. Kurki, MI, Karjalainen, J, Palta, P, Sipilä, TP, Kristiansson, K, Donner, KM, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. (2023) 613:508–18. doi: 10.1038/s41586-022-05473-8
13. Zhang, Y, Dong, S, Ma, Y, and Mou, Y. Burden of psoriasis in young adults worldwide from the global burden of disease study 2019. Front Endocrinol. (2024) 15:1308822. doi: 10.3389/fendo.2024.1308822
14. Katsimbri, P, Korakas, E, Kountouri, A, Ikonomidis, I, Tsougos, E, Vlachos, D, et al. The effect of antioxidant and anti-inflammatory capacity of diet on psoriasis and psoriatic arthritis phenotype: nutrition as therapeutic tool? Antioxidants (Basel, Switzerland). (2021) 10:157. doi: 10.3390/antiox10020157
15. Su, R, Zhao, S, Zhang, J, Cao, M, and Peng, S. Metabolic influences on T cell in psoriasis: a literature review. Front Immunol. (2023) 14:1279846. doi: 10.3389/fimmu.2023.1279846
16. Widanagamage, RD, Ekanayake, S, and Welihinda, J. Carbohydrate-rich foods: glycaemic indices and the effect of constituent macronutrients. Int J Food Sci Nutr. (2009) 60:215–23. doi: 10.1080/09637480902849195
17. Sullivan, VK, Na, M, Proctor, DN, Kris-Etherton, PM, and Petersen, KS. Consumption of dried fruits is associated with greater intakes of Underconsumed nutrients, higher Total energy intakes, and better diet quality in US adults: a cross-sectional analysis of the National Health and nutrition examination survey, 2007-2016. J Acad Nutr Diet. (2021) 121:1258–72. doi: 10.1016/j.jand.2020.08.085
18. Gao, C, and Li, J. Exploring the comorbidity mechanisms between psoriasis and obesity based on bioinformatics. Skin Res Technol. (2024) 30:e13575. doi: 10.1111/srt.13575
19. Mossine, VV, Mawhinney, TP, and Giovannucci, EL. Dried fruit intake and Cancer: a systematic review of observational studies. Adv Nutr. (2020) 11:237–50. doi: 10.1093/advances/nmz085
20. Castaldo, G, Rastrelli, L, Galdo, G, Molettieri, P, Rotondi Aufiero, F, and Cereda, E. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: A proof-of-concept, single-arm, open-label clinical trial. Nutrition. (2020) 74:110757. doi: 10.1016/j.nut.2020.110757
21. Saalbach, A, Seitz, AT, Kohlmann, J, Kalweit, L, Vogt, L, Selig, L, et al. Modulation of dietary fatty acids in an open-label study improves psoriasis and dampens the inflammatory activation status. Nutrients. (2023) 15:1698. doi: 10.3390/nu15071698
22. McRae, MP. Dietary Fiber is beneficial for the prevention of cardiovascular disease: An umbrella review of Meta-analyses. J Chiropr Med. (2017) 16:289–99. doi: 10.1016/j.jcm.2017.05.005
23. D’Unienville, NMA, Hill, AM, Coates, AM, Yandell, C, Nelson, MJ, and Buckley, JD. Effects of almond, dried grape and dried cranberry consumption on endurance exercise performance, recovery and psychomotor speed: protocol of a randomised controlled trial. BMJ Open Sport Exerc Med. (2019) 5:e000560. doi: 10.1136/bmjsem-2019-000560
24. Tian, B, Zhang, Z, Zhao, J, Ma, Q, Liu, H, Nie, C, et al. Dietary whole goji berry (Lycium barbarum) intake improves colonic barrier function by altering gut microbiota composition in mice. Int J Food Sci Technol. (2021) 56:103–14. doi: 10.1111/ijfs.14606
25. Salem, I, Ramser, A, Isham, N, and Ghannoum, MA. The gut microbiome as a major regulator of the gut-skin Axis. Front Microbiol. (2018) 9:1459. doi: 10.3389/fmicb.2018.01459
26. Vinson, JA, Zubik, L, Bose, P, Samman, N, and Proch, J. Dried fruits: excellent in vitro and in vivo antioxidants. J Am Coll Nutr. (2005) 24:44–50. doi: 10.1080/07315724.2005.10719442
27. Rababah, TM, Ereifej, KI, and Howard, L. Effect of ascorbic acid and dehydration on concentrations of total phenolics, antioxidant capacity, anthocyanins, and color in fruits. J Agric Food Chem. (2005) 53:4444–7. doi: 10.1021/jf0502810
28. Ishiwata, K, Yamaguchi, T, Takamura, H, and Matoba, T. DPPH radical-scavenging activity and polyphenol content in dried fruits. Food Sci Technol Res. (2004) 10:152–6. doi: 10.3136/fstr.10.152
29. Cozma, EC, Găman, MA, Orzan, O, Hamed, KV, Voiculescu, VM, and Găman, AM. Oxidative stress and inflammation levels in a population of eastern European Naïve versus treated psoriasis patients. Cureus. (2023) 15:e48177. doi: 10.7759/cureus.48177
Keywords: psoriasis, dietary consumption habits, Mendelian randomization, dry fruit, GWAS
Citation: Minghui L, Changyong G, Runtian Z, Jianhong L, Lingling Y and Xi C (2024) The association between dietary consumption habits and psoriasis: a two-sample Mendelian randomization study. Front. Nutr. 11:1405663. doi: 10.3389/fnut.2024.1405663
Edited by:
Giuseppe Murdaca, University of Genoa, ItalyReviewed by:
Emoke Pall, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, RomaniaBimal Prasanna Mohanty, Indian Council of Agricultural Research, India
Copyright © 2024 Minghui, Changyong, Runtian, Jianhong, Lingling and Xi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Runtian Zhang, ZGVybWFAcXEuY29t
†These authors have contributed equally to this work
 Lu Minghui
Lu Minghui Gao Changyong
Gao Changyong Zhang Runtian1*
Zhang Runtian1*